Experimental Study on Carbon Dioxide Flooding Technology in the Lunnan Oilfield, Tarim Basin
Abstract
1. Introduction
2. Research Methods
2.1. Geological Characteristics
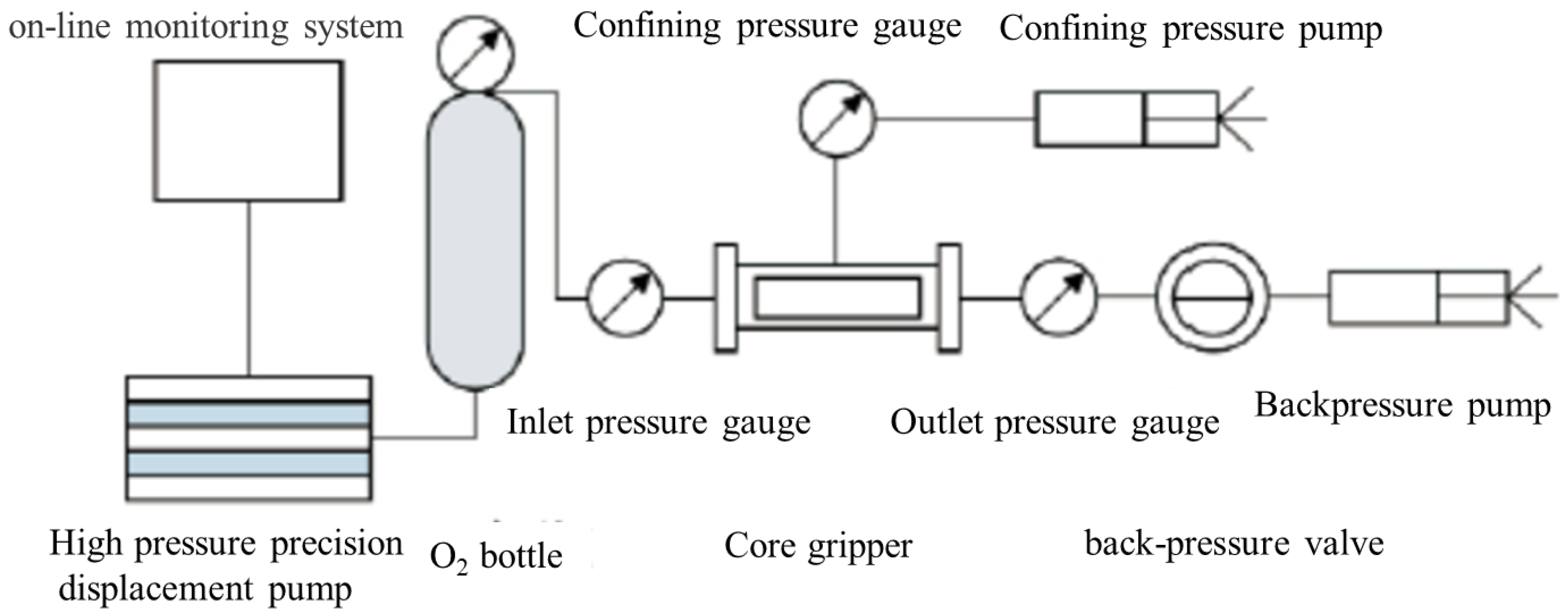
2.2. Experimental Methods
- (1)
- In the experiment, the temperature was set to 128 °C, the pressure was set to 45.4 MPa, pure carbon dioxide was used as the injection gas, and on-site formation water was used as the water sample. By transferring an appropriate amount of mixed oil samples into a high-temperature and high-pressure sampler and adding excess carbon dioxide, the temperature is kept constant at the formation temperature, the pressure is kept at the bubble point pressure, and the oil is stabilized after sufficient stirring [18]. The excess carbon dioxide from the upper part of the sampler is discharged under constant pressure. The fluid in the sampler is the formation of a crude oil sample, which is used to carry out experiments.
- (2)
- During the experiment, the pressure and temperature data in the reaction kettle were continuously collected and recorded through a data collector. At the same time, the flow rate of carbon dioxide gas and the time of chemical reaction were recorded. Based on the data collected from the experiment, physical parameters such as density and viscosity of carbon dioxide gas are calculated.
- (3)
- After extracting and drying the core, measure the gas permeability of the core, sort them in order according to the permeability, connect the core model, and measure the porosity of the saturated formation water in the core model. Oil is injected into the water-saturated core to drive water with oil until there is no water flow at the outlet of the core, and the irreducible water saturation of the core model is calculated.
- (4)
- Connect the cylinder to the container to ensure that the gas enters the container smoothly. Set a pressure sensor and a temperature sensor on the cylinder, connect it to the back pressure valve at the outlet end of the core, and add a predetermined pressure; adjust a certain displacement pressure, and carbon dioxide gas is injected into the core to conduct carbon dioxide gas flooding tight oil experiments until no more crude oil is produced at the outlet end, thereby measuring the cumulative volume of displaced oil and gas and the cumulative volume of injected gas.
- (5)
- Pump the formation oil into the thin tube core until no water comes out from the outlet end, and calculate the irreducible water saturation and original oil saturation in the thin tube model; maintain the back pressure during the experiment, determine the displacement pressure, conduct the displacement experiment under a certain displacement pressure, and record the pump volume and oil production parameters.
- (6)
- By changing the displacement pressure and repeating the experiment, the gas drive recovery rate under different displacement pressures is measured. The minimum pressure corresponding to the gas drive recovery rate of 90–95% is recorded as the minimum miscible pressure [19].
- (7)
- Place the core sample in the permeability measuring device to ensure that the sample can seep smoothly. A pressure sensor and a temperature sensor are provided on the permeability measurement device to monitor pressure and temperature changes during the seepage process.
- (8)
- In the experiment, the permeability was set to 0.1 mD~1000 mD, the porosity was 5~30%, and the pressure gradient was set to 0.1 MPa/m~10 MPa/m. Oil and gas were injected from the oil and gas source into the core sample, and the pressure sensors and temperature sensors monitor the pressure and temperature of oil and gas, and the flow meter monitors the seepage rate of oil and gas. At the same time, data such as the permeability, pressure, and temperature of oil and gas in core samples are continuously collected, and the injection time and amount of oil and gas are recorded through a data collector.
- (9)
- Put the prepared solution into a constant temperature bath, heat it to the set temperature, and stir the solution with a magnetic stirrer to ensure uniformity of the solution; during the dissolution process, monitor the solution in real-time with a pH meter and conductivity meter and monitor the concentration changes of dissolved substances in the solution through spectrophotometer and other instruments, and continuously collect and record the concentration, pH value, conductivity, dissolution time and amount of dissolved substances in the solution (See Figure 2).
3. Results
3.1. High-Temperature and High-Pressure Physical Properties of Carbon Dioxide
3.2. Gas Injection Expansion Characteristics
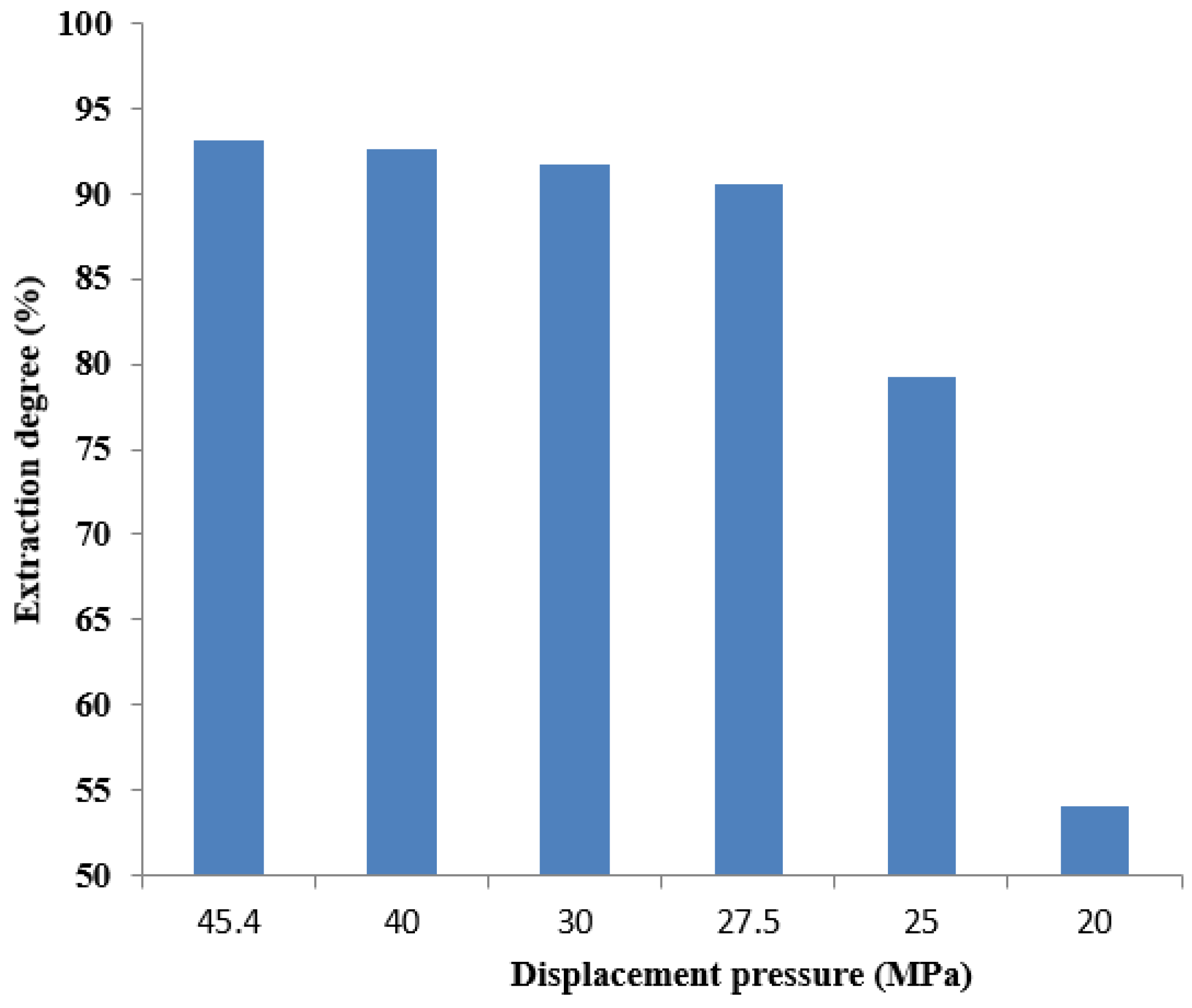
3.3. Minimum Miscibility Pressure
3.4. Long Core Displacement Characteristics
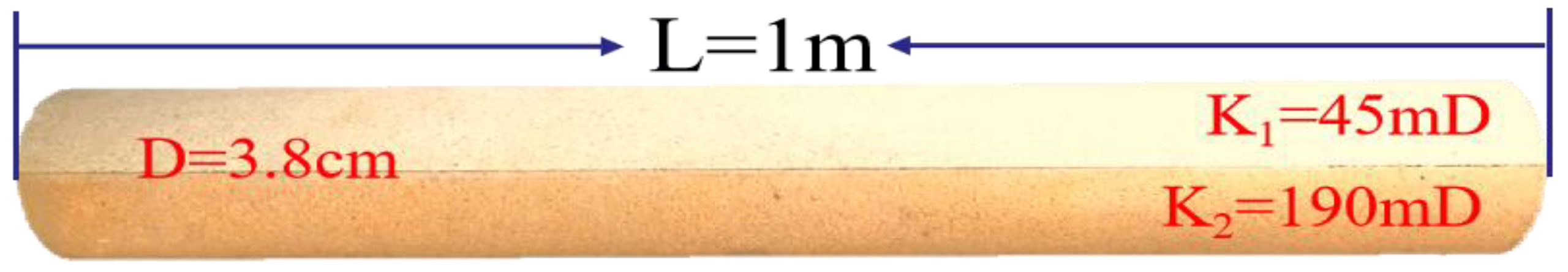
3.5. Oil and Gas Phase Permeability Characteristics
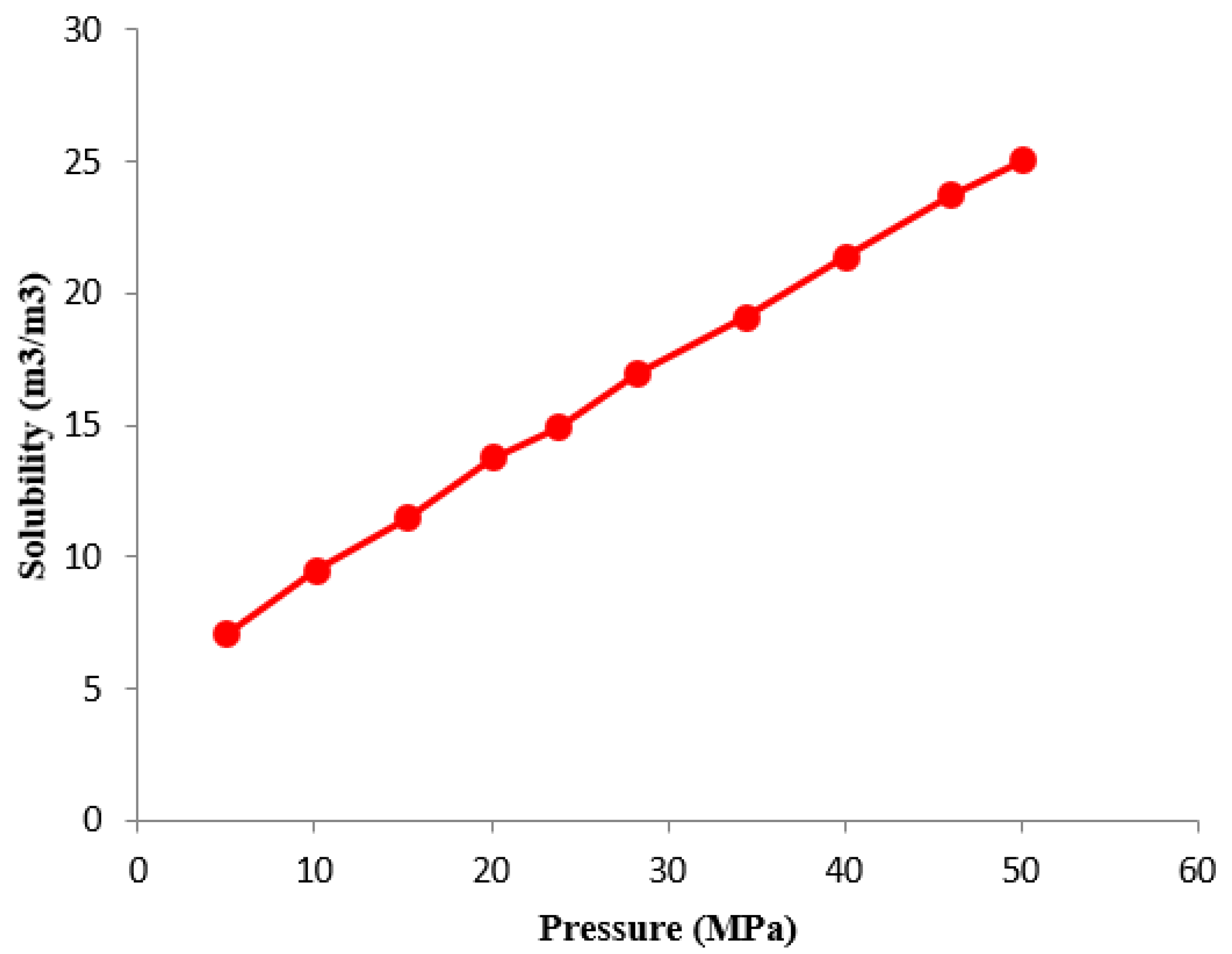
3.6. Water Solubility Characteristics
4. Discussion
5. Conclusions
- (1)
- The crude oil from the Lunnan Oilfield has low viscosity, low solidification point, low medium sulfur content, high wax content, and medium colloidal asphaltene. The carbon dioxide density measured under oil group conditions is 0.74 g/cm3, which is similar to the density of crude oil. But its viscosity is 0.0681 mPa·s, which has good applicability for carbon dioxide flooding.
- (2)
- As the amount of carbon dioxide injected increases, the saturation pressure of crude oil increases from 12.06 MPa to 39.68 MPa. The gas–oil ratio has increased from 42.52 m3/m3 to 594.68 m3/m3. The gas–oil ratio, expansion coefficient, and saturation pressure gradually increase. As the displacement pressure continues to decrease, the carbon dioxide displacement efficiency begins to slowly decrease and then rapidly decline. As the injection rate of carbon dioxide pore volume increases, the growth rate first increases and then slows down, reaching a turning point at 0.8 HCPV., The oil–gas ratio also exhibits a turning point occurring at 0.6 HCPV. The solubility of carbon dioxide in the formation of water is determined to be 24 m3/m3. Beneficial for subsequent oil recovery and carbon storage.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Obaidi, D.A.; Al-Mudhafar, W.J.; Al-Jawad, M.S. Experimental evaluation of Carbon Dioxide-Assisted Gravity Drainage process (CO2-AGD) to improve oil recovery in reservoirs with strong water drive. Fuel 2022, 324, 124409. [Google Scholar] [CrossRef]
- Guerra, A.; McElligott, A.; Du, C.Y.; Marić, M.; Rey, A.D.; Servio, P. Dynamic viscosity of methane and carbon dioxide hydrate systems from pure water at high-pressure driving forces. Chem. Eng. Sci. 2022, 252, 117282. [Google Scholar] [CrossRef]
- Liu, M.; Yang, X.; Wen, J.; Wang, H.; Feng, Y.; Lu, J.; Wang, J. Drivers of China's carbon dioxide emissions: Based on the combination model of structural decomposition analysis and input-output subsystem method. Environ. Impact Assess. Rev. 2023, 100, 107043. [Google Scholar] [CrossRef]
- Kalam, S.; Olayiwola, T.; Al-Rubaii, M.M.; Amaechi, B.I.; Jamal, M.S.; Awotunde, A.A. Carbon dioxide sequestration in underground formations: Review of experimental, modeling, and field studies. J. Pet. Explor. Prod. 2021, 11, 303–325. [Google Scholar] [CrossRef]
- Lv, Z.; Qiao, K.; Chu, F.; Yang, L.; Du, X. Experimental study of divalent metal ion effects on ammonia escape and carbon dioxide desorption in regeneration process of ammonia decarbonization. Chem. Eng. J. 2022, 435, 134841. [Google Scholar] [CrossRef]
- Sun, X.; Cai, J.; Li, X.; Zheng, W.; Wang, T.; Zhang, Y. Experimental investigation of a novel method for heavy oil recovery using supercritical multithermal fluid flooding. Appl. Therm. Eng. 2021, 185, 116330. [Google Scholar] [CrossRef]
- Chuah, L.F.; Bokhari, A.; Asif, S.; Klemeš, J.J.; Dailin, D.J.; El Enshasy, H.; Yusof, A.H.M. A review of performance and emission characteristic of engine diesel fuelled by biodiesel. Chem. Eng. Trans. 2022, 94, 1099–1104. [Google Scholar]
- Varfolomeev, M.A.; Yuan, C.; Bolotov, A.V.; Minkhanov, I.F.; Mehrabi-Kalajahi, S.; Saifullin, E.R.; Shaihutdinov, D.K. Effect of copper stearate as catalysts on the performance of in-situ combustion process for heavy oil recovery and upgrading. J. Pet. Sci. Eng. 2021, 207, 109125. [Google Scholar] [CrossRef]
- Pal, N.; Zhang, X.; Ali, M.; Mandal, A.; Hoteit, H. Carbon dioxide thickening: A review of technological aspects, advances and challenges for oilfield application. Fuel 2022, 315, 122947. [Google Scholar] [CrossRef]
- Ansari, K.B.; Gaikar, V.G.; Trinh, Q.T.; Khan, M.S.; Banerjee, A.; Kanchan, D.R.; Danish, M. Carbon dioxide capture over amine functionalized styrene divinylbenzene copolymer: An experimental batch and continuous studies. J. Environ. Chem. Eng. 2022, 10, 106910. [Google Scholar] [CrossRef]
- Novak Mavar, K.; Gaurina-Međimurec, N.; Hrnčević, L. Significance of enhanced oil recovery in carbon dioxide emission reduction. Sustainability 2021, 13, 1800. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, J.; Li, B.; Zhu, C. Crack propagation patterns and factors controlling complex crack network formation in coal bodies during tri-axial supercritical carbon dioxide fracturing. Fuel 2021, 286, 119381. [Google Scholar] [CrossRef]
- Zhou, M.; Feng, J.; Jiang, T.; Liu, J. Preliminary study on tertiary oil recovery of high temperature and high salinity reservoirs in Tarim Oilfield. Xinjiang Pet. Geol. 2010, 2, 59–62. [Google Scholar]
- Wen, B.; Shi, Z.; Jessen, K.; Hesse, M.A.; Tsotsis, T.T. Convective carbon dioxide dissolution in a closed porous medium at high-pressure real-gas conditions. Adv. Water Resour. 2021, 154, 103950. [Google Scholar] [CrossRef]
- Bagheri, H.; Hashemipour, H.; Rahimpour, E.; Rahimpour, M.R. Particle size design of acetaminophen using supercritical carbon dioxide to improve drug delivery: Experimental and modeling. J. Environ. Chem. Eng. 2021, 9, 106384. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, Y.; Zhang, L.; Chu, G.; Zou, H.; Sun, B.; Zeng, X. Carbon dioxide capture by non-aqueous blend in rotating packed bed reactor: Absorption and desorption investigation. Sep. Purif. Technol. 2021, 269, 118714. [Google Scholar] [CrossRef]
- Ringrose, P.S.; Furre, A.K.; Gilfillan, S.M.; Krevor, S.; Landrø, M.; Leslie, R.; Zahid, A. Storage of carbon dioxide in saline aquifers: Physicochemical processes, key constraints, and scale-up potential. Annu. Rev. Chem. Biomol. Eng. 2021, 12, 471–494. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Lian, Y.; Dong, X.; Liu, J.; Liu, Y.; Wu, Z. Rational planning strategies of urban structure, metro, and car use for reducing transport carbon dioxide emissions in developing cities. Environ. Dev. Sustain. 2023, 25, 6987–7010. [Google Scholar] [CrossRef]
- Mao, F.; Li, Z.; Zhang, K. A comparison of carbon dioxide emissions between battery electric buses and conventional diesel buses. Sustainability 2021, 13, 5170. [Google Scholar] [CrossRef]
- Vaz, S., Jr.; de Souza, A.P.R.; Baeta, B.E.L. Technologies for carbon dioxide capture: A review applied to energy sectors. Clean. Eng. Technol. 2022, 8, 100456. [Google Scholar] [CrossRef]
- Lyu, Q.; Tan, J.; Li, L.; Ju, Y.; Busch, A.; Wood, D.A.; Hu, R. The role of supercritical carbon dioxide for recovery of shale gas and sequestration in gas shale reservoirs. Energy Environ. Sci. 2021, 14, 4203–4227. [Google Scholar] [CrossRef]
- Jia, W.; Jia, X.; Wu, L.; Guo, Y.; Yang, T.; Wang, E.; Xiao, P. Research on regional differences of the impact of clean energy development on carbon dioxide emission and economic growth. Humanit. Soc. Sci. Commun. 2022, 9, 25. [Google Scholar] [CrossRef]
- Voumik, L.C.; Ridwan, M.; Rahman, M.H.; Raihan, A. An investigation into the primary causes of carbon dioxide releases in Kenya: Does renewable energy matter to reduce carbon emission? Renew. Energy Focus 2023, 47, 100491. [Google Scholar] [CrossRef]
- Motie, M.; Bemani, A.; Soltanmohammadi, R. On The Estimation of Phase Behavior Of CO2-Based Binary Systems Using ANFIS Optimized By GA Algorithm. In Fifth CO2 Geological Storage Workshop; European Association of Geoscientists & Engineers: Utrecht, The Netherlands, 2018; Volume 2018, pp. 1–5. [Google Scholar]
- Mahjour, S.K.; Faroughi, S.A. Risks and uncertainties in carbon capture, transport, and storage projects: A comprehensive review. Gas Sci. Eng. 2023, 119, 205117. [Google Scholar] [CrossRef]
- Mahjour, S.K.; Faroughi, S.A. Selecting representative geological realizations to model subsurface CO2 storage under uncertainty. Int. J. Greenh. Gas Control. 2023, 127, 103920. [Google Scholar] [CrossRef]


| Crude Oil | Natural Gas | Formation Water | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ground Density (g/cm3) | Initial Boiling Point (°C) | Viscosity 50 °C(mPa.s) | Wax Content (%) | Sulfur Content (%) | Freezing Point (°C) | Colloidal Asphaltene (%) | Proportion (g/cm3) | Methane Content (%) | Total Mineralization (105 mg/L) | Chloride Ion Content (105 mg/L) |
| 0.8632 | 79.77 | 13.41 | 7.0 | 0.53 | −14.1 | 13.69 | 0.6919 | 78.23 | 1.9 | 1.1 |
| Core Number | Length (cm) | Diameter (cm) | Porosity (%) |
|---|---|---|---|
| 1 | 5.06 | 2.48 | 19.38 |
| 2 | 5.73 | 2.51 | 14.51 |
| 3 | 5.05 | 2.52 | 16.65 |
| 4 | 4.83 | 2.47 | 17.80 |
| 5 | 5.29 | 2.53 | 15.60 |
| 6 | 7.08 | 2.51 | 16.52 |
| 7 | 7.25 | 2.49 | 17.23 |
| Gas/Oil Ratio (m3/m3) | Coefficient of Expansion | Volume Coefficient | Bubble Point Pressure (MPa) | Crude Oil Density (g/cm3) | Crude Oil Viscosity under Formation Pressure (mPa·s) |
|---|---|---|---|---|---|
| 42.52 | 1 | 1.14 | 12.06 | 0.79 | 3.6 |
| Component Name | Well Flow Molar Composition (%) | Component Name | Well Flow Molar Composition (%) |
|---|---|---|---|
| N2 | 1.15 | nC4 | 1.73 |
| carbon dioxide | 0.88 | iC5 | 1.34 |
| C1 | 29.89 | nC5 | 1.36 |
| C2 | 2.15 | C6 | 4.40 |
| C3 | 1.33 | C7+ | 55.11 |
| iC4 | 0.64 | C7+ molecular weight | 362.20 |
| Injected Gas Mole Fraction (Decimal) | Gas/Oil Ratio (m3/m3) | Coefficient of Expansion | Volume Coefficient | Saturation Pressure (MPa) | Surface Degassed Crude Oil Density (g/cm3) | Viscosity under Formation Pressure (mPa·s) |
|---|---|---|---|---|---|---|
| 0 | 42.52 | 1 | 1.14 | 12.06 | 0.79 | 3.62 |
| 0.39 | 126.63 | 1.1 | 1.34 | 19.72 | 0.81 | 1.98 |
| 0.56 | 206.56 | 1.2 | 1.52 | 25.50 | 0.82 | 1.48 |
| 0.67 | 316.56 | 1.3 | 1.76 | 30.65 | 0.82 | 1.27 |
| 0.82 | 594.68 | 1.4 | 2.37 | 39.68 | 0.82 | 1.05 |
| Components Name | Molecular Weight (g/mol) | Equation Coefficient | Equation Coefficient | Critical Temperature (K) | Critical Pressure (b) | Critical Volume | Critical Z Factor | Volume Offset Coefficient | Eccentric Factor |
|---|---|---|---|---|---|---|---|---|---|
| N2 | 28.01 | 0.45 | 0.078 | 126.20 | 33.50 | 90.00 | 0.29 | −0.13 | 0.04 |
| CO2 | 44.01 | 0.45 | 0.078 | 320.08 | 102.96 | 94.00 | 0.36 | 0 | 0.34 |
| C1 | 16.04 | 0.45 | 0.078 | 277.00 | 212.70 | 98.00 | 0.91 | −0.022 | 0.0055 |
| C2~C5 | 53.34 | 0.45 | 0.078 | 397.86 | 39.43 | 237.45 | 0.28 | −0.065 | 0.18 |
| C6~C10 | 110.76 | 0.45 | 0.078 | 578.48 | 35.15 | 447.39 | 0.33 | 0.0 | 0.32 |
| C11+ | 180.03 | 0.45 | 0.078 | 666.99 | 23.52 | 728.18 | 0.31 | 0.0001 | 0.58 |
| C17+ | 365.77 | 0.45 | 0.078 | 859.91 | 12.43 | 1458.12 | 0.25 | 0.0002 | 1.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Feng, Q.; Tang, Y.; Zhou, D.; Lian, L. Experimental Study on Carbon Dioxide Flooding Technology in the Lunnan Oilfield, Tarim Basin. Energies 2024, 17, 386. https://doi.org/10.3390/en17020386
Wu Z, Feng Q, Tang Y, Zhou D, Lian L. Experimental Study on Carbon Dioxide Flooding Technology in the Lunnan Oilfield, Tarim Basin. Energies. 2024; 17(2):386. https://doi.org/10.3390/en17020386
Chicago/Turabian StyleWu, Zangyuan, Qihong Feng, Yongliang Tang, Daiyu Zhou, and Liming Lian. 2024. "Experimental Study on Carbon Dioxide Flooding Technology in the Lunnan Oilfield, Tarim Basin" Energies 17, no. 2: 386. https://doi.org/10.3390/en17020386
APA StyleWu, Z., Feng, Q., Tang, Y., Zhou, D., & Lian, L. (2024). Experimental Study on Carbon Dioxide Flooding Technology in the Lunnan Oilfield, Tarim Basin. Energies, 17(2), 386. https://doi.org/10.3390/en17020386





