Comparison of Gravimetric Determination of Methane Sorption Capacities of Coals for Using Their Results in Assessing Outbursts in Mines
Abstract
1. Introduction
2. Premises for Interlaboratory Comparison
3. Materials and Methods
3.1. Characteristics of Gas Sorption Analyzers and Methodology for Determining Sorption Properties
3.2. Coal Samples Preparation and Experiment Setup
3.3. Application of Sorption–Desorption Method
4. Results and Discussion
- differences in masses of tested samples in both laboratories;
- differences in pretreatment procedure of coal samples in XEMIS analyzers;
- inaccuracies in reading a set sorption filling when determining diffusion coefficient;
- in the case of sorption isotherms, the differences may also be related to a number of determinations of adsorbed gas for isotherms determined using the Langmuir model.
- Mass and grain size distribution of coal samples should be uniform.
- Temperature of the pretreatment stage should be determined. Temperature should increase as the stage’s assumed duration shortens.
- For mining practice, the sorption isotherm should be determined for virgin rock temperature of coal on site.
- Number of methane sorption capacity points within the range of analyzed pressures should be determined. However, it is recommended to exclude the sorption capacity for five values of saturation pressure, of which the first three points should correspond to pressure values of 1, 3, and 5 bar, respectively.
- Maximum time for saturating the sample with methane should be established.
5. Conclusions
- Despite the differences in configuration between XEMIS-001 and XEMIS-100 analyzers and procedures in methodology, the methane sorption results obtained on the six coal samples tested in CLP-B and AGH laboratories are very similar. Apart from one case, the deviation from the average value of sorption capacity () did not exceed 10%. In the case of effective diffusion coefficient (), the deviation from the average value for the first three samples does not exceed 10%, and for the following three samples, it remained within the range of 10–20%.
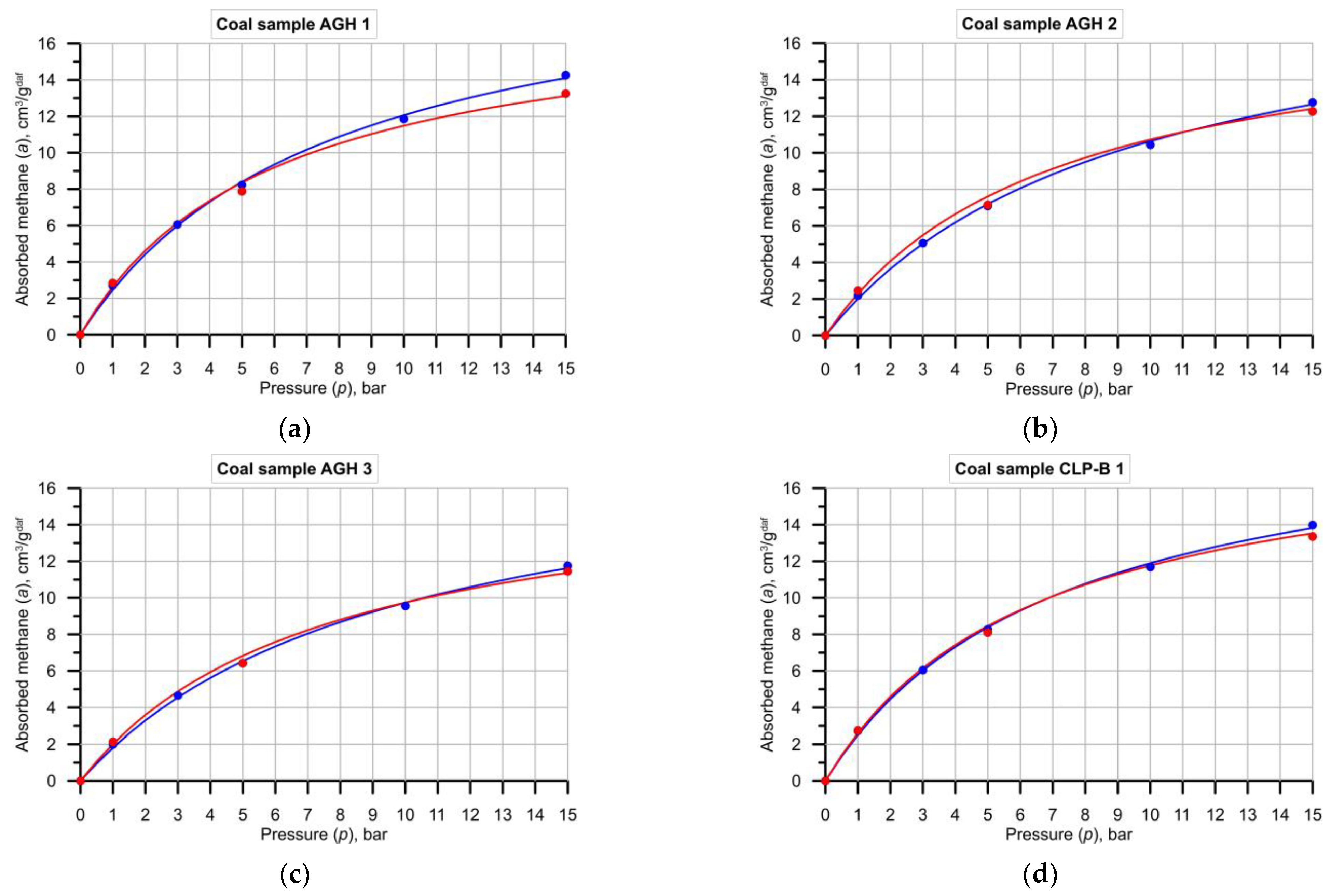
- The most notable discrepancies in the results were observed when matching the Langmuir sorption isotherm. It was found that matching a curve for five isotherm points according to AGH procedure is more beneficial than matching a curve for three points according to CLP-B procedure.
- Slight differences in the procedures for conducting sorption tests on coal samples may influence the results of comparative tests. Key factors affecting the obtained results are related to temperature and duration of the pretreatment stage, sample saturation time of methane, and number of measurement points for determining the Langmuir isotherm.
- The proposed essential methodology factors for determining methane sorption on coal by XEMIS analyzers constitute a foundation for developing a unified procedure. Applying the unified procedure is vital for comparing results in mining practice and assessing the risk of outbursts. Further research in this field is necessary to define the procedure in detail.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ou, J.; Wang, E.; Li, Z.; Li, N.; Liu, H.; Wang, X. Experimental study of coal and gas outburst processes influenced by gas pressure, ground stress and coal properties. Front. Earth Sci. 2023, 11, 1303996. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, Y. Role of coal deformation energy in coal and gas outburst: A review. Fuel 2023, 332, 126019. [Google Scholar] [CrossRef]
- Nguyen, P.M.V.; Litwa, P.; Przybylski, M. Field testing of the methods for prevention and control of coal and gas outburst-a case study in Poland. Arch. Min. Sci. 2023, 68, 639–654. [Google Scholar] [CrossRef]
- Dreger, M.; Celary, P. The Outburst Probability Index (Ww) as a New Tool in the Coal Seam Outburst Hazard Forecasting. J. Sustain. Min. 2024, 23, 5. [Google Scholar] [CrossRef]
- Lama, R.D.; Bodziony, J. Management of outburst in underground coal mines. Int. J. Coal Geol. 1998, 35, 83–115. [Google Scholar] [CrossRef]
- Shang, S.; Zhang, Q.; Zhao, Y.; Diao, Y.; Yin, J.; Che, Y.; Kang, X.; Zhao, B. Experimental research of the geo-stress evolution law and effect in the intact coal and gas outburst process. Front. Earth Sci. 2023, 10, 1036165. [Google Scholar] [CrossRef]
- Dutka, B. Influence of Depth on CO2/CH4 Sorption Ratio in Deep Coal Seams. Sustainability 2024, 16, 43. [Google Scholar] [CrossRef]
- Shan, W.; Chen, X. Study on the gas outburst control effect of goaf on the neighboring working face: Case study of Pingmei No.4 Mine. Energy Sci. Eng. 2024, 12, 1059–1071. [Google Scholar] [CrossRef]
- Makówka, J. (Ed.) Raport Roczny (2022) o Stanie Podstawowych Zagrożeń Naturalnych i Technicznych w Górnictwie Węgla Kamiennego; National Research Institute: Katowice, Poland, 2023. [Google Scholar]
- State Mining Authority. Stan Bezpieczeństwa i Higieny Pracy w Górnictwie; State Mining Authority: Katowice, Poland, 2024. Available online: https://www.wug.gov.pl/bhp/stan_bhp_w_gornictwie (accessed on 30 June 2024). (In Polish)
- Zou, Q.; Liu, H.; Jiang, Z.; Wu, X. Gas flow laws in coal subjected to hydraulic slotting and a prediction model for its permeability-enhancing effect. Energy Sources Part A Recovery Util Environ. Eff. 2021, 1–15. [Google Scholar] [CrossRef]
- Szlązak, N.; Borowski, M.; Obracaj, D.; Swolkień, J.; Korzec, M. Comparison of methane drainage methods used in Polish coal mines. Arch. Min. Sci. 2014, 59, 655–675. [Google Scholar] [CrossRef]
- Zou, Q.; Chen, Z.; Cheng, Z.; Liang, Y.; Xu, W.; Wen, P.; Zhang, B.; Liu, H.; Kong, F. Evaluation and intelligent deployment of coal and coalbed methane coupling coordinated exploitation based on Bayesian network and cuckoo search. Int. J. Min. Sci. Technol. 2022, 32, 1315–1328. [Google Scholar] [CrossRef]
- Zou, Q.; Lin, B.; Liu, T.; Zhou, Y.; Zhang, Z.; Yan, F. Variation of methane adsorption property of coal after the treatment of hydraulic slotting and methane pre-drainage: A case study. J. Nat. Gas Sci. Eng. 2014, 20, 396–406. [Google Scholar] [CrossRef]
- Regulation of the Minister of the Energy on Detailed Requirements for the Operation of Underground Mines. 2017. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20170001118/O/D20171118.pdf (accessed on 30 June 2024).
- Szlązak, N.; Obracaj, D.; Korzec, M. Method for determining the coalbed methane content with determination the uncertainty of measurements. Arch. Min. Sci. 2016, 61, 443–456. [Google Scholar] [CrossRef]
- Skoczylas, N.; Pajdak, A.; Kozieł, K.; Teixeira, L. Methane emission during gas and rock outburst on the basis of the unipore model. Energies 2019, 12, 1999. [Google Scholar] [CrossRef]
- Skoczylas, N. Estimating gas and rock outburst risk on the basis of knowledge and experience—The expert system based on fuzzy logic. Arch. Min. Sci. 2014, 59, 41–52. [Google Scholar] [CrossRef]
- Wierzbiński, K. Zagrożenie wyrzutami gazów i skał. In Bezpieczeństwo Pracy w Kopalniach Węgla Kamiennego; Konopko, W., Ed.; National Research Institute: Katowice, Poland, 2013. [Google Scholar]
- Li, P.; Cao, Y.; Li, X.; Wang, F.; Sun, Z.; Chen, D. Desorption characterization of methane in coal with different moisture contents and its influence on outburst prediction. Adv. Civ. Eng. 2021, 10, 1–10. [Google Scholar] [CrossRef]
- White, C.M.; Smith, D.H.; Jones, K.L.; Goodman, A.L.; Jikich, S.A.; LaCount, R.B.; DuBose, S.B.; Ozdemir, E.; Morsi, B.I.; Schroeder, K.T. Sequestration of Carbon Dioxide in Coal with Enhanced Coalbed Methane Recovery—A Review. Energy Fuels 2005, 19, 659–724. [Google Scholar] [CrossRef]
- Wierzbicki, M.; Skoczylas, N.; Kudasik, M. The Use of a Unipore Diffusion Model to Describe the Kinetics of Methane Release from Coal Spoil in the Longwall Environment. Stud. Geotech. Mech. 2017, 39, 2. [Google Scholar] [CrossRef]
- Godyń, K.; Dutka, B.; Chuchro, M.; Młynarczuk, M. Synergy of parameters determining the optimal properties of coal as a natural sorbent. Energies 2020, 13, 1967. [Google Scholar] [CrossRef]
- Koptoń, H. Alternative methodology to determine effective coefficient of methane diffusion in coal. J. Sustain. Min. 2020, 19, 3. [Google Scholar] [CrossRef]
- Ding, S.; Liu, J.; Xu, B. Factors influencing coal bed methane reservoir in southeastern edge of Ordos Basin, China. Energy Explor. Exploit. 2012, 30, 677–688. [Google Scholar] [CrossRef]
- Dudzińska, A.; Howaniec, N.; Smoliński, A. Experimental Study on Sorption and Desorption of Propylene on Polish Hard Coals. Energy Fuels 2015, 29, 4850–4854. [Google Scholar] [CrossRef]
- Mabuza, M.; Premlall, K.; Onyango, M.; Daramola, M.O. Low-high temperature flue gas direct injection in south African bituminous and anthracite coals: Sorption capacity assessment. Curr. Sci. 2018, 115, 4. [Google Scholar] [CrossRef]
- Skiba, M.; Młynarczuk, M. Estimation of Coal’s Sorption Parameters Using Artificial Neural Networks. Materials 2020, 13, 5422. [Google Scholar] [CrossRef]
- Gasparik, M.; Ghanizadeh, A.; Bertier, P.; Gensterblum, Y.; Bouw, S.; Krooss, B.M. High-Pressure Methane Sorption Isotherms of Black Shales from The Netherlands. Energy Fuels 2012, 26, 4995–5004. [Google Scholar] [CrossRef]
- Wierzbicki, M. Changes in the sorption/diffusion kinetics of a coal-methane system caused by different temperatures and pressures. Gospod. Surowcami Miner. 2013, 29, 155–168. [Google Scholar] [CrossRef]
- Timofiejew, D.P. Adsprptionskinetik; Lipsk VEB: Munich, Germany, 1967. [Google Scholar]
- Karger, J.; Ruthven, D.M. Diffusion in Zeolites and Other Microporous Solids; Wiley & Sons: New York, NY, USA, 1992. [Google Scholar]
- Busch, A.; Gensterblum, Y. CBM and CO2-ECBM related sorption processes in coal: A review. Int. J. Coal Geol. 2011, 87, 49–71. [Google Scholar] [CrossRef]
- Gašparík, M.; Gensterblum, Y.; Ghanizadeh, A.; Weniger, P.; Krooß, B.M. High-pressure/high-temperature methane-sorption measurements on carbonaceous shales by the manometric method: Experimental and data-evaluation considerations for improved accuracy. SPE J. 2015, 20, 790–809. [Google Scholar] [CrossRef]
- Webb, C.J.; Gray, E.M.A. Analysis of uncertainties in gas uptake measurements using the gravimetric method. Int. J. Hydrog. Energy 2014, 39, 7158–7164. [Google Scholar] [CrossRef]
- Liu, H.; Mao, S.; Li, M.; Lyu, P. A GIS Based Unsteady Network Model and System Applications for Intelligent Mine Ventilation. Discret. Dyn. Nat. Soc. 2020, 2020, 1041927. [Google Scholar] [CrossRef]
- Baran, P.; Zarembska, K. Analiza pęcznienia węgli kamiennych i brunatnych w oparciu o analizę izoterm sorpcji CO2. Pr. Inst. Mech. Górotworu PAN 2016, 18, 109–116. [Google Scholar]
- Miknis, F.P.; Netzel, D.A.; Turner, T.F.; Wallace, J.C.; Butcher, C.H. Effect of different drying methods on coal structure and reactivity toward liquefaction. Energy Fuels 1996, 10, 631–640. [Google Scholar] [CrossRef]
- Zhu, C.; Ren, J.; Wan, J.; Lin, B.; Yang, K.; Li, Y. Methane adsorption on coals with different coal rank under elevated temperature and pressure. Fuel 2019, 254, 115686. [Google Scholar] [CrossRef]
- Wang, J.Y.; Mangano, E.; Brandani, S.; Ruthven, D.M. A review of common practices in gravimetric and volumetric adsorption kinetic experiments. Adsorption 2021, 27, 295–318. [Google Scholar] [CrossRef]
- Kudasik, M.; Skoczylas, N.; Pajdak, A. The repeatability of sorption processes occurring in the coal-methane system during multiple measurement series. Energies 2017, 10, 661. [Google Scholar] [CrossRef]
- Salinas, O.; Ma, X.; Litwiller, E.; Pinnau, I. Ethylene/ethane permeation, diffusion and gas sorption properties of carbon molecular sieve membranes derived from the prototype ladder polymer of intrinsic microporosity (PIM-1). J. Membr. Sci. 2016, 504, 133–140. [Google Scholar] [CrossRef]
- Guo, Q.; Fink, R.; Littke, R.; Zieger, L. Methane sorption behaviour of coals altered by igneous intrusion, South Sumatra Basin. Int. J. Coal Geol. 2019, 214, 103250. [Google Scholar] [CrossRef]
- Chattaraj, S.; Mohanty, D.; Kumar, T.; Halder, G. Thermodynamics, kinetics and modeling of sorption behaviour of coalbed methane—A review. J. Unconv. Oil Gas Resour. 2016, 16, 14–33. [Google Scholar] [CrossRef]
- Wierzbicki, M. The effect of temperature on the sorption properties of coal from Upper Silesian Coal Basin, Poland. Arch. Min. Sci. 2013, 58, 1163–1176. [Google Scholar] [CrossRef]
- Mianowski, A.; Marecka, A. The isokinetic effect as related to the activation energy for the gases diffusion in coal at ambient temperatures, Part I. Fick’s diffusion parameter estimated from kinetic curves. J. Therm. Anal. Calorim. 2009, 95, 285–292. [Google Scholar] [CrossRef]



| Parameter | AGH | CLP-B |
|---|---|---|
| Type of analyzer | XEMIS-100 | XEMIS-001 |
| Design pressure range | 1 × 10−6 ÷ 170 bar | 1 × 10−6 ÷ 150 bar |
| Pressure transducer ranges | 0 ÷ 200 bar 0 ÷ 100 mbar 0 ÷ 1 bar 0 ÷ 50 bar | 0 ÷ 200 bar 0 ÷ 50 bar 0 ÷ 1 bar 0 ÷ 100 mbar 0 ÷ 10 mbar |
| Temperature measurement range | −196 ÷ 500 °C | −270 ÷ 500 °C |
| Temperature accuracy | ±0.1 K | ±0.1 K |
| Balance capacity | 5 g | 5 g |
| Weighing resolution | 2 × 10−6 of sample mass | 0.2 μg (100 mg) 2 μg (1 g) |
| Long-term weighing stability (24 h cycle in an inert atmosphere at room temperature): | ±5 µg | ±5 µg |
| Short-term weighing stability | ±1 µg | ±1 µg |
| Dynamic weighing range | 0 ÷ 200 mg | 0 ÷ 200 mg |
| Long-term pressure stability | <±0.08 bar | <±0.08 bar |
| Precision of pressure measurement for the entire measurement range | 0.01 bar | 0.01 bar |
| Cabinet (balance) temperature stability | 0.1 °C | 0.1 °C |
| Measurement modes | static/dynamic | static/dynamic |
| Number of connected gas lines | 5 (CH4, H2, CO2, N2, and corrosive gas) | 1 (CH4) |
| Operating scope of flow controllers | 20 ÷ 1000 mL/min | 20 ÷ 2000 mL/min |
| Accuracy of active pressure regulation | ±0.02% of the range | ±0.02% of the range |
| Precision of pressure measurement | ±0.04% of the transmitter measurement range. | ±0.02% of the transmitter measurement range |
| Reactor temperature control systems | Sample 500 °C furnace and controller (40 ÷ 500 °C), Refrigerated recirculating bath (0 ÷ 80 °C), Liquid Dewar Vessel (−196 °C) | Sample 500 °C furnace and controller (40 ÷ 500 °C), Refrigerated recirculating bath (0 ÷ 90 °C) |
| Valve control | Pneumatic | Mechanical |
| Additional equipment | Vapor generator, Mass spectrometer | - |
| Gas CH4 purity | 99.999% | 99.995% |
| No. | Coal Sample Determination | Virgin Rock Temperature | Methane Content of Coal | Protodiakonov’s Coefficient of Coal Hardness | Desorption Intensity Index | True Density | Ash Content |
|---|---|---|---|---|---|---|---|
| °C | m3/t daf | - | kPa | kg/m3 | % | ||
| 1 | AGH 1 | 38.0 | 4.356 | 0.42 | 0.85 | 1.320 | 6.56 |
| 2 | AGH 2 | 45.5 | 3.215 | 0.39 | 1.32 | 1.328 | 5.49 |
| 3 | AGH 3 | 47.0 | 6.241 | 0.35 | 0.98 | 1.355 | 8.20 |
| 4 | CLP-B 1 | 37.0 | 3.131 | 0.40 | 0.42 | 1.351 | 4.91 |
| 5 | CLP-B 2 | 39.4 | 8.740 | 0.38 | 1.46 | 1.325 | 1.18 |
| 6 | CLP-B 3 | 45.0 | 7.708 | 0.34 | 1.22 | 1.369 | 5.25 |
| Stage | Parameter | AGH | CLP-B |
|---|---|---|---|
| Pretreatment | Sample temperature, °C | 45 | 85 |
| Duration, min | 720 | 240 | |
| Coal sample weight, mg | 130 ± 5 | 135 ± 5 | |
| Test conditions for determining both sorption capacity at the pressure of 1 bar and effective diffusion coefficient | Sample temperature, °C | According to VRT at the place of sampling (see Table 2) | |
| Gas pressure, bar | 1.0 | ||
| Duration, min | 1080 | ||
| Test conditions for determining sorption isotherm parameters | Number of isotherm points | 5 | 3 |
| Gas pressure (sorption), bar | 1.0/3.0/5.0/10.0/15.0 | 1.0/5.0/15.0 | |
| Duration (sorption), min | 1080 for each point | 1080/800/500 | |
| Gas pressure (desorption), bar | 1.0 | 5.0/1.0 | |
| Duration (desorption), min | 1080 | 1000/1000 | |
| Conversion of results into established conditions | Pressure, Pa | 100,000 | |
| Temperature, °C | 25.0 | ||
| No. | Coal Sample Determination | Sorption Capacity at the Pressure of 1 bar | Effective Diffusion Coefficient | Coefficients of Langmuir Model Fitting | Coefficient of Determination for Langmuir Model Fitting | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| cm3/gdaf | ×10−9 cm2/s | cm3/gdaf | 1/bar | ||||||||
| AGH | CLP-B | AGH | CLP-B | AGH | CLP-B | AGH | CLP-B | AGH | CLP-B | ||
| 1 | AGH 1 | 2.71 | 2.84 | 1.44 | 1.42 | 21.34 | 18.32 | 0.130 | 0.168 | 0.9990 | 0.9972 |
| 2 | AGH 2 | 2.19 | 2.46 | 1.31 | 1.36 | 20.39 | 18.12 | 0.109 | 0.145 | 0.9993 | 0.9982 |
| 3 | AGH 3 | 1.99 | 2.13 | 1.01 | 1.07 | 19.02 | 17.01 | 0.105 | 0.134 | 0.9990 | 0.9978 |
| 4 | CLP-B 1 | 2.72 | 2.74 | 1.02 | 1.23 | 20.44 | 19.31 | 0.139 | 0.156 | 0.9992 | 0.9993 |
| 5 | CLP-B 2 | 1.83 | 1.87 | 0.26 | 0.30 | 16.16 | 14.45 | 0.120 | 0.144 | 0.9995 | 0.9996 |
| 6 | CLP-B 3 | 2.20 | 2.36 | 1.85 | 1.67 | 20.37 | 18.45 | 0.110 | 0.146 | 0.9995 | 0.9978 |
| No. | Sorption Capacity at the Pressure of 1 bar | Coefficients of Langmuir Model Fitting | ||||||
|---|---|---|---|---|---|---|---|---|
| cm3/gdaf | ×10−9 cm2/s | |||||||
| AGH | CLP-B | Average | Difference from the Average | AGH | CLP-B | Average | Difference from the Average | |
| 1 | 2.71 | 2.84 | 2.775 | 4.7% | 1.44 | 1.42 | 1.430 | 1.4% |
| 2 | 2.19 | 2.46 | 2.325 | 11.8% | 1.31 | 1.36 | 1.335 | 3.7% |
| 3 | 1.99 | 2.13 | 2.060 | 6.9% | 1.01 | 1.07 | 1.040 | 5.8% |
| 4 | 2.72 | 2.74 | 2.730 | 0.7% | 1.02 | 1.23 | 1.125 | 18.7% |
| 5 | 1.83 | 1.87 | 1.850 | 2.2% | 0.26 | 0.30 | 0.280 | 13.1% |
| 6 | 2.20 | 2.36 | 2.280 | 7.1% | 1.85 | 1.67 | 1.760 | 10.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obracaj, D.; Korzec, M.; Dreger, M. Comparison of Gravimetric Determination of Methane Sorption Capacities of Coals for Using Their Results in Assessing Outbursts in Mines. Energies 2024, 17, 4372. https://doi.org/10.3390/en17174372
Obracaj D, Korzec M, Dreger M. Comparison of Gravimetric Determination of Methane Sorption Capacities of Coals for Using Their Results in Assessing Outbursts in Mines. Energies. 2024; 17(17):4372. https://doi.org/10.3390/en17174372
Chicago/Turabian StyleObracaj, Dariusz, Marek Korzec, and Marcin Dreger. 2024. "Comparison of Gravimetric Determination of Methane Sorption Capacities of Coals for Using Their Results in Assessing Outbursts in Mines" Energies 17, no. 17: 4372. https://doi.org/10.3390/en17174372
APA StyleObracaj, D., Korzec, M., & Dreger, M. (2024). Comparison of Gravimetric Determination of Methane Sorption Capacities of Coals for Using Their Results in Assessing Outbursts in Mines. Energies, 17(17), 4372. https://doi.org/10.3390/en17174372








