Electronically Conductive Polymer Enhanced Solid-State Polymer Electrolytes for All-Solid-State Lithium Batteries
Abstract
1. Introduction
2. Methods and Materials
2.1. Sample Preparation
2.1.1. Preparation of SSPE Membranes
2.1.2. Interfacial Property-Enhancing Polymer Coating on SSPE Membranes
2.1.3. Fabrication of Ni-Rich-Based Cathodes
2.2. Physical Characterizations
2.3. Electrochemical Characterizations
3. Results and Discussion
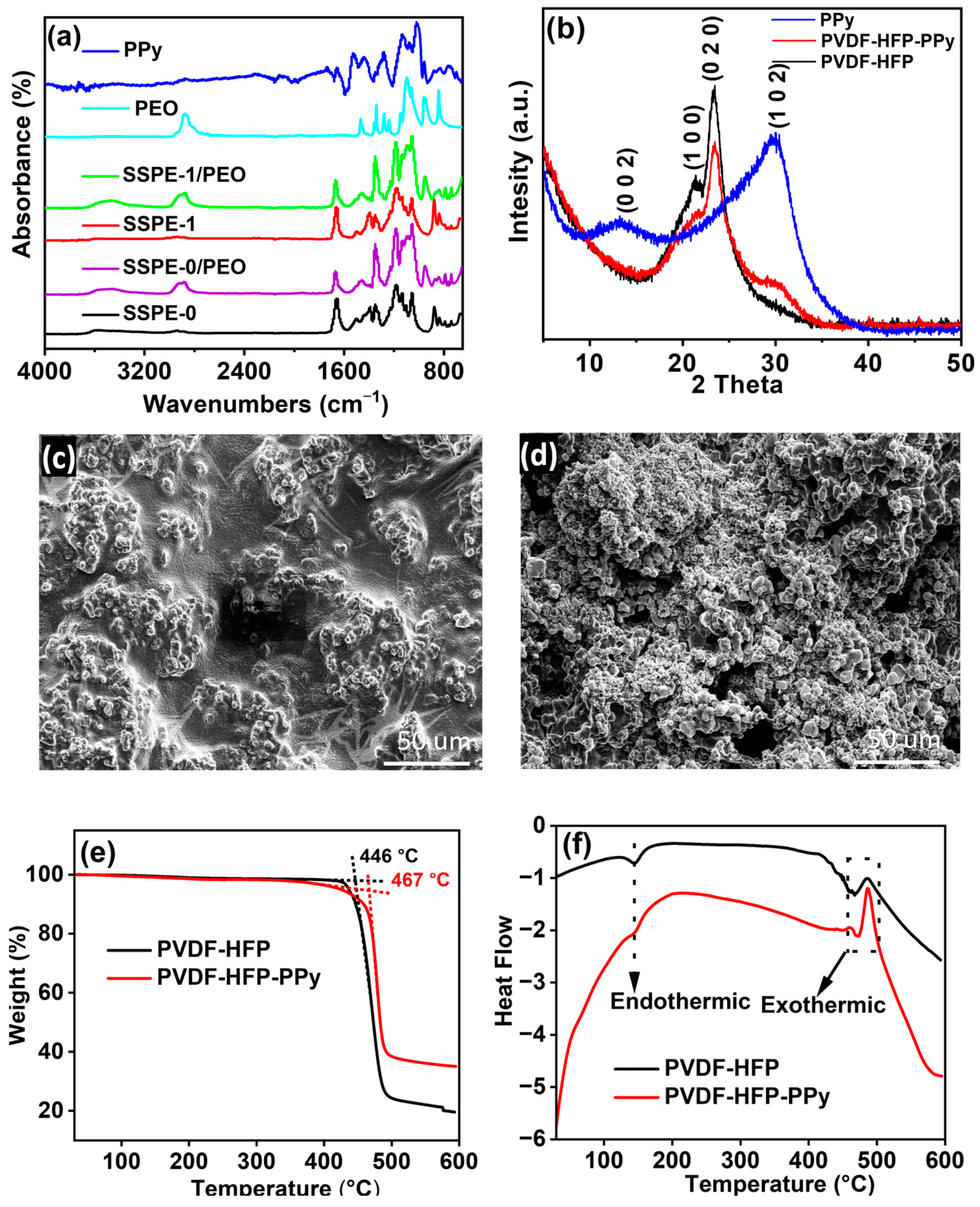
3.1. Physical Characterizations
3.1.1. FTIR Analysis of Polymers and SSPEs
3.1.2. XRD Analysis of Polymer Membranes
3.1.3. SEM Analysis of SSPEs
3.1.4. Thermal Properties of Polymer Membranes
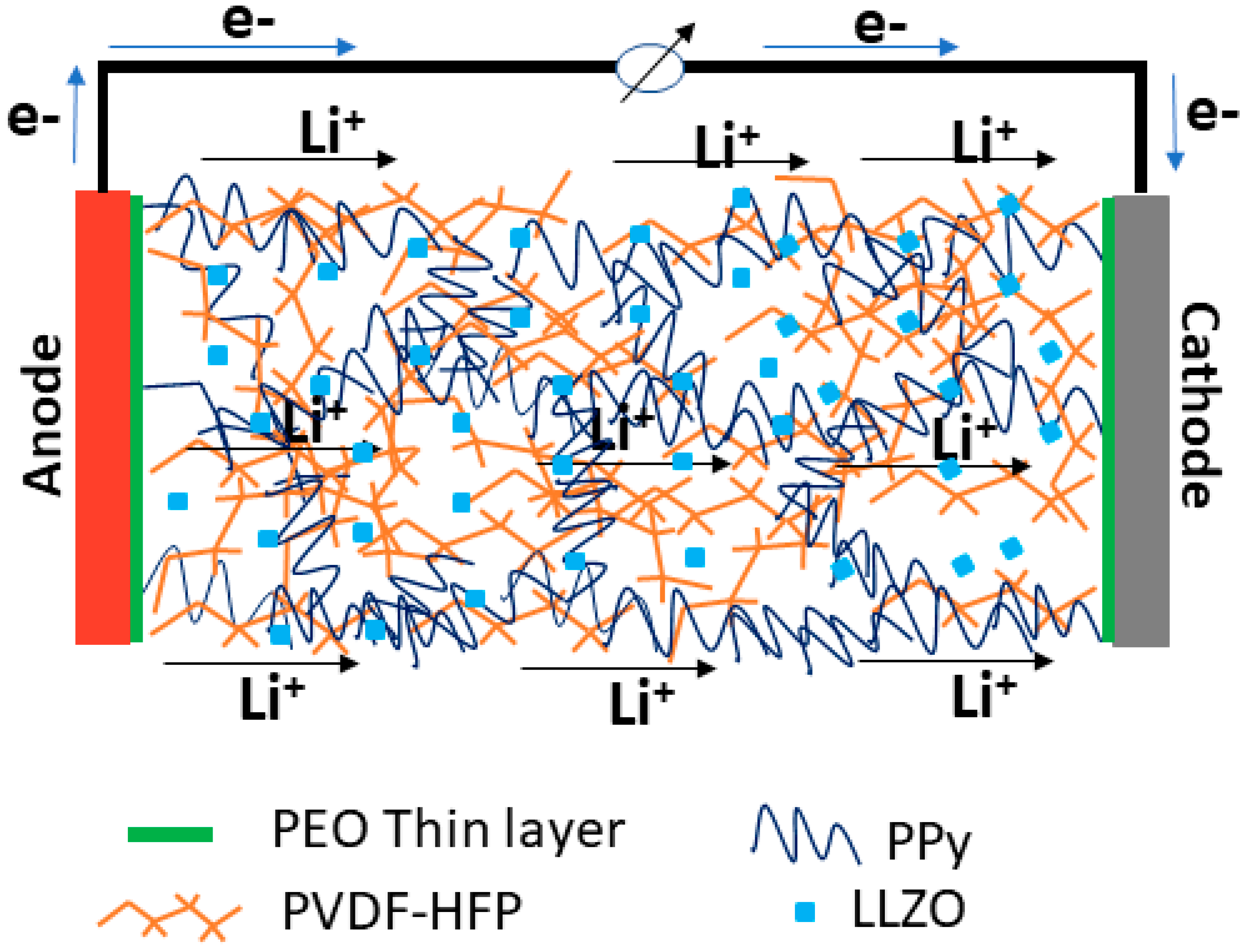
3.2. Electrochemical Characterizations
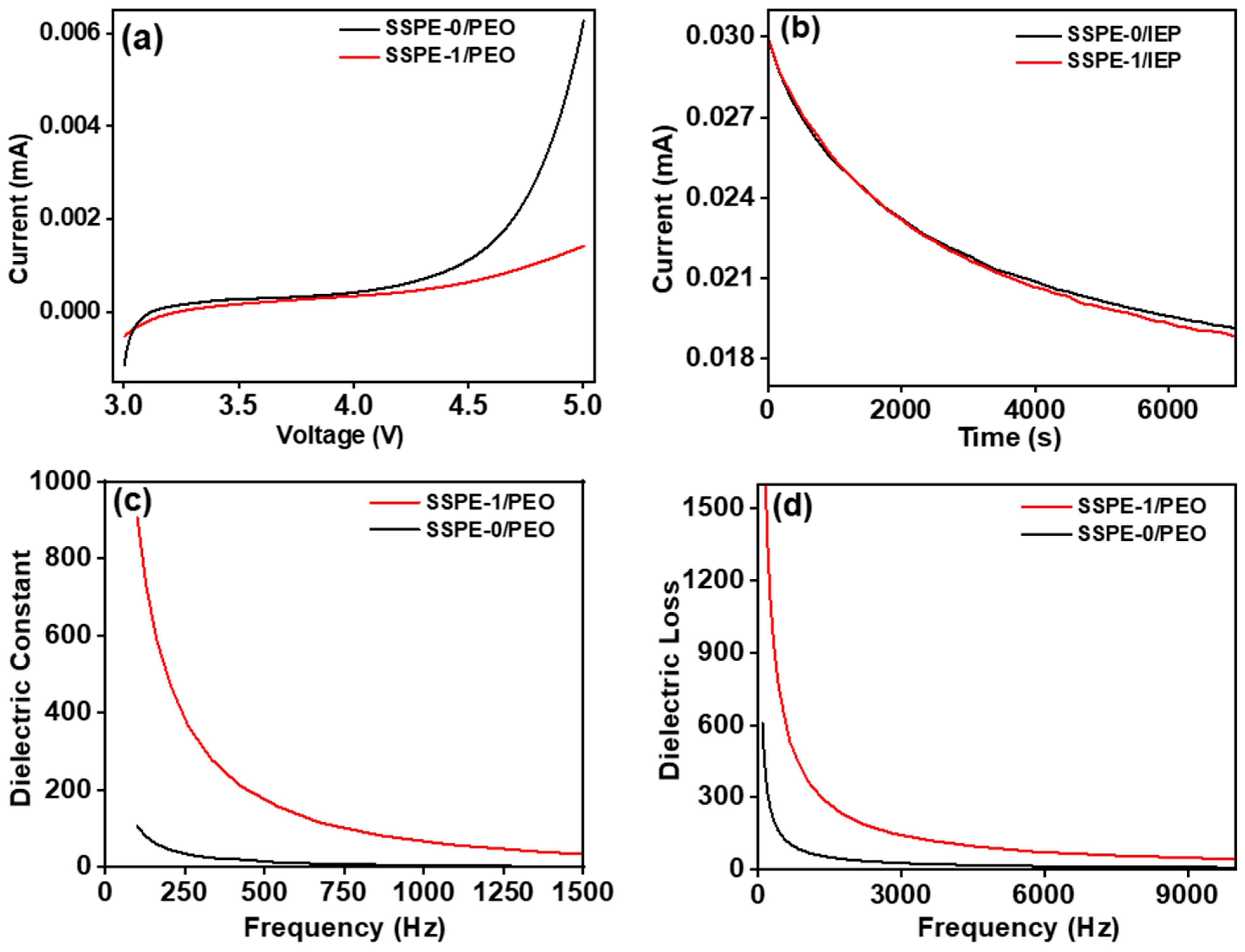
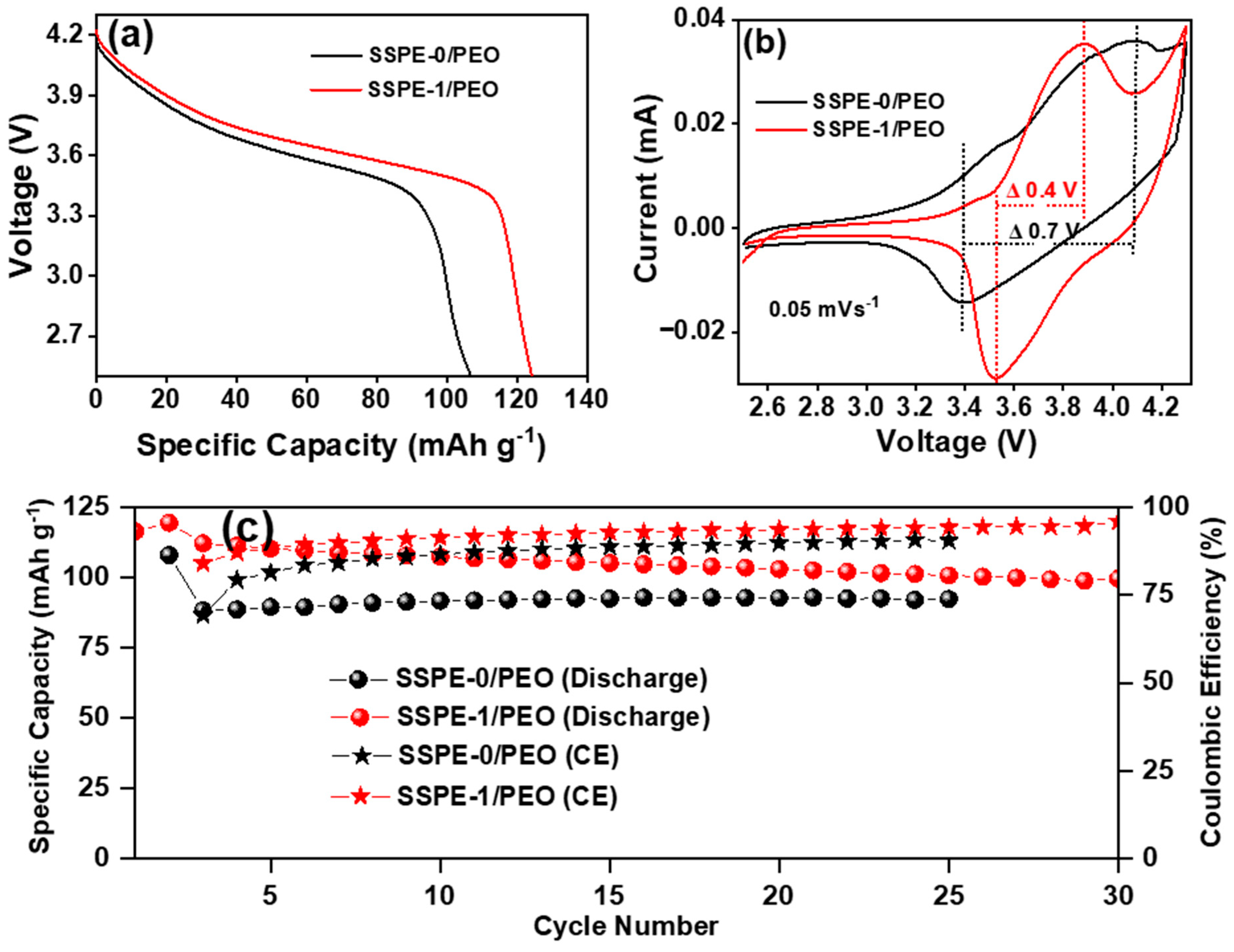
3.2.1. Ionic Conductivity of SSPEs
3.2.2. Linear Sweep Voltammetry (LSV) and Leakage Current Measurement of SSPEs
3.2.3. Dielectric Constant and Dielectric Loss of SSPEs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASSLB | All-solid-state lithium battery |
| GO | Graphen oxide |
| LLZO | Lithium zirconium oxide |
| PEO | Polyethylene oxide |
| PPy | Polypyrrole |
| PVDF-HFP | Polyvinylidene fluoride-co-hexafluoropropylene |
| SSCE | Solid-state ceramic electrolyte |
| SSPE | Polymer–SSCE composite electrolyte |
References
- Liu, Y.; Zhang, R.; Wang, J.; Wang, Y. Current and future lithium-ion battery manufacturing. iScience 2021, 24, 102332. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, M.; Alziyadi, M.O.; Gamal, H.; Hamdy, S. Solid-State Lithium-Ion Battery: The Key Components Enhance The Performance And Efficiency of Anode, Cathode, And Solid Electrolytes. J. Alloys Compd. 2023, 969, 172318. [Google Scholar] [CrossRef]
- Sanches, E.A.; Alves, S.F.; Soares, J.C.; da Silva, A.M.; da Silva, C.G.; de Souza, S.M.; da Frota, H.O. Nanostructured polypyrrole powder: A structural and morphological characterization. J. Nanomater. 2015, 2015, 129678. [Google Scholar] [CrossRef]
- Moradi, Z.; Lanjan, A.; Tyagi, R.; Srinivasan, S. Review on current state, challenges, and potential solutions in solid-state batteries research. J. Energy Storage 2023, 73, 109048. [Google Scholar] [CrossRef]
- Gonzalez Puente, P.; Song, S.; Cao, S.; Rannalter, L.Z.; Pan, Z.; Xiang, X.; Shen, Q.; Chen, F. Garnet-type solid electrolyte: Advances of ionic transport performance and its application in all-solid-state batteries. J. Adv. Ceram. 2021, 10, 933–972. [Google Scholar] [CrossRef]
- Boaretto, N.; Garbayo, I.; Valiyaveettil-SobhanRaj, S.; Quintela, A.; Li, C.; Casas-Cabanas, M.; Aguesse, F. Lithium solid-state batteries: State-of-the-art and challenges for materials, interfaces and processing. J. Power Sources 2021, 502, 229919. [Google Scholar] [CrossRef]
- Yadav, P.; Beheshti, S.H.; Kathribail, A.R.; Ivanchenko, P.; Mierlo, J.V.; Berecibar, M. Improved Performance of Solid Polymer Electrolyte for Lithium-Metal Batteries via Hot Press Rolling. Polymers 2022, 14, 363. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wu, S.; He, Y.-B.; Kang, F.; Chen, L.; Li, H.; Yang, Q.-H. Solid-state lithium batteries: Safety and prospects. eScience 2022, 2, 138–163. [Google Scholar] [CrossRef]
- Wan, J.; Xie, J.; Mackanic, D.G.; Burke, W.; Bao, Z.; Cui, Y. Status, promises, and challenges of nanocomposite solid-state electrolytes for safe and high performance lithium batteries. Mater. Today Nano 2018, 4, 1–16. [Google Scholar] [CrossRef]
- Shi, C.; Hamann, T.; Takeuchi, S.; Alexander, G.V.; Nolan, A.M.; Limpert, M.; Fu, Z.; O’Neill, J.; Godbey, G.; Dura, J.A.; et al. 3D Asymmetric Bilayer Garnet-Hybridized High-Energy-Density Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2023, 15, 751–760. [Google Scholar] [CrossRef]
- Chen, A.; Qu, C.; Shi, Y.; Shi, F. Manufacturing strategies for solid electrolyte in batteries. Front. Energy Res. 2020, 8, 571440. [Google Scholar] [CrossRef]
- Shi, C.; Alexander, G.V.; O’Neill, J.; Duncan, K.; Godbey, G.; Wachsman, E.D. All-Solid-State Garnet Type Sulfurized Polyacrylonitrile/Lithium-Metal Battery Enabled by an Inorganic Lithium Conductive Salt and a Bilayer Electrolyte Architecture. ACS Energy Lett. 2023, 8, 1803–1810. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.Q.; Shen, L.; Liu, Q.; Ma, J.B.; Lv, W.; He, Y.B.; Yang, Q.H. Progress and perspective of ceramic/polymer composite solid electrolytes for lithium batteries. Adv. Sci. 2020, 7, 1903088. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Pareek, T.; Dwivedi, S.; Badole, M.; Kumar, S. LiSn2(PO4)3-based polymer-in-ceramic composite electrolyte with high ionic conductivity for all-solid-state lithium batteries. J. Solid State Electrochem. 2020, 24, 2407–2417. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. A review of composite polymer-ceramic electrolytes for lithium batteries. Energy Storage Mater. 2021, 34, 282–300. [Google Scholar] [CrossRef]
- Sakuda, A.; Hayashi, A.; Tatsumisago, M. Sulfide solid electrolyte with favorable mechanical property for all-solid-state lithium battery. Sci. Rep. 2013, 3, 2261. [Google Scholar] [CrossRef]
- Hayashi, A.; Sakuda, A.; Tatsumisago, M. Development of sulfide solid electrolytes and interface formation processes for bulk-type all-solid-state Li and Na batteries. Front. Energy Res. 2016, 4, 25. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer electrolytes for lithium-based batteries: Advances and prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Gonçalves, R.; Costa, C.M.; Lanceros-Méndez, S. Toward sustainable solid polymer electrolytes for lithium-ion batteries. ACS Omega 2022, 7, 14457–14464. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, X.; Ma, Z.; Mei, P.; Xiao, W.; You, Q.; Zhang, Y. Development of the PEO based solid polymer electrolytes for all-solid state lithium ion batteries. Polymers 2018, 10, 1237. [Google Scholar] [CrossRef]
- Ma, C.; Dai, K.; Hou, H.; Ji, X.; Chen, L.; Ivey, D.G.; Wei, W. High ion-conducting solid-state composite electrolytes with carbon quantum dot nanofillers. Adv. Sci. 2018, 5, 1700996. [Google Scholar] [CrossRef]
- Jian, S.; Cao, Y.; Feng, W.; Yin, G.; Zhao, Y.; Lai, Y.; Zhang, T.; Ling, X.; Wu, H.; Bi, H. Recent progress in solid polymer electrolytes with various dimensional fillers: A review. Mater. Today Sustain. 2022, 20, 100224. [Google Scholar] [CrossRef]
- Yadav, P.; Hosen, M.S.; Dammala, P.K.; Ivanchenko, P.; Van Mierlo, J.; Berecibar, M. Development of composite solid polymer electrolyte for solid-state lithium battery: Incorporating LLZTO in PVDF-HFP/LiTFSI. Solid State Ion. 2023, 399, 116308. [Google Scholar] [CrossRef]
- Li, J.; Zheng, W.; Zhu, L.; Zhou, H.; Zhang, K. Incorporating lithium magnesium silicate into PVDF-HFP based solid electrolyte to achieve advanced solid-state lithium-ion batteries. J. Alloys Compd. 2023, 960, 170640. [Google Scholar] [CrossRef]
- Tanaka, R.; Sakurai, M.; Sekiguchi, H.; Mori, H.; Murayama, T.; Ooyama, T. Lithium ion conductivity in polyoxyethylene/polyethylenimine blends. Electrochim. Acta 2001, 46, 1709–1715. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Gu, T.; Rui, G.; Shi, P.; Fu, W.; Chen, L.; Liu, X.; Zeng, J.; Kang, B.; Yan, Z.; et al. A relaxor ferroelectric polymer with an ultrahigh dielectric constant largely promotes the dissociation of lithium salts to achieve high ionic conductivity. Energy Environ. Sci. 2021, 14, 6021–6029. [Google Scholar] [CrossRef]
- Shi, P.; Ma, J.; Liu, M.; Guo, S.; Huang, Y.; Wang, S.; Zhang, L.; Chen, L.; Yang, K.; Liu, X.; et al. A dielectric electrolyte composite with high lithium-ion conductivity for high-voltage solid-state lithium metal batteries. Nat. Nanotechnol. 2023, 18, 602–610. [Google Scholar] [CrossRef]
- Gao, C.; Li, X.; Wei, G.; Wang, S.; Zhao, X.; Kong, F. "Cellulose acetate propionate incorporated PVDF-HFP based polymer electrolyte membrane for lithium batteries". Composites Communications 2022, 33, 101226. [Google Scholar] [CrossRef]
- Meyer, W.H. Polymer electrolytes for lithium-ion batteries. Adv. Mater. 1998, 10, 439–448. [Google Scholar] [CrossRef]
- Wen, J.; Zhao, Q.; Jiang, X.; Ji, G.; Wang, R.; Lu, G.; Long, J.; Hu, N.; Xu, C. Graphene oxide enabled flexible PEO-based solid polymer electrolyte for all-solid-state lithium metal battery. ACS Appl. Energy Mater. 2021, 4, 3660–3669. [Google Scholar] [CrossRef]
- Yuan, M.; Erdman, J.; Tang, C.; Ardebili, H. High performance solid polymer electrolyte with graphene oxide nanosheets. Rsc Adv. 2014, 4, 59637–59642. [Google Scholar] [CrossRef]
- Guo, X.; Ju, Z.; Qian, X.; Liu, Y.; Xu, X.; Yu, G. A Stable Solid Polymer Electrolyte for Lithium Metal Battery with Electronically Conductive Fillers. Angew. Chem. Int. Ed. 2023, 62, e202217538. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, H.; Dou, Z.; Hao, Z.; Huang, Q.; Guo, Z.; Wang, C.; Miao, K.; Kang, X. Graphene-Oxide-Coated CoP2@C Anode Enables High Capacity of Lithium-Ion Batteries. Electrochem 2023, 4, 473–484. [Google Scholar] [CrossRef]
- St-Onge, V.; Cui, M.; Rochon, S.; Daigle, J.-C.; Claverie, J.P. Reducing crystallinity in solid polymer electrolytes for lithium-metal batteries via statistical copolymerization. Commun. Mater. 2021, 2, 83. [Google Scholar] [CrossRef]
- Harrison, K.L.; Goriparti, S.; Merrill, L.C.; Long, D.M.; Warren, B.; Roberts, S.A.; Perdue, B.R.; Casias, Z.; Cuillier, P.; Boyce, B.L.; et al. Effects of Applied Interfacial Pressure on Li-Metal Cycling Performance and Morphology in 4 M LiFSI in DME. ACS Appl. Mater. Interfaces 2021, 13, 31668–31679. [Google Scholar] [CrossRef]
- Bocharova, V.; Sokolov, A.P. Perspectives for Polymer Electrolytes: A View from Fundamentals of Ionic Conductivity. Macromolecules 2020, 53, 4141–4157. [Google Scholar] [CrossRef]
- Biancolli, A.L.G.; Konovalova, A.; Santiago, E.I.; Holdcroft, S. Measuring the ionic conductivity of solid polymer electrolyte powders. Int. J. Electrochem. Sci. 2023, 18, 100288. [Google Scholar] [CrossRef]
- Gao, H.; Lian, K. A comparative study of nano-SiO2 and nano-TiO2 fillers on proton conductivity and dielectric response of a silicotungstic acid–H3PO4–poly (vinyl alcohol) polymer electrolyte. ACS Appl. Mater. Interfaces 2014, 6, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Ben Ayed, A.; Bouhamed, A.; Nouri, H.; Abdelmoula, N.; Khemakhem, H.; Kanoun, O. Robust and Flexible Piezoelectric Lead-Free Zn-BCZT/PVDF-HFP Nanogenerators for Wearable Energy Harvesting. ACS Appl. Electron. Mater. 2023, 5, 4282–4295. [Google Scholar] [CrossRef]
- Tran, H.K.; Truong, B.T.; Zhang, B.-R.; Jose, R.; Chang, J.-K.; Yang, C.-C. Sandwich-Structured Composite Polymer Electrolyte Based on PVDF-HFP/PPC/Al-Doped LLZO for High-Voltage Solid-State Lithium Batteries. ACS Appl. Energy Mater. 2023, 6, 1475–1487. [Google Scholar] [CrossRef]
- Celik, M.; Kızılaslan, A.; Can, M.; Cetinkaya, T.; Akbulut, H. Electrochemical investigation of PVDF: HFP gel polymer electrolytes for quasi-solid-state Li-O2 batteries: Effect of lithium salt type and concentration. Electrochim. Acta 2021, 371, 137824. [Google Scholar] [CrossRef]
- Martina, P.; Gayathri, R.; Pugalenthi, M.R.; Cao, G.; Liu, C.; Prabhu, M.R. Nanosulfonated silica incorporated SPEEK/SPVdF-HFP polymer blend membrane for PEM fuel cell application. Ionics 2020, 26, 3447–3458. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Ralph, S.A.; Baez, C.; Reiner, R.S.; Verrill, S.P. Effect of sample moisture content on XRD-estimated cellulose crystallinity index and crystallite size. Cellulose 2017, 24, 1971–1984. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, J.; Shi, F.; Lin, D.; Liu, Y.; Liu, W.; Pei, A.; Gong, Y.; Wang, H.; Liu, K.; et al. Vertically Aligned and Continuous Nanoscale Ceramic–Polymer Interfaces in Composite Solid Polymer Electrolytes for Enhanced Ionic Conductivity. Nano Lett. 2018, 18, 3829–3838. [Google Scholar] [CrossRef]
- Fan, R.; Liu, C.; He, K.; Ho-Sum Cheng, S.; Chen, D.; Liao, C.; Li, R.K.Y.; Tang, J.; Lu, Z. Versatile Strategy for Realizing Flexible Room-Temperature All-Solid-State Battery through a Synergistic Combination of Salt Affluent PEO and Li6.75La3Zr1.75Ta0.25O12 Nanofibers. ACS Appl. Mater. Interfaces 2020, 12, 7222–7231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, K.; Ding, F.; Liu, X. Recent advances in solid polymer electrolytes for lithium batteries. Nano Res. 2017, 10, 4139–4174. [Google Scholar] [CrossRef]
- Xue, C.; Qin, Y.; Fu, H.; Fan, J. Thermal Stability, Mechanical Properties and Ceramization Mechanism of Epoxy Resin/Kaolin/Quartz Fiber Ceramifiable Composites. Polym. 2022, 14, 3372. [Google Scholar] [CrossRef] [PubMed]
- Shahabaz, S.M.; Sharma, S.; Shetty, N.; Shetty, S.D.; M.C., G. Influence of Temperature on Mechanical Properties and Machining of Fibre Reinforced Polymer Composites: A Review. Eng. Sci. 2021, 16, 26–46. [Google Scholar] [CrossRef]
- Haque, S.K.M.; Ardila-Rey, J.A.; Umar, Y.; Mas’ud, A.A.; Muhammad-Sukki, F.; Jume, B.H.; Rahman, H.; Bani, N.A. Application and Suitability of Polymeric Materials as Insulators in Electrical Equipment. Energies 2021, 14, 2758. [Google Scholar] [CrossRef]
- Liu, T.; Kum, L.W.; Singh, D.K.; Kumar, J. Thermal, Electrical, and Environmental Safeties of Sulfide Electrolyte-Based All-Solid-State Li-Ion Batteries. ACS Omega 2023, 8, 12411–12417. [Google Scholar] [CrossRef]
- Solarajan, A.K.; Murugadoss, V.; Angaiah, S. Dimensional stability and electrochemical behaviour of ZrO2 incorporated electrospun PVdF-HFP based nanocomposite polymer membrane electrolyte for Li-ion capacitors. Sci. Rep. 2017, 7, 45390. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Ma, J.; Zhang, J.; Zhao, J.; Dong, S.; Liu, Z.; Cui, G.; Chen, L. All solid-state polymer electrolytes for high-performance lithium ion batteries. Energy Storage Mater. 2016, 5, 139–164. [Google Scholar] [CrossRef]
- Jamal, H.; Khan, F.; Kim, J.H.; Kim, E.; Lee, S.U.; Kim, J.H. Compact Solid Electrolyte Interface Realization Employing Surface-Modified Fillers for Long-Lasting, High-Performance All-Solid-State Li-Metal Batteries. Small 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H.; Passerini, S. Effect of fillers on the electrochemical and interfacial properties of PEO–LiN(SO2CF2CF3)2 polymer electrolytes. Electrochim. Acta 2004, 49, 1605–1612. [Google Scholar] [CrossRef]
- Sangeetha, M.; Mallikarjun, A.; Aparna, Y.; Reddy, M.V.; Kumar, J.S.; Sreekanth, T.; Reddy, M.J. Dielectric studies and AC conductivity of PVDF-HFP: LiBF4: EC plasticized polymer electrolytes. Mater. Today Proc. 2021, 44, 2168–2172. [Google Scholar] [CrossRef]
- Chen, S.; Wei, X.; Zhang, G.; Wang, X.; Zhu, J.; Feng, X.; Dai, H.; Ouyang, M. All-temperature area battery application mechanism, performance, and strategies. Innovation 2023, 4, 100465. [Google Scholar] [CrossRef]
| Sample ID | PEO-Coated SSPE | Electrical Resistivity r (Ω × cm) | Ionic Conductivity s (S/cm) |
|---|---|---|---|
| SSPE-0/PEO | PVDF-HFP/PEO | 4.48 × 107 | 1.8 × 10−6 |
| SSPE-1/PEO | PVDF-HFP-PPy/PEO | 4.30 × 107 | 4.5 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smdani, M.G.; Hasan, M.W.; Razzaq, A.A.; Xing, W. Electronically Conductive Polymer Enhanced Solid-State Polymer Electrolytes for All-Solid-State Lithium Batteries. Energies 2024, 17, 4295. https://doi.org/10.3390/en17174295
Smdani MG, Hasan MW, Razzaq AA, Xing W. Electronically Conductive Polymer Enhanced Solid-State Polymer Electrolytes for All-Solid-State Lithium Batteries. Energies. 2024; 17(17):4295. https://doi.org/10.3390/en17174295
Chicago/Turabian StyleSmdani, Md Gulam, Md Wahidul Hasan, Amir Abdul Razzaq, and Weibing Xing. 2024. "Electronically Conductive Polymer Enhanced Solid-State Polymer Electrolytes for All-Solid-State Lithium Batteries" Energies 17, no. 17: 4295. https://doi.org/10.3390/en17174295
APA StyleSmdani, M. G., Hasan, M. W., Razzaq, A. A., & Xing, W. (2024). Electronically Conductive Polymer Enhanced Solid-State Polymer Electrolytes for All-Solid-State Lithium Batteries. Energies, 17(17), 4295. https://doi.org/10.3390/en17174295






