Process Modelling of Integrated Bioethanol and Biogas Production from Organic Municipal Waste
Abstract
1. Introduction
2. Materials and Methods
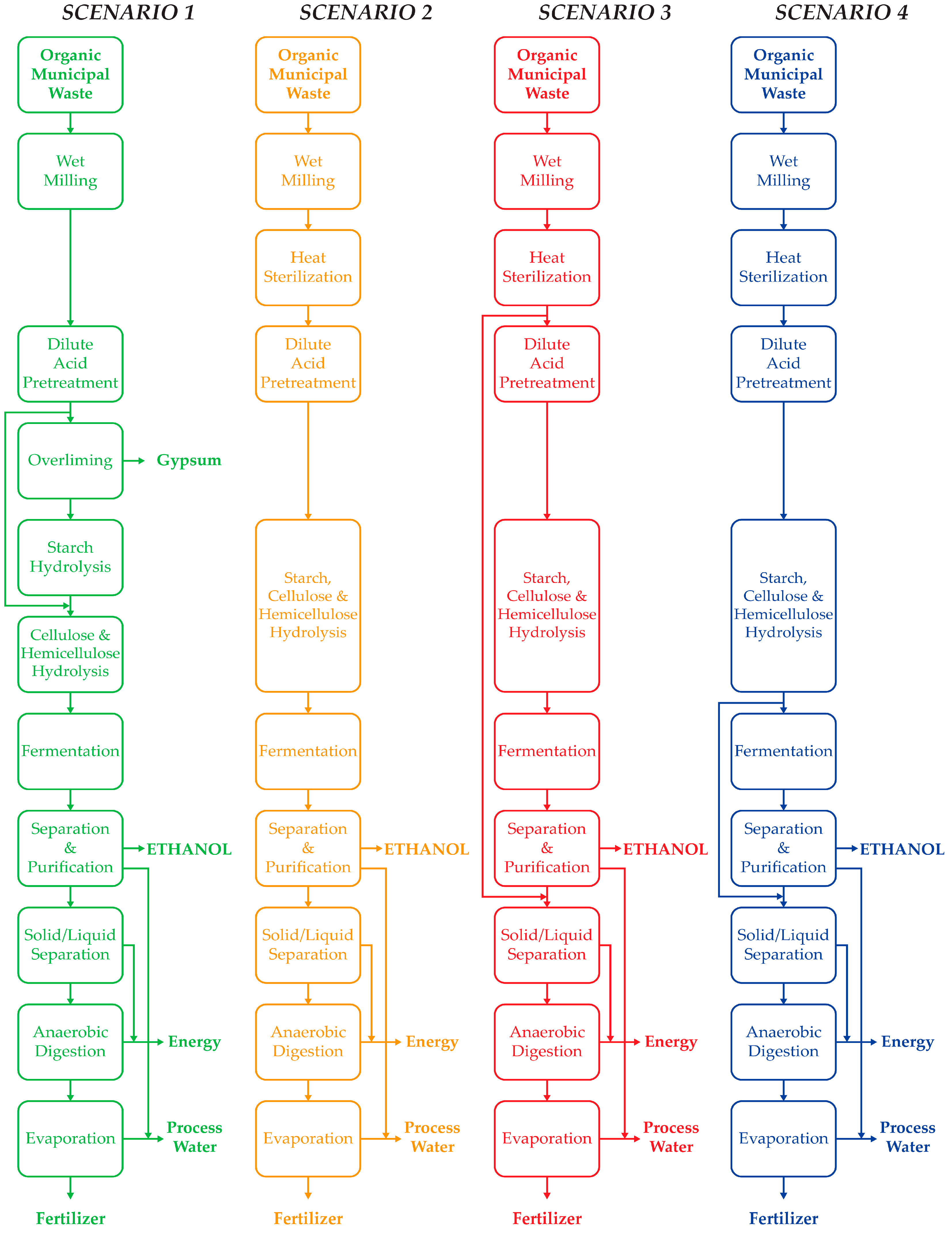
2.1. Process Description
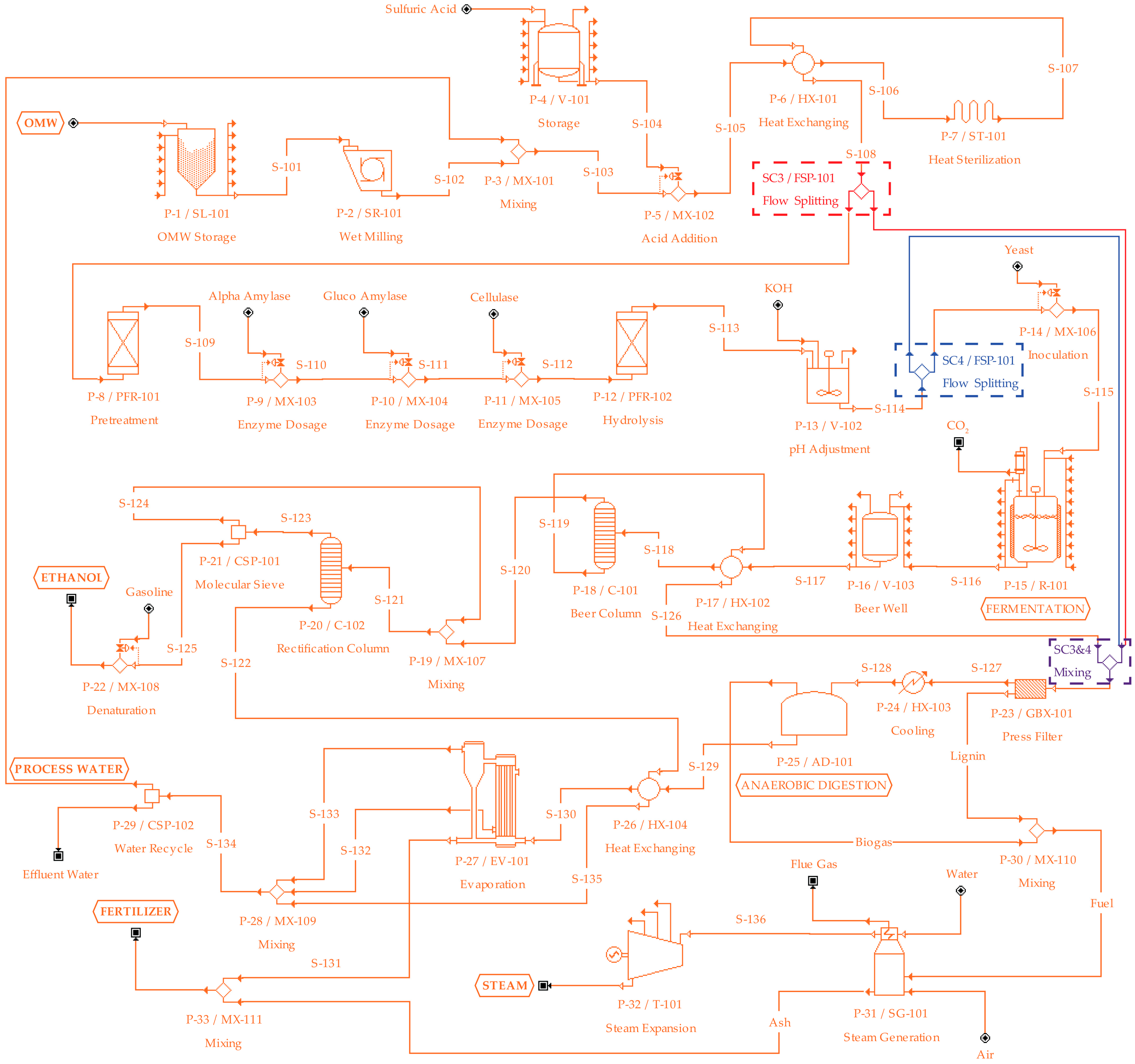
2.2. Process Design
| Procedure | Reaction with Mass Stoichiometric Coefficients | Conversion % | Reference |
|---|---|---|---|
| Pretreatment P-7/PFR-101 | 162 Cellulose + 18 Water → 180 Glucose | 13.04 | [39] |
| 132 Hemicellulose + 18 Water → 150 Xylose | 60.26 | ||
| 180 Glucose → 126 Hydroxymethylfurfural + 54 Water | 5.00 | ||
| 150 Xylose → 96 Furfural + 54 Water | 5.00 | ||
| 1 Lignin → 1 Soluble Lignin | 5.00 | ||
| 162 Starch + 18.02 Water → 180.16 Glucose | 10.00 | ||
| Overliming | 74.09 Ca(OH)2 + 98.08 H2SO4 → 136.14 CaSO4 + 36.03 Water | 100.00 | [2] |
| P-9/V-102 | 136.14 CaSO4 + 36.03 Water → 172.17 Gypsum | 100.00 | |
| Liquefaction | 162 Starch + 18.02 Water → 180.16 Glucose | 35.00 | [15] |
| P-16/PFR-102 | |||
| Saccharification | 162 Starch + 18.02 Water → 180.16 Glucose | 95.00 | [15] |
| P-18/PFR-103 | |||
| Hydrolysis | 162 Cellulose + 18 Water → 180 Glucose | 80.00 | [15] |
| P-21/PFR-104 | 132 Hemicellulose + 18 Water → 150 Xylose | 80.00 | |
| Fermentation | 180 Glucose → 88 Carbon Dioxide + 92 Ethanol | 95.00 | |
| P-23/R-101 | 150 Xylose → 73.33 Carbon Dioxide + 76.67 Ethanol | 70.00 | [15] |
| 0.22 Glucose + 0.41 Proteins → 0.56 Biomass | 70.00 | ||
| Anaerobic Digestion P-33/AD-101 | 1 Glucose + 1 Water → 3.3 Carbon Dioxide + 3.3 Methane | 50.00 | [41] |
| 43.70 Lipids + 24.50 Water → 15.25 Carbon Dioxide + 34.75 Methane | 70.00 | ||
| 1.12 Proteins + 14.50 Water → 3.75 Carbon Dioxide + 16.25 Methane | 70.00 | ||
| 1 Glucose → 5 Sludge + 5 Water | 90.00 | ||
| 1 Xylose → 1 Sludge | 90.00 | ||
| 1 Starch → 5 sludge + 4 Water | 86.00 |
3. Results and Discussion
3.1. Economic Analysis
3.1.1. Scenario 1 vs. Scenario 2
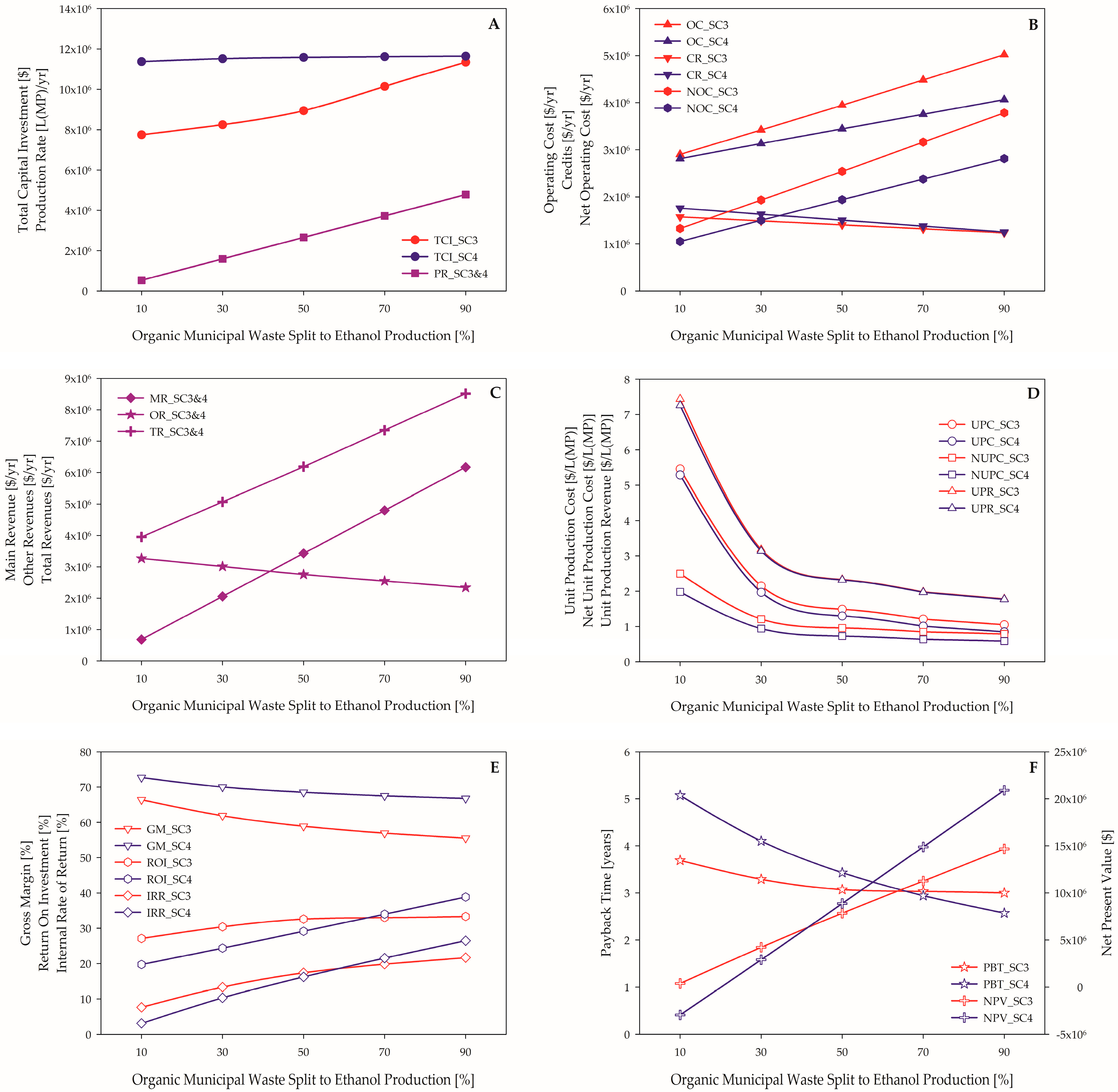
3.1.2. Scenario 3 vs. Scenario 4
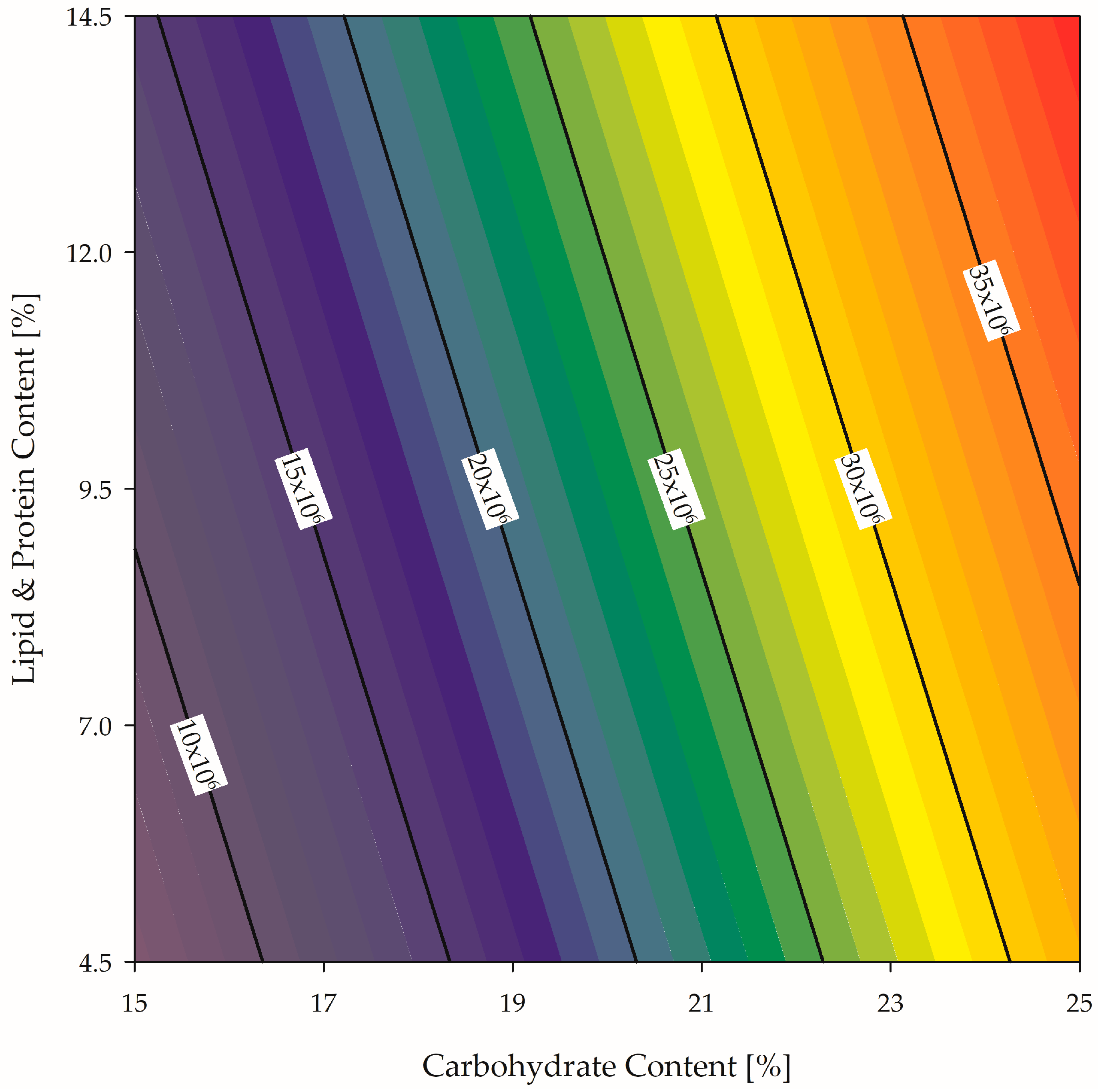
3.2. Sensitivity Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations and Acronyms
| B&H | Bosnia and Herzegovina |
| CFC | Contractor’s Fee and Contingency |
| CR | Credits |
| DFCC | Direct Fixed Capital Cost |
| EU | European Union |
| GHG | Greenhouse Gas |
| GM | Gross Margin |
| IRR | Internal Rate of Return |
| MP | Main Product |
| MR | Main Revenue |
| NOC | Net Operating Cost |
| NPV | Net Present Value |
| NUPC | Net Unit Production Rate |
| OC | Operating Cost |
| OMW | Organic Municipal Waste |
| OR | Other Revenues |
| PBT | Payback Time |
| PC | Purchase Cost |
| PR | Production Rate |
| QA | Quality Analysis |
| QC | Quality Control |
| ROI | Return on Investment |
| SC1 | Scenario 1 |
| SC2 | Scenario 2 |
| SC3 | Scenario 3 |
| SC4 | Scenario 4 |
| TCI | Total Capital Investment |
| TPC | Total Plant Cost |
| TPDC | Total Plant Direct Capital |
| TPIC | Total Plant Indirect Cost |
| TR | Total Revenues |
| UPC | Unit Production Cost |
| UPR | Unit Production Revenue |
References
- Alao, M.A.; Popoola, O.M.; Ayodele, T.R. Waste to energy nexus: An overview of technologies and implementation for sustainable development. Clean. Energy Syst. 2022, 3, 100034. [Google Scholar] [CrossRef]
- Vučurović, D.; Bajić, B.; Vučurović, V.; Jevtić-Mučibabić, R.; Dodić, S. Bioethanol Production from Spent Sugar Beet Pulp—Process Modeling and Cost Analysis. Fermentation 2022, 8, 114. [Google Scholar] [CrossRef]
- Directive (EU) 2023/2413 of the European Parliament and of the Council, Official Journal of the European Union. 2023. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L_202302413 (accessed on 24 April 2024).
- Framework Energy Strategy of Bosnia and Herzegovina until 2035, Official Gazette of BiH, No. 70/18, Sarajevo. 2018. Available online: http://www.sluzbenilist.ba/page/akt/du8gztz5k76kjn45hnsd2ZBw (accessed on 29 April 2024).
- Bajić, B.; Dodić, S.; Vučurović, D.; Dodić, J.; Grahovac, J. Waste-to-energy status in Serbia. Renew. Sust. Energ. Rev. 2015, 50, 1437–1444. [Google Scholar] [CrossRef]
- Naveenkumar, R.; Iyyappan, J.; Pravin, R.; Kadry, S.; Han, J.; Sindhu, R.; Awasthi, M.K.; Rokhum, S.L.; Baskar, G. A strategic review on sustainable approaches in municipal waste management and energy recovery: Role of artificial intelligence, economic stability and life cycle assessment. Bioresour. Technol. 2023, 379, 129044. [Google Scholar] [CrossRef]
- Katinas, V.; Marciukaitis, M.; Perednis, E.; Dzenajaviciene, E.F. Analysis of biodegradable waste use for energy generation in Lithuania. Renew. Sustain. Energy Rev. 2019, 101, 559–567. [Google Scholar] [CrossRef]
- Prasoulas, G.; Gentikis, A.; Konti, A.; Kalantzi, S.; Kekos, D.; Mamma, D. Bioethanol Production from Food Waste Applying the Multienzyme System Produced On-Site by Fusarium oxysporum F3 and Mixed Microbial Cultures. Fermentation 2020, 6, 39. [Google Scholar] [CrossRef]
- Mosquera-Toscano, H.G.; González-Barceló, O.; Valdez-Vazquez, I.; Durán-Moreno, A. Ethanol and Methane Production from the Organic Fraction of Municipal Solid Waste in a Two-Stage Process. BioEnergy Res. 2024, 17, 634–645. [Google Scholar] [CrossRef]
- Mahmoodi, P.; Karimi, K.; Taherzadeh, M.J. Hydrothermal processing as pretreatment for efficient production of ethanol and biogas from municipal solid waste. Bioresour. Technol. 2018, 261, 166–175. [Google Scholar] [CrossRef]
- Varghese, S.; Demeke, M.M.; Verhe, R.; Redant, E.; Cruyssen, C.V.; Thevelein, J.M. Process optimization for saccharification and fermentation of the Organic Fraction of Municipal Solid Waste (OFMSW) to maximize ethanol production performance. Bioresour. Technol. Rep. 2023, 24, 101681. [Google Scholar] [CrossRef]
- Nishimura, H.; Tan, L.; Sun, Z.-Y.; Tang, Y.-Q.; Kida, K.; Morimura, S. Efficient production of ethanol from waste paper and the biochemical methane potential of stillage eluted from ethanol fermentation. Waste Manag. 2016, 48, 644–651. [Google Scholar] [CrossRef]
- Morero, B.; Vicentin, R.; Campanella, E.A. Assessment of biogas production in Argentina from co-digestion of sludge and municipal solid waste. Waste Manag. 2017, 61, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Achinas, S.; Martherus, D.; Krooneman, J.; Euverink, G.J.W. Preliminary Assessment of a Biogas-Based Power Plant from Organic Waste in the North Netherlands. Energies 2019, 12, 4034. [Google Scholar] [CrossRef]
- Mahmoodi, P.; Karimi, K.; Taherzadeh, M.J. Efficient conversion of municipal solid waste to biofuel by simultaneous dilute-acid hydrolysis of starch and pretreatment of lignocelluloses. Energy Convers. Manag. 2018, 166, 569–578. [Google Scholar] [CrossRef]
- Moreno, A.D.; Magdalena, J.A.; Oliva, J.M.; Greses, S.; Lozano, C.C.; Latorre-Sanchez, M.; Negro, M.J.; Susmozas, A.; Iglesias, R.; Llamas, M.; et al. Sequential bioethanol and methane production from municipal solid waste: An integrated biorefinery strategy towards cost-effectiveness. Process Saf. Environ. Prot. 2021, 146, 424–431. [Google Scholar] [CrossRef]
- Cardenas-Fernandez, M.; Bawn, M.; Hamley-Bennett, C.; Bharat, P.K.V.; Subrizi, F.; Suhaili, N.; Ward, D.P.; Bourdin, S.; Dalby, P.A.; Hailes, H.C.; et al. An Integrated Biorefinery Concept for Conversion of Sugar Beet Pulp into Value-Added Chemicals and Pharmaceutical Intermediates. Faraday Discuss. 2017, 202, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Demichelis, F.; Fiore, S.; Pleissner, D.; Venus, J. Technical and economic assessment of food waste valorization through a biorefinery chain. Renew. Sust. Energy Rev. 2018, 94, 38–48. [Google Scholar] [CrossRef]
- Mupondwa, E.; Li, X.; Tabil, L. Integrated bioethanol production from tricitale grain and lignocellulosic straw in Western Canada. Ind. Crops Prod. 2018, 117, 75–87. [Google Scholar] [CrossRef]
- Nishimura, H.; Tan, L.; Kira, N.; Tomiyama, S.; Yamada, K.; Sun, Z.-Y.; Tang, Y.-Q.; Morimura, S.; Kida, K. Production of ethanol from a mixture of waste paper and kitchen waste via a process of successive liquefaction, presaccharification, and simultaneous saccharification and fermentation. Waste Manag. 2017, 67, 86–94. [Google Scholar] [CrossRef]
- Li, X.; Mupondwa, E. Commercial feasibility of an integrated closed-loop ethanol-feedlot-biodigester system based on triticale feedstock in Canadian Prairies. Renew. Sustain. Energy Rev. 2018, 97, 401–413. [Google Scholar] [CrossRef]
- Oleskowicz-Popiel, P.; Kadar, Z.; Heiske, S.; Klein-Marcuschamer, D.; Simmons, B.A.; Blanch, H.W.; Schmidt, J.E. Co-production of ethanol, biogas, protein fodder and natural fertilizer in organic farming—Evaluation of a concept for a farm-scale biorefinry. Biores. Technol. 2012, 104, 440–446. [Google Scholar] [CrossRef]
- Cаnizales, L.; Rojas, F.; Pizzaro, C.A.; Caciedo-Ortega, N.H.; Villegas-Torres, M.F. SuperPro Designer user oriented software used for analyzing the techno-economic feasibility of electronical energy generation from sugarcane vinasse in Columbia. Processes 2020, 8, 1180. [Google Scholar] [CrossRef]
- Khajeeram, S.; Unrean, P. Techno-economic assessment of high-solid simultaneous saccharification and fermentation and economic impacts of yeast consortium and on-site enzyme production technologies. Energy 2017, 122, 194–203. [Google Scholar] [CrossRef]
- Ortiz, I.; Valdez-Vazquez, I.; Hernandez-Vazquez, A.; Olivares-Hernandez, R.; Carrillo-Reyes, J.; Alatriste-Mondragon, F.; Buitron, G.; Razo-Flores, E. Process design and economic evaluation for methane and electricity production from Agave bagasse using different pretreatments. Energy Convers. Manag. 2024, 306, 118319. [Google Scholar] [CrossRef]
- Rahman, S.S.A.; Pasupathi, S.; Karuppiah, S. Statistical optimization, kinetic modeling, and techno-economic analysis for the production of high molecular mass dextran using sugarcane industrial waste—Molasses. Bioresour. Technol. Rep. 2024, 27, 101902. [Google Scholar] [CrossRef]
- Heinzle, E.; Biwer, A.P.; Cooney, C.L. Development of Sustainable Bioprocesses: Modeling and Assessment; John Wiley & Sons Ltd.: Hoboken, NJ, USA; Chichester, UK, 2006; The Atrium, Southern Gate; ISBN 978-0-470-01559-9. [Google Scholar]
- Sokolowski, J.A.; Banks, C.M. Modeling and Simulation Fundamentals: Theoretical Underpinnings and Practical Domains; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; ISBN 978-0-470-48674-0. [Google Scholar]
- Harrison, R.G.; Todd, P.W.; Rudge, S.R.; Petrides, D.P. Bioseparations Science and Engineering, 2nd ed.; Oxford University Press: Oxford, UK, 2015; ISBN 978-0-19-539181-7. [Google Scholar]
- Petrides, D.; Cooney, C.L.; Evans, L.B.; Field, R.P.; Snoswell, M. Bioprocess simulation: An integrated approach to process development. Computers Chem. Eng. 1989, 13, 553–561. [Google Scholar] [CrossRef]
- Agency for Statistics of Bosnia and Herzegovina—BHAS, First Release, Environment, Sarajevo. 2023. Available online: https://bhas.gov.ba/data/Publikacije/Saopstenja/2023/ENV_01_2022_Y1_1_BS.pdf (accessed on 25 April 2024).
- Government of Republic of Srpska. Waste Management Plan for Republic of Srpska. 2019. Available online: https://www.vladars.net/sr-SP-Cyrl/Vlada/Ministarstva/mgr/Documents/%D0%A0%D0%B5%D0%BF%D1%83%D0%B1%D0%BB%D0%B8%D1%87%D0%BA%D0%B8%20%D0%BF%D0%BB%D0%B0%D0%BD%20%D1%83%D0%BF%D1%80%D0%B0%D0%B2%D1%99%D0%B0%D1%9A%D0%B0%20%D0%BE%D1%82%D0%BF%D0%B0%D0%B4%D0%BE%D0%BC%20%D0%B7%D0%B0%20%D0%BF%D0%B5%D1%80%D0%B8%D0%BE%D0%B4%202019-2029_599263879.pdf (accessed on 13 July 2024).
- Bibra, M.; Samanta, D.; Kumar Sharma, N.; Singh, G.; Johnson, G.R.; Sani, R.K. Food waste to bioethanol: Opportunities and Challenges. Fermentation 2023, 9, 8. [Google Scholar] [CrossRef]
- Millati, R.; Niklasson, C.; Taherzadeh, M.J. Effect of pH, time and temperature of overliming on detoxification of dilute-acid hydrolyzates for fermentation by Saccharomyces cerevisiae. Process Biochem. 2002, 38, 515–522. [Google Scholar] [CrossRef]
- Seong, H.A.; Lee, J.S.; Yoon, S.Y.; Song, W.Y.; Shin, S.Y. Fermentation characteristics of acid hydrolysates by different neutralizing agents. Int. J. Hydrogen Energy 2016, 41, 16365–16372. [Google Scholar] [CrossRef]
- Martinez, A.; Rodriguez, M.E.; York, S.W.; Preston, J.F.; Ingram, L.O. Effects of Ca(OH)2 treatments (‘overliming’) on the composition and toxicity of bagasse hemicellulose hydrolysates. Biotechnol. Bioeng. 2000, 69, 526–536. [Google Scholar] [CrossRef]
- Smith, Z.; Isaac, B.; Tumuluru, J.S.; Yancey, N. Grinding and Pelleting Characteristics of Municipal Solid Waste Fractions. Energies 2024, 17, 29. [Google Scholar] [CrossRef]
- Kappe, J.; Sautner, K.H. Process for the Manufacture of Bioethanol. World Intellectual Property Organization. 2009, WO2009072878A1. Available online: https://patents.google.com/patent/WO2009072878A1/en (accessed on 1 July 2024).
- Kumar, D.; Murthy, G.S. Impact of pretreatment and downstream processing technologies on economics and energy in cellulosic ethanol production. Biotechnol. Biofuels 2011, 4, 27. [Google Scholar] [CrossRef]
- Ebrahimian, F.; Khoshnevisan, B.; Mohammadi, A.; Karimi, K.; Birkved, M. A biorefinery platform to valorize organic fraction of municipal solid waste to biofuels: An early environmental sustainability guidance based on life cycle assessment. Energy Convers. Manag. 2023, 283, 116925. [Google Scholar] [CrossRef]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. A Review of the Role of Critical Parameter in the Design and Operation of Biogas Production Plants. Appl. Sci. 2019, 9, 1915. [Google Scholar] [CrossRef]
- Gupte, A.P.; Di Vita, N.; Myburgh, M.W.; Cripwell, R.A.; Basaglia, M.; Van Zyl, W.H.; Viljoen-Bloom, M.; Casella, S.; Favaro, L. Consolidated bioprocessing of the organic fraction of municipal waste into bioethanol. Energy Convers. Manag. 2024, 302, 118105. [Google Scholar] [CrossRef]
- Sánchez-Martín, L.; Ortega Romero, M.; Llamas, B.; Suárez Rodríguez, M.d.C.; Mora, P. Cost Model for Biogas and Biomethane Production in Anaerobic Digestion and Upgrading. Case Study: Castile and Leon. Materials 2023, 16, 359. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Salim, S.; Cha, J.M.; Park, S. Development of Total Capital Investment Estimation Module for Waste Heat Power Plant. Energies 2019, 12, 1492. [Google Scholar] [CrossRef]
- Tax Administration Office of Republic of Srpska. Decision on the Lowest Salary in the Republic of Srpska for 2024. Available online: https://poreskaupravars.org/wp-content/uploads/2024/02/Scan05012024.pdf (accessed on 1 July 2024).
- ChemAnalyst. Commodity Prices Database. Available online: https://www.chemanalyst.com/ (accessed on 1 July 2024).
- Global Petrol Prices. Gasoline Prices. Available online: https://www.globalpetrolprices.com/gasoline_prices/ (accessed on 1 July 2024).
- Republic of Srpska Institute of Statistics. Statistical Yearbook Republic of Srpska 2023. Available online: https://www.rzs.rs.ba/static/uploads/bilteni/godisnjak/2023/StatistickiGodisnjak_2023_WEB.pdf (accessed on 1 July 2024).
- Safaat, M.; Wulan, P.P.D.K. Technoeconomic Analysis of Integrated Bioethanol from Elephant Grass (Pennisetum purpureum) with Utilization of Its Residue and Lignin. Ind. J. Teknol. Dan Manaj. Agroindustri 2022, 11, 64–80. [Google Scholar] [CrossRef]
- Pan, S.; Zabed, H.M.; Zhao, M.; Qi, X.; Wei, Y. Techno-economic and life cycle assessments for bioenergy recovery from acid-hydrolyzed residues of sugarcane bagasse in the biobased xylose production platform. J. Clean. Prod. 2023, 400, 136718. [Google Scholar] [CrossRef]
- Global Petrol Prices. Ethanol Prices. Available online: https://www.globalpetrolprices.com/ethanol_prices/ (accessed on 1 July 2024).
- Statista. Monthly Price for Fertilizers from 2022 to 2024, by Product. Available online: https://www.statista.com/statistics/1288602/quarterly-fertilizer-price-development-by-product/ (accessed on 1 July 2024).



| Component | Flowrate kg/h | Mass Composition % |
|---|---|---|
| Moisture | 3880.800 | 64.6800 |
| Starch | 607.362 | 10.1227 |
| Cellulose | 329.958 | 5.4993 |
| Hemicellulose | 173.988 | 2.8998 |
| Monosaccharides | 107.022 | 1.7837 |
| Lignin | 186.066 | 3.1011 |
| Lipids | 270.834 | 4.5139 |
| Protein | 301.752 | 5.0292 |
| Ash | 142.218 | 2.3703 |
| Total | 6000.000 | 100.0000 |
| Item | SC1 | SC2 |
|---|---|---|
| Total Plant Direct Capital (TPDC) | ||
| 1. Equipment Purchase Cost | 10,430,000 | 6,415,000 |
| 2. Installation | 1,739,000 | 1,042,000 |
| 3. Process Piping | 626,000 | 385,000 |
| 4. Instrumentation | 313,000 | 192,000 |
| 5. Insulation | 104,000 | 64,000 |
| 6. Electrical | 417,000 | 257,000 |
| 7. Buildings | 522,000 | 321,000 |
| 8. Yard Improvement | 209,000 | 128,000 |
| 9. Auxiliary Facilities | 417,000 | 257,000 |
| TPDC | 14,777,000 | 9,061,000 |
| Total Plant Indirect Cost (TPIC) | ||
| 10. Engineering | 443,000 | 272,000 |
| 11. Construction | 1,034,000 | 634,000 |
| TPIC | 1,478,000 | 906,000 |
| Total Plant Cost (TPC = TPDC + TPIC) | ||
| TPC | 16,255,000 | 9,967,000 |
| Contractor’s Fee & Contingency (CFC) | ||
| 12. Contractor’s Fee | 488,000 | 299,000 |
| 13. Contingency | 1,138,000 | 698,000 |
| CFC = 12 + 13 | 1,625,000 | 997,000 |
| Direct Fixed Capital Cost (DFC = TPC + CFC) | ||
| DFC | 17,880,000 | 10,964,000 |
| 14. Working Capital | 393,000 | 340,000 |
| 15. Startup and Validation | 536,000 | 329,000 |
| Total Capital Investment (TCI = DFC + 14 + 15) | ||
| TCI | 18,810,000 | 11,633,000 |
| Cost Item | Unit Cost | Source |
|---|---|---|
| Labor | ||
| Labor | 5.750 $/h | [45] |
| Bulk Materials | ||
| α-amylase | 3.000 $/kg | Local supplier |
| Ca(OH)2 | 0.130 $/kg | [46] |
| Cellulase | 3.500 $/kg | Local supplier |
| Gasoline | 1.320 $/kg | [47] |
| Glucoamylase | 4.500 $/kg | Local supplier |
| H2SO4 (98% w/w) | 0.059 $/kg | [46] |
| KOH | 0.580 $/kg | [46] |
| OMW | 0.000 $/kg | N.A. |
| Water | 0.0003 $/kg | [48] |
| Biomass | 5.000 $/kg | Local supplier |
| Utilities | ||
| Std Power | 0.070 $/kWh | [48] |
| Steam | 12.000 $/MT | [48] |
| Cooling Water | 0.050 $/MT | [48] |
| Chilled Water | 0.400 $/MT | [48] |
| CT Water | 0.070 $/MT | [48] |
| Item | SC1 | SC2 | ||
|---|---|---|---|---|
| Value | Unit | Value | Unit | |
| Revenue/Credit Production Rates | ||||
| Gypsum (Revenue) | 2263 | MT/yr | - | - |
| Ethanol (Main Revenue) | 5,200,807 | L(STP)/yr | 5,314,850 | L(STP)/yr |
| Steam (Revenue) | 42,497 | MT/yr | 41,895 | MT/yr |
| Fertilizer (Revenue) | 9208 | MT/yr | 10,204 | MT/yr |
| Electricity (Credit) | 7,568,590 | kWh/yr | 7,444,602 | kW-h/yr |
| Annual Revenues/Credits | ||||
| Gypsum (Revenue) | 22,632 | $/yr | - | - |
| Ethanol (Main Revenue) | 6,709,041 | $/yr | 6,856,156 | $/yr |
| Steam (Revenue) | 509,966 | $/yr | 502,741 | $/yr |
| Fertilizer (Revenue) | 1,519,370 | $/yr | 1,683,584 | $/yr |
| Electricity (Credit) | 1,210,974 | $/yr | 1,191,136 | $/yr |
| Total Revenues | 8,761,009 | $/yr | 9,042,481 | $/yr |
| Total Credits | 1,210,974 | $/yr | 1,191,136 | $/yr |
| Operating Cost (OC) | ||||
| Actual OC | 4,995,000 | $/yr | 4,220,000 | $/yr |
| Net OC | 3,784,000 | $/yr | 3,029,000 | $/yr |
| Unit Production Cost/Revenue | ||||
| Unit Production Cost (UPC) | 0.96 | $/L(STP) MP | 0.79 | $/L(STP) MP |
| Net Unit Production Cost (NUPC) | 0.73 | $/L(STP) MP | 0.57 | $/L(STP) MP |
| Unit Production Revenue (UPR) | 1.68 | $/L(STP) MP | 1.70 | $/L(STP) MP |
| Gross Profit | 4,977,000 | $/yr | 6,014,000 | $/yr |
| Taxes (20%) | 995,000 | $/yr | 1,203,000 | $/yr |
| Net Profit | 3,982,000 | $/yr | 4,811,000 | $/yr |
| Gross Margin (GM) | 56.81 | % | 66.51 | % |
| Return On Investment (ROI) | 21.17 | % | 41.36 | % |
| Payback Time (PBT) | 4.72 | years | 2.42 | years |
| Internal Rate of Return (IRR) | 13.61 | % | 28.87 | % |
| Net Present Value (NPV) | 9,875,468 | $ | 23,951,483 | $ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gegić, B.; Vučurović, D.; Dodić, S.; Bajić, B. Process Modelling of Integrated Bioethanol and Biogas Production from Organic Municipal Waste. Energies 2024, 17, 4286. https://doi.org/10.3390/en17174286
Gegić B, Vučurović D, Dodić S, Bajić B. Process Modelling of Integrated Bioethanol and Biogas Production from Organic Municipal Waste. Energies. 2024; 17(17):4286. https://doi.org/10.3390/en17174286
Chicago/Turabian StyleGegić, Brankica, Damjan Vučurović, Siniša Dodić, and Bojana Bajić. 2024. "Process Modelling of Integrated Bioethanol and Biogas Production from Organic Municipal Waste" Energies 17, no. 17: 4286. https://doi.org/10.3390/en17174286
APA StyleGegić, B., Vučurović, D., Dodić, S., & Bajić, B. (2024). Process Modelling of Integrated Bioethanol and Biogas Production from Organic Municipal Waste. Energies, 17(17), 4286. https://doi.org/10.3390/en17174286







