Abstract
During liquid hydrogen bunkering into a cryogenic tank, boil-off losses occur due to the high thermal gradient between liquid hydrogen and the warm surface of the tank. This leads to gaseous hydrogen release. Such losses constitute a significant drawback in using hydrogen as a fuel for maritime applications where bunkering operations are regularly carried out, thereby constituting a significant loss along the liquid hydrogen pathway. Due to the inherently low temperature of liquid hydrogen, boil-off losses are always present. Some boil-off losses cannot be eliminated because they are thermodynamically constrained or intrinsic to the system’s design. Boil-off recovery methods can be implemented to capture the boil-off; however, those solutions come with an additional cost and system complexities. Hence, this paper investigates the feasibility of minimizing boil-off losses during the first bunkering of liquid hydrogen or refilling of liquid hydrogen in an empty cryogenic tank by first precooling the cryogenic tank surface to decrease the thermal gradient between the liquid hydrogen and the tank surface/wall. In this paper, different media for precooling a cryogenic tank are evaluated to assess the boil-off reduction potential and the associated costs in order to identify the most suitable solution. The assessment has been carried out based on analytical formulation.
1. Introduction
Hydrogen produced from renewable sources (green hydrogen), among others, shows the potential of enabling carbon-free emissions in several industries, including the maritime sector [1]. The maritime sector is faced with making a transition from conventional/fossil fuel to alternative fuel. This is due to the increasingly stringent regulations on emissions set by the International Maritime Organization (IMO), with a set target of reaching net-zero greenhouse gas (GHG) emissions by 2050 [2].
The majority of commercial hydrogen production comes from fossil fuels using methods like steam natural gas reforming [3], oil/naphtha reforming [4] and coal partial oxidation [5]. Another common method of producing hydrogen is by electrolysis, which splits hydrogen from water using an electric current. Other methods of hydrogen production using biomass, solar energy and microbes are already being researched [6]. Novel technologies utilizing waste plastics and polymers for the production of hydrogen are under currently investigation [4,7,8].
Hydrogen is the lightest element in the periodic table. Although hydrogen is the most prevalent element in the universe, hydrogen gas is not a readily found resource on earth [9]. Hydrogen gas is characterized as a colorless, odorless and tasteless gas with a density of 0.083 kg/m3. At room temperature and pressure, the gravimetric energy density of hydrogen is 33.3 kWh/kg. The volumetric energy density of gaseous hydrogen is only 3 kWh/m3 [10]. Such a low volumetric energy density presents a significant drawback for storage onboard ships, where space is very limited. An alternative storage solution of pure hydrogen includes compressed bundles at 350 bar or 700 bar pressure offering, respectively, a volumetric energy density of 766 kWh/m3 and 1309 kWh/m3 [10]. Liquefaction of hydrogen at a temperature of 20 K (−253 °C) and atmospheric pressure enables a volumetric energy density of 2363 kWh/m3, thus making it an attractive hydrogen storage solution for maritime applications.
There are certain drawbacks to using liquid hydrogen: the process of liquefying hydrogen is costly (costing more than three times the energy of compression to 700 bar), setback distances for liquid hydrogen are stricter, and boil-off losses along the liquid hydrogen pathway may occur [11].
Furthermore, handling cryogenic hydrogen requires maintaining the hydrogen at a temperature lower than its boiling temperature of 20 K (−253 °C). This can be performed by minimizing heat ingress into the liquid. Heat input results in the evaporation of liquid hydrogen, forming gaseous hydrogen. The accumulation of gaseous hydrogen leads to a rise in pressure inside the tank, reaching the tank’s maximum pressure. Venting the boil-off gases is required to maintain safe pressure levels inside the tank. The released hydrogen is then evacuated to the environment. Upon initiation, this sequence can continue indefinitely inside the tank, thereby constituting significant energy wastage. Increasing the efficiency, and, hence, reducing the cost of LH2 storage, requires solutions for evaporation prevention or boil-off gas recovery, such as zero boil-off, reliquefication and direct compression [12]. The feasibility of implementing these measures should be examined as additional infrastructure, space, energy and cost are required. Consequently, it is crucial to initially reduce boil-off losses whenever practicable.
Liquid hydrogen boil-off losses in a cryogenic hydrogen storage system may be generated due to ortho-para conversion, heat ingress from the outer environment, sloshing and flashing [13]. Another source of boil-off occurs during tank refueling due to the large thermal gradient between the tank walls and the LH2. According to [14], this problem is predominant in small cryogenic tanks with low surface-to-volume ratio. Moreover, small cryogenic tanks are subject to high-frequency refilling cycles, which would result in more boil-off if the inner vessel was not adequately cooled down during the fast-filling operation [15]. Furthermore, it has been shown by [11] that the most significant source of boil-off losses occurs during filling operations along the liquid hydrogen pathway.
Precooling the cryogenic tank before refueling with a medium such as liquid nitrogen has been investigated with a focus on boil-off loss reduction [14]. The results showed that boil-off losses dropped significantly with precooling from 38–60% of the total hydrogen mass to 5–9%.
This study investigates the effectiveness of other precooling mediums with a focus on the boil-off reduction potential, the amount of precooling medium required and the economic aspect of the process, in which not only the cost of the precooling mediums is considered in the evaluation but also the cost of the lost liquid hydrogen due to boil-off. The precooling mediums at the center of this study are liquid nitrogen and liquid helium.
2. Materials and Methods
In this section, the methods adopted in evaluating the boil-off losses and estimating the cost are described. In addition, the details of the cryotank and fluids employed in this investigation are provided.
2.1. Method Description
An analytical method is used in this work to estimate the boil-off losses during the bunkering of an empty cryotank. In the first case, the total quantity of heat transfer (invariably the amount of heat that needs to be removed from the cryotank) is estimated, and the boil-off losses due to the heat are evaluated. The tank is then precooled with liquid nitrogen/liquid helium. Similar calculations are performed to assess the implications of using precooling fluids to precool the tank. The mass of boil-off losses is evaluated for three different cases—maximum, mean and minimum. This is dependent on whether the evaporated gases absorb additional heat in the tank or not. The maximum case assumes that the evaporated gases leave the tank without absorbing additional heat. This results in an overestimation of the boil-off losses. The minimum case assumes that the boil-off gases absorb additional heat before exiting the tank. Therefore, it is assumed that the evaporated gases exit the tank at a temperature close to the temperature of the cryotank. For the mean case, the assumption is that the evaporated gases exit the tank at half the temperature interval between the fluid and the cryotank.
The economic impact in terms of the cost of the precooling fluids used is evaluated, as well as the cost of liquid hydrogen lost as boil-off. Figure 1 shows the process flowchart adopted in this study. The material specifications for the tank were first defined, and the boundary/initial conditions used for the analyses were fixed. The amount of boil-off-losses from the tank with and without precooling were then estimated using analytical formulations and compared. Liquid nitrogen and liquid helium are used as the precooling fluids in this study. Furthermore, the cost of precooling the cryogenic tank with precooling fluids is estimated. The cost of the piping and instrumentation is not included in this study; only the cost of the required amount of the precooling fluids (liquid nitrogen and liquid helium) is included. This is so because the study emphasizes the initial bunkering process of the cryogenic tank, and hence, the same piping and instrumentation are assumed to be used for all of the fluids. Moreover, there are also fixed piping and instrumentation for nitrogen and helium found in liquid hydrogen systems used for tank-purging operations.
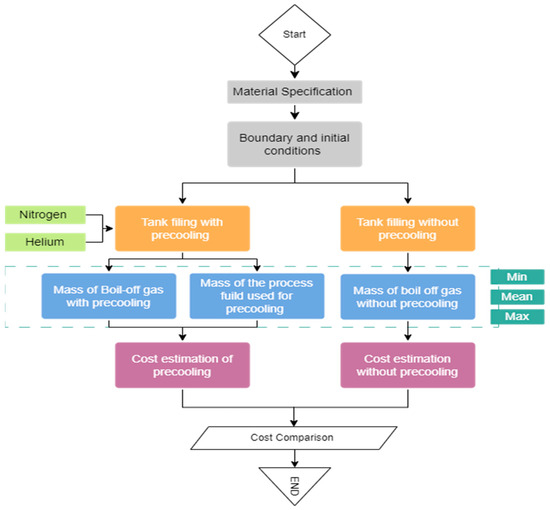
Figure 1.
Flowchart of the evaluation process.
2.2. Material Specification
This section provides more details about the reference tank, the materials/properties of the tank and the fluids utilized in the analysis.
2.2.1. Reference Tank
The reference tank used as a case study in this investigation is a double-wall vacuum-insulated tank with an inner tank volume of 2.66 m3. The inner and outer tanks are modeled as cylinders with elliptical end caps (see Figure 2).
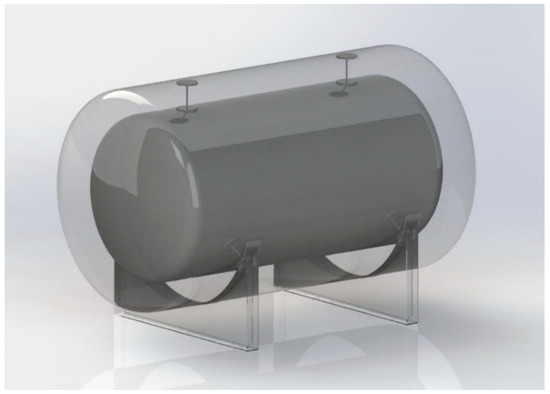
Figure 2.
Three-dimensional rendering of the LH2 tank.
Table 1 shows the inner tank mass for the two wall materials (Section 2.2.2) considered in this work. The thickness of the tank wall is assumed to be the same for both materials. The reference tank is modeled using SOLIDWORKS® Professional 2022 SP4.0 software, and the properties are obtained from the CAD model.

Table 1.
Parameter of the LH2 inner tank.
It is noteworthy that the inner tank’s mass is used for the analysis as its temperature needs to be brought to the temperature of liquid hydrogen during the first filling/bunkering operation.
2.2.2. Tank Materials and Properties
Two different cryogenic tank wall materials were investigated in this study: SS 304 and Al 5083-O. The most relevant tank wall material property considered in this investigation is the specific heat of the materials used. This property is obtained from [16] for both materials. Since specific heat is temperature-dependent, the mean values of the specific heat of the materials are used to carry out the analyses. This is demonstrated for Al 5083-O and SS 304 in Figure 3 for different temperature intervals.
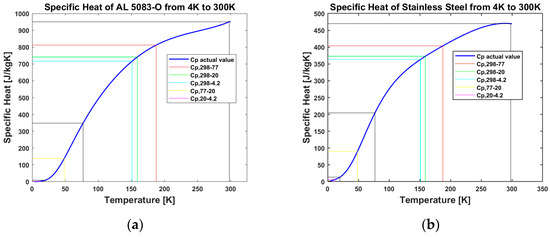
Figure 3.
Diagram of specific heats for tank material: (a) for AL 5083-O tank; (b) for SS 304 tank.
As shown in Figure 3, cp,298-77 represents the mean specific heat of the material between temperatures of 298 K and 77 K. The same approach is applied to the other temperature intervals. The mean specific heat values of the tank wall materials used for this investigation are summarized in Table 2.

Table 2.
Mean specific heat values for tank materials at different temperatures.
2.2.3. Liquid Hydrogen and Precooling Fluids
The relevant properties of liquid hydrogen and the precooling fluids used in this study are tabulated in Table 3. It is assumed that the atmospheric temperature is the initial condition of the tank walls before the bunkering/filling of liquid hydrogen and, in the case of precooling, before the filling of the precooling fluids—liquid nitrogen/liquid helium.

Table 3.
Main thermal properties of the process fluids used in the study.
2.3. Mass of Boil-Off Losses
The mass of the boil-off gas can be estimated using Equation (1) by dividing the heat that needs to be removed from the tank by the enthalpy of the cryogenic fluid.
where is the total amount of heat transfer, and hence the heat to be removed from the cryotank, and is the vaporization enthalpy of the fluids (hydrogen, nitrogen and helium). comprises three parts as shown in Equation (2).
is the heat that needs to be removed from the tank wall, represents the heat that needs to be removed from the insulation, and is the heat that needs to be removed from the gas inside the cryotank. These different heat components can be obtained from the formulation given in Equations (3)–(5).
It has been shown that the heat that needs to be removed due to the insulation and the gas inside the cryotank contributes less than 7% of the total [7], and it is, therefore, not included in the current analysis. Moreover, vacuum insulation is used in this investigation, and the inner tank is assumed to be at atmospheric temperature.
The mass of boil-off losses is calculated for different cases—maximum, mean and minimum—depending on whether the evaporated gases absorb additional heat in the tank or not. Equation (6) is used for the estimation of the maximum case with the assumption that the evaporated gases leave the tank without absorbing additional heat. Such an assumption, therefore, overestimates the boil-off losses.
The minimum boil-off is calculated by assuming that the boil-off gases absorb additional heat before exiting the tank. Therefore, it is assumed that the vapor exits the tank at a temperature close to the temperature of the cryotank. By extending Equation (6), this assumption is implemented, which results in an underestimation of the boil-off losses as shown in Equation (7).
The minimum and maximum boil-off calculations represent the upper and lower limits for boil-off losses. A mean value is also calculated as a third value for the boil-off losses with the assumption that the evaporated gases absorb half of the heat before exiting the tank. Mean boil-off losses are calculated using Equation (8).
where is the specific heat of the fluid being considered.
In the case of precooling, the mass of the boil-off losses of the precooling fluids is regarded as the quantity of the precooling fluids required to precool the tank.
2.4. Cost Analysis
In addition to quantifying the losses of liquid hydrogen due to boil-off, an economic assessment is performed to evaluate the cost implication of the boil-off losses with and without tank precooling. The cost analysis is performed by multiplying the unit cost of the fluids (EUR/kg) by the overall quantity of the fluids (liquid hydrogen, liquid nitrogen and liquid helium) lost in the filling/precooling process. Table 4 lists the unit cost in EUR/kg for liquid helium, liquid hydrogen and liquid nitrogen.

Table 4.
Unit costs for fluids.
Helium, a rare element on earth, is facing a potential supply shortage, a concern echoed by several researchers. This scarcity is a crucial factor contributing to the high volatility in helium prices. Several price indications have been collected from scientists at different research organizations and universities that use liquid helium. The prices in USD/L range from 30 USD/L (27.5 EUR/L) to 45 USD/L (41 EUR/L) and 49 USD/L (45 EUR/L) in Europe [17].
Nitrogen, an abundant product in the air, is generated at air separation plants through the liquefaction of ambient air and subsequent separation of the gas by continuous cryogenic distillation. The nitrogen is then extracted as a cryogenic liquid. The price of liquid nitrogen varies depending on the location and delivered quantities. In the USA, the price is 1.78 USD/L (1.6 EUR/L) [18], and in Germany, based on discussions with suppliers, the cost of liquid nitrogen is around 4 EUR/kg.
Liquid hydrogen results from the liquefication of gaseous hydrogen at −253 °C. Hydrogen can be obtained from different sources. The most conventional one is by natural gas or methane reforming, thus called fossil hydrogen. Hydrogen can be produced from renewable and cleaner sources such as water electrolysis using clean energy (wind and solar). In addition to the difference in the well-to-wake emissions, the production pathway influences the cost. Due to several factors, renewable hydrogen is nowadays more expensive than fossil hydrogen. According to statistics provided by Clean Hydrogen Partnership, hydrogen cost ranges between 2 EUR/kg and 14 EUR/kg [19]. Based on contact with suppliers, the liquid hydrogen cost in Germany is around 17 EUR/kg.
In this work, the cost estimation is only focused on the cost of the fluids. The extra piping and instrumentation that might be used have been neglected in the analysis. It should, however, be noted that it has been assumed that the same piping and instrumentation are used for bunkering/filling both liquid hydrogen and the fluids used for precooling (liquid nitrogen and liquid helium). For emphasis, the unit cost of the product used in this work is obtained from suppliers’ catalogs and literature sources.
3. Results
In this section, the results of our investigations are presented.
3.1. Boil-Off-Losses
The boil-off losses estimated here are the losses of LH2 due to its evaporation during the filling process of the empty tank. Two tank wall materials are used for comparison purposes; one is made of stainless steel (SS 304) and the other of aluminum (Al-5083-O). The tank has a capacity of approximately 170 kg of LH2 at a 90% maximum filling level. In the case of no precooling, 170 kg of LH2 and the amount lost due to boil-off are filled into the tank directly. The filling processes involving precooling are performed in a manner that the precooling fluid is used first to cool down the tank to the boiling temperature of the used fluid, in this case, liquid nitrogen or liquid helium. Once the tank is cooled down, the filling process of liquid hydrogen is initiated.
3.1.1. Without Precooling
The result of the boil-off-losses due to the evaporation of the liquid hydrogen during filling without tank precooling is shown in Figure 4. The maximum, mean and minimum boil-off losses are illustrated for the stainless-steel and aluminum tank. The temperatures of the stainless-steel (SS 304) and aluminum (Al 5083-O) tanks are cooled down from 298 K to 20 K, which is indicated by SS298-20 and Al298-20, respectively.
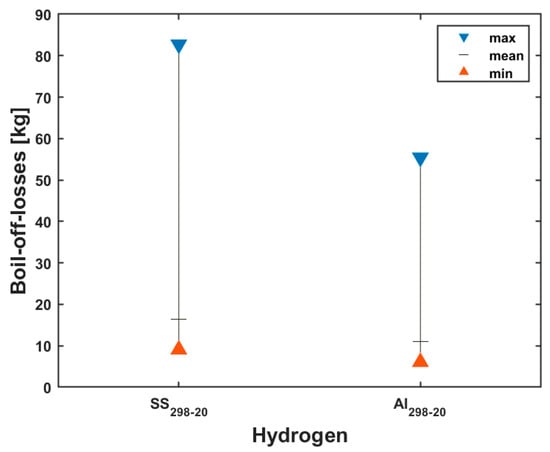
Figure 4.
Hydrogen boil-off losses without precooling for SS 304 and Al 5083-O tank.
From Figure 4, it can be seen that the boil-off losses are higher in the stainless-steel SS 304 tank when compared with the aluminum Al 5083-O tank. This is a result of the difference in the material properties in terms of specific heat and mass. The quantities of LH2 lost due to boil-off are listed in Table 5.

Table 5.
Boil-off quantities in kg without precooling.
To better visualize the maximum amount of hydrogen lost due to boil-off when filling the tank, a pie chart (see Figure 5) is used to illustrate the ratio of liquid hydrogen lost after filling the tank to 90% of its capacity. About 49% of the LH2 filled into the stainless-steel tank is lost, while in the aluminum tank, about 33% of the LH2 is evaporated.
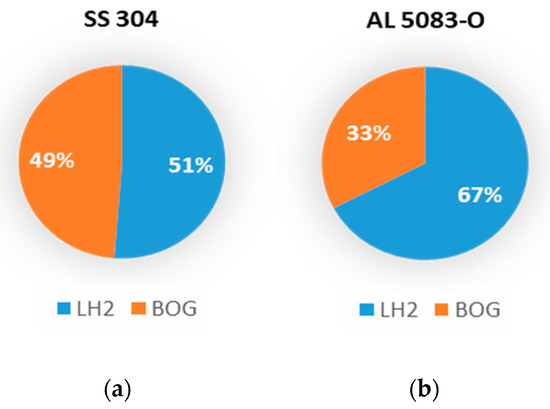
Figure 5.
Breakdown of liquid hydrogen after the filling process without tank precooling: (a) for SS 304 tank and (b) for AL 5083-O tank.
3.1.2. With Precooling
Figure 6 shows the boil-off losses with tank precooling. For brevity, the boil-off losses without precooling earlier described are also included in the plot. This is indicated by SS298-20 and Al298-20 for the stainless-steel and aluminum tanks, respectively. As can be seen in the figure, the boil-off losses are drastically reduced when the tank is precooled with either liquid nitrogen or liquid helium. The amount of boil-off losses when the tank is precooled with nitrogen is represented with SS77-20 and Al77-20 for stainless steel (SS 304) and aluminum (Al 5083-O), respectively. Also, the amount of boil-off losses when the tank is precooled with helium is represented with SS4.2-20 and Al4.2-20 for stainless steel (SS 304) and aluminum (Al 5083-O), respectively.
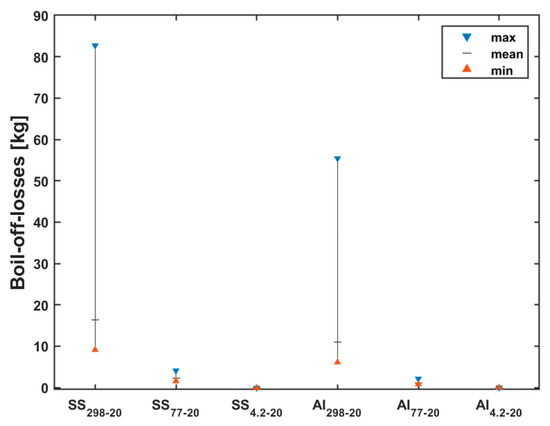
Figure 6.
LH2 boil-off losses for stainless-steel and aluminum tanks with nitrogen and helium precooling.
The quantities of LH2 lost in this case due to evaporation are tabulated in Table 6. As expected, no boil-off losses occur when liquid helium is used for precooling as the tank temperature is brought down to 4.2 K, which is considerably lower than the boiling temperature of liquid hydrogen at 20 K. In the case of precooling with liquid nitrogen, some amount of liquid hydrogen is still lost as the temperature of liquid nitrogen is higher than that of liquid hydrogen. This notwithstanding, the boil-off losses are significantly lower in this case.

Table 6.
Boil-off quantities in kg when precooling the tank with nitrogen and helium.
To better visualize the results, pie charts are used to represent the ratio of liquid hydrogen lost due to boil-off after precooling the tank with liquid nitrogen (Figure 7) and liquid helium (Figure 8).
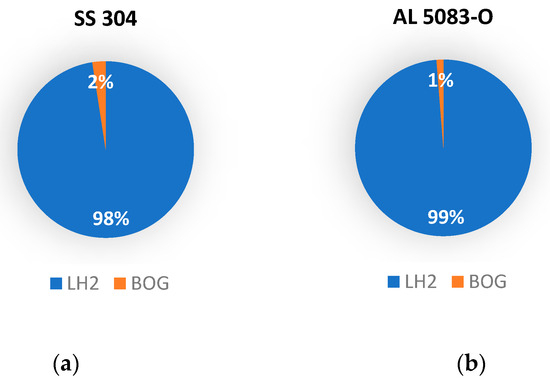
Figure 7.
Breakdown of liquid hydrogen after filling when the tank is precooled with nitrogen: (a) for SS 304 tank; (b) for AL 5083-O tank.
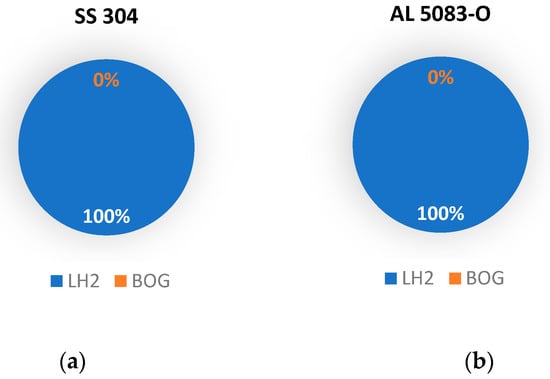
Figure 8.
Breakdown of liquid hydrogen after filling when the tank is precooled with helium: (a) for SS 304 tank; (b) for AL 5083-O tank.
It is found that approximately 2% of LH2 is lost as boil-off gas for the stainless-steel tank, while around 1% of LH2 is lost to boil-off for the aluminum tank in the case of precooling with liquid nitrogen. When liquid helium is used to precool the tank, both stainless steel and aluminum, the temperature of the tank drops below the boiling temperature of liquid hydrogen, thus making the process of filling hydrogen generate zero boil-off losses.
3.2. Quantity of Precooling Fluid Required
This section details the amount of the precooling fluids needed for the two tank wall materials considered in this investigation.
3.2.1. Nitrogen Required
Figure 9 shows the amount of nitrogen required to precool the cryotank from 298 K to 77 K before the bunkering of liquid hydrogen. The plots illustrate the minimum, mean and maximum quantities of nitrogen required for the two tank wall materials considered in this study.
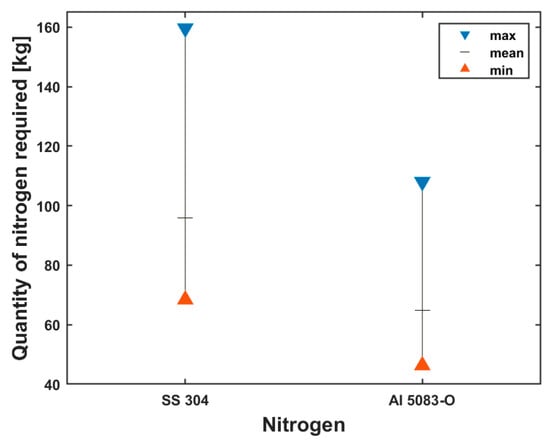
Figure 9.
Min, mean and max quantities of nitrogen required for tank precooling.
A greater amount of nitrogen is required to precool the stainless-steel tank than the aluminum tank as already stated; this is due to the differences in the thermal and mechanical properties of both materials under the assumption of the same wall thickness.
The minimum, mean and maximum quantities of nitrogen required for the stainless-steel tank are 68.43 kg, 95.80 kg and 160 kg, respectively, while those of the aluminum tank are 46.31 kg, 64.83 kg and 108.02 kg, respectively.
3.2.2. Helium Required
The amount of helium required to precool the cryotank from 298 K to 4.2 K before the bunkering of liquid hydrogen is shown in Figure 10. The chart shows the minimum, mean and maximum amounts of helium needed for the two tank wall materials that were taken into consideration for this investigation.
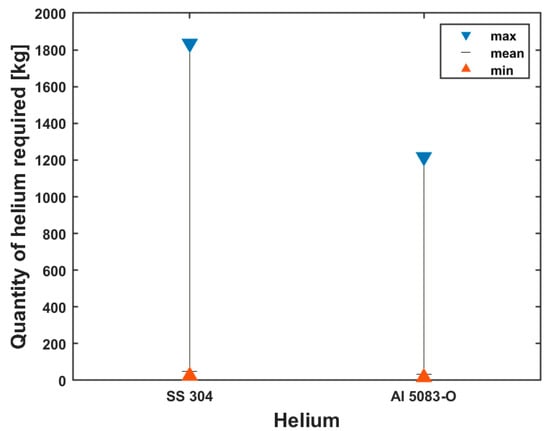
Figure 10.
Min, mean and max quantities of helium required for tank precooling.
As shown in Figure 10, a higher amount of helium is required to precool the stainless-steel tank than the aluminum tank. The minimum, mean and maximum quantities of helium required for precooling the stainless-steel tank are 24.60 kg, 48.55 kg and 1834.48 kg, respectively, while for the aluminum tank, the minimum, mean and maximum quantities of helium required are 16.32 kg, 32.20 kg and 1216.70 kg, respectively.
3.3. Cost Estimation
One of the important aspects of this work is the economic aspect of the tank-filling process with and without precooling. The cost of the hydrogen lost due to boil-off and the precooling fluids (nitrogen and helium) for the calculated quantities in Section 3.2 above is based on the market prices listed in Table 4.
In the case where no precooling is used, the boil-off losses are only composed of gaseous hydrogen. Precooling with liquid helium results in only helium boil-off losses. However, in the case of precooling with liquid nitrogen, the nitrogen will fully evaporate, as well as a small quantity of liquid hydrogen, as already demonstrated in this work. Therefore, the cost in this case is the sum of both the nitrogen and hydrogen boil-off losses.
Figure 11 illustrates the estimated costs associated with filling the tank without precooling and with nitrogen precooling only. Precooling the tank with liquid helium is significantly more expensive than the other two methods due to the high cost of helium. The prices of using liquid helium for the stainless-steel and aluminum tanks are around EUR 550,345 and EUR 365,000, respectively, in the maximum scenario, roughly EUR 14,570 and EUR 9660, respectively, in the mean case and approximately EUR 7380 and EUR 4895, respectively, in the minimum case.
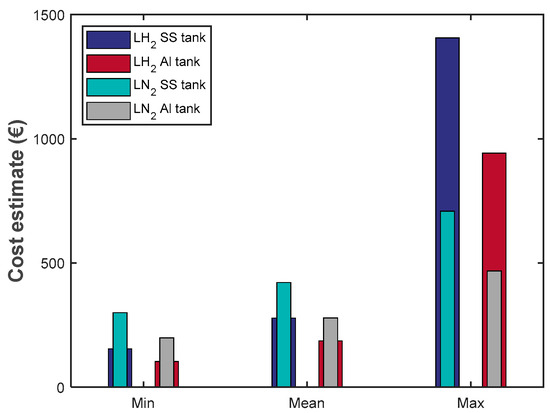
Figure 11.
Cost of boil-off losses in an SS 304 tank and AL 5083-O with and without precooling.
Considering the cases of precooling with liquid nitrogen and without precooling represented in Figure 11, it is found that using nitrogen for precooling is only cost-efficient in the maximum case, where it is assumed that no further heat transfer between the cold boil-off gases occurs. In the mean and minimum cases, the heat absorbed by the boil-off gases is more significant for cold hydrogen gas due to its lower temperature than nitrogen. This drastically reduces the boil-off losses and results in a cost advantage for tank filling without precooling. Furthermore, in terms of the tank wall materials, the cost associated with the use of stainless-steel SS 304 is higher than that of aluminum Al 5083-O in all cases considered in this study.
4. Discussion
This study is built on the premise that it is critical to initially reduce boil-off losses whenever practicable before implementing solutions for boil-off recovery. This is because boil-off recovery measures require additional infrastructure, space, energy and cost. The most significant source of boil-off losses occurs during filling operations along the liquid hydrogen pathway [11]. This is especially noticeable when a cryogenic tank is being filled for the first time.
Precooling of liquid hydrogen tanks during the first filling operation with liquid nitrogen or liquid helium drastically reduces the boil-off losses. In the worst-case scenario, under the assumption that the evaporated gases do not absorb further heat before exiting the tank, the boil-off losses are reduced from 49% to 2% and 33% to 1% when the tank is precooled with liquid nitrogen for the reference stainless-steel and aluminum tanks, respectively. No boil-off losses are generated when the tanks are precooled with liquid helium.
With regards to the economic aspect, the amount of liquid nitrogen needed to precool the tank is 6 times more than liquid hydrogen boil-off losses without precooling (and even higher when the evaporated gas absorbs more heat—minimum case) except in the maximum case, where it is approximately 2 times more, whereas the cost per kilogram of liquid nitrogen is only 4.25 times less than that of liquid hydrogen. Given that the maximum case overestimates the boil-off, precooling with liquid nitrogen is not viable from an economic standpoint. Also, while the amount of liquid helium required to precool the cryogenic tank is approximately 3 times more (and in the worst case, 22 times more) than the liquid hydrogen boil-off losses without precooling, the cost per kilogram is 17.5 times higher than that of liquid hydrogen. Hence, from an economic perspective, precooling with liquid helium is not sustainable. Furthermore, it has been projected that the production costs of hydrogen will drop by around 50% by 2030 and then gradually decline at a somewhat slightly slower rate until 2050 [20]. This further makes liquid hydrogen tank filling without precooling the most economically viable.
It should be noted that helium is inert and rarely reacts with hydrogen. The only challenge is the solidification of liquid hydrogen at the temperature of liquid helium since the liquid hydrogen solidification temperature is 14 K. To overcome this, the temperature of the tank is left to increase to about 15 K, and the tank is flushed with gaseous hydrogen before the filling of liquid hydrogen. In the case of nitrogen, nitrogen is fairly inert and would react with hydrogen to form ammonia only at high pressure and a temperature of about 400 °C. Also, since nitrogen will solidify (solidification temperature = 63 K) at the temperature of liquid hydrogen, the tank could be flushed with gaseous hydrogen at about 70 K before the bunkering of liquid hydrogen. On the other hand, indirect precooling of the tank from the exterior could be adopted. Furthermore, these fluids are used for purging liquid hydrogen systems and hence should not pose any challenge.
Lastly, it is important to emphasize that liquid hydrogen levels should not drop to zero while the tank is in use. Also, the boil-off losses are not necessarily losses per se as they could be captured and used for other purposes.
5. Conclusions
This paper investigated the filling of a liquid hydrogen tank with and without precooling. Two tank wall materials—SS 304 and Al 5083-O—were considered. The precooling was performed using liquid helium at a temperature of 4.2 K and liquid nitrogen at 77 K. The amounts of liquid hydrogen lost due to boil-off were calculated using empirical formulas, considering three assumptions related to the heat transfer between the evaporated gases and the tank. The cost implication of the precooling process was assessed by calculating the cost of liquid hydrogen boil-off losses and the quantity of liquid nitrogen and liquid helium required for precooling using available market prices.
It can, therefore, be concluded that during the filling of an empty cryogenic tank, the following occurs:
- The boil-off losses can be drastically reduced by precooling the tank with either liquid nitrogen or liquid helium.
- Less boil-off losses are generated for the Al 5083-O tank material when compared to the SS 304 tank material. Consequently, less precooling fluid is required for the Al 5083-O tank material.
- The use of liquid helium as a precooling fluid leads to zero liquid hydrogen boil-off; however, it comes at a very high cost, making it economically unfeasible.
- The use of liquid nitrogen as a precooling fluid reduces liquid hydrogen boil-off losses. However, in terms of cost, the use of liquid nitrogen is better only under the assumption that no heat transfer occurs between the cold evaporated gases and the tank. In all other cases, liquid hydrogen tank filling without precooling is economically more viable.
Therefore, Al 5083-O tank material precooled with liquid helium is the most suitable for a reduction in hydrogen boil-off losses. However, in terms of cost, Al 5083-O tank material without precooling is the most economically viable.
Furthermore, additional research should focus on the effects of temperature distribution inside the tank and the dynamics of hydrogen boil-off losses during tank filling operations, taking into account the mass flow rate and time.
Author Contributions
Conceptualization, B.E.O. and C.A.A.; methodology, B.E.O. and C.A.A.; writing—original draft preparation, B.E.O. and C.A.A.; writing—review and editing, C.A.A. and B.E.O.; supervision, L.B. and S.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank our colleagues from the Institute of Maritime Energy Systems for their willingness to proofread this work and for their fruitful discussion on the subject.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- European Maritime Safety Agency. Potential of Hydrogen as Fuel for Shipping; European Maritime Safety Agency (EMSA): Lisbon, Portugal, 2023; pp. 2–7.
- Revised GHG Reduction Strategy for Global Shipping Adopted. Available online: https://www.imo.org/en/MediaCentre/PressBriefings/pages/Revised-GHG-reduction-strategy-for-global-shipping-adopted-.aspx (accessed on 5 July 2024).
- Collodi, G. Hydrogen Production via Steam Reforming with CO2 Capture. Chem. Eng. Trans. 2010, 19, 37–42. [Google Scholar]
- Zhang, Y.; Huang, J.; Williams, P.T. Fe-Ni-MCM-41 Catalysts for Hydrogen-Rich Syngas Production from Waste Plastics by Pyrolysis-Catalytic Steam Reforming. Energy Fuels 2017, 31, 8497–8504. [Google Scholar] [CrossRef]
- Santhanam, K.S.; Press, R.J.; Miri, M.J.; Bailey, A.V.; Takacs, G.A. Introduction to Hydrogen Technology; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Hydrogen Explained, Production of Hydrogen. Available online: https://www.eia.gov/energyexplained/hydrogen/production-of-hydrogen.php (accessed on 12 August 2024).
- Czernik, S.; French, R.J. Production of hydrogen from plastics by pyrolysis and catalytic steam reform. Energy Fuels 2006, 20, 754–758. [Google Scholar] [CrossRef]
- Shchegolkov, A.V.; Shchegolkov, A.V.; Zemtsova, N.V.; Stanishevskiy, Y.M.; Vetcher, A.A. Recent Advantages on Waste Management in Hydrogen Industry. Polymers 2022, 14, 4992. [Google Scholar] [CrossRef] [PubMed]
- Van Hoecke, L.; Laffineur, L.; Campe, R.; Perreault, P.; Verbruggen, S.W.; Lenaerts, S. Challenges in the use of hydrogen for maritime applications. Energy Environ. Sci. 2021, 14, 815–843. [Google Scholar] [CrossRef]
- Depken, J.; Dyck, A.; Roß, L.; Ehlers, S. Safety considerations of hydrogen application in shipping in comparison to LNG. Energies 2021, 15, 3250. [Google Scholar] [CrossRef]
- Petitpas, G. Boil-off Losses along LH2 Pathway; Lawrence Livermore National Lab (LLNL): Livermore, CA, USA, 2018. [Google Scholar] [CrossRef]
- Morales-Ospino, R.; Celzard, A.; Fierro, V. Strategies to recover and minimize boil-off losses during liquid hydrogen storage. Renew. Sustain. Energy Rev. 2023, 182, 113360. [Google Scholar] [CrossRef]
- Sherif, S.A.; Barbir, F.; Veziroglu, T.N. Towards a Hydrogen Economy. Electr. J. 2005, 18, 62–76. [Google Scholar] [CrossRef]
- Ghaffari-Tabrizi, F.; Haemisch, J.; Lindner, D. Reducing Hydrogen Boil-Off Losses during Fuelling by Pre-Cooling Cryogenic Tank. Hydrogen 2022, 3, 255–269. [Google Scholar] [CrossRef]
- Barron, R.F. Cryogenic Systems (Monographs on Cryogenics), 2nd ed.; Oxford University Press: Oxford, UK, 1985. [Google Scholar]
- Material Properties. Cryogenics Material Properties. Available online: https://trc.nist.gov/cryogenics/materials/materialproperties.htm (accessed on 24 June 2024).
- Kramer, D. Helium prices surge to record levels as shortage continues. Phys. Today 2023, 76, 18–20. [Google Scholar] [CrossRef]
- The Price of Liquid Nitrogen in The United States. Available online: https://rb.gy/b1s887 (accessed on 5 July 2024).
- Hydrogen Cost and Sales Price. Available online: https://h2v.eu/analysis/statistics/financing/hydrogen-cost-and-sales-prices (accessed on 5 July 2024).
- The Green Hydrogen Economy. Predicting the Decarbonization Agenda of Tomorrow. Available online: https://www.pwc.com/gx/en/industries/energy-utilities-resources/future-energy/green-hydrogen-cost.html (accessed on 5 August 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).