Investigating the Potential of Grass Biomass (Thysanolaena latifolia) as an Alternative Feedstock for Sugar Platforms and Bioethanol Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Analytical Methods
2.3. Pretreatment Procedure
2.4. Crystallinity and Morphology Analysis
2.5. Biomass Enzyme Saccharification
2.6. Preparation of Biomass Hydrolysate
2.7. Ethanol Fermentation
2.8. Microbial Strain
2.9. Quantitative Analysis
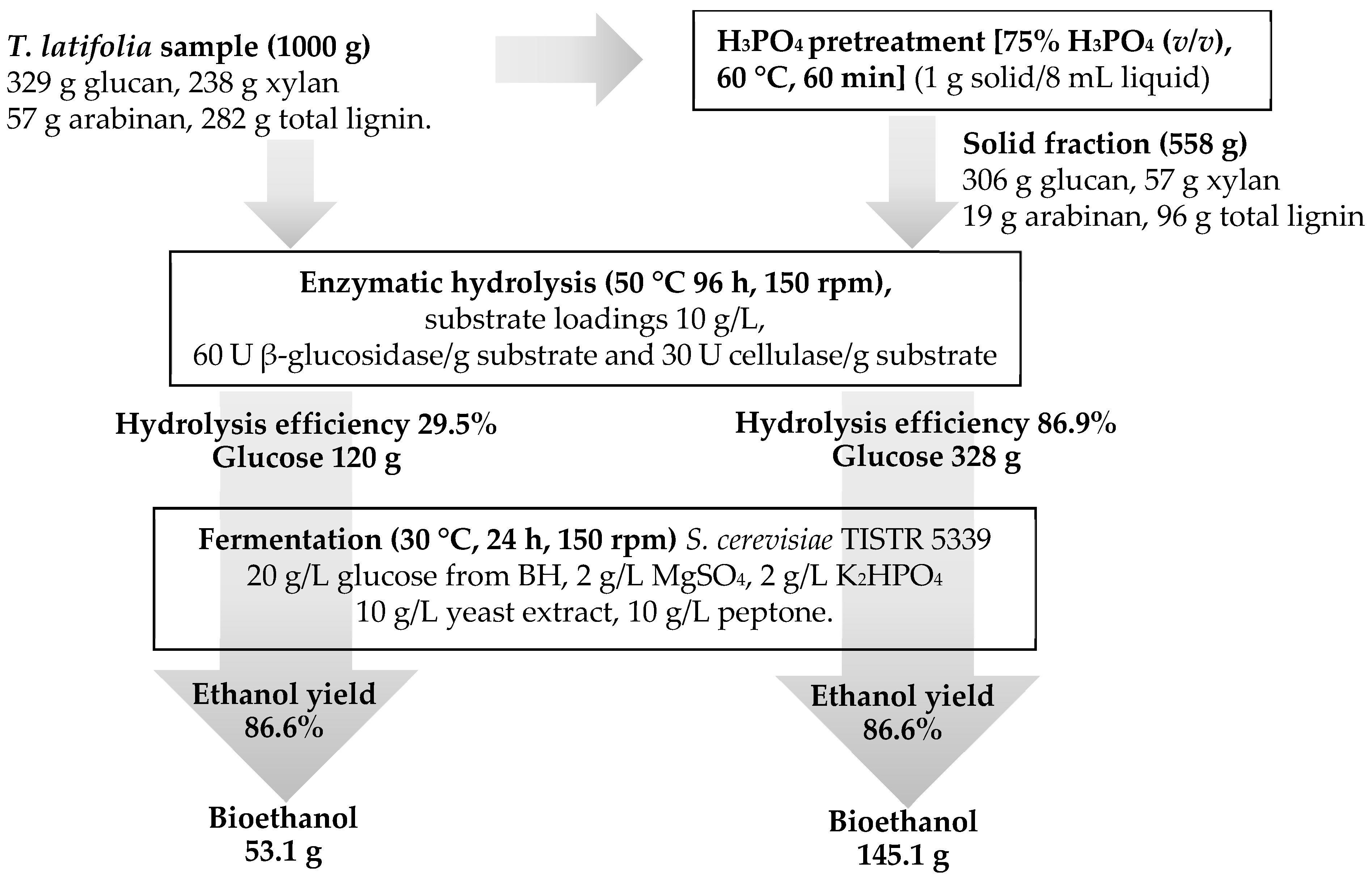
3. Results
3.1. Characterization of T. latifolia Biomass
3.2. Effect of Phosphoric Acid Concentration on Chemical Composition
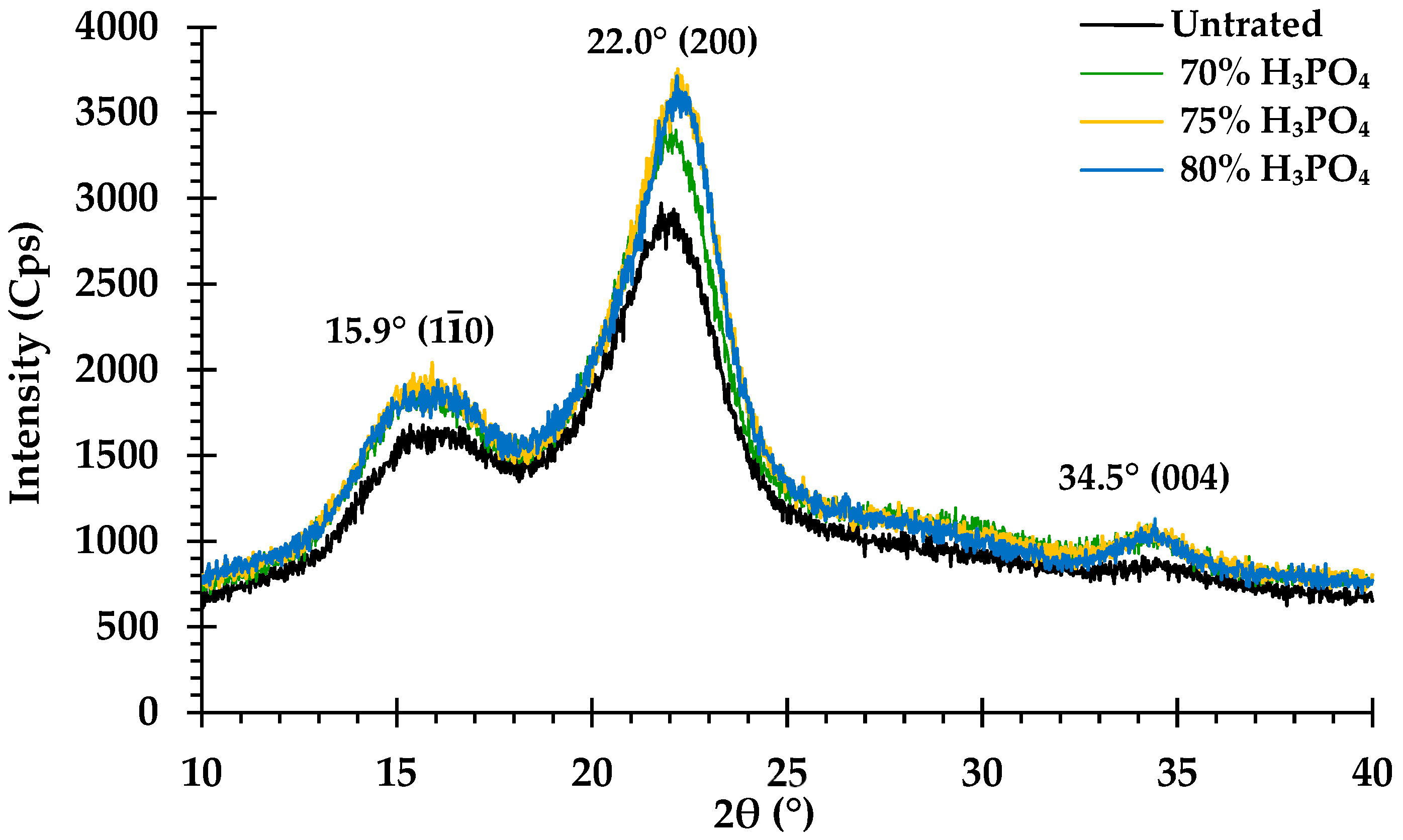
3.3. Impact of Phosphoric Acid Concentration on Cellulose Crystallinity
3.4. Impact of Phosphoric Acid on Biomass Morphology
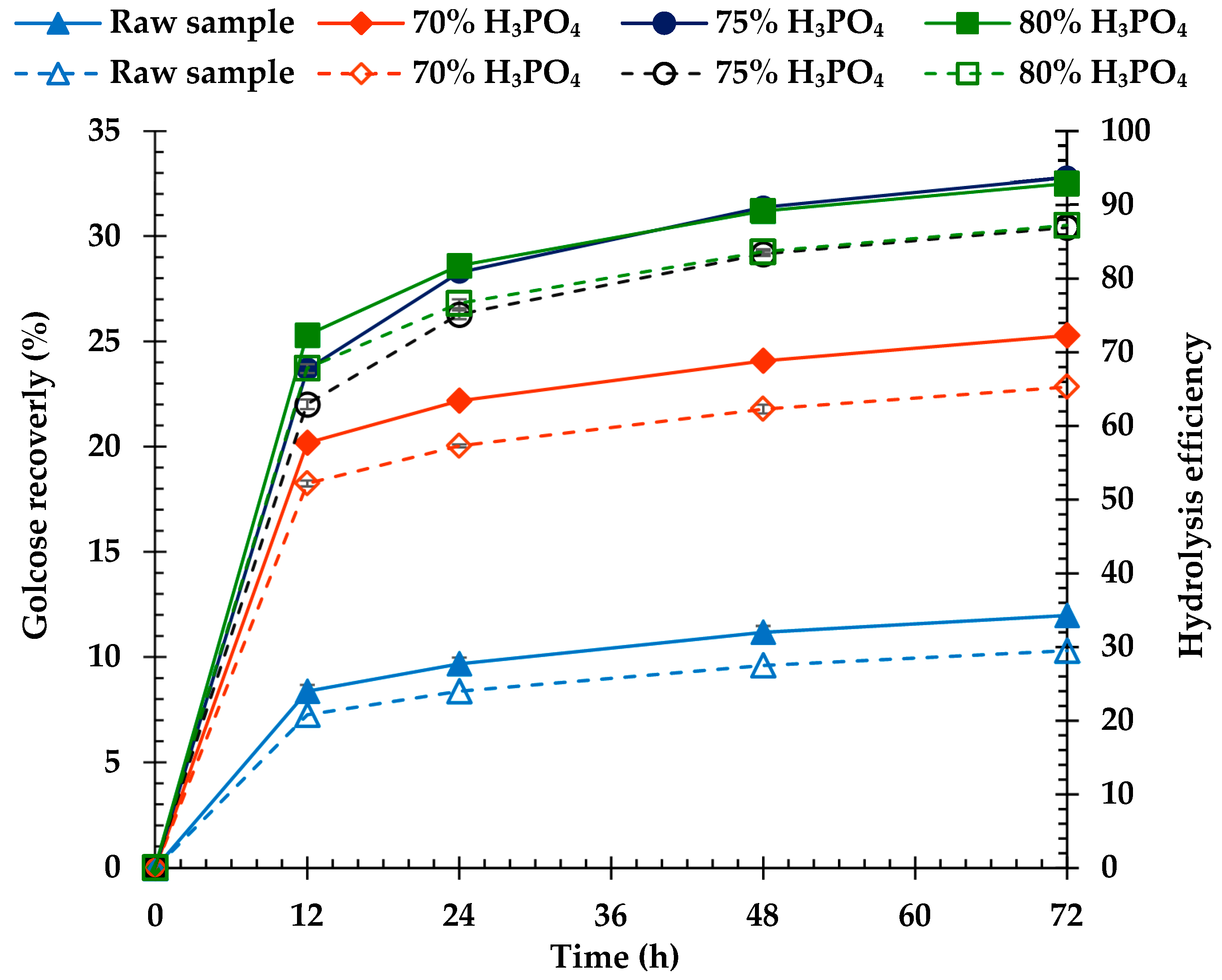
3.5. Enzymatic Saccharification Yields
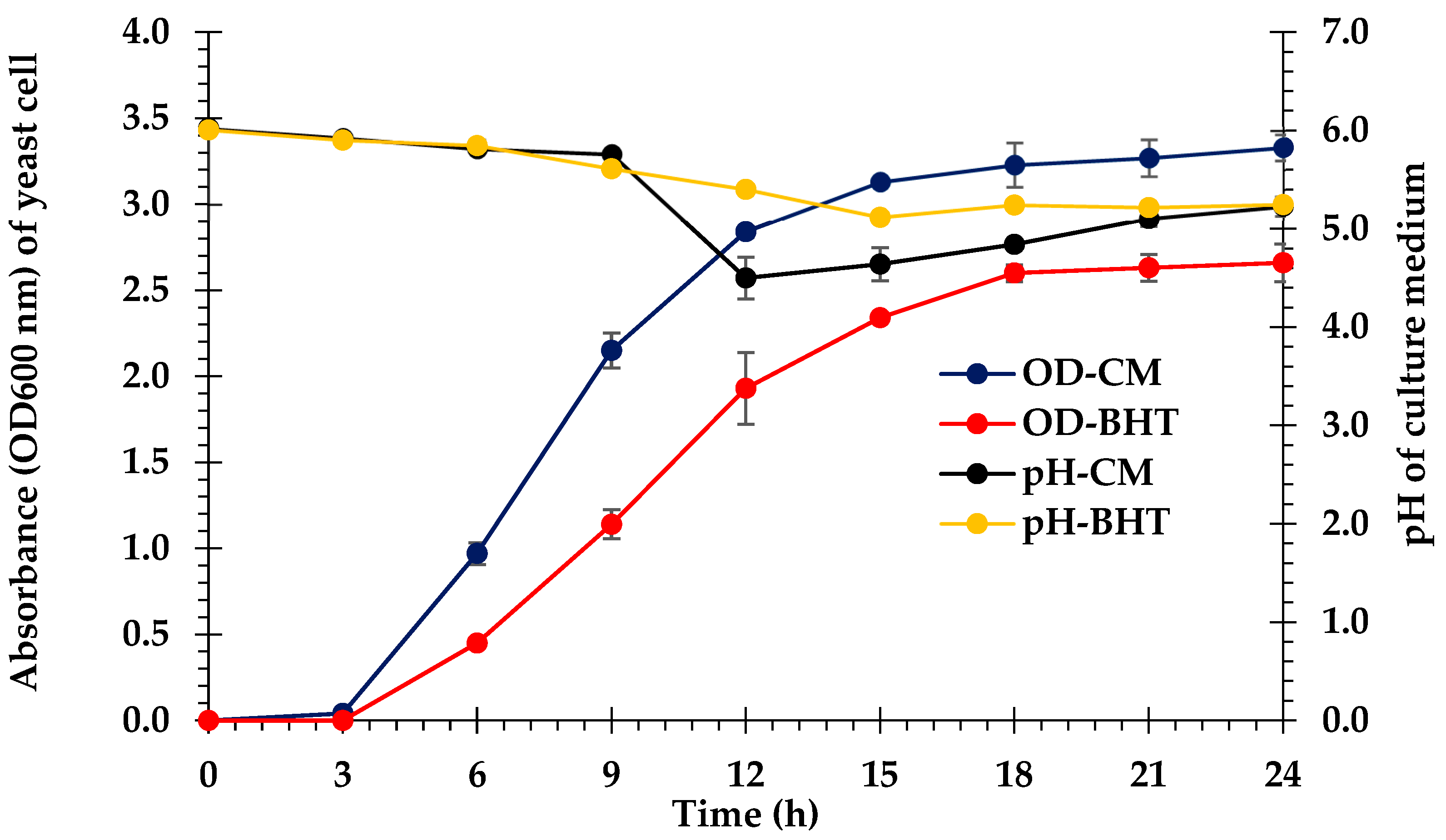
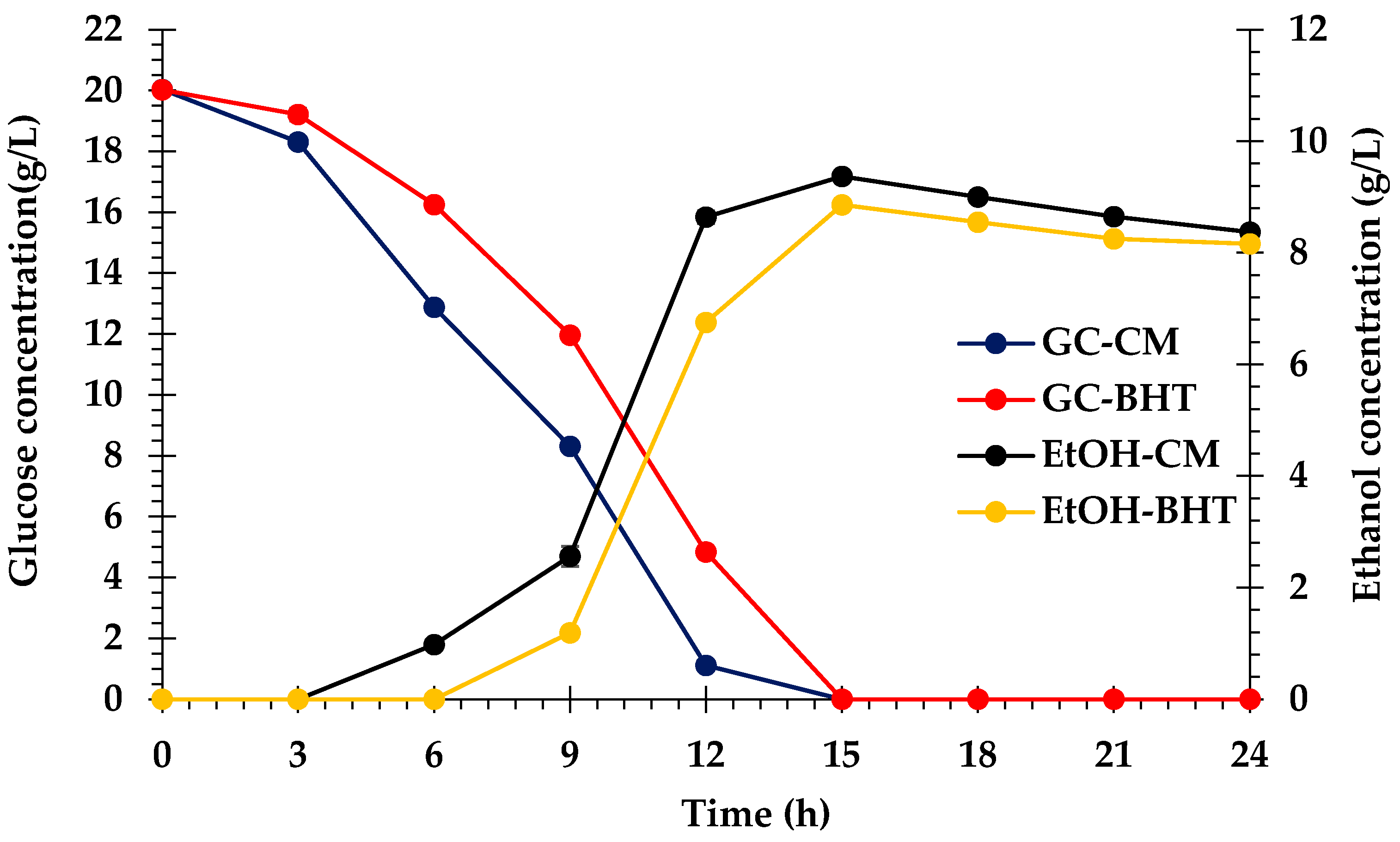
3.6. Bioethanol Fermentation
4. Discussion
4.1. Characterization of T. latifolia Biomass
4.2. Effect of Phosphoric Acid Concentrations on Chemical Composition
4.3. Impact of Phosphoric Acid Concentration on Cellulose Crystallinity
4.4. Impact of Phosphoric Acid Concentration on Biomass Morphology
4.5. Enzymatic Saccharification Yields
4.6. Bioethanol Fermentation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Afraz, M.; Muhammad, F.; Nisar, J.; Shah, A.; Munir, S.; Ali, G.; Ahmad, A. Production of value-added products from biomass waste by pyrolysis: An updated review. Waste Manag. Bull. 2024, 1, 30–40. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Abdulkhani, A.; Aboelazayem, O.; Saha, B. Recent insights into lignocellulosic biomass pyrolysis: A critical review on pretreatment, characterization, and products upgrading. Processes 2020, 8, 799. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.-F.; Zhang, C.-C.; Nan, J.; Ren, N.-Q.; Lee, D.-J.; Chen, C. Advances in pretreatment of lignocellulosic biomass for bioenergy production: Challenges and perspectives. Bioresour. Technol. 2022, 343, 126123. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Kumar, D.; Girdhar, M.; Kumar, A.; Goyal, A.; Malik, T.; Mohan, A. Strategies of pretreatment of feedstocks for optimized bioethanol production: Distinct and integrated approaches. Biotechnol. Biofuels Bioprod. 2023, 16, 44. [Google Scholar] [CrossRef]
- Toor, M.; Kumar, S.S.; Malyan, S.K.; Bishnoi, N.R.; Mathimani, T.; Rajendran, K.; Pugazhendhi, A. An overview on bioethanol production from lignocellulosic feedstocks. Chemosphere 2020, 242, 125080. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Banu, J.R.; Rao, C.V.; Kim, Y.-G.; Yang, Y.-H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef]
- Sharma, S.; Tsai, M.-L.; Sharma, V.; Sun, P.-P.; Nargotra, P.; Bajaj, B.K.; Chen, C.-W.; Dong, C.-D. Environment friendly pretreatment approaches for the bioconversion of lignocellulosic biomass into biofuels and value-added products. Environments 2023, 10, 6. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nizetic, S.; Ong, H.C.; Chong, C.T.; Atabani, A.E.; Pham, V.V. Acid-based lignocellulosic biomass biorefinery for bioenergy production: Advantages, application constraints, and perspectives. J. Environ. Manag. 2021, 296, 113194. [Google Scholar] [CrossRef]
- Sun, W.; Li, X.; Zhao, J.; Qin, Y. Pretreatment strategies to enhance enzymatic hydrolysis and cellulosic ethanol production for biorefinery of corn stover. Int. J. Mol. Sci. 2022, 23, 13163. [Google Scholar] [CrossRef]
- Xiao, K.; Li, H.; Liu, L.; Liu, X.; Lian, Y. Quantitative comparison of the delignification performance of lignocellulosic biomass pretreatment technologies for enzymatic saccharification. Environ. Sci. Pollut. Res. 2023, 30, 22929–22940. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Shafique, O.; Mahmood, S.; Mahmood, T.; Khan, B.A.; Ahmad, I. Biofuels production from weed biomass using nanocatalyst technology. Biomass Bioenergy 2020, 139, 105595. [Google Scholar] [CrossRef]
- Premjet, S.; Pumira, B.; Premjet, D. Determining the potential of inedible weed biomass for bio-energy and ethanol production. Bioresources 2013, 8, 701–716. [Google Scholar] [CrossRef]
- Rana, S.; Tiwari, R.; Kuppusamy, P.; Arora, A.; Singh, S.; Saxena, A.K.; Nain, L. Weedy lignocellulosic biomass, a potential feedstock for bioethanol production: A future perspective. In Recent Advances in Bioenergy Research; Gupta, V.K., Treichel, H., Kuhad, R.C., Rodriguez-Cout, S., Eds.; Sardar Swarns Singh National Institute of Renewable Energy: Kapurthala, India, 2013; pp. 183–192. [Google Scholar]
- Pandiyan, K.; Singh, A.; Singh, S.; Saxena, A.K.; Nain, L. Technological interventions for utilization of crop residues and weedy biomass for second generation bio-ethanol production. Renew. Energy 2019, 132, 723–741. [Google Scholar] [CrossRef]
- Flora & Fauna, Thysanolaena latifolia. Available online: https://www.nparks.gov.sg/florafaunaweb/flora/2/5/2512# (accessed on 20 January 2024).
- Sespene, J.; Fetalvero, E.; Faminial, T. Tiger grass industry in Marigondon Norte, San Andres, Romblon: Implications for research and development. Romblon State Univ. Res. J. 2011, 1, 81–95. [Google Scholar]
- Shrestha, S.; Park, J.H.; Cho, J.G.; Lee, D.Y.; Jeong, R.H.; Song, M.C.; Cho, S.K.; Lee, D.S.; Baek, N.I. Phytochemical constituents from the florets of tiger grass Thysanolaena latifolia from Nepal. J. Asian. Nat. Prod. Res. 2016, 18, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Shukla, R.P.; Lynser, M.B.; Tynsong, H. Growth pattern, production, and marketing of Thysanolaena maxima (Roxb.) Kuntze: An important non-timber forest product of Meghalaya, India. For. Trees Livelihoods 2012, 21, 176–187. [Google Scholar] [CrossRef]
- Saikia, D.C.; Goswami, T.; Chaliha, B.P. Paper from Thysanolaena Maxima. Bioresour. Technol. 1992, 40, 245–248. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P.; Cui, J.; Lynd, L.R.; Kuang, L.R. A transition from cellulose swelling to cellulose dissolution by o-phosphoric acid: Evidence from enzymatic hydrolysis and supramolecular structure. Biomacromolecules 2006, 7, 644–648. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K.; Kumar, R. Cellulose solvent-based pretreatment for enhanced second-generation biofuel production: A review. Sustain. Energ. Fuels. 2019, 3, 11–62. [Google Scholar] [CrossRef]
- Rollin, J.A.; Zhu, Z.; Sathitsuksanoh, N.; Zhang, Y.H. Increasing cellulose accessibility is more important than removing lignin: A comparison of cellulose solvent-based lignocellulose fractionation and soaking in aqueous ammonia. Biotechnol. Bioeng. 2011, 108, 22–30. [Google Scholar] [CrossRef]
- Yu, H.; Xiao, W.; Han, L.; Huang, G. Characterization of mechanical pulverization/phosphoric acid pretreatment of corn stover for enzymatic hydrolysis. Bioresour. Technol. 2019, 282, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, W.; Guo, J.; Song, J.; Xiao, H. Impacts of degree of substitution of quaternary cellulose on the strength improvement of fiber networks. Int. J. Biol. Macromol. 2021, 181, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Tong, D.; Zhan, P.; Zhang, W.; Zhou, Y.; Huang, Y.; Qing, Y.; Chen, J. Surfactant-assisted dilute phosphoric acid plus steam explosion of poplar for fermentable sugar production. ChemistrySelect 2022, 7, e202200423. [Google Scholar] [CrossRef]
- Dey, T.; Bhattacharjee, T.; Nag, P.; Ritika; Ghati, A.; Kuila, A. Valorization of agro-waste into value added products for sustainable development. Bioresour. Technol. Rep. 2021, 16, 100834. [Google Scholar] [CrossRef]
- Ji, L.; Tang, Z.; Yang, D.; Ma, C.; He, Y.-C. Improved one-pot synthesis of furfural from corn stalk with heterogeneous catalysis using corn stalk as biobased carrier in deep eutectic solvent–water system. Bioresour. Technol. 2021, 340, 125691. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Shen, B.; Zhang, D.; Li, R.; Xu, X.; Wang, K.; Lai, C.; Yong, Q. Understanding of promoting enzymatic hydrolysis of combined hydrothermal and deep eutectic solvent pretreated poplars by Tween 80. Bioresour. Technol. 2022, 362, 127825. [Google Scholar] [CrossRef] [PubMed]
- Premejt, S.; Premejt, D.; Takata, E.; Tsutsumi, Y. Phosphoric acid pretreatment of Achyranthes aspera and Sida acuta weed biomass to improve enzymatic hydrolysis. Bioresour. Technol. 2016, 203, 303–308. [Google Scholar] [CrossRef]
- Premjet, S.; Dana, S.; Obeng, A.K.; Premjet, D. Enzymatic response to structural and chemical transformations in Hibiscus sabdariffa var. altissima bark and core during phosphoric acid pretreatment. BioResources 2018, 13, 6778–6789. [Google Scholar] [CrossRef]
- Li, T.; Fang, Q.; Chen, H.; Qi, F.; Ou, X.; Zhao, X.; Liu, D. Solvent-based delignification and decrystallization of wheat straw for efficient enzymatic hydrolysis of cellulose and ethanol production with low cellulase loadings. RSC Adv. 2017, 7, 10609–10617. [Google Scholar] [CrossRef]
- Chen, D.; Tang, W.; Wang, H.; Sheng, Y.; Tan, X.; Shi, Y.; Fan, W.; Ge, S. Phosphoric acid pretreatment of poplar to optimize fermentable sugars production based on orthogonal experimental design. Front. Chem. 2023, 11, 1119215. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2012; pp. 1–15. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass; Technical Report NREL/TP-510-42622; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008; pp. 1–8. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; Technical Report NREL/TP-510-42619; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008; pp. 1–12. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Obeng, A.K.; Premjet, D.; Premjet, S. Combining autoclaving with mild alkaline solution as a pretreatment technique to enhance glucose recovery from the invasive weed Chloris barbata. Biomolecules 2019, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Premjet, S.; Doungporn, P.; Yoo, H.Y.; Kim, S.W. Improvement of sugar recovery from Sida acuta (Thailand Weed) by NaOH pretreatment and application to bioethanol production. Korean J. Chem. Eng. 2018, 35, 2413–2420. [Google Scholar] [CrossRef]
- Wongleang, S.; Premjet, D.; Premjet, S. Cellulosic ethanol production from weed biomass hydrolysate of Vietnamosasa pusilla. Polymers 2023, 15, 1103. [Google Scholar] [CrossRef] [PubMed]
- Binod, P.; Sindhu, R.; Singhania, R.R.; Vikram, S.; Devi, L.; Nagalakshmi, S.; Kurien, N.; Sukumaran, R.K.; Pandey, A. Bioethanol production from rice straw: An overview. Bioresour. Technol. 2010, 101, 4767–4774. [Google Scholar] [CrossRef] [PubMed]
- Komolwanich, T.; Prasertwasu, S.; Khumsupan, D.; Tatijarern, P.; Chaisuwan, T.; Luengnaruemitchai, A.; Wongkasemjit, S. Evaluation of highly efficient monomeric sugar yield from Thai Tiger grass (Thysanolaena maxima). Mat. Res. Innov. 2016, 20, 259–267. [Google Scholar] [CrossRef]
- Khan, M.A.; Dharmalingam, B.; Chuetor, S.; Cheng, Y.-S.; Sriariyanun, M. Comprehensive review on effective conversion of lignocellulosic biomass to levulinic acid. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Lues, R. Anaerobic digestion of lignocellulosic biomass: Substrate characteristics (challenge) and innovation. Fermentation 2023, 9, 755. [Google Scholar] [CrossRef]
- Li, H.-Y.; Chen, X.; Wang, C.-Z.; Sun, S.-N.; Sun, R.-C. Evaluation of the two-step treatment with ionic liquids and alkali for enhancing enzymatic hydrolysis of Eucalyptus: Chemical and anatomical changes. Biotechnol. Biofuels 2016, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Woiciechowski, A.; Dalmas Neto, C.J.; Porto de Souza Vandenberghe, L.; de Carvalho Neto, D.P.; Novak Sydney, A.C.; Letti, L.A.J.; Karp, S.G.; Zevallos Torres, L.A.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance—Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.P.; Ding, S.-Y.; Mielenz, J.R.; Cui, J.-B.; Elander, R.T.; Laser, M.; Himmel, M.E.; McMillan, J.R.; Lynd, L.R. Fractionating recalcitrant lignocellulose at modest reaction conditions. Biotechnol. Bioeng. 2007, 97, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Kundu, C.; Samudrala, S.P.; Kibria, M.A.; Bhattacharya, S. One-step peracetic acid pretreatment of hardwood and softwood biomass for platform chemicals production. Sci. Rep. 2021, 11, 11183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, Y.; Xie, X.; Chernyshev, V.M.; Liu, Y.; Qi, W. Effects of phosphoric acid/hydrogen peroxide, ammonia/hydrogen peroxide and deep eutectic solvent pretreatments on component separation and enzymatic saccharification of Glycyrrhiza residue. Ind. Crops. Prod. 2023, 196, 116525. [Google Scholar] [CrossRef]
- Wongleang, S.; Dana, S.; Premjet, D.; Premjet, S. Phosphoric acid pretreatment of Corchorus capsularis L. biomass for enhancing glucose recovery. NU. Int. J. Sci. 2023, 20, 1–13. [Google Scholar]
- Hossain, A.; Rahaman, M.S.; Lee, D.; Phung, T.K.; Canlas, C.G.; Simmons, B.A.; Renneckar, S.; Reynolds, W.; George, A.; Tulaphol, S.; et al. Enhanced softwood cellulose accessibility by H3PO4 pretreatment: High sugar yield without compromising lignin integrity. Ind. Eng Chem. Res. 2020, 59, 1010–1024. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, D.; Hu, J.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Hu, Y. Fates of hemicellulose, lignin and cellulose in concentrated phosphoric acid with hydrogen peroxide (PHP) pretreatment. RSC Adv. 2018, 8, 12714–12723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Lin, L.; Chen, T.; Zhang, J.; Liu, S.; Li, Z.; Ouyang, P. Dissolution of microcrystalline cellulose in phosphoric acid—Molecular changes and kinetics. Molecules 2009, 14, 5027–5041. [Google Scholar] [CrossRef]
- Cai, D.; Li, P.; Chen, C.; Wang, Y.; Hu, S.; Cui, C.; Qin, P.; Tan, T. Effect of chemical pretreatments on corn stalk bagasse as immobilizing carrier of Clostridium acetobutylicum in the performance of a fermentation-pervaporation coupled system. Bioresour. Technol. 2016, 220, 68–75. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhou, T.; Wang, Y.; Cao, X.; Wu, S.; Zhao, M.; Wang, H.; Xu, M.; Zheng, B.; Zheng, J.; et al. Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep. 2018, 8, 1321. [Google Scholar] [CrossRef]
- Obeng, A.K.; Premjet, D.; Premjet, S. Improved glucose recovery from durian peel by alkaline-catalyzed steam pretreatment. PeerJ 2021, 9, e12026. [Google Scholar] [CrossRef] [PubMed]
- Sathitsuksanoh, N.; George, A.; Zhang, Y.-H.P. New lignocellulose pretreatments using cellulose solvents: A review. J. Chem. Technol. 2013, 88, 169–180. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Nandal, P.; Singh, J.; Verma, M.L. Nanobiotechnological advancements in lignocellulosic biomass pretreatment. Mater. Sci. Energy Technol. 2020, 3, 308–318. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef] [PubMed]
- Sathitsuksanoh, N.; Zhu, Z.; Zhang, Y.H.P. Cellulose solvent- and organic solvent-based lignocellulose fractionation enabled efficient sugar release from a variety of lignocellulosic feedstocks. Bioresour. Technol. 2012, 117, 228–233. [Google Scholar] [CrossRef]
- Gabhane, J.; Prince William, S.P.M.; Vaidya, A.N.; Mahapatra, K.; Chakrabarti, T. Influence of heating source on the efficacy of lignocellulosic pretreatment—A cellulosic ethanol perspective. Biomass Bioenergy 2011, 35, 96–102. [Google Scholar] [CrossRef]
- Gourlay, K.; Arantes, V.; Saddler, J.N. Use of substructure-specific carbohydrate binding modules to track changes in cellulose accessibility and surface morphology during the amorphogenesis step of enzymatic hydrolysis. Biotechnol. Biofuels 2012, 5, 51. [Google Scholar] [CrossRef]
- Jackson de Moraes Rocha, G.; Martin, C.; Soares, I.B.; Souto Maior, A.M.; Baudel, H.M.; Moraes de Abreu, C.A. Dilute mixed-acid pretreatment of sugarcane bagasse for ethanol production. Biomass Bioenergy 2011, 35, 663–670. [Google Scholar] [CrossRef]
- Tong, W.; Fang, H.; Song, K.; Xie, X.; Wang, J.; Jin, Y.; Wu, S.; Hu, J.; Chu, Q. Modified acid pretreatment to alter physicochemical properties of biomass for full cellulose/hemicellulose utilization. Carbohydr. Polym. 2023, 299, 120182. [Google Scholar] [CrossRef] [PubMed]
- Obeng, A.K.; Premjet, D.; Premjet, S. Fermentable sugar production from the peels of two durian (Durio zibethinus Murr.) cultivars by phosphoric acid pretreatment. Resources 2018, 7, 60. [Google Scholar] [CrossRef]
- Premjet, D.; Wongleang, S.; Premjet, S. Enhancing glucose recovery from Hibiscus cannabinus L. through phosphoric acid pretreatment. Energies 2022, 15, 7573. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. Thermochemical pretreatment enhanced bioconversion of elephant grass (Pennisetum purpureum): Insight on the production of sugars and lignin. Biomass Convers. Biorefin. 2022, 12, 1125–1138. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Lee, J.H.; Kim, D.S.; Lee, J.H.; Lee, S.K.; Lee, S.J.; Park, C.; Kim, S.W. Enhancement of glucose yield from canola agricultural residue by alkali pretreatment based on multi-regression models. J. Ind. Eng. Chem. 2017, 51, 303–311. [Google Scholar] [CrossRef]
- Arisht, S.N.; Abdul, P.M.; Liu, C.-M.; Lin, S.-K.; Maaroff, R.M.; Wu, S.-Y.; Jahim, J.M. Biotoxicity assessment and lignocellulosic structural changes of phosphoric acid pre-treated young coconut husk hydrolysate for biohydrogen production. Int. J. Hydrogen Energy 2019, 44, 5830–5843. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Lee, K.H.; Lee, S.K.; Chun, Y.; Kim, S.W.; Park, C.; Yoo, H.Y. Improvement of bioethanol production from waste chestnut shells via evaluation of mass balance-based pretreatment and glucose recovery process. Environ. Technol. Innov. 2022, 28, 102955. [Google Scholar] [CrossRef]
- Nandal, P.; Sharma, S.; Arora, A. Bioprospecting non-conventional yeasts for ethanol production from rice straw hydrolysate and their inhibitor tolerance. Renew. Energy 2020, 147, 1694–1703. [Google Scholar] [CrossRef]

| Composition (% dw) | Raw Material | H3PO4 Concentration (% v/v) | ||
|---|---|---|---|---|
| 70 | 75 | 80 | ||
| Glucan | 32.9 ± 0.5 d | 50.4 ± 0.3 c | 54.9 ± 0.2 b | 59.7 ± 0.5 a |
| Xylan | 23.8 ± 0.2 a | 12.4 ± 0.1 b | 10.2 ± 0.2 c | 9.0 ± 0.0 d |
| Arabinan | 5.7 ± 0.0 a | 3.4 ± 0.0 b | 3.3 ± 0.0 c | 3.2 ± 0.0 d |
| AIL | 22.2 ± 0.5 a | 18.0 ± 0.6 b | 13.6 ± 0.2 c | 12.0 ± 0.6 d |
| ASL | 5.9 ± 0.1 a | 3.7 ± 0.0 b | 3.6 ± 0.1 b | 3.3 ± 0.1 c |
| Total lignin | 28.2 ± 0.4 a | 21.7 ± 0.6 b | 17.1 ± 0.1 c | 15.3 ± 0.7 d |
| Solid recovery | 100.0 ± 0.0 a | 62.2 ± 0.7 b | 55.8 ± 0.6 c | 50.7 ± 0.9 d |
| Glucan recovery | 100.0 ± 0.0 a | 95.3 ± 0.5 b | 93.0 ± 0.3 c | 91.9 ± 0.8 c |
| Xylan recovery | 100.0 ± 0.0 a | 32.4 ± 0.2 b | 23.8 ± 0.4 c | 19.2 ± 0.1 d |
| Arabinan recovery | 100.0 ± 0.0 a | 37.1 ± 0.2 b | 32.4 ± 0.1 c | 28.5 ± 0.3 d |
| AIL recovery | 100.0 ± 0.0 a | 50.4 ± 1.6 b | 34.0 ± 0.5 c | 27.4 ± 1.3 d |
| ASL recovery | 100.0 ± 0.0 a | 39.1 ± 0.5 b | 33.4 ± 0.8 c | 27.9 ± 0.7 d |
| Total lignin recovery | 100.0 ± 0.0 a | 47.9 ± 1.3 b | 33.9 ± 0.2 c | 27.5 ± 1.2 d |
| Total lignin removal | n.d. | 52.0 ± 1.3 d | 66.1 ± 0.2 c | 72.5 ± 1.2 b |
| Untreated | Concentration of Phosphoric Acid | |||
|---|---|---|---|---|
| 70% | 75% | 80% | ||
| CrI (%) | 54.1 | 57.7 | 61.2 | 59.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongleang, S.; Premjet, D.; Premjet, S. Investigating the Potential of Grass Biomass (Thysanolaena latifolia) as an Alternative Feedstock for Sugar Platforms and Bioethanol Production. Energies 2024, 17, 4017. https://doi.org/10.3390/en17164017
Wongleang S, Premjet D, Premjet S. Investigating the Potential of Grass Biomass (Thysanolaena latifolia) as an Alternative Feedstock for Sugar Platforms and Bioethanol Production. Energies. 2024; 17(16):4017. https://doi.org/10.3390/en17164017
Chicago/Turabian StyleWongleang, Suwanan, Duangporn Premjet, and Siripong Premjet. 2024. "Investigating the Potential of Grass Biomass (Thysanolaena latifolia) as an Alternative Feedstock for Sugar Platforms and Bioethanol Production" Energies 17, no. 16: 4017. https://doi.org/10.3390/en17164017
APA StyleWongleang, S., Premjet, D., & Premjet, S. (2024). Investigating the Potential of Grass Biomass (Thysanolaena latifolia) as an Alternative Feedstock for Sugar Platforms and Bioethanol Production. Energies, 17(16), 4017. https://doi.org/10.3390/en17164017






