Abstract
The global challenge of sustainable and affordable wastewater treatment technology looms large as water pollution escalates steadily with the rapid pace of industrialization and population growth. The photocatalytic wastewater treatment is a cutting-edge and environmentally friendly technology that uses photons from light source to degrade and remove organic and inorganic contaminants from water. Thus, utilizing solar energy for photocatalytic wastewater treatment holds great promise as a renewable solution to alleviate pressures on the global water crisis. Employing solar concentrators to intensify sunlight for photocatalysis represents a promising avenue for future applications of a low-cost and rapid sustainable wastewater purification process. This groundbreaking approach will unveil fresh technological avenues for a cost-effective, sustainable, and swift wastewater purification process utilizing sunlight. This review article explores diverse solar concentrating systems and their potential applications in the wastewater treatment process.
1. Introduction
Water is an indispensable component for human survival; hence, fresh and clean water supply has surged in recent decades alongside population growth. Conversely, water pollution has been on the rise due to the swift pace of industrialization and unplanned urban expansion. Agricultural and pharmaceutical runoff, untreated sewage, and toxic industrial discharges constitute significant sources of this pollution [1,2]. Industries like textiles, dyeing, and printing are major contributors, releasing substantial quantities of synthetic organic dyes into waterways [2,3]. Access to fresh water supplies has emerged as a matter of global significance. Additionally, the health risks linked with polluted water are forecasted to escalate into a significant global concern in the coming decades. In developing nations, nearly 70% of all illnesses are linked to water contamination [1]. Hence, the most pressing challenge of the twenty-first century lies in the development of eco-friendly, cost-effective, and efficient wastewater treatment technologies. These advancements are crucial for safeguarding the environment and human health from the harmful effects of pollutants.
Over the past two decades, various treatment techniques have been implemented to treat water for reuse. Techniques such as phase separation, chemical precipitation, coagulation and flocculation, filtration, adsorption, ion exchange, sedimentation, chlorination, and oxidation have been utilized in wastewater treatment [1,2,3,4]. Most of these methods generate sludge, presenting a significant challenge for disposal due to the risk of secondary pollution. Some treatment processes also require high energy input, making water remediation expensive. Additionally, pollutants such as pesticides, hydrocarbons, dyes, and aromatic compounds in wastewater cannot be completely eliminated by a single technique. Consequently, researchers are focusing on developing new, greener technologies capable of degrading a wide range of contaminants. In this context, photocatalysis has emerged as a promising, sustainable, and efficient approach for water treatment [5,6,7,8]. Its capability to harness solar energy, a freely available natural resource, for wastewater treatment underscores its significance. The solar photocatalysis process is effective for the degradation and disinfection of municipal and industrial wastewater [8,9,10]. Operable at ambient room temperature and pressure, photocatalysis offers reduced operating costs and typically eliminates the need for additional processes or secondary treatment to remove reaction byproducts. These advantages make it a highly viable and effective solution for large wastewater treatment plants.
Although solar photocatalysis is considered as a promising technology for reducing chemical and microbiological pollutants in wastewater, it has not yet advanced beyond the bench scale to real-world practical applications. Thousands of research articles have been published on the development of photocatalysts for solar photocatalytic water treatment [11,12,13,14]. Karthikeyan et al. explored various metal oxide semiconductor photocatalysts for solar photocatalysis in their review article [12]. Wang et al. critically reviewed the development of heterojunction in semiconductors for enhanced photocatalysis processing [13]. Beyond the development of photocatalysts, technological development of the utilization solar energy for solar photocatalysis is necessary. Over the past decades, several techniques have been developed to utilize solar energy for photocatalytic water treatment processing. In this context, solar concentrators can a play significant role in enhancing photocatalytic activity under concentrated sunlight irradiation. This review article delineates the fundamental principles of solar concentration techniques and the benefits conferred by employing solar concentrators in the photocatalytic remediation of contaminants in water.
2. Solar Concentrator
Solar energy is the most abundant resource globally, with sunlight providing approximately 1.5 × 1018 kWh of energy annually. In comparison, the combined reserves of oil, coal, and gas amount to only 1.75 × 1015 kWh, 1.4 × 1015 kWh, and 5.5 × 1015 kWh, respectively [8]. This stark contrast highlights solar energy’s potential, offering over a hundred times the energy of the world’s total fossil fuel reserves annually. Researchers are actively pursuing the development of cost-effective and efficient technologies to harness solar energy, both to meet global energy demands and to mitigate environmental impact. The solar concentrating systems emerge as highly promising solutions for various applications. Over the past few decades, significant efforts have been invested in the development of efficient solar concentrators to capitalize on solar energy’s vast potential [8,15,16,17,18].
A solar concentrator is a device that collects solar radiation and focuses it on a smaller area to get higher irradiance power. The device is mainly comprised of a mirror or a lens or a series of lenses or mirror assembly, a receiver, and the tracking system. A paraboloid mirror and Fresnel lens are generally used to design a solar concentrator. The paraboloid mirror reflects the sunlight and concentrates at the focal point of the mirror as shown in Figure 1. The Australian National University has worked for many years on paraboloid dish solar concentrators, and they built a 500 m2 concentrator to convert solar energy into electricity, which is presented in Figure 2 [15]. Many researchers have developed similar kinds of solar concentrators to generate electricity from sunlight. These works have been summarized and described by Baharoon et al. [16] in their review article. Sometimes, a paraboloid cavity mirror is used with another secondary mirror to deliver concentrated sunlight through the cavity of the paraboloid mirror. The working principle of this kind of system is illustrated by ray diagram in Figure 3. The primary mirror (paraboloid cavity mirror) gathers and focuses sunlight onto a secondary mirror (flat mirror). This secondary mirror redirects and intensifies the sunlight onto a receiver or target. We have pioneered the development of such a solar concentrator, which we have coupled with an optical fiber bundle, known as ECoSEnDS (extremely concentrated solar energy delivery system) [8].
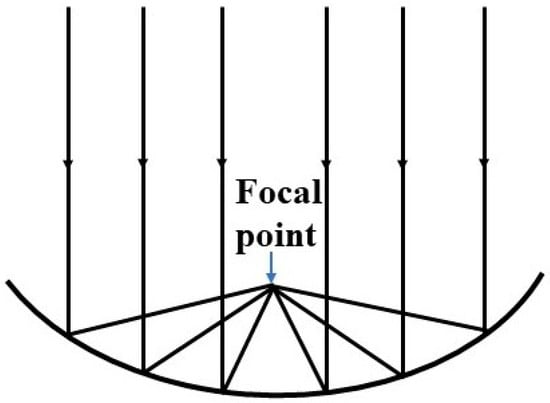
Figure 1.
Schematic ray diagram for sunlight concentration by a parabolic mirror.

Figure 2.
Picture of the 500 m2 paraboloid solar concentrator built by The Australian National University [15].
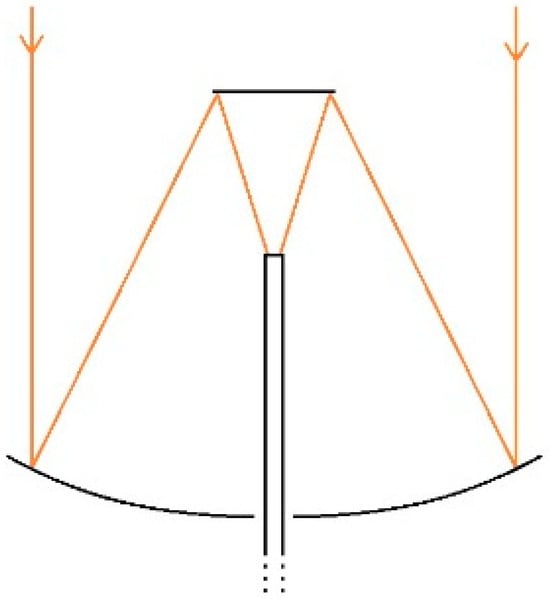
Figure 3.
The schematic ray diagram of a solar concentrator based on a primary paraboloid mirror and a secondary flat mirror (Reprinted with permission from Ref. [8]. 2021, Elsevier).
The picture of the ECoSEnDS is presented in Figure 4. The optical fiber bundle receives concentrated sunlight from the secondary mirror. The ECoSEnDS can deliver concentrated sunlight of 96 suns; i.e., 96 times the solar irradiance power on Earth’s surface with only 50% of the reflecting efficiency of the primary paraboloid mirror. The Fresnel-based solar concentrator has drawn significant attention due to the light weight, cheaper, and good concentration efficiency qualities of the Fresnel lens. The Fresnel lens refracts the sunlight and focuses it at a focal point to get concentrated sunlight. The working principle of a Fresnel lens is shown in Figure 5. Yang et al. [17] developed a Fresnel lens-based solar concentrator for a daylighting system, as shown in Figure 6. The Fresnel lens concentrates the sunlight on the top of the optical fiber bundle and the fiber bundle delivers sunlight inside the buildings.

Figure 4.
The picture of the ECoSEnDS (Reprinted with permission from Ref. [8]. 2021, Elsevier).
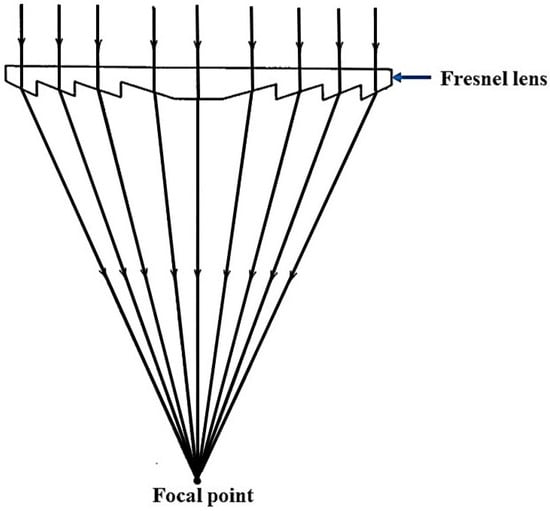
Figure 5.
Schematic ray diagram for sunlight concentration by a Fresnel lens.

Figure 6.
Solar concentrator employing Fresnel lenses coupled with optical fiber (Reprinted with permission from Ref. [17]. 2019, Elsevier]).
Most solar concentrators have been developed for energy harvesting and daylighting systems. There are very few solar concentrators that have been developed for water purification processing [19,20,21]. The utilization of solar concentrators for wastewater purification processing will be discussed in Section 4.
3. Photocatalysis
Photocatalysis is a chemical reaction which is accelerated by a photocatalyst on exposure of light. The key advantage of the photocatalysis is that the photocatalyst facilitates the chemical reaction without undergoing consumption [22,23]. Photocatalysis is categorized into homogeneous and heterogeneous types based on the physical state of the reactants and photocatalysts. In homogeneous photocatalysis, both the reactants and photocatalysts coexist in the same phase, whereas in heterogeneous photocatalysis, they exist in different phases.
Heterogeneous photocatalysis has drawn significant attention as it is very much sustainable and effective for wastewater purification processing. This method offers several advantages, including the utilization of abundant sunlight, the ease of fabrication of semiconductor nanomaterials as photocatalysts, and its rapid reaction rate coupled with low energy consumption. Consequently, heterogeneous photocatalysis emerges as a promising technique for wastewater treatment, leveraging the abundance of solar energy resources. The mechanism of heterogeneous photocatalysis typically involves multiple steps [5,22] and it is represented schematically in Figure 7. The process initiates with the absorption of light, where photons with energy exceeding the band gap of the photocatalyst generate electrons (e−) in the conduction band (CB) and holes (h+) in the valence band (VB) of the semiconductor photocatalysts. These electrons and holes migrate to the photocatalyst’s surface to engage in redox reactions. The holes and electrons participate in oxidation and reduction of H2O and O2 molecules to produce hydroxyl (·OH) and superoxide radicals , respectively. These radicals promptly react with pollutants, leading to their degradation into harmless or less harmful byproducts such as carbon dioxide (CO2), water (H2O), and other intermediate compounds [22]. The complete photocatalytic degradation process can be summarized in Equations (1)–(5) [22,23,24]:
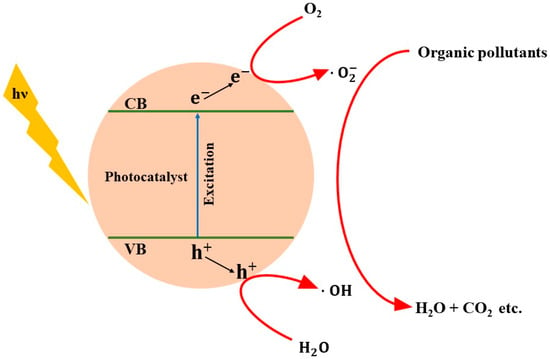
Figure 7.
Schematic representation of the heterogeneous photocatalytic organic pollutant’s degradation mechanism.
The efficiency of the photocatalysis process depends on several parameters including the redox potential of the pollutants, the band edge of semiconductor photocatalysts [25]. The necessary condition for the photocatalytic reaction is that the redox potential of the pollutant should be lower than the conduction band potential and higher than the valence band potential of the photocatalyst [26].
The semiconductor nanomaterials have been extensively researched for their role in heterogeneous photocatalysis. Titanium dioxide (TiO2) and zinc oxide (ZnO) are prominent among these, thanks to their remarkable photocatalytic activity, non-toxic nature, and high stability under light exposure [27,28,29]. However, their effectiveness is mainly confined to UV-light irradiation due to their wide bandgap energy [30,31], limiting their potential for solar photocatalysis since sunlight mainly consists of visible light. To overcome this limitation, various techniques such as anion doping, metal doping, and inducing oxygen deficiency [27,32,33,34,35] have been employed to enhance their activity under visible light. Although doping enables TiO2 or ZnO to absorb visible light, issues like thermal instability and carrier recombination often arise, hindering the development of highly efficient visible-light active photocatalysts. Consequently, researchers have turned to alternative materials like MoS2, BiVO4, Bi2WO6, Ag3VO4, AgVO3, CoFe2O4, Zr2Ni2Cu7, and CdS [5,7,22,36,37,38,39,40,41,42,43,44] to fabricate visible-light active photocatalysts with controlled band structures. These photocatalysts are very much active in visible light and show a higher photocatalytic degradation rate, but some of the photocatalysts like CdS and BiVO4 are highly toxic [45,46,47,48]. Results suggest that high doses can cause significant organ damage, particularly in the liver, kidneys, and lungs. Symptoms observed include inflammation, oxidative stress, and histopathological changes. BiVO4 nanoparticles can also pose risks to the environment. They can accumulate in water bodies and soil, affecting aquatic and terrestrial organisms. Studies have shown that these nanoparticles can be toxic to algae, fish, and invertebrates, potentially disrupting ecosystems. Recent studies indicate that treated water is no longer harmful if toxic photocatalysts are removed following the photocatalysis process [49,50]. However, challenges such as fast electron-hole recombination and limited absorption persist for those photocatalysts. Therefore, numerous strategies have emerged, with photocatalytic heterojunctions being widely explored. These heterojunctions offer promising prospects for enhancing photocatalytic efficiency by spatially separating electron-hole pairs [51,52,53,54]. Low et al. [52] provide a comprehensive discussion on the principles underlying various heterojunction photocatalysts, as depicted in Figure 8.
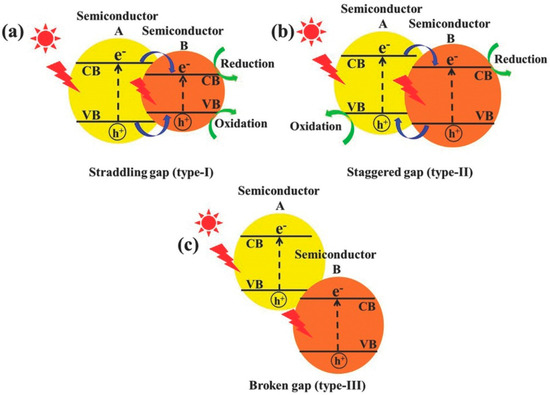
Figure 8.
Schematic depiction of three distinct heterojunction photocatalyst types: (a) type-I, (b) type-II, and (c) type-III heterojunctions (Reprinted with permission from Ref. [52]. 2014, Royal Society of Chemistry).
In recent years, thousands of research articles have been reported in literature on wastewater treatment using photocatalysis under visible light irradiation. However, only a handful of studies have explored the utilization of direct sunlight. Instead, most researchers have opted for artificial sources like Xenon arc lamps, fluorescent lamps, LED lamps, or solar simulators to investigate photocatalytic activity. Leveraging solar energy for pollutant removal in wastewater holds profound importance in energy conservation and environmental remediation efforts.
4. Solar Concentrators for Water Treatment
The photocatalytic treatment of wastewater using solar energy has garnered significant interest due to its environmental and economic benefits. Traditional wastewater treatment processes require substantial electrical power, leading to the emission of greenhouse gases. Studies indicate that conventional wastewater treatment consumes approximately 0.6 kWh per cubic meter, resulting in 185.61 g of CO2 emissions per kWh [55]. In contrast, solar photocatalytic wastewater treatment harnesses freely available solar energy, eliminating the need for external energy sources and reducing greenhouse gas emissions. Therefore, solar concentrators can significantly enhance photocatalytic wastewater treatment efficiency under intense sunlight, offering a promising solution for sustainable and eco-friendly water treatment. The photocatalytic activity hinges significantly on photon count, as photons initiate the creation of electron-hole pairs, as detailed in Section 3. Consequently, photocatalysts generate an increased number of photons under concentrated sunlight irradiation, thereby yielding higher quantities of hydroxyl and superoxide radicals. This cascade effect accelerates the pollutants in water.
In the last few decades, many efforts have been made to develop efficient systems for wastewater purification processing using solar concentrators. Bigoni et al. [20] developed a solar pasteurizer using a parabolic trough concentrator for water purification. The developed system is shown in Figure 9. The sunlight is reflected by a parabolic mirror made with aluminum onto a black steel pipe, serving as the absorber, precisely positioned along the mirror’s focal line. The absorber, measuring 3 m in length with an internal diameter of 3.8 cm and a volume of 3.4 L, effectively captures the concentrated solar energy. They used three pieces of 100 by 200 cm highly reflective sheets to make parabolic reflector. As untreated water flows through the pipe, it undergoes heating from the concentrated solar radiation, reaching pasteurization temperatures. Using this device, Bigoni et al. [20] were able to collect 66 L of treated water in a sunny day. They mainly studied the pathogens removal from the water.
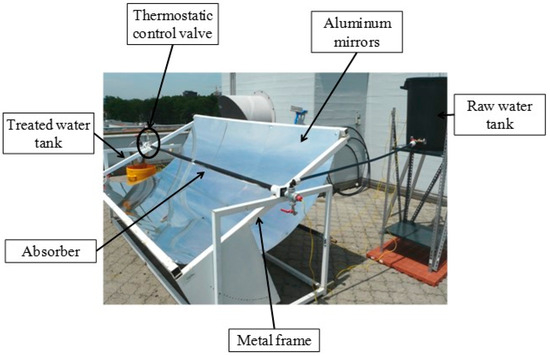
Figure 9.
The water pasteurization system using a parabolic solar concentrator (Reprinted with permission from Ref. [20]. 2014, Elsevier).
Monteagudo et al. [21] implemented a Fresnel lens to concentrate sunlight for photocatalytic wastewater treatment processing. Monteagudo et al. used a Fresnel lens to focus concentrated sunlight on the tank containing wastewater. The schematic representation of the experimental setup is shown in Figure 10. They studied the photocatalytic degradation of orange II dye using titanium dioxide as photocatalysts and observed higher degradation rate than normal sunlight irradiation. The solar concentrator generates higher solar irradiance power at the focal point where sample is kept and hence the photocatalysts get more photons to generate much more electron-hole pairs. Thus, the photocatalysis reaction rate increases and wastewater becomes clean quickly.
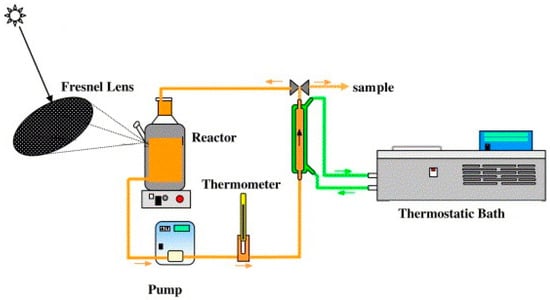
Figure 10.
Experimental setup for photocatalytic dye degradation under concentrated sunlight irradiation using Fresnel lens (Reprinted with permission from Ref. [21]. 2006, Elsevier).
Similar results were observed by Golli et al. [56], who used a parabolic dish solar collector to concentrate sunlight for the photocatalytic wastewater treatment process. The parabolic dish collects and focuses sunlight onto the reactor containing the wastewater. A schematic representation of the experimental setup is shown in Figure 11. They treated 2 L of contaminated water for the photocatalytic degradation of 10 ppm of methylene blue dye using zinc oxide as the photocatalyst and achieved a degradation rate of 94%.
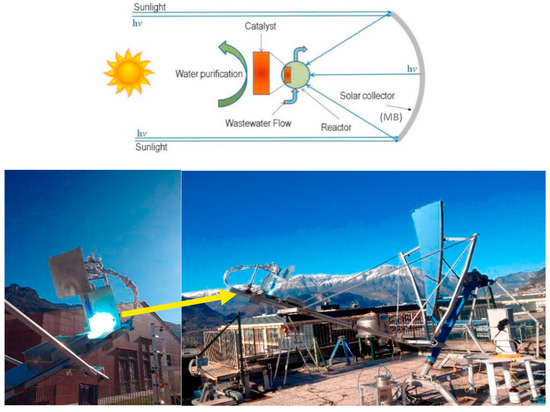
Figure 11.
Parabolic dish solar concentrator-based water treatment setup (Reprinted with permission from Ref. [56]. 2021, Elsevier).
The solar concentrator based on parabolic trough collector (PTC) and compound parabolic collector (CPC) are mostly used for wastewater treatment because of the high solar power concentration ratio. They can be used to degrade various pollutants in water. Some significance reports are summarized in Table 1.

Table 1.
Photocatalytic pollutants removal using a solar concentrator.
The solar concentrator, paired with an optical fiber bundle, efficiently delivers concentrated sunlight precisely where needed. Optical fiber bundles offer flexible installation options and long transmission distances, thanks to minimal light loss due to internal reflections within the fibers. With the aid of a solar tracking system, these devices ensure continuous delivery of highly intense sunlight throughout the day. While many efficient devices have been developed over the past decade to harness sunlight within buildings, none have been specifically tailored for water treatment applications. Addressing this gap, our research group recently introduced a groundbreaking solution: the ECoSEnDS (extremely concentrated solar energy delivery system). In our study, we explored its potential in photocatalytic wastewater treatment. The picture of the ECoSEnDS is presented in Figure 4. The ECoSEnDS comprises two mirrors: a primary paraboloid mirror and a secondary flat mirror, both crafted from glass with aluminum-coated reflecting surfaces. The primary mirror directs sunlight onto the secondary mirror, which then focuses and concentrates the light onto the optical fiber bundle. This setup allows the device to amplify sunlight by a factor of 96 compared to direct sunlight, achieving a concentration ratio of 96 suns with a 50% reflection efficiency of the primary mirror. In our experiments, this concentrated sunlight was utilized for photocatalytic dye degradation processes using various photocatalysts. Figure 12 illustrates how the concentrated light was delivered into the photocatalytic reactor, demonstrating the practical application of the ECoSEnDS in wastewater treatment.

Figure 12.
Photocatalytic reactor with concentrated sunlight delivered using ECoSEnDS.
The photocatalytic wastewater treatment activity of the ECoSEnDS was studied by employing BiVO4 nanoparticles to degrade methylene blue (MB) dye. Results demonstrated degradation rates of 20%, 71%, and 100% within 60 min under solar irradiance levels of 70, 300, and 500 mW/cm2, respectively [8]. Notably, the dye degradation outpaced natural sunlight by fivefold. Figure 13 illustrates the photocatalytic activity.
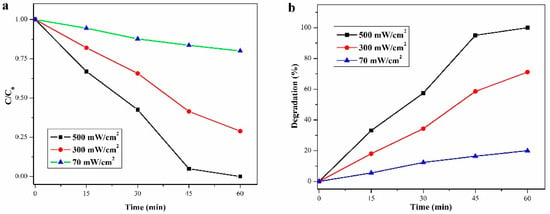
Figure 13.
Photocatalytic MB dye degradation of BiVO4 nanoparticles under concentrated sunlight irradiation: (a) relative degradation over time and (b) % degradation achieved (Reprinted with permission from Ref. [8]. 2021, Elsevier).
Our team further investigated the photocatalytic degradation of MB dye utilizing BiVO4 nanoflakes, employing an advanced solar concentrator coupled with an optical fiber bundle. Interestingly, our findings mirrored those previously mentioned. As depicted in Figure 14, we observed degradation efficiencies of 88.86% and 32.30% after 60 min of sunlight irradiation at intensities of 300 and 74 mW/cm2, respectively [5]. This underscores the enhanced photocatalytic activity of BiVO4 nanoflakes under increased sunlight irradiation intensity.
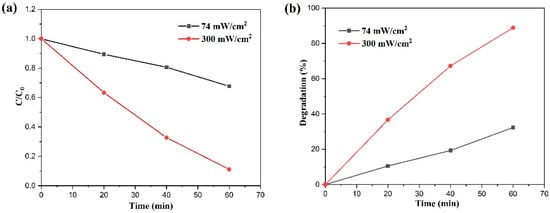
Figure 14.
Photocatalytic MB dye degradation of BiVO4 nanoflakes under concentrated sunlight irradiation: (a) relative degradation over time and (b) % degradation achieved (Reprinted with permission from Ref. [5]. 2021, Elsevier).
5. Advantages and Disadvantages of Using a Solar Concentrator
Using a solar concentrator in photocatalytic wastewater treatment offers several advantages. Solar concentrators focus sunlight onto a smaller area, significantly increasing light intensity, thereby enhancing photocatalytic activity. By concentrating sunlight, the photocatalyst can utilize solar energy more effectively, leading to higher reaction rates and more efficient pollutant degradation. Higher light intensity from concentrated sunlight accelerates the generation of electron-hole pairs, speeding up pollutant degradation. This conclusion can be clearly understood from Figure 13 and Figure 14. Solar energy is free and abundant, reducing the need for external electrical power, which is typically costly and associated with greenhouse gas emissions. By leveraging natural sunlight, operational costs associated with artificial light sources and electricity are minimized. Using solar energy eliminates the greenhouse gas emissions linked to electricity generation for wastewater treatment. Solar photocatalysis is an eco-friendly and sustainable method, promoting green chemistry principles. Solar concentrators can be designed to suit different geographic locations and sunlight conditions, making the technology versatile and adaptable. The use of solar concentrators also encourages the development of innovative reactor designs that can further optimize the photocatalytic process.
Although using a solar concentrator offers many benefits for the photocatalytic wastewater treatment process, there are several disadvantages to consider. The installation of solar concentrators involves significant upfront costs for materials, installation, and maintenance, which can be a barrier, especially for large-scale applications. The costs associated with installing and maintaining water treatment reactors are summarized in Table 2. Additionally, solar energy faces the drawback of being available only during the daytime. Solar concentrators rely on clear, sunny weather to function effectively; cloudy or rainy conditions can significantly reduce their efficiency, leading to inconsistent performance. While solar concentrators enhance light intensity, the overall efficiency of the photocatalytic process can still be limited by the inherent properties of the photocatalyst material, such as its light absorption capacity and charge carrier recombination rates.

Table 2.
Operating and capital costs for selected pilot-scale reactors.
6. Conclusions and Outlook
Photocatalysis is the most promising technique for addressing pressing environmental concerns, particularly in water purification. Leveraging solar energy to eradicate pollutants from wastewater holds profound significance for both energy conservation and environmental remediation. Thus, the photocatalytic wastewater treatment using sunlight irradiation is the most sustainable challenging method to purify wastewater. The solar concentrator has the potential to increase photocatalytic degradation rates of pollutants within wastewater, offering a sustainable and economically viable solution. Furthermore, the synergy of solar concentrators with optical fibers presents a distinct advantage, enabling precise delivery of light to the photocatalytic reactor at the desired location due to the flexibility of the optical fiber bundle. This integration of solar concentrators with optical fibers heralds new technological vistas for harnessing solar energy in wastewater purification processes. Thus, this review article serves as a valuable reference point for comprehending, exploring, and innovating new devices based on solar concentrators, advancing the cause of sustainable wastewater purification.
Author Contributions
Conceptualization: J.S.R.; writing—original draft preparation: J.S.R.; writing—review and editing: Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Roy, J.S.; Bhattacharya, G.; Chauhan, D.; Deshmukh, S.; Upadhyay, R.; Priyadarshini, R.; Sinha Roy, S. Potential use of smartly engineered red mud nanoparticles for removal of arsenate and pathogens from drinking water. SN Appl. Sci. 2020, 2, 796. [Google Scholar] [CrossRef]
- Dutta, D.; Arya, S.; Kumar, S. Industrial wastewater treatment: Current trends, bottlenecks, and best practices. Chemosphere 2021, 285, 131245. [Google Scholar] [CrossRef] [PubMed]
- Gitis, V.; Hankins, N. Water treatment chemicals: Trends and challenges. J. Water Process. Eng. 2018, 25, 34–38. [Google Scholar] [CrossRef]
- Chegrouche, S.; Mellah, A.; Barkat, M. Removal of strontium from aqueous solutions by adsorption onto activated carbon: Kinetic and thermodynamic studies. Desalination 2009, 235, 306–318. [Google Scholar] [CrossRef]
- Roy, J.S.; Morency, S.; Messaddeq, Y. Ultrafast cleaning of methylene blue contaminated water accelarating photocatalytic reaction rate of the BiVO4 nanoflakes under highly intense sunlight irradiation. J. Photochem. Photobiol. 2021, 7, 100037. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.S.; Dugas, G.; Morency, S.; Messaddeq, Y. Rapid degradation of Rhodamine B using enhanced photocatalytic activity of MoS2 nanoflowers under concentrated sunlight irradiation. Phys. E Low-Dimens. Syst. Nanostructures 2020, 120, 114114. [Google Scholar] [CrossRef]
- Roy, J.S.; Morency, S.; Dugas, G.; Messaddeq, Y. Development of an extremely concentrated solar energy delivery system using silica optical fiber bundle for deployment of solar energy: Daylighting to photocatalytic wastewater treatment. Sol. Energy 2021, 214, 93–100. [Google Scholar] [CrossRef]
- Pandey, A.K.; Reji Kumar, R.; Kalidasan, B.; Laghari, I.A.; Samykano, M.; Kothari, R.; Abusorrah, A.M.; Sharma, K.; Tyagi, V.V. Utilization of solar energy for wastewater treatment: Challenges and progressive research trends. J. Environ. Manag. 2021, 297, 113300. [Google Scholar] [CrossRef]
- Muscetta, M.; Ganguly, P.; Clarizia, L. Solar-powered photocatalysis in water purification: Applications and commercialization challenges. J. Environ. Chem. Eng. 2024, 12, 113073. [Google Scholar] [CrossRef]
- Ray, S.K.; Cho, J.; Hur, J. A critical review on strategies for improving efficiency of BaTiO3-based photocatalysts for wastewater treatment. J. Environ. Manag. 2021, 290, 112679. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, C.; Arunachalam, P.; Ramachandran, K.; Al-Mayouf, A.M.; Karuppuchamy, S. Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J. Alloys Compd. 2020, 828, 154281. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Z.; Shen, S.; Zhong, W.; Cao, S. Advances in designing heterojunction photocatalytic materials. Chin. J. Catal. 2021, 42, 710–730. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Graimed, B.H. Recent developments in industrial organic degradation via semiconductor heterojunctions and the parameters affecting the photocatalytic process: A review study. J. Water Proc. Eng. 2022, 47, 102671. [Google Scholar] [CrossRef]
- Lovegrove, K.; Burgess, G.; Pye, J. A new 500 m2 paraboloid dish solar concentrator. Sol. Energy 2011, 85, 620–626. [Google Scholar] [CrossRef]
- Baharoon, D.A.; Rahman, H.A.; Omar, W.Z.W.; Fadhl, S.O. Historical development of concentrating solar power technologies to generate clean electricity efficiently—A review. Renew. Sustain. Energy Rev. 2015, 41, 996–1027. [Google Scholar] [CrossRef]
- Yang, Z.; Li, L.; Wang, J.T.; Wang, W.; Song, J. Realization of high flux daylighting via optical fibers using large Fresnel lens. Sol. Energy 2019, 183, 204–211. [Google Scholar] [CrossRef]
- Chafie, M.; Fadhel, M.; Aissa, B.; Guizani, A. Energetic end exergetic performance of a parabolic trough collector receiver: An experimental study. J. Clean. Prod. 2018, 171, 285–296. [Google Scholar] [CrossRef]
- Tanveer, M.; Guyer, G.T. Solar assisted photo degradation of wastewater by compound parabolic collectors: Review of design and operational parameters. Renew. Sustain. Energy Rev. 2013, 24, 534–543. [Google Scholar] [CrossRef]
- Bigoni, R.; Kötzsch, S.; Sorlini, S.; Egli, T. Solar water disinfection by a Parabolic Trough Concentrator (PTC): Flow-cytometric analysis of bacterial inactivation. J. Clean. Prod. 2014, 67, 62–71. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; Duran, A. Fresnel lens to concentrate solar energy for the photocatalytic decoloration and mineralization of orange II in aqueous solution. Chemosphere 2006, 65, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.S.; Dugas, G.; Morency, S.; Ribeiro, S.J.L.; Messaddeq, Y. Enhanced photocatalytic activity of silver vanadate nanobelts in concentrated sunlight delivered through optical fiber bundle coupled with solar concentrator. SN Appl. Sci. 2020, 2, 185. [Google Scholar] [CrossRef]
- Jia, P.; Tan, H.; Liu, K.; Gao, W. Synthesis, characterization and photocatalytic property of novel ZnO/bone char composite. Mater. Res. Bull. 2018, 102, 45–50. [Google Scholar] [CrossRef]
- Yang, C.; Dong, W.; Cui, G.; Zhao, Y.; Shi, X.; Xia, X.; Tang, B.; Wang, W. Highly efficient photocatalytic degradation of methylene blue by P2ABSA-modified TiO2 nanocomposite due to the photosensitization synergetic effect of TiO2 and P2ABSA. RSC Adv. 2017, 7, 23699–23708. [Google Scholar] [CrossRef]
- Mishra, B.P.; Parida, K. Orienting Z scheme charge transfer in graphitic carbon nitride-based systems for photocatalytic energy and environmental applications. J. Mater. Chem. 2021, 9, 10039–10080. [Google Scholar] [CrossRef]
- Tian, D.; Zhou, H.; Zhang, H.; Zhou, P.; You, J.; Yao, G.; Pan, Z.; Liu, Y.; Lai, B. Heterogeneous photocatalyst-driven persulfate activation process under visible light irradiation: From basic catalyst design principles to novel enhancement strategies. Chem. Eng. J. 2022, 428, 131166. [Google Scholar] [CrossRef]
- Lan, Y.; Lu, Y.; Ren, Z. Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energy 2013, 2, 1031–1045. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Jingyu, H.; Ran, Y.; Zhaohui, L.; Yuanqiang, S.; Lingbo, Q.; Nti Kani, A. In-situ growth zinc oxide globular on the graphitic carbon nitride to fabrication binary heterojunctions and their photocatalytic degradation performance for the tetracycline. Solid State Sci. 2019, 92, 60–67. [Google Scholar] [CrossRef]
- Roy, J.S.; Pal Majumder, T.; Dabrowski, R. Photoluminescence behavior of TiO2 nanoparticles doped with liquid crystals. J. Mol. Struc. 2015, 1098, 351–354. [Google Scholar] [CrossRef]
- Baruah, S.; Afre, R.A.; Pugliese, D. Effect of Size and Morphology of Different ZnO Nanostructures on the Performance of Dye-Sensitized Solar Cells. Energies 2024, 17, 2076. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, Y.; Liu, X.; Xue, H.; Qian, Q.; Chen, Q. Design of Cu–Ce co-doped TiO2 for improved photocatalysis. J. Mater. Sci. 2017, 52, 1265–1271. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Y.; Zhang, W.; Cao, P. Synthesis, characterization, and photocatalytic performance of Cu/Y co-doped TiO2 nanoparticles. Mater. Chem. Phys. 2022, 277, 125558. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Film. 2016, 605, 2–19. [Google Scholar] [CrossRef]
- Pirhashemi, M.; Habibi-Yangjeh, A.; Pouran, S.R. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J. Ind. Eng. Chem. 2018, 62, 1–25. [Google Scholar] [CrossRef]
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A review on BiVO4 photocatalyst: Activity enhancement methods for solar photocatalytic applications. Appl. Catal. A Gen. 2018, 555, 47–74. [Google Scholar]
- Zhang, Z.; Wang, W.; Gao, E. Polypyrrole/Bi2WO6 composite with high charge separation efficiency and enhanced photocatalytic activity. J. Mater. Sci. 2014, 49, 7325–7332. [Google Scholar] [CrossRef]
- Vu, T.A.; Dao, C.D.; Hoang, T.T.T.; Dang, P.T.; Tran, H.T.K.; Nguyen, K.T.; Le, G.H.; Nguyen, T.V.; Lee, G.D. Synthesis of novel silver vanadates with high photocatalytic and antibacterial activities. Mater. Lett. 2014, 123, 176–180. [Google Scholar] [CrossRef]
- Choudhary, S.; Bisht, A.; Mohapatra, S. Facile synthesis, morphological, structural, photocatalytic and optical properties of CoFe2O4 nanostructures. SN Appl. Sci. 2019, 1, 1613. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Ahamad, T.; Al-Saeedi, S.I.; Al-Senani, G.M.; Al-kadhi, N.S.; Stadler, F.J. Facile fabrication of Zr2Ni1Cu7 trimetallic nano-alloy and its composite with Si3N4 for visible light assisted photodegradation of methylene blue. J. Mol. Liq. 2018, 272, 170–179. [Google Scholar] [CrossRef]
- Wu, S.Z.; Li, K.; Zhang, W.D. On the heterostructured photocatalysts Ag3VO4/g-C3N4 with enhanced visible light photocatalytic activity. App. Surf. Sci. 2015, 324, 324–331. [Google Scholar] [CrossRef]
- Wei, Z.; Mogan, T.R.; Wang, K.; Janczarek, M.; Kowalska, E. Morphology-Governed Performance of Multi-Dimensional Photocatalysts for Hydrogen Generation. Energies 2021, 14, 7223. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Singh Bhadwal, A.; Tripathi, R.M.; Gupta, R.K.; Kumar, N.; Singh, R.P.; Shrivastav, A. Biogenic synthesis and photocatalytic activity of CdS nanoparticles. RSC Adv. 2014, 4, 9484–9490. [Google Scholar] [CrossRef]
- Hardman, R. A toxicologic review of quantum dots: Toxicity depends on physicochemical and environmental factors. Environ. Health Perspect. 2006, 114, 165–172. [Google Scholar] [CrossRef]
- Hossain, S.F.; Mukherjee, S.K. Toxicity of cadmium sulfide (CdS) nanoparticles against Escherichia coli and HeLa cells. J. Hazard. Mater. 2013, 260, 1073–1082. [Google Scholar] [CrossRef]
- Llobet, J.M.; Domingo, J.L. Acute toxicity of vanadium compounds in rats and mice. Toxicol. Lett. 1984, 23, 227–231. [Google Scholar] [CrossRef]
- Wörle-Knirsch, J.M.; Kern, K.; Schleh, C.; Adelhelm, C.; Feldmann, C.; Krug, H.F. Nanoparticulate Vanadium Oxide Potentiated Vanadium Toxicity in Human Lung Cells. Environ. Sci. Technol. 2007, 41, 331–336. [Google Scholar] [CrossRef]
- Kamble, G.S.; Ling, Y.-C. Solvothermal synthesis of facet-dependent BiVO4 photocatalyst with enhanced visible-light-driven photocatalytic degradation of organic pollutant: Assessment of toxicity by zebrafish embryo. Sci. Rep. 2020, 10, 12993. [Google Scholar] [CrossRef]
- Ghaware, R.C.; Birajdar, N.B.; Kamble, G.S.; Kolekar, S.S. Degradation of organic Pollutant by Using of BiVO4–NiFe2O4 Heterostructure Photocatalyst under Visible Light Irradiation: Assessment of Detoxicity Study Using Cirrhinus mrigala. Langmuir 2024, 40, 14426–14439. [Google Scholar] [CrossRef] [PubMed]
- Tojo, F.; Ishizaki, M.; Kubota, S.; Kurihara, M.; Hirose, F.; Ahmmad, B. Histidine Decorated Nanoparticles of CdS for Highly Efficient H2 Production via Water Splitting. Energies 2020, 13, 3738. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Rebekah, A.; Lee, K.S. Two-stage hydrothermal process driven visible light sensitive photocatalytic m-ZnWO4/m-WO3 heterojunction composite materials. APL Mater. 2024, 12, 041122. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Z.; Nie, H.; Kong, B. The direct Z-scheme character and roles of S vacancy in BiOCl/Bi2S3-(001) heterostructures for superior photocatalytic activity: A hybrid density functional investigation. Phys. Chem. Chem. Phys. 2024, 26, 10723–10736. [Google Scholar] [CrossRef] [PubMed]
- Guernanou, R.; Sadek, I.; Belgassim, B.; Lamine, A.; Aïcha, S.; Feriel, K.; Fatima, B. Solar wastewater treatment: Advantages and efficiency for reuse in agriculture and industry. In Proceedings of the International Renewable and Sustainable Energy Conference (IRSEC), Agadir, Morocco, 27–30 November 2019. [Google Scholar] [CrossRef]
- Golli, A.E.; Fendrich, M.; Bazzanella, N.; Dridi, C.; Miotello, A.; Orlandi, M. Wastewater remediation with ZnO photocatalysts: Green synthesis and solar concentration as an economically and environmentally viable route to application. J. Environ. Manag. 2021, 286, 112226. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.M.; Fernández, G.; Klamerth, N.; Maldonado, M.I.; Álvarez, P.M.; Malato, S. Efficiency of different solar advanced oxidation processes on the oxidation of bisphenol A in water. Appl. Catal. B Environ. 2010, 95, 228–237. [Google Scholar] [CrossRef]
- Fenoll, J.; Hellín, P.; Martínez, C.M.; Flores, P.; Navarro, S. Semiconductor oxides-sensitized photodegradation of fenamiphos in leaching water under natural sunlight. Appl. Catal. B Environ. 2012, 115–116, 31–37. [Google Scholar] [CrossRef]
- Barwal, A.; Chaudhary, R. Feasibility study for the treatment of municipal wastewater by using a hybrid bio-solar process. J. Environ. Manag. 2016, 177, 271–277. [Google Scholar] [CrossRef]
- Aguas, Y.; Hincapie, M.; Fernández-Ibáñez, P.; Polo-López, M.I. Solar photocatalytic disinfection of agricultural pathogenic fungi (Curvularia sp.) in real urban wastewater. Sci. Total. Environ. 2017, 607–608, 1213–1224. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Polo-López, M.I.; Mosteo, R.; Ormad, M.P.; Fernández-Ibáñez, P. Disinfection of real and simulated urban wastewater effluents using a mild solar photo-Fenton. Appl. Catal. B Environ. 2014, 150–151, 619–629. [Google Scholar] [CrossRef]
- Onotri, L.; Race, M.; Clarizia, L.; Guida, M.; Alfè, M.; Andreozzi, R.; Marotta, R. Solar photocatalytic processes for treatment of soil washing wastewater. Chem. Eng. J. 2017, 318, 10–18. [Google Scholar] [CrossRef]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Solar reclamation of wastewater effluent polluted with bisphenols, phthalates and parabens by photocatalytic treatment with TiO2/Na2S2O8 at pilot plant scale. Chemosphere 2018, 212, 95. [Google Scholar] [CrossRef] [PubMed]
- Durán, A.; Monteagudo, J.M.; San Martín, I. Photocatalytic treatment of an industrial effluent using artificial and solar UV radiation: An operational cost study on a pilot plant scale. J. Environ. Manag. 2012, 98, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, J.; Bayarri, B.; Gonzalez, O.; Malato, S.; Peral, J.; Esplugas, S. Advanced Oxidation Processes at Laboratory Scale: Environmental and Economic Impacts. ACS Sustain. Chem. Eng. 2015, 3, 3188–3196. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).