Influence of the Processing Conditions on the Rheology and Heat of Decomposition of Solution Processed Hybrid Nanocomposites and Implication to Sustainable Energy Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
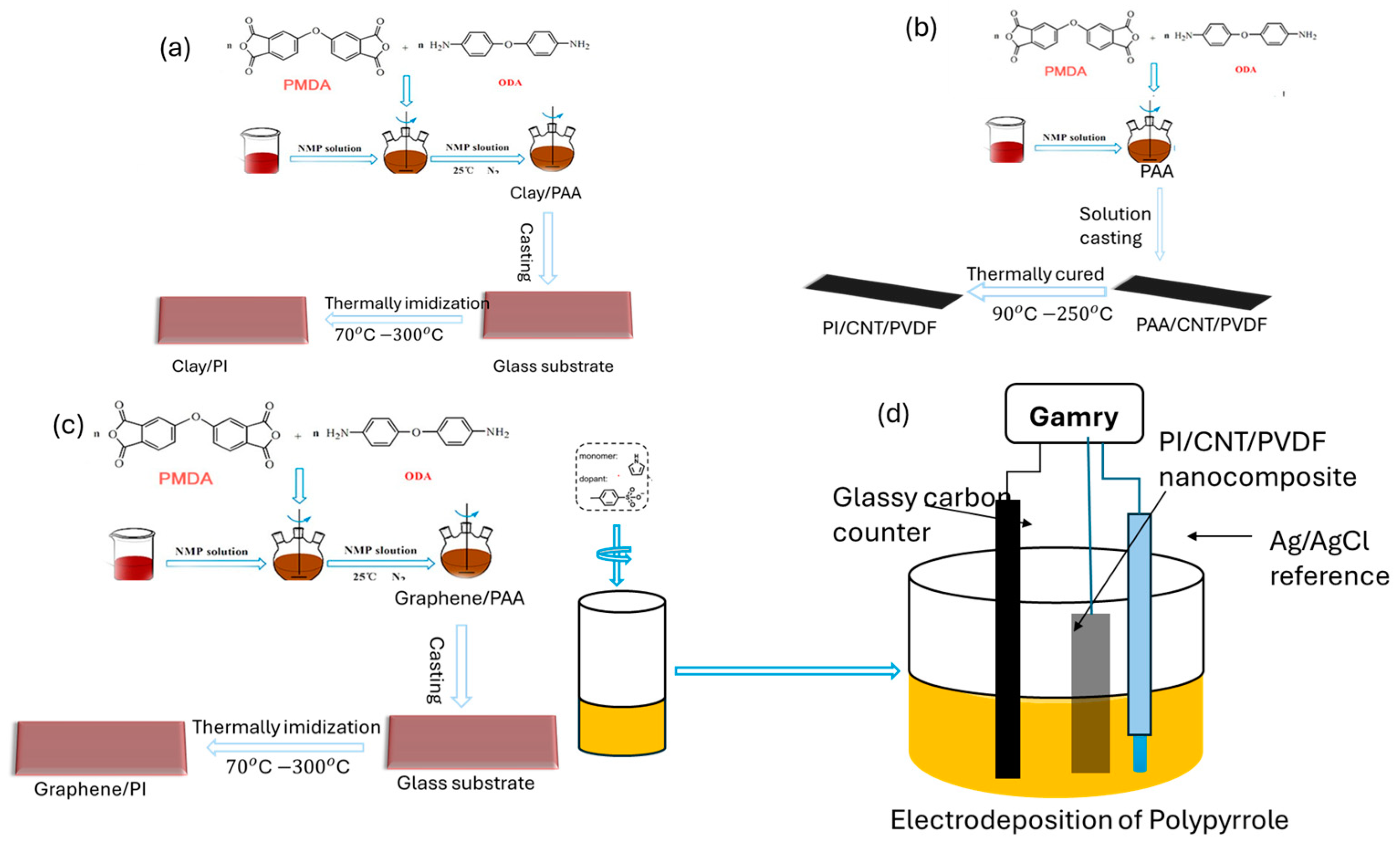
2.2. Synthesis and Fabrication of the Nanocomposites
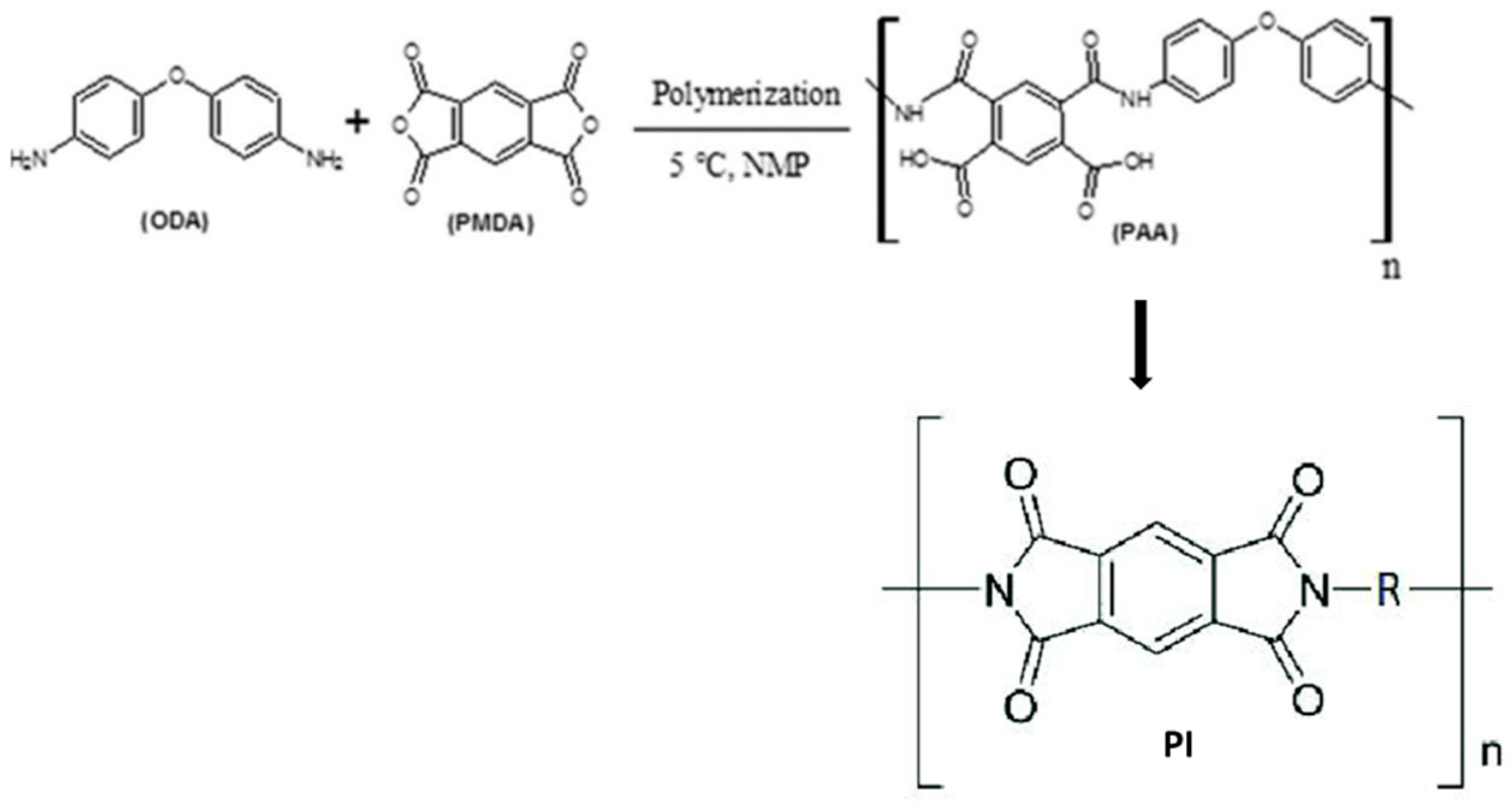
2.2.1. Poly(amic Acid)/Clay Nanocomposites
2.2.2. Preparation of Polyimide–Carbon Nanotube/Poly(vinylidene Fluoride) (PI/CNT-PVDF) Nanocomposites
2.2.3. Polyimide–Nanographene Composite (PI-NGC)
2.2.4. Polypyrrole Electrodeposition and Doping
2.3. Differential Scanning Calorimetry (DSC)
2.4. Cyclic Voltammetry (CV)
3. Results and Discussion
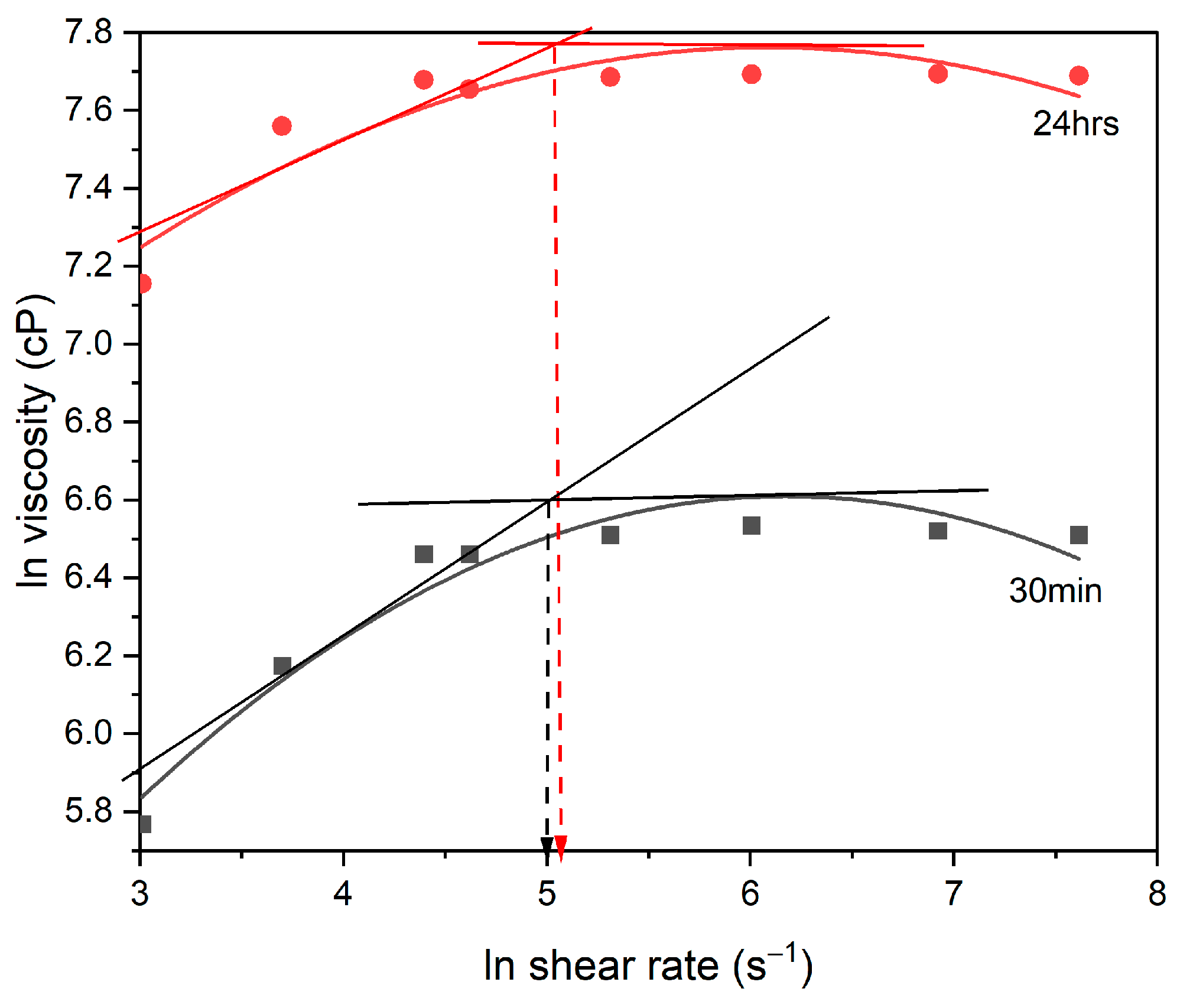
3.1. Rheology
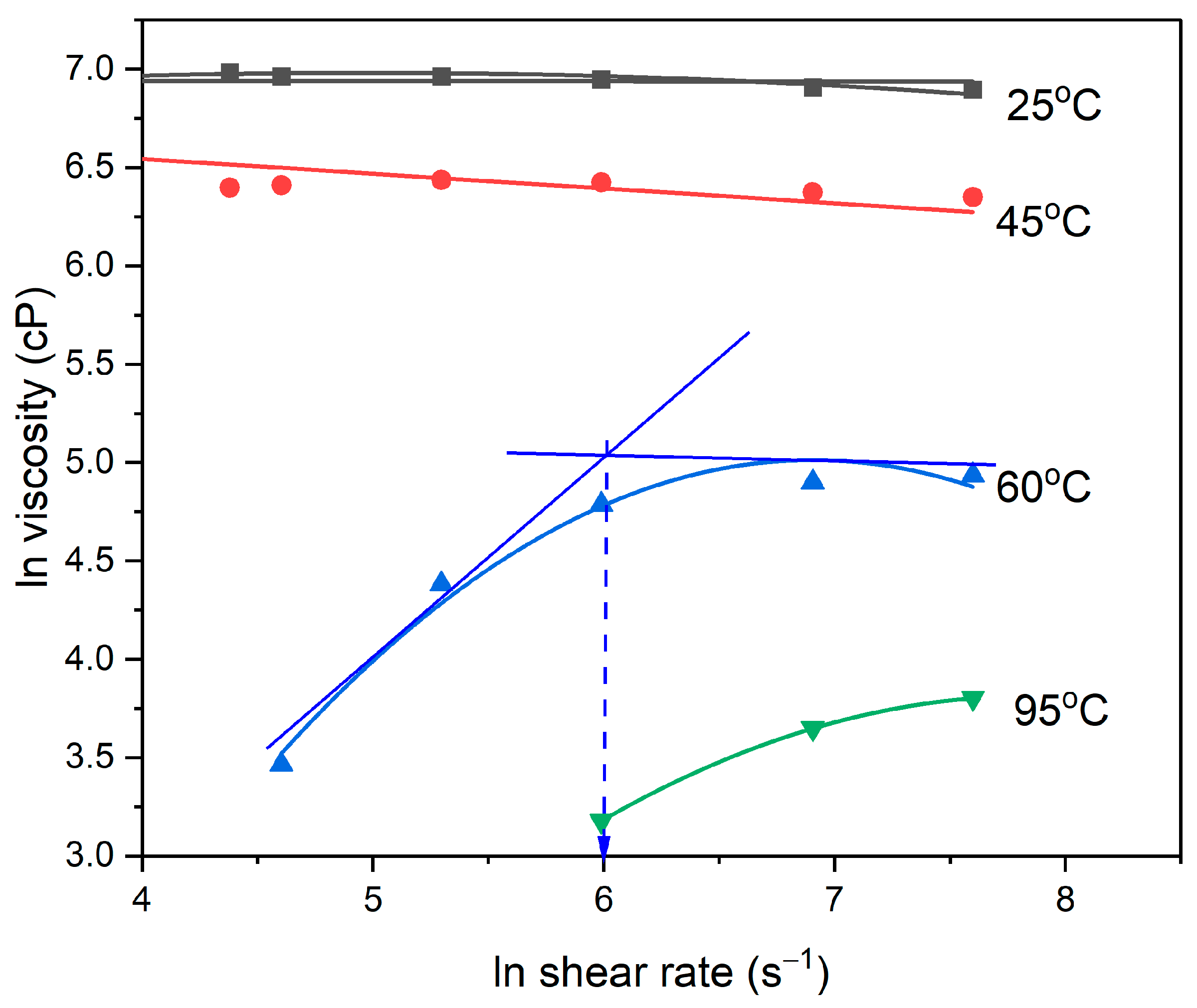
3.1.1. Occurrence of Two Regions of Rheological Behavior
3.1.2. Effect of Polymerization Time
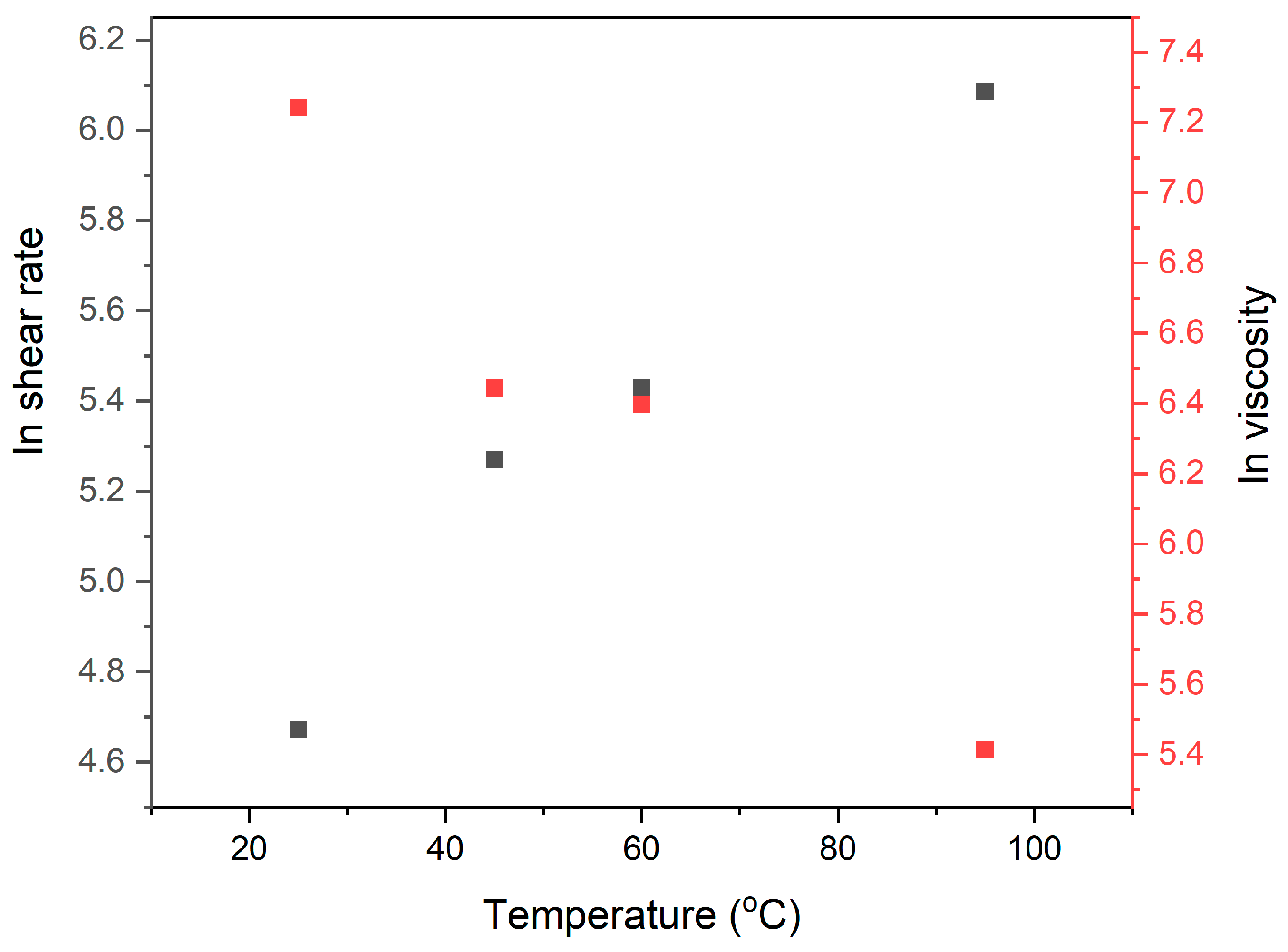
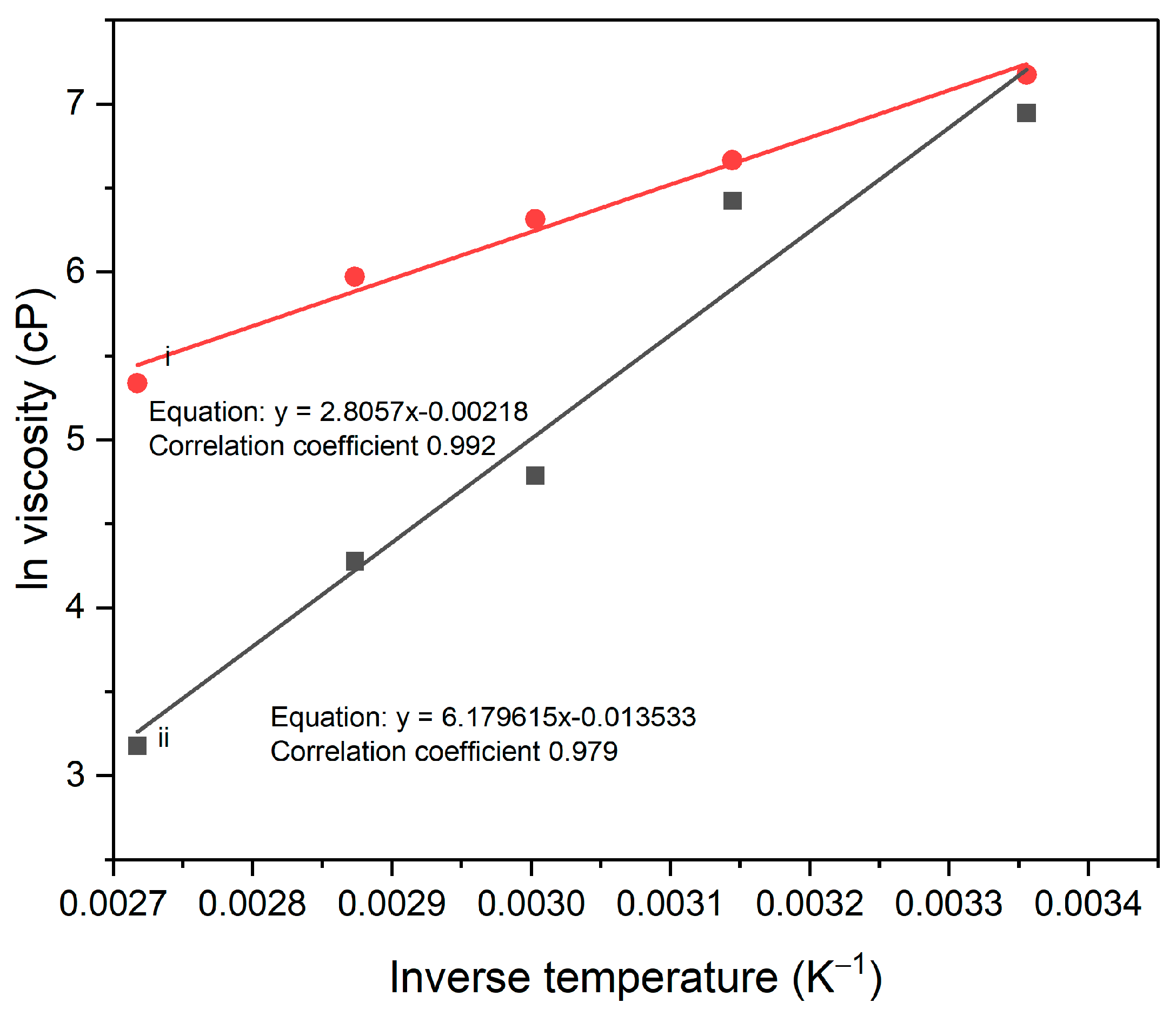
3.1.3. Effect of Testing Temperature
3.1.4. Intrinsic Viscosity of PAA Solution and PAA Suspension Containing Modified Clay
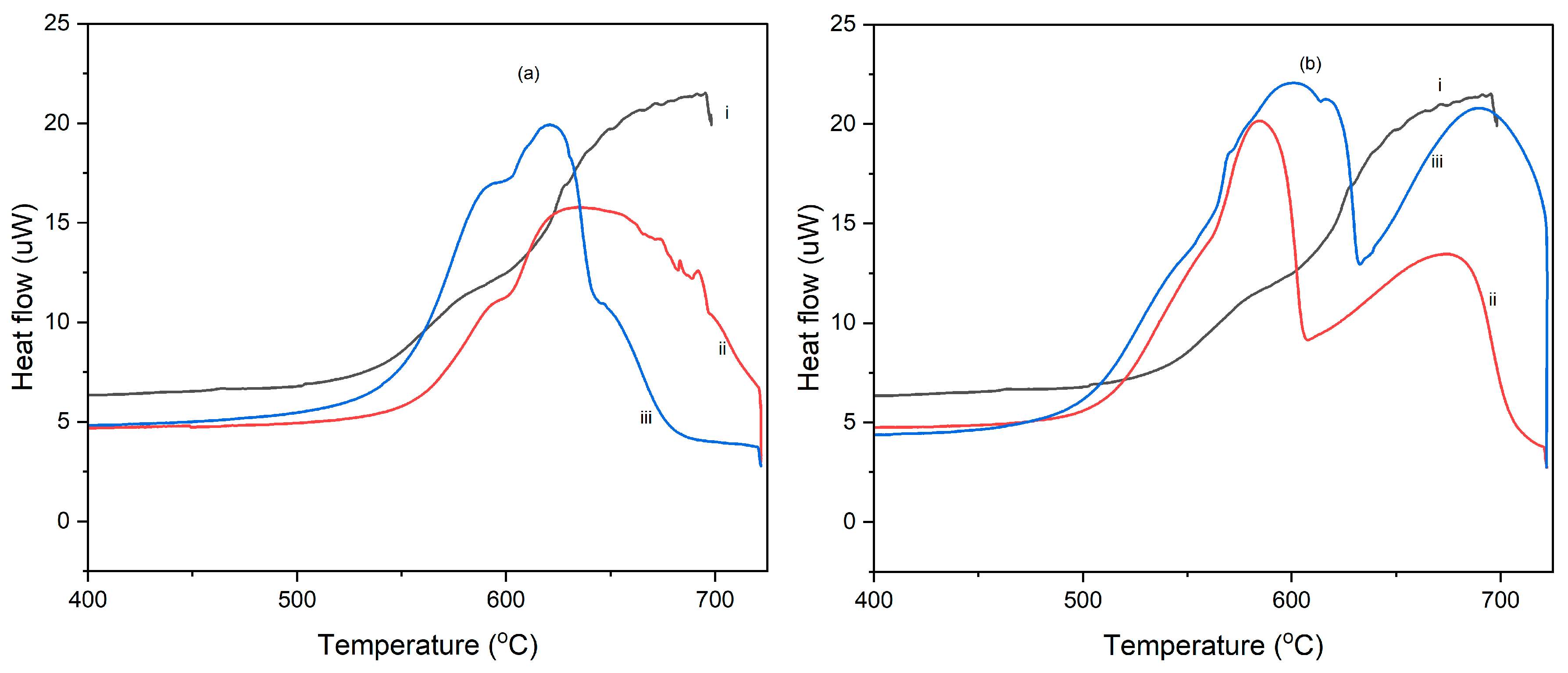
3.2. Differential Scanning Calorimetry (DSC)
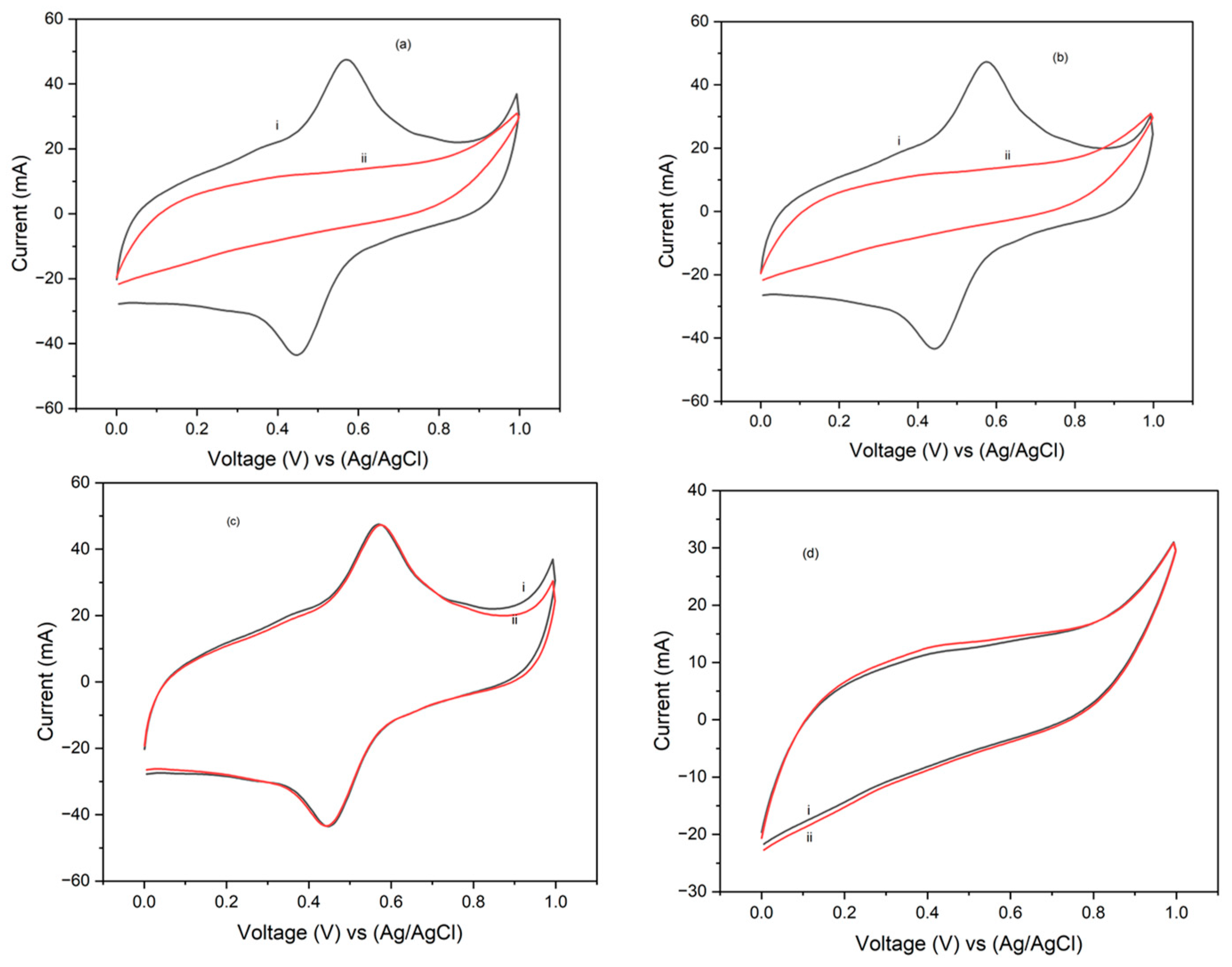
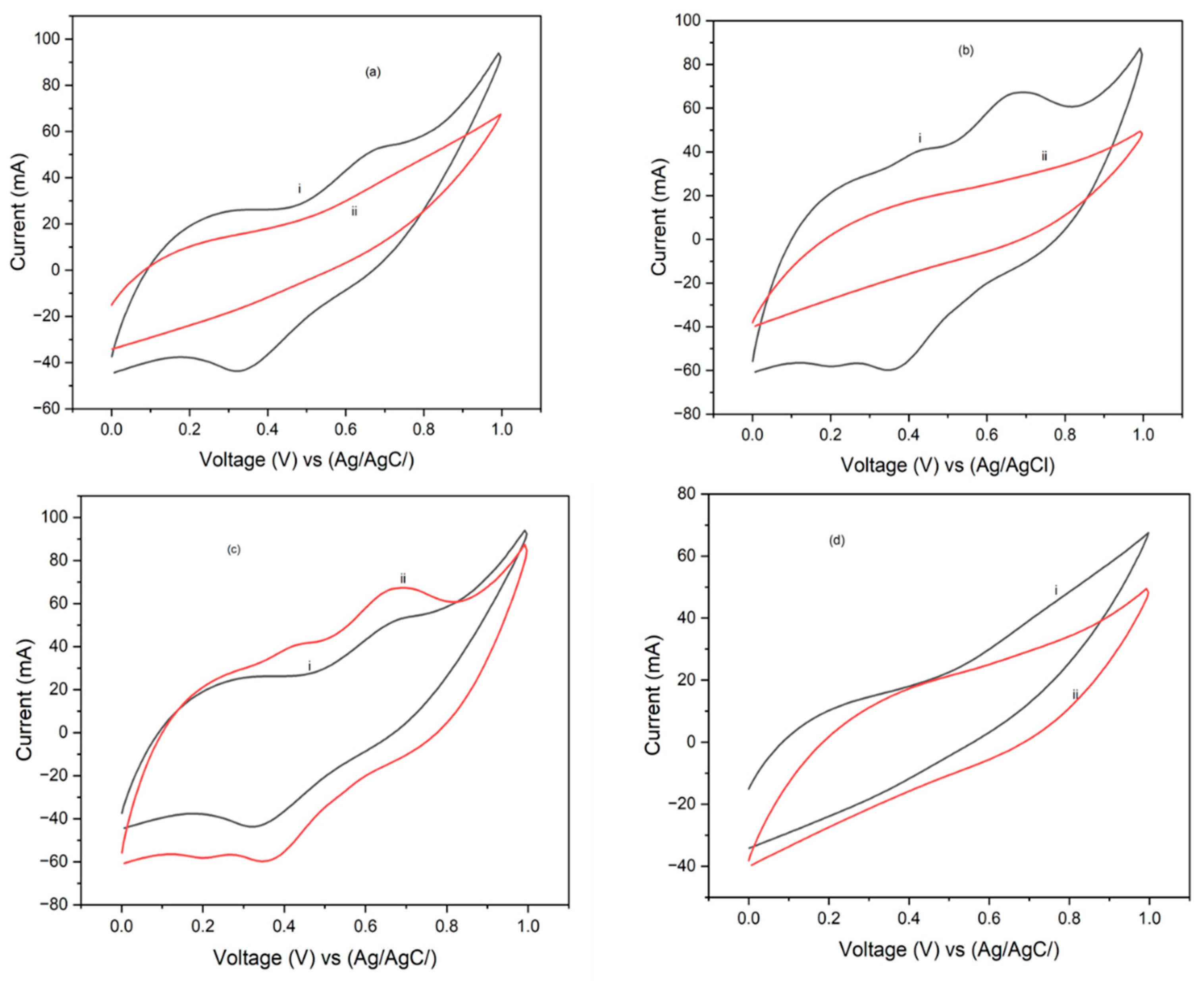
3.3. Cyclic Voltammetry
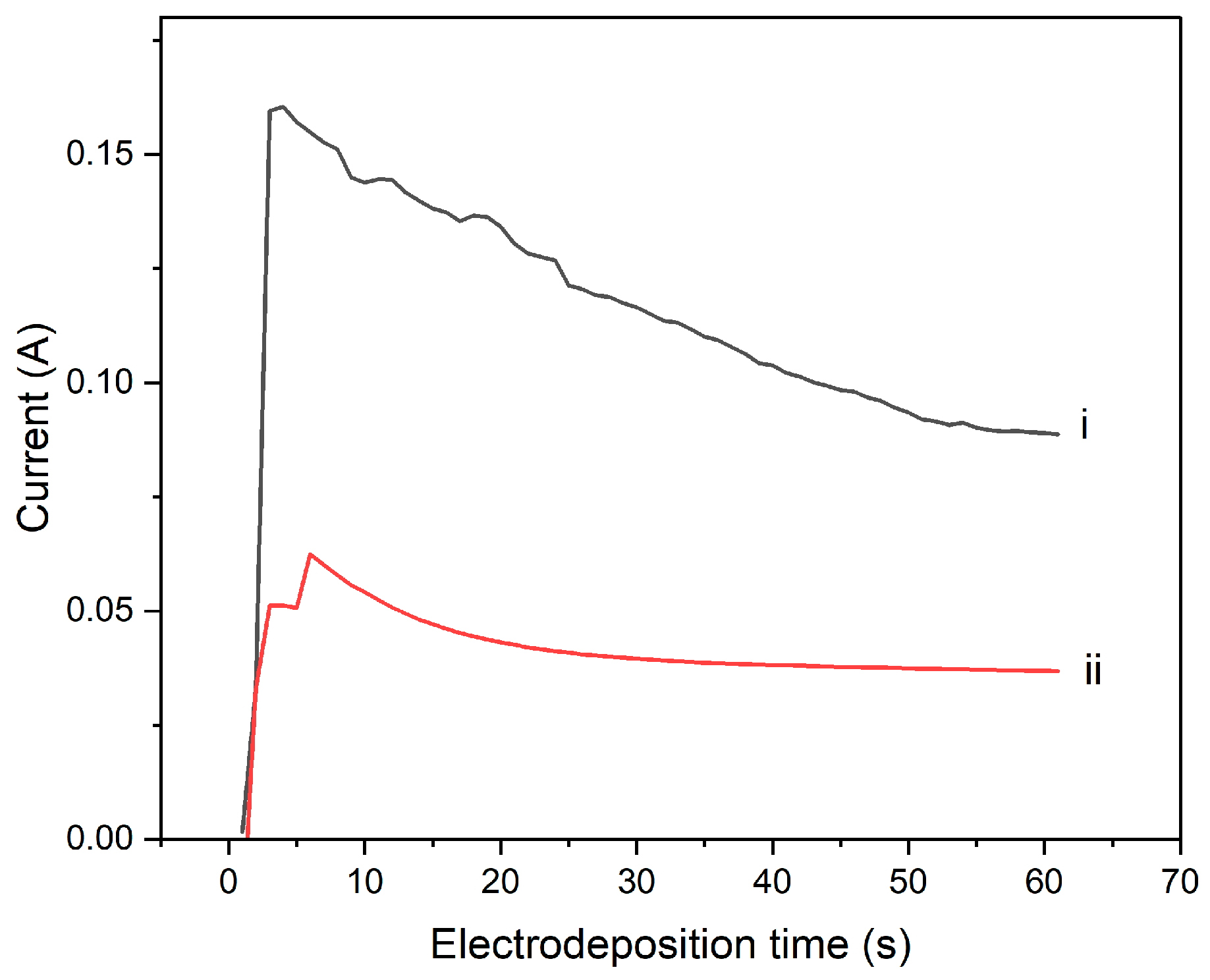
3.4. Current–Time, i–t, Transient Curves
3.5. Discussion of the Effect of Protocol Choices
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ioan, S.; Filimon, A.; Hulubei, C.; Popovici, D. Rheological properties of some complex polymers containing alicyclic structures. In Proceedings of the HEFAT 2012, St Julian’s, Malta, 16–18 July 2012. [Google Scholar]
- Jin, J.-U.; Hahn, J.R.; You, N.-H. Structural Effect of Polyimide Precursors on Highly Thermally Conductive Graphite Films. ACS Omega 2022, 7, 25565–25572. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Iroh, J.O.; Long, A. Controlling the structure and rheology of polyimide/nanoclay composites by condensation polymerization. J. Appl. Polym. Sci. 2012, 125, E486–E494. [Google Scholar] [CrossRef]
- Hicyilmaz, A.S.; Bedeloglu, A.C. Applications of polyimide coatings: A review. SN Appl. Sci. 2021, 3, 1–22. [Google Scholar] [CrossRef]
- Ioan, S.; Filimon, A.; Hulubei, C.; Stoica, I.; Dunca, S. Origin of rheological behavior and surface/interfacial properties of some semi-alicyclic polyimides for biomedical applications. Polym. Bull. 2013, 70, 2873–2893. [Google Scholar] [CrossRef]
- Njuguna, J.; Pielichowski, K.; Fan, J. Polymer nanocomposites for aerospace applications. Adv. Polym. Nanocompos. 2012, 472–539. [Google Scholar] [CrossRef]
- Marashdeh, W.F.; Longun, J.; Iroh, J.O. Relaxation behavior and activation energy of relaxation for polyimide and polyimide–graphene nanocomposite. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- De Oliveira, A.D.; Beatrice, C.A.G. Polymer nanocomposites with different types of nanofiller. Nanocompos.-Recent Evol. 2018, 103–104. [Google Scholar]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/silica nanocomposites: Preparation, characterization, properties, and applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef]
- Kim, J.; Ahmed, R.; Lee, S.J. Synthesis and linear viscoelastic behavior of poly (amic acid)–organoclay hybrid. J. Appl. Polym. Sci. 2001, 80, 592–603. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Mathews, A.S.; Kim, I.; Ha, C.S. Synthesis, characterization, and properties of fully aliphatic polyimides and their derivatives for microelectronics and optoelectronics applications. Macromol. Res. 2007, 15, 114–128. [Google Scholar] [CrossRef]
- Tafreshi, O.A.; Ghaffari-Mosanenzadeh, S.; Karamikamkar, S.; Saadatnia, Z.; Kiddell, S.; Park, C.B.; Naguib, H.E. Novel, flexible, and transparent thin film polyimide aerogels with enhanced thermal insulation and high service temperature. J. Mater. Chem. C 2022, 10, 5088–5108. [Google Scholar] [CrossRef]
- Hergenrother, P.M. The use, design, synthesis, and properties of high performance/high temperature polymers: An overview. High Perform. Polym. 2003, 15, 3–45. [Google Scholar] [CrossRef]
- Ma, P.; Dai, C.; Wang, H.; Li, Z.; Liu, H.; Li, W.; Yang, C. A review on high temperature resistant polyimide films: Heterocyclic structures and nanocomposites. Compos. Commun. 2019, 16, 84–93. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, T.; Qu, L.; Liang, X.; Liu, C.; Qian, C.; Zhu, T.; Zhou, Z.; Liu, C.; Liu, S.; et al. Temperature resistant amorphous polyimides with high intrinsic permittivity for electronic applications. Chem. Eng. J. 2022, 436, 135060. [Google Scholar] [CrossRef]
- Zhang, X.L.; Song, C.; Wei, M.H.; Huang, Z.Z.; Sheng, S.R. Organosoluble and transparent cardo polyimides with high T g derived from 9, 9-bis (4-aminophenyl) xanthene. High Perform. Polym. 2019, 31, 909–918. [Google Scholar] [CrossRef]
- Woo, H.G.; Li, H.; Ha, C.S.; Mathews, A.S. Polyimides and high performance organic polymers. Adv. Funct. Mater. 2011, 1–36. [Google Scholar]
- Agag, T.; Koga, T.; Takeichi, T. Studies on thermal and mechanical properties of polyimide–clay nanocomposites. Polymer 2001, 42, 3399–3408. [Google Scholar] [CrossRef]
- Bourbigot, S.; Devaux, E.; Flambard, X. Flammability of polyamide-6/clay hybrid nanocomposite textiles. Polym. Degrad. Stab. 2002, 75, 397–402. [Google Scholar] [CrossRef]
- Hsiao, S.; Liou, G.; Chang, L. Synthesis and properties of organosoluble polyimide/clay hybrids. J. Appl. Polym. Sci. 2001, 80, 2067–2072. [Google Scholar] [CrossRef]
- Akinyi, C.; Longun, J.; Chen, S.; Iroh, J.O. Decomposition and flammability of polyimide graphene composites. Minerals 2021, 11, 168. [Google Scholar] [CrossRef]
- Longun, J.; Iroh, J.O. Fabrication of High Impact-Resistant Polyimide Nanocomposites with Outstanding Thermomechanical Properties. Polymers 2023, 15, 4427. [Google Scholar] [CrossRef]
- Yu, Y.; Yeh, J.; Liou, S.; Chen, C.; Liaw, D.; Lu, H. Preparation and properties of polyimide–clay nanocomposite materials for anticorrosion application. J. Appl. Polym. Sci. 2004, 92, 3573–3582. [Google Scholar] [CrossRef]
- Khayankarn, O.; Magaraphan, R.; Schwank, J.W. Adhesion and permeability of polyimide–clay nanocomposite films for protective coatings. J. Appl. Polym. Sci. 2003, 89, 2875–2881. [Google Scholar] [CrossRef]
- Ahmadizadegan, H.; Esmaielzadeh, S. Synthesis and characterization of novel polyimide/clay nanocomposites and processing, properties and applications. Int. J. Polym. Anal. Charact. 2020, 25, 604–620. [Google Scholar] [CrossRef]
- Ni, H.-J.; Liu, J.-G.; Wang, Z.-H.; Yang, S.-Y. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Espuche, E.; David, L.; Rochas, C.; Afeld, J.; Compton, J.; Thompson, D.S.; Kranbuehl, D. In situ generation of nanoparticulate lanthanum(III) oxide-polyimide films: Characterization of nanoparticle formation and resulting polymer properties. Polymer 2005, 46, 6657–6665. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Miliozzi, A.; Kenny, J.M. Effects of the nanoparticles on the thermal expansion and mechanical properties of unsaturated polyester/clay nanocomposites. Compos. Part A Appl. Sci. Manuf. 2013, 45, 44–48. [Google Scholar] [CrossRef]
- Hussain, F.; Hojjati, M.; Okamoto, M.; Gorga, R.E. Polymer-matrix nanocomposites, processing, manufacturing, and application: An overview. J. Compos. Mater. 2006, 40, 1511–1575. [Google Scholar] [CrossRef]
- Mokhothu, T.H.; Mtibe, A.; Mokhena, T.C.; Mochane, M.J.; Ofosu, O.; Muniyasamy, S.; Tshifularo, C.A.; Motsoeneng, T.S. Mechanical, thermal and viscoelastic properties of polymer composites reinforced with various nanomaterials. Sustain. Polym. Compos. Nanocompos. 2019, 185–213. [Google Scholar]
- Panwar, V.; Pal, K. Dynamic mechanical analysis of clay–polymer nanocomposites. In Clay-Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 413–441. [Google Scholar]
- Costa, F.R.; Saphiannikova, M.; Wagenknecht, U.; Heinrich, G. Layered double hydroxide based polymer nanocomposites. Wax Cryst. Control. Nanocompos. Stimuli-Responsive Polym. 2008, 210, 101–168. [Google Scholar]
- Judah, H. The Synthesis, Characterisation and Rheological Properties of Clay-Polymer Nanocomposites; Sheffield Hallam University: Sheffield, UK, 2020. [Google Scholar]
- Hamming, L.M.; Qiao, R.; Messersmith, P.B.; Brinson, L.C. Effects of dispersion and interfacial modification on the macroscale properties of TiO2 polymer–matrix nanocomposites. Compos. Sci. Technol. 2009, 69, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Mousa, M.; Evans, N.D.; Oreffo, R.O.C.; Dawson, J.I. Clay nanoparticles for regenerative medicine and biomaterial design: A review of clay bioactivity. Biomaterials 2018, 159, 204–214. [Google Scholar] [CrossRef]
- Szazdi, L.; Pozsgay, A.; Pukanszky, B. Factors and processes influencing the reinforcing effect of layered silicates in polymer nanocomposites. Eur. Polym. J. 2007, 43, 345–359. [Google Scholar] [CrossRef]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.E.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging, automotive and solar energy fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Tripathi, A. Intrinsic viscosity of polymers and biopolymers measured by microchip. Anal. Chem. 2005, 77, 7137–7147. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; An, L.; Wang, Z.-G. Intrinsic viscosity of polymers: General theory based on a partially permeable sphere model. Macromolecules 2013, 46, 5731–5740. [Google Scholar] [CrossRef]
- Nunez, C.M.; Chiou, B.-S.; Andrady, A.L.; Khan, S.A. Solution rheology of hyperbranched polyesters and their blends with linear polymers. Macromolecules 2000, 33, 1720–1726. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z. Graphene-analogous low-dimensional materials. Prog. Mater. Sci. 2013, 58, 1244–1315. [Google Scholar] [CrossRef]
- Yildiz, G.; Bolton-Warberg, M.; Awaja, F. Graphene and graphene oxide for bio-sensing: General properties and the effects of graphene ripples. Acta Biomater. 2021, 131, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, H.; Sui, L.; Jiang, S.; Hou, H. Self-Adhesive Polyimide (PI)@ Reduced Graphene Oxide (RGO)/PI@ Carbon Nanotube (CNT) Hierarchically Porous Electrodes: Maximizing the Utilization of Electroactive Materials for Organic Li-Ion Batteries. Energy Technol. 2020, 8, 2000397. [Google Scholar] [CrossRef]
- Rathod, V.T.; Swamy, J.K.; Jain, A. Polymer and ceramic nanocomposites for aerospace applications. Appl. Nanosci. 2017, 7, 519–548. [Google Scholar] [CrossRef]
- Barbosa, J.; Fidalgo-Marijuan, A.; Dias, J.; Gonçalves, R.; Salado, M.; Costa, C.; Lanceros-Méndez, S. Molecular design of functional polymers for organic radical batteries. Energy Storage Mater. 2023, 60, 102841. [Google Scholar] [CrossRef]
- Choi, C.; Yun, T.G.; Hwang, B. Dispersion Stability of Carbon Nanotubes and Their Impact on Energy Storage Devices. Inorganics 2023, 11, 383. [Google Scholar] [CrossRef]
- Li, X.; Zhong, Y.; Cai, M.; Balogh, M.P.; Wang, D.; Zhang, Y.; Li, R.; Sun, X. Tin-alloy heterostructures encapsulated in amorphous carbon nanotubes as hybrid anodes in rechargeable lithium ion batteries. Electrochim. Acta 2013, 89, 387–393. [Google Scholar] [CrossRef]
- Kulkarni, R.; Lingamdinne, L.P.; Koduru, J.R.; Karri, R.R.; Kailasa, S.K.; Mubarak, N.M.; Chang, Y.-Y.; Dehghani, M.H. Exploring the Recent Cutting-Edge Applications of CNTs in Energy and Environmental Remediation: Mechanistic Insights and Remarkable Performance Advancements. J. Environ. Chem. Eng. 2024, 12, 113251. [Google Scholar] [CrossRef]
- Ismail, K.B.M.; Kumar, M.A.; Mahalingam, S.; Raj, B.; Kim, J. Carbon fiber-reinforced polymers for energy storage applications. J. Energy Storage 2024, 84, 110931. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest advances in supercapacitors: From new electrode materials to novel device designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef]
- Kim, B.S.; Bae, S.H.; Park, Y.H.; Kim, J.H. July. Polyimide/carbon nanotubes composite films: A potential for FPCB. In Proceedings of the 2006 International Conference on Nanoscience and Nanotechnology, Brisbane, QLD, Australia, 3–7 July 2006. [Google Scholar]
- Thuau, D.; Koutsos, V.; Cheung, R. Electrical and mechanical properties of carbon nanotube-polyimide composites. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2009, 27, 3139–3144. [Google Scholar] [CrossRef]
- Guo, W.; Liu, C.; Sun, X.; Yang, Z.; Kia, H.G.; Peng, H. Aligned carbon nanotube/polymer composite fibers with improved mechanical strength and electrical conductivity. J. Mater. Chem. 2012, 22, 903–908. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, J.; Li, W.; Wei, Z.; Jiang, J. The effects of CNT alignment on electrical conductivity and mechanical properties of SWNT/epoxy nanocomposites. Compos. Sci. Technol. 2008, 68, 1644–1648. [Google Scholar] [CrossRef]
- Ma, P.-C.; Liu, M.-Y.; Zhang, H.; Wang, S.-Q.; Wang, R.; Wang, K.; Wong, Y.-K.; Tang, B.-Z.; Hong, S.-H.; Paik, K.-W.; et al. Enhanced electrical conductivity of nanocomposites containing hybrid Fillers of carbon nanotubes and carbon black. ACS Appl. Mater. Interfaces 2009, 1, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, E.; Michalska, A.; Maksymiuk, K. Polypyrrole Nanospheres—Electrochemical Properties and Application as a Solid Contact in Ion-selective Electrodes. Electroanalysis 2017, 29, 123–130. [Google Scholar] [CrossRef]
- Gooneratne, R.; Iroh, J.O. Polypyrrole Modified Carbon Nanotube/Polyimide Electrode Materials for Supercapacitors and Lithium-ion Batteries. Energies 2022, 15, 9509. [Google Scholar] [CrossRef]
- Li, Z.; Gong, L. Research progress on applications of polyaniline (PANI) for electrochemical energy storage and conversion. Materials 2020, 13, 548. [Google Scholar] [CrossRef]
- Thakur, A.K.; Majumder, M.; Choudhary, R.B.; Pimpalkar, S.N. Supercapacitor based on electropolymerized polythiophene and multiwalled carbon nanotubes composites. IOP Conf. Series Mater. Sci. Eng. 2016, 149, 012166. [Google Scholar] [CrossRef]


| Material | Bulk Resistance (Ω), (EIS) | Electrode Resistance (Ω), i–t Transient Curve | Porosity, (EIS) |
|---|---|---|---|
| PI/CNT-PVDF (90 °C) | 4.95 | 20 | 8.41 |
| PI/CNT-PVDF (250 °C) | 15 | 41.24 | 4.33 |
| Material | Specific Capacitance (F/g) | Reference |
|---|---|---|
| PI/CNT-PVDF (90 °C) | 858 | This study |
| PI-CNT-PVDF (250 °C) | 136 | This study |
| PI-SWCNTs | 127 | [59] |
| Graphene nanosheets (GNSs), carbon nanotubes (CNTs), and PANI | 1035 | [60] |
| Polythiophene (PTh)-CNT composites | 125 | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andezai, A.; Iroh, J.O. Influence of the Processing Conditions on the Rheology and Heat of Decomposition of Solution Processed Hybrid Nanocomposites and Implication to Sustainable Energy Storage. Energies 2024, 17, 3930. https://doi.org/10.3390/en17163930
Andezai A, Iroh JO. Influence of the Processing Conditions on the Rheology and Heat of Decomposition of Solution Processed Hybrid Nanocomposites and Implication to Sustainable Energy Storage. Energies. 2024; 17(16):3930. https://doi.org/10.3390/en17163930
Chicago/Turabian StyleAndezai, Andekuba, and Jude O. Iroh. 2024. "Influence of the Processing Conditions on the Rheology and Heat of Decomposition of Solution Processed Hybrid Nanocomposites and Implication to Sustainable Energy Storage" Energies 17, no. 16: 3930. https://doi.org/10.3390/en17163930
APA StyleAndezai, A., & Iroh, J. O. (2024). Influence of the Processing Conditions on the Rheology and Heat of Decomposition of Solution Processed Hybrid Nanocomposites and Implication to Sustainable Energy Storage. Energies, 17(16), 3930. https://doi.org/10.3390/en17163930







