Enhancing Solar Absorption with Double-Layered Nickel Coatings and WS2 Nanoparticles on Copper Substrates
Abstract
1. Introduction
2. Materials and Methods
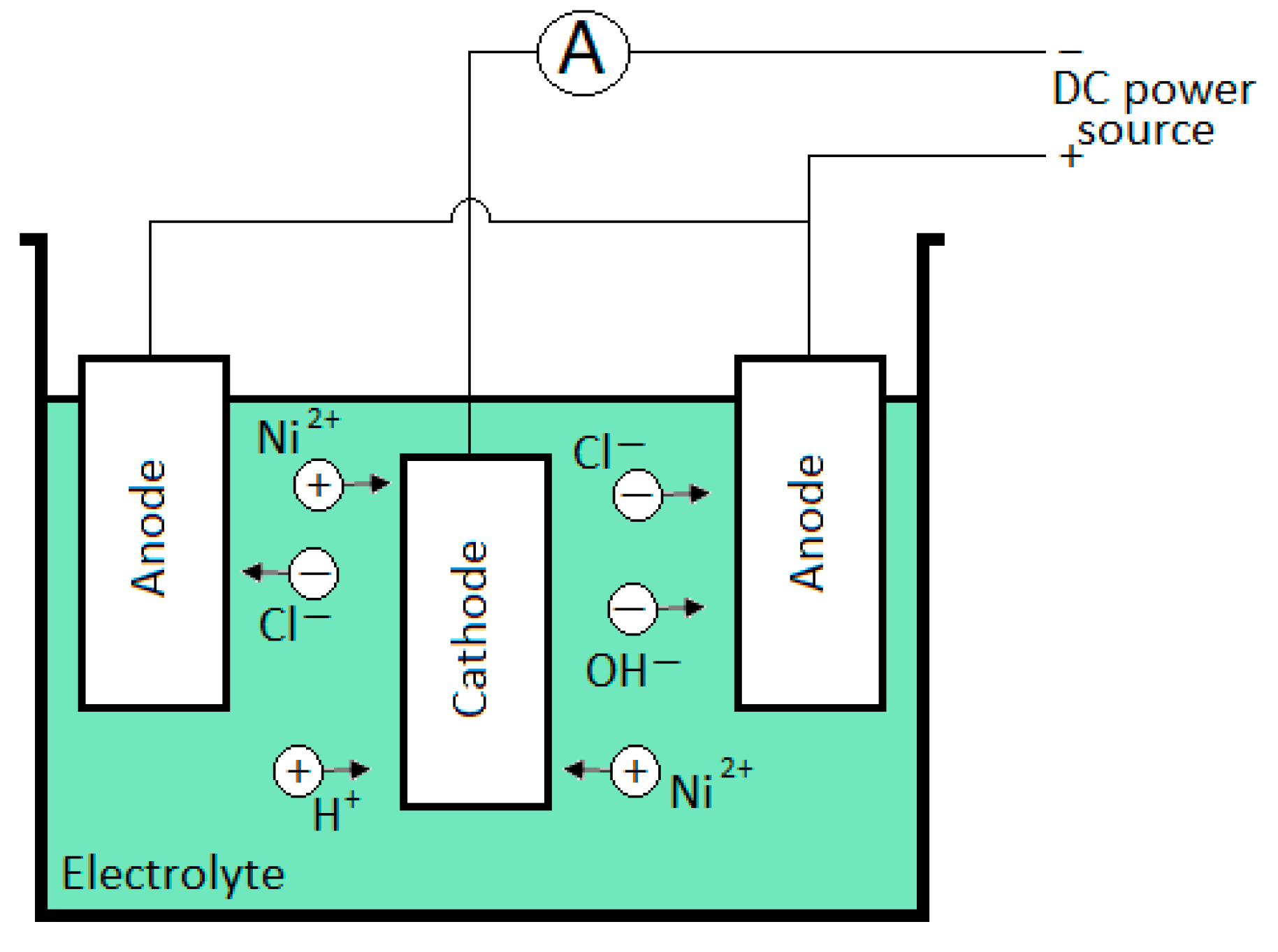
2.1. Electrodeposition Procedure
2.2. Sample Characterization
3. Results and Discussion
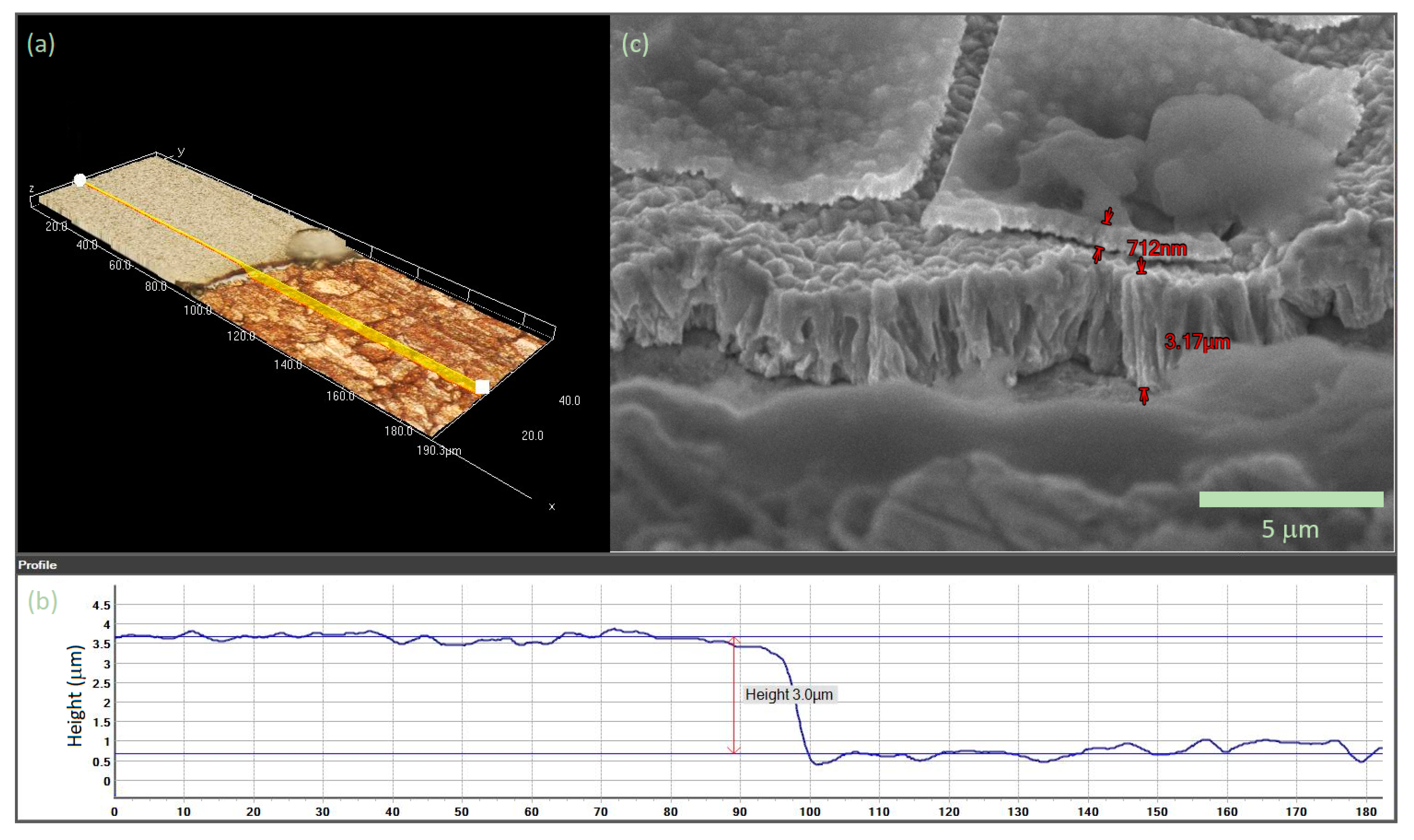
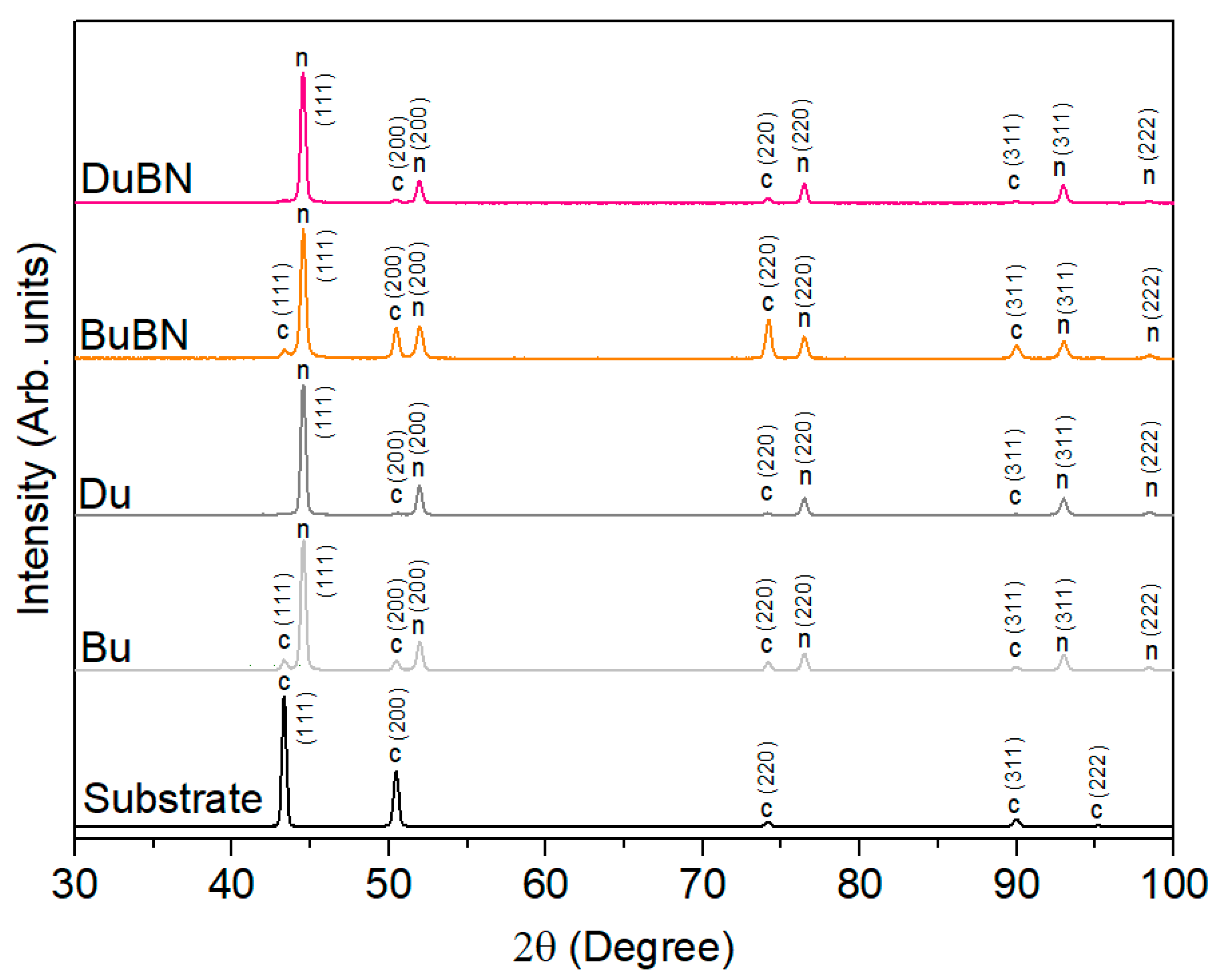
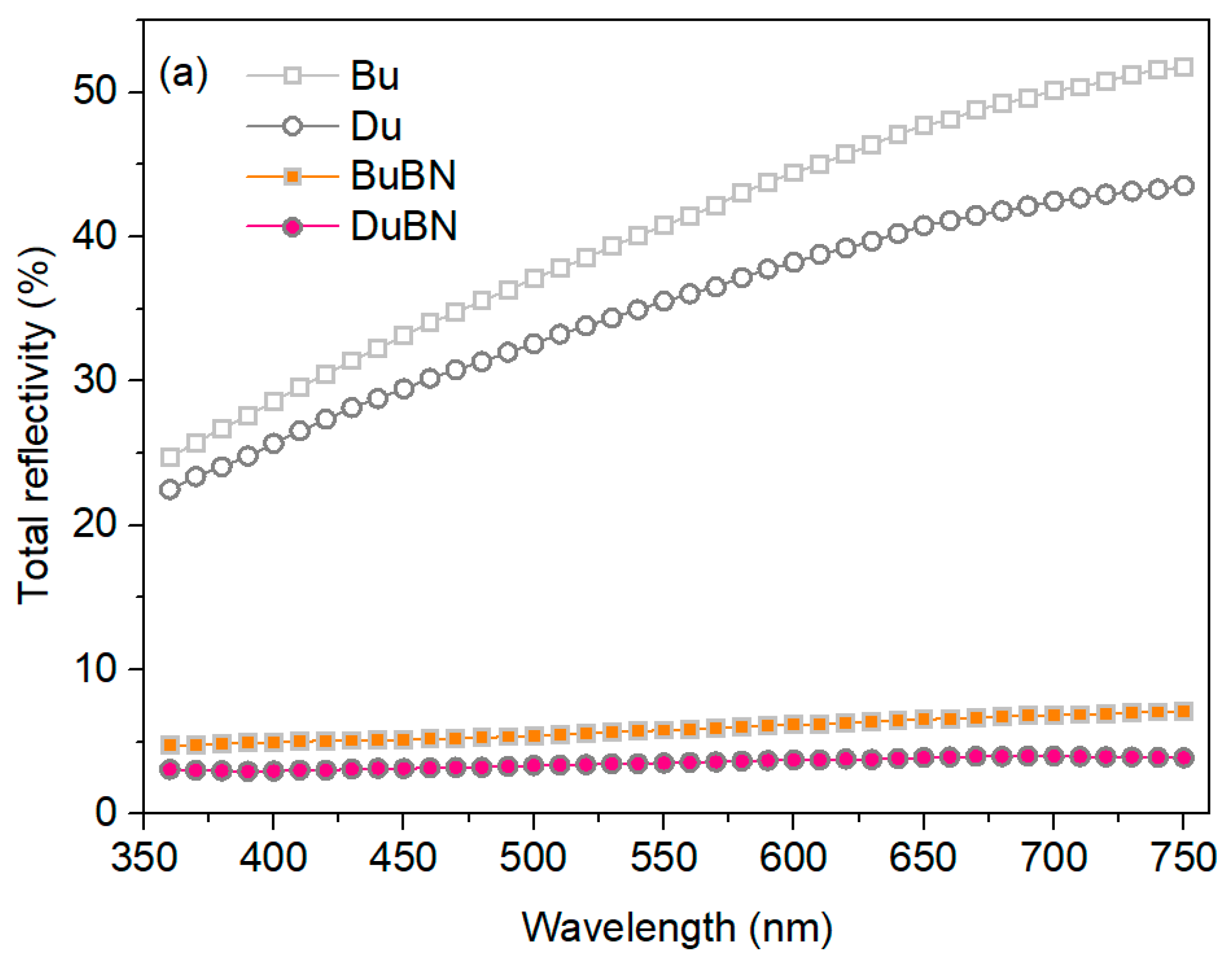
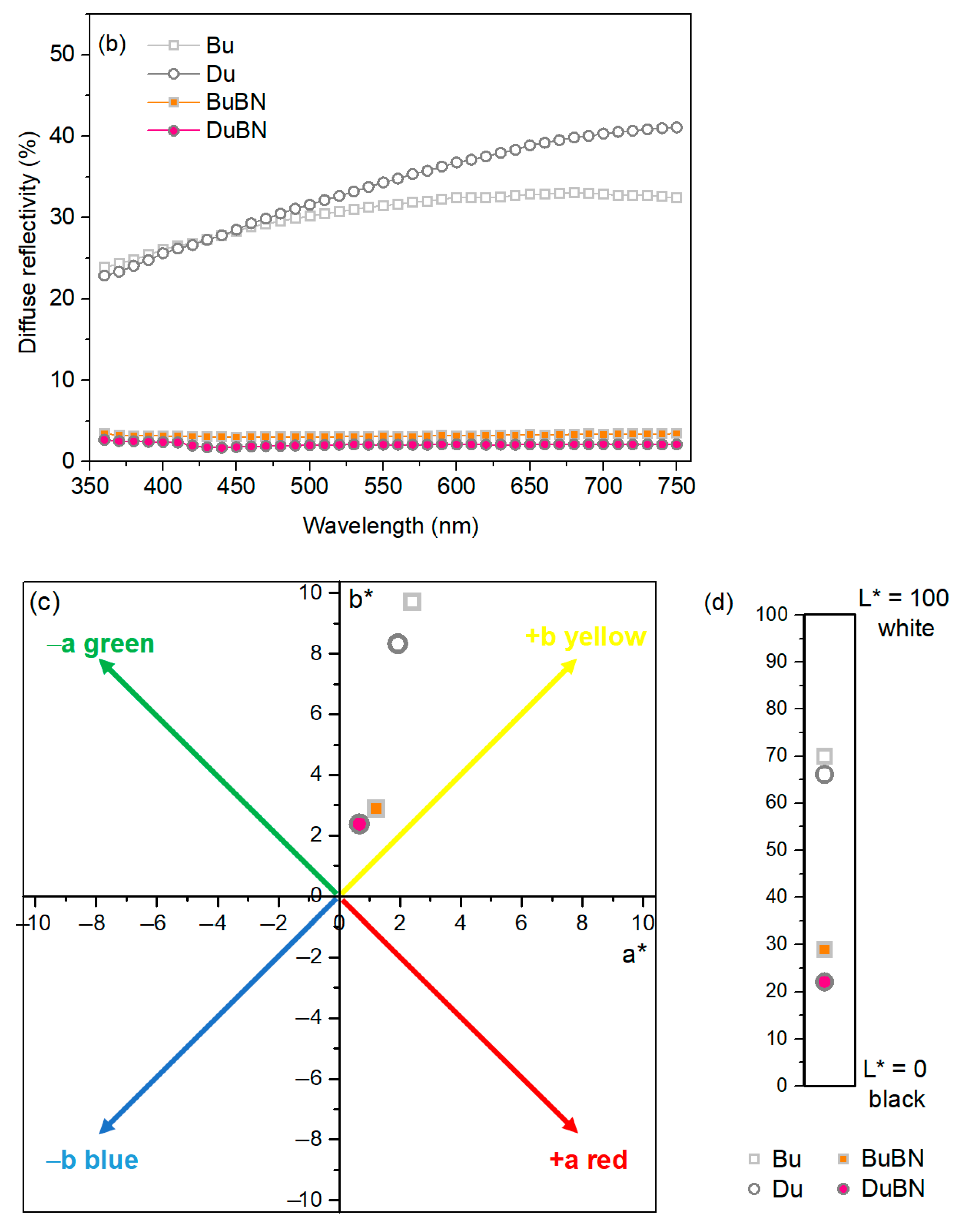
3.1. Double-Layered Nickel Coatings
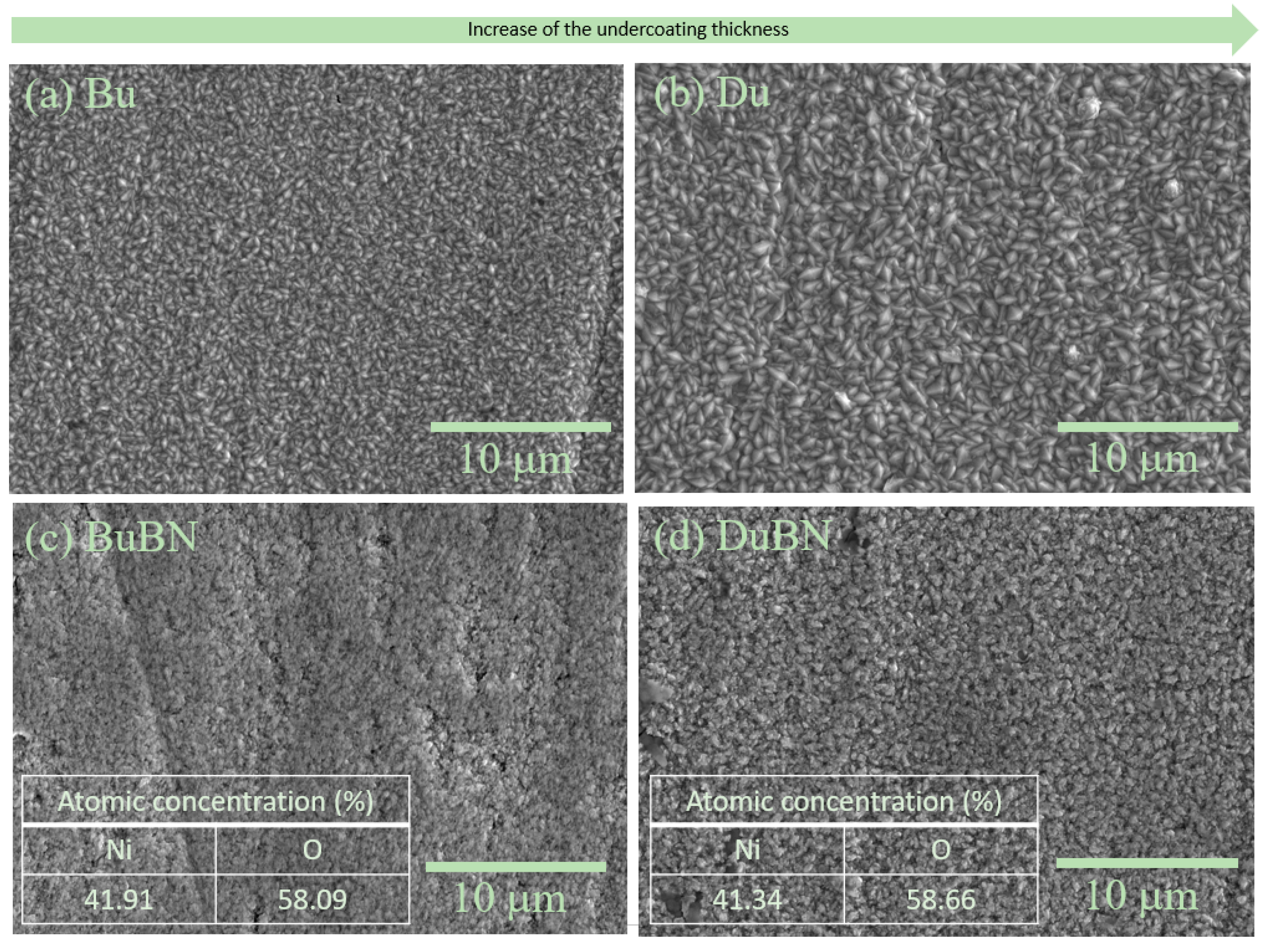
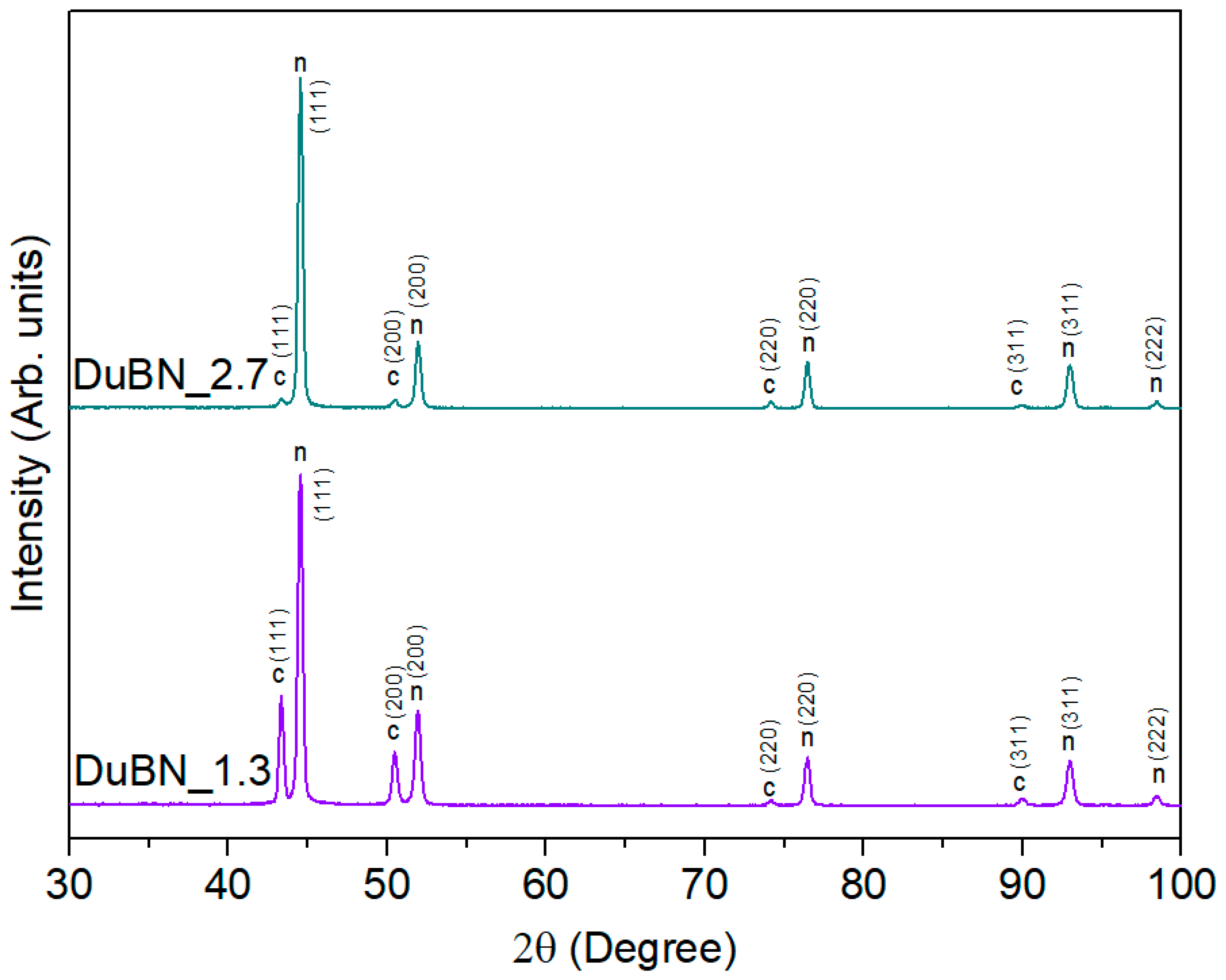
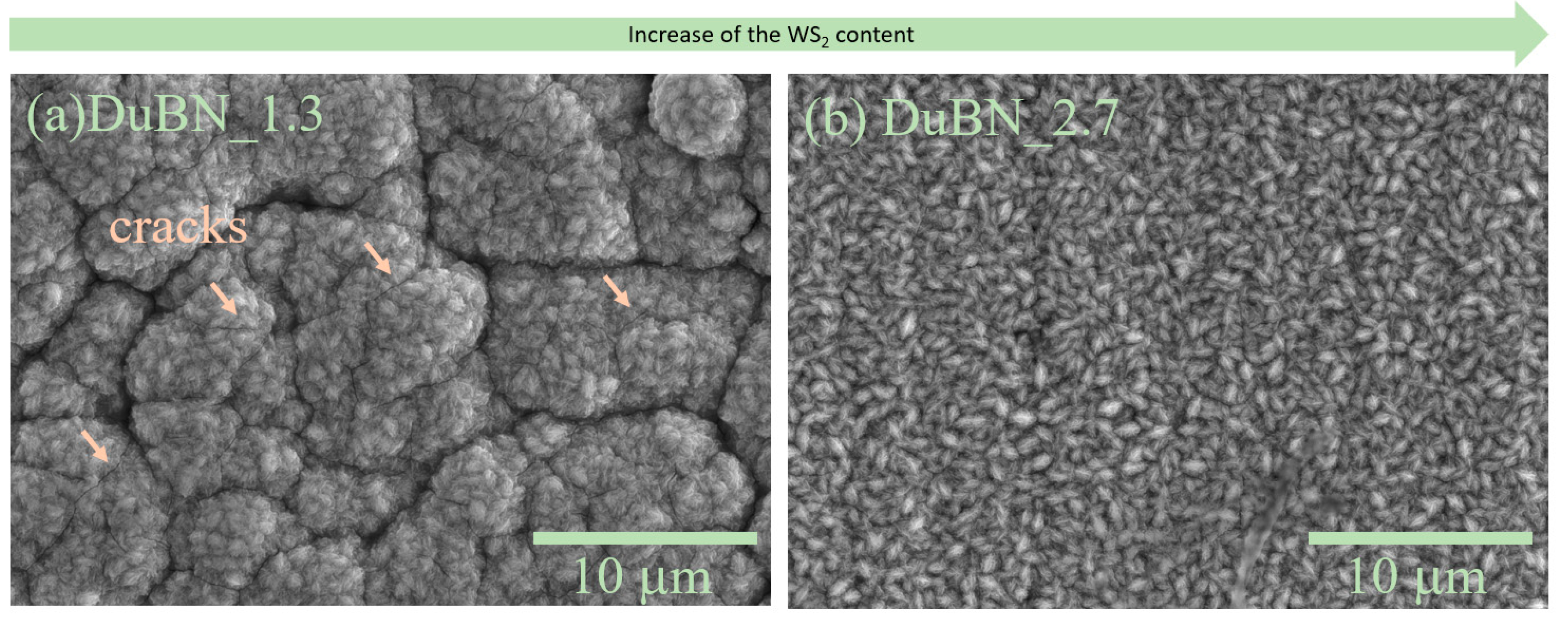
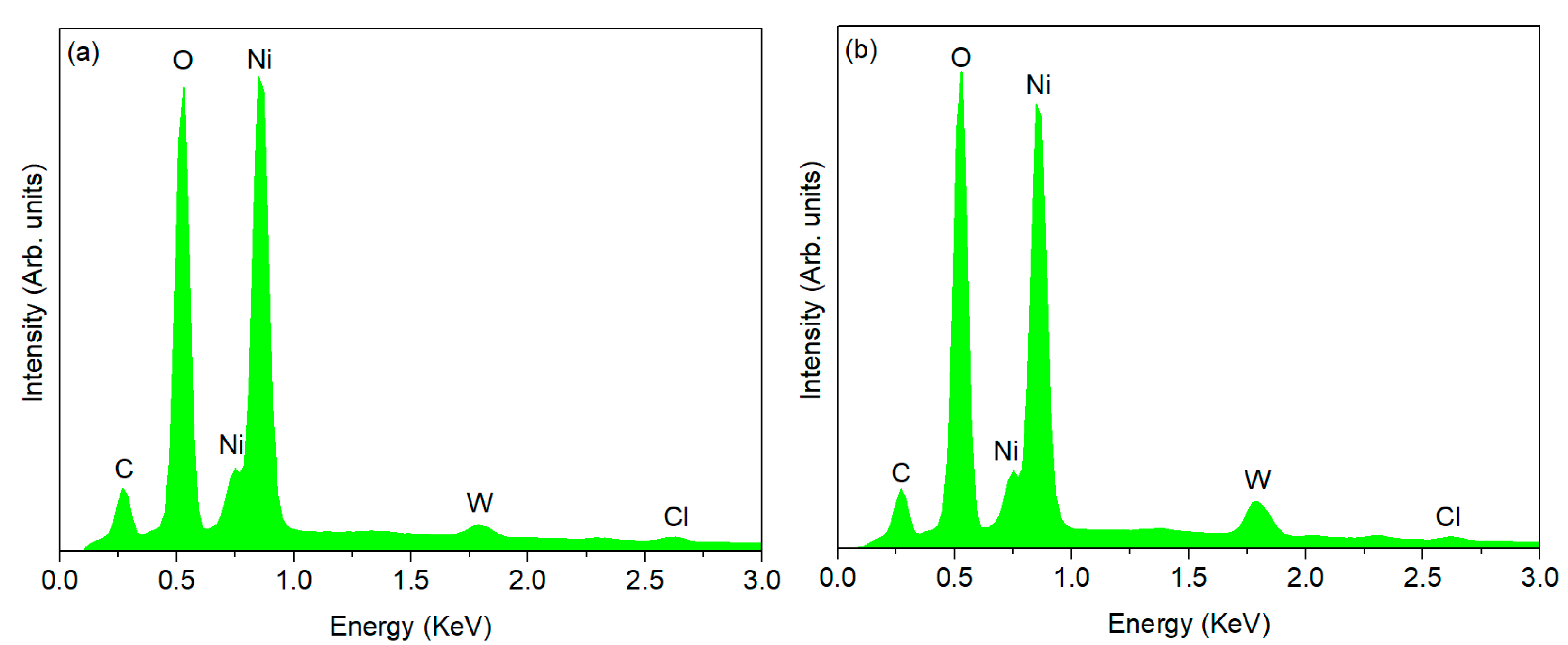
3.2. Double-Layered Nickel Coatings with WS2 Nanoparticles
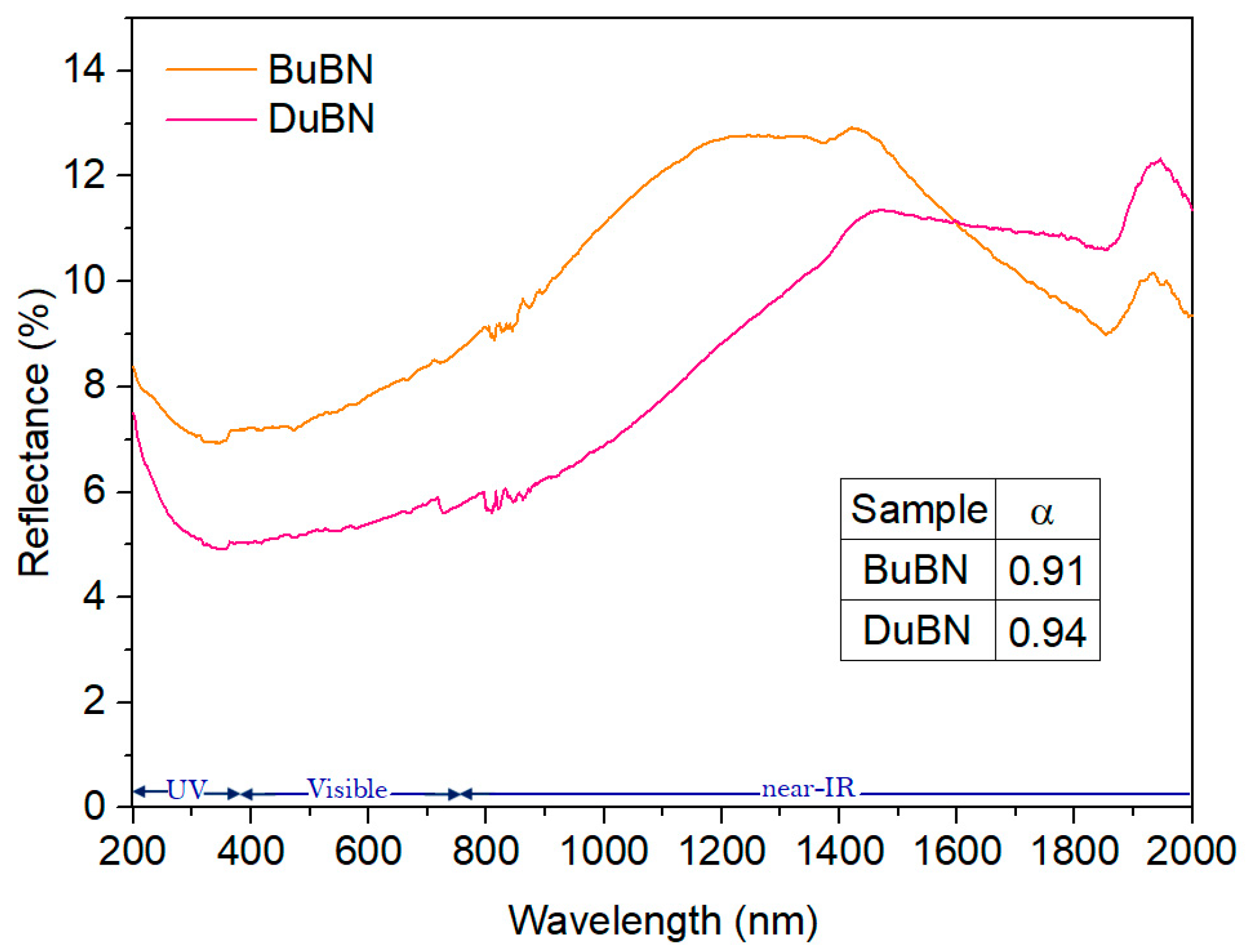
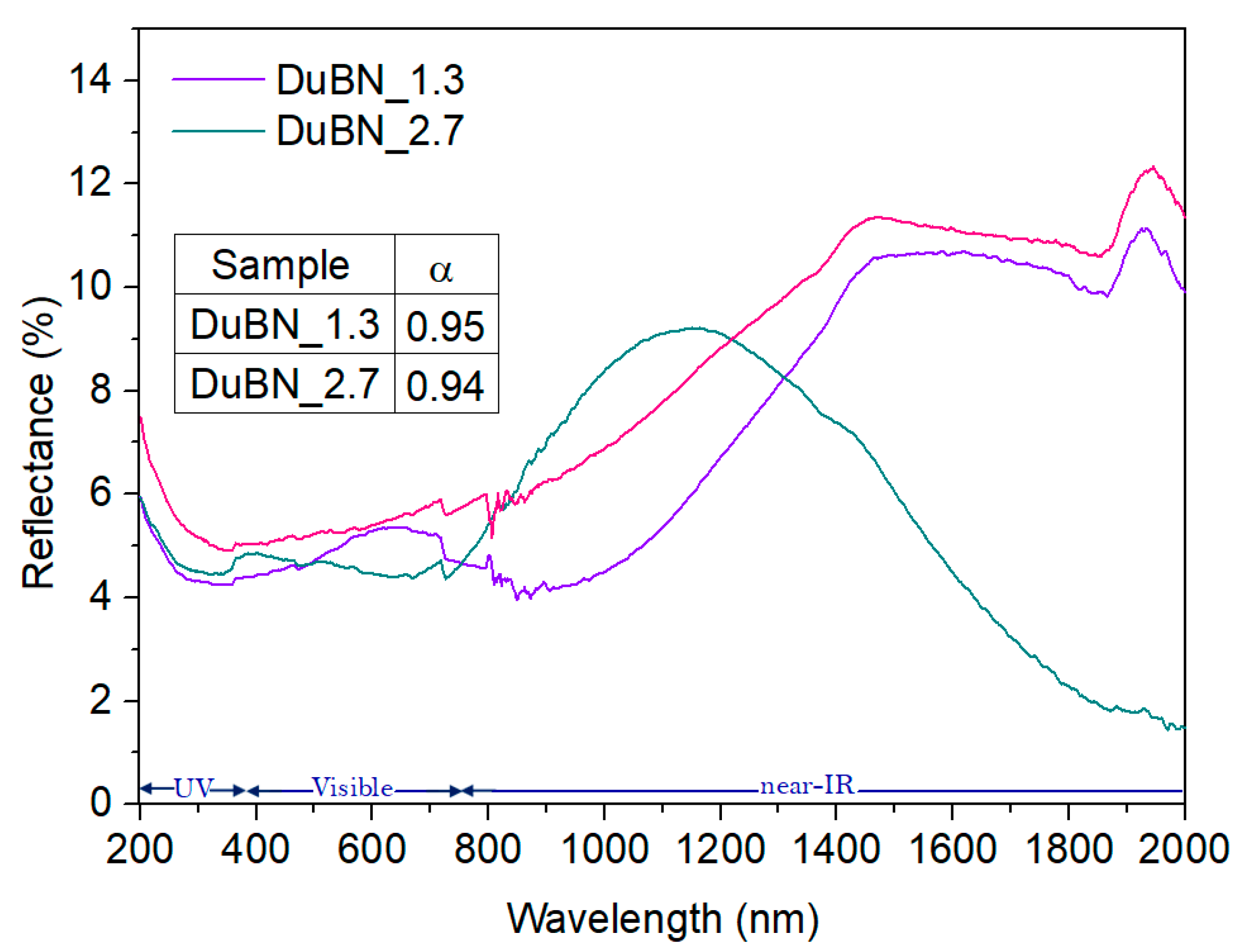
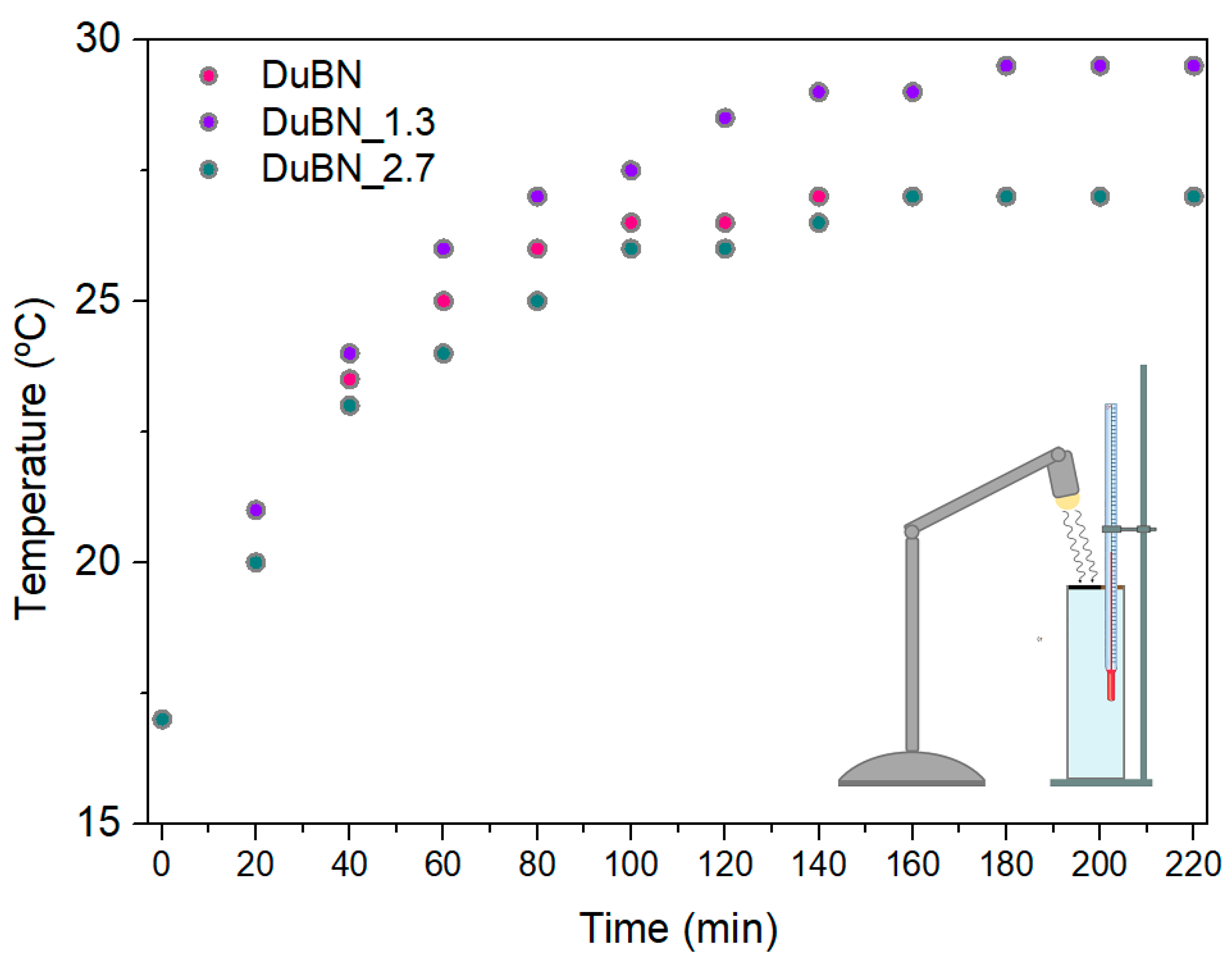
3.3. Conversion of Solar Energy into Thermal Energy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- NASA. Available online: https://Ceres.Larc.Nasa.Gov/News/Education-Overview/#additional-Energy-Budget-Resources (accessed on 23 May 2024).
- Tian, Y.; Zhao, C.Y. A Review of Solar Collectors and Thermal Energy Storage in Solar Thermal Applications. Appl. Energy 2013, 104, 538–553. [Google Scholar] [CrossRef]
- Suman, S.; Khan, M.K.; Pathak, M. Performance Enhancement of Solar Collectors—A Review. Renew. Sustain. Energy Rev. 2015, 49, 192–210. [Google Scholar] [CrossRef]
- Estrella-Gutiérrez, M.A.; Lizama-Tzec, F.I.; Arés-Muzio, O.; Oskam, G. Influence of a Metallic Nickel Interlayer on the Performance of Solar Absorber Coatings Based on Black Nickel Electrodeposited onto Copper. Electrochim. Acta 2016, 213, 460–468. [Google Scholar] [CrossRef]
- El Mahallawy, N.; Shoeib, M.; Ali, Y. Application of CuCoMnO x Coat by Sol Gel Technique on Aluminum and Copper Substrates for Solar Absorber Application. J. Coat. Technol. Res. 2014, 11, 979–991. [Google Scholar] [CrossRef]
- Kadırgan, F. Electrochemical Nano-Coating Processes in Solar Energy Systems. Int. J. Photoenergy 2006, 2006, 084891. [Google Scholar] [CrossRef]
- Lizama-Tzec, F.I.; Macías, J.D.; Estrella-Gutiérrez, M.A.; Cahue-López, A.C.; Arés, O.; de Coss, R.; Alvarado-Gil, J.J.; Oskam, G. Electrodeposition and Characterization of Nanostructured Black Nickel Selective Absorber Coatings for Solar–Thermal Energy Conversion. J. Mater. Sci. Mater. Electron. 2015, 26, 5553–5561. [Google Scholar] [CrossRef]
- Kalogirou, S.A. Solar Thermal Collectors and Applications. Prog. Energy Combust. Sci. 2004, 30, 231–295. [Google Scholar] [CrossRef]
- Wäckelgård, E. Characterization of Black Nickel Solar Absorber Coatings Electroplated in a Nickel Chlorine Aqueous Solution. Sol. Energy Mater. Sol. Cells 1998, 56, 35–44. [Google Scholar] [CrossRef]
- Koltun, M.; Gukhman, G.; Gavrilina, A. Stable Selective Coating “Black Nickel” for Solar Collector Surfaces. Sol. Energy Mater. Sol. Cells 1994, 33, 41–44. [Google Scholar] [CrossRef]
- Shawki, S.; Mikhail, S. Black Nickel Coatings for Solar Collectors. Mater. Manuf. Process. 2000, 15, 737–746. [Google Scholar] [CrossRef]
- Weng Wen, D.K.; Sadiq, T.O.; Idris, J. Deposition of Black Chromium Coating for Solar Thermal Application. J. Eng. Res. 2023, 11, 100023. [Google Scholar] [CrossRef]
- Merlo, A.; Léonard, G. Magnetron Sputtering vs. Electrodeposition for Hard Chrome Coatings: A Comparison of Environmental and Economic Performances. Materials 2021, 14, 3823. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, C.; Walsh, F.C. Alternative Tribological Coatings to Electrodeposited Hard Chromium: A Critical Review. Trans. IMF 2020, 98, 173–185. [Google Scholar] [CrossRef]
- Wang, M.; Xue, Z.; Yan, S.; He, J.; Shao, Q.; Ge, W.; Lu, B. Pulse Electrodeposited Super-Hydrophobic Ni-Co/WS2 Nanocomposite Coatings with Enhanced Corrosion-Resistance. Coatings 2022, 12, 1897. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; Luo, P.; Zhou, H.; Liu, B.; He, Y.; Song, R.; Zhang, Z.; Fan, Y. Construction and Anti-Corrosion Behavior Study of Silanol-Modified Ni-WS2 Superhydrophobic Composite Coating. Surf. Coat. Technol. 2022, 450, 129008. [Google Scholar] [CrossRef]
- Swanson, H.E.; Fuyat, R.K. National Bureau of Standard (U.S.) Circular 539. 1953; Volume 2, p. 49. Available online: https://nvlpubs.nist.gov/nistpubs/Legacy/circ/nbscircular539v2.pdf (accessed on 21 March 2024).
- Duta, A.; Bogatu, C.; Chitanu, G.C.; Pelin, M.I. Electrochemical Deposition of Ni-based Thin Film Cermets Using Polymeric Additives. Phys. Status Solidi C 2008, 5, 3530–3534. [Google Scholar] [CrossRef]
- Oriňáková, R.; Turoňová, A.; Kladeková, D.; Gálová, M.; Smith, R.M. Recent Developments in the Electrodeposition of Nickel and Some Nickel-Based Alloys. J. Appl. Electrochem. 2006, 36, 957–972. [Google Scholar] [CrossRef]
- Okonkwo, B.O.; Jeong, C.; Jang, C. Advances on Cr and Ni Electrodeposition for Industrial Applications—A Review. Coatings 2022, 12, 1555. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, Y.; Zhang, Z. A Study on the Electrocodeposition Processes and Properties of Ni–SiC Nanocomposite Coatings. J. Coat. Technol. Res. 2011, 8, 409–417. [Google Scholar] [CrossRef]
- Ponte, F.; Sharma, P.; Figueiredo, N.M.; Ferreira, J.; Carvalho, S. Decorative Chromium Coatings on Polycarbonate Substrate for the Automotive Industry. Materials 2023, 16, 2315. [Google Scholar] [CrossRef]
- Carneiro, E.; Parreira, N.M.G.; Vuchkov, T.; Cavaleiro, A.; Ferreira, J.; Andritschky, M.; Carvalho, S. Cr-Based Sputtered Decorative Coatings for Automotive Industry. Materials 2021, 14, 5527. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.H.A.; Sun, Y.; Tan, Y.T. Electroplating of Black Nickel: Bath Age and Its Effect on Coating Quality. In Proceedings of the 9th International Conference of Asian Society for Precision Engineering and Nanotechnology, Singapore, 15–18 November 2022; Sharon, N.M.L., Kumar, A.S., Eds.; ASPEN: Singapore, 2022. [Google Scholar]
- Brown, R.J.C.; Brewer, P.J.; Milton, M.J.T. The Physical and Chemical Properties of Electroless Nickel–Phosphorus Alloys and Low Reflectance Nickel–Phosphorus Black Surfaces. J. Mater. Chem. 2002, 12, 2749–2754. [Google Scholar] [CrossRef]
- Kennedy, C.E. Review of Mid- to High-Temperature Solar Selective Absorber Materials; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2002. [Google Scholar]
- ISO 9845-1:2022; Solar Energy—Reference Solar Spectral Irradiance at the Ground at Different Receiving Conditions—Part 1: Direct Normal and Hemispherical Solar Irradiance for Air Mass 1.5. ISO: Geneva, Switzerland, 2022.
- Bogaerts, W.F.; Lampert, C.M. Materials for Photothermal Solar Energy Conversion. J. Mater. Sci. 1983, 18, 2847–2875. [Google Scholar] [CrossRef]
- Lira-Cantú, M.; Morales Sabio, A.; Brustenga, A.; Gómez-Romero, P. Electrochemical Deposition of Black Nickel Solar Absorber Coatings on Stainless Steel AISI316L for Thermal Solar. Cells. Sol. Energy Mater. Sol. Cells 2005, 87, 685–694. [Google Scholar] [CrossRef]



| Reagents and Parameters | Bright Nickel | Dull Nickel | Black Nickel | Black Nickel + WS2 NPs | |
|---|---|---|---|---|---|
| NiCl·6H2O | 75 g/L | 75 g/L | 75 g/L | 75 g/L | 75 g/L |
| NaCl | 30 g/L | 30 g/L | 30 g/L | 30 g/L | 30 g/L |
| H3BO3 | 27 g/L | 27 g/L | ---------- | ---------- | ---------- |
| WS2 NPs | ---------- | ---------- | ---------- | 1.3 g/L | 2.7 g/L |
| T (°C) | Room temperature | ||||
| ω (rot/min) | 200 | ||||
| pH | 4.83–4.93 | 6.40–6.50 | 3.74–3.84 | 3.10–3.20 | |
| J (mA/cm2) | 3.75 | 2.50 | |||
| t (min) | 20 | 45 | 6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devesa, S.; Benzarti, Z.; Santos, G.; Cavaleiro, D.; Cunha, A.; Santos, J.; Carvalho, S. Enhancing Solar Absorption with Double-Layered Nickel Coatings and WS2 Nanoparticles on Copper Substrates. Energies 2024, 17, 3869. https://doi.org/10.3390/en17163869
Devesa S, Benzarti Z, Santos G, Cavaleiro D, Cunha A, Santos J, Carvalho S. Enhancing Solar Absorption with Double-Layered Nickel Coatings and WS2 Nanoparticles on Copper Substrates. Energies. 2024; 17(16):3869. https://doi.org/10.3390/en17163869
Chicago/Turabian StyleDevesa, Susana, Zohra Benzarti, Gabriel Santos, Diogo Cavaleiro, António Cunha, João Santos, and Sandra Carvalho. 2024. "Enhancing Solar Absorption with Double-Layered Nickel Coatings and WS2 Nanoparticles on Copper Substrates" Energies 17, no. 16: 3869. https://doi.org/10.3390/en17163869
APA StyleDevesa, S., Benzarti, Z., Santos, G., Cavaleiro, D., Cunha, A., Santos, J., & Carvalho, S. (2024). Enhancing Solar Absorption with Double-Layered Nickel Coatings and WS2 Nanoparticles on Copper Substrates. Energies, 17(16), 3869. https://doi.org/10.3390/en17163869











