Direct CO2 Hydrogenation over Bifunctional Catalysts to Produce Dimethyl Ether—A Review
Abstract
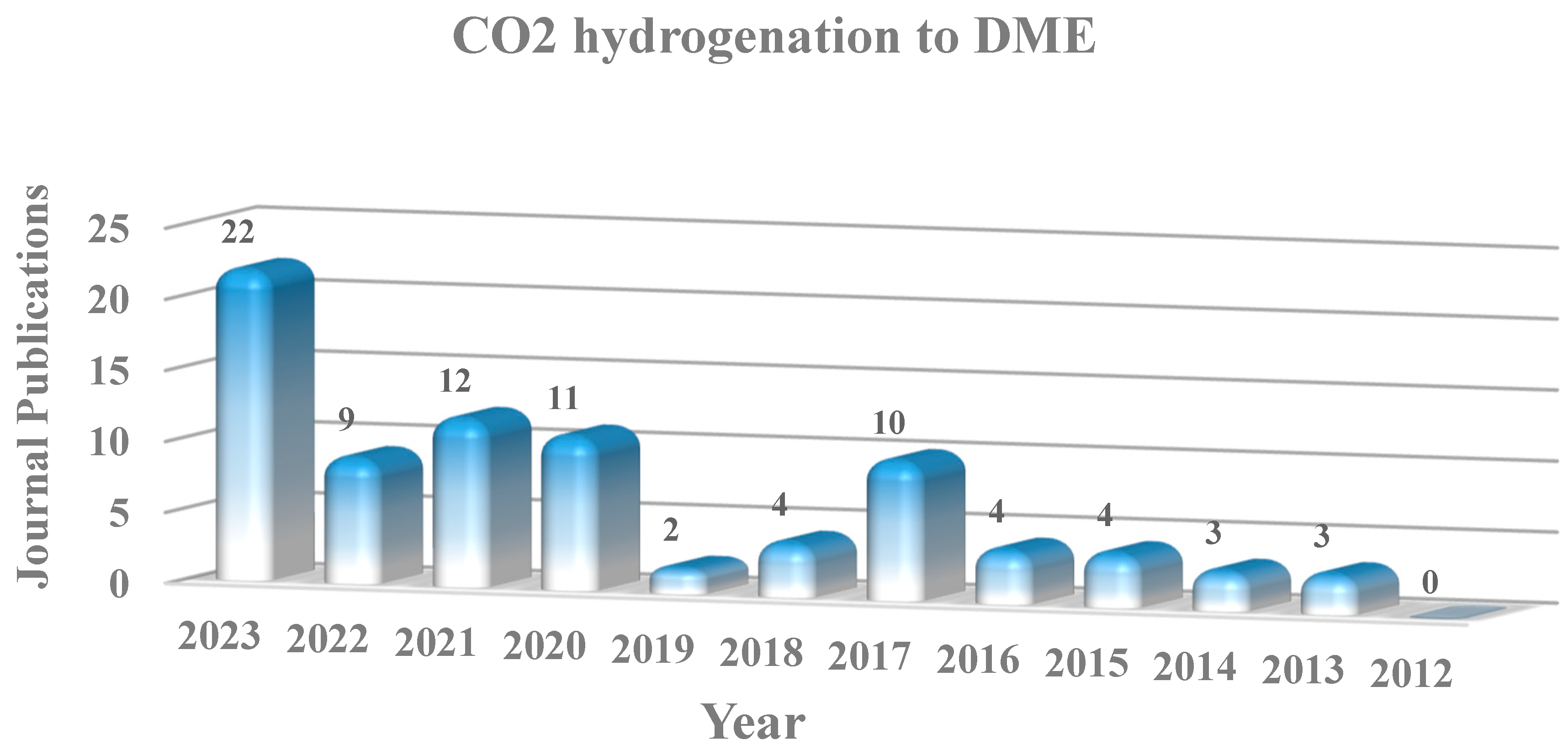
1. Introduction
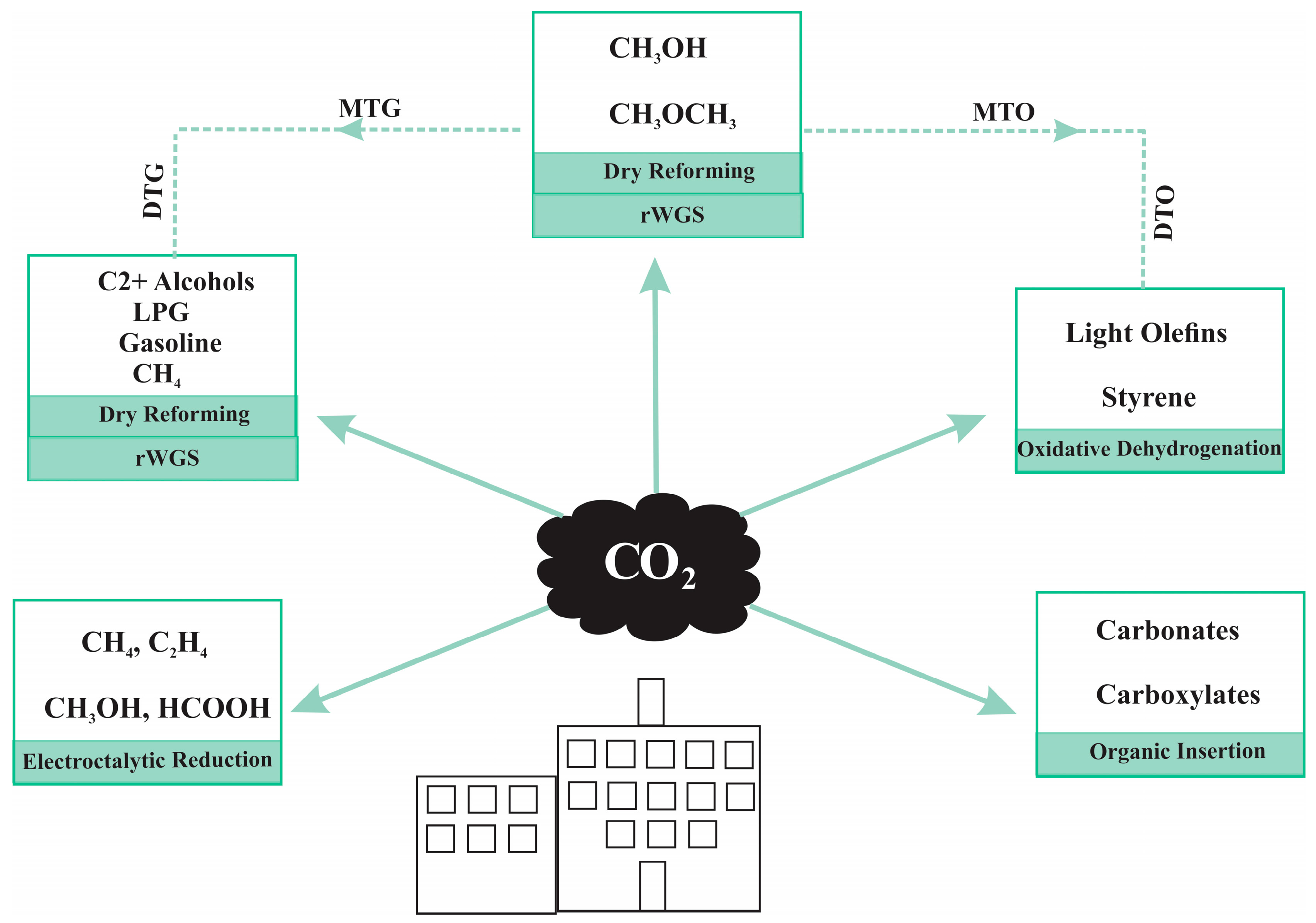
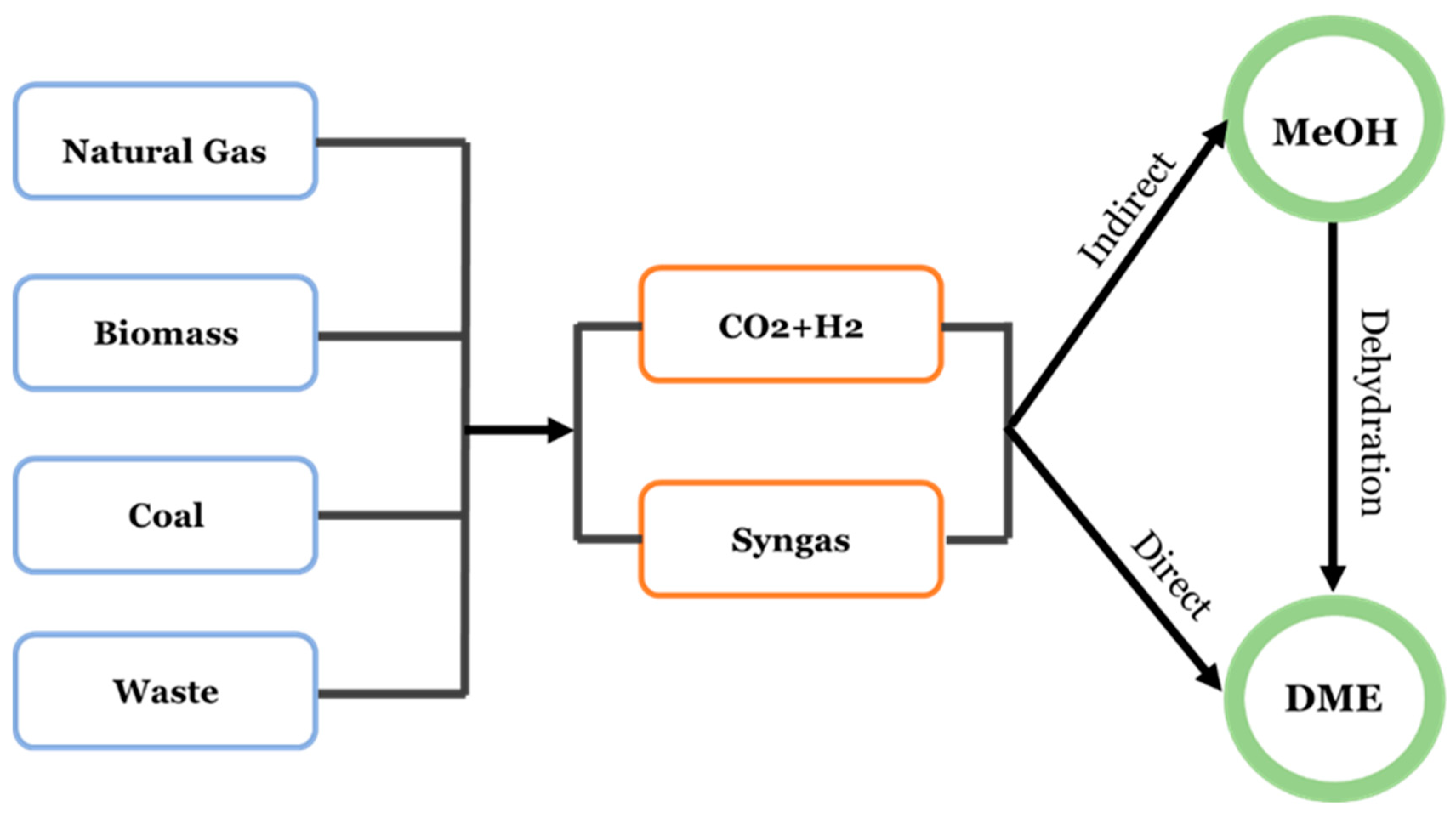
2. CO2 Conversion by Hydrogenation
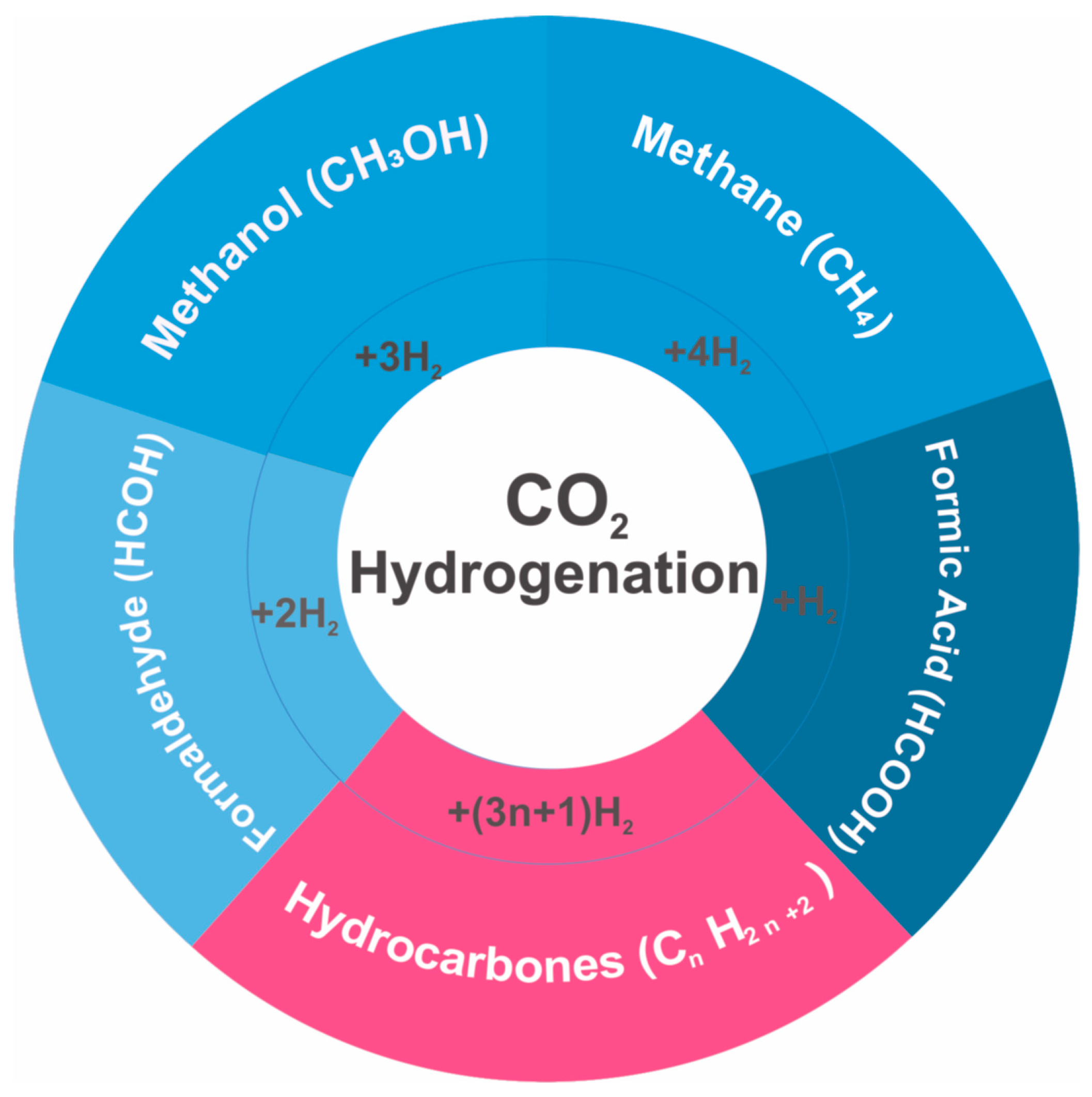
2.1. CO2 Hydrogenation to C1 Compounds
- CO2 Associative Pathway: CO2 is adsorbed with chemisorbed hydrogen (Had) to form an oxygenate, which is then hydrogenated to produce methane.
- CO2 Dissociative Pathway: CO2 undergoes direct dissociation, and the intermediate CO is then hydrogenated to form methane.
2.2. CO2 Hydrogenation to C1+ Hydrocarbons
- CO2—modified Fischer–Tropsch synthesis (CO2-FTS) route
- Methanol-mediated (MeOH) route
2.3. Overview of Reactions in CO/CO2 Hydrogenation to DME
- Conversion of syngas to methanol followed by conversion of methanol to DME, in two stages.
- Direct conversion of syngas to DME.
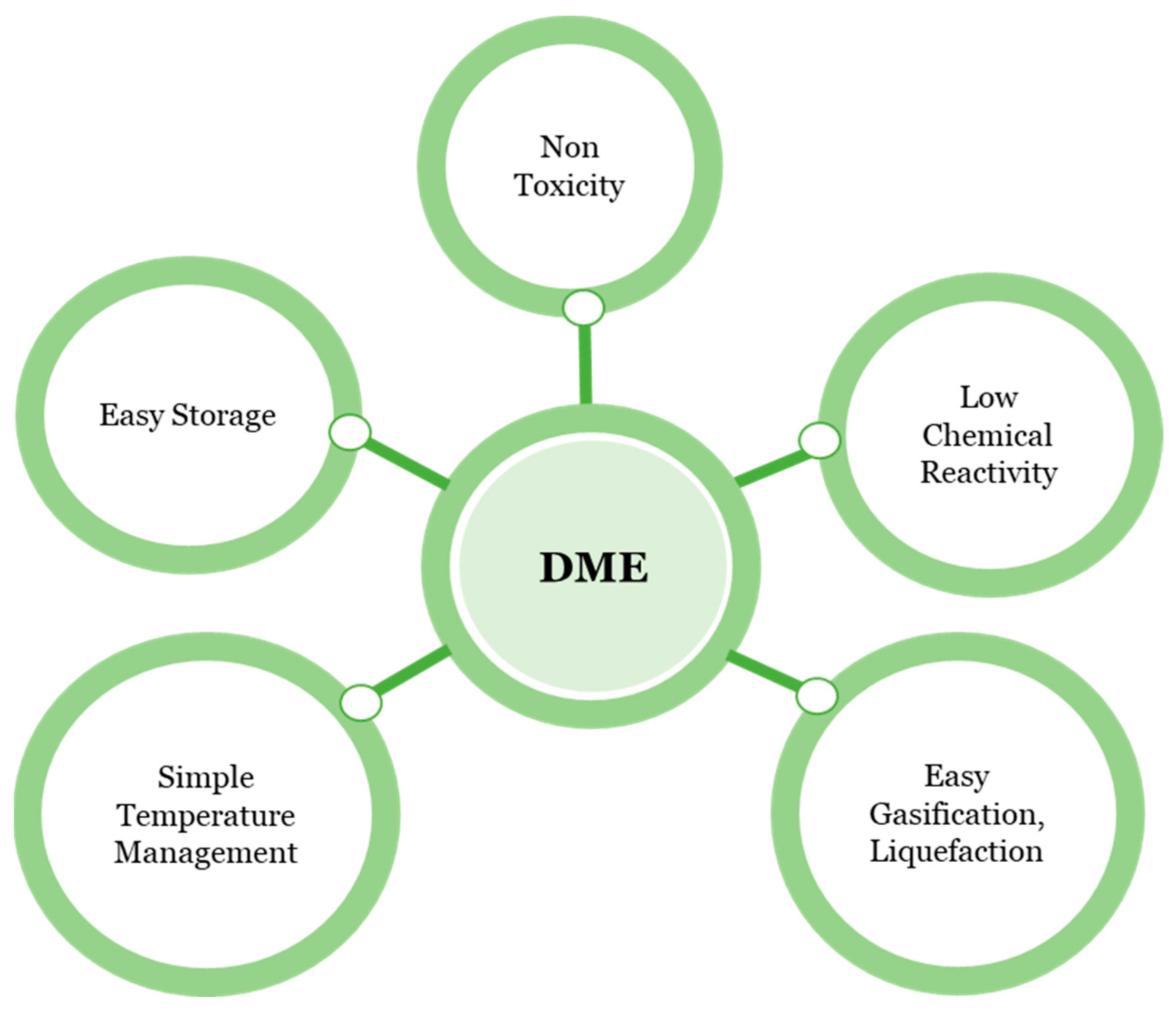
3. Dimethyl Ether (DME) Applications
3.1. Residential Cooking/Heating
3.2. Ignition Engines and Fuels for Transportation
3.3. Gas Turbine Fuel
3.4. Aerosol Propellants
3.5. Replacement to CFCs
3.6. DME as a Hydrogen Carrier
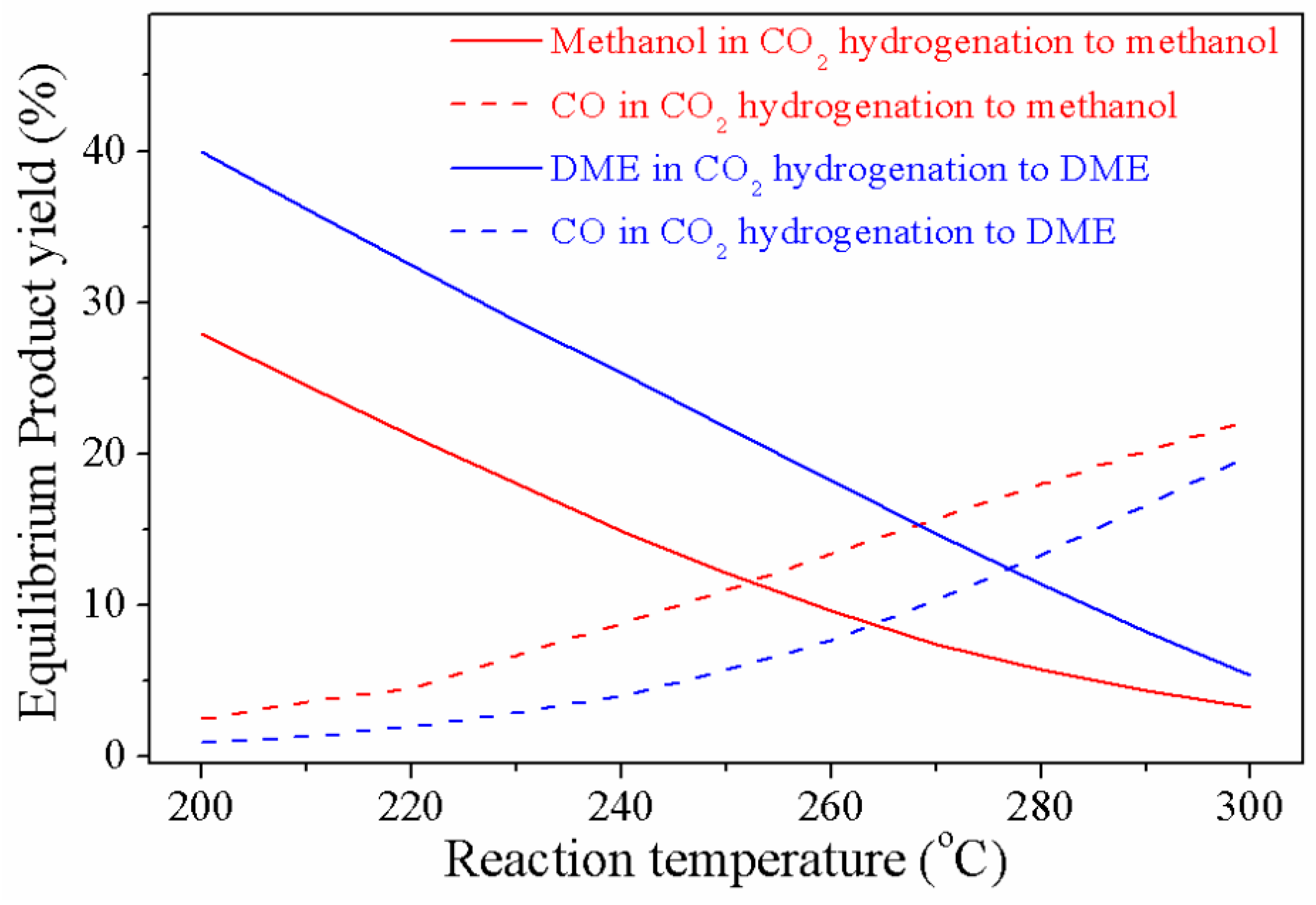
4. Thermodynamic Aspects of CO/CO2 Hydrogenation to MeOH and DME
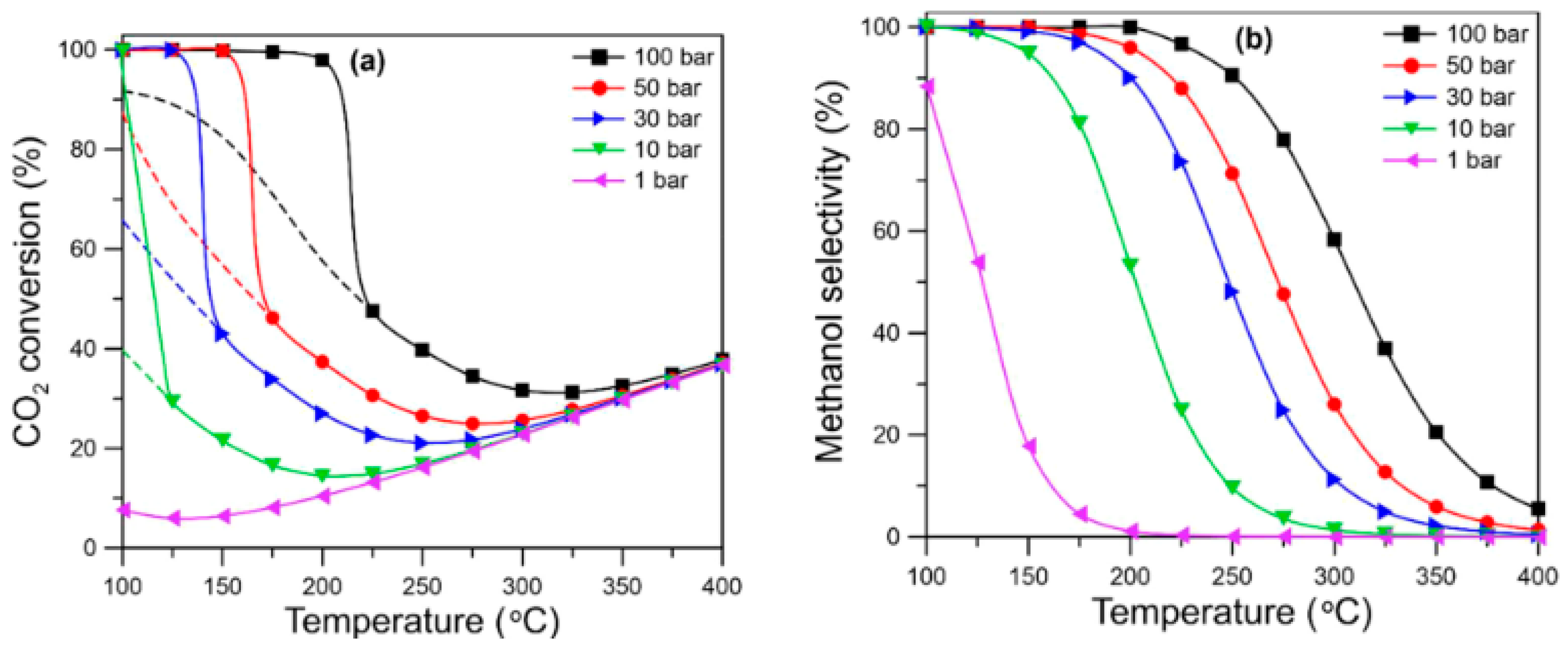
4.1. Temperature
4.2. Pressure
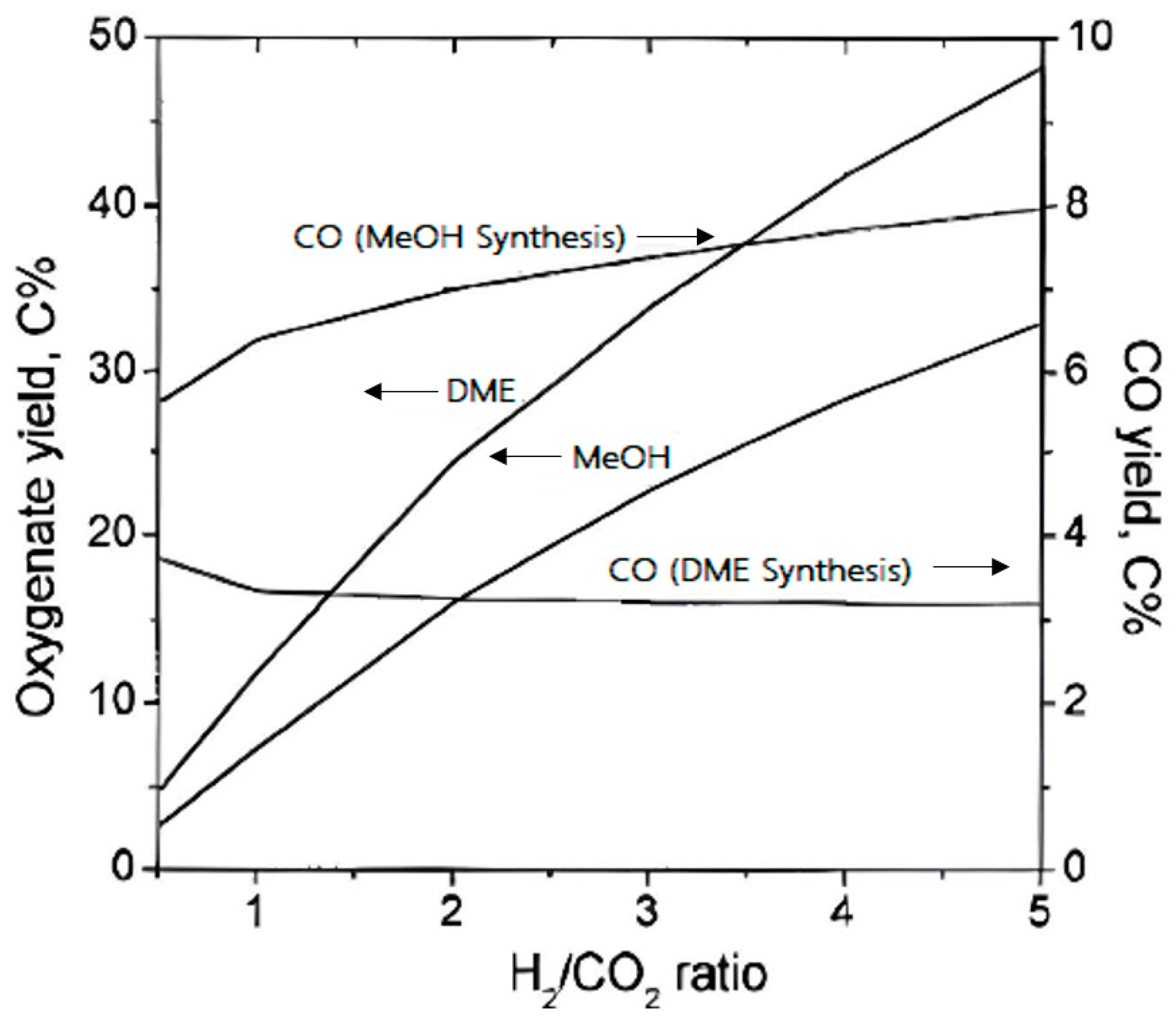
4.3. H2/CO2 Ratio
4.4. Water Content Effects and Current Water Removal Strategies
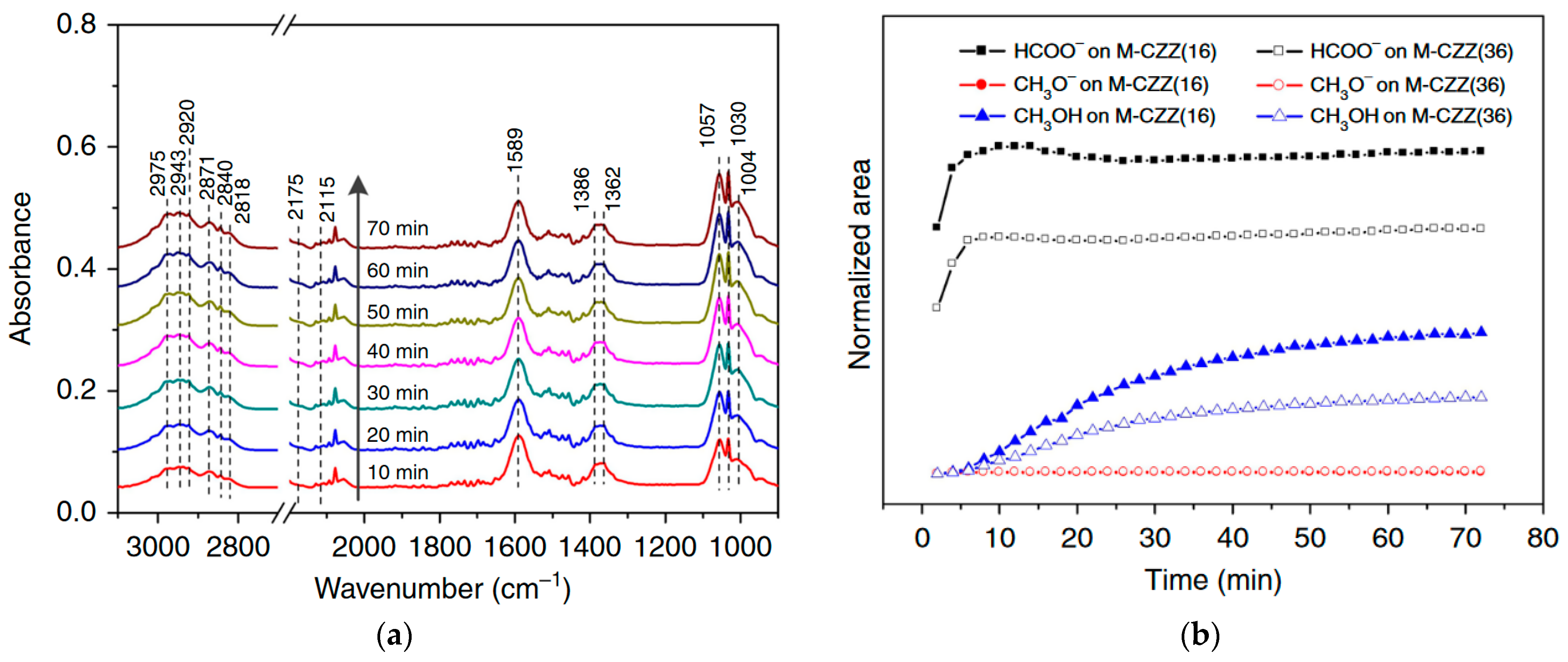
5. Reaction Mechanism
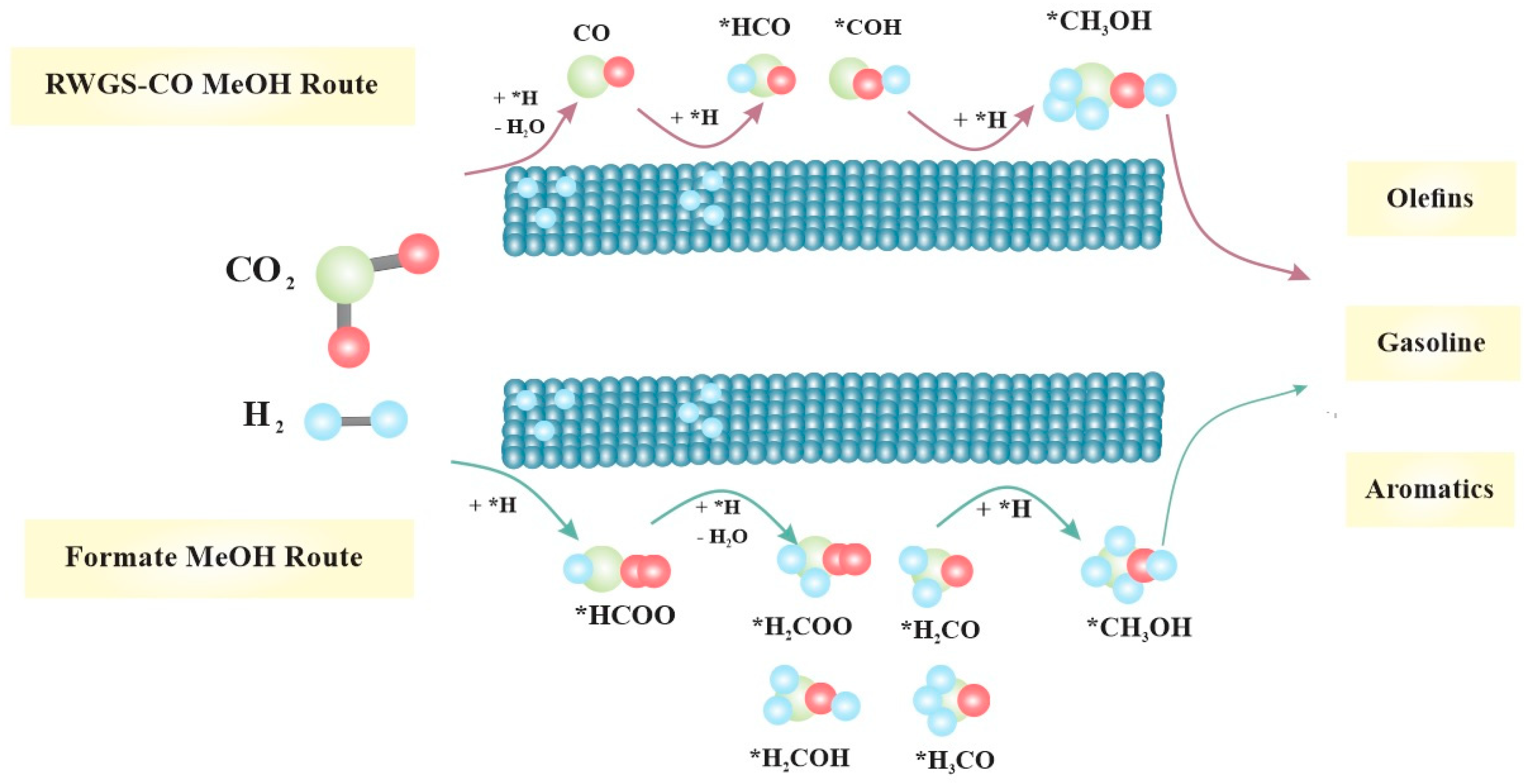
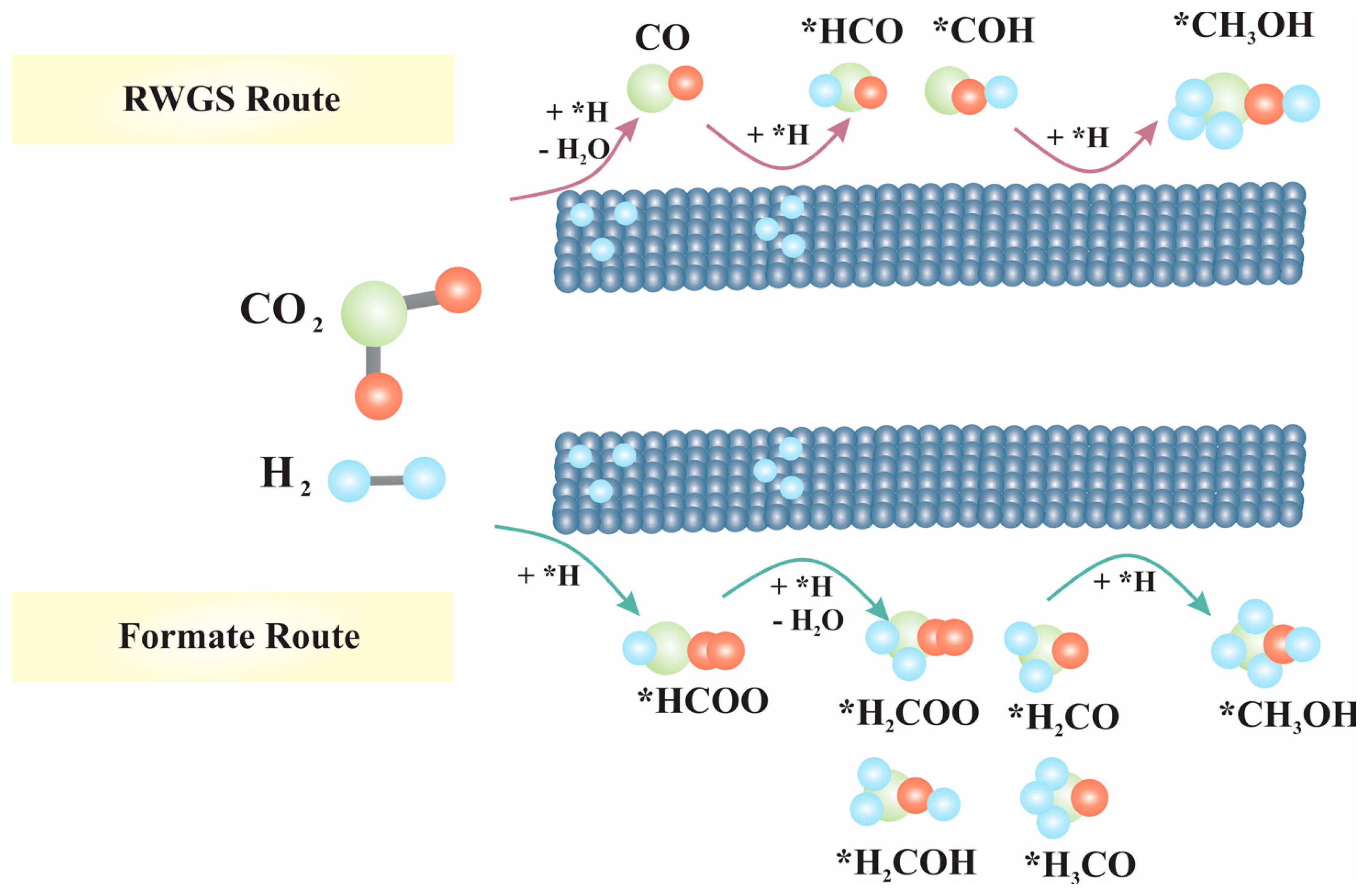
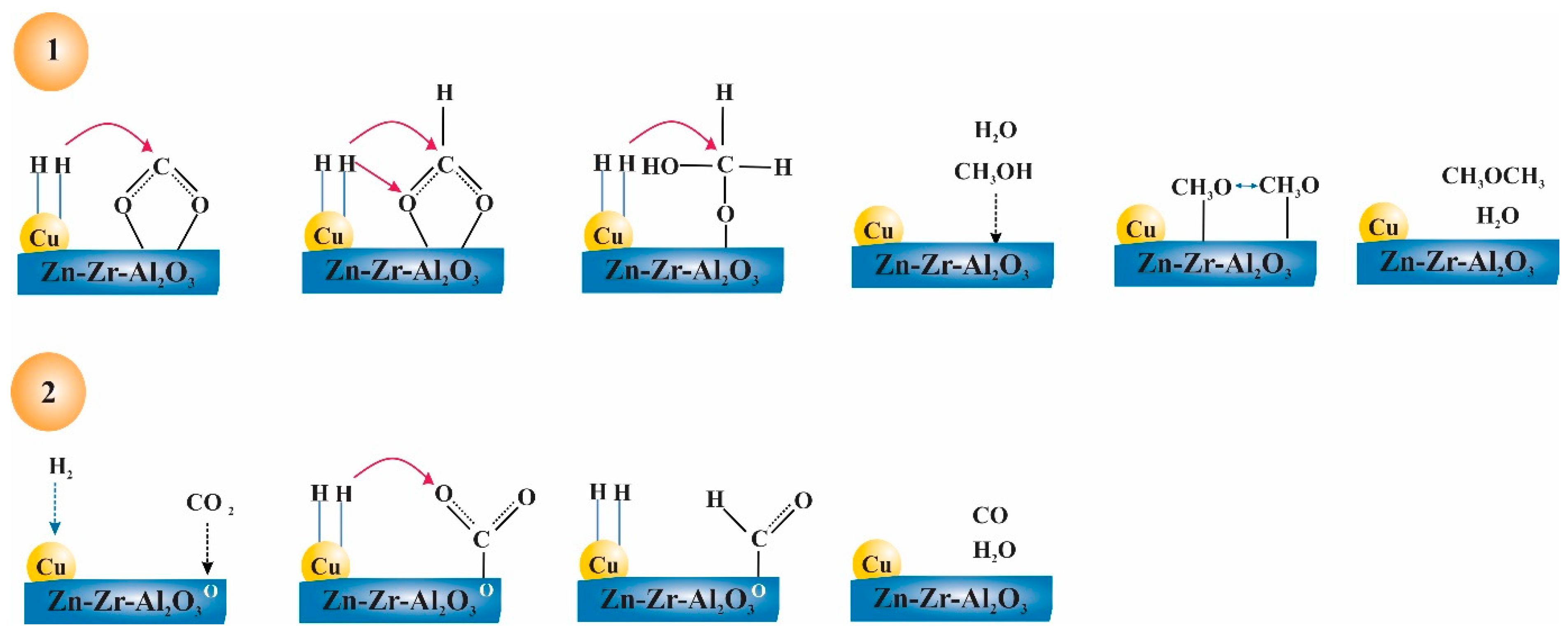
5.1. CO/CO2 Hydrogenation to MeOH
- Mechanism of formate: When CO2 directly combines with H atom from H2, it produces formate molecules (*HCOO or *COOH), which then generate *OCH3 (methoxy) and with further hydrogenation produce MeOH.
- Mechanism of RWGS: CO2 is transformed into CO and subsequently into MeOH through hydrogenation intermediates.
5.2. MeOH Dehydration to DME
- MeOH associate mechanism: Two co-adsorbed methanol molecules at the Bronsted acid sites associate into DME and water.
- MeOH dissociate mechanism: One molecule of adsorbed methanol at the Bronsted acid sites is dissociated into a surface methoxy species by losing water and then reacts with another methanol molecule to form DME.
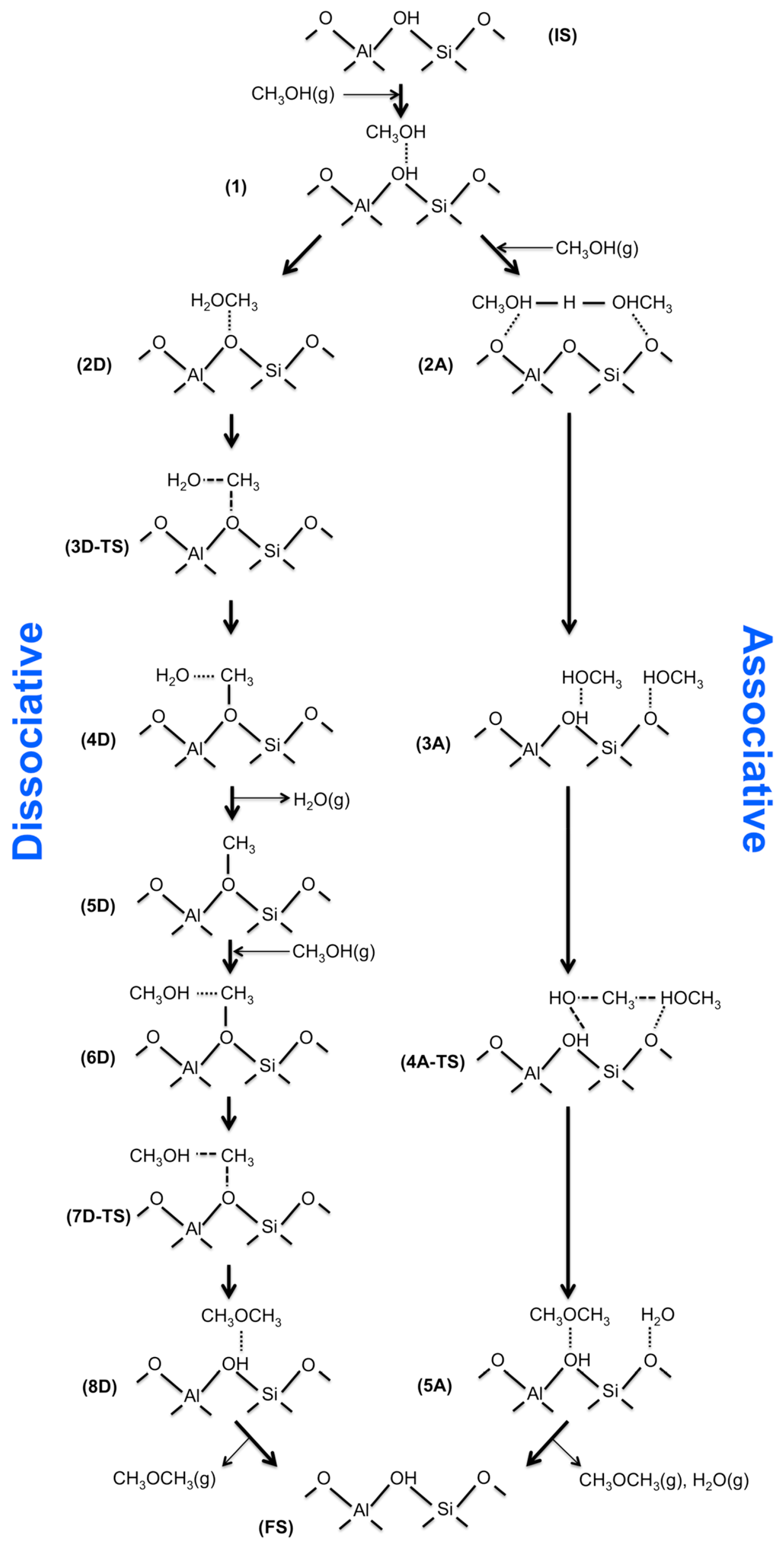
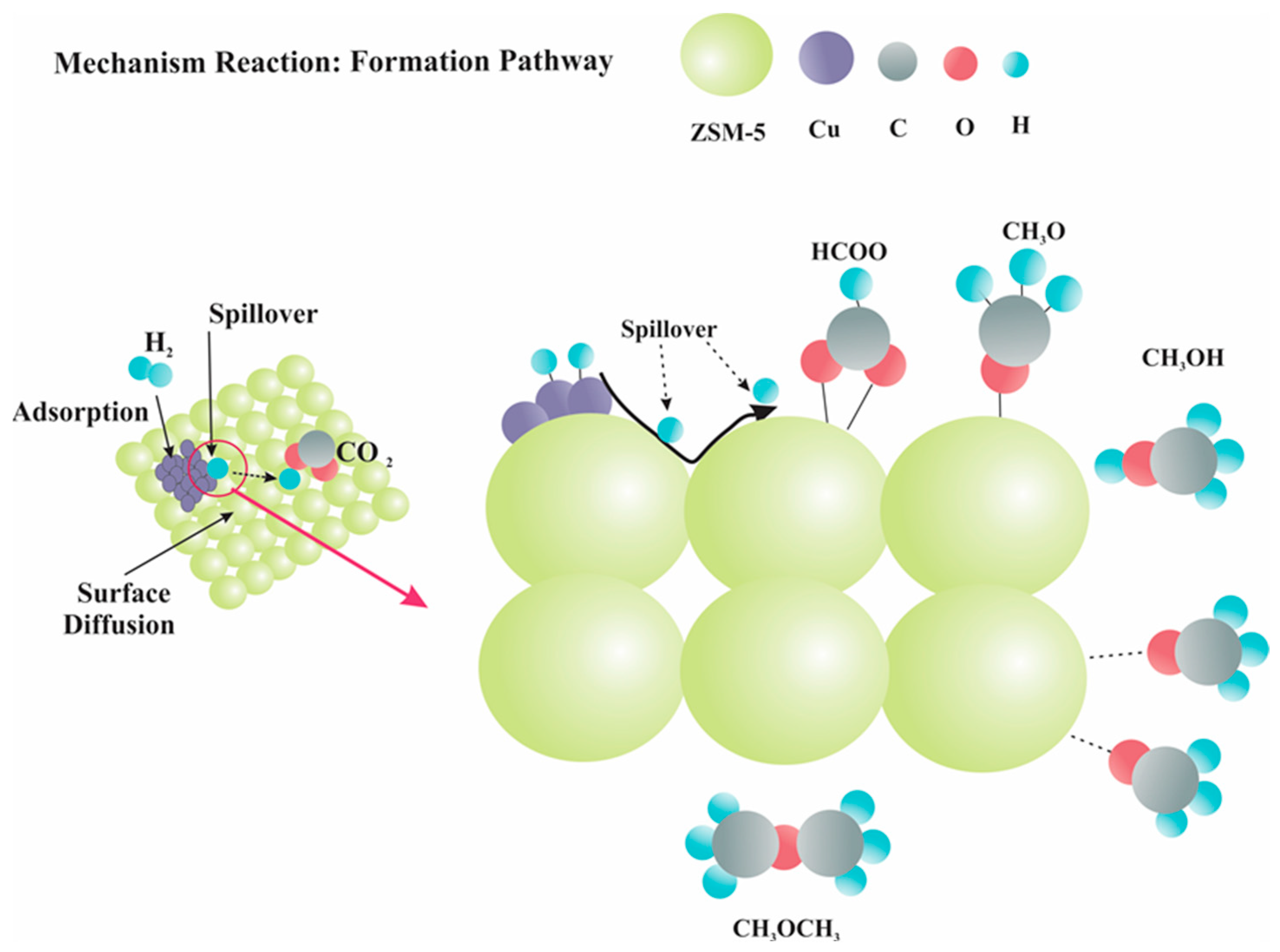
5.3. Spillover of H Atoms
- Effect of hydrogen spillover on CO2 hydrogenation to MeOH
6. Catalyst Development
6.1. Component for MeOH Synthesis
6.2. Component for MeOH Dehydration to DME
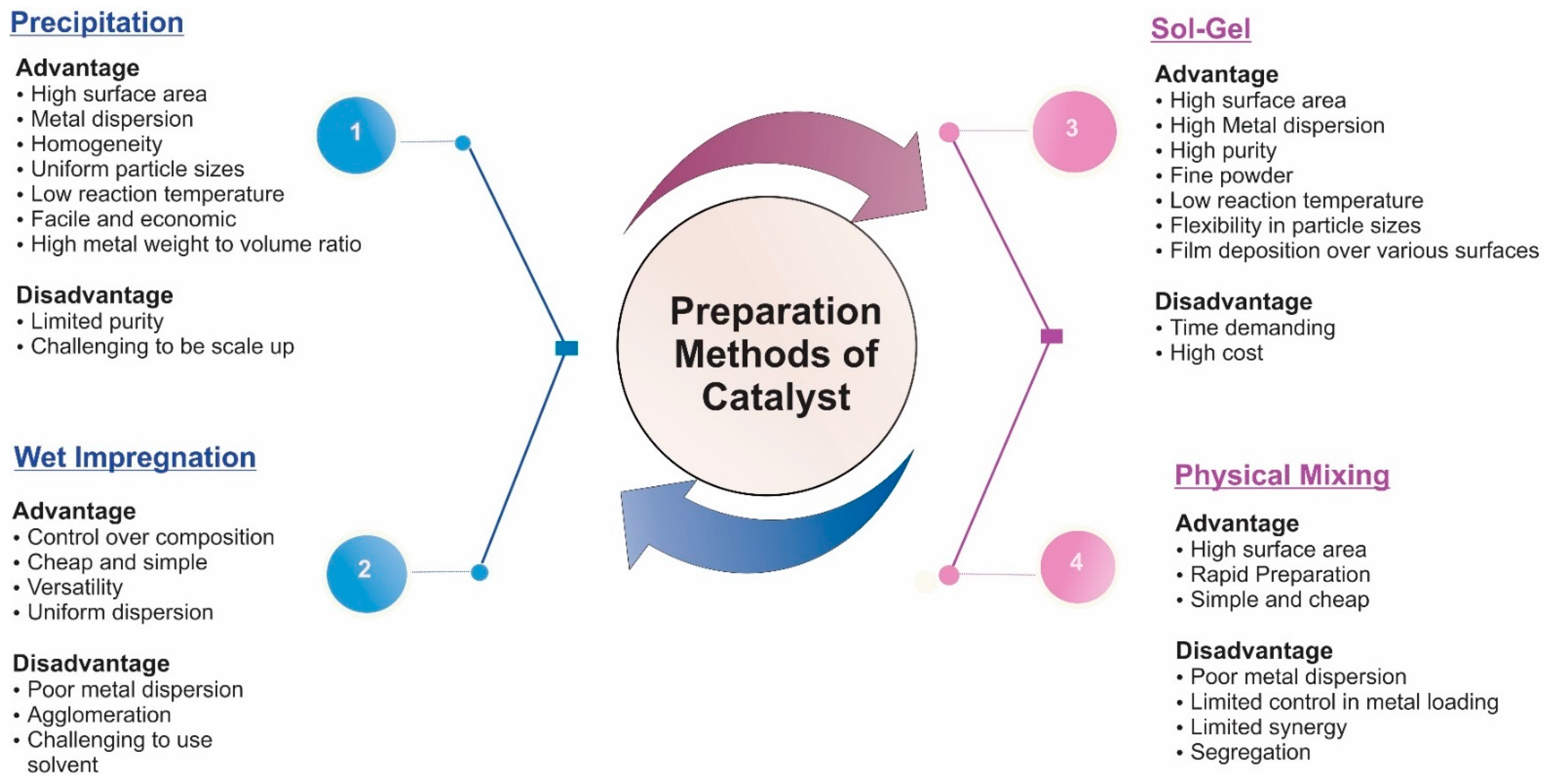
6.3. Catalyst Preparation Technology
6.4. Catalysts Deactivation Issues
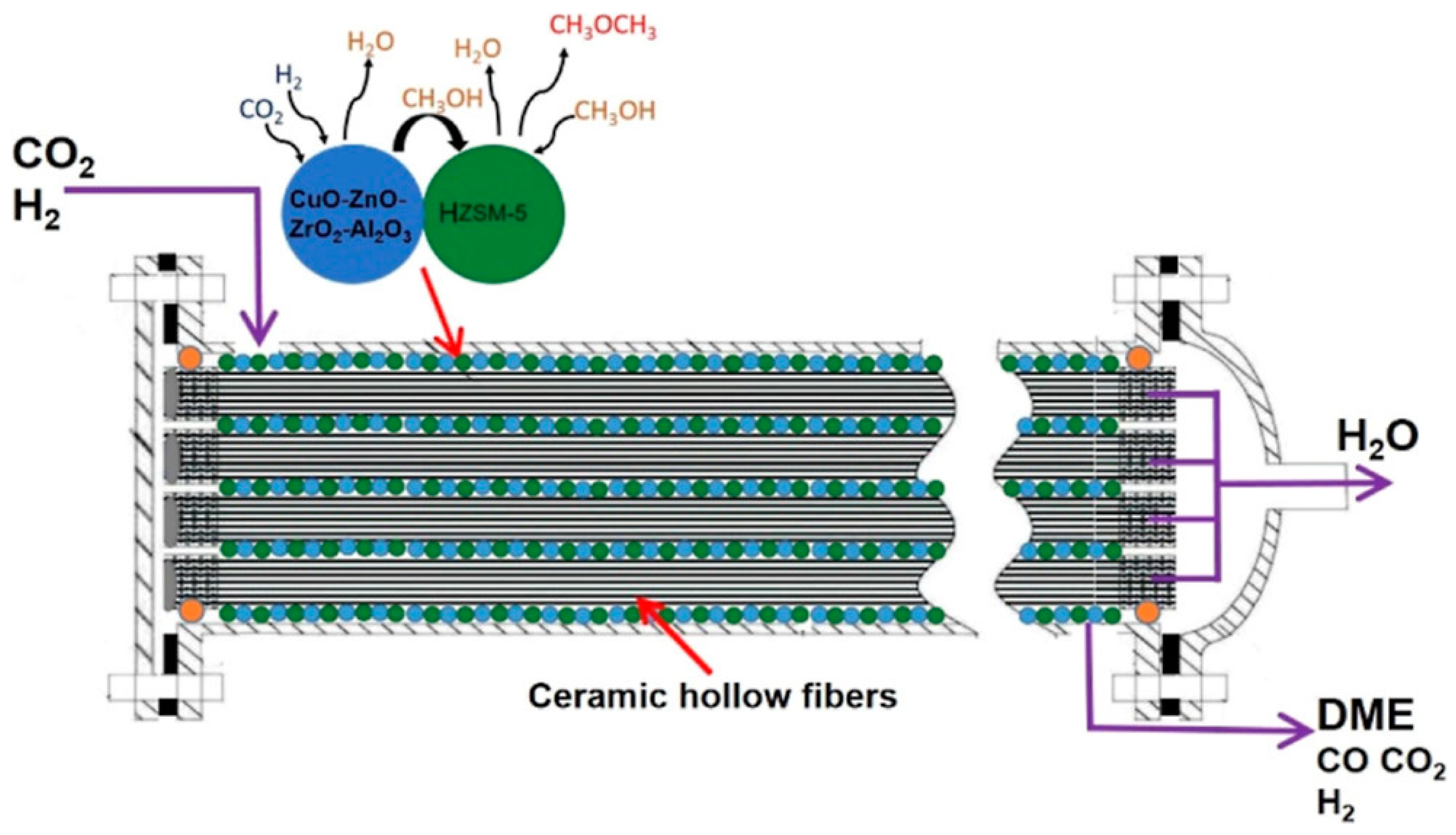
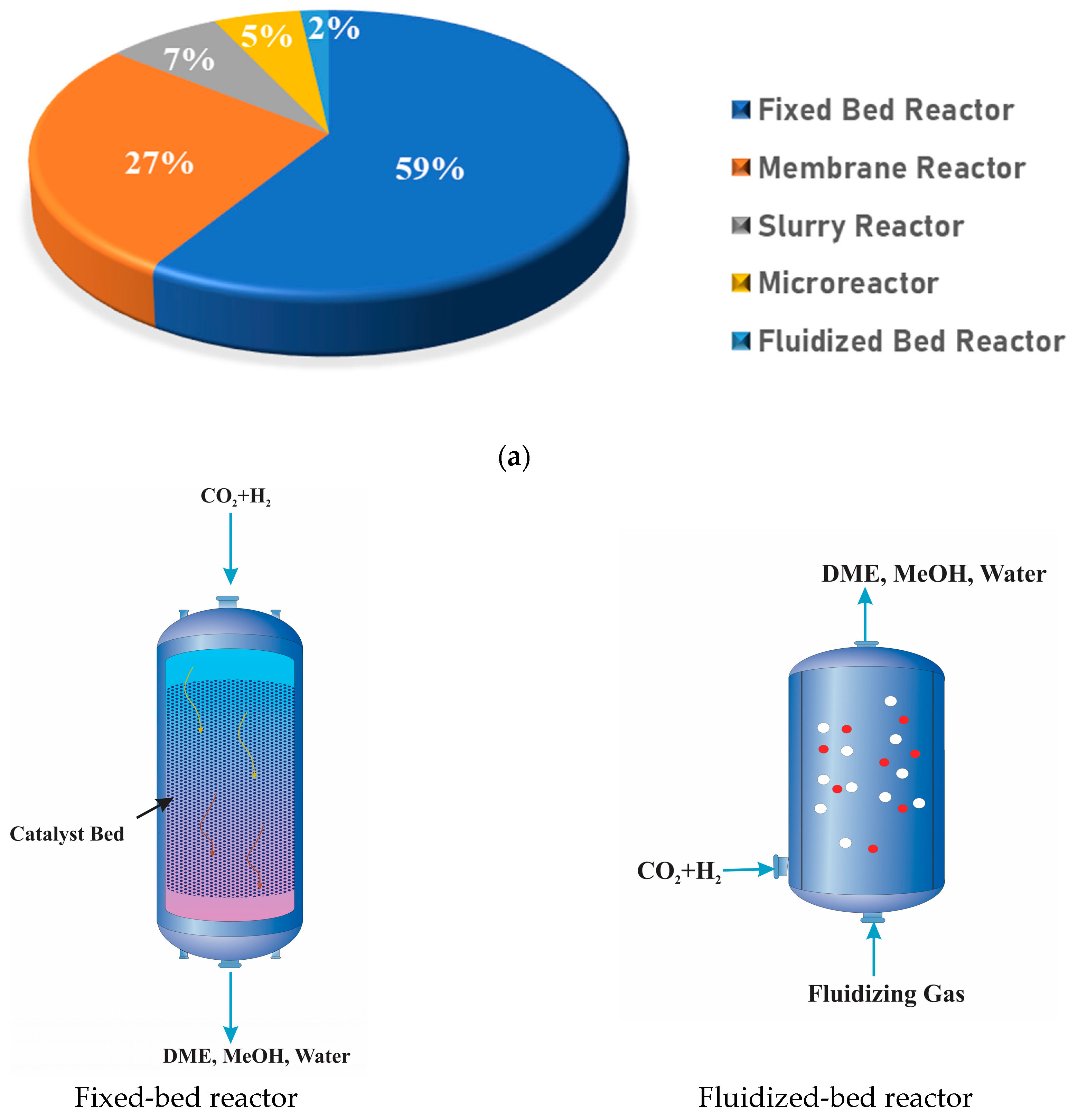
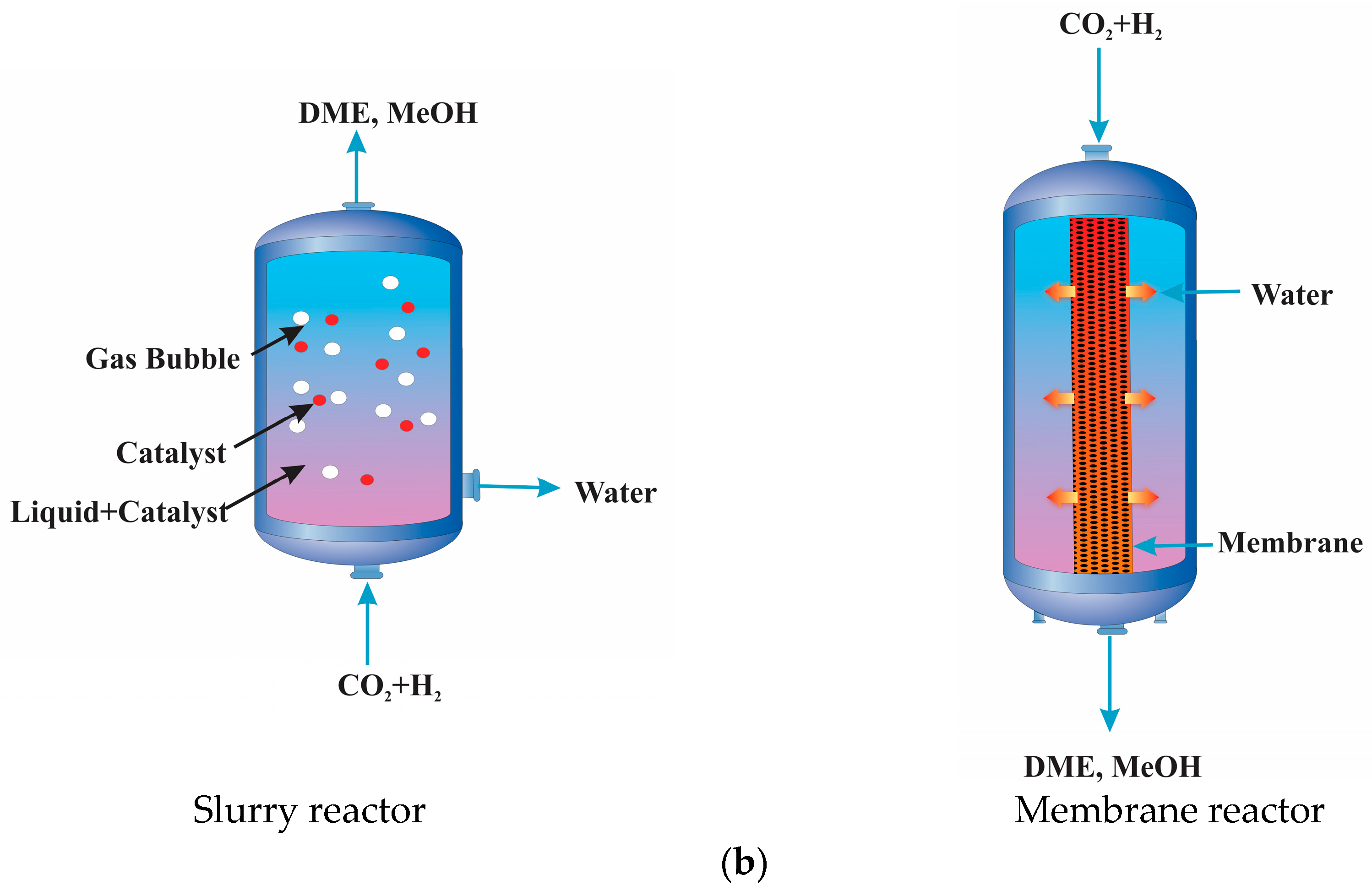
7. Reactor Configuration and Process Operation
8. Conclusions
Funding
Conflicts of Interest
References
- Yoro, K.O.; Daramola, M.O. Chapter 1—CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Rahimpour, M.R., Farsi, M., Makarem, M.A., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 3–28. [Google Scholar]
- Kriprasertkul, W.; Witoon, T.; Kim-Lohsoontorn, P. Dimethyl ether (DME) synthesis from CO2 and H2 through ethanol-assisted methanol synthesis and methanol dehydration. Int. J. Hydrogen Energy 2022, 47, 33338–33351. [Google Scholar] [CrossRef]
- Din, I.U.; Shaharun, M.S.; Alotaibi, M.A.; Alharthi, A.I.; Naeem, A. Recent developments on heterogeneous catalytic CO2 reduction to methanol. J. CO2 Util. 2019, 34, 20–33. [Google Scholar] [CrossRef]
- Using, P.; Carriers, C.H. Recent Progress on Hydrogen Storage and Production Using Chemical Hydrogen Carriers. Energies 2022, 15, 4964. [Google Scholar] [CrossRef]
- Nakyai, T.; Saebea, D. Exergoeconomic comparison of syngas production from biomass, coal, and natural gas for dimethyl ether synthesis in single-step and two-step processes. J. Clean. Prod. 2019, 241, 118334. [Google Scholar] [CrossRef]
- Krim, K.; Sachse, A.; Le Valant, A.; Pouilloux, Y.; Hocine, S. One step dimethyl ether (DME) synthesis from CO2 hydrogenation over hybrid catalysts containing Cu/ZnO/Al2O3 and nano-sized hollow ZSM-5 zeolites. Catal. Lett. 2023, 153, 83–94. [Google Scholar] [CrossRef]
- Zhong, J.; Yang, X.; Wu, Z.; Liang, B.; Huang, Y.; Zhang, T. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol. Chem. Soc. Rev. 2020, 49, 1385–1413. [Google Scholar] [CrossRef]
- Zhu, M.; Ge, Q.; Zhu, X. Catalytic reduction of CO2 to CO via reverse water gas shift reaction: Recent advances in the design of active and selective supported metal catalysts. Trans. Tianjin Univ. 2020, 26, 172–187. [Google Scholar] [CrossRef]
- Schmider, D.; Maier, L.; Deutschmann, O. Reaction kinetics of CO and CO2 methanation over nickel. Ind. Eng. Chem. Res. 2021, 60, 5792–5805. [Google Scholar] [CrossRef]
- Lee, W.J.; Li, C.; Prajitno, H.; Yoo, J.; Patel, J.; Yang, Y.; Lim, S. Recent trend in thermal catalytic low temperature CO2 methanation: A critical review. Catal. Today 2021, 368, 2–19. [Google Scholar] [CrossRef]
- Faria, A.C.; Miguel, C.V.; Madeira, L.M. Thermodynamic analysis of the CO2 methanation reaction with in situ water removal for biogas upgrading. J. CO2 Util. 2018, 26, 271–280. [Google Scholar] [CrossRef]
- Weng, M.H.; Chen, H.-T.; Wang, Y.-C.; Ju, S.-P.; Chang, J.-G.; Lin, M.-C. Kinetics and mechanisms for the adsorption, dissociation, and diffusion of hydrogen in Ni and Ni/YSZ slabs: A DFT study. Langmuir 2012, 28, 5596–5605. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported catalysts for CO2 methanation: A review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Westermann, A.; Azambre, B.; Bacariza, M.; Graça, I.; Ribeiro, M.; Lopes, J.; Henriques, C. The promoting effect of Ce in the CO2 methanation performances on NiUSY zeolite: A FTIR In Situ/Operando study. Catal. Today 2017, 283, 74–81. [Google Scholar] [CrossRef]
- Xu, L.; Wang, F.; Chen, M.; Nie, D.; Lian, X.; Lu, Z.; Chen, H.; Zhang, K.; Ge, P. CO2 methanation over rare earth doped Ni based mesoporous catalysts with intensified low-temperature activity. Int. J. Hydrogen Energy 2017, 42, 15523–15539. [Google Scholar] [CrossRef]
- Atzori, L.; Lai, S.; Cutrufello, M.G.; Ferrara, F.; Pettinau, A.; Mureddu, M.; Rombi, E. Renewable methanol from CO2 over Cu/Zn/Zr/Si oxide catalysts promoted with Mg, Ce, or La. J. Porous Mater. 2024, 31, 281–294. [Google Scholar] [CrossRef]
- Ferraz, C.P.; Tavares, M.; Bordini, L.F.; Garcia, M.A.S.; Ribeiro de Almeida, J.M.A.; Sousa-Aguiar, E.F.; Romano, P.N. Investigating the role of promoters (Ga, Nb, La, and Mg) on In2O3-based catalysts: Advancing on CO2 hydrogenation to C5+ hydrocarbons. Fuel 2024, 358, 130234. [Google Scholar] [CrossRef]
- Kattel, S.; Ramírez, P.J.; Chen, J.G.; Rodriguez, J.A.; Liu, P. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 2017, 355, 1296–1299. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Zhu, J.; Zhang, X.; Ding, F.; Zhang, A.; Guo, X.; Song, C. CO2 hydrogenation to methanol over In2O3-based catalysts: From mechanism to catalyst development. ACS Catal. 2021, 11, 1406–1423. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Tran, T.V.; Singh, S.; Phuong, P.T.; Bach, L.G.; Nanda, S.; Vo, D.-V.N. Conversion of carbon dioxide into formaldehyde. In Conversion of Carbon Dioxide into Hydrocarbons Volume 2 Technology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 159–183. [Google Scholar]
- Ahmad, W.; Chan, F.L.; Shrotri, A.; Palai, Y.N.; Wang, H.; Tanksale, A. Dimethoxymethane production via CO2 hydrogenation in methanol over novel Ru based hierarchical BEA. J. Energy Chem. 2022, 66, 181–189. [Google Scholar] [CrossRef]
- Lee, D.K.; Kim, D.S.; Kim, S.W. Selective formation of formaldehyde from carbon dioxide and hydrogen over PtCu/SiO2. Appl. Organomet. Chem. 2001, 15, 148–150. [Google Scholar] [CrossRef]
- Zhao, S.; Liang, H.Q.; Hu, X.M.; Li, S.; Daasbjerg, K. Challenges and Prospects in the Catalytic Conversion of Carbon Dioxide to Formaldehyde. Angew. Chem. Int. Ed. 2022, 61, e202204008. [Google Scholar] [CrossRef]
- Preti, D.; Resta, C.; Squarcialupi, S.; Fachinetti, G. Carbon dioxide hydrogenation to formic acid by using a heterogeneous gold catalyst. Angew. Chem. 2011, 123, 12759–12762. [Google Scholar] [CrossRef]
- Hao, C.; Wang, S.; Li, M.; Kang, L.; Ma, X. Hydrogenation of CO2 to formic acid on supported ruthenium catalysts. Catal. Today 2011, 160, 184–190. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Lin, K.-S.; Chuang, H.-W. Direct synthesis of formic acid via CO2 hydrogenation over Cu/ZnO/Al2O3 catalyst. J. Clean. Prod. 2018, 172, 1957–1977. [Google Scholar] [CrossRef]
- Weilhard, A.; Argent, S.P.; Sans, V. Efficient carbon dioxide hydrogenation to formic acid with buffering ionic liquids. Nat. Commun. 2021, 12, 231. [Google Scholar] [CrossRef]
- Braga, A.H.; Vidinha, P.; Rossi, L.M. Hydrogenation of carbon dioxide: From waste to value. Curr. Opin. Green Sustain. Chem. 2020, 26, 100386. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Jiang, X.; Zhu, J.; Liu, Z.; Guo, X.; Song, C. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts. RSC Adv. 2018, 8, 7651–7669. [Google Scholar] [CrossRef]
- Liu, B.; Li, W.; Zheng, J.; Lin, Q.; Zhang, X.; Zhang, J.; Jiang, F.; Xu, Y.; Liu, X. CO2 formation mechanism in Fischer–Tropsch synthesis over iron-based catalysts: A combined experimental and theoretical study. Catal. Sci. Technol. 2018, 8, 5288–5301. [Google Scholar] [CrossRef]
- Jia, J.-Y.; Shan, Y.-L.; Tuo, Y.-X.; Yan, H.; Feng, X.; Chen, D. Review of Iron-Based Catalysts for Carbon Dioxide Fischer–Tropsch Synthesis. Trans. Tianjin Univ. 2024, 30, 178–197. [Google Scholar] [CrossRef]
- Wang, D.; Xie, Z.; Porosoff, M.D.; Chen, J.G. Recent advances in carbon dioxide hydrogenation to produce olefins and aromatics. Chem 2021, 7, 2277–2311. [Google Scholar] [CrossRef]
- Wang, Y.; Kazumi, S.; Gao, W.; Gao, X.; Li, H.; Guo, X.; Yoneyama, Y.; Yang, G.; Tsubaki, N. Direct conversion of CO2 to aromatics with high yield via a modified Fischer-Tropsch synthesis pathway. Appl. Catal. B Environ. 2020, 269, 118792. [Google Scholar] [CrossRef]
- Xu, Y.; Zhai, P.; Deng, Y.; Xie, J.; Liu, X.; Wang, S.; Ma, D. Highly selective olefin production from CO2 hydrogenation on iron catalysts: A subtle synergy between manganese and sodium additives. Angew. Chem. 2020, 132, 21920–21928. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, K.; Kang, J.; Zhou, C.; Subramanian, V.; Zhang, Q.; Wang, Y. New horizon in C1 chemistry: Breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels. Chem. Soc. Rev. 2019, 48, 3193–3228. [Google Scholar] [CrossRef]
- Wang, J.; You, Z.; Zhang, Q.; Deng, W.; Wang, Y. Synthesis of lower olefins by hydrogenation of carbon dioxide over supported iron catalysts. Catal. Today 2013, 215, 186–193. [Google Scholar] [CrossRef]
- Wei, J.; Ge, Q.; Yao, R.; Wen, Z.; Fang, C.; Guo, L.; Xu, H.; Sun, J. Directly converting CO2 into a gasoline fuel. Nat. Commun. 2017, 8, 15174. [Google Scholar] [CrossRef]
- Guo, L.; Sun, S.; Li, J.; Gao, W.; Zhao, H.; Zhang, B.; He, Y.; Zhang, P.; Yang, G.; Tsubaki, N. Boosting liquid hydrocarbons selectivity from CO2 hydrogenation by facilely tailoring surface acid properties of zeolite via a modified Fischer-Tropsch synthesis. Fuel 2021, 306, 121684. [Google Scholar] [CrossRef]
- Kallio, P.; Pásztor, A.; Akhtar, M.K.; Jones, P.R. Renewable jet fuel. Curr. Opin. Biotechnol. 2014, 26, 50–55. [Google Scholar] [CrossRef]
- Zhao, H.; Zeng, C.; Tsubaki, N. A mini review on recent advances in thermocatalytic hydrogenation of carbon dioxide to value-added chemicals and fuels. Resour. Chem. Mater. 2022, 1, 230–248. [Google Scholar] [CrossRef]
- Choi, Y.H.; Ra, E.C.; Kim, E.H.; Kim, K.Y.; Jang, Y.J.; Kang, K.N.; Choi, S.H.; Jang, J.H.; Lee, J.S. Sodium-Containing Spinel Zinc Ferrite as a Catalyst Precursor for the Selective Synthesis of Liquid Hydrocarbon Fuels. ChemSusChem 2017, 10, 4764–4770. [Google Scholar] [CrossRef]
- Ramirez, A.; Dutta Chowdhury, A.; Dokania, A.; Cnudde, P.; Caglayan, M.; Yarulina, I.; Abou-Hamad, E.; Gevers, L.; Ould-Chikh, S.; De Wispelaere, K. Effect of zeolite topology and reactor configuration on the direct conversion of CO2 to light olefins and aromatics. ACS Catal. 2019, 9, 6320–6334. [Google Scholar] [CrossRef]
- Weber, D.; He, T.; Wong, M.; Moon, C.; Zhang, A.; Foley, N.; Ramer, N.J.; Zhang, C. Recent advances in the mitigation of the catalyst deactivation of CO2 hydrogenation to light olefins. Catalysts 2021, 11, 1447. [Google Scholar] [CrossRef]
- Gogate, M.R. Methanol-to-olefins process technology: Current status and future prospects. Pet. Sci. Technol. 2019, 37, 559–565. [Google Scholar] [CrossRef]
- He, Y.; Müller, F.H.; Palkovits, R.; Zeng, F.; Mebrahtu, C. Tandem Catalysis for CO2 Conversion to Higher Alcohols: A Review. Appl. Catal. B Environ. 2024, 345, 123663. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Xie, B.; Amal, R.; Lovell, E.C.; Scott, J. Light-Enhanced Conversion of CO2 to Light Olefins: Basis in Thermal Catalysis, Current Progress, and Future Prospects. Small Struct. 2023, 4, 2200285. [Google Scholar] [CrossRef]
- Ruddy, D.A.; Hensley, J.E.; Nash, C.P.; Tan, E.C.; Christensen, E.; Farberow, C.A.; Baddour, F.G.; Van Allsburg, K.M.; Schaidle, J.A. Methanol to high-octane gasoline within a market-responsive biorefinery concept enabled by catalysis. Nat. Catal. 2019, 2, 632–640. [Google Scholar] [CrossRef]
- Fujiwara, M.; Kieffer, R.; Ando, H.; Xu, Q.; Souma, Y. Change of catalytic properties of FeZnO/zeolite composite catalyst in the hydrogenation of carbon dioxide. Appl. Catal. A Gen. 1997, 154, 87–101. [Google Scholar] [CrossRef]
- Nezam, I.; Zhou, W.; Gusmão, G.S.; Realff, M.J.; Wang, Y.; Medford, A.J.; Jones, C.W. Direct aromatization of CO2 via combined CO2 hydrogenation and zeolite-based acid catalysis. J. CO2 Util. 2021, 45, 101405. [Google Scholar] [CrossRef]
- Ni, Y.; Chen, Z.; Fu, Y.; Liu, Y.; Zhu, W.; Liu, Z. Selective conversion of CO2 and H2 into aromatics. Nat. Commun. 2018, 9, 3457. [Google Scholar] [CrossRef]
- Fu, T.; Shao, J.; Li, Z. Catalytic synergy between the low Si/Al ratio Zn/ZSM-5 and high Si/Al ratio HZSM-5 for high-performance methanol conversion to aromatics. Appl. Catal. B Environ. 2021, 291, 120098. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Kazumi, S.; Li, H.; Yang, G.; Tsubaki, N. Direct and oriented conversion of CO2 into value-added aromatics. Chem. A Eur. J. 2019, 25, 5149–5153. [Google Scholar] [CrossRef]
- Tan, K.B.; Xu, K.; Cai, D.; Huang, J.; Zhan, G. Rational design of bifunctional catalysts with proper integration manners for CO and CO2 hydrogenation into value-added products: A review. Chem. Eng. J. 2023, 463, 142262. [Google Scholar] [CrossRef]
- Petrukhina, N.; Maximov, A. Use of Dimethyl Ether in Technologies for Enhancing the Oil Recovery from Reservoirs (A Review). Pet. Chem. 2023, 63, 67–73. [Google Scholar] [CrossRef]
- Lourentius, S. Synthesis of Syngas into Dimethyl Ether Using Cu-Zn-Al/Y-Alumina Bifunctional Catalyst as an Environmentally Friendly Fuel for Substituting Liquified Petroleum Gas. Equilib. J. Chem. Eng. 2022, 5, 117–126. [Google Scholar] [CrossRef]
- Asthana, S.; Samanta, C.; Bhaumik, A.; Banerjee, B.; Voolapalli, R.K.; Saha, B. Direct synthesis of dimethyl ether from syngas over Cu-based catalysts: Enhanced selectivity in the presence of MgO. J. Catal. 2016, 334, 89–101. [Google Scholar] [CrossRef]
- Boretti, A. Carbon dioxide hydrogenation for sustainable energy storage. Int. J. Hydrogen Energy 2024, 58, 1386–1395. [Google Scholar] [CrossRef]
- Mondal, U.; Yadav, G.D. Perspective of dimethyl ether as fuel: Part I. Catalysis. J. CO2 Util. 2019, 32, 299–320. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Kabir, K.B.; Hein, K. Dimethyl ether synthesis from Victorian brown coal through gasification—Current status, and research and development needs. Prog. Energy Combust. Sci. 2013, 39, 577–605. [Google Scholar] [CrossRef]
- Fleisch, T.; Basu, A.; Sills, R. Introduction and advancement of a new clean global fuel: The status of DME developments in China and beyond. J. Nat. Gas Sci. Eng. 2012, 9, 94–107. [Google Scholar] [CrossRef]
- Anggarani, R.; Wibowo, C.S.; Rulianto, D. Application of dimethyl ether as LPG substitution for household stove. Energy Procedia 2014, 47, 227–234. [Google Scholar] [CrossRef]
- Prabowo, B.; Yan, M.; Syamsiro, M.; Setyobudi, R.H.; Biddinika, M.K. State of the Art of Global Dimethyl Ether Production and It’s Potentional Application in Indonesia: Global Dimethyl Ether Production and It’s Potentional Application in Indonesia. Proc. Pak. Acad. Sci. B Life Environ. Sci. 2017, 54, 29–39. [Google Scholar]
- Makmool, U.; Jugjai, S. Thermal efficiency and pollutant emissions of domestic cooking burners using DME-LPG blends as fuel. In Proceedings of the Fourth TSME International Conference on Mechanical Engineering, Parraya, Thailand, 16–18 October 2013; pp. 16–18. [Google Scholar]
- Heywood, J.B. Automotive engines and fuels: A review of future options. Prog. Energy Combust. Sci. 1981, 7, 155–184. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, C.S. Applicability of dimethyl ether (DME) in a compression ignition engine as an alternative fuel. Energy Convers. Manag. 2014, 86, 848–863. [Google Scholar] [CrossRef]
- Bae, C.; Kim, J. Alternative fuels for internal combustion engines. Proc. Combust. Inst. 2017, 36, 3389–3413. [Google Scholar] [CrossRef]
- Wu, J.; Huang, Z.; Qiao, X.; Lu, J.; Zhang, J.; Zhang, L. Study of combustion and emission characteristics of turbocharged diesel engine fuelled with dimethylether. Front. Energy Power Eng. China 2008, 2, 79–85. [Google Scholar] [CrossRef]
- Hua, Y. Ethers and esters as alternative fuels for internal combustion engine: A review. Int. J. Engine Res. 2023, 24, 178–216. [Google Scholar] [CrossRef]
- Styring, P.; Duckworth, E.L.; Platt, E.G. Synthetic fuels in a transport transition: Fuels to prevent a transport underclass. Front. Energy Res. 2021, 9, 707867. [Google Scholar] [CrossRef]
- Moirangthem, K.; Baxter, D. Alternative fuels for marine and inland waterways. Eur. Comm. 2016, 44. [Google Scholar] [CrossRef]
- Fabiś, P.; Flekiewicz, B. Influence of LPG and DME composition on spark ignition engine performance. Energies 2021, 14, 5583. [Google Scholar] [CrossRef]
- Kabir, K.B.; Bhattacharya, S.; Hein, K. Process modelling of dimethyl ether production from Victorian brown coal—Integrating coal drying, gasification and synthesis processes. Comput. Chem. Eng. 2013, 48, 96–104. [Google Scholar] [CrossRef]
- Roh, H.G.; Park, S.; Lee, C.S. Effect of exhaust gas recirculation on the combustion and emissions of dimethyl ether in a passenger vehicle diesel engine. J. Energy Eng. 2018, 144, 04018061. [Google Scholar] [CrossRef]
- Semelsberger, T.A.; Borup, R.L.; Greene, H.L. Dimethyl ether (DME) as an alternative fuel. J. Power Sources 2006, 156, 497–511. [Google Scholar] [CrossRef]
- Lee, J.J.; Kim, T.S. Development of a gas turbine performance analysis program and its application. Energy 2011, 36, 5274–5285. [Google Scholar] [CrossRef]
- Haque, M.A.; Nemitallah, M.A.; Abdelhafez, A.; Mansir, I.B.; Habib, M.A. Review of fuel/oxidizer-flexible combustion in gas turbines. Energy Fuels 2020, 34, 10459–10485. [Google Scholar] [CrossRef]
- Gupta, K.K.; Rehman, A.; Sarviya, R. Bio-fuels for the gas turbine: A review. Renew. Sustain. Energy Rev. 2010, 14, 2946–2955. [Google Scholar] [CrossRef]
- Basu, A.; Gradassi, M.; Sills, R.; Fleisch, T.; Puri, R. Use of DME as a gas turbine fuel. In Turbo Expo: Power for Land, Sea, and Air; American Society of Mechanical Engineers: New York, NY, USA, 2001. [Google Scholar]
- Xi, Z.; Liu, Z.; Shi, X.; Lian, T.; Yang, X.; Zhang, J.; Mei, B.; Li, Y. Enhancement of biogas combustion by co-firing dimethyl ether in a gas turbine model combustor. Fuel 2022, 316, 123446. [Google Scholar] [CrossRef]
- Moghaddam, A.L.; Hazlett, M.J. Methanol dehydration catalysts in direct and indirect dimethyl ether (DME) production and the beneficial role of DME in energy supply and environmental pollution. J. Environ. Chem. Eng. 2023, 11, 110307. [Google Scholar] [CrossRef]
- Wollenhaupt, B.; Le, Q.H.; Herdrich, G. Overview of thermal arcjet thruster development. Aircr. Eng. Aerosp. Technol. 2018, 90, 280–301. [Google Scholar] [CrossRef]
- Kakami, A.; Yokote, J.; Ebara, I.; Tachibana, T. Application of dimethyl ether to arcjet thruster as propellant. Vacuum 2008, 83, 77–81. [Google Scholar] [CrossRef]
- Yount, J.; Piercey, D.G. Electrochemical synthesis of high-nitrogen materials and energetic materials. Chem. Rev. 2022, 122, 8809–8840. [Google Scholar] [CrossRef]
- Asakura, T.; Hayashi, S.; Yano, Y.; Kakami, A. Influence of Injector for Performance of N2O/DME Bipropellant Thruster. Trans. Jpn. Soc. Aeronaut. Space Sci. Aerosp. Technol. Jpn. 2018, 16, 177–180. [Google Scholar]
- Yamamoto, Y.; Tachibana, T. Burning velocities of dimethyl ether (DME)–nitrous oxide (N2O) mixtures. Fuel 2018, 217, 160–165. [Google Scholar] [CrossRef]
- Mondejar, M.; Andreasen, J.; Pierobon, L.; Larsen, U.; Thern, M.; Haglind, F. A review of the use of organic Rankine cycle power systems for maritime applications. Renew. Sustain. Energy Rev. 2018, 91, 126–151. [Google Scholar] [CrossRef]
- Azizi, Z.; Rezaeimanesh, M.; Tohidian, T.; Rahimpour, M.R. Dimethyl ether: A review of technologies and production challenges. Chem. Eng. Process. Process Intensif. 2014, 82, 150–172. [Google Scholar] [CrossRef]
- Apostol, V.; Popescu, G.; Pop, H.; Vasilescu, E.E.; Marinescu, C.; Alionte, C.-G. Thermodynamic Study Regarding the use of dimethylether as Eco-Refrigerant. J. Rev. Chime 2009, 60, 714–718. [Google Scholar]
- Catizzone, E.; Freda, C.; Braccio, G.; Frusteri, F.; Bonura, G. Dimethyl ether as circular hydrogen carrier: Catalytic aspects of hydrogenation/dehydrogenation steps. J. Energy Chem. 2021, 58, 55–77. [Google Scholar] [CrossRef]
- González-Gil, R.; Herrera, C.; Larrubia, M.Á.; Kowalik, P.; Pieta, I.S.; Alemany, L.J. Hydrogen production by steam reforming of DME over Ni-based catalysts modified with vanadium. Int. J. Hydrogen Energy 2016, 41, 19781–19788. [Google Scholar] [CrossRef]
- Pawelczyk, E.; Łukasik, N.; Wysocka, I.; Rogala, A.; Gębicki, J. Recent progress on hydrogen storage and production using chemical hydrogen carriers. Energies 2022, 15, 4964. [Google Scholar] [CrossRef]
- Yarbaş, T.; Ayas, N. A detailed thermodynamic analysis of CO2 hydrogenation to produce methane at low pressure. Int. J. Hydrogen Energy 2024, 49, 1134–1144. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Qin, Z.; Li, Z.; Wang, G.; Dong, M.; Fan, W.; Wang, J. Feasibility, limit, and suitable reaction conditions for the production of alcohols and hydrocarbons from CO and CO2 through hydrogenation, a thermodynamic consideration. Ind. Eng. Chem. Res. 2022, 61, 17027–17038. [Google Scholar] [CrossRef]
- Nie, X.; Jiang, X.; Wang, H.; Luo, W.; Janik, M.J.; Chen, Y.; Guo, X.; Song, C. Mechanistic understanding of alloy effect and water promotion for Pd-Cu bimetallic catalysts in CO2 hydrogenation to methanol. ACS Catal. 2018, 8, 4873–4892. [Google Scholar] [CrossRef]
- Ateka, A.; Rodriguez-Vega, P.; Ereña, J.; Aguayo, A.; Bilbao, J. A review on the valorization of CO2. Focusing on the thermodynamics and catalyst design studies of the direct synthesis of dimethyl ether. Fuel Process. Technol. 2022, 233, 107310. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Z. Perspective on CO2 Hydrogenation for Dimethyl Ether Economy. Catalysts 2022, 12, 1375. [Google Scholar] [CrossRef]
- Shen, W.-J.; Jun, K.-W.; Choi, H.-S.; Lee, K.-W. Thermodynamic investigation of methanol and dimethyl ether synthesis from CO2 hydrogenation. Korean J. Chem. Eng. 2000, 17, 210–216. [Google Scholar] [CrossRef]
- Guo, S.-J.; Han, W.; Qin, Z.-F.; Li, Z.-K.; Wang, G.-F.; Mei, D.; Fan, W.-B.; Wang, J.-G. Conversion of the CO and CO2 mixture to alcohols and hydrocarbons by hydrogenation under the influence of the water-gas shift reaction, a thermodynamic consideration. J. Fuel Chem. Technol. 2023, 51, 482–491. [Google Scholar] [CrossRef]
- Sheng, Q.; Ye, R.-P.; Gong, W.; Shi, X.; Xu, B.; Argyle, M.; Adidharma, H.; Fan, M. Mechanism and catalytic performance for direct dimethyl ether synthesis by CO2 hydrogenation over CuZnZr/ferrierite hybrid catalyst. J. Environ. Sci. 2020, 92, 106–117. [Google Scholar] [CrossRef]
- Fujitani, T.; Nakamura, J. The chemical modification seen in the Cu/ZnO methanol synthesis catalysts. Appl. Catal. A Gen. 2000, 191, 111–129. [Google Scholar] [CrossRef]
- Mota, N.; Ordoñez, E.M.; Pawelec, B.; Fierro, J.L.G.; Navarro, R.M. Direct synthesis of dimethyl ether from CO2: Recent advances in bifunctional/hybrid catalytic systems. Catalysts 2021, 11, 411. [Google Scholar] [CrossRef]
- De Falco, M.; Natrella, G.; Capocelli, M.; Popielak, P.; Sołtysik, M.; Wawrzyńczak, D.; Majchrzak-Kucęba, I. Exergetic Analysis of DME Synthesis from CO2 and Renewable Hydrogen. Energies 2022, 15, 3516. [Google Scholar] [CrossRef]
- Mei, D.; Xu, L.; Henkelman, G. Dimer saddle point searches to determine the reactivity of formate on Cu(111). J. Catal. 2008, 258, 44–51. [Google Scholar] [CrossRef]
- Srivatsa, S.; Bhattacharya, S. Amine-based CO2 capture sorbents: A potential CO2 hydrogenation catalyst. J. CO2 Util. 2018, 26, 397–407. [Google Scholar] [CrossRef]
- Bonura, G.; Todaro, S.; Frusteri, L.; Majchrzak-Kucęba, I.; Wawrzyńczak, D.; Pászti, Z.; Tálas, E.; Tompos, A.; Ferenc, L.; Solt, H.; et al. Inside the reaction mechanism of direct CO2 conversion to DME over zeolite-based hybrid catalysts. Appl. Catal. B Environ. 2021, 294, 120255. [Google Scholar] [CrossRef]
- Liaw, B.J.; Chen, Y.Z. Liquid-phase synthesis of methanol from CO2/H2 over ultrafine CuB catalysts. Appl. Catal. A Gen. 2001, 206, 245–256. [Google Scholar] [CrossRef]
- An, X.; Zuo, Y.; Zhang, Q.; Wang, J. Methanol Synthesis from CO2 Hydrogenation with a Cu/Zn/Al/Zr Fibrous Catalyst. Chin. J. Chem. Eng. 2009, 17, 88–94. [Google Scholar] [CrossRef]
- Tidona, B.; Koppold, C.; Bansode, A.; Urakawa, A.; von Rohr, P.R. CO2 hydrogenation to methanol at pressures up to 950 bar. J. Supercrit. Fluids 2013, 78, 70–77. [Google Scholar] [CrossRef]
- Saravanan, K.; Ham, H.; Tsubaki, N.; Bae, J.W. Recent progress for direct synthesis of dimethyl ether from syngas on the heterogeneous bifunctional hybrid catalysts. Appl. Catal. B Environ. 2017, 217, 494–522. [Google Scholar] [CrossRef]
- Bellotti, D.; Rivarolo, M.; Magistri, L.; Massardo, A.F. Feasibility study of methanol production plant from hydrogen and captured carbon dioxide. J. CO2 Util. 2017, 21, 132–138. [Google Scholar] [CrossRef]
- Stangeland, K.; Li, H.; Yu, Z. Thermodynamic analysis of chemical and phase equilibria in CO2 hydrogenation to methanol, dimethyl ether, and higher alcohols. Ind. Eng. Chem. Res. 2018, 57, 4081–4094. [Google Scholar] [CrossRef]
- Bansode, A.; Urakawa, A. Towards full one-pass conversion of carbon dioxide to methanol and methanol-derived products. J. Catal. 2014, 309, 66–70. [Google Scholar] [CrossRef]
- Cerón, M.L.; Calatayud, M. Application of dual descriptor to understand the activity of Cu/ZrO2 catalysts in the water gas shift reaction. J. Mol. Model. 2017, 23, 1–8. [Google Scholar] [CrossRef]
- Ateka, A.; Erena, J.; Bilbao, J.; Aguayo, A.T. Kinetic modeling of the direct synthesis of dimethyl ether over a CuO-ZnO-MnO/SAPO-18 catalyst and assessment of the CO2 conversion. Fuel Process. Technol. 2018, 181, 233–243. [Google Scholar] [CrossRef]
- Yang, Y.; Mei, D.; Peden, C.H.F.; Campbell, C.T.; Mims, C.A. Surface-Bound Intermediates in Low-Temperature Methanol Synthesis on Copper: Participants and Spectators. ACS Catal. 2015, 5, 7328–7337. [Google Scholar] [CrossRef]
- Arena, F.; Italiano, G.; Barbera, K.; Bordiga, S.; Bonura, G.; Spadaro, L.; Frusteri, F. Solid-state interactions, adsorption sites and functionality of Cu-ZnO/ZrO2 catalysts in the CO2 hydrogenation to CH3OH. Appl. Catal. A Gen. 2008, 350, 16–23. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, C.-W.; Pan, W.; Zhu, Q.-M.; Deng, J.-F. In situ IR studies on the mechanism of methanol synthesis over an ultrafine Cu/ZnO/Al2O3 catalyst. Appl. Catal. A Gen. 1998, 171, 301–308. [Google Scholar] [CrossRef]
- Dong, Q.; Xu, W.L.; Fan, X.; Li, H.; Klinghoffer, N.; Pyrzynski, T.; Meyer, H.S.; Liang, X.; Yu, M.; Li, S. Prototype Catalytic Membrane Reactor for Dimethyl Ether Synthesis via CO2 Hydrogenation. Ind. Eng. Chem. Res. 2022, 61, 14656–14663. [Google Scholar] [CrossRef]
- Yue, W.; Wan, Z.; Li, Y.; He, X.; Caro, J.; Huang, A. Synthesis of Cu–ZnO–Pt@HZSM-5 catalytic membrane reactor for CO2 hydrogenation to dimethyl ether. J. Membr. Sci. 2022, 660, 120845. [Google Scholar] [CrossRef]
- Villora-Picó, J.J.; González-Arias, J.; Pastor-Pérez, L.; Odriozola, J.A.; Reina, T.R. A review on high-pressure heterogeneous catalytic processes for gas-phase CO2 valorization. Environ. Res. 2024, 240, 117520. [Google Scholar] [CrossRef]
- Rodriguez-Vega, P.; Ateka, A.; Kumakiri, I.; Vicente, H.; Erena, J.; Aguayo, A.T.; Bilbao, J. Experimental implementation of a catalytic membrane reactor for the direct synthesis of DME from H2+ CO/CO2. Chem. Eng. Sci. 2021, 234, 116396. [Google Scholar] [CrossRef]
- Saeidi, S.; Najari, S.; Hessel, V.; Wilson, K.; Keil, F.J.; Concepción, P.; Suib, S.L.; Rodrigues, A.E. Recent advances in CO2 hydrogenation to value-added products—Current challenges and future directions. Prog. Energy Combust. Sci. 2021, 85, 100905. [Google Scholar] [CrossRef]
- D’Ambrosio, A.; Bertino, A.; Todaro, S.; Santoro, M.; Cannilla, C.; Frusteri, F.; Bonura, G.; Mazzeo, L.; Piemonte, V. Kinetic Modeling of the Direct Dimethyl Ether (DME) Synthesis over Hybrid Multi-Site Catalysts. Catalysts 2024, 14, 61. [Google Scholar] [CrossRef]
- Pamungkas, W.A.; Budiman, A.W.; Inayati, I.; Margono, M.; Sembodo, B.S.T.; Mersitarini, D.; Ardyatna, D.; Mahendra, I. A Review of DME Manufacturing: Process and Catalyst Studies. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2024. [Google Scholar]
- Wang, Y.; Kattel, S.; Gao, W.; Li, K.; Liu, P.; Chen, J.G.; Wang, H. Exploring the ternary interactions in Cu–ZnO–ZrO2 catalysts for efficient CO2 hydrogenation to methanol. Nat. Commun. 2019, 10, 1166. [Google Scholar] [CrossRef]
- Tabatabaei, J.; Sakakini, B.; Waugh, K. On the mechanism of methanol synthesis and the water-gas shift reaction on ZnO. Catal. Lett. 2006, 110, 77–84. [Google Scholar] [CrossRef]
- Zhao, D.; Han, S.; Kondratenko, E.V. CO2 Hydrogenation to CH3OH over Cu-Based Catalysts: Primary and Side Reactions. ChemCatChem 2023, 15, e202300679. [Google Scholar] [CrossRef]
- Ghosh, A.; Nag, D.; Chatterjee, R.; Singha, A.; Dash, P.S.; Choudhury, B.; Bhaumik, A. CO2 to dimethyl ether (DME): Structural and functional insights of hybrid catalysts. Catal. Sci. Technol. 2024, 14, 1387–1427. [Google Scholar] [CrossRef]
- Pacchioni, G. From CO2 to Methanol on Cu/ZnO/Al2O3 Industrial Catalyst. What Do We Know about the Active Phase and the Reaction Mechanism? ACS Catal. 2024, 14, 2730–2745. [Google Scholar] [CrossRef]
- Carr, R.T.; Neurock, M.; Iglesia, E. Catalytic consequences of acid strength in the conversion of methanol to dimethyl ether. J. Catal. 2011, 278, 78–93. [Google Scholar] [CrossRef]
- Van Speybroeck, V.; Van der Mynsbrugge, J.; Vandichel, M.; Hemelsoet, K.; Lesthaeghe, D.; Ghysels, A.; Marin, G.B.; Waroquier, M. First principle kinetic studies of zeolite-catalyzed methylation reactions. J. Am. Chem. Soc. 2011, 133, 888–899. [Google Scholar] [CrossRef]
- Blaszkowski, S.R.; van Santen, R.A. Theoretical study of the mechanism of surface methoxy and dimethyl ether formation from methanol catalyzed by zeolitic protons. J. Phys. Chem. B 1997, 101, 2292–2305. [Google Scholar] [CrossRef]
- Jones, A.J.; Iglesia, E. Kinetic, spectroscopic, and theoretical assessment of associative and dissociative methanol dehydration routes in zeolites. Angew. Chem. 2014, 126, 12373–12377. [Google Scholar] [CrossRef]
- Ghorbanpour, A.; Rimer, J.D.; Grabow, L.C. Computational assessment of the dominant factors governing the mechanism of methanol dehydration over H-ZSM-5 with heterogeneous aluminum distribution. Acs Catal. 2016, 6, 2287–2298. [Google Scholar] [CrossRef]
- Kramer, R.; Andre, M. Adsorption of atomic hydrogen on alumina by hydrogen spillover. J. Catal. 1979, 58, 287–295. [Google Scholar] [CrossRef]
- Kirchberger, F.M.; Liu, Y.; Plessow, P.N.; Tonigold, M.; Studt, F.; Sanchez-Sanchez, M.; Lercher, J.A. Mechanistic differences between methanol and dimethyl ether in zeolite-catalyzed hydrocarbon synthesis. Proc. Natl. Acad. Sci. USA 2022, 119, e2103840119. [Google Scholar] [CrossRef]
- Grabow, L.C.; Mavrikakis, M. Mechanism of methanol synthesis on cu through CO2 and CO hydrogenation. ACS Catal. 2011, 1, 365–384. [Google Scholar] [CrossRef]
- Arena, F.; Mezzatesta, G.; Spadaro, L.; Trunfio, G. Latest advances in the catalytic hydrogenation of carbon dioxide to methanol/dimethylether. In Transformation and Utilization of Carbon Dioxide; Springer: Berlin/Heidelberg, Germany, 2014; pp. 103–130. [Google Scholar]
- Fujita, S.-i.; Kanamori, Y.; Satriyo, A.M.; Takezawa, N. Methanol synthesis from CO2 over Cu/ZnO catalysts prepared from various coprecipitated precursors. Catal. Today 1998, 45, 241–244. [Google Scholar] [CrossRef]
- Behrens, M.; Studt, F.; Kasatkin, I.; Kühl, S.; Hävecker, M.; Abild-Pedersen, F.; Zander, S.; Girgsdies, F.; Kurr, P.; Kniep, B.-L. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 2012, 336, 893–897. [Google Scholar] [CrossRef]
- Mao, D.; Guo, X. Dimethyl Ether Synthesis from Syngas over the Admixed Cu/ZnO/Al2O3 Catalyst and Alkaline Earth Oxide-Modified HZSM-5 Zeolite. Energy Technol. 2014, 2, 882–888. [Google Scholar] [CrossRef]
- Khoobiar, S. Particle to particle migration of hydrogen atoms on platinum—Alumina catalysts from particle to neighboring particles. J. Phys. Chem. 1964, 68, 411–412. [Google Scholar] [CrossRef]
- Prins, R. Hydrogen spillover. Facts and fiction. Chem. Rev. 2012, 112, 2714–2738. [Google Scholar] [CrossRef]
- Prins, R.; Pálfi, V.K.; Reiher, M. Hydrogen spillover to nonreducible supports. J. Phys. Chem. C 2012, 116, 14274–14283. [Google Scholar] [CrossRef]
- Rozanov, V.V.; Krylov, O.V. Hydrogen spillover in heterogeneous catalysis. Russ. Chem. Rev. 1997, 66, 107. [Google Scholar] [CrossRef]
- Yodsin, N.; Rungnim, C.; Tungkamani, S.; Promarak, V.; Namuangruk, S.; Jungsuttiwong, S. DFT study of catalytic CO2 hydrogenation over Pt-decorated carbon nanocones: H2 dissociation combined with the spillover mechanism. J. Phys. Chem. C 2019, 124, 1941–1949. [Google Scholar] [CrossRef]
- Mori, K.; Hashimoto, N.; Kamiuchi, N.; Yoshida, H.; Kobayashi, H.; Yamashita, H. Hydrogen spillover-driven synthesis of high-entropy alloy nanoparticles as a robust catalyst for CO2 hydrogenation. Nat. Commun. 2021, 12, 3884. [Google Scholar] [CrossRef]
- Hu, B.; Yin, Y.; Liu, G.; Chen, S.; Hong, X.; Tsang, S.C.E. Hydrogen spillover enabled active Cu sites for methanol synthesis from CO2 hydrogenation over Pd doped CuZn catalysts. J. Catal. 2018, 359, 17–26. [Google Scholar] [CrossRef]
- Xu, D.; Hong, X.; Liu, G. Highly dispersed metal doping to ZnZr oxide catalyst for CO2 hydrogenation to methanol: Insight into hydrogen spillover. J. Catal. 2021, 393, 207–214. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, C.; Sun, K.; Liu, C.-J. Improvement in the activity of Ni/In2O3 with the addition of ZrO2 for CO2 hydrogenation to methanol. Catal. Commun. 2022, 162, 106386. [Google Scholar] [CrossRef]
- Wang, X.; Alabsi, M.H.; Zheng, P.; Mei, J.; Ramirez, A.; Duan, A.; Xu, C.; Huang, K.-W. PdCu supported on dendritic mesoporous CexZr1-xO2 as superior catalysts to boost CO2 hydrogenation to methanol. J. Colloid Interface Sci. 2022, 611, 739–751. [Google Scholar] [CrossRef]
- Wang, C. Catalytic Conversion of Short-Chain Alcohols on Atomically Dispersed Au and Pd Supported on Nanoscale Metal Oxides; Tufts University: Medford, MA, USA, 2016. [Google Scholar]
- Fujita, S.-I.; Usui, M.; Ito, H.; Takezawa, N. Mechanisms of methanol synthesis from carbon dioxide and from carbon monoxide at atmospheric pressure over Cu/ZnO. J. Catal. 1995, 157, 403–413. [Google Scholar] [CrossRef]
- Xaba, B.S.; Mahomed, A.S.; Friedrich, H.B. The effect of CO2 and H2 adsorption strength and capacity on the performance of Ga and Zr modified Cu-Zn catalysts for CO2 hydrogenation to methanol. J. Environ. Chem. Eng. 2021, 9, 104834. [Google Scholar] [CrossRef]
- Somorjai, G.A.; Li, Y. Introduction to Surface Chemistry and Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Cai, M.; Palčić, A.; Subramanian, V.; Moldovan, S.; Ersen, O.; Valtchev, V.; Ordomsky, V.V.; Khodakov, A.Y. Direct dimethyl ether synthesis from syngas on copper-zeolite hybrid catalysts with a wide range of zeolite particle sizes Dedicated to Professor Jean-Pierre Gilson on the occasion of his 60th birthday. J. Catal. 2016, 338, 227–238. [Google Scholar] [CrossRef]
- Wender, I. Reactions of synthesis gas. Fuel Process. Technol. 1996, 48, 189–297. [Google Scholar] [CrossRef]
- Liu, X.-M.; Lu, G.Q.; Yan, Z.-F.; Beltramini, J. Recent advances in catalysts for methanol synthesis via hydrogenation of CO and CO2. Ind. Eng. Chem. Res. 2003, 42, 6518–6530. [Google Scholar] [CrossRef]
- Toyir, J.; de la Piscina, P.R.r.; Fierro, J.L.G.; Homs, N.s. Highly effective conversion of CO2 to methanol over supported and promoted copper-based catalysts: Influence of support and promoter. Appl. Catal. B Environ. 2001, 29, 207–215. [Google Scholar] [CrossRef]
- Lee, S. Methanol Synthesis Technology; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Iloy, R.A.; Jalama, K.; Khangale, P.R. Effect of a Second Promoter on the Performance of a Potassium Doped Silica-Supported Cobalt Catalyst During CO2 Hydrogenation to Hydrocarbons. Catal. Lett. 2024, 154, 2818–2828. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, H.; Gao, P.; Zhu, H.; Zhong, L.; Wang, H.; Wei, W.; Sun, Y. Preparation and CO2 hydrogenation catalytic properties of alumina microsphere supported Cu-based catalyst by deposition-precipitation method. J. CO2 Util. 2017, 17, 263–272. [Google Scholar] [CrossRef]
- Ren, H.; Xu, C.H.; Zhao, H.Y.; Wang, Y.X.; Liu, J.; Liu, J.Y. Methanol synthesis from CO2 hydrogenation over Cu/γ-Al2O3 catalysts modified by ZnO, ZrO2 and MgO. J. Ind. Eng. Chem. 2015, 28, 261–267. [Google Scholar] [CrossRef]
- Shi, Z.; Tan, Q.; Tian, C.; Pan, Y.; Sun, X.; Zhang, J.; Wu, D. CO2 hydrogenation to methanol over Cu-In intermetallic catalysts: Effect of reduction temperature. J. Catal. 2019, 379, 78–89. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, A.; Jiang, X.; Liu, M.; Sun, Y.; Song, C.; Guo, X. Selective CO2 Hydrogenation to Hydrocarbons on Cu-Promoted Fe-Based Catalysts: Dependence on Cu-Fe Interaction. ACS Sustain. Chem. Eng. 2018, 6, 10182–10190. [Google Scholar] [CrossRef]
- Álvarez Galván, C.; Schumann, J.; Behrens, M.; Fierro, J.L.G.; Schlögl, R.; Frei, E. Reverse water-gas shift reaction at the Cu/ZnO interface: Influence of the Cu/Zn ratio on structure-activity correlations. Appl. Catal. B Environ. 2016, 195, 104–111. [Google Scholar] [CrossRef]
- Huang, M.H.; Lee, H.M.; Liang, K.C.; Tzeng, C.C.; Chen, W.H. An experimental study on single-step dimethyl ether (DME) synthesis from hydrogen and carbon monoxide under various catalysts. Int. J. Hydrogen Energy 2015, 40, 13583–13593. [Google Scholar] [CrossRef]
- Godini, H.R.; Kumar, S.R.; Tadikamalla, N.; Gallucci, F. Performance analysis of hybrid catalytic conversion of CO2 to DiMethyl ether. Int. J. Hydrogen Energy 2022, 47, 11341–11358. [Google Scholar] [CrossRef]
- Jiang, H.; Bongard, H.; Schmidt, W.; Schüth, F. One-pot synthesis of mesoporous Cu-γ-Al2O3 as bifunctional catalyst for direct dimethyl ether synthesis. Microporous Mesoporous Mater. 2012, 164, 3–8. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Jiang, G.; Liu, H.; Yang, J.; Li, Y. Continuous supercritical hydrothermal synthesis of stabilized ZrO2 nanocomposites: Doping mechanism of typical metals and transition elements. Mater. Today Chem. 2024, 35, 101902. [Google Scholar] [CrossRef]
- Ham, H.W.; Jeong, M.H.; Koo, H.M.; Chung, C.H.; Bae, J.W. The role of the acidity of alumina prepared by aluminum-carbon black composite for CO hydrogenation to dimethyl ether on hybrid Cu-ZnO-Al2O3/alumina. React. Kinet. Mech. Catal. 2015, 116, 173–189. [Google Scholar] [CrossRef]
- Din, I.U.; Alotaibi, M.A.; Alharthi, A.I. Green synthesis of methanol over zeolite based Cu nano-catalysts, effect of Mg promoter. Sustain. Chem. Pharm. 2020, 16, 100264. [Google Scholar] [CrossRef]
- Tian, G.; Wu, Y.; Wu, S.; Huang, S.; Gao, J. Solid-State Synthesis of Pd/In2O3 Catalysts for CO2 Hydrogenation to Methanol. Catal. Lett. 2023, 153, 903–910. [Google Scholar] [CrossRef]
- Corral-Pérez, J.J.; Copéret, C.; Urakawa, A. Lewis acidic supports promote the selective hydrogenation of carbon dioxide to methyl formate in the presence of methanol over Ag catalysts. J. Catal. 2019, 380, 153–160. [Google Scholar] [CrossRef]
- Sápi, A.; Rajkumar, T.; Ábel, M.; Efremova, A.; Grósz, A.; Gyuris, A.; Ábrahámné, K.B.; Szenti, I.; Kiss, J.; Varga, T. Noble-metal-free and Pt nanoparticles-loaded, mesoporous oxides as efficient catalysts for CO2 hydrogenation and dry reforming with methane. J. CO2 Util. 2019, 32, 106–118. [Google Scholar] [CrossRef]
- Shen, C.; Sun, K.; Zhang, Z.; Rui, N.; Jia, X.; Mei, D.; Liu, C.-J. Highly active Ir/In2O3 catalysts for selective hydrogenation of CO2 to methanol: Experimental and theoretical studies. Acs Catal. 2021, 11, 4036–4046. [Google Scholar] [CrossRef]
- Vieira, L.H.; Rasteiro, L.F.; Santana, C.S.; Catuzo, G.L.; da Silva, A.H.; Assaf, J.M.; Assaf, E.M. Noble Metals in Recent Developments of Heterogeneous Catalysts for CO2 Conversion Processes. ChemCatChem 2023, 15, e202300493. [Google Scholar] [CrossRef]
- Jia, G.X.; Ma, H.B.; Tan, Y.S.; Han, Y.Z. Effect of particle size on the hybrid catalyst activity for slurry phase dimethyl ether synthesis. Ind. Eng. Chem. Res. 2005, 44, 2011–2015. [Google Scholar] [CrossRef]
- García-Trenco, A.; Regoutz, A.; White, E.R.; Payne, D.J.; Shaffer, M.S.P.; Williams, C.K. PdIn intermetallic nanoparticles for the Hydrogenation of CO2 to Methanol. Appl. Catal. B Environ. 2018, 220, 9–18. [Google Scholar] [CrossRef]
- Fan, L.; Fujimoto, K. Promotive SMSI Effect for Hydrogenation of Carbon Dioxide to Methanol on a Pd/CeO2 Catalyst. J. Catal. 1994, 150, 217–220. [Google Scholar] [CrossRef]
- Saito, M. R&D activities in Japan on methanol synthesis from CO2 and H2. Catal. Surv. Asia 1998, 2, 175–184. [Google Scholar]
- Alotaibi, M.A.; Din, I.U.; Alharthi, A.I.; Bakht, M.A.; Centi, G.; Shaharun, M.S.; Naeem, A. Green methanol synthesis by catalytic CO2 hydrogenation, deciphering the role of metal-metal interaction. Sustain. Chem. Pharm. 2021, 21, 100420. [Google Scholar] [CrossRef]
- Köppel, R.A.; Stöcker, C.; Baiker, A. Copper- and Silver–Zirconia Aerogels: Preparation, Structural Properties and Catalytic Behavior in Methanol Synthesis from Carbon Dioxide. J. Catal. 1998, 179, 515–527. [Google Scholar] [CrossRef]
- Grabowski, R.; Słoczyński, J.; Śliwa, M.; Mucha, D.; Socha, R.P.; Lachowska, M.; Skrzypek, J. Influence of Polymorphic ZrO2 Phases and the Silver Electronic State on the Activity of Ag/ZrO2 Catalysts in the Hydrogenation of CO2 to Methanol. ACS Catal. 2011, 1, 266–278. [Google Scholar] [CrossRef]
- Ren, S.; Fan, X.; Shang, Z.; Shoemaker, W.R.; Ma, L.; Wu, T.; Li, S.; Klinghoffer, N.B.; Yu, M.; Liang, X. Enhanced catalytic performance of Zr modified CuO/ZnO/Al2O3 catalyst for methanol and DME synthesis via CO2 hydrogenation. J. CO2 Util. 2020, 36, 82–95. [Google Scholar] [CrossRef]
- Li, M.M.J.; Zeng, Z.; Liao, F.; Hong, X.; Tsang, S.C.E. Enhanced CO2 hydrogenation to methanol over CuZn nanoalloy in Ga modified Cu/ZnO catalysts. J. Catal. 2016, 343, 157–167. [Google Scholar] [CrossRef]
- Ihm, S.-K.; Baek, S.-W.; Park, Y.-K.; Jeon, J.-K. CO2 Hydrogenation over Copper-Based Hybrid Catalysts for the Synthesis of Oxygenates; ACS Publications: Washington, DC, USA, 2003. [Google Scholar]
- Sun, K.; Zhang, Z.; Shen, C.; Rui, N.; Liu, C.-j. The feasibility study of the indium oxide supported silver catalyst for selective hydrogenation of CO2 to methanol. Green Energy Environ. 2022, 7, 807–817. [Google Scholar] [CrossRef]
- Tao, J.L.; Jun, K.W.; Lee, K.W. Co-production of dimethyl ether and methanol from CO2 hydrogenation: Development of a stable hybrid catalyst. Appl. Organomet. Chem. 2001, 15, 105–108. [Google Scholar] [CrossRef]
- Kornas, A.; Grabowski, R.; Śliwa, M.; Samson, K.; Ruggiero-Mikołajczyk, M.; Żelazny, A. Dimethyl ether synthesis from CO2 hydrogenation over hybrid catalysts: Effects of preparation methods. React. Kinet. Mech. Catal. 2017, 121, 317–327. [Google Scholar] [CrossRef]
- Wang, S.; Mao, D.; Guo, X.; Wu, G.; Lu, G. Dimethyl ether synthesis via CO2 hydrogenation over CuO-TiO2-ZrO2/HZSM-5 bifunctional catalysts. Catal. Commun. 2009, 10, 1367–1370. [Google Scholar] [CrossRef]
- Liu, R.-W.; Qin, Z.-Z.; Ji, H.-B.; Su, T.-M. Synthesis of dimethyl ether from CO2 and H2 using a Cu–Fe–Zr/HZSM-5 catalyst system. Ind. Eng. Chem. Res. 2013, 52, 16648–16655. [Google Scholar] [CrossRef]
- Qin, Z.Z.; Su, T.M.; Ji, H.B.; Jiang, Y.X.; Liu, R.W.; Chen, J.H. Experimental and theoretical study of the intrinsic kinetics for dimethyl ether synthesis from CO2 over Cu–Fe–Zr/HZSM-5. AIChE J. 2015, 61, 1613–1627. [Google Scholar] [CrossRef]
- Qin, Z.-Z.; Zhou, X.-H.; Su, T.-M.; Jiang, Y.-X.; Ji, H.-B. Hydrogenation of CO2 to dimethyl ether on La-, Ce-modified Cu-Fe/HZSM-5 catalysts. Catal. Commun. 2016, 75, 78–82. [Google Scholar] [CrossRef]
- Singh, R.; Tripathi, K.; Pant, K.K.; Parikh, J.K. Unravelling synergetic interaction over tandem Cu-ZnO-ZrO2/hierarchical ZSM5 catalyst for CO2 hydrogenation to methanol and DME. Fuel 2022, 318, 123641. [Google Scholar] [CrossRef]
- Fang, X.; Jia, H.; Zhang, B.; Li, Y.; Wang, Y.; Song, Y.; Du, T.; Liu, L. A novel in situ grown Cu-ZnO-ZrO2/HZSM-5 hybrid catalyst for CO2 hydrogenation to liquid fuels of methanol and DME. J. Environ. Chem. Eng. 2021, 9, 105299. [Google Scholar] [CrossRef]
- Frusteri, F.; Migliori, M.; Cannilla, C.; Frusteri, L.; Catizzone, E.; Aloise, A.; Giordano, G.; Bonura, G. Direct CO2-to-DME hydrogenation reaction: New evidences of a superior behaviour of FER-based hybrid systems to obtain high DME yield. J. CO2 Util. 2017, 18, 353–361. [Google Scholar] [CrossRef]
- Venugopal, A.; Palgunadi, J.; Deog, J.K.; Joo, O.-S.; Shin, C.-H. Dimethyl ether synthesis on the admixed catalysts of Cu-Zn-Al-M (M = Ga, La, Y, Zr) and γ-Al2O3: The role of modifier. J. Mol. Catal. A Chem. 2009, 302, 20–27. [Google Scholar] [CrossRef]
- Palgunadi, J.; Yati, I.; Jung, K. Catalytic activity of Cu–Zn–Al–Mn admixed with gamma-alumina for the synthesis of DME from syngas: Manganese effect or just method of preparation? React. Kinet. Mech. Catal. 2010, 101, 117–128. [Google Scholar] [CrossRef]
- Zhou, X.; Su, T.; Jiang, Y.; Qin, Z.; Ji, H.; Guo, Z. CuO-Fe2O3-CeO2/HZSM-5 bifunctional catalyst hydrogenated CO2 for enhanced dimethyl ether synthesis. Chem. Eng. Sci. 2016, 153, 10–20. [Google Scholar] [CrossRef]
- Xiao, J.; Mao, D.; Guo, X.; Yu, J. Methanol Synthesis from CO2 Hydrogenation over CuO-ZnO-TiO2 Catalysts: The Influence of TiO2 Content. Energy Technol. 2015, 3, 32–39. [Google Scholar] [CrossRef]
- Ateka, A.; Ereña, J.; Pérez-Uriarte, P.; Aguayo, A.T.; Bilbao, J. Effect of the content of CO2 and H2 in the feed on the conversion of CO2 in the direct synthesis of dimethyl ether over a CuOZnOAl2O3/SAPO-18 catalyst. Int. J. Hydrogen Energy 2017, 42, 27130–27138. [Google Scholar] [CrossRef]
- Xia, S.; Gong, J.; Yin, J.; Zhao, Z.; Tang, F.; Guo, X.; Liu, P. Effects of precursor phase distribution on the performance of Cu-based catalysts for direct CO2 conversion to dimethyl ether. J. Energy Inst. 2023, 109, 101302. [Google Scholar] [CrossRef]
- Navarro-Jaén, S.; Virginie, M.; Thuriot-Roukos, J.; Wojcieszak, R.; Khodakov, A.Y. Structure–performance correlations in the hybrid oxide-supported copper–zinc SAPO-34 catalysts for direct synthesis of dimethyl ether from CO2. J. Mater. Sci. 2022, 57, 3268–3279. [Google Scholar] [CrossRef]
- Mondal, U.; Yadav, G.D. Direct synthesis of dimethyl ether from CO2 hydrogenation over a highly active, selective and stable catalyst containing Cu–ZnO–Al2 O3/Al–Zr (1:1)-SBA-15. React. Chem. Eng. 2022, 7, 1391–1408. [Google Scholar] [CrossRef]
- Cara, C.; Secci, F.; Lai, S.; Mameli, V.; Skrodczky, K.; Russo, P.A.; Ferrara, F.; Rombi, E.; Pinna, N.; Mureddu, M. On the design of mesostructured acidic catalysts for the one-pot dimethyl ether production from CO2. J. CO2 Util. 2022, 62, 102066. [Google Scholar] [CrossRef]
- Asthana, S.; Samanta, C.; Saha, B.; Voolapalli, R.K.; Pant, K.K. Steering the Aspects of MgO-Induced Structure Sensitivity in Cu-Based Catalysts for CO2-Rich Syngas Conversion to Dimethyl Ether: Cu/Zn Ratio and Lattice Parameters. Energy Fuels 2022, 36, 2673–2687. [Google Scholar] [CrossRef]
- Li, L.; Mao, D.; Xiao, J.; Li, L.; Guo, X.; Yu, J. Facile preparation of highly efficient CuO-ZnO-ZrO2/HZSM-5 bifunctional catalyst for one-step CO2 hydrogenation to dimethyl ether: Influence of calcination temperature. Chem. Eng. Res. Des. 2016, 111, 100–108. [Google Scholar] [CrossRef]
- Lin, K.-S.; Hussain, A.; Mdlovu, N.V.; Wang, H.P. Hydrogenation of CO2 to Dimethyl Ether Over Wox-Zro2/Cu-Zno-Zro2 Catalyst S. SSRN 4230053. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4230053 (accessed on 22 September 2022).
- Sun, K.; Lu, W.; Wang, M.; Xu, X. Low-temperature synthesis of DME from CO2/H2 over Pd-modified CuO-ZnO-Al2O3-ZrO2/HZSM-5 catalysts. Catal. Commun. 2004, 5, 367–370. [Google Scholar] [CrossRef]
- Bonura, G.; Cannilla, C.; Frusteri, L.; Mezzapica, A.; Frusteri, F. DME production by CO2 hydrogenation: Key factors affecting the behaviour of CuZnZr/ferrierite catalysts. Catal. Today 2017, 281, 337–344. [Google Scholar] [CrossRef]
- Frusteri, F.; Bonura, G.; Cannilla, C.; Drago Ferrante, G.; Aloise, A.; Catizzone, E.; Migliori, M.; Giordano, G. Stepwise tuning of metal-oxide and acid sites of CuZnZr-MFI hybrid catalysts for the direct DME synthesis by CO2 hydrogenation. Appl. Catal. B Environ. 2015, 176–177, 522–531. [Google Scholar] [CrossRef]
- Wild, S.; Polierer, S.; Zevaco, T.A.; Guse, D.; Kind, M.; Pitter, S.; Delgado, K.H.; Sauer, J. Direct DME synthesis on CZZ/H-FER from variable CO2/CO syngas feeds. RSC Adv. 2021, 11, 2556–2564. [Google Scholar] [CrossRef]
- Wengui, G.; Hua, W.; Yuhao, W.; Wei, G.; Miaoyao, J. Dimethyl ether synthesis from CO2 hydrogenation on La-modified CuO-ZnO-Al2O3/HZSM-5 bifunctional catalysts. J. Rare Earths 2013, 31, 470–476. [Google Scholar]
- Stiefel, M.; Ahmad, R.; Arnold, U.; Döring, M. Direct synthesis of dimethyl ether from carbon-monoxide-rich synthesis gas: Influence of dehydration catalysts and operating conditions. Fuel Process. Technol. 2011, 92, 1466–1474. [Google Scholar] [CrossRef]
- Qi, G.-X.; Fei, J.-H.; Zheng, X.-M.; Hou, Z.-Y. DME synthesis from carbon dioxide and hydrogen over Cu–Mo/HZSM-5. Catal. Lett. 2001, 72, 121–124. [Google Scholar] [CrossRef]
- Jun, K.-W.; Jung, M.-H.; Rao, K.R.; Choi, M.-J.; Lee, K.-W. Effective conversion of CO2 to methanol and dimethyl ether over hybrid catalysts. Stud. Surf. Sci. Catal 1998, 114, 447. [Google Scholar]
- Naik, S.P.; Bui, V.; Ryu, T.; Miller, J.D.; Zmierczak, W. Al-MCM-41 as methanol dehydration catalyst. Appl. Catal. A Gen. 2010, 381, 183–190. [Google Scholar] [CrossRef]
- Bahruji, H.; Abdul Razak, S.; Mahadi, A.H.; Prasetyoko, D.; Sholehah, N.A.; Jiao, Y. PdZn on ZSM-5 nanoparticles for CO2 hydrogenation to dimethyl ether: Comparative in situ analysis with Pd/TiO2 and PdZn/TiO2. React. Kinet. Mech. Catal. 2022, 135, 2973–2991. [Google Scholar] [CrossRef]
- Tan, K.B.; Tian, P.; Zhang, X.; Tian, J.; Zhan, G.; Huang, J.; Li, Q. Green synthesis of microspherical-confined nano-Pd/In2O3 integrated with H-ZSM-5 as bifunctional catalyst for CO2 hydrogenation into dimethyl ether: A carbonized alginate templating strategy. Sep. Purif. Technol. 2022, 297, 121559. [Google Scholar] [CrossRef]
- Baltes, C.; Vukojević, S.; Schüth, F. Correlations between synthesis, precursor, and catalyst structure and activity of a large set of CuO/ZnO/Al2O3 catalysts for methanol synthesis. J. Catal. 2008, 258, 334–344. [Google Scholar] [CrossRef]
- Wang, K.; Li, S.; Yu, M.; Liang, X. Enhancing DMC Production from CO2: Tuning Oxygen Vacancies and In Situ Water Removal. Energies 2024, 17, 839. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [PubMed]
- Bonura, G.; Migliori, M.; Frusteri, L.; Cannilla, C.; Catizzone, E.; Giordano, G.; Frusteri, F. Acidity control of zeolite functionality on activity and stability of hybrid catalysts during DME production via CO2 hydrogenation. J. CO2 Util. 2018, 24, 398–406. [Google Scholar] [CrossRef]
- Wen, Y.; Zhou, C.; Yu, L.; Zhang, Q.; He, W.; Liu, Q. Research Progress on the Effects of Support and Support Modification on the FTO Reaction Performance of Fe-Based Catalysts. Molecules 2023, 28, 7749. [Google Scholar] [CrossRef] [PubMed]
- Bui, S.; Tran, T.; Choi, H.; Oh, S.; Park, J.Y. Influence of Support Acidity of Pt/Nb2O5 Catalysts on Selectivity of—CO2 Hydrogenation. Catal. Lett. 2019, 149, 2823–2835. [Google Scholar]
- Niwa, M.; Katada, N.; Okumura, K. Characterization and Design of Zeolite Catalysts: Solid Acidity, Shape Selectivity and Loading Properties; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010; Volume 141. [Google Scholar]
- Aho, A.; Kumar, N.; Eränen, K.; Salmi, T.; Hupa, M.; Murzin, D.Y. Catalytic pyrolysis of woody biomass in a fluidized bed reactor: Influence of the zeolite structure. Fuel 2008, 87, 2493–2501. [Google Scholar] [CrossRef]
- Rhodes, C.J. Zeolites: Physical aspects and environmental applications. Annu. Rep. Sect. C Phys. Chem. 2007, 103, 287–325. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Yu, J. Recent advances in zeolite chemistry and catalysis. Chem. Soc. Rev. 2015, 44, 7022–7024. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Park, M.J.; Kim, S.J.; Joo, O.-S.; Jung, K.-D. DME synthesis from synthesis gas on the admixed catalysts of Cu/ZnO/Al2O3 and ZSM-5. Appl. Catal. A Gen. 2004, 264, 37–41. [Google Scholar] [CrossRef]
- Mao, D.; Xia, J.; Chen, Q.; Lu, G. Highly effective conversion of syngas to dimethyl ether over the hybrid catalysts containing high-silica HMCM-22 zeolites. Catal. Commun. 2009, 10, 620–624. [Google Scholar]
- Mao, D.; Yang, W.; Xia, J.; Zhang, B.; Song, Q.; Chen, Q. Highly effective hybrid catalyst for the direct synthesis of dimethyl ether from syngas with magnesium oxide-modified HZSM-5 as a dehydration component. J. Catal. 2005, 230, 140–149. [Google Scholar] [CrossRef]
- Xu, Q.L.; Lan, P.; Zhang, S.P.; Li, T.C.; Yan, Y.J. Effect of modified zeolite on one-step process of DME synthesis. Pet. Sci. Technol. 2011, 29, 439–448. [Google Scholar] [CrossRef]
- Zheng, H.; Narkhede, N.; Han, L.; Zhang, H.; Li, Z. Methanol synthesis from CO2: A DFT investigation on Zn-promoted Cu catalyst. Res. Chem. Intermed. 2020, 46, 1749–1769. [Google Scholar] [CrossRef]
- Mao, D.; Xia, J.; Zhang, B.; Lu, G. Highly efficient synthesis of dimethyl ether from syngas over the admixed catalyst of CuO–ZnO–Al2O3 and antimony oxide modified HZSM-5 zeolite. Energy Convers. Manag. 2010, 51, 1134–1139. [Google Scholar] [CrossRef]
- Cored, J.; Lopes, C.W.; Liu, L.; Soriano, J.; Agostini, G.; Solsona, B.; Sánchez-Tovar, R.; Concepción, P. Cu-Ga3+-doped wurtzite ZnO interface as driving force for enhanced methanol production in co-precipitated Cu/ZnO/Ga2O3 catalysts. J. Catal. 2022, 407, 149–161. [Google Scholar] [CrossRef]
- Kang, S.-H.; Bae, J.W.; Jun, K.-W.; Potdar, H.S. Dimethyl ether synthesis from syngas over the composite catalysts of Cu–ZnO–Al2O3/Zr-modified zeolites. Catal. Commun. 2008, 9, 2035–2039. [Google Scholar] [CrossRef]
- Xia, J.; Mao, D.; Zhang, B.; Chen, Q.; Tang, Y. One-step synthesis of dimethyl ether from syngas with Fe-modified zeolite ZSM-5 as dehydration catalyst. Catal. Lett. 2004, 98, 235–240. [Google Scholar] [CrossRef]
- Bonura, G.; Frusteri, F.; Cannilla, C.; Drago Ferrante, G.; Aloise, A.; Catizzone, E.; Migliori, M.; Giordano, G. Catalytic features of CuZnZr–zeolite hybrid systems for the direct CO2-to-DME hydrogenation reaction. Catal. Today 2016, 277, 48–54. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.L.; Chen, Y.X.; Zheng, J.J.; Li, R.F. Synthesis of dimethyl ether from syngas using a hierarchically porous composite zeolite as the methanol dehydration catalyst. Ranliao Huaxue Xuebao/J. Fuel Chem. Technol. 2013, 41, 875–882. [Google Scholar] [CrossRef]
- Ennaert, T.; Van Aelst, J.; Dijkmans, J.; De Clercq, R.; Schutyser, W.; Dusselier, M.; Verboekend, D.; Sels, B.F. Potential and challenges of zeolite chemistry in the catalytic conversion of biomass. Chem. Soc. Rev. 2016, 45, 584–611. [Google Scholar] [CrossRef] [PubMed]
- García-Trenco, A.; Valencia, S.; Martínez, A. The impact of zeolite pore structure on the catalytic behavior of CuZnAl/zeolite hybrid catalysts for the direct DME synthesis. Appl. Catal. A Gen. 2013, 468, 102–111. [Google Scholar] [CrossRef]
- Carvalho, D.F.; Almeida, G.C.; Monteiro, R.S.; Mota, C.J. Hydrogenation of CO2 to methanol and dimethyl ether over a bifunctional Cu·ZnO catalyst impregnated on modified γ-alumina. Energy Fuels 2020, 34, 7269–7274. [Google Scholar] [CrossRef]
- Sung, D.M.; Kim, Y.H.; Park, E.D.; Yie, J.E. Correlation between acidity and catalytic activity for the methanol dehydration over various aluminum oxides. Res. Chem. Intermed. 2010, 36, 653–660. [Google Scholar] [CrossRef]
- Moradi, G.R.; Nosrati, S.; Yaripor, F. Effect of the hybrid catalysts preparation method upon direct synthesis of dimethyl ether from synthesis gas. Catal. Commun. 2007, 8, 598–606. [Google Scholar] [CrossRef]
- Pechenkin, A.; Potemkin, D.; Badmaev, S.; Smirnova, E.; Cherednichenko, K.; Vinokurov, V.; Glotov, A. CO2 hydrogenation to dimethyl ether over In2O3 catalysts supported on aluminosilicate halloysite nanotubes. Green Process. Synth. 2021, 10, 594–605. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Advances in Catalysts and Processes for Methanol Synthesis from CO2; Springer: Berlin/Heidelberg, Germany, 2013; pp. 147–169. [Google Scholar]
- Xia, J.; Mao, D.; Zhang, B.; Chen, Q.; Zhang, Y.; Tang, Y. Catalytic properties of fluorinated alumina for the production of dimethyl ether. Catal. Commun. 2006, 7, 362–366. [Google Scholar] [CrossRef]
- Mao, D.; Yang, W.; Xia, J.; Zhang, B.; Lu, G. The direct synthesis of dimethyl ether from syngas over hybrid catalysts with sulfate-modified γ-alumina as methanol dehydration components. J. Mol. Catal. A Chem. 2006, 250, 138–144. [Google Scholar] [CrossRef]
- Montesano, R.; Chadwick, D. Combined methanol and dimethyl ether synthesis from CO/H2: Phosphorus mediated deactivation. Catal. Commun. 2012, 29, 137–140. [Google Scholar] [CrossRef]
- Takeguchi, T.; Yanagisawa, K.-I.; Inui, T.; Inoue, M. Effect of the property of solid acid upon syngas-to-dimethyl ether conversion on the hybrid catalysts composed of Cu–Zn–Ga and solid acids. Appl. Catal. A Gen. 2000, 192, 201–209. [Google Scholar] [CrossRef]
- Joo, O.-S.; Jung, K.-D.; Han, S.-H. Modification of H-ZSM-5 and γ-alumina with formaldehyde and its application to the synthesis of dimethyl ether from syn-gas. Bull. Korean Chem. Soc. 2002, 23, 1103–1105. [Google Scholar]
- Biswas, S.; Kundu, C.; Ng, W.L.; Samudrala, S.P.; Jarvis, T.; Giddey, S.; Bhattacharya, S. CO2 valorisation to methane on highly stable iron impregnated ceria-zirconia based 3D-printed catalyst. J. CO2 Util. 2023, 22, 102501. [Google Scholar] [CrossRef]
- Li, Y.; Luo, C.; Su, Q. Performance of Cu/ZnO/Al2O3 Catalysts Prepared by Sol–Gel Methods on Methanol Steam Reforming. Energies 2023, 16, 7803. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, P.; Chao, Y.; Yu, J.; Zhu, W.; Liu, Z.; Xu, C. Recent advances in 3D printing for catalytic applications. Chem. Eng. J. 2021, 433, 134341. [Google Scholar] [CrossRef]
- Bogdan, E.; Michorczyk, P. 3D Printing in heterogeneous catalysis—The state of the art. Materials 2020, 13, 4534. [Google Scholar] [CrossRef]
- Ye, R.-P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 5698. [Google Scholar] [CrossRef]
- Ruiz-Morales, J.; Tarancón, A.; Canales-Vázquez, J.; Méndez-Ramos, J.; Hernández-Afonso, L.; Acosta-Mora, P.; Rueda, J.M.; Fernández-González, R. Three dimensional printing of components and functional devices for energy and environmental applications. Energy Environ. Sci. 2017, 10, 846–859. [Google Scholar] [CrossRef]
- Magzoub, F.; Li, X.; Lawson, S.; Rezaei, F.; Rownaghi, A.A. 3D-printed HZSM-5 and 3D-HZM5@SAPO-34 structured monoliths with controlled acidity and porosity for conversion of methanol to dimethyl either. Fuel 2020, 280, 118628. [Google Scholar] [CrossRef]
- Sripada, P.; Kimpton, J.; Barlow, A.; Williams, T.; Kandasamy, S.; Bhattacharya, S. Investigating the dynamic structural changes on Cu/CeO2 catalysts observed during CO2 hydrogenation, conversion, and selectivity. J. Catal. 2020, 380, 415–426. [Google Scholar] [CrossRef]
- Bonura, G.; Todaro, S.; Middelkoop, V.; de Vos, Y.; Abbenhuis, H.; Gerritsen, G.; Koekkoek, A.; Cannilla, C.; Frusteri, F. Effectiveness of the 3D-printing procedure in the synthesis of hybrid catalysts for the direct hydrogenation of CO2 into dimethyl ether. J. CO2 Util. 2023, 70, 102458. [Google Scholar] [CrossRef]
- García-Trenco, A.; Vidal-Moya, A.; Martínez, A. Study of the interaction between components in hybrid CuZnAl/HZSM-5 catalysts and its impact in the syngas-to-DME reaction. Catal. Today 2012, 179, 43–51. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Jin, Y. Slurry reactors for gas-to-liquid processes: A review. Ind. Eng. Chem. Res. 2007, 46, 5824–5847. [Google Scholar] [CrossRef]
- Tozar, U.; Avci, A.K. Strategies for improving CO2 utilization in microchannel enabled production of dimethyl ether. Chem. Eng. Process. Process Intensif. 2020, 151, 107914. [Google Scholar] [CrossRef]
- Allahyari, S.; Haghighi, M.; Ebadi, A. Direct synthesis of DME over nanostructured CuO–ZnO–Al2O3/HZSM-5 catalyst washcoated on high pressure microreactor: Effect of catalyst loading and process condition on reactor performance. Chem. Eng. J. 2015, 262, 1175–1186. [Google Scholar] [CrossRef]
- Sobczak, J.; Wysocka, I.; Murgrabia, S.; Rogala, A. A Review on Deactivation and Regeneration of Catalysts for Dimethyl Ether Synthesis. Energies 2022, 15, 5420. [Google Scholar] [CrossRef]



| Methane | Propane | Butane | DME | |
|---|---|---|---|---|
| Chemical Formula | ||||
| Boiling Point (°C) | −161.5 | −42.07 | −0.6 | −24.9 |
| Explosion Limit (%) | 5–15 | 2.1–9.5 | 1.9–8.5 | 3.4–27 |
| Lower Heating Value (kJ/kg) | 49,900 | 46,360 | 45,740 | 28,620 |
| Auto-Ignition Temperature (°C) | 595 | 450 | 405 | 235 |
| Vapor Pressure at 20 °C (bar) | - | 8.4 | 2.1 | 5.1 |
| Property | Diesel | CNG | LNG | DME | Fischer–Tropsch Diesel | Gasoline |
|---|---|---|---|---|---|---|
| Lower Heating Value (MJ/kg) | 43 | 50 | 50 | 28.6 | 43 | 43 |
| Volumetric Heating Value (MJ/L) | 36.5 | 8 | 21.1 | 18.2 | 33.1 | 32.2 |
| Density (g/mL) | 0.85 | 0.18 @ 207 bar | 0.422 | 0.66 | 0.77 | 0.75 |
| Cetane Number | 45 | - | - | 55+ | 80 | - |
| Catalyst | CO2 Conversion (%) | DME Selectivity (%) | Temperature (°C) | Pressure (MPa) | H2/CO2 | Time (h) | GHSV (mL/g/h) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cu/Zn/Al2O3 | NR | 59 | 220 | 4 | H2/CO=1 | 14 | 15,000 | [167] |
| Cu/Zn/ Al2O3/SAPO-18 | 10 | 68.9 | 275 | 3 | 3 | 30 | NR | [202] |
| C/ Cu/Zn/ Al2O3/HT-1/HZ | 28 | 48 | 260 | 3 | 3 | NR | 2400 | [203] |
| Cu/Zn/Ce/SAPO-34 | 4.8 | 60.8 | 240 | 1 | 3 | 20 | 3000 | [204] |
| Cu/Zn/ZSM-5 | 20 | 65 | 260 | 2 | 3 | NR | 200 1/h | [56] |
| Cu/Zn/Al/(Al–Zr1:1)-SBA-15) | 22 | 70 | 240 | 3 | 3 | 100 | 1500 | [205] |
| Cu/Zn/Al/FER | 19.8 | 37.8 | 250 | 3 | 3 | 36 | 600 | [206] |
| Cu/Zn/Mg/γ-Al2O3 | 50 (XCO + CO2) | 83 | 260 | 4 | H2/(CO + CO2) = 3 | 72 | 2000 gcat/h | [207] |
| Cu/Zn/Zr/Al2O3 | 26.5 | 69.2 | 240 | 2.7 | 3 | 100 | 472 1/h | [185] |
| Cu/Zn/Zr/H-ZSM5 | 14.2 | 60 | 260 | 3 | 3 | 8 | 2400 | [195] |
| Cu/Zn/Zr/HZSM-5 | 34 | 68 | 250 | 5 | 3 | NR | 2400 NL/g/h | [196] |
| Cu/Zn/Zr/HZSM-5 | 22.2 | 67.6 | 250 | 3 | 3 | 3 | 3600 | [208] |
| Cu/Zn/Zr-WOx-ZrO2 | 18.5 | 63.3 | 240 | 3 | 3 | 10 | NR | [209] |
| Cu/Zn/Al/Cr/HZSM-5 | 15 | 90 | 250 | 3 | 3 | 350 | 6150 | [189] |
| Cu/Fe/Ce/HZSM-5 | 20.9 | 63.1 | 260 | 3 | 4 | NR | 1500 | [200] |
| Cu/Ti/Zr/HZSM-5 | 15 | 52 | 250 | 3 | 3 | 4 | 2400 1/h | [201] |
| Cu/Ti/Zr/HZSM-5 | 12.93 | 48.1 | 250 | 3 | 3 | 4 | 1500 1/h | [191] |
| Cu/Zn/Zr/Al/Pd/HZSM-5 | 18.60 | 73.6 | 200 | 3 | 3.3 | NR | 1800 1/h | [210] |
| Cu/Zn/Zr/ferrierite | 23.60 | 47 | 220–260 | 5 | 3 | NR | 8800 Nl/Kg/H | [211] |
| Cu/Al2O3 (Mesoporous) | 72 (XCO) | 69 | 310 | 5 | H2/CO = 2 | 15 | NR | [169] |
| Cu/Zn/Zr/MFI Zeolite | 23.6 | 49.3 | 240 | 5 | 3 | NR | 10,000 | [212] |
| Cu/Zn/Zr/MFI Zeolite | 4.2 | 71 | 200 | 5 | 3 | NR | 8800 NL/Kg/h | [197] |
| Cu/Zn/Zr/MOR Zeolite | 5.2 | 78 | 200 | 5 | 3 | NR | 8800 NL/Kg/h | [197] |
| Cu/Zn/Zr/FER Zeolite | 5.6 | 79 | 200 | 5 | 3 | NR | 8800 NL/Kg/h | [197] |
| Cu/Zn/Zr/FER Zeolite | 8 | 91 | 250 | 5 | 3 | 200 | 18,000 | [213] |
| Cu/Fe/Ce/HZSM-5 | 18.1 | 52 | 260 | 3 | 4 | 15 | 1500 | [194] |
| CuO-Fe2O3-CeO2/HZSM-5 MM | 20.9 | 63.1 | 260 | 4 | 4 | NR | 1500 W | [200] |
| Cu/Fe/La/HZSM-5 | 17.2 | 51.3 | 260 | 3 | 4 | 15 | 1500 | [194] |
| Cu/Fe/Zr/HZSM-5 | 28.4 | 64.5 | 260 | 3 | 5 | 16 | 1500 | [192] |
| Cu/Zn/La/Al2O3/HZSM-5 | 43.8 | 71.2 | 250 | 3 | 3 | 8 | 3000 1/h | [214] |
| Cu/Zn/Al2O3/γ-Al2O3 | 62 (XCO) | 67.4 | 225 | 5 | H2/CO = 2 | NR | NR | [215] |
| 12Cu–6Mo/HZSM-5 | 12.36 | 77.19 | 240 | 2 | 3 | 10 | 1500 1/h | [216] |
| CuO-ZnO-Cr2O3/HY (50:50) MM | 24.2 | 86.6 | 250 | 3 | 3 | NR | 1800 W | [217] |
| MK-121/Al-MCM-41 | 14 | 76 | 260 | 5 | 3 | 5 | 2000 | [218] |
| Pd/Zn/ZSM-5 | 3.5 | 73.4 | 190 | 2 | 3 | 20 | NR | [219] |
| Pd/Zn/Ti/ZSM-5 | 13.3 | 37.6 | 270 | 2 | 3 | 20 | NR | [219] |
| CuO/TiO2/ZrO2/HZSM-5 PM | 15.6 | 47.5 | 250 | 3 | 2.8 | NR | 1500 W | [191] |
| Nano Pd-In2O3/HZSM-5 | 9 | 44.1 | 295 | 3 | 3 | NR | NR | [220] |
| Cu-Ga/Ce-Zr | 14 | 30 | 300 | 5 | 4 | NR | 15,600 1/h | [220] |
| CuO-ZnO-Ga2O3/H-Ga-silicate PM | 19.4 | 19.9 | 250 | 2.8 | 3 | NR | 33.33 gcat·h/mol | [187] |
| CuO-ZnO- Ga2O3/SAPO-34 PM | 19.6 | 19.4 | 250 | 2.8 | 3 | NR | 33.33 gcat·h/mol | [187] |
| Conventional Reactor Types | Advantages | Disadvantages |
|---|---|---|
|
|
|
|
|
|
|
|
|
| Advanced Reactor Types | Advantages | Disadvantages |
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahimian, S.; Bhattacharya, S. Direct CO2 Hydrogenation over Bifunctional Catalysts to Produce Dimethyl Ether—A Review. Energies 2024, 17, 3701. https://doi.org/10.3390/en17153701
Ebrahimian S, Bhattacharya S. Direct CO2 Hydrogenation over Bifunctional Catalysts to Produce Dimethyl Ether—A Review. Energies. 2024; 17(15):3701. https://doi.org/10.3390/en17153701
Chicago/Turabian StyleEbrahimian, Samira, and Sankar Bhattacharya. 2024. "Direct CO2 Hydrogenation over Bifunctional Catalysts to Produce Dimethyl Ether—A Review" Energies 17, no. 15: 3701. https://doi.org/10.3390/en17153701
APA StyleEbrahimian, S., & Bhattacharya, S. (2024). Direct CO2 Hydrogenation over Bifunctional Catalysts to Produce Dimethyl Ether—A Review. Energies, 17(15), 3701. https://doi.org/10.3390/en17153701





