Effect of Supporting Carbon Fiber Anode by Activated Coconut Carbon in the Microbial Fuel Cell Fed by Molasses Decoction from Yeast Production
Abstract
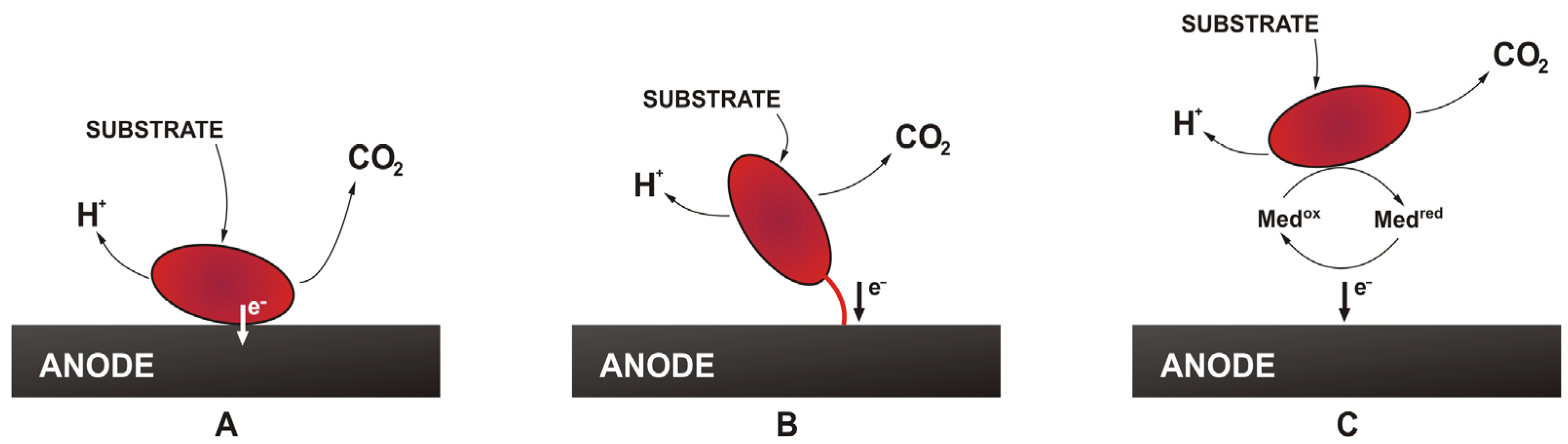
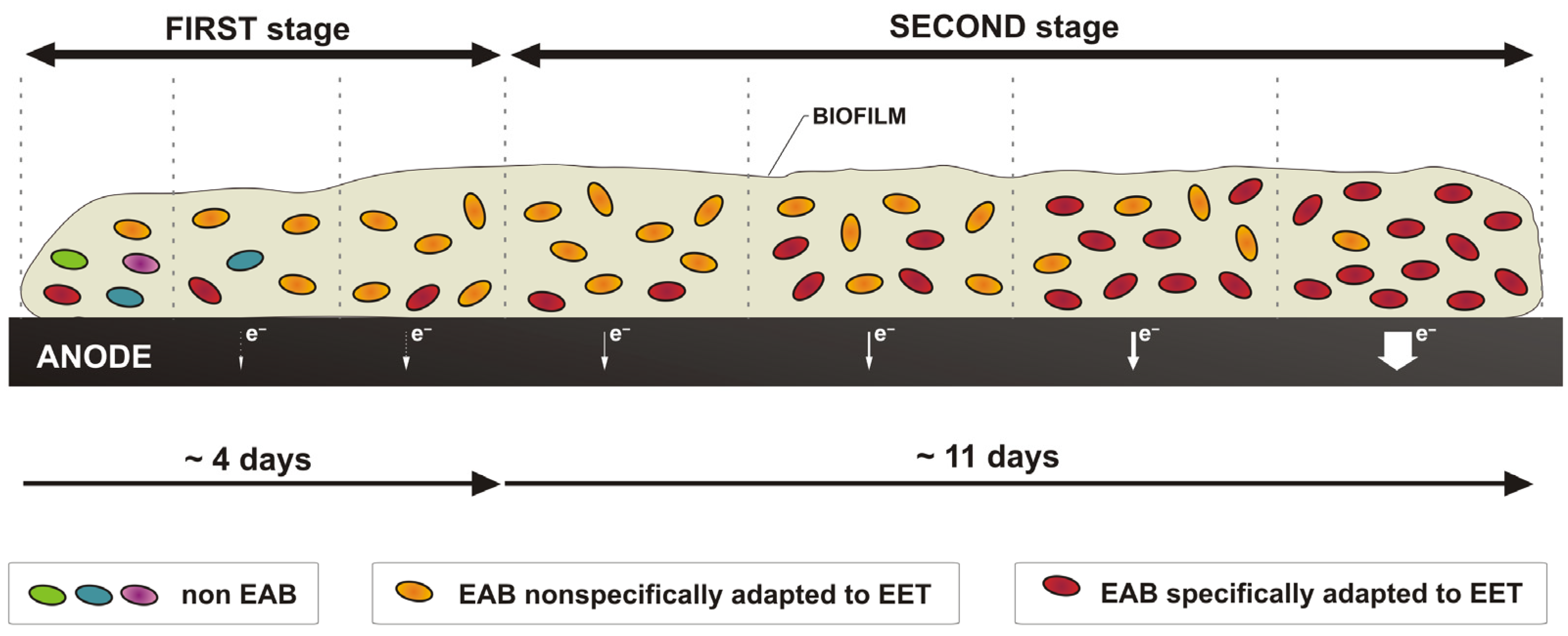
1. Introduction
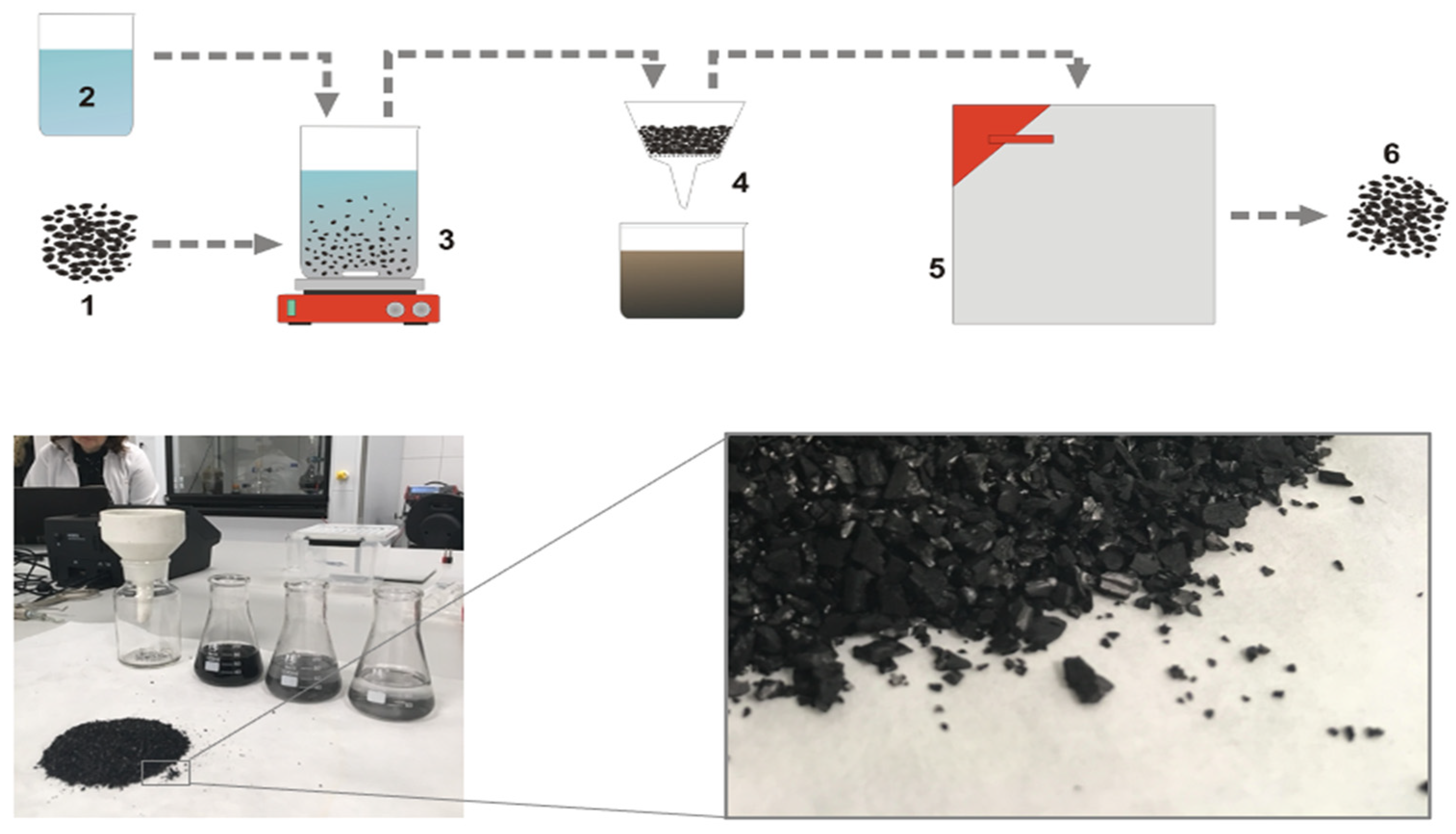
2. Materials and Methods
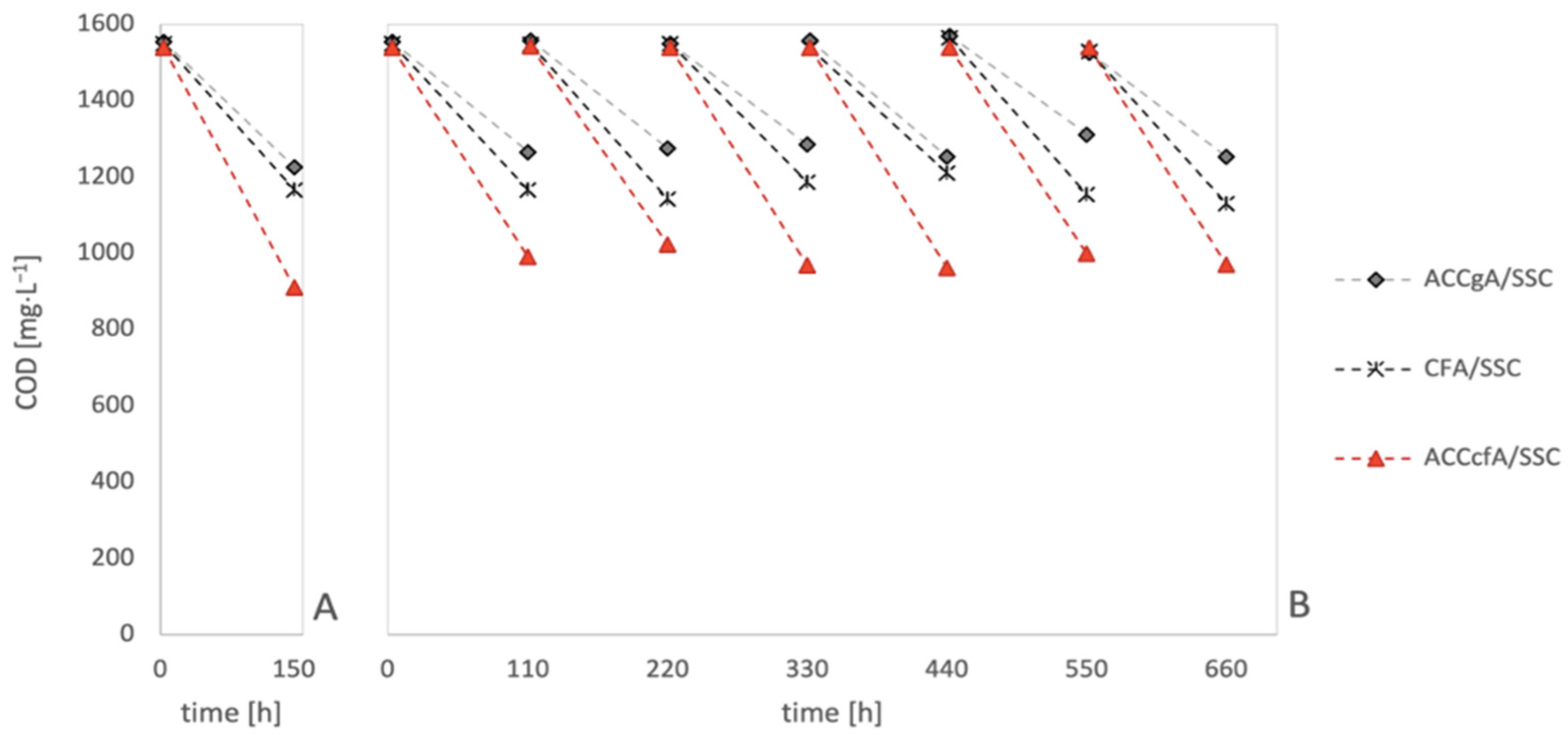
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schroder, U.; Keller, J.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Logan, B.E. Microbial Fuel Cells; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Min, B.; Logan, B.E. Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ. Sci. Technol. 2004, 38, 5809–5814. [Google Scholar] [CrossRef]
- Septiariva, I.Y.; Suryawan, W.K.; Sarwono, A. Energy Conversion of Industrial Wastewater on Microbial Fuel Cell (MFC)-Based with Biocatalysts and Pretreatments: A Review. Indones. J. Environ. Manag. Sustain. 2020, 4, 102–109. [Google Scholar]
- Franks, A.E.; Nevin, K.P. Microbial fuel cells, a current review. Energies 2010, 3, 899–919. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Jujjavarapu, S.E. Bio-Electrochemical Systems: Waste Valorization and Waste Biorefinery; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Kumar, G.; Saratale, R.G.; Kadier, A.; Sivagurunathan, P.; Zhen, G.; Kim, S.-H.; Saratale, G.D. A review on bio-electrochemical systems (BESs) for the syngas and value added biochemicals production. Chemosphere 2017, 177, 84–92. [Google Scholar] [CrossRef]
- Potter, M.C. Electrical effects accompanying the decomposition organic compounds. Proc. R. Soc. Lond. Ser. B 1911, 84, 260–276. [Google Scholar]
- Davis, J.B.; Yarbrough, H.F., Jr. Preliminary experiments on a microbial fuel cell. Science 1962, 137, 615–616. [Google Scholar] [CrossRef]
- Kim, H.J.; Hyun, M.S.; Chang, I.S.; Kim, B.H. A microbial fuel cell type lactate biosensor using a metal-reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 1999, 9, 365–367. [Google Scholar]
- Kim, H.H.; Mano, N.; Zhang, Y.; Heller, A. A miniature membrane-less biofuel cell operating under physiological conditions at 0.5 V. J. Electrochem. Soc. 2003, 150, A209–A213. [Google Scholar] [CrossRef]
- Liu, H.; Ramnarayanan, R.; Logan, B.E. Production of electricity during wastewater treatment using a single chamber microbial fuel Cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar] [CrossRef]
- Liu, H.; Logan, B.E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Min, B.; Cheng, S.; Logan, B.E. Electricity generation using membrane and salt bridge microbial fuel cells. Water Res. 2005, 39, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Microbial fuel cells: Novel microbial physiologies and engineering approaches. Curr. Opin. Biotechnol. 2006, 17, 327–332. [Google Scholar] [CrossRef]
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009, 7, 375–381. [Google Scholar] [CrossRef]
- Chiao, M.; Lam, K.B.; Lin, L. Micromachined microbial and photosynthetic fuel cells. J. Micromech. Microeng. 2006, 16, 2547–2553. [Google Scholar] [CrossRef]
- Lee, J.; Phung, N.T.; Chang, I.S.; Kim, B.H.; Sung, H.C. Use of acetate for enrichment of electrochemically active microorganisms and their 16S rDNA analyses. FEMS Microbiol. Lett. 2003, 223, 185–191. [Google Scholar] [CrossRef]
- Mitra, P.; Hill, G.A. Continuous microbial fuel cell using a photoautotrophic cathode and a fermentative anode. Can. J. Chem. Eng. 2012, 90, 1006–1010. [Google Scholar] [CrossRef]
- Prasad, D.; Arun, S.; Murugesan, M.; Padmanaban, S.; Satyanarayanan, R.S.; Berchmans, S.; Yegnaraman, V. Direct electron transfer with yeast cells and construction of a mediatorless microbial fuel cells. Biosens. Bioelectron. 2007, 22, 2604–2610. [Google Scholar] [CrossRef]
- Schaetzle, O.; Barrière, F.; Baronian, K. Bacteria and yeasts as catalysts in microbial fuel cells: Electron transfer from microorganisms to electrodes for green electricity. Energy Environ. Sci. 2008, 1, 607–620. [Google Scholar] [CrossRef]
- Logan, B.E.; Regan, J.M. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 2006, 14, 512–518. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.J.; Lee, H. Electricity production from beer brewery wastewater using single chamber microbial fuel cell. Water Sci. Technol. 2008, 57, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, P.P.; Włodarczyk, B. Microbial fuel cell with Ni–Co cathode powered with yeast wastewater. Energies 2018, 11, 3194. [Google Scholar] [CrossRef]
- Das, D. Microbial Fuel Cell: A Bioelectrochemical System That Converts Waste to Watts; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Kim, B.-H.; Kim, H.-J.; Hyun, M.-S.; Park, D.-H. Direct electrode reaction of Fe (III)—Reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 1999, 9, 127–131. [Google Scholar]
- Gorby, Y.A.; Yanina, S.; McLean, J.S.; Rosso, K.M.; Moyles, D.; Dohnalkova, A.; Beveridge, T.J.; Chang, I.S.; Kim, B.H.; Kim, K.S.; et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. USA 2006, 103, 11358–11363. [Google Scholar] [CrossRef]
- Lovley, D.R. The microbe electric: Conversion of organic matter to electricity. Curr. Opin. Biotechnol. 2008, 19, 564–571. [Google Scholar] [CrossRef]
- Malvankar, N.S.; Vargas, M.; Nevin, K.P.; Franks, A.E.; Leang, C.; Kim, B.-C.; Inoue, K.; Mester, T.; Covalla, S.F.; Johnson, J.P.; et al. Tunable metallic-like conductivity in microbial nanowire networks. Nat. Nanotechnol. 2011, 6, 573–579. [Google Scholar] [CrossRef]
- Yang, Y.; Kong, G.; Chen, X.; Lian, Y.; Liu, W.; Xu, M. Electricity generation by Shewanella decolorationis S12 without cytochrome c. Front. Microbiol. 2017, 8, 1115. [Google Scholar] [CrossRef]
- Wrighton, K.C.; Thrash, J.C.; Melnyk, R.; Bigi, J.P.; Byrne-Bailey, K.G.; Remis, J.P.; Schichnes, D.; Auer, M.; Chang, C.J.; Coates, J.D. Evidence for direct electron transfer by a gram-positive bacterium isolated from a microbial fuel cell. Appl. Environ. Microbiol. 2011, 77, 7633–7639. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.N.M.; Yaqoob, A.A.; Ahmad, A. Microbial Fuel Cells: Emerging Trends in Electrochemical Applications; Institute of Physics Publishing: Bristol, UK, 2022. [Google Scholar]
- Sun, J.; Li, Y.; Hu, Y.; Hou, B.; Xu, Q.; Zhang, Y.; Li, S. Enlargement of anode for enhanced simultaneous azo dye decolorization and power output in air-cathode microbial fuel cell. Biotechnol. Lett. 2012, 4, 2023–2029. [Google Scholar] [CrossRef]
- Sarma, R.; Tamuly, A.; Kakati, B.K. Recent developments in electricity generation by microbial fuel cell using different substrates. Mater. Today Proc. 2022, 49, 457–463. [Google Scholar] [CrossRef]
- Yang, S.; Du, F.; Liu, H. Characterization of mixed-culture biofilms established in microbial fuel cells. Biomass Bioenergy 2012, 46, 531–537. [Google Scholar] [CrossRef]
- Patil, S.A.; Surakasi, V.P.; Koul, S.; Ijmulwar, S.; Vivek, A.; Shouche, Y.S.; Kapadnis, B.P. Electricity generation using chocolate industry wastewater and its treatment in activated sludge based microbial fuel cell and analysis of developed microbial community in the anode chamber. Bioresour. Technol. 2009, 100, 5132–5139. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Saratale, G.D.; Pugazhendhi, A.; Zhen, G.; Kumar, G.; Kadier, A.; Sivagurunathan, P. Microbiome involved in microbial electrochemical systems (MESs): A review. Chemosphere 2017, 177, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Saratale, R.G.; Shahid, M.K.; Zhen, G.; Kumar, G.; Shin, H.-S.; Choi, Y.-G.; Kim, S.-H. A comprehensive overview on electro-active biofilms, role of exo-electrogens and their microbial niches in microbial fuel cells (MFCs). Chemosphere 2017, 178, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Markowska, K.; Grudniak, A.M.; Wolska, K.I. Mikrobiologiczne Ogniwa Paliwowe: Podstawy technologii, jej ograniczenia i potencjalne zastosowania. Postępy Mikrobiol. 2013, 52, 29–40. [Google Scholar]
- Zheng, S.; Yang, F.; Chen, S.; Liu, L.; Xiong, Q.; Yu, T.; Zhao, F.; Schröder, U.; Hou, H. Binder-free carbon black/stainless steel mesh composite electrode for high-performance anode in microbial fuel cells. J. Power Sources 2015, 284, 252–257. [Google Scholar] [CrossRef]
- Prévoteau, A.; Rabaey, K. Electroactive Biofilms for Sensing: Reflections and Perspectives. ACS Sens. 2017, 2, 1072–1085. [Google Scholar] [CrossRef]
- Pinck, S.; Ostormujof, L.M.; Teychené, S.; Erable, B. Microfluidic Microbial Bioelectrochemical Systems: An Integrated Investigation Platform for a More Fundamental Understanding of Electroactive Bacterial Biofilms. Microorganisms 2020, 8, 1841. [Google Scholar] [CrossRef]
- Greenman, J.; Gajda, I.; You, J.; Mendis, B.A.; Obata, O.; Pasternak, G.; Ieropoulos, I. Microbial Fuel Cells and Their Electrified Biofilms. Biofilm 2021, 3, 100057. [Google Scholar] [CrossRef]
- Hodgson, D.M.; Smith, A.; Dahale, S.; Stratford, J.P.; Li, J.V.; Grüning, A.; Bushell, M.E.; Marchesi, J.R.; Avignone-Rossa, C. Segregation of the Anodic Microbial Communities in a Microbial Fuel Cell Cascade. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Godain, A.; Haddour, N.; Fongarland, P.; Vogel, T.M. Bacterial Competition for the Anode Colonization under Different External Resistances in Microbial Fuel Cells. Catalysts 2022, 12, 176. [Google Scholar] [CrossRef]
- Baudler, A.; Schmidt, I.; Langner, M.; Greiner, A.; Schröder, U. Does it have to be carbon? Metal anodes in microbial fuel cells and related bioelectrochemical systems. Energy Environ. Sci. 2015, 8, 2048–2055. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, X.; Li, H.; Xiong, J.; Li, X.; Li, W. Surface oxygen-rich titanium as anode for high performance microbial fuel cell. Electrochim. Acta 2016, 209, 582–590. [Google Scholar]
- Włodarczyk, B.; Włodarczyk, P.P. Electricity production from yeast wastewater in membrane-less microbial fuel cell with Cu-Ag cathode. Energies 2023, 16, 2734. [Google Scholar] [CrossRef]
- Włodarczyk, P.P.; Włodarczyk, B. Wastewater treatment and electricity production in a microbial fuel cell with Cu–B alloy as the cathode catalyst. Catalysts 2019, 9, 572. [Google Scholar] [CrossRef]
- Włodarczyk, P.P.; Włodarczyk, B. Preparation and analysis of Ni–Co catalyst use for electricity production and COD reduction in microbial fuel cells. Catalysts 2019, 9, 1042. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Hama, A.K.H.; Mustafa, F.S.; Omer, K.M.; Hama, S.; Hamarawf, R.F.; Rahman, K.O. Heavy metal pollution in the aquatic environment: Efficient and low-cost removal approaches to eliminate their toxicity: A review. RSC Adv. 2023, 13, 17595–17610. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, D.; Tommasi, T.; Bocchini, S.; Chiolerio, A.; Chiodoni, A.; Mazzarino, I.; Ruggeri, B. Surface modification of commercial carbon felt used as anode for microbial fuel cells. Energy 2016, 99, 193–201. [Google Scholar] [CrossRef]
- Deng, Q.; Li, X.; Zuo, J.; Ling, A.; Logan, B.E. Power generation using an activated carbon fiber felt cathode in an upflow microbial fuel cell. J. Power Sources 2010, 195, 1130–1135. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Hu, Y.; Li, S.; Xu, Q. Bio-cathode materials evaluation in microbial fuel cells: A comparison of graphite felt, carbon paper and stainless steel mesh materials. Int. J. Hydrogen Energy 2012, 37, 16935–16942. [Google Scholar] [CrossRef]
- Fraiwan, A.; Mukherjee, S.; Sundermier, S.; Lee, H.-S.; Choi, S. A paper-based microbial fuel cell: Instant battery for disposable diagnostic devices. Biosens. Bioelectron. 2013, 49, 410–414. [Google Scholar] [CrossRef]
- Jia, X.; He, Z.; Zhang, X.; Tian, X. Carbon paper electrode modified with TiO2 nanowires enhancement bioelectricity generation in microbial fuel cell. Synth. Met. 2016, 215, 170–175. [Google Scholar] [CrossRef]
- Hutchinson, A.J.; Tokash, J.C.; Logan, B.E. Analysis of carbon fiber brush loading in anodes on startup and performance of microbial fuel cells. J. Power Sources 2011, 196, 9213–9219. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Q.; Wang, X.; Logan, B.E. Treatment of carbon fiber brush anodes for improving power generation in air–cathode microbial fuel cells. J. Power Sources 2010, 195, 1841–1844. [Google Scholar] [CrossRef]
- Włodarczyk, P.P.; Włodarczyk, B. Study of the use of gas diffusion anode with various cathodes (Cu-Ag, Ni-Co, and Cu-B Alloys) in a microbial fuel cell. Energies 2024, 17, 1636. [Google Scholar] [CrossRef]
- Cheng, S.; Wu, J. Air-cathode preparation with activated carbon as catalyst, PTFE as binder and nickel foam as current collector for microbial fuel cells. Bioelectrochemistry 2013, 92, 22–26. [Google Scholar] [CrossRef]
- Liew, K.B.; Daud, W.R.W.; Ghasemi, M.; Leong, J.X.; Lim, S.S.; Ismail, M. Non-Pt catalyst as oxygen reduction reaction in microbial fuel cells: A review. Int. J. Hydrogen Energy 2014, 39, 4870–4883. [Google Scholar] [CrossRef]
- Fraiwan, A.; Kwan, L.; Choi, S. A disposable power source in resource-limited environments: A paper-based biobattery generating electricity from wastewater. Biosens. Bioelectron. 2016, 85, 190–197. [Google Scholar] [CrossRef]
- Neethu, B.; Bhowmick, G.D.; Ghangrekar, M.M. Improving performance of microbial fuel cell by enhanced bacterial-anode interaction using sludge immobilized beads with activated carbon. Process Saf. Environ. Prot. 2020, 143, 285–292. [Google Scholar] [CrossRef]
- Zhao, F.; Rahunen, N.; Varcoe, J.R.; Chandra, A.; Avignone-Rossa, C.; Thumser, A.E.; Slade, R.C.T. Activated carbon cloth as anode for sulfate removal in a microbial fuel cell. Environ. Sci. Technol. 2008, 42, 4971–4976. [Google Scholar] [CrossRef]
- Mohamed, H.O.; Obaid, M.; Sayed, E.T.; Liu, Y.; Lee, J.; Park, M.; Barakat, N.A.M.; Kim, H.Y. Electricity generation from real industrial wastewater using a single-chamber air cathode microbial fuel cell with an activated carbon anode. Bioprocess Biosyst. Eng. 2017, 40, 1151–1161. [Google Scholar] [CrossRef]
- Chen, Q.; Pu, W.; Hou, H.; Hu, J.; Liu, B.; Li, J.; Cheng, K.; Huang, L.; Yuan, X.; Yang, C.; et al. Activated microporous-mesoporous carbon derived from chestnut shell as a sustainable anode material for high performance microbial fuel cells. Bioresour. Technol. 2018, 249, 567–573. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Taguchi, K. Enhancing the performance of E. coli powered MFCs by using porous 3D anodes based on coconut activated carbon. Biochem. Eng. J. 2019, 151, 107357. [Google Scholar] [CrossRef]
- Fan, X.; Zhou, Y.; Jin, X.; Song, R.-B.; Li, Z.; Zhang, Q. Carbon material-based anodes in the microbial fuel cells. Carbon Energy 2021, 3, 449–472. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Guerrero-Barajas, C. Modern trend of anodes in microbial fuel cells (MFCs): An overview. Environ. Technol. Innov. 2021, 23, 101579. [Google Scholar] [CrossRef]
- Moradian, J.M.; Wang, S.; Ali, A.; Liu, J.; Mi, J.; Wang, H. Biomass-Derived Carbon Anode for High-Performance Microbial Fuel Cells. Catalysts 2022, 12, 894. [Google Scholar] [CrossRef]
- Guan, Y.F.; Zhang, F.; Huang, B.C.; Yu, H.Q. Enhancing electricity generation of microbial fuel cell for wastewater treatment using nitrogen-doped carbon dots-supported carbon paper anode. J. Clean. Prod. 2019, 229, 412–419. [Google Scholar] [CrossRef]
- Włodarczyk, B.; Włodarczyk, P.P. Analysis of the Potential of an Increase in Yeast Output Resulting from the Application of Additional Process Wastewater in the Evaporator Station. Appl. Sci. 2019, 9, 2282. [Google Scholar] [CrossRef]
- Buitrón, G.; López-Prieto, I.; Zúñiga, I.T.; Vargas, A. Reduction of start-up time in a microbial fuel cell through Reduction of start-up time in a microbial fuel cell through the variation of external resistance the variation of external resistance. Energy Procedia 2017, 142, 694–699. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; An, C.; Jia, H.; Wang, J. Exploring novel approaches to enhance start-up process in microbial fuel cell: A comprehensive review. J. Water Process Eng. 2024, 63, 105425. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, X.; Du, Q.; Gao, F.; Wang, Z.; Wu, G.; Guo, W.; Ngo, H.H. Assessing the Long-Term performance of an integrated microbial fuel Cell-Anaerobic membrane bioreactor for swine wastewater treatment. Chem. Eng. J. 2024, 493, 152772. [Google Scholar] [CrossRef]
- Song, B.; Wang, Q.; Ali, J.; Wang, Z.; Wang, L.; Wang, J.; Li, J.; Glebov, E.M.; Zhuang, X. Asymmetric reactors as an innovative approach for optimum microbial fuel cells performance. Energy Convers. Manag. 2024, 310, 118475. [Google Scholar] [CrossRef]




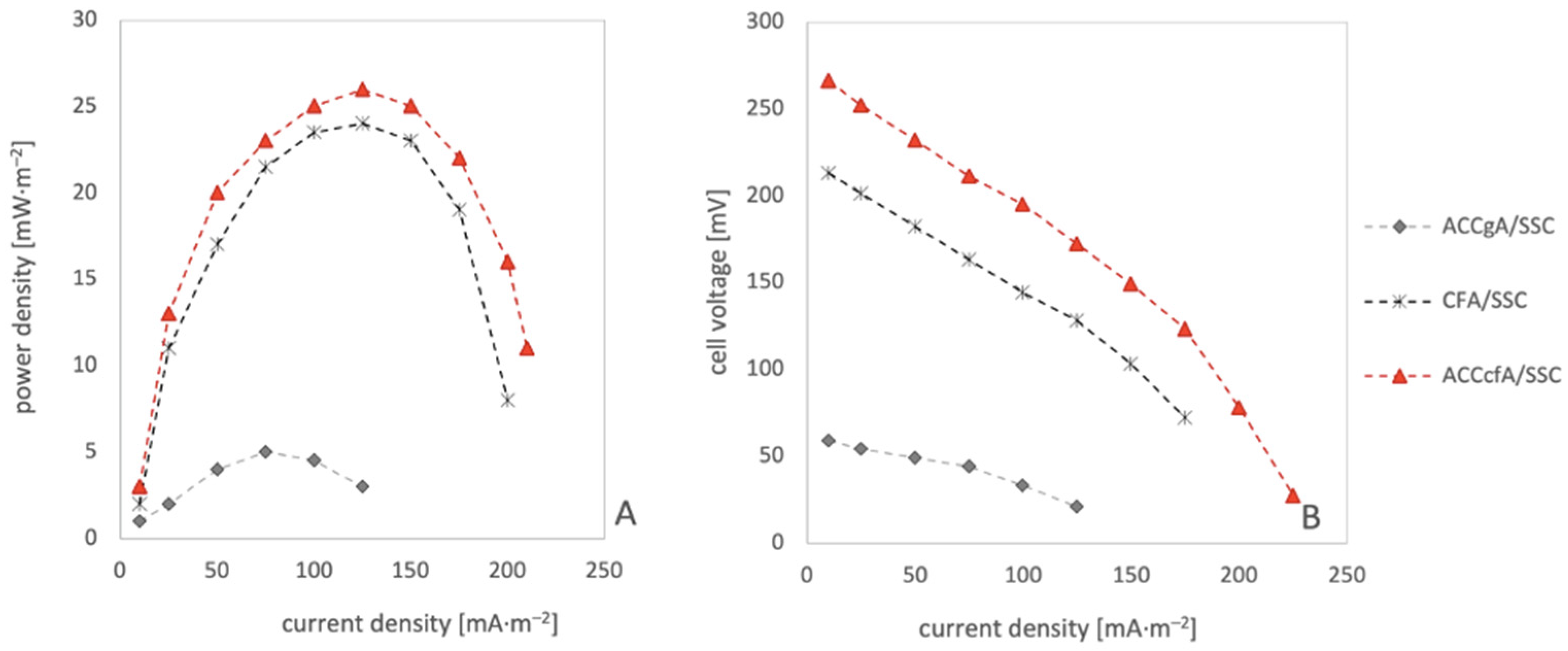
| Electrode System | Average Cell Voltage [mV] | Maximum Power Density [mW·m–2] | Average COD Reduction [%] |
|---|---|---|---|
| ACCgA/SSC | 55 | 5 | 18 |
| CFA/SSC | 231 | 23 | 26 |
| ACCcfA/SSC | 245 | 26 | 33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodarczyk, P.P.; Włodarczyk, B. Effect of Supporting Carbon Fiber Anode by Activated Coconut Carbon in the Microbial Fuel Cell Fed by Molasses Decoction from Yeast Production. Energies 2024, 17, 3607. https://doi.org/10.3390/en17153607
Włodarczyk PP, Włodarczyk B. Effect of Supporting Carbon Fiber Anode by Activated Coconut Carbon in the Microbial Fuel Cell Fed by Molasses Decoction from Yeast Production. Energies. 2024; 17(15):3607. https://doi.org/10.3390/en17153607
Chicago/Turabian StyleWłodarczyk, Paweł P., and Barbara Włodarczyk. 2024. "Effect of Supporting Carbon Fiber Anode by Activated Coconut Carbon in the Microbial Fuel Cell Fed by Molasses Decoction from Yeast Production" Energies 17, no. 15: 3607. https://doi.org/10.3390/en17153607
APA StyleWłodarczyk, P. P., & Włodarczyk, B. (2024). Effect of Supporting Carbon Fiber Anode by Activated Coconut Carbon in the Microbial Fuel Cell Fed by Molasses Decoction from Yeast Production. Energies, 17(15), 3607. https://doi.org/10.3390/en17153607







