The Effect of Hydrogen as a Coolant on the Characteristics of Humidification-Dehumidification Desalination Systems
Abstract
1. Introduction
2. Modelling Details
- The processes operate in a steady state.
- The humidifier and dehumidifier do not lose any heat to the surrounding environment.
- Kinetic and potential energy terms are ignored in the energy balance.
- Pumping and blower power are insignificant in comparison to the heater’s energy input.
- The seawater input temperature is constant.
- The dehumidifier’s condensed water is expected to drain out at a temperature equal to the temperature average of the wet air at the dehumidifier’s inlet and out.
3. Governing Equations
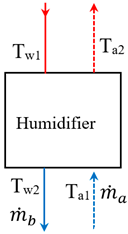
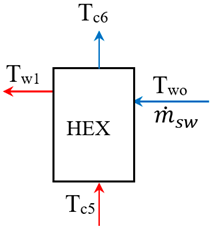
4. Methodology
Validation
5. Results
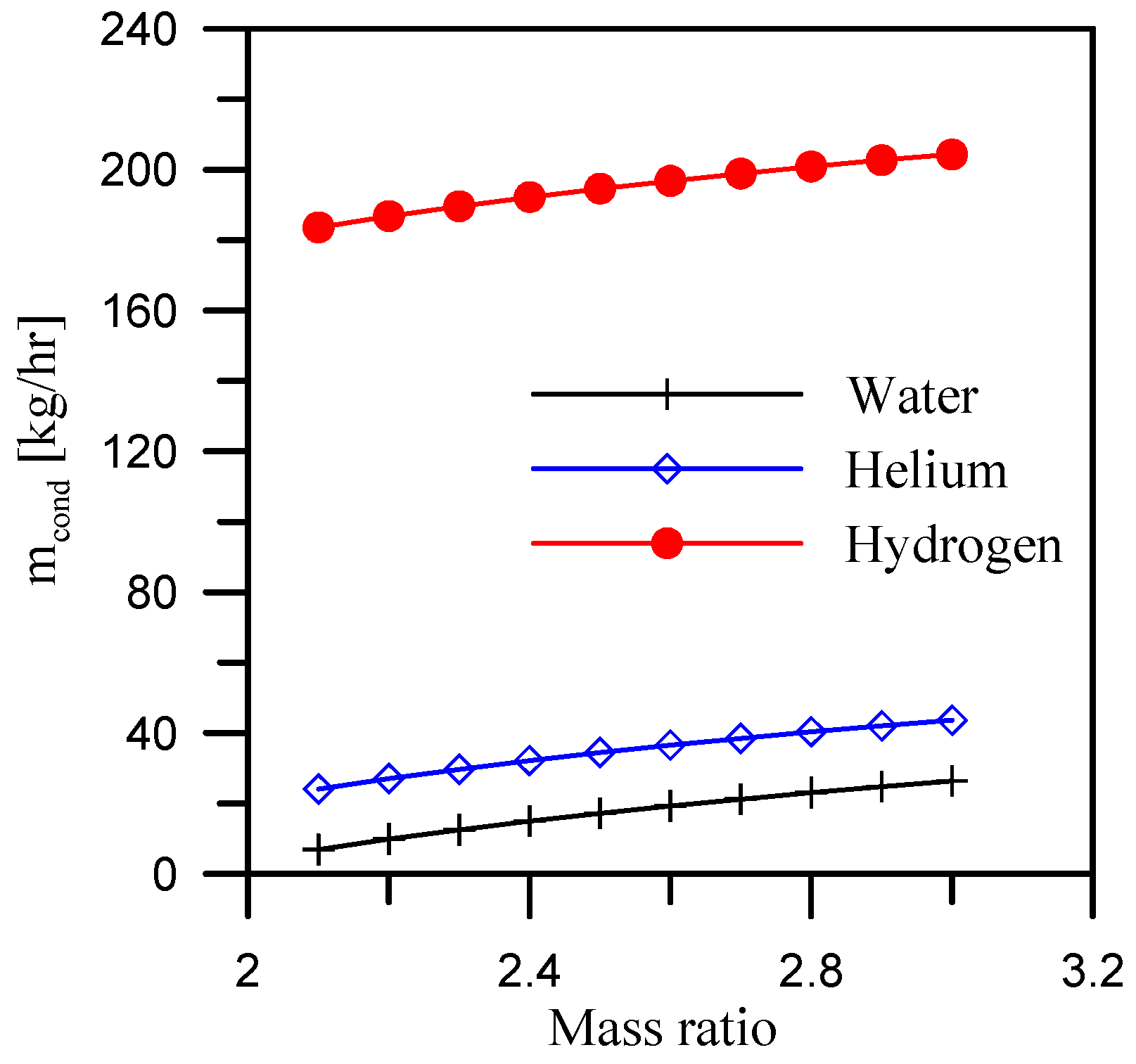
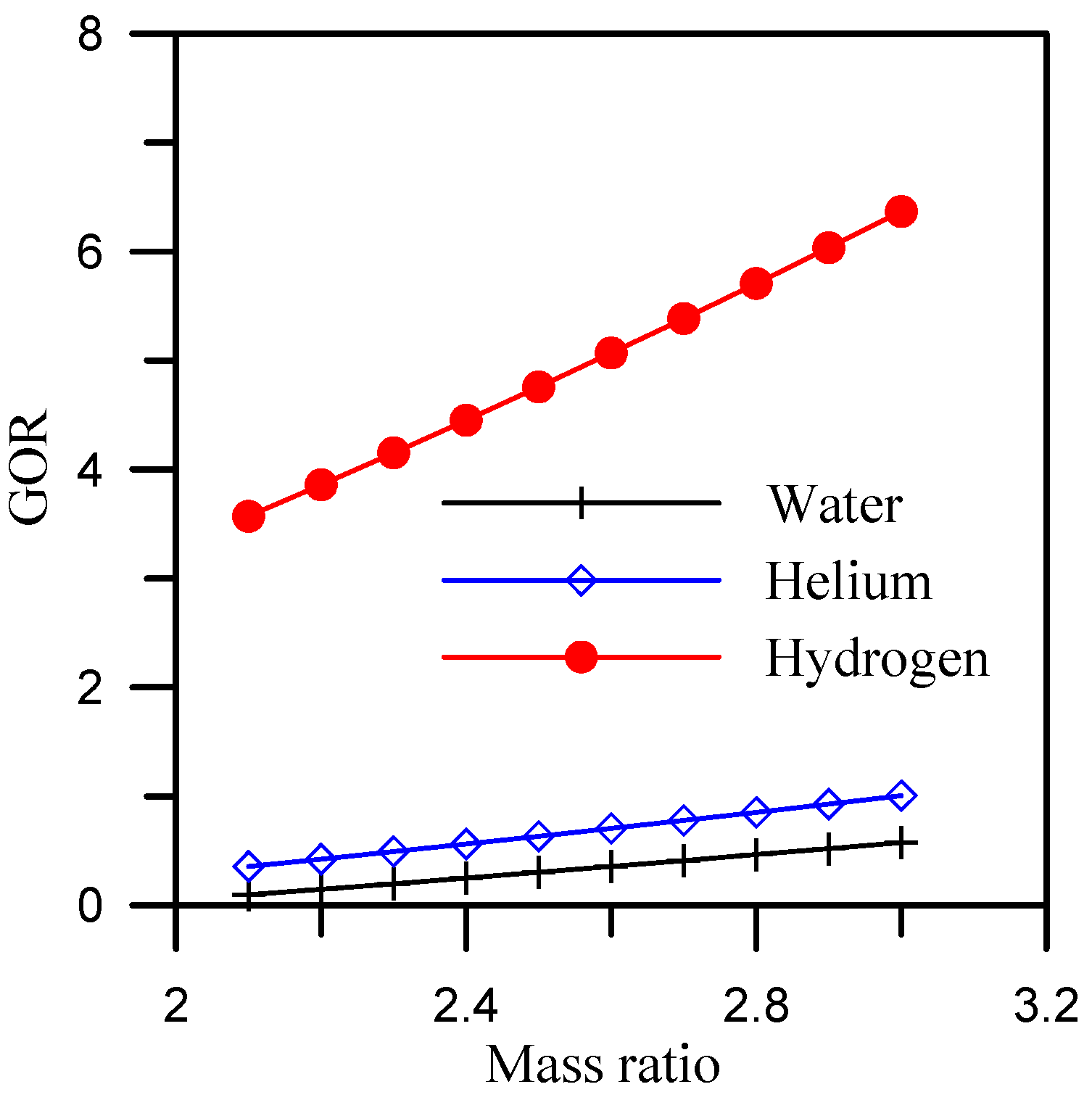
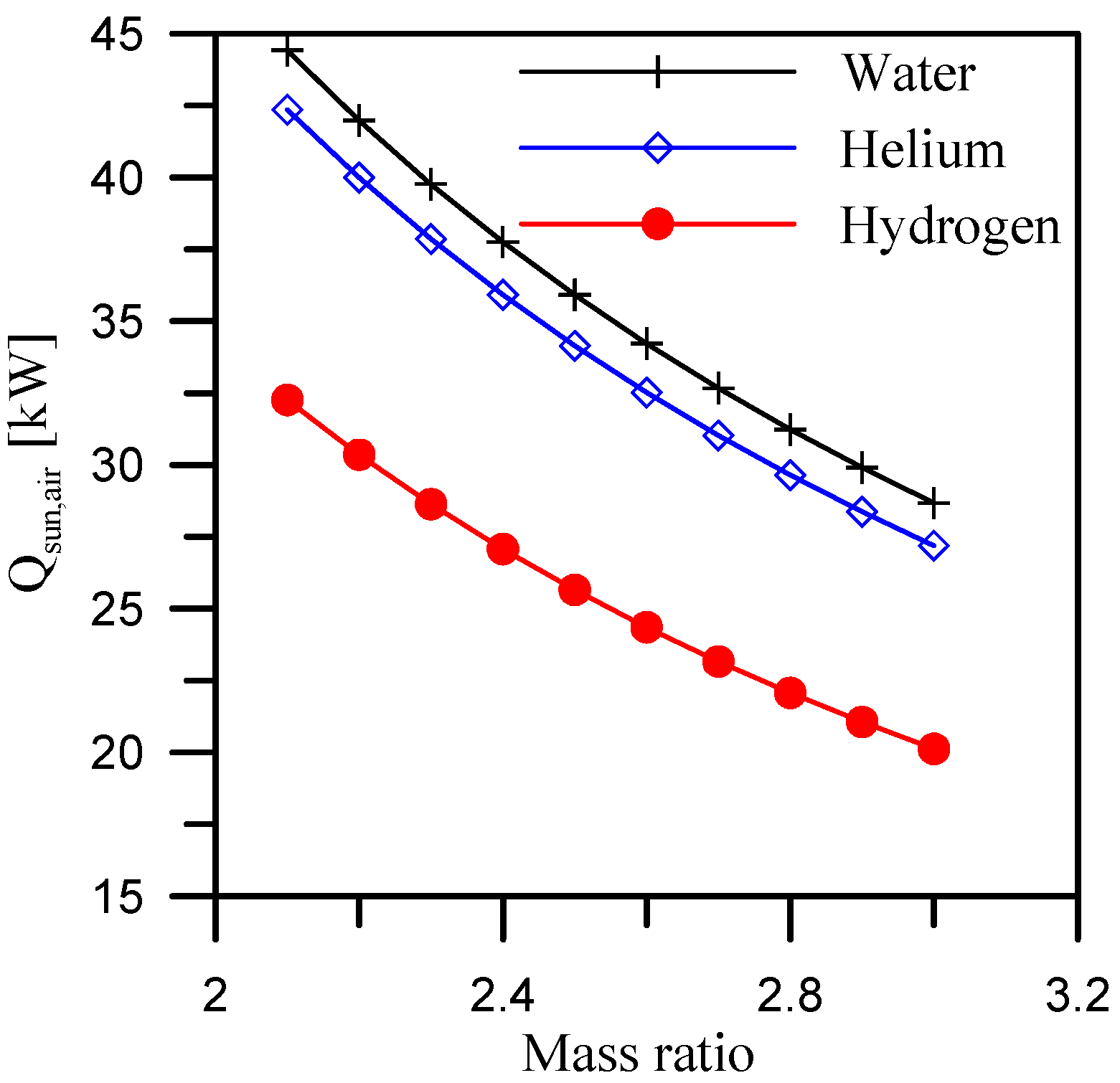
5.1. Effect of Mass Ratio
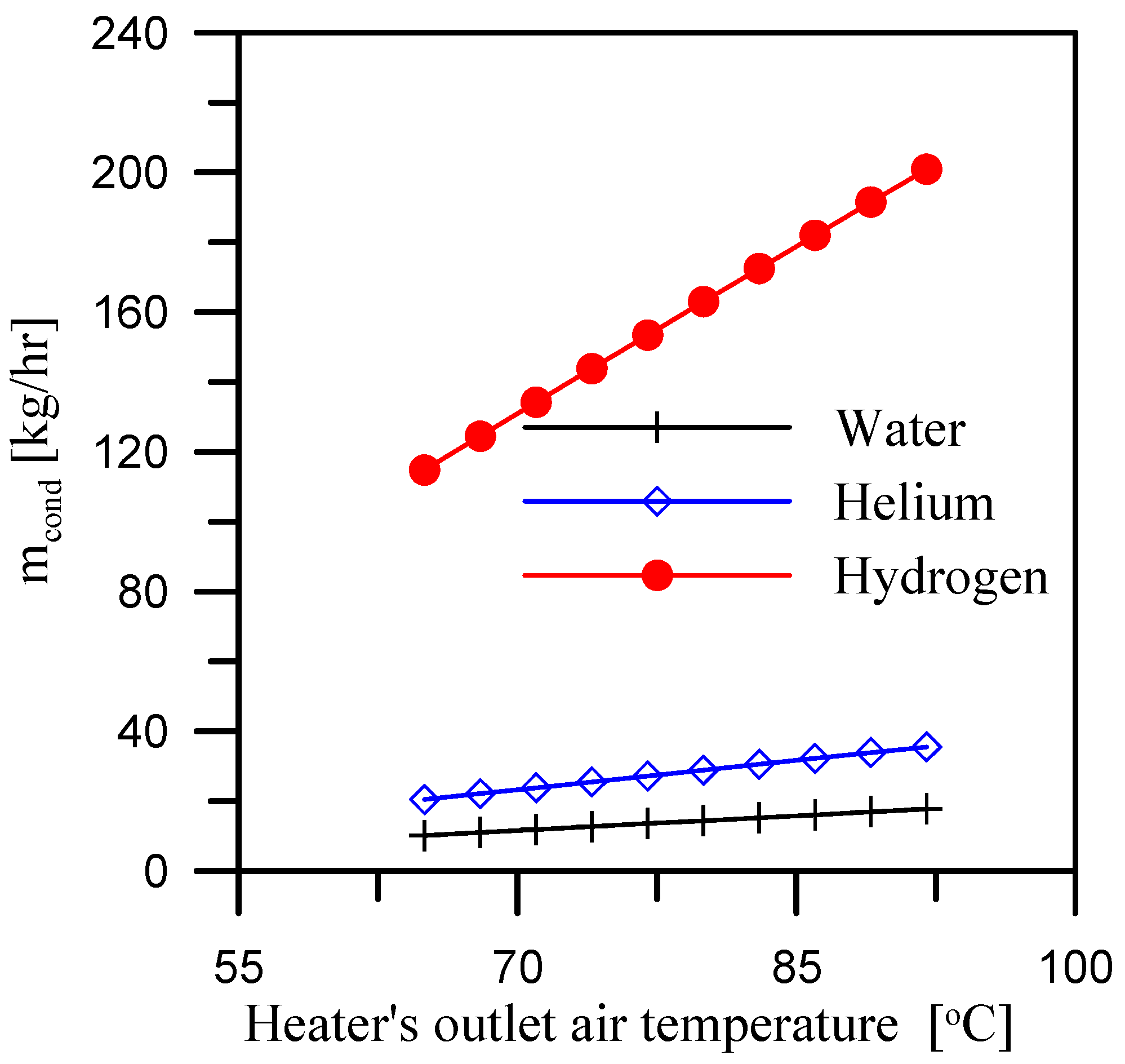
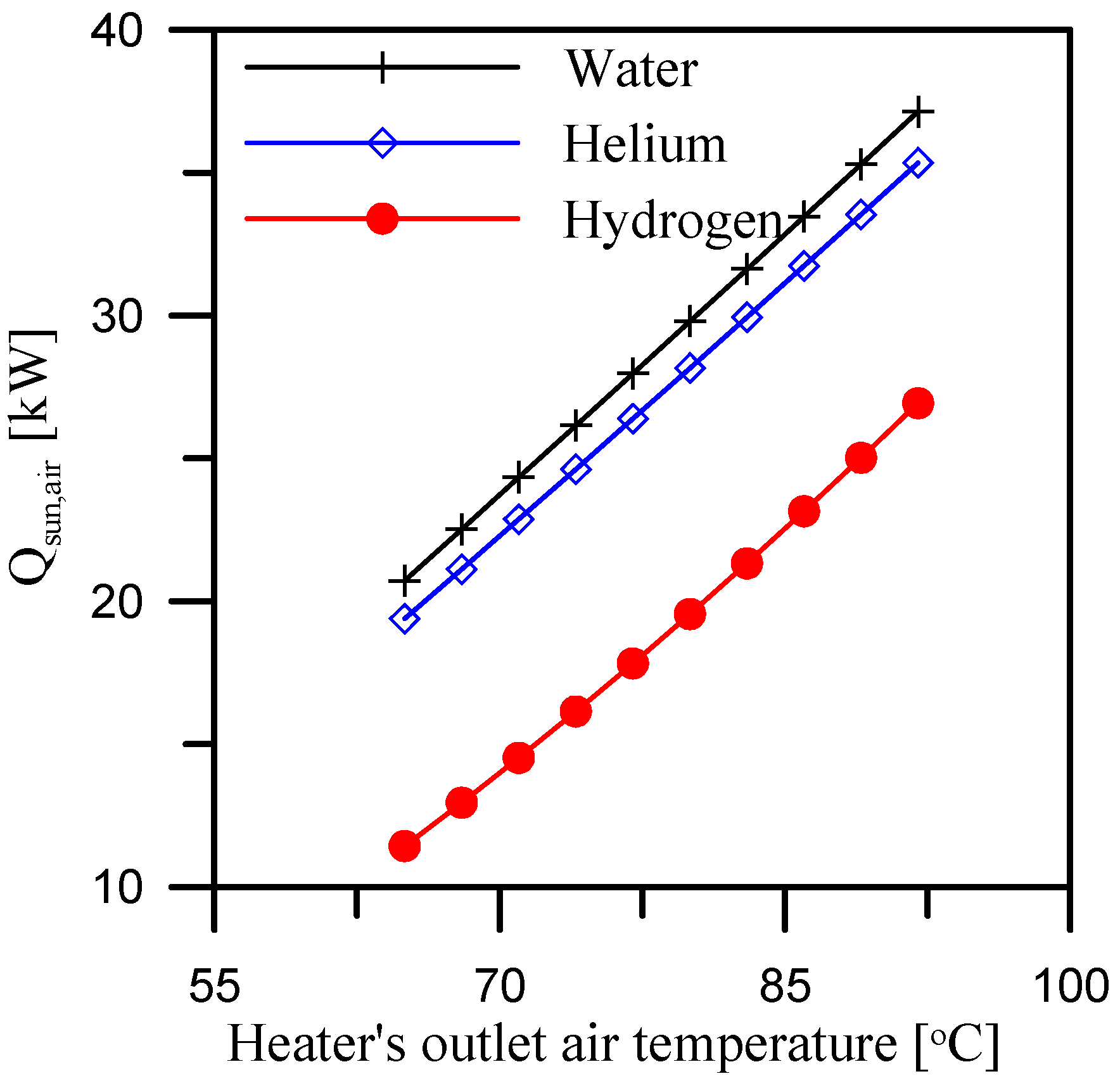
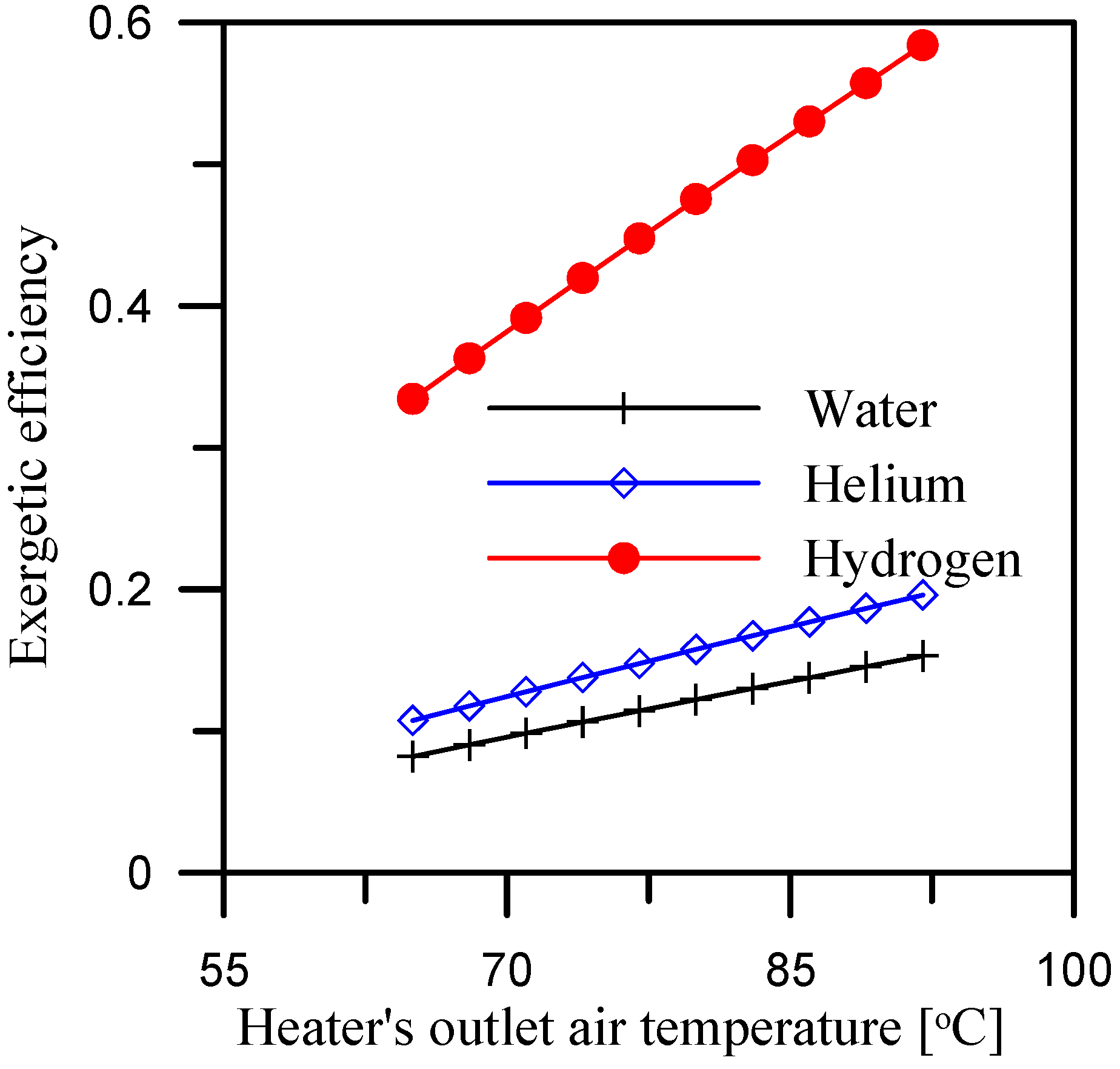
5.2. Effect of Heater’s Outlet Air Temperature
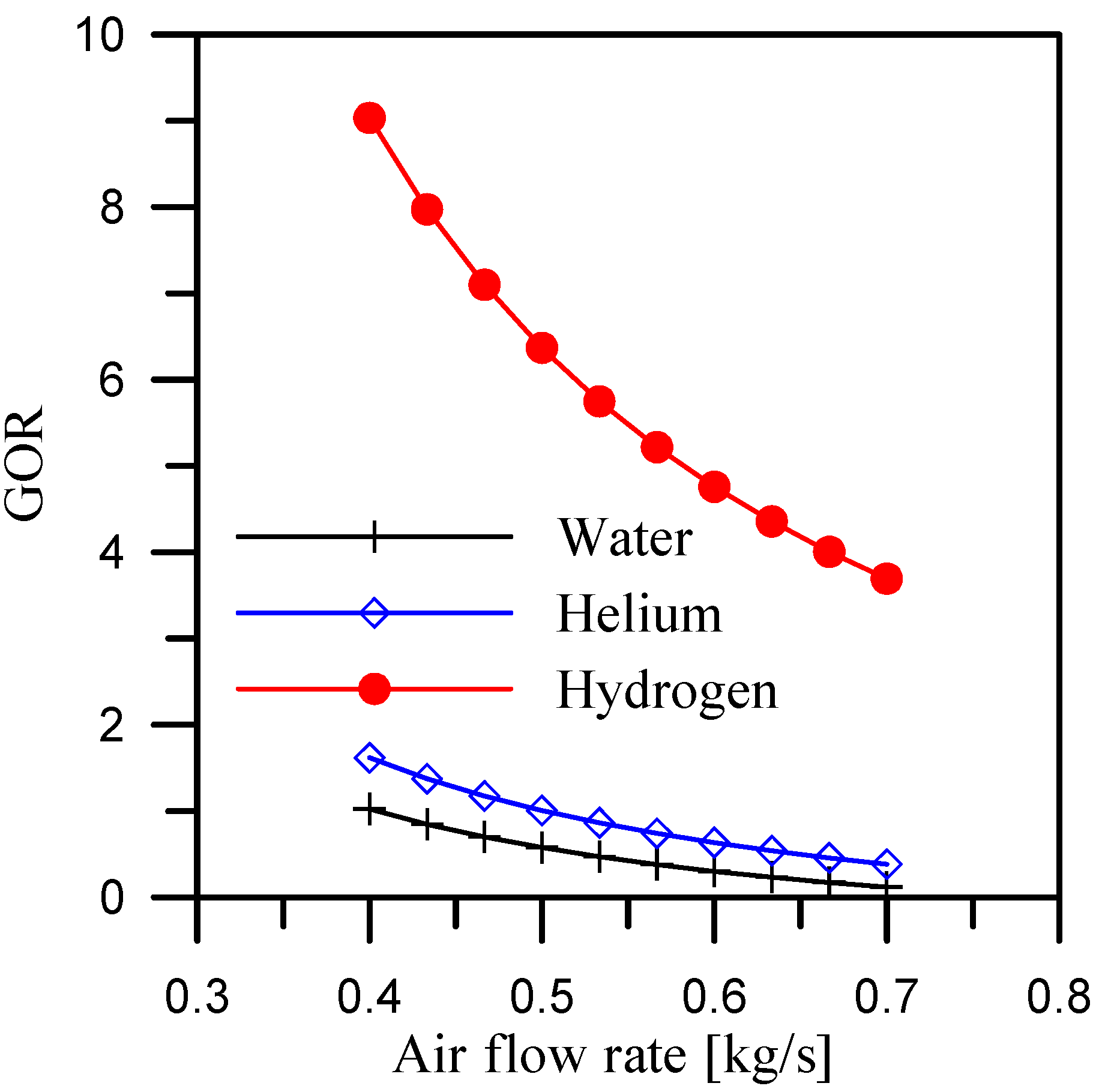
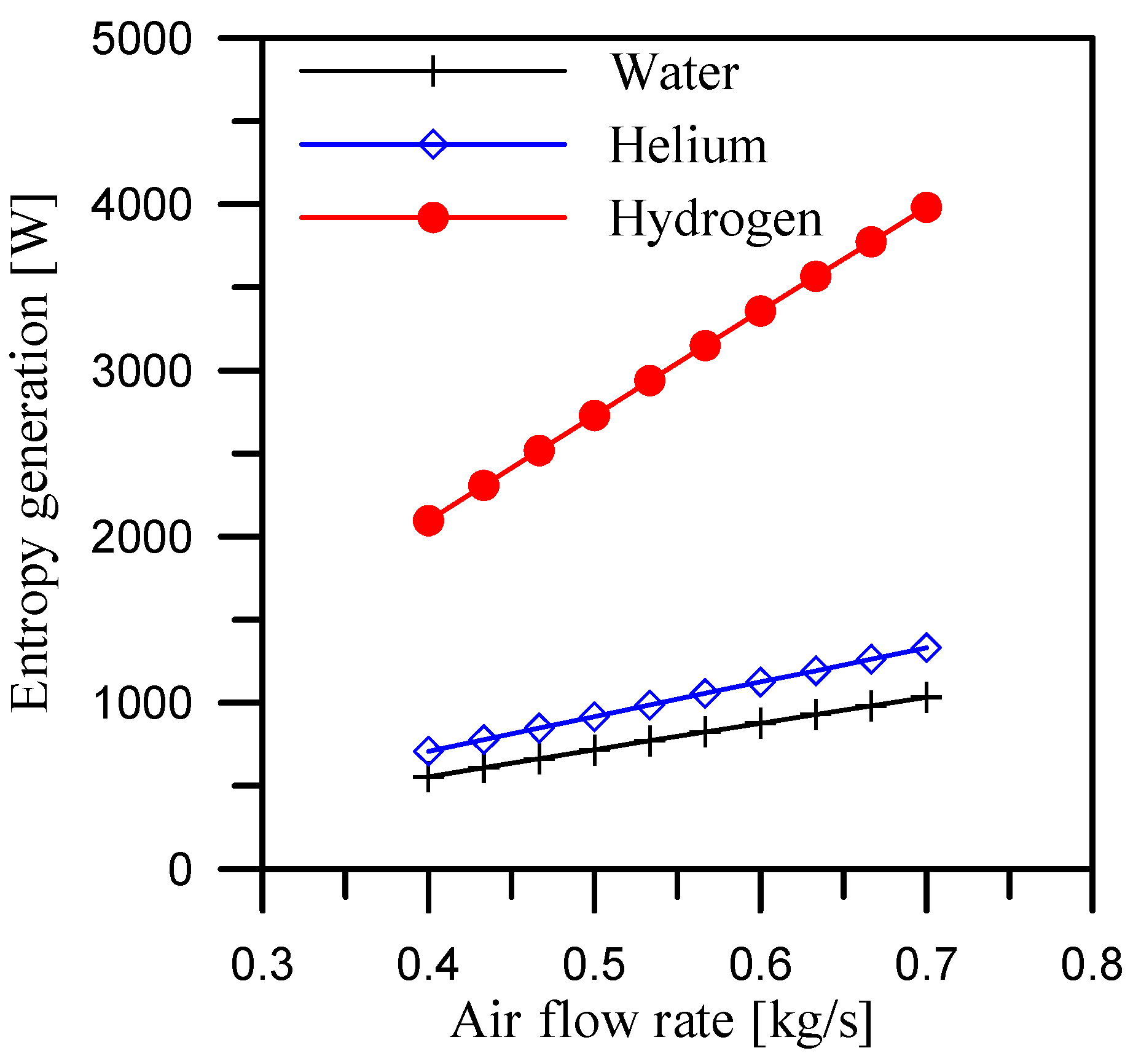
5.3. Effect of Air Flow Rate at Constant Mass Ratio
6. Conclusions
- When hydrogen is used as the coolant for the dehumidifier, the GOR is maximized. The average GOR across the heater outlet temperature range of 65 °C to 92 °C is 5.4, while it reaches 5.8 and 6.37, respectively, for air flow rate and mass ratio ranges of 0.4 to 0.7 kg/s and 2.1 to 3.
- When comparing the requirements for the three dehumidifier coolants, hydrogen has the lowest air heating rate. The average value of heat required for air when using hydrogen as a dehumidifier coolant is 34.7% and 30.9% less than when using water and helium as dehumidifier coolants over the study range of heater outlet air temperatures (65 °C to 92 °C).
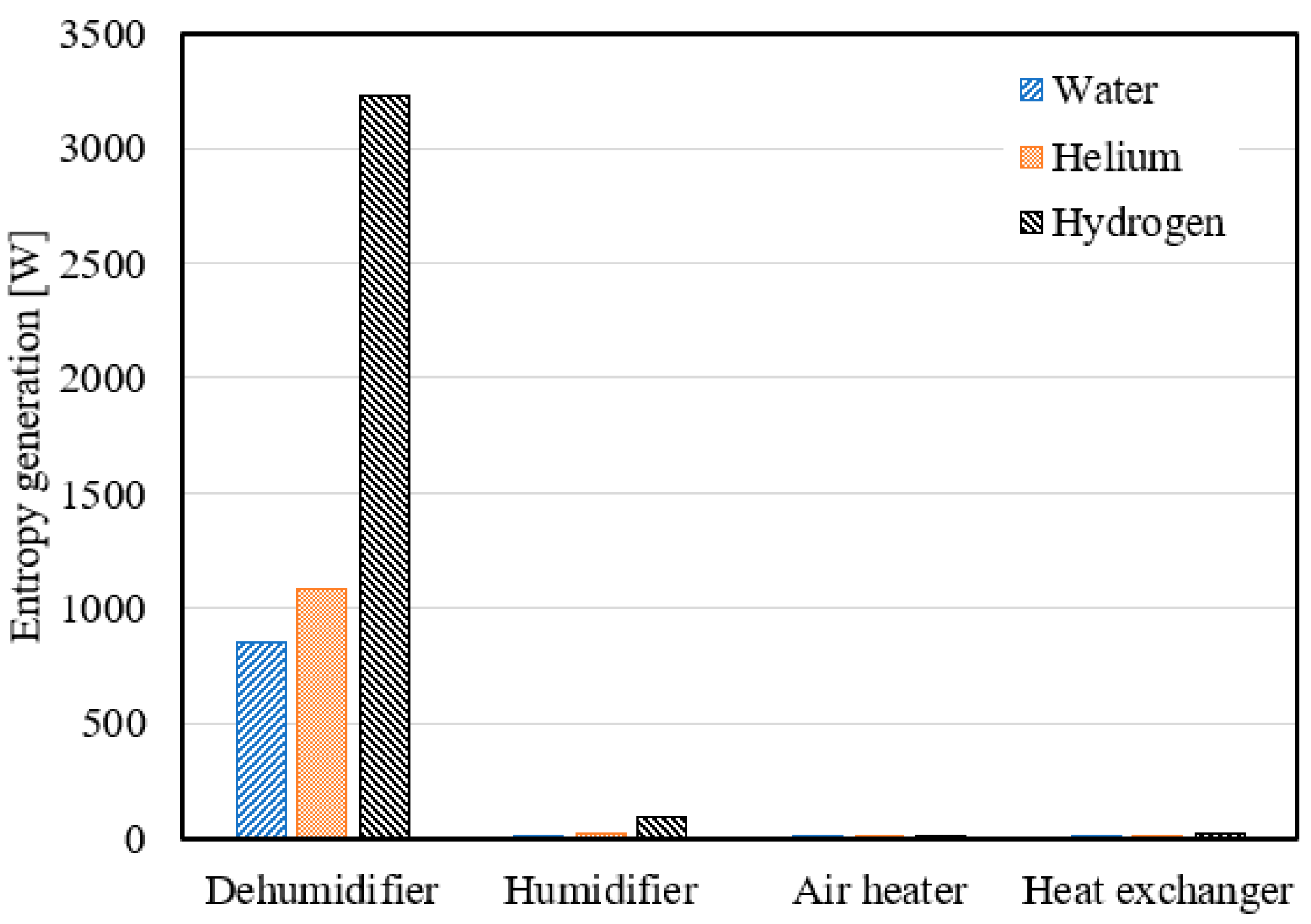
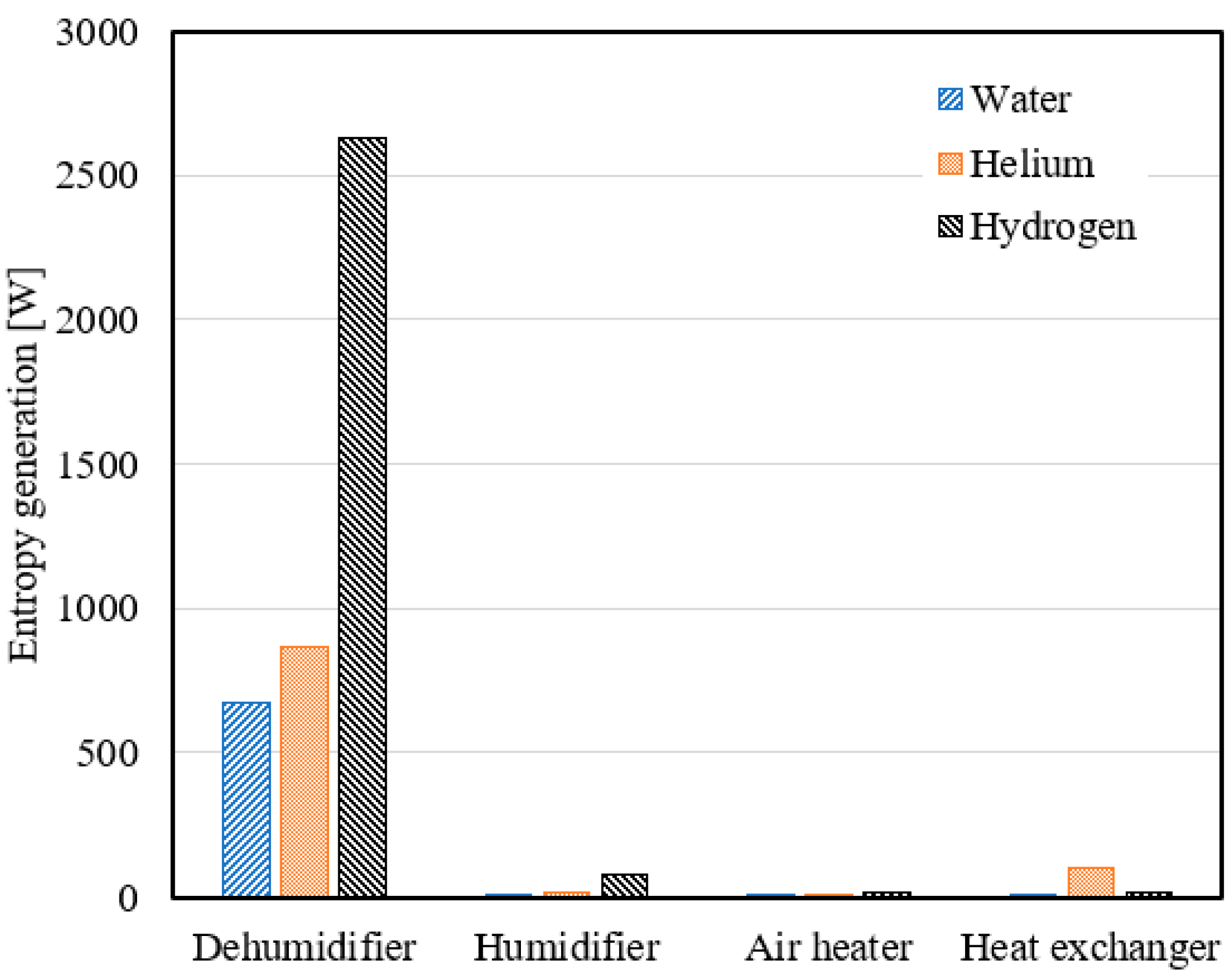
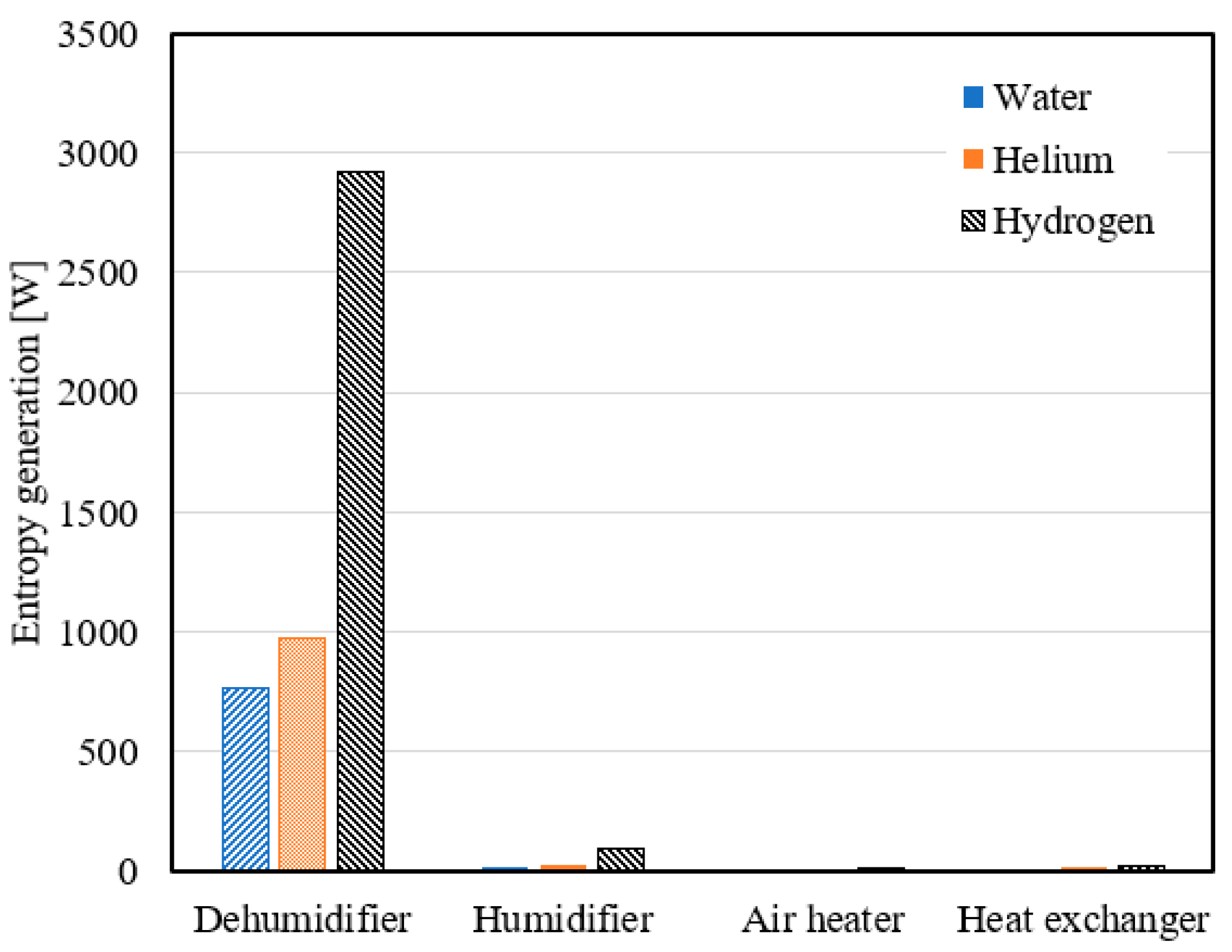
- Using hydrogen as a coolant in the dehumidifier resulted in average entropy generation of 3231.8 W, 2918.3 W, and 2626.9 W at a mass ratio range of 2.1 to 3, heater output temperature range of 65 °C to 92 °C, and air flow rate range of 0.4 to 0.7 kg/s, respectively.
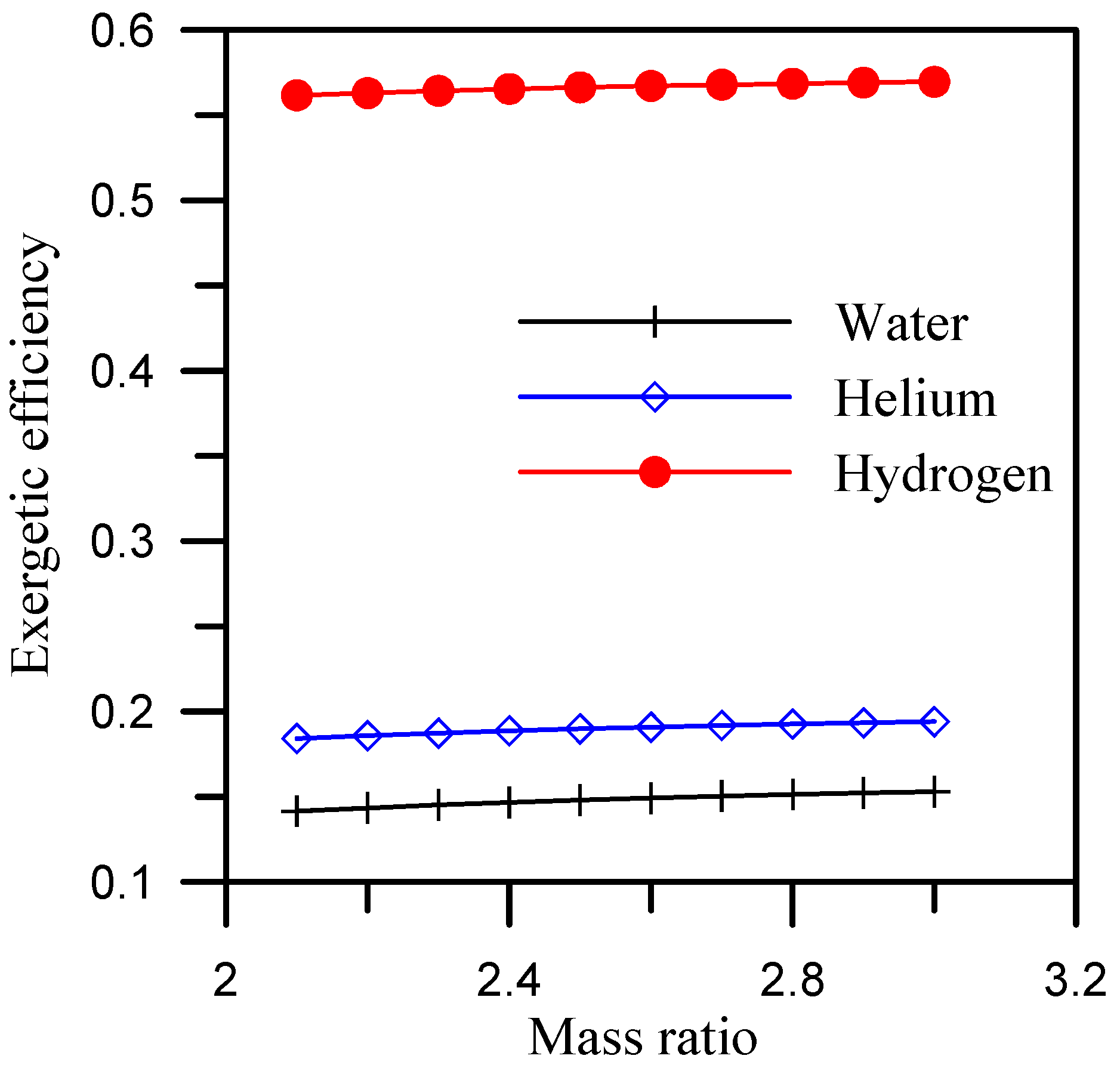
- A hydrogen-cooled dehumidifier has an exergetic efficiency of 0.55 and 0.46 over a range of mass ratio of 2.1 to 3 and range of heater outlet temperature of 65 °C to 92 °C, respectively.
- The system size will be affected when hydrogen is utilized as a coolant in a dehumidifier, but caution must be exercised because hydrogen is dangerous.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Nomenclatural | |
| Mass flow rate [kg/s] | |
| Heat rate [W] | |
| Entropy generation rate [W/K] | |
| Specific heat at constant volume [J/kg.K] | |
| GOR | Gained output ratio [-] |
| h | Specific enthalpy [J/kg] |
| hfg | Latent heat [J/kg] |
| s | Specific entropy [J/kg.K] |
| T | Temperature [K] |
| Absolute humidity [kg/kg dry air] | |
| Subscripts | |
| sw | Seawater |
| a | Air |
| b | Brine |
| f | Fresh water |
| g | Generation |
| o | Ambient |
| sun | Sun |
| in | Inlet |
| h | Humidifier |
| d | Dehumidifier |
| a_h | Air heater |
| HX | Heat exchanger |
| c | Coolant |
References
- Mazzucato, M.; Okonjo-Iweala, N.; Rockström, J.; Shanmugaratnam, T. Turning the Tide: A Call to Collective Action; Global Commission on the Economics of Water: Paris, France, 2023. [Google Scholar]
- Ahmed, M.A.; Zubair, S.M.; Abido, M.A.; Bahaidarah, H.M. An innovative closed-air closed-desiccant HDH system to extract water fromthe air: A case for zero-brine discharge system. Desalination 2018, 445, 236–248. [Google Scholar] [CrossRef]
- Ali, M.T.; Fath, H.E.S.; Armstrong, P.R. A comprehensive techno-economical review of indirect solar desalination. Renew. Sust. Energ. Rev. 2011, 15, 4187–4199. [Google Scholar] [CrossRef]
- Fritzmann, C.; Löwenberg, J.; Wintgens, T.; Melin, T. State-of-the-art of reverse osmosis desalination. Desalination 2007, 216, 1–76. [Google Scholar] [CrossRef]
- Narayan, G.P.; Sharqawy, M.H.; Summers, E.K.; Lienhard, V.J.H.; Zubair, S.M.; Antar, M.A. The potential of solar-driven humidification-dehumidification desalination for small-scale decentralized water production. Renew. Sust. Energ. Rev. 2010, 14, 1187–1201. [Google Scholar] [CrossRef]
- Klausner, J.F.; Mei, R.; Li, Y.; Knight, J.; Jogi, V. Innovative Fresh Water Production Process for Fossil Fuel Plants; University of Florida: Gainesville, FL, USA, 2005. Available online: https://www.osti.gov/servlets/purl/862097 (accessed on 25 June 2024). [CrossRef]
- Al-Enezi, G.; Ettouney, H.; Fawzy, N. Low temperature humidification dehumidification desalination process. Energy Convers. Manag. 2006, 47, 470–484. [Google Scholar] [CrossRef]
- Li, X.; Yuan, G.; Wang, Z.; Li, H.; Xu, Z. Experimental study on a humidification and dehumidification desalination system of solar air heater with evacuated tubes. Desalination 2014, 351, 1–8. [Google Scholar] [CrossRef]
- Yıldırım, C.; Solmus, I. A parametric study on a humidification dehumidification (HDH) desalination unit powered by solar air and water heaters. Energy Convers. Manag. 2014, 86, 568–575. [Google Scholar] [CrossRef]
- Shatat, M.; Omer, S.; Gillott, M.; Riffat, S. Theoretical simulation of small scale psychometric solar water desalination system in semi-arid region. Appl. Therm. Eng. 2013, 59, 232–242. [Google Scholar] [CrossRef]
- Kumar, S.; Dubey, A.; Tiwari, G.N. A solar still augmented with an evacuated tube collector in forced mode. Desalination 2014, 347, 15–24. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Ming, G.; Shen, S.; Cao, G. Thermal and economic analyses of solar desalination system with evacuated tube collectors. Sol. Energy 2013, 93, 144–150. [Google Scholar] [CrossRef]
- Al-Sulaimana, F.A.; Zubair, I.M.; Atif, M.; Gandhidasan, P.; Al-Dini, S.A.; Antar, M.A. Humidification dehumidification desalination system using parabolic trough solar air collector. Appl. Therm. Eng. 2015, 75, 809–816. [Google Scholar] [CrossRef]
- Mohamed, A.M.I.; El-Minshawy, N.A. Theoretical investigation of solar humidification–dehumidification desalination system using parabolic trough concentrators. Energy Convers. Manag. 2011, 52, 3112–3119. [Google Scholar] [CrossRef]
- Dayem, A.M.A.; Fatouh, M. Experimental and numerical investigation of humidification/dehumidification solar water desalination systems. Desalination 2009, 247, 594–609. [Google Scholar] [CrossRef]
- Gabrielli, P.; Gazzanib, M.; Novatic, N.; Sutter, L.; Simonetti, R.; Molinaroli, L.; Manzolini, G.; Mazzotti, M. Combined water desalination and electricity generation through a humidification-dehumidification process integrated with photovoltaic-thermal modules: Design, performance analysis and techno-economic assessment. Energy Convers. Manag. X 2019, 1, 100004. [Google Scholar] [CrossRef]
- Muthusamy, C.; Srithar, K. Energy and exergy analysis for a humidification–dehumidification desalination system integrated with multiple inserts. Desalination 2015, 367, 49–59. [Google Scholar] [CrossRef]
- Raj, P.R.; Jayakumar, J.S. Performance analysis of humidifier packing for humidification dehumidification desalination system. Therm. Sci. Eng. Prog. 2022, 27, 101118. [Google Scholar]
- He, W.F.; Chen, J.J.; Han, D.; Luo, L.T.; Wang, X.C.; Zhang, Q.Y.; Yao, S.Y. Energetic, entropic and economic analysis of an open-air, open-water humidification dehumidification desalination system with a packing bed dehumidifier. Energy Convers. Manag. 2019, 199, 112016. [Google Scholar] [CrossRef]
- Elsafi, A.M. Integration of humidification-dehumidification desalination and concentrated photovoltaic-thermal collectors: Energy and exergy-costing analysis. Desalination 2017, 424, 17–26. [Google Scholar] [CrossRef]
- Alrbai, M.; Enizat, J.; Hayajneh, H.; Qawasmeh, B.; Al-Dahidi, S. Energy and exergy analysis of a novel humidification-dehumidification desalination system with fogging technique. Desalination 2022, 522, 115421. [Google Scholar] [CrossRef]
- Garg, K.; Das, S.; Tyagi, H. Thermal design of a humidification-dehumidification desalination cycle consisting of packed-bed humidifier and finned-tub e dehumidifier. Int. J. Heat Mass Transf. 2022, 183, 122153. [Google Scholar] [CrossRef]
- Saha, P.; Tan, X.; Klausner, J.; Abbasi, B.; Benard, A. Comparative investigation on direct contact crossflow and direct contact counterflow packed beds condensers: Experimental and modeling analyses. Appl. Therm. Eng. 2024, 236, 121777. [Google Scholar] [CrossRef]
- Nada, S.A.; Fouda, A.; Mahmoud, M.A.; Elattar, H.F. Experimental investigation of air-conditioning and HDH desalination hybrid system using new packing pad humidifier and strips-finned helical coil. Appl. Therm. Eng. 2021, 185, 116433. [Google Scholar] [CrossRef]
- Hu, T.; Hassabou, A.H.; Spinnler, M.; Polifke, W. Performance analysis and optimization of direct contact condensation in a PCM fixed bed regenerator. Desalination 2011, 280, 232–243. [Google Scholar] [CrossRef]
- Tan, X.; Saha, P.; Klausner, J.; Abbasi, B.; Benard, A. Modeling and experimental validation of direct contact crossflow packed beds condenser used in HDH desalination systems. Desalination 2023, 548, 116297. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, H.; Liang, S.; Zhang, N.; Ma, X. Experimental research on four-stage cross flow humidification dehumidification (HDH) solar desalination system with direct contact dehumidifiers. Desalination 2019, 467, 147–157. [Google Scholar] [CrossRef]
- He, W.F.; Wu, F.; Wen, T.; Kong, Y.P.; Han, D. Cost analysis of a humidification dehumidification desalination system with a packed bed dehumidifier. Energy Convers. Manag. 2018, 171, 452–460. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, C.; Zhang, H.; Zheng, W.; You, S.; Zhen, Y. Experimental study of a humidification dehumidification desalination system with heat pump unit. Desalination 2018, 442, 108–117. [Google Scholar] [CrossRef]
- Capocelli, M.; Balsamo, M.; Mazzeo, L.; Luberti, M. Thermodynamic analysis of the humidification-dehumidification-adsorption (HDHA) desalination process. Desalination 2023, 554, 116510. [Google Scholar] [CrossRef]
- Capocelli, M.; Balsamo, M.; Lancia, A.; Barba, D. Process analysis of a novel humidification-dehumidification-adsorption (HDHA) desalination method. Desalination 2018, 429, 155–166. [Google Scholar] [CrossRef]
- Klein, S.A. Engineering Equation Solver (EES) V9; F-Chart Software: Madison, WI, USA, 2015; Available online: http://www.fchart.com (accessed on 16 May 2024).
- Narayan, G.P.; Sharqawy, M.H.; Lienhard, V.J.H.; Zubair, S.M. Thermodynamic analysis of humidification dehumidification desalination cycles. Desalination Water Treat. 2010, 16, 339–353. [Google Scholar] [CrossRef]
- Milton, M.; Manuel, R.; Rodrigo, C. Entropy generation analysis for the design of a flat plate solar collector with fins. Dyna 2020, 87, 199–208. [Google Scholar]
- Ge, Z.; Wang, H.; Wang, H.; Zhang, S.; Guan, X. Exergy Analysis of Flat Plate Solar Collectors. Entropy 2014, 16, 2549–2567. [Google Scholar] [CrossRef]







| Properties | Water | Helium | Hydrogen |
|---|---|---|---|
| Molecular weight [g/mole] | 18.02 | 4.003 | 2.016 |
| Density ρ [kg/m3] | 997.1 | 0.164 | 0.0823 |
| Specific heat Cp [J/kg K] | 4183 | 5193 | 14306 |
| viscosity µ [kg/m s] | 8.9 × 10−4 | 1.98 × 10−5 | 9.01 × 10−6 |
| Thermal conductivity k [W/m k] | 0.595 | 0.1553 | 0.1769 |
| Thermal diffusivity | 1.4 × 10−7 | 1.8 × 10−4 | 1.5 × 10−4 |
| Flammability | Non | Non | Extremely |
| Parameters | Range |
|---|---|
| Coolant mass flow rate | 0.2 [kg/s] |
| Coolant inlet temperature (Tc4) | 313 [K] |
| Relative air humidity at the inlet (RHa1) | 1 |
| Relative air humidity at the outlet (RHa2) | 1 |
| Seawater inlet temperature (Two) | 303 [°C] |
| Mass ratio | 2.1:3 |
| Ambient temperature (To) | 27 [°C] |
| Parameters | Naryan et al. [33] | Simulation | Error % |
|---|---|---|---|
| mcond | 0.0059 | 0.00594 | 0.67797% |
| Ta1 | 34.2 | 34.17 | 0.08772% |
| Ta2 | 51.4 | 51.38 | 0.03891% |
| Tw1 | 62.79 | 63.15 | 0.57334% |
| Tw2 | 37.05 | 37.07 | 0.05398% |
| mb | 0.144 | 0.144 | 0% |
| GOR | 2.9 | 2.93 | 1.03448% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdala, A.M.M.; Elwekeel, F.N.M.; Taccani, R. The Effect of Hydrogen as a Coolant on the Characteristics of Humidification-Dehumidification Desalination Systems. Energies 2024, 17, 3593. https://doi.org/10.3390/en17143593
Abdala AMM, Elwekeel FNM, Taccani R. The Effect of Hydrogen as a Coolant on the Characteristics of Humidification-Dehumidification Desalination Systems. Energies. 2024; 17(14):3593. https://doi.org/10.3390/en17143593
Chicago/Turabian StyleAbdala, Antar M. M., Fifi N. M. Elwekeel, and Rodolfo Taccani. 2024. "The Effect of Hydrogen as a Coolant on the Characteristics of Humidification-Dehumidification Desalination Systems" Energies 17, no. 14: 3593. https://doi.org/10.3390/en17143593
APA StyleAbdala, A. M. M., Elwekeel, F. N. M., & Taccani, R. (2024). The Effect of Hydrogen as a Coolant on the Characteristics of Humidification-Dehumidification Desalination Systems. Energies, 17(14), 3593. https://doi.org/10.3390/en17143593







