Abstract
Food loss (FL) and food waste (FW) have become severe global problems, contributing to resource inefficiency and environmental degradation. Approximately 6% of greenhouse gas emissions (GHGs) are derived from FW, which is usually discarded in landfills, emitting methane, a gas that is 28 times more harmful than CO2. Diverting the path of FW towards the energy industry represents a promising avenue to mitigate the environmental impact and save resources while generating energy substitutes. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) approach was utilized to conduct a systematic literature review on 10 different conversion processes used to convert FL and FW into energy. Anaerobic bioconversion integrated with pyrolysis emerges as a potential eco-friendly and promising solution for FW management, nutrient recovery and energy production in various forms, including biogas, heat, biohydrogen and biochar. Despite its potential, the anaerobic digestion of FW still faces some challenges related to the production of intermediate harmful compounds (VOCs, NH3, H2S), which necessitate precise process control and optimization. Nonetheless, converting FW into energy can provide economic and environmental benefits in the context of the circular economy. This review offers insightful information to stakeholders, academics and policymakers who are interested in utilizing FW as a means of producing sustainable energy by summarizing the important findings of ten different waste-to-energy processing methods and their potential for improved energy recovery efficiency.
1. Introduction
Food consumption is an integral part of human survival, but, despite great progress in the global food chain through the expansion of international trade, large-scale agricultural production and increased yield efficiency, a large proportion of food is still discarded as waste. Globally, an estimated 1.3 billion tons of food are lost within the supply chain annually, with an estimated total economic impact of around USD 1 trillion [1]. The increasing trend of food loss (FL) and food waste (FW) in the past decade necessitates the implementation of effective waste reduction techniques [2]. FL and FW represent nearly 40% of the municipal solid waste (MSW) generated globally, carrying significant implications for post-management and processing [3,4].
The U.S. generated approximately 103 million tons of FW in 2018, originating from the industrial (39%), residential (24%), commercial (25%) and institutional (5%) sectors [5]. Food waste management mainly consisted of landfilling (36%), repurposing for animal feed (21%) and anaerobic digestion (10%), and only a small fraction was donated (7%) or used to recover energy through incineration (8%) [5]. Although the majority of FW every year is produced in China (92 million tons) and India (69 million tons), the amount of FW per person is less than 70 kg [6,7]. The European Union (EU) produces 58 million tons of FW per year (10% of available food), with market value of EUR 132 billion [8]. This value is equivalent to around 131 kg/person, while, at the same time, 37 million people are unable to afford a nutritious meal every other day [9].
Agriculture residues, along with vegetables and fruits discarded due to quality degradation in the stages before reaching the consumer (production, post-harvest and processing), are classified as FL [10]. In contrast, FW occurs at the consumption level (household, hospitality sector, retail) and refers to unconsumed edible food, discarded mainly for aesthetic, quality and safety reasons [11]. The EU Directive 2008/851 (2018) defines FW as “Food product (including inedible parts) lost in the food supply chain, excluding food redirected to uses of materials such as bio-products, animal feed, or intended for redistribution” [12].
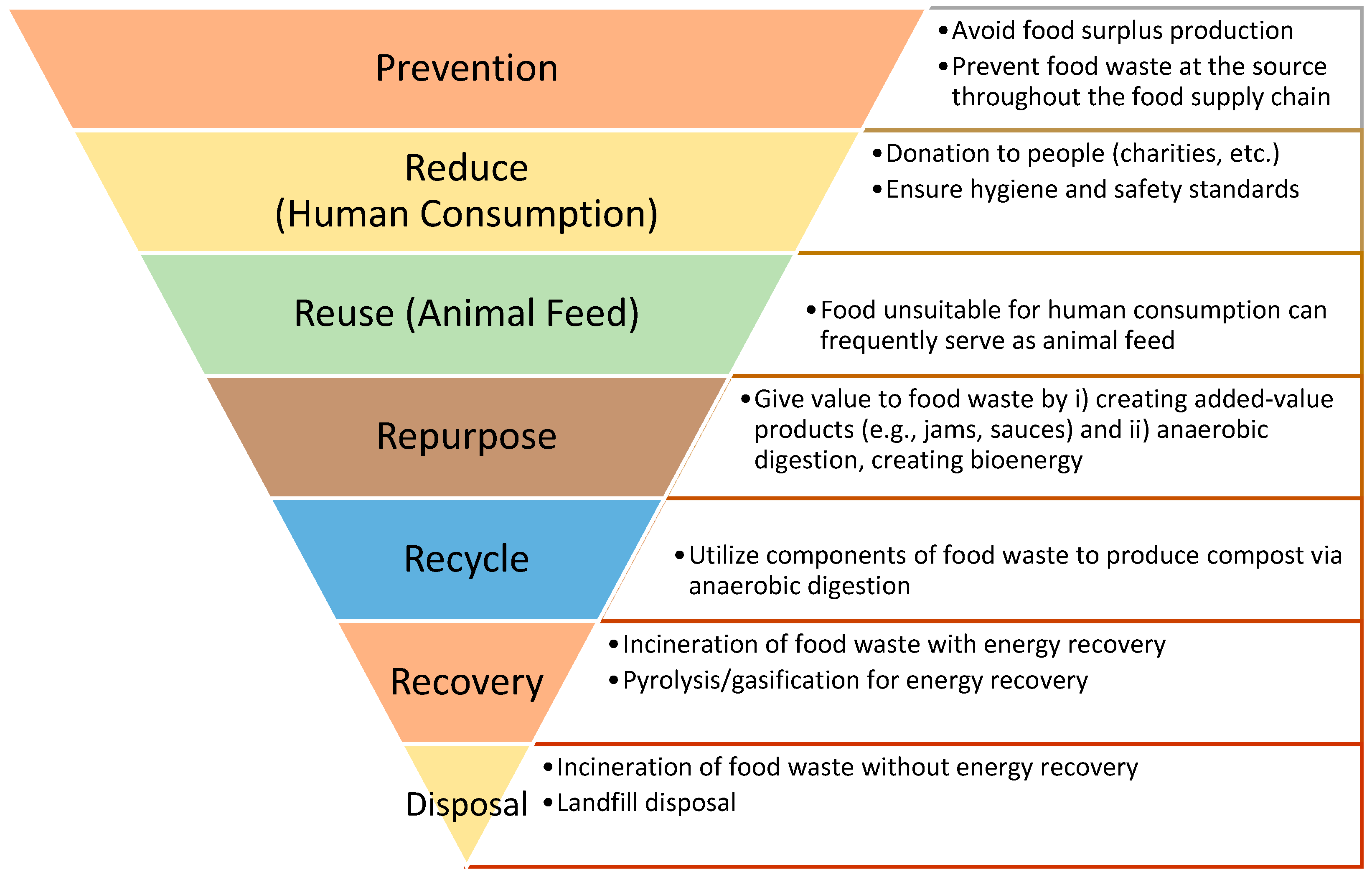
The new Waste Framework Directive proposal requires Member States to implement food waste prevention strategies based on the waste hierarchy framework (Figure 1) in an effort to reduce food waste throughout the food supply chain [13,14]. Adopted in March 2020, Europe’s “Circular Economy Action Plan” focuses on product circularity across its whole life cycle, especially end-of-life treatment [15]. Food waste reduction techniques include (i) reducing the amount sent to landfill, (ii) redistributing food, (iii) treating bio-waste or reusing it as animal feed or fertilizer and (iv) bio-waste treatment measures to decrease the volume sent to landfill [16]. Therefore, as indicated in the frameworks, it is clear that implementing measures to reduce waste and capitalize on by-products and co-products is critical for an effective food waste management system. These measures can improve circularity throughout the food waste management system, while resolving environmental concerns effectively.
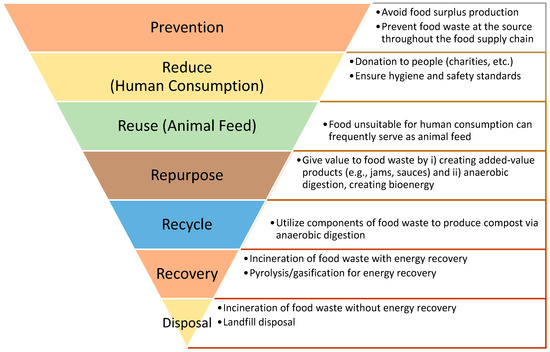
Figure 1.
Food waste hierarchy diagram.
The exploration of new solutions for FL and FW disposal is also in line with the Sustainable Development Goals set by the UN, especially those regarding zero hunger (SDG 2); clean and affordable energy (SDG 7); economic growth and decent employment (SDG 8); infrastructure, industry and innovation (SDG 9); and sustainable cities and communities (SDG 11).
The global issue of food waste has prompted researchers to explore innovative solutions for its management. One promising avenue is the conversion of food waste into energy. This review aims to assess the current technologies available for this purpose and evaluate their effectiveness and environmental benefits. Despite numerous studies on waste-to-energy technologies, there is a lack of comprehensive reviews focusing specifically on food waste as an energy source.
FL and FW Composition
FL and FW are an appealing alternative option for organic feedstock in energy production processes, because it avoids competition with food crops, reduces FW disposal to landfill and lowers the overall carbon footprint. Despite this, the heterogenicity present in FW and FL mixtures makes the energy conversion process challenging [17]. FW feedstocks usually include a wide range of organic waste generated in different value chains, with different moisture levels, nutrient content and physical properties [18]. Seasonal variations in food production, cultural preferences and socio-economic factors can also affect the FW composition [19,20,21]. In general, FW and FL are composed of 41–62% carbohydrates, 15–25% protein and 13–30% lipids and organic acids [22,23]. Fat exhibits the highest theoretical methane potential at 1014 L/kg volatile solids (Vs), followed by protein (496 L/kg Vs) and carbohydrates (415 L/kg Vs) [24].
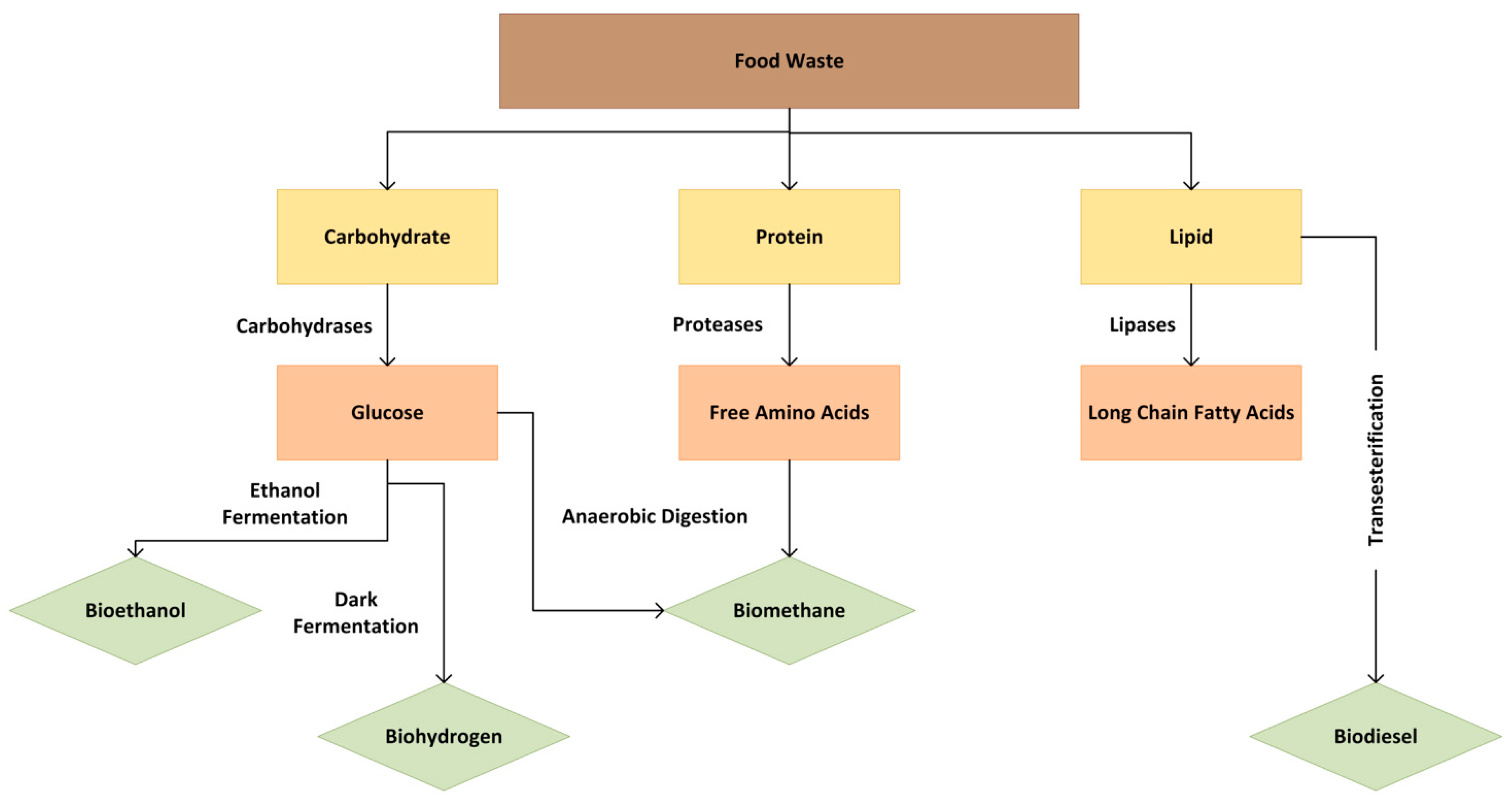
The carbohydrate and protein fractions can be hydrolyzed to obtain fermentable sugars and free amino acids, respectively, while lipids are broken down into long-chain fatty acids (Figure 2). FW energy products include biogas, which can be used for heating and electricity generation; biodiesel and bioethanol, used as automotive or jet fuel; and syngas, which can be further processed into fuels or chemicals. FW processing for energy also produces biochar, a carbon-rich material that can be utilized for soil amendment or energy generation depending on the subsequent treatment.
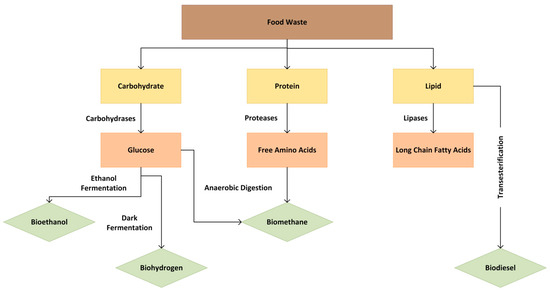
Figure 2.
Converting FW components into energy.
2. Methodology
The Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA), renowned for its systematic approach to literature reviews, was selected to ensure reliability and precision in summarizing the evidence. The PRISMA 2020 checklist comprises 7 sections with 27 items to ensure the comprehensive inclusion of relevant information in the review [25].
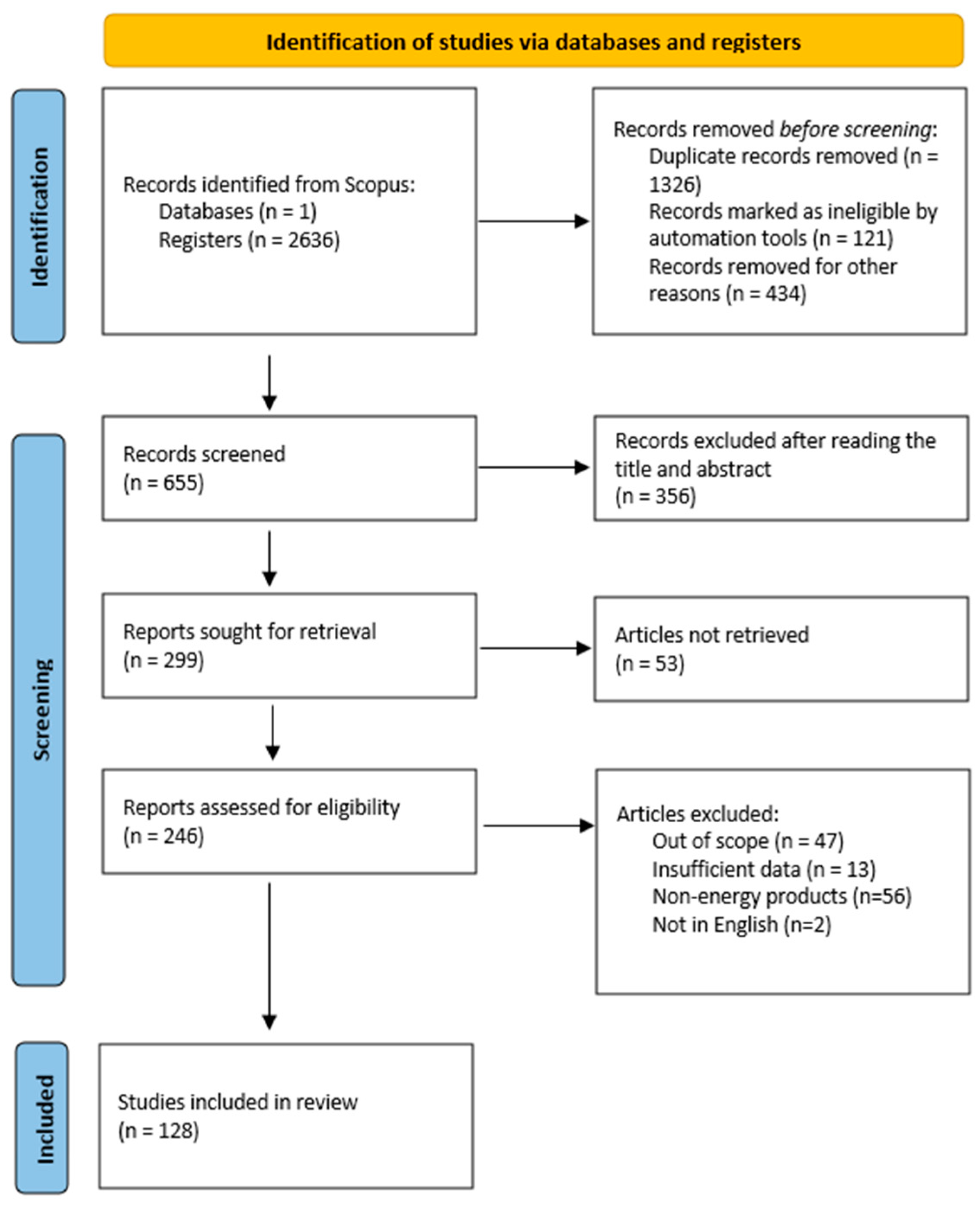
The database of Scopus was chosen to perform the literature review due to its extensive use in scientific research, its advanced search capabilities and the wide range of scientific papers available from various disciplines. The search was conducted in April 2024 using the “title, abstract, keyword” option with the following search string: (“food waste” OR “food loss”) AND (“energy recovery” OR “valorization”). In total, 2636 articles were cross-checked to remove any duplicate publications, using the Mendeley software (Figure 3). Preliminary screening was carried out by reading the titles of the articles and, if required, assessing their abstracts. The selection of studies was based, inter alia, on their relevance, recency and methodological rigor.
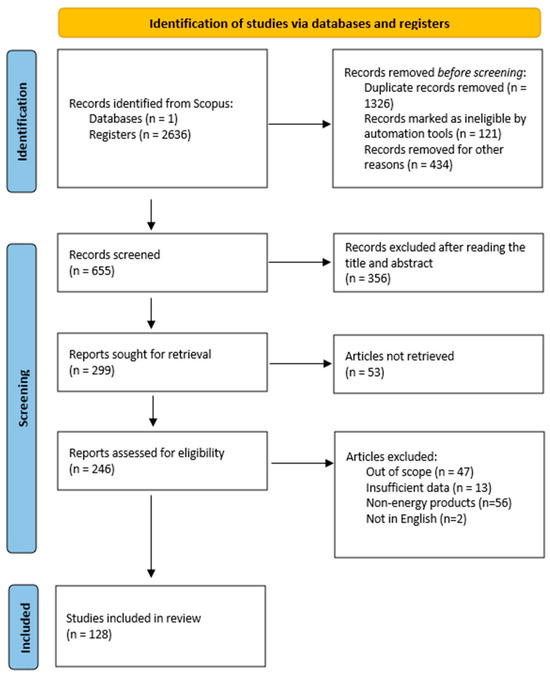
Figure 3.
PRISMA statement report for the current study.
In particular, papers were selected for full-text analysis according to the following eligibility criteria to ensure the inclusion of the most relevant articles. The inclusion criteria encompassed (a) research related to FW-to-energy methods; (b) studies reporting on outcomes related to energy generation, environmental impacts and technological advancements; and (c) articles published from 2018 until the present. The exclusion criteria covered the following: (a) non-peer reviewed literature, (b) studies that did not provide justified results or included insufficient data, (c) studies whose conversion products did noy have energy content and (d) articles not available in English.
3. Energy Recovery from FL and FW
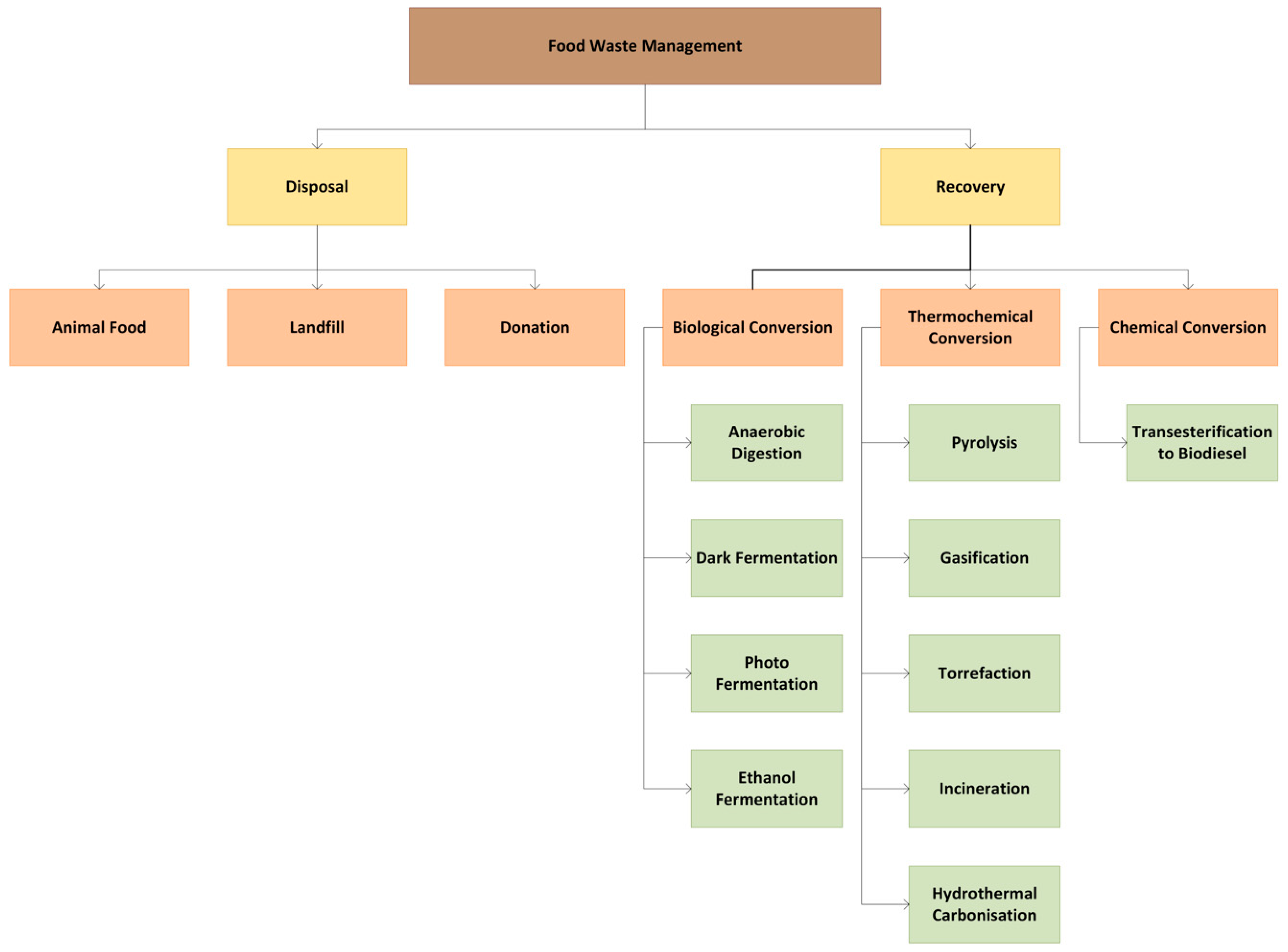
This article includes a comprehensive review on the concept of FL- and FW-to-energy treatment methods, the recovery impacts and the energy yield efficiency of each method. A total of ten energy conversion techniques from organic waste are discussed and categorized into three main categories based on the process’ underlying reactions (Figure 4): (i) thermochemical conversion, (ii) biological conversion and (iii) chemical conversion. The major thermochemical techniques include pyrolysis, incineration, gasification, torrefaction and hydrothermal carbonization. The biological conversion processes discussed are anaerobic digestion, dark fermentation, photofermentation and ethanol fermentation, while chemical conversion is represented solely by transesterification. The complexities and novelties of the aforementioned processes are included to provide insights into sustainable waste management practices, highlighting their potential to enhance the energy recovery efficiency in line with the circular economy principles.
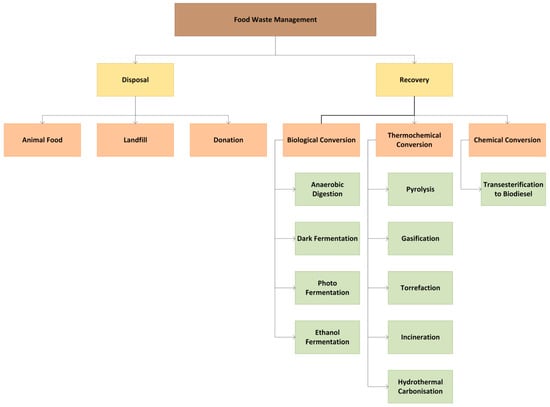
Figure 4.
FW management and treatment options.
Recent studies have examined aspects like technological advancements, policy frameworks, economic viability and the environmental impacts of waste-to-energy conversion or specific waste-to-energy treatment processes [25,26,27]. However, no recent review has provided a comprehensive review of the latest advancements in technological treatments converting FW and FL into energy [28,29].
- Biological Conversion Processes
3.1. Anaerobic Digestion (AD)
Anaerobic digestion (AD) is a complicated biological process in which microorganisms break down organic molecules to simple substances in the absence of oxygen, resulting in the production of biogas and other by-products [30]. The need for sustainable FW management and the growing significance of renewable energy has provided favorable conditions for the development of efficient AD systems, increasing the overall global production of biogas over the years. In the UK, the installed capacity of anaerobic digestion has increased to 300,000 kW from just 40,000 kW in 2013 [31]. There are around 20,000 biogas plants currently operational in Europe, collectively producing 12,173 kilotons of oil equivalents. Germany leads the way, accounting for approximately 55% of this production, followed by France [32,33]. There is potential for sustainable biogas production to provide up to 600 Mtoe of low-carbon energy for a variety of industries, with crop residues (agriculture) providing half of this. The AD performance results in negative GHG emissions in the range of −32g CO2 per ton of FW [34]. AD pathways with combined cycles (CC) and combined heat and power (CHP) have the potential to reduce the GHG emissions by −289.1 kg and −326.6 kg of CO2 per ton of FW, respectively [35]. Several methods and techniques have been applied for the production of energy products, with different production yields and conversion efficiencies (Table 1).

Table 1.
Production yield and efficiency of energy products from FW.
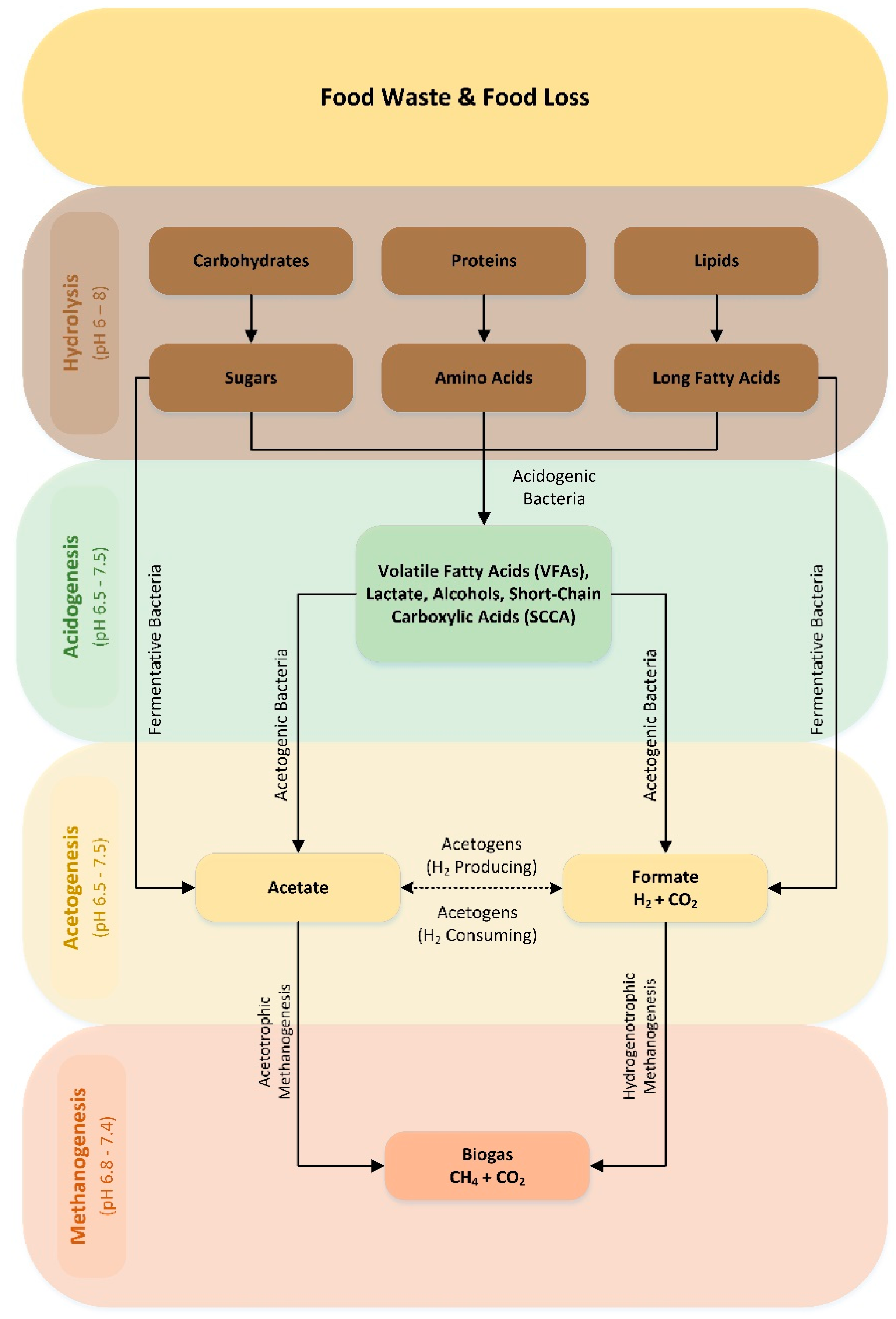
AD is applied as a biological treatment for organic waste such as municipal solid waste, FW, industrial waste, sewage sludge, animal manure and agriculture waste, transforming it into biogas [46]. Biogas is mainly composed of 50–75% methane (CH4) and 25–50% carbon dioxide (CO2), along with hydrogen sulfide (H2S), hydrogen (H2), ammonia (NH3) (1–2%) and small amounts of other gases, like oxygen (O2) and nitrogen (N2) [47]. The purification of biogas can increase the level of methane content to 99%, resulting in the formation of biomethane, which is suitable for injection into natural gas grids, as a vehicle fuel for transportation or simply for electricity production [48]. AD comprises a complex four-stage process that involves multiple stages of physicochemical and biochemical reactions occurring in sequential and parallel routes (Figure 5): (i) hydrolysis, (ii) acidogenesis, (iii) acetogenesis and (iv) methanogenesis [49,50].
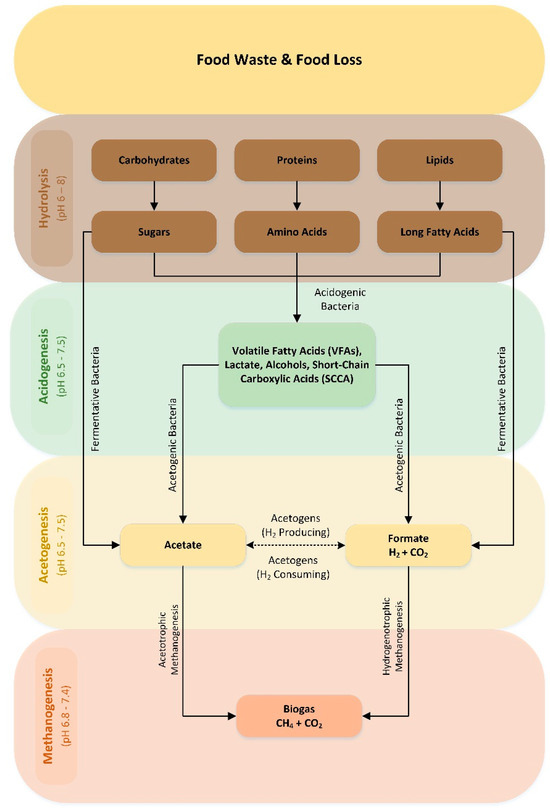
Figure 5.
AD process flow diagram.
The feedstock characteristics, such as the nutrient content, particle size and presence of inhibitory compounds, affect anaerobic digestion. The organic loading rate, pH, temperature, retention time, mixing, hydrogen concentration, moisture content, quality and quantity of inoculum, bioreactor and process configuration are the process parameters that influence biogas production [51,52].
3.1.1. Hydrolysis
AD begins with the hydrolysis of FW substrates, where those with a high molecular weight, like carbohydrates and protein, as well as lipids, are converted into smaller compounds such as peptides, amino acids and soluble sugars or fatty acids [53]. The first stage is carried out by bacteria (Bacteroidetes, Firmicutes and Proteobacteria) that secrete hydrolytic enzymes, followed by anaerobic fungi like Neocalimastix, Piromyces and Orpinomyces, which are able to degrade cellulosic material. The hydrolysis rate is influenced by the bacterial concentration, enzyme production and adsorption on the surface of the substrate. FW pretreatment can increase the available surface area for bacterial activity, enhancing biogas production. The optimum environmental conditions, with a pH ranging between 5 and 7 and a temperature of 30 °C to 50 °C, further enhance the microbial activity [54]. Due to the complex nature of feedstocks, the hydrolysis phase is considered a rate-limiting step.
To address this limitation and speed up the hydrolysis rate, a pretreatment process is used on the substrate before undergoing this step to increase the FW solubility by weakening its cell wall structure [55]. Moreover, this process increases the surface area of the FW substrate, improving the biodegradability of the FW, resulting in higher biogas yields and reducing the retention time. The FW pretreatment methods can be categorized into mechanical, chemical, physical and biological pretreatments. Table 2 shows the methods employed in each category, along with their benefits and limitations [49].

Table 2.
Pretreatment methods involved in AD.
3.1.2. Acidogenesis
The hydrolysis reaction is catalyzed in the next phase by acidogenic bacteria. During acidogenesis, the substrates generated from hydrolysis are converted into intermediate fermented products. Amino acids, soluble sugars and fatty acids are further decomposed into short-chain carboxylic acids (SCCA), alcohols and volatile fatty acids (VFA) such as acetate, butyrate, isobutyrate, propionate and valerate [60]. The by-products from the acidogenesis process are CO2, H2S and NH3. VFA production is carried out by fermentative microorganisms belonging to the genera of Bacillus, Salmonella, Streptococcus, Escherichia and Lactobacillus [61]. An increase in the VFA concentration is responsible for the pH drop to between 4.5 and 5.5, activating the acidogenic and acetogenic bacteria to produce acids.
3.1.3. Acetogenesis
The resulting acids in the acidogenesis steps are used as substrates by acetogenic bacteria, which convert VFAs into acetate compounds, H2 and CO2. Acetogenic bacteria are very sensitive to high concentrations of hydrogen. Hence, this stage is characterized by a syntrophic relationship among acetogenic bacteria and hydrogenotrophic methanogens, which maintain the hydrogen pressure at a low level [62]. The acetogenesis stage can produce 70% of methane during the reduction of acetate. The acetogenic bacteria engaged in this process belong to the Clostredium, Syntrophomonas and Syntrophobacter genera [63,64,65].
3.1.4. Methanogenesis
Methanogenesis is the final stage of AD, in which acetate, H2 and CO2 are utilized by methanogenic bacteria, producing methane (CH4) as a metabolic by-product. Methane production can occur via two pathways: acetotrophic or hydrogenotrophic methanogenesis [66]. The first pathway, as the name implies, utilizes acetate as the main substrate, whereas, in the latter pathway, hydrogen serves as the reducing agent for CO2. Microorganisms belonging to the Methanosarcinaceae and Methanosaetaceae families are the most prevalent acetotrophs. Hydrogenotrophic methanogens’ activity is crucial in maintaining the process’ stability, as they maintain the hydrogen levels at levels conducive to stable acetogenesis [67].
Out of the four stages of AD, this step is the slowest and highly depends on the H2 concentration for the rate of CH4 production. Enriching the feeding bioreactor with H2 molecules can speed up the process by accelerating the activity and the number of hydrogenotrophic methanogens [64]. Nanotechnology can offer innovative solutions in resolving issues related to the methane yields and retention time in anaerobic procedures. Electromethanogenesis is one such cutting-edge approach that utilizes nanomaterials to aid the electron transfer mechanism [64]. Xiao et. al. found that conductive carbon nanotubes can accelerate methanogenesis by stimulating the synthesis of proteins involved in acetate oxidization [68]. The addition of conductive materials such as magnetite and granular active carbon was also found to increase methane production [69]. Acclimatizing sludge with sulfidated nanoscale zerovalent iron (S-nZVI) has shown promising outcomes regarding the treatment of FW. This approach significantly enhanced the microbial activity, which in turn led to increased methane production [70]. The recommended pH concentration level for the activity of acetotrophic and methanogenic bacteria is above 6.5, with pH 8.5 to 9 leading to higher methanogenic activity [71].
3.1.5. One-Stage vs. Two-Stage Anaerobic Digestion
The two-stage AD system holds the potential to further increase the energy recovery and process stability over the single-stage configuration. In two-stage AD, hydrolysis–acidogenesis occurs in the first reactor, followed by acetogenesis and methanogenesis in the second reactor [72]. This facilitates the diverse functioning of the microorganisms involved, as each stage can be regulated to ensure the optimum growing conditions for the microbial communities. Two-stage AD is characterized by a higher capital investment but offers the benefit of increased methane yields (10–30%) and the opportunity to produce value-added products [73]. Similar results were reported by Xiao et al., who estimated the average biogas yield for a two-stage system at 0.810 ± 0.13 L/g and for a single-stage system at 0.775 ± 0.20 L/g [74]. García-Depraect et al. [75] demonstrated that by implementing a lactate-based two-stage AD method, the bioenergy recovery rate from FW can be doubled. However, under certain conditions, energy losses can occur during the hydrolytic–acidogenic step in two-stage AD due to the formation of hydrogen and carbon dioxide, which can lead to the single-stage reactor outperforming its counterpart [76].
3.1.6. Co-Digestion in Anaerobic Treatment (AcoD)
The heterogenous composition and high moisture content of FW can be problematic and challenging for anaerobic digestion. Impurities related to high salt concentrations, protein content (leading to the formation of free ammonia) and hydrogen sulfide can disrupt microbial activity, leading to inefficiencies in biogas production [77,78,79]. Co-digestion has been proven to enhance the biogas quality and quantity by offering solutions to issues related to the FW composition. This treatment is also known as co-substrate digestion and incorporates the simultaneous processing of two or more different types of organic waste, promoting synergistic effects between the substrates [80]. Additionally, the balanced nutrients of co-digestion cause positive shifts in the microbial community, promoting the dilution of toxic substances and improving the AcoD efficiency [81].
Animal manure is a common substrate for co-digestion, due to its high availability, its high buffering capacity against VFA accumulation and its continuous supplementation with bacteria [82]. Studies have explored the mixing of Parthenium hysterophorus weed with different ratios of FW, achieving a maximum yield of 559 mL/L/day at a 60:40 ratio of FW to Parthenium weed [83]. The two-stage co-digestion of FW and activated sludge enabled higher biogas quality (26%) and volatile solid removal (9%) compared to two-stage digestion with a single FW [84]. Biochar, utilized as an additive in an FW and sludge co-digestion system, reportedly enhanced the methane yield and mitigated VFA accumulation due to its alkalinity, high surface area and micro- and macronutrients [85]. Another study created a mixture of FW and sewage sludge combined with a piggery effluent at a ratio of 1:0.39:1, achieving an increase in the methane yield to 294 mL/g total solids (TS), compared with 138 mL/g TS in the single digestion of sewage sludge. The abundance of trace elements found in sewage sludge and swine waste provides a favorable substrate for the establishment of syntrophic and symbiotic interactions within microbial communities [86]. A review carried out by Chow et. al. [87], with agricultural waste and FW co-digested with wastewater sludge, found that the methane yield could be improved from 18 to 67% compared to the mono-digestion of wastewater sludge alone. The AcoD of 95% FW and 5% process water obtained from garden and park waste through hydrothermal carbonization treatment enhanced the diversity of bacteria and the hydrolytic activity, resulting in a higher methane yield and showing synergistic effects (10%) over FW digestion [88].
3.2. Dark Fermentation
Dark fermentation is a biological process in which fermentation solely relying on the activity of microorganisms, like Bacillus cereus, Thermoanaerobacterium spp., Clostridium butyricum and Clostridium acetobutylicum, breaks down the organic substrate to produce hydrogen [89]. Unlike photofermentation, dark fermentation—as the name indicates—is completed in dark and anoxygenic conditions. The optimum yield efficacy varies depending on the feedstock used, bacterial strain, pH, temperature, hydraulic retention time for continuous processes, partial pressure of hydrogen and pretreatment method [90,91]. The values of the pH and partial pressure are the main factors determining the hydrogen yield, with lower pH (<5) conditions and negative pressure (40.52 kPa) favoring hydrogen production due to the inhibitory effect on methanogenesis. Nonetheless, dark fermentation’s main drawback is the very low hydrogen yield [92,93]. This is typically because hydrogen-consuming bacteria reduce H2 to CH4 and organic acids, leading to only 33% conversion rate efficiency in the substrates’ chemical oxygen demand (COD) [94]. Studies on the main agricultural lignocellulosic biomass products found that the biohydrogen production yield had a negative correlation with the lignin content. The values varied significantly, with the lowest hydrogen production (12 mL H2/L) obtained from cashew apple bagasse (35.26% lignin) and the highest of 7019 mL H2/L for sugarcane bagasse (8.3% lignin) [89].
Strategies involving the suppression of methanogenic activity and the promotion of the growth of hydrogen-producing bacteria can enhance the hydrogen yield [95]. Pretreatment methods employed to inhibit hydrogen-consuming bacteria include heat treatment, aeriation and electroporation [96,97,98].
3.3. Photofermentation
Photofermentation is a biological process that involves the fermentation of organic matter in the presence of photosynthetic non-sulfur bacteria, producing cell energy (ATP), hydrogen and CO2 [99]. The process is suitable for anaerobic conditions under sunlight or artificial light, enabling the photosynthetic non-sulfur bacteria to complete the conversion of the substrate into hydrogen energy and other products [100]. Although sunlight is freely available, it is highly affected by external environmental factors (e.g., clouds, warmth), making artificial light the preferred option due to its controllable homogeneity, intensity and minimal heat generation [101]. In photofermentation, certain bacteria, such as Rhodovulum sulfidophilum, Rhodobacter capsulatus, Rhodospirillum rubrum and Rhodopseudomonas palustris, are used [102,103].
In theory, the photofermentation yield efficiency is higher than in dark fermentation (1 mol of H2/mol) as 2 mol acetic acid and 4 mol of water can generate up to eight mol of H2 and 2 mol CO2. A study compared the two processing methods with photofermentation, yielding 870.26 mL H2/g of total volatile fatty acids (TVFA), compared to dark fermentation, which produced 226.24 mL H2/g TS [104]. The photofermentative hydrogen production efficacy is mostly influenced by the light quality and intensity and other factors like the type of substrate, bacterial strain, temperature and pH. Under constant conditions, a high light intensity (7000 Lux) with a higher mixing speed (150 rpm) obtained the highest hydrogen yield, with 78.1 mL/g TS produced [105]. However, dynamic mixing (50-150-50 rpm) and dynamic lighting (4000-7000-4000 Lux) obtained better results, yielding 84.7 mL/g TS hydrogen and light conversion efficiency of 36.3% [105]. Turon et al. [106] employed light-emitting diode lamps at visible and near-infrared wavelengths, which do not generate heat, achieving a hydrogen flow rate of 93 mL/l/h. The optimum conditions for maximum productivity vary depending on the bacterial strain and substate used, with the pH ranging from 6.0 to 7.5 pH and a temperature of 30–35 °C [107,108,109]. A similar study carried out by Barghash et al. (2021) showed that the environmental conditions of pH 6.0, a temperature of 37 °C and 100 rpm agitation were optimal for hydrogen production, attaining 5.75 mL/L [110]. Regarding photofermentation using bread waste as a substrate, the use of Lactobaccillus amylovorus for lactic acid production, followed by R. palustris, yielded 3.1 mol H2/glucose [111].
Photofermentation in combination with dark fermentation has the potential to increase the biohydrogen yield, resulting in a sustainable FW management system. The syntrophic relationship among the dark- and the photofermentative bacteria resulted in a 134% increase in yield and a 67% increase in substrate utilization, due to the increased pH, decreased oxidation–reduction potential (ORP) and volatile fatty acid (VFA) levels observed [112].
- Chemical Conversion Processes
3.4. Transesterification
Biodiesel produced from oily FW is one of the most promising alternative renewable fuel sources as it recovers energy from waste, and it is characterized by its biodegradability, low emission profile and nontoxicity, while reducing the dependence on fossil fuels and mitigating environmental pollution [113].
Transesterification is defined as a chemical process that converts triglycerides from various feedstocks into fatty acid methyl esters (biodiesel), in the presence of alcohols, with glycerol as a secondary product [114]. Methanol and ethanol are the most widely employed alcohols in this process because they are low-cost and readily available. Cooking oil is the most preferred feedstock for biodiesel production, boasting a high production yield of 97.7% w/w, while avoiding the environmental implications associated with its disposal in drainage systems or into soil [115]. The production of oil biodiesel by utilizing vegetable or animal waste has the potential to reduce GHG emissions by 83% (14 g CO2/Mj) in relation to fossil fuels throughout the lifecycle of biodiesel [116]. Despite the high oil content, waste cooking oil faces two major challenges: the high free fatty acid concentration, which triggers emulsion creation, and the high percentage of unsaturated fatty acids, which promotes fast oxidation, compromising the biodiesel’s durability [49]. The use of an acid catalyst through the esterification process has the potential to reduce the free fatty acid concentration before the transesterification process [49].
Other feedstocks suitable for transesterification include nonedible oil seeds, plant oils, animal fats or tallow, FW, microbial lipids or single cells from algae or oleaginous yeasts and oils from filamentous fungi and bacteria [117,118]. Researchers have also investigated fungal hydrolysis using various types of yeast, such as Yarrowia lipolytica and Lipomyces sp., as a pretreatment method for lipid extraction from agricultural products [119,120]. The biodiesel production yield efficiency and cost-efficiency notably depend on the feedstock oil content, which typically ranges between 6 and 30% of dry matter. The biodiesel yield is also highly influenced by the catalyst concentration, reaction time, reaction temperature, methanol ratio and stirring speed [121,122]. A recent study utilized a hexane–methanol (19:1 ratio) mixture to extract lipids from dry microalgae, representing a prominent strategy to be used for the green energy transition [123].
The transesterification process can be employed through two different pathways: catalytic transesterification and non-catalytic transesterification. The first pathway uses a catalyst to facilitate the reaction between triglycerides and alcohols, speeding up the process by decreasing the activation energy and achieving a higher biodiesel yield. The non-catalytic pathway avoids the saponification problems associated with the use of catalysts, eliminating the need for biodiesel purification [124]. Nevertheless, it requires a longer time to achieve conversion, as well as a higher temperature and pressure to ensure efficient output production, raising the operational expenditure.
Catalysts are classified based on their composition as homogenous, heterogeneous or enzymatic or based on their chemical properties as acids, bases or enzymes. Homogeneous acid or alkaline catalysts’ affordability and high yield potential make them an appealing option. However, this category of catalysts poses severe environmental problems caused by the potential for equipment corrosion, excessive wash-water usage and water contamination [125]. On the other hand, due to the simplicity of use, the low cost and the possibility for the reuse and recycling (catalyst–product separation) of heterogenous catalysts, they have long been established as the most preferred option. Lipase is identified as one of the most promising enzymes for biodiesel conversion, suggesting the potential of leveraging biotechnology focused on microorganisms capable of producing lipase enzymes, which can endure extreme processing conditions [121,126].
The transesterification of kitchen waste at a 52.5 °C reaction temperature assisted with the ultrasound reaction, resulting in 93.2% w/w fatty acid methyl ester (FAME) conversion [127]. A rather unique treatment method converted FW nutrients into lipids by using the black soldier fly Hermetia illucens, a fast FW consumer, with the larvae stage directly used as a substrate for biodiesel production (93.8 wt% yield) [128]. Another study created a two-step transesterification process and cultivated the heterotrophic microalgae Auxenochlorella protothecoides, generating 248.21 g FAME per 1 kg FW with 84 to 88% efficiency [129].
- Thermochemical Conversion Processes
3.5. Incineration
Incineration allows for the recovery of energy content from FW, reducing the reliance on landfills when other waste management options are unsuitable or not available [130]. This is exemplified by the Philippines, which is the only country in the world to ban incineration due to air quality concerns, leading to a more severe problem of landfill management [131]. The incineration method involves the burning of organic substances in waste materials at high temperatures, converting them into heat, which can be used to power steam turbines and generate electricity [132]. The burning temperatures exceed 750 °C and can reach up to 1100 °C, reducing the total waste mass by 70% and the total waste volume by up to 80–85%, substantially reducing the volume for disposal. The incineration of FW requires additional fuel or pretreatment, due to its high moisture content and low calorific value [133,134,135].
FW is usually disposed of through the general flow of MSW. This practice offers synergistic advantages, as MSW can increase the calorific value of the mixture, while the high ammonia concentration in FW can react with NOx gases, reducing the emissions released during the process [135]. The incineration process of MSW eventually leads to the production of two main solid by-products: bottom ash and fly ash [136]. Both by-products need to be properly disposed of to avoid the environmental impact associated with the inorganic content, which contains heavy metals [137]. Recent advances have explored the potential for the production of green eco-cement that entirely utilizes MSW incineration residues, as an environmentally friendly alternative [138]. Fly ash and bottom ash are widely used in the construction sector as a substitute or enhancing material for existing construction materials [139,140].
The combustion of waste also generates flue gas, which contains a mixture of air pollutants such as SOx, COx, NOx, polyaromatic hydrocarbons and particulate matter [94]. Exposure to the toxic air emissions from incineration plants has been associated with increased risks of neoplasia among the nearby population, including bowel cancer, soft tissue sarcoma and congenital anomalies [141]. People working in incineration plants have reportedly been found to exhibit significantly higher concentrations of heavy metals in their bloodstreams, as well as increased levels of dioxins. Specifically, the polychlorinated dibenzodioxin (PCDD/dioxin) levels were up to 4.7 times higher and the polychlorinated dibenzofuran (PCDF) levels were 21.2 times higher in male ex-employed workers in Osaka, Japan, compared to the local residents [141]. However, mitigating the potential human health risks and environmental issues associated with air pollution can be achieved with the improvement of air control systems. Technological developments in air pollution control systems serve to reduce the release of dangerous substances into the atmosphere and optimize the electricity-to-heat ratio for maximum energy production [142].
Results indicate that incineration of FW can produce heat valued at 37.7 kJ/g TS heat or 172–338 kWh per ton of FW [134,143]. Ali et. al. estimated that, utilizing the daily fraction of generated MSW, 27.18 MW of energy can be generated, which is enough to power five million houses, assuming the average consumption of 500 W per household [144]. Reportedly, the combustion of MSW worldwide in 2020 amounted to 1.47 exajoules, encompassing both heat and power generation [145].
3.6. Pyrolysis
Pyrolysis offers a promising solution for the management of FW in a sustainable manner, effectively mitigating concerns associated with the release of harmful gases (NOx and SO2) and the volatilization of fly ash during incineration [146]. Pyrolysis involves the thermal decomposition of solid materials in the absence of oxygen at high temperatures, unlike other thermal treatment methods (incineration and gasification), where oxygen is required [147]. Depending on the operating conditions, pyrolysis can be classified into fast, slow, intermediate and flash pyrolysis, obtaining three major energy products, biochar, bio-oil and biogas, as final products (Table 3). The selection of the operating conditions, reactor type, feedstock and catalyst (if any) is driven by the desired final product yield [94]. The moisture in the organic matter needs to be removed (1% w/w content) before initiating the thermal processes of fast and flash pyrolysis. This step can enhance the pyrolysis efficiency and favors the production of bio-oil and biogas over biochar. The heat released during pyrolysis can be utilized for the drying of organic materials, reducing the need for additional energy for drying procedures.
Bio-oil is the most promising energy source compared to other pyrolysis products (biogas and biochar), with high energy content and several applications (steam turbine, vehicle and aviation fuel). Agricultural residues treated at temperatures of 450–500 °C resulted in a 30% biogas yield, effectively pyrolyzing 80% of the biomass [148]. FW collected from catering and households reached the maximum yield potential for biochar (40.3%) and bio-oil (34.9%) at 400 °C, while the maximum yield of biogas (43.1%) was noted at 700 °C [149]. Nonetheless, an increase in temperature over 400 °C improved the quality and stability of the bio-oil, resulting in a three-times-higher aromatic concentration at 700 °C. In general, high temperatures favor biogas formation, while, at low temperatures, the biochar yield is increased, reflecting a contrasting effect [150].
Slow pyrolysis operates at low temperatures, benefiting the production of solid carbonaceous char, known as biochar. Biochar has seen several applications as an enhancement to wastewater treatment, for carbon sequestration, as a soil amendment agent, in flue gas cleaning, as a building material and in metallurgical and agricultural applications, because of its porous structure [151,152,153,154,155,156,157,158]. Biochar produced from the pyrolysis of tomato waste at 350 to 550 °C led to a significant increase in the specific surface area of 288 and 1415%, respectively, making it a suitable soil amendment or sorbent material [159]. Biochar can also be used as a solid fuel in the pyrolysis process, providing the necessary heat required for the drying of FW or the heating of the reactor [160].

Table 3.
Operating conditions and bio-product yield efficiency of four different pyrolysis configurations [155,156].
Table 3.
Operating conditions and bio-product yield efficiency of four different pyrolysis configurations [155,156].
| Slow Pyrolysis | Intermediate Pyrolysis | Fast Pyrolysis | Flash Pyrolysis | |
|---|---|---|---|---|
| Target | High biochar yield | Separable bio-oil, high-quality biochar | High bio-oil yield | High bio-oil yield |
| Heating rate | 0.1–1 °C/min | 1–10 °C/min | 1000 °C/min | >700 °C/s |
| Vapor residence time | 5–30 min | 0.5–20 s | <2 s | <0.5 s |
| Temperature | <400 °C | 400–500 °C | 500 °C | 750–1050 °C |
| Biochar yield (%) | 20–50 | 35–50 | 60–75 | 60–75 |
| Bio-oil yield (%) | 25–35 | 25–40 | 10–25 | 10–25 |
| Biogas yield (%) | 20–50 | 20–30 | 10–30 | 10–30 |
The yield of biochar obtained from FW decreases from 33.3 to 24.8% as the temperature increases from 500 to 800 °C [161]. Studies also have reported that waste cereal pyrolysis resulted in a 11.2 MJ/m3 lower heating value (LHV) for gaseous products [162]. The solid product had a higher heating value (HHV) of 31.44 MJ/kg and LHV of 30.27 MJ/kg. The pyrolysis of FW in a solid digestate resulted in gas with an estimated LHV at 20.52 kJ/Nm3 [162]. FW subjected to thermo-catalytic reforming (TCR) pyrolysis produced bio-oil with a HHV at 36.72 MJ/kg, a value comparable to biodiesel. The performance of CaO, CuO and MgCl2 catalysts was evaluated as a means to enhance the bio-oil yield, with CaO and CaCO3 also proving beneficial for the formation of hydrocarbons [163]. In a case study in Qatar, calculations were carried out using the Aspen Plus software for different FW mixtures. The optimum yield of solid biochar reached 41.81%, with a slight decrease in ash and carbon content when some components—banana peel, mango endocarp, orange peel and tea waste—equally constituted the mixture [164]. The slow pyrolysis of agro-food waste at 300 °C yielded the maximum biochar yield (52.4 wt%) but with the lowest carbon content (51.7 wt%), in contrast to pyrolysis at 600 °C, which resulted in a lower yield (28 wt%) but higher carbon content (60.7 wt%). The same study reported that pyrolysis at 500 °C had the highest production efficiency (42.4 wt%) [165].
The European Biochar Certificate (EBC) has established upper limits for the ratios of hydrogen to carbon (H/C) and oxygen to carbon (O/C) in biochar at <0.7 and <0.4, respectively. Several studies have reported biochar to exhibit a 0.29–0.7 H/C ratio and a 0.12–0.41 O/C ratio, indicating the high quality and applicability of biochar produced from FW pyrolysis. The H/C and O/C ratios improve as the temperature rises above 400 °C [166].
3.6.1. Co-Pyrolysis
Co-pyrolysis is another common type of pyrolysis that is carried out using two or more feedstocks that exhibit a synergistic effect, improving the overall quantity and quality of the bio-oil without modifying the equipment [167]. In a co-digestion configuration, the application of tire waste reduced the oxygenated compounds, while microalgae lowered the acid levels [168]. Both options resulted in an increase in hydrocarbon content, improving the quality of the produced bio-oil. The co-pyrolysis of 87% FW and 13% low-density polyethylene at 550 °C was able to generate 42 wt% of bio-oil [168].
The sustainability of pyrolysis is set to be further improved as adsorbents and materials that can capture the gaseous or liquid mixtures released after the pyrolysis of FW are under development [169]. Researchers have explored the potential of vermicompost-based FW activated carbon, which holds potential as a more environmentally friendly electrode material in supercapacitor applications, acting as an energy storage device [170].
3.6.2. Integrated Pyrolysis and Anaerobic Digestion System
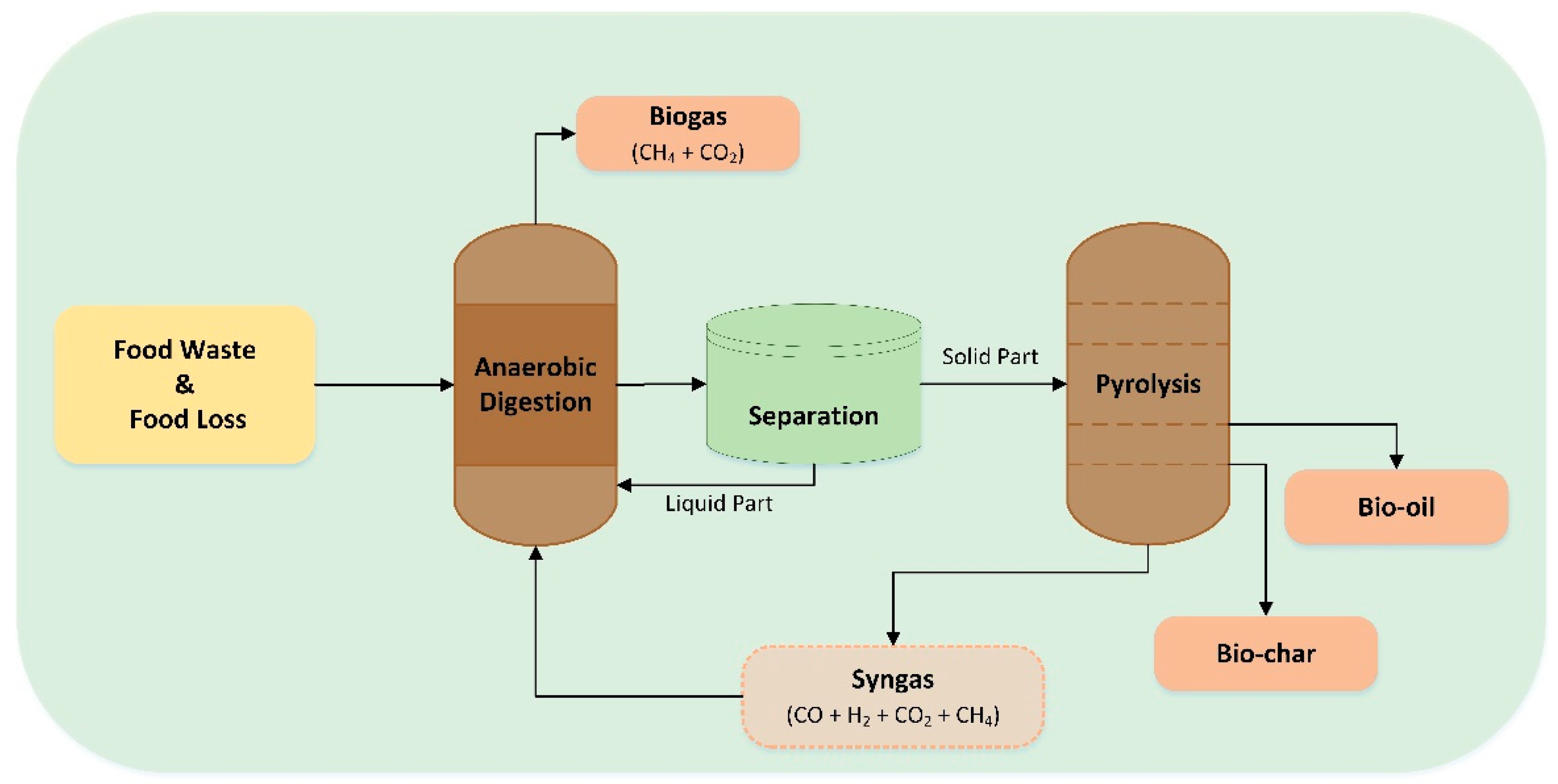
Pyrolyzed biochar integrated with AD can enhance methane production by 47.3%, as it contains spherical crystalline structures of calcium carbonate, which can mitigate the effects of NH4+, N and Na+ on mycelial growth (Figure 6) [171]. A techno-economic analysis study investigated the potential benefits of separately treating the FW liquid and solid fractions, using AcoD and pyrolysis systems, respectively. The FW slurry fraction yielded 572.88 mL/gVsFW of biomethane, almost double compared to treating the whole FW (294.37 mL/GVsFw) [172]. Concerning the system’s efficiency, the LHV of the biogas derived from the FW was slightly higher at 20.52 kJ/Nm3 compared to the FW solid phase (8%). Nonetheless, the pyrolytic oil and biochar derived from the FW solid phase exhibited superior characteristics, with the biochar having a surface area that was 10 times higher and the bio-oil containing elevated levels of aliphatics. Notably, the pyrolysis of the FW solid phase resulted in reduced emissions of nitrogen-containing pollutants, suggesting that this is a more environmentally friendly method [161]. The separation of the solid and liquid phases of FW poses a significant advantage as the pyrolysis pretreatment (drying) of FW and post-treatment AD (digestate handling) are no longer required [173]. This integrated approach results in a negative carbon footprint of −1.726 kg CO2 eq./kgFW when combined and is considered to be a cost-effective strategy to improve AD’s efficacy [174].
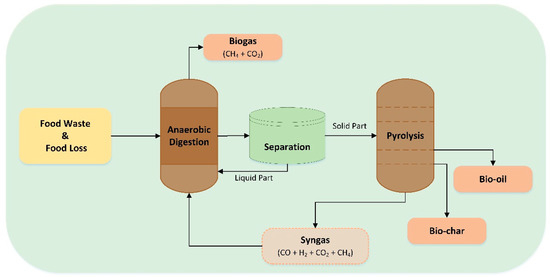
Figure 6.
Integrated pyrolysis and anaerobic digestion system.
3.7. Torrefaction
Torrefaction refers to mild pyrolysis at around 200–350 °C to improve the chemical properties and energy potential of the FW biomass [175]. This process is an appropriate pretreatment for lignocellulosic-rich and high-moisture FW, effectively removing the moisture and volatile compounds from the substrate [176]. Torrefied FW’s advantages are related to its improved grindability, the ease of microbial degradation and the production of a dry and more energy-dense material [177]. Depending on the torrefaction temperature and FW substrate, 56.9–82.4% of oxygen can be removed, resulting in higher yields of gases and solid biochar [178]. Nonetheless, the process comes with the disadvantage that dioxin contaminants may remain in the final biochar product [179]. After treatment at temperatures around 225 °C for 1 h, the mass yield was 85.16% for biochar and 9.68% for bio-oil, while, at 275 °C for 3 h, the biochar yield was 48.32% and the bio-oil yield was 28.45%. Abdul et al. employed torrefaction in a tubular reactor to study the effect of the temperature (220 to 260 °C) and residence time (15–60 min) on the properties of biochar produced from torrefied FW [180]. The results showed that an increase in temperature and residence time leads to an increase in carbon content and a decrease in hydrogen and oxygen. The HHV was also observed to increase from 19.15 MJ/kg to 23.9 MJ/kg after 60 min of torrefaction at 260 °C [180].
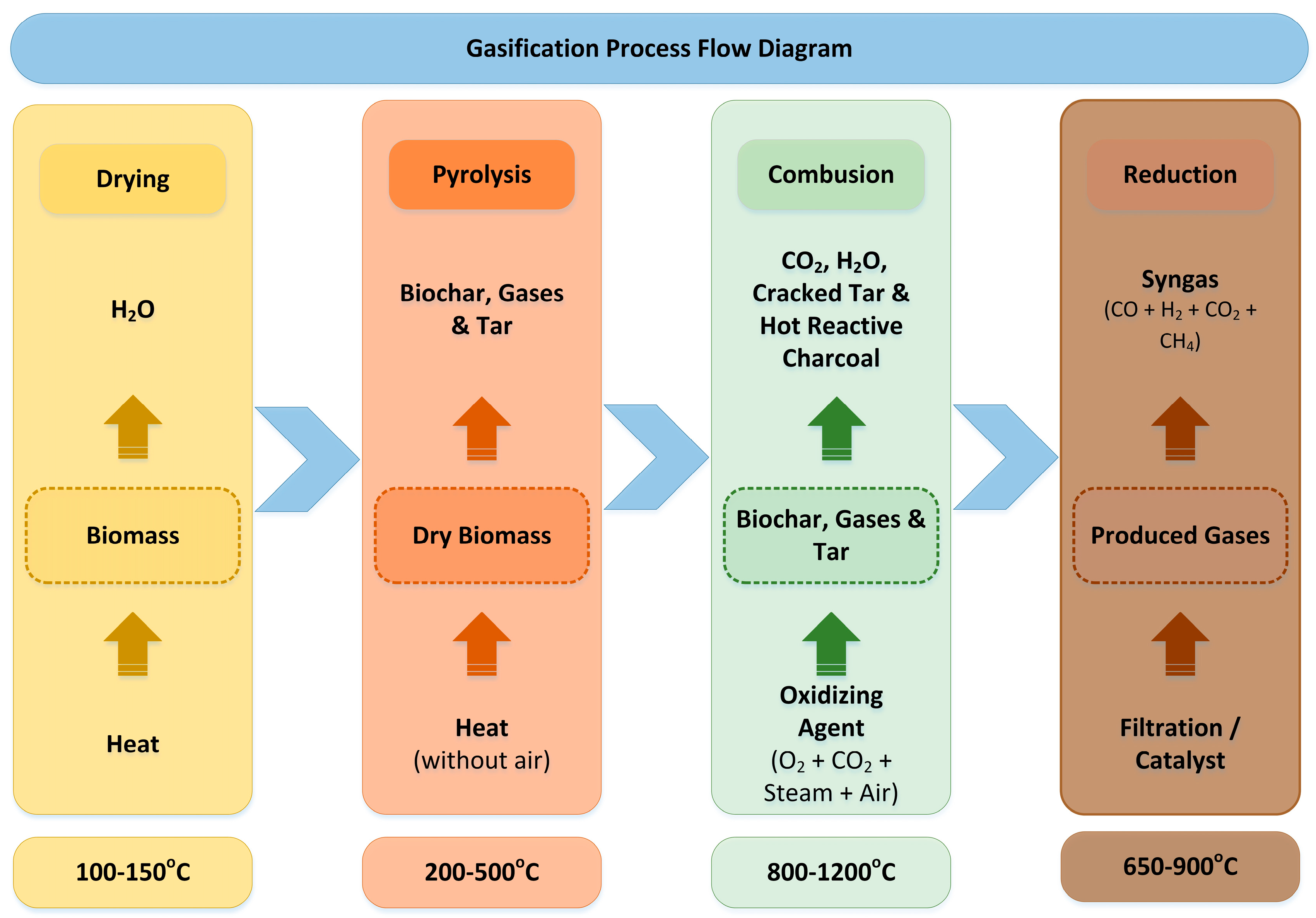
3.8. Gasification
Gasification is characterized as a low-pollution thermochemical process that is similar to pyrolysis [181]. Both methods convert organic feedstocks into valuable syngas composed of CO, H2, CO2, CH4, and H2O and by-products including low-molecular-weight hydrocarbon gases, ash and biochar particles. The difference in gasification occurs under the controlled presence of an oxidization medium (oxygen, steam, air, CO2) at high temperatures ranging from 800 to 1200 °C (Figure 7) [182]. The calorific value of the syngas decreases with the increasing temperature of gasification, regardless of the oxidization medium [183]. The syngas produced from gasification usually contains traces of contaminants or undesired components such as tar, NH3, HCN, H2S, COS, halides (HCl) and trace metals [184]. Subsequently, the produced gas requires purification before its final use as a fuel for gas engines and gas turbines or for the synthesis of chemical products [185]. The gasification process includes a reduction phase, wherein the producer gas is refined into syngas or fuel. Nickel-, iron- and mineral-based catalysts exhibited good removal results for tar, as well as Ru- and Ni-based catalysts for NH3 [184].
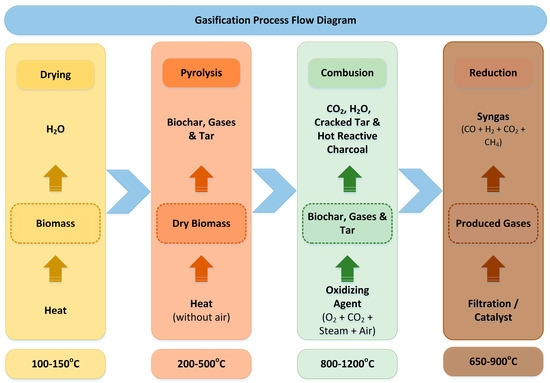
Figure 7.
Gasification process flow diagram.
Koshariya et. al. [186] analyzed the influence of several catalysts, with NaHCO3 recording 12.9 mol/kg hydrogen production and efficiency of around 43%, compared to 30% efficiency with no additives. The results also revealed that the hydrogen and methane production yields generated during gasification had a positive correlation with the temperature and hydrogen reaction, while the effect of the reaction duration and reaction temperature was limited [186]. The presence of high nitrogen and sulfur concentrations has a potent poisoning effect on the catalysts, as they release NOx/SOx emissions. During the gasification process, sulfur is released as H2S and nitrogen generates NH3 emissions [187]. The segregation of organic waste from MSW serves as a promising pretreatment method for electricity production, yielding between 368kW and 770 kW per ton of processed MSW depending on the type of turbine [188]. Operating at lower temperatures in the same system, it is possible to gain larger volumes of syngas with high calorific value.
3.8.1. Steam Gasification
Steam gasification is known for the high reactivity between steam and hydrocarbon, offering a higher yield of hydrogen from FW compared to the common method of air gasification [189,190]. An increase in the steam flow rate promotes methane reformation and the water–gas shift reaction, improving the gas quality [191]. The choice of steam as an oxidization medium is the most environmentally friendly solution, emitting 2.32 kg of CO2/kg of product [189]. Reportedly, steam gasification is highly efficient, producing double the calorific value (11.2 MJ/Nm3) from wood biomass compared to air gasification (5.3 MJ/Nm3) [192]. Huynh [193] et al. examined the injection of 40% pure oxygen prior to mixing with steam, indicating a notable rise in H2 production of between 32 and 70% depending on the type of biomass. Xu et al. reported that the conversion of FW into hydrogen results in a significant hydrogen production capacity of 1.2t/h [194]. Kitchen waste processed with a low-temperature steam gasification method yielded 0.95 m3/kg syngas and 0.62 m3/kg hydrogen at 700 °C, while treatment at 800 °C produced 1.2 m3/kg syngas and 0.70 m3/kg hydrogen [191]. These results indicate that with increasing temperatures, the syngas yield is improved without affecting the hydrogen fraction in the syngas. The steam co-gasification of FW with woody biomass arranged in a tubular reactor achieved 99.7% carbon conversion and produced 888.7 l/kg gas with a reactor temperature of 900 °C and a steam flow rate of 22 g/h [195].
3.8.2. Fischer–Tropsch
The syngas produced from gasification can be fed into a Fischer–Tropsch reactor, where a catalytic reaction converts the CO and H2 in the syngas into liquid hydrocarbons (fuel) [196]. Fischer and Tropsch’s experiments used four different configurations and demonstrated a potential biofuel yield of 91 to 116 g from 1 kg biomass, with the higher yield obtained in the FT system with steam-based gasification and hot gas cleaning [197].
3.8.3. Plasma Gasification
Plasma gasification is an energy-intensive process, converting biomass into high-quality syngas and by-products under extreme temperatures (2000 to 14,000 °C), thus consuming a significant amount of electricity [198]. Nonetheless, the high energy efficiency coupled with the absence of harmful substances in the product makes this new technology attractive [199]. Operating at a plasma energy ratio of 0.25 for the processing of kitchen waste powder, the syngas concentrations peaked, with CO and H2 concentrations of 26.6 and 10.0%, respectively [200]. The estimated running cost of the system is approximately 0.68 Yuan/kWh/day, demonstrating economic viability across various industries [200]. A study compared the efficiency and production costs of hydrogen via plasma gasification (PG–hydrogen) alone and coupled with chemical looping (PG–SCL), using FW as the feedstock. The results showed that PG–SCL achieved higher exergy efficiency (54.31%) compared to PG–hydrogen (31.44%) and higher material consumption (7.14 t/t H2) at a lower production cost (USD 1930.77/t H2) compared to PG–hydrogen (6.47 t/t H2 and 2449.82 USD/t H2, respectively) [201].
3.8.4. Supercritical Water Gasification (SCWG)
Another gasification method suited for FW is supercritical water gasification (SCWG), which, at low temperatures and high pressure, produces hydrogen-rich syngas from wet biomass [202]. The working conditions for SCWG typically range between 375 and 700 °C and higher than 22.12 MPa [203]. A study examined the potential of SCGW, indicating that hydrogen production of 12.73 mol/kg FW can be achieved at 450 °C with the addition of NaOH (5 wt%) as a catalyst [204].
3.9. Hydrothermal Carbonization (HTC)
Hydrothermal carbonization (HTC) is a wet thermochemical conversion method that has the ability to transform biomass with high moisture, such as FW, into valuable products through a combination of high temperatures (170 to 250 °C) and autogenous pressure (2–10 MPa) in a short period of time (5–240 min) and in the absence of oxygen [205,206,207]. The HTC process utilizes the inherent moisture of the feedstock as a solvent. This method generates mainly hydrochar, a carbon-rich product equal to the biochar generated in slow pyrolysis, which can be used as a fuel, as well as a liquid fraction (oils after extraction) and gaseous products [207,208]. Due to the low processing temperature and the absence of biomass-drying pretreatment steps, HTC offers a competitive advantage as the energy requirements are relatively low compared to other thermal processes [209]. Moist agro-industrial waste treated with HTC processing required half of the energy compared to torrefaction treatment [210]. Additionally, the processing conditions facilitate the elimination of pathogens, the inactivation of organic contaminants and a marked reduction in unpleasant odors [211]. The HTC process undergoes a range of simultaneous chemical reactions, including hydrolysis, dehydration, decarboxylation, condensation, polymerization and aromatization, for hydrochar formation [212]. Another advantage of HTC is the potential for its use as a fertilizer, with the solid phase containing nitrogen, phosphorous calcium and magnesium. It was estimated that 0.96% of nitrogen and 2.30% of phosphorus-based fertilizers could be replaced in the US by nutrients integrated within hydrochar and the liquid phases of landfilled waste [213].
The water in HTC is employed as a reaction medium, facilitating the chemical reactions involved and substituting hazardous solvents [214]. Recirculating the process water is an attractive practice that holds the potential to enhance the carbon recovery yields and elevate the energy density of the hydrochar, due to the organic acids concentrated in recycled process water [215].
Restaurant FW treated with the HTC process at 200 °C for 6 h produced high-quality hydrochar with energy content of 23 Mj/kg, presenting a similar composition to that of lignite, making it suitable as a feedstock for co-combustion applications [216]. The HTC of FW also holds the potential to produce hydrochar retaining approximately 70% of the fatty acids, which can be later extracted for industrial purposes using ethanol, with an overall recovery rate of 49% [217]. Another study indicated that hydrochar produced from FW possessed higher carbon content and heating value compared to raw biomass [218]. Additionally, Ohio University evaluated a mixture of FW and noted significantly low ash (1–2%) and low sulfur content (<0.5%) [219]. The highest mass yield was obtained at 200 °C (75%) and the lowest yield at 260 °C (68%); notably, it produced hydrochar with a heating value of 33.1 MJ/kg [219].
3.9.1. Co-Hydrothermal Carbonization (co-HTC)
Co-hydrothermal carbonization (co-HTC), using two different biomasses in the same reactor at the same time, has demonstrated enhancements in biofuel properties, while reducing the concentrations of toxic compounds like sulfides, alkali metals and chlorides [220]. Wang et al. [221] investigated the quality of pellets produced from a mixture of FW and woody biomass. Their research indicated that a higher ratio of FW in the mixture favored a reduction in energy consumption during pelletization and resulted in pellets with a lower ignition temperature and a wider temperature range [220]. Specifically, the incorporation of an FW ratio of 50 to 75% at a temperature of 220 °C was identified as the most suitable condition for pelletization and the production of solid biofuel [220]. A similar study carried out in India, on a co-HTC process with a FW and lignocellulosic yard waste (green waste) mixture, aimed to assess the potential for biofuel pellet production. The results indicated durable and hydrophobic pellets with calorific value of 27.6 MJ/kg, almost double the corresponding calorific value of the raw materials (13.5 MJ/kg) [222]. Under the optimum conditions, the hydrochar produced from the FW and yard waste mixture could replace 11% of the global coal consumption. The co-HTC of FW and sewage sludge at a 30:70 ratio and 230 °C resulted in the production of hydrochar with a HHV of 22.87 MJ/kg and a fuel ratio of 0.36 [223].
3.9.2. Hydrothermal Liquification
Thomsen et al. performed a similar HTC process in a liquid phase, also known as hydrothermal liquification, combined with non-catalytic wet oxidation, used to convert biomass into bio-oil [224]. At 350 °C for 3 h, the almost complete degradation of organic compounds into acetic acid was achieved, increasing the NH4+ and NH3 recovery as nutrients and causing the overall ash concentration to decrease. The hydrochar produced from biomass was well carbonized and presented high stability when treated under 200 °C [225]. Hydrothermal liquefaction for crude bio-oil production can yield up to 45% efficiency with a HHV of 35.1 Mj/kg FW [226].
3.10. Ethanol Fermentation
Ethanol fermentation is a biological metabolic treatment that involves different microbes (bacteria, fungi, algae) or enzymes that are able to break down FW in the absence of oxygen [227]. Various yeast strains, including Saccharomyces cerevisiae, Pichia stipites, Kluyveromyces fragilis and Zymomonas mobilis, are commonly used [228]. This process results in the production of ethanol and carbon dioxide from glucose and fructose, while releasing energy. Further enzymatic hydrolysis through fermentation could generate anhydrous bioethanol, a renewable fuel suitable for the automotive industry [229]. Several countries permit the use of automotive blends containing ethanol and conventional fuel, with the ratio typically set at 15% ethanol and 85% gasoline [230]. In 2022, the global ethanol consumption reached 129 billion liters worldwide, potentially translating into the annual consumption of about 860 million tons of an ethanol–gasoline blend [231]. Ma et al. [232] created an integrated engineering system for bioethanol production, in which Aspergillus oryzae was added to a canteen FW substrate, producing a fungal mash. A 67.5% reduction in the waste volume was achieved, generating 141.5 g/l glucose, which was subsequently fermented to produce 71.8 g/l of bioethanol. Zhang et al. [233] examined the performance of various types of vegetable waste for the production of bioethanol, with potato peels achieving the highest VFA yield (452 mg COD/g Vsfeed), followed by celery (372 mg COD/g Vsfeed), carrots (321 mg COD/g VSfeed) and Chinese cabbage (201 mg COD/g Vsfeed) [233]. A similar study using S. cerevisiae for bioethanol production using sweet potato waste, potato waste and vegetable waste obtained 251.85 mg/g, 240.98 mg/g and 235.4 mg/g, respectively [234]. In a study examining ethanol fermentation in a substrate composed of residues resulting from the saccharification and centrifugation of FW, the highest VFA concentration was achieved with 172.4 mg/g Vs at pH 6.5 [235]. Recent advancements in stillage technology could minimize the use of water in this process, while the use of biotechnology could increase the output efficiency of ethanol [236].
3.11. Ongoing Advancements, Future Research and Recommendations
Recent advancements in FW conversion technologies have addressed significant challenges such as the control of harmful compound production and the development of efficient production systems yielding high-quality products. Extensive research has focused on the pretreatment of FW, co-digestion configurations and the use of catalysts, demonstrating significant synergistic effects in producing high-quality energy products.
Incineration, while the simplest processing method, is the least favorable environmentally due to the release of toxic gases and heavy metals, posing health risks to nearby residents. AD, though widely applied, exhibits a large land footprint and limited volume reduction. The activity of bacteria within AD is highly sensitive to the process conditions, such as the pH, salinity and temperature, which can affect its efficiency. Thermochemical conversion methods offer a viable solution with high efficiency and yields of biogas. Steam gasification, in particular, is a prominent method for the production of high-quality syngas with minimal pollutants. The co-feeding of FW with sewage sludge, manure or other types of biomass can enhance the calorific value of biofuels. The integration of pyrolysis and AD has effectively addressed the challenges posed by the high moisture content of FW, resulting in high-quality biochar and bio-oil, making it one of the most environmentally sustainable options. Additionally, processes such as supercritical water gasification (SCWG), hydrothermal liquefaction and torrefaction are well suited for the processing of wet biomass without the need for prior drying pretreatment.
Future research should focus on the integration of multiple FW conversion technologies to maximize the energy recovery and minimize the environmental impact. Combining AD with thermochemical processes like pyrolysis and gasification can enhance the overall efficiency and stabilize operations. For instance, integrating AD with pyrolysis can utilize the produced biochar to improve the biogas yields and provide a stable digestion process [237]. Similarly, coupling AD with gasification can optimize the energy output by leveraging the strengths of both processes [237]. Additionally, advanced catalysts and materials should be developed to enhance the efficiency of thermochemical processes and improve the quality of biofuels. Research into novel catalysts can lead to better conversion rates and the higher selectivity of the desired products in processes like gasification and pyrolysis.
Moreover, comprehensive life cycle assessments and techno-economic analyses are necessary to evaluate the feasibility and economic viability of these technologies on a commercial scale. Policymakers should be informed about the benefits and challenges of FW-to-energy systems to create supportive regulatory frameworks and incentives. Public awareness and stakeholder engagement are also essential for the successful implementation of these technologies. Educational campaigns and community-based programs can drive behavioral changes and support the development of local and regional FW-to-energy projects. By focusing on these areas, future research can significantly advance the sustainability and effectiveness of FW management, contributing to a circular economy and reducing the environmental impact of food waste.
4. Conclusions
Harnessing FW as an energy source offers a promising solution to the dual challenges of waste management and sustainable energy production. The implementation of food waste-to-energy technologies has the potential to substantially decrease landfill usage and GHG emissions, thereby contributing to broader sustainability goals. This review underscores the significant impact of FW disposal on GHG emissions and environmental degradation, emphasizing the importance of proper management for a sustainable and energy-responsible future. Converting waste to energy not only mitigates these adverse effects but also has the potential to lead to negative CO2 emissions through carbon sequestration under certain conditions. Additionally, it prevents the harmful emissions typically associated with the burning of fossil fuels for energy production.
FW and FL can be treated through various methods, including thermochemical processes (incineration, pyrolysis, gasification, torrefaction and hydrothermal carbonization), biological processes (anaerobic digestion, composting, aerobic fermentation, dark fermentation and photofermentation) and chemical processes (transesterification). These methods generate valuable energy resources, such as biogas, heat, syngas, hydrogen, biodiesel and bioethanol. The variability in the waste composition, such as the moisture content, TS, C/N ratio and the presence of toxic compounds, significantly influences these processes.
Despite the technical advantages of these innovative technologies, further research is necessary to assess the feasibility and economic viability of their application in commercial-scale treatment plants. Future studies should focus on optimizing thermochemical processes to more efficiently handle high-moisture food waste by integrating anaerobic digestion with gasification, pyrolysis and hydrothermal carbonization.
Author Contributions
F.E.: conceptualization, data curation, investigation, writing—original draft, writing—review and editing. I.V.: resources, writing—original draft, writing—review and editing. I.P.: conceptualization, investigation, methodology, writing—original draft, writing—review and editing. V.P.: investigation, validation, writing—original draft, writing—review and editing. P.L.: investigation, validation, writing—original draft, writing—review and editing. V.N.: formal analysis, investigation, writing—original draft, writing—review and editing. P.S.: conceptualization, formal analysis, methodology, writing—original draft, writing—review and editing. M.C.L.: formal analysis, methodology, writing—original draft, writing—review and editing. D.M.Ț.: validation, writing—original draft, writing—review and editing. C.Z.: writing—original draft, writing—review and editing. A.A.Z.: conceptualization, data curation, supervision, resources, investigation, validation, writing—original draft, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to acknowledge the Laboratory of Chemical Engineering and Engineering Sustainability of the Open University of Cyprus, as well as the Science and Technology Driven Policy and Innovation Research Center (STeDI-RC) of the Cyprus Institute, for supporting this research. At the same time, the authors acknowledge the Sanitary Environmental Engineering Division, Department of Civil Engineering of the University of Salerno, along with the Dipartimento di Ingegneria dell’Informazione of the Università Politecnica delle Marche.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO. Global Food Losses and Food Waste 2011. Available online: https://www.fao.org/3/i2697e/i2697e.pdf (accessed on 4 May 2024).
- Zorpas, A.A. Strategy development in the framework of waste management. Sci. Total Environ. 2020, 716, 137088. [Google Scholar] [CrossRef] [PubMed]
- Economou, F.; Chatziparaskeva, G.; Papamichael, I.; Loizia, P.; Voukkali, I.; Navarro-Pedreño, J.; Klontza, E.; Lekkas, D.F.; Naddeo, V.; Zorpas, A.A. The concept of food waste and food loss prevention and measuring tools. Waste Manag. Res. J. A Sustain. Circ. Econ. 2024, 21, 734242X241237187. [Google Scholar] [CrossRef] [PubMed]
- Loizia, P.; Voukkali, I.; Zorpas, A.A.; Navarro Pedreño, J.; Chatziparaskeva, G.; Inglezakis, V.J.; Vardopoulos, I.; Doula, M. Measuring the level of environmental performance in insular areas, through key performed indicators, in the framework of waste strategy development. Sci. Total Environ. 2021, 753, 141974. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency, U.S. 2018 Wasted Food Report Estimates of Generation and Management of Wasted Food in the United States in 2018. 2018. Available online: https://www.epa.gov/sites/default/files/2020-11/documents/2018_wasted_food_report-11-9-20_final_.pdf (accessed on 4 May 2024).
- Tiseo, I. Annual Food Waste by Select Country Worldwide|Statista 2023. Available online: https://www.statista.com/statistics/933083/food-waste-of-selected-countries/ (accessed on 4 May 2024).
- Tiseo, I. Food Waste per Capita of Selected Countries Worldwide 2020|Statista 2023. Available online: https://www.statista.com/statistics/933059/per-capita-food-waste-of-selected-countries/ (accessed on 4 May 2024).
- European Commission. Food Waste—European Commission 2024. Available online: https://food.ec.europa.eu/safety/food-waste_en (accessed on 4 May 2024).
- EUROSTAT. Food Waste and Food Waste Prevention—Estimates—Statistics Explained 2023. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Food_waste_and_food_waste_prevention_-_estimates (accessed on 4 May 2024).
- Economou, F.; Papamichael, I.; Rodríguez-Espinosa, T.; Voukkali, I.; Pérez-Gimeno, A.; Zorpas, A.A.; Navarro-Pedreño, J. The Impact of Food Overproduction on Soil: Perspectives and Future Trends. In Planet Earth: Scientific Proposals to Solve Urgent Issues; Springer International Publishing: Cham, Switzerland, 2024; pp. 263–292. [Google Scholar] [CrossRef]
- Teigiserova, D.A.; Hamelin, L.; Thomsen, M. Towards transparent valorization of food surplus, waste and loss: Clarifying definitions, food waste hierarchy, and role in the circular economy. Sci. Total Environ. 2020, 706, 136033. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and Council of the European Union. Directive 2008/98/EC on Waste 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32018L0851 (accessed on 5 May 2024).
- European Commission. Sustainable EU Food System—New Initiative 2023. Available online: https://ec.europa.eu/info/law/better-regulation/have-your-say/initiatives/13174-Sustainable-EU-food-system-new-initiative_en (accessed on 5 May 2024).
- European Commission. Waste Framework Directive 2023. Available online: https://environment.ec.europa.eu/topics/waste-and-recycling/waste-framework-directive_en (accessed on 5 May 2024).
- European Commission. Circular Economy Action Plan 2023. Available online: https://environment.ec.europa.eu/strategy/circular-economy-action-plan_en (accessed on 5 May 2024).
- Stylianou, M.; Papamichael, I.; Voukkali, I.; Tsangas, M.; Omirou, M.; Ioannides, I.M.; Zorpas, A.A. LCA of Barley Production: A Case Study from Cyprus. Int. J. Environ. Res. Public Health 2023, 20, 2417. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Sanchez, M.; Inocencio-García, P.-J.; Alzate-Ramírez, A.F.; Alzate, C.A.C. Potential and Restrictions of Food-Waste Valorization through Fermentation Processes. Fermentation 2023, 9, 274. [Google Scholar] [CrossRef]
- Nguyen, K.L.P.; Chuang, Y.H.; Chen, H.W.; Chang, C.C. Impacts of socioeconomic changes on municipal solid waste characteristics in Taiwan. Resour. Conserv. Recycl. 2020, 161, 104931. [Google Scholar] [CrossRef]
- Sarker, A.; Ghosh, M.K.; Islam, T.; Bilal, M.; Nandi, R.; Raihan, M.L.; Hossain, M.N.; Rana, J.; Barman, S.K.; Kim, J.E. Sustainable Food Waste Recycling for the Circular Economy in Developing Countries, with Special Reference to Bangladesh. Sustainability 2022, 14, 12035. [Google Scholar] [CrossRef]
- Phasha, L.; Molelekwa, G.F.; Mokgobu, M.I.; Morodi, T.J.; Mokoena, M.M.; Mudau, L.S. Influence of cultural practices on food waste in South Africa—A review. J. Ethn. Foods 2020, 7, 37. [Google Scholar] [CrossRef]
- Roufou, S.; Griffin, S.; Katsini, L.; Polańska, M.; Van Impe, J.F.M.; Valdramidis, V.P. The (potential) impact of seasonality and climate change on the physicochemical and microbial properties of dairy waste and its management. Trends Food Sci. Technol. 2021, 116, 1–10. [Google Scholar] [CrossRef]
- Braguglia, C.M.; Gallipoli, A.; Gianico, A.; Pagliaccia, P. Anaerobic bioconversion of food waste into energy: A critical review. Bioresour. Technol. 2018, 248, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Slopiecka, K.; Liberti, F.; Massoli, S.; Bartocci, P.; Fantozzi, F. Chemical and physical characterization of food waste to improve its use in anaerobic digestion plants. Energy Nexus 2022, 5, 100049. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, L.; Mu, L.; Ma, J.; Li, C.; Li, A. Anaerobic digestion of surfactant and lipid co-existing organic waste: Focusing on the antagonistic enhancement. Chem. Eng. J. 2019, 371, 96–106. [Google Scholar] [CrossRef]
- Shamseer; Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Mayer, F.; Bhandari, R.; Gäth, S. Critical review on life cycle assessment of conventional and innovative waste-to-energy technologies. Sci. Total Environ. 2019, 672, 708–721. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Rasheed, T.; Afreen, M.; Anwar, M.T.; Nawaz, Z.; Anwar, H.; Rizwan, K. Modalities for conversion of waste to energy—Challenges and perspectives. Sci. Total Environ. 2020, 727, 138610. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.-Y.; Du, M.A.; Huang, I.-T.; Liu, I.-H.; Chang, E.-E.; Chiang, P.-C. Strategies on implementation of waste-to-energy (WTE) supply chain for circular economy system: A review. J. Clean. Prod. 2015, 108, 409–421. [Google Scholar] [CrossRef]
- Qazi, W.A.; Abushammala, M.F.M.; Mohammed-HashamAzam Younes, M.K. Waste-to-Energy Technologies: A Literature Review. J. Solid. Waste Technol. Manag. 2018, 44, 387–409. [Google Scholar] [CrossRef]
- Vieira, S.; Schneider, J.; Burgos, M.W.J.; Magalhães, A.; Medeiros, A.B.P.; de Carvalho, J.C.; Vandenberghe, L.P.S.; Soccol, C.D.; Sydney, E.B. Pretreatments of Solid Wastes for Anaerobic Digestion and Its Importance for the Circular Economy. In Handbook of Solid Waste Management; Springer Nature: Singapore, 2022; pp. 69–94. [Google Scholar] [CrossRef]
- Fernández, L. UK: Anaerobic Digestion Installed Capacity 2013–2019|Statista 2019. Available online: https://www.statista.com/statistics/498683/anaerobic-digestion-installed-capacity-quaterly-uk/ (accessed on 4 May 2024).
- EBA. 2023 EBA Statistical Report Tracking Biogas and Biomethane Deployment across Europe 2023. Available online: https://www.europeanbiogas.eu/wp-content/uploads/2023/12/EBA-Statistical-Report-2023-Excerpt.pdf (accessed on 4 May 2024).
- Statista Research Department. Germany: Anaerobic Fermentation Energy Production|Statista 2024. Available online: https://www.statista.com/statistics/863132/anaerobic-fermentation-biogas-energy-production-in-germany/ (accessed on 4 May 2024).
- Slorach, P.C.; Jeswani, H.K.; Cuéllar-Franca, R.; Azapagic, A. Environmental sustainability of anaerobic digestion of household food waste. J. Environ. Manag. 2019, 236, 798–814. [Google Scholar] [CrossRef]
- Chen, Y.; Pinegar, L.; Immonen, J.; Powell, K.M. Conversion of food waste to renewable energy: A techno-economic and environmental assessment. J. Clean. Prod. 2023, 385, 135741. [Google Scholar] [CrossRef]
- Algapani, D.E.; Qiao, W.; Ricci, M.; Bianchi, D.; M Wandera, S.; Adani, F.; Dong, R. Bio-hydrogen and bio-methane production from food waste in a two-stage anaerobic digestion process with digestate recirculation. Renew. Energy 2019, 130, 1108–1115. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, D.; Chen, J.; He, Y.; Dai, Y.; Loh, K.-C.; Ton, W.T. Assessment and optimization of a decentralized food-waste-to-energy system with anaerobic digestion and CHP for energy utilization. Energy Convers. Manag. 2021, 228, 113654. [Google Scholar] [CrossRef]
- Xiao, B.; Qin, Y.; Zhang, W.; Wu, J.; Qiang, H.; Liu, J.; Li, Y.-Y. Temperature-phased anaerobic digestion of food waste: A comparison with single-stage digestions based on performance and energy balance. Bioresour. Technol. 2018, 249, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Zhang, W.; Yi, H.; Qin, Y.; Wu, J.; Liu, J.; Li, Y.-Y. Biogas production by two-stage thermophilic anaerobic co-digestion of food waste and paper waste: Effect of paper waste ratio. Renew. Energy 2019, 132, 1301–1309. [Google Scholar] [CrossRef]
- Pohl, M.; Sánchez-Sánchez, M.; Mumme, J. Anaerobic digestion of wheat straw and rape oil cake in a two-stage solid-state system. Renew. Energy 2019, 141, 359–367. [Google Scholar] [CrossRef]
- Wu, C.; Huang, Q.; Yu, M.; Ren, Y.; Wang, Q.; Sakai, K. Effects of digestate recirculation on a two-stage anaerobic digestion system, particularly focusing on metabolite correlation analysis. Bioresour. Technol. 2018, 251, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Gaby, J.C.; Zamanzadeh, M.; Horn, S.J. The effect of temperature and retention time on methane production and microbial community composition in staged anaerobic digesters fed with food waste. Biotechnol. Biofuels 2017, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Ingrao, C.; Faccilongo, N.; Di Gioia, L.; Messineo, A. Food waste recovery into energy in a circular economy perspective: A comprehensive review of aspects related to plant operation and environmental assessment. J. Clean. Prod. 2018, 184, 869–892. [Google Scholar] [CrossRef]
- Yang, S.; Xue, W.; Liu, P.; Lu, X.; Wu, X.; Sun, L.; Zan, F. Revealing the methanogenic pathways for anaerobic digestion of key components in food waste: Performance, microbial community, and implications. Bioresour. Technol. 2022, 347, 126340. [Google Scholar] [CrossRef]
- Li, R.; Gong, M.; Biney, B.W.; Chen, K.; Xia, W.; Liu, H.; Guo, A. Three-stage pretreatment of food waste to improve fuel characteristics and incineration performance with recovery of process by-products. Fuel 2022, 330, 125655. [Google Scholar] [CrossRef]
- Atelge, M.R.; Krisa, D.; Kumar, G.; Eskicioglu, C.; Nguyen, D.D.; Chang, S.W.; Atabani, A.; Al-Muhtaseb, A.; Ünalan, S. Biogas Production from Organic Waste: Recent Progress and Perspectives. Waste Biomass Valorization 2020, 11, 1019–1040. [Google Scholar] [CrossRef]
- Arelli, V.; Juntupally, S.; Begum, S.; Anupoju, G.R. Solid state anaerobic digestion of organic waste for the generation of biogas and bio manure. In Advanced Organic Waste Management; Elsevier: Amsterdam, The Netherlands, 2022; pp. 247–277. [Google Scholar] [CrossRef]
- Rafiee, A.; Khalilpour, K.R.; Prest, J.; Skryabin, I. Biogas as an energy vector. Biomass Bioenergy 2021, 144, 105935. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, I.K.M.; Tsang, D.C.W.; Bolan, N.S.; Sik Ok, Y.; Igalavithana, A.D.; Kirkham, M.B.; Kim, K.-H.; Vikrant, K. Value-added chemicals from food supply chain wastes: State-of-the-art review and future prospects. Chem. Eng. J. 2019, 375, 121983. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A comprehensive review on food waste anaerobic digestion: Research updates and tendencies. Bioresour. Technol. 2018, 247, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.A.; Tavares, F.; Alves, M.M.; Cavaleiro, A.J.; Pereira, M.A. Garden and food waste co-fermentation for biohydrogen and biomethane production in a two-step hyperthermophilic-mesophilic process. Bioresour. Technol. 2019, 278, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Karim T ul Onik, M.H.; Kumar, D.; Rahman, M.A.; Yousuf, A.; Uddin, M.R. Impact of temperature, inoculum flow pattern, inoculum type, and their ratio on dry anaerobic digestion for biogas production. Sci. Rep. 2022, 12, 6162. [Google Scholar] [CrossRef] [PubMed]
- Menzel, T.; Neubauer, P.; Junne, S. Role of Microbial Hydrolysis in Anaerobic Digestion. Energies 2020, 13, 5555. [Google Scholar] [CrossRef]
- Meegoda, J.; Li, B.; Patel, K.; Wang, L. A Review of the Processes, Parameters, and Optimization of Anaerobic Digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef]
- Park, C.; Lee, C.; Kim, S.; Chen, Y.; Chase, H.A. Upgrading of anaerobic digestion by incorporating two different hydrolysis processes. J. Biosci. Bioeng. 2005, 100, 164–167. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, M.; Shi, W. Evaluation of ultrasound pretreatment and drying methods on selected quality attributes of bitter melon (Momordica charantia L.). Dry. Technol. 2019, 37, 387–396. [Google Scholar] [CrossRef]
- Li, X.; Mettu, S.; Martin, G.J.O.; Ashokkumar, M.; Lin, C.S.K. Ultrasonic pretreatment of food waste to accelerate enzymatic hydrolysis for glucose production. Ultrason. Sonochem. 2019, 53, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Morales-Polo, C.; del Mar Cledera-Castro, M.; Moratilla Soria, B.Y. Reviewing the Anaerobic Digestion of Food Waste: From Waste Generation and Anaerobic Process to Its Perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef]
- Varjani, S.; Sivashanmugam, P.; Tyagi, V.K.; Gunasekaran, M. Breakthrough in hydrolysis of waste biomass by physico-chemical pretreatment processes for efficient anaerobic digestion. Chemosphere 2022, 294, 133617. [Google Scholar] [CrossRef]
- Paritosh, K.; Kushwaha, S.K.; Yadav, M.; Pareek, N.; Chawade, A.; Vivekanand, V. Food Waste to Energy: An Overview of Sustainable Approaches for Food Waste Management and Nutrient Recycling. Biomed. Res. Int. 2017, 2017, 2370927. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.; Braghieri, A.; Capece, A.; Napolitano, F.; Romano, P.; Galgano, F.; Altieri, G.; Genovese, F. Recent Updates on the Use of Agro-Food Waste for Biogas Production. Appl. Sci. 2019, 9, 1217. [Google Scholar] [CrossRef]
- Liebetrau, J.; Sträuber, H.; Kretzschmar, J.; Denysenko, V.; Nelles, M. Anaerobic Digestion. In Biorefineries. Advances in Biochemical Engineering/Biotechnology; Springer: Cham, Switzerland, 2017; pp. 281–299. [Google Scholar] [CrossRef]
- Chew, K.R.; Leong, H.Y.; Khoo, K.S.; Vo, D.-V.N.; Anjum, H.; Chang, C.-K.; Show, P.L. Effects of anaerobic digestion of food waste on biogas production and environmental impacts: A review. Environ. Chem. Lett. 2021, 19, 2921–2939. [Google Scholar] [CrossRef]
- Kumar, V.; Nabaterega, R.; Khoei, S.; Eskicioglu, C. Insight into interactions between syntrophic bacteria and archaea in anaerobic digestion amended with conductive materials. Renew. Sustain. Energy Rev. 2021, 144, 110965. [Google Scholar] [CrossRef]
- Liu, C.; Ren, L.; Yan, B.; Luo, L.; Zhang, J.; Awasthi, M.K. Electron transfer and mechanism of energy production among syntrophic bacteria during acidogenic fermentation: A review. Bioresour. Technol. 2021, 323, 124637. [Google Scholar] [CrossRef]
- Zou, H.; Gao, M.; Yu, M.; Zhang, W.; Zhang, S.; Wu, C.; Tashiro, Y.; Wang, Q. Methane production from food waste via mesophilic anaerobic digestion with ethanol pre-fermentation: Methanogenic pathway and microbial community analyses. Bioresour. Technol. 2020, 297, 122450. [Google Scholar] [CrossRef]
- Zabranska, J.; Pokorna, D. Bioconversion of carbon dioxide to methane using hydrogen and hydrogenotrophic methanogens. Biotechnol. Adv. 2018, 36, 707–720. [Google Scholar] [CrossRef]
- Xiao, L.; Zheng, S.; Lichtfouse, E.; Luo, M.; Tan, Y.; Liu, F. Carbon nanotubes accelerate acetoclastic methanogenesis: From pure cultures to anaerobic soils. Soil Biol. Biochem. 2020, 150, 107938. [Google Scholar] [CrossRef]
- Cavalcante, W.A.; Gehring, T.A.; Zaiat, M. Stimulation and inhibition of direct interspecies electron transfer mechanisms within methanogenic reactors by adding magnetite and granular actived carbon. Chem. Eng. J. 2021, 415, 128882. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, Y.; Wu, S.; Zhou, L. Rapid initiation of methanogenesis in the anaerobic digestion of food waste by acclimatizing sludge with sulfidated nanoscale zerovalent iron. Bioresour. Technol. 2021, 341, 125805. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Du, S.; Xie, L. Effects of pH on ex-situ biomethanation with hydrogenotrophic methanogens under thermophilic and extreme-thermophilic conditions. J. Biosci. Bioeng. 2021, 131, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Karthikeyan, O.P.; Selvam, A.; Palani, S.G.; Ghangrekar, M.M.; Wong, J.W.C. Two-phase anaerobic digestion of food waste: Effect of semi-continuous feeding on acidogenesis and methane production. Bioresour. Technol. 2022, 346, 126396. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Mahapatra, D.; Venkatraman, A.V.; Muthuswamy, S.; Pugazhendhi, A. Advancing anaerobic digestion through two-stage processes: Current developments and future trends. Renew. Sustain. Energy Rev. 2020, 123, 109746. [Google Scholar] [CrossRef]
- Xiao, B.; Qin, Y.; Wu, J.; Chen, H.; Yu, P.; Liu, J.; Li, Y.-Y. Comparison of single-stage and two-stage thermophilic anaerobic digestion of food waste: Performance, energy balance and reaction process. Energy Convers. Manag. 2018, 156, 215–223. [Google Scholar] [CrossRef]
- García-Depraect, O.; Martínez-Mendoza, L.J.; Diaz, I.; Muñoz, R. Two-stage anaerobic digestion of food waste: Enhanced bioenergy production rate by steering lactate-type fermentation during hydrolysis-acidogenesis. Bioresour. Technol. 2022, 358, 127358. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Li, H.; Deng, Z.; Wang, Q.; Zhang, Y.; Zheng, C. Effect of pre-fermentation types on the potential of methane production and energy recovery from food waste. Renew. Energy 2020, 146, 1588–1595. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, H.; Yang, X.; Angelidaki, I.; Zhang, Y. Sulfide restrains the growth of Methylocapsa acidiphila converting renewable biogas to single cell protein. Water Res. 2020, 184, 116138. [Google Scholar] [CrossRef]
- Cheng, F.; Brewer, C.E. Conversion of protein-rich lignocellulosic wastes to bio-energy: Review and recommendations for hydrolysis + fermentation and anaerobic digestion. Renew. Sustain. Energy Rev. 2021, 146, 111167. [Google Scholar] [CrossRef]
- Li, X.; Huang, J.; Liu, Y.; Huang, T.; Maurer, C.; Kranert, M. Effects of Salt on Anaerobic Digestion of Food Waste with Different Component Characteristics and Fermentation Concentrations. Energies 2019, 12, 3571. [Google Scholar] [CrossRef]
- Kunatsa, T.; Xia, X. A review on anaerobic digestion with focus on the role of biomass co-digestion, modelling and optimisation on biogas production and enhancement. Bioresour. Technol. 2022, 344, 126311. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhang, K.; Liu, P.; Khan, A.; Xiong, J.; Tian, F.; Li, X. A critical review on the interaction of substrate nutrient balance and microbial community structure and function in anaerobic co-digestion. Bioresour. Technol. 2018, 247, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yuan, X.; Wang, S.; Sun, F.; Hou, Z.; Hu, Q.; Zhail, L.; Cui, Z.; Zoy, Y. Methane production and characteristics of the microbial community in the co-digestion of spent mushroom substrate with dairy manure. Bioresour. Technol. 2018, 250, 611–620. [Google Scholar] [CrossRef]
- Tayyab, A.; Ahmad, Z.; Mahmood, T.; Khalid, A.; Qadeer, S.; Mahmood, S.; Andleep, S.; Anjum, S. Anaerobic co-digestion of catering food waste utilizing Parthenium hysterophorus as co-substrate for biogas production. Biomass Bioenergy 2019, 124, 74–82. [Google Scholar] [CrossRef]
- Baldi, F.; Pecorini, I.; Iannelli, R. Comparison of single-stage and two-stage anaerobic co-digestion of food waste and activated sludge for hydrogen and methane production. Renew. Energy 2019, 143, 1755–1765. [Google Scholar] [CrossRef]
- Johnravindar, D.; Kaur, G.; Liang, J.; Lou, L.; Zhao, J.; Manu, M.K.; Kumar, R.; Varjani, S.; Wong, J.W.C. Impact of total solids content on biochar amended co-digestion of food waste and sludge: Microbial community dynamics, methane production and digestate quality assessment. Bioresour. Technol. 2022, 361, 127682. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Lee, W.; Kang, S.; Kim, S.-H. Enhancement of Sewage Sludge Digestion by Co-digestion with Food Waste and Swine Waste. Waste Biomass Valorization 2020, 11, 2421–2430. [Google Scholar] [CrossRef]
- Chow, W.L.; Chong, S.; Lim, J.W.; Chan, Y.J.; Chong, M.F.; Tiong, T.J.; Chin, J.K.; Pan, G.-T. Anaerobic Co-Digestion of Wastewater Sludge: A Review of Potential Co-Substrates and Operating Factors for Improved Methane Yield. Processes 2020, 8, 39. [Google Scholar] [CrossRef]
- Suárez, E.; Tobajas, M.; Mohedano, A.F.; de la Rubia, M.A. Biowaste management by hydrothermal carbonization and anaerobic co-digestion: Synergistic effects and comparative metagenomic analysis. Waste Manag. 2024, 180, 1–8. [Google Scholar] [CrossRef]
- Soares, J.F.; Confortin, T.C.; Todero, I.; Mayer, F.D.; Mazutti, M.A. Dark fermentative biohydrogen production from lignocellulosic biomass: Technological challenges and future prospects. Renew. Sustain. Energy Rev. 2020, 117, 109484. [Google Scholar] [CrossRef]
- Toledo-Alarcón, J.; Capson-Tojo, G.; Marone, A.; Paillet, F.; Júnior, A.D.N.F.; Chatellard, L.; Bernet, N.; Trably, E. Basics of Bio-hydrogen Production by Dark Fermentation. In Bioreactors for Microbial Biomass and Energy Conversiony; Springer: Berlin/Heidelberg, Germany, 2018; pp. 199–220. [Google Scholar] [CrossRef]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kaminski, M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Z.; Zhou, Y.; Ng, W.J. The role of hydrogenotrophic methanogens in an acidogenic reactor. Chemosphere 2015, 140, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Renewable hydrogen production by dark-fermentation: Current status, challenges and perspectives. Bioresour. Technol. 2021, 321, 124354. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Basu, S.; Shetti, N.P.; Kamali, M.; Walvekar, P.; Aminabhavi, T.M. Waste-to-energy nexus: A sustainable development. Environ. Pollut. 2020, 267, 115501. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Waste-to-energy nexus for circular economy and environmental protection: Recent trends in hydrogen energy. Sci. Total Environ. 2020, 713, 136633. [Google Scholar] [CrossRef] [PubMed]
- Turhal, S.; Turanbaev, M.; Argun, H. Hydrogen production from melon and watermelon mixture by dark fermentation. Int. J. Hydrogen Energy 2019, 44, 18811–18817. [Google Scholar] [CrossRef]
- Karim, A.; Islam, M.A.; Mohammad Faizal, C.K.; Yousuf, A.; Howarth, M.; Dubey, B.N.; Cheng, C.K.; Khan, M.R. Enhanced Biohydrogen Production from Citrus Wastewater Using Anaerobic Sludge Pretreated by an Electroporation Technique. Ind. Eng. Chem. Res. 2019, 58, 573–580. [Google Scholar] [CrossRef]
- Yang, G.; Yin, Y.; Wang, J. Microbial community diversity during fermentative hydrogen production inoculating various pretreated cultures. Int. J. Hydrogen Energy 2019, 44, 13147–13156. [Google Scholar] [CrossRef]
- Reungsang, A.; Zhong, N.; Yang, Y.; Sittijunda, S.; Xia, A.; Liao, Q. Hydrogen from Photo Fermentation. In Bioreactors for Microbial Biomass and Energy Conversion; Springer: Berlin/Heidelberg, Germany, 2018; pp. 221–317. [Google Scholar] [CrossRef]
- Melitos, G.; Voulkopoulos, X.; Zabaniotou, A. Waste to Sustainable Biohydrogen Production Via Photo-Fermentation and Biophotolysis—A Systematic Review. Renew. Energy Environ. Sustain. 2021, 6, 45. [Google Scholar] [CrossRef]
- Das, S.R.; Basak, N. Molecular biohydrogen production by dark and photo fermentation from wastes containing starch: Recent advancement and future perspective. Bioprocess. Biosyst. Eng. 2021, 44, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Fernandes, A.; Lopes, A.; Grasa, L.; Salafranca, J. Photo-Fermentative Bacteria Used for Hydrogen Production. Appl. Sci. 2024, 14, 1191. [Google Scholar] [CrossRef]
- Wu, Y.-N.; Wen, H.-Q.; Zhu, J.-N.; Ding, J.; Ren, N.-Q.; Liu, B.-F. Best mode for photo-fermentation hydrogen production: The semi-continuous operation. Int. J. Hydrogen Energy 2016, 41, 16048–16054. [Google Scholar] [CrossRef]
- Mıynat, M.E.; Ören, İ.; Özkan, E.; Argun, H. Sequential dark and photo-fermentative hydrogen gas production from agar embedded molasses. Int. J. Hydrogen Energy 2020, 45, 34730–34738. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Li, Y.; Lu, C.; Zhu, S.; He, C.; Ai, F.; Zhang, Q. Investigation of the interaction between lighting and mixing applied during the photo-fermentation biohydrogen production process from agricultural waste. Bioresour. Technol. 2020, 312, 123570. [Google Scholar] [CrossRef]
- Turon, V.; Anxionnaz-Minvielle, Z.; Willison, J.C. Replacing incandescent lamps with an LED panel for hydrogen production by photofermentation: Visible and NIR wavelength requirements. Int. J. Hydrogen Energy 2018, 43, 7784–7794. [Google Scholar] [CrossRef]
- Guo, S.; Lu, C.; Wang, K.; Wang, J.; Zhang, Z.; Jing, Y.; Zhang, Q. Enhancement of pH values stability and photo-fermentation biohydrogen production by phosphate buffer. Bioengineered 2020, 11, 291–300. [Google Scholar] [CrossRef]
- Zagrodnik, R.; Łaniecki, M. The effect of pH on cooperation between dark- and photo-fermentative bacteria in a co-culture process for hydrogen production from starch. Int. J. Hydrogen Energy 2017, 42, 2878–2888. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, D.; Zhang, H.; Jing, Y.; Tahir, N.; Zhang, Y.; Zhang, Q. Comparative study on bio-hydrogen production from corn stover: Photo-fermentation, dark-fermentation and dark-photo co-fermentation. Int. J. Hydrogen Energy 2020, 45, 3807–3814. [Google Scholar] [CrossRef]
- Barghash, H.; Okedu, K.E.; Al Balushi, A. Bio-Hydrogen Production Using Landfill Leachate Considering Different Photo-Fermentation Processes. Front. Bioeng. Biotechnol. 2021, 9. [Google Scholar] [CrossRef]
- Adessi, A.; Venturi, M.; Candeliere, F.; Galli, V.; Granchi, L.; De Philippis, R. Bread wastes to energy: Sequential lactic and photo-fermentation for hydrogen production. Int. J. Hydrogen Energy 2018, 43, 9569–9576. [Google Scholar] [CrossRef]
- Cai, J.; Zhao, Y.; Fan, J.; Li, F.; Feng, C.; Guan, Y.; Wang, R.; Tang, N. Photosynthetic bacteria improved hydrogen yield of combined dark- and photo-fermentation. J. Biotechnol. 2019, 302, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Orege, J.I.; Oderinde, O.; Kifle, G.A.; Ibikunle, A.A.; Raheem, S.A.; Ejeromedoghene, O.; Okeke, E.S.; Olukwi, O.M.; Orege, O.B.; Fagbohun, E.O. Recent advances in heterogeneous catalysis for green biodiesel production by transesterification. Energy Convers. Manag. 2022, 258, 115406. [Google Scholar] [CrossRef]
- Bardhan, P.; Deka, A.; Bhattacharya, S.S.; Mandal, M.; Kataki, R. Economical aspect in biomass to biofuel production. In Value-Chain of Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 395–427. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Hur, J.; Cho, J. Review on transesterification of non-edible sources for biodiesel production with a focus on economic aspects, fuel properties and by-product applications. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar] [CrossRef]
- Biernat, K.; Matuszewska, A.; Samson-Bręk, I.; Owczuk, M. Biological Methods in Biodiesel Production and Their Environmental Impact. Appl. Sci. 2021, 11, 10946. [Google Scholar] [CrossRef]
- Silva, J.D.; Martins, L.H.; Moreira, D.K.; Silva, L.D.; Barbosa, P.D.; Komesu, A.; Ferreira, N.R.; Oliveira, J.A. Microbial Lipid Based Biorefinery Concepts: A Review of Status and Prospects. Foods 2023, 12, 2074. [Google Scholar] [CrossRef] [PubMed]
- Ghazani, S.M.; Marangoni, A.G. Microbial lipids for foods. Trends Food Sci. Technol. 2022, 119, 593–607. [Google Scholar] [CrossRef]
- Vasaki, M.; Sithan, M.; Ravindran, G.; Paramasivan, B.; Ekambaram, G.; Karri, R.R. Biodiesel production from lignocellulosic biomass using Yarrowia lipolytica. Energy Convers. Manag. X 2022, 13, 100167. [Google Scholar] [CrossRef]
- Bušić, A.; Kundas, S.; Morzak, G.; Belskaya, H.; Marđetko, N.; Ivančić Šantek, M.; Komes, D.; Novak, S.; Santek, B. Recent Trends in Biodiesel and Biogas Production. Food Technol. Biotechnol. 2018, 56, 152. [Google Scholar] [CrossRef]
- Chozhavendhan, S.; Vijay Pradhap Singh, M.; Fransila, B.; Praveen Kumar, R.; Karthiga Devi, G. A review on influencing parameters of biodiesel production and purification processes. Curr. Res. Green Sustain. Chem. 2020, 1–2, 1–6. [Google Scholar] [CrossRef]
- Jain, S. Biodiesel production from food waste using insitu transesterification method. Sustain. Energy Technol. Assess. 2023, 58, 103380. [Google Scholar] [CrossRef]
- Oliva, G.; Buonerba, A.; Grassi, A.; Hasan, S.W.; Korshin, G.V.; Zorpas, A.A.; Belgiorno, V.; Naddeo, V.; Zarra, T. Microalgae to biodiesel: A novel green conversion method for high-quality lipids recovery and in-situ transesterification to fatty acid methyl esters. J. Environ. Manag. 2024, 357, 120830. [Google Scholar] [CrossRef] [PubMed]
- Baskar, G.; Kalavathy, G.; Aiswarya, R.; Abarnaebenezer Selvakumari, I. Advances in bio-oil extraction from nonedible oil seeds and algal biomass. In Advances in Eco-Fuels for a Sustainable Environment; Elsevier: Amsterdam, The Netherlands, 2019; pp. 187–210. [Google Scholar] [CrossRef]
- Kumar, S.; Sani, R.K. (Eds.) Biorefining of Biomass to Biofuels; Springer International Publishing: Cham, Switzerland, 2018; Volume 4. [Google Scholar] [CrossRef]
- Moazeni, F.; Chen, Y.-C.; Zhang, G. Enzymatic transesterification for biodiesel production from used cooking oil, a review. J. Clean. Prod. 2019, 216, 117–128. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; Sáez-Bastante, J.; Pinzi, S.; Dorado, M.P. Optimization of solid food waste oil biodiesel by ultrasound-assisted transesterification. Fuel 2019, 255, 115817. [Google Scholar] [CrossRef]
- Jung, S.; Jung, J.-M.; Tsang, Y.F.; Bhatnagar, A.; Chen, W.-H.; Lin, K.-Y.A.; Kwon, E.E. Biodiesel production from black soldier fly larvae derived from food waste by non-catalytic transesterification. Energy 2022, 238, 121700. [Google Scholar] [CrossRef]
- Patel, A.; Hrůzová, K.; Rova, U.; Christakopoulos, P.; Matsakas, L. Sustainable biorefinery concept for biofuel production through holistic volarization of food waste. Bioresour. Technol. 2019, 294, 122247. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y. Turning food waste to energy and resources towards a great environmental and economic sustainability: An innovative integrated biological approach. Biotechnol. Adv. 2019, 37, 107414. [Google Scholar] [CrossRef] [PubMed]
- Bueta, G.R.P. Chapter 5: Circular Economy Policy Initiatives and Experiences in the Philippines: Lessons for Asia and the Pacific and Beyond. 2022. Available online: https://www.adb.org/sites/default/files/publication/774936/adbi-transitioning-linear-circular-economy-developing-asia-web.pdf (accessed on 5 May 2024).
- Kaur, A.; Bharti, R.; Sharma, R. Municipal solid waste as a source of energy. Mater. Today Proc. 2023, 81, 904–915. [Google Scholar] [CrossRef]
- Ren, X.; Song, K.; Xiao, Y.; Chen, W.; Liu, D. Constituent transformation mechanism of concentrated leachate after incineration at different temperatures. Environ. Sci. Pollut. Res. 2019, 26, 34613–34621. [Google Scholar] [CrossRef]
- Pham, T.P.T.; Kaushik, R.; Parshetti, G.K.; Mahmood, R.; Balasubramanian, R. Food waste-to-energy conversion technologies: Current status and future directions. Waste Manag. 2015, 38, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Samadder, S.R. A review on technological options of waste to energy for effective management of municipal solid waste. Waste Manag. 2017, 69, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Assi, A.; Bilo, F.; Federici, S.; Zacco, A.; Depero, L.E.; Bontempi, E. Bottom ash derived from municipal solid waste and sewage sludge co-incineration: First results about characterization and reuse. Waste Manag. 2020, 116, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hu, Y.; Cheng, H. Municipal solid waste (MSW) incineration fly ash as an important source of heavy metal pollution in China. Environ. Pollut. 2019, 252, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.S.; Ghouleh, Z.; Shao, Y. Production of eco-cement exclusively from municipal solid waste incineration residues. Resour. Conserv. Recycl. 2019, 149, 332–342. [Google Scholar] [CrossRef]
- Abdullah, M.H.; Rashid, A.S.A.; Anuar, U.H.M.; Marto, A.; Abuelgasim, R. Bottom ash utilization: A review on engineering applications and environmental aspects. IOP Conf. Ser. Mater. Sci. Eng. 2019, 527, 012006. [Google Scholar] [CrossRef]
- Wang, C.; Liu, K.; Huang, D.; Chen, Q.; Tu, M.; Wu, K.; Shui, Z. Utilization of fly ash as building material admixture: Basic properties and heavy metal leaching. Case Stud. Constr. Mater. 2022, 17, e01422. [Google Scholar] [CrossRef]
- Tait, P.W.; Brew, J.; Che, A.; Costanzo, A.; Danyluk, A.; Davis, M.; Khalaf, A.; McMahon, K.; Watson, L.; Rowcliff, K.; et al. The health impacts of waste incineration: A systematic review. Aust. N. Z. J. Public Health 2020, 44, 40–48. [Google Scholar] [CrossRef]
- Prata, J.C.; Silva, A.L.P.; da Costa, J.P.; Mouneyrac, C.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. Solutions and Integrated Strategies for the Control and Mitigation of Plastic and Microplastic Pollution. Int. J. Environ. Res. Public Health 2019, 16, 2411. [Google Scholar] [CrossRef]
- Slorach, P.C.; Jeswani, H.K.; Cuéllar-Franca, R.; Azapagic, A. Environmental and economic implications of recovering resources from food waste in a circular economy. Sci. Total Environ. 2019, 693, 133516. [Google Scholar] [CrossRef]
- Jabeen, F.; Adrees, M.; Ibrahim, M.; Mahmood, A.; Khalid, S.; Sipra, H.F.K.; Bokhari, A.; Mubashir, M.; Kho, K.S.; Show, P.L. Trash to Energy: A Measure for the Energy Potential of Combustible content of Domestic solid waste generated from an industrialized city of Pakistan. J. Taiwan Inst. Chem. Eng. 2022, 137, 104223. [Google Scholar] [CrossRef]
- Statista Research Department. Global Municipal Waste Energy Production|Statista n.d. Available online: https://www.statista.com/statistics/481722/municipal-waste-to-energy-production-globally/ (accessed on 5 May 2024).
- Kumar, A.; Singh, E.; Mishra, R.; Kumar, S. Solid Waste to Energy: Existing Scenario in Developing and Developed Countries. In Handbook of Solid Waste Management; Springer Nature: Singapore, 2022; pp. 2023–2045. [Google Scholar] [CrossRef]
- Stöcker, M. Perspectives for Thermochemical Conversions of Lignocellulosic Biomass. Small 2023, 2302495. [Google Scholar] [CrossRef] [PubMed]
- Kostas, E.T.; Durán-Jiménez, G.; Shepherd, B.J.; Meredith, W.; Stevens, L.A.; Williams, O.S.A.; Robinson, J.P. Microwave pyrolysis of olive pomace for bio-oil and bio-char production. Chem. Eng. J. 2020, 387, 123404. [Google Scholar] [CrossRef]
- Qing, M.; Long, Y.; Liu, L.; Yi, Y.; Li, W.; He, R.; Yin, Y.; Tian, H.; He, J.; Cheng, S.; et al. Pyrolysis of the food waste collected from catering and households under different temperatures: Assessing the evolution of char structure and bio-oil composition. J. Anal. Appl. Pyrolysis 2022, 164, 105543. [Google Scholar] [CrossRef]
- Świechowski, K.; Matyjewicz, B.; Telega, P.; Białowiec, A. The Influence of Low-Temperature Food Waste Biochars on Anaerobic Digestion of Food Waste. Materials 2022, 15, 945. [Google Scholar] [CrossRef] [PubMed]
- Kazawadi, D.; Ntalikwa, J.; Kombe, G. A Review of Intermediate Pyrolysis as a Technology of Biomass Conversion for Coproduction of Biooil and Adsorption Biochar. J. Renew. Energy 2021, 2021, 5533780. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z.; Hu, Z.; Zhang, J. Recent advances in biochar application for water and wastewater treatment: A review. PeerJ 2020, 8, e9164. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Qin, Y.; Wang, J. Evaluation of the properties and carbon sequestration potential of biochar-modified pervious concrete. Constr. Build. Mater. 2022, 314, 125648. [Google Scholar] [CrossRef]
- Vijay, V.; Shreedhar, S.; Adlak, K.; Payyanad, S.; Sreedharan, V.; Gopi, G.; Voort, T.S.; Malarvizhi, P.P.; Yi, S.; Gebert, J. Review of Large-Scale Biochar Field-Trials for Soil Amendment and the Observed Influences on Crop Yield Variations. Front. Energy Res. 2021, 9, 710766. [Google Scholar] [CrossRef]
- Ahmad Farid, M.A.; Hassan, M.A.; Roslan, A.M.; Samsudin, M.H.; Mohamad, Z.J.J.; Othman, M.R.; Shirai, Y. Carbon monoxide reduction in the flue gas during biochar production from oil palm empty fruit bunch. J. Clean. Prod. 2020, 258, 120580. [Google Scholar] [CrossRef]
- Legan, M.; Gotvajn, A.Ž.; Zupan, K. Potential of biochar use in building materials. J. Environ. Manag. 2022, 309, 114704. [Google Scholar] [CrossRef] [PubMed]
- Adrados, A.; Merchán, M.; Obregón, A.; Artola, A.; Iparraguirre, J.A.; García de Cortázar, M.; Eguizabal, D.; Demey, H. Development of a Sustainable Metallurgical Process to Valorize Copper Smelting Wastes with Olive Stones-Based Biochar. Metals 2022, 12, 1756. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahmed, Z.; Eldin, S.M.; Ali, B.; Bawazeer, S.; Usman, M.; Iqbal, S.; Neupane, D.; Ullah, A.; Khan, A. Biochar-Soil-Plant interactions: A cross talk for sustainable agriculture under changing climate. Front. Environ. Sci. 2023, 11, 1059449. [Google Scholar] [CrossRef]
- Stylianou, M.; Laifi, T.; Bennici, S.; Dutournie, P.; Limousy, L.; Agapiou, A.; Zorpas, A.A. Tomato waste biochar in the framework of circular economy. Sci. Total Environ. 2023, 871, 161959. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.; Andrew Lin, K.-Y.; Hong, E.; Kwon, E.E.; Lee, J. The valorization of food waste via pyrolysis. J. Clean. Prod. 2020, 259, 120816. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Li, J.; Yan, B.; Chen, G. Pyrolysis of food waste and food waste solid digestate: A comparative investigation. Bioresour. Technol. 2022, 354, 127191. [Google Scholar] [CrossRef] [PubMed]
- Grycová, B.; Koutník, I.; Pryszcz, A. Pyrolysis process for the treatment of food waste. Bioresour. Technol. 2016, 218, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Ong, H.C.; Fattah, I.M.R.; Ok, Y.S.; Jang, J.-H.; Wang, C.-T. State-of-the-art of the pyrolysis and co-pyrolysis of food waste: Progress and challenges. Sci. Total Environ. 2022, 809, 151170. [Google Scholar] [CrossRef] [PubMed]
- Elkhalifa, S.; AlNouss, A.; Al-Ansari, T.; Mackey, H.R.; Parthasarathy, P.; Mckay, G. Simulation of Food Waste Pyrolysis for the Production of Biochar: A Qatar Case Study. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 901–906. [Google Scholar] [CrossRef]
- Patra, B.R.; Nanda, S.; Dalai, A.K.; Meda, V. Slow pyrolysis of agro-food wastes and physicochemical characterization of biofuel products. Chemosphere 2021, 285, 131431. [Google Scholar] [CrossRef]
- Wijitkosum, S. Biochar derived from agricultural wastes and wood residues for sustainable agricultural and environmental applications. Int. Soil Water Conserv. Res. 2022, 10, 335–341. [Google Scholar] [CrossRef]
- Park, C.; Lee, N.; Kim, J.; Lee, J. Co-pyrolysis of food waste and wood bark to produce hydrogen with minimizing pollutant emissions. Environ. Pollut. 2021, 270, 116045. [Google Scholar] [CrossRef]
- Neha, S.; Remya, N. Optimization of bio-oil production from microwave co-pyrolysis of food waste and low-density polyethylene with response surface methodology. J. Environ. Manag. 2021, 297, 113345. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.; Kapoor, A.; Senthil Kumar, P.; Ponnuchamy, M.; Balasubramanian, S.; Prabhakar, S. Conversion of food waste to energy: A focus on sustainability and life cycle assessment. Fuel 2021, 302, 121069. [Google Scholar] [CrossRef]
- Sim, C.-K.; Majid, S.R.; Mahmood, N.Z. Electrochemical Performance of Activated Carbon Derived from Treated Food-Waste. Int. J. Electrochem. Sci. 2015, 10, 10157–10172. [Google Scholar] [CrossRef]
- Xu, S.; Zhu, S.; Li, C.; Bu, J.; Wei Tiong, Y.; Sharma, P.; Kong, W.; Shao, C.; Xie, H.; Tong, Y.W. Succession of biochar in integrated pyrolysis, anaerobic digestion, and solid–state fermentation towards closed loop valorization of food waste. Fuel 2024, 369, 131719. [Google Scholar] [CrossRef]
- Okopi, S.I.; Wang, J.; Liang, W.; Kong, W.; Hu, Y.; Cui, J.; Guo, X.; Zhao, W.; Che, L.; Gu, Z. Experimental study and techno-economic analysis of co-processing system for treatment of food waste with various impurities. Bioresour. Technol. 2024, 394, 130020. [Google Scholar] [CrossRef]
- Inalegwu Okopi, S.; Zeng, J.; Fan, X.; Lu, J.; Cui, J.; Hu, Y.; Wang, J.; Chen, J.; Djandja, O.S.; Ma, Y. Environmental sustainability assessment of a new food waste anaerobic digestion and pyrolysis hybridization system. Waste Manag. 2024, 179, 130–143. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, H.; He, S.; Zhao, Q.; Wei, L. A review of biochar in anaerobic digestion to improve biogas production: Performances, mechanisms and economic assessments. Bioresour. Technol. 2021, 341, 125797. [Google Scholar] [CrossRef]
- Piersa, P.; Unyay, H.; Szufa, S.; Lewandowska, W.; Modrzewski, R.; Ślężak, R.; Ledakowicz, S. An Extensive Review and Comparison of Modern Biomass Torrefaction Reactors vs. Biomass Pyrolysis—Part 1. Energies 2022, 15, 2227. [Google Scholar] [CrossRef]
- Hidalgo, D.; Castro, J.; Díez, D.; Martín-Marroquín, J.M.; Gómez, M.; Pérez, E. Torrefaction at low temperature as a promising pretreatment of lignocellulosic biomass in anaerobic digestion. Energy 2023, 263, 125822. [Google Scholar] [CrossRef]
- Pour, F.H.; Makkawi, Y.T. A review of post-consumption food waste management and its potentials for biofuel production. Energy Rep. 2021, 7, 7759–7784. [Google Scholar] [CrossRef]
- Huang, J.; Qiao, Y.; Wang, Z.; Liu, H.; Wang, B.; Yu, Y. Valorization of Food Waste via Torrefaction: Effect of Food Waste Type on the Characteristics of Torrefaction Products. Energy Fuels 2020, 34, 6041–6051. [Google Scholar] [CrossRef]
- Jagodzińska, K.; Czerep, M.; Kudlek, E.; Wnukowski, M.; Pronobis, M.; Yang, W. Torrefaction of Agricultural Residues: Effect of Temperature and Residence Time on the Process Products Properties. J. Energy Resour. Technol. 2020, 142, 070912. [Google Scholar] [CrossRef]
- Abdul Rahman, R.N.U.; Ismail, M.; Rasid, R.A.; Ahamad Nordin, N.I.A. Torrefaction of Food Waste as a Potential Biomass Energy Source. Indones. J. Chem. 2019, 19, 993. [Google Scholar] [CrossRef]
- Frolov, S.M. Organic Waste Gasification: A Selective Review. Fuels 2021, 2, 556–650. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, M.; Raheem, A.; Wang, F.; Wei, J.; Xu, D.; Song, X.; Bao, W.; Huang, A.; Zhang, S. Syngas Production from Biomass Gasification: Influences of Feedstock Properties, Reactor Type, and Reaction Parameters. ACS Omega 2023, 8, 31620–31631. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Lei, T.; Zhang, Q.; Jin, T.; Zhu, S.; Zhu, Y.; Hu, X.; Cheng, C. Production of H 2 -Rich Syngas from Oxygen-Steam Gasification of Biomass Using Modified Red Mud Extract as Catalyst. J. Biobased Mater. Bioenergy 2021, 15, 278–286. [Google Scholar] [CrossRef]
- Abdoulmoumine, N.; Adhikari, S.; Kulkarni, A.; Chattanathan, S. A review on biomass gasification syngas cleanup. Appl. Energy 2015, 155, 294–307. [Google Scholar] [CrossRef]
- de Oliveira, D.C.; Lora, E.E.S.; Venturini, O.J.; Maya, D.M.Y.; Garcia-Pérez, M. Gas cleaning systems for integrating biomass gasification with Fischer-Tropsch synthesis—A review of impurity removal processes and their sequences. Renew. Sustain. Energy Rev. 2023, 172, 113047. [Google Scholar] [CrossRef]
- Koshariya, A.K.; Krishnan, M.S.; Jaisankar, S.; Loganathan, G.B.; Sathish, T.; Ağbulut, Ü.; Saravanan, R.; Tuan, L.T.; Pham, N.D.K. Waste to energy: An experimental study on hydrogen production from food waste gasification. Int. J. Hydrogen Energy 2024, 54, 1–12. [Google Scholar] [CrossRef]
- Sajid, M.; Raheem, A.; Ullah, N.; Asim, M.; Ur Rehman, M.S.; Ali, N. Gasification of municipal solid waste: Progress, challenges, and prospects. Renew. Sustain. Energy Rev. 2022, 168, 112815. [Google Scholar] [CrossRef]
- Lopes, E.J.; Okamura, L.A.; Maruyama, S.A.; Yamamoto, C.I. Evaluation of energy gain from the segregation of organic materials from municipal solid waste in gasification processes. Renew. Energy 2018, 116, 623–629. [Google Scholar] [CrossRef]
- AlNouss, A.; McKay, G.; Al-Ansari, T. A comparison of steam and oxygen fed biomass gasification through a techno-economic-environmental study. Energy Convers. Manag. 2020, 208, 112612. [Google Scholar] [CrossRef]
- Valizadeh, S.; Lam, S.S.; Ko, C.H.; Lee, S.H.; Farooq, A.; Yu, Y.J.; Jeon, K.-J.; Jung, S.-C.; Rhee, G.H.; Park, Y.-K. Biohydrogen production from catalytic conversion of food waste via steam and air gasification using eggshell- and homo-type Ni/Al2O3 catalysts. Bioresour. Technol. 2021, 320, 124313. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Yadav, S.; Bharadwaj, N.; Verma, R. Low temperature steam gasification to produce hydrogen rich gas from kitchen food waste: Influence of steam flow rate and temperature. Int. J. Hydrogen Energy 2020, 45, 20843–20850. [Google Scholar] [CrossRef]
- Shayan, E.; Zare, V.; Mirzaee, I. Hydrogen production from biomass gasification; a theoretical comparison of using different gasification agents. Energy Convers. Manag. 2018, 159, 30–41. [Google Scholar] [CrossRef]
- Huynh CVan Kong, S.-C. Performance characteristics of a pilot-scale biomass gasifier using oxygen-enriched air and steam. Fuel 2013, 103, 987–996. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, H.; Yao, D.; Zhang, J.; Zhu, Z.; Wang, Y.; Cui, P. Modeling and comprehensive analysis of food waste gasification process for hydrogen production. Energy Convers. Manag. 2022, 258, 115509. [Google Scholar] [CrossRef]
- Nagy, G.; Dobó, Z. Experimental investigation of fixed-bed pyrolysis and steam gasification of food waste blended with woody biomass. Biomass Bioenergy 2020, 139, 105580. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Alencar, A.C. Biomass-derived syngas production via gasification process and its catalytic conversion into fuels by Fischer Tropsch synthesis: A review. Int. J. Hydrogen Energy 2020, 45, 18114–18132. [Google Scholar] [CrossRef]
- Piazzi, S.; Patuzzi, F.; Baratieri, M. Energy and exergy analysis of different biomass gasification coupled to Fischer-Tropsch synthesis configurations. Energy 2022, 249, 123642. [Google Scholar] [CrossRef]
- Munir, M.T.; Mardon, I.; Al-Zuhair, S.; Shawabkeh, A.; Saqib, N.U. Plasma gasification of municipal solid waste for waste-to-value processing. Renew. Sustain. Energy Rev. 2019, 116, 109461. [Google Scholar] [CrossRef]
- Okati, A.; Reza Khani, M.; Shokri, B.; Monteiro, E.; Rouboa, A. Parametric studies over a plasma co-gasification process of biomass and coal through a restricted model in Aspen plus. Fuel 2023, 331, 125952. [Google Scholar] [CrossRef]
- Li, H.; Sun, C.; Zhang, Y.; Li, T.; Wei, X. Performance investigation of the gasification for the kitchen waste powder in a direct current plasma reactor. J. Energy Inst. 2022, 100, 170–176. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, Y.; Yin, K.; Zhang, J.; Zhu, Z.; Wang, Y.; Cui, P. Exergy, techno-economic and environment analysis of food waste plasma gasification and syngas chemical looping processes. J. Clean. Prod. 2023, 386, 135835. [Google Scholar] [CrossRef]
- Hu, Y.; Gong, M.; Xing, X.; Wang, H.; Zeng, Y.; Xu, C.C. Supercritical water gasification of biomass model compounds: A review. Renew. Sustain. Energy Rev. 2020, 118, 109529. [Google Scholar] [CrossRef]
- Reddy, S.N.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of biomass for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 6912–6926. [Google Scholar] [CrossRef]
- Su, W.; Cai, C.; Liu, P.; Lin, W.; Liang, B.; Zhang, H.; Ma, Z.; Ma, H.; Xing, Y.; Liu, W. Supercritical water gasification of food waste: Effect of parameters on hydrogen production. Int. J. Hydrogen Energy 2020, 45, 14744–14755. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, S.; Ge, S.; Chen, X.; Ge, X.; Chen, M. Hydrothermal carbonization of medical wastes and lignocellulosic biomass for solid fuel production from lab-scale to pilot-scale. Energy 2017, 118, 312–323. [Google Scholar] [CrossRef]
- Mannarino, G.; Sarrion, A.; Diaz, E.; Gori, R.; De la Rubia, M.A.; Mohedano, A.F. Improved energy recovery from food waste through hydrothermal carbonization and anaerobic digestion. Waste Manag. 2022, 142, 9–18. [Google Scholar] [CrossRef]
- Matsakas, L.; Gao, Q.; Jansson, S.; Rova, U.; Christakopoulos, P. Green conversion of municipal solid wastes into fuels and chemicals. Electron. J. Biotechnol. 2017, 26, 69–83. [Google Scholar] [CrossRef]
- Sangaré, D.; Moscosa-Santillan, M.; Aragón Piña, A.; Bostyn, S.; Belandria, V.; Gökalp, I. Hydrothermal carbonization of biomass: Experimental study, energy balance, process simulation, design, and techno-economic analysis. Biomass Convers. Biorefin 2024, 14, 2561–2576. [Google Scholar] [CrossRef]
- Benavente, V.; Calabuig, E.; Fullana, A. Upgrading of moist agro-industrial wastes by hydrothermal carbonization. J. Anal. Appl. Pyrolysis 2015, 113, 89–98. [Google Scholar] [CrossRef]
- Wüst, D.; Rodriguez Correa, C.; Suwelack, K.U.; Köhler, H.; Kruse, A. Hydrothermal carbonization of dry toilet residues as an added-value strategy—Investigation of process parameters. J. Environ. Manag. 2019, 234, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Pauline, A.L.; Joseph, K. Hydrothermal carbonization of organic wastes to carbonaceous solid fuel—A review of mechanisms and process parameters. Fuel 2020, 279, 118472. [Google Scholar] [CrossRef]
- Idowu, I.; Li, L.; Flora, J.R.V.; Pellechia, P.J.; Darko, S.A.; Ro, K.S.; Berge, N.D. Hydrothermal carbonization of food waste for nutrient recovery and reuse. Waste Manag. 2017, 69, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, S.; Yang, J.; Xu, D.; Li, Y.; Li, J.; Zhang, Y. Hydrothermal liquefaction and gasification of biomass and model compounds: A review. Green. Chem. 2020, 22, 8210–8232. [Google Scholar] [CrossRef]
- Picone, A.; Volpe, M.; Messineo, A. Process Water Recirculation during Hydrothermal Carbonization of Waste Biomass: Current Knowledge and Challenges. Energies 2021, 14, 2962. [Google Scholar] [CrossRef]
- Tradler, S.B.; Mayr, S.; Himmelsbach, M.; Priewasser, R.; Baumgartner, W.; Stadler, A.T. Hydrothermal carbonization as an all-inclusive process for food-waste conversion. Bioresour. Technol. Rep. 2018, 2, 77–83. [Google Scholar] [CrossRef]
- Motavaf, B.; Dean, R.A.; Nicolas, J.; Savage, P.E. Hydrothermal carbonization of simulated food waste for recovery of fatty acids and nutrients. Bioresour. Technol. 2021, 341, 125872. [Google Scholar] [CrossRef]
- Saqib, N.U.; Baroutian, S.; Sarmah, A.K. Physicochemical, structural and combustion characterization of food waste hydrochar obtained by hydrothermal carbonization. Bioresour. Technol. 2018, 266, 357–363. [Google Scholar] [CrossRef]
- McGaughy, K.; Toufiq Reza, M. Hydrothermal carbonization of food waste: Simplified process simulation model based on experimental results. Biomass Convers. Biorefin 2018, 8, 283–292. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Q.; Fang, M.; Wu, D.; Cui, D.; Pan, S.; Bai, J.; Xu, F.; Wang, Z. Hydrothermal carbonization of food waste for sustainable biofuel production: Advancements, challenges, and future prospects. Sci. Total Environ. 2023, 897, 165327. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhai, Y.; Li, H.; Zhu, Y.; Li, S.; Peng, C.; Wang, B.; Wang, Z.; Xi, Y.; Wang, S.; et al. Co-hydrothermal carbonization of food waste-woody biomass blend towards biofuel pellets production. Bioresour. Technol. 2018, 267, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.B.; Dubey, B.K. Co-hydrothermal carbonization of food waste with yard waste for solid biofuel production: Hydrochar characterization and its pelletization. Waste Manag. 2020, 118, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Ma, X.; Yao, Z.; Chen, X. The properties and combustion behaviors of hydrochars derived from co-hydrothermal carbonization of sewage sludge and food waste. Bioresour. Technol. 2019, 285, 121347. [Google Scholar] [CrossRef]
- Silva Thomsen, L.B.; Anastasakis, K.; Biller, P. Wet oxidation of aqueous phase from hydrothermal liquefaction of sewage sludge. Water Res. 2022, 209, 117863. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.; Sohn, D.; Kim, Y.M.; Park, K.Y. Hydrothermal carbonization of lipid extracted algae for hydrochar production and feasibility of using hydrochar as a solid fuel. Energy 2018, 153, 913–920. [Google Scholar] [CrossRef]
- Skaggs, R.L.; Coleman, A.M.; Seiple, T.E.; Milbrandt, A.R. Waste-to-Energy biofuel production potential for selected feedstocks in the conterminous United States. Renew. Sustain. Energy Rev. 2018, 82, 2640–2651. [Google Scholar] [CrossRef]
- Aslanbay Guler, B.; Deniz, I.; Ozmihci, S.; Imamoglu, E. Biological conversion technologies: Enzyme hydrolysis, ethanol fermentation. In Bioenergy Engineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. 171–200. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Feng, K.; Liu, J. Oriented Fermentation of Food Waste towards High-Value Products: A Review. Energies 2020, 13, 5638. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Y.-W.; Dai, Y.-H.; Song, C.; Zhu, Q.-L.; Qin, H.; Tan, F.-R.; Chen, H.-C.; Dai, L.-C.; Hu, G.-Q.; et al. Current status and future prospective of bio-ethanol industry in China. Renew. Sustain. Energy Rev. 2021, 145, 111079. [Google Scholar] [CrossRef]
- Ayas, N. 2.13 Solvent Materials. Comprehensive Energy Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 368–395. [Google Scholar] [CrossRef]
- Statista Research Department, J. Ethanol Consumption Worldwide 2030|Statista 2024. Available online: https://www.statista.com/statistics/1440979/worldwide-consumption-of-ethanol/ (accessed on 5 May 2024).
- Ma, Y.; Cai, W.; Liu, Y. An integrated engineering system for maximizing bioenergy production from food waste. Appl. Energy 2017, 206, 83–89. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Y.; Zhou, X.; Wang, X.; Zhu, J. Effect of different vegetable wastes on the performance of volatile fatty acids production by anaerobic fermentation. Sci. Total Environ. 2020, 748, 142390. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Venkata Mohan, S. Refining of vegetable waste to renewable sugars for ethanol production: Depolymerization and fermentation optimization. Bioresour. Technol. 2021, 340, 125650. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lin, Y.; Wang, P.; Jin, R.; Gao, M.; Wang, Q.; Chang, T.-C.; Ma, C. Volatile fatty acids production from saccharification residue from food waste ethanol fermentation: Effect of pH and microbial community. Bioresour. Technol. 2019, 292, 121957. [Google Scholar] [CrossRef] [PubMed]
- Anwar Saeed, M.; Ma, H.; Yue, S.; Wang, Q.; Tu, M. Concise review on ethanol production from food waste: Development and sustainability. Environ. Sci. Pollut. Res. 2018, 25, 28851–28863. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, R.; Gebremedhin, A.; Sarker, S. Integration of Waste to Bioenergy Conversion Systems: A Critical Review. Energies 2022, 15, 2697. [Google Scholar] [CrossRef]
- Kataya, G.; Cornu, D.; Bechelany, M.; Hijazi, A.; Issa, M. Biomass Waste Conversion Technologies and Its Application for Sustainable Environmental Development—A Review. Agronomy 2023, 13, 2833. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).