Recent Advances of Solvent Effects in Biomass Liquefaction Conversion
Abstract
1. Introduction
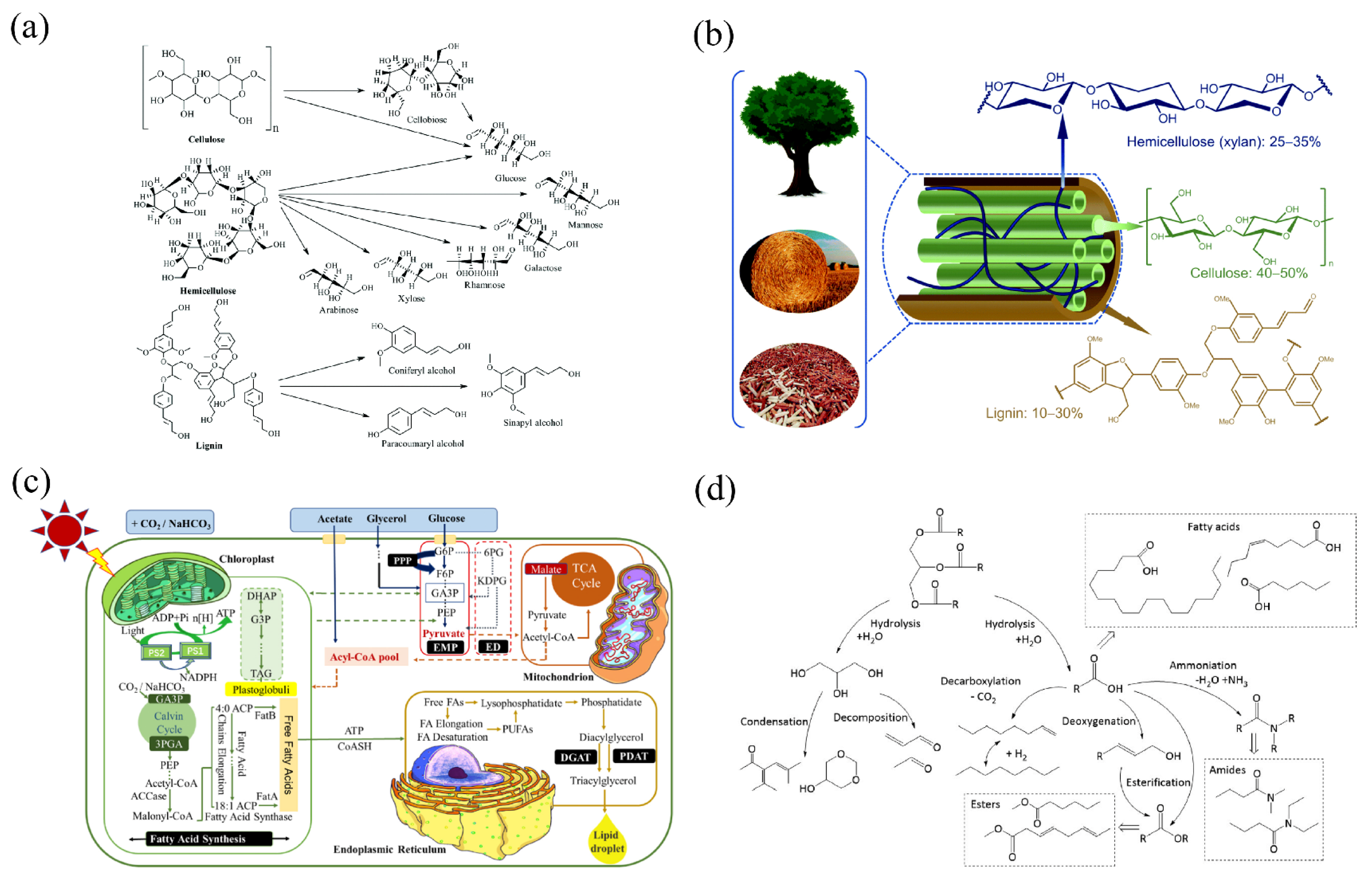
2. Typical Biomass Raw Materials
2.1. Lignocellulose
2.2. Algae
2.3. Sludge
3. Liquefaction Conversion Solvent
3.1. Water
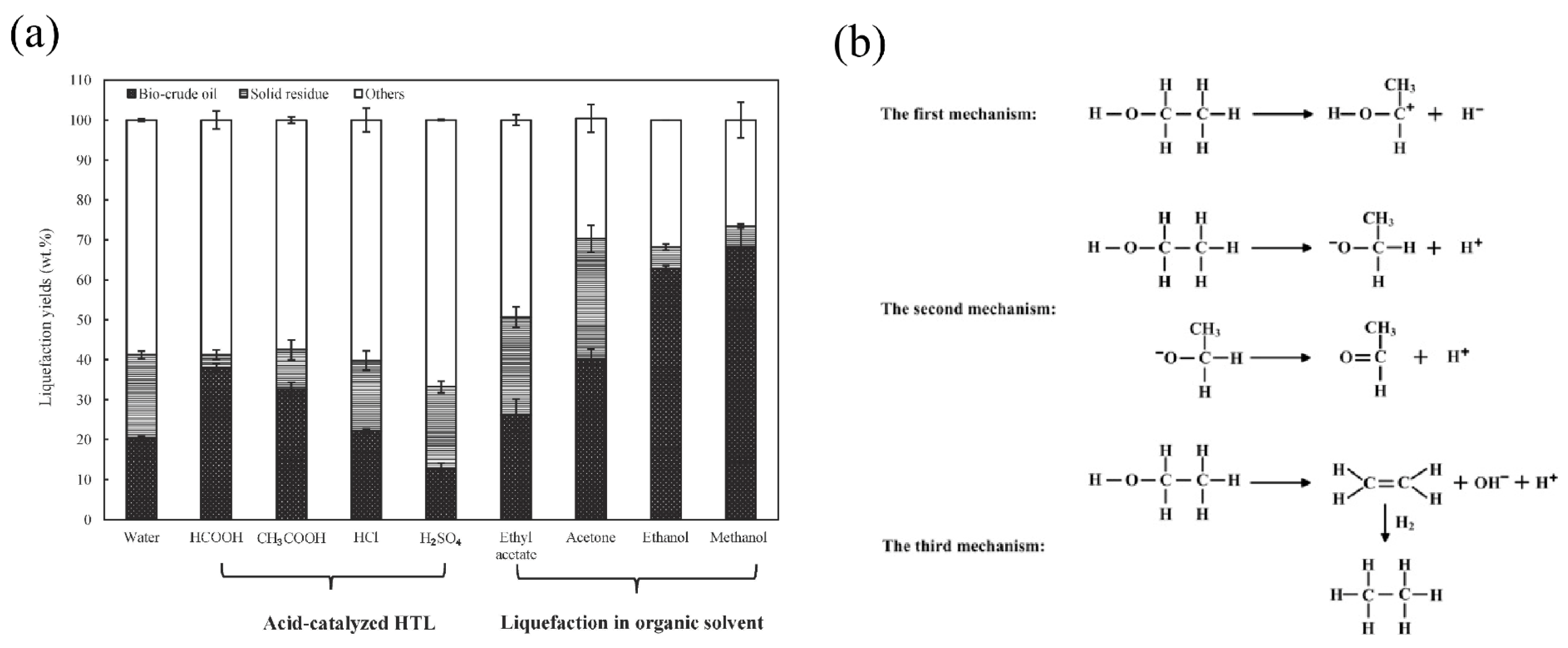
3.2. Organic Solvents
3.2.1. Polar Aprotic Solvents
- (1)
- Aprotic solvents boost the proportion of furanose forms of sugar tautomer by forming intramolecular hydrogen bonds [101,102]. The significance of furanose forms in augmenting 5-HMF production during fructose decomposition in acidic circumstances was validated by recent in situ NMR analyses [103]. Due to the fact that 5-HMF can be easily generated from the breakdown of fructose, it appears that the furanose forms are crucial for the synthesis of 5-HMF from the breakdown of sucrose.
- (2)
- It was reported in a molecular dynamic (MD) simulations study that the direct dehydration of glucose to 5-HMF begins with protonation of the C2-OH and breakdown of the C2−O2 link, followed by the creation of the C2−O5 bond [104]. Nevertheless, it is challenging for these processes to occur in non-acidic environments. An aprotic solvent may alter the local solvent arrangement surrounding the glucose molecules, which would enable the acid-catalyzed processes that lead to the synthesis of 5-HMF [105].
- (3)
- Aprotic solvents increase the stability of 5-HMF formed from sugar decomposition. Aprotic solvents such as DMSO prefer to coordinate around 5-HMF, as shown by earlier simulation studies [106,107]. This creates a shielding effect that stops further rehydration to levulinic acid and formic acid or condensation to produce humins.
3.2.2. Polar Protic Solvents
3.2.3. Non-Polar Solvents
3.2.4. Ionic Liquids
3.3. Co-Solvent
3.3.1. Organic Solvent/Water Co-Solvent
3.3.2. Organic Solvent/Organic Solvent Co-Solvent
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wayth, N.; Davenport, J. Statistical Review of World Energy. Available online: https://www.energyinst.org/statistical-review (accessed on 8 October 2023).
- Saravanan, A.P.; Pugazhendhi, A.; Mathimani, T. A comprehensive assessment of biofuel policies in the BRICS nations: Implementation, blending target and gaps. Fuel 2020, 272, 117635. [Google Scholar] [CrossRef]
- Azwar, E.; Mahari, W.A.W.; Chuah, J.H.; Vo, D.-V.N.; Ma, N.L.; Lam, W.H.; Lam, S.S. Transformation of biomass into carbon nanofiber for supercapacitor application–a review. Int. J. Hydrog. Energy 2018, 43, 20811–20821. [Google Scholar] [CrossRef]
- Sun, C.; Yu, Q.; Zhao, Z.; Zhang, Y. Extracellular electron uptake for CO2 fixation by Rhodopseudomonas palustris during electro-cultivation in darkness. Sci. Total Environ. 2022, 849, 157864. [Google Scholar] [CrossRef]
- Gollakota, A.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sust. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Adekoya, O.B.; Akinbayo, S.B.; Ishola, O.A.; Al-Faryan, M.A.S. Are all the US biomass energy sources green? Energy Policy 2023, 179, 113614. [Google Scholar] [CrossRef]
- Huang, H.; Yan, X.; Song, S.; Du, Y.; Guo, Y. An economic and technology analysis of a new high-efficiency biomass cogeneration system: A case study in DC County, China. Energies 2020, 13, 3957. [Google Scholar] [CrossRef]
- Kanwal, F.; Ahmed, A.; Jamil, F.; Rafiq, S.; Ayub, H.U.; Ghauri, M.; Khurram, M.S.; Munir, S.; Inayat, A.; Abu Bakar, M.S. Co-combustion of blends of coal and underutilised biomass residues for environmental friendly electrical energy production. Sustainability 2021, 13, 4881. [Google Scholar] [CrossRef]
- Zhai, S.; Chen, B.; Sun, E.; Pan, M. Comparative study of liquefaction behavior and products from brown-rotted wood and sound wood. Ind. Crops Prod. 2023, 192, 115982. [Google Scholar] [CrossRef]
- Yu, D.; Xie, G.; Chen, Q.; Yang, Y.; Dong, N. Biofuel potential of liquid products from protein-and lipid-rich biomass hydrothermal liquefaction. Biomass Bioenergy 2022, 165, 106571. [Google Scholar] [CrossRef]
- Meng, S.; Li, W.; Li, Z.; Song, H. Recent progress of the transition metal-based catalysts in the catalytic biomass gasification: A mini-review. Fuel 2023, 353, 129169. [Google Scholar] [CrossRef]
- Weir, A.; del Barco Carrión, A.J.; Queffélec, C.; Bujoli, B.; Chailleux, E.; Uguna, C.; Snape, C.; Airey, G. Renewable binders from waste biomass for road construction: A review on thermochemical conversion technologies and current developments. Constr. Build. Mater. 2022, 330, 127076. [Google Scholar] [CrossRef]
- Kandasamy, S.; Zhang, B.; He, Z.; Chen, H.; Feng, H.; Wang, Q.; Wang, B.; Ashokkumar, V.; Siva, S.; Bhuvanendran, N. Effect of low-temperature catalytic hydrothermal liquefaction of Spirulina platensis. Energy 2020, 190, 116236. [Google Scholar] [CrossRef]
- Gautam, P.; Upadhyay, S.N.; Dubey, S. Bio-methanol as a renewable fuel from waste biomass: Current trends and future perspective. Fuel 2020, 273, 117783. [Google Scholar] [CrossRef]
- Biswas, B.; Kumar, A.A.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Effects of temperature and solvent on hydrothermal liquefaction of Sargassum tenerrimum algae. Bioresour. Technol. 2017, 242, 344–350. [Google Scholar] [CrossRef]
- Pan, Z.-Q.; Huang, H.-J.; Zhou, C.-F.; Xiao, X.-F.; He, X.-W.; Lai, F.-Y.; Xiong, J.-B. Highly efficient conversion of camphor tree sawdust into bio-oil and biochar products by liquefaction in ethanol-water cosolvent. J. Anal. Appl. Pyrolysis 2018, 136, 186–198. [Google Scholar]
- Wu, Y.; Wang, H.; Li, H.; Han, X.; Zhang, M.; Sun, Y.; Fan, X.; Tu, R.; Zeng, Y.; Xu, C.C. Applications of catalysts in thermochemical conversion of biomass (pyrolysis, hydrothermal liquefaction and gasification): A critical review. Renew. Energy 2022, 196, 462–481. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, H.; Zhao, W.; Yang, C.; Ma, P.; Han, S. A review on the operating conditions of producing bio-oil from hydrothermal liquefaction of biomass. Int. J. Energy Res. 2016, 40, 865–877. [Google Scholar] [CrossRef]
- Yan, X.; Ma, J.; Wang, W.; Zhao, Y.; Zhou, J. The effect of different catalysts and process parameters on the chemical content of bio-oils from hydrothermal liquefaction of sugarcane bagasse. BioResources 2018, 13, 997–1018. [Google Scholar] [CrossRef]
- Ni, J.; Qian, L.; Wang, Y.; Zhang, B.; Gu, H.; Hu, Y.; Wang, Q. A review on fast hydrothermal liquefaction of biomass. Fuel 2022, 327, 125135. [Google Scholar] [CrossRef]
- Hao, B.; Xu, D.; Jiang, G.; Sabri, T.A.; Jing, Z.; Guo, Y. Chemical reactions in the hydrothermal liquefaction of biomass and in the catalytic hydrogenation upgrading of biocrude. Green Chem. 2021, 23, 1562–1583. [Google Scholar]
- De Caprariis, B.; De Filippis, P.; Petrullo, A.; Scarsella, M. Hydrothermal liquefaction of biomass: Influence of temperature and biomass composition on the bio-oil production. Fuel 2017, 208, 618–625. [Google Scholar] [CrossRef]
- Nakason, K.; Panyapinyopol, B.; Kanokkantapong, V.; Viriya-empikul, N.; Kraithong, W.; Pavasant, P. Hydrothermal carbonization of unwanted biomass materials: Effect of process temperature and retention time on hydrochar and liquid fraction. J. Energy Inst. 2018, 91, 786–796. [Google Scholar] [CrossRef]
- Guan, H.; Ding, W.; Liu, S.; Zhao, B.; Zhang, H.; Zhong, C.; Chen, B.; Song, A.; Zhu, D.; Li, H. Catalytic hydrothermal liquefaction of Chinese herb residue for the production of high-quality bio-oil. Int. J. Hydrog. Energy 2023, 48, 11205–11213. [Google Scholar] [CrossRef]
- Ağbulut, Ü.; Sirohi, R.; Lichtfouse, E.; Chen, W.-H.; Len, C.; Show, P.L.; Le, A.T.; Nguyen, X.P.; Hoang, A.T. Microalgae bio-oil production through pyrolysis and hydrothermal liquefaction: Mechanism and characteristics. Bioresour. Technol. 2023, 376, 128860. [Google Scholar] [CrossRef]
- Hasanoğlu, A.; Faki, E.; Secer, A.; Üzden, Ş.T. Co–solvent effects on hydrothermal co–gasification of coal/biomass mixtures for hydrogen production. Fuel 2023, 331, 125693. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, H.W.; Lee, S.M.; Jae, J.; Park, Y.-K. Overview of the recent advances in lignocellulose liquefaction for producing biofuels, bio-based materials and chemicals. Bioresour. Technol. 2019, 279, 373–384. [Google Scholar] [CrossRef]
- Solhi, L.; Guccini, V.; Heise, K.; Solala, I.; Niinivaara, E.; Xu, W.; Mihhels, K.; Kröger, M.; Meng, Z.; Wohlert, J. Understanding nanocellulose–water interactions: Turning a detriment into an asset. Chem. Rev. 2023, 123, 1925–2015. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Priya, A.; Thanigaivel, S.; Hoang, T.K.; Soto-Moscoso, M. The conversion of biomass to fuels via cutting-edge technologies: Explorations from natural utilization systems. Fuel 2023, 331, 125668. [Google Scholar] [CrossRef]
- Zhou, S.; Jin, K.; Buehler, M.J. Understanding plant biomass via computational modeling. Adv. Mater. 2021, 33, 2003206. [Google Scholar] [CrossRef]
- Yang, C.; Wang, S.; Yang, J.; Xu, D.; Li, Y.; Li, J.; Zhang, Y. Hydrothermal liquefaction and gasification of biomass and model compounds: A review. Green Chem. 2020, 22, 8210–8232. [Google Scholar] [CrossRef]
- Basar, I.A.; Liu, H.; Carrere, H.; Trably, E.; Eskicioglu, C. A review on key design and operational parameters to optimize and develop hydrothermal liquefaction of biomass for biorefinery applications. Green Chem. 2021, 23, 1404–1446. [Google Scholar] [CrossRef]
- Ma, X.; Mi, Y.; Zhao, C.; Wei, Q. A comprehensive review on carbon source effect of microalgae lipid accumulation for biofuel production. Sci. Total Environ. 2022, 806, 151387. [Google Scholar] [CrossRef]
- Guedes, R.E.; Luna, A.S.; Torres, A.R. Operating parameters for bio-oil production in biomass pyrolysis: A review. J. Anal. Appl. Pyrolysis 2018, 129, 134–149. [Google Scholar] [CrossRef]
- Xu, W.; Ding, K.; Hu, L. A mini review on pyrolysis of natural algae for bio-fuel and chemicals. Processes 2021, 9, 2042. [Google Scholar] [CrossRef]
- Yamada, K.; Nitta, T.; Atsuji, K.; Shiroyama, M.; Inoue, K.; Higuchi, C.; Nitta, N.; Oshiro, S.; Mochida, K.; Iwata, O. Characterization of sulfur-compound metabolism underlying wax-ester fermentation in Euglena gracilis. Sci. Rep. 2019, 9, 853. [Google Scholar] [CrossRef]
- Gao, F.; Yang, H.-L.; Li, C.; Peng, Y.-Y.; Lu, M.-M.; Jin, W.-H.; Bao, J.-J.; Guo, Y.-M. Effect of organic carbon to nitrogen ratio in wastewater on growth, nutrient uptake and lipid accumulation of a mixotrophic microalgae Chlorella sp. Bioresour. Technol. 2019, 282, 118–124. [Google Scholar] [CrossRef]
- Zhu, Q.; Ghoshal, S.; Tyagi, R.; Chakraborty, A. Global IP6K1 deletion enhances temperature modulated energy expenditure which reduces carbohydrate and fat induced weight gain. Mol. Metab. 2017, 6, 73–85. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G. Composition, properties and challenges of algae biomass for biofuel application: An overview. Fuel 2016, 181, 1–33. [Google Scholar] [CrossRef]
- Adedeji, O.M.; Russack, J.S.; Molnar, L.A.; Bauer, S.K. Co-hydrothermal liquefaction of sewage sludge and beverage waste for high-quality bio-energy production. Fuel 2022, 324, 124757. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Huang, Y.-Q.; Xie, J.-J.; Yin, X.-L.; Wu, C.-Z. Hydrothermal reaction of phenylalanine as a model compound of algal protein. J. Fuel Chem. Technol. 2014, 42, 61–67. [Google Scholar] [CrossRef]
- Xu, Y.-H.; Li, M.-F. Hydrothermal liquefaction of lignocellulose for value-added products: Mechanism, parameter and production application. Bioresour. Technol. 2021, 342, 126035. [Google Scholar] [CrossRef]
- Ciuffi, B.; Loppi, M.; Rizzo, A.M.; Chiaramonti, D.; Rosi, L. Towards a better understanding of the HTL process of lignin-rich feedstock. Sci. Rep. 2021, 11, 15504. [Google Scholar] [CrossRef]
- Djandja, O.S.; Salami, A.A.; Yuan, H.; Lin, H.; Huang, Z.; Kang, S. Machine learning prediction of bio-oil yield during solvothermal liquefaction of lignocellulosic biowaste. J. Anal. Appl. Pyrolysis 2023, 175, 106209. [Google Scholar] [CrossRef]
- Yerrayya, A.; Nikunj, A.; Prashanth, P.F.; Chakravarthy, S.; Natarajan, U.; Vinu, R. Optimization of bio-crude yield and its calorific value from hydrothermal liquefaction of bagasse using methanol as co-solvent. Energy 2022, 244, 123192. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, S.; Cao, B.; Hu, Y.; Abomohra, A.E.-F.; Wang, Q.; Qian, L.; Liu, L.; Liu, X.; He, Z. Optimization of hydrothermal co-liquefaction of seaweeds with lignocellulosic biomass: Merging 2nd and 3rd generation feedstocks for enhanced bio-oil production. Energy 2019, 173, 413–422. [Google Scholar] [CrossRef]
- Jiang, H.; Yan, R.; Cai, C.; Chen, X.; Zhao, F.; Fan, L.; Xu, C.C.; Yang, W. Hydrothermal liquefaction of Cd-enriched Amaranthus hypochondriacus L. in ethanol–water co-solvent: Focus on low-N bio-oil and heavy metal/metal-like distribution. Fuel 2021, 303, 121235. [Google Scholar] [CrossRef]
- Jablonsky, M.; Haz, A.; Majova, V. Assessing the opportunities for applying deep eutectic solvents for fractionation of beech wood and wheat straw. Cellulose 2019, 26, 7675–7684. [Google Scholar] [CrossRef]
- Aliyu, M.; Iwabuchi, K.; Itoh, T. Improvement of the fuel properties of dairy manure by increasing the biomass-to-water ratio in hydrothermal carbonization. PLoS ONE 2022, 17, e0269935. [Google Scholar] [CrossRef]
- Ma, X.-Q.; Liao, J.-J.; Chen, D.-B.; Xu, Z.-X. Hydrothermal carbonization of sewage sludge: Catalytic effect of Cl− on hydrochars physicochemical properties. Mol. Catal. 2021, 513, 111789. [Google Scholar] [CrossRef]
- Xu, Z.-X.; Shan, Y.-Q.; Zhang, Z.; Deng, X.-Q.; Yang, Y.; Luque, R.; Duan, P.-G. Hydrothermal carbonization of sewage sludge: Effect of inorganic salts on hydrochar’s physicochemical properties. Green Chem. 2020, 22, 7010–7022. [Google Scholar] [CrossRef]
- Srilek, N.; Aggarangsi, P.; Pattiya, A.; Tippayawong, N. Influence of chloride and propionate anions on properties of corn hydrochar from hydrothermal carbonization and activation. Biomass Convers. Biorefin. 2022, 1–18. [Google Scholar] [CrossRef]
- Biswas, B.; Kumar, A.A.; Bisht, Y.; Krishna, B.B.; Kumar, J.; Bhaskar, T. Role of temperatures and solvents on hydrothermal liquefaction of Azolla filiculoides. Energy 2021, 217, 119330. [Google Scholar] [CrossRef]
- Xu, D.; Savage, P.E. Supercritical water upgrading of water-insoluble and water-soluble biocrudes from hydrothermal liquefaction of Nannochloropsis microalgae. J. Supercrit. Fluids 2018, 133, 683–689. [Google Scholar] [CrossRef]
- Tian, W.; Liu, R.; Wang, W.; Yin, Z.; Yi, X. Effect of operating conditions on hydrothermal liquefaction of Spirulina over Ni/TiO2 catalyst. Bioresour. Technol. 2018, 263, 569–575. [Google Scholar] [CrossRef]
- Azwar, E.; Mahari, W.A.W.; Liew, R.K.; Ramlee, M.Z.; Verma, M.; Chong, W.W.F.; Peng, W.; Ng, H.S.; Naushad, M.; Sonne, C. Remediation and recovery of Kariba weed as emerging contaminant in freshwater and shellfish aquaculture system via solvothermal liquefaction. Sci. Total Environ. 2023, 876, 162673. [Google Scholar] [CrossRef]
- Lai, F.-Y.; Chang, Y.-C.; Huang, H.-J.; Wu, G.-Q.; Xiong, J.-B.; Pan, Z.-Q.; Zhou, C.-F. Liquefaction of sewage sludge in ethanol-water mixed solvents for bio-oil and biochar products. Energy 2018, 148, 629–641. [Google Scholar] [CrossRef]
- Mellmer, M.A.; Sener, C.; Gallo, J.M.R.; Luterbacher, J.S.; Alonso, D.M.; Dumesic, J.A. Solvent effects in acid-catalyzed biomass conversion reactions. Angew. Chem. Int. Ed. 2014, 53, 11872–11875. [Google Scholar] [CrossRef]
- Song, B.; Wu, Z.; Yu, Y.; Wu, H. Hydrothermal reactions of biomass-derived platform molecules: Distinct effect of aprotic and protic solvents on primary decomposition of glucose and fructose in hot-compressed solvent/water mixtures. Ind. Eng. Chem. Res. 2020, 59, 7336–7345. [Google Scholar] [CrossRef]
- Hu, Y.; Qi, L.; Feng, S.; Bassi, A.; Xu, C.C. Comparative studies on liquefaction of low-lipid microalgae into bio-crude oil using varying reaction media. Fuel 2019, 238, 240–247. [Google Scholar] [CrossRef]
- Li, Q.; Liu, D.; Hou, X.; Wu, P.; Song, L.; Yan, Z. Hydro-liquefaction of microcrystalline cellulose, xylan and industrial lignin in different supercritical solvents. Bioresour. Technol. 2016, 219, 281–288. [Google Scholar] [CrossRef]
- Hu, X.; Wang, S.; Westerhof, R.J.; Wu, L.; Song, Y.; Dong, D.; Li, C.-Z. Acid-catalyzed conversion of C6 sugar monomer/oligomers to levulinic acid in water, tetrahydrofuran and toluene: Importance of the solvent polarity. Fuel 2015, 141, 56–63. [Google Scholar] [CrossRef]
- Fan, S.-P.; Zakaria, S.; Chia, C.-H.; Jamaluddin, F.; Nabihah, S.; Liew, T.-K.; Pua, F.-L. Comparative studies of products obtained from solvolysis liquefaction of oil palm empty fruit bunch fibres using different solvents. Bioresour. Technol. 2011, 102, 3521–3526. [Google Scholar] [CrossRef]
- Jablonski, P.; Nikjoo, D.; Warna, J.; Irgum, K.; Mikkola, J.-P.; Khokarale, S.G. Sustainable, highly selective, and metal-free thermal depolymerization of poly-(3-hydroxybutyrate) to crotonic acid in recoverable ionic liquids. Green Chem. 2022, 24, 4130–4139. [Google Scholar] [CrossRef]
- Li, Z.; Su, K.; Ren, J.; Yang, D.; Cheng, B.; Kim, C.K.; Yao, X. Direct catalytic conversion of glucose and cellulose. Green Chem. 2018, 20, 863–872. [Google Scholar] [CrossRef]
- Song, L.; Ouyang, Y.; Huang, S.; Li, Z.; Sun, M. Insight into liquefaction process of sawdust with hydrogen donor solvents. Biomass Bioenergy 2022, 160, 106444. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Biswas, B.; Kumar, J.; Bhaskar, T.; Muraleedharan, U.D. Valorization of the red macroalga Gracilaria corticata by hydrothermal liquefaction: Product yield improvement by optimization of process parameters. Bioresour. Technol. Rep. 2021, 15, 100796. [Google Scholar] [CrossRef]
- Madikizela, M.; Isa, Y.M. Effect of operating conditions on the hydrothermal valorization of sewage sludge. Biofuels Bioprod. Biorefin. 2023, 17, 403–414. [Google Scholar] [CrossRef]
- Han, Y.; Hoekman, S.K.; Cui, Z.; Jena, U.; Das, P. Hydrothermal liquefaction of marine microalgae biomass using co-solvents. Algal Res. 2019, 38, 101421. [Google Scholar] [CrossRef]
- Baloch, H.A.; Siddiqui, M.T.H.; Nizamuddin, S.; Riaz, S.; Haris, M.; Mubarak, N.; Griffin, G.; Srinivasan, M. Effect of solvent on hydro-solvothermal co liquefaction of sugarcane bagasse and polyethylene for bio-oil production in ethanol–water system. Process Saf. Environ. Protect. 2021, 148, 1060–1069. [Google Scholar] [CrossRef]
- Walker, T.W.; Chew, A.K.; Li, H.; Demir, B.; Zhang, Z.C.; Huber, G.W.; Van Lehn, R.C.; Dumesic, J.A. Universal kinetic solvent effects in acid-catalyzed reactions of biomass-derived oxygenates. Energy Environ. Sci. 2018, 11, 617–628. [Google Scholar] [CrossRef]
- Mellmer, M.A.; Sanpitakseree, C.; Demir, B.; Bai, P.; Ma, K.; Neurock, M.; Dumesic, J.A. Solvent-enabled control of reactivity for liquid-phase reactions of biomass-derived compounds. Nat. Catal. 2018, 1, 199–207. [Google Scholar] [CrossRef]
- Ji, C.; He, Z.; Wang, Q.; Xu, G.; Wang, S.; Xu, Z.; Ji, H. Effect of operating conditions on direct liquefaction of low-lipid microalgae in ethanol-water co-solvent for bio-oil production. Energy Convers. Manag. 2017, 141, 155–162. [Google Scholar] [CrossRef]
- Hu, Y.; Feng, S.; Bassi, A.; Xu, C.C. Improvement in bio-crude yield and quality through co-liquefaction of algal biomass and sawdust in ethanol-water mixed solvent and recycling of the aqueous by-product as a reaction medium. Energy Convers. Manag. 2018, 171, 618–625. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, X.-z.; Huang, H.-j.; Wang, X.-l.; Wang, H.; Zeng, G.-m. Thermochemical liquefaction of rice husk for bio-oil production in mixed solvent (ethanol–water). Fuel Process. Technol. 2013, 112, 93–99. [Google Scholar] [CrossRef]
- Wu, X.-F.; Yin, S.-S.; Zhou, Q.; Li, M.-F.; Peng, F.; Xiao, X. Subcritical liquefaction of lignocellulose for the production of bio-oils in ethanol/water system. Renew. Energy 2019, 136, 865–872. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, J.; Jia, S.; Mi, S.; Tong, Z.; Li, Z.; Li, M.; Zhang, Y.; Hu, Y.; Huang, Z. Effect of ethanol on Mulberry bark hydrothermal liquefaction and bio-oil chemical compositions. Energy 2018, 162, 460–475. [Google Scholar] [CrossRef]
- Feng, S.; Wei, R.; Leitch, M.; Xu, C.C. Comparative study on lignocellulose liquefaction in water, ethanol, and water/ethanol mixture: Roles of ethanol and water. Energy 2018, 155, 234–241. [Google Scholar] [CrossRef]
- Cui, Z.; Cheng, F.; Jarvis, J.M.; Brewer, C.E.; Jena, U. Roles of Co-solvents in hydrothermal liquefaction of low-lipid, high-protein algae. Bioresour. Technol. 2020, 310, 123454. [Google Scholar] [CrossRef]
- Li, R.; Ma, Z.; Yang, T.; Li, B.; Wei, L.; Sun, Y. Sub–supercritical liquefaction of municipal wet sewage sludge to produce bio-oil: Effect of different organic–water mixed solvents. J. Supercrit. Fluids 2018, 138, 115–123. [Google Scholar] [CrossRef]
- Arturi, K.R.; Kucheryavskiy, S.; Nielsen, R.P.; Maschietti, M.; Vogel, F.; Bjelić, S.; Søgaard, E.G. Molecular footprint of co-solvents in hydrothermal liquefaction (HTL) of Fallopia Japonica. J. Supercrit. Fluids 2019, 143, 211–222. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, J.; Chen, M.; Zhao, G.; Wu, S. Influence of catalyst and solvent on the hydrothermal liquefaction of woody biomass. Bioresour. Technol. 2022, 346, 126354. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, J.; Li, Y.; Guo, H.; Xiong, L.; Chen, X. Acid-catalyzed liquefaction of bagasse in the presence of polyhydric alcohol. Appl. Biochem. Biotechnol. 2013, 170, 1780–1791. [Google Scholar] [CrossRef]
- Ding, Y.; Shan, B.; Cao, X.; Liu, Y.; Huang, M.; Tang, B. Development of bio oil and bio asphalt by hydrothermal liquefaction using lignocellulose. J. Cleaner Prod. 2021, 288, 125586. [Google Scholar] [CrossRef]
- Supraja, K.V.; Doddapaneni, T.R.K.C.; Ramasamy, P.K.; Kaushal, P.; Ahammad, S.Z.; Pollmann, K.; Jain, R. Critical review on production, characterization and applications of microalgal hydrochar: Insights on circular bioeconomy through hydrothermal carbonization. Chem. Eng. J. 2023, 473, 145059. [Google Scholar] [CrossRef]
- Biswas, B.; Bhaskar, T. Hydrothermal upgradation of algae into value-added hydrocarbons. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 435–459. [Google Scholar]
- Chen, Z.; Chen, H.; Xu, Y.; Hu, M.; Hu, Z.; Wang, J.; Pan, Z. Reactor for biomass conversion and waste treatment in supercritical water: A review. Renew. Sust. Energy Rev. 2023, 171, 113031. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, X.; Hu, X.; Meers, E.; Ong, H.C.; Chen, W.-H.; Duan, P.; Zhang, S.; Lee, K.B.; Ok, Y.S. Co-liquefaction of mixed biomass feedstocks for bio-oil production: A critical review. Renew. Sust. Energy Rev. 2022, 154, 111814. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Cui, D.; Bai, J.; Wang, Z.; Xu, F.; Wang, Z. Advances in supercritical water gasification of lignocellulosic biomass for hydrogen production. J. Anal. Appl. Pyrolysis 2023, 170, 105934. [Google Scholar] [CrossRef]
- Shafizadeh, A.; Shahbeig, H.; Nadian, M.H.; Mobli, H.; Dowlati, M.; Gupta, V.K.; Peng, W.; Lam, S.S.; Tabatabaei, M.; Aghbashlo, M. Machine learning predicts and optimizes hydrothermal liquefaction of biomass. Chem. Eng. J. 2022, 445, 136579. [Google Scholar] [CrossRef]
- Isa, K.M.; Abdullah, T.A.T.; Ali, U.F.M. Hydrogen donor solvents in liquefaction of biomass: A review. Renew. Sust. Energy Rev. 2018, 81, 1259–1268. [Google Scholar] [CrossRef]
- Qian, L.; Wang, S.; Savage, P.E. Hydrothermal liquefaction of sewage sludge under isothermal and fast conditions. Bioresour. Technol. 2017, 232, 27–34. [Google Scholar] [CrossRef]
- Beims, R.F.; Hu, Y.; Shui, H.; Xu, C.C. Hydrothermal liquefaction of biomass to fuels and value-added chemicals: Products applications and challenges to develop large-scale operations. Biomass Bioenergy 2020, 135, 105510. [Google Scholar] [CrossRef]
- Ratha, S.K.; Renuka, N.; Abunama, T.; Rawat, I.; Bux, F. Hydrothermal liquefaction of algal feedstocks: The effect of biomass characteristics and extraction solvents. Renew. Sust. Energy Rev. 2022, 156, 111973. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, Y.; Qi, L.; Gao, J.; Zhao, G.; Ray, M.B.; Xu, C.C. Promotion effects of metallic iron on hydrothermal liquefaction of cornstalk in ethanol-water mixed solvents for the production of biocrude oil. Fuel 2021, 285, 119150. [Google Scholar] [CrossRef]
- Potts, D.S.; Bregante, D.T.; Adams, J.S.; Torres, C.; Flaherty, D.W. Influence of solvent structure and hydrogen bonding on catalysis at solid–liquid interfaces. Chem. Soc. Rev. 2021, 50, 12308–12337. [Google Scholar] [CrossRef]
- Shuai, L.; Luterbacher, J. Organic solvent effects in biomass conversion reactions. ChemSusChem 2016, 9, 133–155. [Google Scholar] [CrossRef]
- Bux, K.; Moin, S.T. Solvation of cholesterol in different solvents: A molecular dynamics simulation study. Phys. Chem. Chem. Phys. 2020, 22, 1154–1167. [Google Scholar] [CrossRef]
- Shukla, M.K.; Tiwari, H.; Verma, R.; Dong, W.-L.; Azizov, S.; Kumar, B.; Pandey, S.; Kumar, D. Role and recent advancements of ionic liquids in drug delivery systems. Pharmaceutics 2023, 15, 702. [Google Scholar] [CrossRef]
- Yu, K.; Ding, W.-L.; Lu, Y.; Wang, Y.; Liu, Y.; Liu, G.; Huo, F.; He, H. Ionic liquids screening for lignin dissolution: COSMO-RS simulations and experimental characterization. J. Mol. Liq. 2022, 348, 118007. [Google Scholar] [CrossRef]
- Dais, P.; Perlin, A.S. Intramolecular hydrogen-bonding and solvation contributions to the relative stability of the β-furanose form of D-fructose in dimethyl sulfoxide. Carbohydr. Res. 1987, 169, 159–169. [Google Scholar] [CrossRef]
- Kimura, H.; Nakahara, M.; Matubayasi, N. Solvent effect on pathways and mechanisms for D-fructose conversion to 5-hydroxymethyl-2-furaldehyde: In situ 13C NMR study. J. Phys. Chem. A 2013, 117, 2102–2113. [Google Scholar] [CrossRef]
- Svenningsen, G.S.; Kumar, R.; Wyman, C.E.; Christopher, P. Unifying mechanistic analysis of factors controlling selectivity in fructose dehydration to 5-hydroxymethylfurfural by homogeneous acid catalysts in aprotic solvents. ACS Catal. 2018, 8, 5591–5600. [Google Scholar] [CrossRef]
- Qian, X. Mechanisms and energetics for acid catalyzed β-D-glucose conversion to 5-hydroxymethylfurfurl. J. Phys. Chem. A 2011, 115, 11740–11748. [Google Scholar] [CrossRef]
- Vasudevan, V.; Mushrif, S.H. Insights into the solvation of glucose in water, dimethyl sulfoxide (DMSO), tetrahydrofuran (THF) and N, N-dimethylformamide (DMF) and its possible implications on the conversion of glucose to platform chemicals. RSC Adv. 2015, 5, 20756–20763. [Google Scholar] [CrossRef]
- Mushrif, S.H.; Caratzoulas, S.; Vlachos, D.G. Understanding solvent effects in the selective conversion of fructose to 5-hydroxymethyl-furfural: A molecular dynamics investigation. Phys. Chem. Chem. Phys. 2012, 14, 2637–2644. [Google Scholar] [CrossRef]
- Tsilomelekis, G.; Josephson, T.R.; Nikolakis, V.; Caratzoulas, S. Origin of 5-hydroxymethylfurfural stability in water/dimethyl sulfoxide mixtures. ChemSusChem 2014, 7, 117–126. [Google Scholar] [CrossRef]
- Huang, H.-j.; Yuan, X.-z. Recent progress in the direct liquefaction of typical biomass. Prog. Energy Combust. Sci. 2015, 49, 59–80. [Google Scholar] [CrossRef]
- Cui, S.; Wei, X.; Chen, X.; Xie, Y. Investigation of chemical linkages between lignin and carbohydrates in cultured poplar cambium tissues via double isotope labeling. Int. J. Biol. Macromol. 2023, 231, 123250. [Google Scholar] [CrossRef]
- Leng, L.; Zhang, W.; Peng, H.; Li, H.; Jiang, S.; Huang, H. Nitrogen in bio-oil produced from hydrothermal liquefaction of biomass: A review. Chem. Eng. J. 2020, 401, 126030. [Google Scholar] [CrossRef]
- Yan, W.-H.; Duan, P.-G.; Wang, F.; Xu, Y.-P. Composition of the bio-oil from the hydrothermal liquefaction of duckweed and the influence of the extraction solvents. Fuel 2016, 185, 229–235. [Google Scholar] [CrossRef]
- Chand, R.; Borugadda, V.B.; Qiu, M.; Dalai, A.K. Evaluating the potential for bio-fuel upgrading: A comprehensive analysis of bio-crude and bio-residue from hydrothermal liquefaction of agricultural biomass. Appl. Energy 2019, 254, 113679. [Google Scholar] [CrossRef]
- Youn, H.S.; Kim, S.J.; Kim, G.H.; Um, B.H. Enhancing the characteristics of hydrochar via hydrothermal carbonization of Korean native kenaf: The effect of ethanol solvent concentration as co-solvent and reaction temperature. Fuel 2023, 331, 125738. [Google Scholar] [CrossRef]
- Mellmer, M.A.; Sanpitakseree, C.; Demir, B.; Ma, K.; Elliott, W.A.; Bai, P.; Johnson, R.L.; Walker, T.W.; Shanks, B.H.; Rioux, R.M. Effects of chloride ions in acid-catalyzed biomass dehydration reactions in polar aprotic solvents. Nat. Commun. 2019, 10, 1132. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Resasco, D.E. Water-mediated heterogeneously catalyzed reactions. ACS Catal. 2019, 10, 1294–1309. [Google Scholar] [CrossRef]
- Chew, A.K.; Walker, T.W.; Shen, Z.; Demir, B.; Witteman, L.; Euclide, J.; Huber, G.W.; Dumesic, J.A.; Van Lehn, R.C. Effect of mixed-solvent environments on the selectivity of acid-catalyzed dehydration reactions. ACS Catal. 2019, 10, 1679–1691. [Google Scholar] [CrossRef]
- Wang, C.; Wu, C.; Hornung, U.; Zhu, W.; Dahmen, N. Suppression of tar and char formation in supercritical water gasification of sewage sludge by additive addition. Chemosphere 2021, 262, 128412. [Google Scholar] [CrossRef]

 represents the biomass: Cyanophyta; the symbol
represents the biomass: Cyanophyta; the symbol  represents the biomass: L. sacharina (Marcoalgae); the symbol
represents the biomass: L. sacharina (Marcoalgae); the symbol  represents the biomass: Spirulina; the symbol
represents the biomass: Spirulina; the symbol  represents the biomass: S. patens C. Agardh; the symbol
represents the biomass: S. patens C. Agardh; the symbol  represents the biomass: Cattle Manure; the symbol
represents the biomass: Cattle Manure; the symbol  represents the biomass: Cellulose; the symbol
represents the biomass: Cellulose; the symbol  represents the biomass: Chitin) Reproduced with permission [21]. Copyright 2021, The Royal Society of Chemistry.
represents the biomass: Chitin) Reproduced with permission [21]. Copyright 2021, The Royal Society of Chemistry.
 represents the biomass: Cyanophyta; the symbol
represents the biomass: Cyanophyta; the symbol  represents the biomass: L. sacharina (Marcoalgae); the symbol
represents the biomass: L. sacharina (Marcoalgae); the symbol  represents the biomass: Spirulina; the symbol
represents the biomass: Spirulina; the symbol  represents the biomass: S. patens C. Agardh; the symbol
represents the biomass: S. patens C. Agardh; the symbol  represents the biomass: Cattle Manure; the symbol
represents the biomass: Cattle Manure; the symbol  represents the biomass: Cellulose; the symbol
represents the biomass: Cellulose; the symbol  represents the biomass: Chitin) Reproduced with permission [21]. Copyright 2021, The Royal Society of Chemistry.
represents the biomass: Chitin) Reproduced with permission [21]. Copyright 2021, The Royal Society of Chemistry.

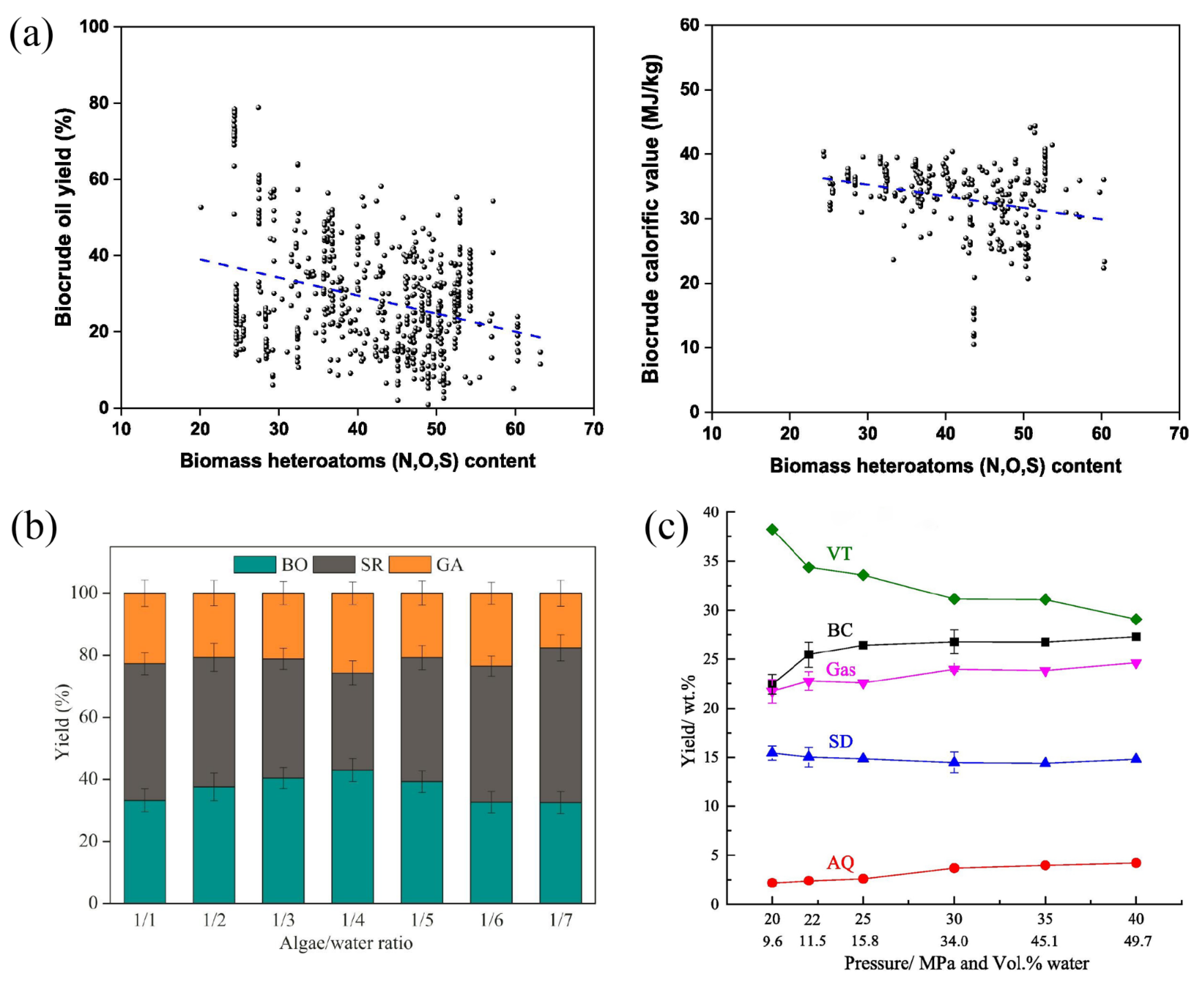



 represents the result of liquefaction of biomass (Sewage sludge) at 340 °C for 20 min;
represents the result of liquefaction of biomass (Sewage sludge) at 340 °C for 20 min;  represents the results of liquefaction of biomass (Spirulina) at 350 °C for 20 min;
represents the results of liquefaction of biomass (Spirulina) at 350 °C for 20 min;  represents the results of liquefaction of biomass (Chlorella pyrenoidosa) at 280 °C for 120 min;
represents the results of liquefaction of biomass (Chlorella pyrenoidosa) at 280 °C for 120 min;  represents the results of liquefaction of biomass (Algae) at 295 °C;
represents the results of liquefaction of biomass (Algae) at 295 °C;  represents the results of liquefaction of biomass (Rice husk) at 260 °C for 20 min;
represents the results of liquefaction of biomass (Rice husk) at 260 °C for 20 min;  represents the results of liquefaction of biomass (Chlorella pyrenoidosa) at 300 °C for 60 min;
represents the results of liquefaction of biomass (Chlorella pyrenoidosa) at 300 °C for 60 min;  represents the liquefaction results of biomass (Sewage sludge) at 280~360 °C;
represents the liquefaction results of biomass (Sewage sludge) at 280~360 °C;  represents the results of liquefaction of biomass (Sewage sludge) at 400 °C for 30 min;
represents the results of liquefaction of biomass (Sewage sludge) at 400 °C for 30 min;  represents the results of liquefaction of biomass (Low-lipid microalgae) at 225 °C for 60 min;
represents the results of liquefaction of biomass (Low-lipid microalgae) at 225 °C for 60 min;  represents the results of liquefaction of biomass (Laminaria Saccharina) at 330 °C for 15 min) (b) Influence of different solvents on biomass liquefaction conversion of extraction solvent on bio-oil N content [111,112]. Reproduced with permission [110]. Copyright 2020, Elsevier.
represents the results of liquefaction of biomass (Laminaria Saccharina) at 330 °C for 15 min) (b) Influence of different solvents on biomass liquefaction conversion of extraction solvent on bio-oil N content [111,112]. Reproduced with permission [110]. Copyright 2020, Elsevier.
 represents the result of liquefaction of biomass (Sewage sludge) at 340 °C for 20 min;
represents the result of liquefaction of biomass (Sewage sludge) at 340 °C for 20 min;  represents the results of liquefaction of biomass (Spirulina) at 350 °C for 20 min;
represents the results of liquefaction of biomass (Spirulina) at 350 °C for 20 min;  represents the results of liquefaction of biomass (Chlorella pyrenoidosa) at 280 °C for 120 min;
represents the results of liquefaction of biomass (Chlorella pyrenoidosa) at 280 °C for 120 min;  represents the results of liquefaction of biomass (Algae) at 295 °C;
represents the results of liquefaction of biomass (Algae) at 295 °C;  represents the results of liquefaction of biomass (Rice husk) at 260 °C for 20 min;
represents the results of liquefaction of biomass (Rice husk) at 260 °C for 20 min;  represents the results of liquefaction of biomass (Chlorella pyrenoidosa) at 300 °C for 60 min;
represents the results of liquefaction of biomass (Chlorella pyrenoidosa) at 300 °C for 60 min;  represents the liquefaction results of biomass (Sewage sludge) at 280~360 °C;
represents the liquefaction results of biomass (Sewage sludge) at 280~360 °C;  represents the results of liquefaction of biomass (Sewage sludge) at 400 °C for 30 min;
represents the results of liquefaction of biomass (Sewage sludge) at 400 °C for 30 min;  represents the results of liquefaction of biomass (Low-lipid microalgae) at 225 °C for 60 min;
represents the results of liquefaction of biomass (Low-lipid microalgae) at 225 °C for 60 min;  represents the results of liquefaction of biomass (Laminaria Saccharina) at 330 °C for 15 min) (b) Influence of different solvents on biomass liquefaction conversion of extraction solvent on bio-oil N content [111,112]. Reproduced with permission [110]. Copyright 2020, Elsevier.
represents the results of liquefaction of biomass (Laminaria Saccharina) at 330 °C for 15 min) (b) Influence of different solvents on biomass liquefaction conversion of extraction solvent on bio-oil N content [111,112]. Reproduced with permission [110]. Copyright 2020, Elsevier.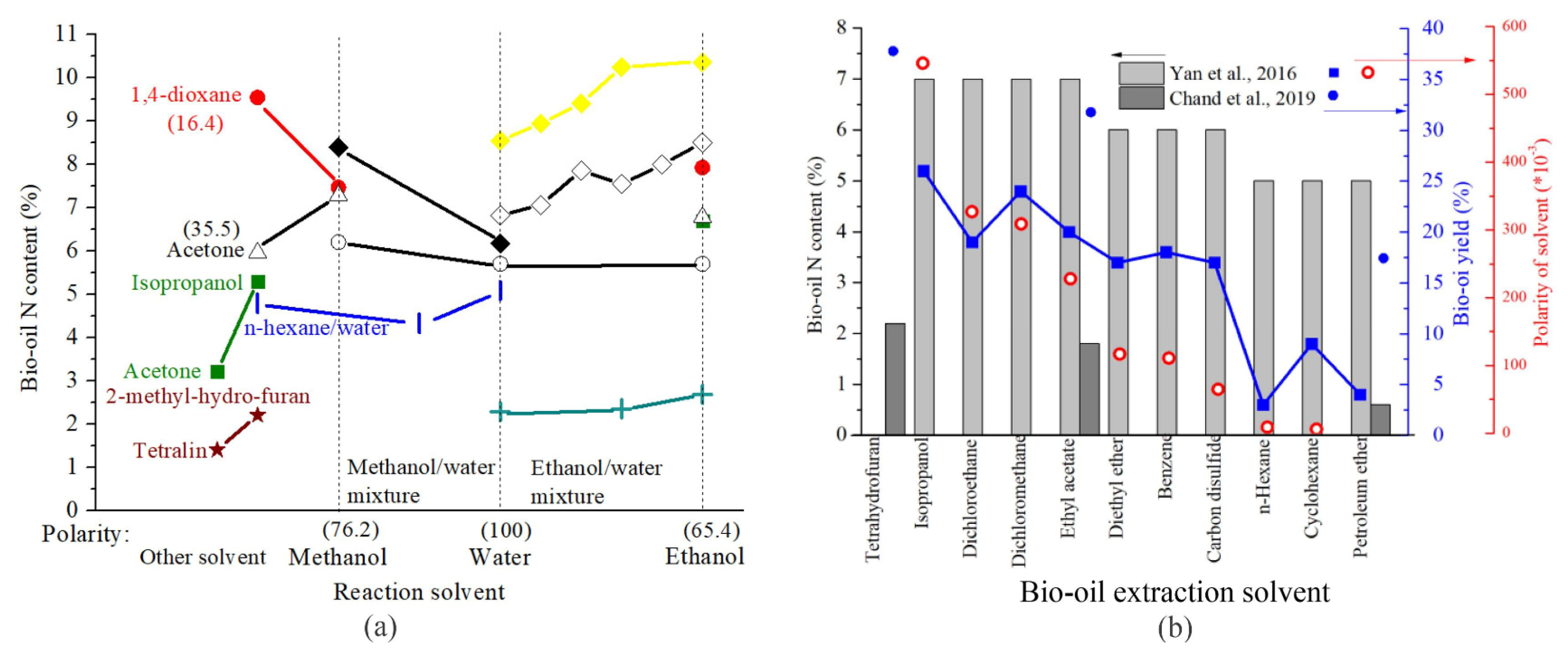
 represents the compound: Fatty acids; the symbol
represents the compound: Fatty acids; the symbol  represents the compound: Fatty acid esters; the symbol
represents the compound: Fatty acid esters; the symbol  represents the compound: Nitrogenated compounds; the symbol
represents the compound: Nitrogenated compounds; the symbol  represents the compound: Hydrocarbons; the symbol
represents the compound: Hydrocarbons; the symbol  represents the compound: Ketones; the symbol
represents the compound: Ketones; the symbol  represents the compound: Alcohols; the symbol
represents the compound: Alcohols; the symbol  represents the compound: Aldehydes; the symbol
represents the compound: Aldehydes; the symbol  represents other compounds.) Reproduced with permission [80]. Copyright 2018, Elsevier.
represents other compounds.) Reproduced with permission [80]. Copyright 2018, Elsevier.
 represents the compound: Fatty acids; the symbol
represents the compound: Fatty acids; the symbol  represents the compound: Fatty acid esters; the symbol
represents the compound: Fatty acid esters; the symbol  represents the compound: Nitrogenated compounds; the symbol
represents the compound: Nitrogenated compounds; the symbol  represents the compound: Hydrocarbons; the symbol
represents the compound: Hydrocarbons; the symbol  represents the compound: Ketones; the symbol
represents the compound: Ketones; the symbol  represents the compound: Alcohols; the symbol
represents the compound: Alcohols; the symbol  represents the compound: Aldehydes; the symbol
represents the compound: Aldehydes; the symbol  represents other compounds.) Reproduced with permission [80]. Copyright 2018, Elsevier.
represents other compounds.) Reproduced with permission [80]. Copyright 2018, Elsevier.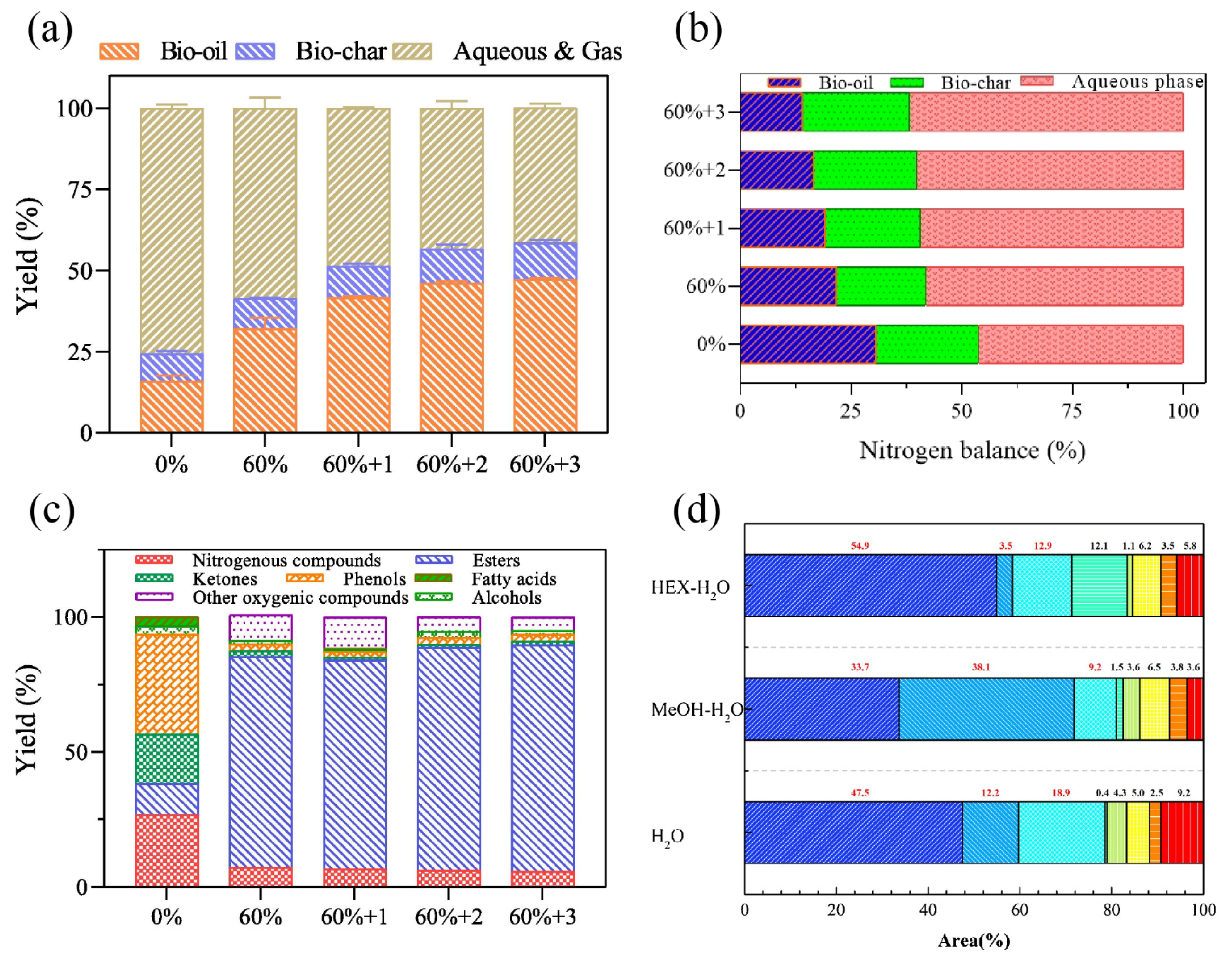

| Solvents | Type of Biomass Feedstock | Reaction Conditions | Results | Bio-Oil Yield | Ref. |
|---|---|---|---|---|---|
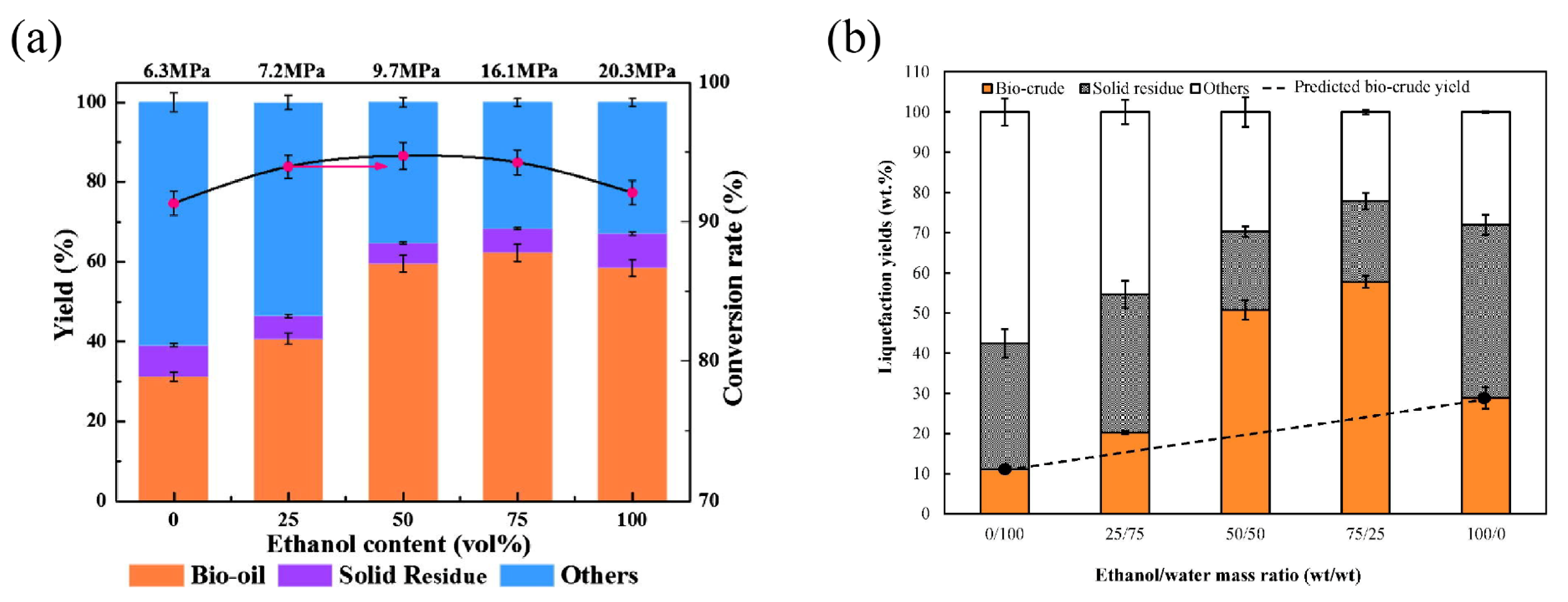
| ethanol/water co-solvent | Cd-enriched Amaranthus hypochondriacus L. (AHL) | 275 °C, 30 min | compared with pure water, ethanol/water co-solvent can transfer organic nitrogen to the aqueous phase, thereby reducing the nitrogen content in bio-oil | the yield of bio-oil obtained after liquefying 60% ethanol content (32.07%) is higher than that obtained after liquefying water (15.93%) | [47] |
| water | Azolla filiculoides | 260 °C, 280 °C and 300 °C, 15~60 min | using water as the solvent, the concentration of total organic carbon is higher | the yield is 13.6–21.5 wt% | [53] |
| water | Nannochloropsis microalgae | 400 °C, 1 h, adding H2 | upgraded treatment with supercritical water almost halves the heteroatom content of the feedstock | the yield of water-insoluble biocrude is 37.6 ± 2.1 wt%, the yield of water-soluble biocrude is 8.2 ± 0.6 wt%, and the total yield of biocrude oil is 45.8 ± 2.9 wt% | [54] |
| water | Spirulina | 260 °C, 30 min | bio-oil production is highest when the ratio of Spirulina to water is 1/4 | the highest bio-oil yield is 43.05 wt% | [55] |
| ethanol or methanol | Kariba weed | 300 °C, 60 min | organic solvents usually have lower dielectric constants than water, which improves the solubility of solvents | the optimal yield is 20.86 wt% | [56] |
| ethanol | sewage sludge | 200~280 °C, 0~60 min | ethanol as a hydrogen-donating solvent can improve the bio-oil yield | the yield is 26.8 wt% | [57] |
| γ-valerolactone | xylose | acid catalysis | γ-valerolactone can decrease the activation energy of the liquefaction reaction and increase the reaction rate and product selectivity | / | [58] |
| γ-valerolactone, acetone, 1,4-dioxane, methanol and ethanol | glucose and fructose | 175~225 °C, 150~200 °C, 5~120 min | polar aprotic solvents decrease raw materials’ decomposition activation energy, but the reaction rate constant for liquefaction is lower than that in polar proton solvents | / | [59] |
| methanol, ethanol, ethyl acetate, acetone, and water | low-lipid microalgae | 275 °C, 60 min | bio-oil yields from polar proton solvents are significantly higher than those from polar aprotic solvents | when the biomass/solvent mass ratio is 1:5, the highest yield of biocrude oil is 85.5 wt% | [60] |
| methanol, acetone, isopropanol, propanol, heptane, and cyclohexane | cellulose | 320 °C, 30 min | more polar solvents may favor the transformation of raw materials; non-polar solvents are unable to break the hydrogen bonds of cellulose during liquefaction, which is not conducive to the conversion of cellulose | the most efficient solvent for liquefaction of cellulose is methanol, and the yield of bio-oil obtained by liquefaction in methanol is up to 32.21% | [61] |
| water, tetrahydrofuran (THF), and toluene | C6 sugar monomer/polymer | 180 °C, 120 min | the polarity of the solvent is critical to the formation of the product | / | [62] |
| acetone, ethylene glycol, ethanol, water, and toluene | oil palm empty fruit bunch (EFB) fibers | 275 °C, 60 min | the polar solvents are more suitable for the dissolution of EFB fibers, resulting in higher yields | / | [63] |
| [EMIM][AcO] | poly 3-hydroxybutyrate (PHB) | 140 °C, 90 min | efficient and selective conversion of poly-3-hydroxybutyrate to crotonic acid with 97% yield by ionic liquids | / | [64] |
| [EMIM]Br | glucose and cellulose | heterogeneous sulfonated poly(phenylene sulfide) (SPPS), 140 °C | the yield of glucose into 5-hydroxymethylfurfural is 87.2%, and the yield of cellulose directly into 5-hydroxymethylfurfural can reach 68.2% | / | [65] |
| ethanol, isopropanol, cyclohexane, cyclohexanol, and tetralin | sawdust | 320 °C, 30 min | the heat and mass transfer capabilities of the solvent will affect the conversion of biomass | the highest liquid yield of cyclohexanol is 79.1%, followed by tetralin (72.0%), ethanol (57.0%), isopropyl alcohol (47.7%), and cyclohexane (44.5%) | [66] |
| water, methanol, ethanol, acetone | red macroalga Gracilaria corticata | 260 °C, 280 °C and 300 °C, 15 min | the maximum bio-oil yield is obtained by acetone liquefaction at 300 °C | the maximum bio-oil yield is 16.16 wt% | [67] |
| ethanol/water co-solvent | sewage sludge | 220 °C, 250 °C, 280 °C, 310 °C, 340 °C and 370 °C, 30 min | the highest bio-oil yield is achieved when water is mixed with ethanol at a ratio of 1:1 | the highest yield reached 40.69 wt% | [68] |
| isopropanol/water co-solvent | marine microalgae | 350 °C, 30 min | bio-oil yield is enhanced by about 14% when using isopropanol as co-solvent | the maximum bio-oil yield (35.4%) is achieved when IPA is added as a co-solvent | [69] |
| ethanol/water co-solvent | bagasse, high-density polyethylene | 280 °C, 90 min | there is a significant synergy between water and ethanol | a high bio-oil yield at the water-to-ethanol volume ratio of 60%:40% | [70] |
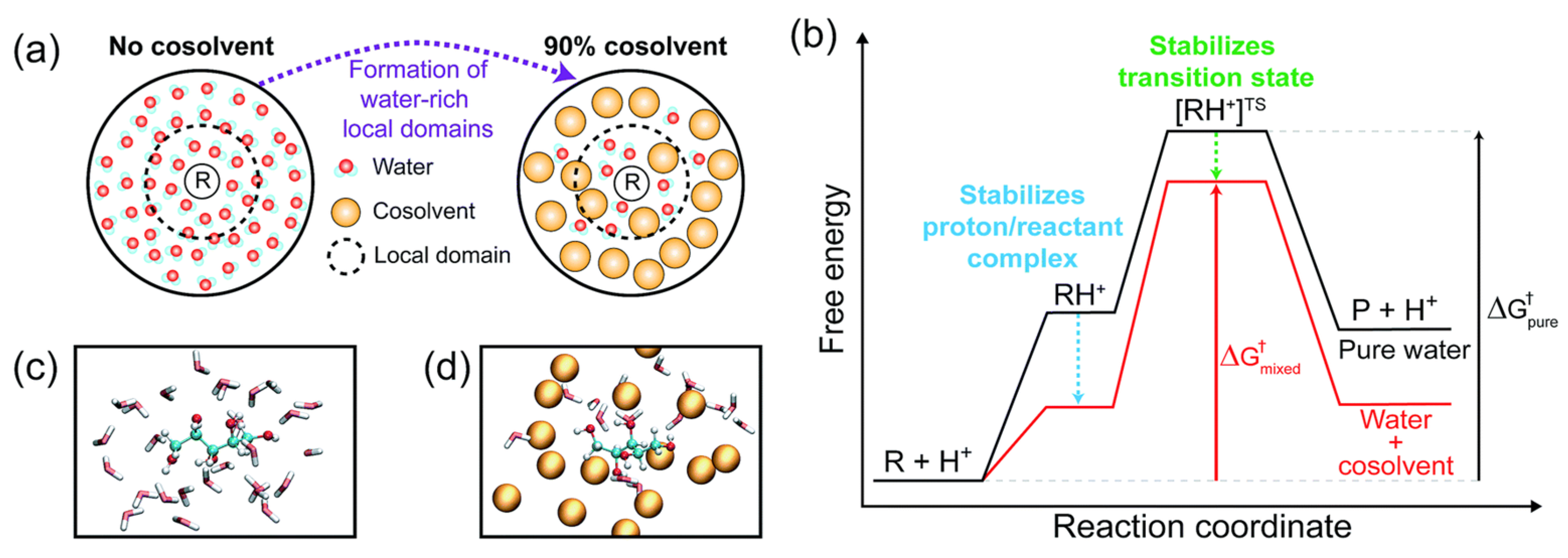
| 1,4-dioxane/water co-solvent, γ-valerolactone/water co-solvent, tetrahydrofuran/water co-solvent | ethyl tert-butyl ether, tert-butanol, levoglucosan, 1,2-propanediol, fructose, cellobiose, and xylitol | acid catalysts | the excellent interaction environment between co-solvent and hydrophilic reactants could promote the generation of water-rich regions near the reactants in the acid-catalyzed biomass-derived oxygenated compounds reaction, in which the stability of protons and transition states could reduce the surface free energy barrier formed in the mixed solvent environment | / | [71] |
| dioxane/water co-solvent, dimethyl sulfoxide/water co-solvent, tetrahydrofuran/water co-solvent, γ-valerolactone/water co-solvent, acetonitrile/water co-solvent | fructose, tert-butanol, and 1,2-propanediol | acid catalysis | the degree of solvation increases the rate of acid-catalyzed reactions | / | [72] |
| ethanol/water co-solvent | low-lipid microalgae | 250 °C, 275 °C, 300 °C, 325 °C and 350 °C, 15 min, 30 min, 45 min, 60 min, and 75 min | as the ethanol content in the solvent mixture increases, the bio-oil production first increases and then decreases | when the reaction temperature is 300 °C, the reaction time is 45 min, the ethanol content is 50 vol%, and the bio-oil yield is 59.5% | [73] |
| ethanol/water co-solvent | algal, sawdust | 200~300 °C, 30~120 min | ethanol/water co-solvent exhibits a synergy, and when the solvent mixture (ethanol/water = 75 wt%:25 wt%) is liquefied at 250 °C, the bio-oil yield is the highest | the highest bio-oil yield of 58 wt% is obtained | [74] |
| ethanol/water co-solvent | rice husk | 533 K, 573 K and 613 K, 20 min | ethanol/water co-solvent combines the advantages of water and ethanol and shows a synergistic effect, with the highest bio-oil yield at 533 K | in ethanol/water co-solvent v/v( 5:5), the highest bio-oil yield of 21.15% is obtained at 533 K | [75] |
| ethanol/water co-solvent | lignocellulose | 260 °C | the heavy oil product contained more esters, ethers, and alcohols and less aldehydes due to the transesterification reaction that occurred with the addition of ethanol | the highest bio-oil yield of 36.62% is achieved when 60% content of ethanol is used to liquefy ligno-cellulose | [76] |
| ethanol/water co-solvent | mulberry bark | 300 °C, 60 min | the phenolic content of bio-oil derived from subcritical water is higher than that of bio-oil derived from subcritical ethanol/water co-solvent, while the ester content of bio-oil derived from subcritical ethanol/water co-solvent is higher | the bio-oil yield of mulberry bark in subcritical ethanol/water co-solvent (30.32 wt%) is slightly higher than that in sub-critical water (28.81 wt%) | [77] |
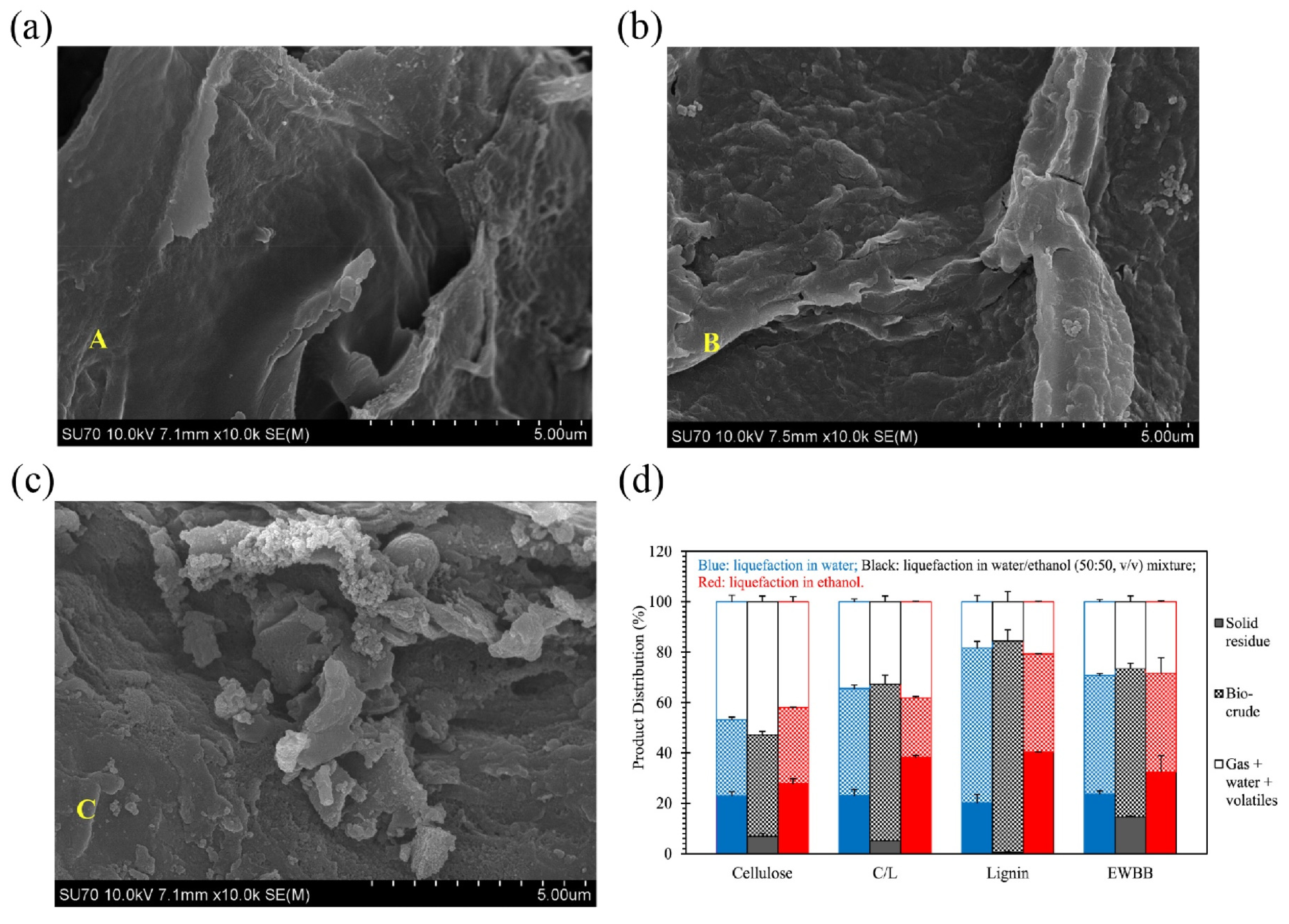
| ethanol/water co-solvent | cellulose, lignin, cellulose/lignin blend, white birch bark | 300 °C, 15 min | ethanol/water co-solvent enhances solvent penetration into lignocellulosic biomass structures and improves the solubility of liquefaction intermediates, thereby increasing bio-crude yields from lignocellulosic liquefaction | the bio-oil yield in water/ethanol (50:50, v/v) co-solvent is as high as 58.8 wt% | [78] |
| ethanol/water co-solvent, isopropanol/water co-solvent, glycerin/water co-solvent | low-lipid, high-protein algae | 310 °C, 330 °C and 350 °C, 30 min | bio-oils are mainly produced by the reaction of alcohol with algae fragments through Maillard, alkylation, and esterification | as the temperature increases from 310 °C to 350 °C, the yield of light bio-oil from dry algae using glycerol as the liquefaction solvent increases from 24.9 wt% to 73.2 wt% | [79] |
| hexane/water co-solvent, methanol/water co-solvent | sewage sludge | 300~380 °C, 0~60 min | synergistic effect between organic solvent/water can help reduce bio-oil nitrogen content while increasing bio-oil yield | the highest yield of bio-oil in methanol/water co-solvent is 46.5 wt% | [80] |
| acetone/tetrahydrofuran co-solvent | Fallopia Japonica | 280 °C, 300 °C and 320 °C | tetralin has both dissolution and scavenging effects, increasing the abundance of monomeric aromatics in the product; acetone facilitates the cleavage of retro-aldol to produce low-molecular-weight oxygenates | / | [81] |
| H2O, C4H10O2/C6H15NO3 co-solvent, C6H15NO3, C4H10O2 | pine tree | 200~300 °C, 10 min, 30 min, 60 min, 90 min and 100 min | C4H10O2/C6H15NO3 co-solvent liquefaction produces the highest bio-oil yield with synergistic effects | the maximum bio-oil yield is 65.0% | [82] |
| polyethylene glycol/glycerin co-solvent | bagasse | 130~170 °C | as the liquefaction reaction proceeds, the acid value of the liquefaction product increases, and the number of hydroxyl groups decreases | / | [83] |
| ethanol, ethylene glycol, ethanol/glycol co-solvent 1 | lignocellulose | 170~290 °C | ethanol/glycol co-solvent exerts a synergistic effect on the liquefaction process, manifesting as higher oil yield and lower biochar yield | the highest bio-oil yield is 52.3% when the solvent ratio is 1:1 | [84] |
| Water | Organic Solvent | Co-Solvent | |
|---|---|---|---|
| advantages | It is environmentally friendly, inexpensive, and can act as a solvent, hydrogenator, and catalyst, facilitating reactions such as pyrolysis and hydration and contributing to the decomposition and extraction of biomass. | (1) Organic solvents can facilitate alkylation and esterification reactions and help dissolve and stabilize intermediates. (2) Compared with water, the reaction conditions of organic solvents are mild, and the liquefaction products have high energy density and low acidity. (3) The protic solvent in the organic solvent can donate hydrogen, inhibit side reactions, and increase bio-oil yield. | There is often a synergistic effect between the co-solvents, which leads to an increase in bio-oil yield. In addition, the use of organic solvents/water as co-solvents to liquefy biomass reduces the nitrogen content of the bio-oil and improves the quality of the bio-oil. |
| disadvantages | It may corrode materials, the bio-oil obtained from its liquefaction has a low calorific value and high viscosity, and some of the organic compounds produced during the liquefaction process may be transferred from the aqueous phase to other phases, thus reducing the total bio-oil yield. | Organic solvents face high solvent costs, difficulties in recovery, and increased biomass drying requirements prior to liquefaction. | The synergistic reaction mechanism between the co-solvents needs to be further investigated in depth. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ming, H.; Yang, X.; Zheng, P.; Zhang, Y.; Jiang, H.; Zhang, L. Recent Advances of Solvent Effects in Biomass Liquefaction Conversion. Energies 2024, 17, 2814. https://doi.org/10.3390/en17122814
Ming H, Yang X, Zheng P, Zhang Y, Jiang H, Zhang L. Recent Advances of Solvent Effects in Biomass Liquefaction Conversion. Energies. 2024; 17(12):2814. https://doi.org/10.3390/en17122814
Chicago/Turabian StyleMing, Hui, Xin Yang, Pu Zheng, Yifan Zhang, Haoxin Jiang, and Libo Zhang. 2024. "Recent Advances of Solvent Effects in Biomass Liquefaction Conversion" Energies 17, no. 12: 2814. https://doi.org/10.3390/en17122814
APA StyleMing, H., Yang, X., Zheng, P., Zhang, Y., Jiang, H., & Zhang, L. (2024). Recent Advances of Solvent Effects in Biomass Liquefaction Conversion. Energies, 17(12), 2814. https://doi.org/10.3390/en17122814








