Simulation Study on Combustion Performance of Ammonia-Hydrogen Fuel Engines
Abstract
1. Introduction
2. Simulation Modeling and Model Verification
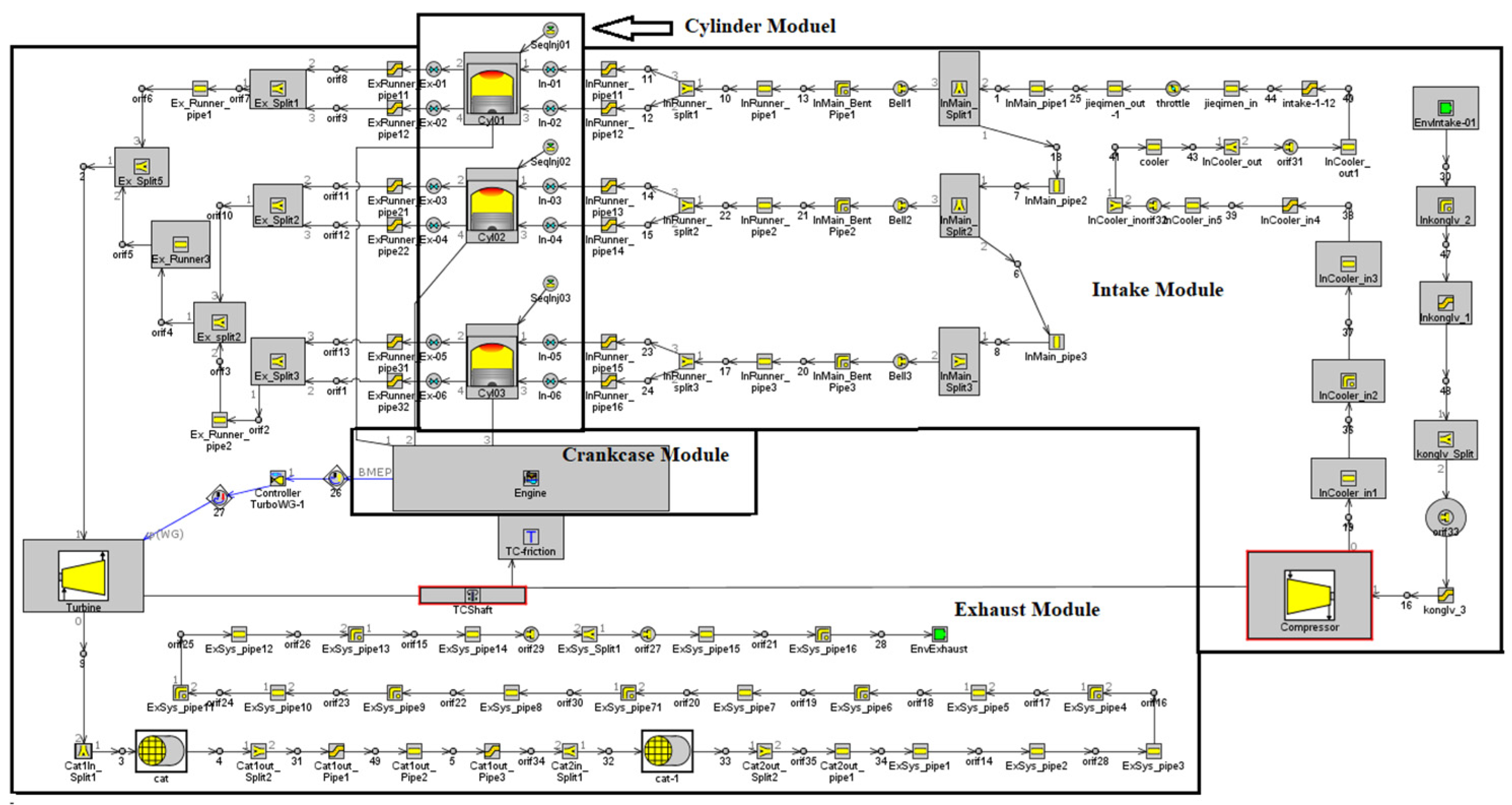
2.1. Simulation Modeling
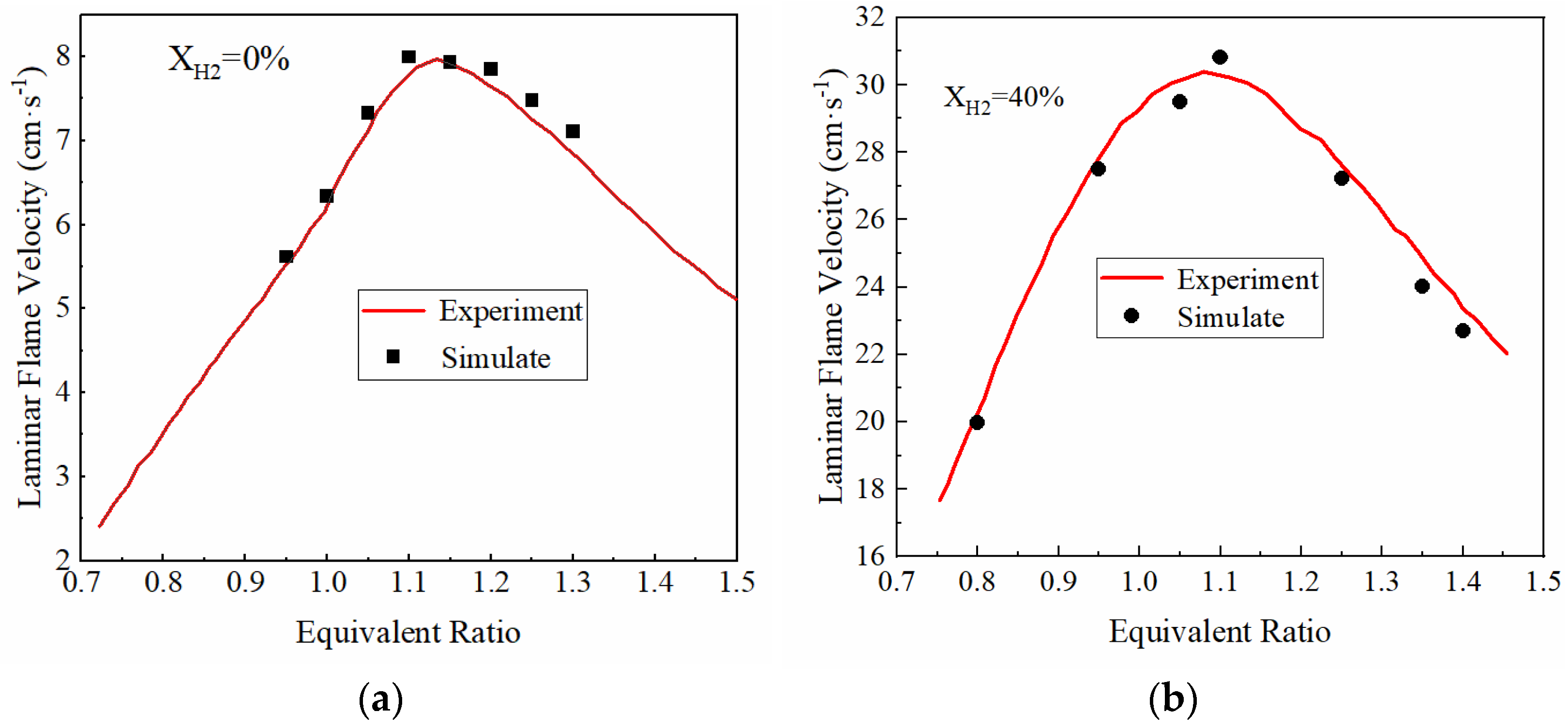
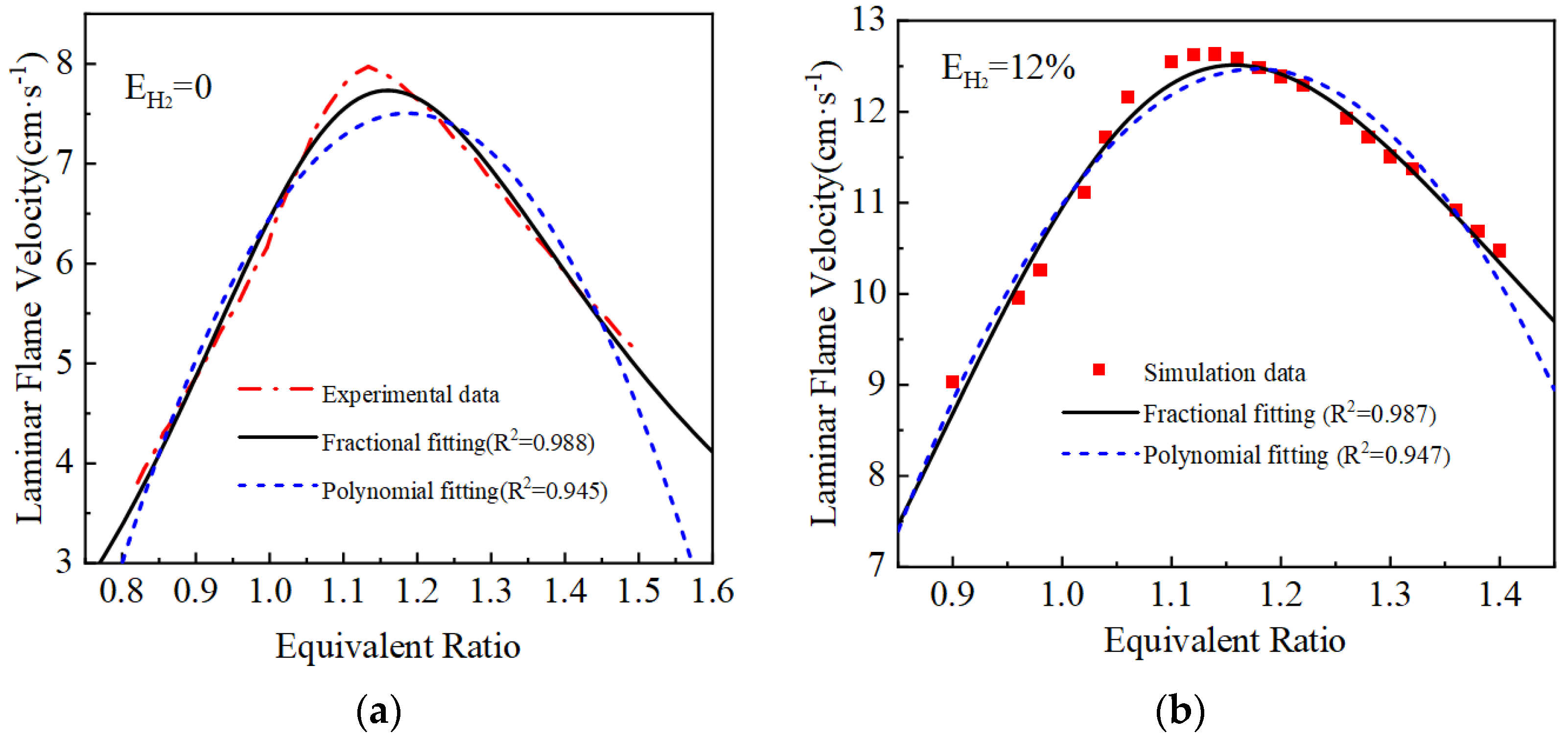
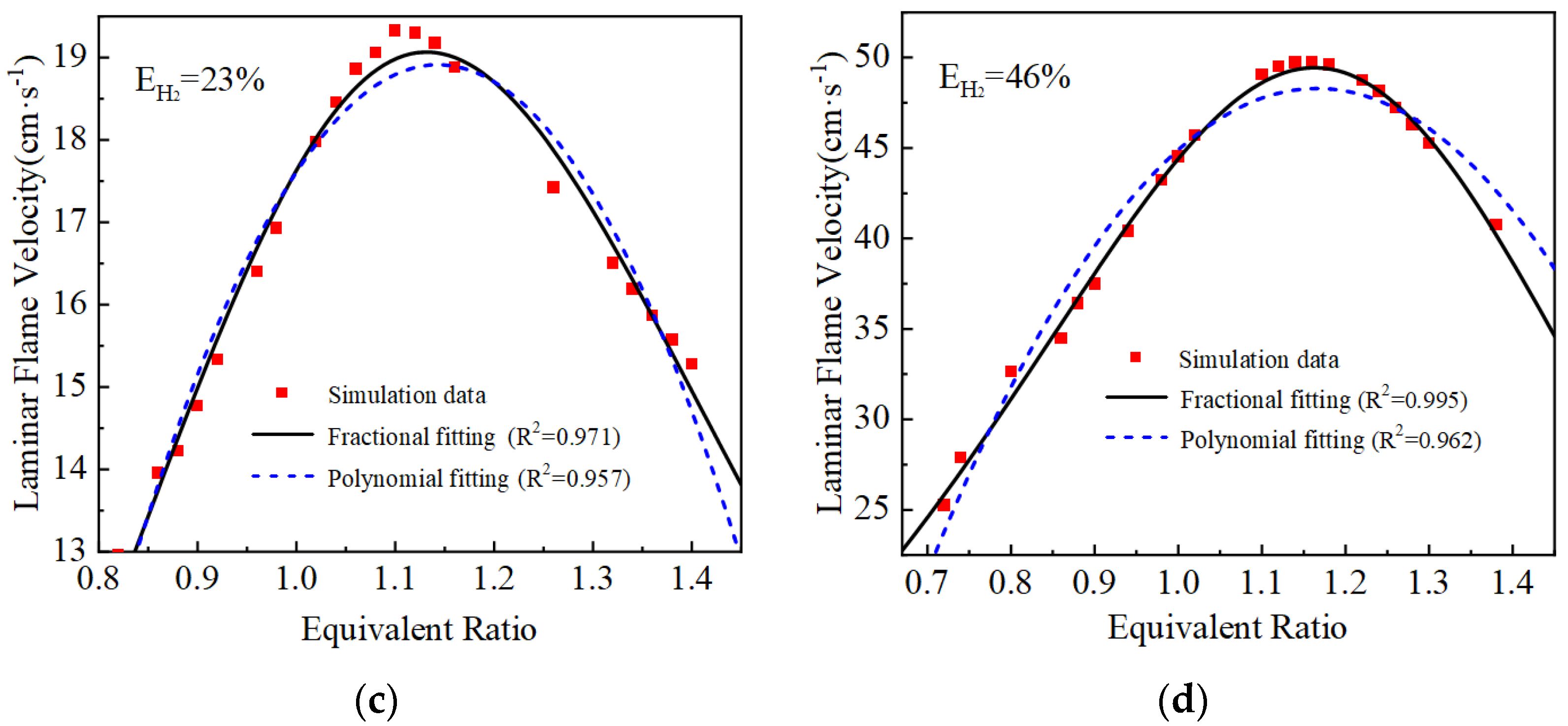
2.2. Laminar Flame Velocity Model Correction and Verification
2.3. GT-Power Model Validation
3. Combustion Characterization of Ammonia-Hydrogen Engine
3.1. Sensitivity Analysis of Ignition Delay
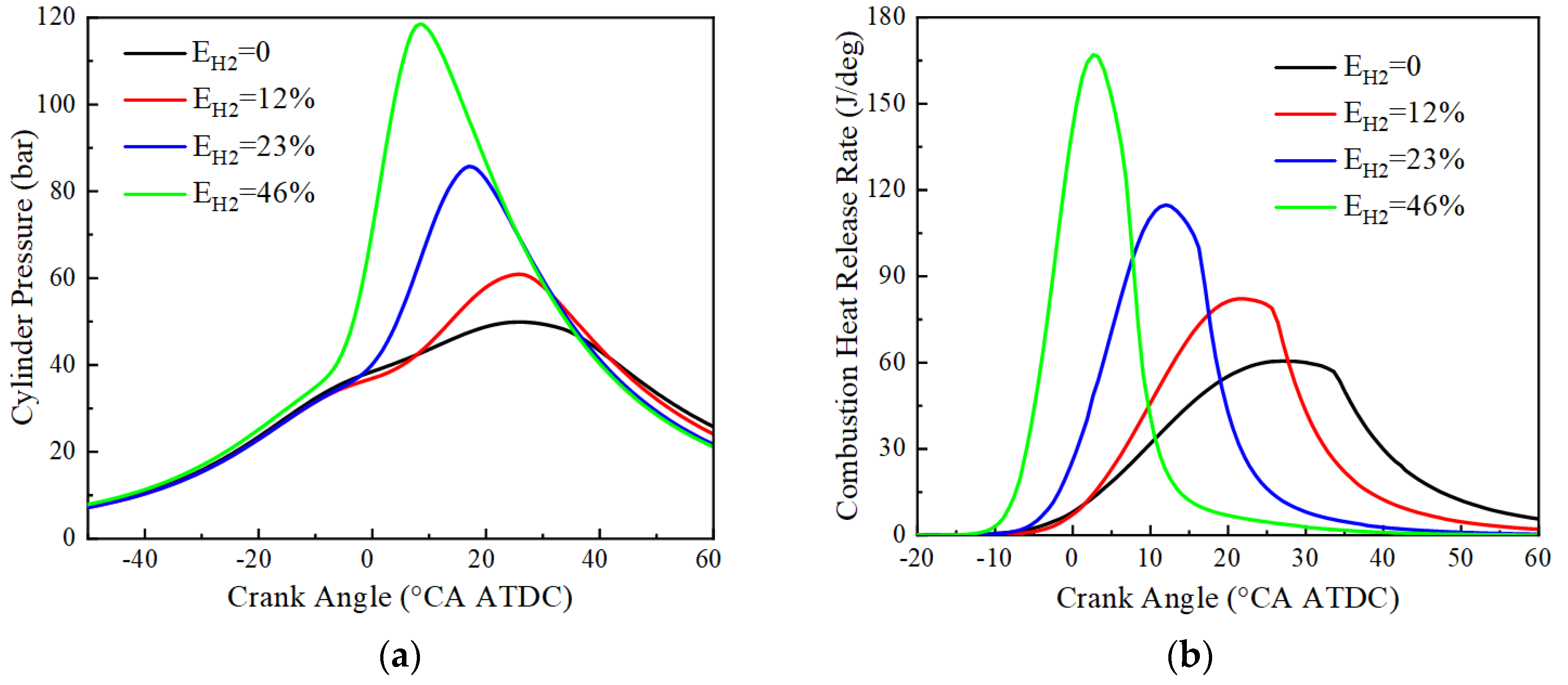
3.2. Effect of Hydrogen Blending Ratio on the Combustion Characteristics
3.3. Effect of Rotational Speed on Combustion Characteristics
3.4. Effect of Load on Combustion Characteristics
4. Conclusions
- (1)
- With the increase of hydrogen blending ratio, the sensitivity of hydroxyl-related elementary reactions (e.g., R3: O + H2 = OH + H, R4: OH + H2 = H + H2O) increases. In the combustion of pure ammonia fuel, the reaction involving nitrogen oxygen and nitrogen hydrogen free radicals has a significant negative effect on the combustion delay period. The addition of hydrogen can inhibit the strong negative sensitive elementary reactions (R64: NH2 + HO2 = NH3 + O2, R69: NH2 + NO = N2 + H2O) in the ammonia combustion process, and can significantly shorten the ignition delay. Under the same ignition timing, with the increase of hydrogen blending ratio, the peak value of cylinder pressure and heat release rate increases, as does the corresponding peak value of the crankshaft. Angle advances and the ignition delay period and combustion duration become shorter.
- (2)
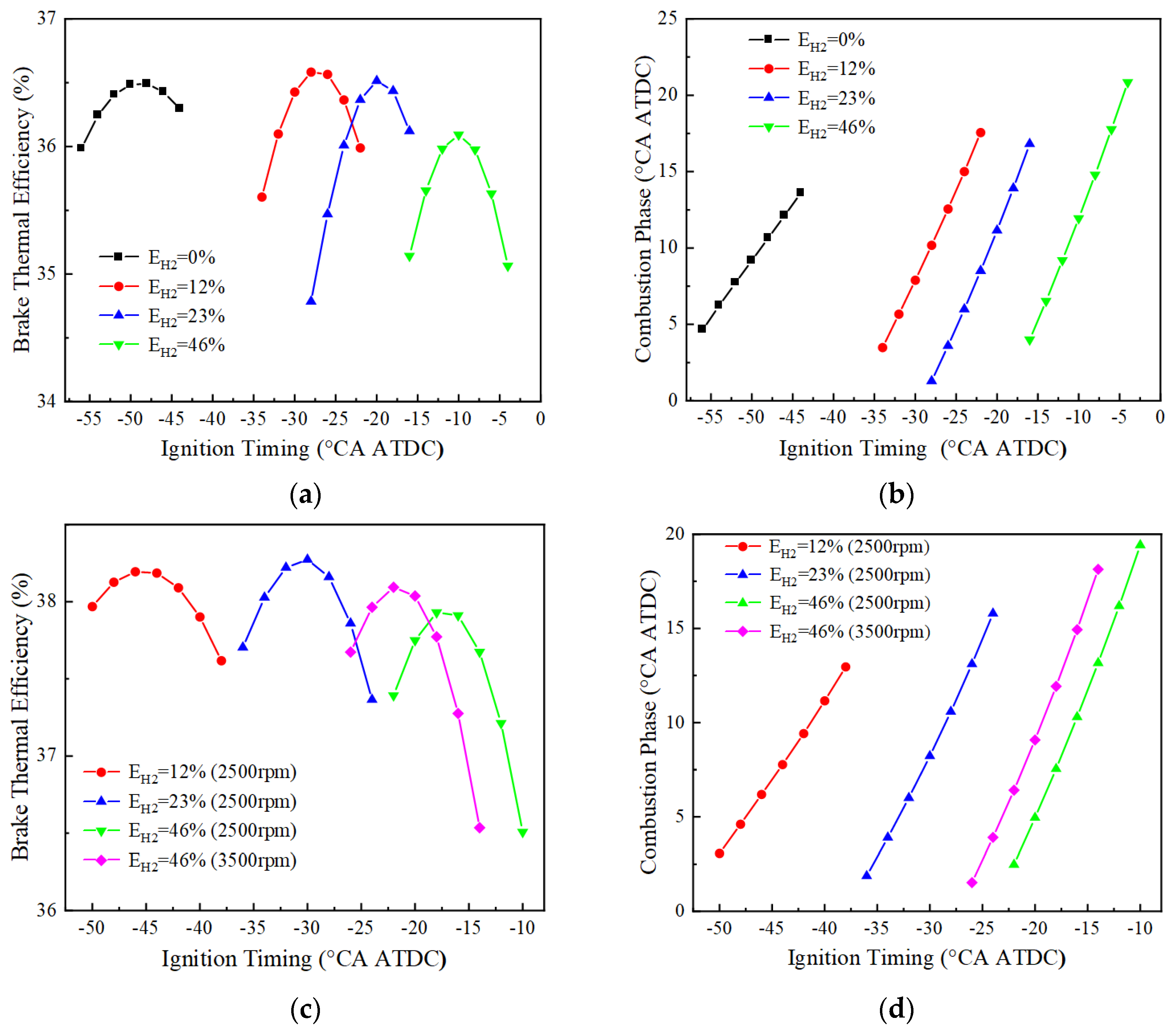
- Ammonia fuel, due to its low reactivity, requires a large ignition advance for successful ignition at 1500 rpm and does not ignite reliably at medium to high engine speeds. The fuel with 12% hydrogen blending has a high BTE of up to 36.6% at low speeds. As the engine speed increases, the ignition difficulty of the pure ammonia and low hydrogen blending ratio increases, and it is necessary to increase the hydrogen blending ratio to ensure reliable ignition.
- (3)
- The BTE of the blended fuel with a 23% hydrogen blending ratio at 2500 r/min has the highest BTE at BMEP = 10, 15, and 20 bar, with optimum thermal efficiencies of 37.1%, 38.5% and 38.3%, respectively. When the hydrogen blending ratio reaches 46%, BTE presents a small reduction. The possible reason is the high ratio of hydrogen blending produces large heat transfer losses due to rapid combustion. Under the speed of 3500 rpm, normal ignition can be ensured only when the hydrogen blending ratio reaches 46%. With the increase of the engine speed, it is necessary to appropriately increase the hydrogen blending ratio in order to obtain a higher BTE.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| ATDC | After Top Dead Center |
| BMEP | Brake Mean Effective Pressure |
| BTDC | Before Top Dead Center |
| BTE | Brake Thermal Efficiency |
| CA | Crank Angle |
References
- El-Adawy, M.; Nemitallah, M.A.; Abdelhafez, A. Towards sustainable hydrogen and ammonia internal combustion engines: Challenges and opportunities. Fuel 2024, 364, 131090. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M.; Rosado, P. Fossil Fuels. Our World in Data. 2020. Available online: https://ourworldindata.org/fossil-fuels (accessed on 4 August 2022).
- Santos, N.D.S.A.; Roso, V.R.; Malaquias, A.C.T.; Baeta, J.G.C. Internal combustion engines and biofuels: Examining why this robust combination should not be ignored for future sustainable transportation. Renew. Sust. Energy Rev. 2021, 148, 111292. [Google Scholar]
- Lešnik, L.; Kegl, B.; Torres-Jiménez, E.; Cruz-Peragón, F. Why we should invest further in the development of internal combustion engines for road applications. Oil Gas. Sci. Technol.—Rev. d’IFP Energies Nouv. 2020, 75, 56. [Google Scholar] [CrossRef]
- Kalghatgi, G. Is it really the end of internal combustion engines and petroleum in transport? Appl. Energy 2018, 225, 965–974. [Google Scholar] [CrossRef]
- Verma, S.; Dwivedi, G.; Verma, P. Life cycle assessment of electric vehicles in comparison to combustion engine vehicles: A review. Mater. Today Proc. 2022, 49, 217–222. [Google Scholar] [CrossRef]
- Yan, F.; Xu, L.; Wang, Y. Application of hydrogen enriched natural gas in spark ignition IC engines: From fundamental fuel properties to engine performances and emissions. Renew. Sust. Energy Rev. 2018, 82, 457–1488. [Google Scholar] [CrossRef]
- Onorati, A.; Payri, R.; Vaglieco, B.M.; Agarwal, A.K.; Bae, C.; Bruneaux, G.; Canakci, M.; Gavaises, M.; Günthner, M.; Hasse, C.; et al. The role of hydrogen for future internal combustion engines. Int. J. Engine Res. 2022, 23, 529–540. [Google Scholar] [CrossRef]
- Stępień, Z. A comprehensive overview of hydrogen-fueled internal combustion engines: Achievements and future challenges. Energies 2021, 14, 6504. [Google Scholar] [CrossRef]
- Sun, Z.; Hong, J.; Zhang, T.; Sun, B.; Yang, B.; Lu, L.; Li, L.; Wu, K. Hydrogen engine operation strategies: Recent progress, industrialization challenges, and perspectives. Int. J. Hydrogen Energy 2023, 48, 366–392. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Kamyab, H.; Zaidi, S.; Khalil, M.J.; Khan, M.A.; Alam, M.A.; Masood, F.; Bazli, L.; Chelliapan, S.; et al. Current trends in hydrogen production, storage and applications in India: A review. Sustain. Energy Technol. 2022, 53, 102677. [Google Scholar] [CrossRef]
- Richter, M.; Schultheis, R.; Dawson, J.R.; Gruber, A.; Barlow, R.S.; Dreizler, A.; Geyer, D. Extinction strain rates of premixed ammonia/hydrogen/nitrogen-air counterflow flames. Proc. Combust. Inst. 2023, 39, 2027–2035. [Google Scholar] [CrossRef]
- Bae, S.H.; Lee, J.S.; Wilailak, S.; Lee, G.Y.; Lee, C.J. Design-based risk assessment on an ammonia-derived urban hydrogen refueling station. Int. J. Energy Res. 2022, 46, 12660–12673. [Google Scholar] [CrossRef]
- Perna, A.; Minutillo, M.; Di Micco, S.; Cigolotti, V.; Pianese, A. Ammonia as hydrogen carrier for realizing distributed on-site refueling stations implementing PEMFC technology. In Proceedings of the E3S Web of Conferences, Kenitra, Morocco, 25–27 December 2020. [Google Scholar]
- Zhou, L.; Zhong, L.; Liu, Z.; Wei, H. Toward highly-efficient combustion of ammonia–hydrogen engine: Prechamber turbulent jet ignition. Fuel 2023, 352, 129009. [Google Scholar] [CrossRef]
- Chiong, M.C.; Chong, C.T.; Ng, J.H.; Mashruk, S.; Chong, W.W.F.; Samiran, N.A.; Mong, G.R.; Valera-Medina, A. Advancements of combustion technologies in the ammonia-fuelled engines. Energy Convers. Manage 2021, 244, 114460. [Google Scholar] [CrossRef]
- Liu, S.; Lin, Z.; Qi, Y.; Lu, G.; Wang, B.; Li, L.; Wang, Z. Combustion and emission characteristics of a gasoline/ammonia fueled SI engine and chemical kinetic analysis of NOx emissions. Fuel 2024, 367, 131516. [Google Scholar] [CrossRef]
- Xu, L.; Xu, S.; Bai, X.S.; Repo, J.A.; Hautala, S.; Hyvönen, J. Performance and emission characteristics of an ammonia/diesel dual-fuel marine engine. Renew. Sust. Energy Rev. 2023, 185, 113631. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Wang, S.; Guiberti, T.F.; Roberts, W.L. Review on the recent advances on ammonia combustion from the fundamentals to the applications. Fuel Commun. 2022, 10, 100053. [Google Scholar] [CrossRef]
- Grannell, S.M.; Assanis, D.N.; Bohac, S.V.; Gillespie, D.E. The operating features of a stoichiometric, ammonia and gasoline dual fueled spark ignition engine. In ASME 2006 International Mechanical Engineering Congress and Exposition; American Society of Mechanical Engineers Digital Collection: New York, NY, USA, 2006. [Google Scholar]
- Gross, C.W.; Kong, S.C. Performance characteristics of a compression-ignition engine using direct-injection ammonia–DME mixtures. Fuel 2013, 103, 1069–1079. [Google Scholar] [CrossRef]
- Niki, Y.; Yoo, D.H.; Hirata, K.; Sekiguchi, H. Effects of ammonia gas mixed into intake air on combustion and emissions characteristics in diesel engine. In Proceedings of the ASME 2016 Internal Combustion Engine Fall Technical Conference, Greenville, SC, USA, 9–12 October 2016. [Google Scholar]
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as effective hydrogen storage: A review on production, storage and utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Wan, Z.; Tao, Y.; Shao, J.; Zhang, Y.; You, H. Ammonia as an effective hydrogen carrier and a clean fuel for solid oxide fuel cells. Energy Convers. Manag. 2021, 228, 113729. [Google Scholar] [CrossRef]
- Yapicioglu, A.; Dincer, I. A review on clean ammonia as a potential fuel for power generators. Renew. Sust. Energy Rev. 2019, 103, 96–108. [Google Scholar] [CrossRef]
- Hasan, M.H.; Mahlia, T.M.I.; Mofijur, M.; Rizwanul Fattah, I.M.; Handayani, F.; Ong, H.C.; Silitonga, A.S. A comprehensive review on the recent development of ammonia as a renewable energy carrier. Energies 2021, 14, 3732. [Google Scholar] [CrossRef]
- Comotti, M.; Frigo, S. Hydrogen generation system for ammonia–hydrogen fuelled internal combustion engines. Int. J. Hydrogen Energy 2015, 40, 10673–10686. [Google Scholar] [CrossRef]
- Frigo, S.; Gentili, R.; Doveri, N. Ammonia Plus Hydrogen as Fuel in a SI Engine: Experimental Results; SAE Technical Paper; SAE: Warrendale, PA, USA, 2012. [Google Scholar]
- Pochet, M.; Jeanmart, H.; Contino, F. A 22: 1 compression ratio ammonia-hydrogen HCCI engine: Combustion, load, and emission performances. Front. Mech. Eng. 2020, 6, 43. [Google Scholar] [CrossRef]
- Kurien, C.; Mittal, M. Review on the production and utilization of green ammonia as an alternate fuel in dual-fuel compression ignition engines. Energy Convers. Manage 2022, 251, 114990. [Google Scholar] [CrossRef]
- Xin, G.; Ji, C.; Wang, S.; Meng, H.; Chang, K.; Yang, J. Effect of different volume fractions of ammonia on the combustion and emission characteristics of the hydrogen-fueled engine. Int. J. Hydrogen Energy 2022, 47, 16297–16308. [Google Scholar] [CrossRef]
- Wang, B.; Yang, C.; Wang, H.; Hu, D.; Duan, B.; Wang, Y. Study on injection strategy of ammonia/hydrogen dual fuel engine under different compression ratios. Fuel 2023, 334, 126666. [Google Scholar] [CrossRef]
- Zhu, T.; Yan, X.; Gao, Z.; Qiu, Y.; Zhu, L.; Huang, Z. Combustion and emission characteristics of ammonia-hydrogen fueled SI engine with high compression ratio. Int. J. Hydrogen Energy 2024, 62, 579–590. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw-Hill Book Co.: New York, NY, USA, 1988. [Google Scholar]
- Zhang, X.; Moosakutty, S.P.; Rajan, R.P.; Younes, M.; Sarathy, S.M. Combustion chemistry of ammonia/hydrogen mixtures: Jet-stirred reactor measurements and comprehensive kinetic modeling. Combust. Flame 2021, 234, 111653. [Google Scholar] [CrossRef]
- Mei, B.; Zhang, X.; Ma, S.; Cui, M.; Guo, H.; Cao, Z.; Li, Y. Experimental and kinetic modeling investigation on the laminar flame propagation of ammonia under oxygen enrichment and elevated pressure conditions. Combust. Flame 2019, 210, 236–246. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Lamoureux, N.; Contino, F.; Mounaïm-Rousselle, C. Experimental investigation on laminar burning velocities of ammonia/hydrogen/air mixtures at elevated temperatures. Fuel 2020, 263, 116653. [Google Scholar] [CrossRef]
- Goldmann, A.; Dinkelacker, F. Approximation of laminar flame characteristics on premixed ammonia/hydrogen/nitrogen/air mixtures at elevated temperatures and pressures. Fuel 2018, 224, 366–378. [Google Scholar] [CrossRef]
- Pyrc, M.; Gruca, M.; Tutak, W.; Jamrozik, A. Assessment of the co-combustion process of ammonia with hydrogen in a research VCR piston engine. Int. J. Hydrogen Energy 2023, 48, 2821–2834. [Google Scholar] [CrossRef]






| Parameters | Value |
|---|---|
| Number of cylinders | 3 |
| Hydrogen supply mode | Direct injection |
| Ammonia supply mode | Port injection |
| Valve timing mechanism | Dual variable valve timing |
| Bore diameter × stroke (mm) | 83 × 92 |
| Connecting rod length (mm) | 144.3 |
| Displacement (L) | 1.493 |
| Compression ratio | 11.3 |
| Rated power (kW) | 108.43 |
| Rated speed (r/min) | 5500 |
| Fuel consumption (g/(kW·h)) | 320.82 |
| Energy Ratio of Hydrogen | Mass Ratio of Hydrogen | Volume Ratio of Hydrogen |
|---|---|---|
| 0 | 0 | 0 |
| 12% | 2.09% | 16.31% |
| 23% | 4.47% | 29.92% |
| 46% | 11.77% | 54.90% |
| Hydrogen-Blending Ratio | Polynomial Fitting Results | Fractional Fitting Results |
|---|---|---|
| EH2 = 0% | ||
| EH2 = 12% | ||
| EH2 = 23% | ||
| EH2 = 46% |
| Parameters | Value |
|---|---|
| Hydrogen blending ratio | 0%, 12%, 23%, 46% |
| Engine speed (r/min) | 1500~5500 |
| BMEP (bar) | 10, 15, 20 |
| Equivalent ratio | 1 |
| Primitive Reaction Number | Elementary Reaction Equation |
|---|---|
| R1 | H + O2 = O + OH |
| R2 | O + H2 = OH + H |
| R3 | O + H2 = OH + H |
| R4 | OH + H2 = H + H2O |
| R16 | H + O2(+M) = HO2(+M) |
| R58 | NH2 + H = NH + H2 |
| R60 | NH2 + O = HNO + H |
| R61 | NH2 + O = HNO + H |
| R63 | NH2 + HO2 = H2NO + OH |
| R64 | NH2 + HO2 = NH3 + O2 |
| R68 | NH2 + NO = NNH + OH |
| R69 | NH2 + NO = N2 + H2O |
| R70 | NH2 + NO = N2 + H2O |
| R103 | N2H3(+M) = N2H2 + H(+M) |
| R161 | NH3 + H = NH2 + H2 |
| R162 | NH3 + OH = H2O + NH2 |
| R164 | NH3 + O = NH2 + OH |
| R219 | H2NO + NH2 = HNO + NH3 |
| R220 | H2NO + HO2 = HNO + H2O2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Gao, W.; Li, Y.; Fu, Z.; Hua, X.; Zhang, Y. Simulation Study on Combustion Performance of Ammonia-Hydrogen Fuel Engines. Energies 2024, 17, 2337. https://doi.org/10.3390/en17102337
Zhao D, Gao W, Li Y, Fu Z, Hua X, Zhang Y. Simulation Study on Combustion Performance of Ammonia-Hydrogen Fuel Engines. Energies. 2024; 17(10):2337. https://doi.org/10.3390/en17102337
Chicago/Turabian StyleZhao, Duanzheng, Wenzhi Gao, Yuhuai Li, Zhen Fu, Xinyu Hua, and Yuxuan Zhang. 2024. "Simulation Study on Combustion Performance of Ammonia-Hydrogen Fuel Engines" Energies 17, no. 10: 2337. https://doi.org/10.3390/en17102337
APA StyleZhao, D., Gao, W., Li, Y., Fu, Z., Hua, X., & Zhang, Y. (2024). Simulation Study on Combustion Performance of Ammonia-Hydrogen Fuel Engines. Energies, 17(10), 2337. https://doi.org/10.3390/en17102337







