Abstract
A reasonable strategy for the development of sludge conditioning methods prior to dewatering appears to be the use of substances that allow the safe management of dewatered sludge. It is also justified to use mineral or organic conditioners instead of synthetic chemicals, e.g., polyelectrolytes, or to try to use other substances, e.g., waste. The properties of iron coagulant (PIX 113) combined with perlite and cellulose can be an environmentally safe method of sludge conditioning. The tests were carried out in accordance with European standards on the efficiency of mechanical dewatering of sewage sludge. The most advantageous method of sludge conditioning was the dosing of the iron coagulant PIX 113. The use of at least a coagulant dose of 0.40 g/g DS enabled the achievement of minimum sludge dewatering parameters, i.e., specific resistance of filtration (SRF) < 5.0 E12 m/kg and final hydration of filtration cake (FH) < 80%. The use of cellulose and perlite as stand-alone conditioners or in combination with PIX 113 resulted in a deterioration of the sludge dewaterability and the quality of the filtrate. It is assumed that the further development of environmentally friendly conditioning methods requires the use of easy-to-use, non-toxic and biodegradable substances. It is important to select conditioners which, in practically acceptable doses, can improve the conditioning effect or show a synergistic effect in combination with previously used conditioners.
1. Introduction
Sewage sludge is a complex mixture of solid and dissolved substances that is produced during the wastewater treatment process. The complex composition of sewage sludge includes valuable organic substances, nutrients and micro and macro elements [1,2], but also emergency contaminants such as heavy metals, polycyclic aromatic hydrocarbons, microplastics or pharmaceuticals [3,4]. Sewage sludge must be managed, but the method of management depends on many factors, such as the chemical composition of the sludge, available technologies, legal requirements, and environmental, energy, and economic aspects [5,6,7].
Generally, there are two ways of sludge management-disposal: land-based applications [3,8] or thermal treatment [9,10]. However, regardless of the method of sewage sludge management, the required condition is sludge dewatering. Sludge dewatering allows for reducing their volume and facilitates storage, transport, and disposal [11]. The dewatering process in sewage sludge technology is very demanding. This is due to the amount and binding strength of water accumulated on the surface of solid particles and the structure of sludge agglomerates [12,13].
The susceptibility of raw sludge to dewatering is usually limited by the hydrophilic organic matter content [14], colloidal particles [15], and extracellular polymeric substances (EPS) [16,17]. Conditioning is a necessity in sludge dewatering technology. Generally, the purpose of conditioning is to change the properties of the sludge using chemical, physical and biological methods in such a way that it enables effective dewatering [11,18].
For practical reasons, the key issue in the sludge dewatering process is the effectiveness and reliability of conditioning methods. Therefore, the dominant methods in sludge conditioning are the use of mineral coagulants such as aluminium and iron salts and, above all, the use of polyelectrolytes [19]. The main disadvantage of the use of polyelectrolytes is the chemicalization of sludge, which causes a potential negative impact on microorganisms and potential environmental and health problems [20,21,22]. Due to the disadvantages of using polyelectrolytes, but also to increase the efficiency of dewatering, research is carried out using many unconventional methods. Unconventional methods often combine the dosing of coagulants and polyelectrolytes with other chemical or waste reagents. Examples include the use of tannic acid [23] and coal fly ash modified by sulfuric acid [24].
A reasonable strategy for the development of conditioning methods seems to be the use of natural coagulants and structure-forming agents as an independent or combined conditioning method. The validity of this strategy is based on the advantages of natural reagents, such as being readily available, economical, easy to use, biodegradable, non-toxic, and eco-friendly [25].
A frequently used natural reagent is lignocellulosic biomass. The use of lignocellulosic materials is particularly justified due to the calorific value and energetic processing of sludge, such as incineration, gasification, pyrolysis, as feedstock or fuel [26] and carbonisation [27]. Cellulose is also treated as a structure-forming material that reduces sludge compressibility. In appropriate doses, cellulose can enhance dewatering and generate a value-added product for the land application of sludge [28,29]. Among the conditioners qualified as a filtration aid, perlite also stands out as a skeleton-forming material. The predominant use of perlite is as a preliminary layer applied to the surface of the filtration barrier [30,31]. The use of perlite is not common in sewage sludge technology. Most applications concern the use of perlite in filtration in industrial applications, especially in the food industry [32,33].
Due to the dewatering of municipal sewage sludge, the main disadvantages of cellulose and perlite are hydrophilicity [28] and water retention [30], respectively. However, as other researchers point out, despite the disadvantages, the use of cellulose and perlite in appropriate doses counteracts the compressibility of sludge and can improve dewatering efficiency [28,29,32,33]. Mineral coagulants such as iron and aluminium salts are assumed to be useful in removing water from the structure of sludge conditioned with cellulose or perlite. Iron coagulants cause destabilisation and agglomeration of solid phase particles [34]. The addition of a mineral coagulant can be one way of counteracting hydrophilicity and water retention. Unlike the use of polyelectrolytes, the only disadvantage of using iron salts is their toxicity to living organisms, which only occurs at high doses [35,36]. The use of a low dose of iron coagulant in combination with perlite and cellulose appears to be an environmentally safe method of sludge conditioning. Safe because of the presence of substances in the dewatered sludge (iron salts, cellulose, perlite), which in particular help to improve the fertilising properties of the dewatered sludge. The main aim of the research is to determine the suitability of the combination of natural reagents (cellulose and perlite) and the commercial iron coagulant PIX 113 (currently used in sewage coagulation) in the effective dewatering of sewage sludge. This simple combination of sludge conditioners has not been tested so far. Additionally, these conditioners are commonly used, and available substances and their chemical characteristics (especially cellulose and perlite) do not have a negative impact on the environment.
2. Materials and Methods
2.1. Research Substrate
Sludge samples were taken from a municipal sewage treatment plant with a capacity of 200 m3∙d−1. This facility is a mechanical-biological treatment plant with activated sludge with dephosphatation, denitrification and nitrification zones. The treatment plant is made of compact, combined steel tanks. All elements of the treatment plant are located in a closed, ventilated hall. The samples collected for testing are sludge after a 25-day aerobic stabilisation process at ambient temperature. The basic physical and chemical parameters of the collected sludge are presented in Table 1.

Table 1.
Physicochemical properties of tested stabilised sludge.
2.2. Reagents
PIX 113 iron coagulant, cellulose and perlite were used to condition the sludge. Commercial product PIX 113 is an aqueous solution of Fe2(SO4)3. The iron content is 11.8% ± 0.4%, the ion iron content is 0.4% ± 0.3%, and the coagulant pH ≤ 1.0. The density of the coagulant solution was 1.5 g/mL. The following doses of PIX 113 were used in the tests: 2.0, 4.0, 6.0, 10.0, 20.0 and 30.0 g/L of tested aerobic sludge. Calculated on the dry solids of sludge, the doses were 0.13, 0.27, 0.40, 0.66, 1.33 and 1.99 g/g DS, respectively.
Cellulose was used as a natural reagent for conditioning the sludge. Fibrous, unrefined pine wood cellulose obtained from a local wood processing factory was used. The second natural conditioner was expanded perlite. A commercial product called EP-100 was used. The applied doses of cellulose and perlite were 0.5, 1.0, 2.0, 4.0, 8.0, 16.0, and 32.0 g/L of sludge. The doses calculated as the weight ratio of the reagent mass to the dry solids of sludge were 0.02, 0.04, 0.09, 0.17, 0.35, 0.71, and 1.42 g/g DS.
2.3. Mixing Sludge with Reagents
The volume of the sludge sample mixed with the reagents was V = 1.0 L, and the mixing time was 40.0 s. Turbulence during the mixing of sludge with reagents was determined on the basis of the calculated Reynolds number, mixing power and the velocity gradient [37]. A 4-blade mixer was used in the mixing process. The length and height of the blade were 0.1 m and 0.015 m, respectively. The rotational speed of the mixer was . It was assumed that the dynamic viscosity coefficient of sludge, including sludge mixed with reagents, is equal to that of water at a temperature of 20 °C and is .
Reynolds number calculations:
Mixing power:
Velocity gradient:
2.4. Analytical Methods
The basic technological parameters of the original sludge samples were tested according to PN-EN 12880—dry solids (DS) and water content (FH)—[38], PN-EN 12879—volatile solids (VS)—[39], PN-EN 14701-1—capillary suction time (CST)—[40], PN-EN 14701-2—specific resistance of filtration (SRT) [41].
Conductance (C) was determined by direct conductivity using the Hydromet CD-210 probe. The turbidity (T) of the filtrate after separation of the solid phase in the vacuum filtration process was determined using a Hach 2100N turbidity meter (Hach Company, Loveland, CO, USA). The rheological tests were carried out using a Brookfield RST rheometer with a CCT-40 measuring system. The tested samples were thermostated at a temperature of 20 °C. A Malvern Zetasize Lab instrument was used to determine the zeta potential of particles in the filtrate. Zeta potential was determined in samples thermostated at 20 °C.
2.5. Rheological Measurements
Rheological models are a group of equations that take into account rheological parameters such as dynamic viscosity, shear rate and shear time. These models are necessary to analytically solve problems related to the flow of non-Newtonian fluids. In order to determine the relationship between shear rate (s−1) and shear stress (Pa s−1), the main rheological models were used (Table 2).

Table 2.
Rheological models used in the research [42].
All experiments were conducted following two steps. The first step was to linearly increase the shear rate from 0 s−1 to 300 s−1 over 300 s. The second step was to linearly decrease the shear rate from 300 s−1 to 0 s−1 over 300 s. Thixotropy was evaluated as the area included between the two flow curves and increasing and decreasing shear rates.
2.6. Research Combinations
The tests were divided into five combinations depending on the reagents used. Single sludge conditioning, i.e., with only one reagent, and dual conditioning were carried out. Dual conditioning was the sequential dosing of two reagents, one after the other (Figure 1).
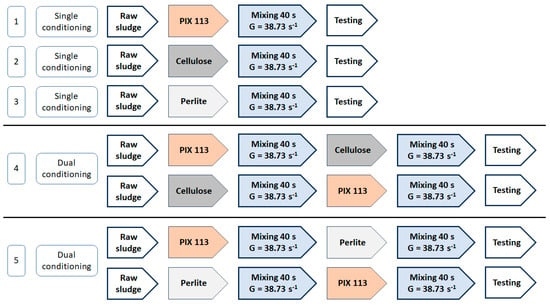
Figure 1.
Research combinations of sludge conditioning.
2.7. Statistical Analysis
Pearson’s R correlation coefficient was used for statistical analysis of single conditioning. The correlation coefficient was used to determine the degree of proportional (linearly dependent) relationships between the variables (CST, FV, SRT, FH, pH, C, T). Correlation coefficients in bold were significant at the p < 0.05 level.
In dual conditioning, analysis of variance (ANOVA) was used to test the significance of differences in the effects of preparation. Post hoc analysis (Tukey’s test) was used for statistically significant data p < 0.05. Values marked with the same letter (a, b, c) are not statistically significant. This means that the sludge treatment methods did not show statistically significant differences in conditioning efficiency.
STATISTICA 13.3, TIBCO Software Inc. (Palo Alto, CA, USA) was used for statistical analysis.
3. Research Results
3.1. Research Results—Combination 1
According to the coagulation theory, the iron reagent PIX 113 caused destabilisation and flocculation of solid particles, which enabled dehydration of the solid particle surface [19,34]. An increase in the reagent dose resulted in a decrease in the capillary suction time and, therefore, accelerated the separation of the sludge liquid (Figure 1). The most rational dose of PIX 113 was found to be 6.0 mL/L of sludge (0.27 g/g DS). The use of higher doses of the reagent than 6.0 mL/L did not accelerate the separation of the sludge liquid, and CST values oscillated around 50 s. The CSK values were inversely proportional to the obtained filtration velocity of the prepared sludge. These two parameters converged to indicate that the optimal coagulant dose was 6.0 mL/L (Figure 2).
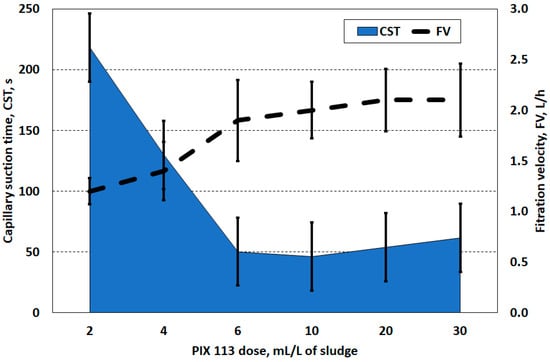
Figure 2.
Capillary suction time and filtration speed of sludge prepared PIX 113.
The dosage of PIX 113 at 6.0–30.0 mL/L (0.40–1.99 g/g DS) caused a decrease in the specific resistance of filtration below 5.0 × 1012 m/kg (Figure 3). This value is considered to be the upper limit of sludge filterability [41]. Depending on the type and characteristics of the sludge and the type and dose of the coagulant, it is possible to achieve substantially different SRF values. In Xu et al. studies on the dewaterability of activated sludge, SRF below 5.0 × 1012 m/kg was obtained using PAC (polyaluminium chloride) at a dose of 5 mg/g DS or FeCl3 at a dose of 3 mg/g DS [43]. Zhang et al. achieved a reduction in SRF of landfill sludge from 45.21 × 1012 m/kg to 2.78 × 1012 m/kg using FeCl3 at a dose of 0.2 g/g DS [44]. It should be noted that the use of doses above 6.0 mL/L (0.40 g/g DS) had no significant effect on changes in filtration resistance, final hydration of sludge cake and filtration velocity. Therefore, it was found that increasing the coagulant dose above 6.0 mL/L is not justified; there is a slight improvement in dewatering effects with high coagulant consumption.
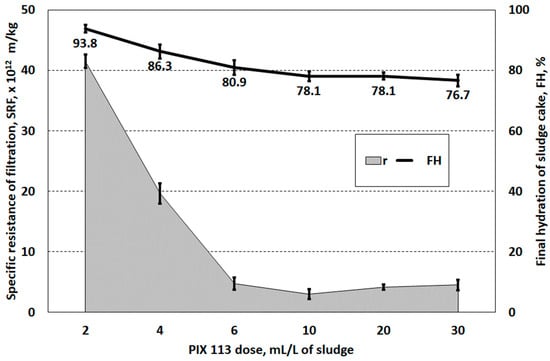
Figure 3.
Specific resistance of and hydration of the filter cake of sludge prepared PIX 113 coagulant.
The supernatant, after the filtration process, was a highly acidified solution. An increase in PIX 113 doses resulted in a decrease in pH even to 2.3. On the other hand, an increase in the reagent dose resulted in an improvement in the quality of the filtrate, which was associated with a decrease in turbidity and conductivity (Table 3).

Table 3.
Parameters of the filtrate after sludge dewatering using vacuum filtration.
The highest coefficient of determination R2 in rheological analysis was obtained using the Ostwald model. The Bingham and Hershel-Bulkley model was characterised by a lower accuracy of fit of the modelled flow curve to the actual measured data (Table 4). In some cases, low R2 values below 0.5 were obtained. In such cases, the accuracy and reliability of the variable rheological models were considered insufficient, and the results were not presented in tables. Analysis of the parameters in the rheological models showed no significant dependence (decreasing or increasing trends) with increasing doses of PIX 113. It can be concluded that the yield stress values are very low, and the hydraulic transport of this type of conditioned sludge will not be problematic [45]. This is confirmed by Wei et al., who specify that the rheological properties of sludge measured in the laboratory may have a specific relationship to the flow behaviour in practice in pipeline systems [46]. Rheological measurements, although in a limited way, can even be used to obtain information on the microbiological composition of activated sludge and settleability properties [47]. It can also be concluded that the prepared sludge belongs to the shear—thinning fluids (n < 1, Oswald model), which means a decrease in viscosity with increasing shear rate and indicates liquefaction of the sludge. A similar shear thinning phenomenon was observed in studies by Santos et al., which analysed the rheological properties of digested sludge and their mixtures with waste [48]. An increase in the applied PIX dose generally resulted in an increase in the thixotropy of the sludge (Table 4). This means that the conditioned sludge becomes a structurally unstable substrate, and its properties are significantly dependent on changes in shear rate.

Table 4.
Results of rheological tests of sludge conditioned in combination 1.
3.2. Research Results—Combination 2
Conditioning of the sludge with cellulose slightly increased the rate of water release from the structure of the prepared sludge. Based on the CST test and filtration velocity, the most favourable cellulose doses were 8.0 and 16.0 g/L (Figure 4). These are very high doses, close to the dry weight of the sludge (22.6 g/L). The CST and FV indicate that the dosage of cellulose significantly limits the release of water from the sludge structure, even in comparison to raw sludge. The observed phenomenon may result from the hydrophilicity of cellulose, while the possible improvement in dewatering ability may result from the three-dimensional structure of the cellulose solution forming a filter for solid particles [49,50].
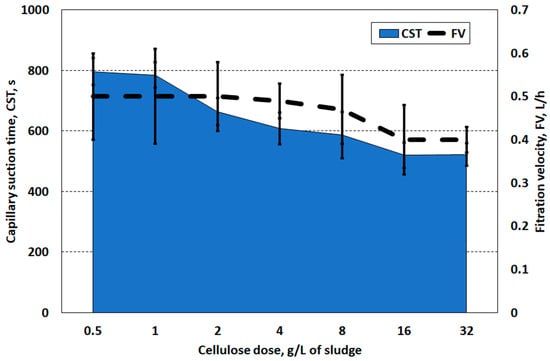
Figure 4.
Capillary suction time and filtration speed of sludge prepared with cellulose.
The values of resistance of filtration (SRT > 70 × 1013 m/kg) and hydration of sludge cake (FH > 94%) did not allow cellulose to be classified as an agent supporting the dewatering process (structure-forming agent) (Figure 5). It can be suggested that the use of cellulose and other lignocellulosic reagents, due to the filtration efficiency, requires the use of high doses. Lin et al. [51] suggest using wood chips at a dose of 60% of the dry weight of the sludge. Similarly, for the effective dewatering of aerobic-stabilised sludge conditioned using wood chips, Jing et al. [52] recommend using a dose of 80% DS of sludge. It is assumed that the use of sludge conditioning methods should result in a reduction of the water content in the dewatered sludge below 80% [43,53].
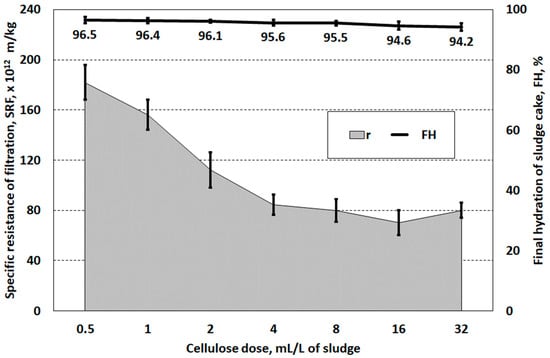
Figure 5.
Specific resistance of and hydration of the filter cake of sludge prepared with cellulose.
The filtrate after the sludge dewatering process was highly contaminated with dissolved and solid phases, as indicated by the values of conductivity (≈100 µS/cm) and turbidity (≈500 NTU). This effect was generally independent of the dose of Regent. Similarly, the pH of the filtrate did not change and was approximately 5.5 (Table 5). Suopajärvi et al. [54] obtained significantly better results in the dewatering of activated sludge and the quality of the supernatant using cationic nanocellulose. Importantly, the small dose of nanocellulose, 1.0 kg/t DS, resulted in an 80% reduction in impurities in the supernatant, including turbidity and chemical oxygen demand (in the current studies, the dose was 0.02–1.4 kg/kg DS).

Table 5.
Parameters of the filtrate after dewatering sludge conditioned with cellulose.
An increase in the cellulose dose resulted in a very heterogeneous increase in thixotropy (Table 6). Compared to sludge prepared with PIX 113, the preparation with cellulose significantly increases the thixotropy of the sludge, and therefore, the resulting structures are very unstable. In addition, modelling shear stresses was very difficult. In most cases, the accuracy of the rheological models was not satisfactory (R2 < 0.5). The irregular results of rheological analysis can be explained by the strong dependence of rheological properties on the physical and chemical parameters of sludge, including dry solids, pH, temperature and protein content in the sludge [55,56]. In the case of the conducted research, the dosed reagents caused both a significant change in pH (PIX 113) and a very significant change in dry solids (dosing of cellulose and perlite at doses up to 32 g/L, 1.42 g/g DS).

Table 6.
Rheological characteristics of sludge conditioned in combination 2.
3.3. Research Results—Combination 3
Perlite conditioning was beneficial only after using the highest doses, i.e., 16.0 and 32.0 g/L (Figure 6). Comparatively, a perlite dose of 32.0 g/L allowed a reduction in CSK (235 s) and an increase in filtration speed (1.2 L/h), which corresponded to the use of the lowest doses of PIX 113 (2.0 and 4.0 mL/L).
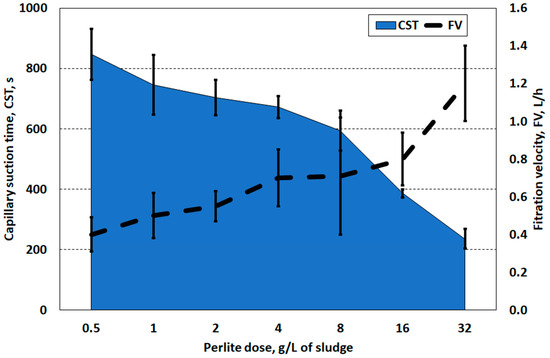
Figure 6.
Capillary suction time and filtration velocity of sludge prepared with perlite.
Filtration of sludge conditioned with perlite also did not show that the tested reagent could be a good structure-forming agent (Figure 7). Only the highest dose of perlite (32.0 g/L, 1.42 g/g DS) improved the solid phase separation efficiency. Only at this dose were acceptable parameters achieved, i.e., SRF = 2.59 × 1012 m/kg and FH = 79.8%. These values are similar to the effects of PIX 113 sludge conditioning at a dose of approximately 2.0–6.0 mL/L (0.13–0.40 g/g DS). For comparison, Xi et al. [57] showed that the use of flu ash in sludge conditioning at a dose of 10–15 g/g DS allows obtaining SRF = 1.03 × 1011 m/kg and FH = 74%. However, to achieve very low hydration of the dewatered sludge of 68%, a dose of 30 g/g DS was necessary. In the research of Qi et al. [58], the filtration efficiency of digested sludge conditioned with lignite was increased six times. However, it required a solid mass ratio to sludge of 1:1.
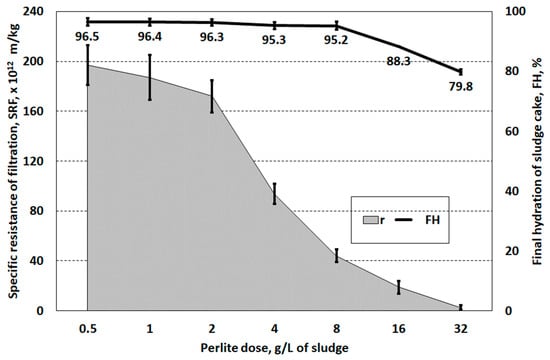
Figure 7.
Specific resistance of and hydration of the filter cake of sludge prepared with perlite.
There was no evidence that the addition of perlite had any chemical effect on the prepared sludge. The pH of the filtrate remained constant at about 5.6. Perlite conditioning, regardless of dose, increased filtrate turbidity (≈600 NTU). However, conductivity measurements of the filtrate showed that conditioning with perlite reduced the content of dissolved solids (Table 7).

Table 7.
Parameters of the filtrate after dewatering sludge conditioned with perlite.
The properties of perlite are a possible explanation for the changes in filtrate quality. Perlite does not react with the filtered liquid, can be crushed during mixing and works as an adsorbent [59,60].
The increase in the perlite dose was not correlated with the sludge thixotropy. The alternating increase and decrease in thixotropy may constitute the basis for determining the problems of rheological measurements, especially due to the heterogeneity of the composition and properties of sludge. The most accurate model describing the rheological properties of the tested sludge was the Ostwald model. Based on this model, it is only possible to determine the phenomenon of sediment thinning by shear (n < 1). However, these changes were not regular and independent of the increasing dose of the reagent (Table 8).

Table 8.
Rheological characteristics of sludge conditioned in combination 3.
Regardless of the conditioning method, the parameters characterising the sludge filtration process (CSK, FV, SRT, FH) were highly correlated, R > 0.75 (Table 9). In the case of sludge conditioning with PIX 113 coagulant, the filtrate parameters (pH, conductance and turbidity) were also found to be proportional to the changes in CSK, FV, SRT, and FH. Conditioning the sludge with cellulose, especially perlite, led to deterioration but also to a lack of correlation between the parameters characterising the filtration efficiency and the quality of the filtrate. The observed correlations may be the basis for the conclusion that the action of natural reagents leads to heterogeneous changes in the properties of the sludge. It can also be related to the non-uniform results of rheological analysis.

Table 9.
Correlation matrix of the examined parameters of conditioned and dewatered sewage sludge.
3.4. Research Results—Combination 4 and 5
Cellulose and perlite doses of 8.0 g/L and 16.0 g/L were selected for research on the dual conditioning of sludge. This choice is due to the fact that the action of cellulose and perlite can only be effective in large doses. In dual conditioning, the PIX 113 iron coagulant was used at a previously determined optimal dose of 6.0 mL/L.
Sequential dual conditioning of sludge with PIX 113 and cellulose or perlite (regardless of the order of dosing) did not result in favourable changes in CSK values. The independent use of PIX 113 coagulum at a dose of 6.0 mL/L was more effective in reducing capillary suction time (Figure 8). Similarly, the results obtained for vacuum filtration speed showed that the amount of filtrate during separation was lower than when using only PIX 113 (Figure 9).
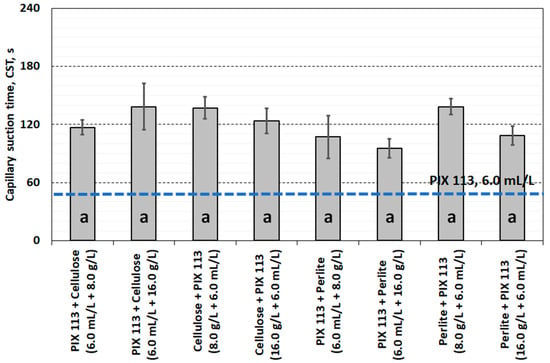
Figure 8.
Capillary suction time of dually conditioned sludge.
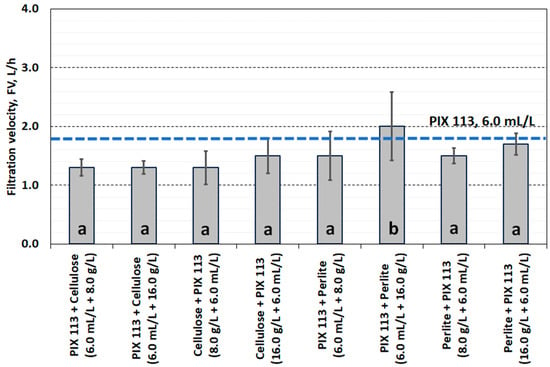
Figure 9.
Filtration velocity of dually conditioned sludge.
Conditioning the sludge with PIX 113 and cellulose (or in the reverse order) did not improve the filtration ability. The obtained SRF values (>5.0 × 1012 m/kg) do not qualify the conditioning method as justified for use. A more advantageous method of dual conditioning was the use of iron coagulant and perlite, especially at a dose of 16.0 g/L (Figure 10). Similar dependencies were observed in the hydration of the final filter cake. In dual conditioning with cellulose, FH values exceeded 80%. Replacing cellulose with perlite slightly reduced the water content in the filter cake to approximately 78% (Figure 11).
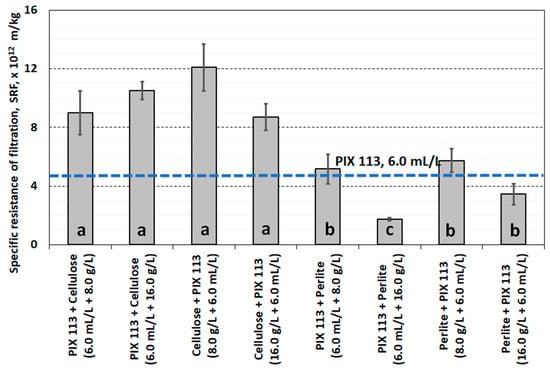
Figure 10.
Specific resistance of filtration of sludge conditioned PIX 113, cellulose and perlite.
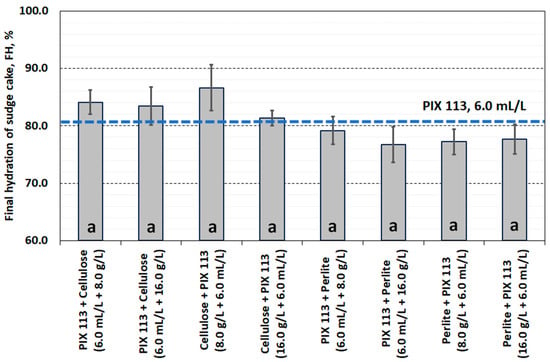
Figure 11.
Water content in the filter cake of sludge-conditioned PIX 113, cellulose and perlite.
The pH of the filtrate separated during dewatering on a vacuum filter was constant and independent of the combinations used and was approximately 4.6 (Table 10). This value corresponds to the pH of the filtrate using PIX 113 at a dose of 6.0 mL/L. The addition of PIX 113 and cellulose resulted in an increase in conductivity (average 240 μS/cm) compared to the interaction of PIX 113 alone (6.0 mL/L–140 μS/cm). Even higher conductance (>300 μS/cm) was recorded for sediment samples dually conditioned with coagulant and perlite. Filtrate turbidity was comparable for all conditioning combinations and ranged from 140 to 160 NTU. It can be mentioned that the turbidity of the filtrate after dual conditioning with perlite was approximately 40 NTU lower than for sludge conditioned individually with PIX 113 (Table 10).

Table 10.
Quality parameters of the filtrate after separation of dually conditioned sludge.
Statistical analysis of the filtration parameters of sludge and filtrate showed that there were no significant differences in the effects of using cellulose or perlite. Differences in dewatering efficiency were observed only in the case of specific resistance of filtration, which resulted from the use of cellulose (group a) or perlite (group b). Moreover, only the quality of the filtrate (conductivity, turbidity) after conditioning the sludge with the PIX 113 coagulant was different from the other combinations used. This was probably related to the change in the amount of dissolved particles, colloids and fine suspensions entering the filtrate as a result of conditioning the sludge with cellulose and perlite.
As in the previous combinations, the Oswald rheological model described the shear stresses most accurately. Based on the variables of this model, a lower flow behaviour index n was noted for sludge prepared with cellulose than with perlite. Thus, cellulose-conditioned sludge was more susceptible to the shear-thinning phenomenon. Moreover, increasing the dose of cellulose or perlite increased the n values, which limited the shear thinning phenomenon. The obtained n values were not greater than 0.40. Comparatively, at similar dry solids concentrations, in the studies by Wójcik and Stachowicz [61], n = 0.4–0.7 were obtained for sludge prepared with biomass ash. In the conducted research, but also in the research of Ruiz-Hernando et al. [62] and Cao et al. [63], Oswald’s rheological model was the most accurate. On the contrary, other authors [64,65] analysing the rheological properties of wastewater sludge indicated the Herschel and Bulkley model as more appropriate.
The dually conditioned sludge had greater thixotropy than the individually conditioned PIX 113 (Table 11 and Table 12). Additionally, an increased dose of cellulose or perlite (18.0 g/L) caused the sludge to be more susceptible to structure destruction by shear forces. It was also observed that higher thixotropy occurred when sludge was prepared in the PIX 113 + cellulose sequence than in the reverse order. The perlite dosing sequence in dual conditioning changed the thixotropy of the sludge in the opposite way (Table 11 and Table 12).

Table 11.
Rheological properties of sludge conditioned dually with PIX 113 and cellulose.

Table 12.
Rheological properties of sludge conditioned dually with PIX 113 and perlite.
The analysis of thixotropy, similar to the analysis of the rheological models, did not provide a basis for identifying clear trends. However, the characteristics of sludge, the method of conditioning and the specificity of rheological tests make them one of the most difficult measurements [66].
The filtrate after dewatering the conditioned sludge was also used to determine the zeta potential. An increase in the dose of iron coagulant resulted in a rapid reduction in zeta potential. Already at a PIX 113 dose of 4.0 mL/L, the zeta potential of filtrate particles was definitely close to the isoelectric point (Table 13). It is possible to associate the increase in zeta potential with the decomposition of negatively charged organic matter and the neutralisation of negative charges by positively charged iron ions [67,68]. Cellulose dosage resulted in an increase in potential, which could be related to the hydrophobicity of cellulose. Differently, dosing perlite reduced the zeta potential, although only by 4 mV compared to raw sludge. In dual conditioning, regardless of the type and dose of reagents used, the zeta potential ranged from 2.0 to 4.0 mV. The results presented indicate that in dual conditioning, the dosage and dose of the coagulant had the greatest impact on the potential reduction in zeta. Differences in zeta potential in dual conditioning combinations may result from changes in the pH of the solution and particle size changes due to the dosing of natural reagents [43,69].

Table 13.
Zeta potential determined in the filtrate after the separation process of single/dual conditioned sludge.
4. Conclusions
- Dosing the commercial iron coagulant PIX 113 iron coagulant was the most beneficial method of sludge conditioning. Therefore, the greatest influence on the dewatering effect was the conditioner dosage, which caused destabilisation and flocculation of the sludge particles.
- Cellulose and perlite cannot be described as structure-forming agents supporting dewatering. Sludge single conditioned with cellulose or perlite excessively bound water in the structure of the sludge and ultimately limited filtration.
- The combination of reagents (PIX 113, cellulose, perlite) in dual conditioning resulted in the deterioration of sludge dewatering. Dual conditioning can only be effective if a combination of factors whose single action (single conditioning) is beneficial for the technological properties of the sludge.
- Dewatering of single/dual conditioned sludge, regardless of the doses and combinations used, generated a highly contaminated filtrate. The pH, conductivity and turbidity of the filtrate indicated that the resulting filtrate may be a problematic substrate for further purification.
- The use of rheological tests as a tool useful in determining the properties of sludge, and especially the effectiveness of conditioning methods, was limited. A high variability of the rheological parameters and no dependence on the technological parameters of the sludge were observed. The conditioned sludge were structurally unstable substrates, and their properties significantly depended on changes in shear rate. A conclusion can be drawn regarding increasing the mixing gradient or homogenising the mixture of sludge, especially with cellulose or perlite. However, it is necessary to check whether such processes affect the efficiency of dewatering and the quality of rheological analyses.
- The zeta potential was consistent with sediment dewatering effects. It seems justified to use zeta potential measurements for the initial selection of conditioners and their mixtures with sludge prior to dewatering. Minimising the zeta potential could justify the use of types and doses of conditioners of a coagulant and sorbent nature. However, it is recognised that a practical assessment of the susceptibility of sludge to dewatering can only be made on the basis of key technological parameters such as specific resistance of filtration resistance and sludge cake hydration.
Author Contributions
T.K., conceptualisation, methodology, supervision, writing—review and edit; project administration; M.W., methodology, investigation, writing—review and edit; M.K., methodology, investigation, writing—review and edit; All authors have read and agreed to the published version of the manuscript.
Funding
The scientific research was funded by the statute subvention of Czestochowa University of Technology, Faculty of Infrastructure and Environment.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to a request requiring scientific justification.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Meng, X.; Huang, Q.; Xu, J.; Gao, H.; Yan, J. A review of phosphorus recovery from different thermal treatment products of sewage sludge. Waste Dispos. Sustain. Energy 2019, 1, 99–115. [Google Scholar] [CrossRef]
- Marin, E.; Rusanescu, C.O. Agricultural Use of Urban Sewage Sludge from the Wastewater Station in the Municipality of Alexandria in Romania. Water 2023, 15, 458. [Google Scholar] [CrossRef]
- Lamastra, L.; Suciu, N.A.; Trevisan, M. Sewage sludge for sustainable agriculture: contaminants’ contents and potential use as fertilizer. Chem. Biol. Technol. Agric. 2018, 5, 10. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, M.; Srivastava, S. Recent Advances in Wastewater Sludge Valorization. In Bio-Valorization of Waste: Trends and Perspectives; Shah, S., Venkatramanan, V., Prasad, R., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2021; Chapter 10; pp. 225–247. [Google Scholar]
- Shaddel, S.; Bakhtiary-Davijany, H.; Kabbe, C.; Dadgar, F.; Østerhus, S.W. Sustainable Sewage Sludge Management: From Current Practices to Emerging Nutrient Recovery Technologies. Sustainability 2019, 11, 3435. [Google Scholar] [CrossRef]
- Christodoulou, A.; Stamatelatou, K. Overview of legislation on sewage sludge management in developed countries worldwide. Water Sci. Technol. 2016, 73, 453–462. [Google Scholar] [CrossRef]
- Domini, M.; Bertanza, G.; Vahidzadeh, R.; Pedrazzani, R. Sewage Sludge Quality and Management for Circular Economy Opportunities in Lombardy. Appl. Sci. 2022, 12, 10391. [Google Scholar] [CrossRef]
- Hušek, M.; Moško, J.; Pohořelý, M. Sewage sludge treatment methods and P-recovery possibilities: Current state-of-the-art. J. Environ. Manag. 2022, 315, 115090. [Google Scholar] [CrossRef]
- Schnell, M.; Horst, T.; Quicker, P. Thermal treatment of sewage sludge in Germany: A review. J. Environ. Manag. 2020, 263, 110367. [Google Scholar] [CrossRef]
- Kwapinski, W.; Kolinovic, I.; Leahy, J.J. Sewage Sludge Thermal Treatment Technologies with a Focus on Phosphorus Recovery: A Review. Waste Biomass Valor. 2021, 12, 5837–5852. [Google Scholar] [CrossRef]
- Mowla, D.; Tran, H.N.; Allen, D.G. A review of the properties of biosludge and its relevance to enhanced dewatering processes. Biomass Bioenergy 2013, 58, 365–378. [Google Scholar] [CrossRef]
- Tian, G.; Shen, Y.; Hu, X.; Zhang, T.; Zhang, L.; Bian, B. The change of water content and role of microbe in the sludge drying process. J. Environ. Manag. 2021, 286, 112254. [Google Scholar] [CrossRef]
- Wu, B.; Dai, X.; Chai, X. Critical review on dewatering of sewage sludge: Influential mechanism, conditioning technologies and implications to sludge re-utilizations. Water Res. 2020, 180, 115912. [Google Scholar] [CrossRef]
- Wu, Y.J.; Xu, Y.; Zhang, X.D.; Lu, Y.T.; He, X.Y.; Song, B.J.; Zhang, Y.D.; Ji, J.W. Experimental study on treating landfill sludge by preconditioning combined with vacuum preloading: Effects of freeze-thaw and FeCl3 preconditioning. Sci. Total Environ. 2020, 747, 141092. [Google Scholar] [CrossRef]
- Mikkelsen, L.H.; Keiding, K. Physico-chemical characteristics of full scale sewage sludges with implications to dewatering. Water Res. 2002, 36, 2451–2462. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, H.H.P. Influences of extracellular polymeric substances (EPS) on flocculation, settling, and dewatering of activated sludge. Crit. Rev. Environ. Sci. Technol. 2003, 33, 237–273. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, B.; Wang, D.; Ma, T.; Yu, D. Variations in distribution and composition of extracellular polymeric substances (EPS) of biological sludge under potassium ferrate conditioning: Effects of pH and ferrate dosage. Biochem. Eng. J. 2016, 106, 37–47. [Google Scholar] [CrossRef]
- Wei, H.; Gao, B.; Ren, J.; Li, A.; Yang, H. Coagulation/flocculation in dewatering of sludge: A review. Water Res. 2018, 143, 608–631. [Google Scholar] [CrossRef]
- Burton, F.L.; Tchobanoglous, G.; Tsuchihashi, R.; Stensel, H.D.; Metcalf & Eddy, Inc. Wastewater Engineering: Treatment and Resource Recovery; McGraw-Hill Education: New York, NY, USA, 2013. [Google Scholar]
- Khazaie, A.; Mazarji, M.; Samali, B.; Osborne, D.; Minkina, T.; Sushkova, S.; Mandzhieva, S.; Soldatov, A. A Review on Coagulation/Flocculation in Dewatering of Coal Slurry. Water 2022, 14, 918. [Google Scholar] [CrossRef]
- Saveyn, H.; Pauwels, G.; Timmerman, R.; Van der Meeren, P. Effect of polyelectrolyte conditioning on the enhanced dewatering of activated sludge by application of an electric field during the expression phase. Water Res. 2005, 39, 3012–3020. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Zhang, T.; Zhang, W.; Wang, D. Enhanced technology based for sewage sludge deep dewatering: A critical review. Water Res. 2021, 189, 116650. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ru, S.; Yuan, C.; Wu, B.; Ji, Y.; Dai, Z.; Lei, Z.; Zhang, Z.; Yuan, T.; Li, F.; et al. An all-organic conditioning method to achieve deep dewatering of waste activated sludge and the underlying mechanism. J. Environ. Manag. 2023, 327, 116923. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, P.; Zeng, G.; Deng, J.; Zhou, Y.; Lu, H. Sewage sludge conditioning with coal fly ash modified by sulfuric acid. Chem. Eng. J. 2010, 158, 616–622. [Google Scholar] [CrossRef]
- Koul, B.; Bhat, N.; Abubakar, M.; Mishra, M.; Arukha, A.P.; Yadav, D. Application of Natural Coagulants in Water Treatment: A Sustainable Alternative to Chemicals. Water 2022, 14, 3751. [Google Scholar] [CrossRef]
- Netzer, C.; Løvås, T. Chemical Model for Thermal Treatment of Sewage Sludge. ChemEngineering 2022, 6, 16. [Google Scholar] [CrossRef]
- Parmar, K.R.; Brown, A.E.; Hammerton, J.M.; Camargo-Valero, M.A.; Fletcher, L.A.; Ross, A.B. Co-Processing Lignocellulosic Biomass and Sewage Digestate by Hydrothermal Carbonisation: Influence of Blending on Product Quality. Energies 2022, 15, 1418. [Google Scholar] [CrossRef]
- Shi, Q.; Lu, Y.; Guo, W.; Wang, T.; Zhu, Q.; Zhang, Y.; Li, C. Application of a cellulose filter aid in municipal sewage sludge dewatering and drying: Jar, pilot, and factory scale. Water Environ. Res. 2020, 92, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Ramirez, J.A.; Outram, J.G.; Dunn, K.; Jensen, P.D.; O’Hara, I.M.; Zhang, Z. Effects of lignocellulosic biomass type on the economics of hydrothermal treatment of digested sludge for solid fuel and soil amendment applications. Waste Manag. 2023, 156, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, K. Filters and Filtration Handbook, 5th ed.; Butterworth-Heinemann: Oxford, UK, 2008. [Google Scholar]
- Wu, C.; Li, Z.; Su, X.; Jia, Y.; Lu, X. Novel ultrafiltration membrane fouling control method with in-situ filter aid of perlite particles. Desalination Water Treat. 2016, 57, 5365–5375. [Google Scholar] [CrossRef]
- Wang, Z.; Jackson, L.S.; Jablonski, J.E. Factors Affecting the Levels of Heavy Metals in Juices Processed with Filter Aids. J. Food Prot. 2017, 80, 892–902. [Google Scholar] [CrossRef]
- Zafisaha, N.S.; Ang, W.L.; Johnson, D.J.; Mohammad, A.W.; Hilal, N. Effect of different filter aids used in cake filtration process on the removal of suspended solids in anaerobically digested palm oil mill effluent (POME). Desalination Water Treat. 2018, 110, 362–370. [Google Scholar] [CrossRef]
- Duan, J.; Gregory, J. Coagulation by hydrolysing metal salts. Adv. Colloid Interface Sci. 2003, 100–102, 475–502. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N. Removal of iron for safe drinking water. Desalination 2012, 303, 1–11. [Google Scholar] [CrossRef]
- Elías, F.A.; Marcos, A.G.; Bobadilla, M.C.; de Santo Domingo, E.D. Valorization of bio-waste for the removal of aluminum from industrial wastewater. J. Clean. Prod. 2020, 264, 121608. [Google Scholar] [CrossRef]
- Koch, R.; Noworyta, A. Mechanical Processes in Chemical Engineering; Scientific and Technical Publishing House: Warsaw, Poland, 1998. (In Polish) [Google Scholar]
- PN-EN 12880:2004; Characterization of Sewage Sludge—Determination of Dry Solids Residue and Water Content. Polish Committee for Standardization: Warsaw, Poland, 2004. (In Polish)
- PN-EN 12879:2004; Characterization of Sewage Sludge—Determination of Losses on Ignition of Dry Sludge Mass. Polish Committee for Standardization: Warsaw, Poland, 2004. (In Polish)
- PN-EN 14701-1:2007; Characterization of Sewage Sludge—Filtration Properties—Part 1: Capillary Suction Time (CST). Polish Committee for Standardization: Warsaw, Poland, 2007. (In Polish)
- PN-EN 14701-2:2013-07; Characterization of Sewage Sludge—Filtration Properties—Part 2: Determination of Specific Filtration Resistance. Polish Committee for Standardization: Warsaw, Poland, 2013.
- Ratkovich, N.; Horn, W.; Helmus, F.P.; Rosenberger, S.; Naessens, W.; Nopens, I.; Bentzen, T.R. Activated sludge rheology: A critical review on data collection and modeling. Water Res. 2013, 47, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, Q.; Zhang, W.; Yang, P.; Du, Y.; Wang, D. Highly effective enhancement of waste activated sludge dewaterability by altering proteins properties using methanol solution coupled with inorganic coagulants. Water Res. 2018, 138, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Lu, Y.T.; Yao, J.; Wu, Y.J.; Tran, Q.C.; Vu, Q.V. Insight into conditioning landfill sludge with ferric chloride and a Fenton reagent: Effects on the consolidation properties and advanced dewatering. Chemosphere 2020, 252, 126528. [Google Scholar] [CrossRef]
- Lotito, V.; Lotito, A.M. Rheological measurements on different types of sewage sludge for pumping design. J. Environ. Manag. 2014, 137, 189–196. [Google Scholar] [CrossRef]
- Wei, P.; Uijttewaal, W.; Lier, J.B.; Kreuk, M. Impacts of shearing and temperature on sewage sludge: Rheological characterisation and integration to flow assessment. Sci. Total Environ. 2021, 774, 145005. [Google Scholar] [CrossRef]
- Papa, M.; Pedrazzani, R.; Nembrini, S.; Bertanza, G. Should Rheological Properties of Activated Sludge be Measured? Appl. Rheol. 2015, 25, 24590. [Google Scholar] [CrossRef]
- Santos, A.F.; Ferreira, A.G.M.; Quina, M.J. Efficient Management of Sewage Sludge from Urban Wastewaters with the Addition of Inorganic Waste: Focus on Rheological Properties. Clean Technol. 2022, 4, 841–853. [Google Scholar] [CrossRef]
- Nilsson, M.; Mihranyan, A.; Valizadeh, S.; Strømme, M. Mesopore Structure of Microcrystalline Cellulose Tablets Characterized by Nitrogen Adsorption and SEM: The Influence on Water-Induced Ionic Conduction. J. Phys. Chem. B 2006, 110, 15776–15781. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Morita, S.; Ozaki, Y. A Study on Water Adsorption onto Microcrystalline Cellulose by Near-Infrared Spectroscopy with Two-Dimensional Correlation Spectroscopy and Principal Component Analysis. Appl. Spectrosc. 2006, 60, 054–1061. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Jing, S.R.; Lee, D.Y. Recycling of wood chips and wheat dregs for sludge processing. Bioresour. Technol. 2001, 76, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.R.; Lin, Y.F.; Lin, Y.M.; Hsu, C.S.; Huang, C.S.; Lee, D.Y. Evaluation of effective conditioners for enhancing sludge dewatering and subsequent detachment from filter cloth. J. Environ. Sci. Health A 1999, 34, 1517–1531. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, P.; Wu, Y. Enhanced technology for sewage sludge advanced dewatering from an engineering practice perspective: A review. J. Environ. Manag. 2022, 321, 115938. [Google Scholar] [CrossRef]
- Suopajärvi, T.; Sirviö, J.A.; Liimatainen, H. Cationic nanocelluloses in dewatering of municipal activated sludge. J. Environ. Chem. Eng. 2017, 5, 86–92. [Google Scholar] [CrossRef]
- Hong, E.; Yeneneh, A.M.; Sen, T.K.; Ang, H.M.; Kayaalp, A. A comprehensive review on rheological studies of sludge from various sections ofmunicipal wastewater treatment plants for enhancement of process performance. Adv. Colloid Interface Sci. 2018, 257, 19–30. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Carnevale Miino, M.; Bellazzi, S.; Caccamo, F.M.; Durante, A.; Abbà, A. Review of rheological behaviour of sewage sludge and its importance in the management of wastewater treatment plants. Water Pract. Technol. 2022, 17, 483–491. [Google Scholar] [CrossRef]
- Xia, J.; Rao, T.; Ji, J.; He, B.; Liu, A.; Sun, Y. Enhanced Dewatering of Activated Sludge by Skeleton-Assisted Flocculation Process. Int. J. Environ. Res. Public Health 2022, 19, 6540. [Google Scholar] [CrossRef]
- Qi, Y.; Thapa, K.B.; Hoadley, A.F.A. Benefit of lignite as a filter aid for dewatering of digested sewage sludge demonstrated in pilot scale trials. Chem. Eng. J. 2011, 166, 504–510. [Google Scholar] [CrossRef]
- Angelopoulos, P.M.; Maliachova, C.; Papakonstantinou, K.; Taxiarchou, M.; Diplas, S. Structural and physical characteristics of fine perlite expanded with a novel method in a vertical electric furnace. Miner. Process. Extr. Metall. 2016, 125, 71–80. [Google Scholar] [CrossRef]
- Lee, K.-S.; Lee, D.-S.; Lim, C.-S.; Lee, S.-P.; Yang, J.-E.; Chung, D.-Y. Water Retention Characteristics of Various Sizes of Expanded Perlite Produced from Two Different Types of Rocks. Horticulturae 2022, 8, 805. [Google Scholar] [CrossRef]
- Wójcik, M.; Stachowicz, F. Influence of sewage sludge conditioning with use of biomass ash on its rheological characteristics. Arch. Environ. Prot. 2019, 45, 92–102. [Google Scholar] [CrossRef]
- Ruiz-Hernando, M.; Martín-Díaz, J.; Labanda, J.; Mata-Alvarezc, J.; Llorens, J.; Lucena, F.; Astals, S. Effect of ultrasound, low-temperature thermal and alkali pre-treatments on waste activated sludge rheology, hygienization and methane potential. Water Res. 2014, 61, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Jiang, Z.; Cui, W.; Wang, Y.; Yang, P. Rheological properties of municipal sewage sludge: Dependency on solid concentration and temperature. Procedia Environ. Sci. 2019, 31, 113–121. [Google Scholar] [CrossRef]
- Baroutian, S.; Eshtiaghi, N.; Gapes, D.J. Rheology of a primary and secondary sewage sludge mixture: Dependency on temperature and solid concentration. Bioresour. Technol. 2013, 140, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Hii, K.; Parthasarathy, R.; Baroutian, S.; Gapes, D.J.; Eshtiaghi, N. Rheological measurements as a tool for monitoring the performance of highpressure and high temperature treatment of sewage sludge. Water Res. 2017, 114, 254–263. [Google Scholar] [CrossRef]
- Eshtiaghi, N.; Markis, F.; Yap, S.D.; Baudez, J.-C.; Slatter, P. Rheological characterisation of municipal sludge: A review. Water Res. 2013, 47, 5493–5510. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, Q.; Wang, Y.; Zhu, Y.; Fang, D.; Wu, G.; Zeng, Z.; Peng, H. Improved dewaterability of waste activated sludge by Fe(II)-activated potassium periodate oxidation. Int. J. Environ. Res. Public Health 2022, 19, 14726. [Google Scholar] [CrossRef]
- Shi, C.; Sun, W.; Sun, Y.; Chen, L.; Xu, Y.; Tang, M. Synthesis, Characterization, and Sludge Dewaterability Evaluation of the Chitosan-Based Flocculant CCPAD. Polymers 2019, 11, 95. [Google Scholar] [CrossRef]
- Wana, S.; Hanb, Y.; Zhoua, M.; Zhouc, X.; Chena, Y.; Donga, Y.; Huanga, F.; Luoa, T.; Zhengc, Y.; Hou, H. Improvement of sewage sludge dewaterability and immobilization of the heavy metals by using pretreated steel slag. Desalination Water Treat. 2020, 182, 135–143. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).