Renewable Energy Potential and CO2 Performance of Main Biomasses Used in Brazil
Abstract
1. Introduction
2. Biomass Potential in Brazil
3. Biomass Composition and Properties
Ash Composition and Ash Fusibility Trends
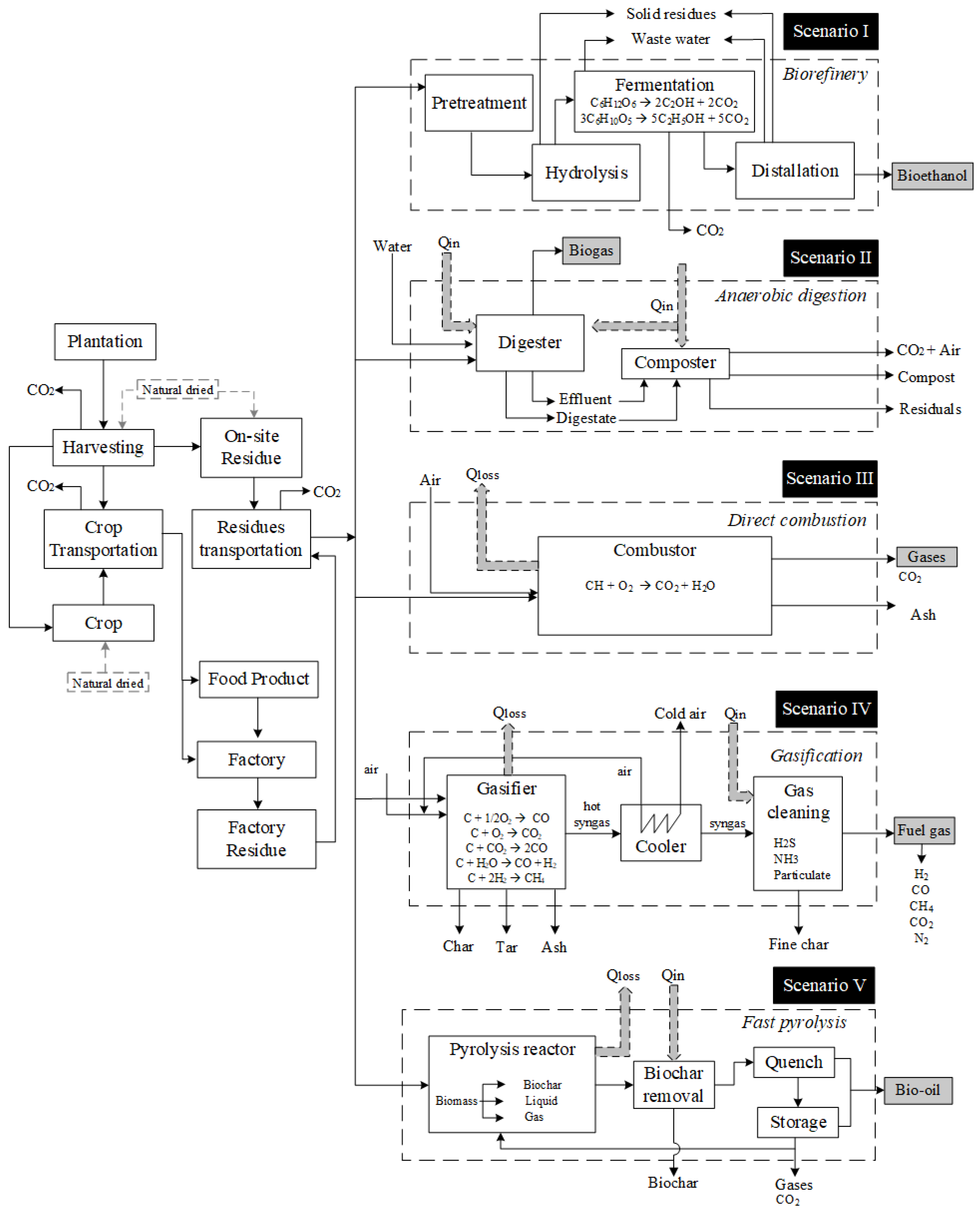
4. Conversion Technologies Routes
5. Carbon Potential
- (I)
- Biomass to bioethanol to replace gasoline;
- (II)
- Anaerobic digestion for biogas production;
- (III)
- Direct combustion for power generation;
- (IV)
- Gasification to replace natural gas;
- (V)
- Fast pyrolysis for bio-oil production as substitutes for fuel oil.
6. Summary, Conclusions and Outline
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IEA. International Energy Agency—Data and Statistics; IEA: Paris, France, 2022. [Google Scholar]
- Zou, C.; Xiong, B.; Xue, H.; Zheng, D.; Ge, Z.; Wang, Y.; Jiang, L.; Pan, S.; Wu, S. The role of new energy in carbon neutral. Pet. Explor. Dev. 2021, 48, 480–491. [Google Scholar] [CrossRef]
- Antar, M.; Lyu, D.; Nazari, M.; Shah, A.; Zhou, X.; Smith, D.L. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization. Renew. Sustain. Energy Rev. 2021, 139, 110691. [Google Scholar] [CrossRef]
- Kim, S.H.; Kumar, G.; Chen, W.H.; Khanal, S.K. Renewable hydrogen production from biomass and wastes (ReBioH2-2020). Bioresour. Technol. 2021, 331, 125024. [Google Scholar] [CrossRef]
- Edenhofer, O.; Madruga, R.P.; Sokona, Y.; Seyboth, K.; Matschoss, P.; Kadner, S.; Zwickel, T.; Eickemeier, P.; Hansen, G.; Schlömer, S.; et al. Renewable Energy Sources and Climate Change Mitigation: Special Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Agência Nacional de Energia Elétrica, Sistema de Informações de Geração da ANEEL-SIGA. 2023. Available online: https://www.gov.br/aneel/pt-br/ (accessed on 24 April 2023).
- Mendoza Martinez, C.L.; Alves Rocha, E.P.; Oliveira Carneiro, A.D.C.; Borges Gomes, F.J.; Ribas Batalha, L.A.; Vakkilainen, E.; Cardozo, M. Characterization of residual biomasses from the coffee production chain and assessment the potential for energy purposes. Biomass Bioenergy 2019, 120, 68–76. [Google Scholar] [CrossRef]
- Climate Watch. Historical GHG Emissions. 2021. Available online: https://www.climatewatchdata.org/ (accessed on 25 April 2023).
- Building an Environmental Powerhouse Brazil 2045—Volume 1—Environmental Policy Proposals for 2023–2024; Observatorio Do Clima: Rio de Janeiro. 2022. Available online: https://www.oc.eco.br/wp-content/uploads/2022/05/2045-EN%E2%80%94VF.pdf (accessed on 24 April 2023).
- Mapbiomas Brasil. Available online: https://mapbiomas.org/ (accessed on 24 April 2023).
- Tišma, M.; Bucić-Kojić, A.; Planinić, M. Bio-based Products from Lignocellulosic Waste Biomas. Chem. Biochem. Eng. Q 2021, 35, 139–156. [Google Scholar] [CrossRef]
- Usmani, Z.; Sharma, M.; Karpichev, Y.; Pandey, A.; Chandra Kuhad, R.; Bhat, R.; Punia, R.; Aghbashlo, M.; Tabatabaei, M.; Gupta, V.K. Advancement in valorization technologies to improve utilization of bio-based waste in bioeconomy context. Renew. Sustain. Energy Rev. 2020, 131, 109965. [Google Scholar] [CrossRef]
- Oasmaa, A.; Fonts, I.; Pelaez-Samaniego, M.R.; Garcia-Perez, M.E.; Garcia-Perez, M. Pyrolysis Oil Multiphase Behavior and Phase Stability: A Review. Energy Fuels 2016, 30, 6179–6200. [Google Scholar] [CrossRef]
- Anex, R.P.; Aden, A.; Kazi, F.K.; Fortman, J.; Swanson, R.M.; Wright, M.M.; Satrio, J.A.; Brown, R.C.; Daugaard, D.E.; Platon, A. Techno-economic comparison of biomass-to-transportation fuels via pyrolysis, gasification, and biochemical pathways. Fuel 2010, 89, S29–S35. [Google Scholar] [CrossRef]
- Statista. Agricultural Sector’s Share of GDP in Brazil 2020. 2022. Available online: https://www.statista.com/statistics/1075019/brazil-agriculture-share-gdp/ (accessed on 24 April 2023).
- IBÁ. Annual Report 2020 Brazilian Tree Industry; IBÁ: Sao Paulo, Brazil, 2020. [Google Scholar]
- IBA. Brazilian Tree Industry-Annual Report, Brazilian Tree Industry; IBÁ: Sao Paulo, Brazil, 2016. [Google Scholar]
- IBGE. Average Yield, per Harvest Year and Crop Yield; IBGE: Rio de Janeiro, Brazil, 2022. Available online: https://www.ibge.gov.br/ (accessed on 24 April 2023). (In Portuguese)
- FAOSTAT. Crops and Livestock Products. 2022. Available online: https://www.fao.org/faostat/en/#data/ (accessed on 24 April 2023).
- IBGE. Agriculture and Livestock Census 2017; IBGE: Rio de Janeiro, Brazil, 2018. Available online: https://www.ibge.gov.br/ (accessed on 24 April 2023).
- Ullah, K.; Kumar Sharma, V.; Dhingra, S.; Braccio, G.; Ahmad, M.; Sofia, S. Assessing the lignocellulosic biomass resources potential in developing countries: A critical review. Renew. Sustain. Energy Rev. 2015, 51, 682–698. [Google Scholar] [CrossRef]
- Singh, J.; Panesar, B.S.; Sharma, S.K. Energy potential through agricultural biomass using geographical information system—A case study of Punjab. Biomass Bioenergy 2008, 32, 301–307. [Google Scholar] [CrossRef]
- CONAB. Brazilian Grain Harvest; Ministry of Agriculture, Livestock and Supply: Manaus, Brazil, 2021. [Google Scholar]
- Sruamsiri, S. Agricultural wastes as dairy feed in Chiang Mai. Anim. Sci. J. 2007, 78, 335–341. [Google Scholar] [CrossRef]
- Roozen, A. Availability of Sustainable Lignocellulosic Biomass Residues in Brazil for Export to the EU; Universiteit Utrecht: Utrecht, The Netherlands, 2015. [Google Scholar]
- Ferreira-Leitao, V.; Gottschalk, L.M.F.; Ferrara, M.A.; Nepomuceno, A.L.; Molinari, H.B.C.; Bon, E.P.S. Biomass residues in Brazil: Availability and potential uses. Waste Biomass Valorization 2010, 1, 65–76. [Google Scholar] [CrossRef]
- Meena, S.K.; Sahu, R.; Ayothiraman, R. Utilization of Waste Wheat Straw Fibers for Improving the Strength Characteristics of Clay. J. Nat. Fibers 2019, 18, 1404–1418. [Google Scholar] [CrossRef]
- CONAB. Brazilian Coffee Harvest; Ministry of Agriculture, Livestock and Supply: Manaus, Brazil, 2021. [Google Scholar]
- De Castro Pereira Brainer, M.S. Coconut: Production and market. Cad Setorial ETENE 2021, 206, 1–13. (In Portuguese) [Google Scholar]
- CONAB. Brazilian Sugarcane Harvest; Ministry of Agriculture, Livestock and Supply: Manaus, Brazil, 2021. [Google Scholar]
- IBGE. Systematic Survey of Agricultural Production; IBGE: Rio de Janeiro, Brazil, 2022. Available online: https://www.ibge.gov.br/ (accessed on 24 April 2023).
- Wadhwa, M.; Bakshi, M.P.S.; Makkar, H.P.S. Waste to worth: Fruit wastes and by-products as animal feed. CAB Rev. 2015, 2015, 10. [Google Scholar] [CrossRef]
- CitrusBR. Brazilian Orange Juice: En Route to Sustainability; CitrusBR: São Paulo, Brazil, 2012; Available online: https://issuu.com/citrusbr/docs/folder_citrus__ingles_vers_o_site (accessed on 25 April 2023).
- Bundhoo, Z.M.A. Potential of bio-hydrogen production from dark fermentation of crop residues: A review. Int. J. Hydrog. Energy 2019, 44, 17346–17362. [Google Scholar] [CrossRef]
- World Agricultural production. World Rice Production by Country 2022/2023. 2022. Available online: http://www.worldagriculturalproduction.com/crops/rice.aspx/ (accessed on 5 February 2023).
- Ahmad, S.; Zhu, X.; Wei, X.; Zhang, S. Influence of process parameters on hydrothermal modification of soybean residue: Insight into the nutrient, solid biofuel, and thermal properties of hydrochars. J. Environ. Manag. 2021, 283, 111981. [Google Scholar] [CrossRef]
- USDA. Production, Supply and Distribution—Market and Trade Data; USDA: Washington, D.C., USA, 2023. [Google Scholar]
- Ratna, A.S.; Ghosh, A.; Mukhopadhyay, S. Advances and prospects of corn husk as a sustainable material in composites and other technical applications. J. Clean. Prod. 2022, 371, 133563. [Google Scholar] [CrossRef]
- Aghaei, S.; Karimi Alavijeh, M.; Shafiei, M.; Karimi, K. A comprehensive review on bioethanol production from corn stover: Worldwide potential, environmental importance, and perspectives. Biomass Bioenergy 2022, 161, 106447. [Google Scholar] [CrossRef]
- Zhao, Y.; Damgaard, A.; Christensen, T.H. Bioethanol from corn stover—A review and technical assessment of alternative biotechnologies. Prog. Energy Combust. Sci. 2018, 67, 275–291. [Google Scholar] [CrossRef]
- FAO. Land use in agriculture by the numbers. Sustainable Food and Agriculture. Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/ (accessed on 24 April 2023).
- Pighinelli, A.L.M.T.; Boateng, A.A.; Mullen, C.A.; Elkasabi, Y. Evaluation of Brazilian biomasses as feedstocks for fuel production via fast pyrolysis. Energy Sustain. Dev. 2014, 21, 42–50. [Google Scholar] [CrossRef]
- ANP. Oil, Natural Gas and Biofuels Statistical Yearbook 2022—Português (Brasil); ANP: Brasilia, DF, Brazil, 2022. [Google Scholar]
- Mendoza Martinez, C.L.; Saari, J.; Melo, Y.; Cardoso, M.; de Almeida, G.M.; Vakkilainen, E. Evaluation of thermochemical routes for the valorization of solid coffee residues to produce biofuels: A Brazilian case. Renew. Sustain. Energy Rev. 2021, 137, 110585. [Google Scholar] [CrossRef]
- Pereira, B.S.; de Freitas, C.; Vieira, R.M.; Brienzo, M. Brazilian banana, guava, and orange fruit and waste production as a potential biorefinery feedstock. J. Mater. Cycles Waste Manag. 2022, 24, 2126–2140. [Google Scholar] [CrossRef]
- Mendes, F.B.; Delmondes, K.L.; Hassan, D.; Barros, J.H.T. Economic, Social and Environmental Perspectives on Organic Residues from Brazilian Amazonian Fruits (Acre). In Proceedings of the World Congress on Engineering and Computer Science, San Francisco, CA, USA, 22–24 October 2019. [Google Scholar]
- Redondo-Gómez, C.; Quesada, M.R.; Astúa, S.V.; Zamora, J.P.M.; Lopretti, M.; Vega-Baudrit, J.R. Biorefinery of Biomass of Agro-Industrial Banana Waste to Obtain High-Value Biopolymers. Molecules 2020, 25, 3829. [Google Scholar] [CrossRef]
- Anniwaer, A.; Chaihad, N.; Zhang, M.; Wang, C.; Yu, T.; Kasai, Y.; Abudala, A.; Guan, G. Hydrogen-rich gas production from steam co-gasification of banana peel with agricultural residues and woody biomass. Waste Manag. 2021, 125, 204–214. [Google Scholar] [CrossRef]
- Sawarkar, A.N.; Kirti, N.; Tagade, A.; Tekade, S.P. Bioethanol from various types of banana waste: A review. Bioresour. Technol. Rep. 2022, 18, 101092. [Google Scholar] [CrossRef]
- Prastuti, O.P.; Septiani, E.L.; Kurniati, Y.; Widiyastuti, S.H. Banana Peel Activated Carbon in Removal of Dyes and Metals Ion in Textile Industrial Waste. Mater. Sci. Forum 2019, 966, 204–209. [Google Scholar] [CrossRef]
- Umaru, I.J.; Umaru, H.A.; Umaru, K.I. Extraction of essential oils from coconut agro-industrial waste. In Extraction of Natural Products from Agro-industrial Wastes, 1st ed.; Elservier: New Delhi, India, 2023; pp. 303–318. [Google Scholar] [CrossRef]
- De la Torre, I.; Martin-Dominguez, V.; Acedos, M.G.; Esteban, J.; Santos, V.E.; Ladero, M. Utilisation/upgrading of orange peel waste from a biological biorefinery perspective. Appl. Microbiol. Biotechnol. 2019, 103, 5975–5991. [Google Scholar] [CrossRef]
- Biel-Nielsen, T.L.; Li, K.; Sørensen, S.O.; Sejberg, J.J.P.; Meyer, A.S.; Holck, J. Utilization of industrial citrus pectin side streams for enzymatic production of human milk oligosaccharides. Carbohydr. Res. 2022, 519, 108627. [Google Scholar] [CrossRef]
- Christofi, A.; Tsipiras, D.; Malamis, D.; Moustakas, K.; Mai, S.; Barampouti, E.M. Biofuels production from orange juice industrial waste within a circular economy vision. J. Water Process. Eng. 2022, 49, 103028. [Google Scholar] [CrossRef]
- Simões, L.M.S.; Setter, C.; Sousa, N.G.; Cardoso, C.R.; de Oliveira, T.J.P. Biomass to biofuel densification of coconut fibers: Kinetic triplet and thermodynamic evaluation. Biomass Convers. Biorefinery 2022, 12, 1–18. [Google Scholar] [CrossRef]
- Brookes, G. Farm income and production impacts from the use of genetically modified (GM) crop technology 1996-2020. GM Crops Food 2022, 13, 171–195. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Hydrothermal gasification of soybean straw and flax straw for hydrogen-rich syngas production: Experimental and thermodynamic modeling. Energy Convers. Manag. 2020, 208, 112545. [Google Scholar] [CrossRef]
- Xian, S.; Xu, Q.; Feng, Y. Simultaneously remove organic pollutants and improve pyrolysis gas quality during the co-pyrolysis of soybean straw and oil shale. J. Anal. Appl. Pyrolysis 2022, 167, 105665. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Gan, Z.; Zhuang, X.; Ba, Y. Comparative study of electric-heating torrefaction and solar-driven torrefaction of biomass: Characterization of property variation and energy usage with torrefaction severity. Appl. Energy Combust. Sci. 2022, 9, 100051. [Google Scholar] [CrossRef]
- Leng, S.; Li, W.; Han, C.; Chen, L.; Chen, J.; Fan, L.; Lu, Q.; Li, J.; Leng, L.; Zhou, W. Aqueous phase recirculation during hydrothermal carbonization of microalgae and soybean straw: A comparison study. Bioresour. Technol. 2020, 298, 122502. [Google Scholar] [CrossRef]
- Jiang, Y.; Havrysh, V.; Klymchuk, O.; Nitsenko, V.; Balezentis, T.; Streimikiene, D. Utilization of Crop Residue for Power Generation: The Case of Ukraine. Sustainability 2019, 11, 7004. [Google Scholar] [CrossRef]
- Namsaraev, Z.B.; Gotovtsev, P.M.; Komova, A.V.; Vasilov, R.G. Current status and potential of bioenergy in the Russian Federation. Renew. Sustain. Energy Rev. 2018, 81, 625–634. [Google Scholar] [CrossRef]
- Vijay, V.; Kapoor, R.; Singh, P.; Hiloidhari, M.; Ghosh, P. Sustainable utilization of biomass resources for decentralized energy generation and climate change mitigation: A regional case study in India. Environ. Res. 2022, 212, 113257. [Google Scholar] [CrossRef]
- Krička, T.; Matin, A.; Voća, N.; Pospišil, A.; Grubor, M.; Šaronja, I.; Jurišić, V. Changes in nutritional and energy properties of soybean seed and hull after roasting. Res. Agric. Eng. 2018, 64, 96–103. [Google Scholar] [CrossRef]
- Toro-Trochez, J.L.; DE Haro Del Río, D.A.; Sandoval-Rangel, L.; Bustos-Martínez, D.; García-Mateos, F.J.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T.; Carrillo-Pedraza, E.S. Catalytic fast pyrolysis of soybean hulls: Focus on the products. J. Anal. Appl. Pyrolysis 2022, 163, 105492. [Google Scholar] [CrossRef]
- Fitri Faradilla, R.H.; Lucia, L.; Hakovirta, M. Hydrothermal carbonization of soybean hulls for the generation of hydrochar: A promising valorization pathway for low value biomass. Environ Nanotechnology. Monit. Manag. 2021, 16, 100571. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chen, W.R.; Chiueh, P.T.; Kuan, W.H.; Lo, S.L. Microwave torrefaction of rice straw and pennisetum. Bioresour. Technol. 2012, 123, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, W.; Chen, W.H.; Ho, S.H.; Pétrissans, A.; Pétrissans, M. Effect of torrefaction on the structure and reactivity of rice straw as well as life cycle assessment of torrefaction process. Energy 2022, 240, 122470. [Google Scholar] [CrossRef]
- Kai, X.; Meng, Y.; Yang, T.; Li, B.; Xing, W. Effect of torrefaction on rice straw physicochemical characteristics and particulate matter emission behavior during combustion. Bioresour. Technol. 2019, 278, 122730. [Google Scholar] [CrossRef]
- Chen, C.; Qu, B.; Wang, W.; Wang, W.; Ji, G.; Li, A. Rice husk and rice straw torrefaction: Properties and pyrolysis kinetics of raw and torrefied biomass. Environ. Technol. Innov. 2021, 24, 101872. [Google Scholar] [CrossRef]
- Chen, D.; Chen, F.; Cen, K.; Cao, X.; Zhang, J.; Zhou, J. Upgrading rice husk via oxidative torrefaction: Characterization of solid, liquid, gaseous products and a comparison with non-oxidative torrefaction. Fuel 2020, 275, 117936. [Google Scholar] [CrossRef]
- Naqvi, M.; Yan, J.; Dahlquist, E.; Naqvi, S.R. Off-grid electricity generation using mixed biomass compost: A scenario-based study with sensitivity analysis. Appl. Energy 2017, 201, 363–370. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, Z.; Wang, Z.; Ma, C.; Chen, S. A novel on-site wheat straw pretreatment method: Enclosed torrefaction. Bioresour. Technol. 2019, 281, 48–55. [Google Scholar] [CrossRef]
- Bai, X.; Wang, G.; Sun, Y.; Yu, Y.; Liu, J.; Wang, D.; Wang, Z. Effects of combined pretreatment with rod-milled and torrefaction on physicochemical and fuel characteristics of wheat straw. Bioresour. Technol. 2018, 267, 38–45. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Z.; Coward, B.; Wang, J.; Tian, H.; Yin, Y.; Cheng, Y. Improvement of wheat (T. aestivum) straw catalytic fast pyrolysis for valuable chemicals production by coupling pretreatment of acid washing and torrefaction. Ind. Crop. Prod. 2022, 187, 115475. [Google Scholar] [CrossRef]
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of agricultural biomass residues: Comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Gautam, S. Physicochemical Performance of Wood Chips Char and Wheat Husk Char for Utilisation as an Alternate Source of Energy. Int. J. Recent Technol. Eng. 2020, 8, 2876–2880. [Google Scholar] [CrossRef]
- Rajkumar, P.; Murugavelh, S. Co-pyrolysis of wheat husk and residual tyre: Techno-economic analysis, performance and emission characteristics of pyro oil in a diesel engine. Bioresour. Technol. Rep. 2022, 19, 101164. [Google Scholar] [CrossRef]
- Brlek, T.; Bodroza-Solarov, M.; Vuckovic, J.; Levic, J. Utilization of Spelt Wheat Hull as a Renewable Energy Source by Pelleting758 Agricultural Academy. Bulg. J. Agric. Sci. 2012, 18, 752. [Google Scholar]
- Santos, J.; Ouadi, M.; Jahangiri, H.; Hornung, A. Integrated intermediate catalytic pyrolysis of wheat husk. Food Bioprod. Process. 2019, 114, 23–30. [Google Scholar] [CrossRef]
- Han, S.; Bai, L.; Chi, M.; Xu, X.; Chen, Z.; Yu, K. Conversion of Waste Corn Straw to Value-Added Fuel via Hydrothermal Carbonization after Acid Washing. Energies 2022, 15, 1828. [Google Scholar] [CrossRef]
- Liu, Y.; Rokni, E.; Yang, R.; Ren, X.; Sun, R.; Levendis, Y.A. Torrefaction of corn straw in oxygen and carbon dioxide containing gases: Mass/energy yields and evolution of gaseous species. Fuel 2021, 285, 119044. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, S.; Zhang, X.; Liu, H.; Sun, B.; Guo, S. Influence of air oxidative and non-oxidative torrefaction on the chemical properties of corn stalk. Bioresour. Technol. 2021, 332, 125120. [Google Scholar] [CrossRef]
- Chen, D.; Cen, K.; Cao, X.; Li, Y.; Zhang, Y.; Ma, H. Restudy on torrefaction of corn stalk from the point of view of deoxygenation and decarbonization. J. Anal. Appl. Pyrolysis 2018, 135, 85–93. [Google Scholar] [CrossRef]
- Maj, G.; Szyszlak-Bargłowicz, J.; Zajac, G.; Słowik, T.; Krzaczek, P.; Piekarski, W. Energy and Emission Characteristics of Biowaste from the Corn Grain Drying Process. Energies 2019, 12, 4383. [Google Scholar] [CrossRef]
- Walmsley, T.G.; Varbanov, P.S.; Su, R.; Klemeš, J.J.; Tippayawong, N.; Rerkkriangkrai, P.; Aggarangsi, P.; Pattiya, A. Characterization of Biochar from Pyrolysis of Corn Residues in a Semi-continuous Carbonizer. Chem. Eng. Trans. 2018, 70. [Google Scholar] [CrossRef]
- Klaas, M.; Greenhalf, C.; Ouadi, M.; Jahangiri, H.; Hornung, A.; Briens, C.; Aggarangsi, P.; Pattiya, A. The effect of torrefaction pre-treatment on the pyrolysis of corn cobs. Results Eng. 2020, 7, 100165. [Google Scholar] [CrossRef]
- Phuakpunk, K.; Chalermsinsuwan, B.; Assabumrungrat, S. Comparison of chemical reaction kinetic models for corn cob pyrolysis. Energy Rep. 2020, 6, 168–178. [Google Scholar] [CrossRef]
- Setter, C.; Silva, F.T.M.; Assis, M.R.; Ataíde, C.H.; Trugilho, P.F.; Oliveira, T.J.P. Slow pyrolysis of coffee husk briquettes: Characterization of the solid and liquid fractions. Fuel 2020, 261, 116420. [Google Scholar] [CrossRef]
- Mukherjee, A.; Okolie, J.A.; Niu, C.; Dalai, A.K. Experimental and Modeling Studies of Torrefaction of Spent Coffee Grounds and Coffee Husk: Effects on Surface Chemistry and Carbon Dioxide Capture Performance. ACS Omega 2022, 7, 638–653. [Google Scholar] [CrossRef]
- Tadesse, Y.; Kassahun, S.K.; Kiflie, Z. Effects of operational parameters on torrefaction performance of coffee husk and cotton stalk mixed biomass: A surface response methodology approach. Biomass. Convers. Biorefinery 2021, 2021, 1–16. [Google Scholar] [CrossRef]
- Singh, R.K.; Pandey, D.; Patil, T.; Sawarkar, A.N. Pyrolysis of banana leaves biomass: Physico-chemical characterization, thermal decomposition behavior, kinetic and thermodynamic analyses. Bioresour. Technol. 2020, 310, 123464. [Google Scholar] [CrossRef]
- Alves, J.L.F.; da Silva, J.C.G.; Sellin, N.; de Prá, F.B.; Sapelini, C.; Souza, O.; Marangoni, C. Upgrading of banana leaf waste to produce solid biofuel by torrefaction: Physicochemical properties, combustion behaviors, and potential emissions. Environ. Sci. Pollut. Res. 2021, 29, 25733–25747. [Google Scholar] [CrossRef]
- Mosqueda, A.; Medrano, K.; Gonzales, H.; Takahashi, F.; Yoshikawa, K. Hydrothermal treatment of banana leaves for solid fuel combustion. Biofuels 2019, 12, 1123–1129. [Google Scholar] [CrossRef]
- Espinosa, E.; Tarrés, Q.; Domínguez-Robles, J.; Delgado-Aguilar, M.; Mutjé, P.; Rodríguez, A. Recycled fibers for fluting production: The role of lignocellulosic micro/nanofibers of banana leaves. J. Clean Prod. 2018, 172, 233–238. [Google Scholar] [CrossRef]
- Taib, R.M.; Abdullah, N.; Aziz, N.S.M. Bio-oil derived from banana pseudo-stem via fast pyrolysis process. Biomass Bioenergy 2021, 148, 106034. [Google Scholar] [CrossRef]
- Rambo, M.K.D.; Schmidt, F.L.; Ferreira, M.M.C. Analysis of the lignocellulosic components of biomass residues for biorefinery opportunities. Talanta 2015, 144, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, G.; Nges, I.A.; Deng, L.; Nistor, M.; Liu, J. Fresh banana pseudo-stems as a tropical lignocellulosic feedstock for methane production. Energy Sustain. Soc. 2016, 6, 27. [Google Scholar] [CrossRef]
- Prawisudha, P.; Azka, G.R.; Triyono, B.; Pasek, A.D. Multi-production of solid fuel and liquid fertilizer from organic waste by employing wet torrefaction process. AIP Conf. Proc. 2018, 1984, 30008. [Google Scholar] [CrossRef]
- Virmond, E.; De Sena, R.F.; Albrecht, W.; Althoff, C.A.; Moreira, R.F.P.M.; José, H.J. Characterisation of agroindustrial solid residues as biofuels and potential application in thermochemical processes. Waste Manag. 2012, 32, 1952–1961. [Google Scholar] [CrossRef]
- Alves, J.L.F.; da Trindade, E.O.; da Silva, J.C.G.; Mumbach, G.D.; Alves, R.F.; Barbosa Filho, J.M.; De Athayde-Filho, P.F.; De Sena, R.F. Lignocellulosic Residues from the Brazilian Juice Processing Industry as Novel Sustainable Sources for Bioenergy Production: Preliminary Assessment Using Physicochemical Characteristics. J. Braz. Chem. Soc. 2020, 31, 1939–1948. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Biswas, A.B. Pyrolysis of orange bagasse: Comparative study and parametric influence on the product yield and their characterization. J. Environ. Chem. Eng. 2019, 7, 102903. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Biswas, A.B. Physicochemical analysis and kinetic study of orange bagasse at higher heating rates. Fuel 2020, 271, 117642. [Google Scholar] [CrossRef]
- Bardone, E.; Bravi, M.; Keshavarz, T.; Zanella, K.; Gonçalves, J.L.; Taranto, O.P. Charcoal Briquette Production Using Orange Bagasse and Corn Starch. Chem. Eng. Trans. 2016, 49, 313–318. [Google Scholar] [CrossRef]
- Jerzak, W.; Kuźnia, M. Examination of inorganic gaseous species and condensed phases during coconut husk combustion based on thermodynamic equilibrium predictions. Renew. Energy 2021, 167, 497–507. [Google Scholar] [CrossRef]
- Nakason, K.; Pathomrotsakun, J.; Kraithong, W.; Khemthong, P.; Panyapinyopol, B. Torrefaction of Agricultural Wastes: Influence of Lignocellulosic Types and Treatment Temperature on Fuel Properties of Biochar. Int. Energy J. 2019, 19, 253–266. [Google Scholar]
- Wang, Q.; Sarkar, J. Pyrolysis behaviors of waste coconut shell and husk biomasses. Int. J. Energy Prod. Manag. 2018, 3, 34–43. [Google Scholar] [CrossRef]
- Lee, Y.T.; Ng, H.K.; Gan, S.; Jourabchi, S.A. Thermochemical upgrading of coconut husk and rubber seed to coal co-firing feedstock via torrefaction. IOP Conf. Ser. Earth Environ. Sci. 2019, 354, 12074. [Google Scholar] [CrossRef]
- Meriño Stand, L.; Valencia Ochoa, G.; Duarte Forero, J. Energy and exergy assessment of a combined supercritical Brayton cycle-orc hybrid system using solar radiation and coconut shell biomass as energy source. Renew. Energy 2021, 175, 119–142. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmahdi, S.M.; Mohamed, A.R.; Abidin, C.Z.A.; Kasim, N.N. Pretreatment of Coconut Shell by Torrefaction for Pyrolysis Conversion. IOP Conf. Ser. Earth Environ. Sci. 2021, 920, 12002. [Google Scholar] [CrossRef]
- Da Silva, J.C.G.; Alves, J.L.F.; de Araujo Galdino, W.V.; de Sena, R.F.; Andersen, S.L.F. Pyrolysis kinetics and physicochemical characteristics of skin, husk, and shell from green coconut wastes. Energy Ecol. Environ. 2019, 4, 125–132. [Google Scholar] [CrossRef]
- Akogun, O.A.; Waheed, M.A. Property Upgrades of Some Raw Nigerian Biomass through Torrefaction Pre-Treatment- A Review. J. Phys. Conf. Ser. 2019, 1378, 32026. [Google Scholar] [CrossRef]
- Nanda, S.; Isen, J.; Dalai, A.K.; Kozinski, J.A. Gasification of fruit wastes and agro-food residues in supercritical water. Energy Convers. Manag. 2016, 110, 296–306. [Google Scholar] [CrossRef]
- Toscano Miranda, N.; Lopes Motta, I.; Maciel Filho, R.; Wolf Maciel, M.R. Sugarcane bagasse pyrolysis: A review of operating conditions and products properties. Renew. Sustain. Energy Rev. 2021, 149, 111394. [Google Scholar] [CrossRef]
- Canettieri, E.V.; da Silva, V.P.; Neto, T.G.S.; Hernández-Pérez, A.F.; da Silva, D.D.V.; Dussán, K.J.; Maria de Carvalho, J.A. Physicochemical and thermal characteristics of sugarcane straw and its cellulignin. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 416. [Google Scholar] [CrossRef]
- dos Reis Ferreira, R.A.; da Silva Meireles, C.; Assunção, R.M.N.; Reis Soares, R. Heat required and kinetics of sugarcane straw pyrolysis by TG and DSC analysis in different atmospheres. J. Therm. Anal. Calorim. 2018, 132, 1535–1544. [Google Scholar] [CrossRef]
- Halder, P.; Kundu, S.; Patel, S.; Parthasarathy, R.; Pramanik, B.; Paz-Ferreiro, J.; Maria de Carvalho, J.A. TGA-FTIR study on the slow pyrolysis of lignin and cellulose-rich fractions derived from imidazolium-based ionic liquid pre-treatment of sugarcane straw. Energy Convers. Manag. 2019, 200, 112067. [Google Scholar] [CrossRef]
- Schmitt, C.C.; Moreira, R.; Neves, R.C.; Richter, D.; Funke, A.; Raffelt, K.; Shah, K. From agriculture residue to upgraded product: The thermochemical conversion of sugarcane bagasse for fuel and chemical products. Fuel Process Technol. 2020, 197, 106199. [Google Scholar] [CrossRef]
- Kanwal, S.; Chaudhry, N.; Munir, S.; Sana, H. Effect of torrefaction conditions on the physicochemical characterization of agricultural waste (sugarcane bagasse). Waste Manag 2019, 88, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Manatura, K. Inert torrefaction of sugarcane bagasse to improve its fuel properties. Case Stud. Therm. Eng. 2020, 19, 100623. [Google Scholar] [CrossRef]
- Da Veiga, P.A.S.; Cerqueira, M.H.; Gonçalves, M.G.; da Matos, T.T.S.; Pantano, G.; Schultz, J.; De Andrade, J.D.; Mangrich, A.S. Upgrading from batch to continuous flow process for the pyrolysis of sugarcane bagasse: Structural characterization of the biochars produced. J. Environ. Manag. 2021, 285, 112145. [Google Scholar] [CrossRef]
- Pena-Vergara, G.; Castro, L.R.; Gasparetto, C.A.; Bizzo, W.A. Energy from planted forest and its residues characterization in Brazil. Energy 2022, 239, 122243. [Google Scholar] [CrossRef]
- Rocha, E.P.A.; Gomes, F.J.B.; Sermyagina, E.; Cardoso, M.; Colodette, J.L. Analysis of Brazilian Biomass Focusing on Thermochemical Conversion for Energy Production. Energy Fuels 2015, 29, 7975–7984. [Google Scholar] [CrossRef]
- Silva, F.T.M.; Ataíde, C.H. Valorization of eucalyptus urograndis wood via carbonization: Product yields and characterization. Energy 2019, 172, 509–516. [Google Scholar] [CrossRef]
- Sette, C.R.; Hansted, A.L.S.; Novaes, E.; Lima, P.A.F.; Rodrigues, A.C.; de Santos, D.R.S.; Yamaji, F.M. Energy enhancement of the eucalyptus bark by briquette production. Ind. Crop. Prod. 2018, 122, 209–213. [Google Scholar] [CrossRef]
- de Paula Protásio, T.; Scatolino, M.V.; de Araújo, A.C.C.; de Oliveira, A.F.C.F.; de Figueiredo, I.C.R.; de Assis, M.R.; Trugilho, P.M. Assessing Proximate Composition, Extractive Concentration, and Lignin Quality to Determine Appropriate Parameters for Selection of Superior Eucalyptus Firewood. BioEnergy Res. 2019, 12, 626–641. [Google Scholar] [CrossRef]
- Loxley, T.A. Within-Tree Fuel Quality of Loblolly Pine (Pinus taeda); Auburn University: Auburn, AL, USA, 2018. [Google Scholar]
- Acquah, G.E.; Via, B.K.; Gallagher, T.; Billor, N.; Fasina, O.O.; Eckhardt, L.G. High Throughput Screening of Elite Loblolly Pine Families for Chemical and Bioenergy Traits with Near Infrared Spectroscopy. Forests 2018, 9, 418. [Google Scholar] [CrossRef]
- Park, J.; Hung, I.; Gan, Z.; Rojas, O.J.; Lim, K.H.; Park, S. Activated carbon from biochar: Influence of its physicochemical properties on the sorption characteristics of phenanthrene. Bioresour. Technol. 2013, 149, 383–389. [Google Scholar] [CrossRef]
- Pacheco, J.M.; Lima, R.; Sehgal, D.; Anderson, R.; Mangueira, F.; Coelho, S.T.; Filho, A.F. Quantification analysis unravels significance of residual biomass of Pinus taeda L. Aust. J. Basic Appl. Sci. 2020, 14, 1–9. [Google Scholar] [CrossRef]
- Tanger, P.; Field, J.L.; Jahn, C.E.; DeFoort, M.W.; Leach, J.E. Biomass for thermochemical conversion: Targets and challenges. Front. Plant Sci. 2013, 4, 218. [Google Scholar] [CrossRef]
- Demirbas, A. Thermochemical Conversion Processes. In Green Energy Technologies, 1st ed.; Springe: London, UK, 2009; pp. 261–304. [Google Scholar] [CrossRef]
- Brito, F.M.S.; Paes, J.B.; da Oliveira, J.T.S.; Arantes, M.D.C.; Neto, H.F. Anatomical and Physical Characterization of the Giant Bamboo (Dendrocalamus giganteus Munro) (In Portuguese). Floresta Ambient 2015, 22, 559–566. [Google Scholar] [CrossRef]
- Leoncio Paiva, H.; Neutzling Bierhals, A.; dos Santos Guimarrães, V.; Carlos Marafon, A.; Dias Santiago, A.; Felipe Câmara Amaral, A. Drying of elephant grass biomass for direct combustion. (In Portuguese). In Proceedings of the Congresso Acadêmico Integrado de Inovação e Tecnologia—CAIITE, Alagoas, Brazil, 8–10 November 2016. [Google Scholar]
- dos Santos, G.R.V.; Jankowsky, I.P.; de Andrade, A. Characteristic drying curve for Eucalyptus grandis lumber. Sci. For. 2003, 63, 214–220. [Google Scholar]
- Fernandes, E.R.K.; Marangoni, C.; Souza, O.; Sellin, N. Thermochemical characterization of banana leaves as a potential energy source. Energy Convers. Manag. 2013, 75, 603–608. [Google Scholar] [CrossRef]
- Mendoza Martinez, C.L.; Sermyagina, E.; Saari, J.; Silva de Jesus, M.; Cardoso, M.; Matheus de Almeida, G.; Vakkilainen, E. Hydrothermal carbonization of lignocellulosic agro-forest based biomass residues. Biomass Bioenergy 2021, 147, 106004. [Google Scholar] [CrossRef]
- Van Swaaij, W.P.; Kersten, S.R.; Palz, W. Biomass Power for the World; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Heinek, S.; Polanz, S.; Huber, M.B.; Hofmann, A.; Monthaler, G.; Fuchs, H.P.; Larch, C.; Giovannini, A. Biomass Conditioning Degradation Of Biomass During The Storage Of Woodchips. In Proceedings of the 21st European Biomass Conference and Exhibition, Copenhagen, Denmark, 3–7 June 2013. [Google Scholar]
- Obernberger, I.; Brunner, T.; Bärnthaler, G. Chemical properties of solid biofuels—Significance and impact. Biomass Bioenergy 2006, 30, 973–982. [Google Scholar] [CrossRef]
- Wzorek, M. Solar drying of granulated waste blends for dry biofuel production. Environ. Sci. Pollut. Res. 2021, 28, 34290–34299. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Vo, D.V.N. Recent advances and sustainable development of biofuels production from lignocellulosic biomass. Bioresour. Technol. 2022, 344, 126203. [Google Scholar] [CrossRef] [PubMed]
- Vedovatto, F.; Ugalde, G.; Bonatto, C.; Bazoti, S.F.; Treichel, H.; Mazutti, M.A.; Zabot, G.L.; Tres, M.V. Subcritical water hydrolysis of soybean residues for obtaining fermentable sugars. J. Supercrit. Fluids 2021, 167, 105043. [Google Scholar] [CrossRef]
- Šelo, G.; Planinić, M.; Tišma, M.; Tomas, S.; Koceva Komlenić, D.; Bucić-Kojić, A. A Comprehensive Review on Valorization of Agro-Food Industrial Residues by Solid-State Fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef]
- Robles Barros, P.J.; Ramirez Ascheri, D.P.; Siqueira Santos, M.L.; Morais, C.C.; Ramirez Ascheri, J.L.; Signini, R.; dos Santos, D.M.; de Campos, A.J.; Alessandro Devilla, I. Soybean hulls: Optimization of the pulping and bleaching processes and carboxymethyl cellulose synthesis. Int. J. Biol. Macromol. 2020, 144, 208–218. [Google Scholar] [CrossRef]
- Debiagi, F.; Faria-Tischer, P.C.S.; Mali, S. Nanofibrillated cellulose obtained from soybean hull using simple and eco-friendly processes based on reactive extrusion. Cellul 2019, 27, 1975–1988. [Google Scholar] [CrossRef]
- Toro-Trochez, J.L.; Carrillo-Pedraza, E.S.; Bustos-Martínez, D.; García-Mateos, F.J.; Ruiz-Rosas, R.R.; Rodríguez-Mirasol, J.; Cordero, T. Thermogravimetric characterization and pyrolysis of soybean hulls. Bioresour. Technol. Rep. 2019, 6, 183–189. [Google Scholar] [CrossRef]
- Osorio, L.L.D.R.; Flórez-López, E.; David Grande-Tovar, C.; Flórez-López, E.; Grande-Tovar, C.D.; Trombetta, D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef]
- da Silva Vilar, D.; Cruz, I.A.; Torres, N.H.; Figueiredo, R.T.; de Melo, L.; de Resende, I.T.F.; Eguiluz, K.I.B.; Bharagava, R.N.; Ferreira, L.F.R. Agro-industrial Wastes: Environmental Toxicology, Risks, and Biological Treatment Approaches. In Microorganisms for Sustainability; Bharagava, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–23. [Google Scholar] [CrossRef]
- Romaní, A.; Rocha, C.M.R.; Michelin, M.; Domingues, L.; Teixeira, J.A. Valorization of Lignocellulosic-Based Wastes. In Current Developments in Biotechnology and Bioengineering: Resource Recovery from Wastes; Elservier: Amsterdam, The Netherlands, 2020; pp. 383–410. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Mamun, A.A.; Volk, J. Physical, chemical and surface properties of wheat husk, rye husk and soft wood and their polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 480–488. [Google Scholar] [CrossRef]
- Sobhy, M.S.; Tammam, M.T. The influence of fiber length and concentration on the physical properties of wheat husk fibers rubber composites. Int. J. Polym. Sci. 2010, 2010, 528173. [Google Scholar] [CrossRef]
- Terzioğlu, P.; Yücel, S.; Kuş, Ç. Review on a novel biosilica source for production of advanced silica-based materials: Wheat husk. Asia-Pac. J. Chem. Eng. 2019, 14, e2262. [Google Scholar] [CrossRef]
- Ahmad, S.; Iqbal, Y.; Muhammad, R. Effects of coal and wheat husk additives on the physical, thermal and mechanical properties of clay bricks. Boletín Soc Española Cerámica Vidr. 2017, 56, 131–138. [Google Scholar] [CrossRef]
- Wang, P.; Liu, C.; Chang, J.; Yin, Q.; Huang, W.; Liu, Y.; Dang, X.; Gao, T.; Lu, F. Effect of physicochemical pretreatments plus enzymatic hydrolysis on the composition and morphologic structure of corn straw. Renew. Energy 2019, 138, 502–508. [Google Scholar] [CrossRef]
- Othman, S.H.; Najhah Tarmiti, N.A.; Shapi’i, R.A.; Mohd Zahiruddin, S.M.; Amin Tawakkal, I.S.M.; Basha, R.K. Starch/banana pseudostem biocomposite films for potential food packaging applications. BioResources 2020, 15, 3984–3998. [Google Scholar] [CrossRef]
- Mohamad, N.A.N.; Jai, J. Response surface methodology for optimization of cellulose extraction from banana stem using NaOH-EDTA for pulp and papermaking. Heliyon 2022, 8, e09114. [Google Scholar] [CrossRef]
- Alvarez, J.; Hooshdaran, B.; Cortazar, M.; Amutio, M.; Lopez, G.; Freire, F.B.; Haghshenasfard, M.; Hosseini, S.H.; Olazar, M. Valorization of citrus wastes by fast pyrolysis in a conical spouted bed reactor. Fuel 2018, 224, 111–120. [Google Scholar] [CrossRef]
- Mantovan, J.; Yamashita, F.; Mali, S. Modification of Orange Bagasse with Reactive Extrusion to Obtain Cellulose-Based Materials. Polysaccharides 2022, 3, 401–410. [Google Scholar] [CrossRef]
- Gonçalves, F.A.; Ruiz, H.A.; Nogueira, C.D.C.; dos Santos, E.S.; Teixeira, J.A.; De Macedo, G.R. Comparison of delignified coconuts waste and cactus for fuel-ethanol production by the simultaneous and semi-simultaneous saccharification and fermentation strategies. Fuel 2014, 131, 66–76. [Google Scholar] [CrossRef]
- Azeta, O.; Ayeni, A.O.; Agboola, O.; Elehinafe, F.B. A review on the sustainable energy generation from the pyrolysis of coconut biomass. Sci. Afr. 2021, 13, e00909. [Google Scholar] [CrossRef]
- Batista, G.; Souza, R.B.A.; Pratto, B.; dos Santos-Rocha, M.S.R.; Cruz, A.J.G. Effect of severity factor on the hydrothermal pretreatment of sugarcane straw. Bioresour. Technol. 2019, 275, 321–327. [Google Scholar] [CrossRef]
- Halysh, V.; Sevastyanova, O.; de Carvalho, D.M.; Riazanova, A.V.; Lindström, M.E.; Gomelya, M. Effect of oxidative treatment on composition and properties of sorbents prepared from sugarcane residues. Ind. Crop. Prod. 2019, 139, 111566. [Google Scholar] [CrossRef]
- Formann, S.; Hahn, A.; Janke, L.; Stinner, W.; Sträuber, H.; Logroño, W.; Nikolausz, M. Beyond Sugar and Ethanol Production: Value Generation Opportunities Through Sugarcane Residues. Front. Energy Res. 2020, 8, 267. [Google Scholar] [CrossRef]
- Loaiza, J.M.; Palma, A.; Díaz, M.J.; Ruiz-Montoya, M.; García, M.T.; García, J.C. Effect of autohydrolysis on hemicellulose extraction and pyrolytic hydrogen production from Eucalyptus urograndis. Biomass Convers. Biorefinery 2020, 12, 4021–4030. [Google Scholar] [CrossRef]
- da Silva Morais, A.P.; Sansígolo, C.A.; de Oliveira Neto, M. Effects of autohydrolysis of Eucalyptus urograndis and Eucalyptus grandis on influence of chemical components and crystallinity index. Bioresour. Technol. 2016, 214, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Negrão, D.R.; da Silva, T.A.Ô.F., Jr.; de Passos, J.R.S.; Sansígolo, C.A.; de Minhoni, M.T.A.; Furtado, E.L. Biodegradation of eucalyptus urograndis wood by fungi. Int. Biodeterior. Biodegrad. 2014, 89, 95–102. [Google Scholar] [CrossRef]
- de Medeiros, L.C.D.; Pimenta, A.S.; Braga, R.M.; de Carnaval, T.K.A.; Neto, P.N.M.; de Melo, D.M.A. Effect of pyrolysis heating rate on the chemical composition of wood vinegar from Eucalyptus Urograndis and Mimosa Tenuiflora. Rev. Árvore 2019, 43, 1–11. [Google Scholar] [CrossRef]
- Kandhola, G.; Djioleu, A.; Rajan, K.; Labbé, N.; Sakon, J.; Carrier, D.J.; Kim, J.W. Maximizing production of cellulose nanocrystals and nanofibers from pre-extracted loblolly pine kraft pulp: A response surface approach. Bioresour. Bioprocess 2020, 7, 19. [Google Scholar] [CrossRef]
- Huang, F.; Ragauskas, A. Extraction of hemicellulose from loblolly pine woodchips and subsequent kraft pulping. Ind. Eng. Chem. Res. 2013, 52, 1743–1749. [Google Scholar] [CrossRef]
- Lengowski, E.C.; De Muñiz, G.I.B.; Klock, U.; Nisgoski, S. Potential use of NIR and visible spectroscopy to analyze chemical properties of thermally treated wood. Maderas Cienc Tecnol 2018, 20, 627–640. [Google Scholar] [CrossRef]
- de Morais, S.A.L.; do Nascimento, E.A.; de Melo, D.C. Chemical analysis of Pinus oocarpa wood part I: Quantification of macromolecular components and volatile extractives. Rev. Árvore 2005, 29, 461–470. [Google Scholar] [CrossRef]
- Rajan, K.; Djioleu, A.; Kandhola, G.; Labbé, N.; Sakon, J.; Carrier, D.J.; Kim, J.W. Investigating the effects of hemicellulose pre-extraction on the production and characterization of loblolly pine nanocellulose. Cellul 2020, 27, 3693–3706. [Google Scholar] [CrossRef]
- Febrero, L.; Granada, E.; Regueiro, A.; Míguez, J.L. Influence of Combustion Parameters on Fouling Composition after Wood Pellet Burning in a Lab-Scale Low-Power Boiler. Energies 2015, 8, 9794–9816. [Google Scholar] [CrossRef]
- Pronobis, M. Evaluation of the influence of biomass co-combustion on boiler furnace slagging by means of fusibility correlations. Biomass Bioenergy 2005, 28, 375–383. [Google Scholar] [CrossRef]
- Teixeira, P.; Lopes, H.; Gulyurtlu, I.; Lapa, N.; Abelha, P. Evaluation of slagging and fouling tendency during biomass co-firing with coal in a fluidized bed. Biomass Bioenergy 2012, 39, 192–203. [Google Scholar] [CrossRef]
- Yin, H.B.; Yao, M. Analysis of the nonuniform slag film, mold friction, and the new cracking criterion for round billet continuous casting. Metall. Mater. Trans. B 2005, 36, 857–864. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, G.; Xu, Z.; Sun, H.; Lam, P.K.S. The retention mechanism, transformation behavior and environmental implication of trace element during co-combustion coal gangue with soybean stalk. Fuel 2017, 189, 32–38. [Google Scholar] [CrossRef]
- Seckler, D.; Dea, C.M.; Rios, E.A.M.; de Godoi, M.; da Rampon, D.S.; D’Oca, M.G.M.; D’Oca, C.R.M. Rice straw ash extract/glycerol: An efficient sustainable approach for Knoevenagel condensation. New. J. Chem. 2022, 46, 4570–4578. [Google Scholar] [CrossRef]
- Shen, X.; Zeng, J. Prediction of rice straw ash fusion behaviors and improving its ash fusion properties by layer manure addition. J. Mater. Cycles Waste Manag. 2020, 22, 965–974. [Google Scholar] [CrossRef]
- Kwan, W.H.; Wong, Y.S. Acid leached rice husk ash (ARHA) in concrete: A review. Mater. Sci. Energy Technol. 2020, 3, 501–507. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Song, Y.C.; Li, W.Y.; Feng, J. Ash contents and ash-forming elements of biomass and their significance for solid biofuel combustion. Fuel 2017, 208, 377–409. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, H.; Wang, X.; Du, W.; Mikulčić, H.; Duić, N. Study on extracting available salt from straw/woody biomass ashes and predicting its slagging/fouling tendency. J. Clean. Prod. 2017, 155, 164–171. [Google Scholar] [CrossRef]
- Ma, Q.; Han, L.; Huang, G. Evaluation of different water-washing treatments effects on wheat straw combustion properties. Bioresour. Technol. 2017, 245, 1075–1083. [Google Scholar] [CrossRef]
- Sharma, V.; Rathore, P.K.; Sharma, A. Soil Stabilization by Using Wheat Husk Ash. J. Civ. Eng. Environ. Technol. 2018, 5, 31–35. [Google Scholar]
- Song, X.; Lin, Z.; Bie, R.; Wang, W. Effects of Additives Blended in Corn Straw to Control Agglomeration and Slagging in Combustion. BioResources 2019, 14, 8963–8972. [Google Scholar] [CrossRef]
- Atahu, M.K.; Saathoff, F.; Gebissa, A. Strength and compressibility behaviors of expansive soil treated with coffee husk ash. J. Rock. Mech. Geotech. Eng. 2019, 11, 337–348. [Google Scholar] [CrossRef]
- Kanning, R.C.; Portella, K.F.; Bragança, M.O.G.P.; Bonato, M.M.; Dos Santos, J.C.M. Banana leaves ashes as pozzolan for concrete and mortar of Portland cement. Constr. Build. Mater. 2014, 54, 460–465. [Google Scholar] [CrossRef]
- Yathushan, V.; Puswewala, U.G.A. Effectiveness of Pozzolanic Leaf Ashes and Plastics on Geotechnical Characteristics. Int. J. Eng. Technol. Innov. 2022, 12, 155–166. [Google Scholar] [CrossRef]
- Torres de Sande, V.; Sadique, M.; Pineda, P.; Bras, A.; Atherton, W.; Riley, M. Potential use of sugar cane bagasse ash as sand replacement for durable concrete. J. Build. Eng. 2021, 39, 102277. [Google Scholar] [CrossRef]
- Rizvi, T.; Xing, P.; Pourkashanian, M.; Darvell, L.I.; Jones, J.M.; Nimmo, W. Prediction of biomass ash fusion behaviour by the use of detailed characterisation methods coupled with thermodynamic analysis. Fuel 2015, 141, 275–284. [Google Scholar] [CrossRef]
- Birley, R.I.; Jones, J.M.; Darvell, L.I.; Williams, A.; Waldron, D.J.; Levendis, Y.A.; Rokni, E.; Panahi, A. Fuel flexible power stations: Utilisation of ash co-products as additives for NOx emissions control. Fuel 2019, 251, 800–807. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Bohn, L.R.; Dresch, A.P.; Cavali, M.; Vargas, A.C.G.; Führ, J.F.; Tironi, S.P.; Fogolari, O.; Mibielli, G.M.; Alves Jr, S.L.; Bender, J.P. Alkaline pretreatment and enzymatic hydrolysis of corn stover for bioethanol production. Res. Soc. Dev. 2021, 10, e149101118914. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L. Technologies for Biochemical Conversion of Biomass; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Nanda, S.; Kozinski, J.A.; Dalai, A.K. Lignocellulosic Biomass: A Review of Conversion Technologies and Fuel Products. Curr. Biochem. Eng. 2016, 3, 24–36. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Adams, P.; Bridgwater, T.; Lea-Langton, A.; Ross, A.; Watson, I. Biomass Conversion Technologies. Greenh Gas. Balanc. Bioenergy Syst., 1st ed.; Elservier: London, UK, 2018; pp. 107–139. [Google Scholar] [CrossRef]
- Soares, J.F.; Confortin, T.C.; Todero, I.; Luft, L.; Ugalde, G.A.; Tovar, L.P.; Mayer, F.D.; Mazutti, M.A. Estimation of Bioethanol, Biohydrogen, and Chemicals Production from Biomass Wastes in Brazil. Clean—Soil Air Water 2022, 50, 2200155. [Google Scholar] [CrossRef]
- Kim, S. Evaluation of Alkali-Pretreated Soybean Straw for Lignocellulosic Bioethanol Production. Int. J. Polym. Sci. 2018, 2018, 5241748. [Google Scholar] [CrossRef]
- Govumoni, S.P.; Koti, S.; Kothagouni, S.Y.; Venkateshwar, S.; Linga, V.R. Evaluation of pretreatment methods for enzymatic saccharification of wheat straw for bioethanol production. Carbohydr. Polym. 2013, 91, 646–650. [Google Scholar] [CrossRef]
- Rabeya, T.; Jehadin, F.; Asad, M.A.; Ayodele, O.O.; Adekunle, A.E.; Islam, M.S. Alkali Intensified Heat Treat. Corn Stalk Bioethanol Production. Sugar Tech. 2020, 23, 643–650. [Google Scholar] [CrossRef]
- Gouvea, B.M.; Torres, C.; Franca, A.S.; Oliveira, L.S.; Oliveira, E.S. Feasibility of ethanol production from coffee husks. Biotechnol. Lett. 2009, 31, 1315–1319. [Google Scholar] [CrossRef]
- Menezes, E.G.T.; do Carmo, J.R.; Alves, J.G.L.F.; Menezes, A.G.T.; Guimarães, I.C.; Queiroz, F.; Pimenta, C.J. Optimization of alkaline pretreatment of coffee pulp for production of bioethanol. Biotechnol. Prog. 2014, 30, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Suhag, M.; Kumar, A.; Singh, J. Saccharification and fermentation of pretreated banana leaf waste for ethanol production. SN Appl. Sci. 2020, 2, 1448. [Google Scholar] [CrossRef]
- Uchôa, P.Z.; Porto, R.C.T.; Battisti, R.; Marangoni, C.; Sellin, N.; Souza, O. Ethanol from residual biomass of banana harvest and commercialization: A three-waste simultaneous fermentation approach and a logistic-economic assessment of the process scaling-up towards a sustainable biorefinery in Brazil. Ind. Crop. Prod. 2021, 174, 114170. [Google Scholar] [CrossRef]
- Nogueira, D.P.; Ferreira-Rosa, P.R.; Seolatto, A.A.; Galeano-Suarez, C.A.; Ferreira-Freitas, F. Sacarificación de bagazo de naranja pretratado con hidroxido de calcio usando un cóctel enzimático y acido diluido. Rev. ION 2019, 32, 75–85. [Google Scholar] [CrossRef]
- Saadatinavaz, F.; Karimi, K.; Denayer, J.F.M. Hydrothermal pretreatment: An efficient process for improvement of biobutanol, biohydrogen, and biogas production from orange waste via a biorefinery approach. Bioresour. Technol. 2021, 341, 125834. [Google Scholar] [CrossRef] [PubMed]
- Cabral, M.M.S.; de Abud, A.K.S.; de Silva, C.E.F.; Almeida, R.M.R.G. Bioethanol production from coconut husk fiber. Ciência Rural 2016, 46, 1872–1877. [Google Scholar] [CrossRef]
- Bronzato, G.R.F.; dos Reis, V.A.C.A.; Borro, J.A.; Leão, A.L.; Cesarino, I. Second generation ethanol made from coir husk under the biomass Cascade approach. Mol. Cryst. Liq. Cryst. 2020, 693, 107–114. [Google Scholar] [CrossRef]
- de Carvalho Silvello, M.A.; Martínez, J.; Goldbeck, R. Increase of reducing sugars release by enzymatic hydrolysis of sugarcane bagasse intensified by ultrasonic treatment. Biomass Bioenergy 2019, 122, 481–489. [Google Scholar] [CrossRef]
- Perez, C.L.; Pereira, L.P.R.d.C.; Milessi, T.S.; Sandri, J.P.; Demeke, M.; Foulquié-Moreno, M.R.; Thevelein, J.M.; Zangirolami, T.C. Towards a practical industrial 2G ethanol production process based on immobilized recombinant S. cerevisiae: Medium and strain selection for robust integrated fixed-bed reactor operation. Renew. Energy 2022, 185, 363–375. [Google Scholar] [CrossRef]
- Rochón, E.; Cabrera, M.N.; Scutari, V.; Cagno, M.; Guibaud, A.; Martínez, S.; Böthig, S.; Guchin, N.; Ferrari, M.D.; Lareo, C. Co-production of bioethanol and xylosaccharides from steam-exploded eucalyptus sawdust using high solid loads in enzymatic hydrolysis: Effect of alkaline impregnation. Ind. Crop. Prod. 2022, 175, 114253. [Google Scholar] [CrossRef]
- Oliveira, R.J.; Santos, B.; Mota, M.J.; Pereira, S.R.; Branco, P.C.; Pinto, P.C.R. The impact of acid hydrolysis conditions on carbohydrate determination in lignocellulosic materials: A case study with Eucalyptus globulus bark. Holzforschung 2021, 75, 957–967. [Google Scholar] [CrossRef]
- Farías-Sánchez, J.C.; Velázquez-Valadez, U.; Pineda-Pimentel, M.G.; López-Miranda, J.; Castro-Montoya, A.J.; Carrillo-Parra, A.; Vargas-Santillán, A.; Rutiaga-Quiñones, J.G. Simultaneous saccharification and fermentation of pine sawdust (Pinus pseudostrobus L.) pretreated with nitric acid and sodium hydroxide for bioethanol production. BioResources 2017, 12, 1052–1063. [Google Scholar] [CrossRef]
- Carrillo-Varela, I.; Vidal, C.; Vidaurre, S.; Parra, C.; Machuca, A.; Briones, R.; Mendonça, R.T. Alkalization of Kraft Pulps from Pine and Eucalyptus and Its Effect on Enzymatic Saccharification and Viscosity Control of Cellulose. Polymers 2022, 14, 3127. [Google Scholar] [CrossRef] [PubMed]
- Vedovatto, F.; Bonatto, C.; Bazoti, S.F.; Venturin, B.; Alves, S.L.; Kunz, A.; Steinmetz, R.L.R.; Treichel, H.; Mazutti, M.A.; Zabot, G.L.; et al. Production of biofuels from soybean straw and hull hydrolysates obtained by subcritical water hydrolysis. Bioresour. Technol. 2021, 328, 124837. [Google Scholar] [CrossRef]
- Rodrigues, B.C.G.; De Mello, B.S.; Gonsales Da Costa Araujo, M.L.; Ribeiro Da Silva, G.H.; Sarti, A. Soybean molasses as feedstock for sustainable generation of biomethane using high-rate anaerobic reactor. J. Environ. Chem. Eng. 2021, 9, 105226. [Google Scholar] [CrossRef]
- Nadaleti, W.C. Utilization of residues from rice parboiling industries in southern Brazil for biogas and hydrogen-syngas generation: Heat, electricity and energy planning. Renew. Energy 2019, 131, 55–72. [Google Scholar] [CrossRef]
- Leite, S.A.F.; Leite, B.S.; Ferreira, D.J.O.; Baêta, B.E.L.; Dangelo, J.V.H. The effects of agitation in anaerobic biodigesters operating with substrates from swine manure and rice husk. Chem. Eng. J. 2023, 451, 138533. [Google Scholar] [CrossRef]
- Simioni, T.; Agustini, C.B.; Dettmer, A.; Gutterres, M. Enhancement of biogas production by anaerobic co-digestion of leather waste with raw and pretreated wheat straw. Energy 2022, 253, 124051. [Google Scholar] [CrossRef]
- Albornoz, S.; Wyman, V.; Palma, C.; Carvajal, A. Understanding of the contribution of the fungal treatment conditions in a wheat straw biorefinery that produces enzymes and biogas. Biochem. Eng. J. 2018, 140, 140–147. [Google Scholar] [CrossRef]
- Vaz, A.; Mattos, A.P.; Nascimento, A.; Ana, V.; Mattos, P. Energy and carbon credits generation from the production of biogas from the ethanol stillage of corn and sugar cane. In Proceedings of the 18th Brazilian Congress of Thermal Science and Engineering, Online Event, Brazil, 8–10 December 2020. [Google Scholar]
- Venturin, B.; Frumi Camargo, A.; Scapini, T.; Mulinari, J.; Bonatto, C.; Bazoti, S.; Pereira-Siqueira, D.; Maria-Colla, L.; Alves, S.L.; Paulo-Bender, J.; et al. Effect of pretreatments on corn stalk chemical properties for biogas production purposes. Bioresour. Technol. 2018, 266, 116–124. [Google Scholar] [CrossRef]
- dos Santos, L.C.; Adarme, O.F.H.; Baêta, B.E.L.; Gurgel, L.V.A.; de Aquino, S.F. Production of biogas (methane and hydrogen) from anaerobic digestion of hemicellulosic hydrolysate generated in the oxidative pretreatment of coffee husks. Bioresour. Technol. 2018, 263, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Da Pin, B.V.R.; Barros, R.M.; Silva Lora, E.E.; Almazan del Olmo, O.; Silva dos Santos, I.F.; Ribeiro, E.M.; Victor de Freitas Rocha, J. Energetic use of biogas from the anaerobic digestion of coffee wastewater in southern Minas Gerais, Brazil. Renew. Energy 2020, 146, 2084–2094. [Google Scholar] [CrossRef]
- Benish, P.M.R.; Mozhiarasi, V.; Nagabalaji, V.; Weichgrebe, D.; Srinivasan, S.V. Optimization of process parameters for enhanced methane production from banana peduncle by thermal pretreatment. Biomass Convers. Biorefinery 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Oyaro, D.K.; Oonge, Z.I.; Odira, P.M. Anaerobic Digestion of Banana Wastes for Biogas Production. J. Civ. Environ. Eng. 2020, 10, 3. [Google Scholar] [CrossRef]
- Jiménez-Castro, M.P.; Buller, L.S.; Zoffreo, A.; Timko, M.T.; Forster-Carneiro, T. Two-stage anaerobic digestion of orange peel without pre-treatment: Experimental evaluation and application to São Paulo state. J. Environ. Chem. Eng. 2020, 8, 104035. [Google Scholar] [CrossRef]
- Echeverri, A.; Forero-Rojas, L.F.; Durán-Aranguren, D.; Carazzone, C.; Sierra, R. A Biorefinery for the Valorization of Orange Residues. In Proceedings of the 29th, European Biomass Conference and Exhibition, Online Event, 26–29 April 2021. [Google Scholar]
- Ndubuisi-Nnaji, U.U.; Ofon, U.A.; Offiong, N.A.O. Anaerobic co-digestion of spent coconut copra with cow urine for enhanced biogas production. Waste Manag. Res. 2020, 39, 594–600. [Google Scholar] [CrossRef]
- Cheng, J.R.; Liu, X.M.; Chen, Z.Y.; Zhang, Y.S.; Zhang, Y.H. A Novel Mesophilic Anaerobic Digestion System for Biogas Production and In Situ Methane Enrichment from Coconut Shell Pyroligneous. Appl. Biochem. Biotechnol. 2016, 178, 1303–1314. [Google Scholar] [CrossRef]
- Soares, L.A.; Solano, M.G.; Lindeboom, R.E.F.; van Lier, J.B.; Silva, E.L.; Varesche, M.B.A. Valorization of sugarcane bagasse through biofuel and value-added soluble metabolites production: Optimization of alkaline hydrothermal pretreatment. Biomass Bioenergy 2022, 165, 106564. [Google Scholar] [CrossRef]
- Silva Rabelo, C.A.B.; Camargo, F.P.; Sakamoto, I.K.; Varesche, M.B.A. Metataxonomic characterization of an autochthonous and allochthonous microbial consortium involved in a two-stage anaerobic batch reactor applied to hydrogen and methane production from sugarcane bagasse. Enzym. Microb. Technol. 2023, 162, 110119. [Google Scholar] [CrossRef]
- Poveda-Giraldo, J.A.; Cardona Alzate, C.A. Biorefinery potential of eucalyptus grandis to produce phenolic compounds and biogas. Can. J. Res. 2021, 51, 89–100. [Google Scholar] [CrossRef]
- Eftaxias, A.; Passa, E.A.; Michailidis, C.; Daoutis, C.; Kantartzis, A.; Diamantis, V. Residual Forest Biomass in Pinus Stands: Accumulation and Biogas Production Potential. Energies 2022, 15, 5233. [Google Scholar] [CrossRef]
- Ali, S.S.; Abomohra, A.E.F.; Sun, J. Effective bio-pretreatment of sawdust waste with a novel microbial consortium for enhanced biomethanation. Bioresour. Technol. 2017, 238, 425–432. [Google Scholar] [CrossRef]
- Armesto, L.; Bahillo, A.; Veijonen, K.; Cabanillas, A.; Otero, J. Combustion behaviour of rice husk in a bubbling fluidised bed. Biomass Bioenergy 2002, 23, 171–179. [Google Scholar] [CrossRef]
- Pottmaier, D.; Melo, C.R.; Sartor, M.N.; Kuester, S.; Amadio, T.M.; Fernandes, C.A.H.; Marinha, D.; Alarcon, O.E. The Brazilian energy matrix: From a materials science and engineering perspective. Renew. Sustain. Energy Rev. 2013, 19, 678–691. [Google Scholar] [CrossRef]
- Saenger, M.; Hartge, E.U.; Werther, J.; Ogada, T.; Siagi, Z. Combustion of coffee husks. Renew. Energy 2001, 23, 103–121. [Google Scholar] [CrossRef]
- de Oliveira Maia, B.G.; de Oliveira, A.P.N.; de Oliveira, T.M.N.; Marangoni, C.; Souza, O.; Sellin, N. Characterization and production of banana crop and rice processing waste briquettes. Environ. Prog. Sustain. Energy 2018, 37, 1266–1273. [Google Scholar] [CrossRef]
- De Oliveira Maia, B.G.; Souza, O.; Marangoni, C.; Hotza, D.; De Oliveira, A.P.N.; Sellin, N. Production and Characterization of Fuel Briquettes from Banana Leaves Waste. Chem. Eng. Trans. 2014, 37, 439–444. [Google Scholar] [CrossRef]
- Vamvuka, D.; Trikouvertis, M.; Pentari, D.; Alevizos, G. Evaluation of ashes produced from fluidized bed combustion of residues from oranges’ plantations and processing. Renew. Energy 2014, 72, 336–343. [Google Scholar] [CrossRef]
- Osman, A.I. Mass spectrometry study of lignocellulosic biomass combustion and pyrolysis with NOx removal. Renew. Energy 2020, 146, 484–496. [Google Scholar] [CrossRef]
- Obeng, G.Y.; Amoah, D.Y.; Opoku, R.; Sekyere, C.K.K.; Adjei, E.A.; Mensah, E. Coconut Wastes as Bioresource for Sustainable Energy: Quantifying Wastes, Calorific Values and Emissions in Ghana. Energies 2020, 13, 2178. [Google Scholar] [CrossRef]
- Abelha, P.; Leiser, S.; Pels, J.R.; Cieplik, M.K. Combustion properties of upgraded alternative biomasses by washing and steam explosion for complete coal replacement in coal-designed power plant applications. Energy 2022, 248, 123546. [Google Scholar] [CrossRef]
- Centeno-González, F.O.; Silva Lora, E.E.; Villa Nova, H.F.; Mendes Neto, L.J.; Martínez Reyes, A.M.; Ratner, A.; Ghamari, M. CFD modeling of combustion of sugarcane bagasse in an industrial boiler. Fuel 2017, 193, 31–38. [Google Scholar] [CrossRef]
- Aziz, I.; Bin Babar, Z.; Haider, R.; Saleem, M.; Munir, S.; Sattar, H. A comparative study of thermal and combustion kinetics for raw and bio-chars of eucalyptus wood and bark. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 3313–3329. [Google Scholar] [CrossRef]
- Guerrero, F.; Yáñez, K.; Vidal, V.; Cereceda-Balic, F. Effects of wood moisture on emission factors for PM2.5, particle numbers and particulate-phase PAHs from Eucalyptus globulus combustion using a controlled combustion chamber for emissions. Sci. Total Environ. 2019, 648, 737–744. [Google Scholar] [CrossRef]
- Xu, X.; Pan, R.; Chen, R. Combustion Characteristics, Kinetics, and Thermodynamics of Pine Wood Through Thermogravimetric Analysis. Appl. Biochem. Biotechnol. 2021, 193, 1427–1446. [Google Scholar] [CrossRef]
- Singh, D.; Tassew, D.D.; Nelson, J.; Chalbot, M.C.G.; Kavouras, I.G.; Demokritou, P.; Tesfaigzi, Y. Development of an Integrated Platform to Assess the Physicochemical and Toxicological Properties of Wood Combustion Particulate Matter. Chem. Res. Toxicol. 2022, 35, 1541–1557. [Google Scholar] [CrossRef]
- Motta, I.L.; Marchesan, A.N.; Maciel Filho, R.; Wolf Maciel, M.R. Correlating biomass properties, gasification performance, and syngas applications of Brazilian feedstocks via simulation and multivariate analysis. Ind. Crop. Prod. 2022, 181, 114808. [Google Scholar] [CrossRef]
- Susastriawan, A.A.P.; Saptoadi, H.; Purnomo. Comparison of the gasification performance in the downdraft fixed-bed gasifier fed by different feedstocks: Rice husk, sawdust, and their mixture. Sustain. Energy Technol. Assess. 2019, 34, 27–34. [Google Scholar] [CrossRef]
- Ismail, T.M.; Abd El-Salam, M.; Monteiro, E.; Rouboa, A. Eulerian—Eulerian CFD model on fluidized bed gasifier using coffee husks as fuel. Appl Therm. Eng 2016, 106, 1391–1402. [Google Scholar] [CrossRef]
- Tacuri, D.; Andrade, C.; Álvarez, P.; Abril-González, M.; Pinos-Vélez, V.; Jara, L.; Montero-Izquierdo, A.; Zalamea, S. Design and Development of a Catalytic Fixed-Bed Reactor for Gasification of Banana Biomass in Hydrogen Production. Catalysts 2022, 12, 395. [Google Scholar] [CrossRef]
- Aluri, S.; Syed, A.; Flick, D.W.; Muzzy, J.D.; Sievers, C.; Agrawal, P.K. Pyrolysis and gasification studies of model refuse derived fuel (RDF) using thermogravimetric analysis. Fuel Process Technol. 2018, 179, 154–166. [Google Scholar] [CrossRef]
- Prestipino, M.; Chiodo, V.; Maisano, S.; Zafarana, G.; Urbani, F.; Galvagno, A. Hydrogen rich syngas production by air-steam gasification of citrus peel residues from citrus juice manufacturing: Experimental and simulation activities. Int. J. Hydrog. Energy 2017, 42, 26816–26827. [Google Scholar] [CrossRef]
- Arun, K.; Venkata Ramanan, M.; Mohanasutan, S. Comparative studies and analysis on gasification of coconut shells and corn cobs in a perforated fixed bed downdraft reactor by admitting air through equally spaced conduits. Biomass Convers. Biorefinery 2022, 12, 1257–1269. [Google Scholar] [CrossRef]
- Ram, M.; Mondal, M.K. Conversion of unripe coconut husk into refined products using humidified air in packed bed gasification column. Biomass Convers. Biorefinery 2020, 10, 409–421. [Google Scholar] [CrossRef]
- Shukla, A.; Sudhir, D.; Kumar, Y. A Comparative study of Sugarcane Bagasse gasification and Direct Combustion. Int. J. Appl. Eng. Res. 2017, 12, 14739–14745. [Google Scholar]
- Parsa, S.; Jafarmadar, S.; Neshat, E.; Javani, N. Thermodynamic analysis of a novel biomass-driven trigeneration system using different biomass resources. Biomass Convers. Biorefinery 2022, 12, 1–17. [Google Scholar] [CrossRef]
- Parrillo, F.; Ardolino, F.; Calì, G.; Marotto, D.; Pettinau, A.; Arena, U. Fluidized bed gasification of eucalyptus chips: Axial profiles of syngas composition in a pilot scale reactor. Energy 2021, 219, 119604. [Google Scholar] [CrossRef]
- Huang, F.; Jin, S. Investigation of biomass (pine wood) gasification: Experiments and Aspen Plus simulation. Energy Sci. Eng. 2019, 7, 1178–1187. [Google Scholar] [CrossRef]
- Soares, J.; Oliveira, A.C. Experimental assessment of pine wood chips gasification at steady and part-load performance. Biomass Bioenergy 2020, 139, 105625. [Google Scholar] [CrossRef]
- Oliveira, T.J.P.; Cardoso, C.R.; Ataíde, C.H. Fast pyrolysis of soybean hulls: Analysis of bio-oil produced in a fluidized bed reactor and of vapor obtained in analytical pyrolysis. J. Therm. Anal. Calorim. 2015, 120, 427–438. [Google Scholar] [CrossRef]
- Boateng, A.A.; Mullen, C.A.; Goldberg, N.M.; Hicks, K.B.; Devine, T.E.; Lima, I.M.; McMurtrey, J.E. Sustainable production of bioenergy and biochar from the straw of high-biomass soybean lines via fast pyrolysis. Environ. Prog. Sustain. Energy 2010, 29, 175–183. [Google Scholar] [CrossRef]
- Raymundo, L.M.; Espindola, J.S.; Borges, F.C.; Lazzari, E.; Trierweiler, J.O.; Trierweiler, L.F. Continuous fast pyrolysis of rice husk in a fluidized bed reactor with high feed rates. Chem. Eng. Commun. 2020, 208, 1553–1563. [Google Scholar] [CrossRef]
- Pittman, C.U.; Mohan, D.; Eseyin, A.; Li, Q.; Ingram, L.; Hassan, E.B.M.; Mitchell, B.; Guo, H.; Steele, P.H. Characterization of Bio-oils Produced from Fast Pyrolysis of Corn Stalks in an Auger Reactor. Energy Fuels 2012, 26, 3816–3825. [Google Scholar] [CrossRef]
- da Silva, J.P. Caracterização da Casca de Café (coffea arábica L.) in Natura, e de seus Produtos Obtidos pelo Processo de Pirólise em Reator Mecanicamente Agitado; Universidade Estadual de Campinas: Campinas, SP, Brazil, 2012. [Google Scholar]
- Singh, R.K.; Patil, T.; Pandey, D.; Tekade, S.P.; Sawarkar, A.N. Co-pyrolysis of petroleum coke and banana leaves biomass: Kinetics, reaction mechanism, and thermodynamic analysis. J. Environ. Manag. 2022, 301, 113854. [Google Scholar] [CrossRef] [PubMed]
- Sellin, N.; Krohl, D.R.; Marangoni, C.; Souza, O. Oxidative fast pyrolysis of banana leaves in fluidized bed reactor. Renew. Energy 2016, 96, 56–64. [Google Scholar] [CrossRef]
- Wei, X.; Xue, X.; Wu, L.; Yu, H.; Liang, J.; Sun, Y. High-grade bio-oil produced from coconut shell: A comparative study of microwave reactor and core-shell catalyst. Energy 2020, 212, 118692. [Google Scholar] [CrossRef]
- Babatabar, M.A.; Yousefian, F.; Mousavi, M.V.; Hosseini, M.; Tavasoli, A. Pyrolysis of lignocellulosic and algal biomasses in a fixed-bed reactor: A comparative study on the composition and application potential of bioproducts. Int. J. Energy Res. 2022, 46, 9836–9850. [Google Scholar] [CrossRef]
- Fardhyanti, D.S.; Megawati, C.A.; Prasetiawan, H.; Raharjo, P.T.; Habibah, U.; Abasaeed, A.E. Production of bio-oil from sugarcane bagasse by fast pyrolysis and removal of phenolic compounds. Biomass Convers. Biorefinery 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Varma, A.K.; Mondal, P. Pyrolysis of sugarcane bagasse in semi batch reactor: Effects of process parameters on product yields and characterization of products. Ind. Crop. Prod. 2017, 95, 704–717. [Google Scholar] [CrossRef]
- Li, M.; Yu, Z.; Bin, Y.; Huang, Z.; He, H.; Liao, Y.; Zheng, A.; Ma, X. Microwave-assisted pyrolysis of eucalyptus wood with MoO3 and different nitrogen sources for coproducing nitrogen-rich bio-oil and char. J. Anal. Appl. Pyrolysis 2022, 167, 105666. [Google Scholar] [CrossRef]
- Matos, M.; Mattos, B.D.; de Cademartori, P.H.G.; Lourençon, T.V.; Hansel, F.A.; Zanoni, P.R.S.; Yamamoto, C.I.; Magalhães, W.L.E. Pilot-Scaled Fast-Pyrolysis Conversion of Eucalyptus Wood Fines into Products: Discussion Toward Possible Applications in Biofuels, Materials, and Precursors. Bioenergy Res. 2020, 13, 411–422. [Google Scholar] [CrossRef]
- Xu, B.; Argyle, M.D.; Shi, X.; Goroncy, A.K.; Rony, A.H.; Tan, G.; Fan, M. Effects of mixture of CO2 /CH4 as pyrolysis atmosphere on pine wood pyrolysis products. Renew. Energy 2020, 162, 1243–1254. [Google Scholar] [CrossRef]
- Torr, K.M.; De Miguel Mercader, F.; Murton, K.D.; Harbers, T.J.M.; Cooke-Willis, M.H.; Van De Pas, D.J.; Suckling, I.D. Fast Pyrolysis of Pine Wood Pretreated by Large Pilot-Scale Thermomechanical Refining for Biochemical Production. Ind. Eng. Chem. Res. 2020, 59, 21294–21304. [Google Scholar] [CrossRef]
- EPA Center for Corporate Climate Leadership. Greenhouse Gas Inventory Guidance: Direct Emissions from Stationary Combustion Sources, December 2020. Available online: https://www.epa.gov/sites/default/files/2020-12/documents/stationaryemissions.pdf/ (accessed on 2 April 2023).
- Metz, B.; Davidson, O.R.; Bosch, P.R.; Dave, R.; Meyer, L.A. Contribution of working group III to the fourth assessment report of the intergovernmental panel on climate change. IPCC Fourth Assess Rep. 2007. Available online: https://archive.ipcc.ch/publications_and_data/publications_ipcc_fourth_assessment_report_wg3_report_mitigation_of_climate_change.htm/ (accessed on 3 April 2023).
- Zhu, J.Y.; Zhuang, X.S. Conceptual net energy output for biofuel production from lignocellulosic biomass through biorefining. Prog. Energy Combust. Sci. 2012, 38, 583–598. [Google Scholar] [CrossRef]
- Hudiburg, T.W.; Wang, W.; Khanna, M.; Long, S.P.; Dwivedi, P.; Parton, W.J.; Hartman, M.; Delucia, E.H. Impacts of a 32-billion-gallon bioenergy landscape on land and fossil fuel use in the US. Nat. Energy 2016, 1, 15005. [Google Scholar] [CrossRef]
- Rana, R.; Ingrao, C.; Lombardi, M.; Tricase, C. Greenhouse gas emissions of an agro-biogas energy system: Estimation under the Renewable Energy Directive. Sci. Total Environ. 2016, 550, 1182–1195. [Google Scholar] [CrossRef]
- IPEA. Diagnóstico dos Resíduos Orgânicos do Setor Agrossilvopastoril e Agroindústrias Associadas; IPEA: Brasilia, Brazil, 2012. Available online: https://repositorio.ipea.gov.br/bitstream/11058/7687/1/RP_Diagn%C3%B3stico_2012.pdf (accessed on 24 April 2023).
- Board CIA. Power Generation from Coal: Measuring and Reporting Efficiency Performance and CO2 Emissions; CIAB International Energy Agency; Rep OECD/IEA2010 2010:1–114; Board CIA: Paris, France, 2010. [Google Scholar]
- FAO. Food and Agricultural Commodities Production. Countries by Commodity, Sugarcane; FAO: Rome, Italy, 2018. [Google Scholar]
- Basu, P.; Butler, J.; Leon, M.A. Biomass co-firing options on the emission reduction and electricity generation costs in coal-fired power plants. Renew. Energy 2011, 36, 282–288. [Google Scholar] [CrossRef]
- World Nuclear Association. Heat Values of Various Fuels; World Nuclear Association: London, UK, 2018. [Google Scholar]
- Kersten, S.; Garcia-Perez, M. Recent developments in fast pyrolysis of ligno-cellulosic materials. Curr. Opin. Biotechnol. 2013, 24, 414–420. [Google Scholar] [CrossRef]
- Mesa-Pérez, J.M.; Rocha, J.D.; Barbosa-Cortez, L.A.; Penedo-Medina, M.; Luengo, C.A.; Cascarosa, E. Fast oxidative pyrolysis of sugar cane straw in a fluidized bed reactor. Appl. Therm. Eng 2013, 56, 167–175. [Google Scholar] [CrossRef]
| Agro-Industrial Biomass | ||||||||
|---|---|---|---|---|---|---|---|---|
| Raw Material | Planted Areas (Million ha) | Production 2021/2022 Harvest (Million Tons) | Type of Residue | RPR [%] a [19,20,21] | Amount of Residue (Million Tons) | LHV [MJ/kg] | EP (PJ/yr) b | Competitive Uses |
| Soybean | 40.95 [23] | 124.05 [23] | Stalk and straw | 20 | 248.10 | 17.15 | 4254.92 |
|
| Husk | 8 | 9.90 | 14.14 | 140.37 | ||||
| Rice | 1.62 [23] | 10.80 [23] | Straw | 154 | 16.63 | 17.14 | 285.07 |
|
| Husk | 26 | 2.81 | 16.43 | 46.12 | ||||
| Wheat | 2.92 [23] | 9.03 [23] | Straw | 155 | 14.00 | 15.10 | 211.35 |
|
| Corn | 21.66 [23] | 115.66 [23] | Leaves | 21 | 24.29 | 22.43 | 544.79 |
|
| Corn cob | 15 | 17.35 | 19.32 | 355.18 | ||||
| Coffee | 1.84 [28] | 3.21 [28] | Husk | 33 | 1.06 | 18.20 | 19.25 |
|
| Coconut | 0.19 [29] | 2.45 [29] | Husk | 70 | 1.71 | 19.91 | 34.05 |
|
| Shell | 10 | 0.24 | 15.94 | 3.83 | ||||
| Sugarcane | 8.21 [30] | 596.01 [30] | Straw | 34 | 202.64 | 18.07 | 3661.77 |
|
| Bagasse | 30 | 178.80 | 18.40 | 3289.98 | ||||
| Banana | 0.47 [31] | 7.11 [31] | Leaves | 48 | 3.41 | 16.13 | 55.05 |
|
| Stem | 300 | 21.33 | 15.73 | 335.52 | ||||
| Orange | 16.47 [31] | 0.63 [31] | Bagasse | 50 | 0.32 | 15.82 | 4.98 |
|
| Forestry Biomass | ||||||||
| Planted Areas in 2019 (Million ha) [12] | Productivity (m3/ha yr) [12] | Type of Residue | RPR [%] a [34,35] | Amount of Residue (Million tons) c | LHV [MJ/kg] | EP (PJ/yr) | Competitive Uses | |
| Eucalyptus d | 6.97 | 35.30 | Bark | 0.08 | 0.56 | 18.26 | 10.23 |
|
| Branches | 0.03 | 0.21 | 18.05 | 3.79 | ||||
| Leaves | 0.02 | 0.14 | 16.05 | 2.25 | ||||
| Tips e | 0.13 | 0.91 | - | - | ||||
| Pinus f | 1.64 | 31.30 | Bark | 0.10 | 0.69 | 16.81 | 11.60 |
|
| Branches | 0.30 | 2.09 | 18.08 | 37.79 | ||||
| Leaves | 0.05 | 0.35 | 17.81 | 6.23 | ||||
| Resource | Residue | Proximate Analysis [wt%] | Ultimate Analysis [wt%] | LHVdaf | HHVdaf | Ref | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MC | VM | FC | AC | C | H | O | N | S | MJ/kg | MJ/kg | |||
| Agro-Industrial Biomass | |||||||||||||
| Soybean | Straw | 5.20 | 81.30 | 8.90 | 4.60 | 41.98 | 5.05 | 47.46 | 0.46 | 0.45 | - | 16.40 | [57] |
| Straw | 7.98 | 70.03 | 13.78 | 8.21 | 41.34 | 4.23 | 45.26 | 0.85 | 0.11 | - | - | [58] | |
| Straw | - | 85.50 | 10.60 | 3.90 | 44.30 | 5.80 | 45.20 | 0.70 | 0.10 | - | 16.10 | [59] | |
| Stalk | - | - | - | 8.87 | 41.05 | 5.52 | 41.39 | 2.90 | 0.28 | - | 16.39 | [60] | |
| Straw | 7.60–10.90 | - | - | 2.6–5.9 | - | - | - | - | - | 15.92 | - | [61] | |
| Straw | - | - | - | - | - | - | - | - | - | 15.92 | - | [62] | |
| Stalk | - | - | - | - | - | - | - | - | - | 16.99 | - | [63] | |
| Husk (gordana) | 13.93 | 68.18 | 8.15 | 5.81 | 49.54 | 4.72 | 52.73 | 2.51 | 0.18 | 15.71 | 16.17 | [64] | |
| Husk (sivka) | 14.85 | 66.91 | 9.41 | 4.74 | 48.41 | 4.28 | 54.77 | 2.21 | 0.11 | 16.18 | 17.12 | [64] | |
| Husk (slavonka) | 16.25 | 66.24 | 10.08 | 4.00 | 49.33 | 5.14 | 53.42 | 2.11 | 0.14 | 15.34 | 16.46 | [64] | |
| Husk | 6.80 | 82.20 | 6.80 | 94.00 | - | - | - | - | - | - | - | [65] | |
| Husk | - | 70.60 | 24.50 | 4.90 | - | - | - | - | - | - | - | [66] | |
| Rice | Straw | 5.46 | 88.78 | 1.43 | 9.82 | 46.24 | 6.21 | 46.23 | 1.32 | - | - | 16.16 | [67] |
| Straw | 4.91 | 71.82 | 8.07 | 4.91 | 46.11 | 6.83 | 46.03 | 1.03 | - | - | 16.14 | [68] | |
| Straw | 4.35 | 74.86 | 11.56 | 9.23 | 42.57 | 5.84 | 49.33 | 2.13 | 0.13 | - | - | [69] | |
| Straw | 8.08 | 69.99 | 13.40 | 8.79 | 39.61 | 5.83 | 43.80 | 1.21 | - | 14.21 | - | [70] | |
| Straw | - | - | - | - | - | - | - | - | - | 15.54 | - | [63] | |
| Husk | 7.73 | 64.20 | 15.50 | 12.57 | 38.62 | 5.67 | 41.38 | 0.48 | - | 15.39 | - | [70] | |
| Husk | - | 68.70 | 16.30 | 15.00 | 40.80 | 5.30 | 38.20 | 0.60 | 0.10 | - | 15.30 | [59] | |
| Husk | - | 70.70 | 15.40 | 13.90 | 40.10 | - | 39.70 | - | - | - | 16.79 | [71] | |
| Husk | - | - | - | - | - | - | - | - | - | 15.54 | - | [63] | |
| Husk | - | - | - | - | - | - | - | - | - | 12.80 | - | [72] | |
| Wheat | Straw | 7.10 | 76.70 | 7.10 | 9.20 | 45.50 | 5.70 | 47.90 | 1.00 | - | - | 16.50 | [73] |
| Straw | 6.46 | 77.03 | 19.47 | 3.50 | 44.00 | 5.76 | 48.92 | 0.94 | 0.38 | - | 17.52 | [74] | |
| Straw | 10.11 | 68.92 | 12.74 | 8.23 | 38.96 | 5.27 | 55.27 | 0.50 | - | - | 13.37 | [75] | |
| Straw | 12.81 | 83.08 | 10.29 | 6.63 | 38.34 | 5.47 | 55.59 | 0.60 | 0.37 | - | 16.68 | [76] | |
| Stalk | 10–20 | - | - | 2.6–9.6 | - | - | - | - | - | 17.20 | - | [61] | |
| Stalk | - | - | - | - | - | - | - | - | - | 17.15 | - | [63] | |
| Husk | 3.37 | 72.78 | 13.70 | 12.14 | 38.70 | 5.50 | 54.73 | 0.66 | 0.41 | - | 13.64 | [77] | |
| Husk | 13.31 | 69.53 | 13.20 | 3.96 | 52.84 | 6.10 | 15.12 | 2.55 | - | 22.91 | [78] | ||
| Husk | 8.13 | 80.54 | 15.73 | 3.43 | 45.97 | 6.81 | 52.62 | 1.41 | 0.11 | 17.11 | 18.59 | [79] | |
| Husk | 7.00 | 71.40 | 19.30 | 2.30 | 42.00 | 6.30 | 47.40 | 1.90 | 0.10 | 17.80 | [80] | ||
| Corn | Straw | 26.00 | 67.60 | 17.80 | 14.60 | 41.90 | 5.76 | 35.75 | - | - | - | 16.30 | [81] |
| Straw | 6.18 | 71.21 | 16.12 | 6.49 | 45.84 | 5.11 | 34.89 | 1.28 | 0.21 | - | 16.80 | [82] | |
| Stalk | - | 78.12 | 17.99 | 3.89 | 44.36 | 5.73 | 45.35 | 0.67 | - | - | - | [83] | |
| Stalk | - | 75.38 | 17.95 | 6.67 | 42.53 | 6.17 | 43.59 | 0.93 | 0.11 | 16.59 | [84] | ||
| Stalk | 15–45 | - | - | 3.50–9.00 | - | - | - | - | - | 8–17. | - | [61] | |
| Stalk | - | - | - | - | - | - | - | - | - | 13.70 | - | [62] | |
| Cob | 7.83 | 69.24 | 17.29 | 5.64 | 48.51 | 5.90 | 39.14 | 0.29 | 0.52 | 14.94 | 17.05 | [85] | |
| Cob | 9.60 | 71.60 | 17.20 | 1.60 | 44.40 | 6.50 | 48.80 | 0.30 | 0.00 | - | 16.80 | [86] | |
| Cob | 11.00 | 70.00 | 9.20 | 9.80 | 36.40 | 6.20 | 47.10 | 0.50 | 0.05 | - | 15.40 | [87] | |
| Cob | - | 83.10 | 13.78 | 3.12 | 43.40 | 6.55 | 48.88 | 0.65 | 0.49 | - | - | [88] | |
| Coffee | Husk | 9.95 | 84.20 | 14.30 | 1.50 | 48.98 | 5.32 | 44.92 | 0.78 | 0.29 | - | 18.04 | [44] |
| Husk | 9.06 | 77.09 | 19.36 | 3.55 | 46.41 | 6.33 | 44.51 | 2.66 | 0.09 | - | 18.50 | [89] | |
| Husk | 2.70 | 77.70 | 17.90 | 1.70 | 48.50 | 5.90 | 40.60 | 2.80 | 0.60 | - | 18.30 | [90] | |
| Husk | 8.33 | 78.44 | 18.93 | 5.63 | 44.41 | 5.78 | 49.80 | - | - | - | 18.26 | [91] | |
| Banana | Leaves | 8.40 | 73.05 | 11.29 | 7.26 | 43.28 | 6.68 | 48.31 | 1.28 | 0.30 | - | 17.80 | [92] |
| Leaves | - | 77.79 | 11.31 | 10.90 | 44.85 | 6.23 | 48.17 | 0.58 | 0.17 | 14.69 | 15.90 | [93] | |
| Leaves | - | 72.60 | 18.00 | 9.10 | 41.40 | 5.40 | 41.40 | 2.50 | 0.29 | 16.10 | 16.30 | [94] | |
| Leaves | - | 70.14 | 14.51 | 15.35 | - | - | - | - | - | - | - | [95] | |
| Stem | 10.20 | 80.60 | 6.90 | 12.50 | 33.60 | 7.30 | 36.90 | 22.1 | 0.20 | 10.8 | 12.40 | [96] | |
| Stem | 12.56 | 80.27 | 9.96 | 8.00 | 39.00 | 5.44 | 54.84 | 0.82 | - | - | 16.13 | [97] | |
| Stem | - | - | - | - | 38.44 | 5.03 | 43.10 | 1.24 | 0.09 | - | - | [98] | |
| Stem | - | 73.98 | 17.94 | 8.08 | - | - | - | - | - | - | 14.09 | [99] | |
| Orange | Bagasse | 7.62 | 76.45 | 23.55 | 7.62 | 44.93 | 7.10 | 46.31 | 1.42 | 0.14 | 14.31 | 15.86 | [100] |
| Bagasse | 9.23 | 73.20 | 20.60 | 6.20 | 46.40 | 5.54 | 40.15 | 1.17 | 0.01 | 17.03 | 18.16 | [101] | |
| Bagasse | 6.15 | 70.33 | 20.86 | 2.66 | 43.57 | 4.40 | 51.78 | 0.17 | 0.09 | - | 17.26 | [102] | |
| Bagasse | 1.50 | 74.10 | 23.60 | 2.30 | 42.70 | 6.40 | 47.60 | 1.00 | - | - | 19.40 | [103] | |
| Bagasse | 2.71 | 81.84 | 11.44 | 6.71 | 44.33 | 6.09 | 48.46 | 1.64 | - | - | 17.61 | [104] | |
| Coconut | Husk | 8.50 | 61.50 | 33.11 | 5.39 | 49.59 | 5.30 | 36.87 | 0.38 | 0.01 | 18.22 | 19.31 | [105] |
| Husk | 6.70 | 61.78 | 31.52 | 6.70 | 49.03 | 5.37 | 38.36 | 0.41 | 0.13 | - | 19.33 | [106] | |
| Husk | 9.96 | 72.60 | 15.21 | 2.23 | 48.95 | 5.40 | 43.10 | 0.40 | - | - | - | [107] | |
| Husk | - | 82.94 | 16.14 | 0.92 | 47.00 | 6.07 | 46.60 | 0.21 | 0.12 | - | 15.44 | [77] | |
| Husk | - | 73.38 | 22.95 | 4.66 | - | - | - | - | - | - | 16.75 | [108] | |
| Shell | 8.83 | 92.16 | 7.35 | 0.49 | 47.70 | 5.44 | 46.25 | 0.06 | 0.03 | - | 24.29 | [109] | |
| Shell | 5.67 | 73.89 | 19.55 | 8.89 | 48.35 | 6.21 | 45.25 | 0.18 | 0.01 | - | 17.17 | [110] | |
| Shell | 3.29 | 73.80 | 19.40 | 6.78 | 46.77 | 5.61 | 46.83 | 0.79 | - | - | 18.64 | [111] | |
| Shell | 15.98 | 72.90 | 19.40 | 0.80 | 46.60 | 7.10 | 41.80 | 0.32 | - | - | 14.10 | [112] | |
| Shell | 7.82 | 79.91 | 12.04 | 0.23 | 39.20 | 4.50 | 55.90 | 0.20 | - | - | - | [107] | |
| Shell | - | - | - | - | 49.50 | 6.10 | 40.10 | 0.80 | 0.06 | - | 18.90 | [113] | |
| Sugarcane | Straw | 16.80 | 80.50 | 19.50 | 20.10 | 50.60 | 6.40 | 44.60 | 2.60 | 0.28 | - | 19.00 | [114] |
| Straw | 9.54 | - | - | - | 45.69 | 5.80 | 48.38 | 0.13 | - | - | 17.38 | [115] | |
| Straw | 3.12 | 87.61 | 3.22 | 9.17 | 41.88 | 5.87 | 41.72 | 0.47 | - | - | 16.42 | [116] | |
| Straw | 8.30 | 71.10 | 14.60 | 6.00 | 42.60 | 5.29 | 43.40 | 0.51 | 0.14 | - | - | [117] | |
| Bagasse | 2.80 | 80.32 | 10.14 | 6.75 | 47.40 | 6.14 | 46.18 | 0.28 | 0.10 | - | 18.51 | [118] | |
| Bagasse | - | 79.01 | 16.09 | 4.90 | 32.50 | 5.01 | 61.55 | 0.38 | 0.56 | - | 16.53 | [119] | |
| Bagasse | - | 83.46 | 14.26 | 2.17 | 46.37 | 6.29 | 46.79 | 0.55 | 0.11 | - | 14.33 | [120] | |
| Bagasse | - | - | - | - | 42.52 | 5.92 | 50.38 | 1.18 | - | - | - | [121] | |
| Bagasse | - | - | - | - | - | - | - | - | - | 20.00 | - | [63] | |
| Forestry Biomass | |||||||||||||
| Eucalyptus | Leaves | 48.40 (in nature) | 80.10 | 16.60 | 3.20 | 54.70 | 6.00 | 34.70 | 1.20 | 0.20 | - | 21.10 | [122] |
| Bark | 61.70 (in nature) | 80.40 | 15.10 | 4.50 | 48.10 | 5.50 | 41.70 | 0.10 | 0.10 | - | 20.47 | [122] | |
| Wood | 13.18 | 75.21 | 11.00 | 0.10 | 49.29 | 5.91 | 44.68 | 0.09 | 0.03 | - | 18.10 | [123] | |
| Wood | 7.60 | 87.95 | 11.59 | 0.46 | 46.13 | 5.90 | 47.83 | 0.14 | - | - | 20.25 | [124] | |
| Wood | 12.00 | 83.10 | 16.70 | 0.30 | - | - | - | - | - | - | 19.48 | [125] | |
| Wood | 12.00 | 85.49 | 14.16 | 0.34 | - | - | - | - | - | 15.50 | 19.32 | [126] | |
| Wood | - | 81.60 | 18.20 | 0.21 | 48.60 | 6.10 | 44.60 | 0.49 | - | 17.89 | 19.28 | [126] | |
| Wood | - | 87.00 | 12.80 | 0.30 | 52.30 | 5.90 | 41.40 | 0.00 | 0.10 | - | 19.10 | [122] | |
| Pinus | Wood | - | 88.30 | 9.80 | 1.90 | 50.30 | 6.90 | 40.80 | 0.10 | - | - | 18.50 | [59] |
| Wood | - | 82.40 | 16.43 | 1.17 | 52.80 | 6.10 | 40.50 | 0.50 | 0.09 | - | 20.80 | [127] | |
| Wood | 6.28 | 83.43 | 16.32 | 0.26 | - | - | - | - | - | - | - | [128] | |
| Wood | - | 87.40 | 11.00 | 1.55 | - | - | - | - | - | - | - | [129] | |
| Wood | 67.00 (in nature) | - | - | - | - | - | - | - | - | 18.08 | 19.44 | [130] | |
| Resource | Residue | Extractive [wt.%] | Lignin [wt.%] | Cellulose [wt.%] | Hemicelluloses [wt.%] | Ref. |
|---|---|---|---|---|---|---|
| Agro-Industrial Residues | ||||||
| Soybean | Straw | 15.50 | 15.20 | 37.60 | 27.80 | [59] |
| Straw | - | 21.80 | 35.30 | 16.90 | [142] | |
| Straw | - | 24.12 | 22.69 | 17.73 | [143] | |
| Straw | - | 21.60 | 34.10 | 16.10 | [57] | |
| Stalks | - | 19.80 | 34.50 | 24.80 | [144] | |
| Husk | 4.80 | 7.80 | 40.60 | 33.80 | [145] | |
| Husk | - | 2.10 | 32.90 | 3.10 | [146] | |
| Husk | - | 3.70 | 52.30 | 18.50 | [147] | |
| Husk | - | 2.10 | 5.10 | 19.40 | [148] | |
| Rice | Straw | 1.62 | 25.79 | 42.32 | 19.50 | [67] |
| Straw | - | 18.70 | 47.20 | 31.80 | [149] | |
| Straw | - | 8.3–9.9 | 19.6–36.2 | 19.0–50.4 | [144] | |
| Straw | - | 14–28 | 32–40 | 24.00 | [150] | |
| Husk | - | 21.10 | 38.57 | 21.30 | [70] | |
| Husk | - | 26.00 | 33.00 | 7.00 | [142] | |
| Husk | - | 22.00 | 40.00 | 21.00 | [148] | |
| Wheat | Husk | 20.00 | 16.00 | 36.00 | 18.00 | [151] |
| Husk | 2.40 | 16.40 | 30.50 | 28.90 | [152] | |
| Husk | - | 14.00 | 23.00 | 21.00 | [153] | |
| Husk | - | 16.00 | 39.00 | 30.00 | [154] | |
| Straw | 20.10 | 20.20 | 34.00 | 23.15 | [150] | |
| Straw | - | 16.00 | 30.00 | 26.00 | [149] | |
| Straw | - | 8.9–22.1 | 32.9–49.8 | 23.7–25.0 | [144] | |
| Corn | Straw | - | 7.02 | 32.72 | 33.35 | [155] |
| Straw | - | 6.87 | 24.58 | 25.97 | [155] | |
| Stalks | - | 7.0–7.3 (db) | 35.0–39.0 | 16.8–42.0 | [144] | |
| Stalks | - | 17.18 | 49.22 | 25.57 | [149] | |
| Husk | 19.60 | 15.50 | 32.50 | 30.40 | [150] | |
| Husk | - | 14.30 | 31.00 | 34.00 | [148] | |
| Cob | 14.25 | 18.50 | 35.75 | 30.70 | [150] | |
| Cob | - | 9.40 | 27.71 | 38.78 | [142] | |
| Cob | - | 6.10 | 33.70 | 31.90 | [144] | |
| Coffee | Husk | 38.00 | 24.30 | 31.50 | 43.80 | [44] |
| Husk | - | 27.57 | 41.60 | 21.90 | [91] | |
| Husk | 20.53 | 24.15 | 47.29 (hollocellulose) | [89] | ||
| Banana | Leaves | 7.32 | 15.00 | 43.34 | 34.34 | [92] |
| leaves | 7.59 | 25.25 | 35.20 | 20.28 | [95] | |
| stem | 7.60 | 22.30 | 55.50 | 5.40 | [156] | |
| stem | 4.90 | 15.30 | 69.40 | 8.80 | [157] | |
| Stem | - | 6.08 | 27.79 | 30.08 | [98] | |
| Orange | Baggase | 35.30 | 28.70 | 17.10 | 16.60 | [158] |
| Bagasse | 21.96 | 29.04 | 40.33 | 8.66 | [101] | |
| Bagasse | 29.80 | 9.52 | 28.98 | 31.70 | [102] | |
| Bagasse | - | 8.50 | 12.40 | 7.50 | [159] | |
| Coconut | Husk | - | 26.69 | 31.60 | 26.33 | [160] |
| husk | 5.44 | 43.34 | 26.27 | 26.00 | [106] | |
| Husk | - | 46.36 | 21.26 | 17.33 | [142] | |
| shell | 4.20 | 29.70 | 29.58 | 23.80 | [161] | |
| Shell | 13.96 | 5.35 | 1.70 | 61.96 | [109] | |
| Shell | 2.71 | 33.15 | 30.47 | 25.42 | [160] | |
| Sugarcane | Straw | 25.00 | 27.00 | 54.00 | 39.00 | [114] |
| Straw | 15.31 | 18.21 | 33.13 | 26.25 | [162] | |
| Straw | 8.91 | 31.14 | 31.46 | 27.03 | [115] | |
| Straw | 4.30 | 19.60 | 37.20 | 30.60 | [163] | |
| Straw | - | 16.00 | 30.00 | 22.50 | [164] | |
| Bagasse | 12.70 | 19.20 | 36.90 | 26.30 | [119] | |
| Bagasse | 6.49 | 26.72 | 44.46 | 20.53 | [121] | |
| Bagasse | 4.80 | 19–25 | 35–45 | 25–32 | [150] | |
| Bagasse | - | 25.00 | 50.00 | 25.00 | [142] | |
| Bagasse | - | 20.30 | 41.60 | 25.10 | [149] | |
| Bagasse | - | 11.70 | 36.50 | 26.50 | [164] | |
| Forestry Biomass | ||||||
| Eucalyptus | Wood | 3.70 | 24.40 | 47.00 | 24.90 | [165] |
| Wood | 1.81 | 23.24 | 42.83 | 43.42 | [166] | |
| Wood | 4.80 | 23.30 | 38.10 | 36.60 | [167] | |
| Wood | - | 14.58 | 48.54 | 28.36 | [168] | |
| Wood | - | 31.08 | 68.92 (hollocellulose) - | [124] | ||
| Pinus | Wood | 9.00 | 26.30 | 41.10 | 13.70 | [59] |
| Wood | 3.06 | 31.56 | 37.20 | 22.88 | [128] | |
| Wood | 14.00 | 34.50 | - | - | [169] | |
| Wood | 3.20 | 28.00 | 45.50 | 23.10 | [170] | |
| Wood | 2.54 | 22.06 | 69.49 (hollocellulose) - | [171] | ||
| Wood | 7.10 | 26.50 | 59.00 | 21.10 | [172] | |
| Wood | - | 36.10 | 37.80 | 26.10 | [173] | |
| Index | Range | Slagging and Fouling Inclinations |
|---|---|---|
| B/A | <0.5 | Low |
| 0.5–1.0 | Medium | |
| 1.0–1.75 | High | |
| >1.75 | Extremely High | |
| Fu | <0.6 | Low |
| 0.6–40 | Medium | |
| >40 | High | |
| SR | >72 | Low |
| 65–72 | Medium | |
| <65 | High | |
| SI | >0.6 | Low |
| 0.6–2 | Medium | |
| <2 | High |
| Resource | Residue | Ash Composition | Ash Fusibility Trends | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F2O3 | CaO | MgO | Na2O | K2O | SiO2 | Al2O3 | TiO2 | P2O5 | B/A | B/A + P | Fu | SR | SI | Ref | ||
| Agro-Industrial Residues | ||||||||||||||||
| Soybean | Stalk | 0.83 | 33.20 | 9.83 | 0.91 | 18.80 | 30.40 | 2.15 | 0.05 | 2.62 | 1.95 | 2.03 | 38.43 | 40.94 | 2.18 | [178] |
| Husk | 0.25 | 0.70 | 0.61 | - | 1.06 | 94.87 | 0.84 | 0.03 | 1.25 | 0.03 | 0.04 | - | 98.38 | - | [100] | |
| Rice | Straw | 0.73 | 1.61 | 1.89 | 1.85 | 11.30 | 74.31 | 1.40 | 0.02 | 2.65 | 0.23 | 0.26 | 3.02 | 94.61 | 0.27 | [179] |
| Straw | 0.46 | 5.92 | 3.61 | 2.08 | 22.92 | 51.02 | 0.23 | 0.04 | 2.83 | 0.68 | 0.74 | 17.05 | 83.63 | 0.38 | [180] | |
| Husk | 0.05 | 0.67 | 0.40 | 1.26 | 0.62 | 95.77 | 0.05 | - | 0.46 | - | - | - | 98.84 | 0.57 | [181] | |
| Husk | 0.21 | 0.91 | 0.26 | 0.13 | 2.42 | 94.26 | 0.29 | 0.02 | 0.55 | 0.04 | 0.05 | 0.11 | 98.56 | 0.46 | [182] | |
| Wheat | Straw | 1.60 | 12.20 | 7.03 | 0.42 | 20.49 | 38.43 | 3.41 | 0.27 | 3.39 | 0.99 | 1.07 | 20.73 | 64.85 | 0.92 | [183] |
| Straw | 0.49 | 6.11 | 4.95 | 0.31 | 25.08 | 25.08 | 1.05 | 0.07 | 1.81 | 1.41 | 1.48 | 35.80 | 68.47 | 0.44 | [184] | |
| Husk | 0.84 | 5.46 | 0.99 | 0.16 | 11.30 | 43.22 | - | - | - | - | - | - | 85.57 | 0.56 | [185] | |
| Husk | 0.08 | 1.20 | 0.80 | - | 0.70 | 95.56 | 0.14 | - | 0.80 | - | - | - | 97.87 | - | [185] | |
| Corn | Straw | 1.31 | 21.66 | 15.96 | 0.90 | 20.24 | 26.79 | 1.73 | 0.18 | 2.75 | 2.09 | 2.19 | 44.25 | 40.76 | 1.78 | [186] |
| Cob | 1.20 | 10.43 | 0.11 | 2.24 | 31.13 | 19.63 | 1.23 | 0.35 | 7.19 | 2.13 | 2.47 | 70.97 | 62.58 | 0.32 | [85] | |
| Coffee | Husk | 2.06 | 13.05 | 4.32 | 0.66 | 52.45 | 14.65 | 1.07 | 0.27 | 4.94 | 4.54 | 4.85 | 240.94 | 42.99 | 0.33 | [182] |
| Husk | 0.56 | 17.70 | 4.51 | 0.14 | 46.46 | 1.24 | 0.58 | 0.08 | 3.85 | 36.51 | 38.54 | 1701.39 | 5.16 | 0.48 | [187] | |
| Banana | Leaves | 1.14 | - | - | 0.21 | - | 48.70 | 2.60 | - | - | - | - | - | - | - | [188] |
| Leaves | 1.11 | 18.75 | 9.43 | 0.39 | 10.73 | 49.14 | 1.49 | 0.18 | 3.07 | 0.80 | 0.86 | 8.84 | 62.65 | 2.53 | [189] | |
| Orange | Bagasse | 2.91 | 22.22 | 6.34 | 0.26 | 31.58 | 3.18 | 5.24 | 0.19 | 10.71 | 7.35 | 8.60 | 234.12 | 9.18 | 0.90 | [100] |
| Bagasse | 0.09 | 29.47 | 4.78 | 1.98 | 30.90 | 0.29 | 0.33 | 0.02 | 8.34 | - | - | 3453.43 | 0.84 | 1.04 | [158] | |
| Coconut | Husk | 11.90 | 2.33 | 2.19 | 4.82 | 27.50 | 31.60 | 3.00 | 0.30 | 1.60 | 1.40 | 1.44 | 45.14 | 65.81 | 0.14 | [105] |
| Shell | 6.16 | 2.41 | 1.54 | 4.62 | 8.48 | 66.75 | 8.48 | 0.01 | 1.54 | 0.31 | 0.33 | 4.04 | 86.85 | 0.30 | [182] | |
| Sugarcane | Bagasse | 5.55 | 9.60 | 2.36 | 1.18 | 2.08 | 53.09 | 6.94 | 0.57 | 0.25 | 0.34 | 0.35 | 1.12 | 75.20 | 3.67 | [190] |
| Bagasse | 5.42 | 4.00 | 0.63 | 0.19 | 0.96 | 64.12 | 20.01 | 1.12 | 0.38 | 0.13 | 0.14 | 0.15 | 86.45 | 4.03 | [182] | |
| Forestry Biomass | ||||||||||||||||
| Eucalyptus | Wood | 2.05 | 48.19 | 3.78 | 2.68 | 29.92 | 3.46 | 0.47 | 0.16 | 4.88 | 21.18 | 22.37 | 690.42 | 6.02 | 1.59 | [182] |
| Pinus | Wood | 5.93 | 20.04 | 4.55 | 1.42 | 9.76 | 45.23 | 10.6 | 0.64 | 1.29 | 0.74 | 0.76 | 8.26 | 59.71 | 2.20 | [191] |
| Wood | 5.8 | 11.7 | 3.3 | 1.3 | 5.9 | 47.4 | 18.1 | 0.8 | 1.2 | 0.42 | 0.44 | 3.04 | 69.50 | 2.08 | [192] | |
| Feedstock | Source | Operation Parameters | Optimum Obtained Results | Ref. |
|---|---|---|---|---|
| Scenario I—Bioethanol Production | ||||
| Soybean | Waste agro-industrial residue (Brazil) | Hydrolysis conditions:
|
| [199] |
| Soybean strawfarm residue (Korea) |
|
| [200] | |
| Rice | Rice huskagro-industrial residue (Brazil) | Hydrolysis conditions
|
| [199] |
| Rice branagro-industrial residue (Brazil) |
|
| [199] | |
| Wheat | Waste agro-industrial residue (Brazil) | Hydrolysis conditions
|
| [199] |
| Wheat straw (India) | Pretreatment (100 °C, 2 h—RT overnight):
|
| [201] | |
| Corn | Corn stover collected from field after corn harvest (Brazil) |
|
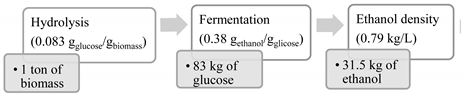 | [194] |
| Corn stalk agricultural farm (Bangladesh) |
|
| [202] | |
| Coffee |
|
|
Theoretical yield [%]: 67.64 ± 1.39 (coffee husk); 62.78 ± 4.56 (ground coffee husk); 48.07 ± 0.96 (aqueous extract) Sugar conversion [%]: 92.10 ± 0.40 (coffee husk); 92.67 ± 0.52(ground coffee husk); 91.43 ± 0.38 (aqueous extract) Productivity [g/L h]: 1.22 ± 0.02 (coffee husk); 1.15 ± 0.08 (ground coffee husk); 1.03 ± 0.03 (aqueous extract) | [203] |
| Coffee pulp Semidry processing coffee (Brazil) |
|
| [204] | |
| Banana | Banana leaf waste (India) | Steam, alkali (0.1 N NaOH) and acid pretreatment (0.1 N H2SO4) pretreatment [%w/v]: 1:10 (121 °C, 1 h) Sacharification condition: Cellulase (enzyme): produced by Aspergillus niger JD-11 Enzyme loading [FPU/g]: 5–15 Temperature [°C]: 40, 45 and 50 Substrate [%wt./v]: 2 to 6 Surfactant [%vol]: 0.05–0.15 (Tween 80 and PEG 6000)Time [h]: 70 Fermentation condition: Inoculum: S. cerevisiae (40 g/L reduced sugars, pH 5.5, 30 °C for 30 h) | Pretreatment effect on hydrolysis: Higher reduced sugars[mg/g]: 358.11 (acid pretreatment). Main increase at 40 h Enzyme load effect on hydrolysis: Higher reduced sugars [mg/g]: 397.57 (15 FPU/g). 38% more compared with 5 FPU/g. Temperature effect on hydrolysis: Higher reduced sugars [mg/g]: 455.91 (at 45 °C) Surfactant effect on hydrolysis: Higher reduced sugars [mg/g]: 524.83 (0.15% vol of PEG 6000) Substrate concentration effect on hydrolysis: Higher reduced sugars [mg/g]: 524.83 (2% wt./vol of substrate) Higher ethanol production [g/L]: 15.43Conversion factor reduced sugars to ethanol [g/g]: 0.38 Volumetric productivity [g/L·h]: 1.28 (at 12 h) | [205] |
| Banana pulp, peels and pseudostem bagasse (Brazil—Simulation study) | Biomass proportions: 1:2:10 (pulp:peels:pseudestem) Inoculum: S. cerevisiae and Pachysolen tannophilus (ATCC32691). pH fermentation: 5 Fermentation time: 36–48 Hydrolysis Temperature [°C]: 120 Hydrolysis Time [min]: 15 | Best ethanol performance at 48 h of fermentation: Reduced sugars before chem pretreatment [g/L]: 151.6 Reduced sugars in hydrolyzed broth [g/L]: 19.5 Ethanol at the beginning of fermentation [g/L]: 0.8 Ethanol at the after fermentation [g/L]: 53.1 Volumetric productivity [g/L·h]: 1.09 Conversion factor reduced sugars to ethanol [g/g]: 0.4 | [206] | |
| Orange | Peels (Brazil) | Pretreatment: Ca(OH)2, biomass and destilate water (1:4:20 w/w/v), at 60 °C for 120 min. Enzymatic and dilute acid hydrolysis Enzymes: cellulase and xylanase Acid: HCl | Best cellulose activity [FPU/mL]: 24.08 (ph 4.8, 60 °C) Best xylanase activity [U/kg]:1.99.58 × 10−3 (pH 5.2, 50 °C) Acid Hydrolysis effect:Total reduced sugars [mg/g]: 30.15 (acid concentration 3.5%, 55.82 °C, 45 min) Enzymes effect: Total reduced sugars [mg/g]: 99.66 (7.02 PFU/mL of cellulase, 2.5 U/g xylanase, 36 h) | [207] |
| Bagasse (Iran) | ABE Production (acetone-butanol-ethanol) Pretreatment: High-pressure reactor: Biomass to water 1:10 (w/w); T [°C]: 100, 140 and 180; t [min]: 30, 60 and 120 Enzymatic hydrolysis conditions: Cellulase:Hemicellulase 9:1 Cellulase to biomass [FPU/g]: 15; Solid loading [%wt./v]: 5; T [°C]: 45; t [h]: 72 (solid residue from Enzymatic hydrolysis to Anaerobic digestion) Fermentation: C. acetobutylicum NRRL B-591 | Pretreatment effect: Best performance at 180 °C and 120 min Solid solubility [%]: 68.2 Hemicellulosic sugar removal [%]: 86.4 Enzymatic hydrolysis: Total sugar concentration [g/L]: 25.7 (23.3 glucose, 2.4 xylose) (Pre: 140 °C, 120 min) Highest ABE production [g/L]: 4.68 (Pre: 140 °C, 30 min) Per kg of Baggase: 42.3 g biobutanol, 33.1 g acetona, 13.4 g ethanol, 104.54 L biohydrogen, 28.3 L biomethane | [208] | |
| Coconut | Husk (Brazil) | Pretreatment: NaOH 5% [mL]: 100, 121 °C, 1 atm for 40 min Enzymatic hydrolysis conditions: Enzyme: Accellerase 1500. Temperature [°C]: 50 Time [h]: 72 Fermentation: PD medium (10 g/L yeast extract; 20 g/L bacteriological peptone, 20 g/L glucose) Time [h]: 18 | Reducing sugars yield [g/100 g]: 45.86 Reducing sugars concentration [g/L]: 8.84 Enzymatic conversion [%]: 88.40 Overall enzymatic yield [g/100 g]: 22.71 Ethanol production [g/L·h]: 0.015 Theoretical maximum yield [gEtOH/g biomass]: 0.078 | [209] |
| Husk (Brazil) | Two way strategies SHF and SSF. Pretreatment: solution NaCl at 10% of the acetic acid. | Highest ethanol yield [L EtOH/ton biomass]: 52.7 (SSF) | [210] | |
| Sugar cane | Bagasse (Brazil) | Ultrasound (US)-assisted enzymatic hydrolysis. Ultrasound parameters: Temperature [°C]: 25, 30, 37.5, 45, 50. Time [s]: 10, 75, 170, 265, 330. Intensity [W/cm2: 120.6, 150.7, 192.5, 234.4, 263.7 Enzymatic hydrolysis conditions: Enzymes: Celluclast 1.5 L and Cellic CTec2 Temperature [°C]: 50 Time [h]: 24 | Reduced sugar concentration (No US) [g/L]: 2.09 (Celluclast 1.5 L) and 3.20 (Cellic CTeC2) Enzyme and US effect on reduced sugars: Celluclast 1.5 L [% Relative RS Concentration]: 189.37 (330 s, 150.7 W/cm2, 25 °C). Theoretical cellulose yield [%]: 45 (0.487 g/L cellobiose and 3.985 g/L glucose) Cellic CTec2 [% Relative RS Concentration]: 195.39 (75 s, 150.7 W/cm2, 30 °C) Theoretical cellulose yield [%]: 66.31 (0.487 g/L cellobiose and 3.985 g/L glucose) | [211] |
| Bagasse (Brazil) | Hydrolysis conditions: Temperature [°C]: 121, Time [min]: 20, Acid hydrolysis with H2SO4: 100 mg/g dry bagasse and solid ratio of 10% Yeast: S. cerevisiae MDS130 immobilized in Ca-alginat Medium: Sugarcane bagasse hemicellulose hydrolysate and molasses Fermentation device: Fixed-bed reactor Operation mode: 20 repeated batches | Ethanol production [g/L·h]: 14.06–22.80 Ethanol yield [gEtOH/gTRS] = 0.36–0.51 Highest ethanol concentration [g/L]: 46.98 | [212] | |
| Eucalyptus | Sawdust (Uruguay) | Bioethanol and xylosaccharides Pretreatment: Steam explosion with and without NaOH impregnation (10–20%). T[°C]: 180, 190 and 200; t [min]: 10. Enzymatic hydrolysis: Enzyme loading [FPU/mL]: 125; Solid loading [%wt/w]: 15; T [°C]: 50; t [h]: 96 and 168, pH: 4.85 Fermentation: SHF (separate hydrolysis and fermentation), PSSF (simultaneous saccharification and fermentation) and SSF (simultaneous saccharification and fermentation) | Pretreatment effect: Highest Glucose concentration [g/L]: 105 (200 °C, 0% NaOH); Highest Hydrolysis efficiency [%]: 96 (200 °C, 0% NaOH) Ethanol conversion [%]: 78 (SHF), 82 (PSSF), 83 (SSF) Ethanol production [g/L]: 71.8 (SHF), 70.2 (PSSF), 75.6 (SSF) | [213] |
| Bark (Portugal) | Two sequential steps of acid hydrolysis: 1) 0.4 mL of 72% wt. H2SO4, T [°C]: room temperature, t [min]: 180 2) 4.4 mL of water was added to obtain a 9% wt. acid solution, T [°C]: 90, 100 and 120 | Highest Glucose concentration [%wt.]: 48.6 (100 °C, 2.5 h) Highest Xylose concentration [%wt.]: 15.2 (90 °C—2 h or 120 °C—0.5 h) Hypothetical ethanol yield [L/ton bark]: 248 | [214] | |
| Pinus | Sawdust (Mexico) | Two way strategies SHF and SSF. Pretreatment: HNO3 and NaOH. Concentration [%wt.]: 6 and 12; T [°C]: 100 and 130; t [min]: 30. Enzymatic hydrolysis: Enzyme loading [FPU/g]: 25; t [h]: 72; T [°C]: 48; pH: 4.8 Fermentation: Saccharomyces cerevisiae ITD-00185; pH: 5.5 | (SHF) Highest reducing sugar conversion [%]: 98.64 (10.9% HNO3 at 115 °C and 30 min) Highest ethanol yield [g/L]: 17.1 (40 h) Fermentation yield [%]: 84.1 Hypothetical ethanol yield [L/ton biomass]: 235.3 (SSF) Highest ethanol yield [g/L]: 15.0 Hypothetical ethanol yield [L/ton biomass]: 160 | [215] |
| Sawdust (Chile) | Two way strategies SHF and SSF. Pretreatment: Soda ethanol Liquor-to-biomass ratio: 5.44:1; T [°C]: 170; t [min]: 60; EtOH:H2O ratio [%vol]: 35–65 Enzymatic hydrolysis: Enzyme loading [FPU/g]: 30; t [h]: 48; T [°C]: 37; pH: 5 Fermentation: Saccharomyces cerevisiae IMR 1181 (SC 1181) | (SHF): Highest reducing sugar conversion [%]: ~98 Highest bioethanol concentration [g/L]: 3.40 (13 h) Fermentation yield [%]: 89.3 (SSF): Highest bioethanol concentration [g/L]: 5.68 (72 h) Fermentation yield [%]: 100 | [216] | |
| Scenario II-Biogas Production | ||||
| Soybean | Straw and hull (Brazil) | Subcritical water hydrolysis: Temperature [°C]: 220 Liquid/solid mass ratio: 18 g water/g straw; 15 g water/g hull. Flow rate [mL/min]: 30 Reaction time [min]: 4 (straw); 3 (hull) Fermentation: Yeast: Wickerhamomyces sp. UFFS-CE-3.1.2 10 mL of inoculum and 90 mL of hydrolysate Procedure: orbital shaker at 30 °C and 50 rpm. Hydrolysates supplemented with glucose (10 g/L). Biochemical biogas and methane: Starter inoculant: anaerobic sludge treated with swine manure, fresh dairy cattle manure, and anaerobic mesophilic granular sludge from a gelatin manufactory. Temperature [°C]: 37 Procedure: 250 mL glass reactors. 2 g of straw or hull and 30 g for the samples of hydrolysates or fermented hydrolysates were mixed. Inoculum/Substrate ratio: 2 | Soybean straw hydrolysate [g/L]: 2.16 (glucose); 1.33 (xylose); 0.08 (arabinose); 4.76 (formic acid); 8.22 (acetic acid); 0.28 (HMF); 0.48 (furfural) Soybean hull hydrolysate [g/L]: 0.96 (glucose); 1.11 (xylose); 0.43 (arabinose); 0.09 (cellobiose); 3.24 (formic acid); 3.14 (acetic acid); 0.16 (HMF); 0.31 (furfural) Fermentation of straw hydrolysate (72h) [g/L]: 0.69 ± 0.06 (ethanol); 2.04 ± 0.17 (glucose); 1.08 ± 0.02 (xylose); 7.35 ± 0.65 (acetic acid); 4.17 ± 0.19 (formic acid); 0.30 ± <0.01 (HMF); 0.30 ± <0.01 (furfural) Fermentation of hull hydrolysate (96 h) [g/L]: 0.72 ± 0.01 (ethanol); 1.02 ± 0.25 (glucose); 0.90 ± 0.04 (xylose); 0.40 ± <0.01 (arabinose); 0.09 ± <0.01 (cellobiose) 2.85 ± 0.05 (acetic acid); 2.96 ± 0.02 (formic acid); 0.19 ± <0.02 (HMF); 0.19 ± <0.04 (furfural) Biogas potential:
| [217] |
| Molasses (Brazil) | Reactor: Lab-scale up-flow anaerobic sludge blanket reactor (UASB) 12 L Organic loads rates [kg COD/m3d]: 0.28–6.98 Time [days]: 134 Temperature [°C]: 23 ± 1–25 ± 1 pH [-]: 7.3–7.8 Mass of soybean molasses [g]: 17.5–140 Flow rate [L/h]: 0.25–1 Inoculum: 3.5 L of anaerobic granular sludge (28.5 gTS/L and 24.4 gTVS/L) Mesophilic conditions | Characterization raw soybean molasses (dry basis): 50g/kg (crude protein); 250 g/kg (moisture); 150 g/kg (ashes); 5g/kg (fat); 3 g/kg (crude fiber); 5.45 pH; 1.35 g/cm3 (Density); 9000–14,000 cP (viscosity); 119 g/kg (stachyose); 50g/kg (raffinose); 199 g/kg (sucrose); 25 g/kg (fructose); 4.64 g/100g (galactose); 6 g/kg (glucose); 400 mg/kg (total sugars); 5.32 g/kg (total carbohydrate); 500 mg/kg (sulfite); 5.5 mg/kg (manganese); 100 mg/kg (calcium); 462 mg/kg (iron); 400 mg/kg (sodium); 0.74 mg/kg (cobalt); 1.30 g/kg (magnesium); 4150 mg/kg (phosphorous). OLR [kg COD/m3d]: 0.28 ± 0.02–6.98 ± 0.35 Biogas production [mL/d]: 12 ± 5–1456 ± 426 Biogas production [mL CH4/g COD]: 23.3–356.1 Methane [%]: 75.5–82.1 | [218] | |
| Rice | Husk (Brazil) | Chernicharo methodology COD monitored between 2016–2017, total of 12 samples. The theoretical production of methane: | COD [mg/L]: 3968.9–7540.2 Total rice production [tons/year]: 6.4 × 106 Parboiled rice [tons/year]: 2.3 × 106 Effluent flow [m3/d]: 1.5 × 104 Flow of methane [Nm3/d]: 1.7 × 104 Chemical energy from husk [MJ/y]: 2.1 × 1010 Parboiling energy demand [MJ/y]: 2.4 × 109 Electrical energy fromCH4 [KWh/y]: 2.2 × 107 Thermal energy from CH4 [KWh/y]: 3.1 × 107 Flow of syngas [Nm3/d]: 9 × 106 Electrical energy from syngas [KWh/y]: 7.3 × 108 Total termal energy from genset and gasifier [KWh/y]: 2 × 109 Total energy [KWh/y]: 2 × 1012 | [219] |
| Husk (Brazil) | Biodigester: Flow condition: unsteady Flow regime: laminar Simulation time [s]: 1800 Time Step [s]: 60 Reference pressure [atm]: 1 Inlet [m/s]: 0.18 Temperature [°C]: 25 Feeding: 7 L swine manure—150 g rice husk—400 mL inoculum Anaerobic digestion conducted for 21 days | Generation of biogas [mL/g (VSad)]: 85.5–94.3 Biogas on batch step [mL/g (VSad)]: 14.08–15.52 Biogas on complete process [mL/g (VSad)]: 86.30–71.48 CH4 on biomass [%v/v]: 33.6 ± 2.4–34.9 ± 5 Methane on complete process [mL/g(VSad)]: 62.3–53.6 | [220] | |
| Wheat | Wheat (Triticum aestivum) straw (Brazil) | Pretreatment methods to wheat straw: acid; alkaline; thermal; acid+thermal; alkaline+thermal Biodigestor capacity [mL]: 300 Nutrient solution: 200 mL of 2 g/L of yeast extract, 7 g/L of K2HPO4, 3 g/L of KH2PO4. Temperature [°C]: 25 Operation time [days]: 274 | Characterization of waste used in bioreactor: Sludge [g/L]: 0.31 (chromium); 1.21 (TKN); 11.27 (TOC); 1.89 (IC); 10.88 (C/N ratio); 34.42% (VS); 7.45 [-] (pH) Leather shavings [%g/g]: 1.14 (chromium); 2.95 (TKN); 32.29 (TOC); 0 (IC); 10.95 (C/N ratio); 90.24% (VS); 4.09 [-] (pH) Wheat straw [%g/g]: 0.60 (TKN); 41.32 (TOC); 0 (IC); 68.87 (C/N ratio); 92.37% (VS); 5.84 [-] (pH) Biogas cumulative volume of VSS added [mL/g]: 4.01–43.15 Methane cumulative volume of VSS [mL/g]: 0.14–10.06 Maximum yield of methane [%]: 7.71–40.61 Days of maximum yield of methane: 161–266 | [221] |
| Straw (Chile) | Fungi: white rot fungi incubated in agar Petri dishes for 10 d at 30 °C in MEA medium. Inoculum: Industrial anaerobic reactor treating brewery wastewater. Reactor volume [mL]: 250 pH [-]: 7–7.2 Total solids [%]: 18 Substrate/inoculum ratio [gVS/gVS]: 1 Temperature [°C]: 30 | 0.15 and 30 d of fungal treatment using Pleurotus ostreatus: Biogas yield [mLSTP/g VS]: 235 ± 2–337 ± 3 Biogas yield rate [mLSTP/g VSd]: 13.6 ± 0.9–25.8 ± 1.3 | [222] | |
| Corn | Vinasse (Brazil) | The vinasse from corn uses the volume of ethanol produced in Brazil in 2019/20 to estimate the bioenergy. Temperature [°C]: 32–37 Reactor: UASB | Corn vinasse: 67.5 kg/m3 (COD); 87 m3/kgCOD (ECOD); 71.25% (CH4 in biogas); 0.295 m3 CH4//kgDQOrem; 25.44 MJ/kg (LHV biogas); 150 days (season period). Biogas flow rate [m3/h]: 8.52 × 108 Potential power generates from corn biogas [MW/year]: 2.27 × 108 Potential the bioenergy from biogas [MWh/year]: 7.35 × 105 Carbon credits from corm use [tCO2eq/y]: 1.22 × 106–4.29 × 105 | [223] |
| Stalk (Brazil) | Pretreatment: humid steam in autoclave in the presence of H2SO4 and H2O2 in an orbital shaker Reactor volume [mL]: 250 pH [-]: 7–7.2 Volatile solids [%]: 10 Substrate/inoculum ratio [gVS/gVS]: 1 Temperature [°C]: 37 | Biogas [LNbiogas/kgVSad]: 650 (Pretreatment with H2O2); 550 (not sifted and untreated); 540 (Sifted and untreated); 350 (pretreated with H2SO4) Stalk pretreated with H2O2 produced about 86% more LNbiogas/kgVSad when compared to the biomass pretreated with H2SO4. | [224] | |
| Coffee | Husk(Brazil) | Ozone pretreatment to generate hydrolysates for biogas Inoculum: mixture of bovine manure and anaerobic sludge (1:1 [w/w]) Temperature [°C]: 35 Anaerobic digestion: single stage; two stage; single stage with PAC. | Single-stage anaerobic digestion: Maximum methane production [NmL CH4/g CH]: 36 with hydrolysate (10 mL/g) (LSR); 11 (pH); 18.5 mg O3/g CH (SAOL); 0.064 kJ/g CH (energy recovery). Two-stage anaerobic digestion: Maximum methane production [NmL CH4/g CH]: 49, produced 0.26 kJ/g CH (energy recovery). | [225] |
| Wastewater (Brazil) | Mesophilic anaerobic biodigestion 4 digestors filled with 1.5 L of substrate Temperature [°C]: 35–40 Reactor volume [L]: 2 | Physicochemical parameters of coffee wastewater (INPUT): 3.87–4.50 (pH); 2082–2485 mg/L (COD); 602–1503 mg/L (BOD); 6640–7269 mg/L (TS); 535–1046 mg/L (FS); 6105–6223 mg/L (VS); 11–25 mg/L (TN); 86–92% (VS:TS); 1.39–4.13 (COD:DBO) Biogas composition: 10–38 (Hydraulic Retention Times); 0.2–11.4% (CH4); 6.4–35.7 (CO2); 9.1–17.3% (O2); 54→2000 ppm (CO); 9–1648 ppm (H2S); 53.1–76.4% (Balance) | [226] | |
| Banana | Peduncle (India) | Pretreatments: Thermal, alkali and extrusion Biomethane potential (BMP): automated methane potential test system II (AMPTS II), Temperature [°C]: 37 Inoculum: Seed sludge | Specific CH4 yield [mL/g volatile solids]: 527.6 (Thermal 120 °C, 60 min), 298.9 (Alkali 5% NaOH, 1 h) and 248.02 (extruded, twin screw). Optimized Yields [mL/g volatile solids]: 527.6 (thermal 120 °C, 60 min), 298 | [227] |
| Leaf, stem, and peduncle (Kenya) | Time [day]: 51 Temperature [°C]: 37 Inoculum: digested sludge | CH4 production [mLN/g organic dry matter]: 63.34 Net biogas production [ml]: 400 CH4 yield [m3/kgoDM]: 0.062 Biogas composition [wt.]: 65.335 (CH4), 34.665 (CO2) | [228] | |
| Orange | Peels (Brazil) | Two-stage anaerobic digestion Stage I (acidogenic, pH 5–6), Stage II (methanogenic, pH 7–8) Anaerobic digestion conditions: Temperature [°C]: 35, time [days]: 25.8 Inoculum characteristics: Mesophilic anaerobic sludge, pH: 7.53, TS an VS [%]: 9.07 and 8.03 Reactor mix: [%v/v]: 35 (biomass), 26 (inoculum) and 39 (water) | Highest cumulative CH4 yield [L/gVS]: 0.79 (in methanogenic stage), 38% more than simple stage reactor. Cumulative biogas volume [cm3]: 13,000 (stage I), 10,000 (stage II) Total Biogas yield [m3/ton biomass]: 18.21 Potential electricity generation [MWh/year]: 97.5 × 103 in São Paulo State Potential emission mitigation [tCO2eq/year]: 7.5 × 103 and 9.05 × 103 in São Paulo State | [229] |
| Peels, seeds, bagasse | Treatment: pectin and essential oil extraction. Anaerobic digestion conditions: VDI 4630 procedure, time [h]: 500 Inoculum characteristics: pig manure, VS [g/kg dry matter)]: 45 Inoculum to substrate ratio: 2:1 | Highest cumulative CH4 yield [mL/gVS]: 223 (oils extraction), 222 (pectin extraction), 190 (untreated) | [230] | |
| Coconut | Spent copra (Nigeria) | Pretreatment: Mix with cow urine (CU) at different ratios. CU to copra [mL to g]: 1:15, 1:7, 1:5 Anaerobic digestion conditions: Temperature [°C]: 45, time [days]: 42 Inoculum characteristics: anaerobic digester sludge, pH: 7.1, TS and VS [%]: 9.5 and 5.5 | Highest cumulative biogas yield [mL/gVS]: 786 (CU to copra ratio 1:7), 225 (unpretreated copra) Highest cumulative CH4 yield [mL/gVS]: 648.5 (CU to copra ratio 1:7), 99.9 (unpretreated copra) Highest CH4 yield [mL/gVS]: 77 (for all pretreatment ratios at day 12), 38 (unpretreated copra at day 24) | [231] |
| Shell | Pretreatment: pyrolysis at 600 °C Pyroligneous detoxification: oxidation by H2O2: 0–12%, temperature [°C]: 10, time [h]: 4 Anaerobic digestion conditions: Temperature [°C]: 37, time [days]: 4 Inoculum characteristics: Anaerobic granular sludge, pH: 7.74, TS and VS [mg/L]: 13.69 and 9.33 Inoculum to substrate ratio: 3:2 | Highest biogas volume [mL]: 1190 ((pretreatment 10% H2O2)) Highest CH4 yield [L/gCOD]: 0.317 (pretreatment 4% H2O2) | [232] | |
| Sugar cane | Bagasse (Brazil) | Hydrothermal pretreatment: NaOH [M]: 0.7–2.3, Temperature [°C]: 146.4–213.6, time [min]: 3.2–36.8 Anaerobic co-digestion conditions: Temperature [°C]: 55 time [days]: 52 Inoculum characteristics: from industrial biogas plant Inoculum to substrate [gVS/gVS]: 2:1 | Higher CH4 content in the biogas [%]: 70 (pretreatment conditions of: 200 °C, 2.0 M NaOH, 30 min; 160 °C, 2.0 M, NaOH, 30 min; 180 °C, 2.34 M NaOH, 20 min) | [233] |
| Bagasse (Brazil) | Enzymatic pretreatment and two stages anaerobic process Pretreatment (Trametes versicolor laccase): Temperature [°C]: 50, time [min]: 120 Stage I (acidogenic/fermentative): pH: 6.8, Temperature [°C]: 37, time [days]: 8 Inoculum characteristics: Pure Paraclostridium sp. isolated from sugarcane bagasse. Stage II (methanogenic): Temperature [°C]: 37, time [day]: 10Inoculum characteristics: Microbial consortium from anaerobic sludge. TVS [g TVS/g]: 0.84. | Stage I: H2 production rate [mL/L·h]: 3.2 H2 production [mL/L·h]: 166.8 Stage II: CH4 production rate [mL/L·h]: 2.31 CH4 production [mL/L·h]: 870.8 | [234] | |
| Eucalyptus | Wood (Colombia) | Alkali pretreatment: solution NaOH (8% wt./v), solid liquid ratio 1:5 (wt./v), Temperature [°C]: 130, time [min]: 60 Anaerobic digestion (Remanent solid): pH: 7, Temperature [°C]: 37, time [days]: 20 Inoculum: sludge form water treatment Inoculum characteristics: TS and VS [%]: 6.4 and 5.7 | Highest daily biogas production [mL/gVS.d]: 13.1 Highest cumulative biogas yield [mL/gVS]: 163 Highest cumulative CH4 yield [ML/gVS]: 87.9 | [235] |
| Pinus | Fresh needles, needle litter, bark and branches (Greece) | Mesophilic anaerobic digestion: Temperature[°C]: 38, time [days]: 30 Inoculum: took from a full-scale digester treating agro-industrial wastes and energy crops. Inoculum characteristics: pH: 7.8, ammonia nitrogen and orthophosphates [mg/L]: 24,411 and 83, TS and VS [g/L]: 34.9 and 22.3 | CH4 yield [ mLN/g VS]: 164 (fresh needles after 26 days), 138 (branches after 30 days), 85 (bark after 30 days), 77 (needle litter after 26 days) CH4 production potential [Nm3/km]: 500 (needle litter accumulated on adjacent forest roads) | [236] |
| Sawdust (Egypt) | Anaerobic digestion: Temperature [°C]: 30, time [days]: 35 Pretreatment: lignocellulosic degradation microbial consortium (LCDC) from rotten sawdust. Inoculum characteristics: pH: 7.01, total dissolved solids [mg/L]: 910, TS and VS [% db]: 9.33 and 5.68 | Highest daily biogas production [L/kgVS.d]: 15.7 (untreated after 19 days) and 15.9 (pretreated after 13 days) Highest significant cumulative biogas yield [L/kgVS]: 248.4 (untreated after 28 days) and 312.0 (pretreated residue after 28 days) Highest significant cumulative CH4 yield [L/kgVS]: 155.2 (pretreated residue after 28 days), 72.6% more than untreated. | [237] | |
| Scenario III—Combustion | ||||
| Rice | Husk (Brazil) | Reactor: atmospheric bubbling fluidized bed pilot Bed material: sand (particle size 0.5 –1 mm) and 95% silica content. Temperature [°C]: 834–877 O2 [%]: 5–9.9 6% O2 excess | Main characteristic of the feedstock: high volatile matter (74 wt.%) and medium ash content (12.8 wt.%). Silicon (87.7% as SiO2), potassium (5.4% as K2O) and phosphorous (3.7% as P2O5). CO2 [%]: 11.6–14.4 CO [mg/Nm3]: 1085–1808 NOx [mg/Nm3]: 100–430 Combustion efficiency [%]: 97.2–98.9 | [238] |
| Wheat | Straw (Brazil) | Technique: TGA curve analysis Isothermal conditions Heating rates [°C/min]: 5–100 Maximum temperature [°C]: 900 | Kinetics parameters: 85.4 [kJ/mol] (Activation energy); 3.1 × 106 [1/min] (Pre-exponential factor) Combustion scheme: evolution of volatiles (up to 300 °C); ignition of volatiles (500–650 °C), burning of volatiles (650–800 °C), and burning of char (700–850 °C) Direct combustion at low heating rates is favored with respect to the devolatilization/char burnout schemes. Alkali K2O crosses the stability regions of CO and CO2 at a temperature as low as 427 °C. | [239] |
| Coffee | Husk (Kenya) | Reactor: pilot-scale fluidized bed (FBC) Reactor bed material: quartz sand (0.48 mm) T [°C]: 500–900 Flue gas (O2) concentration [vol%]: 10–16 | Exhaust gas composition (mg/m3): NOx = 450–525; N2O = 3–27 N concentration in volatiles [%]: 54.2 (at 500 °C); 52 (at 600 °C); 53.2 (at 700 °C); 60.2 (at 800 °C); 67.6 (at 900 °C) Ash concentration [wt.%]: SiO2 = 16.6; FeO3 = 2.4; P2O5 = 3.4; Al2O3 = 4.5; CaO = 9.8; MgO = 3.7; Na2O = 0.5; K2O = 36.9 Note: over 700 °C sintering observed | [240] |
| Banana | Leaves and stem (Brazil) | Technique: TGA curve analysis Oxidative atmosphere (syntetic air) Comparison between loose and briquetted biomass Heating rate [°C/min]: 10 Operational temperature [°C]: 25–900 | Temperature ranges are the same, but there is a lower rate of mass loss in the first stage in loose biomass compared to briquettes First stage temperature range. Leaves: 180–400 °C; Tm [°C]: 280. Stem: 180–360 °C; Tm [°C]: 275 Second stage temperature range: Leaves: 400–580 °C. Stem [°C]: 360–600 | [241] |
| Leaves (Brazil) | Technique: TGA curve analysis Oxidative atmosphere (syntetic air) Heating rate [°C/min]: 10 Operational temperature [°C]: 22–900 Optical dilatometer at a heating rate [°C/min]: 5 Emissions quantification was carried out in open grill and using a multi flue gas analyzer (5 measurements in 15 min) | Single degradation stage Ti [°C]: ~200; Tm [°C]: ~300; Tb [°C]: ~550 From optical dilatometer: Remaining mass [wt.%]: 98.72 (100 °C); 43.59 (400 °C); 36.32 (899 °C) Max CO2 release [%]: 0.48 (6 min) Max CO [ppm]: 200 (6 min); 700 (15 min) | [242] | |
| Orange | Bagasse (Greece) | Technique: lab-scale fluidized bed reactor; 2 m height. Reaction time [h]: 4 Minimum fluidization velocity [m/s]: 0.25 Air flow rates [m3/h]: 4.53–5.94; Excess air ratios [-]: 1.3–1.7 Biomass feed rate [g/min]: 0.84 Bed temperature [°C]: 805–988 Freeboard temperatures [°C]: 810–838 | The orange bagasse has a high slagging and fouling tendency. Ash composition [%wt.]: 2.4 (SiO2); 3.0 (Al2O3); 0.2 (Fe2O3); 9.4 (MgO); 15.1 (CaO); 4.2 (Na2O); 37.1 (K2O); 0.01 (TiO2); 3.5 (P2O5); 0.01 (MnO); 4.7 (SO3) HHV [MJ/kg]: 16.7 CO heat loses [%]: 1.13 Efficiency [%]: 97.6 Low levels of heavy metals such as Cr, As, Hg and Pb. Toxic elements As, Cd, Hg, Co and Pb ranged from <0.2 ppm to 36 ppm Unburned carbon ashes [%]: 0.50 (bottom ash); 0.70 (fly ash) | [243] |
| Bagasse (United Kingdom) | Technique: fixed bed reactor coupled with a mass spectrometer (MS) | EDX orange bagasse [%wt.]: C = 60.2; O = 38.6; K = 0.7; Ca = 0.3; S = 0.2 EDX ashes [%wt.]: C = 35.9; O = 34.5; K = 17.5; Ca = 7.6; P = 1.6: S = 1.4; Mg = 1.0; Cl = 0.4; Si = 0.1 Two stages combustion: 160–370 °C and 440–600 °C Main emissions: N2O, H2O, CO2 and O2 Low level gas emissions: H4, H2, C2H6, CH3CHO, NO and NO2 Surface area [m2/g]: 1.89 Pore volume [cm3/g]: 0.002 Heating rate [°C/min]: 10 Ti [°C]: 260; Tb [°C]: 529; Release heat [W/g]: 1828.6 Heating rate [°C/min]: 20 Ti [°C]: 268; Tb [°C]: 572; Release heat [W/g]: 3294.1 Heating rate [°C/min]: 30 Ti [°C]: 275; Tb [°C]: 632; Release heat [W/g]: 3881.2 | [244] | |
| Coconut | Husk | Technique: Lab scale combustion Particle size [µm]: 250–300 Operational temperature [°C]: 650 | When Tc (600–750) flue gas main component was HCl (inhibits CO and CO2 oxidation) When Tc (1000) in flue gas [KCl] 5 times higher than [HCl] Liquid salt solution formation at 600 °C (KCl–NaCle–K2SO4–Na2SO4) reporting its maximum amount at 720 °C and disappearing at 980 °C Three groups of condensed phases were identified in ash: alkali metal salts (solid and liquid), other solid salts, and solid oxides. | [105] |
| Husk-shell (Ghana) | Technique: Pilot scale biochar unit Biomass [kg]: 5 Sample drying time [days]: 3, 6, 9, 12, 15, 18 Direct gas detection from the chimney (no filters) | HHV [MJ/kg]: 11.54 (Uncharred biomass); 21.30 (Charred biomass) CO [ppm](drying time, MC): 9.7 (3 days, 36.4 MC%); 7 (18 days, 10.3 MC%) CO emissions 40% higher than the standard WHO 24-hr AQG (6ppm) Change in smoke color indicates reduction on the volatiles amount and water vapor: thick white (3 days), light smoke (>15 days). PM2.5 [µg/m3]: 1200 (3 days); 994 (6–12 days); 1169 (18 days) PM2.5 120% higher than the value indicated by quality guidelines (10 µg/m3) | [245] | |
| Sugar cane | Bagasse | Reactor type: Lab-Scale Combustion and gasification Simulator Biomass flow rate [g/h]: 3 Primary air–fuel ration (λ): 0.85 Raw SCB: steam explosion (SE) treated and pelletized Pelletization carried out using a Khal pelletizer 14–175 with a flat-die type AKN1. Grinded pellets size [mm]: 1.0 | After steam explosion the SCB ash content increased from 2.2 to 4.7 wt.% SCB (both raw and SE) are primarily composed of Si (35–45 wt.%), K (10–15 wt.%) and Ca, Al, Fe and Mg (5–10 wt.%). SCB slagging propensity is qualified as severe. The slag deposit formed by SE-SCB was less molten and more sintered. SE has positive impact on slagging behavior. Element deposit composition of SCB [wt.%]: 44.3 (O); 29.3 (Si); 7.3 (Fe); 5.5 (Al); 4.6 (K); 2.9 (Ca); 2.6 (C); 1.6 (Ti); 1.2 (Mg) Element deposit composition of SE-SCB [wt.%]: 47.8 (O); 31.8 (Si); 6.4 (Fe); 2.9 (Al); 2.9 (K); 2.5 (Ca); 2.0 (C); 1.6 (Ti); 1.2 (Mg) Specific fouling factor [(K.m2)/(W.MJ)]: 1.66 (SCB); 0.62 (SE-SCB); low fouling factors NOx concentration [g(NO)/GJ fuel]: ~150 (SCB); ~155 (SE-SCB) | [246] |
| Bagasse (Chile) | Technique: CFD model. Continuous phase (gas mix): (volatiles, O2, CO2, water vapor, CO and N2) Biomass flow rate [kg/s]: 22.63 at 300 K Air flows [kg/s]: 33.64 at 544 K (primary air); 42.04 at 625 k (secondary air); 7.76 at 544 K (pneumatic air) | Furnace outlet T [°C]: 900 Larger particle size yield a more complete and efficient combustion, but are more likely to reach the rear wall and increase the possibility of slagging. For particle size (1.78 mm): Moisture [%] = 23.50 (grate), 0.00 (exit); Volatiles [%]: 86.60 (grate), 0.11 (exit); Char [%]: 96.67 (grate), 1.67 (exit) Gas flow at furnace exit [g/s]: 5.37 Gas flow components at furnace exit [kg/s]: 7.77 (O2); 15.27 (CO2); 18.37 (H2O); 4.19 (CO); 64.28 (N2) | [247] | |
| Eucalyptus | Wood and bark (Pakistan) | Technique: TGA curve analysis Heating rate [°C/min]: 25, 35, 45 Operational temperature [°C]: 25–950 | Heating rate [25]: Ti [°C]: 260; Tm [°C]: 420; Tb [°C]: 900; CCF: 1.1; Rm [%/s·°C]: 1.9 Heating rate [35]: Ti [°C]: 270; Tm [°C]: 370; Tb [°C]: 930; CCF: 1.5; Rm [%/s·°C]: 3.5 Heating rate [45]: Ti [°C]: 280; Tm [°C]: 540; Tb [°C]: 940; CCF: 1.2; Rm %/s·°C]: 1.8 Insignificant contents of sulfur and nitrogen were detected in the wood, which would reduce the environmental impacts in terms of SOx and NOx emissions. Rm (Mean Reactivity); CCF (Combustion characterization factor) | [248] |
| Wood (Chile) | Technique: Combustion in controlled combustion chamber for emissions Wood MC [wt.%]: 0 and 25 | Gas pollutants emissions [vol%]: At 0% MC: Max CO2: 10.66% vol; max CO: 2077 ppm; higher T: 537 °C Combustion efficiency [%]: 93.6 Emissions factor [g/kg]: 38.98 (CO); 1701.62 (CO2) Emission factor for PM2.5 [g/kg]: 2.01 Emission factors of total PAHs [ng/g]: 5215.47 At 25% MC: Max CO2: 1.25% vol; max CO: 3742 ppm; higher T: 236 °C Combustion efficiency [%]: 49.3 Emissions factor [g/kg wood): 104.84 (CO); 795.04 (CO2) Emission factor for PM2.5 [g/kg]: 22.90 Emission factors of total PAHs[ng/g]: 7644.48 | [249] | |
| Pinus | Wood (China) | Technique: TGA curve analysis Heating rate [°C/min]: 5 and 40 Operational temperature [°C]: 50–600 Air flow rate [mL/min]: 60 | In the study, combustion performance of pine wood was compared with bamboo branches and Lentinus edodes, having the pine wood the best combustion performance. Combustion index [×10−7%/min2 K]: 1.97 (5 °C/min), 70.37 (40 °C/min) Flammability index [10−4%/min K2]: 1.12 (5 °C/min), 5.88 (40 °C/min) Ignition index [−2%/min3]: 0.27 (5 °C/min), 86.70 (40 °C/min) Burn out index [−2%/ min4]: 0.002 (5 °C/min), 5.296 (40 °C/min) | [250] |
| Wood (USA) | Reactor: Integrated Exposure Generation System (platform developed by the authors) Wood MC [%]: 6 and 24 Combustion condition: Flaming (F), Smoldering (S), Incomplete combustion (IC). Final temperature [°C]: 400 (F and IC) and 250 (S), Heating rate [°C/min]: 20 (F, S and IC) O2 [%vol]: 20.9 (F and S) and 5 (IC) | Bulk inorganic element concentration [wt.%]:0.0468 Relative wood inorganics [%wt.]: 49 (Ca); 15 (K); 14 (Mg); 12 (Al); 4 (S); 2 (Mn); 2 (Na). Gas pollutants: CO emissions [ppm] Moisture effect on [CO ppm]: 63 (6% MC); 49 (24% MC) Combustion condition effect on [CO ppm]: 63 (F); ∼0.3 (S); 13 (IC) VOC emissions [ppb] Moisture effect on [VOC ppb]: 2415 (6% MC); 2436 (24% MC) Combustion condition effect on [VOC ppb]: 2415 (F); 580 (S); 3021 (IC) | [251] | |
| Scenario IV—Gasification | ||||
| Soybean | Straw (Brazil) | CFB gasifier in Aspen PlusTM Assumptions: zero-dimensional; steady-state; isothermal conditions; drying and pyrolysis occur instantaneously; inert ashes; char is 100% carbon; fuel-bound N, S, and Cl are converted into NH3, H2S, and HCl, respectively; heat loss neglected; thermodynamic model: Peng-Robinson with Boston-Mathias (PR-BM); feedstock particle size and density not influence; gasifier operated below the ash melting point. Temperature [°C]: 779–920.71 | Syngas composition [%vol]: 14.07–45.24 (H2); 5.68–20.88 (CO); 20.58–37.29 (CO2); 13.12–40.97 (CH4) HHV syngas [MJ/m3]: 13.13–18.33 Flow rate syngas [kg/h]: 19.62–21.69 Heat duty gasifier [MJ/kg]: 4.59–8.82 H2/CO: 1.44–3.92 The cold gas efficiency [%]: 68.46–77.22 | [252] |
| Straw (Canada) | Fixed bed tubular batch reactor Conditions: subcritical water (300 °C) and supercritical water (400 and 500 °C). Biomass-to-water ratio: 1:5 and 1:10 Biomass particle size [mm]: 0.13 and 0.8 Residence time [min]: 30–60 Pressure range [MPa]: 22–25 Hydrothermal gasification process using Aspen Plus program. | Maximum H2 yield [mmol/g]: 6.62 Total gas yields [mmol/g]: 14.91 Carbon gasification efficiency [%]: 20.2 Lower heating value [kJ/Nm3]: 1592 Hydrogen selectivity [%]: 63.0 Product yield [wt.%]: 6.28 ± 0.33–8.13 ± 0.30 (Solid product); 57.92 ± 1.41–75.46 ± 0.64 (Liquid product); 3.14 ± 0.16–4.54 ± 0.01 (gas product). ratio of the experimental yield to the equilibrium yield values of CH4, CO2 and H2 yields for the non-catalytic gasification of soybean straw at 500 °C were 17.9%, 27% and 57.6%. | [57] | |
| Rice | Husk (Brazil) | CFB gasifier in Aspen PlusTM Temperature [°C]: 779–920.71 | Syngas composition [%vol]: 15.72–47.72 (H2); 4.26–19.93 (CO); 21.31–39.57 (CO2); 11.23–39.19 (CH4) HHV syngas [MJ/m3]: 12.42–17.94 Flow rate syngas [kg/h]: 17.03–19.58 Heat duty gasifier [MJ/kg]: 3.46–7.36 H2/CO: 1.65–4.48 The cold gas efficiency [%]: 66.55–76.29 | [252] |
| Husk (Indonesia) | Fixed bed downdraft reactor Air at equivalence ratio: 0.15, 0.20, 0.25 Air flows [m3/h] = 1.07, 1.43, 1.79 Temperature [°C] = 600–800 Reaction time [1/min]: 10–30 | Syngas composition; H2 (8.05%,), CO (15.41%,), CH4 (<2%). Cold gas efficiency of the gasifier = 72.73% gas yield: 4.33 Nm3/gas. Tar formed from 5.8 to 53.3 g/Nm3 | [253] | |
| Wheat | Straw (Brazil) | CFB gasifier in Aspen PlusTM Temperature [°C]: 779–920.71 | Syngas composition [%vol]: 11.16–41.95 (H2); 4.70–22.95 (CO); 19.51–38.51 (CO2); 16.29–43.82 (CH4) HHV syngas [MJ/m3]: 13.97–19.50 Flow rate syngas [kg/h]: 20.96–23.03 Heat duty gasifier [MJ/kg]: 8.22–12.91 H2/CO: 1.00–3.25 The cold gas efficiency [%]: 71.57–81.41 | [252] |
| Corn | Straw (Brazil) | CFB gasifier in Aspen PlusTM Temperature [°C]: 779–920.71 | Syngas composition [%vol]: 13.88–44.68 (H2); 4.51–21.24 (CO); 22.52–42.02 (CO2); 11.81–37.04 (CH4) HHV syngas [MJ/m3]: 12.34–17.27 Flow rate syngas [kg/h]: 19.37–21.78 Heat duty gasifier [MJ/kg]: 3.14–7.08 H2/CO: 1.38–3.82 The cold gas efficiency [%]: 68.09–77.29 | [252] |
| Coffee | Husk | Reactor type: Fluidized bed gasifier T [°C]: 790 Airrate/Biomassadmission [kg/h/Nm3/h]: 0.48 | Syngas Composition (vol%): H2 = 12.4; CO = 11.4; CH4 = 1.6; CO2 = 18.7; N2 = 52.3; C2H4 = ~4.36; C2H6 = ~1.01; C2H2 = ~3.86. HHV (MJ·Nm−3): 3.34 | [254] |
| Banana | Stem | Reactor type: pilot-scale plant Operational temperature [°C]: 368 Biomass [g]: 11.75 Particle size [mm]: 1.84 Atmospheric pressure Catalyzer: Ni/Al2O3 [Ni% w/w]: 1.5, 2.5 and 5 Fluidization agent: superheated water vapor | Gas composition [% molar]: Ni [0%]: 25.79 (H2); 47.15 (CO2); 3.87 (CO); 20.32 (C2H4); 2.21 (CH4). HHV [kcal/kg]: 3342.5, LHV [kcal/kg]: 3077.4 Best hydrogen yield: Ni [2.5%]: 51.78 (H2); 22.54 (CO2); 0 (CO); 25.01 (C2H4); 0.44 (CH4). HHV [kcal/kg]: 5057.0, LHV [kcal/kg]: 4604.0 | [255] |
| Peel | Reactor type: fixed bed Heating rate [°C/min]: 10 Gasification agent: Steam (200 °C) Biomass [g]: 1 Operational temperature [°C]: 650–850 Operational time [h]: 2 | Ash composition: 3.5 (Ca); 67.3 (K); 2.4 (Na); 2.8 (Si); 21.8 (Cl); 2.2 (other) 50% weight loss (T50) [°C]: 386.1 Best hydrogen yield at 850 °C Carbo conversion efficiency increase as temperature increase having a max near to 70% at 850 °C | [48] | |
| Orange | Bagasse (USA) | Technique: Gasification under TGA curve analysis For gasification: (1) the pyrolysis step at 20 K/min to 800 °C, (2) isothermal step of 15 min to stabilize the weight in the TGA Gasifying agent: 100% CO2 Particle size [mm]: 0.6–0.8 | Reactivity [min−1]: ~0.15 The high reactivity of orange peel char is attributed to its high potassium content (catalytic role) Ash content and inorganic elements [%]: ~3 (ash feed basis); 12 (ash char basis); 0.68 (Ca); 0.007 (Fe); 3.8 (K); 0.11 (Mg); 0.005 (Al); 0.23 (P); 0.14 (S); 0.07 (Si) Inorganic index: 3.65 Time for 95% conversion [min]: 3 | [256] |
| Bagasse (Italy) | Reactor type: bench-scale fluidized bed reactor Operational temperature [°C]: 700, 750 and 850 Steam to biomass [wt/wt]: 0.5, 0.75, 1 and 1.25 Particle size [mm]: 0.4–1 Biomass flow rate [g/min]: 0.3–2 Gasifying agent: air-steam | Syngas composition and H2 yields [Nm3/kgbiom] are function of (S/B) and T. Max H2 concentration [%vol]: 26.5 (S/B: 1.5; T: 750 °C) Max H2 yield [Nm3/kgbiom]: 0.69 (S/B: 1.5; T: 750 °C) Max syngas yield [Nm3/kgbiom]: 2.45 (S/B: 1.5; T: 750 °C) At 750 °C, as the S/B increases from 0.5 to 1.25, the N2% vol decreases from 44% to 41% Max carbon efficiency: ~ 0.90 (S/B: 0.5; T: 850 °C) Max cold gas efficiency: 0.64 (S/B: 0.5; T: 850 °C) | [257] | |
| Coconut | Shell (India) | Reactor type: fixed bed downdraft reactor Gasifying medium: air Equivalence ratio (ER): 0.1–0.45 | Gas composition: CO [%]: ~11 (ER: 0.1) to ~18 (ER:0.35) H2 [%]: ~11 (practically constant throughout the process) CH4 [%]: ~10 (ER: 0.1) to ~ 4 (ER: 0.35) Max HHV [MJ/Nm3]: 4.229 (ER: 0.35) Specific gas generation [m3 of gas/kg of fuel]: 2.1 (ER: 0.1); 3.05 (ER: 0.45) Max cold gas efficiency [%]: 72.47 (ER: 0.35) Max hot gas efficiency [%]: 78.37 (ER: 0.35) Optimun operational T [°C]: 900 (ER: 0.35) Tar in gas at optimum operational condition [g/m3]: 0.62; Particle matter in gas at optimum operational condition [g/m3]: 0.215 | [258] |
| Husk (India) | Reactor type: packed bed gasification column Biomass [g]: 20 Particle size [mm]: 0.25, 0.72, 2 and 3 Operational temperature [°C]: 700–850 Heating rate [°C/min]: 50 Gasifying medium: air Air relative humidity [%]: 55–95 Equivalence ratio (ER): 0.1–0.4 | Gasification started after 700 °C T effect (p.s: 0.72, ER: 0.1): Max H2 in flue gas [mole]: 7.67 (800–850 °C) Max CO in flue gas [mole]: ~16 (850 °C) Max CH4 in flue gas [mole]: 7.17 (700 °C); Max GY [Nm3/kg]: 0.78 Max carbon conversion (C-conv) [%]: 22.18 Max HHV [MJ/Nm3]: 4.9 (850 °C) ER effect (p,s: 0.72, T: 800 °C): Max H2 in flue gas [mole]: ~ 8 (0.1 ER) Max CO in flue gas [mole]: ~18 (0.2 ER) Max CH4 in flue gas [mole]: 7.41 (0.3 ER); Max GY [Nm3/kg]: 2.89 Max carbon conversion (C-conv)[%]: 77.53 Max HHV [MJ/Nm3]: ~5.4 (0.2–0.3) RH effect (p.s: 0.72, T: 800 °C, ER: 0.1): Max H2 in flue gas [mole]: 10.26 (0.1 ER) Max CO in flue gas [mole]: ~18 (0.2 ER) Max CH4 in flue gas [mole]: 13.13 (0.3 ER); Max GY [Nm3/kg]: 1 Max carbon conversion (C-conv)[%]: 42.56 Max HHV [MJ/Nm3]: 8.81 (95% RH) Biochar specific surface [m2/g]: 173.42 Total pore vol [cm3/g]: 0.074733 | [259] | |
| Sugarcane | Bagasse | Technique: Simulation in ASPEN Gasification process divided in four steps: heating and drying, pyrolysis, gas–solid reactions, and gas phase reactions. Zero-dimensional and time independent reactions were considered. The model is considered in thermodynamic equilibrium, it is not necessary the use of reaction kinetics or hydrodynamics of the reactor. Gasification temperature [°C]: 100–1000Steam to biomass [wt/wt]: 0.3–1 | The higher the steam temperature, the higher the LHV [MJ/kg]: ~14.8 (100 °C); ~15.0 (600 °C); ~15.2 (1000 °C) The higher the air temperature, the higher the LHV [MJ/kg]:~14.55 (10 °C); ~15.0 (40 °C); ~15.3 (60 °C) The higher the gasification temperature, the higher the LHV [MJ/kg]: ~14.38 (600 °C); ~14.85 (600 °C); ~15.1 (1200 °C) Max LHV as a function of S/B between 0.5 and 0.6: ~15.05 Average LHV [MJ/kg]: 14.9 Syngas composition [%]: 45–40 (CO2); 31–35 (CO); 16–19 (CH4); 3–6 (H2); 0.3 (N2) Dry flue gas composition [%]: 69.24 (N2); 19.61 (CO2); 11.15 (H2O) | [260] |
| Bagasse | Technique: Simulation (tri-generation system) The biomass was assumed free of ash, dry, N and S and comprising C, H and O. Biomasses are gasified into the gasifier using the waste heat of the Homogenous Charge Compression Ignition (HCCI) engine Operational temperature [°C]: 600 LHV [kJ/mole]: 467 Qin. Gasification [kW]: 2.33 | Syngas composition [%wt.]: 48.08 (H2); 18.86 (CO); 24.29 (CO2); 8.77 (CH4) Cold gas efficiency [%]: 73 Hydrogen efficiency [%]: 34 Exergy efficiency of gasifier [%]: 90 Exergy results [kW]: 3538 (biomass); 83.51 (steam); 1.536 (gasifier inlet heat); 3243 (syngas) | [261] | |
| Eucalyptus | Wood | Reactor: Batch using NiFe2O4 as a catalyzer Operational temperature [°C]: 400, 450 and 500 Residence time [min]: 30, 40 and 60 Catalyst amount (Cat) [g]: 0, 1 and 2 Gasification agent: Super critical water (SCW) | Under same T, as increase the catalyst increase the GY Rx (450 °C, 30 min): best GY[wt.%]: 58.28 (1 g cat); best conversion[%]: 89.12 (2 g cat] Rx (450 °C, 40 min): best GY[wt.%]: 52.57 (1 g cat); best conversion[%]: 92.84 (2 g cat] Rx (450 °C, 60 min): best GY[wt.%]: 48.19 (1 g cat); best conversion[%]: 95.49 (2 g cat] Highest GY [wt.%]: 65.94 (60 min, 500 °C, 2 g cat) Highest H2 [mol%]: 22.69 (60 min, 450 °C, 2 g cat) HGE (60 min, 450 °C) [%]: 11.1 (0 g cat); 30.62 (2 g cat) CGE (60 min, 450 °C) [%]: 69.6 (0 g cat); 97.03 (2 g cat) | [248] |
| Wood | Reactor: pilot scale bubbling fluidized bed Biomass flow rate (bfr) [kg/h]: 57.8 to 94 Bed reactor temperature [°C]: 700–900 Gasification agent: air | Highest H2 [mol%]: 14.7 (94 kg/h bfr, 764 °C) Highest cold gas efficiency [%]: 0.74 (64.5 kg/h bfr, 846 °C) Highest CGE [%]: 0.94 (kg/h bfr, 887 °C) Highest syngas LHV [MJ/m3): 5.9 (95.5 kg/h bfr, 795 °C) | [262] | |
| Pinus | Wood | Technique: Aspen Plus simulation Operational temperature [°C]: 700, 750, 800, 850 and 900 Particle size [mesh]: 60, 80, 100 Steam-to-biomass mass (S/B): 0, 0.7, 1.4, 2.1 and 2.8 Gasification agent: Steam (200 °C) Biomass flow rate [g/min]: 3 | Syngas composition [%vol] at 900 °C: 100 mesh: 25.24 (CO); 32.74 (H2); 2.28 (CH4); 12.12 (CO2) 80 mesh: 52.83 (CO); 30.53 (H2); 1.95 (CH4); 14.69 (CO2). Best performance 60 mesh: 54.49 (CO); 25.89 (H2); 1.79 (CH4); 16.96 (CO2) Temperature effect at mesh 80 (700 to 900 °C): CO and CH4 decreases by 4.17% and 6.95%, respectively. H2 and CO2 increased by 5.43% and 5.69%, respectively. Optimal T [°C]: 850. S/B effect at mesh 80 and 850°C (0.7 to 2.8): CO and CH4 decreases by 19.52% and 1.9%, respectively. H2 and CO2 increased by 6.78% and 13.74%, respectively. Optimal S/B: 1.4 | [263] |
| Wood (Brazil) | Reactor: downdraft gasifier and combustion engine coupled to a power generator. Not SteadyState entirely operation Particle size [cm]:1–2.5 | Syngas composition [%wt.]: 12.72 (H2); 24.78 (CO); 11.1 (CO2); 2.1 (CH4). LHV [MJ/kg]: 5.51 1 kg of produced gas requires about 0.64 kg of air. Average E/R: 0.26 Average Cold gas efficiency [%]: 69.4 (18% lower than manufacturer announcement) | [264] | |
| Scenario V—Fast Pyrolysis (Substitutes for Fuel Oil) | ||||
| Soybean | Hull (Brazil) | Reactor: fluidized bed reactor Reactor loaded with 800 g of inert material (sand) Temperature [°C]: 550 Velocity of the fluidizing gas (nitrogen) [cm/s]: 150 Biomass feeding rate [kg/h]: 40 | Average yield [%]: 45 (bio-oil); 33 (char); 22 (non-condensable gases). In the organic phase, the three main compounds identified in soybean hull bio-oil were: phenol (14.88%), 2-methylphenol (7.59%) and 4-methylphenol (12.55%). Bio-oil organic: 64.66 wt.% (C); 6.68 wt.% (H); 5.80 wt.% (N); 1.17 wt.% (S); 21.69 wt.% (O); 24.28 MJ/kg (HHV); 22.83 MJ/kg (LHV) Bio-oil aqueous: 13.31 wt.% (C); 2.15 wt.% (H); 3.01 wt.% (N); 0.42 wt.% (S); 81.11 wt.% (O); 6.89MJ/kg (HHV); 6.42 MJ/kg (LHV) | [265] |
| Straw | Bubbling fluidized bed reactor Temperature [°C]: 500 | Average yield [%]: 67 (biooil); 28.5 (biochar); 4.25 (syngas) Bio-oil organic: 67.24 wt.% (C); 47.37 wt.% (H); 50.34 wt.% (O) | [266] | |
| Rice | Husk (Brazil) | Reactor: Laboratory-scale fluidized bed with a SiC bed. Feed rates [g/L]: 875 Carrier gas to biomass ratio [wt/wt]: 0.8 Temperatures [°C]: 450, 525, and 600 SiC bed heights [cm]: 4.9 and 6.5 | Product yield: 43% (max liquid); 31% (organics); 12% (water); 32% (solids); 25% (gas and losses). | [267] |
| Corn | Stalk (USA) | Temperature [°C]: 400–450 Acid pretreated and untreated corn stalks were pyrolyzed Feed rate [kg/h]: 1–2.5 | Average yield [%]: 35–46 (biooil); 20.4–29 (biochar); 4.4–32 (syngas) Elemental analysis untreated stalk bio-oil: 19.05% C, 9.31% H, 0.17% N, 71.46% remaining. Acid-treated stalk bio-oil: 24.88% C, 5.33% H, 0% N, 69.79% remaining | [268] |
| Coffee | Husk (Brazil) | Biomass in [g]: 100 g Stirring rate [rpm]: 64 Heating rate [°C/min]: 20 T [°C]: 500 | Yield [%]: SY (26.2–28.7); LY (47.5–56.5); NCGY (18.6–24.8) Biochar composition [% db]: C (73.75 ± 0.5); H (1.99 ± 0.1); N (1.90 ± 0.2); O (6.00 ± 0.3) HHV biochar [MJ/kg]: 24.6 ± 0.28 LHV biochar [MJ/kg]: 23.16 ± 0.26 Proximate analysis biochar [wt.% db]: VM (9.5 ± 1.18); FC (73.5 ± 1.48); AC (17 ± 0.63) MC [wt.% wb]: 1.89 ± 0.14 Apparent density biochar [kg/m3]: 401 ± 6 Specific density biochar [kg/m3]: 770 ± 10 Porosity biochar [−]: 0.48 Moisture aqueous phase (at different temperature ranges) [%wb]: 82.05 ± 0.24 (25–200 °C); 77.22 ± 0.22 (200–250 °C); 61.09 ± 0.29 (250–300 °C); 55.31 ± 0.37 (300–350 °C); 29.55 ± 0.23(350–400 °C); 22.76 ± 0.22 (400–500 °C) HHV aqueous phase (at different temperature ranges) [MJ/kg]: 16.77 ± 0.45 (25–200 °C); 17.17 ± 0.40 (200–250 °C); 21.75 ± 0.33 (250–300 °C); 27.87 ± 0.38 (300–350 °C); 30.63 ± 0.42(350–400 °C); 33.51 ± 0.29 (400–500 °C) pH aqueous phase (at different temperature ranges) [−]: 3.63 ± 0.01 (25–200 °C); 4.12 ± 0.01 (200–250 °C); 4.74 ± 0.01 (250–300 °C); 6.44 ± 0.01 (300–350 °C); 7.87 ± 0.01 (350–400 °C); 8.19 ± 0.01 (400–500 °C) | [269] |
| Banana | Leaves (India) | Technique: TGA curve analysis Particle size [µm]: 250 Heating rate [°C/min]: 10, 20, 30 Operational temperature [°C]: 22–900 | Heating rate [10]: Ti [°C]: 151.5; Tb [°C]: 493; Tm [°C]: 297 Max mass decomposition [μg/min]: 437 Heating rate [20]: Ti [°C]: 159; Tb [°C]: 499; Tm [°C]: 304 Max mass decomposition [μg/min]: 652 Heating rate [30]: Ti [°C]: 163; Tb [°C]: 506; Tm [°C]: 316 Max mass decomposition [μg/min]: 1131 | [270] |
| Leaves (Brazil) | Reactor type: pilot-scale plant Fluidization agent: air Flow gas rate [Nm3/h]: 15 Operational temperature [°C]: 500 Biomass feed rate [kg/h]: 0.84 | Tm [°C]: 340 LY [wt.%]: 27; SY [wt.%]: 23.3; GY [wt.%]: 49.6 Bio-oil: two phases (light and heavy) Heavy oil composition [%]: 55.9 (CO2); 7.8 (H2); 0.87 (N2); 0.08 (S); 35.3 (O2). HHV [MJ/kg]: 25.0 Light oil composition [%]: 16.9 (CO2); 8.8 (H2); (N2); 0.01 (S); 74.3 (O2). HHV [MJ/kg]: 1.2 Biochar: Proximate analysis [wt.%]: 1.68 (MC); 53.2 (VM); 23.2 (FC); 23.5 (AC) Ultimate analysis [wt.%]: 48.0 (C); 3.2 (H); 1.2 (N); 0.33 (S)HHV [MJ/kg]: 18.2 Total process energy consumption: 5.58 kWh | [271] | |
| Orange | Bagasse | Reactor type: Conical Spouted Bed Reactor. Residence time[min]: 50 min Specifications: Reactor, cyclone and filter are located in a hot box heated to 290 C to avoid condensation of heavy compounds. Particle size [µm]: 1000 Biomass flow rate [g.min−1]: 1 N2 flow rate [L.min−1]: 7 Operational temperature [°C]: 425, 500 and 600 | SY [wt%]: 33 (425 °C); 29 (500 °C); 27 (600 °C); LY [wt%]: 54.6 (425 °C); 54.9 (500 °C); 49 (600 °C) GY [wt%]: 12 (425 °C); 16 (500°); 24 (600 °C); Gas: Composition (vol%): Mainly CO2 and CO (45–80%); C1-C4, H2 and CH4 (detected but not specified). LHV [MJ.m−3]: 8.5 (600 °C). Bio-oil (500 °C): Composition [%wt]: Alcohols = 4.74; Ketone = 13.98; Furans = 21.47; Phenols = 1.71; Saccharides = 2.88; Nitrogenous compounds = 0.51; Hydrocarbons = 0.02; Unidentified = 7.2; Water = 40.81 Bio-char (600 °C): Composition [wt%]: C= 72.9; H= 2.6; N= 1.4; O= 12.2; Proximate analysis [wt%]: VM= 26.9; FC= 72.2; AC= 10.9. HHV [MJ/kg]: 27.5 Surface area [m2/g]: 4.8 Pore volume [m3/g]: 0.003 | [158] |
| Bagasse | Reactor type: Pyrex glass semi-batch. Specifications: Ice cold water is feed directly to the straight condenser using a miniature submersible pump to condense the pyrolysis vapors into liquid. Three different batches were performed, varying final temperature, heat rate and gas flow rate. Particle size [µm]: 425 Biomass [g]: 30 Operational temperature [°C]: 350, 375, 400, 425, 450, 475, 500, 525, 550, 575 and 600 Heating rate [°C/min]: 25, 50, 75 and 100 N2 flow rate [L/min]: 0.1, 0.2, 0.3, 0.4 and 0.5 | Batch 1: highest pyrolysis oil yield of 28.04 wt% at 525 °C, heating rate of 25 °C/min and N2 gas flow rate of 0.1 L/min. T [350–600 °C]: SY [wt%]: 56.71–28.26; LY [wt%]: 16.38–26.20; GY [wt%]: 20.76–37.83 Mass loss [wt%]: 1.2–3.24 Pyrolysis-gas: H2/C molar ratio: 0.04; Flow rate [L/min]: 0.09; Volume [mL]: 2030; GCV [MJ/m3]: 5.19 Batch 2: highest pyrolysis oil yield of 34.03 wt% at 75 °C/min, constant temperature of 525 °C and N2 gas flow rate of 0.1 L/min Heating rate [25–100 °C/min]: SY [wt%]: 33.02–24.87; LY [wt%]: 28.04–33.62; GY [wt%]: 31.37–34.89 Mass loss [wt%]: 2.61–3.11 Pyrolysis-gas: H2/C molar ratio: 0.04; Flow rate [L/min]: 0.12; Volume [ml]: 2270; GCV [MJ/m3]: 5.47 Batch 3: highest pyrolysis oil yield of 35.53 wt% at N2 gas flow rate of 0.2 L/min at 525 °C and heating rate of 75 °C/min N2 flow rate [0.1–0.5 °C/min]: SY [wt%]: 22.66–22.37; LY [wt%]: 34.03–30.41; GY [wt%]: 31.90–39.58 Mass loss [wt%]: 2.94–4.96 Pyrolysis-gas: H2/C molar ratio: 0.04; Flow rate [L/min]: 0.15; Volume [mL]: 2360; GCV [MJ/m3]: 5.49 Bio-char: Composition [wt%]: C = 70.13; H = 4.26; N = 0.61; O = 24.97; S = 0.03; proximate analysis [wt%]: MC = 2.14; VM = 41.26; FC = 53.58; AC = 3.02. HHV [MJ/kg]: 27.67 Molecular weight [g/mol]: 13.13; Surface area [m2/g]: 23.17; Pore Volume [m3/g]: 1.52 × 10−5 Other elements [%wt]: Si = 1.01; Mn = 0.82; Fe = 0.36; Co = 0.05; Al = 78.76 Bio-oil: Composition [wt%]: C = 54.20; H = 5.99; N = 0.02; O = 39.75; S = 0.04; AC = 1.31 HHV [MJ/kg]: 21.72. Molecular weight [g/mol]: 22.79; Total Acid Number [mgKOH/mL]: 24.73; pH = 3.21; Water content [%wt] = 21.30; Kinematic viscosity [40 °C, cSt] = 23.58; Kinematic viscosity [100 °C, cSt] = 10.11; Density [gm/cc, 15 °C] = 0.98; Flash point [°C] = 71; Fire point [°C] = 91; IBP [°C] = 93; FBP [°C] = 321 | [102] | |
| Coconut | Shell (China) | Reactors: microwave and fixed-bed reactor Catalyst (cat): Conventional ZSM-5 zeolites and ZSM-5 (25) @SBA-15 | LY [%]: 42 (ZSM-5), 68 (ZSM-5 (25)@SBA-15) Hydrocarbon yield [%]: 146 (ZSM-5), 200 (ZSM-5 (25)@SBA-15) The phenol selectivity was greater than 70% of the area, regardless of the catalyst. Microwave reactor enhanced the conversion of phenols to hydrocarbons For phenolic-rich bio-oil (14.3 wt.%) is recommended the combination of the fixed-bed reactor and core–shell hierarchical ZSM-5@SBA-15. For hydrocarbon-rich bio-oil (6 wt.%) is recommended the combination of microwave reactor and core–shell hierarchical ZSM-5@SBA-15. | [272] |
| Shell (Iran) | Reactor type: fixed-bed reactor Particle size [µm]: <150 Heating rate [°C.min-1]: 100 Flow gas rate (Ar) [mL/min]: 30 Operational temperature [°C]: 500 Reaction time [min]: 30 | Tm [°C]: 333 LY [wt%]: 50.25; SY [wt%]: 29.0; GY [wt%]: 20.75 Gas product composition [vol%]: 58.0 (CO2); 18.5 (CO); 10.9 (H2); 9.9 (CH4); 2.7 (C2–C4) Gas product LHV [MJ/Nm3]: 8.85; H2/CO ratio [-]: 0.59 Bio-oil relative components concentration [%]: 23.5 (hydrocarbon); 6.1 (alcohol); 4.2 (acid); 35.3 (phenol); 10.7 (ketone); 7.1 (ester); 5 (ether); 3.5 (furfural) Biochar specific surface [m2/g]: 26.22 Av pore diameter [nm]: 9.35 Total pore vol [cm3/g]: 0.084 | [273] | |
| Sugarcane | Bagasse | Type: reaction (semi batch reactor) Operational temperature [°C]: 500–700 Heating rate [°C/min]: 10 Particle size [mm]: 0.5 N2 flow rate [ml/min]: 200 | Best oil characteristics performance at 700 °C Density [kg/m3]: 988 Viscosity [cSt]: 9.4 Acid number [mg KO/g]: 44.7 pH: 3 Flash point [°C]: 130 Heating value [MJ/kg]: 4.3 Total phenol content [%]: 58.89 | [274] |
| Bagasse | Type: reaction (semi batch reactor) Operational temperature [°C]: 350–650 Heating rate [°C/min]: 10 and 50 Particle size [mm]: <0.25–1.7 N2 flow rate [cm3/min] | Heating rate [°C/min]: 10 Ti [°C]: 160; Tb [°C]: 500; Tm [°C]: 311 (peak 1); 440 (peak 2); LY [wt.%]: 29.41 (350 °C); 42.29 (500 °C); 38.82 (650 °C) SY [wt.%]:49.45 (350 °C); 23.47 (650 °C) GY [wt.%]: 21.14 (350 °C); 37.71 (650 °C) Heating rate [°C/min]: 10 LY [wt.%]: 31.25 (350 °C); 45.23 (500 °C); 40.39 (650 °C) SY [wt.%]:47.41 (350 °C); 24.88 (650 °C) GY [wt.%]: 21.34 (350 °C); 34.73 (650 °C) Max LY[wt.%]:45.23 (500 °C, 50 °C/min); 45.03 (particle size 0.5 mm); 44.95 (N2 flow 100 cm3/min) Bio-oil composition [wt.%]: 65.64 (C); 26.67 (O); 6.97 (H); 0.96 (N); 0.03 (S) Bio-oil density [kg/m3]: 1039 Bio-oil kinetic viscosity [cSt, 40 °C]: 14.20 HHV [MJ/kg]: 27.75 | [275] | |
| Eucalyptus | Wood | Microwave-assisted pyrolysis (for high nitrogen-containing compounds (NCCs)) Catalyst (cat): MoO3 Cat ratios (Wood/MoO3): 1/1, 2/1 and 3/1 Operational temperature [°C]: 550 | Raw wood yields [wt%]: LY: 34.12; SY: 23.78; GY: 42.1. HHV [MJ/kg]: 17.4. NCCs in bio-oil [%]: 7.81 (raw); 15.32 (1/1); Highest LY [wt%]: 41.66 (2/1) Highest GY [wt%]: 54.37 (1/1) | [276] |
| Wood (Brazil) | Pilot scale: fluidized bed Biomass flow rate [kg/h]: 20 Poor O2 atmosphere Operational temperature [°C]: 500 Fluidization gas flow [Nm3/h]: 15 | SY [wt%]: 14. Composition [wt%]: 0.38 (N2), 67.18 (C), 3.86 (H2). HHV [MJ/kg]: 26.38 LY [wt%]: 53. Composition [wt%]: 0.17 (N2), 53.63 (C), 7.37 (H2). HHV [MJ/kg]: 22.39 Bio-oil properties: 30% heavy fraction and 22% light fraction. Sulfur content [mg.kg-1]: 85. Density (at 20) [kg/m3]: 1225.6. pH: 3.3. Water content [wt%]: 14.2 Volatile organic compounds [wt%]: 0.40 (methanol), 0.27 (ethanol), 0.04 (acetone), 11.22 (acetic acid), 0.01 (furfural) | [277] | |
| Wood (Brazil) | Auto-thermal SDB-20 pilot-scale plant Feed rate [kg/h]: 15.06 Temperature [°C]: 480 ± 8 Fluidization agent: Air supplied by a blower at 13 Nm3/h, and recirculation gases, supplied by a fan at 7 Nm3/h. Quartz sand (Quartzo Brasil Minas 403/050) of 1300 kg/m3 | Heavy bio-oil energy yield of 30% and 21.4 MJ kg−1 lower heating value | [278] | |
| Pinus | Wood | Reactor: fixed bed reactor Operational temperature [°C]: 500 Fluidization agent: N2, H2, CO2 and CH4 Biomass [g]: 5 Particle size [µm]: >125 Heat rate [°C·min−1]: 10 | Fluidization agents: CH4: Highest biomass conversion (76.90%). CO content of 93.58% H2: Promote non-condensable gases formation but lower LY CH4 and CO2: LY of 27.77 wt% N2: Max HHV of 22.62 MJ/kg | [279] |
| Wood | Pilot scale. Thermomechanical pretreatment. Pretreatment: wood heated at 173 °C, for 3, 24, and 72 min, impregnated or not with acid citric (CA) solution 1.5 wt% Pyrolysis: Reactor: bubbling fluidized bed Biomass flow rate [kg/h]:0.4–0.7 Operational temperature [°C]: 450 | Raw wood yields [wt%]: LY: 54.70; SY: 17.18; GY: 19.18. HHV [MJ·kg−1]: 17.4 Highest LYs [wt%]: 60.56 (72 min, no CA), HHV [MJ/kg]: 18.6; 61.62 (3 min + CA), HHV [MJ/kg]: 18.1 highest HHV [MJ/kg]: 18.9 (24 min + CA) | [270] | |
| Agricultural Residues | Wood Residues | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Soybean | Corn | Sugarcane | Rice | Wheat | Coffee | Banana | Orange | Coconut | Eucalyptus | Pinus | |
| Carbon content (Tg) | 84.8 | 6.0 | 163.7 | 7.8 | 4.1 | 0.4 | 8.3 | 0.9 | 0.6 | 74.3 | 16.9 |
| Carbon sequestration (Tg-CO2) | 310.9 | 22.2 | 599.8 | 28.7 | 15.0 | 1.5 | 30.4 | 3.4 | 2.4 | 273 | 61.9 |
| Scenario I—Bioethanol production | |||||||||||
| Biothanol potential (v/w%) | 29.27 | 35.04 | 40.15 | 33.65 | 37.43 | 37.06 | 24.43 | 15.58 | 31.47 | 39.7 | 38.9 |
| Ethanol (GL) | 56.01 | 4.74 | 143.69 | 5.91 | 3.59 | 0.33 | 5.25 | 0.34 | 0.39 | 60.0 | 13.9 |
| Equivalent gasoline (GL) | 40.27 | 3.41 | 103.31 | 4.25 | 2.58 | 0.24 | 3.77 | 0.24 | 0.28 | 43.1 | 10.0 |
| CO2 emission from biethanol production | 5.60 | 0.47 | 14.37 | 0.59 | 0.36 | 0.03 | 0.52 | 0.03 | 0.04 | 6.0 | 1.4 |
| Carbon sequestration (Tg-CO2) | 85.37 | 7.23 | 219.02 | 9.01 | 5.47 | 0.51 | 8.00 | 0.52 | 0.59 | 91.4 | 21.1 |
| Scenario II—biogas production | |||||||||||
| Biogas potential (Gm3) | 105.2 | 7.6 | 226.4 | 9.0 | 5.8 | 0.6 | 12.4 | 4.8 | 0.8 | 91.6 | 21.6 |
| Equivalent energy (PJ) | 2240.0 | 162.5 | 4821.9 | 191.9 | 122.6 | 11.8 | 264.3 | 102.3 | 18.0 | 1950 | 460 |
| Carbon sequestration (Tg CO2-eq) | 136.6 | 9.9 | 294.1 | 11.7 | 7.5 | 0.7 | 16.1 | 6.2 | 1.1 | 119 | 28.1 |
| Scenario III—Combustion | |||||||||||
| Heat value (MJ/kg) | 16.9 | 17.2 | 16.3 | 16.6 | 14.6 | 16.9 | 15.5 | 15.5 | 17.8 | 17.8 | 17.8 |
| Harvested Energy (PJ) | 3230.5 | 232.3 | 5814.9 | 290.7 | 140.0 | 15.1 | 333.8 | 33.6 | 21.8 | 2694 | 636 |
| Equivalent Coal (Tg) | 125.7 | 9.0 | 226.3 | 11.3 | 5.4 | 0.6 | 13.0 | 1.3 | 0.8 | 105 | 24.7 |
| Carbon sequestration (Tg CO2) | 289.1 | 20.8 | 520.4 | 26.0 | 12.5 | 1.4 | 29.9 | 3.0 | 2.0 | 241 | 56.9 |
| Scenario IV—Gasification (ar) | |||||||||||
| Heat value of gas (MJ/Nm3) | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.0 | 6.00 | 6.00 |
| Harvested Energy (PJ) | 2295.9 | 162.5 | 4294.1 | 210.8 | 115.0 | 10.8 | 257.7 | 26.1 | 14.7 | 1813 | 428 |
| Equivalent Natural gas (Nm3) | 62.1 | 4.4 | 116.1 | 5.7 | 3.1 | 0.3 | 7.0 | 0.7 | 0.4 | 49.0 | 11.6 |
| Carbon sequestration (Tg) | 133.4 | 9.4 | 249.5 | 12.2 | 6.7 | 0.6 | 15.0 | 1.5 | 0.9 | 105 | 24.9 |
| Scenario V—fast-pyrolysis (substitutes for fuel oil) | |||||||||||
| Heat value of bio-oil (MJ/kg) | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Harvested Energy (PJ) | 1913.3 | 135.4 | 3578.4 | 175.6 | 95.9 | 9.0 | 214.7 | 21.7 | 12.2 | 1511 | 357 |
| Equivalent fuel oil (Tg) | 44.5 | 3.1 | 83.2 | 4.1 | 2.2 | 0.2 | 5.0 | 0.5 | 0.3 | 35.1 | 8.3 |
| Carbon sequestration (Tg) (fuel oil) | 133.4 | 9.4 | 249.4 | 12.2 | 6.7 | 0.6 | 15.0 | 1.5 | 0.9 | 105 | 24.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, E.P.R.; Salcedo-Puerto, O.; Nuncira, J.; Emebu, S.; Mendoza-Martinez, C. Renewable Energy Potential and CO2 Performance of Main Biomasses Used in Brazil. Energies 2023, 16, 3959. https://doi.org/10.3390/en16093959
Alves EPR, Salcedo-Puerto O, Nuncira J, Emebu S, Mendoza-Martinez C. Renewable Energy Potential and CO2 Performance of Main Biomasses Used in Brazil. Energies. 2023; 16(9):3959. https://doi.org/10.3390/en16093959
Chicago/Turabian StyleAlves, Elem Patricia Rocha, Orlando Salcedo-Puerto, Jesús Nuncira, Samuel Emebu, and Clara Mendoza-Martinez. 2023. "Renewable Energy Potential and CO2 Performance of Main Biomasses Used in Brazil" Energies 16, no. 9: 3959. https://doi.org/10.3390/en16093959
APA StyleAlves, E. P. R., Salcedo-Puerto, O., Nuncira, J., Emebu, S., & Mendoza-Martinez, C. (2023). Renewable Energy Potential and CO2 Performance of Main Biomasses Used in Brazil. Energies, 16(9), 3959. https://doi.org/10.3390/en16093959








