Ice-Templated Method to Promote Electrochemical Energy Storage and Conversion: A Review
Abstract
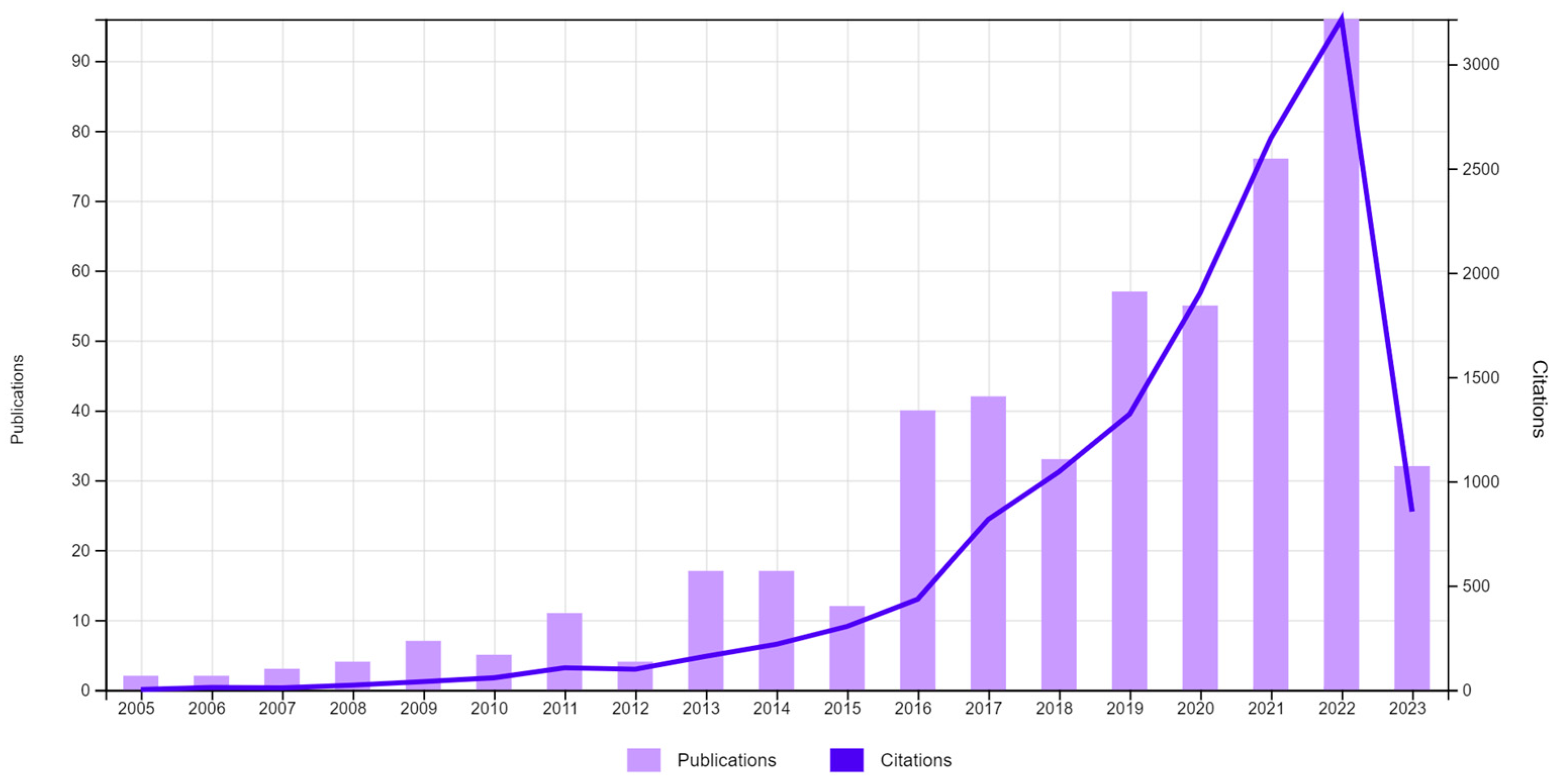
1. Introduction
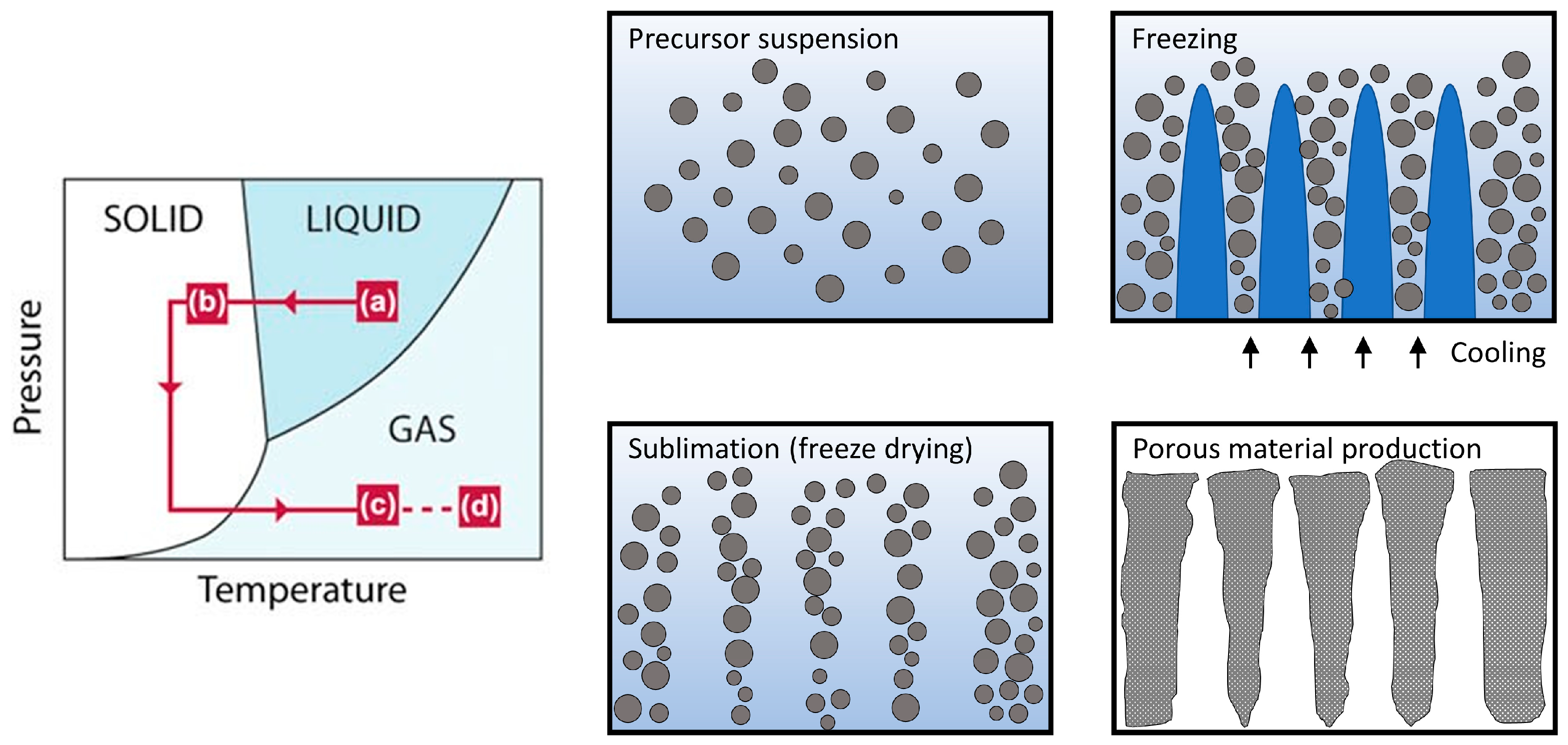
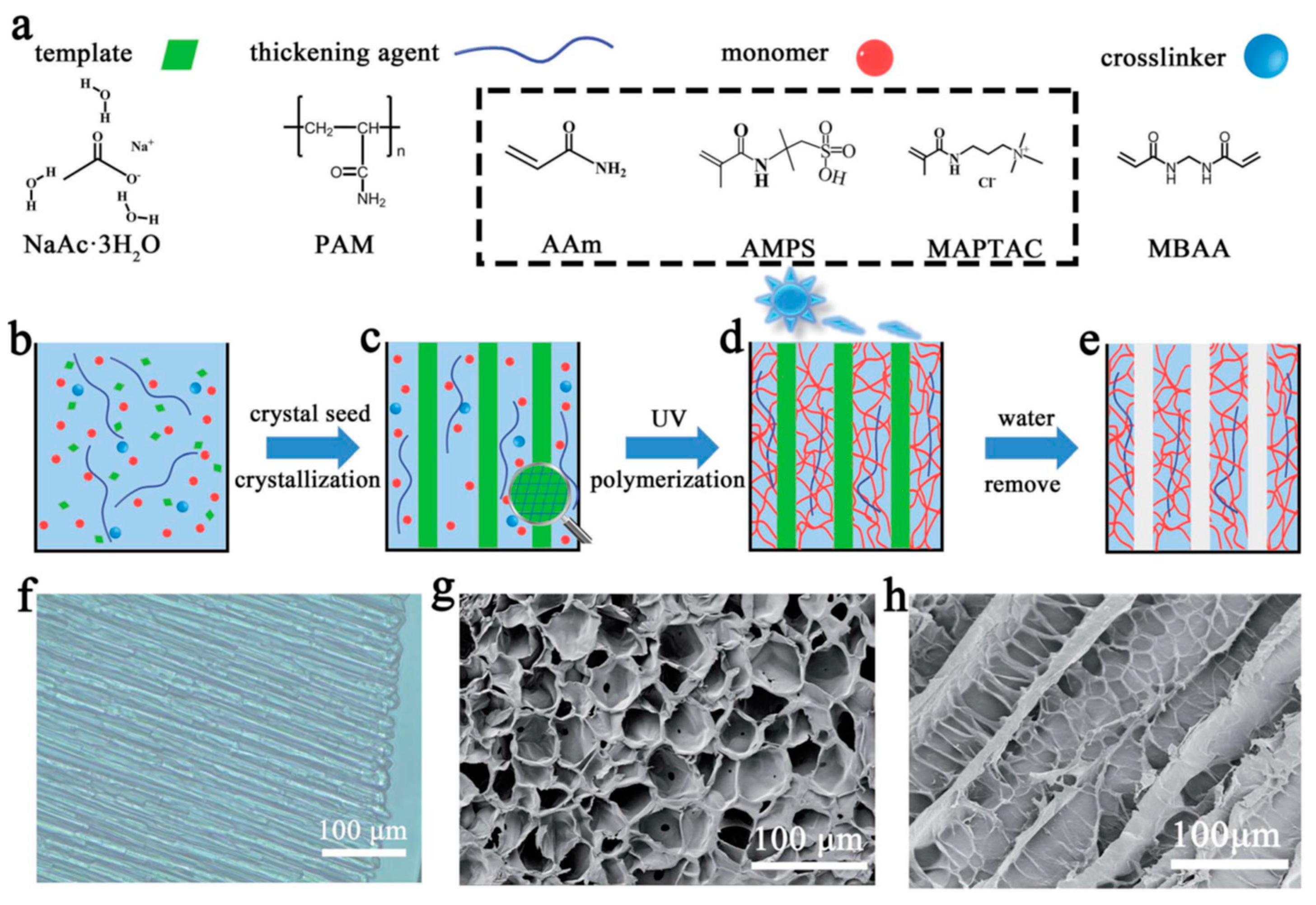
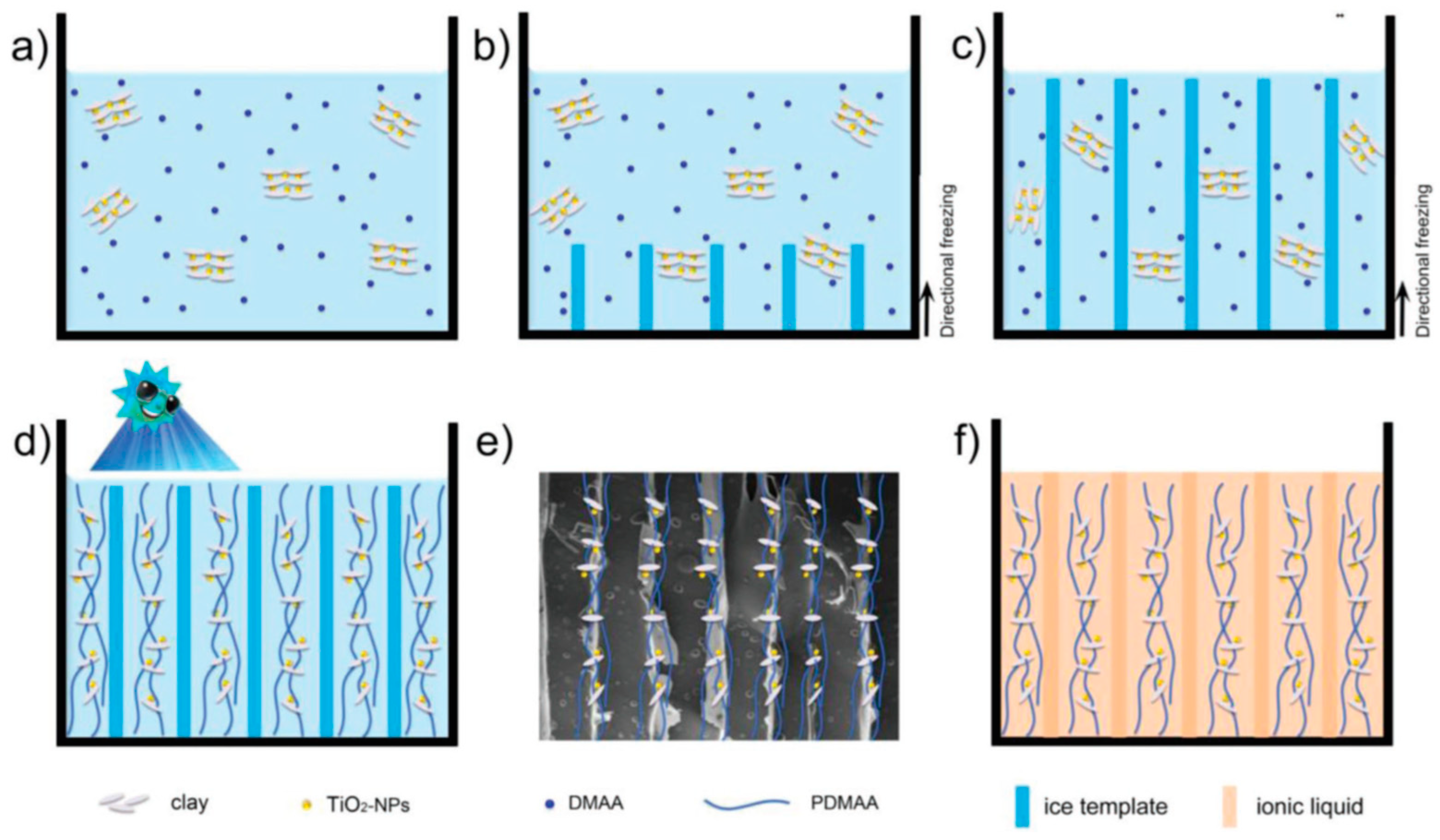
2. Methodology of Ice-Templated Method
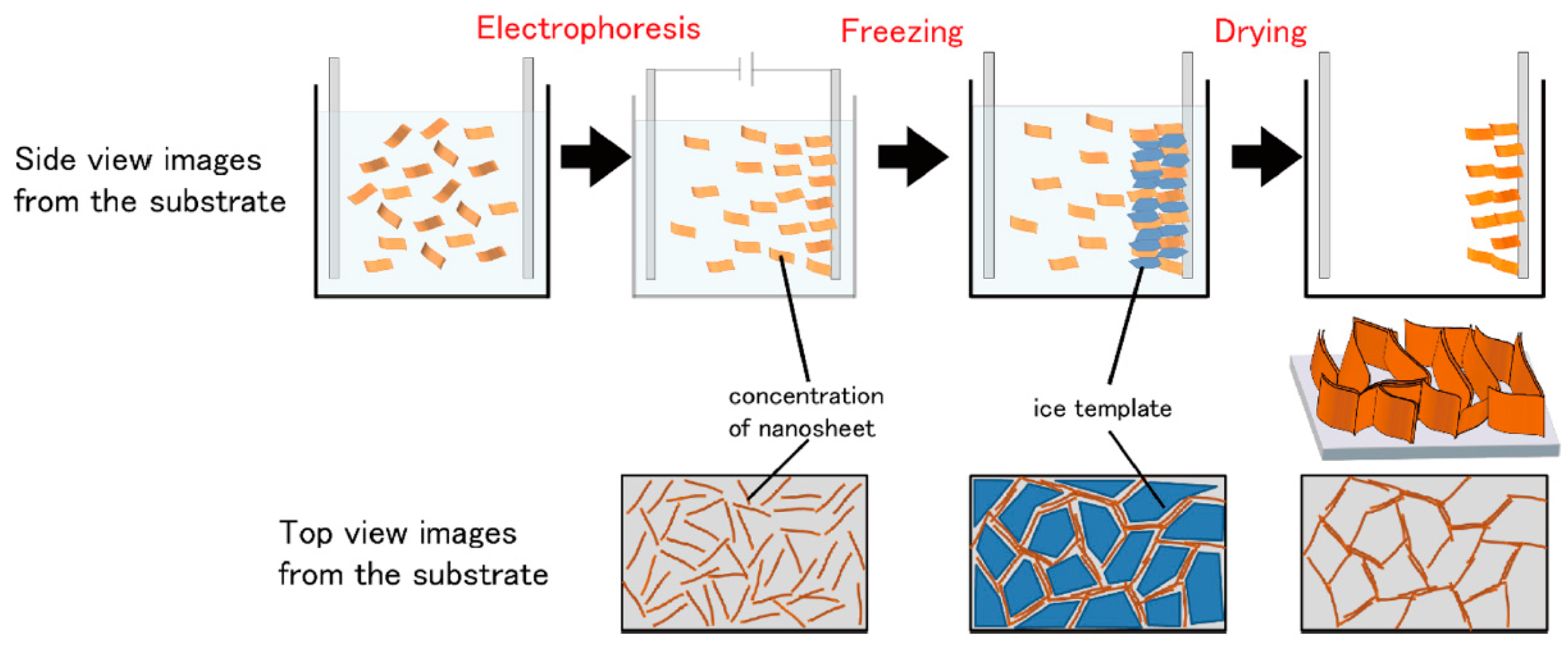
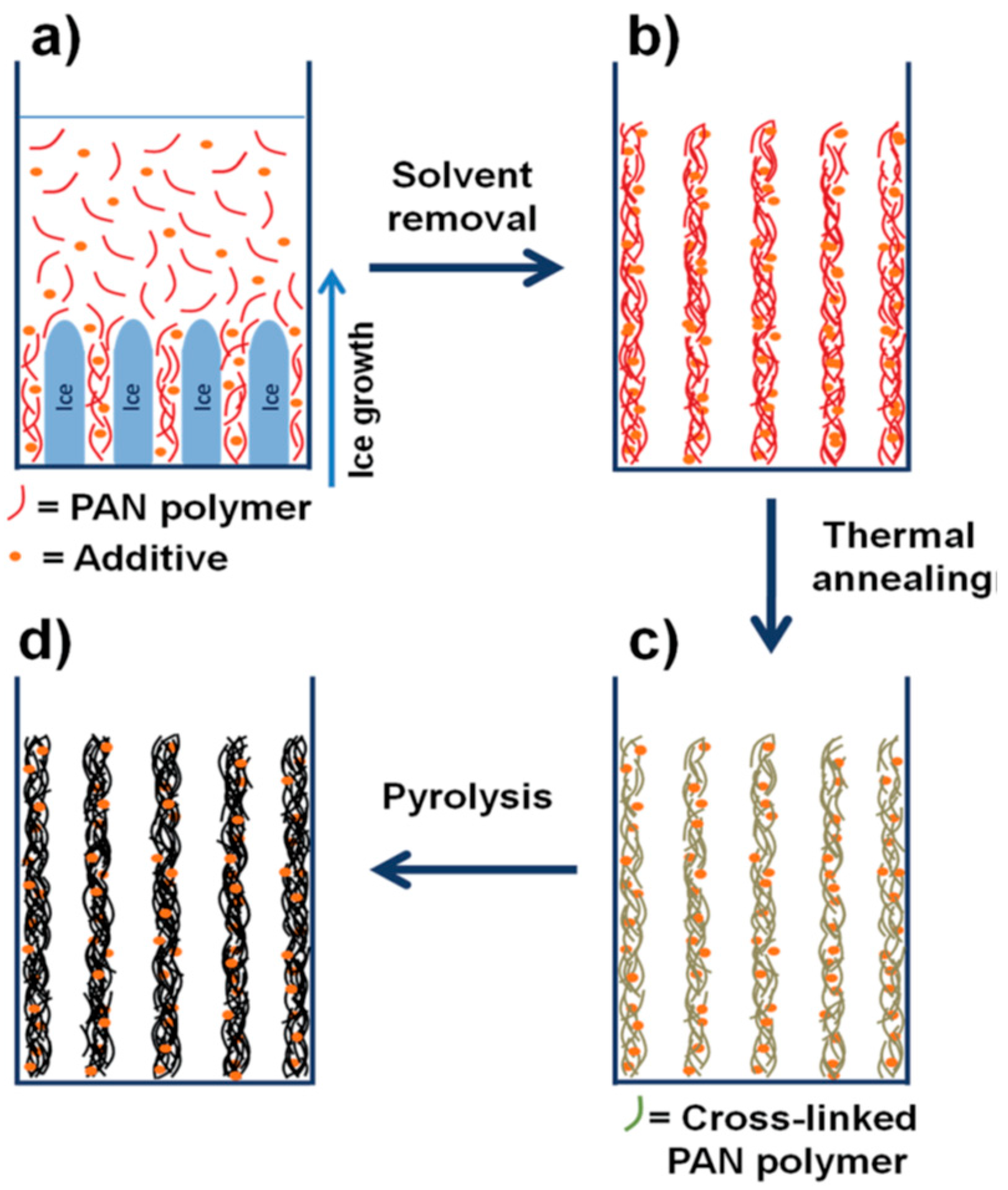
2.1. ITM Processing Steps
2.2. Type of Second Phase
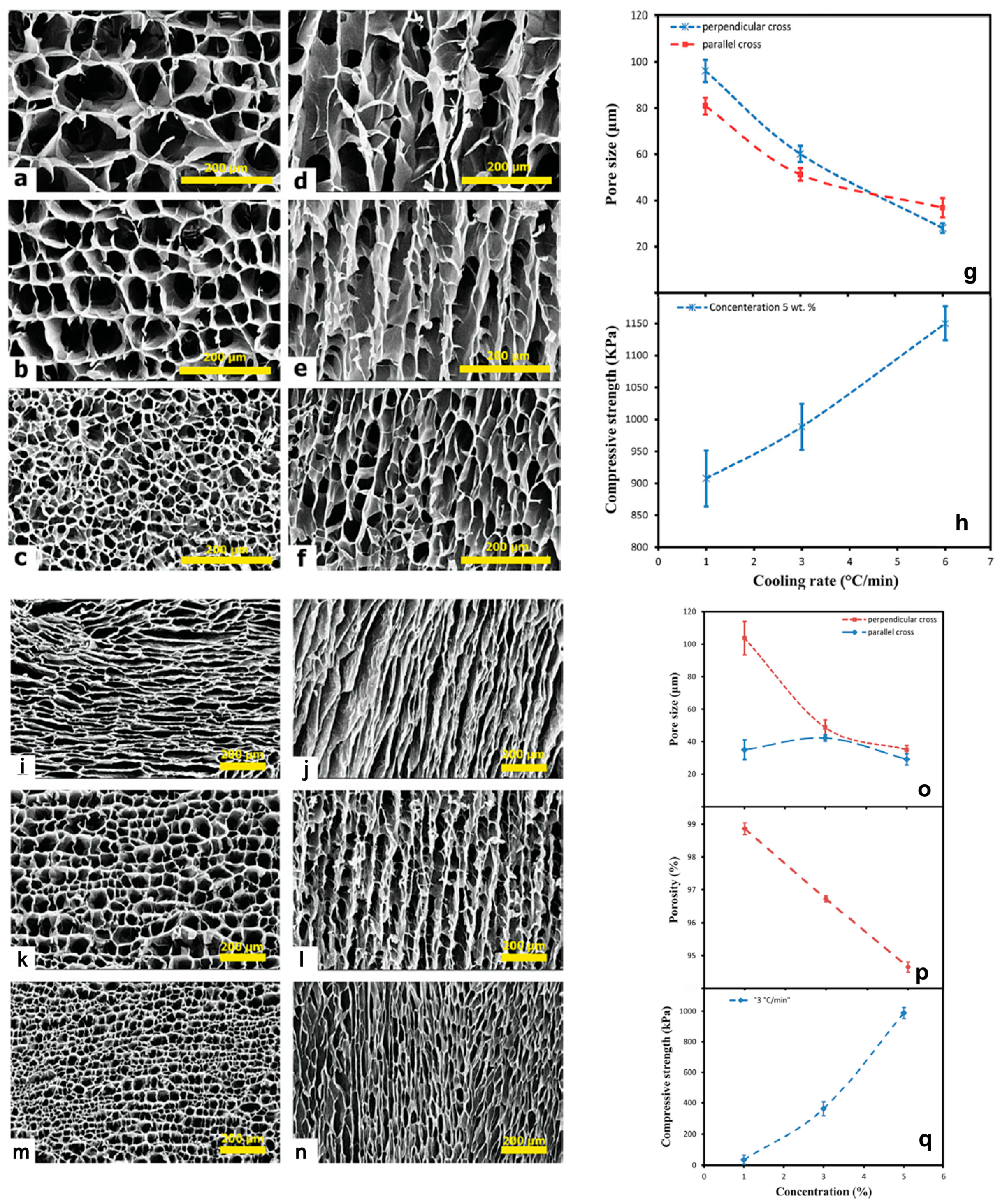
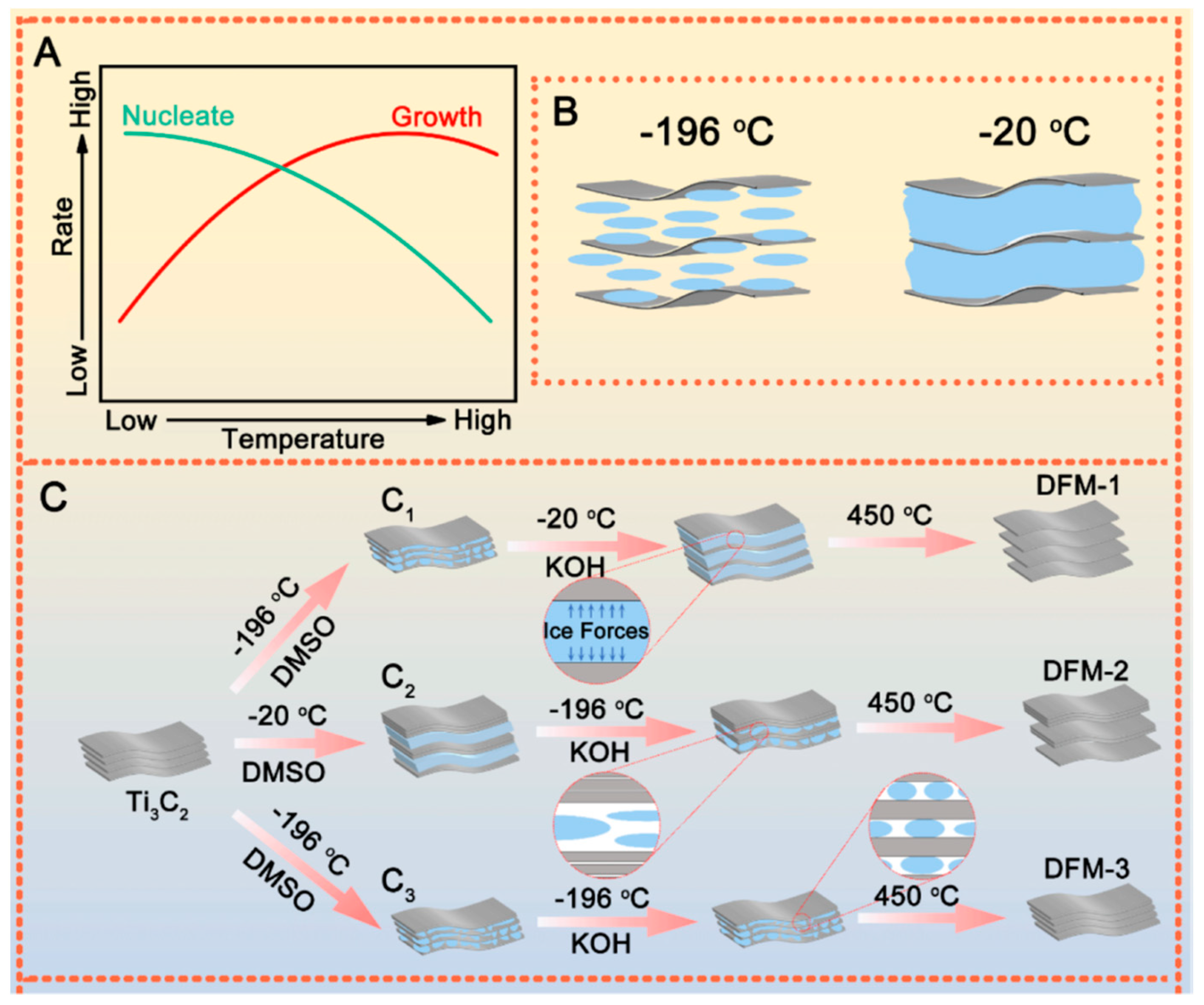
2.3. Controlling of Reaction Conditions
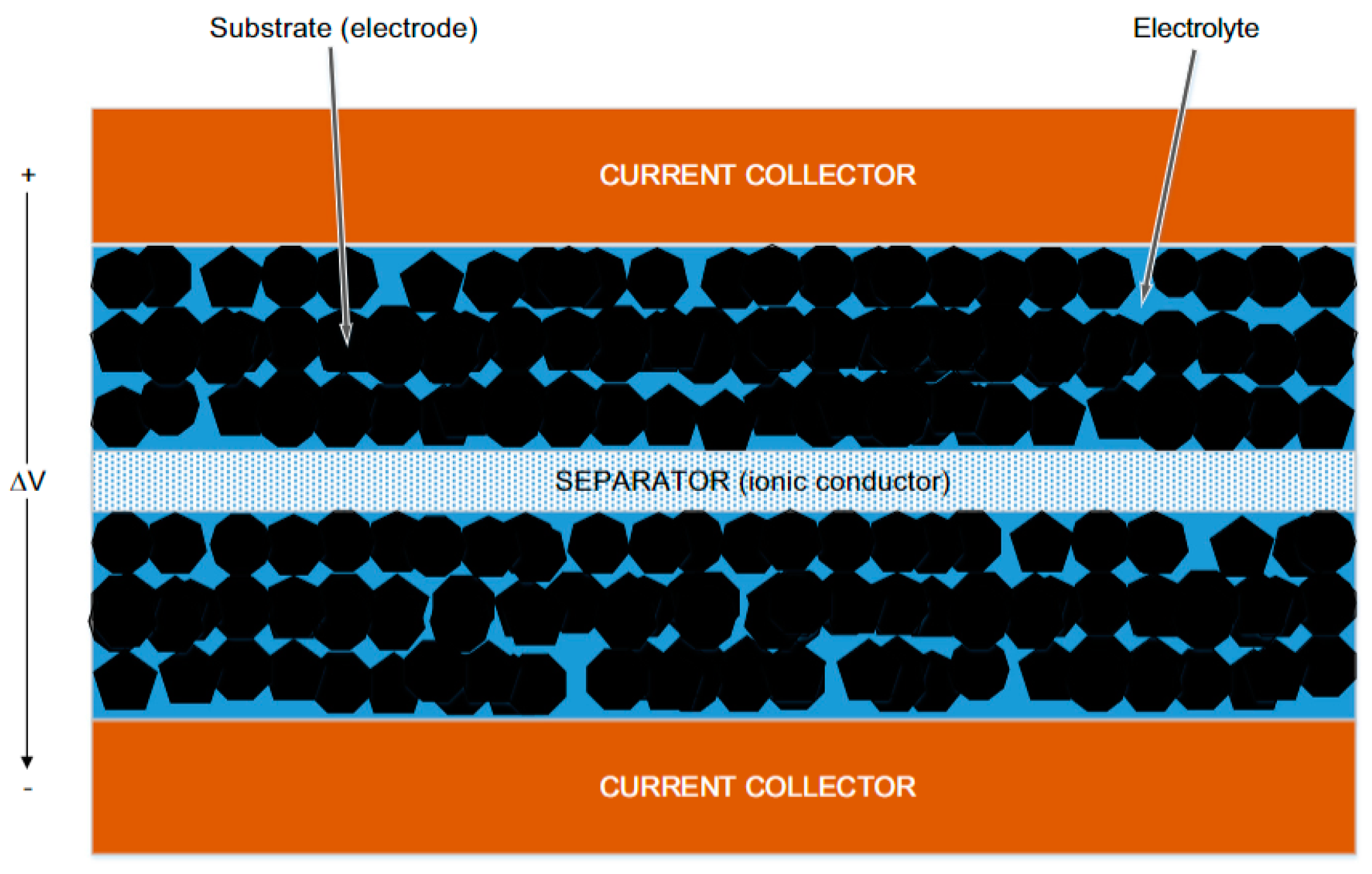
3. Applications in Electrochemistry for Energy Storage
3.1. Supercapacitors
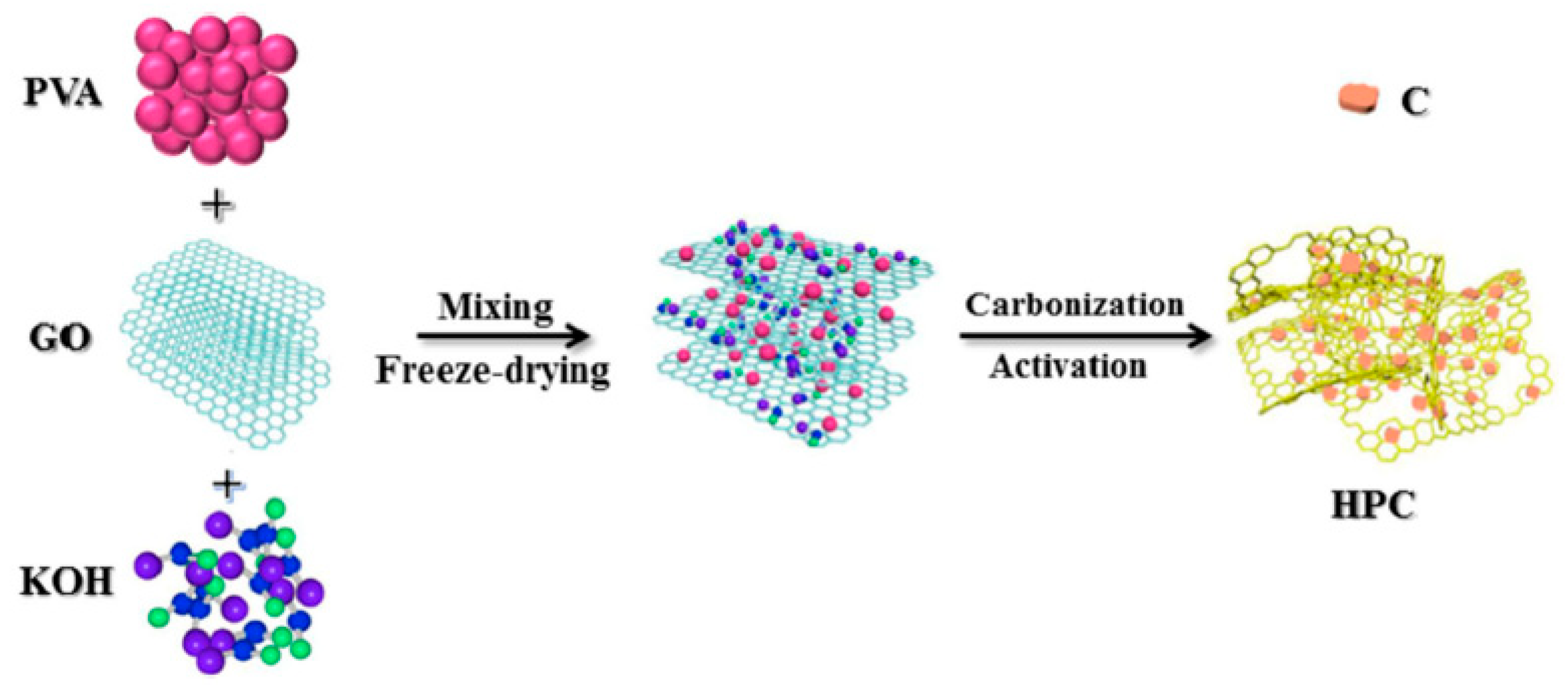
3.1.1. Carbon-Based Materials as Electrodes
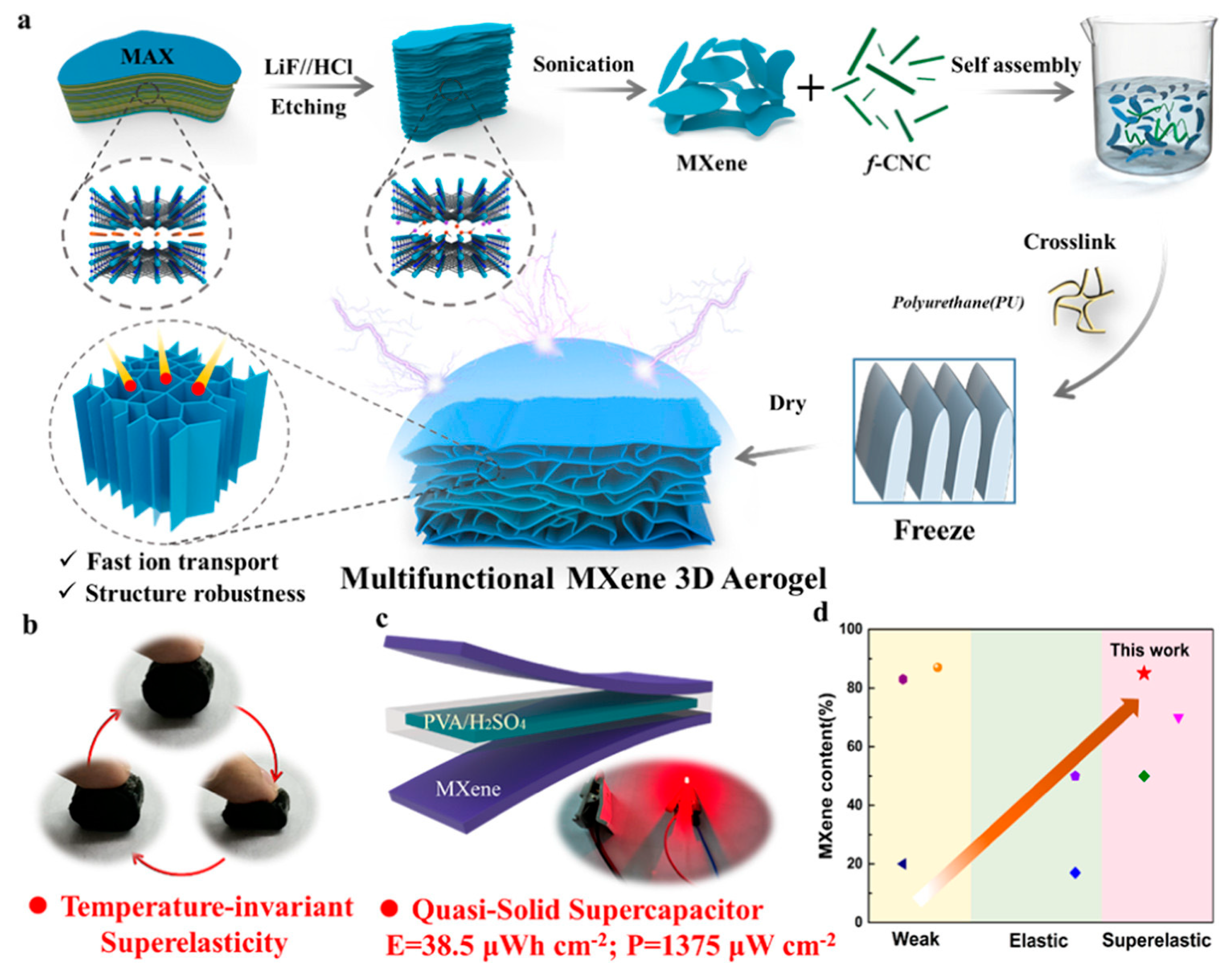
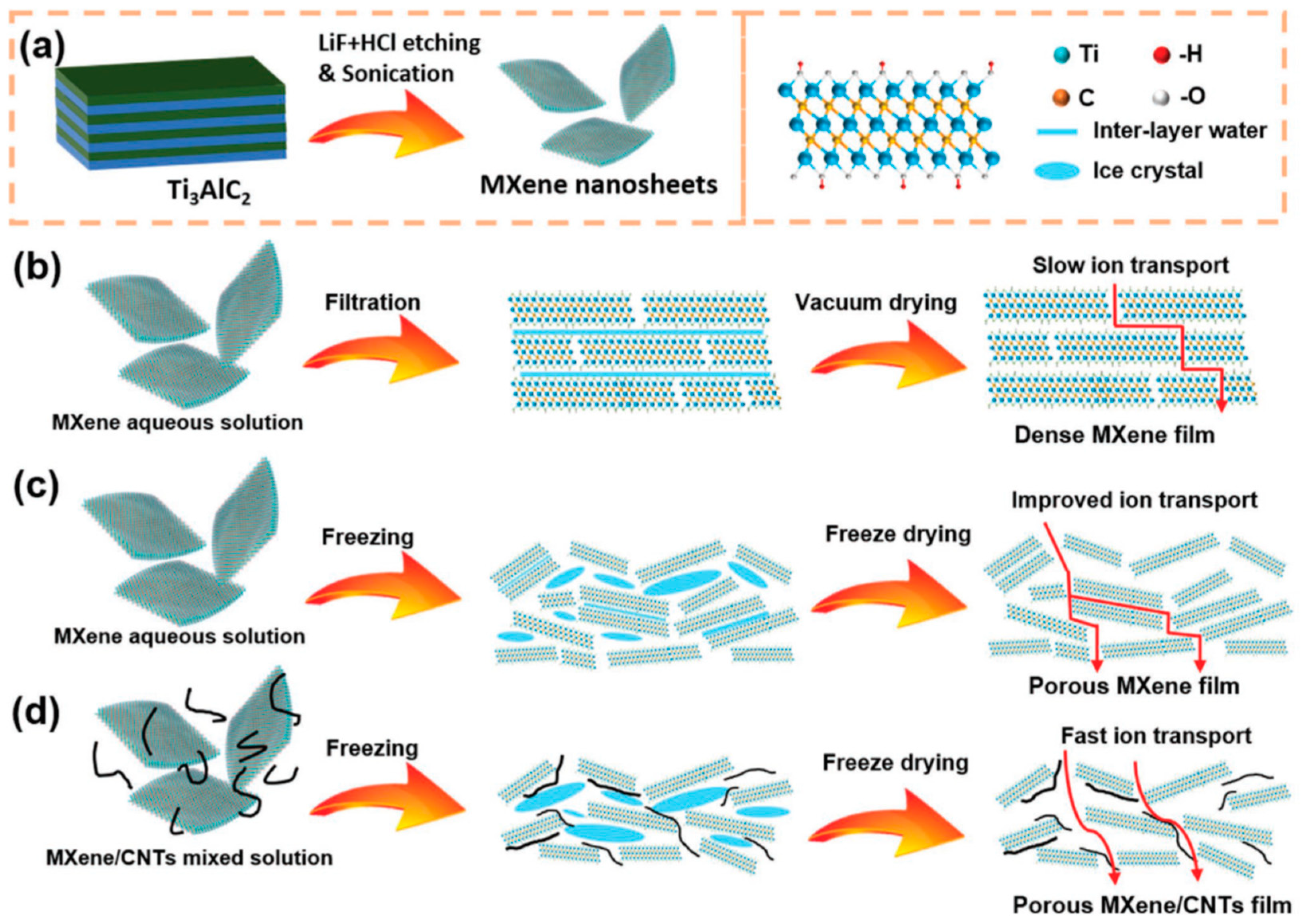
3.1.2. MXene as Electrodes
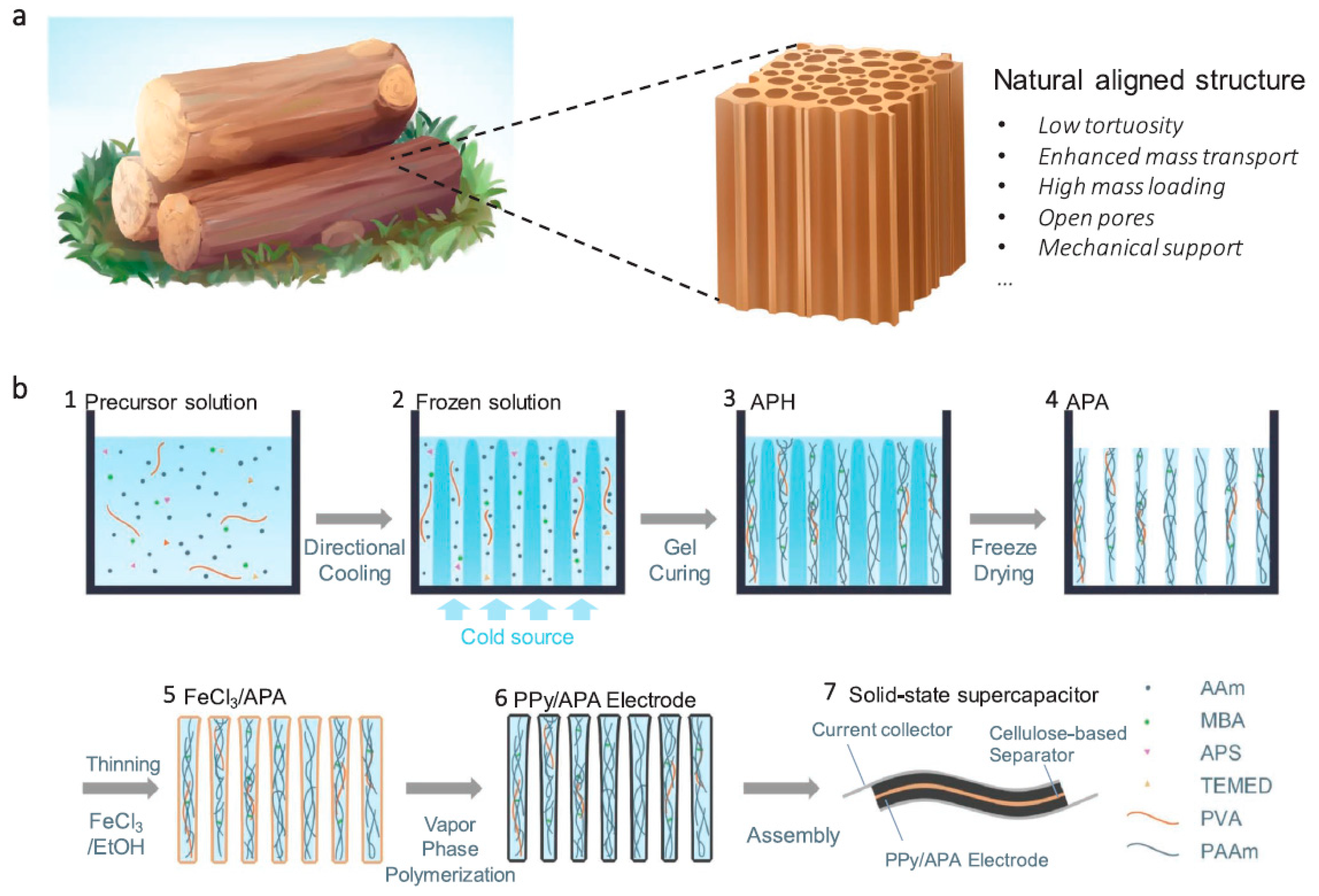
3.1.3. Polymer-Based Supercapacitors
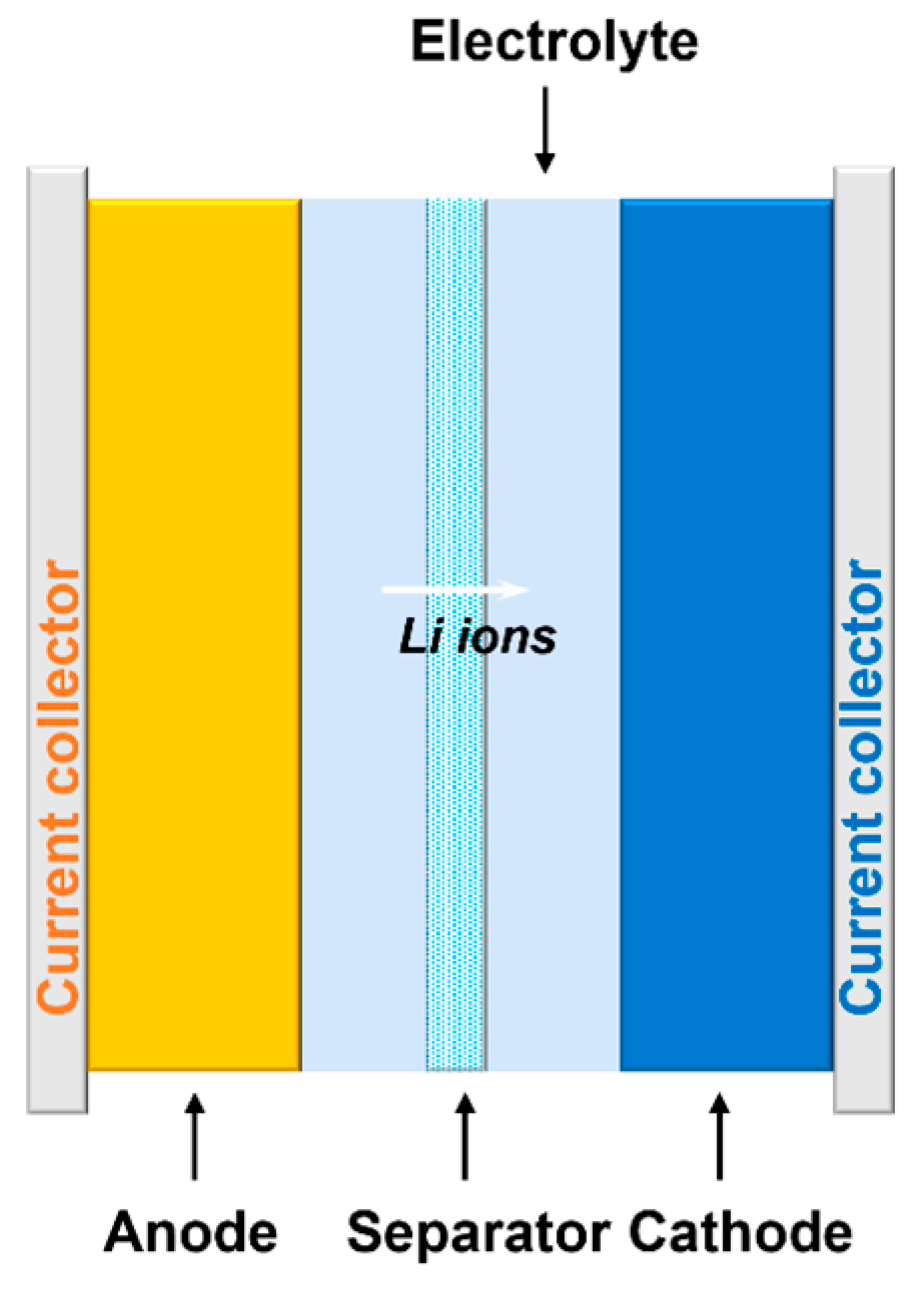
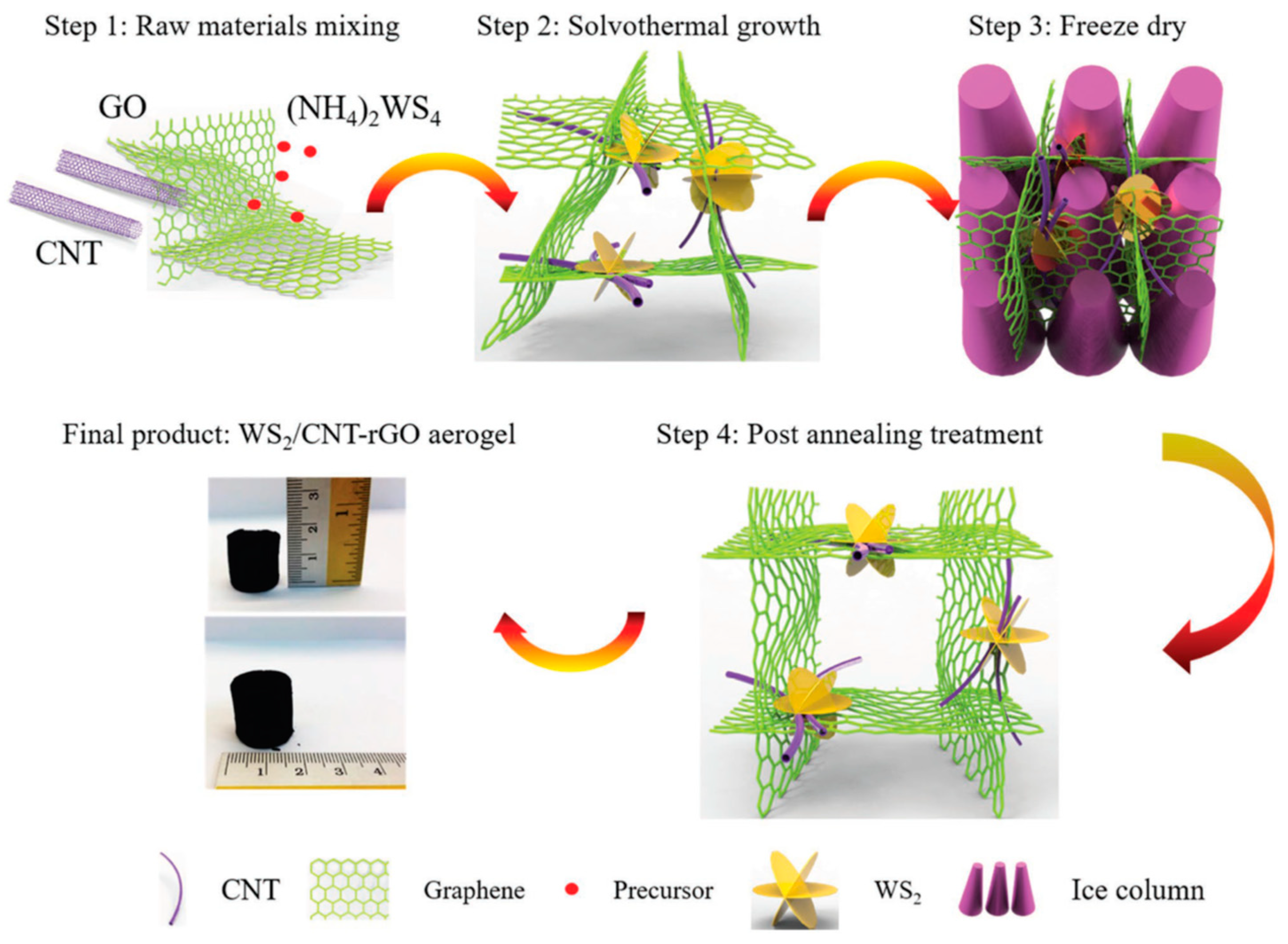
3.2. Li Ion Batteries (LIBs)
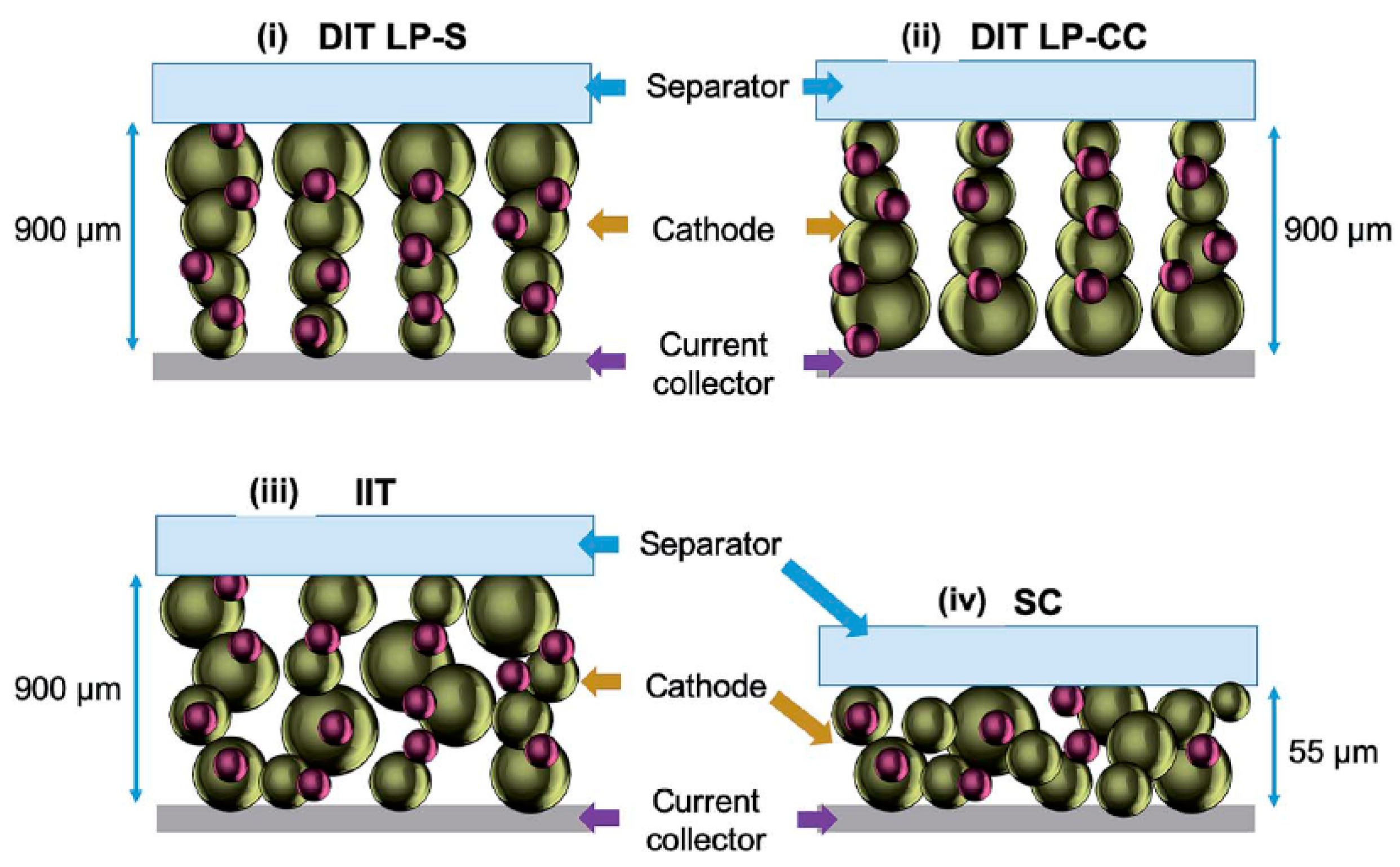
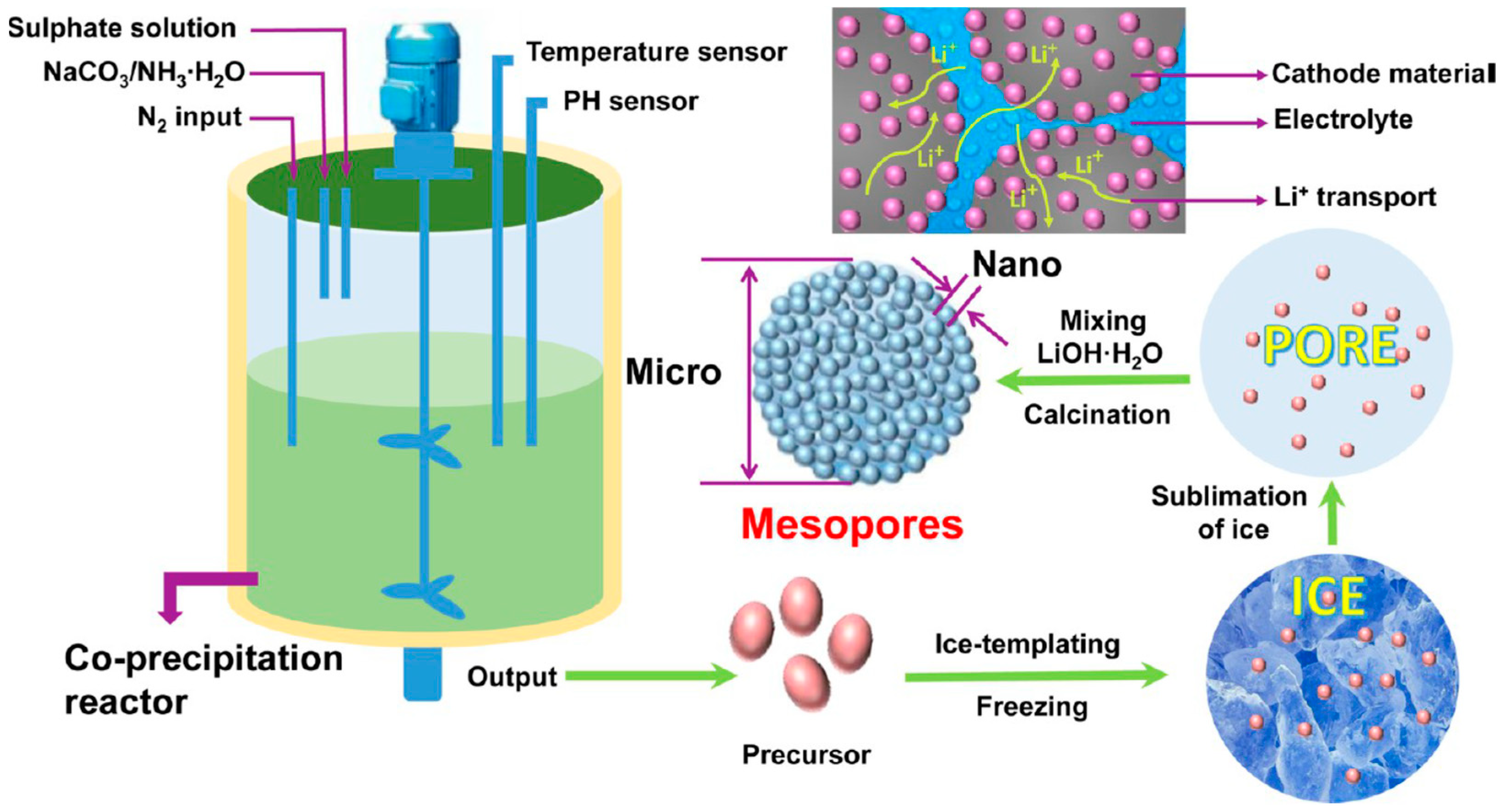
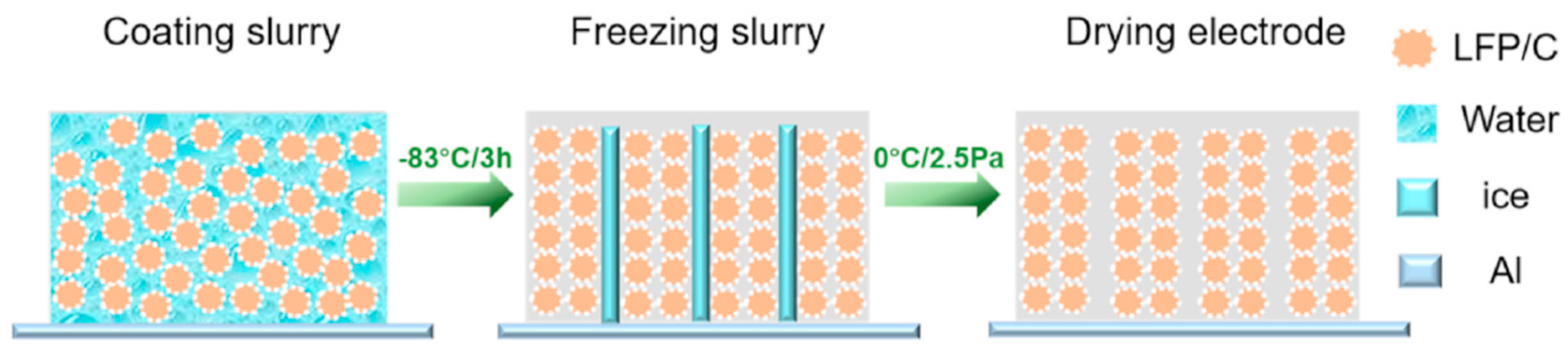
3.2.1. Cathode Material in LIBs
3.2.2. Anode Material in LIBs
3.2.3. Electrolyte Material in LIBs
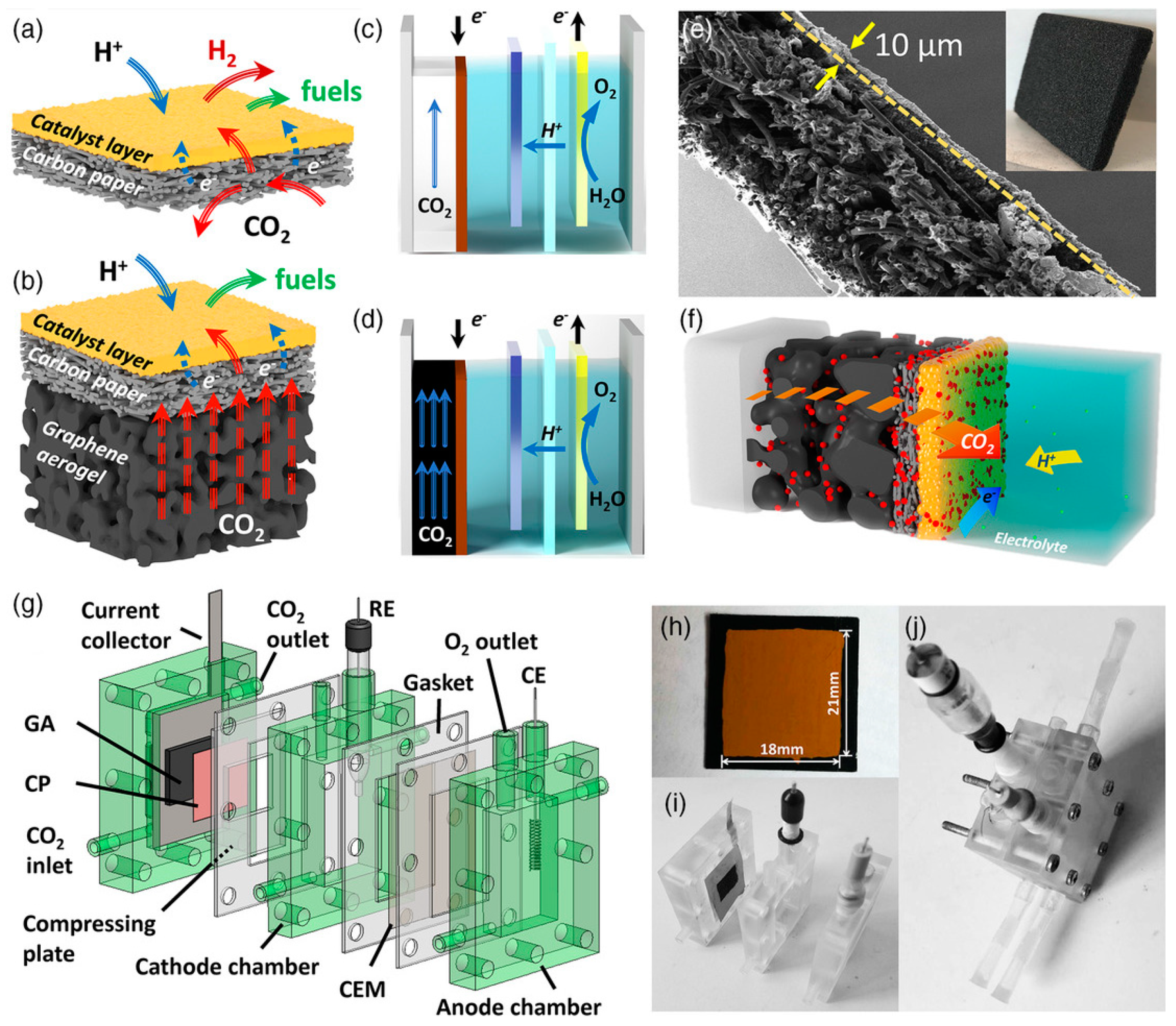
3.3. Fuel Cell Type Electrochemical Reactor
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kannan, N.; Vakeesan, D. Solar energy for future world: A review. Renew. Sustain. Energy Rev. 2016, 62, 1092–1105. [Google Scholar] [CrossRef]
- Herbert, G.J.; Iniyan, S.; Sreevalsan, E.; Rajapandian, S. A review of wind energy technologies. Renew. Sustain. Energy Rev. 2007, 11, 1117–1145. [Google Scholar] [CrossRef]
- He, X.; Zhang, X. A comprehensive review of supercapacitors: Properties, electrodes, electrolytes and thermal management systems based on phase change materials. J. Energy Storage 2022, 56, 106023. [Google Scholar] [CrossRef]
- Li, L.; Lu, S.; Dai, Y.; Li, H.; Wang, X.; Zhang, Y. Controlled Synthesis of Hierarchical Nanostructured Metal Ferrite Microspheres for Enhanced Electrocatalytic Oxygen Evolution Reaction. ACS Appl. Nano Mater. 2023, 6, 2184–2192. [Google Scholar] [CrossRef]
- Şahin, A.D. Progress and recent trends in wind energy. Prog. Energy Combust. Sci. 2004, 30, 501–543. [Google Scholar] [CrossRef]
- Lu, S.; Hummel, M.; Gu, Z.; Gu, Y.; Cen, Z.; Wei, L.; Zhou, Y.; Zhang, C.; Yang, C. Trash to treasure: A novel chemical route to synthesis of NiO/C for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 16144–16153. [Google Scholar] [CrossRef]
- Jia, H.; Lu, S.; Shin, S.H.R.; Sushko, M.L.; Tao, X.; Hummel, M.; Thallapally, P.K.; Liu, J.; Gu, Z. In situ anodic electrodeposition of two-dimensional conductive metal-organic framework@nickel foam for high-performance flexible supercapacitor. J. Power Sources 2022, 526, 231163. [Google Scholar] [CrossRef]
- Lu, S.; Wang, Y.; Xiang, H.; Lei, H.; Xu, B.B.; Xing, L.; Yu, E.H.; Liu, T.X. Mass transfer effect to electrochemical reduction of CO2: Electrode, electrocatalyst and electrolyte. J. Energy Storage 2022, 52, 104764. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, X.; Fang, L.; Lu, S. Urea Electrooxidation in Alkaline Environment: Fundamentals and Applications. ChemElectroChem 2023, e202300138. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and mechanisms of asymmetric supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Yan, C.; Han, E.; Yang, X.; Hu, K.; Xu, H.; Li, Y.; He, Y.; Lu, S. Engineering sulfur vacancies on Mo-doped nickel sulfide for enhanced electrochemical energy storage. Ceram. Int. 2023, 49, 14155–14165. [Google Scholar] [CrossRef]
- Yan, C.; Yang, X.; Lu, S.; Han, E.; Chen, G.; Zhang, Z.; Zhang, H.; He, Y. Hydrothermal synthesis of vanadium doped nickel sulfide nanoflower for high-performance supercapacitor. J. Alloys Compd. 2022, 928, 167189. [Google Scholar] [CrossRef]
- Eriksson, E.; Gray, E.M. Optimization and integration of hybrid renewable energy hydrogen fuel cell energy systems—A critical review. Appl. Energy 2017, 202, 348–364. [Google Scholar] [CrossRef]
- Nie, M.; Zhang, L.; Jiang, C.; Tian, X.; Li, Q.; Liu, X.; Du, S.; Lu, S.; Lei, D.; Wang, X. New energy and new power–the prospect of increasing use of polymers in fuel cells. Plast. Rubber Compos. 2016, 45, 31–42. [Google Scholar] [CrossRef]
- Yan, C.; Shen, Y.; Lu, S.; Yuan, J.; Li, Y.; Yang, X.; Han, E.; He, Y. Surfactant-Assisted rGO-PbO2 Electrode to Boost Acrylamide Degradation in Industrial Sewage. Ind. Eng. Chem. Res. 2023. [Google Scholar] [CrossRef]
- Lu, S.; Hummel, M.; Gu, Z.; Wang, Y.; Wang, K.; Pathak, R.; Zhou, Y.; Jia, H.; Qi, X.; Zhao, X. Highly efficient urea oxidation via nesting nano-nickel oxide in eggshell membrane-derived carbon. ACS Sustain. Chem. Eng. 2021, 9, 1703–1713. [Google Scholar] [CrossRef]
- Xue, F.; Kang, S.; Dai, Y.; Li, T.; Shen, P.K.; Zhu, J.; Lu, S.; Fu, X.; Wang, L.; Feng, S. Hierarchical lead grid for highly stable oxygen evolution in acidic water at high temperature. J. Power Sources 2021, 493, 229635. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, L.; He, W.; Li, L.; Lu, S. Heteroatom-Doped Nickel Sulfide for Efficient Electrochemical Oxygen Evolution Reaction. Energies 2023, 16, 881. [Google Scholar] [CrossRef]
- He, X.; Ling, Z.; Peng, X.; Yang, X.; Ma, L.; Lu, S. Facile synthesis of Cu2SnS3 nanocrystals for efficient nitrogen reduction reaction. Electrochem. Commun. 2023, 148, 107441. [Google Scholar] [CrossRef]
- Fang, L.; Wang, S.; Song, C.; Lu, S.; Yang, X.; Qi, X.; Liu, H. Boosting nitrate electroreduction to ammonia via in situ generated stacking faults in oxide-derived copper. Chem. Eng. J. 2022, 446, 137341. [Google Scholar] [CrossRef]
- Wang, Y.; Lei, H.; Xiang, H.; Fu, Y.; Xu, C.; Jiang, Y.; Xu, B.B.; Yu, E.H.; Gao, C.; Liu, T.X. Porous Bilayer Electrode-Guided Gas Diffusion for Enhanced CO2 Electrochemical Reduction. Adv. Energy Sustain. Res. 2021, 2, 2100083. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, J.; Baumann, D.; Peng, L.; Xu, Y.; Shakir, I.; Huang, Y.; Duan, X. Hierarchical 3D electrodes for electrochemical energy storage. Nat. Rev. Mater. 2019, 4, 45–60. [Google Scholar] [CrossRef]
- Deville, S. Ice-templating, freeze casting: Beyond materials processing. J. Mater. Res. 2013, 28, 2202–2219. [Google Scholar]
- Lai, K.C.; Lee, L.Y.; Hiew, B.Y.Z.; Thangalazhy-Gopakumar, S.; Gan, S. Environmental application of three-dimensional graphene materials as adsorbents for dyes and heavy metals: Review on ice-templating method and adsorption mechanisms. J. Environ. Sci. 2019, 79, 174–199. [Google Scholar]
- Hiew, B.Y.Z.; Lee, L.Y.; Lee, X.J.; Thangalazhy-Gopakumar, S.; Gan, S.; Lim, S.S.; Pan, G.-T.; Yang, T.C.-K.; Chiu, W.S.; Khiew, P.S. Review on synthesis of 3D graphene-based configurations and their adsorption performance for hazardous water pollutants. Process Saf. Environ. Prot. 2018, 116, 262–286. [Google Scholar] [CrossRef]
- Shehzad, K.; Xu, Y.; Gao, C.; Duan, X. Three-dimensional macro-structures of two-dimensional nanomaterials. Chem. Soc. Rev. 2016, 45, 5541–5588. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Shi, Y.; Yang, D.; Liu, Y.; Qu, J.; Yu, Z.-Z. Graphene oxide/chitosan aerogel microspheres with honeycomb-cobweb and radially oriented microchannel structures for broad-spectrum and rapid adsorption of water contaminants. ACS Appl. Mater. Interfaces 2017, 9, 21809–21819. [Google Scholar] [PubMed]
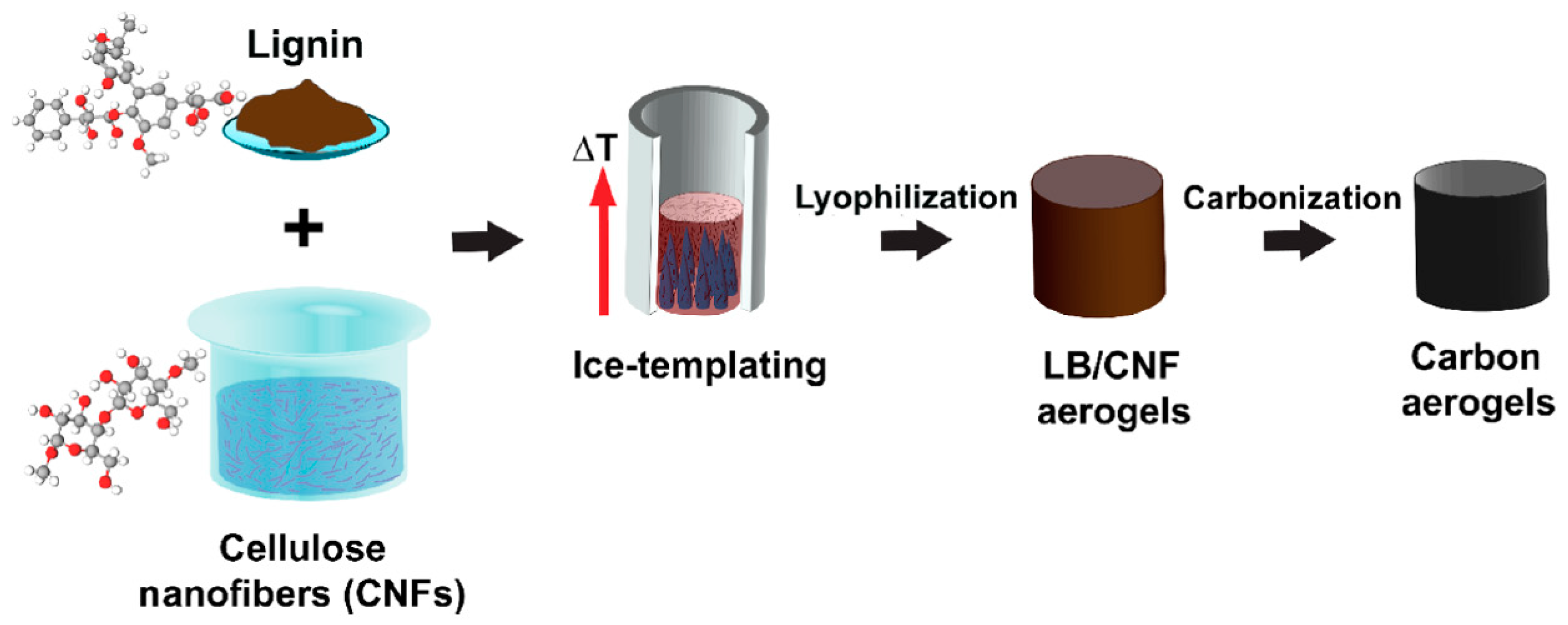
- Thomas, B.; Geng, S.; Wei, J.; Lycksam, H.; Sain, M.; Oksman, K. Ice-Templating of Lignin and Cellulose Nanofiber-Based Carbon Aerogels: Implications for Energy Storage Applications. ACS Appl. Nano Mater. 2022, 5, 7954–7966. [Google Scholar] [CrossRef]
- White, M.A.; Conrad, J.; Ellis, S.N.; Chen, R. Investigations of ice-structuring agents in ice-templated ceramics. J. Am. Ceram. Soc. 2017, 100, 5066–5074. [Google Scholar]
- Yang, Q.; Liu, Q.; Ling, W.; Dai, H.; Chen, H.; Liu, J.; Qiu, Y.; Zhong, L. Porous Electrode Materials for Zn-Ion Batteries: From Fabrication and Electrochemical Application. Batteries 2022, 8, 223. [Google Scholar]
- Judez, X.; Zhang, H.; Li, C.; Eshetu, G.G.; Zhang, Y.; González-Marcos, J.A.; Armand, M.; Rodriguez-Martinez, L.M. Polymer-rich composite electrolytes for all-solid-state Li–S cells. J. Phys. Chem. Lett. 2017, 8, 3473–3477. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Li, S.; Xiong, R.; Jiang, Z.; Sun, M.; Hu, W.; Peng, L.; He, R.; Zhou, H.; Yu, C. Low Tortuosity and Reinforced Concrete Type Ultra-Thick Electrode for Practical Lithium–Sulfur Batteries. Adv. Funct. Mater. 2022, 32, 2108669. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, X.; Liu, B.; Chen, S.; Liu, X.; Liu, Y.; Liu, J.; Chen, J. Orderly and highly dense polyaniline nanorod arrays fenced on carbon nanofibers for all-solid-state flexible electrochemical energy storage. Electrochim. Acta 2020, 338, 135846. [Google Scholar] [CrossRef]
- Qie, L.; Chen, W.M.; Wang, Z.H.; Shao, Q.G.; Li, X.; Yuan, L.X.; Hu, X.L.; Zhang, W.X.; Huang, Y.H. Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv. Mater. 2012, 24, 2047–2050. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xu, N.; Jiang, Z.; Zheng, A.; Shi, Q.; Lv, R.; Ni, L.; Diao, G.; Chen, M. Space and interface confinement effect of necklace-box structural FeS2/WS2 carbon nanofibers to enhance Na+ storage performance and electrochemical kinetics. Chem. Eng. J. 2022, 427, 131002. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, D.; Shi, W.; Liu, B.; Sim, G.J.; Ge, Q.; Yang, H.Y. Ice templated free-standing hierarchically WS2/CNT-rGO aerogel for high-performance rechargeable lithium and sodium ion batteries. Adv. Energy Mater. 2016, 6, 1601057. [Google Scholar] [CrossRef]
- Yu, X.; Pei, C.; Feng, L. Surface modulated hierarchical graphene film via sulfur and phosphorus dual-doping for high performance flexible supercapacitors. Chin. Chem. Lett. 2019, 30, 1121–1125. [Google Scholar] [CrossRef]
- Dong, X.; Xu, C.; Lu, S.; Wang, R.; Shi, Z.; Cui, Q.; You, T. ZIF-8 coupling with reduced graphene oxide to enhance the electrochemical sensing of dopamine. J. Electrochem. Soc. 2021, 168, 116517. [Google Scholar] [CrossRef]
- Lu, S.; Hummel, M.; Chen, K.; Zhou, Y.; Kang, S.; Gu, Z. Synthesis of Au@ ZIF-8 nanocomposites for enhanced electrochemical detection of dopamine. Electrochem. Commun. 2020, 114, 106715. [Google Scholar] [CrossRef]
- Nie, M.; Lu, S.; Lei, D.; Yang, C.; Zhao, Z. Rapid synthesis of ZIF-8 nanocrystals for electrochemical detection of dopamine. J. Electrochem. Soc. 2017, 164, H952. [Google Scholar] [CrossRef]
- Chen, W.; Huang, Y.-X.; Li, D.-B.; Yu, H.-Q.; Yan, L. Preparation of a macroporous flexible three dimensional graphene sponge using an ice-template as the anode material for microbial fuel cells. RSC Adv. 2014, 4, 21619–21624. [Google Scholar] [CrossRef]
- Li, S.; Xiong, R.; Han, Z.; He, R.; Li, S.; Zhou, H.; Yu, C.; Cheng, S.; Xie, J. Unveiling low-tortuous effect on electrochemical performance toward ultrathick LiFePO4 electrode with 100 mg cm−2 area loading. J. Power Sources 2021, 515, 230588. [Google Scholar] [CrossRef]
- Colard, C.A.; Cave, R.A.; Grossiord, N.; Covington, J.A.; Bon, S.A. Conducting nanocomposite polymer foams from ice-crystal-templated assembly of mixtures of colloids. Adv. Mater. 2009, 21, 2894–2898. [Google Scholar] [CrossRef]
- Joukhdar, H.; Seifert, A.; Jüngst, T.; Groll, J.; Lord, M.S.; Rnjak-Kovacina, J. Ice templating soft matter: Fundamental principles and fabrication approaches to tailor pore structure and morphology and their biomedical applications. Adv. Mater. 2021, 33, 2100091. [Google Scholar] [CrossRef] [PubMed]
- Arabi, N.; Zamanian, A. Effect of cooling rate and gelatin concentration on the microstructural and mechanical properties of ice template gelatin scaffolds. Biotechnol. Appl. Biochem. 2013, 60, 573–579. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, B. Emerging crystalline porous materials as a multifunctional platform for electrochemical energy storage. Chem. Soc. Rev. 2017, 46, 6927–6945. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Chen, K.; Pathak, R.; Hummel, M.; Reza, K.M.; Ghimire, N.; Pokharel, J.; Lu, S.; Gu, Z.; Qiao, Q. High-mass-loading Sn-based anode boosted by pseudocapacitance for long-life sodium-ion batteries. Chem. Eng. J. 2021, 414, 128638. [Google Scholar] [CrossRef]
- Adhamash, E.; Pathak, R.; Chen, K.; Rahman, M.T.; El-Magrous, A.; Gu, Z.; Lu, S.; Qiao, Q.; Zhou, Y. High-energy plasma activation of renewable carbon for enhanced capacitive performance of supercapacitor electrode. Electrochim. Acta 2020, 362, 137148. [Google Scholar] [CrossRef]
- Nie, M.; Lu, S.; Li, Q.; Liu, X.; Du, S. Facile solvothermal synthesis of HKUST-1 as electrocatalyst for hydrogen evolution reaction. Sci. Sin. Chim. 2016, 46, 357–364. [Google Scholar]
- Wang, Y.; Lei, H.; Lu, S.; Yang, Z.; Xu, B.B.; Xing, L.; Liu, T.X. Cu2O nano-flowers/graphene enabled scaffolding structure catalyst layer for enhanced CO2 electrochemical reduction. Appl. Catal. B Environ. 2022, 305, 121022. [Google Scholar] [CrossRef]
- Nie, Y.; Qi, X.; Wu, R.; Yang, R.; Wang, H.; Deng, M.; Zhang, S.; Lu, S.; Gu, Z.; Liu, X. Structurally ordered PtFe intermetallic nanocatalysts toward efficient electrocatalysis of methanol oxidation. Appl. Surf. Sci. 2021, 569, 151004. [Google Scholar] [CrossRef]
- Nie, M.; Du, S.; Li, Q.; Hummel, M.; Gu, Z.; Lu, S. Tungsten carbide as supports for trimetallic AuPdPt electrocatalysts for methanol oxidation. J. Electrochem. Soc. 2020, 167, 044510. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, J.; Nagaraju, D.H.; Jiang, L.; Marinov, V.R.; Lubineau, G.; Alshareef, H.N.; Oh, M. Flexible, highly graphitized carbon aerogels based on bacterial cellulose/lignin: Catalyst-free synthesis and its application in energy storage devices. Adv. Funct. Mater. 2015, 25, 3193–3202. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Ong, W.K.; Lu, X. Ultrafast-freezing-assisted mild preparation of biomass-derived, hierarchically porous, activated carbon aerogels for high-performance supercapacitors. ACS Sustain. Chem. Eng. 2018, 7, 403–411. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Ni, Y.; Qi, J.; Zhou, B.; Zhu, L.; Ren, Y.; Zhang, D. Controllable synthesis of multilayered porous carbon by ice templating with graphene addition for supercapacitors. J. Mater. Sci. 2021, 56, 7533–7546. [Google Scholar] [CrossRef]
- Mochizuki, D.; Tanaka, R.; Makino, S.; Ayato, Y.; Sugimoto, W. Vertically aligned reduced graphite oxide nanosheet film and its application in a high-speed charge/discharge electrochemical capacitor. ACS Appl. Energy Mater. 2019, 2, 1033–1039. [Google Scholar] [CrossRef]
- Dey, R.S.; Hjuler, H.A.; Chi, Q. Approaching the theoretical capacitance of graphene through copper foam integrated three-dimensional graphene networks. J. Mater. Chem. A 2015, 3, 6324–6329. [Google Scholar] [CrossRef]
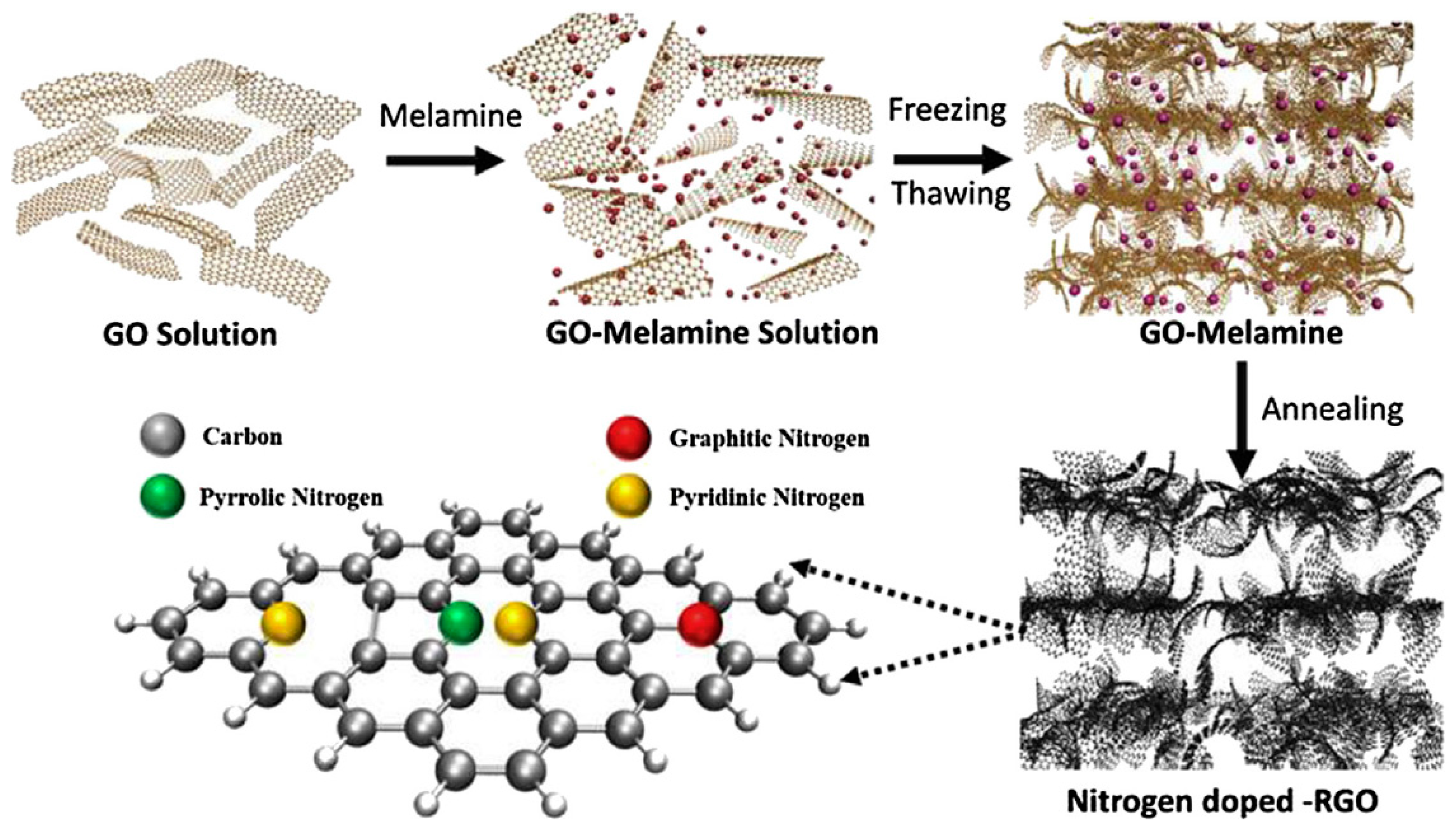
- Kota, M.; Yu, X.; Yeon, S.-H.; Cheong, H.-W.; Park, H.S. Ice-templated three dimensional nitrogen doped graphene for enhanced supercapacitor performance. J. Power Sources 2016, 303, 372–378. [Google Scholar] [CrossRef]
- Guo, S.; Kang, S.; Feng, S.; Lu, W. MXene-enhanced deep ultraviolet photovoltaic performances of crossed Zn2GeO4 nanowires. J. Phys. Chem. C 2020, 124, 4764–4771. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Xie, X.; Ren, C.E.; Makaryan, T.; Anasori, B.; Wang, G.; Gogotsi, Y. Hollow MXene spheres and 3D macroporous MXene frameworks for Na-ion storage. Adv. Mater. 2017, 29, 1702410. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Yang, S.; Wang, S.; Li, L. Construction of 3D porous MXene supercapacitor electrode through a dual-step freezing strategy. Scr. Mater. 2022, 213, 114605. [Google Scholar] [CrossRef]
- Cai, C.; Wei, Z.; Deng, L.; Fu, Y. Temperature-invariant superelastic multifunctional MXene aerogels for high-performance photoresponsive supercapacitors and wearable strain sensors. ACS Appl. Mater. Interfaces 2021, 13, 54170–54184. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, Q.; Soomro, R.A.; He, S.; Sun, N.; Qiao, N.; Xu, B. In situ ice template approach to fabricate 3D flexible MXene film-based electrode for high performance supercapacitors. Adv. Funct. Mater. 2020, 30, 2000922. [Google Scholar] [CrossRef]
- Xia, Z.; Sun, R.; Jing, F.; Wang, S.; Sun, H.; Sun, G. Modeling and optimization of Scaffold-like macroporous electrodes for highly efficient direct methanol fuel cells. Appl. Energy 2018, 221, 239–248. [Google Scholar] [CrossRef]
- Zhao, Y.; Alsaid, Y.; Yao, B.; Zhang, Y.; Zhang, B.; Bhuskute, N.; Wu, S.; He, X. Wood-inspired morphologically tunable aligned hydrogel for high-performance flexible all-solid-state supercapacitors. Adv. Funct. Mater. 2020, 30, 1909133. [Google Scholar] [CrossRef]
- Wei, J.; Yin, C.; Wang, H.; Wang, Q. Polyampholyte-doped aligned polymer hydrogels as anisotropic electrolytes for ultrahigh-capacity supercapacitors. J. Mater. Chem. A 2018, 6, 58–64. [Google Scholar] [CrossRef]
- Liu, X.; Taiwo, O.O.; Yin, C.; Ouyang, M.; Chowdhury, R.; Wang, B.; Wang, H.; Wu, B.; Brandon, N.P.; Wang, Q. Aligned ionogel electrolytes for high-temperature supercapacitors. Adv. Sci. 2019, 6, 1801337. [Google Scholar] [CrossRef]
- Kim, S.W.; Seo, D.H.; Ma, X.; Ceder, G.; Kang, K. Electrode materials for rechargeable sodium-ion batteries: Potential alternatives to current lithium-ion batteries. Adv. Energy Mater. 2012, 2, 710–721. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, M.; Chen, W.; Liu, Y.; Zhang, L.; Dongfang, N.; Ruan, Y.; Zhang, J.; Wang, P.; Dong, L. Sandwich, vertical-channeled thick electrodes with high rate and cycle performance. Adv. Funct. Mater. 2019, 29, 1809196. [Google Scholar] [CrossRef]
- Huang, C.; Dontigny, M.; Zaghib, K.; Grant, P.S. Low-tortuosity and graded lithium ion battery cathodes by ice templating. J. Mater. Chem. A 2019, 7, 21421–21431. [Google Scholar] [CrossRef]
- Roberts, A.D.; Wang, S.; Li, X.; Zhang, H. Hierarchical porous nitrogen-rich carbon monoliths via ice-templating: High capacity and high-rate performance as lithium-ion battery anode materials. J. Mater. Chem. A 2014, 2, 17787–17796. [Google Scholar] [CrossRef]
- Zhai, H.; Xu, P.; Ning, M.; Cheng, Q.; Mandal, J.; Yang, Y. A flexible solid composite electrolyte with vertically aligned and connected ion-conducting nanoparticles for lithium batteries. Nano Lett. 2017, 17, 3182–3187. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Xing, L.; Jiang, H.; Wang, Y.; Xu, B.B.; Xuan, J.; Liu, T.X. Designing graded fuel cell electrodes for proton exchange membrane (PEM) fuel cells with recurrent neural network (RNN) approaches. Chem. Eng. Sci. 2023, 267, 118350. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, Y.; Zheng, X.; Lu, S. Ice-Templated Method to Promote Electrochemical Energy Storage and Conversion: A Review. Energies 2023, 16, 3865. https://doi.org/10.3390/en16093865
Wang Y, Wu Y, Zheng X, Lu S. Ice-Templated Method to Promote Electrochemical Energy Storage and Conversion: A Review. Energies. 2023; 16(9):3865. https://doi.org/10.3390/en16093865
Chicago/Turabian StyleWang, Yucheng, Yanan Wu, Xingqun Zheng, and Shun Lu. 2023. "Ice-Templated Method to Promote Electrochemical Energy Storage and Conversion: A Review" Energies 16, no. 9: 3865. https://doi.org/10.3390/en16093865
APA StyleWang, Y., Wu, Y., Zheng, X., & Lu, S. (2023). Ice-Templated Method to Promote Electrochemical Energy Storage and Conversion: A Review. Energies, 16(9), 3865. https://doi.org/10.3390/en16093865






