Geochemical and Microstructural Characteristics of Clay Minerals and Their Effects on the Pore Structure of Coal-Measure Shale: A Case Study in Qinshui Basin, China
Abstract
1. Introduction
2. Samples and Methods
3. Results
3.1. Mineralogical Compositions
3.2. Elemental Composition
3.3. FTIR
3.4. Petrography
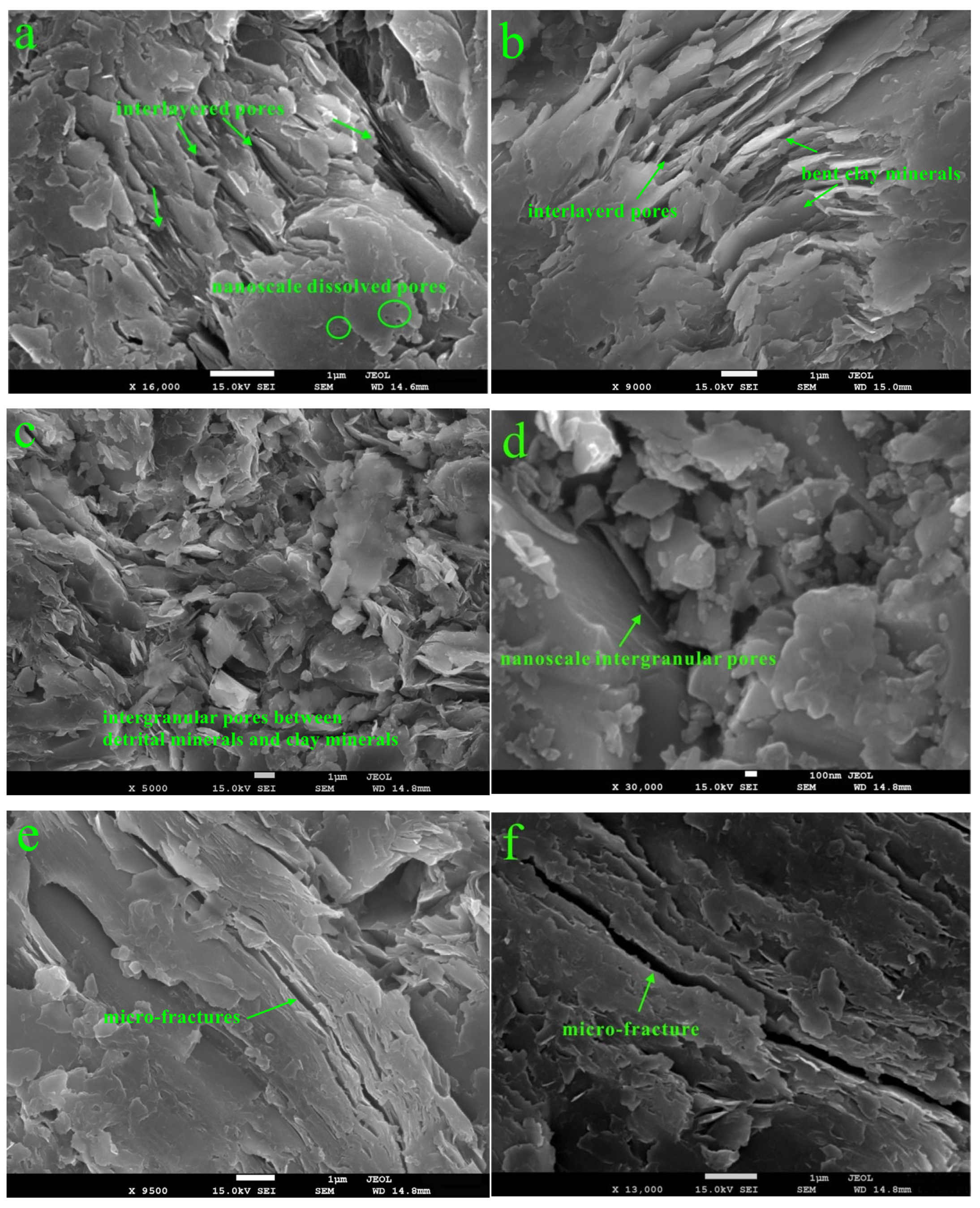
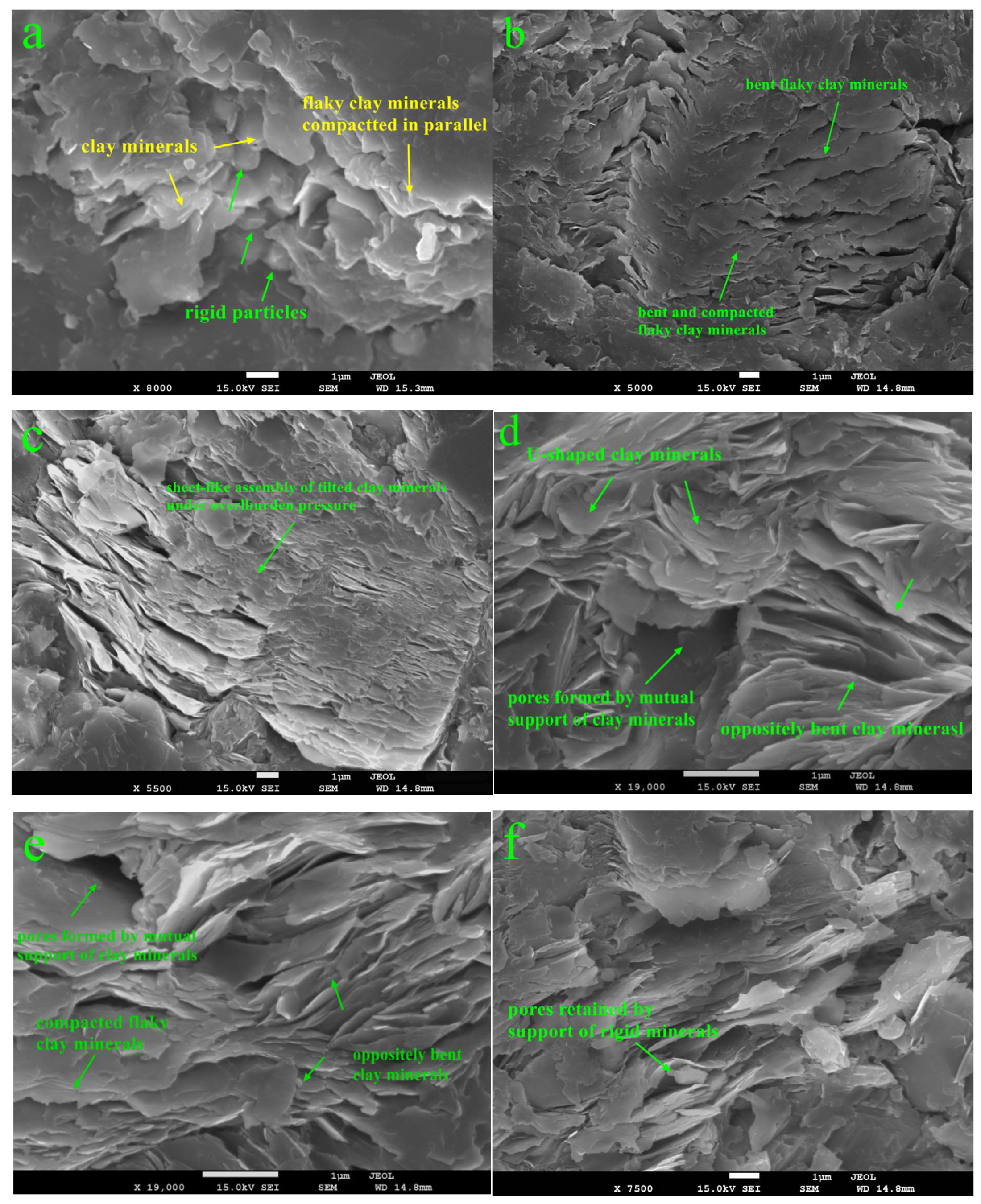
3.5. FE-SEM
3.6. Clay-Related Pores
4. Discussion
4.1. Effect of Mechanical Compaction on Clay-Related Pores
4.2. The Role of Clay Mineral Cementation in Pore Development
4.2.1. Kaolinite
4.2.2. Illite
5. Conclusions
- (1)
- Clay minerals are dominant in the compositions of TYS. They mainly consist of kaolinite, illite, and chlorite. Most kaolinite is detrital in origin with a low crystallinity and low degree of ordering, while most illite is formed from diagenesis with 1Md polytype.
- (2)
- An XRF analysis suggests that shale is probably dominated by the strong chemical weathering of first-cycle deposits.
- (3)
- A considerable number of multisized, micro/nano pores are developed in clay minerals. Pores associated with clay minerals in TYS can be divided into interlayered pores, intergranular pores, and microfractures. Controlled by the crystalline structure and particle morphology of minerals, clay-related pores mostly present in a slitlike or irregular shape.
- (4)
- Mechanical compaction causes clay minerals to be arranged into multiple morphologies, including parallel, bent, tilted, and mutually supporting structures, etc., which is a key factor for the high permeability anisotropy of shale.
- (5)
- Compaction has a decisive effect on the pore structure and morphology of clay-related pores. Additionally, it is also the main reason for the strong heterogeneity, low porosity, and high permeability anisotropy of shale. The cementation of clay minerals has a limited influence on pore structure.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, L.W.; Yang, K.; Wei, H.; Liu, L.; Li, X.; Chen, L.; Xu, T.; Wang, X. Full-Scale Pore Structure Characteristics and the Main Controlling Factors of Mesoproterozoic Xiamaling Shale in Zhangjiakou, Hebei, China. Nanomaterials 2021, 11, 527. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Fan, Y.; Xuan, Q.; Zheng, G. A review of shale pore structure evolution characteristics with increasing thermal maturities. Adv. Geo-Energy Res. 2020, 4, 247–259. [Google Scholar] [CrossRef]
- Ross, D.J.K.; Marc Bustin, R. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Cullers, R.L.; Podkovyrov, V.N. The source and origin of terrigenous sedimentary rocks in the Mesoproterozoic Ui group, southeastern Russia. Precambr. Res. 2002, 117, 157–183. [Google Scholar] [CrossRef]
- Jiang, S. Clay Minerals from the Perspective of Oil and Gas Exploration. In Clay Minerals in Nature—Their Characterization, Modification and Application; BoD—Books on Demand: Norderstedt, Germany, 2012. [Google Scholar]
- Chen, S.; Han, Y.; Fu, C.; Zhang, H.; Zhu, Y.; Zuo, Z. Micro and nano-size pores of clay minerals in shale reservoirs: Implication for the accumulation of shale gas. Sediment. Geol. 2016, 342, 180–190. [Google Scholar] [CrossRef]
- Han, M.; Han, C.; Han, Z.; Song, Z.; Zhong, W.; Li, H.; Xu, W. Mineral compositional controls on the porosity of black shales from the Wufeng and Longmaxi Formations (Southern Sichuan Basin and its surroundings) and insights into shale diagenesis. Energy Explor. Exploit. 2018, 36, 665–685. [Google Scholar] [CrossRef]
- Romero-Sarmiento, M.-F.; Rouzaud, J.-N.; Bernard, S.; Deldicque, D.; Thomas, M.; Littke, R. Evolution of Barnett Shale organic carbon structure and nanostructure with increasing maturation. Org. Geochem. 2014, 71, 7–16. [Google Scholar] [CrossRef]
- Wang, G.; Ju, Y. Organic shale micropore and mesopore structure characterization by ultra-low pressure N2 physisorption: Experimental procedure and interpretation model. J. Nat. Gas Sci. Eng. 2015, 27, 452–465. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Liu, S.; Zhang, R. Pore characterization and its impact on methane adsorption capacity for organic-rich marine shales. Fuel 2016, 181, 227–237. [Google Scholar] [CrossRef]
- Hackley, P.C.; Jubb, A.M.; McAleer, R.J.; Valentine, B.J.; Birdwell, J.E. A review of spatially resolved techniques and applications of organic petrography in shale petroleum systems. Int. J. Coal Geol. 2021, 241, 103745. [Google Scholar] [CrossRef]
- Pan, L.; Xiao, X.; Tian, H.; Zhou, Q.; Chen, J.; Li, T.; Wei, Q. A preliminary study on the characterization and controlling factors of porosity and pore structure of the Permian shales in Lower Yangtze region, Eastern China. Int. J. Coal Geol. 2015, 146, 68–78. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, X.; Zhang, Q.; Cheng, W. Research on the organic geochemical and mineral composition properties and its influence on pore structure of coal-measure shales in Yushe-Wuxiang Block, South Central Qinshui Basin, China. J. Pet. Sci. Eng. 2019, 173, 1065–1079. [Google Scholar] [CrossRef]
- Mukasa-Tebandeke, I.Z.; Ssebuwufu, P.J.M.; Nyanzi, S.A.; Schumann, A.; Nyakairu, G.W.A.; Ntale, M.; Lugolobi, F. The Elemental, Mineralogical, IR, DTA and XRD Analyses Characterized Clays and Clay Minerals of Central and Eastern Uganda. Adv. Mater. Phys. Chem. 2015, 5, 67–86. [Google Scholar] [CrossRef]
- Li, K.; Kong, S.; Xia, P.; Wang, X. Microstructural characterisation of organic matter pores in coal-measure shale. Adv. Geo-Energy Res. 2020, 4, 372–391. [Google Scholar] [CrossRef]
- Peltonen, C.; Marcussen, Ø.; Bjørlykke, K.; Jahren, J. Clay mineral diagenesis and quartz cementation in mudstones: The effects of smectite to illite reaction on rock properties. Mar. Pet. Geol. 2009, 26, 887–898. [Google Scholar] [CrossRef]
- Ural, N. The significance of scanning electron microscopy (SEM) analysis on the microstructure of improved clay: An overview. Open Geosci. 2021, 13, 197–218. [Google Scholar] [CrossRef]
- Sun, Y.; Ju, Y.; Zhou, W.; Qiao, P.; Tao, L.; Xiao, L. Nanoscale pore and crack evolution in shear thin layers of shales and the shale gas reservoir effect. Adv. Geo-Energy Res. 2022, 6, 221–229. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, X.; Liang, L.; Wei, X.; Xu, P. Investigation of the factors influencing methane adsorption on illite. Energy Sci. Eng. 2019, 7, 3317–3331. [Google Scholar] [CrossRef]
- Zou, C.; Dong, D.; Wang, Y.; Li, X.; Huang, J.; Wang, S.; Guan, Q.; Zhang, C.; Wang, H.; Liu, H.; et al. Shale gas in China: Characteristics, challenges and prospects (I). Pet. Explor. Dev. 2015, 42, 753–767. [Google Scholar] [CrossRef]
- Zou, C.N.; Yang, Z.; Huang, S.P.; Ma, F.; Sun, Q.P.; Li, F.H.; Pan, S.Q.; Tian, W.G. Resource types, formation, distribution and prospects of coal-measure gas. Pet. Explor. Dev. 2019, 46, 451–462. [Google Scholar] [CrossRef]
- Li, K.J.; Zeng, F.G.; Cai, J.C.; Sheng, G.L.; Xia, P.; Zhang, K. Fractal Characteristics of Pores in Taiyuan Formation Shale from Hedong Coal Field, China. Fractals 2018, 26, 1840006. [Google Scholar] [CrossRef]
- Hazra, B.; Varma, A.K.; Bandopadhyay, A.K.; Chakravarty, S.; Buragohain, J.; Samad, S.K.; Prasad, A.K. FTIR, XRF, XRD and SEM characteristics of Permian shales, India. J. Nat. Gas Sci. Eng. 2016, 32, 239–255. [Google Scholar] [CrossRef]
- Liang, J.; Huang, W.; Wang, H.; Blum, M.J.; Chen, J.; Wei, X.; Yang, G. Organic geochemical and petrophysical characteristics of transitional coal-measure shale gas reservoirs and their relationships with sedimentary environments: A case study from the Carboniferous-Permian Qinshui Basin, China. J. Pet. Sci. Eng. 2020, 184, 106510. [Google Scholar] [CrossRef]
- Yin, L.; Guo, S. Full-Sized Pore Structure and Fractal Characteristics of Marine-Continental Transitional Shale: A Case Study in Qinshui Basin, North China. Acta Geol. Sin. Engl. Ed. 2019, 93, 675–691. [Google Scholar] [CrossRef]
- Shao, L.; Xiao, Z.; Lu, J.; He, Z.; Wang, H.; Zhang, P. Permo-Carboniferous coal measures in the Qinshui basin: Lithofacies paleogeography and its control on coal accumulation. Front. Earth Sci. China 2007, 1, 106–115. [Google Scholar] [CrossRef]
- Chinese Oil and Gas Industry Standard, SY/T 5368-2016; Identification of Rock Flakes. National Energy Administration: Beijing, China, 2016.
- Weaver, C.E. A Discussion on the Origin of Clay Minerals in Sedimentary Rocks. Clays Clay Miner. 1956, 5, 159–173. [Google Scholar] [CrossRef]
- Aparicio, P.; Emilio, G. Mineralogical Interference on Kaolinite Crystallinity Index Measurements. Clays Clay Miner. 1999, 47, 12–27. [Google Scholar] [CrossRef]
- Liu, Q.F.; Liang, X.H.; Zhang, P.F. Crystallinity difference for various origin of kaolinites in coal measures. J. China Coal Soc. 2000, 25, 576–580. [Google Scholar]
- Grathoff, G.H.; Moore, D.M. Illite Polytype Quantification Using Wildfire© Calculated X-ray Diffraction Patterns. Clays Clay Miner. 1996, 44, 835–842. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution. Blackwell 1985, 122, 349. [Google Scholar]
- Li, B.; Zhuang, X.; Liu, X.; Wu, C.; Zhou, J.; Ma, X. Mineralogical and geochemical composition of Middle Permian Lucaogou Formation in the southern Junggar Basin, China: Implications for paleoenvironment, provenance, and tectonic setting. Arab. J. Geosci. 2016, 9, 1–16. [Google Scholar] [CrossRef]
- Cox, R.; Lowe, D.R.; Cullers, R.L. The influence of sediment recycling and basement composition on evolution of mudrock chemistry in the southwestern United States. Geochim. Cosmochim. Acta 1995, 59, 2919–2940. [Google Scholar] [CrossRef]
- Cullers, R.L.; Podkovyrov, V.N. Geochemistry of the Mesoproterozoic Lakhanda shales in southeastern Yakutia, Russia: Implications for mineralogical and provenance control, and recycling. Precambr. Res. 2000, 104, 77–93. [Google Scholar] [CrossRef]
- Hayashi, K.I.; Fujisawa, H.; Holland, H.D.; Ohmoto, H. Geochemistry of approximately 1.9 Ga sedimentary rocks from northeastern Labrador, Canada. Geochim. Cosmochim. Acta 1997, 61, 4115–4137. [Google Scholar] [CrossRef]
- Fedo, C.M.; Wayne Nesbitt, H.; Young, G.M. Unraveling the effects of potassium metasomatism in sedimentary rocks and paleosols, with implications for paleoweathering conditions and provenance. Geology 1995, 23, 921–924. [Google Scholar] [CrossRef]
- Balan, E.; Lazzeri, M.; Saitta, A.M.; Allard, T.; Fuchs, Y.; Mauri, F. First-principles study of OH-stretching modes in kaolinite, dickite, and nacrite. Am. Mineral. 2005, 90, 50–60. [Google Scholar] [CrossRef]
- Khang, V.C.; Korovkin, M.V.; Ananyeva, L.G. Identification of clay minerals in reservoir rocks by FTIR spectroscopy. IOP Conf. Ser. Earth Environ. Sci. 2016, 43, 012004. [Google Scholar] [CrossRef]
- Chmielová, M.; Weiss, Z. Determination of structural disorder degree using an XRD profile fitting procedure: Application to Czech kaolins. Appl. Clay Sci. 2002, 22, 65–74. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, H.; Cai, D.; Guo, H.; Zhu, G.; Zhu, X.; Hu, X. The Secondary Porosity and Permeability Characteristics of Tertiary Strata and Their Origins, Liaodong Bay Basin, China. Energy Explor. Exploit. 2010, 28, 207–222. [Google Scholar] [CrossRef]
- Dou, W.; Liu, L.; Wu, K.; Xu, Z.; Feng, X. Origin and significance of secondary porosity: A case study of upper Triassic tight sandstones of Yanchang Formation in Ordos basin, China. J. Pet. Sci. Eng. 2017, 149, 485–496. [Google Scholar] [CrossRef]
- Sposito, G. The Chemistry of Soils, 3rd ed.; Oxford University Press: Oxford, UK, 2016; pp. 96–104. [Google Scholar]
- Xiong, J.; Tang, J.; Zhou, X.; Liu, X.; Liang, L.; Hou, L. Molecular Dynamics Simulation and Experimental Studies of the Wettability Behaviors of Shales. Energy Fuels 2022, 36, 3526–3538. [Google Scholar] [CrossRef]
- Voltolini, M.; Wenk, H.R.; Mondol, N.H.; Bjørlykke, K.O.; Jahren, J.J.G. Anisotropy of experimentally compressed kaolinite-illite-quartz mixtures. Geophysics 2009, 74, D13–D23. [Google Scholar] [CrossRef]
- Daniels, E.J.; Altaner, S.P. Clay mineral authigenesis in coal and shale from the anthracite region, Pennsylvania. Am. Mineral. 1990, 75, 825–839. [Google Scholar]
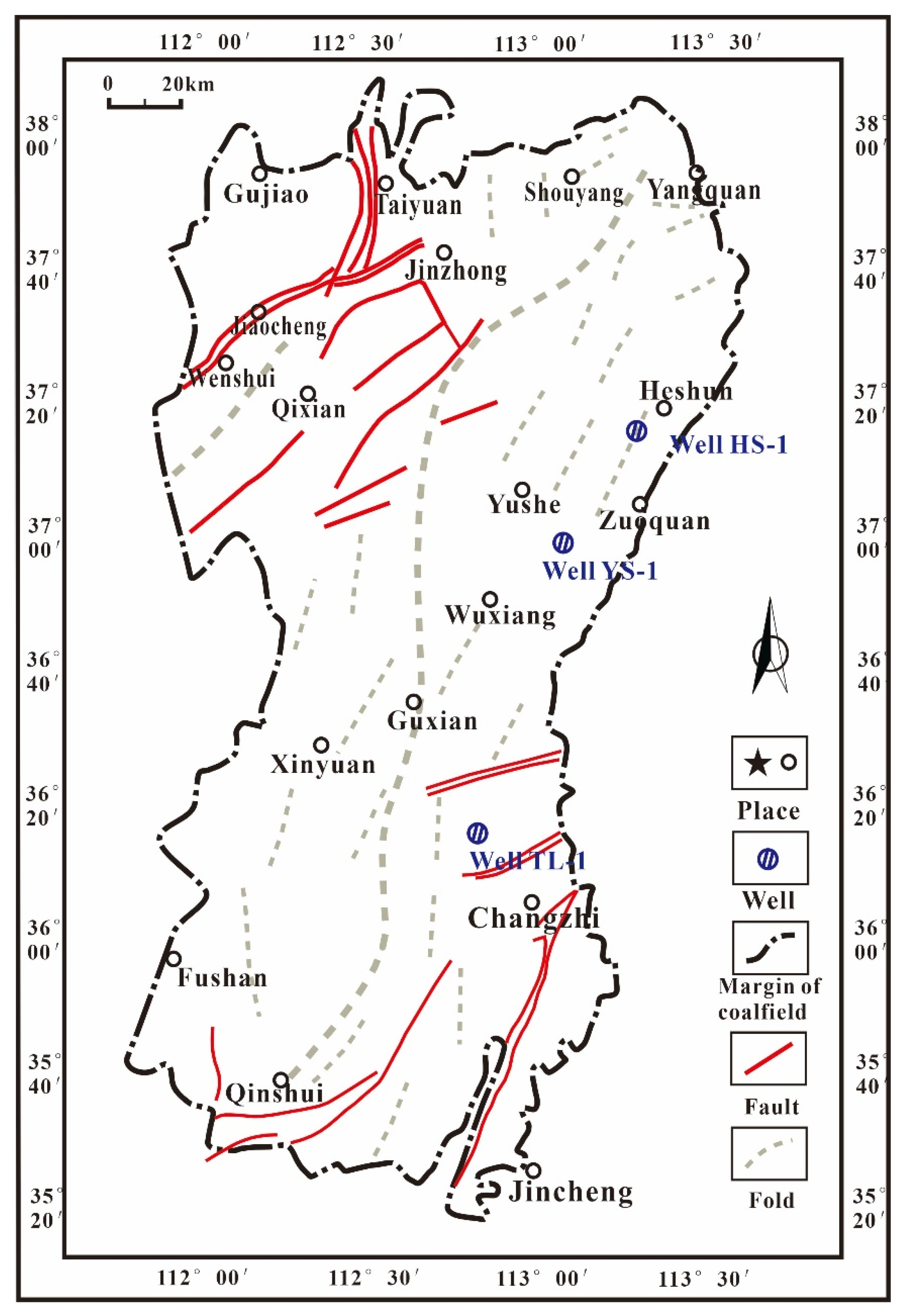
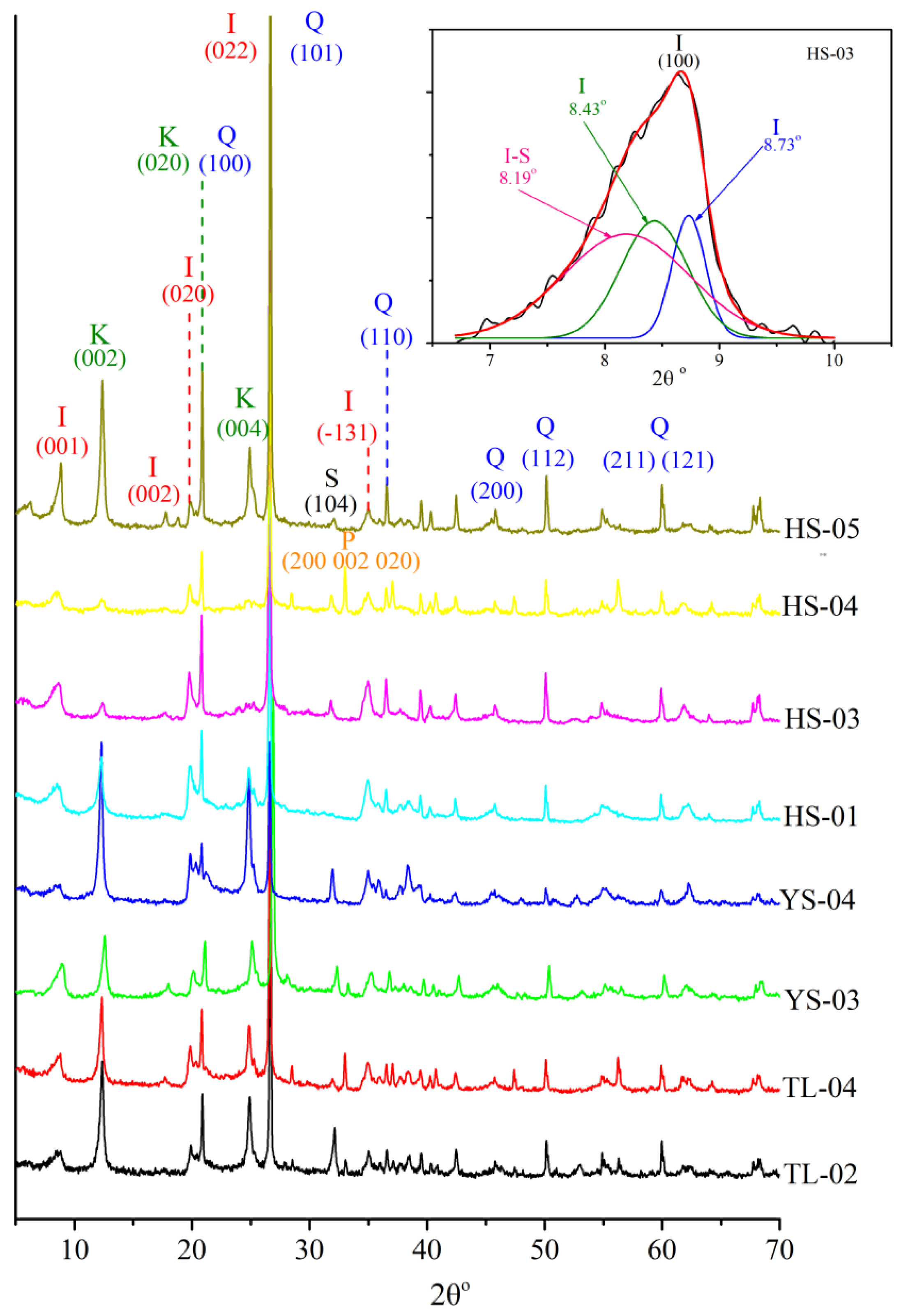
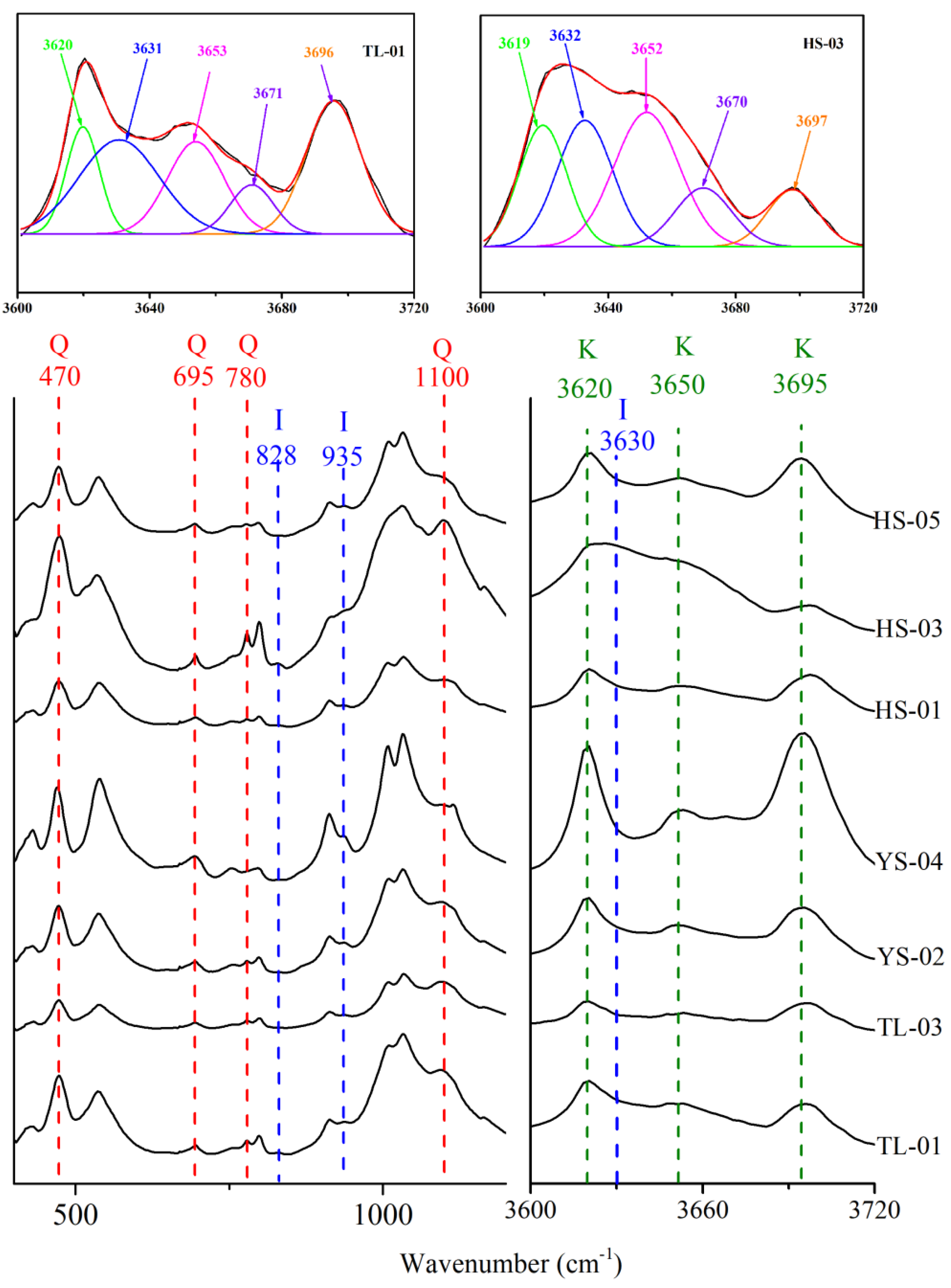

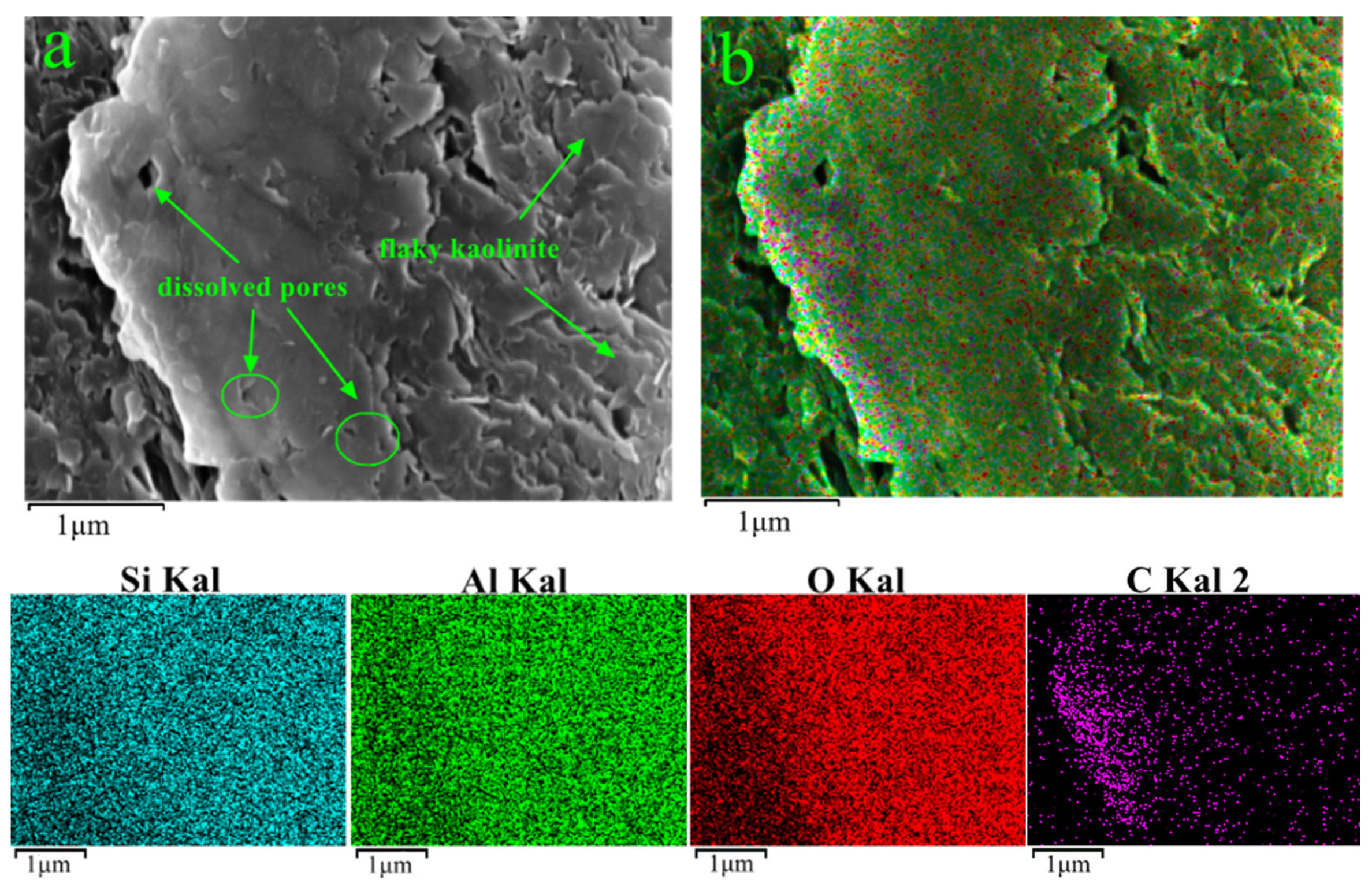

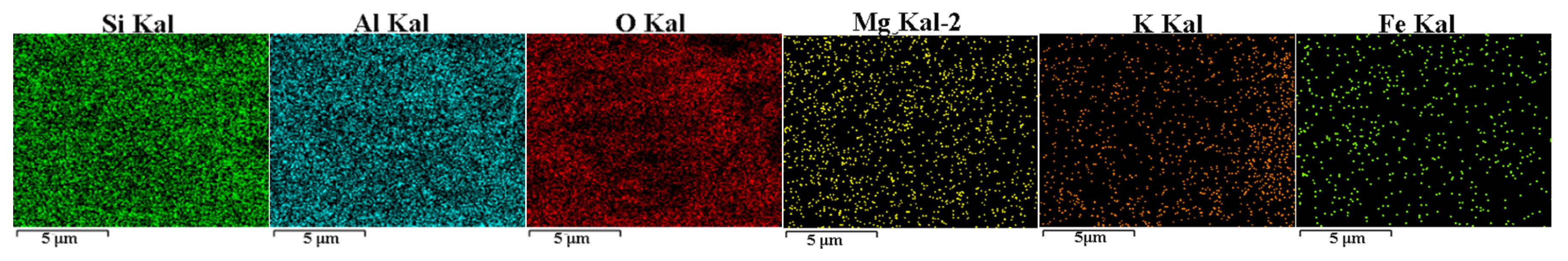


| Samples | Quartz (%) | Pyrite (%) | Siderite (%) | Dolomite (%) | Kaolinite (%) | Illite (%) | Chlorite (%) |
|---|---|---|---|---|---|---|---|
| TL-01 | 22.0 | 1.6 | 37.4 | 39.0 | 0.0 | ||
| TL-02 | 39.0 | 1.6 | 8.8 | 36.7 | 14.0 | 0.0 | |
| TL-03 | 50.0 | 31.9 | 14.3 | 3.8 | |||
| TL-04 | 60.0 | 4.0 | 19.7 | 15.0 | 1.3 | ||
| YS-01 | 47.0 | 1.2 | 4.0 | 31.0 | 14.4 | 2.4 | |
| YS-02 | 43.4 | 2.0 | 32.6 | 19.4 | 2.6 | ||
| YS-03 | 16.5 | 0.7 | 22.0 | 58.9 | 1.9 | ||
| YS-04 | 14.2 | 2.7 | 71.4 | 10.1 | 1.6 | ||
| HS-01 | 46.0 | 20.0 | 31.0 | 3.0 | |||
| HS-02 | 45.0 | 3.0 | 7.2 | 23.0 | 18.3 | 3.5 | |
| HS-03 | 48.0 | 29.0 | 20.0 | 3.0 | |||
| HS-04 | 61.3 | 8.8 | 13.9 | 13.5 | 2.5 | ||
| HS-05 | 31.2 | 0.8 | 49.0 | 15.6 | 3.4 |
| Samples | SiO2 (%) | Al2O3 (%) | CaO (%) | Fe2O3 (%) | K2O (%) | MgO (%) | Na2O (%) | TiO2 (%) | MnO (%) | P2O5 (%) | SiO2/ Al2O3 | ICV | K2O/ Al2O3 | TiO2/ Al2O3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TL-01 | 62.30 | 26.42 | 0.46 | 4.14 | 3.55 | 1.14 | 1.30 | 0.53 | 0.09 | 0.08 | 2.36 | 0.42 | 0.13 | 0.02 |
| TL-02 | 58.77 | 25.88 | 0.62 | 10.08 | 2.00 | 1.16 | 0.75 | 0.54 | 0.20 | 0.04 | 2.27 | 0.59 | 0.08 | 0.02 |
| TL-03 | 68.06 | 25.54 | 0.23 | 1.91 | 2.07 | 0.80 | 0.68 | 0.68 | 0.00 | 0.02 | 2.66 | 0.25 | 0.08 | 0.03 |
| TL-04 | 61.01 | 28.00 | 0.26 | 5.87 | 2.90 | 0.55 | 0.42 | 0.80 | 0.00 | 0.04 | 2.18 | 0.39 | 0.10 | 0.03 |
| YS-01 | 60.95 | 24.62 | 1.12 | 5.70 | 2.50 | 1.31 | 1.04 | 0.57 | 0.11 | 0.08 | 2.48 | 0.50 | 0.10 | 0.02 |
| YS-02 | 61.88 | 26.32 | 0.59 | 5.55 | 3.44 | 0.78 | 0.58 | 0.77 | 0.06 | 0.04 | 2.35 | 0.45 | 0.13 | 0.03 |
| YS-03 | 61.52 | 25.95 | 0.60 | 5.19 | 3.41 | 1.22 | 1.31 | 0.56 | 0.17 | 0.08 | 2.37 | 0.48 | 0.13 | 0.02 |
| YS-04 | 53.73 | 37.52 | 0.29 | 4.79 | 1.56 | 0.57 | 0.53 | 0.89 | 0.09 | 0.04 | 1.43 | 0.23 | 0.04 | 0.02 |
| HS-01 | 58.67 | 33.99 | 0.31 | 1.46 | 2.99 | 0.67 | 1.25 | 0.62 | 0.00 | 0.03 | 1.73 | 0.22 | 0.09 | 0.02 |
| HS-02 | 56.13 | 26.74 | 0.69 | 10.13 | 3.43 | 1.10 | 1.06 | 0.51 | 0.16 | 0.06 | 2.10 | 0.64 | 0.13 | 0.02 |
| HS-03 | 66.91 | 22.58 | 0.46 | 3.48 | 4.12 | 0.90 | 1.02 | 0.43 | 0.04 | 0.06 | 2.96 | 0.46 | 0.18 | 0.02 |
| HS-04 | 59.34 | 19.89 | 0.54 | 14.89 | 3.16 | 0.83 | 0.79 | 0.42 | 0.07 | 0.07 | 2.98 | 1.04 | 0.16 | 0.02 |
| HS-05 | 62.75 | 26.82 | 0.51 | 4.34 | 2.80 | 1.33 | 0.65 | 0.70 | 0.04 | 0.05 | 2.34 | 0.39 | 0.10 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Kong, S.; Liang, Y.; Ali, M.; Zhang, Y.; Zhao, Y. Geochemical and Microstructural Characteristics of Clay Minerals and Their Effects on the Pore Structure of Coal-Measure Shale: A Case Study in Qinshui Basin, China. Energies 2023, 16, 3804. https://doi.org/10.3390/en16093804
Li K, Kong S, Liang Y, Ali M, Zhang Y, Zhao Y. Geochemical and Microstructural Characteristics of Clay Minerals and Their Effects on the Pore Structure of Coal-Measure Shale: A Case Study in Qinshui Basin, China. Energies. 2023; 16(9):3804. https://doi.org/10.3390/en16093804
Chicago/Turabian StyleLi, Kunjie, Shaoqi Kong, Yanxia Liang, Muhammad Ali, Yongfa Zhang, and Yuqiong Zhao. 2023. "Geochemical and Microstructural Characteristics of Clay Minerals and Their Effects on the Pore Structure of Coal-Measure Shale: A Case Study in Qinshui Basin, China" Energies 16, no. 9: 3804. https://doi.org/10.3390/en16093804
APA StyleLi, K., Kong, S., Liang, Y., Ali, M., Zhang, Y., & Zhao, Y. (2023). Geochemical and Microstructural Characteristics of Clay Minerals and Their Effects on the Pore Structure of Coal-Measure Shale: A Case Study in Qinshui Basin, China. Energies, 16(9), 3804. https://doi.org/10.3390/en16093804







