Application of an NDIR Sensor System Developed for Early Thermal Runaway Warning of Automotive Batteries
Abstract
1. Introduction
2. Materials and Methods
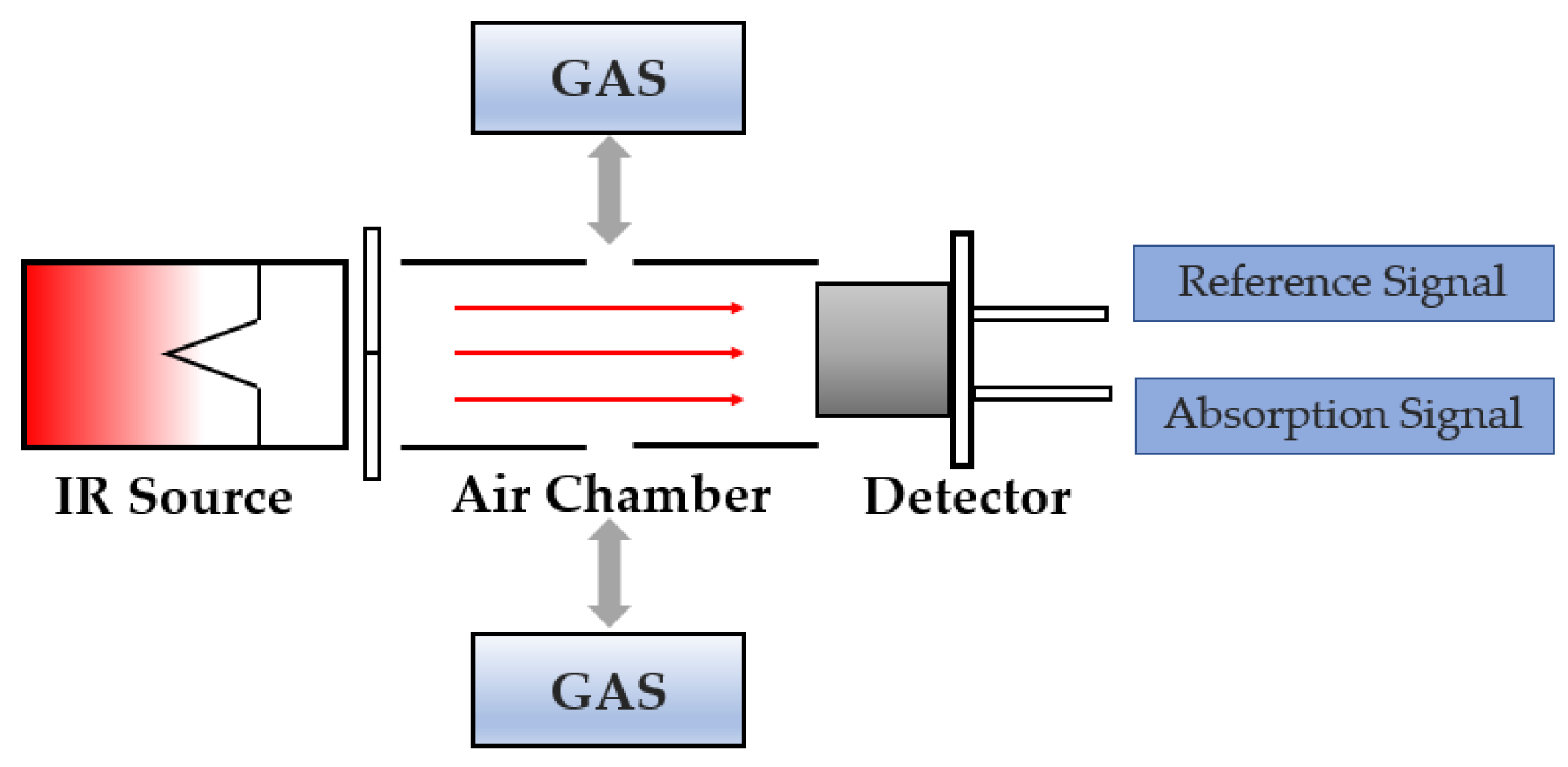
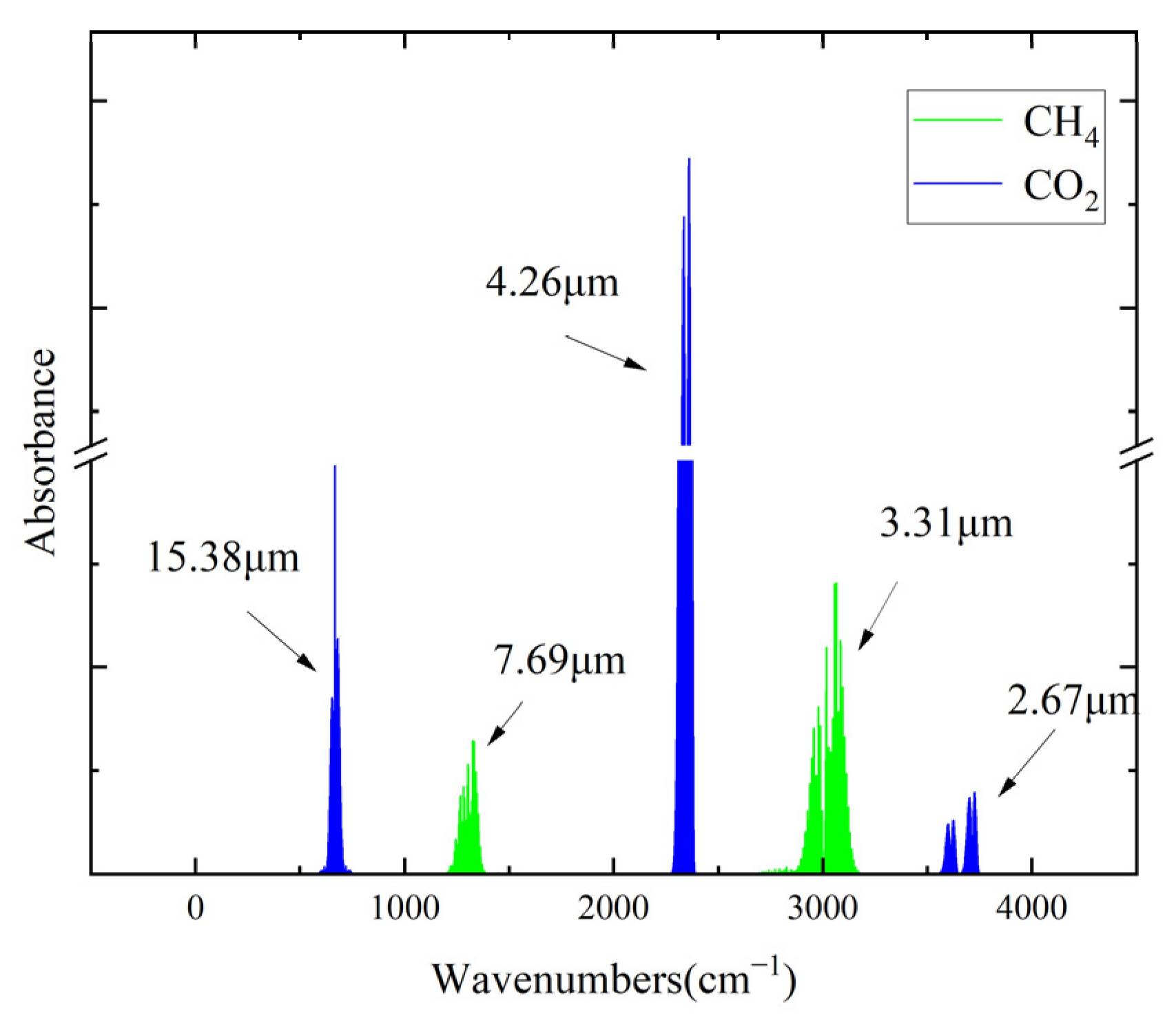
2.1. Development of NDIR Gas Sensors
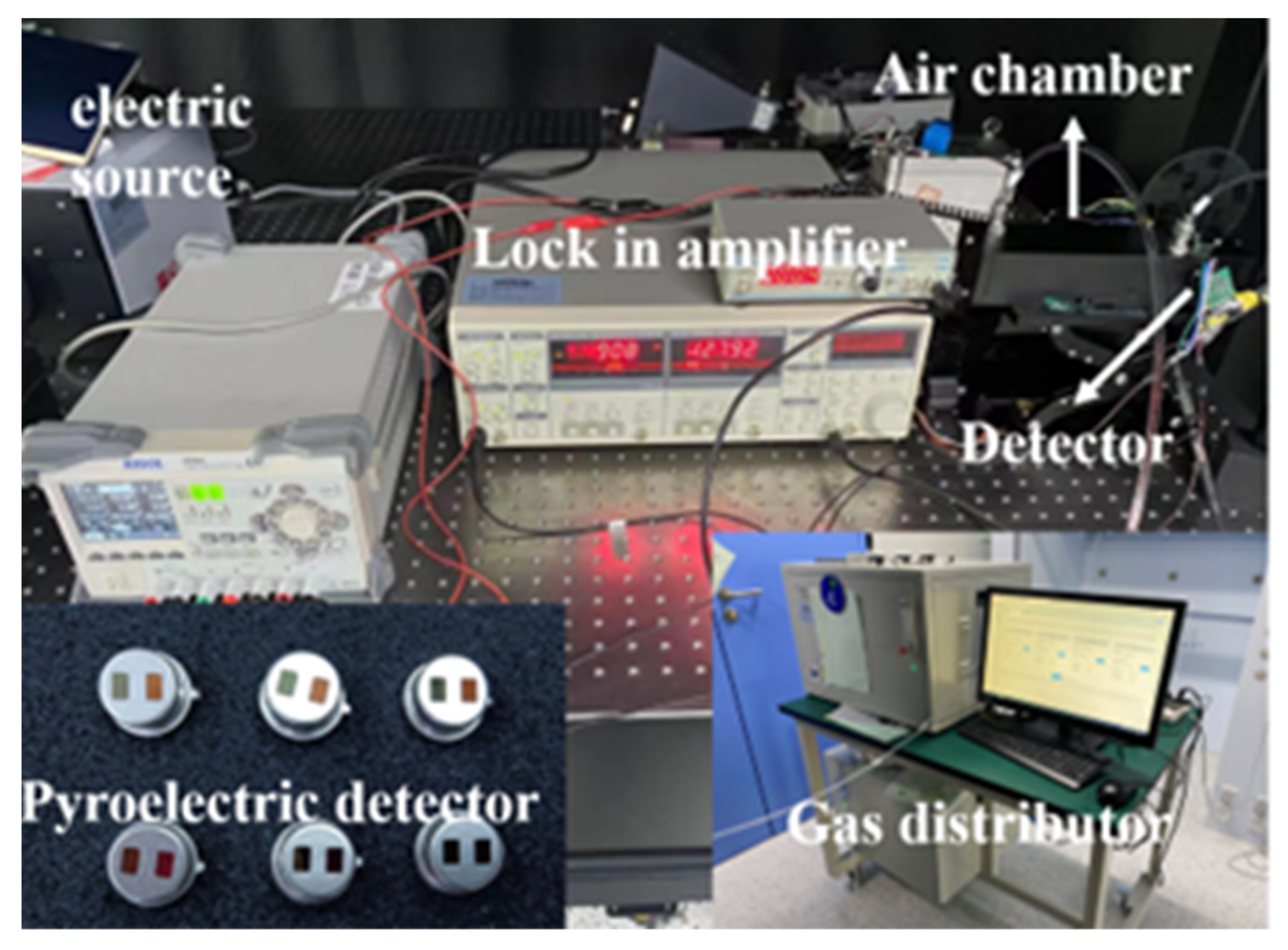
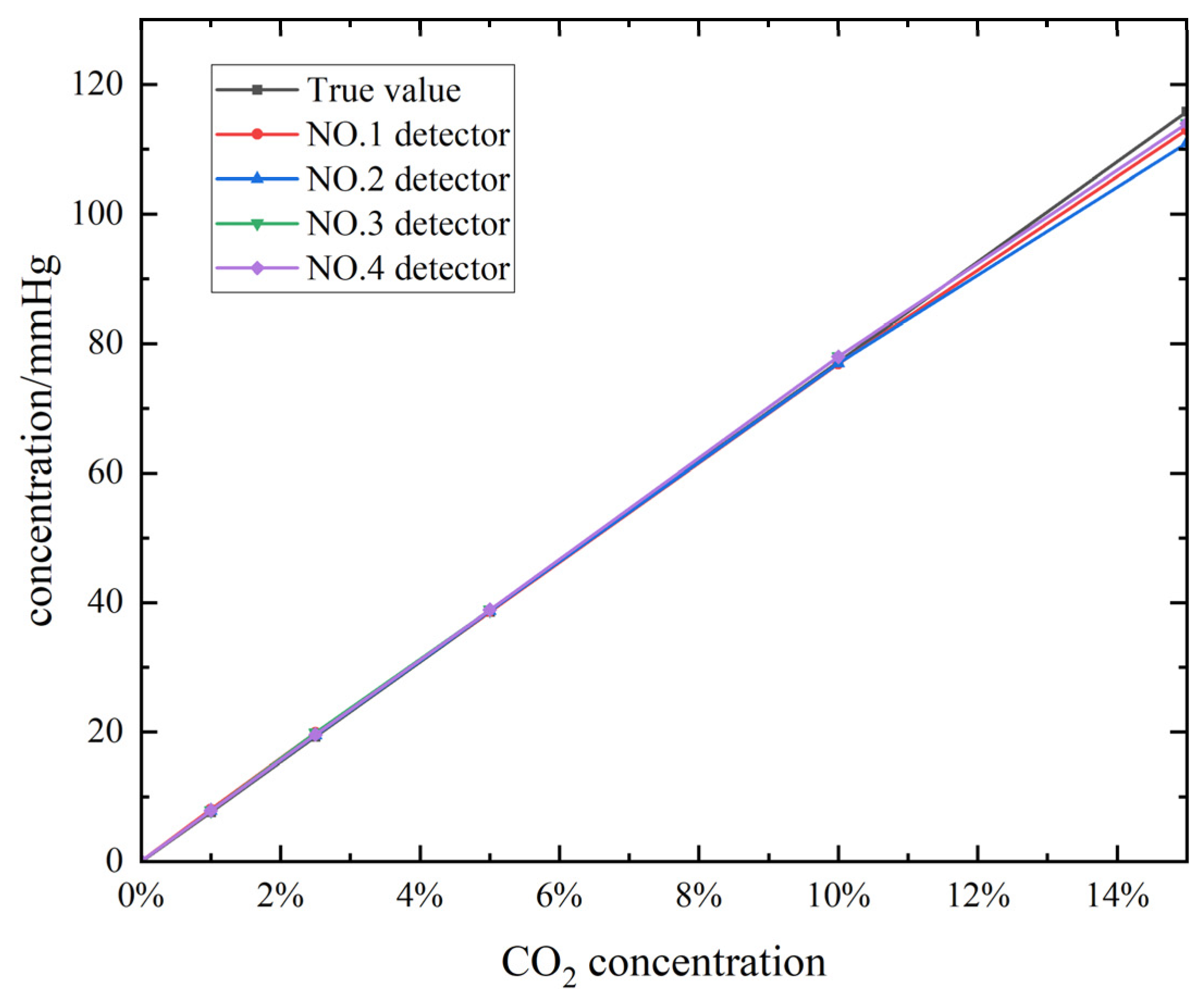
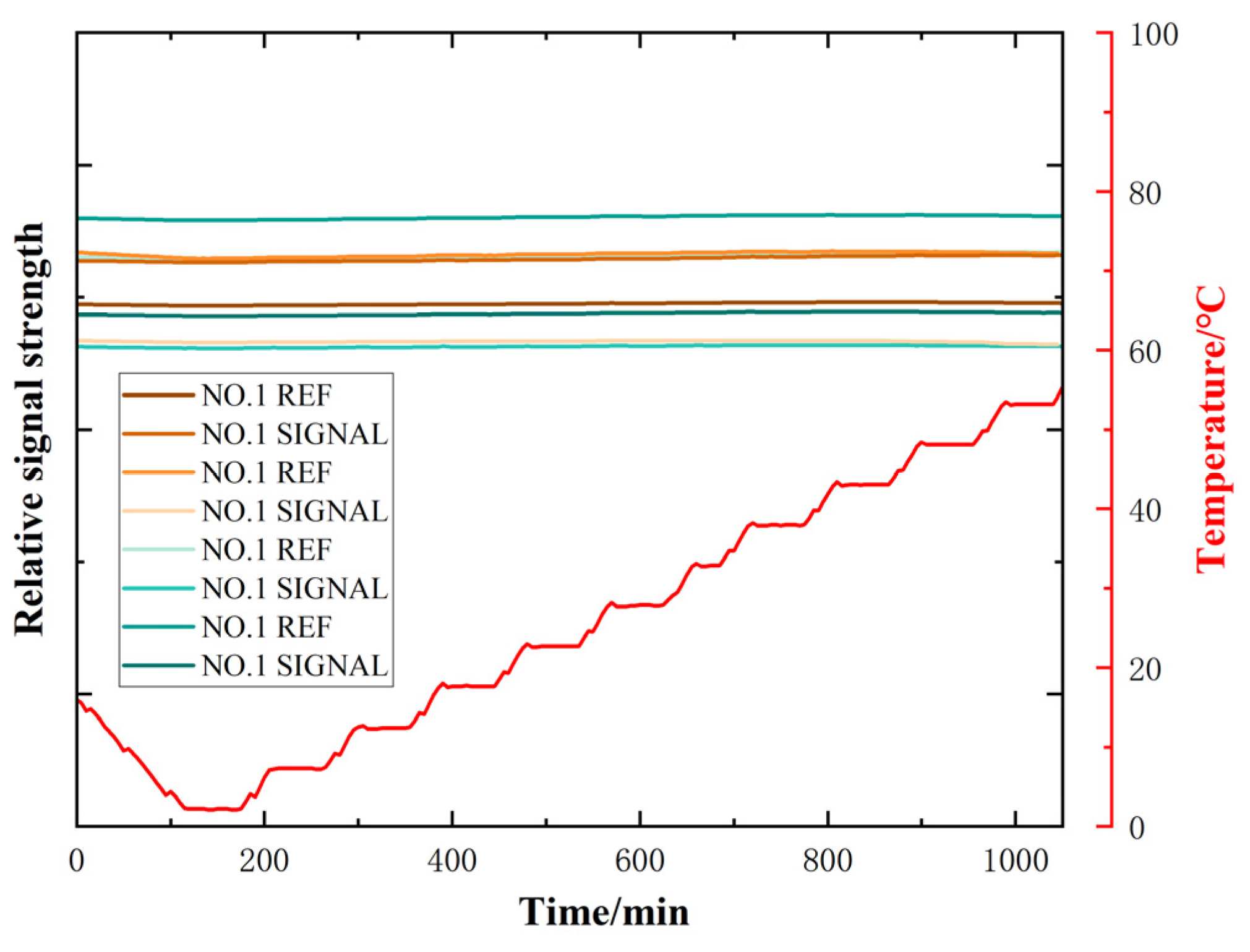
2.2. NDIR Gas Sensor Performance Testing
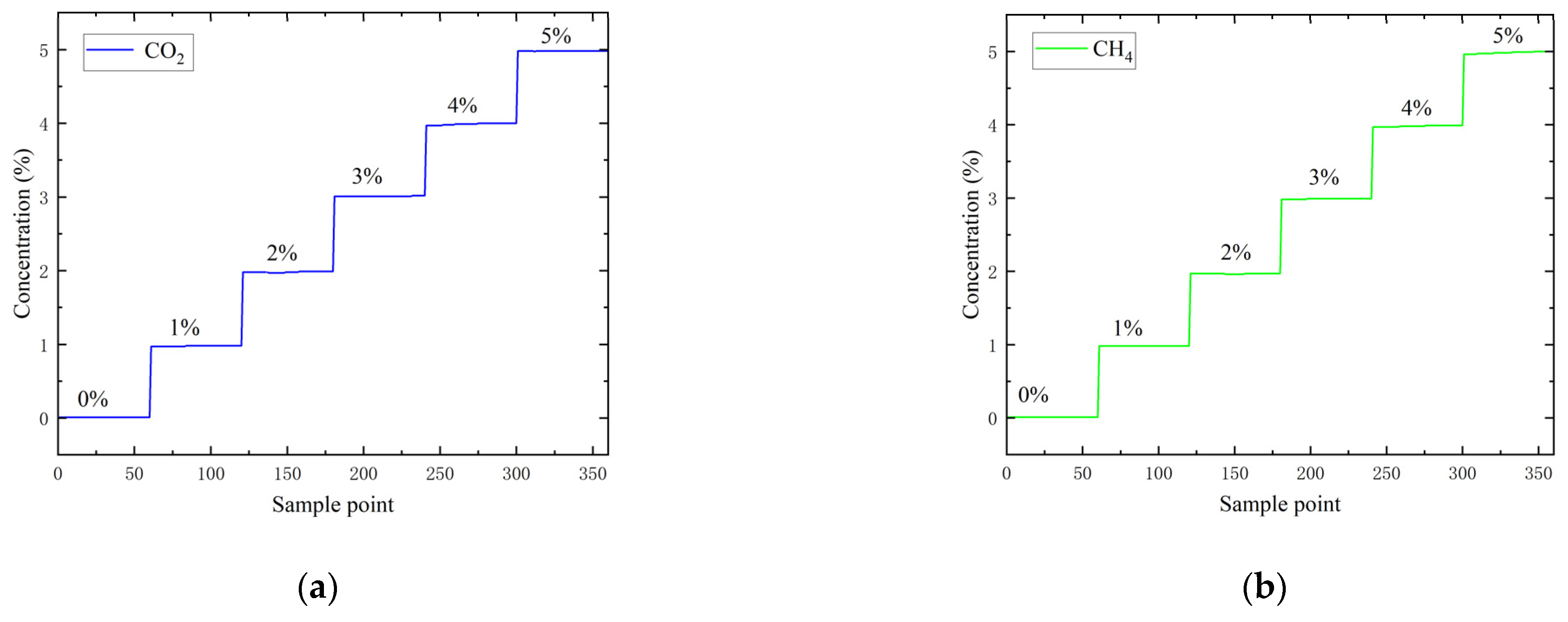
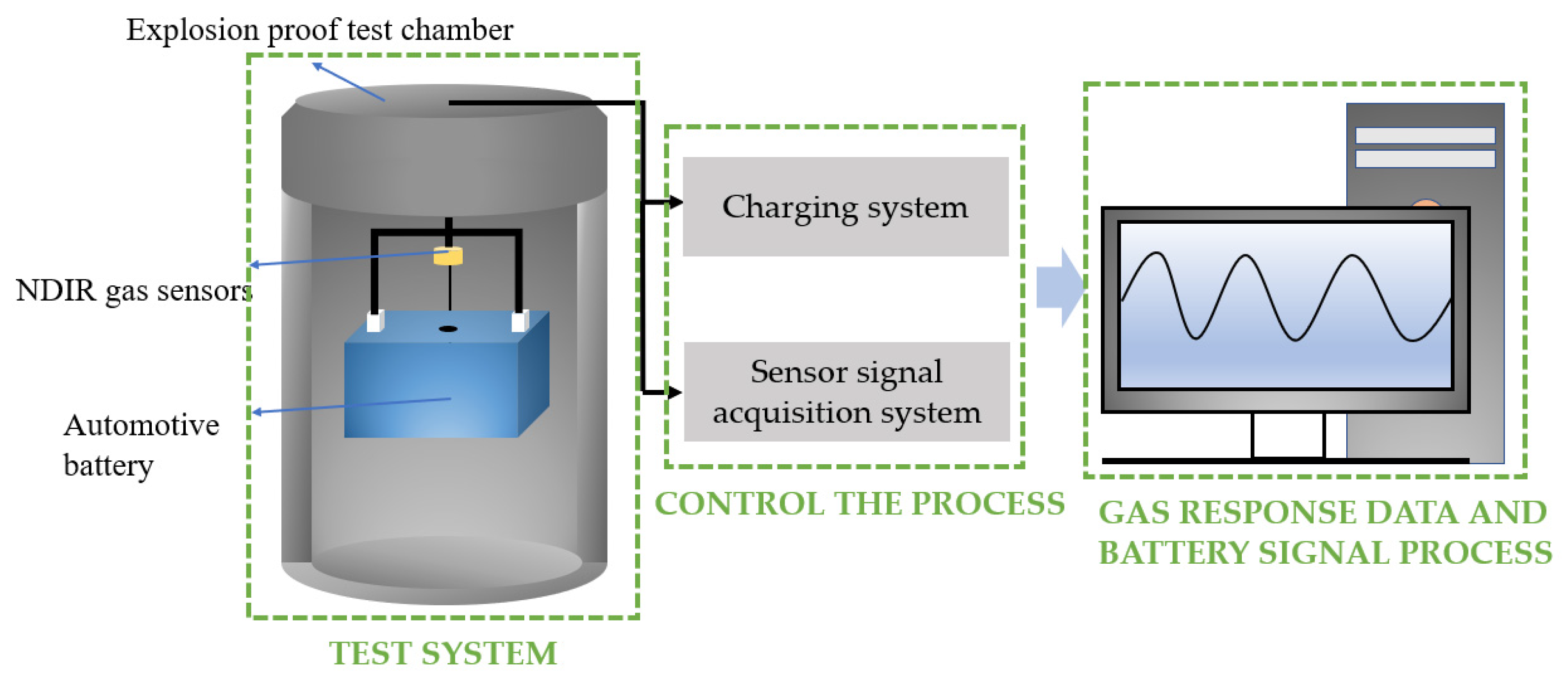
3. Experiments
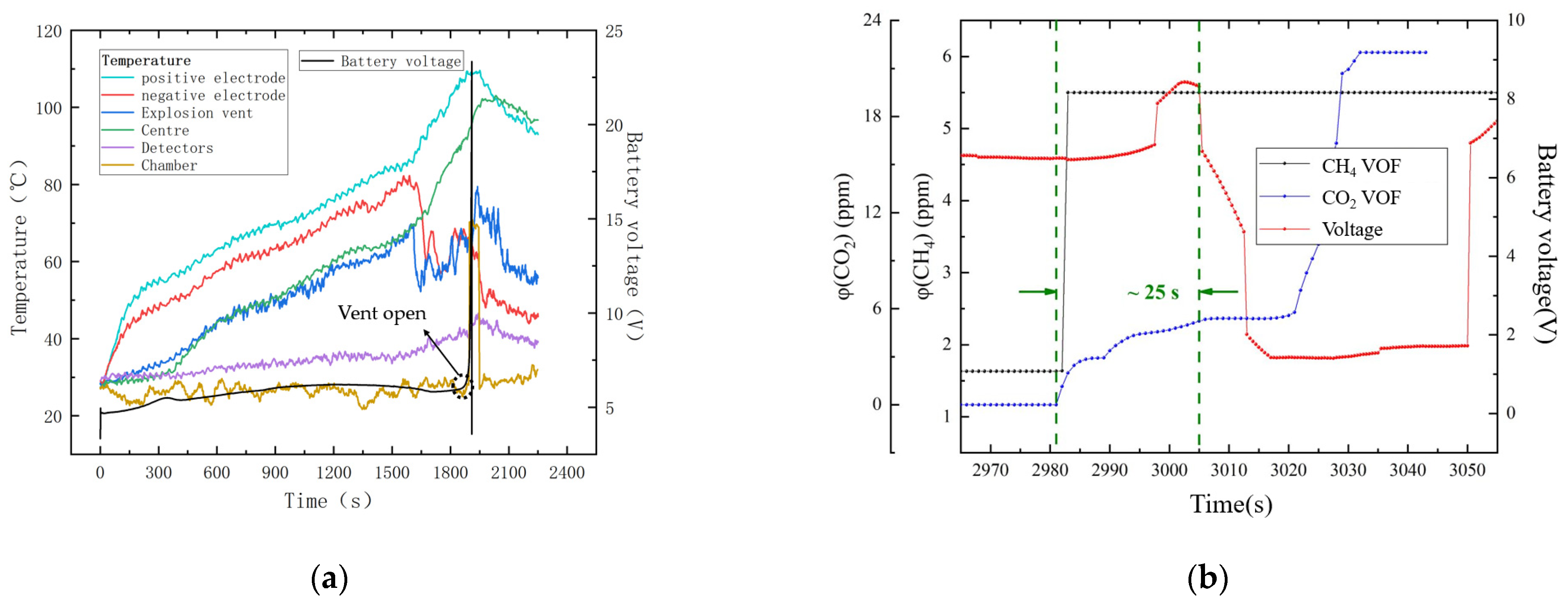
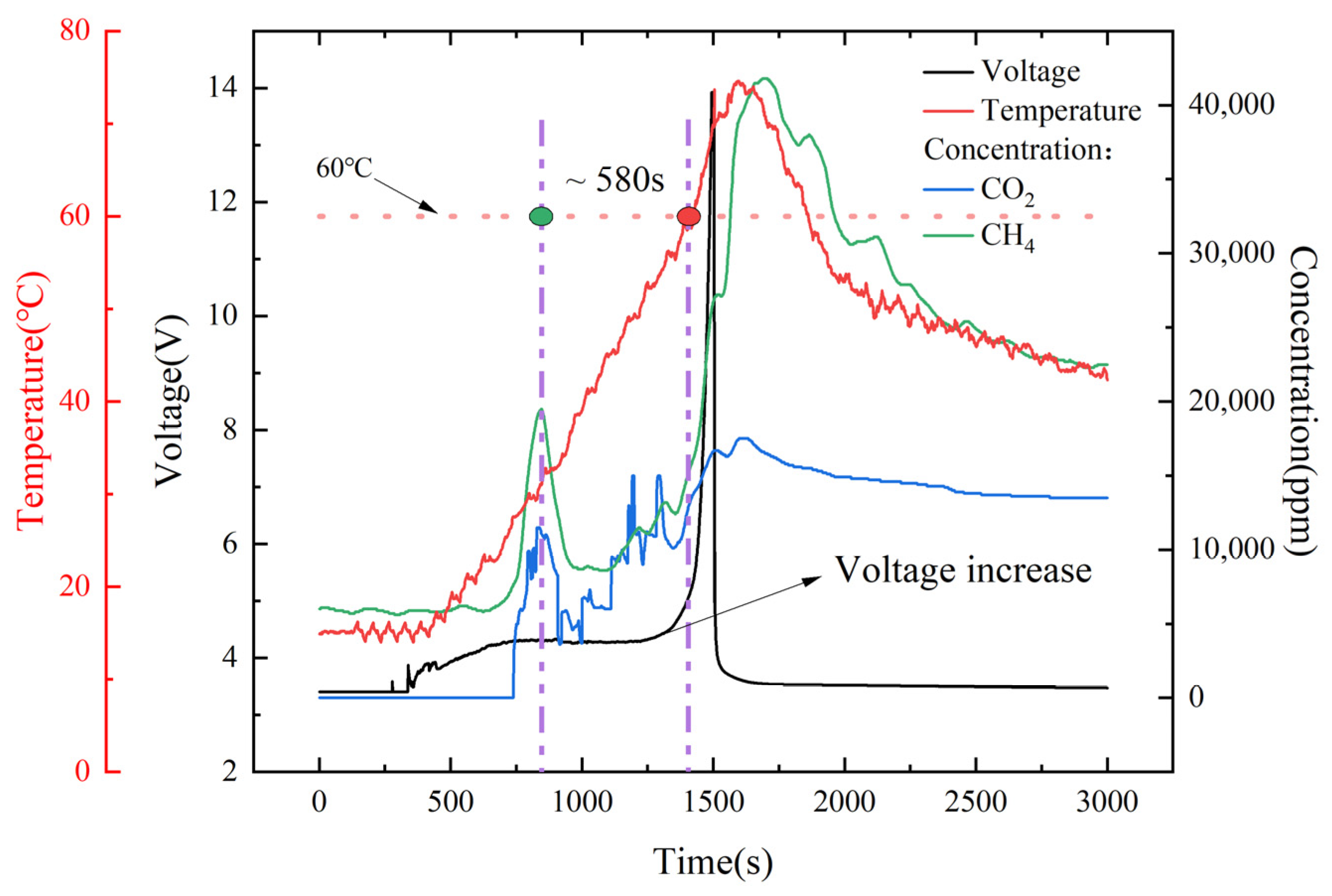
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, W.; Feng, X.; Han, X.; Zhang, W.; Jiang, F. Questions and Answers Relating to Lithium-Ion Battery Safety Issues. Cell Rep. Phys. Sci. 2021, 2, 100285. [Google Scholar] [CrossRef]
- Zaghib, K.; Dubé, J.; Dallaire, A.; Galoustov, K.; Guerfi, A.; Ramanathan, M.; Benmayza, A.; Prakash, J.; Mauger, A.; Julien, C.M. Enhanced thermal safety and high power performance of carbon-coated LiFePO4 olivine cathode for Li-ion batteries. J. Power Sources 2012, 219, 36–44. [Google Scholar] [CrossRef]
- Wen, J.; Yu, Y.; Chen, C. A Review on Lithium-Ion Batteries Safety Issues: Existing Problems and Possible Solutions. Mater. Express 2012, 2, 197–212. [Google Scholar] [CrossRef]
- Doh, C.H.; Kim, D.H.; Kim, H.S.; Shin, H.M.; Jeong, Y.D.; Moon, S.I.; Jin, B.S.; Eom, S.W.; Kim, H.S.; Kim, K.W.; et al. Thermal and electrochemical behaviour of C/LixCoO2 cell during safety test. J. Power Sources 2008, 175, 881–885. [Google Scholar] [CrossRef]
- Zeng, Y.; Wu, K.; Wang, D.; Wang, Z.; Chen, L. Overcharge investigation of lithium-ion polymer batteries. J. Power Sources 2006, 160, 1302–1307. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Yan, W. Identification and characteristic analysis of powder ejected from a lithium ion battery during thermal runaway at elevated temperatures. J. Hazard. Mater. 2020, 400, 123169. [Google Scholar] [CrossRef] [PubMed]
- Beletskii, E.V.; Fedorova, A.A.; Lukyanov, D.A.; Kalnin, A.Y.; Ershov, V.A.; Danilov, S.E.; Spiridonova, D.V.; Alekseeva, E.V.; Levin, O.V. Switchable resistance conducting-polymer layer for Li-ion battery overcharge protection. J. Power Sources 2021, 490, 229548.1–229548.10. [Google Scholar] [CrossRef]
- Kumai, K.; Miyashiro, H.; Kobayashi, Y.; Takei, K.; Ishikawa, R. Gas generation mechanism due to electrolyte decomposition in commercial lithium-ion cell. J. Power Sources 1999, 81–82, 715–719. [Google Scholar] [CrossRef]
- Essl, C.; Seifert, L.; Rabe, M.; Fuchs, A. Early Detection of Failing Automotive Batteries Using Gas Sensors. Batteries 2021, 7, 25. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Z.; Wang, Y.; Wang, H.; Wang, C.; Tong, L.; Yi, M. Overcharge investigation of large format lithium-ion pouch cells with Li(Ni0.6Co0.2Mn0.2)O2 cathode for electric vehicles: Thermal runaway features and safety management method. Energy 2019, 169, 868–880. [Google Scholar] [CrossRef]
- Kong, W.; Li, H.; Huang, X.; Chen, L. Gas evolution behaviors for several cathode materials in lithium-ion batteries. J. Power Sources 2005, 142, 285–291. [Google Scholar] [CrossRef]
- Galushkin, N.E.; Yazvinskaya, N.N. Echanism of Gases Generation during Lithium-Ion Batteries Cycling. J. Electrochem. Soc. 2019, 166, A897–A908. [Google Scholar] [CrossRef]
- Yu, Y.; Lee, S.J.; Theerthagiri, J.; Lee, Y.; Choi, M.Y. Architecting the AuPt alloys for hydrazine oxidation as an anolyte in fuel cell: Comparative analysis of hydrazine splitting and water splitting for energy-saving H2 generation. Appl. Catal. B Environ. 2022, 316, 121603. [Google Scholar] [CrossRef]
- Pinnangudi, B.N.; Dalal, S.B.; Medora, N.K.; Arora, A.; Swart, J. Thermal shutdown characteristics of insulating materials used in lithium ion batteries. In Proceedings of the 2010 IEEE Symposium on Product Compliance Engineering Proceedings, Boston, MA, USA, 18–20 October 2010; pp. 1–5. [Google Scholar]
- Rahimi-Eichi, H.; Ojha, U.; Baronti, F.; Chow, M.Y. Battery management system: An overview of its application in the smart grid and electric vehicles. IEEE Ind. Electron. Mag. 2013, 7, 4–16. [Google Scholar] [CrossRef]
- Liu, K.; Li, K.; Peng, Q.; Zhang, C. A brief review on key technologies in the battery management system of electric vehicles. Front. Mech. Eng. 2019, 14, 47–64. [Google Scholar] [CrossRef]
- GB 38031-2020; National Standard of the People’s Republic China (GB): Electric Vehicles Traction Battery Safety Requirements. Standardization Administration: Beijing, China, 2020; pp. 1–42.
- Kenichiroh, K. Journey to a New Regulatory Option. In OICA Submission to IWG for GTR 20, Phase 2; United Nations Economic Commission for Europe (UNECE): Berlin, Germany, 2019. [Google Scholar]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Chaudhary, S.; Umar, A.; Bhasi, K.; Baskoutas, S. Chemical Sensing Applications of ZnO Nanomaterials. Materials 2018, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Tamvakos, A.; Calestani, D.; Tamvakos, D.; Pullini, D.; Sgroi, M.; Pruna, A. Low concentration CO gas sensing properties of hybrid ZnO architecture. Microelectron. Eng. 2016, 160, 12–17. [Google Scholar] [CrossRef]
- Gibson, D.; MacGregor, C. A Novel Solid State Non-Dispersive Infrared CO2 Gas Sensor Compatible with Wireless and Portable Deployment. Sensors 2013, 13, 7079–7103. [Google Scholar] [CrossRef]
- Zhou, R.; Vaihinger, S.; Geckeler, K.; Göpel, W. Reliable CO2 sensors with silicon-based polymers on quartz microbalancetransducers. Sens. Actuat. B Chem. 1994, 19, 415–420. [Google Scholar] [CrossRef]
- Cai, T.; Stefanopoulou, A.G.; Siegel, J.B. Early Detection for Li-Ion Batteries Thermal Runaway Based on Gas Sensing Ting. ECS Trans. 2019, 89, 85–97. [Google Scholar] [CrossRef]
- Koch, S.; Birke, K.P.; Kuhn, R. Fast thermal runaway detection for lithium-ion cells in large scale traction batteries. Batteries 2018, 4, 16. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, L.; Liu, J.; Wang, J.; Yan, W. Gas Sensing Technology for the Detection and Early Warning of Battery Thermal Runaway: A Review. Energy Fuels 2022, 36, 6038–6057. [Google Scholar] [CrossRef]
- Sun, X.; Ming, A.; Zhang, J.; Liu, W.; Meng, Y.; Qin, D.; Yao, J.; Wang, W.; Chen, D. Pyroelectric infrared detector based on LiTaO3 crystal with novelty nanostructured amorphous carbon film. In Proceedings of the IEEE 12th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Los Angeles, CA, USA, 9–12 April 2017; pp. 623–626. [Google Scholar]
- Hodgkinson, J.; Ralph, P.T. Optical gas sensing: A review. Meas. Sci. Technol. 2013, 24, 012004. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Smith, R. Non-dispersive infra-red (NDIR) measurement of carbon dioxide at 4.2 μm in a compact and optically efficient sensor. Sens. Actuators B Chem. 2013, 186, 580–588. [Google Scholar] [CrossRef]
- Vincent, T.A.; Gardner, J.W. A low cost MEMS based NDIR system for the monitoring of carbon dioxide in breath analysis at ppm levels. Sens. Actuators B Chem. 2016, 236, 954–964. [Google Scholar] [CrossRef]
- Vincent, T.A.; Urasinska-Wojcik, B. Development of a lowcost NDIR system for ppm detection of carbon dioxide in exhaled breath analysis. Procedia Eng. 2015, 120, 388–391. [Google Scholar] [CrossRef]
- Mendes, L.B.; Ogink, N.W.; Edouard, N.; Van Dooren, H.J.; Tinôco, I.D.; Mosquera, J. NDIR gas sensor for spatial monitoring of carbon dioxide concentrations in naturally ventilated livestock buildings. Sensors 2015, 15, 11239–11257. [Google Scholar] [CrossRef]
- Kwon, J.; Ahn, G.; Kim, G.; Kim, J.-C.; Kim, H. A study on NDIR-based CO2 sensor to apply remote air quality monitoring system. In Proceedings of the ICCAS-SICE 2009—ICROS-SICE International Joint Conference, Fukuoka, Japan, 18–21 August 2009. [Google Scholar]
- Popa, D.; Udrea, F. Towards integrated mid-infrared gas sensors. Sensors 2019, 19, 2076. [Google Scholar] [CrossRef]
- Dinh, T.V.; Choi, I.Y. A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction. Sens. Actuators B Chem. 2016, 231, 529–538. [Google Scholar] [CrossRef]
- Rothman, L.S.; Jacquemart, D. The HITRAN 2004 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2005, 96, 139–204. [Google Scholar] [CrossRef]
- Lee, R.; Kester, W. Complete gas sensor circuit using nondispersive infrared (NDIR). Anal. Dialog. 2016, 50, 10–18. [Google Scholar]

| Type | LFP Battery |
|---|---|
| Chemical composition | positive pole: LFP |
| negative pole: LiC6 | |
| Rated capacity | 50 Ah |
| Size | 147 × 115 × 28 mm |
| Mass | 986 g |
| Standard voltage | 3.0 V |
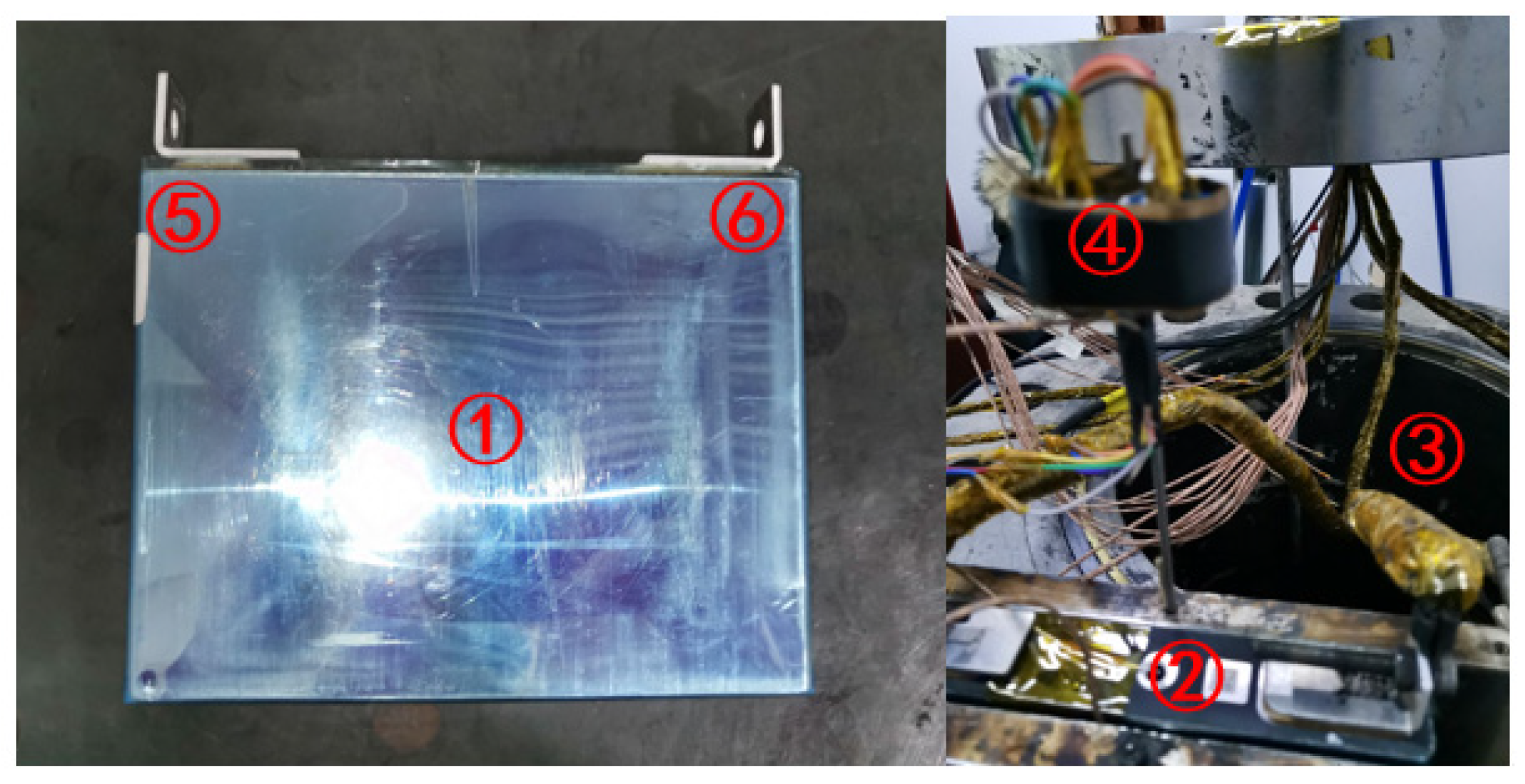
| Thermopile Sensors | Position Settings |
|---|---|
| 1 | The center of the battery surface |
| 2 | The battery explosion vent |
| 3 | The ambient temperature |
| 4 | The gas sensor |
| 5 | The positive poles of the battery |
| 6 | The negative poles of the battery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Zhao, Y.; Ming, A.; Fang, Y.; Fang, S.; Bi, S.; Chen, J.; Xu, R.; Wei, F.; Mao, C. Application of an NDIR Sensor System Developed for Early Thermal Runaway Warning of Automotive Batteries. Energies 2023, 16, 3620. https://doi.org/10.3390/en16093620
Han Y, Zhao Y, Ming A, Fang Y, Fang S, Bi S, Chen J, Xu R, Wei F, Mao C. Application of an NDIR Sensor System Developed for Early Thermal Runaway Warning of Automotive Batteries. Energies. 2023; 16(9):3620. https://doi.org/10.3390/en16093620
Chicago/Turabian StyleHan, Yulu, Yongmin Zhao, Anjie Ming, Yanyan Fang, Sheng Fang, Shansong Bi, Jiezhi Chen, Ran Xu, Feng Wei, and Changhui Mao. 2023. "Application of an NDIR Sensor System Developed for Early Thermal Runaway Warning of Automotive Batteries" Energies 16, no. 9: 3620. https://doi.org/10.3390/en16093620
APA StyleHan, Y., Zhao, Y., Ming, A., Fang, Y., Fang, S., Bi, S., Chen, J., Xu, R., Wei, F., & Mao, C. (2023). Application of an NDIR Sensor System Developed for Early Thermal Runaway Warning of Automotive Batteries. Energies, 16(9), 3620. https://doi.org/10.3390/en16093620






