Abstract
The dewatering process reduces the water quantity in sludge, allowing the decrease in its volume, which facilitates its storage, transport, stabilization, and improves the post-treatment efficiency. Chemical polymers including aluminum sulphate and polyaluminum chloride were applied as flocculants in the conditioning process in order to prepare sludge for dewatering. However, these synthetic polymers may cause risks for human health, and should be substituted with ecofriendly and safe materials. These materials include plant-based flocculants, animal-based flocculants, and microbial-based flocculants. Sludge dewaterability was evaluated by considering many parameters, such as moisture content (MC), dry solids (DS), specific resistance to filtration (SRF), capillary suction time (CST), and sludge volume index (SVI). The use of microorganisms for sludge dewatering is an available option, since many strains (R. erythropolis, A. ferrooxidans, P. mirabilis, T. flavus, etc.) demonstrated their ability to produce polymers useful for dewatering sludge from various origins (chemically treated primary sludge, activated sludge, anaerobically digested sludge, etc.). For plant-based flocculants, only okra (Abelmoschus esculentus), cactus (Opuntia ficus Indica), moringa (M. oleifera), and aloe (A. vera) plants are examined for sludge dewatering. Compared to synthetic polymers, plant-based flocculants showed a viable alternative to chemicals and a step forward in green sludge treatment technology. Among the animal-based flocculants, chitosan and aminated chitosan were able to reduce the SRF (SRF reduction rate > 80%) of the anaerobically digested sludge. A new strategy using methylated hemoglobin also showed a significant enhancement in cake solid content of sludge (47%) and a decrease in sludge bound water content of 17.30%. Generally, extensive investigations are needed to explore and optimize all the related parameters (operating conditions, preparation procedure, production cost, etc.) and to choose the appropriate materials for large-scale application.
1. Introduction
Because of the intensification of industrial activities and the improvement in people’s living standards, an increasing quantity of wastewater is generated, causing a serious health problem, mainly when it is discharged in the environment without treatment [1]. To manage wastewater from various origins (industrial and urban, etc.), wastewater treatment facilities are designed to remove pollutants using various methods, including physical, chemical, and biological processes. Generally, wastewater treatment processes generate large amounts of sludge, creating a potential threat to the environment and human health [2,3]. The obtained sludge with a lower solid content (under 8%) should be treated for final safe disposal [4,5]. Sludge handling and disposal is a significant step of the whole system, which costs as much as 50% of the total wastewater treatment cost [6,7]. However, sludge management cost is governed mainly by the efficiency of the methods used to separate liquids and solids in sludge [8], allowing the decrease in its volume and enhancing the post-treatment efficiency [9,10]. Generally, after mechanical dehydration, the sludge water content remains more than 70% [11], which should be reduced to meet the subsequent sludge reuse. The performance of sludge dewatering is controlled by various factors related to its composition, the particle size, the surface charge, the presence of extracellular polymers, etc. [12,13]. Because of their composition (mainly hydrophilic proteins and polysaccharides), extracellular polymers bind water molecules, allowing for high water content in sludge and making the sludge dewatering process difficult [14,15,16,17]. In order to enhance the efficiency of sludge dewatering, various methods are applied. These methods are classified into biological, chemical, and physical methods [18,19,20,21,22,23]. The biological methods are based on the use of enzymes that degrade proteins, allowing sludge floc fragmentation [18]. In the physical methods, the sludge physicochemical characteristics are modified (formation of particle skeletons, building of drainage channels, change in the particle size, etc.), using various approaches (thermal, freeze–thaw, microwave, ultrasonic, skeleton builders, etc.), allowing the improvement in the sludge dewaterability [24,25,26,27,28]. However, the chemical methods are based on the addition of many reagents (flocculants, coagulants, acids, alkalis, surfactants, Fenton’s, oxidants, etc.) [22,29,30,31,32,33,34]. Flocculation is the most universally used method because of the many advantages (low cost, high efficiency, simple operation, and applicability for various sludge type) related to its use [35,36,37,38]. The addition of flocculants to sludge allows small colloidal particles to form large flocs and compacted cakes. Hence, the flocculants attack the stable colloid system and compress the double electric layer, allowing the fragmentation of the sludge extracellular polymers. Consequently, the linked water is released, enhancing the sludge dewatering rates and its solid content [39,40,41,42]. Generally, inorganic, organic synthetic, and natural flocculants are often used to improve sludge dewaterability before mechanical processing. The organic synthetic polymers include polyacrylic acid and polyacrylamide derivatives. Despite their effectiveness and their lower costs, their residual monomers are toxic and associated with serious diseases (cancer, neurological diseases, Alzheimer’s, etc.) [43,44]. Similarly, the inorganic polymers, including aluminum sulphate and polyaluminum chloride with residual metal ions, remain in sludge after treatment and may cause other risks for human health [45]. Therefore, there is a necessity to develop and use ecofriendly and safe flocculants. In this context, bioflocculants are potential sustainable materials which can substitute synthetic polymers. Generally, bioflocculants are made from non-toxic, biodegradable, and renewable materials, which fit well with the notion of sustainability [46,47]. However, an economical and efficient bioflocculant should be naturally abundant and renewable. Interestingly, the improvement in sludge dewatering by natural materials was reported in the literature. These materials include plant-based flocculants, animal-based flocculants, and microbial-based flocculants. The literature reported the use of numerous methods to prepare bioflocculants. To conclude about their efficiencies regarding sludge dewaterability, these natural materials were tested for sludge dewatering by assessing various sludge parameters including moisture content (MC), dry solids (DS), specific resistance to filtration (SRF), capillary suction time (CST), settling velocity (Vs), sludge volume index (SVI), and bound water content (BWC) [48,49,50,51,52,53]. This paper will review and discuss the potential use of bioflocculants as an alternative to synthetic polymers to enhance wastewater sludge dewaterability.
2. Microbial-Based Flocculants
Various microorganisms (fungi, bacteria, and microalgae) are able to produce flocculating materials, such as polysaccharides, proteins, and glycoproteins. The ability of microorganisms to produce these molecules is identified based on many parameters, including the morphology and the existence of slimy extracellular polysaccharides. For this purpose, various methods (colorimetric, 16S rRNA gene sequence, etc.) and reagents (chelating agents, CuSO4 solution crystal violet, etc.) are applied to isolate suitable microorganisms from soil, rivers, seawater, sludge, etc. [54]. The general process of the preparation of microbial-based flocculants is illustrated in Figure 1.
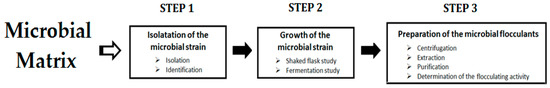
Figure 1.
General process of the preparation of the microbial-based flocculants.
Generally, the microbial bioflocculants have been successfully applied for the removal of various pollutants (suspended solids, chemical oxygen demand, heavy metals, dyes, etc.) with high efficiency levels (>90%), allowing a significant flocculating activity (>70%) [54,55]. Interestingly they have the potential to improve sludge dewaterability, as indicated in Table 1.

Table 1.
Applications of microbial-based flocculants for sludge dewatering.
The bioflocculant produced by Rhodococcus erythropolis in alkaline thermal pre-treated sludge allowed a significant increase in both SRF and DS, reaching 3.4 × 1012 m/kg and 22.5%, respectively [57]. In the same study, the use of R. erythropolis supplemented with synthetic polymers (PAC and Al2(SO4)3) increased the charge neutralization and bridging effect, allowing the enlargement of the flocs and, consequently, improving the sludge dewaterability [57]. However, for specific microbial strains there is a need for an energy substance (Fe2+) for efficient production of biogenic flocculants [62,63,64,65]. For example, Acidithiobacillus ferrooxidans in the presence of Fe2+ (10% v/v) significantly improved the dewaterability of anaerobically digested sludge, and the values of SRF and CST passed from 16.1 × 1012 m/kg to less than 1 × 1012 m/kg and from 30.4 s to less than 20 s [64]. The same strain improved the dewaterability of various sludges (chemically treated primary sludge, activated sludge, and anaerobically digested sludge) and the highest reduction was observed for chemically treated primary sludge, with final values for SRF and CST of 5 × 1012 m/kg and 20 s, respectively [63]. Moreover, the biopolymer produced by the same strain (Acidithiobacillus ferrooxidans) reduced the SRF and the CST of municipal anaerobically digested sludge with an interesting reduction rate of MC (70.3%), SRF, and CST. The SRF and CST values passed from 3.29 × 1013 m/kg to 0.36 × 1013 m/kg and from 38.7 s to 10.1 s, respectively. The obtained reduction rates are higher than those reported for polyacrylamide (PAM) [56]. Similarly, the use of filamentous fungal strains for the dewatering of chemically treated primary sludge allowed the decrease in CST from 86.9 to 35.5 s in the presence of metal cations [66]. More recently, the strain A. ferrooxidans ILS-2 was added to municipal digested sludge in the presence of ferrous iron (10–21%), allowing a significant reduction in CST and MC values. However, this reduction increased when increasing Fe2+ loading, and the highest reduction was obtained with ferrous iron at 21%. Fe2+ loading at 21% reduced CST from 339.1 s (without strain and ferrous addition) to 26 s, and MC from 82.4% (without strain and ferrous addition) to 84.6% [68]. Therefore, higher loading of ferrous iron could improve the growth of A. ferrooxidans in sludge, and this strain transforms ferrous iron to biogenic ferric iron that acts as bioflocculant, allowing the enhancement of sludge dewaterability by the release of bound/stagnant water in extracellular polymeric substances in sludge.
A bioflocculant TJ-F1 obtained by growing P. mirabilis was tested for the dewaterability of a secondary sludge showing a higher reduction in SRF compared to a synthetic polymer P(AM-DMC). In the presence of 7 mg of the bioflocculant supplemented with 12.5 mg/g dw (dry weight) of the synthetic polymer and at pH 7.5, the SRT of the sludge reduced by 69% which is significantly higher than that obtained by P(AM-DMC) [61]. In the same context, the exopolysaccharide Klebsiella sp. at a dosage of 6 mg/g dw and at pH 8 allowed a reduction in the secondary sludge SRF by 69%, giving a final DS of about 17.5% [67]. In the same study, the use of the bioflocculant supplemented with alum reduced the SRF by 84.2% and achieved a DS of 21.3% [67]. In this context, Serratia flocculant used for sludge dewatering allowed for a sludge volume index of 54 mg/L, obtained at a dosage of 0.3 g/L of the bioflocculant. However, with a synthetic flocculant, such as cationic polymers, a sludge volume index of 56 mg/L at a dosage of 0.3 g/L was achieved [71]. Similarly, the polysaccharidic bioflocculant produced by Rhodococcus erythropolis cultivated in rice stover hydrolysate showed better sludge dewaterability performances than synthetic polymer in terms of DS and SRF [69]. More recently, the spores of the filamentous fungus Talaromyces flavus S1 were used to inoculate activated sludge. This inoculation improved the dewaterability by 48% [70]. This improvement may be related to the polysaccharides produced by the fungal mycelium [72]. It was reported in the literature that extracellular polymeric substances have the ability to enhance the formation of biofloc, allowing higher settleability of sludge [73]. The content of the extracellular polymeric substances significantly affects their role in sludge dewaterability. Thus, higher carbohydrate content and lower protein content may increase sludge dewatering [74,75]. Likewise, it is very important to point out that sludge characteristics (sludge origin, pH, organic content, cationic content, etc.) affect the facility of extracellular polymeric substances to act in sludge conditioning [76]. Indeed, the use of microbial flocculant could increase the sludge calorific value, as reported by Kurade et al. [56,65]. Moreover, microbial flocculants act at lower dosages when compared to synthetic polymers, such as FeCl3 and Al2(SO4)3 [57].
According to the literature, sludge dewatering can be achieved by adding the microbial strain into sludge and the bioflocculant will be produced during the growth or by the application of a pure bioflocculant purified after its production by a selected microbial strain growing in an appropriate growth medium [77]. However, the microbial bioflocculant production is controlled by various factors including the culture medium and the operating conditions (C/N ratio, oligoelements, pH, temperature, aeration etc.) [54,78]. For large-scale production, optimization studies should be carried out in order to maximize the bioflocculant production. Moreover, the purification process and the preservation method should be taken into consideration in bioflocculant recovery. The optimization of the growth media and the purification process are considered as the main factors that control the product commercialization. For economical production, a low-cost medium should be developed and/or high-yield strains should be selected. In this context, various agricultural and industrial wastes (molasses, poultry processing waste, corn, rice, peanut, potato, corn, etc.) [79,80] and wastewaters generated by many industries (potato starch, brewery, corn ethanol, swine, palm oil mill, livestock, ramie biodegumming, etc.) have demonstrated their ability to replace standard microbial growth media for bioflocculant production [55]. This may considerably reduce the microbial flocculant production cost, as reported by Siddeeg et al. (2019) [55]. In the same way, another strategy was developed based on the screening of new microbial strains able to grow and produce flocculant in a culture medium low in nutrients [81]. Is also important to promote the selection of strains with the ability to produce bioflocculants that act without metal activation [81,82,83]. Furthermore, the microbial bioflocculant yield could be improved using genetic engineering [84]. The microbial diversity and the variability of the carbon sources may affect the nature and the characteristics of the produced bioflocculant (structure, composition, flocculating activity, etc.) [55]. Although these variations may limit the universal use of the produced microbial bioflocculant, these biopolymers seem suitable to replace synthetic polymers in the coagulation/flocculation process in wastewater treatment and sludge dewatering [54]. Generally, the research activities reported for sludge conditioning are limited and more investigations are needed to evaluate the flocculating activity at a large scale for sludge from various origins. A techno-economic feasibility should be conducted, taking into consideration the various parameters, such as the growth conditions (culture medium composition, operating parameters, extraction and purification of bioflocculants, etc.).
3. Plant-Based Flocculants
As reported in the literature, various plant-based flocculants were prepared using several parts of plants (moringa seeds, tamarind pods seeds, banana fruits peels, acorn leaves, cactus cladodes, hyacinth beans, okra, Lobularia maritima seeds, etc.) [85,86,87,88,89,90,91] and applied for wastewater treatment for the removal of various pollutants, such as turbidity, chemical oxygen demand (COD), heavy metals, dye, etc. Most of the research papers treated contaminated waters through the coagulation/flocculation process, and a limited number of studies are devoted to sludge dewatering. To the best of our knowledge, okra (Abelmoschus esculentus), cactus (Opuntia ficus Indica), moringa (M. oleifera), and aloe (A. vera) are the plants that have been used to prepare flocculants for sludge dewatering, and have been compared to synthetic polymers. Looking for efficient plants for the flocculation process is always a difficult procedure for scientists, and the limited list of plants explored by researchers may be related to the fact that these natural products are renewable, adaptable, abundant in nature, and easily retrievable. The general process of the preparation of plant-based flocculants is summarized in Figure 2. The steps for the preparation included slicing, peeling, drying, grinding, and solvent extraction.
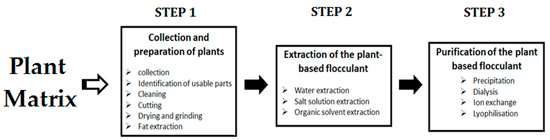
Figure 2.
General process of the preparation of the plant-based flocculants.
As listed in Table 2, different preparation strategies for plant-based flocculants were applied depending on the natural material.

Table 2.
Applications of plant-based flocculants for sludge dewatering.
According to Table 2, the gel obtained from Aloe vera was tested as a bioflocculant to dewater sludge collected from a municipal wastewater treatment plant. Aleo vera leaves was washed, their skin was removed, and the remained samples were mixed, homogenized, and used fresh at a rate of 3%, allowing an efficient solid–liquid separation (45% turbidity removal and an improvement of the settling rate of 22.72% of sludge) [93]. In the same experiment, the mixing of Aleo vera with water glass (3%) increased the settling rate to 90% (an improvement of 63.63%). Interestingly, this bioflocculant allowed the removal of the sludge odor (caused by volatile organic compounds analysis) [93]. However, more investigations are needed to confirm these results by measuring other parameters, such as the DS, SRF, and CST, which should be compared to synthetic polymers. Likewise, cactus juice prepared by using cladodes of Opuntia ficus Indica (the cactus cladodes were cut, blended, and sieved, and the obtained juice was dried at 60 °C for 3 days) was tested to dewater municipal wastewater sludge and compared to chemical polymers, such as Chimfloc C4346, Sedipur NF 102, Sedipu AF 400, FeCl3, and Al2(SO4)3 [94]. High efficiency of sludge dewatering (SRF = 0.13 × 1012 m/Kg, Dryness of filtration cake (DC) = 20.5% and filtrate turbidity = 2.5 NTU) was obtained with cactus juice powder at lower dose of 0.4 g/Kg. These values are comparable to those obtained for synthetic polymers, such as the cationic polymer Chimfloc C4346 (SRF = 0.3 × 1012 m/Kg, DC = 20.5%, and filtrate turbidity = 1.5 NTU), FeCl3 (SRF = 1 × 1012 m/Kg, DC = 22.0% and filtrate turbidity = 2.4 NTU), and Al2(SO4)3 (SRF = 1 × 1012 m/Kg, DC = 21.5% and filtrate turbidity = 2.2 NTU) [94]. These results confirm the utility of cactus juice for wastewater treatment as reported by [101,102].
For both cactus and Aleo vera, the action of the biological material in the coagulation/bioflocculation process is related to their high content in polysaccharides (mainly composed of L-arabinose, d-galactose, l-rhamnose, d-xylose and galacturonic acid), and the presence of minerals (Ca and K). Because of the presence of carboxyl (–COOH), hydroxyl (–OH), and amino or amine (–NH2) functional groups, galacturonic acid is the main compound implicated in the coagulation flocculation process [103,104,105].
In the same context, a number of studies reported the use of the active components of Moringa oleifera as effective flocculants for sludge dewatering that reduce SRF and CST and improve the solid content and the settling rate [95,96,97,98,99]. Wai et al. (2009) [96] evaluated the performances of three forms of Moringa oleifera seeds (dry powder, water extract, and salted water extract) when they were applied to settle activated sludge collected from a municipal wastewater treatment plant. The salted water extract was found to be the most active form, with SRF and CST reduction values of 56.52% and 18.96%, respectively. Generally, the results are comparable to those assigned to the chemical reagent Zetag 7653. However, a higher dosage of Moringa oleifera (2000–4000 mg/L) was necessary to compete with Zetag 7653 (dosage 50 mg/L) in reducing both SRF and CST [96]. The highest rate of enhancement in solid content (31.56%) of sludge obtained with seed dry powder is associated with the added dose (3000 mg/L). As reported in the literature, the active compound extracted from Moringa seeds is a soluble dimeric cationic protein (13 kDa) known as A low charge density cationic polymer acting with the bridging mechanism in the flocculation process, allowing low sludge filterability when compared to Zetag 7653 [106,107,108]. The efficiency offered by Zetag 7653 is related to its nature. Zetag 7653 is a cationic polyacrylamide with a high molecular weight, which has the ability to bind strongly to the negatively charged surfaces of particles in sludge, allowing efficient filterability [108]. Later, Tat et al. (2010) [95] investigated the effect of the dosage of Moringa oleifera seeds (in the range of 1000 to 5000 mg/L) on SRF and CST for the same sludge, and the operating conditions were optimized. The lowest values of SRF (1.22 × 1011 m/kg) and CST (4.5 s) were achieved under the optimum conditions of sludge dewatering (100 rpm, 1 h, and at a dosage of 4695 mg/L). The obtained results are in agreement with those reported by Muyibi et al. (2001) [97]. Interestingly, Muyibi et al. (2001) [97] pointed out the potential of using seed powder free of oil, which performed as well as the untreated seed powder. Likewise, the extraction of oil from seeds may enhance the potential of sludge conditioning [109]. In the same perspective, salted water extract of moringa seeds can be applied as an effective flocculant for sludge from drinking water treatment plants. A dosage of 125 kg/t dry solids allowed acceptable reductions in SRF and CST of 34.75 and 57.35%, respectively. These values were enhanced by mixing moringa seeds with alum (reductions in SRF and CST were respectively 81.08% and 71.42%). This combination allowed the formation of stronger flocs compared to polyelectrolytes used alone [100]. The partial replacement of alum may reduce the pollutant load of chemicals in sludge.
Plant-based flocculants for sludge dewatering represent a viable alternative to chemicals and a step forward in green sludge treatment technology, reducing environmental pollution and health risks while also advancing green technology in wastewater treatment processing. This strategy is interesting for regions of the world favorable to the cultivation of specific plants, such as cactus, which is abundant, cheap, and has little commercial use [110].
4. Animal-Based Flocculants
Animal-based flocculants with an interesting flocculating activity are generated from animal sources (chitin, animal gelatin, animal blood, and blood protein components) [111,112,113,114,115,116]. To the best of our knowledge, only chitosan and hemoglobin are applied to improve sludge dewaterability (Table 3). The preparation steps of these animal-based flocculants are illustrated in Figure 3.

Table 3.
Applications of animal-based flocculants for sludge dewatering.
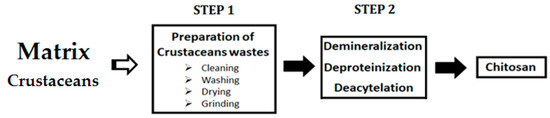
Figure 3.
Process of chitosan preparation.
Chitosan is a biopolymer obtained by chitin deacetylation (Figure 3). Chitin is a natural polysaccharide having various origins (shrimps, crabs, sponges, diatoms, fungi, etc.) [120]. Chitosan is biodegradable, safe, biocompatible, sustainable, and an economical material used in various fields [121,122]. Because of these characteristics, chitosan is well studied as coagulant/flocculant to remove pollutants from municipal and industrial wastewaters [112]. In the coagulation/flocculation process of acidic wastewater, chitosan generates positive charges, allowing the destabilization of the negative charges of colloidal particles [123,124,125]. Likewise, the blood protein (hemoglobin), which is considered as a by-product of meat processing, has demonstrated its ability to act as a bioflocculant for kaolin and lignin at low dosages [113,114,115,116].
As reported in Table 3, Lau et al. (2017) [118] investigated the capability of chitosan for dewatering anaerobically digested sludge. Both low molecular weight and medium molecular weight chitosan allowed higher dewatering performances than synthetic polymers (PAM and EMA 8845) with CST reduction values exceeding 80% against 43% and 41% for PAM and EMA 8845, respectively [118]. In the same work, the results reported that chitosan sludge dewatering performances is controlled by the pH, since pH may affect the ionization state of the functional groups of biopolymers. At low pH, the amine groups (–NH2) in chitosan may generate positive charges (–NH3+), allowing the improvement of sludge flocculation and dewatering at acidic pH [118,126]. In the same context, Zhang et al. (2019) [117] reported the enhancement of the dewaterability of the anaerobically digested sludge while using aminated and virgin chitosan (Table 3). Aminated chitosan performed well in terms of SRF (reduction rate of 88.90%) and CST (reduction rate of 95.60%) obtained at a dosage of 35 mg/gTSS (total suspended solids). This study reported that chitosan-based polymers interact with extracellular polymeric substances in sludge, and a densification of the gel-like structure and an augmentation of floc strength of sludge were confirmed using confocal laser scanning microscopy. The observed behavior may offer abundant huge pores in flocs, providing channels for water liberation during the dewatering process using a filter press [117]. Recently, as indicated in Table 3, Ghazisaidi et al. (2020) [119] reported the ability of methylated hemoglobin to enhance sludge dewaterability. The use of untreated hemoglobin showed no enhancement in sludge dewatering performances. A non-significant enhancement in cake solid content was recorded (2.9%). However, the application of methylated hemoglobin increases the sludge dewatering ability with an enhancement of 47% in cake solid content. Moreover, the decrease in sludge bound water content reached 17.30%. The methylation process allowed the elimination of the carboxylic acid groups in proteins, decreasing the number of negatively charged groups. This fact will raise the basicity and the net positive charges on the protein, leading to the enhancement of the bioflocculation performance. The zeta potential measurements illustrate the decrease in the negative surface charge of the particles in sludge after adding methylated hemoglobin. Therefore, the charge neutralization allowed extracellular polymeric substances surrounding the sludge flocs to become detached, releasing the imprisoned water and, consequently, increasing the dehydration process [119]. Interestingly, blood processing of swine, cattle, etc., has the potential to be an excellent source of bioflocculants. However, this strategy requires rendering facilities to collect blood from the industry. As such, more investigation is needed to draw conclusions about the large-scale applicability of this strategy.
5. Future Prospective of Bioflocculant for Sludge Dewatering at Large Scale
The potential of using natural flocculants from various origins has been proved mainly for water and wastewater treatment by many researchers [54,55,127]. However, a limited number of studies dealing with sludge dewatering using bioflocculants. Therefore, more studies are required to demonstrate the possibility of dewatering sludge using biological materials, since this fact will allow the safe use of sludge as a fertilizer for soils [128,129]. The research of new available biological materials with a flocculating activity and the ability to enhance the sludge dewatering remains an interesting approach that should continue to be extensively investigated.
Generally, the efficiency of the used bioflocculants may depend on what type of sludge is being treated. This fact is well discussed for the coagulation/flocculation of wastewater from various origins. For instance, a polymer applied for the flocculation of food processing wastewater might not work efficiently for other industrial effluents, since the effluent characteristics (pH, temperature, solids, pollutants, multivalent cations, etc.) affect the action of the flocculant and, consequently, the required optimal dose [110,130,131,132]. Similarly, the variability of efficiencies of natural based-flocculants in sludge dewatering (Table 1, Table 2 and Table 3) may be associated with two main factors; the first is the bioflocculant origin and its preparation process, and the second is the sludge origin and characteristics (pH, microbial composition, pollutant composition, solids, etc.). However, the lack of data related to the dewatering of different sludges from various origins (food industry, pharmaceutical industry, chemical industry, etc.) with bioflocculants limits the conclusion about the major factors controlling the sludge dewaterability. Therefore, more investigations are needed to determine and analyze the specific operational parameters (pH, dosage, mixing speed, etc.) for each bioflocculant and for sludges from various origins. Moreover, the flocculation operating conditions should be statistically optimized to maximize the dewatering performance. More studies are also required to compare bioflocculants from various origins and to clarify the flocculation mechanisms occurring in the presence of different sludges [78].
The production of flocculants using microbial stains is an available option, as the process of microbial growth as well the extraction and the purification of microbial polymers are well established and valid for large-scale production. However, production costs limit the large-scale production, and the cost reducing strategy should take into account several points including the selected strain (the bioflocculant biosynthesis pathway, high-yield strains, genetically modified strains, etc.), the growth media (composition, the availability of low-cost medium, operating conditions), bioflocculant harvesting methods (the extraction, purification, preservation, etc.), and flocculation mechanism [133,134,135]. However, the use of the chosen plant-based flocculants for large-scale application is feasible, since the plant species are abundant. Useful plants are specific to some geographical regions, making the availability of the produced bioflocculant limited over the world. Moreover, the plant-based flocculant characteristics (forms, production process, cost, etc.) vary depending on origin (geographical location). However, these plants that produce active bioflocculant may have the potential of commercial value as industrial crops, and a continuous investigation into the behavior of these natural material may help their large-scale application [136].
Finally, animal-based flocculants, such as chitosan, are one of the most environmentally beneficial and economical biological polymers with the ability to clean wastewater [137]. However, the investigation of new animal-based flocculants, such as animal blood, is proposed as a valuable strategy for wastewater treatment, and extensive research should be conducted to explore this sustainable approach [119,138]. Moreover, other wastes, such as fish bones, which showed its ability to flocculate microalgae [139], should be investigated for sludge dewatering.
6. Conclusions
Efficient and effective bioflocculants for sludge dewatering are needed to ensure environmental and public health. Flocculants from natural sources (microorganisms, plants, and animals) provide a relevant opportunity to replace chemical reagents in the dewatering processing. The reviewed data have shown important results for sludge dewatering in terms of moisture content, dry solids, specific resistance to filtration, capillary suction time, etc. The efficiency of the microbial flocculant for sludge dewatering was proved by adding the microbial strain producing flocculant into sludge or by applying a purified bioflocculant after its production by a selected strain cultivated in an appropriate growth medium. However, the production cost associated with the growth media may limit the application of microbial flocculant at a large scale. Plant-based flocculants were also successfully applied for sludge dewatering. However, studies are limited for specific plants (okra, cactus, moringa, and aloe). For animal-based flocculants, chitosan and blood processing showed the potential to be excellent sources of bioflocculants with a significant reduction in sludge dewaterability parameters. Generally, the efficiency of sludge dewaterability seems to be controlled by the bioflocculation nature (origin and the preparation process) and the sludge characteristics. Thus, it is important to determine which bioflocculant is appropriate for large-scale application. In this context, various factors need to be considered, including the product origin and availability, the preparation methods (drying, microbial growth, extraction, purification, etc.), the operating conditions (dosage, pH, temperature, etc.), and the dewatering efficiency compared to chemical reagents. Therefore, extensive research is necessary to compare the suitability of natural materials from different origins for various sludges while also using statistical analysis. Moreover, optimized operating conditions should be determined for each bioflocculant, which should be linked to another study aimed at understanding the mechanism of the bioflocculant process. After that, the optimized operating conditions could be verified at a large scale. Finally, for the application at real scales, a techno-economic feasibility should be conducted.
Author Contributions
Conceptualization, F.B.R. and W.M.; writing—original draft preparation, F.B.R. and W.M.; writing—review and editing, F.B.R.; visualization, F.B.R.; supervision, W.M.; project administration, W.M.; funding acquisition, W.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at the University of Bisha for funding this research through the general research project under grant number (UB-GRP-66-1444).
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at University of Bisha for funding this research through the general research project under grant number (UB-GRP-66-1444).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
BWC: bound water content; COD: chemical oxygen demand; CST: capillary suction time; DC: Dryness of filtration cake; DS: dry solids; dw: dry weight; EMA: ethylene methyl acrylate; MBF: microbial bioflocculant; MC: moisture content; MW: molecular weight; NTU: nephelometric turbidity units; PAC: polyaluminum chloride; PAM: polyacrylamide; P(AM-DMC): poly (acrylamide-[2-(methacryloyloxy) ethyl] trimethyl ammonium chloride); pH: potential of hydrogen; rRNA: ribosomal ribonucleic acid; TSS: total suspended solids; SC: superconductivity; SRF: specific resistance to filtration; SS: suspended solids; SVI: sludge volume index; TSS: total suspended solids; Vs: settling velocity.
References
- Kesari, K.K.; Soni, R.; Jamal, Q.M.S.; Tripathi, P.; Lal, J.A.; Jha, N.K.; Siddiqui, M.H.; Kumar, P.; Tripathi, V.; Ruokolainen, J. Wastewater treatment and reuse: A review of its applications and health implications. Water Air Soil Pollut. 2021, 232, 208. [Google Scholar] [CrossRef]
- Elmi, A.; AlOlayan, M. Sewage sludge land application: Balancing act between agronomic benefits and environmental concerns. J. Clean. Prod. 2020, 250, 119512. [Google Scholar] [CrossRef]
- Ekane, N.; Barquet, K.; Rosemarin, A. Resources and risks: Perceptions on the application of sewage sludge on agricultural land in Sweden, a case study. Front. Sustain. Food Syst. 2021, 5, 647780. [Google Scholar] [CrossRef]
- Anjum, M.; Al-Makishah, N.H.; Barakat, M.A. Wastewater sludge stabilization using pre-treatment methods. Process Saf. Environ. Prot. 2016, 102, 615–632. [Google Scholar] [CrossRef]
- Castellanos-Rozo, J.; Galvis-López, J.A.; Castellanos, N.A.M.; Manjarres-Hernández, E.H.; Rojas, A.L. Assessment of two sludge stabilization methods in a wastewater treatment plant in Sotaquirá, Colombia. Univ. Sci. 2020, 25, 17–36. [Google Scholar] [CrossRef]
- Roldán, M.; Bouzas, A.; Seco, A.; Mena, E.; Mayor, Á.; Barat, R. An integral approach to sludge handling in a WWTP operated for EBPR aiming phosphorus recovery: Simulation of alternatives, LCA and LCC analyses. Water Res. 2020, 175, 115647. [Google Scholar] [CrossRef]
- Flores-Alsina, X.; Ramin, E.; Ikumi, D.; Harding, T.; Batstone, D.; Brouckaert, C.; Sotemann, S.; Gernaey, K.V. Assessment of sludge management strategies in wastewater treatment systems using a plant-wide approach. Water Res. 2021, 190, 116714. [Google Scholar] [CrossRef]
- Mowla, D.; Tran, H.N.; Allen, D.G. A review of the properties of biosludge and its relevance to enhanced dewatering processes. Biomass Bioenergy 2013, 58, 365–378. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Champagne, P.; Mabee, W. Overview of current biological and thermo-chemical treatment technologies for sustainable sludge management. Waste Manag. Res. 2014, 32, 586–600. [Google Scholar] [CrossRef]
- Wu, B.; Dai, X.; Chai, X. Critical review on dewatering of sewage sludge: Influential mechanism, conditioning technologies and implications to sludge re-utilizations. Water Res. 2020, 180, 115912. [Google Scholar] [CrossRef]
- Zhen, Z.; Jinxiang, Y.; Renhui, D. A review on the physical dewatering methods of sludge pretreatment in recent ten years. IOP Conf. Ser. Earth Environ. Sci. 2020, 455, 012189. [Google Scholar] [CrossRef]
- Wang, H.F.; Hu, H.; Wang, H.J.; Bai, Y.N.; Shen, X.F.; Zhang, W.; Zeng, R.J. Comprehensive investigation of the relationship between organic content and waste activated sludge dewaterability. J. Hazard. Mater. 2020, 394, 122547. [Google Scholar] [CrossRef]
- Xiao, K.; Li, N.; Yang, C.; Zhu, Y.; Yu, Z.; Yu, W.; Liang, S.; Hou, H.; Liu, B.; Hu, J.; et al. Deciphering the impacts of composition of extracellular polymeric substances on sludge dewaterability: An often overlooked role of amino acids. Chemosphere 2021, 284, 131297. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, T.; Lv, L.; Chen, Y.; Tang, W.; Tang, S. Destroying the structure of extracellular polymeric substance to improve the dewatering performance of waste activated sludge by ionic liquid. Water Res. 2021, 199, 117161. [Google Scholar] [CrossRef]
- Meyer, T.; Amin, P.; Allen, D.G.; Tran, H. Dewatering of pulp and paper mill biosludge and primary sludge. J. Environ. Chem. Eng. 2018, 6, 6317–6321. [Google Scholar] [CrossRef]
- Yin, X.; Han, P.; Lu, X.; Wang, Y. A review on the dewaterability of bio-sludge and ultrasound pretreatment. Ultrason. Sonochem. 2004, 11, 337–348. [Google Scholar] [CrossRef]
- Li, Y.B.; Song, J.L.; Yao, Q.J.; Chen, Z.X.; Wei, Y.; Li, H.L.; Wang, M.X.; Wang, B.J.; Zhou, J.M. Effects of dissolved oxygen on the sludge dewaterability and extracellular polymeric substances distribution by bioleaching. Chemosphere 2021, 281, 130906. [Google Scholar]
- Kang, X.; Li, C.; Ding, W.; Ma, Y.; Gao, S.; Zhou, X.; Chen, Y.; Liu, W.; Jiang, G. Optimization of operating conditions in the biological enzymes for efficient waste activated sludge dewatering. Process Saf. Environ. Prot. 2023, 170, 545–552. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, P.; Wu, Y. Enhanced technology for sewage sludge advanced dewatering from an engineering practice perspective: A review. J. Environ. Manag. 2022, 321, 115938. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, T.; Zhang, W.; Wang, D. Enhanced technology based for sewage sludge deep dewatering: A critical review. Water Res. 2021, 189, 116650. [Google Scholar] [CrossRef]
- Guo, J.; Wen, X. Performances and mechanisms of sludge dewatering by a biopolymer from piggery wastewater and application of the dewatered sludge in remediation of Cr (VI)-contaminated soil. J. Environ. Manag. 2020, 259, 109678. [Google Scholar] [CrossRef] [PubMed]
- Hyrycz, M.; Ochowiak, M.; Krupińska, A.; Włodarczak, S.; Matuszak, M. A review of flocculants as an efficient method for increasing the efficiency of municipal sludge dewatering: Mechanisms, performances, influencing factors and perspectives. Sci. Total Environ. 2022, 820, 153328. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Qin, L.; Gao, J.; Nan, R.; Gao, J. Protein extraction and sludge dewatering performance of ultrasound-assisted enzymatic hydrolysis of excess sludge. Environ. Sci. Pollut. Res. 2020, 27, 18317–18328. [Google Scholar] [CrossRef] [PubMed]
- Tunçal, T.; Mujumdar, A.S. Modern techniques for sludge dewaterability improvement. Drying Technol. 2022, 41, 339–351. [Google Scholar] [CrossRef]
- Hui, K.; Song, L.; Yin, Z.; Song, H.; Wang, Z.; Gao, W.; Xuan, L. Freeze–thaw combined with activated carbon improves electrochemical dewaterability of sludge: Analysis of sludge floc structure and dewatering mechanism. Environ. Sci. Pollut. Res. 2022, 29, 20333–20346. [Google Scholar] [CrossRef]
- Kocbek, E.; Garcia, H.A.; Hooijmans, C.M.; Mijatović, I.; Lah, B.; Brdjanovic, D. Microwave treatment of municipal sewage sludge: Evaluation of the drying performance and energy demand of a pilot-scale microwave drying system. Sci. Total Environ. 2020, 742, 140541. [Google Scholar] [CrossRef]
- Guo, J.; Gao, Q.; Jiang, S. Insight into dewatering behavior and heavy metals transformation during waste activated sludge treatment by thermally-activated sodium persulfate oxidation combined with a skeleton builder—Wheat straw biochar. Chemosphere 2020, 252, 126542. [Google Scholar] [CrossRef]
- Huang, J.; Liang, J.; Yang, X.; Zhou, J.; Liao, X.; Li, S.; Zheng, L.; Sun, S. Ultrasonic coupled bioleaching pretreatment for enhancing sewage sludge dewatering: Simultaneously mitigating antibiotic resistant genes and changing microbial communities. Ecotoxicol. Environ. Saf. 2020, 193, 110349. [Google Scholar] [CrossRef]
- Lin, W.; Liu, X.; Ding, A.; Ngo, H.H.; Zhang, R.; Nan, J.; Ma, J.; Li, G. Advanced oxidation processes (AOPs)-based sludge conditioning for enhanced sludge dewatering and micropollutants removal: A critical review. J. Water Process Eng. 2022, 45, 102468. [Google Scholar] [CrossRef]
- Ge, D.; Wu, W.; Li, G.; Wang, Y.; Li, G.; Dong, Y.; Yuan, H.; Zhu, N. Application of CaO2-enhanced peroxone process to adjust waste activated sludge characteristics for dewaterability amelioration: Molecular transformation of dissolved organic matters and realized mechanism of deep-dewatering. Chem. Eng. J. 2022, 437, 135306. [Google Scholar] [CrossRef]
- Chen, N.; Tao, S.; Xiao, K.; Liang, S.; Yang, J.; Zhang, L. A one-step acidification strategy for sewage sludge dewatering with oxalic acid. Chemosphere 2020, 238, 124598. [Google Scholar] [CrossRef]
- Wei, H.; Gao, B.; Ren, J.; Li, A.; Yang, H. Coagulation/flocculation in dewatering of sludge: A review. Water Res. 2018, 143, 608–631. [Google Scholar] [CrossRef]
- Ma, C.X.; Pei, H.Y.; Hu, W.Y.; Cheng, J.; Xu, H.Z.; Jin, Y. Significantly enhanced dewatering performance of drinking water sludge from a coagulation process using a novel chitosanealuminum chloride composite coagulant in the treatment of cyanobacteria-laden source water. RSC Adv. 2016, 6, 61047–61056. [Google Scholar] [CrossRef]
- Mahmoud, A.; Hoadley, A.F.A.; Citeau, M.; Sorbet, J.M.; Olivier, G.; Vaxelaire, J.; Olivier, J. A comparative study of electro-dewatering process performance for activated and digested wastewater sludge. Water Res. 2018, 129, 66–82. [Google Scholar] [CrossRef]
- Dao, V.H.; Cameron, N.R.; Saito, K. Synthesis, properties and performance of organic olymers employed in flocculation applications. Polym. Chem. 2016, 7, 11–25. [Google Scholar] [CrossRef]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosanbased flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, K.; Guibal, E.; Jia, S.; Shen, J.; Zhang, X.; Yang, W. Removal of trace nonylphenol from water in the coexistence of suspended inorganic particles and NOMs by using a cellulosebased flocculant. Chemosphere 2016, 161, 482–490. [Google Scholar] [CrossRef]
- Wang, J.P.; Yuan, S.J.; Wang, Y.; Yu, H.Q. Synthesis, characterization and application of a novel starch-based flocculant with high flocculation and dewatering properties. Water Res. 2013, 47, 2643–2648. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.J.; Wang, D.S.; Ma, T.; Bai, R.Y. Enhancement of activated sludge dewatering performance by combined composite enzymatic lysis and chemical re-flocculation with inorganic coagulants: Kinetics of enzymatic reaction and re-flocculation morphology. Water Res. 2015, 83, 367–376. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, H.L.; Guan, Q.Q.; Teng, H.K.; Zhao, C.L.; Zhao, C. Fabricating a flocculant with controllable cationic microblock structure: Characterization and sludge conditioning behavior evaluation. Ind. Eng. Chem. Res. 2016, 55, 2892–2902. [Google Scholar] [CrossRef]
- Cao, B.D.; Zhang, W.J.; Wang, Q.D.; Huang, Y.R.; Meng, C.R.; Wang, D.S. Wastewater sludge dewaterability enhancement using hydroxyl aluminum conditioning: 478 Role of aluminum speciation. Water Res. 2016, 105, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Kameswari, K.S.B.; Kalyanaraman, C.; Varma, V.S.; Porselvam, S.; Thanasekaran, K. Significance of chemical conditioning to improve dewaterability of biosludge generated from tanneries. Clean Technol. Environ. Policy 2013, 15, 945–953. [Google Scholar] [CrossRef]
- Mortula, M.; Bard, S.M.; Walsh, M.E.; Gagnon, G.A. Aluminum toxicity and ecological risk assessment of dried alum residual into surface water disposal. Can. J. Civ. Eng. 2009, 36, 127–136. [Google Scholar] [CrossRef]
- Exley, C.; Clarkson, E. Aluminium in human brain tissue from donors without neurodegenerative disease: A comparison with Alzheimer’s disease, multiple sclerosis and autism. Sci. Rep. 2020, 10, 7770. [Google Scholar] [CrossRef] [PubMed]
- Krupińska, I. Aluminium drinking water treatment residuals and their toxic impact on human health. Molecules 2020, 25, 641. [Google Scholar] [CrossRef]
- Li, R.; Gao, B.; Huang, X.; Dong, H.; Li, X.; Yue, Q.; Wang, Y.; Li, Q. Bioresource Technology Compound bioflocculant and polyaluminum chloride in kaolin-humic acid coagulation: Factors influencing coagulation performance and floc characteristics. Bioresour. Technol. 2014, 172, 8–15. [Google Scholar] [CrossRef]
- Bo, X.; Gao, B.; Peng, N.; Wang, Y.; Yue, Q.; Zhao, Y. Effect of dosing sequence and solution pH on floc properties of the compound bioflocculant-aluminum sulfate dual-coagulant in kaolin-humic acid solution treatment. Bioresour. Technol. 2012, 113, 89–96. [Google Scholar] [CrossRef]
- Lu, Y.; Zheng, G.Y.; Wu, W.Z.; Cui, C.H.; Zhou, L.X. Significances of deflocculated sludge flocs as well as extracellular polymeric substances in influencing the compression dewatering of chemically acidified sludge. Separ. Purif. Technol. 2017, 176, 243–251. [Google Scholar] [CrossRef]
- Novak, J.T. Dewatering of sewage sludge. Dry. Technol. 2006, 24, 1257–1262. [Google Scholar] [CrossRef]
- Pan, J.R.; Huang, C.; Cherng, M.; Li, K.C.; Lin, C.F. Correlation between dewateringindex and dewatering performance of three mechanical dewatering devices. Adv. Environ. Res. 2003, 7, 599–602. [Google Scholar] [CrossRef]
- Sawalha, O.; Scholz, M. Modeling the relationship between capillary suction time and specific resistance to filtration. J. Environ. Eng. 2010, 136, 983–991. [Google Scholar] [CrossRef]
- Scholz, M. Review of recent trends in capillary suction time (CST) dewaterability testing research. Ind. Eng. Chem. Res. 2005, 44, 8157–8163. [Google Scholar] [CrossRef]
- Yukseler, H.; Tosun, I.; Yetis, U. A new approach in assessing slurry filterability. J. Membr. Sci. 2007, 303, 72–79. [Google Scholar] [CrossRef]
- Ben Rebah, F.; Mnif, W.; Siddeeg, S.M. Microbial flocculants as an alternative to synthetic polymers for wastewater treatment: A review. Symmetry 2018, 10, 556. [Google Scholar] [CrossRef]
- Siddeeg, S.M.; Tahoon, M.A.; Rebah, F.B. Agro-industrial waste materials and wastewater as growth media for microbial bioflocculants production: A review. Mater. Res. Express 2019, 7, 012001. [Google Scholar] [CrossRef]
- Kurade, M.B.; Murugesan, K.; Selvam, A.; Yu, S.A.M.; Wong, J.W.C. Sludge conditioning using biogenic flocculant produced by Acidithiobacillus ferrooxidans for enhancement in dewaterability. Bioresour. Technol. 2016, 217, 179–185. [Google Scholar] [CrossRef]
- Guo, J.; Ma, J. Bioflocculant from pre-treated sludge and its applications in sludge dewatering and swine wastewater pretreatment. Bioresour. Technol. 2015, 196, 736–740. [Google Scholar] [CrossRef]
- Guo, J.; Nengzi, K.L.; Zhao, J.; Zhang, Y. Enhanced dewatering of sludge with the composite of bioflocculant MBFGA1 and P(AM-DMC) as a conditioner. Appl. Microbiol. Biotechnol. 2015, 99, 2989–2998. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Zhao, J.; Zhang, Y.; Xiao, X.; Wang, B.; Shu, B. Characterization of a bioflocculant from potato starch wastewater and its application in sludge dewatering. Appl. Microbiol. Biotechnol. 2015, 99, 5429–5437. [Google Scholar] [CrossRef]
- Liu, J.; Ma, J.; Liu, Y.; Yang, Y.; Yue, D.; Wang, H. Optimized production of a novel bioflocculant M-C11 by Klebsiella sp. and its application in sludge dewatering. J. Environ. Sci. 2014, 26, 2076–2083. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Xia, S.Q.; Zhang, J. Enhanced dewatering of waste sludge with microbial flocculant TJ-F1 as a novel conditioner. Water Res. 2010, 44, 3087–3092. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.W.C.; Murugesan, K.; Yu, S.M.; Kurade, M.B.; Selvam, A. Improved dewatering of CEPT sludge by biogenic flocculant from Acidithiobacillus ferrooxidans. Water Sci. Technol. 2016, 73, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.W.C.; Murugesan, K.; Selvam, A.; Ravindran, B.; Kurade, M.B.; Yu, S.M. Dewatering of saline sewage sludge using iron-oxidizing bacteria: Effect of substrate concentration. Bioresour. Technol. 2016, 213, 31–38. [Google Scholar] [CrossRef]
- Murugesan, K.; Ravindran, B.; Selvam, A.; Kurade, M.B.; Yu, S.M.; Wong, J.W. Enhanced dewaterability of anaerobically digested sewage sludge using Acidithiobacillusferrooxidans culture as sludge conditioner. Bioresour. Technol. 2014, 169, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Kurade, M.B.; Murugesan, K.; Selvam, A.; Yu, S.M.; Wong, J.W.C. Ferric biogenic flocculant produced by Acidithiobacillus ferrooxidans enable rapid dewaterability of municipal sewage sludge: A comparison with commercial cationic polymer. Int. Biodeterior. Biodegrad. 2014, 96, 105–111. [Google Scholar] [CrossRef]
- Murugesan, K.; Selvam, A.; Wong, J.W. Flocculation and dewaterability of chemically enhanced primary treatment sludge by bioaugmentation with filamentous fungi. Bioresour. Technol. 2014, 168, 198–203. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, K.; Liao, D.X.; Li, X.M.; Wang, D.B.; Liu, X.; Zeng, G.M.; Li, X. A novel bioflocculant produced by Klebsiella sp. and its application to sludge dewatering. Water Environ. J. 2012, 26, 560–566. [Google Scholar] [CrossRef]
- Cai, G.; Ebrahimi, M.; Zheng, G.; Kaksonen, A.H.; Morris, C.; O’Hara, I.M.; Zhang, Z. Effect of ferrous iron loading on dewaterability, heavy metal removal and bacterial community of digested sludge by Acidithiobacillus ferrooxidans. J. Environ. Manag. 2021, 295, 113114. [Google Scholar] [CrossRef]
- Guo, J.; Chen, C. Sludge conditioning using the composite of a bioflocculant and PAC for enhancement in dewaterability. Chemosphere 2017, 185, 277–283. [Google Scholar] [CrossRef]
- Liu, H.; Shi, J.; Xu, X.; Zhan, X.; Fu, B.; Li, Y. Enhancement of sludge dewaterability with filamentous fungi Talaromyces flavus S1 by depletion of extracellular polymeric substances or mycelium entrapment. Bioresour. Technol. 2017, 245, 977–983. [Google Scholar] [CrossRef]
- Subramanian, S.B.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular polymeric substances (EPS) producing bacterial strains of municipal wastewater sludge: Isolation, molecular identification, EPS characterization and performance for sludge settling and dewatering. Water Res. 2010, 44, 2253–2266. [Google Scholar] [CrossRef]
- Liu, W.; Hao, Y.; Jiang, J.; Zhu, A.; Zhu, J.; Dong, Z. Production of a bioflocculant from Pseudomonas veronii L918 using the hydrolyzate of peanut hull and its application in the treatment of ash-flushing wastewater generated from coal fired power plant. BioresourTechnol. 2016, 218, 318. [Google Scholar] [CrossRef]
- Houghton, J.J.; Quarmby, J.; Stephenson, T. Municipal wastewater sludge dewaterability and the presence of microbial extracellular polymer. Water Sci.Technol. 2001, 44, 373–379. [Google Scholar] [CrossRef]
- Cetin, S.; Erdincler, A. The role of carbohydrate and protein parts of extracellular polymeric substances on the dewaterability of biological sludges. Water Sci. Technol. 2004, 50, 49–56. [Google Scholar] [CrossRef]
- Sponza, D.T. Extracellular polymer substances and physicochemical properties of flocs in steady and unsteady-state activated sludge systems. Process Biochem. 2002, 37, 983–998. [Google Scholar] [CrossRef]
- Faye, M.C.A.S.; Zhang, K.K.; Sun, P.; Zhang, Y. Sludge dewaterability: The variation of extracellular polymeric substances during sludge conditioning with two natural organic conditioners. J. Environ. Manag. 2019, 251, 109559. [Google Scholar] [CrossRef]
- Nkosi, N.C.; Basson, A.K.; Ntombela, Z.G.; Maliehe, T.S.; Pullabhotla, R.V.S.R. Isolation, Identification and Characterization of Bioflocculant-Producing Bacteria from Activated Sludge of Vulindlela Wastewater Treatment Plant. Appl. Microbiol. 2021, 1, 586–606. [Google Scholar] [CrossRef]
- Li, H.; Wu, S.; Du, C.; Zhong, Y.; Yang, C. Preparation, performances, and mechanisms of microbial flocculants for wastewater treatment. Int. J. Environ. Res. Public Health 2020, 17, 1360. [Google Scholar] [CrossRef]
- Qi, Z.; Zhu, Y.; Guo, H.; Wang, X.; Zhao, Y.; Zhou, Y.; Chen, Y.; Yang, Y.; Qin, W.; Shao, Q. Production of glycoprotein bioflocculant from untreated rice straw by a cazyme-rich bacterium, Pseudomonas sp. HP2. J. Biotechnol. 2019, 306, 185–192. [Google Scholar] [CrossRef]
- Sam, S.; Kucukasik, F.; Yenigun, O.; Nicolaus, B.; Oner, E.T.; Yukselen, M.A. Flocculating performances of exopolysaccharides produced by a halophilic bacterial strain cultivated on agro-industrial waste. Bioresour. Technol. 2011, 102, 1788–1794. [Google Scholar] [CrossRef]
- Liu, W.; Wang, K.; Li, B.; Yuan, H.; Yang, J. Production and characterization of an intracellular bioflocculant by Chryseobacterium daeguense W6 cultured in low nutrition medium. Bioresour. Technol. 2010, 101, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Song, L.; Li, D.; Qiao, J.; Zhao, T.; Zhao, H. Production, characterization, and flocculation mechanism of cation independent, pH tolerant, and thermally stable bioflocculant from Enterobacter sp. ETH-2. PLoS ONE 2014, 9, e114591. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.J.; Tian, Z.M.; Tang, W.; Li, L.; Song, L.Y.; Mcelmurry, S.P. Production and characterization of high efficiency bioflocculant isolated from Klebsiella sp. ZZ-3. Bioresour. Technol. 2014, 171, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, P.; Li, Z.; Yu, W.; Wang, Z.; Yao, H.; Wang, Y.; Li, Q.; Deng, X.; He, N. Identification of key genes involved in polysaccharide bioflocculant synthesis in Bacillus licheniformis. Biotechnol. Bioeng. 2017, 114, 645–655. [Google Scholar] [CrossRef]
- Seghosime, A.; Awudza, M.; Akpabla, J.; Richard, B. Comparative studies on proximate composition and phytochemical screening of mango, key lime, African star apple and african pear seeds as possible coagulant aids for water treatment. Am. J. Env. Sci. 2017, 13, 325–333. [Google Scholar] [CrossRef]
- Buenaño, B.; Vera, E.; Aldás, M.B. Study of coagulating/flocculating characteristics of organic polymers extracted from biowaste for water treatment. Ing. Investig. 2019, 39, 24–35. [Google Scholar]
- Benalia, A.; Derbal, K.; Panico, A.; Pirozzi, F. Use of Acorn Leaves as a natural coagulant in a drinking water treatment plant. Water 2019, 11, 57. [Google Scholar] [CrossRef]
- Kumar, M.R.; Istalingamurthy, D.B. Natural bio-flocculant in water treatment: Investigation of the performance of cactus extracts as a natural flocculant. Int. J. Innov. Res. Sci. Eng. Technol. 2017, 5, 12609–12614. [Google Scholar]
- Zaidi, N.S. Potential of fruit peels in becoming natural coagulant for water treatment. Int. J. Integr. Eng. 2019, 11, 140–150. [Google Scholar] [CrossRef]
- Shilpaa, B.S.; Akankshaa, K.; Girish, P. Evaluation of cactus andhyacinth bean peels as natural coagulants. Int. J. Chem. Environ. Eng. 2012, 3, 189–191. [Google Scholar]
- Eichhorn, C.; Weckmüller, S.; Urban, W. Natural Flocculant from a Combination of Moringa Oleifera Seeds and Cactus Cladodes (Opuntia Ficus-Indica) to Optimize Flocculation Properties. Water 2022, 14, 3570. [Google Scholar] [CrossRef]
- Lee, C.S.; Chong, M.F.; Robinson, J.; Binner, E. Optimisation of extraction and sludge dewatering efficiencies of bio-flocculants extracted from Abelmoschus esculentus (okra). J. Environ. Manag. 2015, 157, 320–325. [Google Scholar] [CrossRef]
- Jaouadi, T.; Hajji, M.; Kasmi, M.; Kallel, A.; Chatti, A.; Hamzaoui, H.; Mnif, A.; Tizaoui, C.; Trabelsi, I. Aloe sp. leaf gel and water glass for municipal wastewater sludge treatment and odour removal. Water Sci. Technol. 2020, 81, 479–490. [Google Scholar] [CrossRef]
- Betatache, H.; Aouabed, A.; Drouiche, N.; Lounici, H. Conditioning of sewage sludge by prickly pear cactus (Opuntia ficus Indica) juice. Ecol. Eng. 2014, 70, 465–469. [Google Scholar] [CrossRef]
- Tat, W.K.; Idris, A.; Noor, M.J.M.M.; Mohamed, T.A.; Ghazali, A.H.; Muyibi, S.A. Optimization study on sewage sludge conditioning using Moringa oleifera seeds. Desalination Water Treat. 2010, 16, 402–410. [Google Scholar] [CrossRef]
- Wai, K.T.; Idris, A.; Johari, M.M.N.M.; Mohammad, T.A.; Ghazali, A.H.; Muyibi, S.A. Evaluation on different forms of Moringa oleifera seeds dosing on sewage sludge conditioning. Desalination Water Treat. 2009, 10, 87–94. [Google Scholar] [CrossRef]
- Muyibi, S.A.; Noor, M.J.M.M.; Ong, D.T.; Kai, K.W. Moringa oleifera seeds as a flocculant in waste sludge treatment. Int. J. Environ. Stud. 2001, 58, 185–195. [Google Scholar] [CrossRef]
- Abdulazeez, Q.M.; Jami, M.S.; Alam, M.Z.; Iwata, M. Analysis of the efficiency of sludge dewatering using Moringa oleifera as natural phytocoagulant. Int. J. Res. Chem. Metall. Civ. Eng. 2015, 2, 111–117. [Google Scholar]
- Abdulazeez, Q.M.; Jami, M.S.; Alam, M.Z. Effective sludge dewatering using moringa oleifera seed extract combined with aluminium sulfate. J. Eng. Appl. Sci. 2016, 11, 372–381. [Google Scholar]
- Ghebremichael, K.A.; Hultman, B. Alum sludge dewatering using Moringa oleifera as a conditioner. Water Air Soil Pollut. 2004, 158, 153–167. [Google Scholar] [CrossRef]
- Rebah, F.B.; Siddeeg, S.M. Cactus an eco-friendly material for wastewater treatment: A review. J. Mater. Environ. Sci. 2017, 8, 1770–1782. [Google Scholar]
- Al-Saati, N.H.A.; Hwaidi, E.H.; Jassam, S.H. Comparing cactus (Opuntia spp.) and alum as coagulants for water treatment at Al-Mashroo Canal: A case study. Int. J. Environ. Sci. Technol. 2016, 13, 2875–2882. [Google Scholar] [CrossRef]
- Hamman, J.H. Composition and applications of Aloe veraleaf gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, G.; Sivakumar, T.; Kumar, A.V. Application of plant based coagulants for waste watertreatment. Int. J. Adv. Eng. Res. Stud. 2011, 1, 88–92. [Google Scholar]
- Sepúlveda, E.; Sáenz, C.; Aliaga, E.; Aceituno, C. Extraction and characterization of mucilage in Opuntia spp. J. Arid Environ. 2007, 68, 534–545. [Google Scholar] [CrossRef]
- Camacho, F.P.; Sousa, V.S.; Bergamasco, R.; Teixeira, M.R. The use of Moringa oleifera as a natural coagulant in surface water treatment. Chem. Eng. J. 2017, 313, 226–237. [Google Scholar] [CrossRef]
- Shebek, K.; Schantz, A.B.; Sines, I.; Lauser, K.; Velegol, S.; Kumar, M. The flocculating cationic polypetide from Moringa oleifera seeds damages bacterial cell membranes by causing membrane fusion. Langmuir 2015, 31, 4496–4502. [Google Scholar] [CrossRef]
- Kansal, S.K.; Kumari, A. Potential of M. oleifera for the treatment of water and wastewater. Chem. Rev. 2014, 114, 4993–5010. [Google Scholar] [CrossRef]
- Ademiluyi, J.O.; Eze, R.M. Improving the sludge conditioning potential of moringa seed. Environ. Manag. 1990, 14, 125–129. [Google Scholar] [CrossRef]
- Sellami, M.; Zarai, Z.; Khadhraoui, M.; Jdidi, N.; Leduc, R.; Ben Rebah, F. Cactus juice as bioflocculant in the coagulation–flocculation process for industrial wastewater treatment: A comparative study with polyacrylamide. Water Sci. Technol. 2014, 70, 1175–1181. [Google Scholar] [CrossRef]
- Piazza, G.J.; Garcia, R.A. Meat and bone meal extract and gelatin as renewable flocculants. Bioresour. Technol. 2010, 101, 781–787. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Karthikeyan, P.; Sirajudheen, P.; Meenakshi, S. Optimization of sustainable chitosan/Moringa. oleifera as coagulant aid for the treatment of synthetic turbid water–A systemic study. Environ. Chem. Ecotoxicol. 2020, 2, 132–140. [Google Scholar] [CrossRef]
- Garcia, R.A.; Bumanlag, L.P.; Piazza, G.J. The relationship between extent of hemoglobin purification and the performance characteristics of a blood-based flocculant. J. Sci. Food Agric. 2017, 97, 4822–4826. [Google Scholar] [CrossRef]
- Piazza, G.J.; Lora, J.H.; Garcia, R.A. Flocculation of kaolin and lignin by bovine blood and hemoglobin. J. Chem. Technol. Biotechnol. 2015, 90, 1419–1425. [Google Scholar] [CrossRef]
- Garcia, R.A.; Qi, P.X.; Essandoh, M.; Bumanlag, L.P. Enhancement of protein flocculant properties through carboxyl group methylation and the relationship with protein structural changes. J. Dispers. Sci. Technol. 2021, 42, 2063–2074. [Google Scholar] [CrossRef]
- Essandoh, M.; Garcia, R.A.; Strahan, G.D. Methylation of hemoglobin to enhance flocculant performance. J. Chem. Technol. Biotechnol. 2017, 92, 2032–2037. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Li, L.; Li, D.; Wang, Q.; Xu, Q.; Wang, D. Impact of molecular structure and charge property of chitosan based polymers on flocculation conditioning of advanced anaerobically digested sludge for dewaterability improvement. Sci. Total Environ. 2019, 670, 98–109. [Google Scholar] [CrossRef]
- Lau, S.W.; Sen, T.K.; Chua, H.B.; Ang, H.M. Conditioning of synthetic sludge and anaerobically digested sludge using chitosan, organic polyelectrolytes and inorganic metal cations to enhance sludge dewaterability. Water Air Soil Pollut. 2017, 228, 358. [Google Scholar] [CrossRef]
- Ghazisaidi, H.; Garcia, R.A.; Tran, H.; Yuan, R.; Allen, D.G. Enhancing biosludge dewaterability with hemoglobin from waste blood as a bioflocculant. Polymers 2020, 12, 2755. [Google Scholar] [CrossRef]
- Kumari, S.; Kishor, R. Chitin and chitosan: Origin, properties, and applications. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–33. [Google Scholar]
- Chang, X.X.; Mubarak, N.M.; Mazari, S.A.; Jatoi, A.S.; Ahmad, A.; Khalid, M.; Walvekar, R.; Abdullah, E.; Karri, R.R.; Siddiqui, M.; et al. A review on the properties and applications of chitosan, cellulose and deep eutectic solvent in green chemistry. J. Ind. Eng. Chem. 2021, 104, 362–380. [Google Scholar] [CrossRef]
- Maliki, S.; Sharma, G.; Kumar, A.; Moral-Zamorano, M.; Moradi, O.; Baselga, J.; Stadler, F.J.; García-Peñas, A. Chitosan as a Tool for Sustainable Development: A Mini Review. Polymers 2022, 14, 1475. [Google Scholar] [CrossRef] [PubMed]
- Meraz, K.A.S.; Vargas, S.M.P.; Maldonado, J.T.L.; Bravo, J.M.C.; Guzman, M.T.O.; Maldonado, E.A.L. Eco-friendly innovation for nejayote coagulation–flocculation process using chitosan: Evaluation through zeta potential measurements. Chem. Eng. J. 2016, 284, 536–542. [Google Scholar] [CrossRef]
- Iber, B.T.; Okomoda, V.T.; Rozaimah, S.A.; Kasan, N.A. Eco-friendly approaches to aquaculture wastewater treatment: Assessment of natural coagulants vis-a-vis chitosan. Bioresour. Technol. Rep. 2021, 15, 100702. [Google Scholar] [CrossRef]
- Wilson, L.D. An overview of coagulation-focculation technology. Water Cond. Purif. Int. Mag. 2014, 56, 28–34. [Google Scholar]
- Christensen, M.L.; Keiding, K.; Halkj, P. Dewatering in biological wastewater treatment: A review. Water Res. 2015, 82, 14–24. [Google Scholar] [CrossRef]
- Othmani, B.; Rasteiro, M.G.; Khadhraoui, M. Toward green technology: A review on some efficient model plant-based coagulants/flocculants for freshwater and wastewater remediation. Clean Technol. Environ. Policy 2020, 22, 1025–1040. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Szopa, D.; Izydorczyk, G.; Moustakas, K.; Witek-Krowiak, A. Management of biological sewage sludge: Fertilizer nitrogen recovery as the solution to fertilizer crisis. J. Environ. Manag. 2023, 326, 116602. [Google Scholar] [CrossRef]
- Engida, T.; Mekonnen, A.; Wu, J.M.; Xu, D.; Wu, Z.B. Review paper on beverage agro-industrial wastewater treatment plant bio-sludge for fertilizer potential in Ethiopia. Appl. Ecol. Environ. Res. 2020, 18, 33–57. [Google Scholar] [CrossRef]
- Khadhraoui, M.; Sellami, M.; Zarai, Z.; Saleh, K.; Ben Rebah, F.; Roland Leduc, R. Cactus juice preparations as bioflocculant: Properties, characteristics and application. Environ. Eng. Manag. J. 2019, 18, 137–146. [Google Scholar]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef]
- Suresh, A.; Grygolowicz-Pawlak, E.; Pathak, S.; Poh, L.S.; bin Abdul Majid, M.; Dominiak, D.; Bugge, T.V.; Gao, X.; Ng, W.J. Understanding and optimization of the flocculation process in biological wastewater treatment processes: A review. Chemosphere 2018, 210, 401–416. [Google Scholar] [CrossRef]
- Bakar, S.N.H.A.; Hasan, H.A.; Abdullah, S.R.S.; Kasan, N.A.; Muhamad, M.H.; Kurniawan, S.B. A review of the production process of bacteria-based polymeric flocculants. J. Water Process Eng. 2021, 40, 101915. [Google Scholar] [CrossRef]
- Liu, C.; Sun, D.; Liu, J.; Zhu, J.; Liu, W. Recent advances and perspectives in efforts to reduce the production and application cost of microbial flocculants. Bioresour. Bioprocess. 2021, 8, 51. [Google Scholar] [CrossRef]
- Pi, S.; Qiu, J.; Li, A.; Feng, L.; Wu, D.; Zhao, H.P.; Ma, F. Applied microbiology and biotechnology uncovering the biosynthetic pathway of polysaccharide-based microbial flocculant in Agrobacterium tumefaciens F2. Appl. Microbiol. Biotechnol. 2020, 104, 8479–8488. [Google Scholar] [CrossRef]
- Patchaiyappan, A.; Devipriya, S.P. Application of plant-based natural coagulants in water treatment. In Cost Effective Technologies for Solid Waste and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2022; pp. 51–58. [Google Scholar]
- Keshvardoostchokami, M.; Majidi, M.; Zamani, A.; Liu, B. A review on the use of chitosan and chitosan derivatives as the bio-adsorbents for the water treatment: Removal of nitrogen-containing pollutants. Carbohydr. Polym. 2021, 273, 118625. [Google Scholar] [CrossRef]
- Liang, C.; Qi, P.X.; Garcia, R.A.; Lee, C. Molecular basis for the performance and mechanisms of methylated decolorized bovine hemoglobin flocculants. Sep. Purif. Technol. 2022, 292, 121017. [Google Scholar] [CrossRef]
- Suparmaniam, U.; Shaik, N.B.; Lam, M.K.; Lim, J.W.; Uemura, Y.; Shuit, S.H.; Show, P.L.; Tan, I.S.; Lee, K.T. Valorization of fish bone waste as novel bioflocculant for rapid microalgae harvesting: Experimental evaluation and modelling using back propagation artificial neural network. J. Water Process Eng. 2022, 47, 102808. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).