Influence of Water on the Methane Adsorption Capacity of Organic-Rich Shales and Its Controlling Factors: A Review
Abstract
1. Introduction
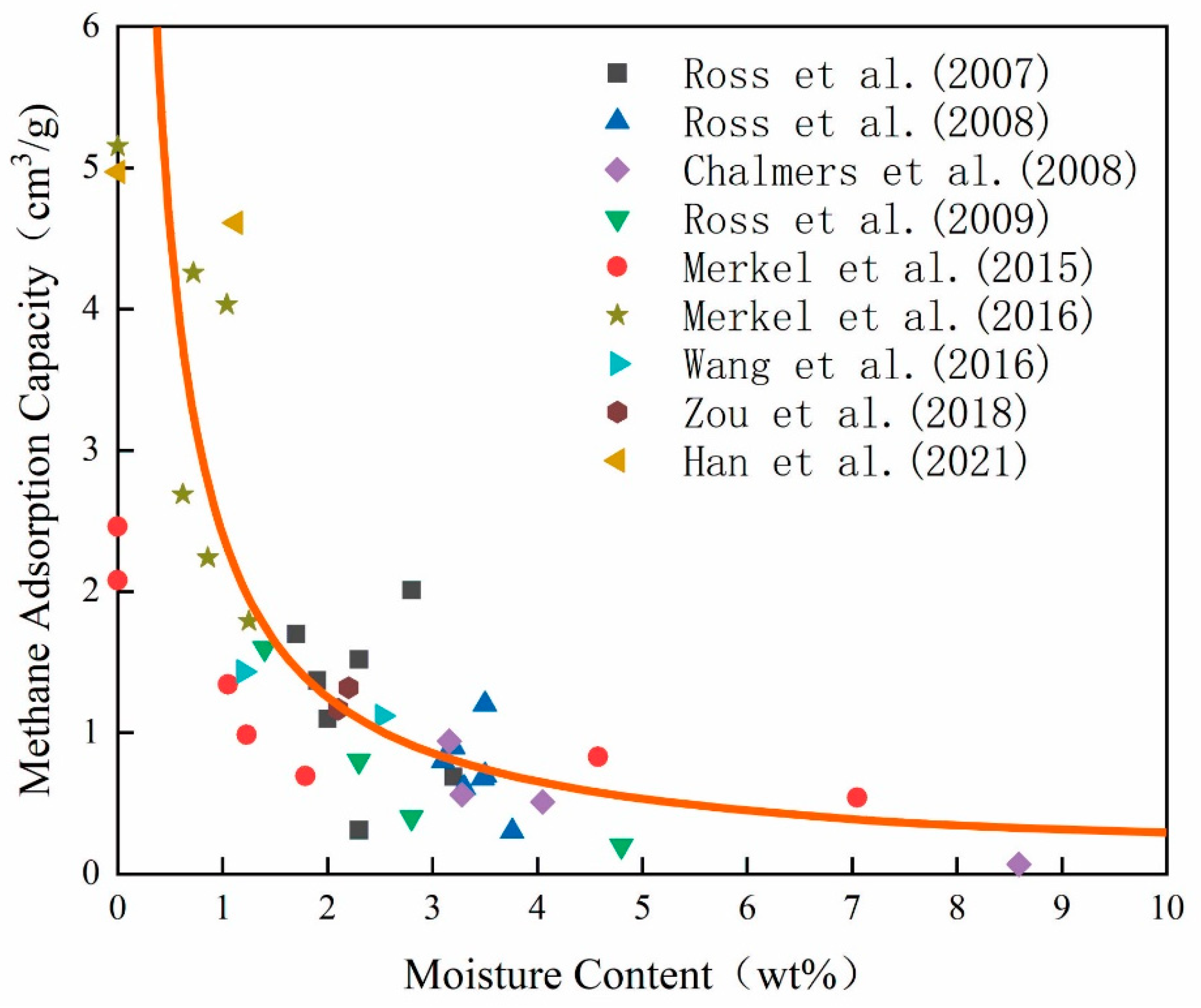
2. Influence of Water on Methane Adsorption Capacity
3. Effects of Organic Matter Attributes on the Adsorption of Water and Methane
3.1. Total Organic Carbon Content

3.2. Organic Matter Type
3.3. Maturity of Organic Matter
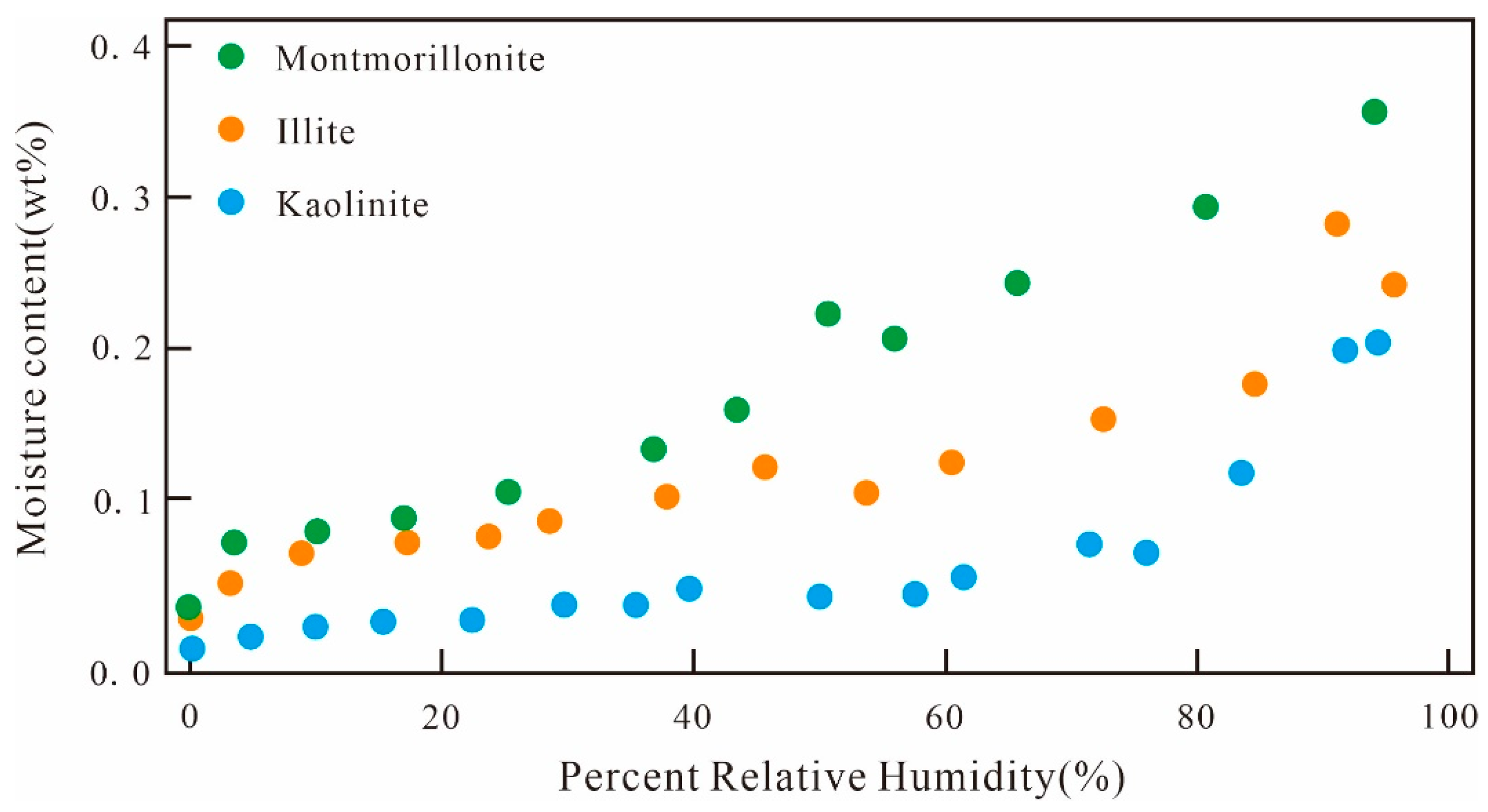
4. Effect of Mineral Composition on the Adsorption of Water and Methane
5. Pore Property
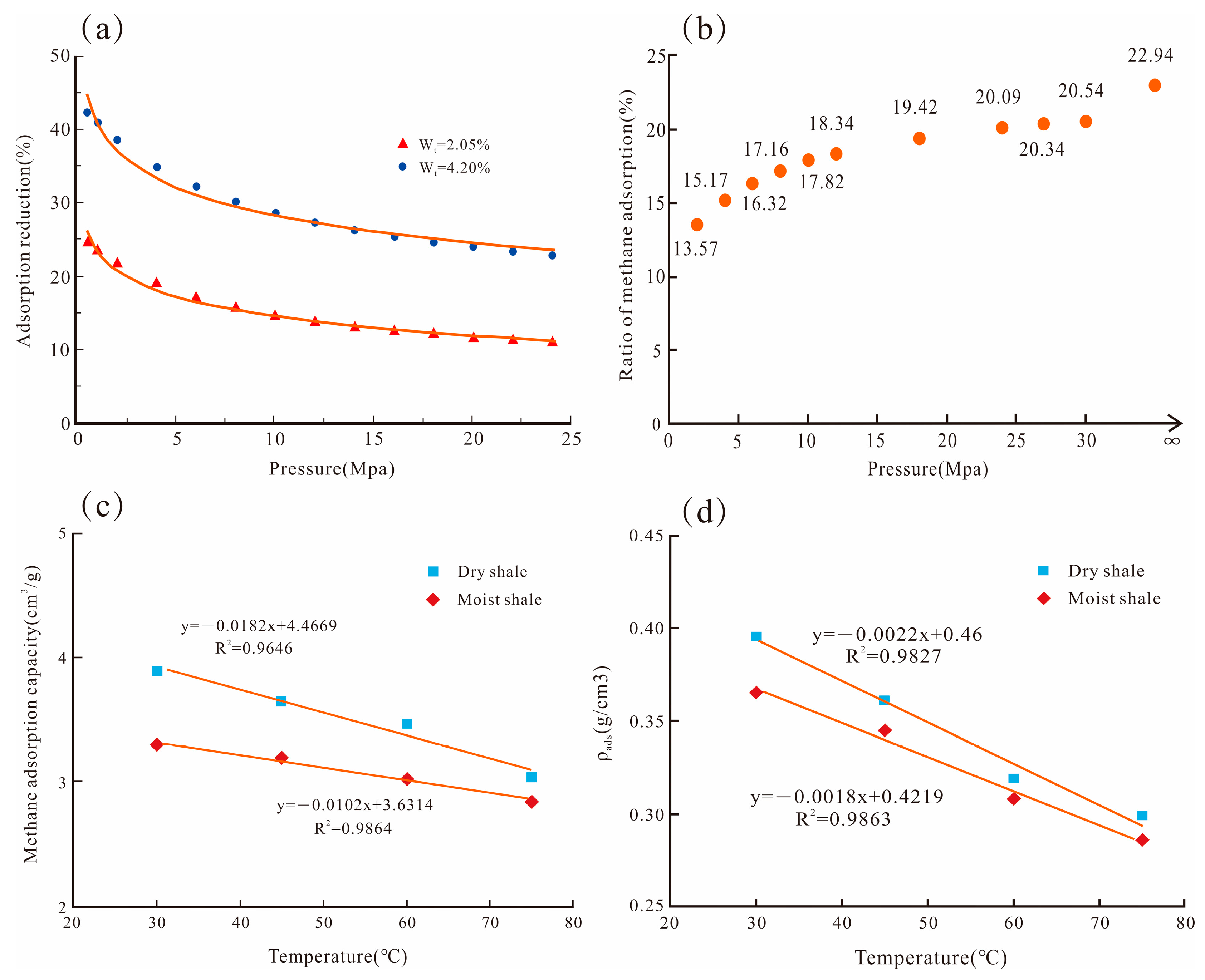
6. Temperature and Pressure
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weijermars, R.; Drijkoningen, G.; Heimovaara, T.; Rudolph, E.; Weltje, G.; Wolf, K. Unconventional gas research initiative for clean energy transition in Europe. J. Nat. Gas Sci. Eng. 2011, 3, 402–412. [Google Scholar] [CrossRef]
- Wood, D. Establishing credible reaction-kinetics distributions to fit and explain multi-heating rate S2 pyrolysis peaks of kerogens and shales. Adv. Geo-Energy Res. 2018, 3, 1–28. [Google Scholar] [CrossRef]
- Dai, J.; Qin, S.; Hu, G.; Ni, Y.; Gan, L.; Huang, S.; Hong, F. Major progress in the natural gas exploration and development in the past seven decades in China. Pet. Explor. Dev. 2019, 46, 1100–1110. [Google Scholar] [CrossRef]
- Zou, C.; Pan, S.; Jin, Z.; Gao, J.; Yang, Z.; Wu, S.; Zhao, Q. Shale oil and gas revolution and its impact. Acta Pet. Sin. 2020, 41, 1–12, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Ross, D.; Bustin, R. Shale gas potential of the Lower Jurassic Gordondale Member, northeastern British Columbia, Canada. Bull. Can. Pet. Geol. 2007, 55, 51–75. [Google Scholar] [CrossRef]
- Zhang, T.; Ellis, G.; Ruppel, S.; Milliken, K.; Yang, R. Effect of organic-matter type and thermal maturity on methane adsorption in shale-gas systems. Org. Geochem. 2012, 47, 120–131. [Google Scholar] [CrossRef]
- Ambrose, R.; Hartman, R.; Diaz-Campos, M.; Yucel Akkutlu, I.; Sondergeld, C. Shale Gas-in-Place Calculations Part I: New Pore-Scale Considerations. SPE J. 2012, 17, 219–229. [Google Scholar] [CrossRef]
- Hao, F.; Zou, H.; Lu, Y. Mechanisms of shale gas storage: Implications for shale gas exploration in China. AAPG Bull. 2013, 97, 1325–1346. [Google Scholar] [CrossRef]
- Ross, D.; Marc, B. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Nelson, P. Pore-throat sizes in sandstones, tight sandstones, and shales. AAPG Bull. 2009, 93, 329–340. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, T.; Milliken, K.; Qu, J.; Zhang, X. Experimental investigation of main controls to methane adsorption in clay-rich rocks. Appl. Geochem. 2012, 27, 2533–2545. [Google Scholar] [CrossRef]
- Zou, C.; Zhao, Q.; Dong, D.; Yang, Z.; Qiu, Z.; Liang, F.; Wang, N.; Huang, Y.; Duan, A.; Zhang, Q.; et al. Geological characteristics, main challenges and future prospect of shale gas. Nat. Gas Geosci. 2017, 28, 1781–1796. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Wu, K.; Wang, K.; Luo, J.; Feng, D.; Qu, S.; Li, X. A multi-site model to determine supercritical methane adsorption in energetically heterogeneous shales. Chem. Eng. J. 2018, 349, 438–455. [Google Scholar] [CrossRef]
- Curtis, J.B. Fractured shale-gas systems. AAPG Bull. 2002, 86, 1921–1938. [Google Scholar]
- Jarvie, D.M. Shale Resource Systems for Oil and Gas: Part2-Shale-Oil Resource Systems. Shale Reservoirs-Giant Resources for the 21st Century; Worldwide Geochemistry, LLC.: Humble, TX, USA, 2012. [Google Scholar]
- Chen, L.; Zuo, L.; Jiang, Z.; Jiang, S.; Liu, K.; Tang, J.; Zhang, L. Mechanisms of shale gas adsorption: Evidence from thermodynamics and kinetics study of methane adsorption on shale. Chem. Eng. J. 2019, 361, 559–570. [Google Scholar] [CrossRef]
- Wu, S.; Guo, J.; Li, Z.; Qin, M.; Huang, Y.; He, H. Identification and optimization of shale gas “sweet spots” in marine Niutitang Formation, South China. Oil Gas Geol. 2020, 41, 1048–1059. [Google Scholar]
- Gou, Q.; Xu, S.; Hao, F.; Zhang, B.; Shu, Z.; Yang, F.; Wang, Y.; Li, Q. Quantitative calculated shale gas contents with different lithofacies: A case study of Fuling gas shale, Sichuan Basin, China. J. Nat. Gas Sci. Eng. 2020, 76, 103222. [Google Scholar] [CrossRef]
- Qiao, J.; Littke, R.; Zieger, L.; Jiang, Z.; Fink, R. Controls on gas storage characteristics of Upper Paleozoic shales from the southeastern Ordos Basin. Mar. Pet. Geol. 2020, 117, 104377. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, S.; Tian, C.; Li, D.; Li, D.; Li, Y.; Wu, W.; Cai, C.; Chen, Y. Methane adsorption characteristics and controlling factors of deep shale gas in southern Sichuan Basin, China. J. Nat. Gas Geosci. 2021, 32, 1735–1748. [Google Scholar]
- Lu, C.; Chen, L.; Jing, C.; Tan, X.; Nie, Z.; Chen, X.; Heng, D. Gas-Bearing Characteristics of the Longmaxi Formation Shale in the Changning Area, Southern Sichuan Basin, SW China. Front. Earth Sci. 2022, 10, 755690. [Google Scholar] [CrossRef]
- Gasparik, M.; Ghanizadeh, A.; Bertier, P.; Gensterblum, Y.; Bouw, S.; Krooss, B.M. High-Pressure Methane Sorption Isotherms of Black Shales from The Netherlands. Energy Fuels 2012, 26, 4995–5004. [Google Scholar] [CrossRef]
- Rexer, T.F.; Mathia, E.J.; Aplin, A.C.; Thomas, K.M. High-pressure methane adsorption and characterization of pores in Posidonia shales and isolated kerogens. Energy Fuels 2014, 28, 2886–2901. [Google Scholar] [CrossRef]
- Ji, W.; Song, Y.; Jiang, Z.; Chen, L.; Li, Z.; Yang, X.; Meng, M. Estimation of marine shale methane adsorption capacity based on experimental investigations of Lower Silurian Longmaxi formation in the Upper Yangtze Platform, south China. Mar. Pet. Geol. 2015, 68, 94–106. [Google Scholar] [CrossRef]
- Jarvie, D.M.; Liu, B.; Schieber, J.; Mastalerz, M.; Cichon-Pupienis, A.; Littke, R.; Froidl, F.; Kong, X.; Jiang, Z.; Han, C. Shale resource systems for oil and gas: Part 1—Shale-gas resource systems. AAPG Mem. 2012, 97, 69–87. [Google Scholar]
- Ahmad, M.; Haghighi, M. Water Saturation Evaluation of Murteree and Roseneath Shale Gas Reservoirs, Cooper Basin, Australia Using Wire-line Logs, Focused Ion Beam Milling and Scanning Electron Microscopy. In Proceedings of the SPE Unconventional Resources Conference and Exhibition-Asia Pacific, Brisbane, Australia, 11–13 November 2013. [Google Scholar]
- Fang, C.; Huang, Z.; Wang, Q.; Zheng, D.; Liu, H.-L. Cause and significance of the ultra-low water saturation in gas-enriched shale reservoir. Nat. Gas Geosci. 2014, 25, 471–476. [Google Scholar]
- Merkel, A.; Fink, R.; Littke, R. The role of pre-adsorbed water on methane sorption capacity of Bossier and Haynesville shales. Int. J. Coal Geol. 2015, 147, 1–8. [Google Scholar] [CrossRef]
- Hu, Z.; Duan, X.; He, Y. Influence of reservoir primary water on shale gas occurrence and flow capacity. Nat. Gas Ind. B 2019, 6, 71–78. [Google Scholar] [CrossRef]
- Jin, Z.; Firoozabadi, A. Effect of water on methane and carbon dioxide sorption in clay minerals by Monte Carlo simulations. Fluid Phase Equilibria 2014, 382, 10–20. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Wang, X.; Li, Y.; Wu, K.; Shi, J.; Yang, L.; Feng, D.; Zhang, T.; Yu, P. Water distribution characteristic and effect on methane adsorption capacity in shale clay. Int. J. Coal Geol. 2016, 159, 135–154. [Google Scholar] [CrossRef]
- Whitelaw, P.; Uguna, C.; Stevens, L.; Meredith, W.; Snape, C.; Vane, C.; Moss-Hayes, V.; Carr, A. Shale gas reserve evaluation by laboratory pyrolysis and gas holding capacity consistent with field data. Nat. Commun. 2019, 10, 3659. [Google Scholar] [CrossRef]
- Chalmers, G.; Bustin, R. Lower Cretaceous gas shales in northeastern British Columbia, Part I: Geological controls on methane sorption capacity. Bull. Can. Pet. Geol. 2008, 56, 1–21. [Google Scholar] [CrossRef]
- Gensterblum, Y.; Merkel, A.; Busch, A. High-pressure CH4 and CO2 sorption isotherms as a function of coal maturity and the influence of moisture. Int. J. Coal Geol. 2013, 118, 45–57. [Google Scholar] [CrossRef]
- Gasparik, M.; Bertier, P.; Gensterblum, Y.; Ghanizadeh, A.; Krooss, B.; Littke, R. Geological controls on the methane storage capacity in organic-rich shales. Int. J. Coal Geol. 2014, 123, 34–51. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Fu, C.; Zhang, H.; Zhu, Y.; Zuo, Z. Micro and nano-size pores of clay minerals in shale reservoirs: Implication for the accumulation of shale gas. Sediment Geol. 2016, 342, 180–190. [Google Scholar] [CrossRef]
- Zou, J.; Rezaee, R.; Yuan, Y.; Liu, K.; Xie, Q.; You, L. Distribution of adsorbed water in shale: An experimental study on isolated kerogen and bulk shale samples. J. Pet. Sci. Eng. 2020, 187, 106858. [Google Scholar] [CrossRef]
- Wang, M.; Lun, Z.; Zhao, C.; Wang, H.; Luo, C.; Fu, X.; Li, C.; Zhang, D. Influences of Primary Moisture on Methane Adsorption within Lower Silurian Longmaxi Shales in the Sichuan Basin, China. Energy Fuels 2020, 34, 10810–10824. [Google Scholar] [CrossRef]
- Sun, J.; Xiao, X.; Cheng, P. Methane absorption of coal-measure shales with and without pore water from the Qinshui Basin, North China: Based on high-pressure methane absorption experiments. Int. J. Coal Geol. 2022, 263, 104116. [Google Scholar] [CrossRef]
- Xu, L.; Wei, H.; Chen, L.; Liu, L.; Jiang, Z.; Yang, K.; Li, X. Storing characteristics and main controlling factors of connate water in lower Paleozoic shales in southeast Chongqing, China. J. Pet. Sci. Eng. 2022, 215, 110543. [Google Scholar] [CrossRef]
- Bennion, D.; Bietz, R.; Thomas, F. Reductions in the productivity of oil and low permeability gas-reservoirs due to aqueous-phase trapping. J. Can. Pet. Technol. 1994, 33, 45–54. [Google Scholar] [CrossRef]
- Wang, F.; Reed, R.; John, A.; Katherine, G. Pore Networks and Fluid Flow in Gas Shales. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4–7 October 2009. [Google Scholar]
- Day-Stirrat, R.; Milliken, K.; Dutton, S.; Loucks, R.; Hillier, S.; Aplin, A.; Schleicher, A. Open-system chemical behavior in deep Wilcox Group mudstones, Texas Gulf Coast, USA. Mar. Pet. Geol. 2010, 27, 1804–1818. [Google Scholar] [CrossRef]
- Dehghanpour, H.; Lan, Q.; Saeed, Y.; Fei, H.; Qi, Z. Spontaneous Imbibition of Brine and Oil in Gas Shales: Effect of Water Adsorption and Resulting Microfractures. Energy Fuels 2013, 27, 3039–3049. [Google Scholar] [CrossRef]
- Makhanov, K.; Habibi, A.; Dehghanpour, H.; Kuru, E. Liquid Uptake of Gas Shales: A Workflow to Estimate Water Loss during Shut-in Periods after Fracturing Operations; Department of Geophysics, Stanford University: Stanford, CA, USA, 2014; Volume 7, pp. 22–32. [Google Scholar]
- Vidic, R.D.; Brantley, S.; Vandenbossche, J.; Yoxtheimer, D.; Abad, J. Impact of Shale Gas Development on Regional Water Quality. Science 2013, 340, 1235009. [Google Scholar] [CrossRef] [PubMed]
- Vengosh, A.; Jackson, R.; Warner, N.; Darrah, T.; Kondash, A. A Critical Review of the Risks to Water Resources from Unconventional Shale Gas Development and Hydraulic Fracturing in the United States. Environ. Sci. Technol. 2014, 48, 8334–8348. [Google Scholar] [CrossRef]
- Testamanti, M.N.; Rezaee, R. Determination of NMR T2 cut-off for clay bound water in shales: A case study of Carynginia Formation, Perth Basin, Western Australia. J. Pet. Sci. Eng. 2017, 149, 497–503. [Google Scholar] [CrossRef]
- Joubert, J.; Grein, C.; Bienstock, D. Effect of moisture on the methane capacity of American coals. Fuel 1974, 53, 186–191. [Google Scholar] [CrossRef]
- Ambrose, R.; Hartman, R.; Diaz-Campos, M.; Akkutlu, I.; Sondergeld, C. New Pore-scale Considerations for Shale Gas in Place Calculations. In Proceedings of the SPE Unconventional Gas Conference, Pittsburgh, PA, USA, 23–25 February 2010. [Google Scholar]
- Xiao, X.; Wang, M.; Wei, Q.; Tian, H.; Pan, L.; Li, T. Evaluation of Lower Paleozoic shale with shale gas prospect in south China. Nat. Gas Geosci. 2015, 26, 1433–1445. [Google Scholar]
- Bennion, D.; Thomas, F. Formation damage issues impacting the productivity of low permeability, low initial water saturation gas producing formations. J. Energy Resour. Technol. 2006, 127, 240–247. [Google Scholar] [CrossRef]
- Bowker, K.A. Barnett Shale gas production, Fort Worth Basin: Issues and discussion. AAPG Bull. 2007, 91, 523–533. [Google Scholar] [CrossRef]
- Boyer, C.; Kieschnick, J.; Suarez-Rivera, R.; Lewis, R.; Waters, G. Producing gas from its source. Oilfield Rev. 2008, 18, 36–49. [Google Scholar]
- Newsham, K.E.; Rushing, J.A.; Lasswell, P.M. Use of Vapor Desorption Data to Characterize High Capillary Pressures in a Basin-Centered Gas Accumulation with Ultra-Low Connate Water Saturations. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 5–8 October 2003. [Google Scholar]
- Liu, H.; Wang, H. Characteristics of ultra-low water saturation of marine shale in southern China and selection index of overpressure core area. Nat. Gas Ind. 2013, 33, 140–144, (In Chinese with English abstract). [Google Scholar]
- Cheng, P.; Xiao, X.; Tian, H.; Wang, X. Water Content and Equilibrium Saturation and Their Influencing Factors of the Lower Paleozoic Overmature Organic-Rich Shales in the Upper Yangtze Region of Southern China. Energy Fuels 2018, 32, 11452–11466. [Google Scholar] [CrossRef]
- Yee, D.; Seidle, J.; Hanson, W.; Law, B.; Rice, D. Gas Sorption on Coal and Measurement of Gas Content. In Hydrocarbons from Coal; American Association of Petroleum Geologists: Tulsa, OK, USA, 1993; Volume 38. [Google Scholar]
- Krooss, B.; Bergen, F.; Gensterblum, Y.; Siemons, N.; Pagnier, H.; David, P. High-pressure methane and carbon dioxide adsorption on dry and moisture-equilibrated Pennsylvanian coals. Int. J. Coal Geol. 2002, 51, 69–92. [Google Scholar] [CrossRef]
- Kadoura, A.; Narayanan Nair, A.; Sun, S. Adsorption of carbon dioxide, methane, and their mixture by montmorillonite in the presence of water. Microporous Mesoporous Mater. 2016, 225, 331–341. [Google Scholar] [CrossRef]
- Fan, K.; Li, Y.; Elsworth, D.; Dong, M.; Yin, C.; Li, Y.; Chen, Z. Three stages of methane adsorption capacity affected by moisture content. Fuel 2018, 231, 352–360. [Google Scholar] [CrossRef]
- Hartman, R.; Lasswell, P.; Bhatta, N. Recent Advances in the Analytical Methods Used for Shale Gas Reservoir Gas-in-Place Assessment. Search Discov. 2008, 40317, 20–23. [Google Scholar]
- Handwerger, D.; Suarez-Rivera, R.; Vaughn, K.; Keller, J. Improved Petrophysical Core Measurements on Tight Shale Reservoirs Using Retort and Crushed Samples. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 30 October–2 November 2011. [Google Scholar]
- Cheng, P.; Tian, H.; Xiao, X.; Gai, H.; Li, T.; Wangt, X. Water Distribution in Overmature Organic-Rich Shales: Implications from Water Adsorption Experiments. Energy Fuels 2017, 31, 13120–13132. [Google Scholar] [CrossRef]
- Cheng, P.; Xiao, X.; Tian, H.; Gai, H.; Zhou, Q.; Li, T.; Fan, Q. Differences in the distribution and occurrence phases of pore water in various nanopores of marine-terrestrial transitional shales in the Yangquan area of the northeast Qinshui Basin, China. Mar. Pet. Geol. 2022, 137, 105510. [Google Scholar] [CrossRef]
- Yuan, Y.; Rezaee, R.; Verrall, M. Pore characterization and clay bound water assessment in shale with a combination of NMR and low-pressure nitrogen gas adsorption. Int. J. Coal Geol. 2018, 194, 11–21. [Google Scholar] [CrossRef]
- Hu, M.; Wang, J. Laboratory measurement of water imbibition into low-permeability welded tuff. J. Hydrol. 2001, 242, 64–78. [Google Scholar] [CrossRef]
- ASTM D1412-07; ASTM Standard Test Method for Equilibrium Moisture of Coal at 96 to 97 Percent Relative Humidity and 30 °C. American Society for Testing and Materials: West Conshohocken, PA, USA, 2007; p. 5.
- Gasparik, M.; Ghanizadeh, A.; Gensterblum, Y.; Krooss, B. ‘Multi-temperature’ method for high-pressure sorption measurements on moist shales. Rev. Sci. Instrum. 2013, 84, 85116. [Google Scholar] [CrossRef]
- Wang, L.; Wan, J.; Tokunaga, T. Experimental and modeling study of methane adsorption onto partially saturated shales. Water Resour. Res. 2018, 54, 5017–5029. [Google Scholar] [CrossRef]
- Tian, H.; Wang, M.; Liu, S. Influence of Pore Water on the Gas Storage of Organic-Rich Shale. Energy Fuels 2020, 34, 5293–5306. [Google Scholar] [CrossRef]
- Feng, D.; Li, X.; Wang, X.; Li, J.; Sun, F.; Sun, Z.; Zhang, T.; Li, P.; Chen, Y.; Zhang, X. Water adsorption and its impact on the pore structure characteristics of shale clay. Appl. Clay Sci. 2018, 155, 126–138. [Google Scholar] [CrossRef]
- Li, P.; Zhang, J.; Rezaee, R.; Dang, W.; Tang, X.; Nie, H.; Chen, S. Effect of adsorbed moisture on the pore size distribution of marine-continental transitional shales: Insights from lithofacies differences and clay swelling. Appl. Clay Sci. 2021, 201, 105926. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Q.; Ye, X.; Dong, L. Adsorption Characteristics and Pore Structure of Organic-Rich Shale with Different Moisture Contents. Front. Earth Sci. 2022, 10, 863691. [Google Scholar] [CrossRef]
- Gao, H.; Cheng, P.; Wu, W.; Liu, S.; Luo, C.; Li, T.; Zhong, K.; Tian, H. Pore Water and Its Influences on the Nanopore Structures of Deep Longmaxi Shales in the Luzhou Block of the Southern Sichuan Basin, China. Energies 2022, 15, 4053. [Google Scholar] [CrossRef]
- Labus, M.A.; Labus, K.a.; Bujok, P.B. Determination of the pore space parameters in microporous rocks by means of thermal methods. J. Pet. Sci. Eng. 2015, 127, 482–489. [Google Scholar] [CrossRef]
- Shen, B.; Li, Z.; Zheng, Z.; Li, C.; Lei, H.; Zhang, L.; Zhu, H.; Lu, S.; Du, M. Status and relative content of water in shale determined by thermogravimetry-mass spectrometry analysis. J. Pet. Sci. Eng. 2021, 196, 107739. [Google Scholar] [CrossRef]
- Dfaz, A.; Roegiers, J. Water distribution: A key factor to characterize shale. In Proceedings of the DC Rocks 2001, The 38th U.S. Symposium on Rock Mechanics (USRMS), Washington, DC, USA, 7–10 July 2001. [Google Scholar]
- Mu, Y.; Hu, Z.; Chang, J.; Duan, X.; Li, Y.; Li, Y.; Niu, W. Effect of water occurrence on shale seepage ability. J. Pet. Sci. Eng. 2021, 204, 108725. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Wang, Y.; Zhang, Y.; Wang, Y.; Wu, J.; Zhang, J.; Khan, J. Influence of Pore Structure Particularity and Pore Water on the Occurrence of Deep Shale Gas: Wufeng–Longmaxi Formation, Luzhou Block, Sichuan Basin. Nat. Resour. Res. 2022, 31, 1403–1423. [Google Scholar] [CrossRef]
- Zolfaghari, A.; Dehghanpour, H.; Xu, M. Water sorption behaviour of gas shales: II. Pore size distribution. Int. J. Coal Geol. 2017, 179, 187–195. [Google Scholar] [CrossRef]
- Shen, W.; Li, X.; Lu, X.; Wan, Y.; Guo, W.; Zuo, L. Study on Moisture Transport Characteristics of Shale Based on Isothermal Adsorption. Lixue Xuebao 2019, 51, 932–939. [Google Scholar]
- Ren, W.; Guo, J.; Zeng, F.; Wang, T. Modeling of High-Pressure Methane Adsorption on Wet Shales. Energy Fuels 2019, 33, 7043–7051. [Google Scholar] [CrossRef]
- Chalmers, G.; Bustin, R. The organic matter distribution and methane capacity of the Lower Cretaceous strata of Northeastern British Columbia, Canada. Int. J. Coal Geol. 2007, 70, 223–239. [Google Scholar] [CrossRef]
- Ross, D.; Bustin, R. Characterizing the shale gas resource potential of Devonian-Mississippian strata in the Western Canada sedimentary basin: Application of an integrated formation evaluation. AAPG Bull. 2008, 43, 87–125. [Google Scholar] [CrossRef]
- Yuan, W.; Pan, Z.; Li, X.; Yang, Y.; Zhao, C.; Connell, L.; Li, S.; He, J. Experimental study and modelling of methane adsorption and diffusion in shale. Fuel 2014, 117, 509–519. [Google Scholar] [CrossRef]
- Merkel, A.; Fink, R.; Littke, R. High pressure methane sorption characteristics of lacustrine shales from the Midland Valley Basin, Scotland. Fuel 2016, 182, 361–372. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Q. The effect of moisture on the methane adsorption capacity of shales: A study case in the eastern Qaidam Basin in China. J. Hydrol. 2016, 542, 487–505. [Google Scholar] [CrossRef]
- Zou, J.; Rezaee, R.; Xie, Q.; You, L.; Liu, K.; Saeedi, A. Investigation of moisture effect on methane adsorption capacity of shale samples. Fuel 2018, 232, 323–332. [Google Scholar] [CrossRef]
- Crosdale, P.; Moore, T.; Mares, T. Influence of moisture content and temperature on methane adsorption isotherm analysis for coals from a low-rank, biogenically-sourced gas reservoir. Int. J. Coal Geol. 2008, 76, 166–174. [Google Scholar] [CrossRef]
- Yang, F.; Xie, C.; Ning, Z.; Krooss, B. High-Pressure Methane Sorption on Dry and Moisture-Equilibrated Shales. Energy Fuels 2016, 31, 482–492. [Google Scholar] [CrossRef]
- Day, S.; Sakurovs, R.; Weir, S. Supercritical gas sorption on moist coals. Int. J. Coal Geol. 2008, 74, 203–214. [Google Scholar] [CrossRef]
- Sun, J.; Xiao, X.; Wei, Q.; Cheng, P.; Tian, H. Occurrence of Irreducible Water and Its Influences on Gas-Bearing Property of Gas Shales from Shallow Longmaxi Formation in the Xishui Area, Guizhou, Southern China. Front. Earth Sci. 2021, 9, 654136. [Google Scholar] [CrossRef]
- Zhao, T.; Li, X.; Zhao, H.; Li, M. Molecular simulation of adsorption and thermodynamic properties on type II kerogen: Influence of maturity and moisture content. Fuel 2017, 190, 198–207. [Google Scholar] [CrossRef]
- Chen, Z.; Ning, Z.; Wang, Q.; Huang, L.; Qi, R.; Wang, J. Experimental study on methane adsorption characteristics of water-bearing shale. Fault-Block Oil Gas Field 2018, 25, 510–514+548, (In Chinese with English abstract). [Google Scholar]
- Han, W.; Li, A.; Memon, A.; Ma, M. Synergetic Effect of Water, Temperature, and Pressure on Methane Adsorption in Shale Gas Reservoirs. ACS Omega 2021, 6, 2215–2229. [Google Scholar] [CrossRef]
- Xiang, J.; Zeng, F.; Liang, H.; Li, B.; Song, X. Molecular simulation of the CH4/CO2/H2O adsorption onto the molecular structure of coal. Sci. China Earth Sci. 2014, 57, 1749–1759. [Google Scholar] [CrossRef]
- Habibi, A.; Dehghanpour, H. Wetting Behavior of Tight Rocks: From Core Scale to Pore Scale. Water Resour. Res. 2018, 54, 9162–9186. [Google Scholar] [CrossRef]
- Schettler, P.; Parmely, C. Contributions to Total Storage Capacity in Devonian Shales. In Proceedings of the SPE Eastern Regional Meeting, Lexington, KY, USA, 22–25 October 1991. [Google Scholar]
- Lu, X.; Li, F.; Watson, A. Adsorption measurements in Devonian shales. Fuel 1995, 74, 599–603. [Google Scholar] [CrossRef]
- Wang, S.; Song, Z.; Cao, T.; Song, X. The methane sorption capacity of Paleozoic shales from the Sichuan Basin, China. Mar. Pet. Geol. 2013, 44, 112–119. [Google Scholar] [CrossRef]
- Yang, F.; Ning, Z.; Zhang, R.; Zhao, H.; Krooss, B. Investigations on the methane sorption capacity of marine shales from Sichuan Basin, China. Int. J. Coal Geol. 2015, 146, 104–117. [Google Scholar] [CrossRef]
- Li, T. Effect of Water Content on Methane Adsorption in Shale; China University of Geosciences: Beijing, China, 2019; (In Chinese with English abstract). [Google Scholar]
- Sang, G.; Liu, S.; Elsworth, D. Water Vapor Sorption Properties of Illinois Shales Under Dynamic Water Vapor Conditions: Experimentation and Modeling. Water Resour. Res. 2019, 55, 7212–7228. [Google Scholar] [CrossRef]
- Yang, R.; Jia, A.; He, S.; Hu, Q.; Dong, T.; Hou, Y.; Yan, J. Water adsorption characteristics of organic-rich Wufeng and Longmaxi Shales, Sichuan Basin (China). J. Pet. Sci. Eng. 2020, 193, 107387. [Google Scholar] [CrossRef]
- Huang, L.; Ning, Z.; Wang, Q.; Zhang, W.; Cheng, Z.; Wu, X.; Qin, H. Effect of organic type and moisture on CO2/CH4 competitive adsorption in kerogen with implications for CO2 sequestration and enhanced CH4 recovery. Appl. Energy 2018, 210, 28–43. [Google Scholar] [CrossRef]
- Branson, K.; Newman, A. Water sorption on Ca-saturated clays; I, Multilayer sorption and microporosity in some illites. Clay Miner. 1983, 18, 277–287. [Google Scholar] [CrossRef]
- Hatch, C.; Wiese, J.; Crane, C.; Harris, K.; Kloss, H.; Baltrusaitis, J. Water adsorption on clay minerals as a function of relative humidity: Application of BET and Freundlich adsorption models. Langmuir ACS J. Surf. Colloids 2012, 28, 1790–1803. [Google Scholar] [CrossRef]
- Passey, Q.; Bohacs, K.; Esch, W.; Klimentidis, R.; Sinha, S. From Oil-Prone Source Rock to Gas-Producing Shale Reservoir—Geologic and Petrophysical Characterization of Unconventional Shale-Gas Reservoirs. In Proceedings of the International Oil and Gas Conference and Exhibition in China, Beijing, China, 8–10 June 2010. [Google Scholar]
- Odusina, E.; Sondergeld, C.; Rai, C. An NMR Study on Shale Wettability. In Proceedings of the Canadian Unconventional Resources Conference, Alberta, Canada, 15–17 November 2011. [Google Scholar]
- Sulucarnain, I.; Sondergeld, C.; Rai, C. An NMR Study of Shale Wettability and Effective Surface Relaxivity; Society of Petroleum Engineers: Richardson, TX, USA, 2012. [Google Scholar]
- Borysenko, A.; Clennell, B.; Sedev, R.; Burgar, I.; Ralston, J.; Raven, M.; Dewhurst, D.; Liu, K. Experimental investigations of the wettability of clays and shales. J. Geophys. Res. Solid Earth 2009, 114, B07202. [Google Scholar] [CrossRef]
- Dehghanpour, H.; Zubair, H.; Chhabra, A.; Ullah, A. Liquid Intake of Organic Shales. Energy Fuels 2012, 26, 5750–5758. [Google Scholar] [CrossRef]
- Korb, J.; Nicot, B.; Louis-Joseph, A.; Bubici, S.; Ferrante, G. Dynamics and Wettability of Oil and Water in Oil Shales. J. Phusical Chem. 2014, 118, 23212–23218. [Google Scholar] [CrossRef]
- Zolfaghari, A.; Dehghanpour, H.; Holyk, J. Water sorption behaviour of gas shales: I. Role of clays. Int. J. Coal Geol. 2017, 179, 130–138. [Google Scholar] [CrossRef]
- Seemann, T.; Bertier, P.; Krooß, B.; Stanjek, H. Water vapour sorption on mudrocks. Geol. Soc. Spec. Publ. 2017, 454, 201–233. [Google Scholar] [CrossRef]
- Charrière, D.; Behra, P. Water sorption on coals. J. Colloid Interface Sci. 2010, 344, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Devegowda, D.; Striolo, A.; Phan, A.; Ho, T.; Civan, F.; Sigal, R. Microscopic Dynamics of Water and Hydrocarbon in Shale-Kerogen Pores of Potentially Mixed Wettability. SPE J. 2015, 20, 112–124. [Google Scholar] [CrossRef]
- Hu, Y.; Devegowda, D.; Sigal, R. A microscopic characterization of wettability in shale kerogen with varying maturity levels. J. Nat. Gas Sci. Eng. 2016, 33, 1078–1086. [Google Scholar] [CrossRef]
- Kuila, U.; McCarty, D.; Derkowski, A.; Fischer, T.; Topor, T.; Prasad, M. Nano-scale texture and porosity of organic matter and clay minerals in organic-rich mudrocks. Fuel 2014, 135, 359–373. [Google Scholar] [CrossRef]
- Gu, X.; Mildner, D.; Cole, D.; Rother, G.; Slingerland, R.; Brantley, S. Quantification of Organic Porosity and Water Accessibility in Marcellus Shale Using Neutron Scattering. Energy Fuels 2016, 30, 4438–4449. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Lu, S.; Zhang, P.; Cai, J.; Zhao, J.; Li, W. Microdistribution and mobility of water in gas shale: A theoretical and experimental study. Mar. Pet. Geol. 2019, 102, 496–507. [Google Scholar] [CrossRef]
- Gao, Z.; Fan, Y.; Hu, Q.; Jiang, Z.; Cheng, Y.; Xuan, Q. A review of shale wettability characterization using spontaneous imbibition experiments. Mar. Pet. Geol. 2019, 109, 330–338. [Google Scholar] [CrossRef]
- Shao, D.; Zhang, L.; Zhang, Y.; Zhang, Y.; Luo, H.; Qiao, B.; Yan, J.; Zhang, T. Water absorption characteristics of organic-rich shale of the Lower Cambrian in the Middle and Upper Yangtze region and its implications for shale gas exploration. Nat. Gas Geosci. 2020, 31, 1004–1015, (In Chinese with English abstract). [Google Scholar]
- Sun, J. Gas Bearing Properties and Controlling Factors of Shallow Longmaxi Shale in Northern Guizhou; Guangzhou Institute of Geochemistry, Chinese Academy of Sciences: Beijing, China, 2020; (In Chinese with English abstract). [Google Scholar]
- Bekyarova, E.; Hanzawa, Y.; Kaneko, K.; Silvestre-Albero, J.; Sepulveda-Escribano, A.; Rodriguez-Reinoso, F.; Kasuya, D.; Yudasaka, M.; Iijima, S. Cluster-mediated filling of water vapor in intratube and interstitial nanospaces of single-wall carbon nanohorns. Chem. Phys. Lett. 2002, 366, 463–468. [Google Scholar] [CrossRef]
- Bahadur, J.; Contescu, C.; Rai, D.; Gallego, N.; Melnichenko, Y. Clustering of water molecules in ultramicroporous carbon: In-situ small-angle neutron scattering. Carbon 2017, 111, 681–688. [Google Scholar] [CrossRef]
- Mamontov, E.; Yue, Y.; Bahadur, J.; Guo, J.; Contescu, C.; Gallego, N.; Melnichenko, Y. Hydration level dependence of the microscopic dynamics of water adsorbed in ultramicroporous carbon. Carbon 2017, 111, 705–712. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, T.; Jiang, S.; Yang, X.; Ding, H.; Meng, M.; Wei, D. Methane Adsorption Capacities of the Lower Paleozoic Marine Shales in the Yangtze Platform, South China. Fuels 2015, 29, 4160–4167. [Google Scholar] [CrossRef]
- Shabani, M.; Moallemi, S.; Krooss, B.; Amann-Hildenbrand, A.; Zamani-Pozveh, Z.; Ghalavand, H.; Littke, R. Methane sorption and storage characteristics of organic-rich carbonaceous rocks, Lurestan province, southwest Iran. Int. J. Coal Geol. 2018, 186, 51–64. [Google Scholar] [CrossRef]
- Tan, J.; Weniger, P.; Krooss, B.; Merkel, A.; Horsfield, B.; Zhang, J.; Boreham, C.; van Graas, G.; Tocher, B. Shale gas potential of the major marine shale formations in the Upper Yangtze Platform, South China, Part II: Methane sorption capacity. Fuel 2014, 129, 204–218. [Google Scholar] [CrossRef]
- Ma, J.; Cai, Y.; Hu, Z.; Lu, C.; Wang, W. Water content in coal measure shale of Taiyuan Formation in Yushe Block, Qinshui Basin and its influence on pore characteristics. Nat. Gas Geosci. 2021, 32, 145–154, (In Chinese with English abstract). [Google Scholar]
- Gao, P.; Xiao, X.; Hu, D.; Liu, R.; Cai, Y.; Yuan, T.; Meng, G. Water Distribution in the Ultra-Deep Shale of the Wufeng–Longmaxi Formations from the Sichuan Basin. Energies 2022, 15, 2215. [Google Scholar] [CrossRef]
- Tong, J.; Han, X.; Wang, S.; Jiang, X. Evaluation of Structural Characteristics of Huadian Oil Shale Kerogen Using Direct Techniques (Solid-State C-13 NMR, XPS, FT-IR, and XRD). Energy Fuels 2011, 25, 4006–4013. [Google Scholar] [CrossRef]
- Billemont, P.; Benoit, C.; Weireld, G. Adsorption of carbon dioxide, methane, and their mixtures in porous carbons: Effect of surface chemistry, water content, and pore disorder. Langmuir 2013, 29, 3328–3338. [Google Scholar] [CrossRef]
- Wei, L.; Mastalerz, M.; Schimmelmann, A.; Chen, Y. Influence of Soxhlet-extractable bitumen and oil on porosity in thermally maturing organic-rich shales. Int. J. Coal Geol. 2014, 132, 38–50. [Google Scholar] [CrossRef]
- Lan, Q.; Xu, M.; Binazadeh, M.; Dehghanpour, H.; Wood, J. A comparative investigation of shale wettability: The significance of pore connectivity. J. Nat. Gas Sci. Eng. 2015, 27, 1174–1188. [Google Scholar] [CrossRef]
- Vandenbroucke, M.; Largeau, C. Kerogen origin, evolution and structure. Org. Geochem. 2007, 38, 719–833. [Google Scholar] [CrossRef]
- Glorioso, J.; Rattia, A. Unconventional Reservoirs: Basic Petrophysical Concepts for Shale Gas; European Association of Geoscientists & Engineers: Vienna, Austria, 2012. [Google Scholar]
- Bai, J.; Kang, Y.; Chen, M.; Chen, Z.; You, L. Impact of the water adsorption monolayer on methane ad-/desorption behavior in gas shale nanopores. Ind. Eng. Chem. Res. 2021, 60, 3130–3141. [Google Scholar] [CrossRef]
- Li, P. Wettability of Shale and its Control Mechanism for Methane Adsorption; China University of Geosciences: Beijing, China, 2021; (In Chinese with English abstract). [Google Scholar]
- Arnold, B.; Aplan, F. The hydrophobicity of coal macerals. Fuel 1989, 68, 651–658. [Google Scholar] [CrossRef]
- Xv, M.; Bai, Y.; Xia, J. Discussion on Heterogeneous Wettability of Coal and Rock and Its Influencing Factors. In Proceedings of the Sixth National Natural Gas Reservoir Efficient Development Technology Seminar, Beijing, China, 11–14 May 2015; pp. 395–401+429, (In Chinese with English abstract). [Google Scholar]
- Larsen, J.; Aida, M. Kerogen Chemistry 1. Sorption of Water by Type II Kerogens at Room Temperature. Energy Fuels 2004, 18, 1603–1604. [Google Scholar] [CrossRef]
- Do, D.; Junpirom, S.; Do, H. A new adsorption–desorption model for water adsorption in activated carbon. Carbon 2009, 47, 1466–1473. [Google Scholar] [CrossRef]
- Ungerer, P. State of the art of research in kinetic modelling of oil formation and expulsion. Org. Geochem. 1990, 16, 1–25. [Google Scholar] [CrossRef]
- Li, J. Study on the Occurrence Mode and Production Mechanism of Shale Gas; China University of Petroleum: Beijing, China, 2017; (In Chinese with English abstract). [Google Scholar]
- Xiang, J.; Zeng, F.; Liang, H.; Li, B.; Song, X. Molecular simulation of CH4/CO2/H2O adsorption in coal molecular structure. Sci. China Earth Sci. 2014, 44, 1418–1428, (In Chinese with English abstract). [Google Scholar]
- Xia, Y.; Liu, X.; Liu, S. Adsorption mechanism of water molecules by oxygen-containing functional groups on lignite surface. Coal Conversion. 2016, 39, 9, (In Chinese with English abstract). [Google Scholar]
- Tissot, P.B.; Welte, H.D. Petroleum Formation and Occurrence; Springer: Berlin, Germany, 1984; p. 699. [Google Scholar]
- Helgeson, H.; Richard, L.; McKenzie, W.; Norton, D.; Schmitt, A. A chemical and thermodynamic model of oil generation in hydrocarbon source rocks. Geochim. Cosmochim. Acta 2009, 73, 594–695. [Google Scholar] [CrossRef]
- Heuchel, M.; Davies, G.; Buss, E.; Seaton, N. Adsorption of Carbon Dioxide and Methane and Their Mixtures on an Activated Carbon: Simulation and Experiment. Langmuir 1999, 15, 8695–8705. [Google Scholar] [CrossRef]
- Guo, J.; Zou, Y.; Yang, Y.; Qv, Z.; Wang, X.; Cai, Y.; Peng, P. Evolution characteristics of kerogen molecular structure in low maturity stage: Based on infrared spectrum analysis. Geochimica 2014, 43, 529–537, (In Chinese with English abstract). [Google Scholar]
- Yang, C.; Xiong, Y.; Zhang, J. Differences in the development of hydrocarbon-generating organic pores in shale of different sedimentary types in China. Geochemistry 2019, 48, 544–554, (In Chinese with English abstract). [Google Scholar]
- Zhao, Y.; Gao, P.; Zhou, Q.; Xiao, X.; Xing, Y.; Liu, W. A Review of the Heterogeneity of Organic-Matter-Hosted Pores in Shale Reservoirs. Energies 2022, 15, 8805. [Google Scholar] [CrossRef]
- Cheng, P.; Xiao, X.; Wang, X.; Sun, J.; Wei, Q. Evolution of water content in organic-rich shales with increasing maturity and its controlling factors: Implications from a pyrolysis experiment on a water-saturated shale core sample. Mar. Pet. Geol. 2019, 109, 291–303. [Google Scholar] [CrossRef]
- Gensterblum, Y.; Ghanizadeh, A.; Cuss, R.; Amann-Hildenbrand, A.; Krooss, B.; Clarkson, C.; Harrington, J.; Zoback, M. Gas transport and storage capacity in shale gas reservoirs—A review. Part A: Transport processes. J. Unconv. Oil Gas Resour. 2015, 12, 87–122. [Google Scholar] [CrossRef]
- Liu, H.; Wang, G.; Fang, C.; Guo, W.; Sun, S. The formation mechanism of over pressure reservoir and target screening index of the marine shale in the South China. Earth Sci. Front. 2016, 23, 48–54. [Google Scholar]
- Srodon, J.; Clauer, N.; Huff, W.; Dudek, T.; Banas, M. K-Ar dating of the Lower Palaeozoic K-bentonites from the Baltic Basin and the Baltic Shield: Implications for the role of temperature and time in the illitization of smectite. Clay Miner. 2009, 44, 361–387. [Google Scholar] [CrossRef]
- Goulty, N.; Sargent, C.; Andras, P.; Aplin, A. Compaction of diagenetically altered mudstones—Part 1: Mechanical and chemical contributions. Mar. Pet. Geol. 2016, 77, 703–713. [Google Scholar] [CrossRef]
- Ola, P.; Aidi, A.; Bankole, O. Clay mineral diagenesis and source rock assessment in the Bornu Basin, Nigeria: Implications for thermal maturity and source rock potential. Mar. Pet. Geol. 2018, 89, 653–664. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Zhu, R. Water consumption in hydrocarbon generation and its significance to reservoir formation. Pet. Explor. Dev. 2013, 40, 259–267. [Google Scholar] [CrossRef]
- Lahann, R. Impact of smectite diagenesis on compaction modeling and compaction equilibrium. AAPG Memoir. 2004, 76, 61–72. [Google Scholar]
- Begum, M.; Yassin, M.; Dehghanpour, H. Effect of kerogen maturity on organic shale wettability: A Duvernay case study. Mar. Pet. Geol. 2019, 110, 483–496. [Google Scholar] [CrossRef]
- Jagadisan, A.; Heidari, Z. Experimental Quantification of the Effect of Thermal Maturity of Kerogen on Its Wettability. SPE Reserv. Eval. Eng. 2019, 22, 1323–1333. [Google Scholar] [CrossRef]
- Jagadisan, A.; Heidari, Z. Molecular dynamic simulation of the impact of thermal maturity and reservoir temperature on the contact angle and wettability of kerogen. Fuel 2022, 309, 122039. [Google Scholar] [CrossRef]
- Yang, A.; Firdaus, G.; Heidari, Z. Electrical resistivity and chemical properties of kerogen isolated from organic-rich mudrocks. Geophysics 2016, 81, D643–D655. [Google Scholar] [CrossRef]
- Jagadisan, A.; Heidari, Z. Application of X-Ray Photoelectron Spectroscopy in Connecting Thermal Maturity of Kerogen to Its Dielectric Constant in Organic-Rich mudrocks. In Proceedings of the SPWLA 58th Annual Logging Symposium, Oklahoma City, OK, USA, 17–21 June 2017. [Google Scholar]
- Valdes, C.; Heidari, Z.; Gonzalez, A. Quantifying the Impacts of Thermal Maturity on Elastic Properties of Kerogen. In Proceedings of the SPWLA 58th Annual Logging Symposium, Oklahoma City, OK, USA, 17–21 June 2017. [Google Scholar]
- Kozbial, A.; Li, Z.; Sun, J.; Gong, X.; Zhou, F.; Wang, Y.; Xu, H.; Liu, H.; Li, L. Understanding the intrinsic water wettability of graphite. Carbon 2014, 74, 218–225. [Google Scholar] [CrossRef]
- Wei, Y.; Jia, C. Intrinsic wettability of graphitic carbon. Carbon 2015, 87, 10–17. [Google Scholar] [CrossRef]
- Chen, J.; Gai, H.; Xiao, Q. Effects of composition and temperature on water sorption in overmature Wufeng-Longmaxi shales. Int. J. Coal Geol. 2021, 234, 103673. [Google Scholar] [CrossRef]
- Curtis, M.; Cardott, B.; Sondergeld, C. Development of organic porosity in the Woodford Shale with increasing thermal maturity. Int. J. Coal Geol. 2012, 103, 26–31. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, X. Evolution of nanoporosity in organic-rich shales during thermal maturation. Fuel 2014, 129, 173–181. [Google Scholar] [CrossRef]
- Cao, T.; Song, Z.; Wang, S.; Xia, J. Comparative study on specific surface area and pore structure of different shales and kerogen. Sci. China 2015, 45, 139–151, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Powers, M. Fluid-release mechanisms in compacting marine mudrocks and their importance in oil exploration. AAPG Bull. 1967, 51, 1240–1254. [Google Scholar]
- Gensterblum, Y.; Busch, A.; Krooss, B. Molecular concept and experimental evidence of competitive adsorption of H2O, CO2 and CH4 on organic material. Fuel 2014, 115, 581–588. [Google Scholar] [CrossRef]
- Huang, L.; Ning, Z.; Wang, Q.; Qi, R.; Zeng, Y.; Qin, H.; Ye, H.; Zhang, W. Molecular simulation of adsorption behaviors of methane, carbon dioxide and their mixtures on kerogen: Effect of kerogen maturity and moisture content. Fuel 2018, 211, 159–172. [Google Scholar] [CrossRef]
- Sui, H.; Zhang, F.; Wang, Z.; Wang, D.; Wang, Y. Effect of Kerogen Maturity, Water Content for Carbon Dioxide, Methane, and Their Mixture Adsorption and Diffusion in Kerogen: A Computational Investigation. Langmuir ACS J. Surf. Colloids 2020, 36, 9756–9769. [Google Scholar] [CrossRef] [PubMed]
- Sondergeld, C.; Newsham, K.; Comisky, J.; Rice, M.; Rai, C. Petrophysical Considerations in Evaluating and Producing Shale Gas Resources. In Proceedings of the SPE Unconventional Gas Conference, Pittsburgh, PA, USA, 23–25 February 2010. [Google Scholar]
- Lahn, L.; Bertier, P.; Seemann, T.; Stanjek, H. Distribution of sorbed water in the pore network of mudstones assessed from physisorption measurements. Microporous Mesoporous Mater. 2020, 295, 109902. [Google Scholar] [CrossRef]
- Zhang, Y. Study on the Influence of Water-Bearing on Shale Gas Adsorption; China University of Petroleum: Beijing, China, 2017; (In Chinese with English abstract). [Google Scholar]
- Sun, J.; Xiao, X.; Wei, Q.; Cheng, P.; Tian, H.; Wu, Y. Gas in place and its controlling factors of the shallow Longmaxi shale in the Xishui area, Guizhou, China. J. Nat. Gas Sci. Eng. 2020, 77, 103272. [Google Scholar] [CrossRef]
- Jagadisan, A.; Heidari, Z. Impacts of competitive water adsorption of kerogen and clay minerals on wettability of organic-rich mudrocks. SPE Reserv. Eval. Eng. 2020, 23, 1180–1189. [Google Scholar] [CrossRef]
- Bi, H.; Jiang, Z.; Li, P.; Cheng, L.; Zeng, C.; Xv, Y.; Zhang, Y. Adsorption characteristics and influencing factors of Longmaxi Formation shale in southeast Chongqing. Nat. Gas Geosci. 2014, 25, 302–310, (In Chinese with English abstract). [Google Scholar]
- Li, P.; Zhang, J.; Rezaee, R.; Dang, W.; Li, X.; Fauziah, C.; Nie, H.; TANG, X. Effects of swelling-clay and surface roughness on the wettability of transitional shale. J. Pet. Sci. Eng. 2021, 196, 108007. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, X. Study of the Characteristics of Marine-Terrigenous Facies Shale from the Permo-Carboniferous System in the Guxian Block, Southwest Qinshui Basin. Energy Fuels 2018, 32, 1096–1109. [Google Scholar] [CrossRef]
- He, Q.; Dong, T.; He, S.; Zhai, G. Methane adsorption capacity of marine-continental transitional facies shales: The case study of the Upper Permian Longtan Formation, northern Guizhou Province, Southwest China. J. Pet. Sci. Eng. 2019, 183, 106406. [Google Scholar] [CrossRef]
- Tabrizy, V.; Denoye, R.; Hamouda, A. Characterization of wettability alteration of calcite, quartz and kaolinite: Surface energy analysis. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 98–108. [Google Scholar] [CrossRef]
- Chen, Z.; Song, Y.; Li, Z.; Liu, S.; Li, Y.; Liu, G.; Yang, W.; Wang, Q.; Yang, Y.; Gao, F. The occurrence characteristics and removal mechanism of residual water in marine shales: A case study of Wufeng-Longmaxi shale in Changning-Weiyuan area, Sichuan basin. Fuel 2019, 253, 1056–1070. [Google Scholar] [CrossRef]
- Wu, Q.; Deng, Y.; Fan, X.; Lu, H.; Wang, X.; Gang, Z.; Wang, F. Effect of Mineral Surface Properties on Water Behaviors in Pores Constructed by Calcite and Silica Particles. J. Phys. Chem. C 2019, 123, 13288–13294. [Google Scholar] [CrossRef]
- Chai, R.; Liu, Y.; Wang, J.; Xin, J.; Pi, J.; Li, C. Molecular dynamics simulation of wettability of calcite and dolomite. Jisuan Wuli/Chin. J. Comput. Phys. 2019, 36, 474–482. [Google Scholar]
- Wu, C.; Xue, H.; Lu, S.; Tian, S. Measurement and discussion of oil-water-mineral contact Angle for several common minerals. Geoscience 2018, 32, 842–849, (In Chinese with English abstract). [Google Scholar]
- Li, J.; Chen, Z.; Li, X.; Wang, X.; Wu, K.; Feng, D.; QV, S. Quantitative study of liquid water distribution in shale and clay nano-pores. Sci. China Technol. Sci. 2018, 48, 1219–1233, (In Chinese with English abstract). [Google Scholar]
- Sun, J.; Xiao, X.; Cheng, P. Influence of water on shale pore heterogeneity and the implications for shale gas-bearing property—A case study of marine Longmaxi Formation shale in northern Guizhou. Mar. Pet. Geol. 2021, 134, 105379. [Google Scholar] [CrossRef]
- Ruppert, L.; Sakurovs, R.; Blach, T.; He, L.; Melnichenko, Y.; Mildner, D.; Alcantar-Lopez, L. A USANS/SANS Study of the Accessibility of Pores in the Barnett Shale to Methane and Water. Energy Fuels 2013, 27, 772–779. [Google Scholar] [CrossRef]
- Li, H.; Guo, H.; Li, H.; Liu, W.; Jiang, B.; Hua, J. Thickness analysis of bound water film in tight reservoir. Nat. Gas Geosci. 2015, 26, 186–192. [Google Scholar]
- Pan, L.; Xiao, X.; Tian, H.; Zhou, Q.; Chen, J.; Li, T.; Wei, Q. A preliminary study on the characterization and controlling factors of porosity and pore structure of the Permian shales in Lower Yangtze region, Eastern China. Int. J. Coal Geol. 2015, 146, 68–78. [Google Scholar] [CrossRef]
- Chen, L.; Jiang, Z.; Liu, Q.; Jiang, S.; Liu, K.; Tan, J.; Gao, F. Mechanism of shale gas occurrence: Insights from comparative study on pore structures of marine and lacustrine shales. Mar. Pet. Geol. 2019, 104, 200–216. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, D.; Wang, H.; Li, X. A modified BET equation to investigate supercritical methane adsorption mechanisms in shale. Mar. Pet. Geol. 2019, 105, 284–292. [Google Scholar] [CrossRef]
- Yang, R.; Hu, Q.; Yi, J.; Zhang, B.; He, S.; Guo, X.; Hou, Y.; Dong, T. The effects of mineral composition, TOC content and pore structure on spontaneous imbibition in Lower Jurassic Dongyuemiao shale reservoirs. Mar. Pet. Geol. 2019, 109, 268–278. [Google Scholar] [CrossRef]
- Li, A.; Han, W.; Fang, Q.; Memon, A.; Ma, M. Experimental investigation of methane adsorption and desorption in water-bearing shale. Capillarity 2020, 3, 45–55. [Google Scholar] [CrossRef]
- Huang, H.; Li, R.; Lyu, Z.; Cheng, Y.; Zhao, B.; Jiang, Z.; Zhang, Y.; Xiong, F. Comparative study of methane adsorption of Middle-Upper Ordovician marine shales in the western Ordos Basin, NW China: Insights into impacts of moisture on thermodynamics and kinetics of adsorption. Chem. Eng. J. 2022, 446, 137411. [Google Scholar] [CrossRef]
- Zou, J.; Rezaee, R.; Xie, Q.; You, L. Characterization of the combined effect of high temperature and moisture on methane adsorption in shale gas reservoirs. J. Pet. Sci. Eng. 2019, 182, 106353. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Wu, K.; Feng, D.; Zhang, T.; Zhang, Y. Thickness and stability of water film confined inside nanoslits and nanocapillaries of shale and clay. Int. J. Coal Geol. 2017, 179, 253–268. [Google Scholar] [CrossRef]
- Long, S.; Feng, D.; Li, F.; Du, W. Prospect analysis of the deep marine shale gas exploration and development in the Sichuan Basin, China. J. Nat. Gas Geosci. 2022, 3, 181–189. [Google Scholar] [CrossRef]
- Guo, X.; Hu, D.; Huang, R.; Wei, Z.; Duan, J.; Wei, X.; Fan, X.; Miao, Z. Deep and ultra-deep natural gas exploration in the Sichuan Basin: Progress and prospect. Nat. Gas Ind. B 2020, 7, 419–432. [Google Scholar] [CrossRef]
- Ma, X.; Wang, H.; Zhou, S.; Shi, Z.; Zhang, L. Deep shale gas in China: Geological characteristics and development strategies. Energy Rep. 2021, 7, 1903–1914. [Google Scholar] [CrossRef]






| Sample | Seam Rank | Maceral | Contact Angles θ (°) | Ro (%) |
|---|---|---|---|---|
| Carbon Co., UT | Liptinites | 120 | 0.5 | |
| subA | Vitrinite | 35 | 0.5 | |
| Fusinite | 25 | 0.5 | ||
| Parke Co., IN | Liptinites | 90 | 0.6 | |
| hvBb | Vitrinite | 57 | 0.6 | |
| Fusinite | 41 | 0.6 |
| Adsorption Site | * Ecoal/water/(kJ/mol) | * Eads/(kJ/mol) |
|---|---|---|
| Carboxyl | 7,039,986.00 | −69.25 |
| Phenolic hydroxyl | 7,039,962.37 | −45.63 |
| Alcoholic hydroxyl | 7,039,957.81 | −41.06 |
| Carbonyl | 7,039,953.30 | −36.55 |
| Ether linkage | 7,039,939.84 | −23.10 |
| Sample | Water Adsorption Capacity (mL/100 g) |
|---|---|
| * K1 | 5.31 |
| * K2 | 1.90 |
| * K3 | 0.09 |
| Illite | 4.01 |
| Kaolinite | 2.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Y.; Xiao, X.; Zhou, Q.; Liu, W.; Zhao, Y. Influence of Water on the Methane Adsorption Capacity of Organic-Rich Shales and Its Controlling Factors: A Review. Energies 2023, 16, 3305. https://doi.org/10.3390/en16083305
Xing Y, Xiao X, Zhou Q, Liu W, Zhao Y. Influence of Water on the Methane Adsorption Capacity of Organic-Rich Shales and Its Controlling Factors: A Review. Energies. 2023; 16(8):3305. https://doi.org/10.3390/en16083305
Chicago/Turabian StyleXing, Yijie, Xianming Xiao, Qin Zhou, Wei Liu, and Yanming Zhao. 2023. "Influence of Water on the Methane Adsorption Capacity of Organic-Rich Shales and Its Controlling Factors: A Review" Energies 16, no. 8: 3305. https://doi.org/10.3390/en16083305
APA StyleXing, Y., Xiao, X., Zhou, Q., Liu, W., & Zhao, Y. (2023). Influence of Water on the Methane Adsorption Capacity of Organic-Rich Shales and Its Controlling Factors: A Review. Energies, 16(8), 3305. https://doi.org/10.3390/en16083305








