Anaerobic Digestion of Olive Mill Wastewater in the Presence of Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Olive Mill Wastewater
2.1.2. Inoculum
2.1.3. Biochar
2.2. Anaerobic Digestion Tests
2.3. Phytotoxicity Tests
2.4. Modelling of Biogas Production Curve
3. Results
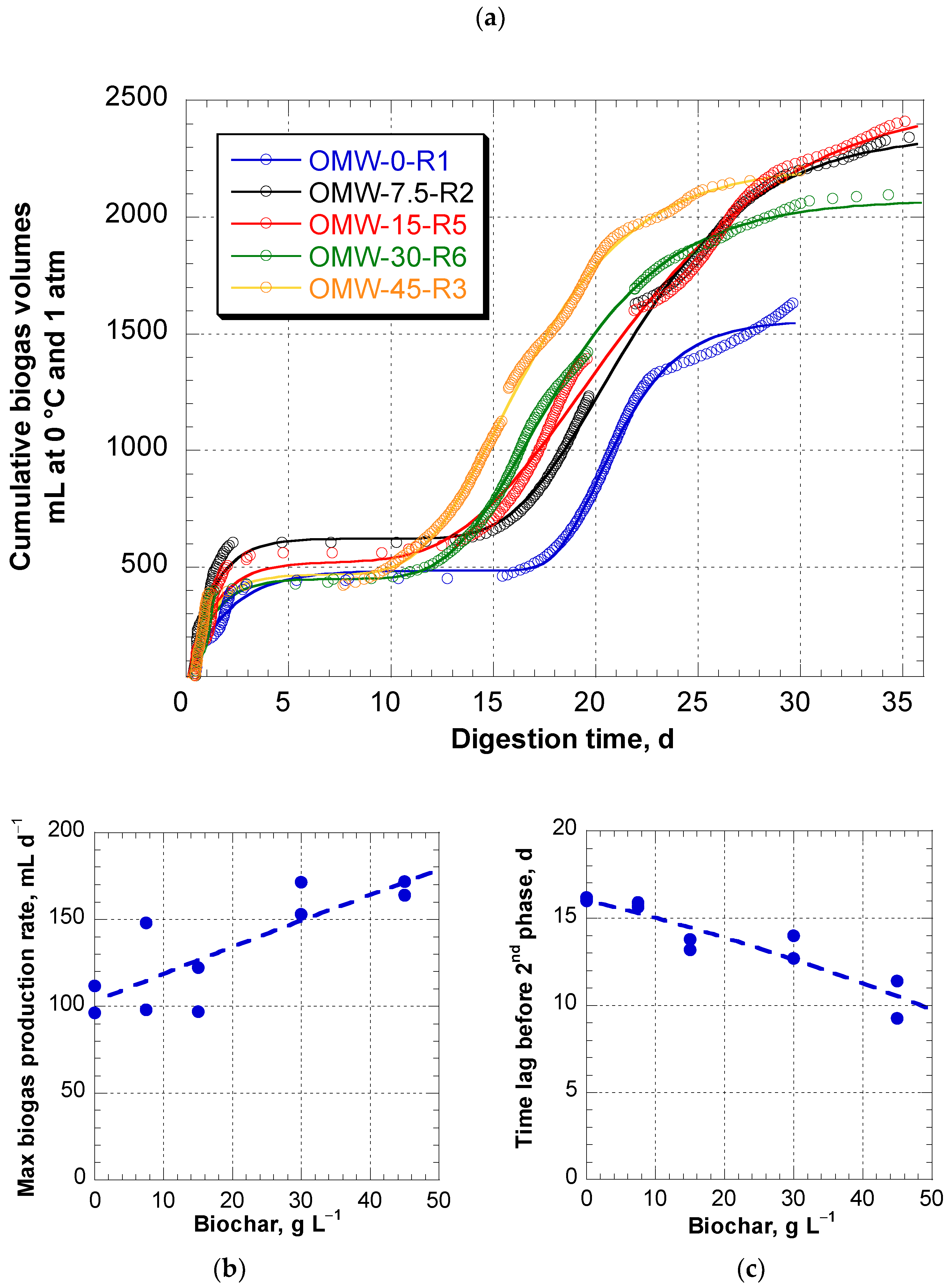
3.1. Time Course of Biogas Production
3.2. Overall Biogas Production
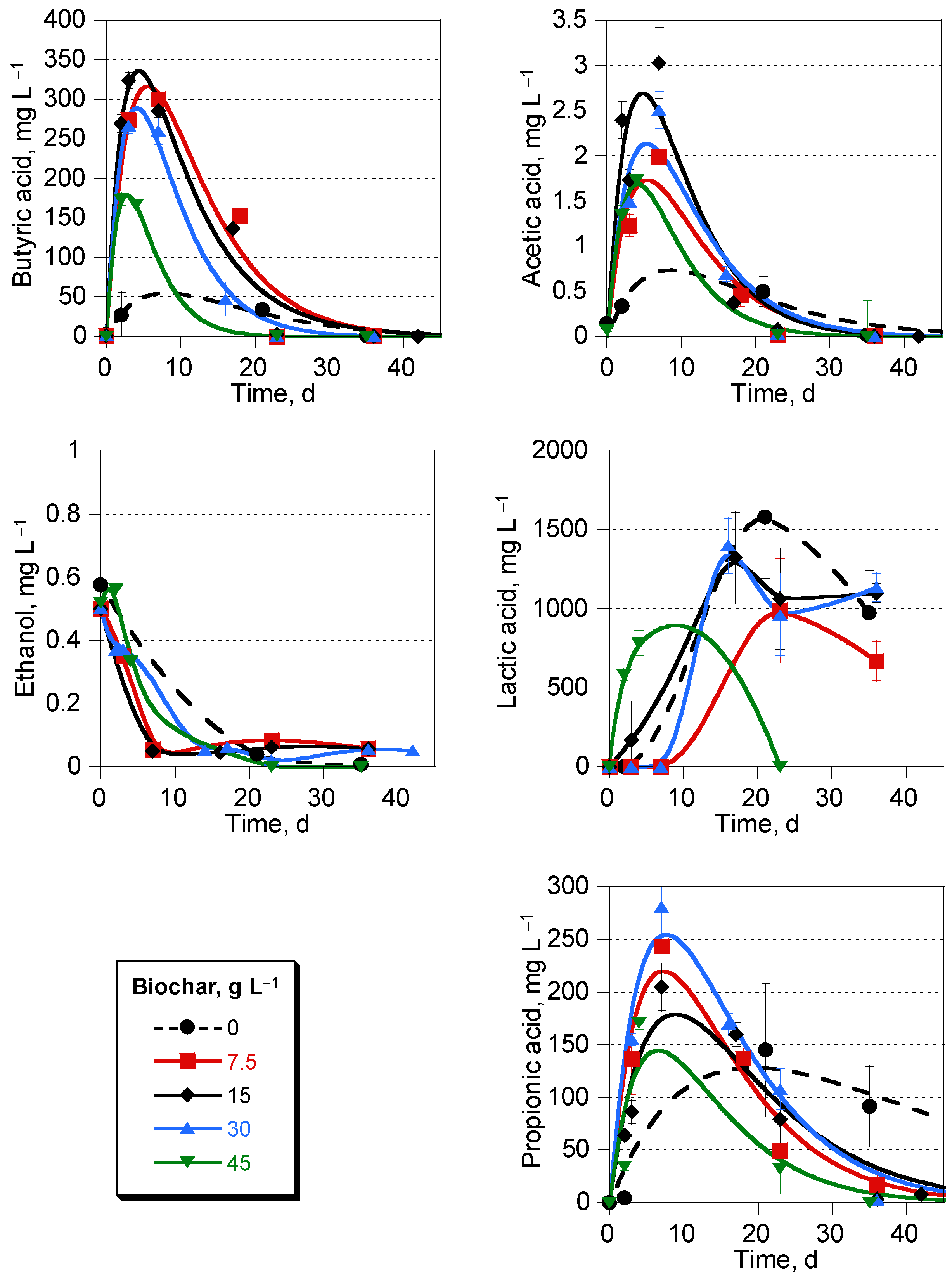
3.3. Volatile Acid Trends during Digestion
3.4. Digestate Characterization
4. Discussion
- Adsorption of polyphenolic compounds. It has been hypothesized that adsorption by biochar is involved in the attenuation of the phenol inhibition of methanogenesis [45].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Magdich, S.; Ahmed, C.B.; Jarboui, R.; Rouina, B.B.; Boukhris, M.; Ammar, E. Dose and Frequency Dependent Effects of Olive Mill Wastewater Treatment on the Chemical and Microbial Properties of Soil. Chemosphere 2013, 93, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- McNamara, C.J.; Anastasiou, C.C.; O’Flaherty, V.; Mitchell, R. Bioremediation of Olive Mill Wastewater. Int. Biodeterior. Biodegrad. 2008, 61, 127–134. [Google Scholar] [CrossRef]
- Paredes, C.; Cegarra, J.; Roig, A.; Sanchez-Monedero, M.A.; Bernal, M.P. Characterization of Olive Mill Wastewater (Alpechin) and Its Sludge for Agricultural Purposes. Bioresour. Technol. 1999, 67, 111–115. [Google Scholar] [CrossRef]
- Ioannou-Ttofa, L.; Michael-Kordatou, I.; Fattas, S.C.; Eusebio, A.; Ribeiro, B.; Rusan, M.; Amer, A.R.B.; Zuraiqi, S.; Waismand, M.; Linder, C.; et al. Treatment Efficiency and Economic Feasibility of Biological Oxidation, Membrane Filtration and Separation Processes, and Advanced Oxidation for the Purification and Valorization of Olive Mill Wastewater. Water Res. 2017, 114, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Morillo, J.A.; Antizar-Ladislao, B.; Monteoliva-Sánchez, M.; Ramos-Cormenzana, A.; Russell, N.J. Bioremediation and Biovalorisation of Olive-Mill Wastes. Appl. Microbiol. Biotechnol. 2009, 82, 25–39. [Google Scholar] [CrossRef]
- Greco, G.; Colarieti, M.L.; Toscano, G.; Iamarino, G.; Rao, M.A.; Gianfreda, L. Mitigation of Olive Mill Wastewater Toxicity. J. Agric. Food Chem. 2006, 54, 6776–6782. [Google Scholar] [CrossRef]
- Hamdi, M. Toxicity and Biodegradability of Olive Mill Wastewaters in Batch Anaerobic Digestion. Appl. Biochem. Biotechnol. 1992, 37, 155–163. [Google Scholar] [CrossRef]
- Gonçalves, M.R.; Costa, J.C.; Pereira, M.A.; Abreu, A.A.; Alves, M.M. On the Independence of Hydrogen Production from Methanogenic Suppressor in Olive Mill Wastewater. Int. J. Hydrog. Energy 2014, 39, 6402–6406. [Google Scholar] [CrossRef] [Green Version]
- Calabrò, P.S.; Fòlino, A.; Tamburino, V.; Zappia, G.; Zema, D.A. Increasing the Tolerance to Polyphenols of the Anaerobic Digestion of Olive Wastewater through Microbial Adaptation. Biosyst. Eng. 2018, 172, 19–28. [Google Scholar] [CrossRef]
- Borja, R.; Alba, J.; Banks, C.J. Impact of the Main Phenolic Compounds of Olive Mill Wastewater (OMW) on the Kinetics of Acetoclastic Methanogenesis. Process Biochem. 1997, 32, 121–133. [Google Scholar] [CrossRef]
- Tabatabaei, M.; Rahim, R.A.; Abdullah, N.; Wright, A.-D.G.; Shirai, Y.; Sakai, K.; Sulaiman, A.; Hassan, M.A. Importance of the Methanogenic Archaea Populations in Anaerobic Wastewater Treatments. Process Biochem. 2010, 45, 1214–1225. [Google Scholar] [CrossRef]
- Gunay, A.; Karadag, D. Recent Developments in the Anaerobic Digestion of Olive Mill Effluents. Process Biochem. 2015, 50, 1893–1903. [Google Scholar] [CrossRef]
- Gonçalves, M.R.; Freitas, P.; Marques, I.P. Bioenergy Recovery from Olive Mill Effluent in a Hybrid Reactor. Biomass Bioenergy 2012, 39, 253–260. [Google Scholar] [CrossRef] [Green Version]
- González-González, A.; Cuadros, F. Effect of Aerobic Pretreatment on Anaerobic Digestion of Olive Mill Wastewater (OMWW): An Ecoefficient Treatment. Food Bioprod. Process. 2015, 95, 339–345. [Google Scholar] [CrossRef]
- Borja, R.; Martín, A.; Alonso, V.; García, I.; Banks, C.J. Influence of Different Aerobic Pretreatments on the Kinetics of Anaerobic Digestion of Olive Mill Wastewater. Water Res 1995, 29, 489–495. [Google Scholar] [CrossRef]
- Azbar, N.; Keskin, T.; Yuruyen, A. Enhancement of Biogas Production from Olive Mill Effluent (OME) by Co-Digestion. Biomass Bioenergy 2008, 32, 1195–1201. [Google Scholar] [CrossRef]
- Mantzavinos, D.; Kalogerakis, N. Treatment of Olive Mill Effluents: Part I. Organic Matter Degradation by Chemical and Biological Processes–An Overview. Environ. Int. 2005, 31, 289–295. [Google Scholar] [CrossRef]
- Ye, M.; Liu, J.; Ma, C.; Li, Y.-Y.; Zou, L.; Qian, G.; Xu, Z.P. Improving the Stability and Efficiency of Anaerobic Digestion of Food Waste Using Additives: A Critical Review. J. Clean. Prod. 2018, 192, 316–326. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: London, UK; Routledge: New York, NY, USA, 2015; ISBN 978-1-134-48953-4. [Google Scholar]
- Zhang, J.; Zhao, W.; Zhang, H.; Wang, Z.; Fan, C.; Zang, L. Recent Achievements in Enhancing Anaerobic Digestion with Carbon-Based Functional Materials. Bioresour. Technol. 2018, 266, 555–567. [Google Scholar] [CrossRef]
- Fagbohungbe, M.O.; Herbert, B.M.J.; Hurst, L.; Ibeto, C.N.; Li, H.; Usmani, S.Q.; Semple, K.T. The Challenges of Anaerobic Digestion and the Role of Biochar in Optimizing Anaerobic Digestion. Waste Manag. 2017, 61, 236–249. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Ai, J.; Shen, F.; Yang, G.; Zhang, Y.; Deng, S.; Zhang, J.; Zeng, Y.; Song, C. Improving Anaerobic Digestion of Easy-Acidification Substrates by Promoting Buffering Capacity Using Biochar Derived from Vermicompost. Bioresour. Technol. 2017, 227, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Liu, F.; Fan, W.; Nevin, K.P.; Lovley, D.R. Promoting Interspecies Electron Transfer with Biochar. Sci. Rep. 2014, 4, 5019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, C.; Lü, F.; Shao, L.; He, P. Application of Eco-Compatible Biochar in Anaerobic Digestion to Relieve Acid Stress and Promote the Selective Colonization of Functional Microbes. Water Res. 2015, 68, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Holmes, D.E.; Dang, Y.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Potential Enhancement of Direct Interspecies Electron Transfer for Syntrophic Metabolism of Propionate and Butyrate with Biochar in Up-Flow Anaerobic Sludge Blanket Reactors. Bioresour. Technol. 2016, 209, 148–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; de Wilde, V.; et al. Towards a Standardization of Biomethane Potential Tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef]
- APAT. Metodi Analitici Delle Acque; Agenzia per la Protezione dell’Ambiente e per i Servizi Tecnici (APAT): Roma, Italy, 2003; ISBN 88-448-0083-7. [Google Scholar]
- De Pasquale, C.; Marsala, V.; Berns, A.E.; Valagussa, M.; Pozzi, A.; Alonzo, G.; Conte, P. Fast Field Cycling NMR Relaxometry Characterization of Biochars Obtained from an Industrial Thermochemical Process. J. Soils Sediments 2012, 12, 1211–1221. [Google Scholar] [CrossRef]
- Wiedner, K.; Rumpel, C.; Steiner, C.; Pozzi, A.; Maas, R.; Glaser, B. Chemical Evaluation of Chars Produced by Thermochemical Conversion (Gasification, Pyrolysis and Hydrothermal Carbonization) of Agro-Industrial Biomass on a Commercial Scale. Biomass Bioenergy 2013, 59, 264–278. [Google Scholar] [CrossRef]
- Rao, M.A.; Simeone, G.D.R.; Scelza, R.; Conte, P. Biochar Based Remediation of Water and Soil Contaminated by Phenanthrene and Pentachlorophenol. Chemosphere 2017, 186, 193–201. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L.; Eddy, M.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse, 4th ed.; McGraw-Hill: New York, NY, USA, 2004; ISBN 0-07-124140-X. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [Green Version]
- Ruggeri, B.; Tommasi, T.; Sanfilippo, S. BioH₂ & BioCH₄ Through Anaerobic Digestion. In Green Energy and Technology; Springer: London, UK, 2015; ISBN 978-1-4471-6431-9. [Google Scholar]
- Baty, F.; Ritz, C.; Charles, S.; Brutsche, M.; Flandrois, J.-P.; Delignette-Muller, M.-L. A Toolbox for Nonlinear Regression in R: The Package Nlstools. J. Stat. Softw. 2015, 66, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Bundhoo, M.A.Z.; Mohee, R. Inhibition of Dark Fermentative Bio-Hydrogen Production: A Review. Int. J. Hydrog. Energy 2016, 41, 6713–6733. [Google Scholar] [CrossRef]
- Cai, J.; He, P.; Wang, Y.; Shao, L.; Lü, F. Effects and Optimization of the Use of Biochar in Anaerobic Digestion of Food Wastes. Waste Manag. Res. 2016, 34, 409–416. [Google Scholar] [CrossRef]
- Sunyoto, N.M.S.; Zhu, M.; Zhang, Z.; Zhang, D. Effect of Biochar Addition on Hydrogen and Methane Production in Two-Phase Anaerobic Digestion of Aqueous Carbohydrates Food Waste. Bioresour. Technol. 2016, 219, 29–36. [Google Scholar] [CrossRef]
- Wang, G.; Li, Q.; Gao, X.; Wang, X.C. Synergetic Promotion of Syntrophic Methane Production from Anaerobic Digestion of Complex Organic Wastes by Biochar: Performance and Associated Mechanisms. Bioresour. Technol. 2018, 250, 812–820. [Google Scholar] [CrossRef]
- Micoli, L.; Di Rauso Simeone, G.; Turco, M.; Toscano, G.; Rao, M.A. Anaerobic Digestion of Olive Miil Wastewater after Biochar Pretreatment. Chem. Eng. Trans. 2023. submitted. [Google Scholar]
- Braghiroli, F.L.; Bouafif, H.; Hamza, N.; Neculita, C.M.; Koubaa, A. Production, Characterization, and Potential of Activated Biochar as Adsorbent for Phenolic Compounds from Leachates in a Lumber Industry Site. Environ. Sci. Pollut. Res. 2018, 25, 26562–26575. [Google Scholar] [CrossRef]
- Kasozi, G.N.; Zimmerman, A.R.; Nkedi-Kizza, P.; Gao, B. Catechol and Humic Acid Sorption onto a Range of Laboratory-Produced Black Carbons (Biochars). Environ. Sci. Technol. 2010, 44, 6189–6195. [Google Scholar] [CrossRef]
- Komnitsas, K.A.; Zaharaki, D. Morphology of Modified Biochar and Its Potential for Phenol Removal from Aqueous Solutions. Front. Environ. Sci. 2016, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Di Rauso Simeone, G.; Cesarano, G.; Micoli, L.; Toscano, G.; Turco, M.; Rao, M.A. Chemical and Biochemical Changes in Soil after Biochar-Treated OMW Digestate Amendment. In Proceedings of the EGU General Assembly 2021, online, 19–30 April 2021. EGU21-10407. [Google Scholar]
- Wang, G.; Gao, X.; Li, Q.; Zhao, H.; Liu, Y.; Wang, X.C.; Chen, R. Redox-Based Electron Exchange Capacity of Biowaste-Derived Biochar Accelerates Syntrophic Phenol Oxidation for Methanogenesis via Direct Interspecies Electron Transfer. J. Hazard. Mater. 2020, 390, 121726. [Google Scholar] [CrossRef]



| Properties | OMW | Inoculum |
|---|---|---|
| pH | 5.00 ± 0.03 | 7.60 ± 0.01 |
| EC, mS cm–1 | 9.30 ± 0.04 | 12.9 ± 0.12 |
| Dry matter, % | 8.0 ± 0.2 | 6.0 ± 0.1 |
| Organic C, g kg−1 | 55.0 ± 0.8 | 22.1 ± 3.0 |
| TKN, g N L−1 | 0.82 ± 0.01 | 2.5 ± 0.2 |
| C/N | 67.4 | 8.7 |
| tCOD a, g L− | 98.6 ± 0.7 | 22.5 ± 0.7 |
| sCOD b, g L−1 | 91.7 ± 0.5 | 1.15 ± 0.07 |
| BOD5, g L−1 | 65.0 ± 0.5 | 2.0 ± 0.3 |
| TPC, g L−1 | 3.3 ± 0.1 | 0.20 ± 0.02 |
| Batch Label | Biochar (g L−1) | H2 (L L−1) | CH4 (L L−1) | COD Removal (g L−1) |
|---|---|---|---|---|
| OMW-0-R1 | 0 | 0.32 | 2.55 | 7.52 |
| OMW-0-R2 | 0 | 0.28 | 2.60 | 7.64 |
| OMW-7.5-R2 | 7.5 | 0.39 | 2.59 | 7.67 |
| OMW-7.5-R3 | 7.5 | 0.57 | 2.84 | 8.53 |
| OMW-15-R4 | 15 | 0.38 | 3.15 | 9.27 |
| OMW-15-R5 | 15 | 0.44 | 2.56 | 7.62 |
| OMW-30-R6 | 30 | 0.35 | 2.59 | 7.66 |
| OMW-30-R7 | 30 | 0.27 | 2.12 | 6.23 |
| OMW-45-R3 | 45 | 0.36 | 2.78 | 8.19 |
| OMW-45-R4 | 45 | 0.41 | 2.39 | 7.12 |
| Mean ± standard deviation | 0.38 ± 0.09 | 2.62 ± 0.27 | 7.74 ± 0.81 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micoli, L.; Di Rauso Simeone, G.; Turco, M.; Toscano, G.; Rao, M.A. Anaerobic Digestion of Olive Mill Wastewater in the Presence of Biochar. Energies 2023, 16, 3259. https://doi.org/10.3390/en16073259
Micoli L, Di Rauso Simeone G, Turco M, Toscano G, Rao MA. Anaerobic Digestion of Olive Mill Wastewater in the Presence of Biochar. Energies. 2023; 16(7):3259. https://doi.org/10.3390/en16073259
Chicago/Turabian StyleMicoli, Luca, Giuseppe Di Rauso Simeone, Maria Turco, Giuseppe Toscano, and Maria A. Rao. 2023. "Anaerobic Digestion of Olive Mill Wastewater in the Presence of Biochar" Energies 16, no. 7: 3259. https://doi.org/10.3390/en16073259
APA StyleMicoli, L., Di Rauso Simeone, G., Turco, M., Toscano, G., & Rao, M. A. (2023). Anaerobic Digestion of Olive Mill Wastewater in the Presence of Biochar. Energies, 16(7), 3259. https://doi.org/10.3390/en16073259






