Improving the Bio-Oil Quality of Residual Biomass Pyrolysis by Chemical Activation: Effect of Alkalis and Acid Pre-Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Methodology
2.2. Materials
2.3. Pre-Treatnent of Açaí Seeds
2.3.1. Physical Pre-Treatment of Açaí Seeds
2.3.2. Chemical Activation of Açaí Seeds
2.4. Experimental Apparatus and Procedures
2.4.1. Experimental Apparatus
2.4.2. Experimental Procedures
2.5. Physical-Chemistry Analysis and Chemical Composition of Bio-Oils and Aqueous Phase
2.5.1. Physical-Chemistry Analysis of Bio-Oils and Aqueous Phase
2.5.2. Chemical Composition of Bio-Oils and Aqueous Phase
GC-MS Analysis
FT-IR Analysis
2.6. Characterization of Bio-Char
XRD Analysis
2.7. Mass Balances by Pyrolysis of Açaí Seeds
3. Results
3.1. Characterization of Bio-Char
3.1.1. XRD Analysis
Effect of KOH Activation
Effect of HCl Activation
3.2. Pyrolysis of Activated Açaí Seeds
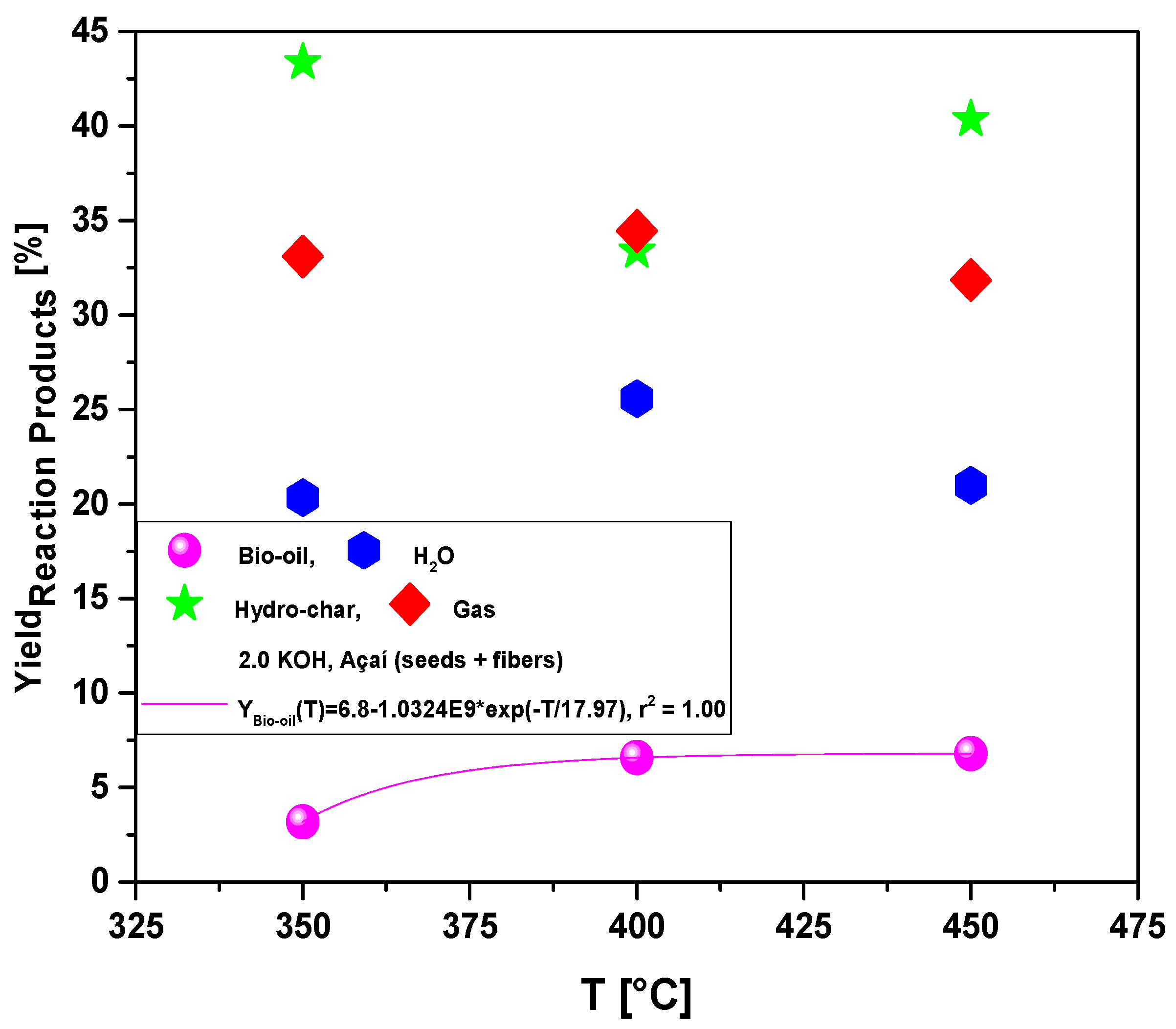
3.2.1. Process Conditions, Mass Balances, and Yields of Reaction Products by Pyrolysis of Activated Açaí Seeds with KOH
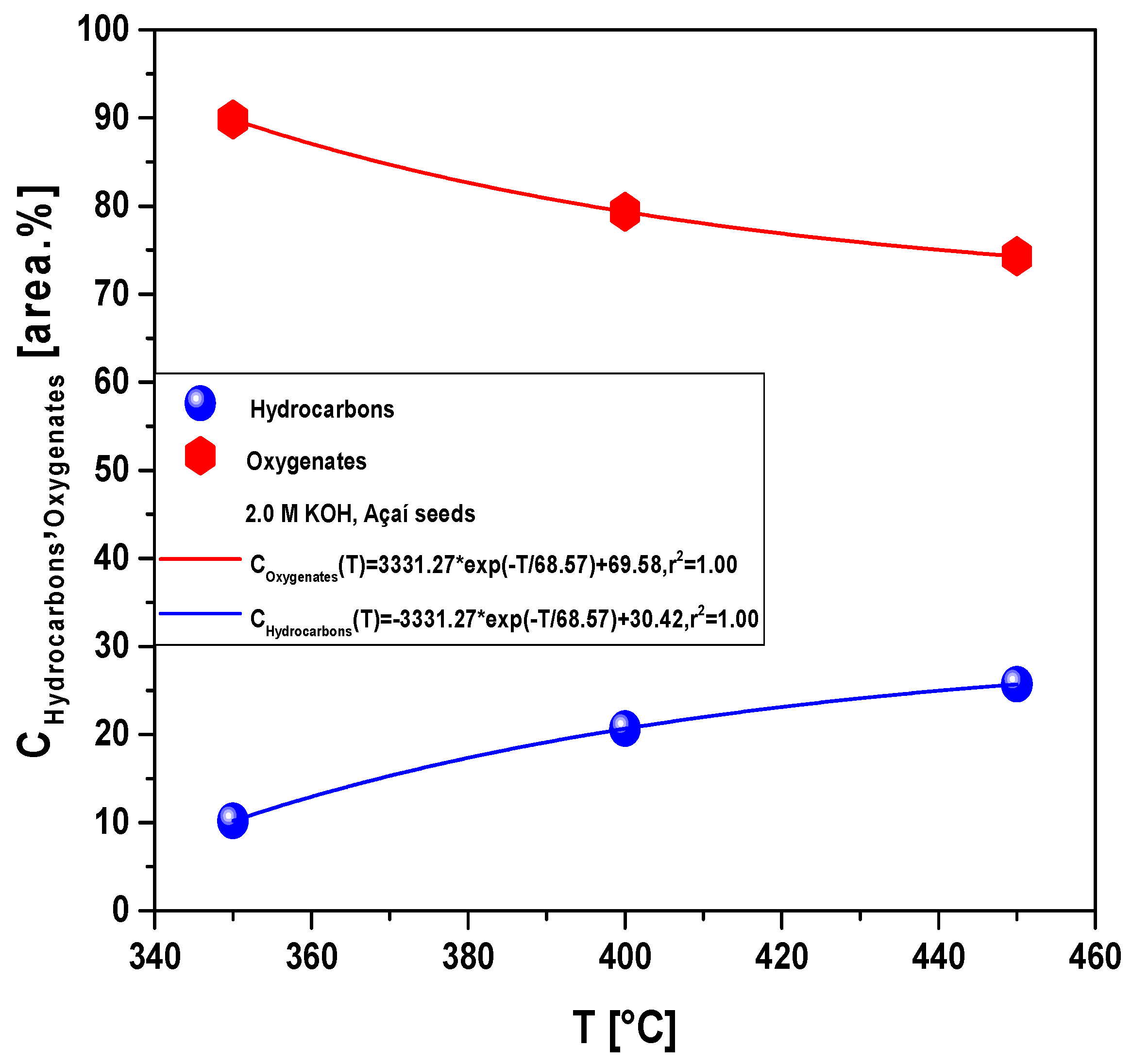
Effect of Temperature on the Composition of Hydrocarbons and Oxygenates in Bio-Oil
Effect of Temperature on the Composition of Hydrocarbons and Oxygenates in the Aqueous Phase
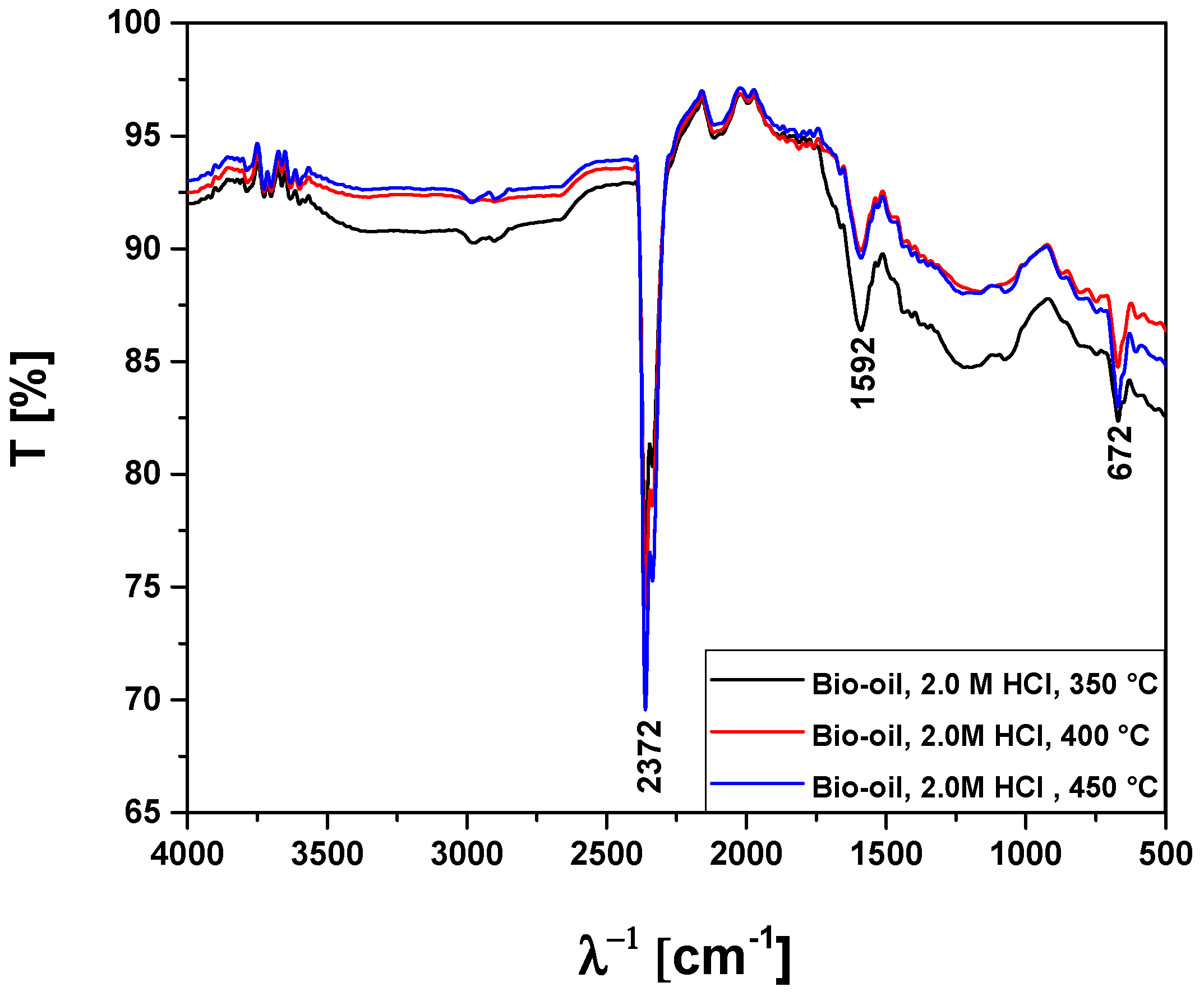
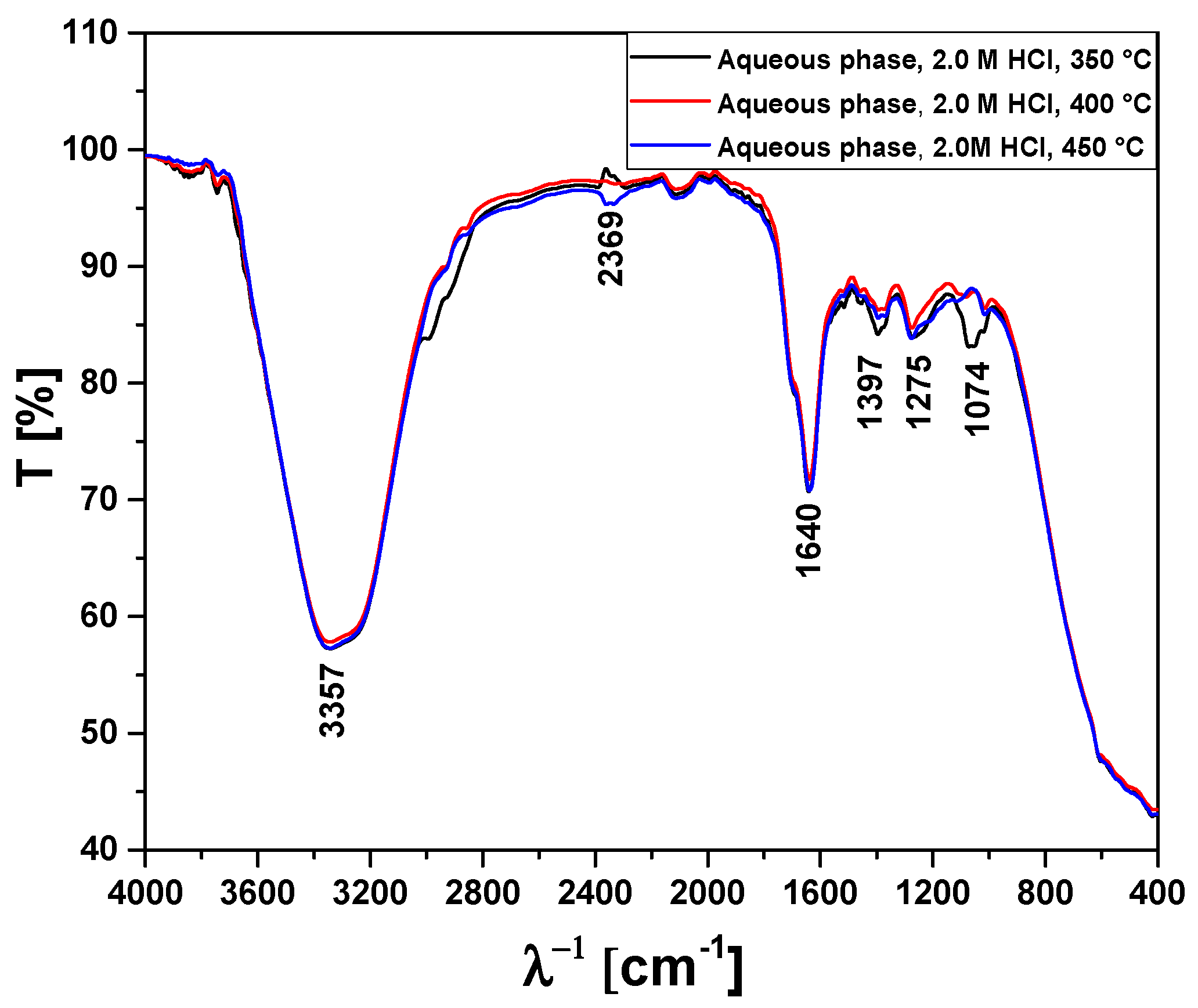
3.2.2. Process Conditions, Mass Balances, and Yields of Reaction Products by Pyrolysis of Activated Açaí Seeds with HCl
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mia, S.; Uddin, E.; Kader, A.; Ahsan, A.; Mannan, M.; Hossain, M.M.; Solaiman, Z.M. Pyrolysis and co-composting of municipal organic waste in Bangladesh: A quantitative estimate of recyclable nutrients, greenhouse gas emissions, and economic benefits. Waste Manag. 2018, 75, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Pompeu, D.; Silva, E.; Rogez, H. Optimisation of the solvent extraction of phenolic antioxidants from fruits of Euterpe oleracea using Response Surface Methodology. Bioresour. Technol. 2009, 100, 6076–6082. [Google Scholar] [CrossRef] [PubMed]
- Ilyushin, Y.V.; Fetisov, V. Experience of virtual commissioning of a process control system for the production of high-paraffin oil. Sci. Rep. 2022, 12, 18415. [Google Scholar] [CrossRef] [PubMed]
- Eremeeva, A.M.; Kondrasheva, N.K.; Khasanov, A.F.; Oleynik, I.L. Environmentally Friendly Diesel Fuel Obtained from Vegetable Raw Materials and Hydrocarbon Crude. Energies 2023, 16, 2121. [Google Scholar] [CrossRef]
- Heinrich, M.; Dhanji, T.; Casselman, I. Açai (Euterpe oleracea Mart.)—A phytochemical and phar-macological assessment of the species’ health claims. Phytochem. Lett. 2011, 4, 10–21. [Google Scholar] [CrossRef]
- Sabbe, S.; Verbeke, W.; Deliza, R.; Matta, V.; Van Damme, P. Effect of a health claim and personal charac-teristics on consumer acceptance of fruit juices with different concentrations of açaí (Euterpe oleracea Mart.). Appetite 2009, 53, 84–92. [Google Scholar] [CrossRef]
- Del Pozo-Insfran, D.; Percival, S.S.; Talcott, S.T. Açaí (Euterpe oleracea Mart.) Poly-phenolics in Their Glycoside and Aglycone Forms Induce Apoptosis of HL-60 Leukemia Cells. J. Agric. Food Chem. 2006, 54, 1222–1229. [Google Scholar] [CrossRef]
- de Castro, D.A.R.; da Silva Ribeiro, H.J.; Ferreira, C.C.; de Andrade Cordeiro, M.; Guerreiro, L.H.; Pereira, A.M.; Dos Santos, W.G.; Santos, M.C.; de Carvalho, F.B.; Junior, J.O.; et al. Fractional Distillation of Bio-Oil Produced by Pyrolysis of Açaí (Euterpe oleracea) Seeds. In Fractionation; Al-Haj Ibrahim, H., Ed.; Intechopen: London, UK, 2019; ISBN 978-1-78984-965-3. [Google Scholar] [CrossRef]
- Guerreiro, L.H.H.; Baia, A.C.F.; Assunção, F.P.D.C.; Rodrigues, G.D.O.; e Oliveira, R.L.; Junior, S.D.; Pereira, A.M.; de Sousa, E.M.P.; Machado, N.T.; de Castro, D.A.R.; et al. Investigation of the Adsorption Process of Biochar Açaí (Euterpea olerácea Mart.) Seeds Produced by Pyrolysis. Energies 2022, 15, 6234. [Google Scholar] [CrossRef]
- Bufalino, L.; Guimaraes, A.A.; de Silva, B.M.; de Souza, R.L.F.; de Melo, I.C.N.A.; de Oliveira, D.N.P.S.; Trugilho, P.F. Local variability of yield and physical properties of açaí waste and improvement of its energetic attributes by separation of lignocel-lulosic fibers and seeds. J. Renew. Sustain. Energy 2018, 10, 053102. [Google Scholar] [CrossRef]
- de Castro, D.R.; Ribeiro, H.D.S.; Guerreiro, L.H.; Bernar, L.P.; Bremer, S.J.; Santo, M.C.; Almeida, H.D.S.; Duvoisin, S.; Borges, L.P.; Machado, N.T. Production of Fuel-Like Fractions by Fractional Distillation of Bio-Oil from Açaí (Euterpe oleracea Mart.) Seeds Pyrolysis. Energies 2021, 14, 3713. [Google Scholar] [CrossRef]
- Silva, C.D.M.S.D.; de Castro, D.A.R.; Santos, M.C.; Almeida, H.D.S.; Schultze, M.; Lüder, U.; Hoffmann, T.; Machado, N.T. Process Analysis of Main Organic Compounds Dissolved in Aqueous Phase by Hydrothermal Processing of Açaí (Euterpe oleraceae, Mart.) Seeds: Influence of Process Temperature, Biomass-to-Water Ratio, and Production Scales. Energies 2021, 14, 5608. [Google Scholar] [CrossRef]
- de Lima, A.C.P.; Bastos, D.L.R.; Camarena, M.A.; Bon, E.P.S.; Cammarota, M.C.; Teixeira, R.S.S.; Gutarra, M.L.E. Physicochemical characterization of residual biomass (seed and fiber) from açaí (Euterpe oleracea) processing and assessment of the potential for energy production and bioproducts. Biomass-Convers. Biorefinery 2021, 11, 925–935. [Google Scholar] [CrossRef]
- Ferdinand, F.W.; Van de Steene, L.; Blaise, K.K.; Siaka, T. Prediction of pyrolysis oils higher heating value with gas chromatography–mass spectrometry. Fuel 2012, 96, 141–145. [Google Scholar] [CrossRef]
- Bernar, L.P.; Ferreira, C.C.; Costa, A.F.d.F.; Ribeiro, H.J.D.S.; dos Santos, W.G.; Pereira, L.M.; Pereira, A.M.; Moraes, N.L.; Assunção, F.P.D.C.; da Mota, S.A.P.; et al. Catalytic Upgrading of Residual Fat Pyrolysis Vapors over Activated Carbon Pellets into Hydrocarbons-like Fuels in a Two-Stage Reactor: Analysis of Hydrocarbons Composition and Physical-Chemistry Properties. Energies 2022, 15, 4587. [Google Scholar] [CrossRef]
- Ferreira, C.C.; Bernar, L.P.; Costa, A.F.d.F.; Ribeiro, H.J.D.S.; Santos, M.C.; Moraes, N.L.; Costa, Y.S.; Baia, A.C.F.; Mendonça, N.M.; da Mota, S.A.P.; et al. Improving Fuel Properties and Hydrocarbon Content from Residual Fat Pyrolysis Vapors over Activated Red Mud Pellets in Two-Stage Reactor: Optimization of Reaction Time and Catalyst Content. Energies 2022, 15, 5595. [Google Scholar] [CrossRef]
- Sato, M.K.; de Lima, H.V.; Costa, A.N.; Rodrigues, S.; Pedroso, A.J.S.; de Freitas Maia, C.M.B. Biochar from Acai agroindustry waste: Study of pyrolysis conditions. Waste Manag. 2019, 96, 158–167. [Google Scholar] [CrossRef]
- Sato, M.K.; de Lima, H.V.; Costa, A.N.; Rodrigues, S.; Mooney, S.J.; Clarke, M.; Pedroso, A.J.; de Freitas Maia, C.M. Biochar as a sustainable alternative to ac¸ aí waste disposal in Amazon, Brazil. Process Saf. Environ. Prot. 2020, 139, 36–46. [Google Scholar] [CrossRef]
- Ortiz, L.R.; Torres, E.; Zalazar, D.; Zhang, H.; Rodriguez, R.; Mazza, G. Influence of pyrolysis temperature and bio-waste composition on biochar characteristics. Renew. Energy 2020, 155, 837–847. [Google Scholar] [CrossRef]
- Queiroz, L.S.; de Souza, L.K.; Thomaz, K.T.C.; Lima, E.T.L.; Filho, G.N.D.R.; Nascimento, L.A.S.D.; Pires, L.H.D.O.; Faial, K.D.C.F.; da Costa, C.E. Activated carbon obtained from amazonian biomass tailings (acai seed): Modification, characterization, and use for removal of metal ions from water. J. Environ. Manag. 2020, 270, 110868. [Google Scholar] [CrossRef]
- Pessôa, T.S.; Ferreira, L.E.D.L.; da Silva, M.P.; Neto, L.M.P.; Nascimento, B.F.D.; Fraga, T.J.M.; Jaguaribe, E.F.; Cavalcanti, J.V.; Sobrinho, M.A.D.M. Açaí waste beneficing by gasification process and its employment in the treatment of synthetic and raw textile wastewater. J. Clean. Prod. 2019, 240, 118047. [Google Scholar] [CrossRef]
- Zavarize, D.G. Insights on preparation and characteristics of KOH-doped carbons derived from an abundant agroindustrial waste in Brazil: Amazon açaí berry seeds. Bioresour. Technol. Rep. 2020, 13, 100611. [Google Scholar] [CrossRef]
- de Souza, L.K.; Gonçalves, A.A.; Queiroz, L.S.; Chaar, J.S.; Filho, G.N.D.R.; da Costa, C.E. Utilization of acai stone biomass for the sustainable production of nanoporous carbon for CO2 capture. Sustain. Mater. Technol. 2020, 25, e00168. [Google Scholar] [CrossRef]
- de Souza, T.N.V.; Vieira, M.G.A.; da Silva, M.G.C.; Brasil, D.D.S.B.; de Carvalho, S.M.L. H3PO4-activated carbons produced from açai stones and Brazil nut shells: Removal of basic blue 26 dye from aqueous solutions by adsorption. Environ. Sci. Pollut. Res. 2019, 26, 28533–28547. [Google Scholar] [CrossRef] [PubMed]
- Araujo, R.O.; Chaar, J.D.S.; Queiroz, L.S.; Filho, G.N.D.R.; da Costa, C.E.F.; da Silva, G.C.; Landers, R.; Costa, M.J.; Gonçalves, A.A.; de Souza, L.K. Low temperature sulfonation of acai stone biomass derived carbons as acid catalysts for esterification reactions. Energy Convers. Manag. 2019, 196, 821–830. [Google Scholar] [CrossRef]
- de Souza, T.N.V.; de Carvalho, S.M.L.; Vieira, M.G.A.; da Silva, M.G.C.; do Brasil, D.S.B. Adsorption of basic dyes onto activated carbon: Experimental and theoretical investigation of chemical reactivity of basic dyes using DFT-based descriptors. Appl. Surf. Sci. 2018, 448, 662–670. [Google Scholar] [CrossRef]
- Nascimento, B.F.D.; de Araujo, C.M.B.; Nascimento, A.C.D.; da Silva, F.L.H.; de Melo, D.J.N.; Jaguaribe, E.F.; Cavalcanti, J.V.F.L.; Sobrinho, M.A.D.M. Detoxification of sisal bagasse hydrolysate using activated carbon produced from the gasification of açaí waste. J. Hazard. Mater. 2020, 409, 124494. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.A.D.S.; Thim, G.P.; Alvarez-Mendez, M.O.; Coutinho, A.D.R.; de Moraes, N.P.; Rodrigues, L.A. Preparation, characterization, and application of low-cost açaí seed-based activated carbon for phenol adsorption. Int. J. Environ. Res. 2018, 12, 755–764. [Google Scholar] [CrossRef]
- de Souza, L.K.C.; Martins, J.C.; Oliveira, D.P.; Ferreira, C.S.; Gonçalves, A.A.S.; Araujo, R.O.; Chaar, J.D.S.; Costa, M.J.F.; Sampaio, D.V.; Passos, R.R.; et al. Hierarchical porous carbon derived from acai seed biowaste for supercapacitor electrode materials. J. Mater. Sci. Mater. Electron. 2020, 31, 12148–12157. [Google Scholar] [CrossRef]
- de Andrade Cordeiro, M.; de Almeida, O.; de Castro, D.A.R.; da Silva Ribeiro, H.J.; Machado, N.T. Produção de Etanol através da Hidrólise Enzimática do Caroço de Açaí (Euterpe oleracea, Mart.). Rev. Bras. Energ. Renov. 2019, 8, 122–152. [Google Scholar]
- Almeida, H.D.S.; Corrêa, O.; Eid, J.; Ribeiro, H.; de Castro, D.; Pereira, M.; Pereira, L.; Mâncio, A.D.A.; Santos, M.; Souza, J.D.S.; et al. Production of biofuels by thermal catalytic cracking of scum from grease traps in pilot scale. J. Anal. Appl. Pyrolysis 2016, 118, 20–33. [Google Scholar] [CrossRef]
- Almeida, H.D.S.; Corrêa, O.; Eid, J.; Ribeiro, H.; de Castro, D.; Pereira, M.; Pereira, L.; Aâncio, A.D.A.; Santos, M.; da Mota, S.; et al. Performance of thermochemical conversion of fat, oils, and grease into kerosene-like hydrocarbons in different production scales. J. Anal. Appl. Pyrolysis 2016, 120, 126–143. [Google Scholar] [CrossRef]
- Almeida, H.D.S.; Corrêa, O.; Ferreira, C.; Ribeiro, H.; de Castro, D.; Pereira, M.; Mâncio, A.D.A.; Santos, M.; da Mota, S.; Souza, J.D.S.; et al. Diesel-like hydrocarbon fuels by catalytic cracking of fat, oils, and grease (FOG) from grease traps. J. Energy Inst. 2017, 90, 337–354. [Google Scholar] [CrossRef]
- da Mota, S.; Mancio, A.; Lhamas, D.; de Abreu, D.; da Silva, M.; dos Santos, W.; de Castro, D.; de Oliveira, R.; Araújo, M.; Borges, L.E.; et al. Production of green diesel by thermal catalytic cracking of crude palm oil (Elaeis guineensis Jacq) in a pilot plant. J. Anal. Appl. Pyrolysis 2014, 110, 1–11. [Google Scholar] [CrossRef]
- Mancio, A.; da Mota, S.; Ferreira, C.; Carvalho, T.; Neto, O.; Zamian, J.; Araújo, M.; Borges, L.; Machado, N. Separation and characterization of biofuels in the jet fuel and diesel fuel ranges by fractional distillation of organic liquid products. Fuel 2018, 215, 212–225. [Google Scholar] [CrossRef]
- Mâncio, A.; da Costa, K.; Ferreira, C.; Santos, M.; Lhamas, D.; da Mota, S.; Leão, R.; de Souza, R.; Araújo, M.; Borges, L.; et al. Process analysis of physicochemical properties and chemical composition of organic liquid products obtained by thermochemical conversion of palm oil. J. Anal. Appl. Pyrolysis 2017, 123, 284–295. [Google Scholar] [CrossRef]
- Santos, M.C.; Lourenço, R.M.; de Abreu, D.H.; Pereira, A.M.; de Castro, D.A.R.; Pereira, M.S.; Almeida, H.S.; Mâncio, A.A.; Lhamas, D.E.L.; da Mota, S.A.P.; et al. Gasoline-like hydrocar-bons by catalytic cracking of soap phase residue of neutralization process of palm oil (Elaeis guineensis Jacq). J. Taiwan Inst. Chem. Eng. 2017, 71, 106–119. [Google Scholar] [CrossRef]
- Ferreira, C.; Costa, E.; de Castro, D.; Pereira, M.; Mâncio, A.; Santos, M.; Lhamas, D.; da Mota, S.; Leão, A.; Duvoisin, S.; et al. Deacidification of organic liquid products by fractional distillation in laboratory and pilot scales. J. Anal. Appl. Pyrolysis 2017, 127, 468–489. [Google Scholar] [CrossRef]
- Manoj, B.; Kunjomana, A.G. Study of Stacking Structure of Amorphous Carbon by X-Ray Diffraction Technique. Int. J. Elec-trochem. Sci. 2012, 7, 3127–3134. [Google Scholar]
- Prakongkep, N.; Gilkes, R.J.; Wiriyakitnateekul, W. Agronomic benefits of durian shell biochar. J. Met. Mater. Miner. 2014, 24, 7–11. [Google Scholar] [CrossRef]
- Lee, J.H.; Cha, Y.L.; Kang, Y.-M.; Roh, K.C. Study on the reaction mechanism of the potassium bicarbonate alkali activation process in black liquor. APL Mater. 2022, 10, 101105. [Google Scholar] [CrossRef]
- Díaz-Terán, J.; Nevskaia, D.; Fierro, J.; López-Peinado, A.; Jerez, A. Study of chemical activation process of a lignocellulosic material with KOH by XPS and XRD. Microporous Mesoporous Mater. 2003, 60, 173–181. [Google Scholar] [CrossRef]
- Serrão, A.C.M.; Silva, C.M.S.; Assunção, F.P.D.C.; Ribeiro, H.J.D.S.; Santos, M.C.; Almeida, H.D.S.; Junior, S.D.; Borges, L.E.P.; de Castro, D.A.R.; Machado, N.T. Process analysis of pyrolysis of Açaí (Euterpe Oleracea, Mart) seeds: Influence of temperature on the yield of reaction products and physico-chemical properties of Bio-Oil. Braz. J. Dev. 2021, 7, 18200–18220. [Google Scholar] [CrossRef]
- Das, P.; Ganesh, A. Bio-oil from pyrolysis of cashew nutshell—A near fuel. Biomass Bioenergy 2003, 25, 113–117. [Google Scholar] [CrossRef]
- Asadullah, M.; Rahman, M.; Ali, M.; Motin, M.; Sultan, M.; Alam, M. Production of bio-oil from fixed bed pyrolysis of bagasse. Fuel 2007, 86, 2514–2520. [Google Scholar] [CrossRef]
- Zheng, J.-L. Bio-oil from fast pyrolysis of rice husk: Yields and related properties and improvement of the pyrolysis system. J. Anal. Appl. Pyrolysis 2007, 80, 30–35. [Google Scholar] [CrossRef]
- Sukiran, M.A.; Chin, C.M.; Bakar, N.K. Bio-oils from Pyrolysis of Oil Palm Empty Fruit Bunches. Am. J. Appl. Sci. 2009, 6, 869–875. [Google Scholar] [CrossRef]
- Kim, S.-J.; Jung, S.-H.; Kim, J.-S. Fast pyrolysis of palm kernel shells: Influence of operation parameters on the bio-oil yield and the yield of phenol and phenolic compounds. Bioresour. Technol. 2010, 101, 9294–9300. [Google Scholar] [CrossRef]
- Kumar, G.; Panda, A.K.; Singh, R. Optimization of process for the production of bio-oil from eucalyptus wood. J. Fuel Chem. Technol. 2010, 38, 162–167. [Google Scholar] [CrossRef]
- Heo, H.S.; Park, H.J.; Dong, J.I.; Park, S.H.; Kim, S.; Suh, D.J.; Suh, Y.W.; Kim, S.S.; Park, Y.K. Fast pyrolysis of rice husk under different reaction conditions. J. Ind. Eng. Chem. 2010, 16, 27–31. [Google Scholar] [CrossRef]
- Ortega, J.V.; Renehan, A.M.; Liberatore, M.W.; Herring, A. Physical and chemical characteristics of aging pyrolysis oils produced from hardwood and softwood feedstocks. J. Anal. Appl. Pyrolysis 2011, 91, 190–198. [Google Scholar] [CrossRef]
- Sharma, R.; Sheth, P.N. Thermo-Chemical Conversion of Jatropha Deoiled Cake: Pyrolysis vs. Gasification. Int. J. Chem. Eng. Appl. 2015, 6, 376–380. [Google Scholar] [CrossRef]
- Paenpong, C.; Pattiya, A. Effect of pyrolysis and moving-bed granular filter temperatures on the yield and prop-erties of bio-oil from fast pyrolysis of biomass. J. Anal. Appl. Pyrolysis 2016, 119, 40–51. [Google Scholar] [CrossRef]
- Garg, R.; Anand, N.; Kumar, D. Pyrolysis of babool seeds (Acacia nilotica) in a fixed bed reactor and bio-oil char-acterization. Renew. Energy 2016, 96 Pt A, 167–171. [Google Scholar] [CrossRef]
- Varma, A.K.; Mondal, P. Pyrolysis of sugarcane bagasse in semi batch reactor: Effects of process parameters on product yields and characterization of products. Ind. Crop. Prod. 2017, 95, 704–717. [Google Scholar] [CrossRef]
- de Sousa, J.L.; Guerreiro, L.H.H.; Bernar, L.P.; Ribeiro, H.J.D.S.; e Oliveira, R.L.; Santos, M.C.; Almeida, H.D.S.; Junior, S.D.; Borges, L.E.P.; de Castro, D.A.R.; et al. Análise da composição química do bio-óleo produzido via pirólise de sementes de açaí (euterpe oleracea, mart)/chemical analysis of bio-oil produced by pyrolise of açaí (euterpe oleracea, mart) seeds. Braz. J. Dev. 2021, 7, 15549–15565. [Google Scholar] [CrossRef]
- George, K.M.; Ruthenburg, T.C.; Smith, J.; Yu, L.; Zhang, Q.; Anastasio, C.; Dillner, A.M. FT-IR quantifi-cation of the carbonyl functional group in aqueous-phase secondary organic aerosol from phenols. Atmos. Environ. 2015, 100, 230–237. [Google Scholar] [CrossRef]
- Atabani, A.E.; Al-Rubaye, O.K. Valorization of spent coffee grounds for biodiesel production: Blending with higher alcohols, FT-IR, TGA, DSC, and NMR characterizations. Biomass-Convers. Biorefinery 2022, 12, 577–596. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.; Li, T.; Ren, Z. Upgrading of liquid fuel from the pyrolysis of biomass. Bioresour. Technol. 2005, 96, 545–550. [Google Scholar] [CrossRef]
- Torri, C.; Fabbri, D. Biochar enables anaerobic digestion of aqueous phase from intermediate pyrolysis of biomass. Bioresour. Technol. 2014, 172, 335–341. [Google Scholar] [CrossRef]
- Zhou, H.; Brown, R.C.; Wen, Z. Anaerobic digestion of aqueous phase from pyrolysis of biomass: Reducing toxicity and improving microbial tolerance. Bioresour. Technol. 2019, 292, 121976. [Google Scholar] [CrossRef]
- Wang, X.; Leng, S.; Bai, J.; Zhou, H.; Zhong, X.; Zhuang, G.; Wang, J. Role of pretreatment with acid and base on the distribution of the products obtained via lignocellulosic biomass pyrolysis. RSC Adv. 2015, 5, 24984–24989. [Google Scholar] [CrossRef]
- Bru, K.; Blin, J.; Julbe, A.; Volle, G. Pyrolysis of metal impregnated biomass: An innovative catalytic way to produce gas fuel. J. Anal. Appl. Pyrolysis 2007, 78, 291–300. [Google Scholar] [CrossRef]

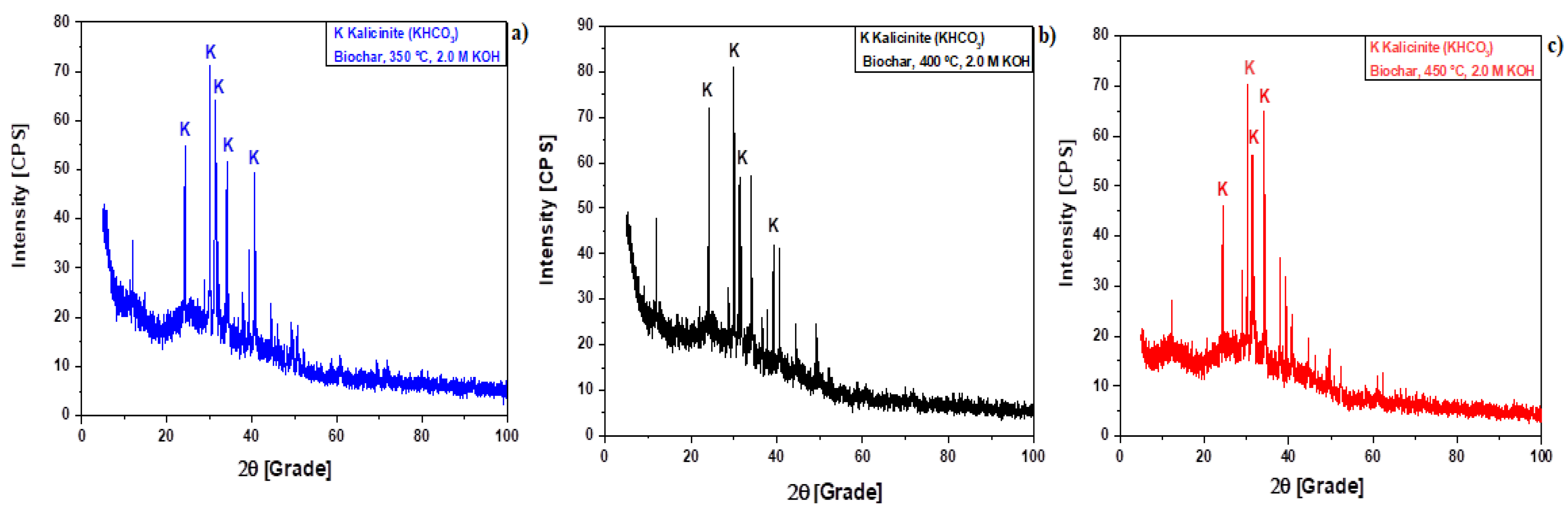
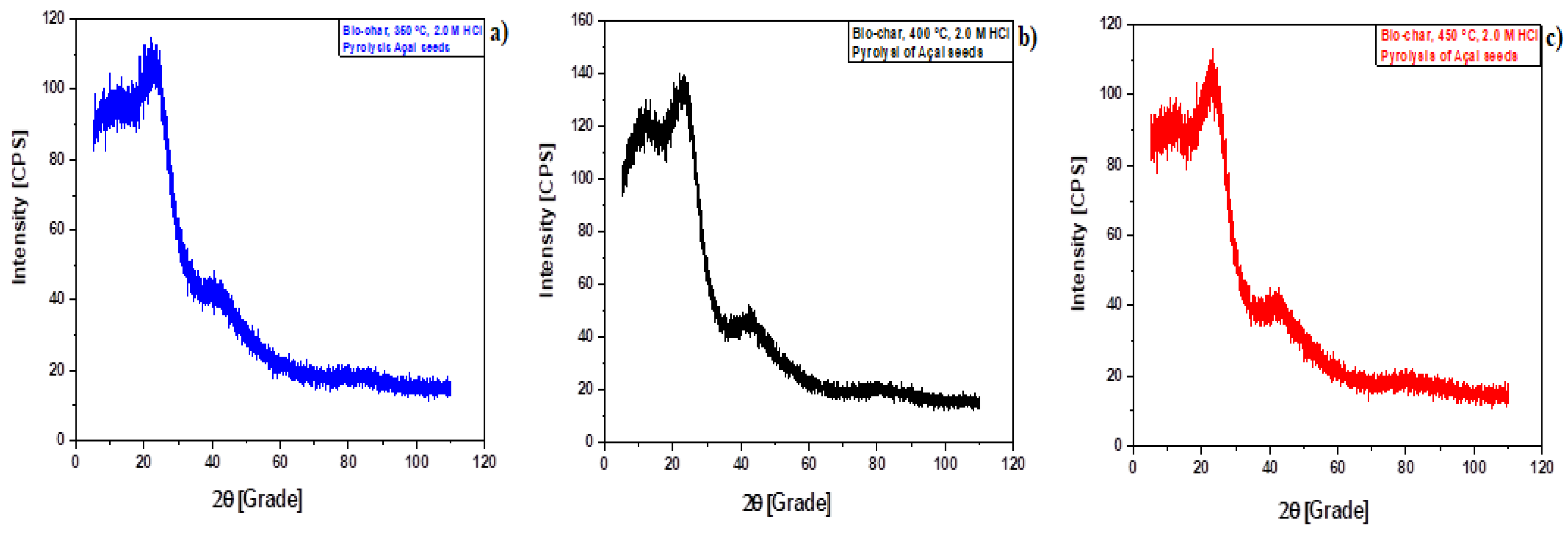
| Temperature | Peaks Intensity, Position (2θ), and Percentage (%) | |||||
|---|---|---|---|---|---|---|
| 350 °C | Medium | Medium | High | |||
| 2θ | (%) | 2θ | (%) | 2θ | (%) | |
| 24.2 | 66.8 | 40.6 | 68.6 | 30.0 | 100 | |
| 400 °C | Medium | High | High | |||
| 2θ | (%) | 2θ | (%) | 2θ | (%) | |
| 31.3 | 62.2 | 24.1 | 81.73 | 30.0 | 100 | |
| 450 °C | High | High | High | |||
| 2θ | (%) | 2θ | (%) | 2θ | (%) | |
| 30.2 | 100.0 | 31.3 | 79.9 | 34.2 | 92.1 | |
| Process Parameters | 2.0 M KOH | ||
|---|---|---|---|
| 350 °C | 400 °C | 450 °C | |
| Mass of Açaí seeds (g) | 40.12 | 40.12 | 40.06 |
| Cracking time (min) | 72 | 72 | 72 |
| Yield of Bio-oil (wt.%) | 3.19 | 6.58 | 6.79 |
| Yield of H2O (wt.%) | 20.34 | 25.57 | 20.99 |
| Yield of Hydro-char (wt.%) | 43.37 | 33.40 | 40.36 |
| Yield of Gas (wt.%) | 33.10 | 34.45 | 31.85 |
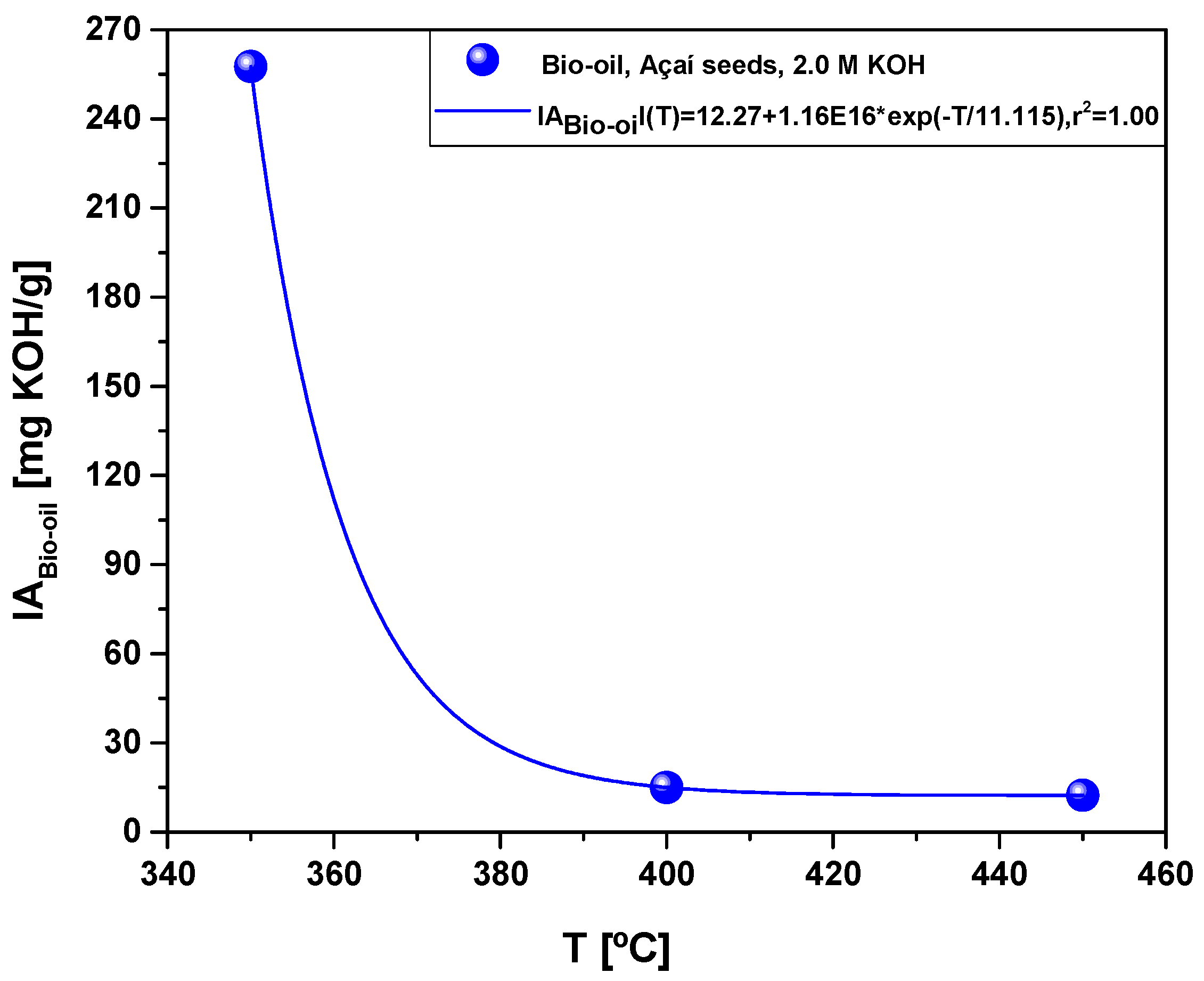
| Acidity (mg KOH/g) | 257.6 | 15.0 | 12.3 |
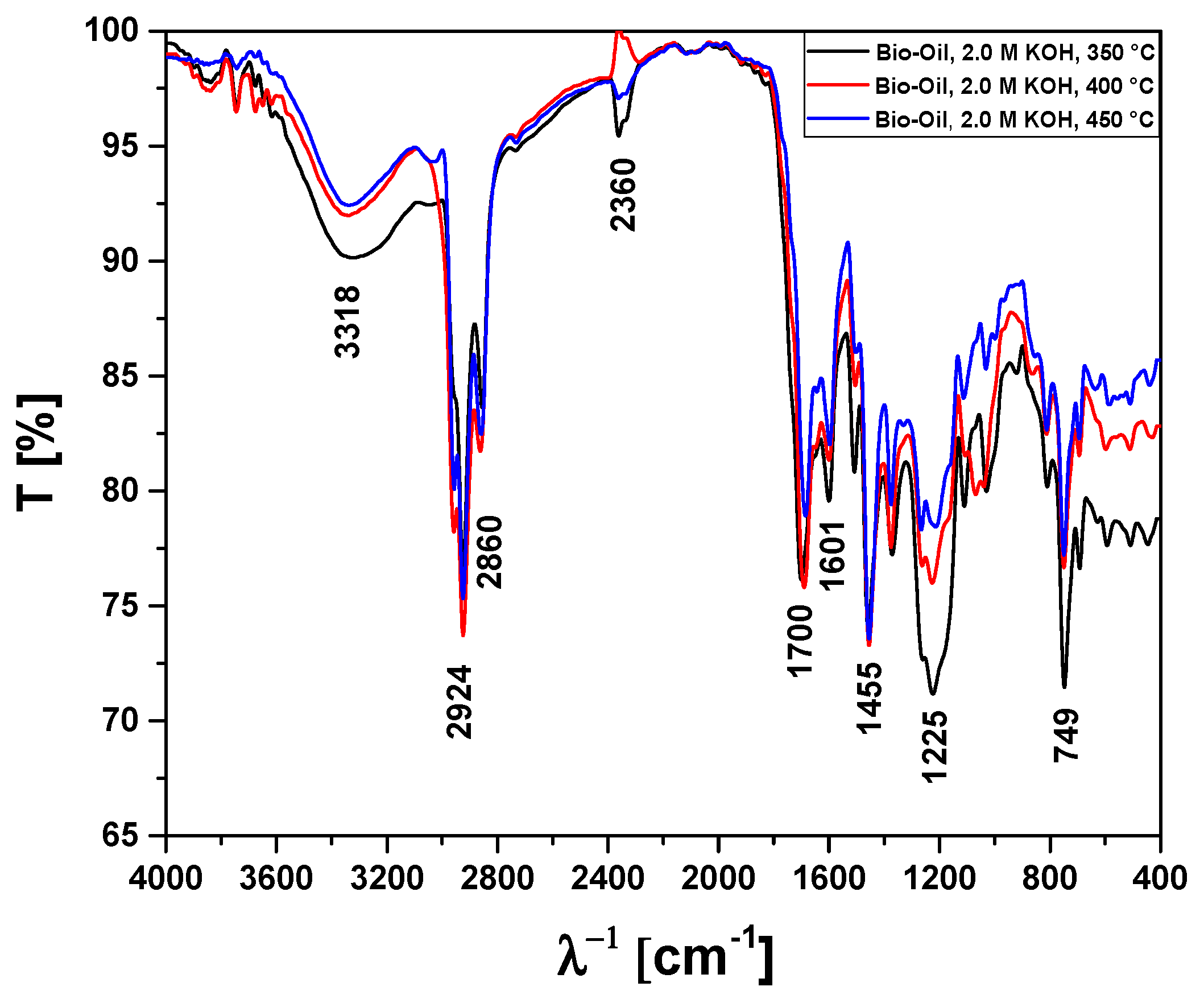
| Absorption Bands | Chemical Functions/Chemical Bonds |
|---|---|
| 3400–3200 cm−1 | ν-OH, hydrogen bonds of alcohol and H2O. |
| 2870–2840 cm−1 | νs-CH2, methylene group CH2. |
| 2930–2920 cm−1 | νas-CH2, methylene group CH2. |
| 1709 cm−1 | ν-C=O, carbonyl group of carboxylic acids and ketones. |
| 1601 cm−1 | νC=C-C, C=C-C ring-related stretching associated to phenols. |
| 1465–1440 cm−1 | δasCH3, methyl group (C-H). |
| 1200–1125 cm−1 | ν-C-O, saturated alcohols (C-O). |
| 1000–650 cm−1 | γ=C-H, Alkenes (=C-H). |
| Chemical Composition Ci (area.%) | 2.0 M KOH | ||
|---|---|---|---|
| 350 °C | 400 °C | 450 °C | |
| Alcohols | 2.34 | 20.74 | 26.62 |
| Carboxylic Acids | 4.05 | 15.02 | 9.23 |
| Ketones | 52.81 | 44.38 | 19.69 |
| Oxygenates | 40.80 | 19.86 | 44.46 |
| 100.00 | 100.00 | 100.00 | |
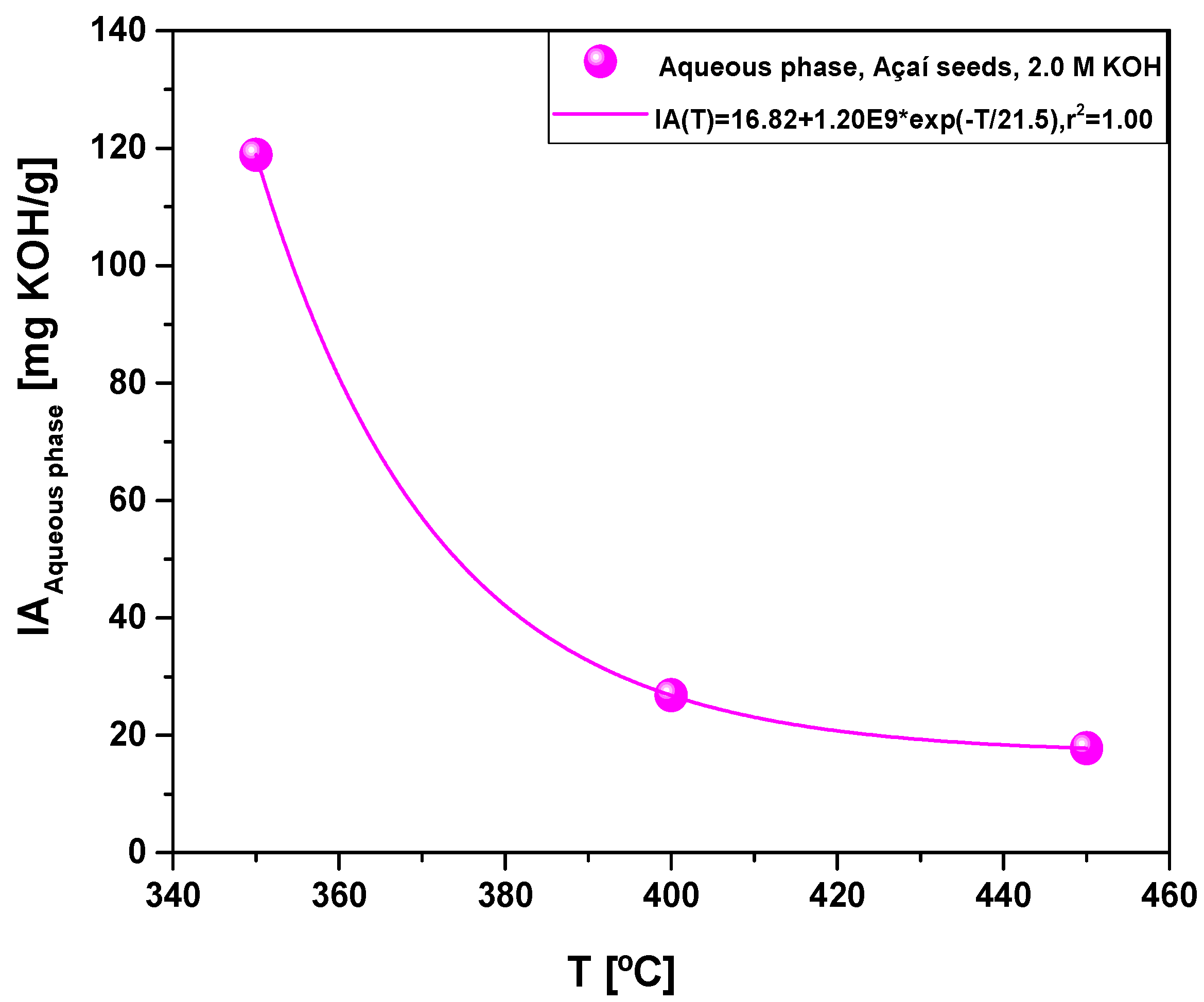
| Acidity (mg KOH/g) | 118.9 | 26.8 | 17.9 |
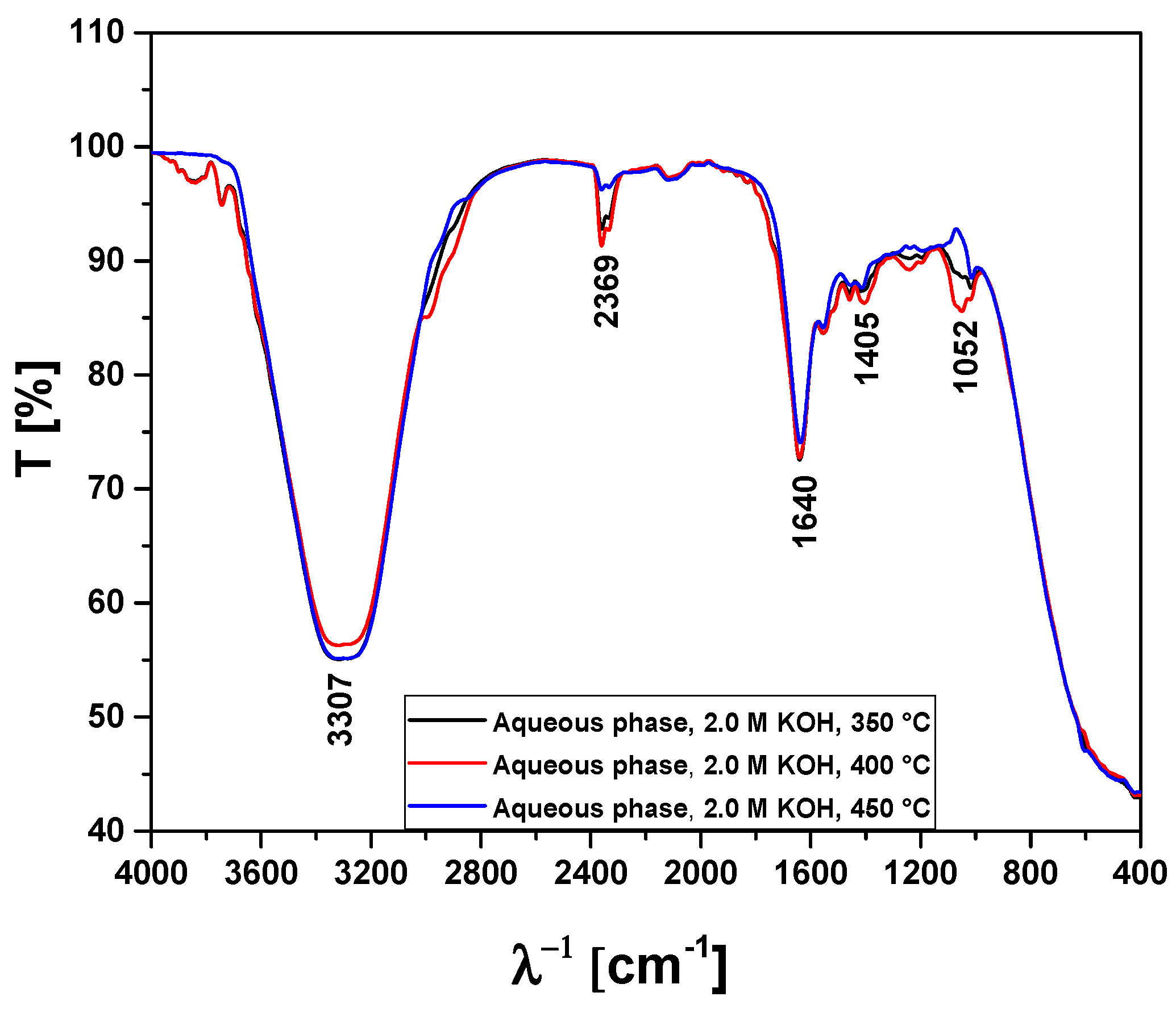
| Absorption Bands | Chemical Functions/Chemical Bonds |
|---|---|
| 3400–3200 cm−1 | ν-OH, hydrogen bond of alcohols and H2O. |
| 2369 cm−1 | νass-CO2, axial asymmetric deformation of CO2. |
| 1648–1636 cm−1 | νC=C-C, C=C-C ring-related stretching associated to phenols. |
| 1052 cm−1 | νC-O-C, νC-O, νC-H, C-O-C bond of esters, C-O bonds, and C-H bonds of benzene rings. |
| Process Parameters. | 2.0 M HCl | ||
|---|---|---|---|
| 350 °C | 400 °C | 450 °C | |
| Mass of Açaí seeds (g) | 42.100 | 40.480 | 40.433 |
| Cracking time (min) | 72 | 72 | 72.0 |
| Yield of Bio-oil (wt.%) | 3.37 | 2.84 | 2.13 |
| Yield of H2O (wt.%) | 31.19 | 32.85 | 22.91 |
| Yield of Hydro-char (wt.%) | 47.53 | 35.08 | 37.32 |
| Yield of Gas (wt.%) | 17.91 | 29.22 | 37.64 |
| Acidity (mg KOH/g) | 127.1 | 128.9 | 218.5 |
| Chemical Composition Ci (area.%) | 2.0 M HCl | ||
|---|---|---|---|
| 350 °C | 400 °C | 450 °C | |
| Carboxylic Acids | 53.056 | 43.540 | 61.175 |
| Phenols | 35.945 | 32.700 | 28.682 |
| Oxygenates (alcohols, aldehydes, ketones, cresols) | 10.999 | 23.860 | 10.143 |
| 100.00 | 100.00 | 100.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniel Valdez, G.; Valois, F.P.; Bremer, S.J.; Bezerra, K.C.A.; Hamoy Guerreiro, L.H.; Santos, M.C.; Bernar, L.P.; Feio, W.P.; Moreira, L.G.S.; Mendonça, N.M.; et al. Improving the Bio-Oil Quality of Residual Biomass Pyrolysis by Chemical Activation: Effect of Alkalis and Acid Pre-Treatment. Energies 2023, 16, 3162. https://doi.org/10.3390/en16073162
Daniel Valdez G, Valois FP, Bremer SJ, Bezerra KCA, Hamoy Guerreiro LH, Santos MC, Bernar LP, Feio WP, Moreira LGS, Mendonça NM, et al. Improving the Bio-Oil Quality of Residual Biomass Pyrolysis by Chemical Activation: Effect of Alkalis and Acid Pre-Treatment. Energies. 2023; 16(7):3162. https://doi.org/10.3390/en16073162
Chicago/Turabian StyleDaniel Valdez, Gérson, Flávio Pinheiro Valois, Sammy Jonatan Bremer, Kelly Christina Alves Bezerra, Lauro Henrique Hamoy Guerreiro, Marcelo Costa Santos, Lucas Pinto Bernar, Waldeci Paraguassu Feio, Luiz Gabriel Santos Moreira, Neyson Martins Mendonça, and et al. 2023. "Improving the Bio-Oil Quality of Residual Biomass Pyrolysis by Chemical Activation: Effect of Alkalis and Acid Pre-Treatment" Energies 16, no. 7: 3162. https://doi.org/10.3390/en16073162
APA StyleDaniel Valdez, G., Valois, F. P., Bremer, S. J., Bezerra, K. C. A., Hamoy Guerreiro, L. H., Santos, M. C., Bernar, L. P., Feio, W. P., Moreira, L. G. S., Mendonça, N. M., de Castro, D. A. R., Duvoisin, S., Jr., Borges, L. E. P., & Machado, N. T. (2023). Improving the Bio-Oil Quality of Residual Biomass Pyrolysis by Chemical Activation: Effect of Alkalis and Acid Pre-Treatment. Energies, 16(7), 3162. https://doi.org/10.3390/en16073162






