Numerical Investigation on the Performance of IT-SOEC with Double-Layer Composite Electrode
Abstract
1. Introduction
2. Model Development
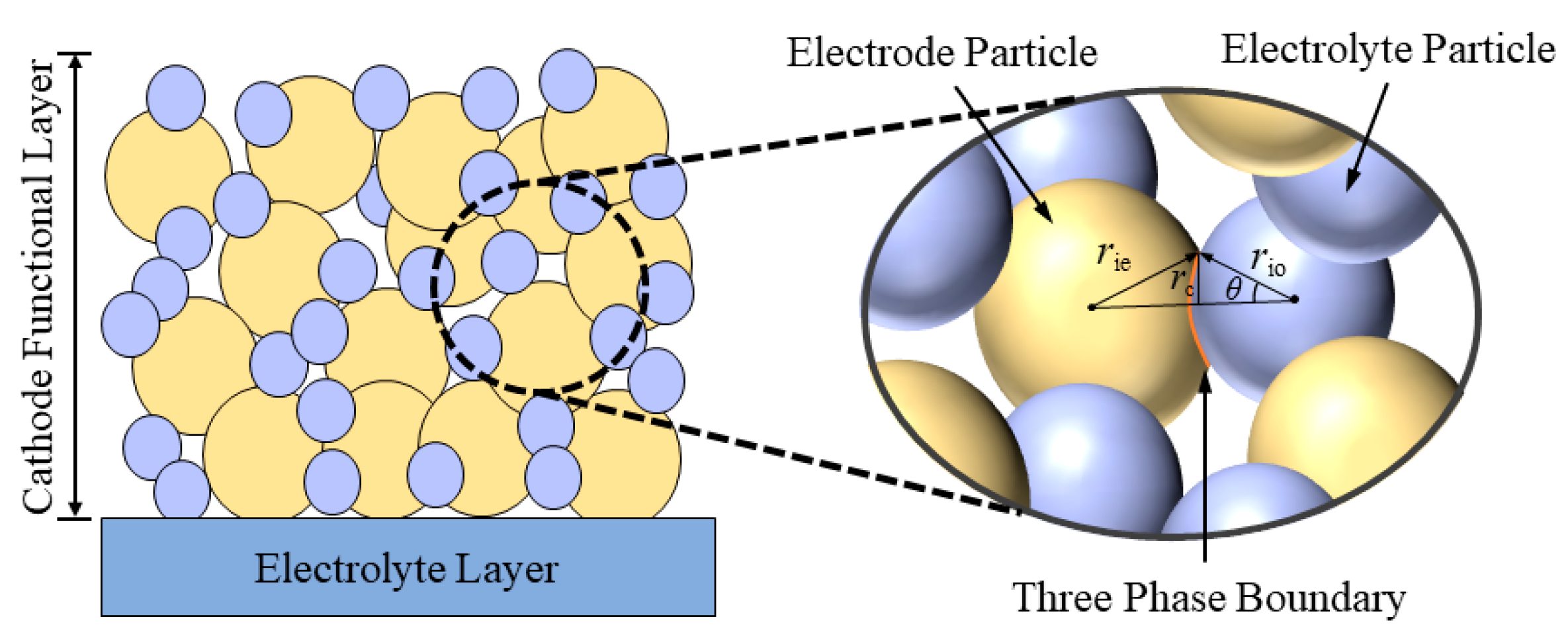
2.1. Geometric Model
2.2. Assumptions and Governing Equations
- Steady state.
- The gases are assumed to be ideal gas.
- IT-SOEC operates at the adiabatic state.
- Electrochemical reactions take place in the anode and cathode function layers.
- The radiation heat exchange inside the cell is ignored.
- The effect of gravity is ignored.
2.2.1. Electrochemical Reactions Model
2.2.2. Energy Conservation Model
2.2.3. Momentum, Mass and Component Conservation Model
2.3. Mesostructure Model of Composite Electrode
2.4. Boundary Conditions and Model Parameters
2.5. Model Solution
3. Results and Discussion
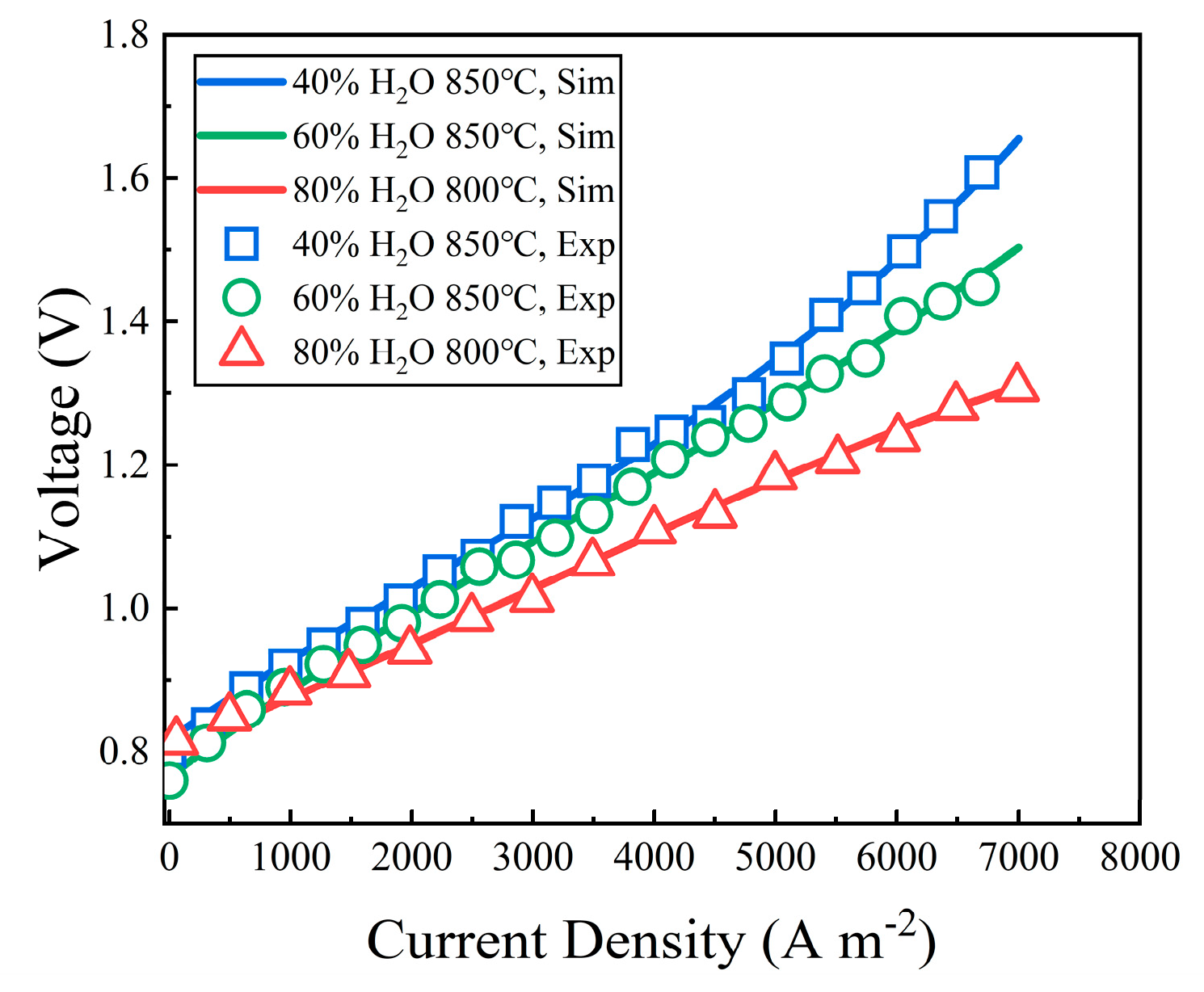
3.1. Model Validation
3.2. Effects of CDL Porosity
3.2.1. Effects on Mass Transport Process
3.2.2. Effects on Electrolysis Performance
3.3. Effects of Internal Mesostructure of CFL
3.3.1. Effects on Mass Transport Process
3.3.2. Effects on Electrolysis Performance
4. Conclusions
- The increase of the CDL porosity promotes the development of the internal mass transport process of the CFL, but hinders the development of the overall mass transport process in the CDL. The convection flux decreases by 1.12% when the porosity of the CDL increases from 0.3 to 0.7. The increase of the CDL porosity also increases the overall energy consumption. The energy consumption at the porosity of 0.5 decreases by 9.64% compared with the porosity of 0.7. The appropriate value of the CDL porosity is determined in the range of 0.3–0.5.
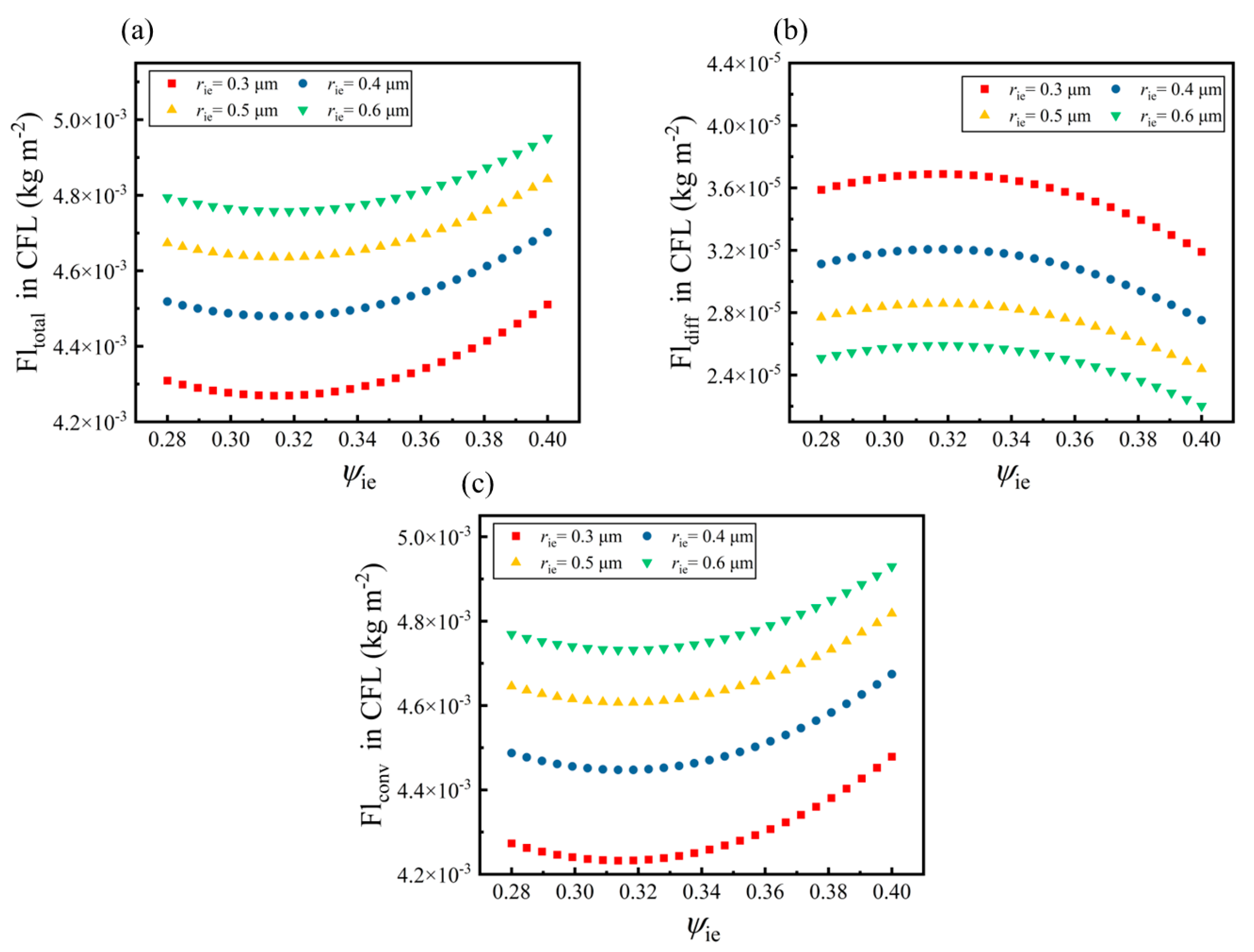
- The equal volume fractions of the electrode phase (ψie) and electrolyte phase (ψio) in the CFL can enhance the mass transport process along the wall-normal direction from the channel to the cathode. The total mass flux along the wall-normal direction reaches the maximum value in the CFL when ψie = 0.32.
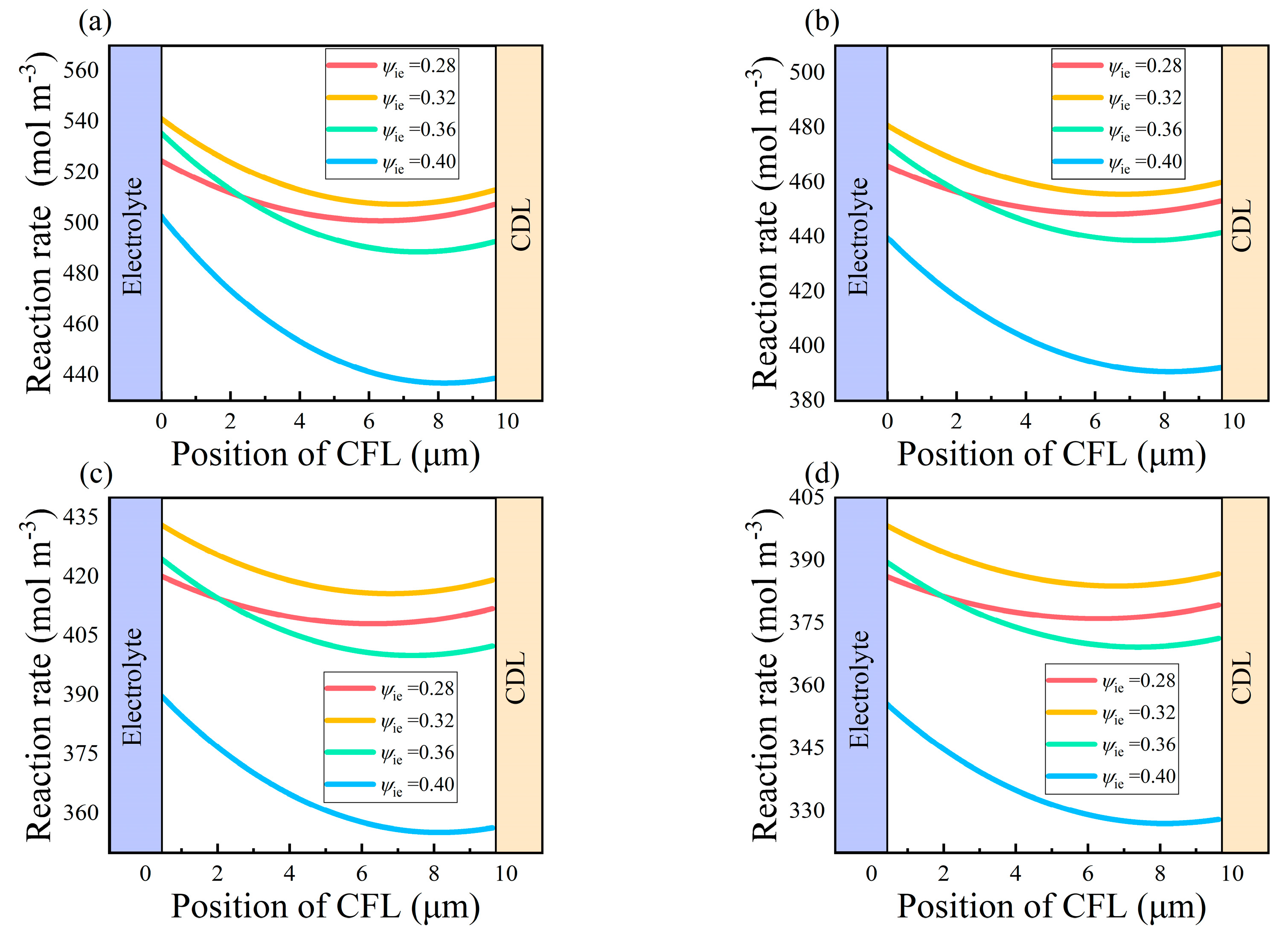
- The maximum reaction rate inside the CFL increases by 32.64% when the radius of the electrode particle is reduced from 0.5 μm to 0.3 μm. It reaches the maximum in the area near the electrolyte. The optimal value of ψie in the CFL is determined to be 0.32 according to its effects on the mass transport processes and electrochemical reactions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| AeffTPB | Effective TPB area of per unit volume (m2/m3) |
| Cp | Specific heat capacity (J/(kg·K)) |
| D | Binary diffusion coefficient (m2/s) |
| Eact | Reaction activation energy (J/mol) |
| F | Faraday constant (C/mol) |
| iion | Ionic current density (A/m2) |
| ielec | Local current density (A/m2) |
| i0 | Exchange current density (A/m2) |
| j | Adjustable parameters |
| k | Adjustable parameters |
| M | Mole mass (kg/mol) |
| m | Adjustable parameters |
| n | Electrons numbers involved in the reaction |
| PTPB | Pressure on the TPB interface (atm) |
| Pref | Pressure under the reference state (atm) |
| Pie | Probability of electrode particle belonging to percolated clusters |
| Pio | Probability of electrolyte particle belonging to percolated clusters |
| p | Operating pressure (atm) |
| Qion | Net reaction ion current density (A/m2) |
| Qheat | Heat source (W/m3) |
| Qohm | Ohmic heat source (W/m3) |
| Qrev | Reversible heat source (W/m3) |
| Qirr | Irreversible heat source (W/m3) |
| R | Universal gas constant (J/(mol·K)) |
| r | Particle radius (μm) |
| S | Molar entropy (J/(mol·K)) |
| T | Operating temperature (K) |
| Greek Symbols | |
| α | Transfer coefficient |
| γ | Pre-exponential factor (A/m2) |
| δie | Electronic conductivity (S/m) |
| δio | Ionic conductivity (S/m) |
| δeff | Effective conductivity (S/m) |
| η | Overpotential (V) |
| ηact | Activation overpotential (V) |
| θ | Contact angle between the electronic and ionic particles (°) |
| λ | Thermal conductivity (W/(m·K)) |
| λeff | Effective thermal conductivity (W/(m·K)) |
| τ | Fluid tortuosity factor |
| Superscripts and Subscripts | |
| an | Anode |
| ca | Cathode |
| elec | Electronic |
| ie | Electrode |
| io | Electrolyte |
| ion | Ionic |
| i | Gas species |
| p | Gas-phase |
| TPB | Three-phase boundary |
References
- Ebbesen, S.D.; Knibbe, R.; Mogensen, M. Co-electrolysis of steam and carbon dioxide in solid oxide cells. J. Electrochem. Soc. 2012, 159, F482–F489. [Google Scholar] [CrossRef]
- Shimada, H.; Fujimaki, Y.; Fujishiro, Y. Highly active and durable La0.4Sr0.6MnO3-δ and Ce0.8Gd0.2O1.9 nanocomposite electrode for high-temperature reversible solid oxide electrochemical cells. Ceram. Int. 2020, 46, 19617–19623. [Google Scholar] [CrossRef]
- Tan, K.; Yan, X.M.; Zhu, Z.Y.; Zhou, M.Y.; Tian, F.Y.; Liu, J. Solid oxide cells with cermet of silver and gadolinium-doped-ceria symmetrical electrodes for high-performance power generation and water electrolysis. Int. J. Hydrogen Energy 2022, 47, 25090–25103. [Google Scholar] [CrossRef]
- Jolaoso, L.A.; Asadi, J.; Duan, C.; Kazempoor, P. A novel green hydrogen production using water-energy nexus framework. Energy Convers. Manag. 2023, 276, 116344. [Google Scholar] [CrossRef]
- Hauch, A.; Küngas, R.; Blennow, P.; Hansen, A.B.; Hansen, J.B.; Mathiesen, B.V.; Mogensen, M.B. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, eaba6118. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. ‘Renewable’ hydrogen: Prospects and challenges. Renew. Sust. Energy Rev. 2011, 15, 3034–3040. [Google Scholar] [CrossRef]
- Min, G.B.; Choi, S.; Hong, J.S. A review of solid oxide steam-electrolysis cell systems: Thermodynamics and thermal integration. Appl. Energy 2022, 328, 120145. [Google Scholar] [CrossRef]
- Lei, B.; Boulfrad, S.; Traversa, E. Steam electrolysis by solid oxide electrolysis cells (SOECs) with proton-conducting oxides. Chem. Soc. Rev. 2014, 43, 8255–8270. [Google Scholar] [CrossRef]
- Meng, H.J.; Tian, N.; Qu, Y.M.; Wang, M.; Zhao, S.; Yu, J. Improved performance of a lanthanum strontium manganite–based oxygen electrode for an intermediate-temperature solid oxide electrolysis cell realized via ionic conduction enhancement. Ceram. Int. 2019, 45, 7945–7949. [Google Scholar] [CrossRef]
- Wang, Z.W.; Mori, M.; Araki, T. Steam electrolysis performance of intermediate-temperature solid oxide electrolysis cell and efficiency of hydrogen production system at 300 Nm3 h−1. Int. J. Hydrogen Energy 2010, 35, 4451–4458. [Google Scholar] [CrossRef]
- Chen, T.; Liu, M.Q.; Yuan, C.; Zhou, Y.C.; Ye, X.F.; Zhang, Z.L.; Xia, C.R.; Wang, S.R. High performance of intermediate temperature solid oxide electrolysis cells using Nd2NiO4+δ impregnated scandia stabilized zirconia oxygen electrode. J. Power Sources 2015, 276, 1–6. [Google Scholar] [CrossRef]
- Liu, S.M.; Yu, B.; Zhang, W.Q.; Zhai, Y.C.; Chen, J. Electrochemical performance of Co-containing mixed oxides as oxygen electrode materials for intermediate-temperature solid oxide electrolysis cells. Int. J. Hydrogen Energy 2016, 41, 15952–15959. [Google Scholar] [CrossRef]
- Gaudillere, C.; Navarrete, L.; Serra, J.M. Syngas production at intermediate temperature through H2O and CO2 electrolysis with a Cu-based solid oxide electrolyzer cell. Int. J. Hydrogen Energy 2014, 39, 3047–3054. [Google Scholar] [CrossRef]
- Liu, C.L.; Ma, B.; Lin, Z.; Zhou, Y.K.; Wu, K.M. Exploring (1-x)CuFe2O4-xGd0.1Ce0.9O1.95 composites as the cathode materials for solid oxide fuel cells. Mater. Lett. 2022, 325, 132860. [Google Scholar] [CrossRef]
- Guo, M.T.; He, Q.J.; Cheng, C.; Zhao, D.Q.; Ni, M. New interconnector designs for electrical performance enhancement of solid oxide fuel cells: A 3D modelling study. J. Power Sources 2022, 533, 231373. [Google Scholar] [CrossRef]
- Fu, Z.G.; Wang, Z.J.; Li, Y.W.; Li, J.F.; Shao, Y.; Zhu, Q.Z.; Weng, P.F. Effects of composite electrode structure on performance of intermediate-temperature solid oxide electrolysis cell. Energies 2022, 15, 7173. [Google Scholar] [CrossRef]
- Li, H.; Su, C.X.; Wang, C.Q.; Lü, Z. Electrochemical performance evaluation of FeCo2O4 spinel composite cathode for solid oxide fuel cells. J. Alloys Compd. 2020, 829, 154493. [Google Scholar] [CrossRef]
- Araújo, A.J.M.; Loureiro, F.J.A.; Raimundo, R.A.; Macedo, D.A.; Paskocimas, C.A.; Fagg, D.P. The effects of polarisation on the performance of the Ba2Co9O14-Ce0.8Gd0.2O2-δ composite electrode for fuel cells and electrolysers. Int. J. Hydrogen Energy 2022, 47, 11270–11278. [Google Scholar] [CrossRef]
- Nechache, A.; Hody, S. Alternative and innovative solid oxide electrolysis cell materials: A short review. Renew. Sust. Energ. Rev. 2021, 149, 111322. [Google Scholar] [CrossRef]
- Dumortier, M.; Sanchez, J.; Keddam, M.; Lacroix, O. Theoretical considerations on the modelling of transport in a three-phase electrode and application to a proton conducting solid oxide electrolysis cell. Int. J. Hydrogen Energy 2012, 37, 11579–11594. [Google Scholar] [CrossRef]
- Rashidi, R.; Karimi, N.; Sunden, B.; Kim, K.C.; Olabi, A.G.; Mahian, O. Progress and challenges on the thermal management of electrochemical energy conversion and storage technologies: Fuel cells, electrolysers, and supercapacitors. Prog. Energy Combust. 2022, 88, 100966. [Google Scholar] [CrossRef]
- Kim, S.; Joh, D.W.; Lee, D.Y.; Lee, J.; Kim, H.S.; Khan, M.Z.; Hong, J.E.; Lee, S.B.; Park, S.J.; Song, R.H.; et al. Microstructure tailoring of solid oxide electrolysis cell air electrode to boost performance and long-term durability. Chem. Eng. J. 2021, 410, 128318. [Google Scholar] [CrossRef]
- Mehran, M.T.; Khan, M.Z.; Lee, S.B.; Lim, T.H.; Park, S.; Song, R.H. Improving sulfur tolerance of Ni-YSZ anodes of solid oxide fuel cells by optimization of microstructure and operating conditions. Int. J. Hydrogen Energy 2018, 43, 11202–11213. [Google Scholar] [CrossRef]
- Bertei, A.; Nucci, B.; Nicolella, C. Microstructural modeling for prediction of transport properties and electrochemical performance in SOFC composite electrodes. Chem. Eng. Sci. 2013, 101, 175–190. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C. Micro-scale modelling of solid oxide fuel cells with micro-structurally graded electrodes. J. Power Sources 2007, 168, 369–378. [Google Scholar] [CrossRef]
- Vijay, P.; Tadé, M.O.; Shao, Z.P. Model based evaluation of the electrochemical reaction sites in solid oxide fuel cell electrodes. Int. J. Hydrogen Energy 2019, 44, 8439–8459. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Xu, H.R.; Xuan, J. Advancing the multiscale understanding on solid oxide electrolysis cells via modelling approaches: A review. Renew. Sust. Energy Rev. 2021, 141, 110863. [Google Scholar] [CrossRef]
- Monaco, F.; Effori, E.; Hubert, M.; Siebert, E.; Geneste, G.; Morel, B.; Djurado, E.; Montinaro, D.; Laurencin, J. Electrode kinetics of porous Ni-3YSZ cermet operated in fuel cell and electrolysis modes for solid oxide cell application. Electrochim. Acta 2021, 389, 138765. [Google Scholar] [CrossRef]
- Théodon, L.; Laurencin, J.; Hubert, M.; Cloetens, P.; Debayle, J. A stochastic geometrical 3D model for time evolution simulation of microstructures in SOC-electrodes. Comp. Mater. Sci. 2022, 212, 111568. [Google Scholar] [CrossRef]
- Hubert, M.; Laurencin, J.; Cloetens, P.; Sliva, J.C.D.; Lefebvre-Joud, F.; Bleuet, P.; Nakajo, A.; Siebert, E. Role of microstructure on electrode operating mechanisms for mixed ionic electronic conductors: From synchrotron-based 3D reconstruction to electrochemical modeling. Solid State Ion. 2016, 294, 90–107. [Google Scholar] [CrossRef]
- Beale, S.B.; Andersson, M.; Boigues-Muñoz, C.; Frandsen, H.L.; Lin, Z.J.; Mcphail, S.J.; Ni, M.; Sundén, B.; Weber, A.; Weber, A.Z. Continuum scale modelling and complementary experimentation of solid oxide cells. Prog. Energy Combust. Sci. 2021, 85, 100902. [Google Scholar] [CrossRef]
- Kushi, T. Heat balance of dry reforming in solid oxide fuel cell systems. Int. J. Hydrogen Energy 2017, 42, 11779–11787. [Google Scholar] [CrossRef]
- Chen, D.F.; Bi, W.X.; Kong, W.; Lin, Z.J. Combined micro-scale and macro-scale modeling of the composite electrode of a solid oxide fuel cell. J. Power Sources 2010, 195, 6598–6610. [Google Scholar] [CrossRef]
- Moussaoui, H.; Sharma, R.K.; Debayle, J.; Gavet, Y.; Delette, G.; Laurencin, J. Microstructural correlations for specific surface area and triple phase boundary length for composite electrodes of solid oxide cells. J. Power Sources 2019, 412, 736–748. [Google Scholar] [CrossRef]
- Wei, Y.H.; Bao, C.; Jiang, Z.Y.; Zhang, X.X. Numerical study on TPB density and percolation properties of microstructure reconstruction of nickel/yttria stabilized zirconia cermet anode based on discrete element method. Int. J. Hydrogen Energy 2022, 47, 28061–28073. [Google Scholar] [CrossRef]
- Li, W.Y.; Shi, Y.X.; Luo, Y.; Cai, N.S. Theoretical modeling of air electrode operating in SOFC mode and SOEC mode: The effects of microstructure and thickness. Int. J. Hydrogen Energy 2014, 39, 13738–13750. [Google Scholar] [CrossRef]
- Shi, Y.X.; Cai, N.S.; Li, C.; Bao, C.; Croiset, E.; Qian, J.Q.; Hu, Q.; Wang, S.R. Modeling of an anode-supported Ni-YSZ|Ni-ScSZ|ScSZ|LSM-ScSZ multiple layers SOFC cell: Part II. Simulations and discussion. J. Power Sources 2007, 172, 235–245. [Google Scholar] [CrossRef]
- Wu, C.R.; Yang, Z.R.; Huo, S.; Najmi, A.U.H.; Du, Q.; Jiao, K. Modeling and optimization of electrode structure design for solid oxide fuel cell. Int. J. Hydrogen Energy 2018, 43, 14648–14664. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C. Mathematical modeling of the coupled transport and electrochemical reactions in solid oxide steam electrolyzer for hydrogen production. Electrochim. Acta 2007, 52, 6707–6718. [Google Scholar] [CrossRef]
- Navasa, M.; Graves, C.; Chatzichristodoulou, C.; Theis, L.S.; Sundén, B.; Frandsen, H.L. A three-dimensional multiphysics model of a solid oxide electrochemical cell: A tool for understanding degradation. Int. J. Hydrogen Energy 2018, 43, 11913–11931. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.R.; Wang, Y.; Yang, Z.B.; Du, Q.; Jiao, K. Assessment of CO2 enrichment mechanism in integrated coal gasification fuel cell combined cycle system with carbon capture. Front. Energy Res. 2023, 10, 1017829. [Google Scholar] [CrossRef]
- Ni, M. An electrochemical model for syngas production by co-electrolysis of H2O and CO2. J. Power Sources 2012, 202, 209–216. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, W.J.; Ji, S.Y.; Sun, A.; Shuai, W.; Zheng, N.; Han, Y.; Xiao, G.; Ni, M.; Xu, H.R. Dynamic behavior of high-temperature CO2/H2O co-electrolysis coupled with real fluctuating renewable power. Sustain. Energy Technol. 2022, 52, 102344. [Google Scholar] [CrossRef]
- Luo, Y.; Shi, Y.X.; Li, W.Y.; Cai, N.S. Comprehensive modeling of tubular solid oxide electrolysis cell for co-electrolysis of steam and carbon dioxide. Energy 2014, 70, 420–434. [Google Scholar] [CrossRef]
- Newman, J.S.; Thomas-Alyea, K.E. Electrochemical Systems, 3rd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2004; pp. 449–486. [Google Scholar]
- Astaneh, M.; Maggiolo, D.; Ström, H. Finite-size effects on heat and mass transfer in porous electrodes. Int. J. Therm. Sci. 2022, 179, 107610. [Google Scholar] [CrossRef]
- Todd, B.; Young, J.B. Thermodynamic and transport properties of gases for use in solid oxide fuel cell modelling. J. Power Sources 2002, 110, 186–200. [Google Scholar] [CrossRef]
- Mehran, M.T.; Yu, S.B.; Lee, D.Y.; Hong, J.E.; Lee, S.B.; Park, S.J.; Song, R.H.; Lim, T.H. Production of syngas from H2O/CO2 by high-pressure coelectrolysis in tubular solid oxide cells. Appl. Energy 2018, 212, 759–770. [Google Scholar] [CrossRef]
- Recalde, M.; Amladi, A.; Venkataraman, V.; Woudstra, T.; Aravind, P.V. Thermodynamic analysis of supercritical water gasification combined with a reversible solid oxide cell. Energy Convers. Manag. 2022, 270, 116208. [Google Scholar] [CrossRef]
- Weng, F.B.; Dlamini, M.M.; Jung, G.B.; Lian, C.X. Analyses of reversible solid oxide cells porosity effects on temperature reduction. Int. J. Hydrogen Energy 2020, 45, 12170–12184. [Google Scholar] [CrossRef]
- Völker, B.; McMeeking, R.M. Impact of particle size ratio and volume fraction on effective material parameters and performance in solid oxide fuel cell electrodes. J. Power Sources 2012, 215, 199–215. [Google Scholar] [CrossRef]
- Timurkutluk, B.; Ciflik, Y.; Altan, T.; Genc, O. Synthetical designing of solid oxide fuel cell electrodes: Effect of particle size and volume fraction. Int. J. Hydrogen Energy 2022, 47, 31446–31458. [Google Scholar] [CrossRef]







| Parameters | Value |
|---|---|
| Channel width | 0.5 mm |
| Channel height | 0.5 mm |
| Channel length | 10 cm |
| Rib width | 0.25mm |
| Cathode width | 1 mm |
| Thickness of CDL | 300 μm |
| Thickness of CFL | 10 μm |
| Thickness of anode diffusion layer (ADL) | 30 μm |
| Thickness of AFL | 15 μm |
| Thickness of electrolysis layer | 10 μm |
| Operating pressure | 1 atm |
| Operating temperature | 973.15 K |
| Parameters | Value |
|---|---|
| Thermal conductivity of composite cathode | 6.23 W/(m·K) |
| Thermal conductivity of composite anode | 9.6 W/(m·K) |
| Thermal conductivity of electrolyte | 2.7 W/(m·K) |
| Heat capacity of composite cathode | 420 J/(kg·K) |
| Heat capacity of composite anode | 390 J/(kg·K) |
| Heat capacity of electrolyte | 420 J/(kg·K) |
| Porosity of CDL | Electrode Phase Volume Fraction | Particle Radius | |
|---|---|---|---|
| ψp of CDL | 0.3–0.7 | 0.4 | 0.4 |
| ψie | 0.32 | 0.28–0.4 | 0.28–0.4 |
| rie (μm) | 1 | 1 | 0.3–0.6 |
| Parameters | Value |
|---|---|
| Electrolyte thickness | 9.82 μm [48], 7.27 μm [22] |
| Composite cathode thickness | 11.7 μm [48], 48.2 μm [22] |
| Composite anode thickness | 16.8 μm [48], 37.5 μm [22] |
| Operating pressure | 1 atm [22,48] |
| Cathode flowrate | 150 mL/min [48], 500 mL/min [22] |
| Anode flowrate | 300 mL/min [48], 300 mL/min [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Y.; Li, Y.; Fu, Z.; Li, J.; Zhu, Q. Numerical Investigation on the Performance of IT-SOEC with Double-Layer Composite Electrode. Energies 2023, 16, 2525. https://doi.org/10.3390/en16062525
Shao Y, Li Y, Fu Z, Li J, Zhu Q. Numerical Investigation on the Performance of IT-SOEC with Double-Layer Composite Electrode. Energies. 2023; 16(6):2525. https://doi.org/10.3390/en16062525
Chicago/Turabian StyleShao, Yan, Yongwei Li, Zaiguo Fu, Jingfa Li, and Qunzhi Zhu. 2023. "Numerical Investigation on the Performance of IT-SOEC with Double-Layer Composite Electrode" Energies 16, no. 6: 2525. https://doi.org/10.3390/en16062525
APA StyleShao, Y., Li, Y., Fu, Z., Li, J., & Zhu, Q. (2023). Numerical Investigation on the Performance of IT-SOEC with Double-Layer Composite Electrode. Energies, 16(6), 2525. https://doi.org/10.3390/en16062525







