Hydrothermal Carbonization of Sewage Sludge into Solid Biofuel: Influences of Process Conditions on the Energetic Properties of Hydrochar
Abstract
1. Introduction
2. Materials and Methods
2.1. Sewage Sludge Sample Preparation
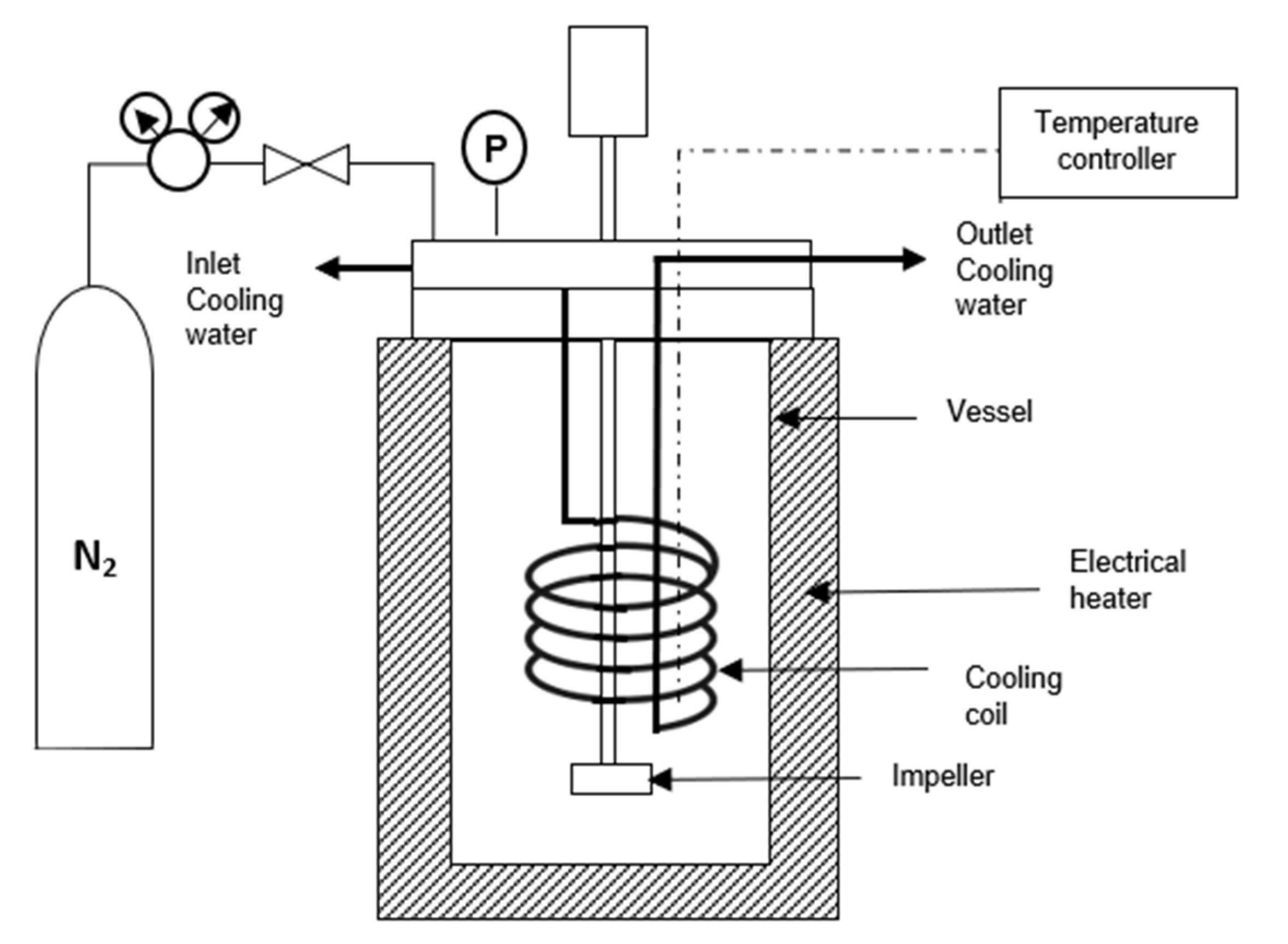
2.2. HTC Experiment
2.3. Sewage Sludge and Products Characterization
2.4. Fuel Properties of Hydrochar
3. Results and Discussion
3.1. Characteristics of Raw Sewage Sludge
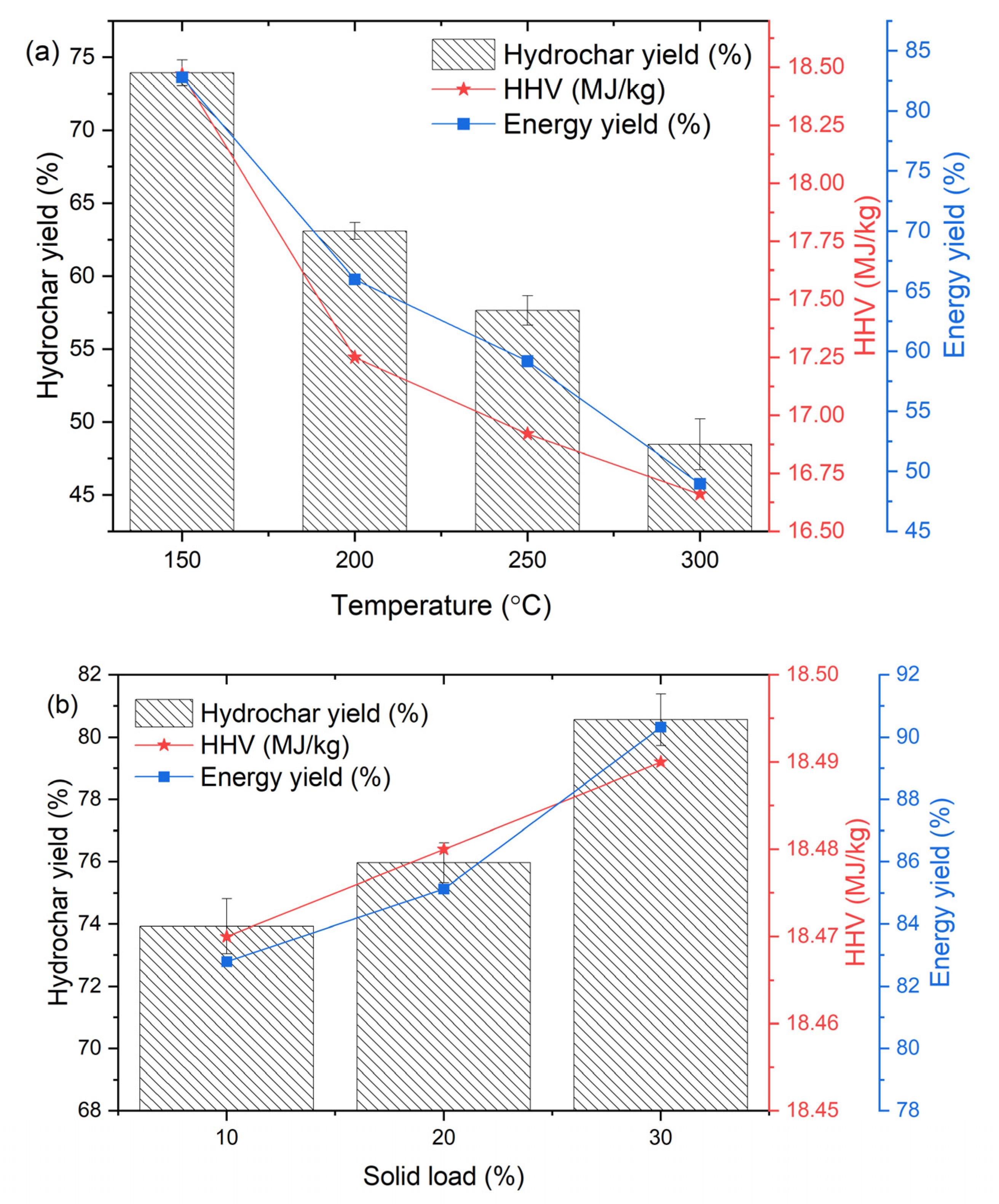
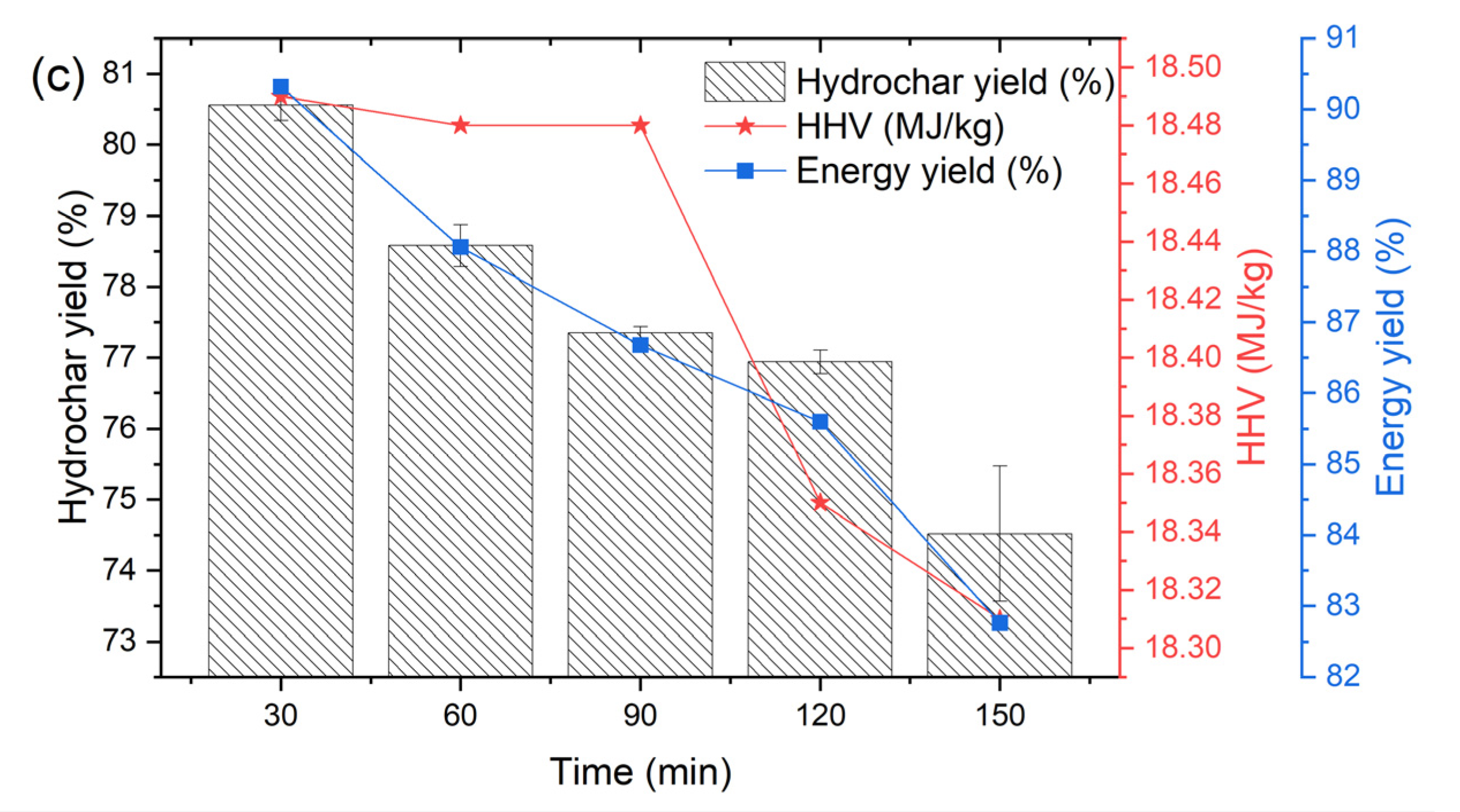
3.2. Effect of HTC Conditions on Hydrochar Characteristics
3.3. Fuel Characteristics
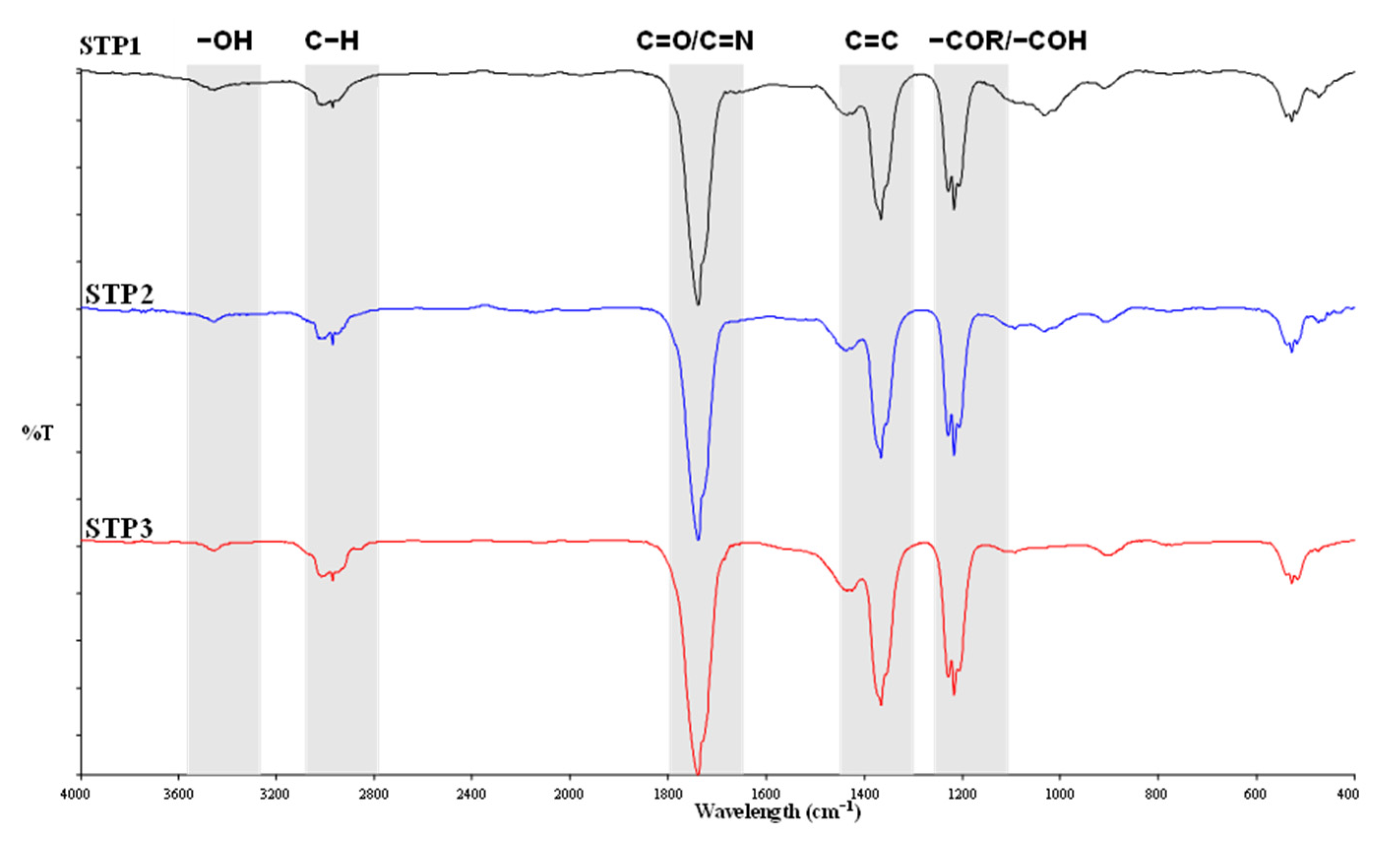
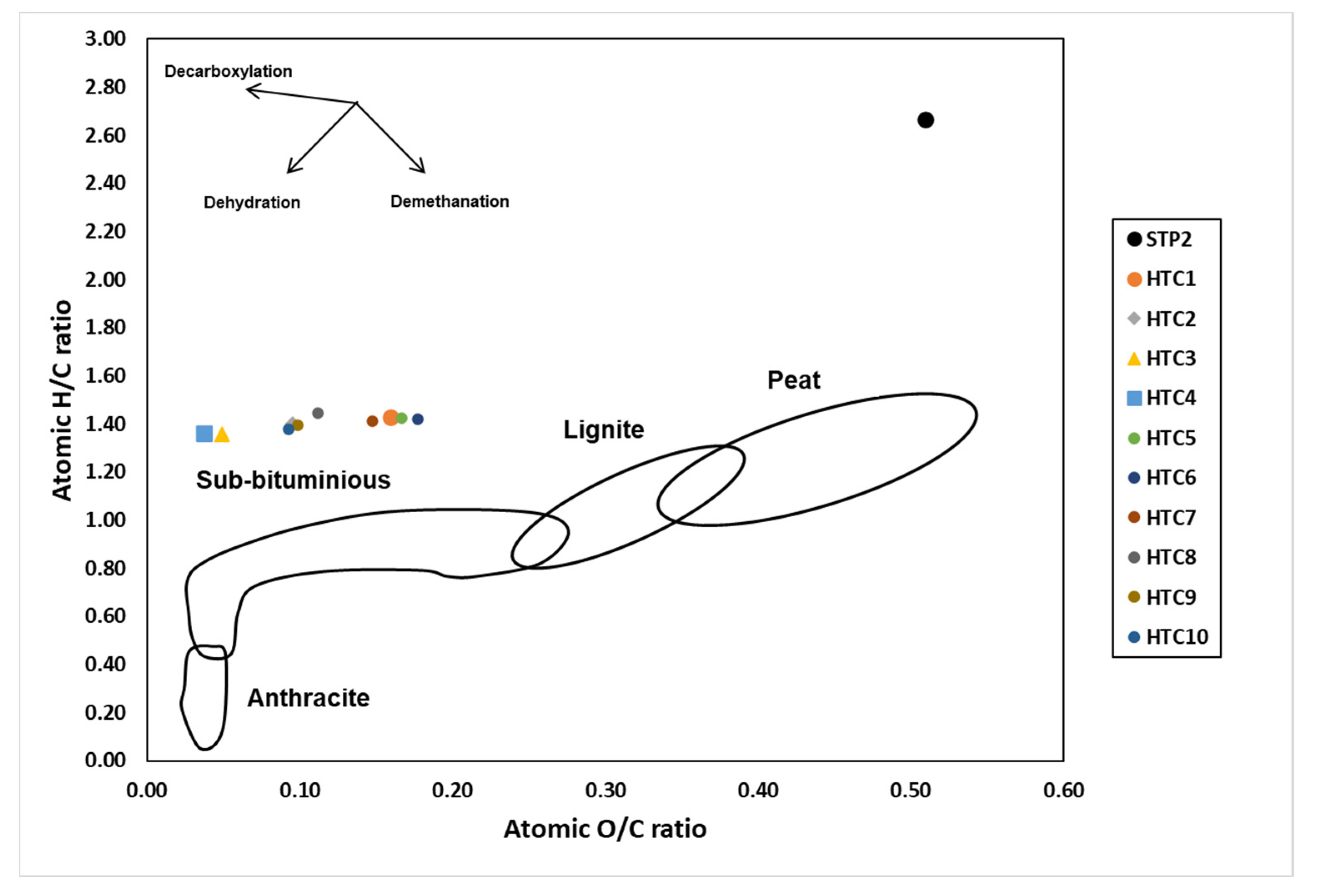
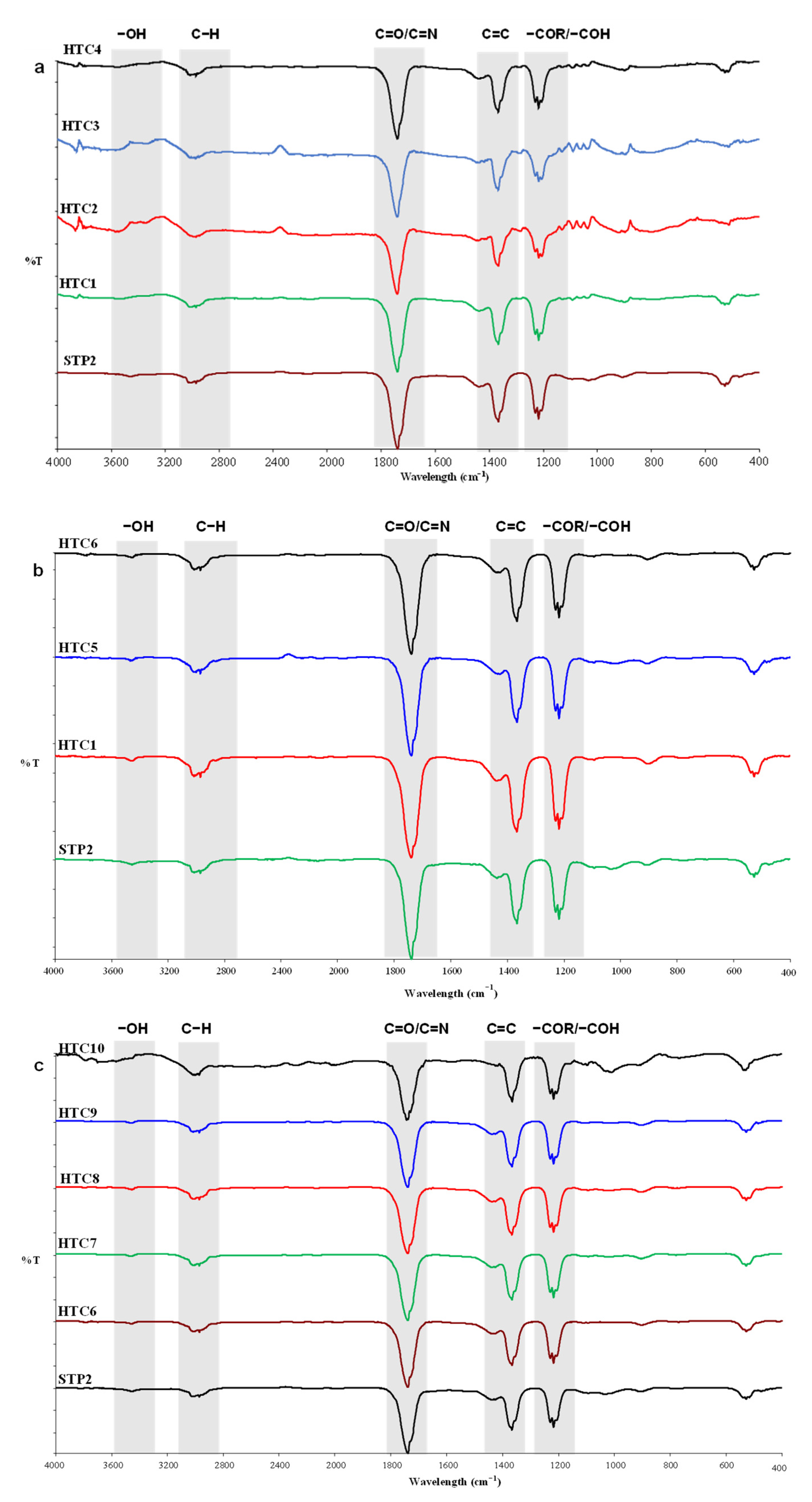
3.4. Surface Functionalities of Hydrochar
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roslan, S.N.; Ghazali, S.S.; Asli, N.M. Study on the Characteristics and Utilization of Sewage Sludge at Indah Water Konsortium (IWK) Sungai Udang, Melaka. Int. J. Environ. Ecol. Eng. 2013, 7, 544–548. [Google Scholar]
- Spinosa, L. Wastewater Sludge: A Global Overview of the Current Status and Future Prospects, 2nd ed.; IWA Publishing: London, UK, 2011; Volume 10, ISBN 9781780401195. [Google Scholar]
- Chojnacka, K.; Skrzypczak, D.; Szopa, D.; Izydorczyk, G.; Moustakas, K.; Witek-Krowiak, A. Management of Biological Sewage Sludge: Fertilizer Nitrogen Recovery as the Solution to Fertilizer Crisis. J. Environ. Manag. 2023, 326, 116602. [Google Scholar] [CrossRef] [PubMed]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage Sludge Disposal Strategies for Sustainable Development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef]
- Syed-Hassan, S.S.A.; Wang, Y.; Hu, S.; Su, S.; Xiang, J. Thermochemical Processing of Sewage Sludge to Energy and Fuel: Fundamentals, Challenges and Considerations. Renew. Sustain. Energy Rev. 2017, 80, 888–913. [Google Scholar] [CrossRef]
- Kakwani, N.S.; Kalbar, P.P. Review of Circular Economy in Urban Water Sector: Challenges and Opportunities in India. J. Environ. Manag. 2020, 271, 111010. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Peng, C.; Xu, B.; Wang, T.; Li, C.; Zeng, G.; Zhu, Y. Hydrothermal Carbonisation of Sewage Sludge for Char Production with Different Waste Biomass: Effects of Reaction Temperature and Energy Recycling. Energy 2017, 127, 167–174. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Hoekman, S.K.; Balasubramanian, R. Chemical, Structural and Combustion Characteristics of Carbonaceous Products Obtained by Hydrothermal Carbonization of Palm Empty Fruit Bunches. Bioresour. Technol. 2013, 135, 683–689. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Mubarak, N.M.; Tiripathi, M.; Jayakumar, N.S.; Sahu, J.N.; Ganesan, P. Chemical, Dielectric and Structural Characterization of Optimized Hydrochar Produced from Hydrothermal Carbonization of Palm Shell. Fuel 2016, 163, 88–97. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, F.; Shen, X.; Yi, W.; Li, Z.; Li, Y.; Tian, C. Comparison Study on Fuel Properties of Hydrochars Produced from Corn Stalk and Corn Stalk Digestate. Energy 2018, 165, 527–536. [Google Scholar] [CrossRef]
- Mäkelä, M.; Benavente, V.; Fullana, A. Hydrothermal Carbonization of Industrial Mixed Sludge from a Pulp and Paper Mill. Bioresour. Technol. 2016, 200, 444–450. [Google Scholar] [CrossRef]
- Saqib, N.U.; Baroutian, S.; Sarmah, A.K. Physicochemical, Structural and Combustion Characterization of Food Waste Hydrochar Obtained by Hydrothermal Carbonization. Bioresour. Technol. 2018, 266, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Danso-Boateng, E.; Shama, G.; Wheatley, A.D.; Martin, S.J.; Holdich, R.G. Hydrothermal Carbonisation of Sewage Sludge: Effect of Process Conditions on Product Characteristics and Methane Production. Bioresour. Technol. 2015, 177, 318–327. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.I.; Grasham, O.; Ross, A.B.; Dupont, V.; Camargo-Valero, M.A. Hydrothermal Carbonization of Sewage Digestate at Wastewater Treatment Works: Influence of Solid Loading on Characteristics of Hydrochar, Process Water and Plant Energetics. Renew. Energy 2020, 157, 959–973. [Google Scholar] [CrossRef]
- Wang, L.; Chang, Y.; Li, A. Hydrothermal Carbonization for Energy-Efficient Processing of Sewage Sludge: A Review. Renew. Sustain. Energy Rev. 2019, 108, 423–440. [Google Scholar] [CrossRef]
- Pauline, A.L.; Joseph, K. Hydrothermal Carbonization of Organic Wastes to Carbonaceous Solid Fuel-A Review of Mechanisms and Process Parameters. Fuel 2020, 279, 118472. [Google Scholar] [CrossRef]
- Knez, Ž.; Markočič, E.; Hrnčič, M.K.; Ravber, M.; Škerget, M. High Pressure Water Reforming of Biomass for Energy and Chemicals: A Short Review. J. Supercrit. Fluids 2015, 96, 46–52. [Google Scholar] [CrossRef]
- Zhao, P.; Shen, Y.; Ge, S.; Chen, Z.; Yoshikawa, K. Clean Solid Biofuel Production from High Moisture Content Waste Biomass Employing Hydrothermal Treatment. Appl. Energy 2014, 131, 345–367. [Google Scholar] [CrossRef]
- Maniscalco, M.P.; Volpe, M.; Messineo, A. Hydrothermal Carbonization as a Valuable Tool for Energy and Environmental Applications: A Review. Energies 2020, 13, 4098. [Google Scholar] [CrossRef]
- Djandja, O.S.; Yin, L.-X.; Wang, Z.-C.; Duan, P.-G. From Wastewater Treatment to Resources Recovery through Hydrothermal Treatments of Municipal Sewage Sludge: A Critical Review. Process Saf. Environ. Prot. 2021, 151, 101–127. [Google Scholar] [CrossRef]
- Silva, R.D.V.K.; Lei, Z.; Shimizu, K.; Zhang, Z. Hydrothermal Treatment of Sewage Sludge to Produce Solid Biofuel: Focus on Fuel Characteristics. Bioresour. Technol. Rep. 2020, 11, 100453. [Google Scholar] [CrossRef]
- Hansen, L.J.; Fendt, S.; Spliethoff, H. Impact of Hydrothermal Carbonization on Combustion Properties of Residual Biomass. Biomass Convers. Biorefinery 2020, 12, 2541–2552. [Google Scholar] [CrossRef]
- ASTM D 3174-02; Standard Test Method for Ash in the Analysis Sample of Coal and Coke from Coal. American Society for Testing and Materials International: West Conshohocken, PA, USA, 2002.
- ASTM D3175-07; Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke. American Society for Testing and Materials International: West Conshohocken, PA, USA, 2007. [CrossRef]
- ASTM D 3176-89; Standard Practice for Ultimate Analysis of Coal and Coke. American Society for Testing and Materials International: West Conshohocken, PA, USA, 2002.
- ASTM D 2015-00; Standard Test Method for Gross Calorific Value of Coal and Coke by the Adiabatic Bomb Calorimeter. American Society for Testing and Materials International: West Conshohocken, PA, USA, 2015.
- Lu, X.; Ma, X.; Qin, Z.; Ke, C.; Chen, L.; Chen, X. Co-Hydrothermal Carbonization of Sewage Sludge with Wood Chip: Fuel Properties and Heavy Metal Transformation Behavior of Hydrochars. Energy Fuels 2021, 35, 15790–15801. [Google Scholar] [CrossRef]
- Wang, S.; Persson, H.; Yang, W.; Jönsson, P.G. Pyrolysis Study of Hydrothermal Carbonization-Treated Digested Sewage Sludge Using a Py-GC/MS and a Bench-Scale Pyrolyzer. Fuel 2020, 262, 116335. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, Y.; Zhu, X.; Brewer, C.E.; Brown, R.C. Ex-Situ Catalytic Pyrolysis of Wastewater Sewage Sludge-A Micro-Pyrolysis Study. Biomass Convers. Biorefinery 2017, 232, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.C.; Wüst, D.; Köhler, H.; Lautenbach, A.; Kruse, A. Novel Approach of Phosphate-Reclamation as Struvite from Sewage Sludge by Utilising Hydrothermal Carbonization. J. Environ. Manag. 2019, 238, 119–125. [Google Scholar] [CrossRef]
- Deng, W.; Tao, C.; Cobb, K.; Zhou, H.; Su, Y.; Ruan, R. Catalytic Oxidation of NO at Ambient Temperature over the Chars from Pyrolysis of Sewage Sludge. Chemosphere 2020, 251, 126429. [Google Scholar] [CrossRef]
- Tytła, M. Assessment of Heavy Metal Pollution and Potential Ecological Risk in Sewage Sludge from Municipal Wastewater Treatment Plant Located in the Most Industrialized Region in Poland—Case Study. Int. J. Environ. Res. Public Health 2019, 16, 2430. [Google Scholar] [CrossRef]
- Peng, C.; Zhai, Y.; Zhu, Y.; Xu, B.; Wang, T.; Li, C.; Zeng, G. Production of Char from Sewage Sludge Employing Hydrothermal Carbonization: Char Properties, Combustion Behavior and Thermal Characteristics. Fuel 2016, 176, 110–118. [Google Scholar] [CrossRef]
- Zhang, J.H.; Lin, Q.M.; Zhao, X.R. The Hydrochar Characters of Municipal Sewage Sludge Under Different Hydrothermal Temperatures and Durations. J. Integr. Agric. 2014, 13, 471–482. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.; Ross, A.B.; Camargo-Valero, M.A. Evaluation and Comparison of Product Yields and Bio-Methane Potential in Sewage Digestate Following Hydrothermal Treatment. Appl. Energy 2017, 208, 1357–1369. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal Carbonization of Biomass: A Summary and Discussion of Chemical Mechanisms for Process Engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Zabaleta, I.; Marchetti, P.; Lohri, C.R.; Zurbrügg, C. Influence of Solid Content and Maximum Temperature on the Performance of a Hydrothermal Carbonization Reactor. Environ. Technol. 2017, 38, 2856–2865. [Google Scholar] [CrossRef]
- Setyawati, W.; Damanhuri, E.; Lestari, P.; Dewi, K. Correlation Equation to Predict HHV of Tropical Peat Based on Its Ultimate Analyses. Procedia Eng. 2015, 125, 298–303. [Google Scholar] [CrossRef]
- Grammelis, P.; Margaritis, N.; Karampinis, E. Solid Fuel Types for Energy Generation: Coal and Fossil Carbon-Derivative Solid Fuels. In Fuel Flexible Energy Generation; Elsevier: Amsterdam, The Netherlands, 2016; pp. 29–58. ISBN 9781782423997. [Google Scholar] [CrossRef]
- Pulka, J.; Manczarski, P.; Koziel, J.A.; Białowiec, A. Torrefaction of Sewage Sludge: Kinetics and Fuel Properties of Biochars. Energies 2019, 12, 565. [Google Scholar] [CrossRef]
- Barry, D.; Barbiero, C.; Briens, C.; Berruti, F. Pyrolysis as an Economical and Ecological Treatment Option for Municipal Sewage Sludge. Biomass Bioenergy 2019, 122, 472–480. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, A.; Pandey, J.K.; Chen, W.; Patel, A.; Ashokkumar, V. Pyrolysis of Sewage Sludge for Sustainable Biofuels and Value-Added Biochar Production. J. Environ. Manag. 2021, 298, 113450. [Google Scholar] [CrossRef]
- Akhtar, J.; Aishah, N.; Amin, S. A Review on Process Conditions for Optimum Bio-Oil Yield in Hydrothermal Liquefaction of Biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Lee, J.-Y.; Mao, X.; Ye, L.; Xu, W.; Ning, X.; Zhang, N.; Teng, H.; Wang, C. Hydrothermal Carbonization of Maize Straw for Hydrochar Production and Its Injection for Blast Furnace. Appl. Energy 2020, 266, 114818. [Google Scholar] [CrossRef]
- He, C.; Giannis, A.; Wang, J.Y. Conversion of Sewage Sludge to Clean Solid Fuel Using Hydrothermal Carbonization: Hydrochar Fuel Characteristics and Combustion Behavior. Appl. Energy 2013, 111, 257–266. [Google Scholar] [CrossRef]
- Xu, Z.-X.; Xu, L.; Cheng, J.-H.; He, Z.-X.; Wang, Q.; Hu, X. Investigation of Pathways for Transformation of N-Heterocycle Compounds during Sewage Sludge Pyrolysis Process. Fuel Process. Technol. 2018, 182, 37–44. [Google Scholar] [CrossRef]
- Volpe, M.; Fiori, L. From Olive Waste to Solid Biofuel through Hydrothermal Carbonisation: The Role of Temperature and Solid Load on Secondary Char Formation and Hydrochar Energy Properties. J. Anal. Appl. Pyrolysis 2017, 124, 63–72. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.-H.; Yang, H.-P.; Chen, H.-P. Characterization of Products from Hydrothermal Treatments of Cellulose. Energy 2012, 42, 457–465. [Google Scholar] [CrossRef]
- Fei, Y.-H.; Zhao, D.; Liu, Y.; Zhang, W.; Tang, Y.-Y.; Huang, X.; Wu, Q.; Wang, Y.-X.; Xiao, T.; Liu, C. Feasibility of Sewage Sludge Derived Hydrochars for Agricultural Application: Nutrients (N, P, K) and Potentially Toxic Elements (Zn, Cu, Pb, Ni, Cd). Chemosphere 2019, 236, 124841. [Google Scholar] [CrossRef]
- Chen, C.; Liu, G.; An, Q.; Lin, L.; Shang, Y.; Wan, C. From Wasted Sludge to Valuable Biochar by Low Temperature Hydrothermal Carbonization Treatment: Insight into the Surface Characteristics. J. Clean. Prod. 2020, 263, 121600. [Google Scholar] [CrossRef]
- Liang, W.; Wang, G.; Xu, R.; Ning, X.; Zhang, J.; Guo, X.; Ye, L.; Li, J.; Jiang, C.; Wang, P.; et al. Hydrothermal Carbonization of Forest Waste into Solid Fuel: Mechanism and Combustion Behavior. Energy 2022, 246, 123343. [Google Scholar] [CrossRef]
- Malhotra, M.; Garg, A. Hydrothermal Carbonization of Centrifuged Sewage Sludge: Determination of Resource Recovery from Liquid Fraction and Thermal Behaviour of Hydrochar. Waste Manag. 2020, 117, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Özçimen, D.; Ersoy-Meriçboyu, A. Characterization of Biochar and Bio-Oil Samples Obtained from Carbonization of Various Biomass Materials. Renew. Energy 2010, 35, 1319–1324. [Google Scholar] [CrossRef]
| Temperature (°C) | Solid Loads (wt.%) | Reaction Time (min) |
|---|---|---|
| 150, 200, 250, 300 | 10 | 30 |
| 150 | 20, 30 | 30 |
| 150 | 30 | 60, 90, 120, 150 |
| Analysis | STP1 | STP2 | STP3 | |
|---|---|---|---|---|
| Moisture content (%, as received) | 73.03 | 83.70 | 80.97 | |
| Proximate (wt.%, db) | Volatile matter | 61.00 | 52.52 | 57.00 |
| Fixed carbon | 10.29 | 12.58 | 12.31 | |
| Ash | 28.71 | 34.90 | 30.69 | |
| Ultimate analysis (wt.%, db) | C | 31.70 | 30.90 | 31.80 |
| H | 7.66 | 6.87 | 7.85 | |
| N | 5.61 | 5.06 | 5.16 | |
| O | 25.02 | 20.99 | 23.24 | |
| S | 1.30 | 1.28 | 1.26 | |
| HHV (MJ/kg) | 13.74 | 13.10 | 13.76 | |
| Major ash forming elements, (wt.%, db) | Si | 5.37 | 5.60 | 5.44 |
| Al | 0.73 | 1.30 | 1.02 | |
| Ti | 0.18 | 0.20 | 0.21 | |
| Fe | 1.69 | 2.35 | 1.90 | |
| Ca | 0.55 | 0.41 | 0.45 | |
| Mg | 0.06 | 0.04 | 0.04 | |
| Na | 0.09 | 0.10 | 0.12 | |
| K | 0.12 | 0.21 | 0.19 | |
| P | 1.38 | 1.13 | 1.42 | |
| Sample ID | Proximate Analysis (wt.%, db) | Ultimate Analysis (wt.%, db) | Solid Yield (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| VM | FC | Ash | C | H | O * | N | S | ||
| HTC1:150 °C:10%:30 min | 41.70 | 16.30 | 42.00 | 39.30 | 4.68 | 8.35 | 3.89 | 1.78 | 73.93 |
| HTC2:200 °C:10%:30 min | 32.15 | 16.85 | 51.00 | 35.80 | 4.18 | 4.55 | 2.85 | 1.62 | 63.09 |
| HTC3:250 °C:10%:30 min | 28.10 | 16.90 | 55.00 | 34.70 | 3.93 | 2.28 | 2.64 | 1.45 | 57.65 |
| HTC4:300 °C:10%:30 min | 23.29 | 19.71 | 57.00 | 33.95 | 3.85 | 1.69 | 2.13 | 1.38 | 48.46 |
| HTC5:150 °C:20%:30 min | 42.08 | 16.61 | 41.31 | 39.52 | 4.69 | 8.78 | 3.91 | 1.79 | 75.97 |
| HTC6:150 °C:30%:30 min | 42.96 | 16.65 | 40.39 | 39.78 | 4.71 | 9.38 | 3.93 | 1.81 | 80.56 |
| HTC7:150 °C:30%:60 min | 40.53 | 16.76 | 42.71 | 39.25 | 4.62 | 7.70 | 3.92 | 1.80 | 78.58 |
| HTC8:150 °C:30%:90 min | 36.80 | 17.41 | 45.79 | 38.23 | 4.61 | 5.68 | 3.89 | 1.80 | 77.35 |
| HTC9:150 °C:30%:120 min | 35.69 | 17.50 | 46.81 | 38.21 | 4.44 | 5.03 | 3.75 | 1.76 | 76.94 |
| HTC10:150 °C:30%:150 min | 35.05 | 17.02 | 47.93 | 38.19 | 4.38 | 4.70 | 3.61 | 1.63 | 74.52 |
| Sample ID | Energy Densification | Fuel Ratio (FC/VM) | Energy Yield (%) |
|---|---|---|---|
| HTC1:150 °C:10%:30 min | 1.12 | 0.39 | 82.79 |
| HTC2:200 °C:10%:30 min | 1.05 | 0.52 | 65.98 |
| HTC3:250 °C:10%:30 min | 1.03 | 0.60 | 59.16 |
| HTC4:300 °C:10%:30 min | 1.01 | 0.85 | 48.96 |
| HTC5:150 °C:20%:30 min | 1.12 | 0.39 | 85.13 |
| HTC6:150 °C:30%:30 min | 1.12 | 0.39 | 90.32 |
| HTC7:150 °C:30%:60 min | 1.12 | 0.41 | 88.06 |
| HTC8:150 °C:30%:90 min | 1.12 | 0.47 | 86.68 |
| HTC9:150 °C:30%:120 min | 1.11 | 0.49 | 85.60 |
| HTC10:150 °C:30%:150 min | 1.11 | 0.49 | 82.76 |
| Sludge Sample Location | Carbonization Method | Condition | Solid Yield (%) | HHV (MJ/kg) | Energy Yield (%) | References |
|---|---|---|---|---|---|---|
| Shah Alam | Hydrothermal carbonization | Temperature:150 °C | 80.56 | 18.49 | 90.32 | This study |
| Time: 30 min | ||||||
| Solid load: 30% | ||||||
| Changsha, China | Hydrothermal carbonization | Temperature:150 °C Time: 30 min | 58.51 | 12.06 | 64.32 | [33] |
| Jastrzebie-Zdrój, Poland | Torrefaction | Temperature: 240 °C | - | 12.06 | - | [40] |
| Time: 60 min | ||||||
| Time: 50 min | ||||||
| London, Ontario, Canada | Slow pyrolysis | Temperature: 300 °C Time: 30 min | 50 | 18.6 | 65.96 | [41] |
| Dehradun, India | Pyrolysis | Temperature: 500 °C Time: 60 min | 58.70 | 10.04 | 50.41 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roslan, S.Z.; Zainudin, S.F.; Mohd Aris, A.; Chin, K.B.; Musa, M.; Mohamad Daud, A.R.; Syed Hassan, S.S.A. Hydrothermal Carbonization of Sewage Sludge into Solid Biofuel: Influences of Process Conditions on the Energetic Properties of Hydrochar. Energies 2023, 16, 2483. https://doi.org/10.3390/en16052483
Roslan SZ, Zainudin SF, Mohd Aris A, Chin KB, Musa M, Mohamad Daud AR, Syed Hassan SSA. Hydrothermal Carbonization of Sewage Sludge into Solid Biofuel: Influences of Process Conditions on the Energetic Properties of Hydrochar. Energies. 2023; 16(5):2483. https://doi.org/10.3390/en16052483
Chicago/Turabian StyleRoslan, Siti Zaharah, Siti Fairuz Zainudin, Alijah Mohd Aris, Khor Bee Chin, Mohibah Musa, Ahmad Rafizan Mohamad Daud, and Syed Shatir A. Syed Hassan. 2023. "Hydrothermal Carbonization of Sewage Sludge into Solid Biofuel: Influences of Process Conditions on the Energetic Properties of Hydrochar" Energies 16, no. 5: 2483. https://doi.org/10.3390/en16052483
APA StyleRoslan, S. Z., Zainudin, S. F., Mohd Aris, A., Chin, K. B., Musa, M., Mohamad Daud, A. R., & Syed Hassan, S. S. A. (2023). Hydrothermal Carbonization of Sewage Sludge into Solid Biofuel: Influences of Process Conditions on the Energetic Properties of Hydrochar. Energies, 16(5), 2483. https://doi.org/10.3390/en16052483







