Analysis of the Combustion Process in a Hydrogen-Fueled CFR Engine
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Test Execution and Data Manipulation
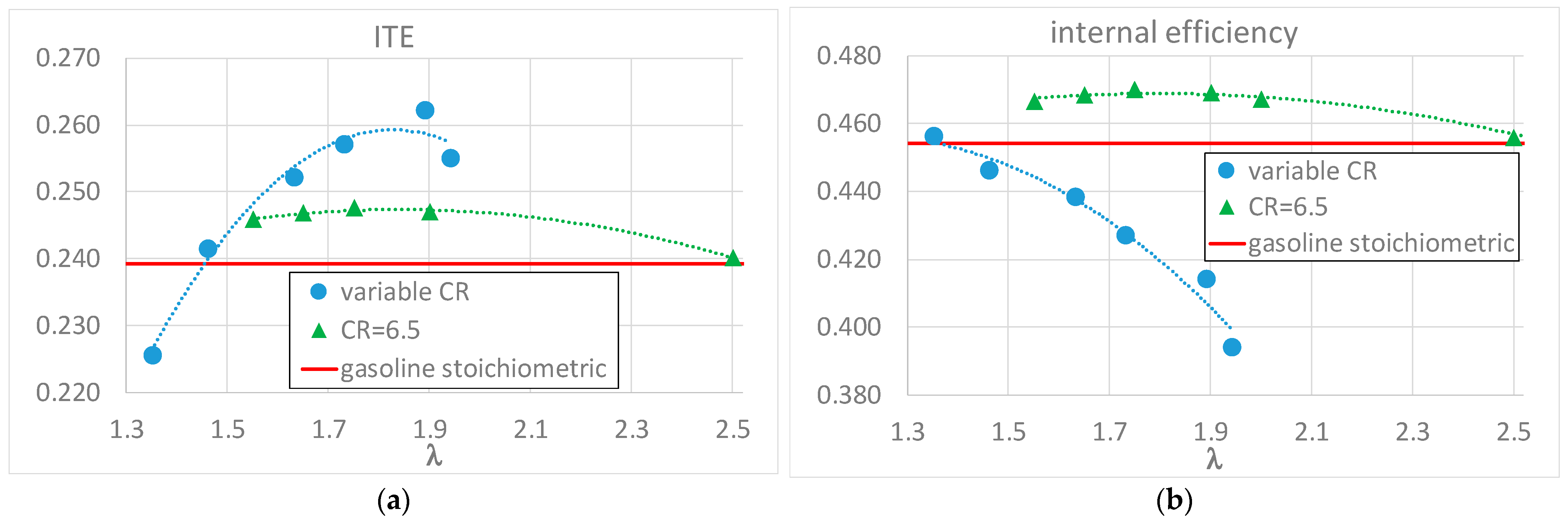
3. Results and Discussion
4. Conclusions
- Increasing λ produces an increasing effect on the duration of both combustion parts;
- Increasing CR produces a decrease of the laminar combustion duration and a slight increase in the turbulent one;
- The whole combustion duration undergoes an increasing effect by λ and a decreasing effect by CR;
- The heat loss to the combustion chamber wall undergoes a slight reduction by increasing λ (due to the combustion temperature reduction) and a great increase by increasing CR (due to the increase of both combustion temperature and convective heat transfer);
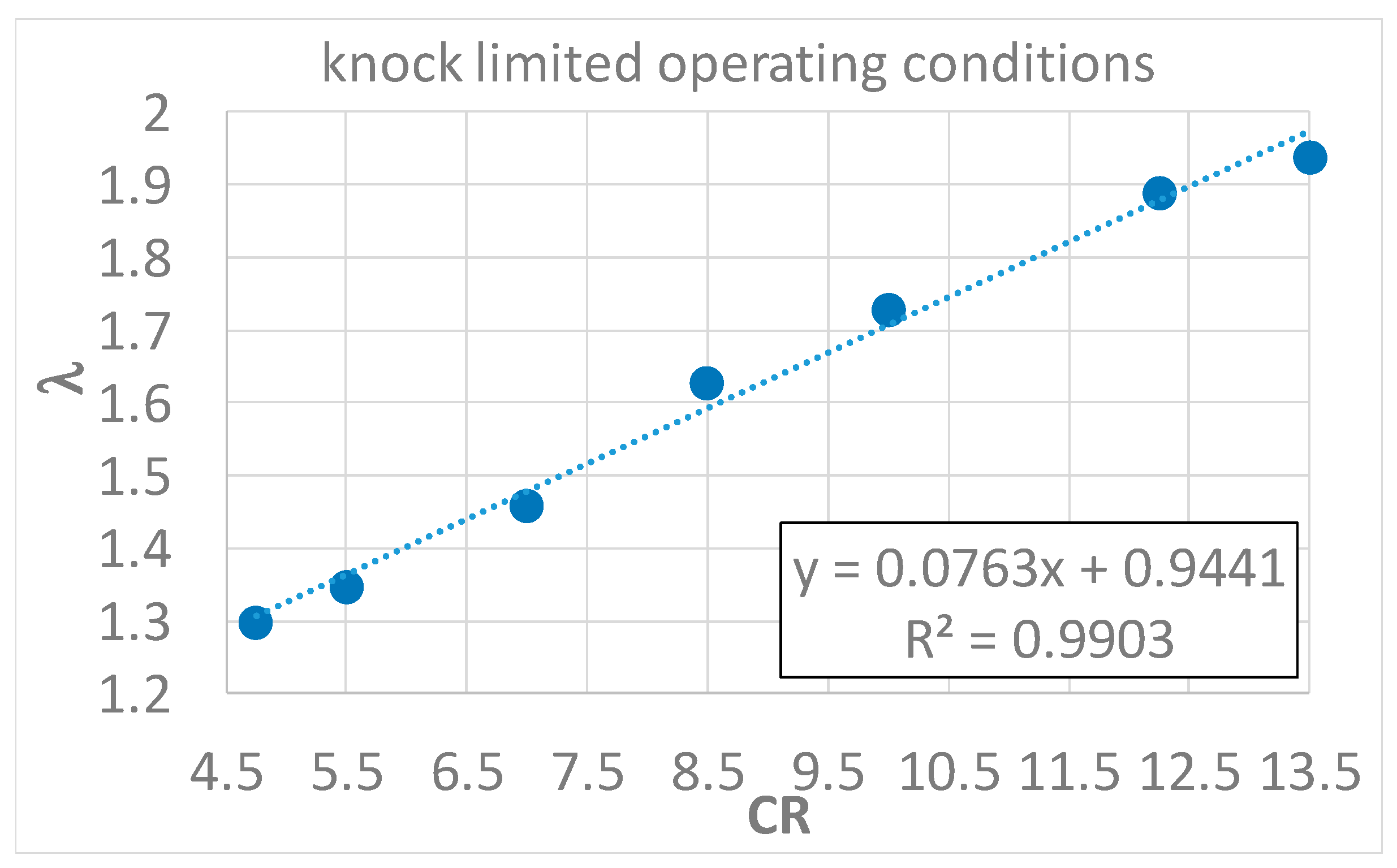
- An experimental correlation between CR and λ was determined, in order to obtain knock-free operating conditions, and a linear trend between the two parameters was found.
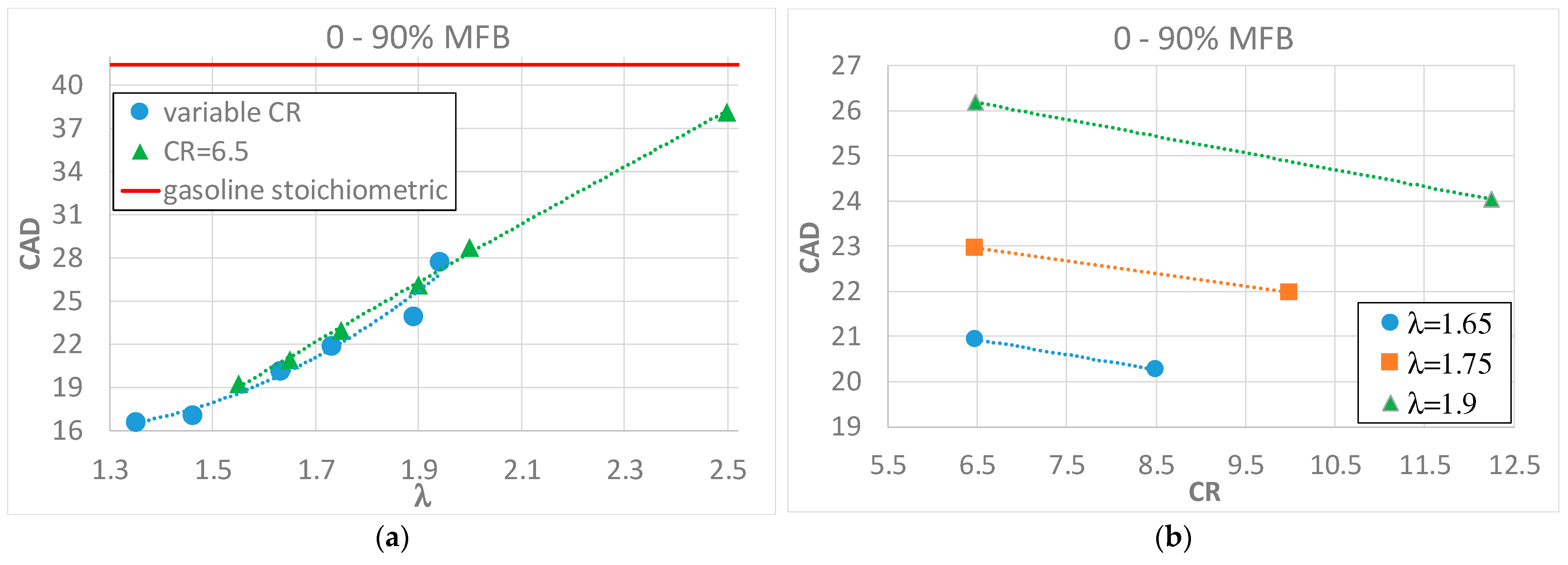
- Hydrogen combustion always shows a shorter duration with respect to gasoline combustion, in both the laminar and the turbulent part;
- For λ > 1.4, the NOx levels are lower than the gasoline ones and for λ > 1.9, the measured NOx are near zero;
- The heat transfer to the chamber wall is higher when fueling with hydrogen with respect to gasoline operation in particular for higher CR.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shu, X.; Guo, Y.; Yang, W.; Wei, K.; Zhu, G. Life-cycle assessment of the environmental impact of the batteries used in pure electric passenger cars. Energy Rep. 2021, 7, 2302–2315. [Google Scholar] [CrossRef]
- Picatoste, A.; Justel, D.; Mendoza, J.M.F. Circularity and life cycle environmental impact assessment of batteries for electric vehicles: Industrial challenges, best practices and research guidelines. Renew. Sustain. Energy Rev. 2022, 169, 112941. [Google Scholar] [CrossRef]
- Mazzeo, D.; Herdem, M.S.; Matera, N.; Wen, J.Z. Green hydrogen production: Analysis for different single or combined large-scale photovoltaic and wind renewable systems. Renew. Energy 2022, 200, 360–378. [Google Scholar] [CrossRef]
- Lee, H.; Choe, B.; Lee, B.; Gu, J.; Cho, H.S.; Won, W.; Lim, H. Outlook of industrial-scale green hydrogen production via a hybrid system of alkaline water electrolysis and energy storage system based on seasonal solar radiation. J. Clean. Prod. 2022, 377, 134210. [Google Scholar] [CrossRef]
- White, C.M.; Steeper, R.R.; Lutz, A.E. The hydrogen-fueled internal combustion engine: A technical review. Int. J. Hydrog. Energy 2006, 31, 1292–1305. [Google Scholar] [CrossRef]
- Verhelst, S. Recent progress in the use of hydrogen as a fuel for internal combustion engines. Int. J. Hydrog. Energy 2014, 39, 1071–1085. [Google Scholar] [CrossRef]
- Verhelst, S.; Wallner, T. Hydrogen-fueled internal combustion engines. Prog. Energy Combust. Sci. 2009, 35, 490–527. [Google Scholar] [CrossRef]
- Saafi, M.A.; Ou, S.; Jiang, Y.; Li, H.; He, X.; Lin, Z.; Gan, Y.; Lu, Z.; Dawson, S.T.M. Exploring the potential of hydrogen in decarbonizing China’s light-duty vehicle market. Int. J. Hydrog. Energy 2022, 47, 36355–36371. [Google Scholar] [CrossRef]
- Sopena, C.; Diéguez, P.M.; Sáinz, D.; Urroz, J.C.; Guelbenzu, E.; Gandía, L.M. Conversion of a commercial spark ignition engine to run on hydrogen: Performance comparison using hydrogen and gasoline. Int. J. Hydrog. Energy 2010, 35, 1420–1429. [Google Scholar] [CrossRef]
- Keller, J.; Lutz, A. Hydrogen Fueled Engines in Hybrid Vehicles. SAE Trans. 2001, 110, 481–486. Available online: http://www.jstor.org/stable/44724324 (accessed on 26 February 2023).
- Wang, Y.; Biswas, A.; Rodriguez, R.; Keshavarz-Motamed, Z.; Emadi, A. Hybrid electric vehicle specific engines: State-of-the-art review. Energy Rep. 2022, 8, 832–851. [Google Scholar] [CrossRef]
- Arat, H.T. Simulation of diesel hybrid electric vehicle containing hydrogen enriched CI engine. Int. J. Hydrog. Energy 2019, 44, 10139–10146. [Google Scholar] [CrossRef]
- Arat, H.T. Alternative fuelled hybrid electric vehicle (AF-HEV) with hydrogen enriched internal combustion engine. Int. J. Hydrog. Energy 2019, 44, 19005–19016. [Google Scholar] [CrossRef]
- He, X.; Maxwell, T.; Parten, M.E. Development of a Hybrid Electric Vehicle With a Hydrogen-Fueled IC Engine. IEEE Trans. Veh. Technol. 2006, 55, 1693–1703. [Google Scholar] [CrossRef]
- Nakajima, Y.; Yamane, K.; Shudo, T.; Hiruma, M.; Takagi, Y. Research and Development of a Hydrogen-Fueled Engine for Hybrid Electric Vehicles. SAE Trans. 2000, 109, 1175–1179. Available online: http://www.jstor.org/stable/44634296 (accessed on 26 February 2023).
- Dahoe, A.E. Laminar burning velocities of hydrogen–air mixtures from closed vessel gas explosions. J. Loss Prev. Process Ind. 2005, 18, 152–166. [Google Scholar] [CrossRef]
- Shudo, T.; Nakajima, Y.; Futakuchi, T. Thermal efficiency analysis in a hydrogen premixed combustion engine. JSAE Rev. 2000, 21, 177–182. [Google Scholar] [CrossRef]
- Nagalingam, B.; Dübel, M.; Schmillen, K. Performance of the Supercharged Spark Ignition Hydrogen Engine; SAE Technical Paper 831688; SAE International: Warrendale, PA, USA, 1983. [Google Scholar] [CrossRef]
- Berckmüller, M.; Rottengruber, H.; Eder, A.; Brehm, N.; Elsässer, G.; Müller-Alander, G.; Schwarz, C. Potentials of a Charged SI-Hydrogen Engine; SAE Technical Paper 2003-01-3210; SAE International: Warrendale, PA, USA, 2003. [Google Scholar] [CrossRef]
- Natkin, R.; Tang, X.; Boyer, B.; Oltmans, B.; Denlinger, A.; Heffel, J.W. Hydrogen IC Engine Boosting Performance and NOx Study; SAE Technical Paper 2003-01-0631; SAE International: Warrendale, PA, USA, 2003. [Google Scholar] [CrossRef]
- Salvi, B.L.; Subramanian, K.A. A novel approach for experimental study and numerical modeling of combustion characteristics of a hydrogen fueled spark ignition engine. Sustain. Energy Technol. Assess. 2022, 51, 101972. [Google Scholar] [CrossRef]
- ASTM Standard D2700; Standard Test Method for Motor Octane Number of Spark Ignition Engine Fuel. ASTM International: West Conshohocken, PA, USA, 2022. [CrossRef]
- Beccari, S.; Pipitone, E.; Genchi, G. Knock onset prediction of propane, gasoline and their mixtures in spark ignition engines. J. Energy Inst. 2016, 89, 101–114. [Google Scholar] [CrossRef]
- Beccari, S.; Pipitone, E.; Genchi, G. Calibration of a Knock Prediction Model for the Combustion of Gasoline-LPG Mixtures in Spark Ignition Engines. Combust. Sci. Technol. 2015, 187, 721–738. [Google Scholar] [CrossRef]
- Pipitone, E.; Beccari, S. A Comprehensive Model for the Auto-Ignition Prediction in Spark Ignition Engines Fueled With Mixtures of Gasoline and Methane-Based Fuel. J. Eng. Gas Turbines Power 2019, 141, 041009. [Google Scholar] [CrossRef]
- Rassweiler, G.; Withrow, L. Motion Pictures of Engine Flames Correlated with Pressure Cards; SAE Technical Paper 380139; SAE International: Warrendale, PA, USA, 1938. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw-Hill: New York, NY, USA, 1988. [Google Scholar]
- Falfari, S.; Brusiani, F.; Pelloni, P. 3D CFD Analysis of the Influence of Some Geometrical Engine Parameters on Small PFI Engine Performances—The Effects on the Tumble Motion and the Mean Turbulent Intensity Distribution. Energy Procedia 2014, 45, 701–710. [Google Scholar] [CrossRef]






| Specification | H2 | CH4 | C8H18 | H2-Air | CH4-Air | C8H18-Air |
|---|---|---|---|---|---|---|
| laminar flame speed at 360 K [cm/s] | - | - | - | 290 | 48 | 45 |
| lower heating value [MJ/kg] | 120 | 50 | 44.3 | - | - | - |
| density [kg/m3] | 0.08 | 0.65 | 692 | - | - | - |
| volumetric energy content [kJ/m3] | - | - | - | 3189 | 3041 | 3704 |
| minimum ignition energy [mJ] | - | - | - | 0.02 | 0.28 | 0.28 |
| adiabatic flame temperature [K] | - | - | - | 2390 | 2226 | 2276 |
| minimum quencing distance [mm] | - | - | - | 0.64 | 2.03 | 3.5 |
| stoichiometric air-fuel ratio [kg/kg] | 34.2 | 17.1 | 15 | - | - | - |
| flammability limits (λ) | 10–0.14 | 2–0.6 | 1.51–0.26 | - | - | - |
| Specification | Value |
|---|---|
| Manufacturer | Dresser waukesha |
| Model | F1/F2 Octane |
| Compression ratio | 4.5–16 |
| Inlet mixture temperature | variable |
| Spark advance | variable |
| Bore | 82.6 [mm] |
| Stroke | 114.3 [mm] |
| Connecting rod length | 254.0 [mm] |
| Displacement | 611.2 [cm3] |
| Speed (fixed) | 900 [rpm] |
| Sensor | Accuracy |
|---|---|
| Gasoline mass flow meter | ±1% of reading |
| Hydrogen mass flow meter | ±1% of reading |
| Air mass flow meter | ±1% of reading |
| Combustion chamber pressure sensor | linearity error < ±0.3% FSO |
| Combustion chamber pressure sensor | thermal sensitivity shift < ±0.5% at temperature between 200 and 300 °C |
| NOx sensor | ±4% or 25 ppm absolute |
| Fuel | A/F Mixture Inlet Temperature (°C) | Engine CR | λ | SA (CAD BTDC) | Average Combustion Temperature T′c [K] |
|---|---|---|---|---|---|
| gasoline | 42 | 6.5 | 1.00 | 25 | 1237 |
| hydrogen | 43 | 4.75 | 1.3 | 5 | 1053 |
| hydrogen | 43 | 5.5 | 1.35 | 4 | 1037 |
| hydrogen | 43 | 7.0 | 1.46 | 4 | 1060 |
| hydrogen | 44 | 8.5 | 1.63 | 5 | 1030 |
| hydrogen | 44 | 10.0 | 1.73 | 5 | 1022 |
| hydrogen | 44 | 12.3 | 1.89 | 6 | 982 |
| hydrogen | 45 | 13.5 | 1.94 | 3 | 951 |
| hydrogen | 44 | 6.5 | 1.55 | 4 | 1034 |
| hydrogen | 44 | 6.5 | 1.65 | 7 | 1006 |
| hydrogen | 45 | 6.5 | 1.75 | 9 | 973 |
| hydrogen | 45 | 6.5 | 1.90 | 12 | 945 |
| hydrogen | 45 | 6.5 | 2.00 | 15 | 916 |
| hydrogen | 45 | 6.5 | 2.50 | 21 | 835 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beccari, S.; Pipitone, E.; Caltabellotta, S. Analysis of the Combustion Process in a Hydrogen-Fueled CFR Engine. Energies 2023, 16, 2351. https://doi.org/10.3390/en16052351
Beccari S, Pipitone E, Caltabellotta S. Analysis of the Combustion Process in a Hydrogen-Fueled CFR Engine. Energies. 2023; 16(5):2351. https://doi.org/10.3390/en16052351
Chicago/Turabian StyleBeccari, Stefano, Emiliano Pipitone, and Salvatore Caltabellotta. 2023. "Analysis of the Combustion Process in a Hydrogen-Fueled CFR Engine" Energies 16, no. 5: 2351. https://doi.org/10.3390/en16052351
APA StyleBeccari, S., Pipitone, E., & Caltabellotta, S. (2023). Analysis of the Combustion Process in a Hydrogen-Fueled CFR Engine. Energies, 16(5), 2351. https://doi.org/10.3390/en16052351







