Thermal Degradation Studies and Machine Learning Modelling of Nano-Enhanced Sugar Alcohol-Based Phase Change Materials for Medium Temperature Applications
Abstract
1. Introduction
2. Materials and Methodology
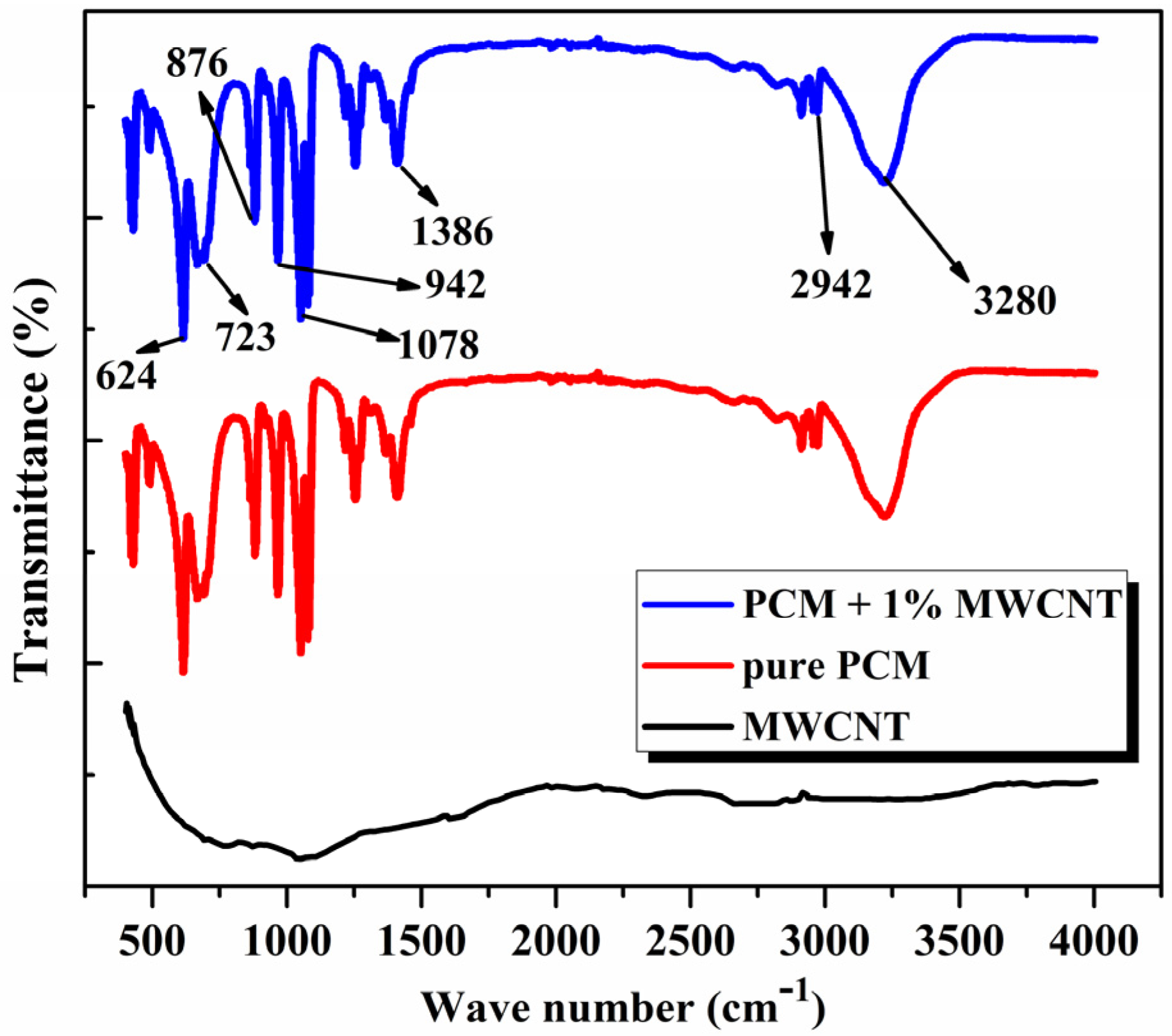
2.1. Sample Preparation and Chemical Stability of Nano PCM Composites
2.2. Thermogravimetric Analysis of Nano-PCM Samples
2.2.1. Model-Free Kinetic Methods
2.2.2. Kissinger–Akahira–Sunose (KAS)
2.2.3. Flynn-Wall-Ozawa (FWO)
2.2.4. Starink Method
2.3. Description of Machine Learning Models
2.3.1. Linear Regression
2.3.2. Support Vector Machine
2.3.3. Random Forest Regression
2.3.4. Gaussian Process Regression
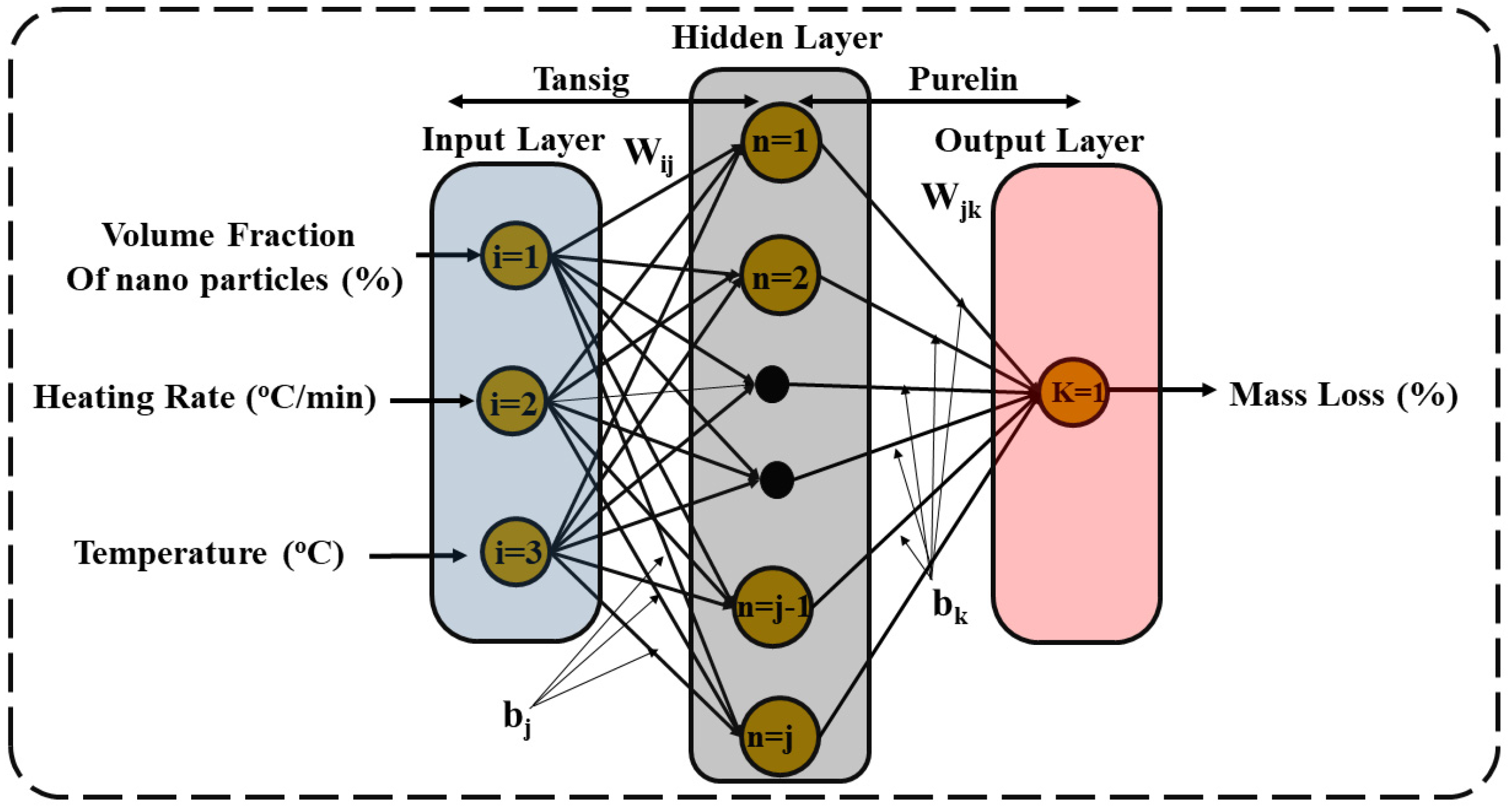
2.3.5. ANN Modelling
3. Results and Discussion
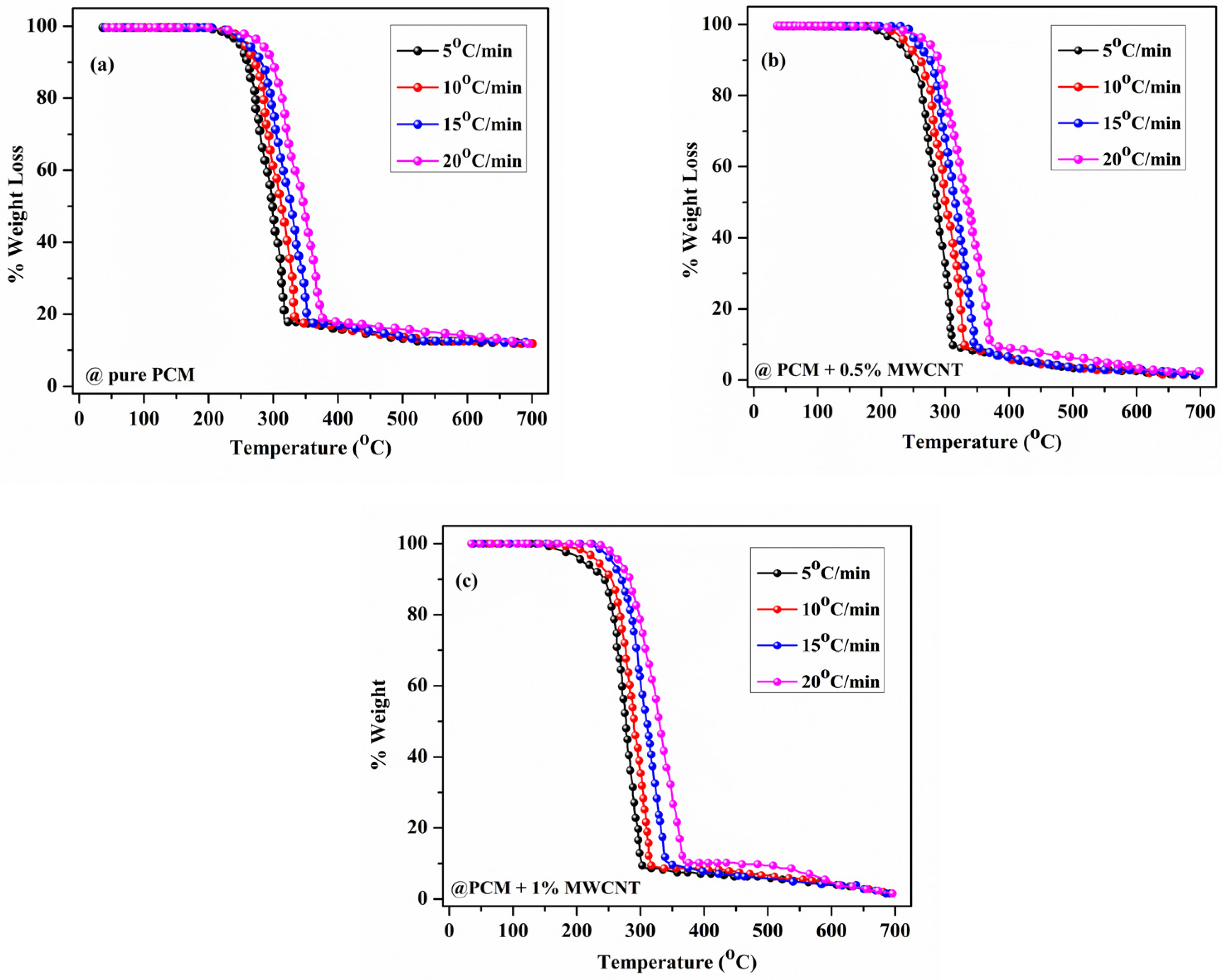
3.1. Thermogravimetric Analysis of Prepared Nano-Enhanced PCM Samples
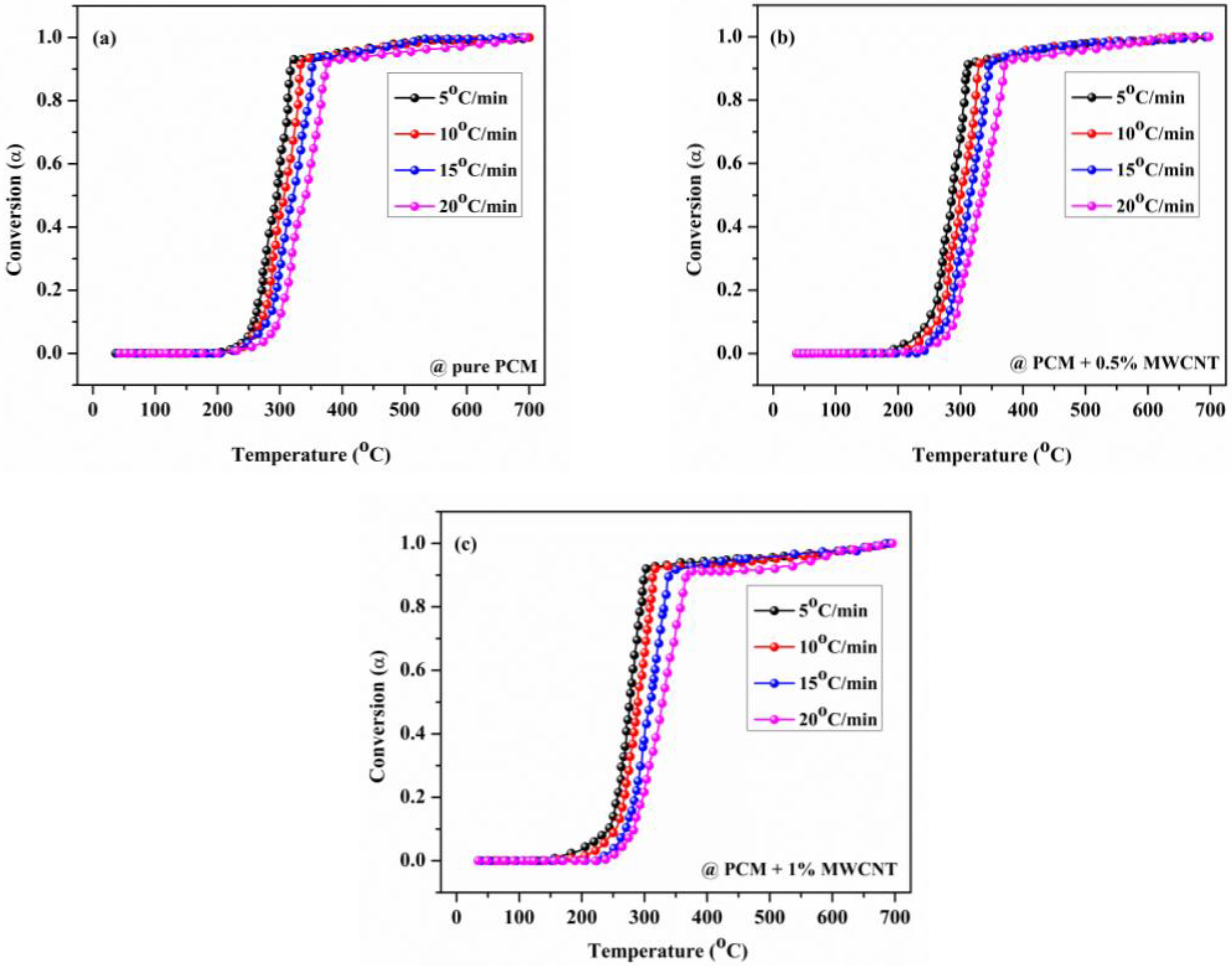
3.2. Kinetic Characterization of Nano Enhanced PCM Samples
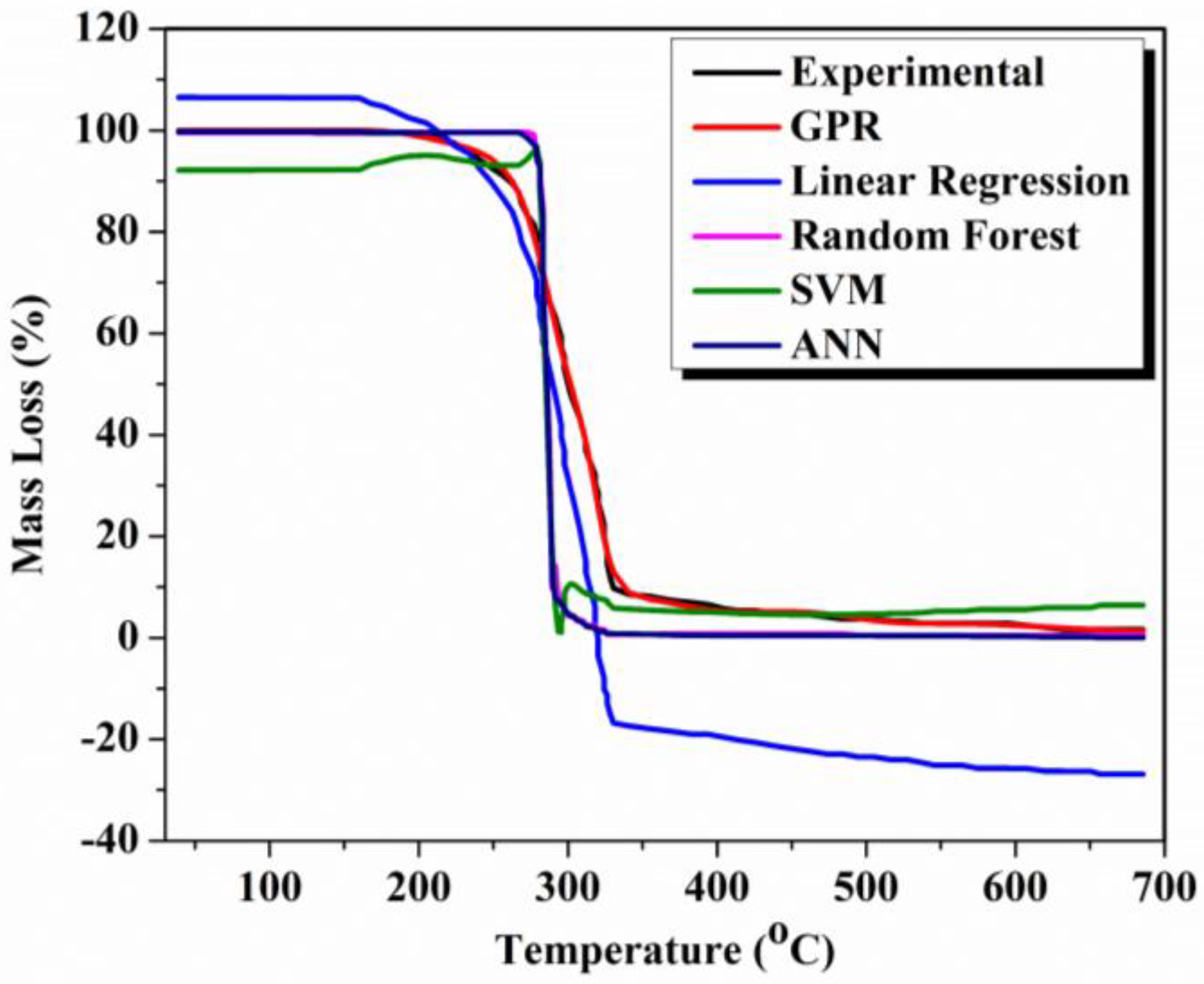
3.3. Machine Learning Modelling of Thermal Degradation of Nano Enhanced PCM Samples
3.4. Hyper-Parameter Tuning of Developed Machine Learning Models
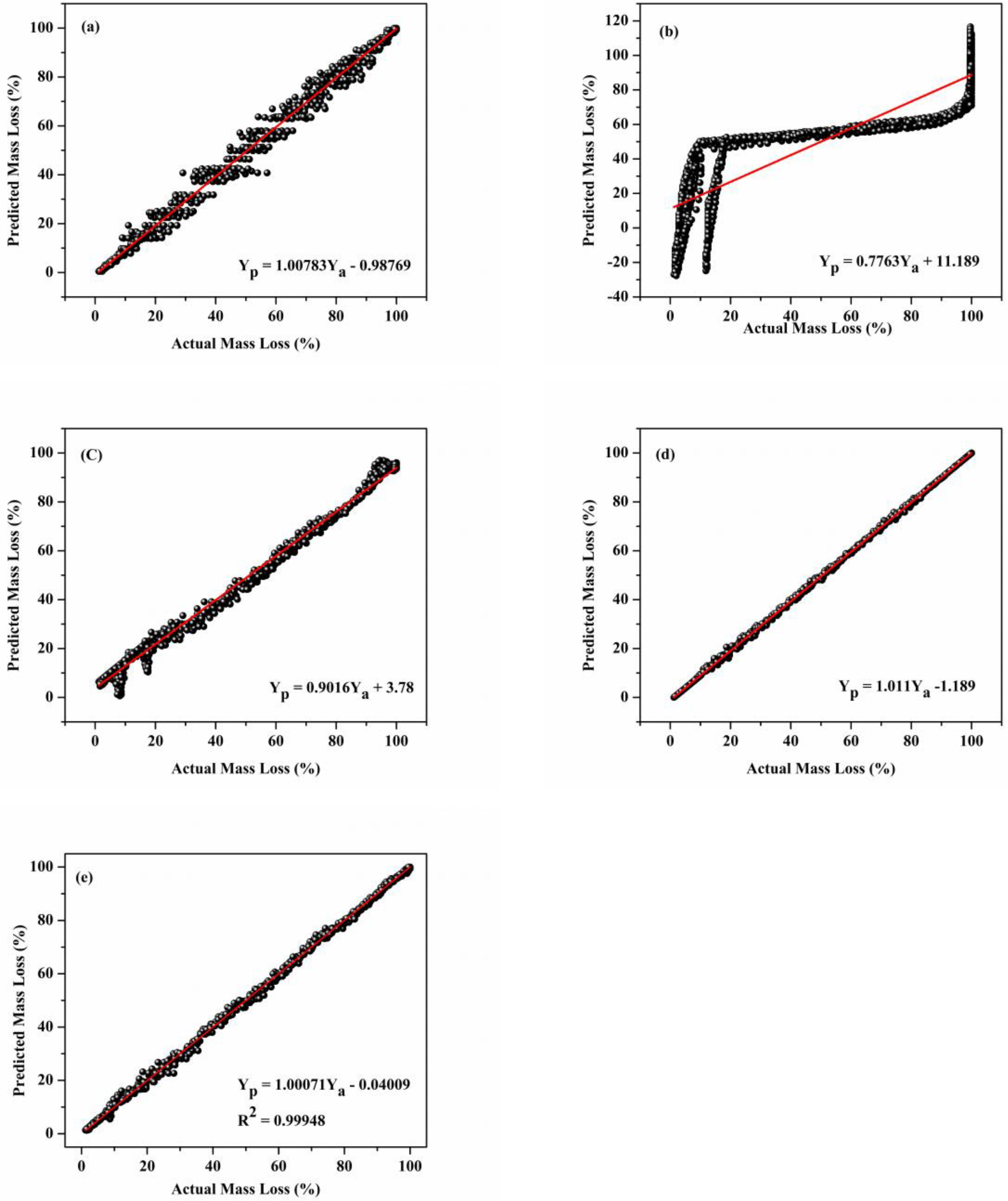
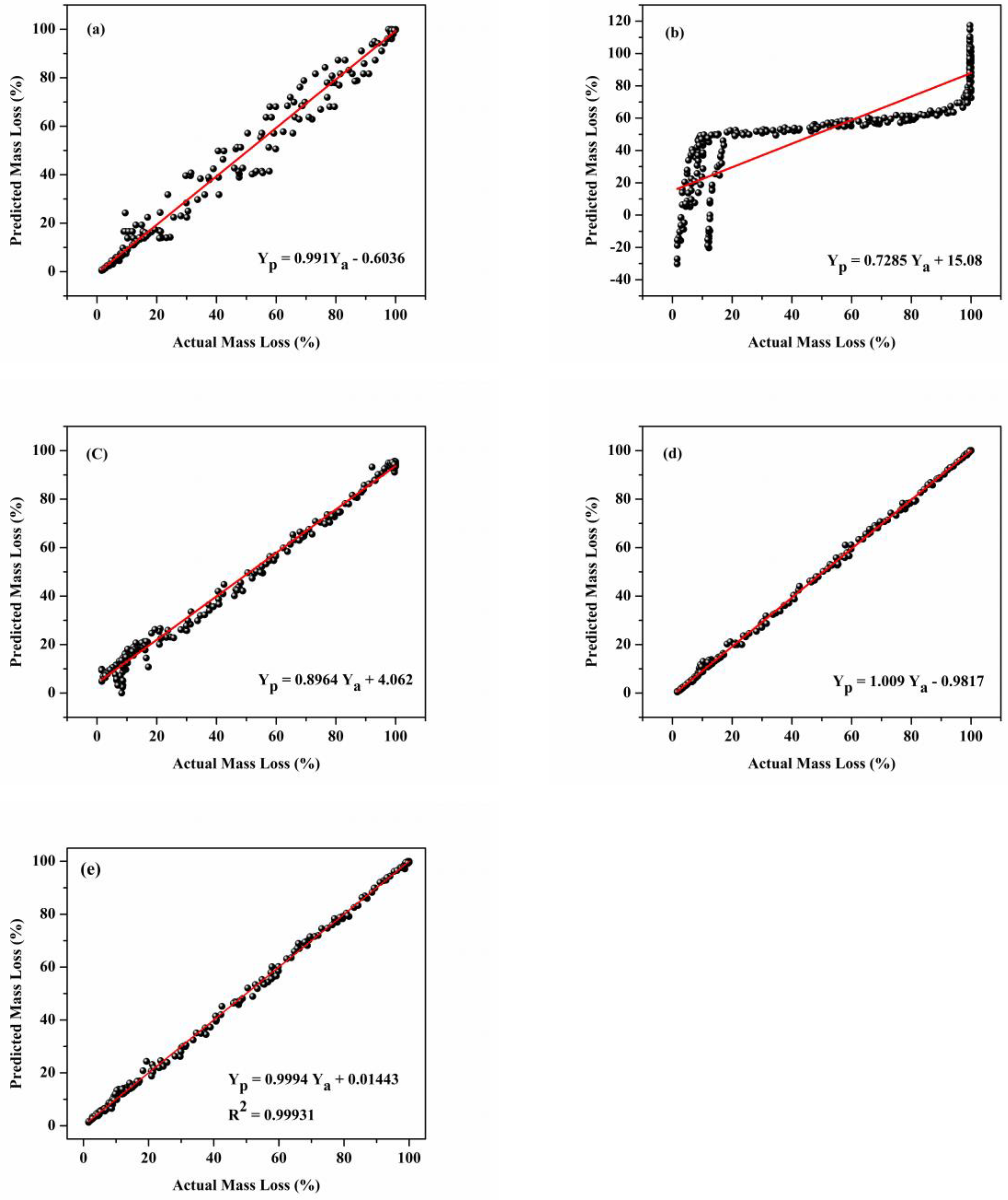
3.5. Comparison of Model Accuracy of Developed Machine Learning Models
3.6. Sensitivity Analysis
3.7. Model Accuracy Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jinshah, B.S.; Ramaraj, B.K.; Kottala, R.K.; Sivapirakasam, S.P. A complete numerical model for low temperature composite form-stable phase change material slab based on dynamically simplified temperature transforming method. Energy Sources A Recovery Util. Environ. Eff. 2021, 1–26. [Google Scholar] [CrossRef]
- Balasubramanian, K.R.; Jinshah, B.S.; Ravikumar, K.; Divakar, S. Thermal and hydraulic characteristics of a parabolic trough collector based on an open natural circulation loop: The effect of fluctuations in solar irradiance. Sustain. Energy Technol. Assess. 2022, 52, 102290. [Google Scholar] [CrossRef]
- Jinshah, B.S.; Kottala, R.K.; Balasubramanian, K.R.; Francis, A. Experimental analysis of phase change material integrated single-phase natural circulation loop. Mater. Today Proc. 2021, 46, 10000–10005. [Google Scholar] [CrossRef]
- Jinshah, B.S.; Balasubramanian, K.R.; Kottala, R.; Divakar, S. Influence of power step on the behavior of an Open Natural Circulation Loop as applied to a Parabolic Trough Collector. Renew. Energy 2022, 181, 1046–1061. [Google Scholar] [CrossRef]
- Marudaipillai, S.K.; Karuppudayar Ramaraj, B.; Kottala, R.K.; Lakshmanan, M. Experimental study on thermal management and performance improvement of solar PV panel cooling using form-stable phase change material. Energy Sources A Recovery Util. Environ. Eff. 2020, 45, 160–177. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Balasubramanian, K.R.; Kottala, R.K.; Sivapirakasam, S.P.; Maheswari, L. Characterization of form-stable phase-change material for solar photovoltaic cooling. J. Therm. Anal. Calorim. 2020, 141, 2487–2496. [Google Scholar] [CrossRef]
- Goud, M. A comprehensive investigation and artificial neural network modeling of shape stabilized composite phase change material for solar thermal energy storage. J. Energy Storage 2022, 48, 103992. [Google Scholar] [CrossRef]
- Goud, M.; Raval, F. A sustainable biochar-based shape stable composite phase change material for thermal management of a lithium-ion battery system and hybrid neural network modeling for heat flow prediction. J. Energy Storage 2022, 56, 106163. [Google Scholar] [CrossRef]
- Peter, R.J.; Balasubramanian, K.R.; Kumar, K.R. Comparative study on the thermal performance of microencapsulated phase change material slurry in tortuous geometry microchannel heat sink. Appl. Therm. Eng. 2023, 218, 119328. [Google Scholar] [CrossRef]
- Luo, J.; Zou, D.; Wang, Y.; Wang, S.; Huang, L. Battery thermal management systems (BTMs) based on phase change material (PCM): A comprehensive review. J. Chem. Eng. 2022, 430, 132741. [Google Scholar] [CrossRef]
- Karthick, A.; Manokar Athikesavan, M.; Pasupathi, M.K.; Manoj Kumar, N.; Chopra, S.S.; Ghosh, A. Investigation of inorganic phase change material for a semi-transparent photovoltaic (STPV) module. Energies 2020, 13, 3582. [Google Scholar] [CrossRef]
- Ye, R.; Fang, X.; Zhang, Z. Numerical Study on Energy-Saving Performance of a New Type of Phase Change Material Room. Energies 2021, 14, 3874. [Google Scholar] [CrossRef]
- Hua, W.; Xu, X.; Zhang, X.; Yan, H.; Zhang, J. Progress in corrosion and anti-corrosion measures of phase change materials in thermal storage and management systems. J. Energy Storage 2022, 56, 105883. [Google Scholar] [CrossRef]
- Bell, S.; Jones, M.W.M.; Graham, E.; Peterson, D.J.; van Riessen, G.A.; Hinsley, G.; Will, G. Corrosion mechanism of SS316L exposed to NaCl/Na2CO3 molten salt in air and argon environments. Corros. Sci. 2022, 195, 109966. [Google Scholar] [CrossRef]
- Moreno, P.; Miro, L.; Sole, A.; Barreneche, C.; Sole, C.; Martorell, I.; Cabeza, L.F. Corrosion of metal and metal alloy containers in contact with phase change materials (PCM) for potential heating and cooling applications. Appl. Energy 2014, 125, 238–245. [Google Scholar] [CrossRef]
- Ravi Kumar, K.; Balasubramanian, K.R.; Jinshah, B.S.; Abhishek, N. Experimental analysis and neural network model of MWCNTs enhanced phase change materials. Int. J. Thermophys. 2022, 43, 11. [Google Scholar] [CrossRef]
- Kottala, R.K.; Ramaraj, B.K.; BS, J.; Vempally, M.G.; Lakshmanan, M. Experimental investigation and neural network modeling of binary eutectic/expanded graphite composites for medium temperature thermal energy storage. Energy Sources A Recovery Util. Environ. Eff. 2022, 1–24. [Google Scholar] [CrossRef]
- Png, Z.M.; Soo, X.Y.D.; Chua, M.H.; Ong, P.J.; Suwardi, A.; Tan, C.K.I.; Zhu, Q. Strategies to reduce the flammability of organic phase change Materials: A review. Sol. Energy 2022, 231, 115–128. [Google Scholar] [CrossRef]
- Saleel, C.A. A review on the use of coconut oil as an organic phase change material with its melting process, heat transfer, and energy storage characteristics. J. Therm. Anal. Calorim. 2022, 147, 4451–4472. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Chopra, K.; Sharma, R.K.; Pandey, A.K.; Tyagi, S.K.; Ahmad, M.S.; Kothari, R. A comprehensive review on phase change materials for heat storage applications: Development, characterization, thermal and chemical stability. Sol. Energy Mater. Sol. Cells 2022, 234, 111392. [Google Scholar] [CrossRef]
- Ma, C.; Wang, J.; Wu, Y.; Wang, Y.; Ji, Z.; Xie, S. Characterization and thermophysical properties of erythritol/expanded graphite as phase change material for thermal energy storage. J. Energy Storage 2022, 46, 103864. [Google Scholar] [CrossRef]
- Ramaraj, B.K.; Kottala, R.K. Preparation and characterisation of binary eutectic phase change material/activated porous bio char/multi walled carbon nano tubes as composite phase change material. Fuller. Nanotub. Carbon Nanostructures 2022, 31, 75–89. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, H.; Feng, Y.; Qiu, L.; Lin, L.; Zhang, X.; Ohara, T. Excellent heat transfer and phase transformation performance of erythritol/graphene composite phase change materials. Compos. B Eng. 2022, 228, 109435. [Google Scholar] [CrossRef]
- Wang, K.; Sun, C.; Biney, B.W.; Li, W.; Al-shiaani, N.H.; Chen, K.; Guo, A. Polyurethane template-based erythritol/graphite foam composite phase change materials with enhanced thermal conductivity and solar-thermal energy conversion efficiency. Polymer 2022, 256, 125204. [Google Scholar] [CrossRef]
- Lin, W.; Lai, J.; Xie, K.; Liu, D.; Wu, K.; Fu, Q. D-Mannitol/Graphene Phase-Change Composites with Structured Conformation and Thermal Pathways Allow Durable Solar–Thermal–Electric Conversion and Electricity Output. ACS Appl. Mater. Interfaces 2022, 14, 38981–38989. [Google Scholar] [CrossRef]
- Jame, L.; Perumal, S.; Moorthy, M.; Kumar, J.R. Nanocomposites of GO/D-Mannitol Assisted Thermoelectric Power Generator for Transient Waste Heat Recovery. J. Nanomater. 2022, 2022, 2460364. [Google Scholar] [CrossRef]
- Balasubramanian, K.R.; Ravi Kumar, K.; Sathiya Prabhakaran, S.P.; Jinshah, B.S.; Abhishek, N. Thermal degradation studies and hybrid neural network modelling of eutectic phase change material composites. Int. J. Energy Res. 2022, 46, 15733–15755. [Google Scholar] [CrossRef]
- Venkitaraj, K.P.; Suresh, S. Experimental thermal degradation analysis of pentaerythritol with alumina nano additives for thermal energy storage application. J. Energy Storage 2019, 22, 8–16. [Google Scholar] [CrossRef]
- Sun, L.; Qu, Y.; Li, S. Co-microencapsulate of ammonium polyphosphate and pentaerythritol and kinetics of its thermal degradation. Polym. Degrad. Stab. 2012, 97, 404–409. [Google Scholar] [CrossRef]
- Fabiani, C.; Pisello, A.L.; Barbanera, M.; Cabeza, L.F. Palm oil-based bio-PCM for energy efficient building applications: Multipurpose thermal investigation and life cycle assessment. J. Energy Storage 2020, 28, 101129. [Google Scholar] [CrossRef]
- Xiang, L.; Luo, D.; Yang, J.; Sun, X.; Qi, Y.; Qin, S. Preparation and comparison of properties of three phase change energy storage materials with hollow fiber membrane as the supporting carrier. Polymers 2019, 11, 1343. [Google Scholar] [CrossRef]
- Cheepu, M. Machine Learning Approach for the Prediction of Defect Characteristics in Wire Arc Additive Manufacturing. Trans. Indian Inst. Met. 2022, 13, 1–9. [Google Scholar] [CrossRef]
- Kumar, C.B.; Anandakrishnan, V. Experimental investigations on the effect of wire arc additive manufacturing process parameters on the layer geometry of Inconel 825. Mater. Today Proc. 2020, 21, 622–627. [Google Scholar] [CrossRef]
- Chigilipalli, B.K.; Veeramani, A. An experimental investigation and neuro-fuzzy modeling to ascertain metal deposition parameters for the wire arc additive manufacturing of Incoloy 825. CIRP J. Manuf. Sci. Technol. 2022, 38, 386–400. [Google Scholar] [CrossRef]
- Kumar, K.R.; Balasubramanian, K.R.; Kumar, G.P.; Bharat Kumar, C.; Cheepu, M.M. Experimental Investigation of Nano-encapsulated Molten Salt for Medium-Temperature Thermal Storage Systems and Modeling of Neural Networks. Int. J. Thermophys. 2022, 43, 145. [Google Scholar] [CrossRef]
- Ozveren, U.; Kartal, F.; Sezer, S.; Ozdogan, Z.S. Investigation of steam gasification in thermogravimetric analysis by means of evolved gas analysis and machine learning. Energy 2022, 239, 122232. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Z.; Jin, Y.; Zaini, I.N.; Li, Y.; Tang, C.; Yang, W. A machine learning model to predict the pyrolytic kinetics of different types of feedstocks. Energy Convers. Manag. 2022, 260, 115613. [Google Scholar] [CrossRef]
- Balsora, H.K.; Kartik, S.; Dua, V.; Joshi, J.B.; Kataria, G.; Sharma, A.; Chakinala, A.G. Machine learning approach for the prediction of biomass pyrolysis kinetics from preliminary analysis. J. Environ. Chem. Eng. 2022, 10, 108025. [Google Scholar] [CrossRef]
- Wei, H.; Luo, K.; Xing, J.; Fan, J. Predicting co-pyrolysis of coal and biomass using machine learning approaches. Fuel 2022, 310, 122248. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Starink, M.J. A new method for the derivation of activation energies from experiments performed at constant heating rate. Thermochim. Acta 1996, 288, 97–104. [Google Scholar] [CrossRef]
- Mavromatidis, L.E.; Bykalyuk, A.; Lequay, H. Development of polynomial regression models for composite dynamic envelopes’ thermal performance forecasting. Appl. Energy 2013, 104, 379–391. [Google Scholar] [CrossRef]
- Ahmad, M.W.; Reynolds, J.; Rezgui, Y. Predictive modelling for solar thermal energy systems: A comparison of support vector regression, random forest, extra trees and regression trees. J. Clean. Prod. 2018, 203, 810–821. [Google Scholar] [CrossRef]

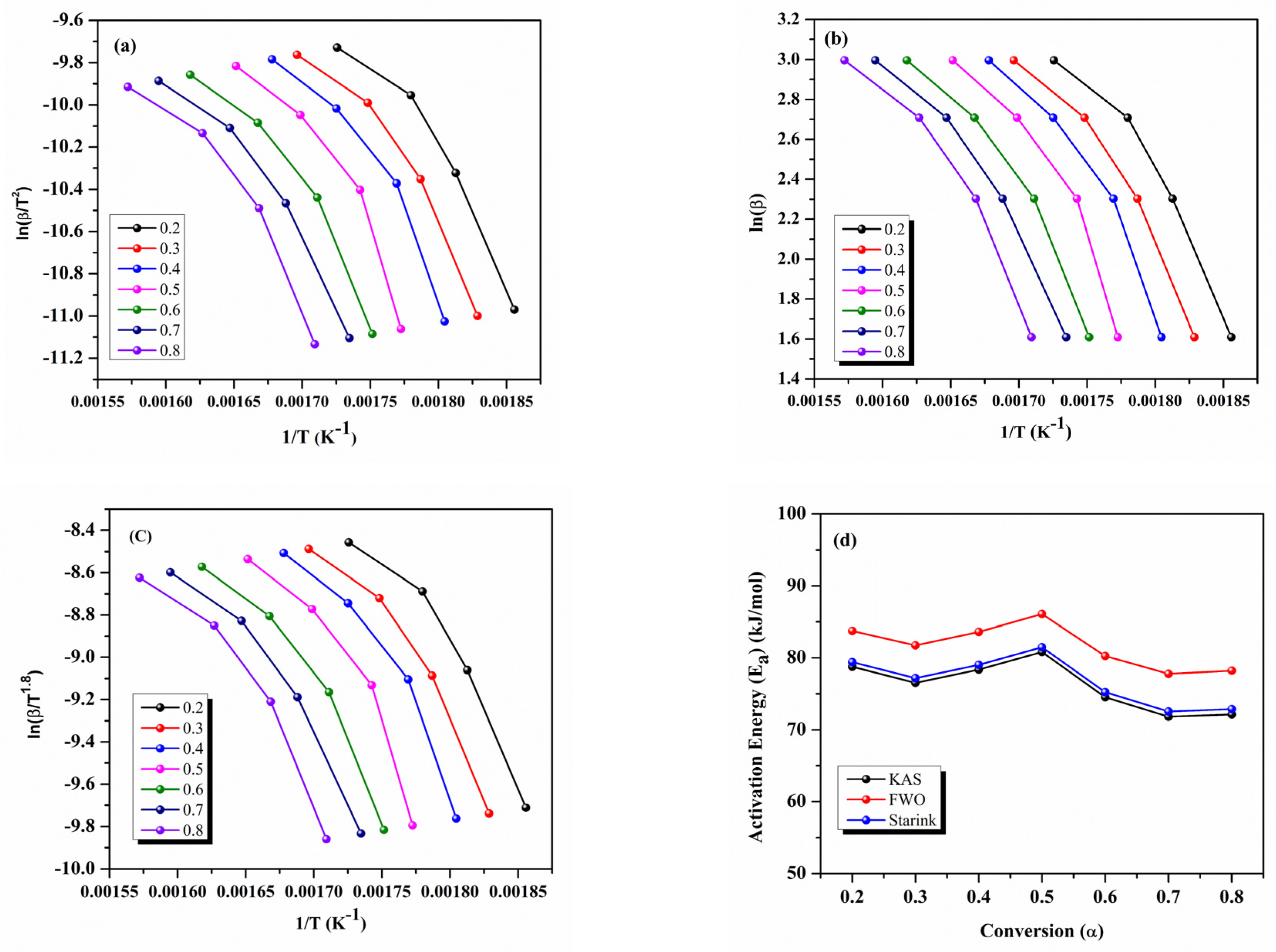

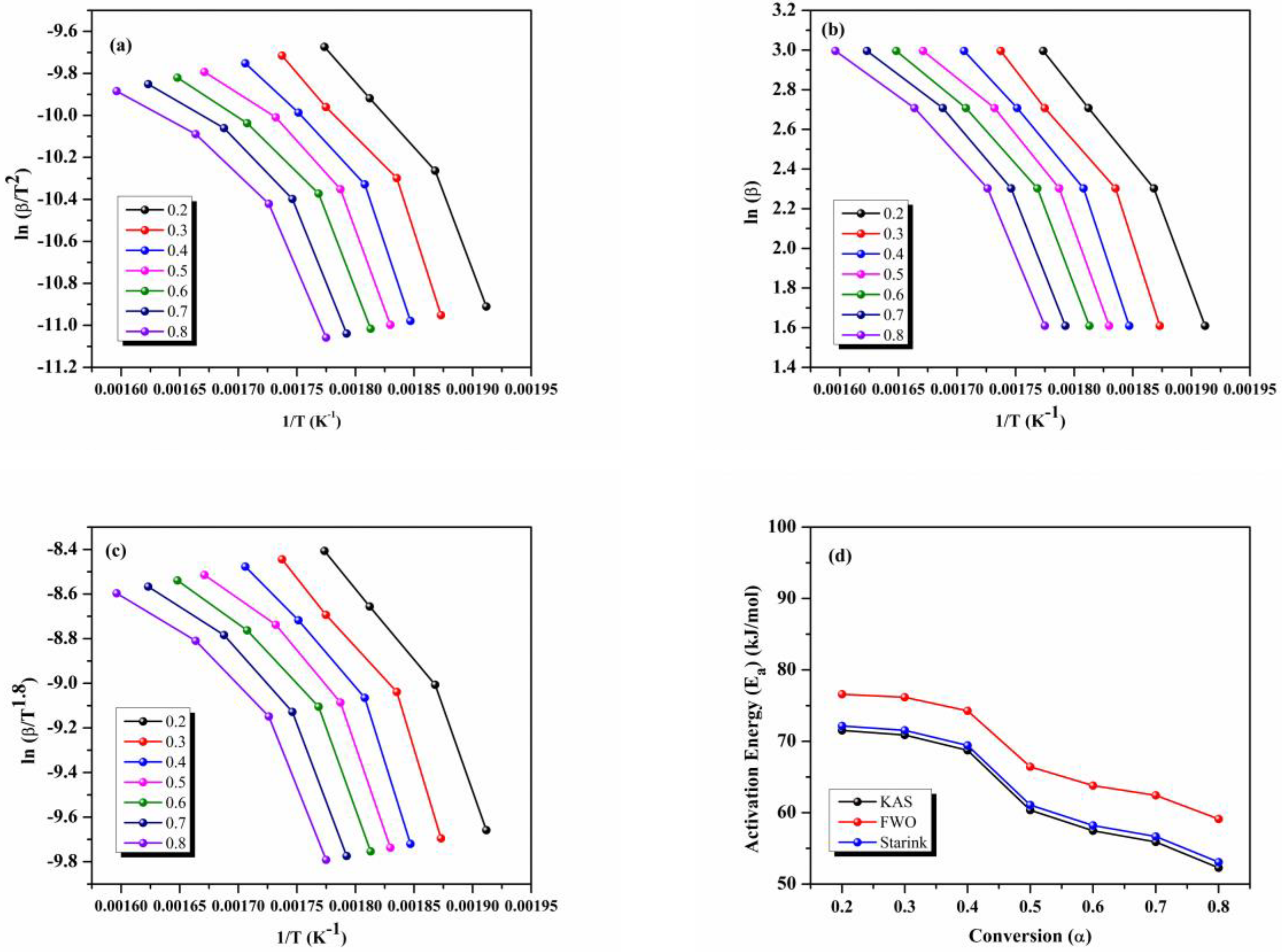


| Conversion (α) | Activation Energy (Ea) (kJ/mol) | ||
|---|---|---|---|
| KAS Method | OFW Method | Starink Method | |
| Pure PCM | |||
| 0.2 | 65.89 | 71.10 | 66.53 |
| 0.3 | 78.76 | 83.74 | 79.40 |
| 0.4 | 76.52 | 81.74 | 77.17 |
| 0.5 | 78.36 | 83.60 | 79.02 |
| 0.6 | 80.80 | 86.08 | 81.47 |
| 0.7 | 74.51 | 80.25 | 75.22 |
| 0.8 | 71.79 | 77.77 | 72.52 |
| PCM + 0.5% MWCNT | |||
| 0.2 | 79.36 | 84.14 | 79.97 |
| 0.3 | 86.36 | 90.97 | 86.97 |
| 0.4 | 77.64 | 82.82 | 78.29 |
| 0.5 | 75.47 | 80.92 | 76.15 |
| 0.6 | 70.06 | 75.90 | 70.77 |
| 0.7 | 68.74 | 74.79 | 69.47 |
| 0.8 | 66.87 | 73.13 | 67.62 |
| PCM + 1% MWCNT | |||
| 0.2 | 71.52 | 76.59 | 72.15 |
| 0.3 | 70.88 | 76.17 | 71.54 |
| 0.4 | 68.74 | 74.27 | 69.41 |
| 0.5 | 60.35 | 66.43 | 61.07 |
| 0.6 | 57.46 | 63.78 | 58.20 |
| 0.7 | 55.90 | 62.43 | 56.67 |
| 0.8 | 52.28 | 59.11 | 53.07 |
| Model | Parameter | Value |
|---|---|---|
| Support vector machine | kernel | rbf |
| degree | 3 | |
| Random Forest Regression | n-estimators | 55 |
| Min-samples-split | 3 | |
| Min-samples-leaf | 1 | |
| Max-depth | 9 | |
| Gaussian Process Regression | Basis function | 0 |
| Sigma | 0.143 | |
| Kernel function | Isotropic matern 3/2 | |
| ANN | Number of hidden layers | 1 |
| Number of hidden neurons | 33 | |
| Activation functions | Tanh, Purelin |
| Model | Training | Testing | Validation | |||
|---|---|---|---|---|---|---|
| RMSE | R2 | RMSE | R2 | RMSE | R2 | |
| Random Forest Regression | 2.6325 | 0.9983 | 4.2996 | 0.9957 | 19.9326 | 0.9029 |
| Linear Regression | 18.6926 | 0.9162 | 19.4342 | 0.9154 | 20.0745 | 0.8854 |
| Support vector machine Regression | 4.7426 | 0.9946 | 4.9398 | 0.9944 | 18.9254 | 0.9113 |
| Artificial Neural Network | 0.8437 | 0.9998 | 1.0541 | 0.9997 | 20.1265 | 0.9010 |
| Gaussian Process Regression | 0.8871 | 0.9998 | 1.000 | 0.9998 | 1.0632 | 0.9997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kottala, R.K.; Chigilipalli, B.K.; Mukuloth, S.; Shanmugam, R.; Kantumuchu, V.C.; Ainapurapu, S.B.; Cheepu, M. Thermal Degradation Studies and Machine Learning Modelling of Nano-Enhanced Sugar Alcohol-Based Phase Change Materials for Medium Temperature Applications. Energies 2023, 16, 2187. https://doi.org/10.3390/en16052187
Kottala RK, Chigilipalli BK, Mukuloth S, Shanmugam R, Kantumuchu VC, Ainapurapu SB, Cheepu M. Thermal Degradation Studies and Machine Learning Modelling of Nano-Enhanced Sugar Alcohol-Based Phase Change Materials for Medium Temperature Applications. Energies. 2023; 16(5):2187. https://doi.org/10.3390/en16052187
Chicago/Turabian StyleKottala, Ravi Kumar, Bharat Kumar Chigilipalli, Srinivasnaik Mukuloth, Ragavanantham Shanmugam, Venkata Charan Kantumuchu, Sirisha Bhadrakali Ainapurapu, and Muralimohan Cheepu. 2023. "Thermal Degradation Studies and Machine Learning Modelling of Nano-Enhanced Sugar Alcohol-Based Phase Change Materials for Medium Temperature Applications" Energies 16, no. 5: 2187. https://doi.org/10.3390/en16052187
APA StyleKottala, R. K., Chigilipalli, B. K., Mukuloth, S., Shanmugam, R., Kantumuchu, V. C., Ainapurapu, S. B., & Cheepu, M. (2023). Thermal Degradation Studies and Machine Learning Modelling of Nano-Enhanced Sugar Alcohol-Based Phase Change Materials for Medium Temperature Applications. Energies, 16(5), 2187. https://doi.org/10.3390/en16052187







