Characteristics and Significance of Acid-Soluble Organic Matter in Marine Carbonate Source Rocks
Abstract
1. Introduction
2. Samples and Methods
2.1. Samples
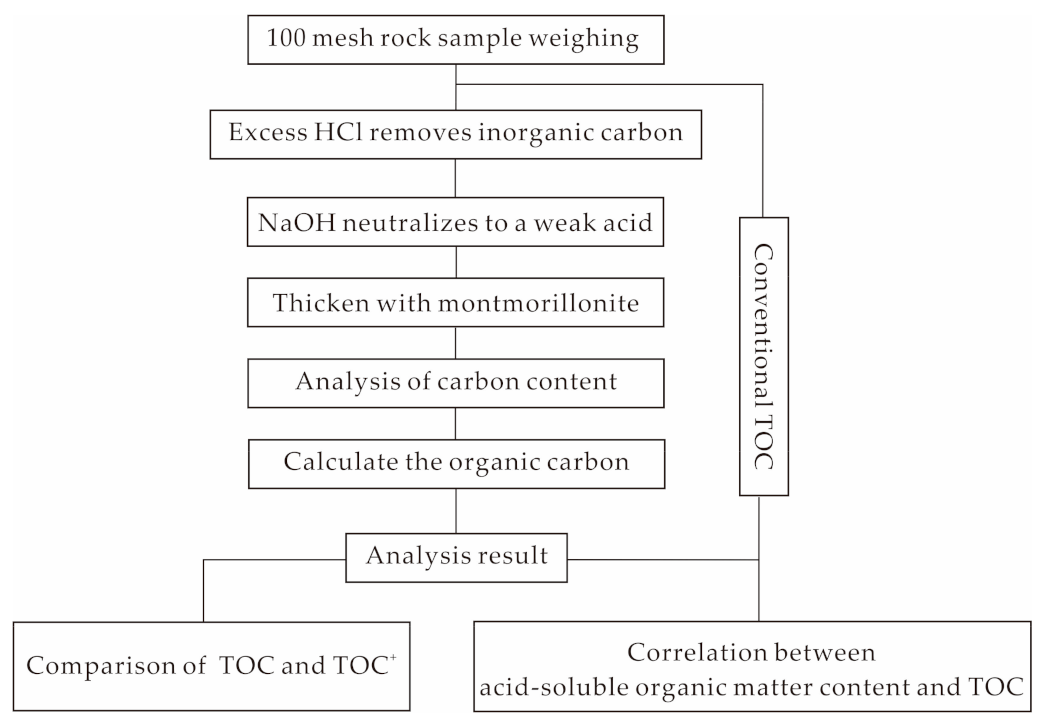
2.2. Instruments and Experimental Methods
3. Results and Discussion
3.1. Comparison of TOC Results Obtained Using Different Methods
3.2. Relationship between Acid-Soluble Organic Matter Content and TOC
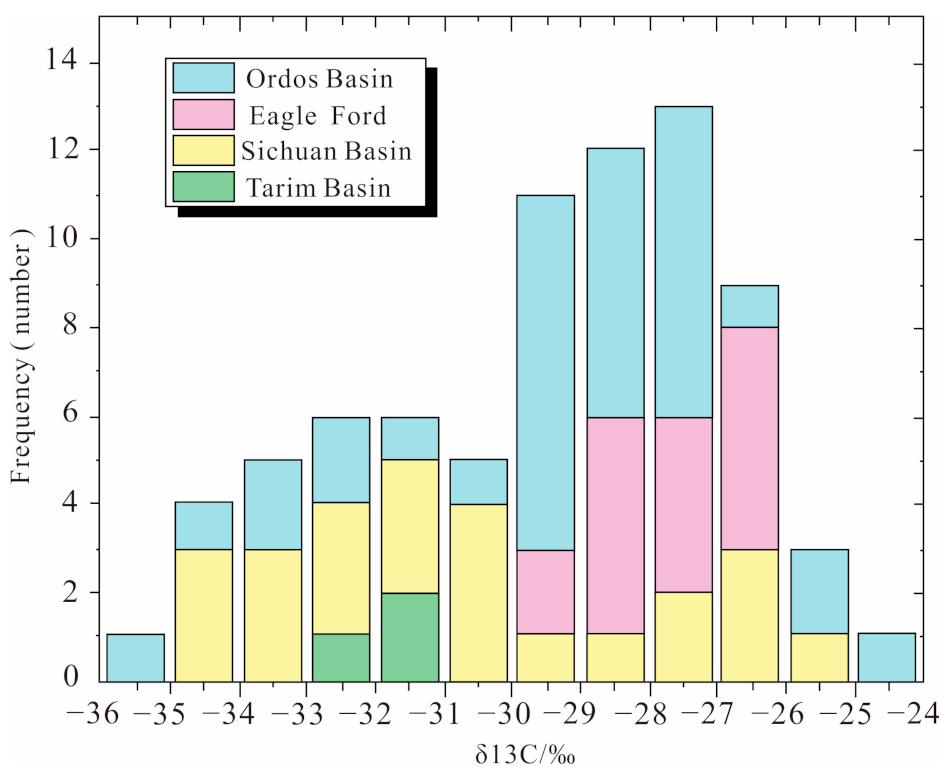
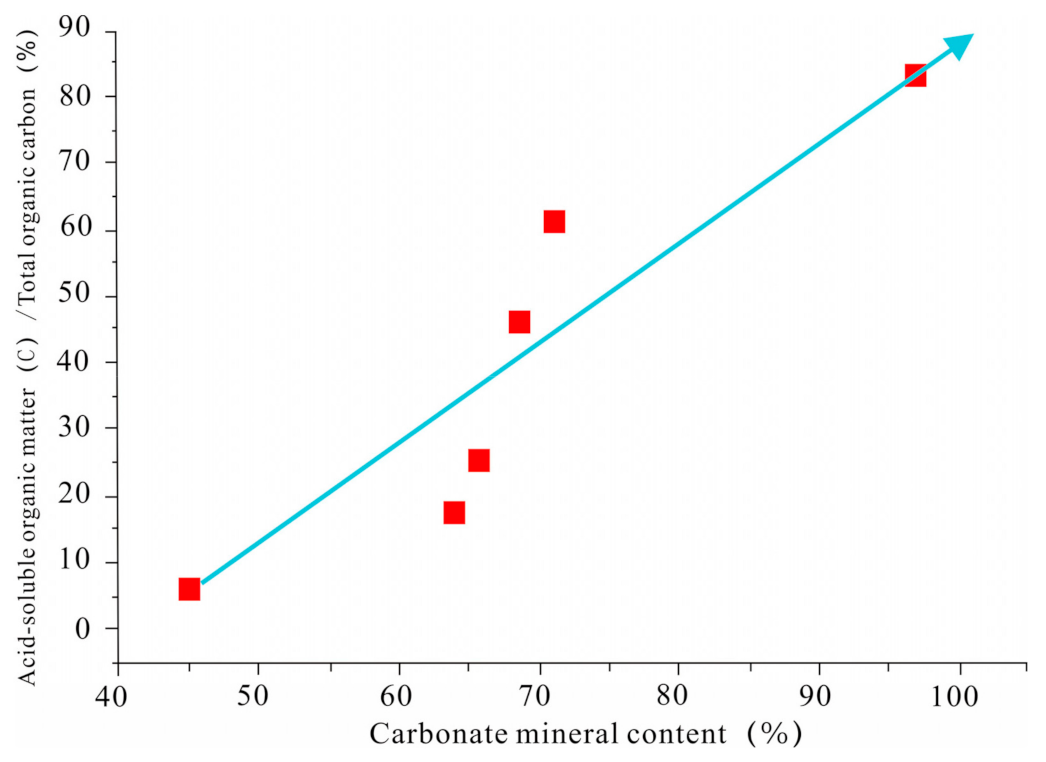
3.3. Factors Affecting the Abundance of Acid-Soluble Organic Matter
3.3.1. Thermal Evolution
3.3.2. Carbonate Minerals
3.3.3. Depositional Environment
4. Conclusions
- (1)
- In this study, the characteristics and significance of acid-soluble organic matter in marine carbonate source rocks are discussed. The problem of low TOC measurements caused by the loss of large amounts of organic matter during traditional TOC pretreatment is solved, and a new idea for the evaluation of marine carbonate source rocks is provided.
- (2)
- For most samples, the TOC+ values measured by the new method are higher than the conventional TOC values. The loss of organic matter during conventional TOC testing of some carbonate rock samples from the Eagle Ford Formation accounted for more than half of the total organic matter.
- (3)
- There is no obvious positive correlation between the content of acid-soluble organic matter and TOC. Furthermore, there is a pronounced negative correlation between the content of acid-soluble organic matter of carbonate rocks in the Permian Maokou Formation in the Sichuan Basin and conventional TOC. Additionally, the relative proportion of acid-soluble organic matter to total organic matter has an obvious positive correlation with the mineral content of carbonate rocks.
- (4)
- The content of acid-soluble organic matter is affected by many factors. There is a certain negative correlation between the content of acid-soluble organic matter and the thermal maturity of organic matter, but low-maturity carbonate rocks are not necessarily rich in organic acid salts. The development of acid-soluble organic matter should be considered along with other influencing factors.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, S.G.; Sun, W.; Li, Z.W.; Deng, B.; Zhong, Y.; Song, J.M.; Ran, B.; Luo, Z.L.; Han, K.Y.; Jiang, L.; et al. Distribution characteristics of marine carbonate reservoirs and their tectonic controlling factors across the Sichuan Superimposed Basin. Lithol. Reserv. 2016, 28, 1–17. [Google Scholar] [CrossRef]
- Jin, Z.J. Particularity of petroleum exploration on marine carbonate strata in China sedimentary basins. Earth Sci. Front. 2005, 12, 15–22. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, G.X.; Wu, S.G.; Zhu, Y.H.; Wu, C.; Zhang, L.; Liu, S.F.; Yan, W.; Sun, M.; Zhang, Y.M.; et al. Geological distribution of the Miocene carbonate platform in the Xisha Sea area of the South China Sea, and its implications for hydrocarbon exploration. Appl. Sci. 2022, 12, 11831. [Google Scholar] [CrossRef]
- Zhao, W.Z.; Wang, Z.C.; Zhang, S.C.; Wang, H.J. Accumulation conditions and enrichment zones of deep marine oil and gas in superimposed basins of China. Chin. Sci. Bull. 2007, 52, 9–18. [Google Scholar] [CrossRef]
- Geng, X.H.; Geng, A.S.; Xiong, Y.Q. Review on the evaluation of lower Paleozoic carbonate source rocks in China. Bull. Mineral. Petrol. Geochem. 2004, 23, 344–349. [Google Scholar] [CrossRef]
- Zhao, W.Z.; Hu, S.Y.; Liu, W.; Wang, T.S.; Li, Y.X. Petroleum geological features and exploration propect in deep marine carbonate strata onshore China: A further discussion. Nat. Gas Ind. 2014, 34, 14–23. [Google Scholar] [CrossRef]
- He, Z.L.; Jin, X.H.; Wo, Y.J.; Li, H.L.; Bai, Z.R.; Jiao, C.L.; Zhang, Z.P. Hydrocarbon accumulation characteristics and exploration domains of ultra-deep marine carbonates in China. China Pet. Explor. 2016, 21, 3–14. [Google Scholar] [CrossRef]
- Zhao, W.Z.; Wang, Z.Y.; He, H.Q.; Zhang, M.J.; Wang, H.J.; Wang, Y.P.; Qin, Y. Gas formation mechanism of marine carbonate source rocks in China. Sci. China Ser. D Earth Sci. 2005, 35, 638–648. [Google Scholar] [CrossRef]
- Huang, J.Z.; Lü, Z.G. How to judge carbonate rock as source rock: A case of Sichuan Basin. Mar. Orig. Pet. Geol. 2011, 16, 8–14. [Google Scholar] [CrossRef]
- Liu, W.H.; Borjigin, T.; Wang, X.F.; Li, M.W.; Hu, G.; Wang, J.; Lu, L.F.; Zhao, H.; Chen, Q.L.; Luo, H.Y. New knowledge of hydrocarbon generating theory of organic matter in Chinese marine carbonates. Pet. Explor. Dev. 2017, 44, 159–169. [Google Scholar] [CrossRef]
- Huo, Z.P.; Pang, X.Q.; Zhang, B.S.; Chen, J.F.; Fan, B.J.; Li, S.M. Evidences on effective carbonate source rock of low organic matter abundance and its lower limit of TOC. Geol. Rev. 2013, 59, 1165–1176. [Google Scholar] [CrossRef]
- Lei, T.Z.; Xia, Y.Q.; Qiu, J.L.; Jin, M.; Meng, Q.X.; Fang, L.H. Organic matter loss during the acidolysis of hydrocarbon source rock. Nat. Gas Geosci. 2009, 20, 957–960. [Google Scholar] [CrossRef]
- Liu, P.; Wang, X.F.; Fang, X.; Zheng, J.J.; Li, X.F.; Meng, Q. A new method to measure the value of organic abundance in carbonate rocks. Acta Sedimentol. Sin. 2016, 34, 200–206. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Jin, Z.J.; Liu, W.H.; Lu, L.F.; Meng, Q.X.; Tao, Y.; Han, P.L. Presence of carboxylate salts in marine carbonate strata of the Ordos Basin and their impact on hydrocarbon generation evaluation of low TOC, high maturity source rocks. Sci. China Ser. D Earth Sci. 2013, 56, 2141–2149. [Google Scholar] [CrossRef]
- Sun, M.Z.; Meng, Q.X.; Zheng, J.J.; Wang, G.C.; Fang, X.; Wang, Z.D. Analysis of organic acid salts of marine carbonate rocks in Tarim Basin. J. Cent. South Univ. 2013, 44, 216–222. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Jia, R.F. Organic geochemical and petrological characteristics of carbonate rocks as a source rock for petroleum. Geochimica 1974, 4, 278–298. [Google Scholar] [CrossRef]
- Fu, J.M.; Shi, J.Y. Theory and practice of petroleum evolution(II)—The practical model of petroleum evolution and its significance. Geochimica 1977, 2, 87–104. [Google Scholar] [CrossRef]
- Chen, P.J. Comment on several topics in the geochemistry carbonate source rock. Exp. Pet. Geol. 1985, 7, 3–12. [Google Scholar] [CrossRef]
- Hunt, J.M. The Origin of Petroleum in Carbonate Rocks. In Developments in Sedimentology; Elsevier: Amsterdam, The Netherlands, 1967; Volume 9, pp. 225–251. [Google Scholar] [CrossRef]
- Liu, B.Q.; Liang, D.G.; Fang, J.; Jia, R.F.; Fu, J.M. Organic matter maturity and oil/gas prospects in middle-upper Proterozoic and lower Paleozoic carbonate rocks in northern China. Chin. J. Geochim. 1986, 1, 55–70. [Google Scholar] [CrossRef]
- Palacas, J.G. Petroleum in carbonate rocks. In Petroleum Geochemistry and Source Rock Potential of Carbonate Rocks; American Association of Petroleum Geologists: Tulsa, OK, USA, 1984; pp. 1–208. [Google Scholar] [CrossRef]
- Tissot, B.P.; Welte, D.H. Petroleum Formation and Occurrence; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar] [CrossRef]
- Hao, S.S.; Jia, Z.Y. Hydrocarbon Formation and Distribution in Carbonate Rocks; Petroleum Industry Press: Beijing, China, 1989; Available online: https://xueshu.baidu.com/usercenter/paper/show?paperid=2cc08aed7272fc122a779fa762eb1e0e&site=xueshu_se (accessed on 3 March 2022).
- Liang, D.G.; Zhang, S.C.; Zhang, B.M.; Wang, F.Y. Understanding on marine oil generation in China based on Tarim Basin. Earth Sci. Front. 2000, 7, 534–547. [Google Scholar] [CrossRef]
- Xia, X.Y.; Dai, J.X. A critical review on the evaluation of hydrocarbon potential of marine carbonate rocks in China. Acta Pet. Sin. 2000, 21, 36–41. [Google Scholar] [CrossRef]
- Peng, P.A.; Liu, D.Y.; Qin, Y.; Yu, C.L.; Zhang, S.W.; Sui, F.G.; Li, J.Y. Low limits of organic carbon in carbonate as oil and gas source socks. Geochimica 2008, 37, 415–442. [Google Scholar] [CrossRef]
- Deaf, A.S.; El-Soughier, M.I.; Gentzis, T.; Makled, W.A. Hydrocarbon source rock potential of the lower Eocene carbonates from the Abu Darag sub-basin, Gulf of Suez, Egypt: Integrated organic geochemical and petrographic analyses. Mar. Pet. Geol. 2021, 132, 105235. [Google Scholar] [CrossRef]
- Rashid, A.; Siddiqui, N.A.; Bavoh, C.B.; Haque, A.E.; Usman, M.; Kasim, S.A.; ElGhali, M.A.K.; Ridha, S. Organic matter distribution and characteristics among rock formations in Malaysia: Implications on hydrocarbon generation potential. Appl. Sci. 2022, 12, 9470. [Google Scholar] [CrossRef]
- Hu, S.Q.; Zhang, H.W.; Zhang, R.J.; Jin, L.X.; Liu, Y.M. Quantitative interpretation of TOC in complicated lithology based on well log data: A case of Majiagou Formation in the Eastern Ordos Basin. China Appl. Sci. 2021, 11, 8724. [Google Scholar] [CrossRef]
- Fu, J.M.; Jia, R.F. Main forms of disseminated organic matter in carbonate rocks, their evolutionary characteristics and significance in oil-gas evaluation. Geochimica 1984, 1, 1–9+105. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Ye, J.S.; Sheng, G.Y.; Jia, R.F. Organic matter enclosed in carbonate minerals and its implication in petroleum generation. Geochimica 1983, 3, 276–284. [Google Scholar] [CrossRef]
- Zhang, S.C.; Tong, Z.Y. The composition and hydrocarbon-generation evolution of organic matter associated with carbonate minerals. Acta Sedimentol. Sin. 1992, 10, 76–82. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Cheng, K.M. The hydrocarbon generation mechanism and the three-stage type model of hydrocarbon generation for carbonate source rocks. Sci. China Ser. D: Earth Sci. 1997, 3, 60–64. [Google Scholar] [CrossRef]
- Xie, Q.L.; Zhou, Z.Y.; Lu, M.Y. Organic matter enclosed in carbonate minerals—A kind of important hydrocarbon-producing matter. Acta Mineral. Sin. 2000, 20, 59–62. [Google Scholar] [CrossRef]
- Zeng, F.G. A study on hydrocarbon-generating mechanism and model for lower Palaeozoic marine carbonate rocks. Geol. Geochem. 1998, 26, 79–84. Available online: http://qikan.cqvip.com/Qikan/Article/Detail?id=3212361 (accessed on 9 September 2021).
- Qin, S.F.; Qin, Y.; Zhong, N.N. Classification on occurrence of organic matter in carbonate rocks. Pet. Explor. Dev. 1996, 23, 23–27. [Google Scholar] [CrossRef]
- Liu, W.H.; Hu, G.; Tenger; Wang, J.; Lu, L.F.; Xie, X.M. Organism assemblages in the Paleozoic source rocks and their implications. Oil Gas Geol. 2016, 37, 617–626. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, W.H.; Li, Y.J.; Liu, Q.Y.; Zhou, B.; Luo, H.Y.; Wang, J.; Lu, L.F.; Meng, Q.Q.; Wu, X.Q. A preliminary study on hydrocarbon generation and evaluation of marine gypsum/salt-bearing source rocks. Mar. Orig. Pet. Geol. 2019, 24, 3. [Google Scholar] [CrossRef]
- Roberts, A.A.; Palacas, J.G.; Frost, I.C. Determination of organic carbon in modern carbonate sediments. J. Sediment. Petrol. 1973, 43, 1157–1159. [Google Scholar] [CrossRef]
- Chong, S.-L.; Mckay, J.F. Extractable metal salts of carboxylic acids in Green River oil shale. Fuel 1984, 63, 303–309. [Google Scholar] [CrossRef]
- Vandegrift, G.F.; Winans, R.E.; Scott, R.G.; Horwitz, E.P. Quantitative study of the carboxylic acids in Green River oil shale bitumen. Fuel 1980, 59, 627–633. [Google Scholar] [CrossRef]
- Ren, D.C. The Natural Gas Resources Potential Evaluate of the Middle Majiagou Formation in the East Side of the Ordos Basin; Chengdu University of Technology: Chengdu, China, 2017; Available online: https://www.zhangqiaokeyan.com/academic-degree-domestic_mphd_thesis/020314349881.html (accessed on 6 June 2022).
- Zhang, P.X.; He, X.P.; Gao, Q.F.; Gao, Y.Q.; Sun, B.; Cai, X.; He, G.S.; Zhang, Z.P.; Liu, N.N. Geological characteristics and enrichment pattern of Permian Mao 1 Member shale gas reservoirs at the southeastern margin of Sichuan Basin. Oil Gas Geol. 2021, 42, 146–157. [Google Scholar] [CrossRef]
- Wang, Z.X.; Lü, X.X.; Qian, W.W. Geochemical characteristica of the Cambrian marine carbonate elements and its petroleum geological significance: Case study of Xiaoerbulake Formation in Keping area of Tarim Basin. Nat. Gas Geosci. 2017, 28, 1085–1095. [Google Scholar] [CrossRef]
- Gao, H.; He, M.Q.; Zhao, P.Y.; Dou, L.B.; Wang, C. Comparison of geological characteristics of Chang 7 shale oil in Ordos Basin and typical shale oil in North America. Exp. Pet. Geol. 2018, 40, 133–140. [Google Scholar] [CrossRef]
- Lei, S.M.; Hao, Q.; Xiong, B.H.; Zeng, X.B. Characteristics of montmorillonite and its application prospect. Resour. Environ. Eng. 2006, 20, 565–569. [Google Scholar] [CrossRef]
- Liu, W.H.; Wang, J.; Tenger; Qin, J.Z.; Rao, D.; Tao, C.; Lu, L.F. Multiple hydrocarbon generation of marine strata and its tracer technique in China. Acta Pet. Sin. 2012, 33, 115–125. [Google Scholar] [CrossRef]
- Wu, T.H.; Guan, P.; Liu, W.H. Organic acid salt as the possible hydrocarbon source matter in carbonate rocks. Nat. Gas Ind. 2005, 25, 11–13. [Google Scholar] [CrossRef]
- Zhou, S.X.; Xia, Y.Q.; Luo, B.J.; Cheng, X.H.; Cui, M.Z.; Li, Y.; Wang, C.J. Study on laboratory simulation of the hydrocarbon formation from salts of fatty acids. Acta Sedimentol. Sin. 1997, 15, 118–121. [Google Scholar] [CrossRef]
- Wang, Q.T.; Liu, W.H.; Pei, L.X.; Cai, Z.H.; Luo, H.Y.; Wang, X.F.; Zhang, D.D.; Liu, J.Z. Hydrocarbon generation from calcium stearate: Insights from closed-system pyrolysis. Mar. Pet. Geol. 2021, 126, 104923. [Google Scholar] [CrossRef]
- Lei, T.Z.; Xia, Y.Q.; Jin, M.; Qiu, J.L.; Liu, Z.Y.; Fang, L.H. The geological significance and characteristics of aromatic fraction during organic acid salt generating hydrocarbon. Acta Sedimentol. Sin. 2010, 28, 1250–1253. [Google Scholar] [CrossRef]
- Lei, H. Genesis and reservoir microscopic characteristics of Eyelid-Eyeball-Shaped limestone in the 1st member of Maokou Formation, Sichuan Basin. China Univ. Geosci. 2021, 4. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Q.G.; Luo, K.P.; Li, L.L.; Liu, J.L. Reservoir exploration of the Permian Maokou Formation in the Sichuan Basin and enlightenment obtained. Oil Gas Geol. 2022, 43, 610–620. [Google Scholar] [CrossRef]
- Gao, G.H.; Cao, J.; Luo, B.; Xiao, D.; Zhang, Y.; Chen, C. Evidence of the middle Permian marine mixed type source rocks in the northwestern Sichuan Basin and its contribution to large gas reservoirs in Shuangyushi area. Acta Pet. Sin. 2020, 41, 433–445. [Google Scholar] [CrossRef]
- Hu, D.F.; Wang, L.J.; Zhang, H.R.; Duan, J.B.; Xia, W.Q.; Liu, Z.J.; Wei, Q.C.; Wang, K.; Pan, L. Discovery of carbonate source rock gas reservoir and its petroleum geological implications: A case study of the gas reservoir in the first Member of Middle Permian Maokou Formation in the Fuling area, Sichuan Basin. Nat. Gas Ind. 2020, 40, 23–33. [Google Scholar] [CrossRef]
- Liu, P.; Wang, X.F.; Li, X.F.; Zhang, T.; Liu, W.H. Geochemical characteristics of released organic matters by acid decomposition of hydrocarbon source rocks from different sedimentary basins. Geofluids 2019, 2019, 4816218. [Google Scholar] [CrossRef]
- Liang, C.; Cao, Y.C.; Liu, K.Y.; Jiang, Z.X.; Wu, J.; Hao, F. Diagenetic variation at the lamina scale in lacustrine organic-rich shales: Implications for hydrocarbon migration and accumulation. Geochem. Cosmochim. Acta. 2018, 229, 112–128. [Google Scholar] [CrossRef]
- Yahaira, G.-B.A.; Eduardo, G.-P.; Alejandro, C.-C.; Josué, E.-C.J. Diagenetic studies of the Eagle Ford and Indidura Formations in the Sabinas basin: Implications for source rock maturation. J. S. Am. Earth Sci. 2022, 120, 104092. [Google Scholar] [CrossRef]
- Li, Y. Analysis of hydrocarbon generation petential of marine carbonate in the upper Sinian-lower Cambrian strata in Aksu area, Tarim Basin. China Univ. Geosci. 2021, 12. [Google Scholar] [CrossRef]
- Gao, J.J.; Li, M.Z.; Hu, T.L.; Ge, B.X. Hydrocarbon generation potential of carbonate rocks in shallow platform. Oil Gas Geol. 1996, 17, 128–133. [Google Scholar] [CrossRef]
- Xia, X.Y.; Hong, F.; Zhao, L.; Zhang, W.Z. Organic facies type and hydrocarbon potential of carbonates in Majiagou lower Ordovician in Ordos Basin. Acta Sedimentol. Sin. 1999, 4, 638–643+650. [Google Scholar] [CrossRef]
- Edgell, H.S. Proterozoic salt basins of the Persian Gulf area and their role in hydrocarbon generation. Precambrian Res. 1991, 54, 1–14. [Google Scholar] [CrossRef]
- Tänavsuu-Milkeviciene, K.; Sarg, J.F. Evolution of an organic-rich lake basin—Stratigraphy, climate and tectonics: Piceance Creek basin, Eocene Green River Formation. Sedimentology 2012, 59, 1735–1768. [Google Scholar] [CrossRef]
- Gocke, K. Seasonal variations of bacterial abundance and biomass and their relation to phytoplankton in the hypertrophic tropical lagoon Cienaga Grande de Santa Marta, Colombia. J. Plankton Res. 2004, 26, 1429–1439. [Google Scholar] [CrossRef]
- Wang, X.F.; Feng, D.T.; Liu, W.H.; Bao, H.P.; Zhang, D.D.; Wei, L.B.; Luo, H.Y.; Li, Y.N. Hydrocarbon generation characteristics and evaluation methods of highly maturity carbonate source rocks. J. Northwest. Teach. Univ. Nat. Sci. 2022, 52, 954–967. [Google Scholar] [CrossRef]
- Carothers, W.W.; Kharaka, Y.K. Aliphatic acid anions in oil-field waters; implications for origin of natural gas. Am. Assoc. Pet. Geol. Bull. 1978, 62, 2441–2453. [Google Scholar] [CrossRef]



| Scholar | TOC Lower Limit/% | Scholar | TOC Lower Limit/% |
|---|---|---|---|
| Hunt [19] | 0.29 | Liu B. Q. et al. [20] | 0.05 |
| Palacas [21] | 0.40 | Chen P. J. et al. [18] | 0.1 |
| Tissot et al. [22] | 0.30 | Hao S. S. et al. [23] | 0.2 |
| Fu J.M. et al. [17] | 0.1–0.2 | Liang D. G. et al. [24] | 0.5 |
| Xia X.Y. et al. [25] | 0.4–0.5 | Peng P. A. et al. [26] | 0.1 |
| Sample | Strata | Stage of Thermal Evolution | TOC Distribution Range (%) | Mineral Content of Carbonate Rock (%) | Depositional Environment | Organic Matter Type |
|---|---|---|---|---|---|---|
| Majiagou Formation | Ordovician | High-over mature (1.6–2.5%) | 0.01–1.67 | 40.2–99.1 | Restricted open carbonate platform | Type I |
| Maokou Formation | Permian | Low mature-mature (0.8–1.5%) | 0.16–2.36 | 9.4–69.4 | Open carbonate platform; slope facies | Type II |
| Xiaoerbulake Formation | Cambrian | High-over mature (1.8–2.5%) | 0–0.61 | 45–78 | Carbonate gentle slope facies | Type I/II |
| the Eagle Ford carbonate rocks | K | Low mature-mature (0.6–1.2%) | 0.09–6.16 | >45 | Littoral shallow marine environment | Type II |
| Samples | Lithology | Formation | TOC (Conventional)/% | TOC+ (New Method)/% | δ13C/‰ |
|---|---|---|---|---|---|
| Tsgt-3 | Oily bituminous limestone | Xiaoerbulake Formation | 0.08 | 0.60 | −31.9 |
| Tsgt-4 | Shale interlayers | Xiaoerbulake Formation | 0.61 | 1.41 | −31.3 |
| Tsgt-5 | argillaceous limestone | Xiaoerbulake Formation | 0.47 | 1.18 | −32.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, D.; Wang, X.; Liu, W.; Zhang, D.; Wang, J.; Luo, H.; Liu, P. Characteristics and Significance of Acid-Soluble Organic Matter in Marine Carbonate Source Rocks. Energies 2023, 16, 2017. https://doi.org/10.3390/en16042017
Feng D, Wang X, Liu W, Zhang D, Wang J, Luo H, Liu P. Characteristics and Significance of Acid-Soluble Organic Matter in Marine Carbonate Source Rocks. Energies. 2023; 16(4):2017. https://doi.org/10.3390/en16042017
Chicago/Turabian StyleFeng, Danting, Xiaofeng Wang, Wenhui Liu, Dongdong Zhang, Jie Wang, Houyong Luo, and Peng Liu. 2023. "Characteristics and Significance of Acid-Soluble Organic Matter in Marine Carbonate Source Rocks" Energies 16, no. 4: 2017. https://doi.org/10.3390/en16042017
APA StyleFeng, D., Wang, X., Liu, W., Zhang, D., Wang, J., Luo, H., & Liu, P. (2023). Characteristics and Significance of Acid-Soluble Organic Matter in Marine Carbonate Source Rocks. Energies, 16(4), 2017. https://doi.org/10.3390/en16042017






