Relationship between Odor Adsorption Ability and Physical–Hydraulic Properties of Torrefied Biomass: Initial Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. Initial Preparation and Torrefaction Process
2.3. Proximate Analysis and Torrefaction Process Performance
2.4. Physical–Hydraulic Properties
2.4.1. Mass Loss and Density Determination
2.4.2. pH and Electroconductivity
2.4.3. Sorption Parameters
2.4.4. Water-Holding Capacity and Hydrophobicity
2.5. Odor Adsorption Experiment
2.5.1. Odor Mixture
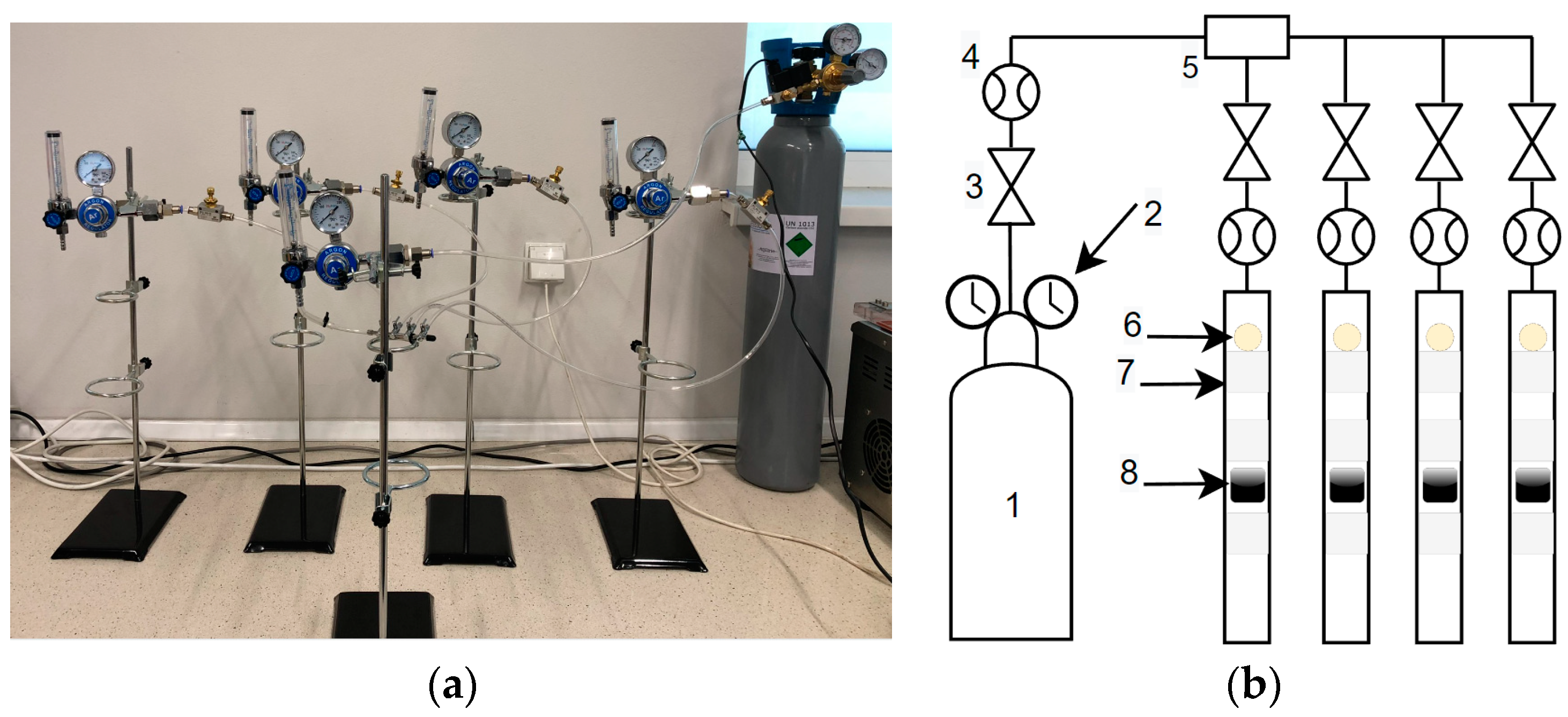
2.5.2. Laboratory Stand
2.5.3. Experimental Procedure
2.6. Statistical Analysis
3. Results and Discussion
3.1. Proximate Analysis of Materials
3.2. Torrefaction Performance Determination
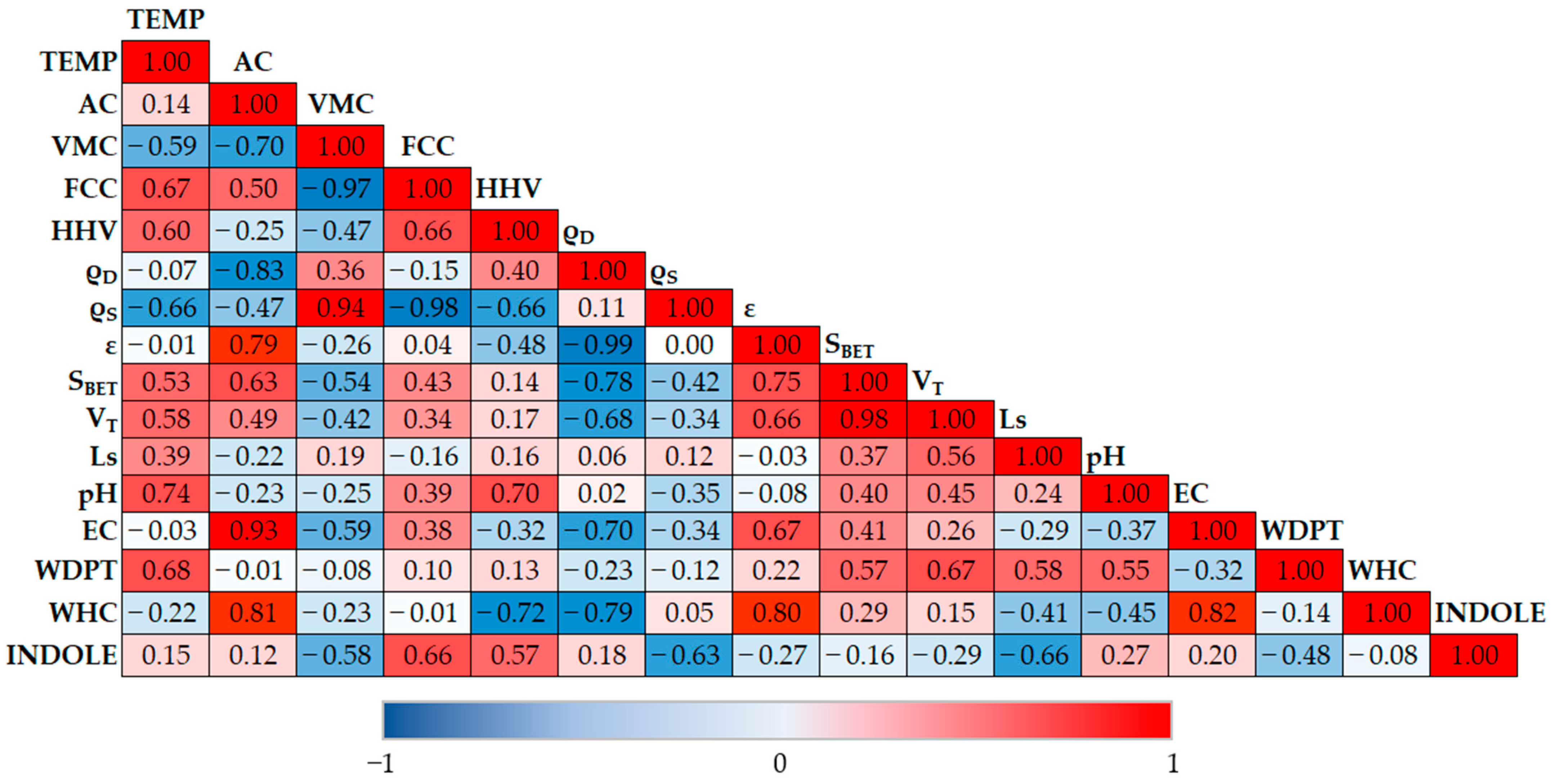
3.3. Physical Parameter Determination
3.4. pH and Electroconductivity
3.5. Hydraulic Parameter Determination
3.6. Odor Adsorption Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boesveldt, S.; Parma, V. The Importance of the Olfactory System in Human Well-Being, through Nutrition and Social Behavior. Cell Tissue Res. 2021, 383, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Capelli, L.; Bax, C.; Diaz, C.; Izquierdo, C.; Arias, R.; Seoane, N.S. Review on Odour Pollution, Odour Measurement, Abatement Techniques; D-NOSES, H2020-SwafS-23-2017-789315; Publications Office of the European Union: Luxembourg, 2019; pp. 3–80. [Google Scholar]
- Departament Ochrony Powietrza i Klimatu (Department of Air and Climate Protection). Kodeks Przeciwdziałania Uciążliwości Zapachowej; Ministerstwo Środowiska (Environment Ministry): Warsaw, Poland, 2016; pp. 1–57. [Google Scholar]
- Li, J.; Zou, K.; Li, W.; Wang, G.; Yang, W. Olfactory Characterization of Typical Odorous Pollutants Part I: Relationship Between the Hedonic Tone and Odor Concentration. Atmosphere 2019, 10, 524. [Google Scholar] [CrossRef]
- Nicell, J.A. Assessment and Regulation of Odour Impacts. Atmos. Environ. 2009, 43, 196–206. [Google Scholar] [CrossRef]
- Vasile, A.; Danciulescu, V.; Gheorghita, T.; Kim, L.; Dediu, V. Environmental Impact Assessment of Odours from Solid Waste Landfills Located in Highly Urbanized Areas. Rev. Chim. 2017, 68, 1749–1751. [Google Scholar] [CrossRef]
- Sucker, K.; Both, R.; Bischoff, M.; Guski, R.; Krämer, U.; Winneke, G. Odor Frequency and Odor Annoyance Part II: Dose-Response Associations and Their Modification by Hedonic Tone. Int. Arch. Occup. Environ. Health 2008, 81, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, S.S.; Williams, C.M. Science of Odor as a Potential Health Issue. J. Environ. Qual. 2005, 34, 129–138. [Google Scholar]
- Gałwa-Widera, M.; Kwarciak-Kozłowska, A. Reduction of Odor Nuisance from the Composting Process. J. Ecol. Eng. 2019, 20, 84–89. [Google Scholar] [CrossRef]
- Ghinea, G.; Ademoye, O.A. Olfaction-Enhanced Multimedia: Perspectives and Challenges. Multimed. Tools Appl. 2011, 55, 601–626. [Google Scholar] [CrossRef]
- Wojewódzki Inspektorat Ochrony Środowiska w Rzeszowie (Voivodship Inspectorate for Environmental Protection in Rzeszów). Counteracting the Odor Nuisance of the Air (Przeciwdziałanie Uciążliwości Zapachowej Powietrza); Information Brochure: Rzeszów, Poland, 2015. [Google Scholar]
- Płuska, E.; Przybyła, T.; Rackiewicz, I.; Rosicki, T.; Schönfelder, T.; Sobecki, I.; Miller, U.; Sówka, I. Safe Distances from Buildings for Projects Whose Functioning Is Associated with the Risk of Odor Nuisance (Bezpieczne Odległości Od Zabudowań Dla Przedsięwzięć, Których Funkcjonowanie Wiąże Się z Ryzykiem Powstawania Uciążliwości Zapachowej); Expertise: Warsaw, Poland, 2020. [Google Scholar]
- Oniszk-Popławska, A.; Kulig, A. Application of Odour Predictions to Spatial Planning, the Case of Agricultural Biogas. Chem. Eng. Trans. 2014, 40, 277–282. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Calheiros, C.S.C.; Villanueva, F.; Alonso-Cuevilla, N.P.; Gabriel, M.F.; Silva, G.V. Indoor Air Quality: A Review of Cleaning Technologies. Environments 2022, 9, 118. [Google Scholar] [CrossRef]
- Szklarczyk, M.; Zwoździak, J.; Sówka, I. Przemysłowe Źródła Emisji Zapachów. In Współczesna Problematyka Odorów; WNT: Warsaw, Poland, 2010; pp. 54–84. [Google Scholar]
- Food and Agriculture Organization of the United Nations. FAO Food Wastage Footprint: Impacts on Natural Resources—Summary Report 2013; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Dias, B.O.; Silva, C.A.; Higashikawa, F.S.; Roig, A.; Sánchez-Monedero, M.A. Use of Biochar as Bulking Agent for the Composting of Poultry Manure: Effect on Organic Matter Degradation and Humification. Bioresour. Technol. 2010, 101, 1239–1246. [Google Scholar] [CrossRef]
- Sethupathi, S.; Zhang, M.; Rajapaksha, A.; Lee, S.; Mohamad Nor, N.; Mohamed, A.; Al-Wabel, M.; Lee, S.; Ok, Y. Biochars as Potential Adsorbers of CH4, CO2 and H2S. Sustainability 2017, 9, 121. [Google Scholar] [CrossRef]
- Baltrėnas, P.; Baltrėnaitė, E.; Kleiza, J.; Švedienė, J. A Biochar-Based Medium in the Biofiltration System: Removal Efficiency, Microorganism Propagation, and the Medium Penetration Modeling. J. Air Waste Manag. Assoc. 2016, 66, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Meiirkhanuly, Z.; Koziel, J.A.; Bialowiec, A.; Banik, C.; Brown, R.C. The Proof-of-the Concept of Biochar Floating Cover Influence on Swine Manure PH: Implications for Mitigation of Gaseous Emissions From Area Sources. Front. Chem. 2020, 8, 656. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-X.; Ta, N.; Wang, X.-D. Effect of Temperature on the Structural and Physicochemical Properties of Biochar with Apple Tree Branches as Feedstock Material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef]
- Deng, H.; Li, Y.F.; Tao, S.Q.; Li, A.Y.; Li, Q.Y.; Hu, L.N. Efficient Adsorption Capability of Banana and Cassava Biochar for Malachite Green: Removal Process and Mechanism Exploration. Environ. Eng. Res. 2021, 27, 200575. [Google Scholar] [CrossRef]
- Moradi-Choghamarani, F.; Moosavi, A.A.; Sepaskhah, A.R.; Baghernejad, M. Physico-Hydraulic Properties of Sugarcane Bagasse-Derived Biochar: The Role of Pyrolysis Temperature. Cellulose 2019, 26, 7125–7143. [Google Scholar] [CrossRef]
- Dyjakon, A.; Noszczyk, T. Alternative Fuels from Forestry Biomass Residue: Torrefaction Process of Horse Chestnuts, Oak Acorns, and Spruce Cones. Energies 2020, 13, 2468. [Google Scholar] [CrossRef]
- Drozd, J.; Licznar, M.; Licznar, S.E.; Weber, J. Gleboznawstwo: Z Elementami Mineralogii i Petrografii; Wyd. 3; Wydaw. Akademii Rolniczej: Wrocław, Poland, 2002; ISBN 978-83-87866-78-5. [Google Scholar]
- Brewer, C.E.; Chuang, V.J.; Masiello, C.A.; Gonnermann, H.; Gao, X.; Dugan, B.; Driver, L.E.; Panzacchi, P.; Zygourakis, K.; Davies, C.A. New Approaches to Measuring Biochar Density and Porosity. Biomass Bioenergy 2014, 66, 176–185. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis Temperature Induced Changes in Characteristics and Chemical Composition of Biochar Produced from Conocarpus Wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- Gautam, R.; Ashwath, N. Hydrophobicity of 43 Potting Media: Its Implications for Raising Seedlings in Revegetation Programs. J. Hydrol. 2012, 430–431, 111–117. [Google Scholar] [CrossRef]
- Bisdom, E.B.A.; Dekker, L.W.; Schoute, J.F.T. Water Repellency of Sieve Fractions from Sandy Soils and Relationships with Organic Material and Soil Structure. Geoderma 1993, 56, 105–118. [Google Scholar] [CrossRef]
- Yu, O.-Y.; Raichle, B.; Sink, S. Impact of Biochar on the Water Holding Capacity of Loamy Sand Soil. Int. J. Energy Environ. Eng. 2013, 4, 44. [Google Scholar] [CrossRef]
- Usevičiūtė, L.; Baltrėnaitė-Gedienė, E. Dependence of Pyrolysis Temperature and Lignocellulosic Physical-Chemical Properties of Biochar on Its Wettability. Biomass Convers. Bioref. 2021, 11, 2775–2793. [Google Scholar] [CrossRef]
- Odor Nutty Description. Available online: http://www.thegoodscentscompany.com/odor/nutty.html (accessed on 25 January 2023).
- Indole Odor Description. Available online: https://www.scentspiracy.com/fragrance-ingredients/indol (accessed on 25 January 2023).
- Hwang, O.; Lee, S.-R.; Cho, S.; Ro, K.S.; Spiehs, M.; Woodbury, B.; Silva, P.J.; Han, D.-W.; Choi, H.; Kim, K.-Y.; et al. Efficacy of Different Biochars in Removing Odorous Volatile Organic Compounds (VOCs) Emitted from Swine Manure. ACS Sustain. Chem. Eng. 2018, 6, 14239–14247. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Hills, C.D.; Tripathi, N.; Singh, R.S.; Carey, P.J.; Lowry, F. Valorisation of Agricultural Biomass-Ash with CO2. Sci. Rep. 2020, 10, 13801. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J.; Lin, Y.-Y.; Chu, Y.-S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.-S.; Ho, S.-H.; Culaba, A.B.; et al. Progress in Biomass Torrefaction: Principles, Applications and Challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Mamvura, T.A.; Danha, G. Biomass Torrefaction as an Emerging Technology to Aid in Energy Production. Heliyon 2020, 6, e03531. [Google Scholar] [CrossRef]
- Rivas-Cantu, R.C.; Jones, K.D.; Mills, P.L. A Citrus Waste-Based Biorefinery as a Source of Renewable Energy: Technical Advances and Analysis of Engineering Challenges. Waste Manag. Res. 2013, 31, 413–420. [Google Scholar] [CrossRef]
- Piersa, P.; Unyay, H.; Szufa, S.; Lewandowska, W.; Modrzewski, R.; Ślężak, R.; Ledakowicz, S. An Extensive Review and Comparison of Modern Biomass Torrefaction Reactors vs. Biomass Pyrolysis—Part 1. Energies 2022, 15, 2227. [Google Scholar] [CrossRef]
- Kazimierski, P.; Januszewicz, K.; Godlewski, W.; Fijuk, A.; Suchocki, T.; Chaja, P.; Barczak, B.; Kardaś, D. The Course and the Effects of Agricultural Biomass Pyrolysis in the Production of High-Calorific Biochar. Materials 2022, 15, 1038. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.; Pradhan, R.R.; Dutta, A. Qualitative and Kinetic Analysis of Torrefaction of Lignocellulosic Biomass Using DSC-TGA-FTIR. AIMS Energy 2015, 3, 760–773. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification and Pyrolysis; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 978-0-12-374988-8. [Google Scholar]
- Demirbaş, A. Calculation of Higher Heating Values of Biomass Fuels. Fuel 1997, 76, 431–434. [Google Scholar] [CrossRef]
- Sobol, Ł.; Wolski, K.; Radkowski, A.; Piwowarczyk, E.; Jurkowski, M.; Bujak, H.; Dyjakon, A. Determination of Energy Parameters and Their Variability between Varieties of Fodder and Turf Grasses. Sustainability 2022, 14, 11369. [Google Scholar] [CrossRef]
- Bangkha, N.; Saechua, W.; Nuamyakul, T.; Jongyingcharoen, J.S. Effect of Torrefaction Temperature on Energy Properties of Spent Coffee Ground. E3S Web Conf. 2020, 187, 03009. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Liu, S.-C. Effect of Temperature on Thermochemical Property and True Density of Torrefied Coffee Residue. J. Anal. Appl. Pyrolysis 2013, 102, 47–52. [Google Scholar] [CrossRef]
- Orisaleye, J.I.; Jekayinfa, S.O.; Pecenka, R.; Ogundare, A.A.; Akinseloyin, M.O.; Fadipe, O.L. Investigation of the Effects of Torrefaction Temperature and Residence Time on the Fuel Quality of Corncobs in a Fixed-Bed Reactor. Energies 2022, 15, 5284. [Google Scholar] [CrossRef]
- Peng, J.H. A Study of Softwood Torrefaction and Densification for the Production of High Quality Wood Pellets. Doctoral Thesis, University of British Columbia: Vancouver, BC, Canada, 2012. [Google Scholar] [CrossRef]
- Peng, J.; Wang, J.; Bi, X.T.; Lim, C.J.; Sokhansanj, S.; Peng, H.; Jia, D. Effects of Thermal Treatment on Energy Density and Hardness of Torrefied Wood Pellets. Fuel Process. Technol. 2015, 129, 168–173. [Google Scholar] [CrossRef]
- Ramos-Carmona, S.; Pérez, J.F.; Pelaez-Samaniego, M.R.; Barrera, R.; Garcia-Perez, M. Effect of Torrefaction Temperature on Properties of Patula Pine. Maderas Cienc. Tecnol. 2017, 19, 39–50. [Google Scholar] [CrossRef]
- Ibitoye, S.E.; Jen, T.-C.; Mahamood, R.M.; Akinlabi, E.T. Improving the Combustion Properties of Corncob Biomass via Torrefaction for Solid Fuel Applications. J. Compos. Sci. 2021, 5, 260. [Google Scholar] [CrossRef]
- Dias Júnior, A.F.; Suuchi, M.A.; Sant’Anna Neto, A.; da Silva, J.G.M.; da Silva, Á.M.; de Souza, N.D.; Protásio, T.d.P.; Brito, J.O. Blends of Charcoal Fines and Wood Improve the Combustibility and Quality of the Solid Biofuels. Bioenerg. Res. 2021, 14, 344–354. [Google Scholar] [CrossRef]
- Kihedu, J. Torrefaction and Combustion of Ligno-Cellulosic Biomass. Energy Procedia 2015, 75, 162–167. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Bajwa, S.G. Properties of Densified Solid Biofuels in Relation to Chemical Composition, Moisture Content, and Bulk Density of the Biomass. BioResources 2019, 14, 4996–5015. [Google Scholar] [CrossRef]
- Yu, S.; Park, J.; Kim, M.; Kim, H.; Ryu, C.; Lee, Y.; Yang, W.; Jeong, Y. Improving Energy Density and Grindability of Wood Pellets by Dry Torrefaction. Energy Fuels 2019, 33, 8632–8639. [Google Scholar] [CrossRef]
- Dyjakon, A.; Sobol, Ł.; Noszczyk, T.; Mitręga, J. The Impact of Torrefaction Temperature on the Physical-Chemical Properties of Residual Exotic Fruit (Avocado, Mango, Lychee) Seeds. Energies 2022, 15, 612. [Google Scholar] [CrossRef]
- Nurek, T.; Roman, K. Effect of Mineral Matter Content on Specific Density of Forest Biomass. Ann. Wars. Univ. Life Sci.-SGGW Agricult. 2014, 64, 109–116. [Google Scholar]
- Bergna, D.; Varila, T.; Romar, H.; Lassi, U. Comparison of the Properties of Activated Carbons Produced in One-Stage and Two-Stage Processes. C 2018, 4, 41. [Google Scholar] [CrossRef]
- Victorin, M.; Davidsson, Å.; Wallberg, O. Characterization of Mechanically Pretreated Wheat Straw for Biogas Production. Bioenerg. Res. 2020, 13, 833–844. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An Overview on Engineering the Surface Area and Porosity of Biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Chen, D.; Chen, X.; Sun, J.; Zheng, Z.; Fu, K. Pyrolysis Polygeneration of Pine Nut Shell: Quality of Pyrolysis Products and Study on the Preparation of Activated Carbon from Biochar. Bioresour. Technol. 2016, 216, 629–636. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Xu, R.-K.; Zhang, H. The Forms of Alkalis in the Biochar Produced from Crop Residues at Different Temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Melo, L.C.A.; Coscione, A.R.; Abreu, C.A.; Puga, A.P.; Camargo, O.A. Influence of Pyrolysis Temperature on Cadmium and Zinc Sorption Capacity of Sugar Cane Straw–Derived Biochar. BioResources 2013, 8, 4992–5004. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Pal, K.; Johan, R.B.; Yehya Dabdawb, W.A.; Ali, M.E.; Faizur Rafique, R. Comparative Evaluation of Physiochemical Properties of a Solid Fuel Derived from Adansonia Digitata Trunk Using Torrefaction. BioResources 2017, 12, 3816–3833. [Google Scholar] [CrossRef]
- Gezahegn, S.; Sain, M.; Thomas, S. Variation in Feedstock Wood Chemistry Strongly Influences Biochar Liming Potential. Soil Syst. 2019, 3, 26. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental Application of Biochar: Current Status and Perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Rehrah, D.; Reddy, M.R.; Novak, J.M.; Bansode, R.R.; Schimmel, K.A.; Yu, J.; Watts, D.W.; Ahmedna, M. Production and Characterization of Biochars from Agricultural By-Products for Use in Soil Quality Enhancement. J. Anal. Appl. Pyrolysis 2014, 108, 301–309. [Google Scholar] [CrossRef]
- Gabhi, R.S.; Kirk, D.W.; Jia, C.Q. Preliminary Investigation of Electrical Conductivity of Monolithic Biochar. Carbon 2017, 116, 435–442. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E. Effect of Feedstock and Pyrolysis Temperature on Properties of Biochar Governing End Use Efficacy. Biomass Bioenergy 2017, 105, 136–146. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J.; Colin, B.; Chang, J.-S.; Pétrissans, A.; Bi, X.; Pétrissans, M. Hygroscopic Transformation of Woody Biomass Torrefaction for Carbon Storage. Appl. Energy 2018, 231, 768–776. [Google Scholar] [CrossRef]
- Dyjakon, A.; Noszczyk, T.; Sobol, Ł.; Misiakiewicz, D. Influence of Torrefaction Temperature and Climatic Chamber Operation Time on Hydrophobic Properties of Agri-Food Biomass Investigated Using the EMC Method. Energies 2021, 14, 5299. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; Yang, H.; Yang, Q.; Chen, H. Evolution of Functional Groups and Pore Structure During Cotton and Corn Stalks Torrefaction and its Correlation with Hydrophobicity. Fuel 2014, 137, 41–49. [Google Scholar] [CrossRef]
- Chen, D.; Gao, A.; Cen, K.; Zhang, J.; Cao, X.; Ma, Z. Investigation of Biomass Torrefaction Based on Three Major Components: Hemicellulose, Cellulose and Lignin. Energy Convers. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Ru, B.; Zhao, Y.; Wang, X.; Xiao, G.; Luo, Z. Influence of Torrefaction on the Characteristics and Pyrolysis Behaviour of Cellulose. Energy 2017, 120, 864–871. [Google Scholar] [CrossRef]
- Baltrėnaitė, E.; Baltrėnas, P.; Bhatnagar, A.; Vilppo, T.; Selenius, M.; Koistinen, A.; Dahl, M.; Penttinen, O.-P. A Multicomponent Approach to Using Waste-Derived Biochar in Biofiltration: A Case Study Based on Dissimilar Types of Waste. Int. Biodeterior. Biodegrad. 2017, 119, 565–576. [Google Scholar] [CrossRef]
- Hien, T.T.T.; Tsubota, T.; Taniguchi, T.; Shinogi, Y. Enhancing Soil Water Holding Capacity and Provision of a Potassium Source via Optimization of the Pyrolysis of Bamboo Biochar. Biochar 2021, 3, 51–61. [Google Scholar] [CrossRef]
- Piekarski, J.; Dąbrowski, T.; Dąbrowski, J.; Ignatowicz, K. Preliminary Studies on Odor Removal in the Adsorption Process on Biochars Produced Form Sewage Sludge and Beekeeping Waste. Arch. Environ. Prot. 2021, 47. [Google Scholar] [CrossRef]
- Ro, K.S.; Woodbury, B.; Spiehs, M.; Szogi, A.A.; Silva, P.J.; Hwang, O.; Cho, S. Pilot-Scale H2S and Swine Odor Removal System Using Commercially Available Biochar. Agronomy 2021, 11, 1611. [Google Scholar] [CrossRef]
- Stetina, K. Control of Fecal Malodor by Adsorption onto Biochar. Doctoral Thesis, University of Colorado at Boulder, Boulder, CO, USA, 2017. [Google Scholar]
- Granados, D.A.; Basu, P.; Chejne, F.; Nhuchhen, D.R. Detailed investigation into torrefaction of wood in a two-stage inclined rotary torrefier. Energy Fuels 2017, 31, 647–658. [Google Scholar] [CrossRef]
- Tang, J.X.; Jin, Y.T.; He, Z.L.; Hou, Q.Y.; Zhao, C.T. A Review Of Researches On Biochar Adsorbing Organic Contaminants And Its Mechanism And Influence Factors. IOP Conf. Ser. Mater. Sci. Eng. 2018, 392, 052030. [Google Scholar] [CrossRef]
| Time of Drop of Water Penetration | Hydrophobic Properties |
|---|---|
| below 5 s | Hydrophilic |
| from 5 s to 60 s | Slightly hydrophobic |
| from 60 s to 600 s | Strongly hydrophobic |
| from 600 s to 3600 s | Severely hydrophobic |
| above 3600 s | Extremely hydrophobic |
| Compound | Structural Formula * | Odor Description |
|---|---|---|
| Indole | 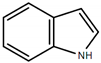 | Pungent, floral, slightly naphtha- and mothball-like with a fecal and animalic musty character |
| 2,3-dimethyl pyrazine | 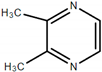 | Nutty, nut skin, cocoa, peanut butter, coffee, walnut, caramelly, roasted |
| 2,3,5-trimethyl pyrazine | 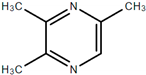 | Nutty, nut skin, earthy, powdery, cocoa, baked potato, roasted peanut/hazelnut, musty |
| Biomass Residue | Temperature | AC | VMC | FCC | HHV |
|---|---|---|---|---|---|
| °C | % | % | % | kJ∙kg−1 | |
| Walnut shells | 105 | 0.99 a ± 0.03 | 82.11 ab ± 0.76 | 16.90 abc ± 0.73 | 19,327 ab ± 269 |
| 200 | 1.18 a ± 0.07 | 80.27 a ± 0.10 | 18.56 b ± 0.17 | 19,794 a ± 159 | |
| Orange peels | 105 | 3.11 d ± 0.05 | 80.91 ab ± 0.26 | 15.98 ac ± 0.29 | 16,210 ± 127 |
| 200 | 4.55 e ± 0.14 | 68.35 c ± 0.86 | 27.11 e ± 0.92 | 20,692 ± 93 | |
| Peach stones | 105 | 0.68 b ± 0.06 | 80.89 ab ± 1.48 | 18.43 ab ± 1.44 | 19,794 ± 159 |
| 200 | 0.62 b ± 0.07 | 77.32 d ± 1.24 | 22.06 d ± 1.31 | 21,640 ± 333 | |
| Apple wood chips | 105 | 1.62 c ± 0.06 | 83.06 b ± 0.46 | 15.32 c ± 0.44 | 19,113 b ± 198 |
| 200 | 1.52 c ± 0.10 | 80.41 a ± 1.14 | 18.07 ab ± 1.04 | 19,321 ab ± 98 |
| Torreficate | ML | dF | ED | EY |
|---|---|---|---|---|
| % | - | - | - | |
| Walnut shells | 11.70 a ± 0.51 | 0.98 a ± 0.01 | 1.02 a ± 0.02 | 0.90 a ± 0.01 |
| Orange peels | 29.17 c ± 3.71 | 0.84 b ± 0.01 | 1.28 c ± 0.01 | 0.90 a ± 0.04 |
| Peach stones | 9.66 a ± 2.23 | 0.96 a ± 0.01 | 1.09 b ± 0.03 | 0.99 b ± 0.03 |
| Apple wood chips | 1.26 b ± 0.12 | 0.97 a ± 0.02 | 1.01 a ± 0.01 | 1.00 b ± 0.01 |
| Biomass Residue | Temp. | ρD | ρS | ε |
|---|---|---|---|---|
| °C | kg∙m−3 | kg∙m−3 | - | |
| Walnut shells | 105 | 653 b ± 24 | 1340 ab ± 35 | 0.512 b ± 0.013 |
| 200 | 650 b ± 12 | 1337 ab ± 12 | 0.513 b ± 0.004 | |
| Orange peels | 105 | 286 a ± 5 | 1427 ac ± 51 | 0.799 de ± 0.007 |
| 200 | 200 d ± 6 | 1102 d ± 25 | 0.819 e ± 0.004 | |
| Peach stones | 105 | 720 c ± 32 | 1367 ac ± 15 | 0.458 a ± 0.025 |
| 200 | 703 c ± 15 | 1252 b ± 12 | 0.438 a ± 0.005 | |
| Apple wood chips | 105 | 325 a ± 8 | 1449 c ± 57 | 0.775 c ± 0.009 |
| 200 | 322 a ± 6 | 1352 a ± 34 | 0.762 c ± 0.006 |
| Biomass Residue | Temp. | SBET | VT | LS |
|---|---|---|---|---|
| °C | m2∙g−1 | cm3∙g−1 | nm | |
| Walnut shells | 105 | 0.23 | 0.0003 | 2.91 |
| 200 | 0.41 | 0.0008 | 3.92 | |
| Orange peels | 105 | 0.27 | 0.0003 | 2.43 |
| 200 | 0.85 | 0.0012 | 2.84 | |
| Peach stones | 105 | 0.08 | 0.0001 | 2.71 |
| 200 | 0.23 | 0.0003 | 2.58 | |
| Apple wood chips | 105 | 0.57 | 0.0009 | 3.23 |
| 200 | 0.73 | 0.0012 | 3.34 |
| Biomass Residue | Temp. | pH | EC |
|---|---|---|---|
| °C | - | mS∙cm−1 | |
| Walnut shells | 105 | 4.82 b ± 0.04 | 0.558 ab ± 0.006 |
| 200 | 5.16 a ± 0.05 | 0.665 b ± 0.008 | |
| Orange peels | 105 | 4.61 d ± 0.02 | 1.291 e ± 0.130 |
| 200 | 5.24 a ± 0.02 | 1.491 f ± 0.051 | |
| Peach stones | 105 | 4.92 b ± 0.09 | 0.434 ac ± 0.056 |
| 200 | 5.81 c ± 0.08 | 0.514 ab ± 0.017 | |
| Apple wood chips | 105 | 5.27 a ± 0.02 | 0.848 d ± 0.026 |
| 200 | 5.67 c ± 0.14 | 0.356 c ± 0.011 |
| Biomass Residue | Temp. | WDPT | Properties | WHC |
|---|---|---|---|---|
| °C | s | - | % | |
| Walnut shells | 105 | 25 a ± 2 | slightly hydrophobic | 129 b ± 11 |
| 200 | 7020 b ± 635 | extremely hydrophobic | 79 ab ± 28 | |
| Orange peels | 105 | 3 a ± 1 | hydrophilic | 819 e ± 36 |
| 200 | 3500 ab ± 173 | severely hydrophobic | 495 d ± 22 | |
| Peach stones | 105 | 39 a ± 3 | slightly hydrophobic | 73 ab ± 10 |
| 200 | 1800 a ± 520 | severely hydrophobic | 55 a ± 2 | |
| Apple wood chips | 105 | 34 a ± 3 | slightly hydrophobic | 288 c ± 26 |
| 200 | 15,200 c ± 4257 | extremely hydrophobic | 245 c ± 15 |
| Biomass Residue | Temp. | 2,3-Dimethylpyrazine | 2,3,5-Trimethylpyrazine | Indole |
|---|---|---|---|---|
| °C | % | % | % | |
| Walnut shells | 105 | 1.40 ab ± 0.40 | 20.80 a ± 7.75 | 77.74 a ± 8.14 |
| 200 | 1.95 b ± 0.48 | 21.99 a ± 6.84 | 76.06 a ± 6.37 | |
| Orange peels | 105 | 0.50 ab ± 0.65 | 25.55 a ± 7.11 | 73.95 a ± 7.58 |
| 200 | 5.14 c ± 1.15 | 24.23 a ± 6.22 | 70.63 a ± 5.23 | |
| Peach stones | 105 | 0.86 ab ± 0.46 | 29.15 a ± 3.31 | 72.50 a ± 4.58 |
| 200 | 1.09 ab ± 0.84 | 26.42 a ± 4.35 | 68.62 a ± 5.82 | |
| Apple wood chips | 105 | n.d. a | 23.67 a ± 3.95 | 76.33 a ± 3.95 |
| 200 | n.d. a | 21.16 a ± 3.08 | 78.84 a ± 3.08 |
| Biomass Residue | Temp. | 2,3-Dimethylpyrazine | 2,3,5-Trimethylpyrazine | Indole |
|---|---|---|---|---|
| °C | µg∙mL−1 | µg∙mL−1 | µg∙mL−1 | |
| Walnut shells | 105 | n.d. | n.d. | 14.87 a ± 3.55 |
| 200 | n.d. | n.d. | n.d. a | |
| Orange peels | 105 | n.d. | n.d. | 15.92 a ± 14.57 |
| 200 | n.d. | n.d. | 45.64 a ± 40.02 | |
| Peach stones | 105 | n.d. | n.d. | 27.18 a ± 11.61 |
| 200 | n.d. | n.d. | 61.26 a ± 49.55 | |
| Apple wood chips | 105 | n.d. | n.d. | 24.50 a ± 10.98 |
| 200 | n.d. | n.d. | n.d. a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobol, Ł.; Łyczko, J.; Dyjakon, A.; Sroczyński, R. Relationship between Odor Adsorption Ability and Physical–Hydraulic Properties of Torrefied Biomass: Initial Study. Energies 2023, 16, 1780. https://doi.org/10.3390/en16041780
Sobol Ł, Łyczko J, Dyjakon A, Sroczyński R. Relationship between Odor Adsorption Ability and Physical–Hydraulic Properties of Torrefied Biomass: Initial Study. Energies. 2023; 16(4):1780. https://doi.org/10.3390/en16041780
Chicago/Turabian StyleSobol, Łukasz, Jacek Łyczko, Arkadiusz Dyjakon, and Ryszard Sroczyński. 2023. "Relationship between Odor Adsorption Ability and Physical–Hydraulic Properties of Torrefied Biomass: Initial Study" Energies 16, no. 4: 1780. https://doi.org/10.3390/en16041780
APA StyleSobol, Ł., Łyczko, J., Dyjakon, A., & Sroczyński, R. (2023). Relationship between Odor Adsorption Ability and Physical–Hydraulic Properties of Torrefied Biomass: Initial Study. Energies, 16(4), 1780. https://doi.org/10.3390/en16041780









