Effects of Lignin Gasification Impurities on the Growth and Product Distribution of Butyribacterium methylotrophicum during Syngas Fermentation
Abstract
1. Introduction
2. Materials and Methods
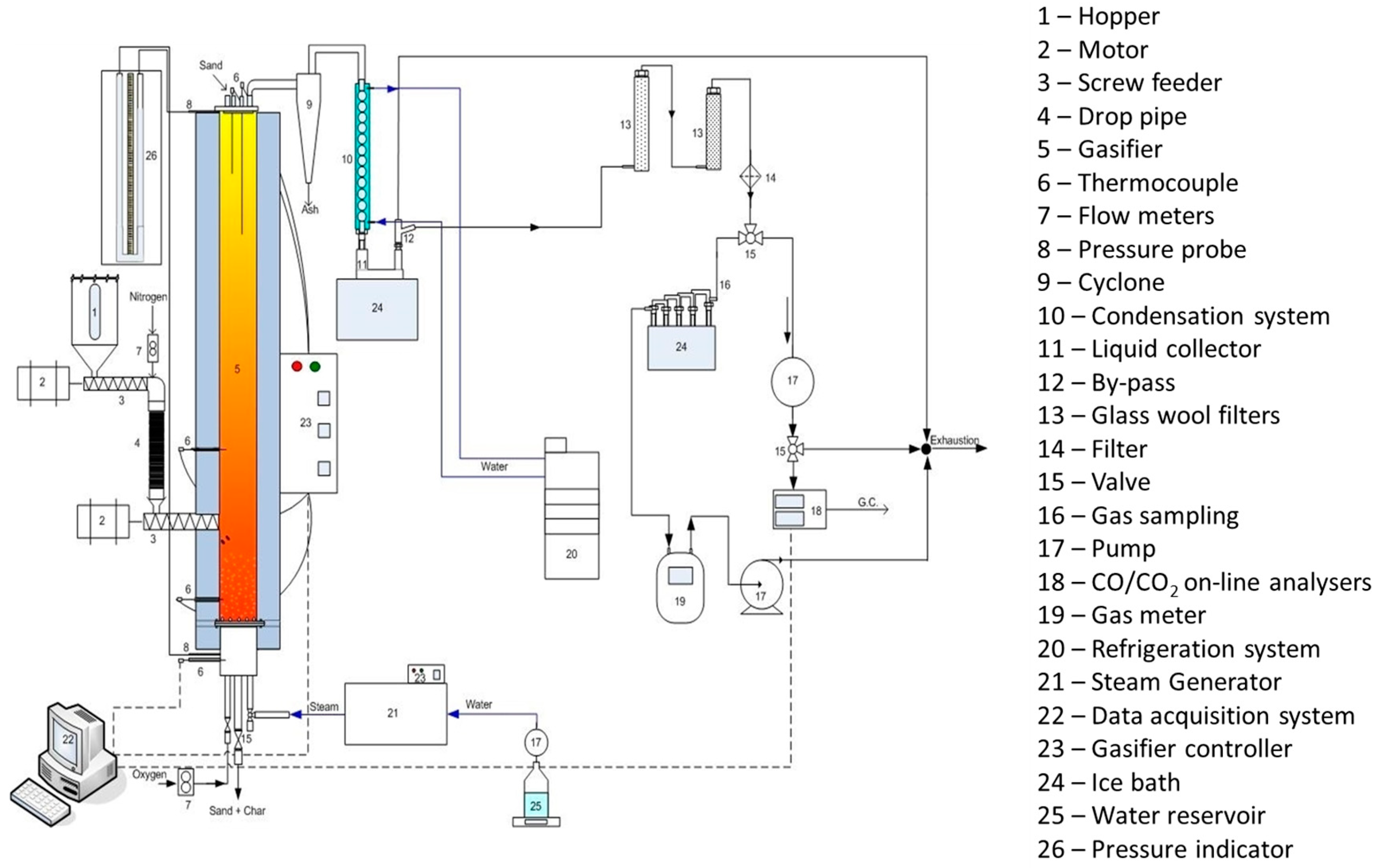
2.1. Gasification—Operating Conditions
2.2. Syngas Fermentation—Strain and Culture Medium
2.3. Analytical Methods
3. Results and Discussion
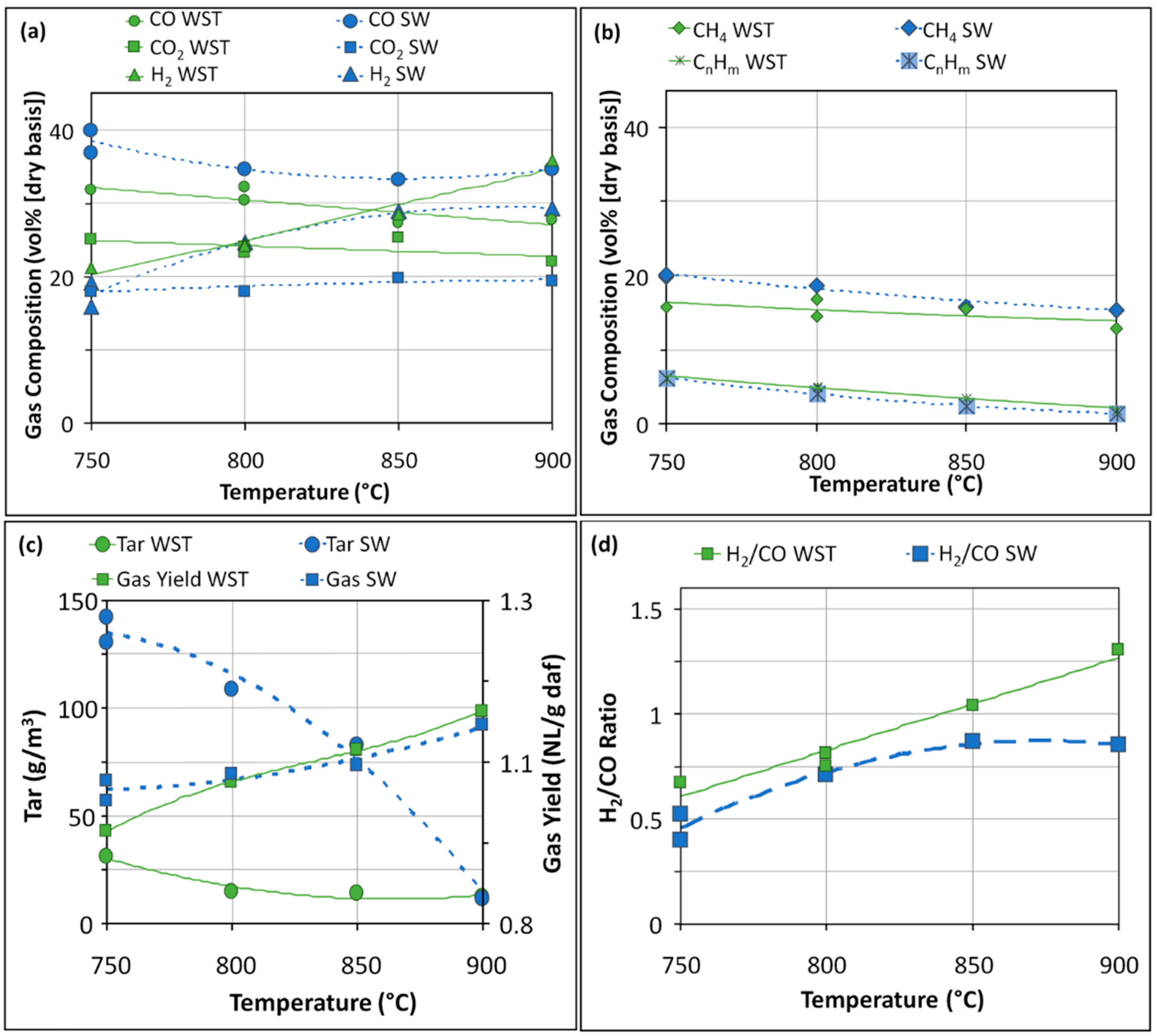
3.1. Effect of the Lignin Type
3.1.1. Syngas and Condensable Compounds
3.1.2. B. methylotrophicum Growth and Product Distribution
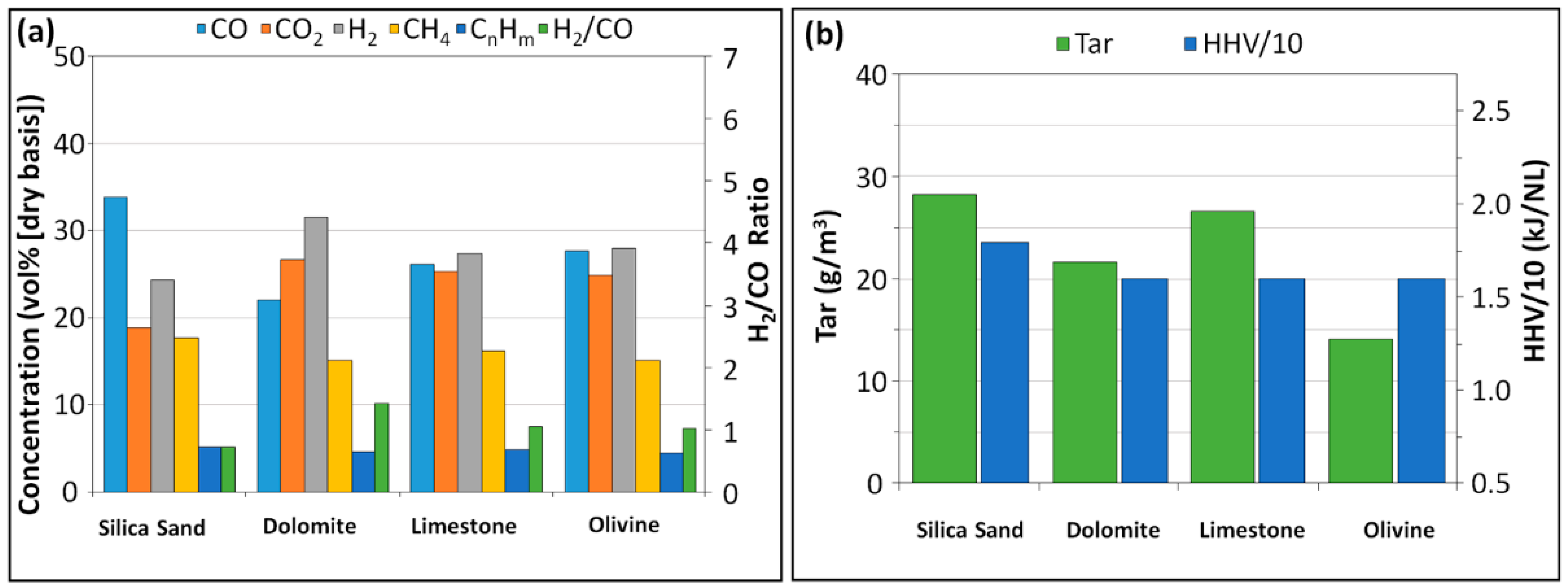
3.2. Effect of the Gasification Catalyst
3.2.1. Syngas and Condensable Compounds
3.2.2. B. methylotrophicum Growth and Product Distribution
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.Y.; Sankaran, R.; Chew, K.W.; Tan, C.H.; Krishnamoorthy, R.; Chu, D.-T.; Show, P.-L. Waste to bioenergy: A review on the recent conversion technologies. BMC Energy 2019, 1, 4. [Google Scholar] [CrossRef]
- Liakakou, E.T.; Infantes, A.; Neumann, A.; Vreugdenhil, B.J. Connecting gasification with syngas fermentation: Comparison of the performance of lignin and beech wood. Fuel 2020, 290, 120054. [Google Scholar] [CrossRef]
- Liakakou, E.; Vreugdenhil, B.; Cerone, N.; Zimbardi, F.; Pinto, F.; André, R.; Marques, P.; Mata, R.; Girio, F. Gasification of lignin-rich residues for the production of biofuels via syngas fermentation: Comparison of gasification technologies. Fuel 2019, 251, 580–592. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.; Marques, P.; Mata, R.; Pacheco, M.; Moura, P.; Gírio, F. Production of Syngas Suitable to Be Used in Fermentation to Obtain Biochemical Added-Value Compounds. Chem. Eng. Trans. 2019, 76, 1399–1404. [Google Scholar] [CrossRef]
- Chiche, D.; Diverchy, C.; Lucquin, A.-C.; Porcheron, F.; Defoort, F. Synthesis Gas Purification. Oil Gas Sci. Technol. 2013, 68, 707–723. [Google Scholar] [CrossRef]
- Daniell, J.; Köpke, M.; Simpson, S.D. Commercial Biomass Syngas Fermentation. Energies 2012, 5, 5372–5417. [Google Scholar] [CrossRef]
- Benevenuti, C.; Amaral, P.; Ferreira, T.; Seidl, P. Impacts of Syngas Composition on Anaerobic Fermentation. Reactions 2021, 2, 391–407. [Google Scholar] [CrossRef]
- Latif, H.; Zeidan, A.A.; Nielsen, A.T.; Zengler, K. Trash to treasure: Production of biofuels and commodity chemicals via syngas fermenting microorganisms. Curr. Opin. Biotechnol. 2014, 27, 79–87. [Google Scholar] [CrossRef]
- Oliveira, L.; Rückel, A.; Nordgauer, L.; Schlumprecht, P.; Hutter, E.; Weuster-Botz, D. Comparison of Syngas-Fermenting Clostridia in Stirred-Tank Bioreactors and the Effects of Varying Syngas Impurities. Microorganisms 2022, 10, 681. [Google Scholar] [CrossRef]
- Bertsch, J.; Müller, V. Bioenergetic constraints for conversion of syngas to biofuels in acetogenic bacteria. Biotechnol. Biofuels 2015, 8, 1–12. [Google Scholar] [CrossRef]
- Kennes, D.; Abubackar, H.N.; Diaz, M.; Veiga, M.C.; Kennes, C. Bioethanol production from biomass: Carbohydrate vs syngas fermentation. J. Chem. Technol. Biotechnol. 2015, 91, 304–317. [Google Scholar] [CrossRef]
- Kumar Panda, S.; Sahu, L.; Kumar Behera, S.; Ray, R.C. Chapter 9-Research and Production of Organic Acids and Industrial Potential. In Bioprocessing for Biomolecules Production; John Wiley & Sons, Ltd.: Chichester, UK, 2019; pp. 195–209. [Google Scholar]
- Kumar, S.; Dineshkumar, R.M.; Vinayakaselvi, M.A.; Ramanathan, A. Bio-Ethanol Production from Syngas-Derived Biomass: A Review. Mater. Today Proc. 2021, 46, 9989–9993. [Google Scholar] [CrossRef]
- Munasinghe, P.C.; Khanal, S.K. Biomass-derived syngas fermentation into biofuels: Opportunities and challenges. Bioresour. Technol. 2010, 101, 5013–5022. [Google Scholar] [CrossRef]
- Xu, D.; Tree, D.R.; Lewis, R.S. The effects of syngas impurities on syngas fermentation to liquid fuels. Biomass-Bioenergy 2011, 35, 2690–2696. [Google Scholar] [CrossRef]
- Infantes, A.; Kugel, M.; Neumann, A. Evaluation of Media Components and Process Parameters in a Sensitive and Robust Fed-Batch Syngas Fermentation System with Clostridium ljungdahlii. Fermentation 2020, 6, 61. [Google Scholar] [CrossRef]
- Pacheco, M.; Pinto, F.; Ortigueira, J.; Silva, C.; Gírio, F.; Moura, P. Lignin Syngas Bioconversion by Butyribacterium methylotrophicum: Advancing towards an Integrated Biorefinery. Energies 2021, 14, 7124. [Google Scholar] [CrossRef]
- Rückel, A.; Hannemann, J.; Maierhofer, C.; Fuchs, A.; Weuster-Botz, D. Studies on Syngas Fermentation With Clostridium carboxidivorans in Stirred-Tank Reactors With Defined Gas Impurities. Front. Microbiol. 2021, 12, 655390. [Google Scholar] [CrossRef]
- Oswald, F.; Zwick, M.; Omar, O.; Hotz, E.N.; Neumann, A. Growth and Product Formation of Clostridium ljungdahlii in Presence of Cyanide. Front. Microbiol. 2018, 9, 1213. [Google Scholar] [CrossRef]
- Infantes, A.; Kugel, M.; Raffelt, K.; Neumann, A. Side-by-Side Comparison of Clean and Biomass-Derived, Impurity-Containing Syngas as Substrate for Acetogenic Fermentation with Clostridium ljungdahlii. Fermentation 2020, 6, 84. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.N.; Carolino, C.; Miranda, M.; Abelha, P.; Direito, D.; Dohrup, J.; Sørensen, H.R.; Girio, F. Effects of Experimental Conditions and of Addition of Natural Minerals on Syngas Production from Lignin by Oxy-Gasification: Comparison of Bench- and Pilot Scale Gasification. Fuel 2015, 140, 62–72. [Google Scholar] [CrossRef]
- Worden, R.M.; Grethlein, A.J.; Zeikus, J.G.; Datta, R. Butyrate production from carbon monoxide by Butyribacterium methylotrophicum. Appl. Biochem. Biotechnol. 1989, 20–21, 687–698. [Google Scholar]
- Humphreys, J.R.; Hebdon, S.D.; Rohrer, H.; Magnusson, L.; Urban, C.; Chen, Y.-P.; Lo, J. Establishing Butyribacterium methylotrophicum as a Platform Organism for the Production of Biocommodities from Liquid C1 Metabolites. Appl. Environ. Microbiol. 2022, 88, e02393-21. [Google Scholar] [CrossRef] [PubMed]
- Oswald, F.; Dörsam, S.; Veith, N.; Zwick, M.; Neumann, A.; Ochsenreither, K.; Syldatk, C. Sequential Mixed Cultures: From Syngas to Malic Acid. Front. Microbiol. 2016, 7, 891. [Google Scholar] [CrossRef]
- Alabdrabalameer, H.A.; Taylor, M.J.; Kauppinen, J.; Soini, T.; Pikkarainen, T.; Skoulou, V. Big problem, little answer: Overcoming bed agglomeration and reactor slagging during the gasification of barley straw under continuous operation. Sustain. Energy Fuels 2020, 4, 3764–3772. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Q.; Gong, Y.; Ding, L.; Wang, J.; Yu, G. A review of the effects of alkali and alkaline earth metal species on biomass gasification. Fuel Process. Technol. 2021, 214, 106723. [Google Scholar] [CrossRef]
- Jordan, C.A.; Akay, G. Speciation and Distribution of Alkali, Alkali Earth Metals and Major Ash Forming Elements during Gasification of Fuel Cane Bagasse. Fuel 2012, 91, 253–263. [Google Scholar] [CrossRef]
- Widjaya, E.R.; Chen, G.; Bowtell, L.; Hills, C. Gasification of non-woody biomass: A literature review. Renew. Sustain. Energy Rev. 2018, 89, 184–193. [Google Scholar] [CrossRef]
- Zhu, C.; Maduskar, S.; Paulsen, A.D.; Dauenhauer, P.J. Alkaline-Earth-Metal-Catalyzed Thin-Film Pyrolysis of Cellulose. ChemCatChem 2016, 8, 818–829. [Google Scholar] [CrossRef]
- Ge, Z.; Kong, L.; Bai, J.; Chen, X.; He, C.; Li, H.; Bai, Z.; Li, P.; Li, W. Effect of CaO/Na2O on slag viscosity behavior under entrained flow gasification conditions. Fuel Process. Technol. 2018, 181, 352–360. [Google Scholar] [CrossRef]
- Holmgren, P.; Broström, M.; Backman, R. Slag Formation during Entrained Flow Gasification: Silicon-Rich Grass Fuel with a KHCO3 Additive. Energy Fuels 2018, 32, 10720–10726. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Chemical Composition of Biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Guo, Q.; Cheng, Z.; Chen, G.; Yan, B.; Li, J.; Hou, L.; Ronsse, F. Assessment of biomass demineralization on gasification: From experimental investigation, mechanism to potential application. Sci. Total. Environ. 2020, 726, 138634. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Hu, S.; Wang, Y.; Su, S.; Sun, L.; Xu, B.; He, L.; Xiang, J. Catalytic effects of inherent alkali and alkaline earth metallic species on steam gasification of biomass. Int. J. Hydrog. Energy 2015, 40, 15460–15469. [Google Scholar] [CrossRef]
- Skoulou, V.; Kantarelis, E.; Arvelakis, S.; Yang, W.; Zabaniotou, A. Effect of biomass leaching on H2 production, ash and tar behavior during high temperature steam gasification (HTSG) process. Int. J. Hydrogen Energy 2009, 34, 5666–5673. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, C.; Onwudili, J.A.; Meng, A.; Zhang, Y.; Williams, P.T. Polycyclic Aromatic Hydrocarbon Formation from the Pyrolysis/Gasification of Lignin at Different Reaction Conditions. Energy Fuels 2014, 28, 6371–6379. [Google Scholar] [CrossRef]
- Neumann, A.; Dörsam, S.; Oswald, F.; Ochsenreither, K. Chapter 4-Microbial Production of Value-Added Chemicals from Pyrolysis Oil and Syngas. In Sustainable Production of Bulk Chemicals; Xian, M., Ed.; Springer: Dordrecht, The Netherlands, 2016; pp. 69–105. ISBN 9789401774758. [Google Scholar]
- Broniatowski, M.; Binczycka, M.; Wójcik, A.; Flasiński, M.; Wydro, P. Polycyclic aromatic hydrocarbons in model bacterial membranes—Langmuir monolayer studies. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 2402–2412. [Google Scholar] [CrossRef]
- Dyrda, G.; Boniewska-Bernacka, E.; Man, D.; Barchiewicz, K.; Słota, R. The effect of organic solvents on selected microorganisms and model liposome membrane. Mol. Biol. Rep. 2019, 46, 3225–3232. [Google Scholar] [CrossRef]
- Park, S.; Yasin, M.; Jeong, J.; Cha, M.; Kang, H.; Jang, N.; Choi, I.-G.; Chang, I.S. Acetate-assisted increase of butyrate production by Eubacterium limosum KIST612 during carbon monoxide fermentation. Bioresour. Technol. 2017, 245, 560–566. [Google Scholar] [CrossRef]
- Pinto, F.; Lopes, H.; André, R.N.; Gulyurtlu, I.; Cabrita, I. Effect of catalysts in the quality of syngas and by-products obtained by co-gasification of coal and wastes. 1. Tars and nitrogen compounds abatement. Fuel 2007, 86, 2052–2063. [Google Scholar] [CrossRef]
- Shahbaz, M.; Yusup, S.; Inayat, A.; Patrick, D.O.; Ammar, M. The influence of catalysts in biomass steam gasification and catalytic potential of coal bottom ash in biomass steam gasification: A review. Renew. Sustain. Energy Rev. 2017, 73, 468–476. [Google Scholar] [CrossRef]
- Corella, J.; Toledo, J.M.; Padilla, R. Olivine or Dolomite as In-Bed Additive in Biomass Gasification with Air in a Fluidized Bed: Which Is Better? Energy Fuels 2004, 18, 713–720. [Google Scholar] [CrossRef]
- Bugg, T.; Foght, J.M.; Pickard, M.A.; Gray, M.R. Uptake and Active Efflux of Polycyclic Aromatic Hydrocarbons by Pseudomonas fluorescens LP6a. Appl. Environ. Microbiol. 2000, 66, 5387–5392. [Google Scholar] [CrossRef] [PubMed]
- Flesher, J.W.; Lehner, A.F. Structure, function and carcinogenicity of metabolites of methylated and non-methylated polycyclic aromatic hydrocarbons: A comprehensive review. Toxicol. Mech. Methods 2016, 26, 151–179. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 2675. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Acute Toxicity and Synergistic and Antagonistic Effects of the Aromatic Compounds of Some Essential Oils against Culex Quinquefasciatus Say Larvae. Parasitol Res 2015, 114, 3835–3853. [Google Scholar] [CrossRef]
- Vitale, C.M.; Gutovitz, S. Aromatic Toxicity; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]

| WST | SW | |
|---|---|---|
| Proximate analysis (wt.%, dry basis) | ||
| Volatile matter | 64.6 | 72.1 |
| Ash 550 °C | 14.0 | 0.1 |
| Ultimate analysis (wt.%, dry basis) | ||
| C | 47.2 | 57.7 |
| H | 5.6 | 6.2 |
| O | 33.0 | 33.8 |
| N | 1.3 | 0.8 |
| S | 0.18 | 0.13 |
| Cl | 0.020 | 0.002 |
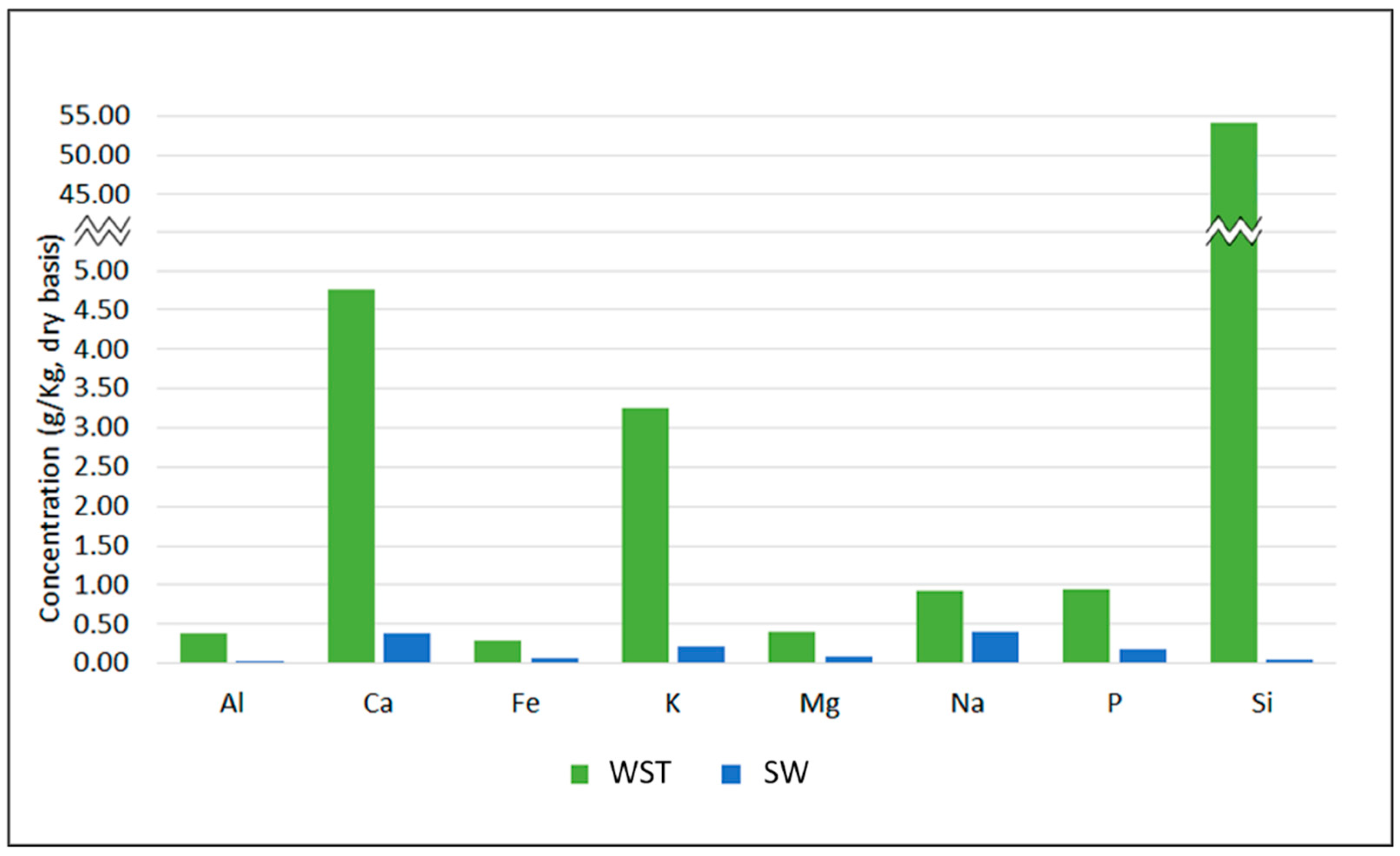
| ICP-AES 1 analysis (mg/kg, dry basis) | ||
| Al | 380 | 17 |
| Ca | 4750 | 380 |
| Fe | 290 | 48 |
| K | 3250 | 210 |
| Mg | 385 | 68 |
| Na | 906 | 390 |
| P | 930 | 160 |
| S | 1750 | 1300 |
| Si | 54,000 | <30 |
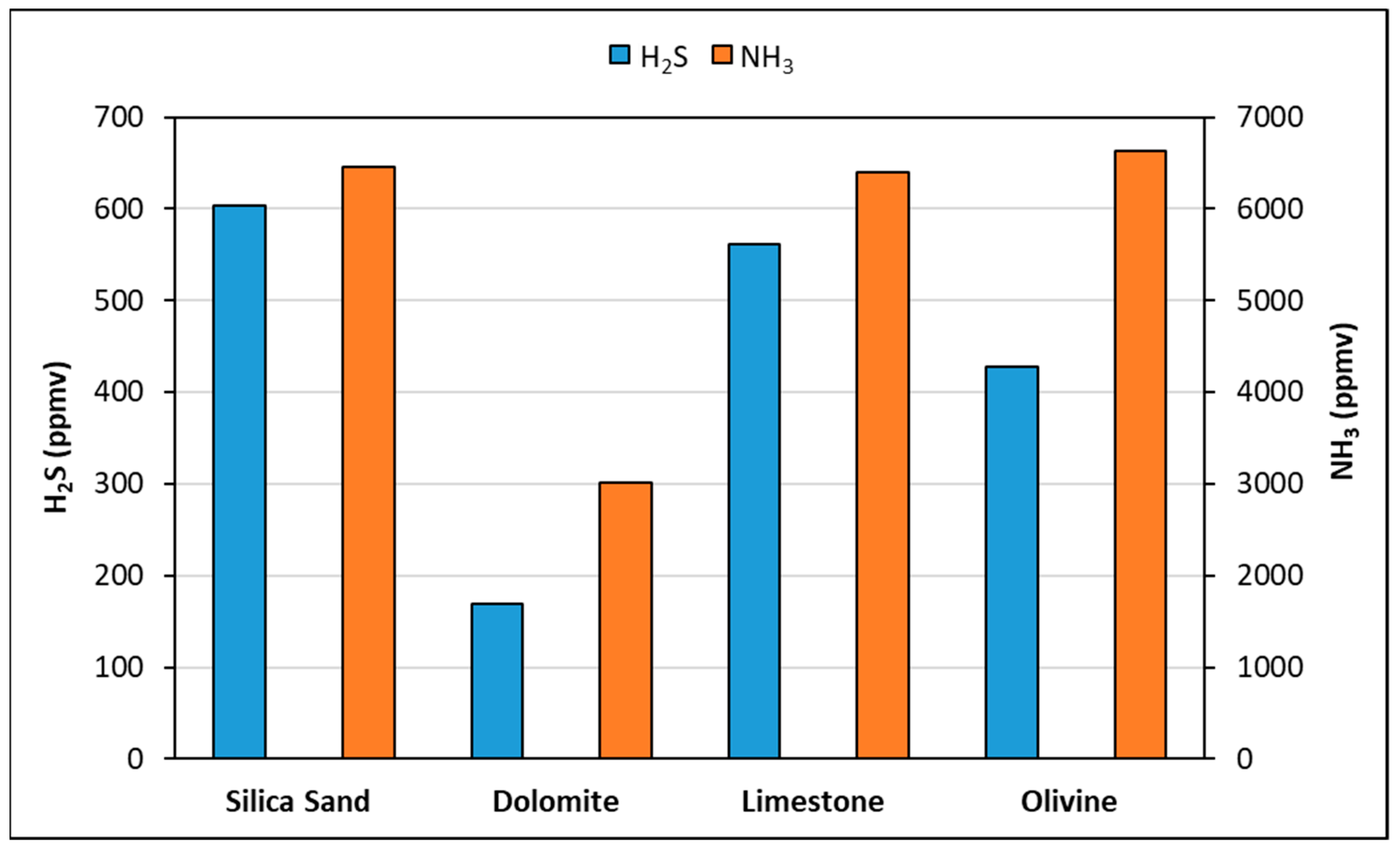
| Condensate | Lignin | Gasification Temperature (°C) | Catalyst |
|---|---|---|---|
| Cond1 | WST | 850 | - |
| Cond2 | SW | 750 | - |
| Cond3 | WST | 800 | - |
| Cond4 | WST | 800 | Dolomite |
| Cond5 | WST | 800 | Limestone |
| Cond6 | WST | 800 | Olivine |
| Group Specification | Cond1 | Cond2 | |||||
|---|---|---|---|---|---|---|---|
| No. of Compounds 1 | Total Percentage (%) | Relative Percentage (%) | No. of Compounds 1 | Total Percentage (%) | Relative Percentage (%) | ||
| Ions | 1 | 20 | 7 | 1 | 11 | 4 | |
| Carboxylic acids | 1 | 7 | - | 0 | |||
| Secondary Amines | - | 0 | 1 | 4 | |||
| Nitriles | 1 | 7 | 1 | 4 | |||
| Identified compounds | 3 | 3 | |||||
| Aromatics | Hydrocarbons | 1 | 80 | 7 | 1 | 89 | 4 |
| Benzene | 2 | 13 | 4 | 14 | |||
| Pyrroles | - | 0 | 1 | 4 | |||
| Pyridines | 5 | 33 | 6 | 21 | |||
| Pyrimidines | 2 | 13 | 4 | 14 | |||
| Pyrazines | - | 0 | 2 | 7 | |||
| Quinolines | 1 | 7 | - | 0 | |||
| Indoles | - | 0 | 2 | 7 | |||
| Phenols | 1 | 7 | 4 | 14 | |||
| Furans | - | 0 | 1 | 4 | |||
| Identified aromatics | 12 | 25 | |||||
| Total identified compounds | 15 | 28 | |||||
| Without Condensate | Cond1 | Cond2 | |||
|---|---|---|---|---|---|
| 1:100 (vol.) | 1:1000 (vol.) | 1:100 (vol.) | 1:1000 (vol.) | ||
| Max. CDW (g/L) | 1.19 ± 0.03 | 1.04 ± 0.00 | 1.15 ± 0.06 | 1.10 ± 0.02 | 1.13 ± 0.02 |
| OD (600 nm) | 0.75 | 0.11 | 0.67 | 0.59 | 0.76 |
| Acetate (mM) | 12.6 ± 0.9 | n.d 1 | 8.7 ± 1.3 | 15.5 ± 2.6 | 10.8 ± 1.9 |
| Butyrate (mM) | 3.0 ± 0.0 | 0.4 ± 0.0 | 4.5 ± 0.2 | 1.3 ± 0.3 | 4.5 ± 0.1 |
| Butyrate/Acetate (mol/mol) | 0.2 ± 0.0 | - | 0.5 ± 0.1 | 0.1 ± 0.0 | 0.4 ± 0.1 |
| Group Specification | Cond5 (Limestone + Silica Sand) | Cond6 (Olivine + Silica Sand) | |||||
|---|---|---|---|---|---|---|---|
| No. of Compounds 1 | Total Percentage (%) | Relative Percentage (%) | No. of Compounds1 | Total Percentage (%) | Relative Percentage (%) | ||
| Ions | 1 | 16 | 3 | 1 | 14 | 3 | |
| Carboxylic/Fatty acids | 2 | 5 | - | 0 | |||
| Nitriles | 3 | 8 | 4 | 11 | |||
| Identified compounds | 6 | 6 | |||||
| Aromatics | Hydrocarbons | 2 | 84 | 5 | 3 | 86 | 8 |
| Benzene | 4 | 10 | 5 | 13 | |||
| Imidazoles | 1 | 3 | - | 0 | |||
| Pyrroles | 1 | 3 | 1 | 3 | |||
| Pyridines | 14 | 37 | 14 | 38 | |||
| Pyrimidines | 3 | 8 | 3 | 8 | |||
| Pyrazines | 2 | 5 | 1 | 3 | |||
| Quinolines | 1 | 3 | 1 | 3 | |||
| Indoles | 1 | 3 | 1 | 3 | |||
| Phenols | 1 | 3 | 1 | 3 | |||
| Furans | 2 | 5 | 2 | 5 | |||
| Identified Aromatics | 32 | 32 | |||||
| Total identified compounds | 38 | 37 | |||||
| Group Specification | Cond3 (Silica Sand) | Cond4 (Dolomite + Silica Sand) | |||||
|---|---|---|---|---|---|---|---|
| No. of Compounds 1 | Total Percentage (%) | Relative Percentage (%) | No. of Compounds 1 | Total Percentage (%) | Relative Percentage (%) | ||
| Ions | 1 | 10 | 3 | 1 | 38 | 13 | |
| Carboxylic acids | - | 0 | 1 | 13 | |||
| Nitriles | 3 | 8 | 1 | 13 | |||
| Identified compounds | 4 | 3 | |||||
| Aromatics | Hydrocarbons | 2 | 90 | 5 | 1 | 63 | 13 |
| Benzene | 6 | 15 | 3 | 38 | |||
| Imidazoles | - | 0 | - | 0 | |||
| Pyrroles | 3 | 8 | - | 0 | |||
| Pyridines | 15 | 38 | 1 | 13 | |||
| Pyrimidines | 3 | 8 | - | 0 | |||
| Pyrazines | 1 | 3 | - | 0 | |||
| Quinolines | 2 | 5 | - | 0 | |||
| Indoles | 1 | 3 | - | 0 | |||
| Phenols | 1 | 3 | - | 0 | |||
| Furans | 1 | 3 | - | 0 | |||
| Identified Aromatics | 35 | 5 | |||||
| Total identified compounds | 39 | 8 | |||||
| Without Condensate | Cond3 | Cond4 | Cond5 | Cond6 | |
|---|---|---|---|---|---|
| ΔPressure (atm) 1 | 0.36 ± 0.06 | 0.11 ± 0.01 | 0.42 ± 0.00 | 0.30 ± 0.07 | 0.41 ± 0.00 |
| Max. cellular density (600 nm) | 0.28 | 0.26 | 0.32 | 0.27 | 0.31 |
| Acetate (mM) | 7.0 ± 0.5 | 8.8 ± 0.4 | 3.9 ± 0.9 | 5.3 ± 0.2 | 3.6 ± 0.4 |
| Butyrate (mM) | 3.2 ± 0.0 | 1.9 ± 0.3 | 3.9 ± 0.3 | 3.4 ± 0.5 | 4.5 ± 0.1 |
| Butyrate/Acetate (mol/mol) | 0.5 ± 0.0 | 0.2 ± 0.0 | 1.0 ± 0.2 | 0.6 ± 0.1 | 1.3 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, M.; Pinto, F.; Brunsvik, A.; André, R.; Marques, P.; Mata, R.; Ortigueira, J.; Gírio, F.; Moura, P. Effects of Lignin Gasification Impurities on the Growth and Product Distribution of Butyribacterium methylotrophicum during Syngas Fermentation. Energies 2023, 16, 1722. https://doi.org/10.3390/en16041722
Pacheco M, Pinto F, Brunsvik A, André R, Marques P, Mata R, Ortigueira J, Gírio F, Moura P. Effects of Lignin Gasification Impurities on the Growth and Product Distribution of Butyribacterium methylotrophicum during Syngas Fermentation. Energies. 2023; 16(4):1722. https://doi.org/10.3390/en16041722
Chicago/Turabian StylePacheco, Marta, Filomena Pinto, Anders Brunsvik, Rui André, Paula Marques, Ricardo Mata, Joana Ortigueira, Francisco Gírio, and Patrícia Moura. 2023. "Effects of Lignin Gasification Impurities on the Growth and Product Distribution of Butyribacterium methylotrophicum during Syngas Fermentation" Energies 16, no. 4: 1722. https://doi.org/10.3390/en16041722
APA StylePacheco, M., Pinto, F., Brunsvik, A., André, R., Marques, P., Mata, R., Ortigueira, J., Gírio, F., & Moura, P. (2023). Effects of Lignin Gasification Impurities on the Growth and Product Distribution of Butyribacterium methylotrophicum during Syngas Fermentation. Energies, 16(4), 1722. https://doi.org/10.3390/en16041722






