Abstract
In recent years, the interest in renewable fuels has increased mainly due to regulations regulating the permissible limits of toxic components of exhaust gases emitted by reciprocating engines. This paper presents the results of a comparison of the effects of fueling a compression-ignition piston engine with a mixture of diesel fuel and n-butanol, as well as RME (Rapeseed Oil Methyl Esters) biodiesel and n-butanol. The tests were carried out for a constant load and a wide energetic share of fuels in the mixture. The main focus was on the assessment of combustion stability, the uniqueness of the combustion stages, and the assessment of the fuel type influence on the CA50 angle. The tests show that RME offers the possibility of efficient combustion with n-butanol with up to 80% energy share. The share of n-butanol has a positive effect on the engine’s efficiency and very effectively reduces soot emissions. Without the influence on COVIMEP, the share of n-butanol up to 40% in the mixture with diesel fuel and up to 80% in the mixture with RME was recorded. Combustion of RME with n-butanol was more stable. The share of n-butanol in the mixture with diesel fuel caused an increase in NOx emissions, and co-combustion with RME caused a decrease in emissions.
1. Introduction
The choice of the combustion system in a compression ignition engine is limited due to the compromise required between NOx emissions and soot emissions. Carrying out the combustion process at low temperature helps to reduce NOx emissions, while contributing to an increase in the emission of soot, which is not oxidized [1]. On the other hand, compression-ignition engines are characterized by high efficiency and durability. They are still eagerly used to drive machines and devices where these properties of the engine are required [2]. In order to reduce the negative environmental impact of a compression ignition engine, alternative fuels to diesel fuel can be used. Both liquid fuels and all kinds of alcohols [3,4] and gaseous fuels, such as CNG or hydrogen, are used here [5,6]. Liquid fuels, if their physical properties allow it, are mixed with typical engine fuel and fed to the engine using a typical injection engine [7]. Gaseous as well as liquid fuels can be co-combusted in a dual-fuel engine, where alternative fuel is fed to the intake manifold and direct injection is used to initiate ignition [4,8]. The simplest way of co-combusting fuels is to create a stable mixture and bring it to the combustion chamber through the already existing injection system.
Biofuels made from agricultural products not only offer benefits in terms of exhaust gas emissions and environmental impact, but also provide the opportunity to diversify energy carriers, reducing dependence on oil suppliers [9]. Another advantageous aspect is the possibility of supporting the agricultural industry and thus greater energy security of many countries. Among the biofuels for powering piston engines, bioalcohols and vegetable oils or their derivatives in the form of biodiesel are considered [10,11]. The use of biofuels to power a reciprocating engine has been studied for many years and the results of these studies encourage their wider use [4,12]. The most popular alcohols used to power internal combustion engines are ethanol and methanol [13]. Ethanol, due to its high octane number, is considered as a good fuel for spark ignition engines, and it is also believed that, similarly to methanol, it can partially eliminate diesel fuel when fed to the compression ignition engine [14]. Ethanol is a renewable fuel that can be produced by the alcoholic fermentation of sugar contained in plant raw materials [15]. In the case of compression ignition engines, the advantage of ethanol over methanol is mainly due to its better miscibility with diesel fuel [14]. The use of both fuels to power a compression ignition engine is caused by the desire to reduce the smoke opacity [3]. The use of most alcoholic fuels in the CI (compression ignition) engine also has some disadvantages caused by poor miscibility with diesel fuel. Thus, in order to obtain a stable mixture, additives ensuring the solubility of these fuels should be used [16]. In addition, ethanol is a hygroscopic fuel and tends to absorb water from the air. Another limiting feature of these fuels in the context of engine fueling is their low viscosity, which may adversely affect the durability of the injection system, including injectors and fuel pumps [17]. These fuels also have a much lower calorific value in relation to diesel fuel as well as a low cetane number [18,19]. Their participation in combustion contributes to the increase of the ignition delay time [15].
Compared to petrol engines, the disadvantage of compression-ignition engines powered by diesel fuel is the increased emission of soot particles and nitrogen oxides. Higher alcohols, such as butanol, pentanol, hexanol, and octanol, have great potential in this situation. Earlier work on using lower alcohols as a blend with diesel fuel in compression ignition engines suggested a reduction in emissions. However, problems, such as phase separation and increased fuel consumption, have also been encountered when using lower alcohols, e.g., ethanol, in diesel engines. Higher alcohols can be used with diesel fuel to obtain a homogeneous mixture without phase separation. They also contain molecular oxygen, which improves combustion and reduces emissions [20].
Butanol can be a good alternative to ethanol and methanol to power a compression ignition engine [21,22]. Butanol is a fuel with higher calorific value, higher cetane number, and better miscibility with diesel fuel than ethanol. Like ethanol, butanol is a renewable fuel obtained from the processing of biomass. Among other things, it is a by-product, like glycerol, in the production of biodiesel [23].
When comparing the properties of fuels, such as alcohols and biodiesel, with diesel fuel, it is necessary to point out the fundamental difference consisting in the different share of oxygen in the molecular structure of these biofuels. Butanol has a 4-carbon structure and is a more complex alcohol than ethanol. 1-Butanol, also known as n-butanol (normal butanol), has a straight chain structure with a hydroxyl (-OH) group [24]. Diesel fuel can be replaced with biodiesel, which creates stable mixtures with higher alcohols such as butanol and pentanol [25]. Biodiesel and n-butanol are typical renewable biofuels and are used as additives in diesel fuel in most of the existing research work on compression ignition engines. Compared to ethanol, butanol has more favorable properties, its calorific value (LHV) is about 30% higher than that of ethanol, it is not as corrosive as ethanol and forms stable mixtures with diesel fuel or biodiesel [26]. In addition, the share of butanol in the combustion process has a positive effect on the combustion itself as well as reducing exhaust emissions.
Sahin et al. [27] conducted a comparative study of two technologies for co-combustion of n-butanol and diesel fuel. The first one involved combusting a previously prepared mixture of both fuels, while the second one involved co-combusting diesel fuel with n-butanol injected into the collector. They found that combustion in a dual-fuel engine (with n-butanol injection) is characterized by lower soot emissions than in the case of combustion in the form of a mixture, but both methods favor the elimination of soot. Summarizing their research, they found that combustion of a fuel mixture had a more favorable effect on engine performance than combustion in a dual-fuel engine. Huang et al. [28] examined the use of biodiesel/n-butanol blends in an agricultural diesel engine in terms of combustion parameters, efficiency, and emissions. The combustion of biodiesel and n-butanol blends containing 10%, 20%, and 30% by weight of n-butanol and marked as BBu10 (10% by weight of n-butanol + 90% by weight of biodiesel), BBu20 (20% by weight of n-butanol + 80% by weight of biodiesel), and BBu30 (30% by weight of n-butanol + 70% by weight of biodiesel) was tested. It was found that adding 30% n-butanol to biodiesel can reduce the viscosity by 39.3% and increase the latent heat of vaporization by 57.3%. The engine test results showed that when n-butanol was added to biodiesel, the maximum cylinder pressure and temperature decreased slightly, and the peak pressure rate and heat release rate peaks increased. In addition, fuel ignition was delayed and the combustion time reduced. BBu20 had ignition characteristics similar to diesel fuel. Both the thermal efficiency and specific fuel consumption of the BBu30 increased by an average of 2.7% and 14.9%, while the NOx, soot, and CO emissions of the BBu30 decreased by 17.6%, 34.1%, and 15%, respectively. The above differences became more visible with the increase in the share of n-butanol [28]. Singh et al. [29] presented the results of combustion studies in an engine fueled with biodiesel (eucalyptus oil) mixed with diesel fuel and butanol. Two-component mixtures containing 20% biodiesel and 80% diesel fuel and three-component mixtures containing 20% biodiesel, 65–75% diesel fuel, and 5–15% butanol were tested. It was found that the share of butanol caused an increase in BSFC (brake specific fuel consumption) and a slight increase in engine power, while at the same time there was a 10% decrease in CO emissions for the share of butanol, a significant reduction in THC emissions by more than 40% and about a 20% reduction in NOx emissions. They concluded that a biodiesel/diesel/butanol blend could be a good alternative to diesel. Divakar Shetty et al. [30] presented experimental studies of combustion of three-component mixtures of butanol, biodiesel and diesel fuel (BBD) in terms of combustion phases, performance and emission characteristics of a diesel engine with direct injection (DI). The blends were prepared in various proportions of butanol, biodiesel, and diesel fuel with different properties, such as calorific value, viscosity, auto-ignition temperature, cloud point, and pour point. The engine test was carried out at different speed and load. Based on the results obtained for the fuel properties, it can be observed that the flash point, combustion point, and solidification point, as well as viscosity and density, decrease with increasing percentage of butanol in BBD blends. It was also observed that the operating parameters, such as thermal efficiency and exhaust gas temperature, increased with an increase in the share of butanol in the BBD mixture. However, the brake specific fuel consumption (BFSC) decreases as the proportion of butanol in the BBD blend increases. The increase in butanol in BBD blends also increases the emission of carbon monoxide (CO), hydrocarbons (HC), and nitrogen oxides (NOx) [30]. Xiao et al. [26,31] presented the results of the characteristics of the combustion process of a mixture of biodiesel and butanol in a compression ignition engine at various loads. They found that the addition of butanol improves the degree of fuel evaporation and atomization, and thus has a positive effect on the combustion process itself. The increase in the proportion of butanol resulted in an increase in the ignition delay and shortened the combustion time. When assessing the exhaust emissions, they found an increase in THC emissions for the entire analyzed load range and, at the same time, a reduction in CO emissions. NOx emissions increased significantly. Another conclusion was that the increase in the share of butanol causes an increase in PM emission in the range <10 µm and a significant reduction in the emission of larger particles. Imtenan et al. [32] presented the results of the impact of 5–10% of butanol in a mixture with Jatropha biodiesel on the combustion process in a compression ignition engine. The influence of butanol on the beginning of combustion was found, and the ignition delay increased. The fuel with butanol participation generated higher pmax values due to the change in the cetane number value of the mixture. The specific fuel consumption increased due to the lower calorific value of butanol. As in the previously cited studies, decreased emissions of both CO and soot were noted. Moreover, THC emissions were significantly reduced compared to the engine running on diesel fuel alone.
Siwale et al. [33] investigated the influence of n-butanol in a mixture with diesel fuel in the range from 5 to 20% v/v on supercharged engine operation at various loads. They confirmed that the share of n-butanol contributes to the reduction of exhaust emissions. Moreover, it was noted that the share of n-butanol in the mixture causes the intensification of the kinetic combustion phase along with increase in its share. Another important finding was that combustion of the mixture with n-butanol improves the stability of the engine. Mickevicius et al. [34] presented the results of combustion of the RME biodiesel mixture with butanol in a naturally aspirated compression ignition engine. Three mixtures B5, B10 and B15 were tested, respectively 5%, 10 and 20% v/v of butanol in the mixture with RME. Higher BSFCs have been reported for mixtures compared to RME alone, but this is obvious as butanol has a lower calorific value. CO emissions have decreased. A slight reduction in NOx emissions was also achieved with a simultaneous reduction in soot opacity, which is a great advantage of using renewable fuels. Makarevičienė et al. [35] used a three-component mixture of diesel fuel (D), biodiesel (RME), and butanol (B). It was found that such a mixture meets the standards of summer fuels which are used in compression ignition engines. The fuel filter plugging point for such ternary mixtures is below 5 °C. In the period with the temperature below 30 °C, it is possible to use mixtures containing up to 14% RME.
The use of alternative fuels very often adversely affects the stability of the engine. As the test results show, in a reciprocating engine, each subsequent cycle of engine operation differs from the previous one, which is a characteristic of that type of machine. This uniqueness of successive cycles of engine operation significantly impacts the torque curve on the engine shaft. As shown by the studies of the combustion chamber aerodynamics [36], in the engine powered without a fuel supply, the velocity field is unique in subsequent cycles.
The difference in speed occurs not only in its maximum value but also in the angle of the crankshaft position. As seen from the data in Figure 1, the speed peak value occurs before the piston reaches TDC, i.e., within the CA range, which is essential for developing the initial combustion phase. Many factors in the cylinder of a reciprocating engine contribute to the uniqueness of its successive cycles. These factors affect the stability of the combustion process. Combustion instability can be divided into several categories:
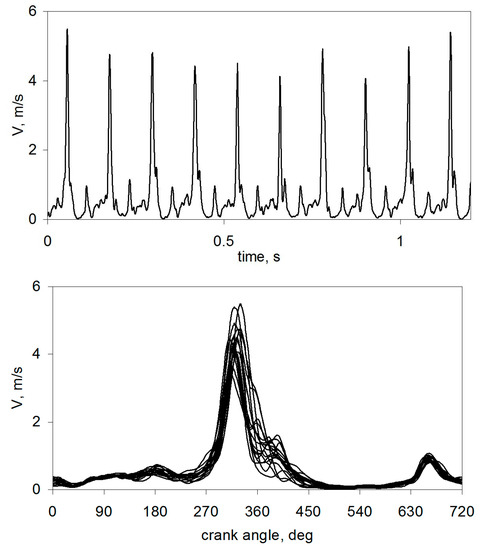
Figure 1.
Speeds of the fresh charge in the combustion chamber of the SI engine [36].
- −
- Chamber instability is caused by the fact that combustion in the engine takes place in a closed space, and additionally, this volume changes during the combustion process. In this closed volume, the spreading combustion front is influenced by flow processes, such as charge swirling, the so-called process of squeezing the charge from above the piston crown to the center of the combustion chamber, and the turbulence of the charge.
- −
- Internal instability is controlled by the combustion kinetics. It is essential in the co-combustion of fuels of different reactivity, as is the case with the combustion of fuel mixtures and in a dual-fuel engine. Diffusion and hydrodynamic phenomena are also of great importance here.
- −
- System instability: This type of instability depends on the engine power system, the interaction of the intake and exhaust manifolds during the overlap period, and the operation of the internal EGR.
In the combustion chamber of internal combustion reciprocating engines, all types of instability occur simultaneously and affect the engine’s operation, deteriorating its operation (Figure 2).
Figure 2.
The effect of the uniqueness of engine work cycles.
The effect of combustion instability, as shown in Figure 2, can cause the increased emission of toxic exhaust gas components, the high uniqueness of the subsequent cycles will reduce the engine’s efficiency, and thus the engine power will decrease. It follows that the stable combustion process determines the stable operation of the engine. The paper presents the results of the analysis of the parameters of a compression ignition engine fueled by a mixture with a large share of n-butanol. The obtained results were related to the areas of instability, showing what values can reach the engine performance parameters in individual cycles of its operation.
The paper presents the results of comparative experimental tests of a compression ignition engine powered by a mixture of diesel fuel/n-butanol and biodiesel/n-butanol, where biodiesel is RME (Rapeseed Methyl Esters) produced based on rapeseed oil [37]. The tests were carried out on the same engine for comparable working and load conditions. The maximum acceptable engine proportion of butanol in the mixture was determined for both primary fuels, diesel fuel and biodiesel.
Particular attention was paid to the assessment of combustion stability by analyzing the stability of the combustion process based on the analysis of the unrepeatability coefficient of the indicated mean effective pressure (COVIMEP), the spread of characteristic combustion stages (CA10, CA50, and CA90), the distribution of the maximum pressure pmax, maximum temperature Tmax, and IMEP as a function of the CA50 angle, and the IMEP probability density function was analyzed. Reference is also made to changes in the emission of toxic exhaust components depending on the share of co-combusted fuels.
2. Materials and Methods
2.1. Research Engine and Apparatus
Experimental research was carried out on a stationary, industrial, compression-ignition engine designed to drive various types of machines and devices, mainly in the agricultural industry. It is a single cylinder, naturally aspirated, air-cooled, four-stroke engine with a nominal power of 7 kW, working at maximum load (IMEP = 0.68 MPa) and constant speed (1500 rpm). The tests were carried out for the nominal engine load. A dynamometer with a DC generator was used to measure and stabilize the power, load and rotational speed of the engine. The indicator system included: a piezoquartz pressure sensor (Kistler 6061), placed in the combustion chamber, a charge amplifier (Kistler 5011), a crank angle marker (encoder), installed on the engine crankshaft and a digital data acquisition system with an A/D card (Measurement Computing USB-1608HS), a PC and specialized software (National Instruments LabVIEW). The encoder enabled detection of the top dead center (TDC) signal and the angle of rotation of the crankshaft with a resolution of 1 deg CA and its synchronization with the pressure signal. Measurement of air consumption was carried out using a rotor flow meter (Common CGR-01) through a tank damping pulsations in the intake manifold. Fuel consumption during engine operation was determined by measuring the time of emptying an additional glass fuel tank of a known volume. The system made it possible to correct the measured consumption with a dose of fuel returned from the injection pump and the injector. For each operating point, the pressure was recorded in 200 consecutive cycles, after the engine had been thermally stabilized. As part of the research, engine exhaust emissions were also measured. Concentrations of NO, THC, CO, and CO2 were recorded using a five-gas exhaust gas analyzer (Bosch BEA 350), and the concentration of soot using an opacimeter (AVL Smokemeter 415SE). The method of measuring HC, CO, and CO2 emissions was the technology of nondispersive infrared (NDIR) spectrometry, while the measurement of NO emissions was based on the use of electrochemical sensors. The smoke opacity was measured by the filter paper method on the FSN (Filter Smoke Number) scale (according to ISO 10054), which enables the mapping of the soot concentration in mg/m3. To ensure the correctness of the engine tests, each measurement was repeated three times, and all the experiments were carried out in the same ambient conditions with the same pressure and temperature. The engine test stand, instrumentation, and data recording system are presented in Figure 3 and Table 1.
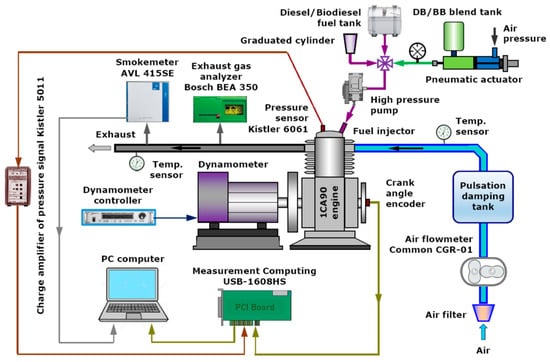
Figure 3.
The engine test stand.

Table 1.
Technical specifications of the test devices.
The obtained values of measurement uncertainties and errors were calculated on the basis of the error analysis contained in the work [38].
2.2. Fuels Characteristics
As part of the research, the engine was powered by mixtures of diesel fuel and n-butanol as well as RME and n-butanol. Fuel mixtures were prepared according to energy shares every 10%. The properties of the base fuels are presented in Table 2. Diesel fuel is a standard, commercially available fuel that meets the EN590 standard. Biodiesel RME is a fuel that meets the EN 14214 standard, applicable to the markets of the European Union.

Table 2.
Fuels specification.
The RME biodiesel is a biofuel produced based on rapeseed oil, intended for diesel engines. RME (Rapeseed Methyl Esters) are formed in the chemical process of transesterification of vegetable oil, as a result of which triglycerides react with methanol in the presence of a catalyst to form fatty acid methyl esters and glycerol. Transesterification involves the exchange of chemically bound glycerol in the triacylglycerol molecule to the added methyl alcohol in the presence of an alkaline catalyst. The catalyst for the reaction is the alkoxide ions [39]. According to the manufacturer, RME contains min. 96.5% m/m fatty acid methyl esters. Most biodiesel stocks consist of high viscosity methyl esters, for which rapeseed methyl ester (RME) is often used as the model compound [40]. RME and conventional diesel have similar physical and chemical characteristics. The offered biofuel has a beneficial effect on the operation and durability of the engine, and thanks to its good lubricating properties, it protects the engine’s injection apparatus against excessive wear. A very important advantage of RME is practically zero sulfur content, which is a beneficial feature both for ecological reasons and due to corrosive wear of engine components. The higher oxygen content in biodiesel than traditional diesel fuel ensures better combustion, and the high cetane number contributes to trouble-free ignition initiation. Biodiesel is characterized by a slightly lower calorific value than diesel fuel, adversely affecting the specific fuel consumption and engine efficiency. The use of biodiesel protects the environment. It significantly reduces the emission of harmful substances and greenhouse gases during biofuel production and its use. It is a fuel safe for human health and additionally biodegradable.
Butanol is a higher-order alcohol with a longer carbon chain, which, compared to lower-order alcohols such as ethanol and methanol, is characterized by a much higher cetane number, higher flame rate and calorific value, and is also an ecological fuel, possible to be obtained from natural sources. Compared to ethanol, butanol shows better fuel properties in terms of energy content, lubricity and corrosiveness of metal construction materials (tank, cylinder wall). In addition, butanol forms a phase-stable mixture with diesel fuel that does not require additional equipment and homogenizing agents, and its lower corrosiveness and lower water absorption (hydrophobicity) can help reduce corrosion problems in fuel delivery and injection systems. Butanol can be produced using a variety of technologies and a wide variety of sources (e.g., fossil fuels). Currently, more and more often, the raw material for the production of butanol are derived from natural sources [41,42]. It can be produced, e.g., by plant fermentation from lignocellulosic biomass and wood material.
Diesel fuel was selected as the reference fuel and the results obtained for RME and the analyzed mixtures were related to this fuel.
Figure 4a,b show graphically the list of energy doses of fuels. These fuel doses in the engine at the same injection start angle ensured the same load. For the engine powered by a mixture of diesel fuel and n-butanol, for the entire range of fuel share, the energy dose was lower than that required for the engine powered by the reference fuel, i.e., diesel fuel. The lowest energy dose was recorded for the engine powered with 40% of n-butanol energy and it was 907 J/cycle. For a larger share of n-butanol, the requirements for the energy dose increased, which was caused by the deterioration of the combustion process and the increasing uniqueness of cycles.
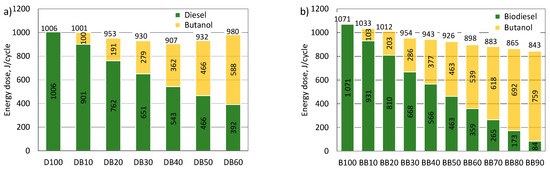
Figure 4.
The effect of the addition of n-butanol to diesel (a) and RME biodiesel (b) on the energy dose of fuel delivered in each engine cycle.
For the engine powered by the RME-n-butanol mixture, it was found that with the increase in the energy content of n-butanol, the required energy dose to power the engine, in order to obtain the same engine power, decreased. The combustion of the BB90 mixture was already very unstable. Therefore, 80% was considered the final share of n-butanol. For the BB80, the required fuel energy dose was 865 J/cycle and was nearly 20% lower than for the engine powered by RME.
The tests were carried out with a constant load. Changing the fuel caused a different course of the combustion process; the values of ignition delay and combustion duration changed. An increase in engine efficiency was noted with an increase in alcohol content. A higher efficiency value, while maintaining the same load, means lower fuel consumption by the engine. Hence, a smaller dose of energy is contained in the fuel, ensuring the same engine power.
2.3. Calculation Methodology
Engine operational indicators, such as indicated mean effective pressure (IMEP), were constantly monitored for each cycle. This was ensured by the data acquisition software (using a data acquisition software). The IMEP value was determined individually for each subsequent cycle. The efficiency of the engine was determined on the basis of the knowledge of the IMEP value, engine displacement volume and the dose of energy contained in the fuel per engine operation cycle. The energy dose per cycle was determined based on the measurement of fuel consumption. The heat release rate (HRR) was determined from the pressure in the based on the first law of thermodynamics and the equation of state [43].
The basic parameter for the assessment of the uniqueness of the engine operation is the COVIMEP index, as the ratio of the standard deviation of IMEP to the average value of 200 cycles [44]. The probability density distribution function was also used to assess the stability of the combustion process [43].
In practice, experimental measurement of physical properties is typically connected with a measurement error and uncertainty. Engine indication is a typical measurement that depends to a different degree on measurement accuracy and uncertainty of the obtained measurement. Therefore, the evaluation of the characteristic values for the engine based on the indicator diagram must be connected with the analysis of measurement errors and uncertainty of the experiments.
2.3.1. Indicated Mean Effective Pressure—IMEP
The indicated pressure is evaluated based on the recorded changes in the cylinder pressure and represents one of the indices that characterize the operation of combustion engines in terms of the opportunities to ensure high and expected functional performance. Indicated mean effective pressure for an engine cycle:
where p is in the cylinder pressure, V is cylinder volume, and Vd is cylinder displacement volume.
where i is the number of engine cycles.
Heat release rate profiles in the cylinder of the engine can also be derived from the developed indicator diagram through the calculation of changes in internal energy and indicated work of the medium. Heat release rate:
where κ is the ratio of specific heats, V is cylinder volume, and p is in-cylinder pressure.
The character of compression-ignition engine operation is substantially affected by the pressure rise rate. Pressure rise rate dp/dφ was determined from:
where p is in cylinder pressure and φ is crank angle.
2.3.2. Measurement Error of IMEP
The error of indicated mean effective pressure (IMEP) and rate of heat release (HRR) are affected by the error of in-cylinder pressure measurement and the error of measurement of instantaneous displacement. The IMEP error can be determined from the equation:
where δp is measuring error of the in-cylinder pressure and δV is the measurement error of instantaneous cylinder displacement.
The error of in-cylinder pressure measurement consists of the errors of pressure sensor, charge amplifier, and the data acquisition from the data logging module:
where δpt is the error of pressure sensor, δa is the error of charge amplifier, and δ(A/D) is the error of A/D data acquisition.
The measurement error of the instantaneous cylinder volume is affected by the errors of crank angle encoder and data acquisition measurement:
where δk is the error of CA crank angle encoder and δ(A/D) is the error of A/D data acquisition.
2.3.3. The Uncertainty Designation of IMEP
The uncertainty designation of the indicated mean effective pressure is the dispersion (spread) of the average calculation results of the IMEP in the individual cycles of the three measurements containing 200 recorded engine cycles. It was assumed that the uncertainty designation of the IMEP has a normal distribution, and it is calculated from the equation:
where ts is coefficient of the t-Student distribution for n − 1 degrees of freedom and for the most commonly adopted technique in the 95% confidence level, STDIMEP is standard deviation of the IMEP.
2.3.4. Indicated Thermal Efficiency—ITE
A crucial parameter in evaluating energy transitions in a piston engine is indicated by thermal efficiency (ITE), expressed in %. Thermal efficiency reflects engine thermal characteristics and is defined as a ratio of indicated work to the amount of energy (heat) supplied to the engine during one cycle.
The average value of the indicated thermal efficiency of the engine:
where IMEP is the indicated mean effective pressure, Vd is the displaced cylinder volume, and Qe is the total heat in the fuel supplied to the engine.
2.3.5. Measurement Error of ITE
Indicated efficiency is the ratio of indicated work performed in an engine operating cycle to the heat supplied to the engine in this cycle. During the experiment, measurement of heat supplied in a single cycle was impossible since the amount of heat in the fuel was measured in ca. 1 min (750 cycles). Indicated efficiency was defined as a ratio of the mean indicated work during the measurement of fuel consumption to mean heat. Indicated work is the product of indicated pressure (IMEP) and cylinder displacement. Therefore, the measurement error for the indicated mean efficiency represents the sum of the measurement error of indicated mean pressure and the measurement error of total heat supplied to the engine.
where δIMEP is the error of indicated mean effective pressure and δQe is the error of total heat supplied to the engine.
The heat in the fuel supplied to the engine:
where Ve is the volume of fuel supplied to the engine, ρf is density of the fuel, LHVf is lower heating value, n is engine speed, and t is the time of consumption for the fuel in engine cylinder.
The error of heat in the fuel supplied to the engine:
where δVe is measurement error of volume of fuel delivered to the engine, δρf is estimated error of fuel density, δLHVf is estimated error of LHV for the fuel, δn is measurement error of engine speed in rpm, and δt is measurement error of consumption time for the fuel supplied to the engine.
2.3.6. The Uncertainty of ITE
The determination of indicated efficiency uncertainty is affected by the uncertainty of determination of indicated pressure, rotational speed, and fuel consumption time in the engine cylinder. Uncertainty of determination of indicated efficiency was determined based on three measurements of engine speed, time of fuel consumption in the engine, and three mean values of indicated pressure. Uncertainty of indicated efficiency was calculated from the formula:
where ∆IMEP is uncertainty of indicated mean effective pressure, ∆n is uncertainty of engine speed, and ∆t is uncertainty of time of consumption of fuel in the engine cylinder.
2.3.7. Coefficient of Variation in Indicated Mean Effective Pressure—COVIMEP
One of the essential criteria for evaluating the correct operation of combustion engines is the non-repeatability of the work cycles. The coefficient of variation of indicated pressure COVIMEP, expressed in percentages and calculated as a ratio of the standard deviation of the engine indicated pressure to its mean value from three measurements containing 200 recorded engine operation cycles, was adopted as a measure of non-repeatability.
where STDIMEP is the standard deviation of indicated mean effective pressure.
3. Results and Discussion
As part of the research, a comparative analysis of the combustion stability and exhaust emissions of a compression-ignition piston engine powered by a mixture of diesel fuel and n-butanol as well as RME biodiesel and n-butanol was carried out. The assessment of combustion stability was based on the results of recording changes in pressure in the engine cylinder for the next 200 cycles for each analyzed measuring point. Stability was assessed on the basis of the uniqueness of the mean indicated pressure, as well as on the basis of the uniqueness of the characteristic combustion stages. Moreover, the influence of the fuel type on the 50% heat release angle (CA50) was assessed.
3.1. Assessment of Combustion Stability
The assessment of the combustion process taking place in a reciprocating engine is carried out on the basis of the recorded pressure courses in the engine cylinder. Indication of a piston engine gives the most reliable diagnostic results. The engine operation indicators determined on this basis are considered to be the closest to the real ones. In such a research process, an averaged pressure course obtained from a set of several dozen consecutive engine operation cycles is usually used.
Figure 5 and Figure 6 show the results of a comparative analysis of the co-combustion of diesel fuel and n-butanol (DB) and RME biodiesel/n-butanol (BB) for the full energy share of n-butanol acceptable to the engine. The obtained results refer to the constant engine load and the constant injection timing into the combustion chamber. This made it possible to evaluate the influence of the type of fuel on the combustion process and then to assess the combustion stability.
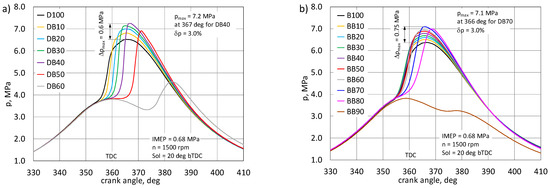
Figure 5.
Comparison of the combustion pressure courses for an engine powered by a mixture of diesel fuel with n-butanol (DB) (a) and RME biodiesel with n-butanol (BB) (b).
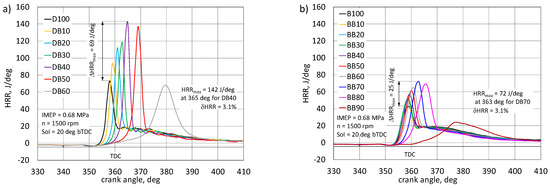
Figure 6.
Comparison of the heat release rate for an engine powered by a mixture of diesel fuel with n-butanol (DB) (a) and RME biodiesel with n-butanol (BB) (b).
In the engine powered by DB, the highest energy share of n-butanol, at which the engine could still be controlled, was 60%. For the engine powered by BB, the maximum energy share of n-butanol was as much as 80%. Cycles with combustion as well as cycles without combustion occurred with a higher share of n-butanol. The nature of the pressure changes in the engine powered by DB shows the influence of n-butanol content on the delay of self-ignition of the mixture, the effect of which is a significant difference in the rate of heat release, the peak value of which increases with the ignition delay. Burning becomes more violent [4]. For the engine powered by DB, the highest combustion pressure was obtained for a 40% share of n-butanol and reached 7.25 MPa at 7 deg aTDC. On the basis of tests in a constant-volume chamber, it was found that an increase in the share of n-butanol in a mixture with diesel fuel causes an increase in the maximum combustion pressure [16,19,22].
For the same proportion of n-butanol, the maximum HRR value was 142 J/deg and was higher by 69 J/deg in relation to the diesel fuel combustion effect. Combustion in the same engine powered by BB has a different character. Here, up to a 60% share of n-butanol was recorded, practically only an increase in the maximum pressure value, which for the mentioned share was 0.7 MPa and the maximum pressure was close to TDC. Exceeding this share of n-butanol resulted in a visible delay in auto-ignition. This effect is also visible on HRR traces. For the BB-powered engine, the HRR gain was only 25 J/deg. RME, thanks to its properties and good miscibility with n-butanol, gives great possibilities for co-combustion with alcohols, especially with higher alcohols [20,22,38]. In the work [28], a similar effect of butanol share on the nature of heat release rate was observed. The increase in the share of butanol resulted in an increase in the HRRmax value. An important aspect of the assessment of the combustion process in a reciprocating engine is the analysis of its characteristic stages: ignition delay (CA10) and duration of combustion (CA90), as well as the assessment of the crank angle after the TDC for which 50% heat release (CA50) occur. These characteristic combustion stages are determined on the basis of the normalized heat release (Qnorm) [27,45]. The first stage of combustion is the ignition delay, defined as the time (usually expressed in degrees of CA) from the start of injection to the moment when 10% of heat is released. It is assumed that at this stage the flame front is already so developed that it quickly penetrates into the combustion chamber. The ignition delay time is Influenced by many factors, both physical and chemical [22,46]. Consequently, the ignition delay period is often divided into physical and chemical retardation. Both steps usually occur simultaneously. The physical delay is mainly influenced by the injection process, namely the angle of the jet and its range in the combustion chamber, the ability of the jet to atomize, vaporize the fuel, and mix with air within the flammability limits [22]. This feature can be indirectly observed in the course of the heat release rate. In the engine, where this phenomenon occurs intensively, the HRR waveform shows a large share of the kinetic combustion phase. The second stage of the ignition delay is the chemical delay, the duration of which depends on the chemical properties of the fuel [45]. In the case of co-combustion of fuels, with the increasing share of n-butanol, the physicochemical properties of the fuel mixture will change, and this will affect the period of ignition delay, both physically and chemically. Figure 7 shows the normalized heat release curves for the DB and BB powered engine.
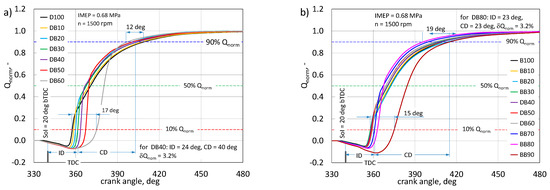
Figure 7.
Normalized heat release curves for the DB (a) and BB (b) powered engine.
The curves for the analyzed cases were obtained as the average of 200 consecutive engine operation cycles. Characteristic stages of combustion are marked. From the nature of the Qnorm run, it can be seen that the greatest differences for both fuel mixtures will be noticeable for the initial combustion phase. Basic fuels, i.e., diesel fuel and RME biodiesel, differ, inter alia, in the cetane number and thus in their ability to self-ignite [19].
Reciprocating engines powering working machines, especially those powering electricity generators, must meet the requirements for work stability. As reported in the literature [45], the IMEP uniqueness index should not exceed 5%.
Figure 8 presents the obtained results of the IMEP uniqueness assessment for the engine powered by DB and BB. For both variants, a set of pressure cycles was also shown, respectively. For the highest obtained COVIMEP values for DB, the maximum COVIMEP value was 4% and for BB as much as 78%.
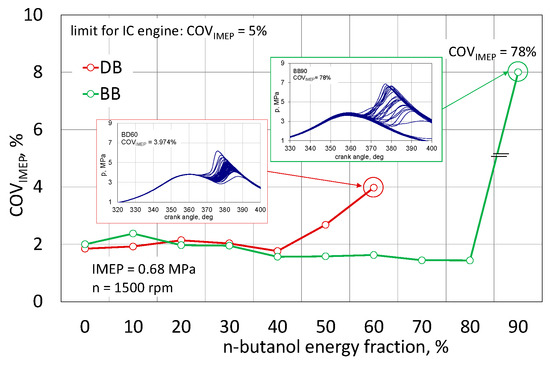
Figure 8.
Uniqueness of IMEP.
For both analyzed cases, up to 40% of the energy content of n-butanol, the COVIMEP index was at the same level of approximately 2%, i.e., the engine performance was stable. After exceeding this share for the DB mixture, the IMEP uniqueness began to increase, reaching the value of 4% for DB60. This is not exceeding the permissible limit, but above this proportion of n-butanol, the engine clearly had problems with the spontaneous combustion of the mixture. In an engine powered by a mixture with RME biodiesel, up to 80% of n-butanol content, the COVIMEP index remained constant below 2%, only exceeding this limit of n-butanol content practically prevented the engine from working. The assessment of the engine operation stability based only on COVIMEP does not provide full knowledge about this phenomenon [43,44,47]. In the assessment of the engine operation, characteristic stages of the combustion process are determined, i.e., the ignition delay period, the duration of combustion, and for optimization reasons, the angle after TDC of 50% of heat is released [7,21,46]. These parameters are determined from the MFB or normalized heat development determined for the averaged engine cycle. Figure 9 shows the nature of changes in the characteristic combustion stages for both fuels.
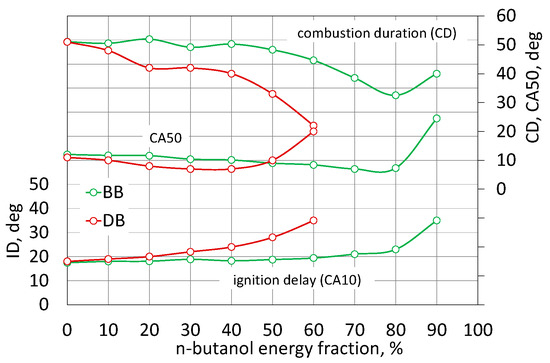
Figure 9.
The stages of CA10, CA50 and CA90 combustion.
The first stage is the ignition time in the engine, marked as ID, calculated from the injection start angle to the 10% heat release angle. The ignition delay time (ID) is defined as the period from the start of fuel injection to the of 10% of heat released. Combustion duration (CD) is also calculated by reading the time between the crank angle of 10% heat release and 90% of heat release. It is clearly visible here that the combustion of DB is characterized by an increase in the ignition delay with the increasing share of n-butanol. In the engine fueled with the BB mixture, the increase in the ignition delay angle up to BB80 is negligible (ID increase by 5 deg of CA in relation to RME biodiesel). In the DB-powered engine, for a 60% share of n-butanol, the ID increment was 7 deg of CA. The increase in ignition delay in an engine powered by DB was accompanied by a shortening of the combustion time along with an increase in the share of n-butanol. The same character of the influence of butanol content on the combustion stages is presented in [7,31]. The increase in butanol content increased ignition delay. This is due to the fact that a long ignition delay gives the fuel stream longer time to evaporate and thus more of the mixture burns in the kinetic phase [7,19,22,26]. For DB60, the burning time was reduced from 51 to 22 deg of CA, while for BB60 from 51 to 45 deg of CA, respectively. Analyzing the angle of 50% heat release after TDC, which is important for optimization of the engine cycle, with up to 50% of n-butanol share, in both cases, there are no significant changes in this value [46]. After exceeding this share for DB, this CA50 value clearly lags behind GMP. In the engine powered by BB, with up to an 80% share of n-butanol, the CA50 value is lower by 4 deg of CA in relation to rape oil alone.
The heat release traces presented in Figure 7 represent an average value and the values for individual cycles are important for engine control. Figure 10 presents four selected cases for the traces of normalized heat release for engine fueled with DB, BB, DB60, and BB60 mixtures. The spreads of conventional combustion stages are marked: ignition time (sCA10), angle of 50% heat release (sCA50), and conventional end of combustion (sCA90).
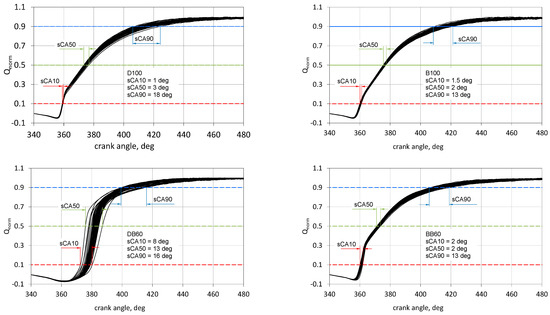
Figure 10.
Spread the characteristic stages of combustion for DB, BB, and DB60 and BB60 fuel mixtures.
Comparing the data for the engine powered by DB and BB, it can be seen that the spreads of the characteristic combustion stages are similar for both fuels, and for the engine powered by RME biodiesel there is a slightly higher repeatability of the cycles. CA90 spread was 5 deg smaller. Comparing the Qnorm waveforms for the engine powered with 60% n-butanol content, the stability of the combustion stages has clearly shifted towards the engine powered by RME biodiesel. Detailed results of the assessment of the uniqueness of these combustion stages are presented in Figure 11.
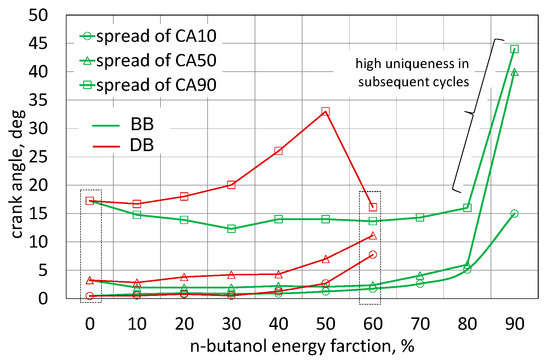
Figure 11.
Spread the stages of combustion.
When analyzing the CA10 dispersion, it was found that up to 40% of the n-butanol energy content, this stage is similar for both fuels, and the dispersion is within 1.5 deg CA. The increase in the proportion of n-butanol causes an increase in the spread of CA10 and so for the selected 60% share of n-butanol in the DB-powered engine this spread was close to 8 deg CA and for the BB engine only 2.5 deg CA. A significantly lower scattering of the CA50 step was obtained for the engine powered by the mixture with RME biodiesel as compared to the engine powered by the mixture with diesel fuel. For the 60% n-butanol share for DB, it was 11.5 deg CA, and for the BB engine it was 2.5 deg CA. The engine powered by BB up to 80% of n-butanol content achieved a smaller CA50 spread than the engine powered by DB60. When assessing the spread of the CA90 notional end-of-combustion, it was found that in a comparable range of n-butanol share, the BB engine shows a smaller end-of-combustion spread. In a BB powered engine, the uniqueness of all combustion stages was no longer acceptable and they are shown for information purposes only. Changing the combustion stages will affect the exhaust emissions [7,16,18,25]. Engine operation cycles with a shorter combustion time will generate greater increases in pressure and thus also in temperature, which will lead to an increase in NOx emissions. Cycles with extended combustion time will run under lower temperature conditions, and thus may, in effect, favor the emission of soot [28,31,48].
The correlation between the maximum pressure, maximum temperature, and IMEP values in relation to the CA50 angle was analyzed in detail. Figure 12 shows the dependence of the occurrence of the maximum pressure value as a function of the CA50 angle.
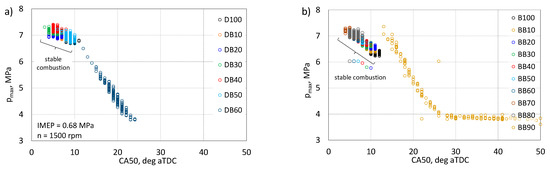
Figure 12.
Correlation of peak pressure and CA50 for DB (a) and BB (b).
For an engine powered by DB up to a 50% share of n-butanol, the angle CA50 is close to the optimal limits for a reciprocating engine. It should be noted here that the tests were carried out at a constant angle of the fuel injection start. After exceeding this share, the pmax value is successively decreased and the CA angle is shifted towards higher values after TDC. For the BB-powered engine up to 80% of n-butanol content, CA 50 is located near the optimal values, and for the BB90, there is a decrease in pmax and a delay in the CA50 angle, as was found for the DB60-fed engine. For the engine combusting BB90 fuel, many cycles of pmax of about 3.8 MPa were obtained, and these are cycles in which combustion was lost.
Figure 13 shows the dependence of the maximum temperature as a function of the CA50 angle. The temperature value is related to the combustion pressure in the combustion chamber of the engine, while according to the data in Figure 8, the nature of the changes is slightly different. In the DB combustion engine, the highest combustion temperature values were obtained for the share of 50% n-butanol, which may be the cause of NOx emission. It is known that an increase in combustion temperature with excess air causes an increase in NOx emissions [45,47]. On the other hand, for DB60 combustion, there was no significant increase in combustion temperature, but values were obtained approximately 10 deg (for CA50) later after TDC. In the BB-powered engine, only for a 90% share of n-butanol, part of the cycles with higher temperature compared to the reference fuel (B100) was achieved, and these Tmax values were also for the delayed cycles. For the maximum proportion of n-butanol, the ignition delay was so large that either there were cycles with a large increase in pressure and temperature Tmax or cycles where combustion was not initiated. IMEP is important for the evaluation of the operational properties of the engine, and the evaluation of this parameter related to the CA50 angle is presented in Figure 14.
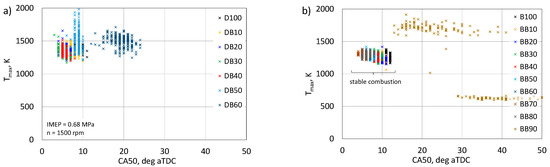
Figure 13.
Relationship of maximum temperature and CA50 for DB (a) and BB (b).
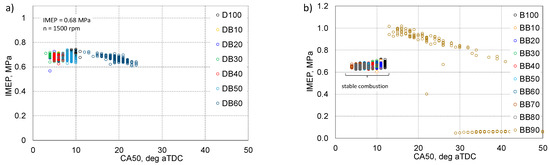
Figure 14.
Interdependence of IMEP and CA50 for DB (a) and BB (b).
The engine was tested under constant load and rotational speed, i.e., the IMEP value should be constant at 0.68 MPa. It is known that optimal engine operation is related to the CA50 angle, i.e., 50% heat release of approximately 8–10 deg aTDC. Analyzing the obtained data, it can be seen that for both analyzed cases, for stable engine operation, the IMEP values are concentrated within the CA50 range of 5–12 deg aTDC. For the DB60 powered engine, IMEP values were obtained for CA50 already within 20 deg aTDC. In the BB90 powered engine, as already mentioned, there were cycles with a higher IMEP than assumed and cycles that did not generate power. Figure 15 shows the relationship between the maximum pressure and IMEP.
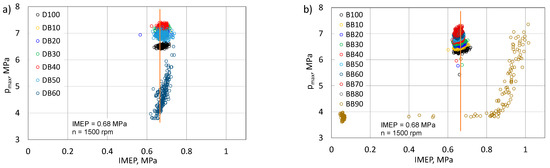
Figure 15.
Correlation of maximum pressure and IMEP for DB (a) and BB (b).
The IMEP value is closely related to the pressure in the engine cylinder. In the optimal cycle of a piston engine, the maximum pressure is several degrees after the TDC [45]. The change of the physicochemical properties of the fuel supplied to the engine, and each of the mixtures, is a different fuel. This affects the value of the maximum pressure and the angle after TDC of its occurrence. The speed of flame propagation changes, and thus the combustion time [47]. Figure 15 shows how the type of fuel (fuel mixture) affects the value of the maximum pressure at nearly the same IMEP. For the DB-powered engine, the increase in the proportion of n-butanol meant that the same IMEP was achieved at a higher pmax than was the case for the D100-powered engine. Only for the DB60 can one see a drop in pmax, but this was due to the high uniqueness of the cycles and there were clear problems with maintaining a constant engine load. For the BB fed engine, there is a greater focus around the set point. Similarly, for RME biodiesel, the share of n-butanol caused an increase in pmax. For the BB90 fuel, a very large dispersion of the pmax value in relation to the IMEP value can be seen, but it was already beyond the stable combustion range in the engine.
The last considered stability assessment criterion in this paper is the IMEP probability density function, presented in Figure 16.
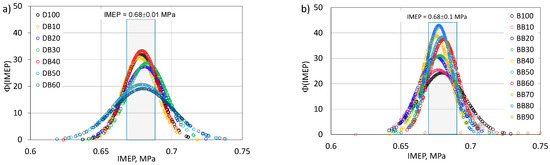
Figure 16.
Probability density function of IMEP for DB (a) and BB (b).
The Φ(IMEP) distribution shows that the engine powered by diesel fuel is characterized by a greater repeatability of IMEP compared to the engine powered by RME biodiesel. For the engine powered by D100, the value of Φ(IMEP) is 32 and for the engine powered by B100 it is equal to 25. The increase in the proportion of n-butanol in the mixture with diesel fuel adversely affects the combustion stability, determined by the Φ(IMEP) function. The situation is different for the engine fueled with a mixture with B100. Here, an improvement in stability can be seen up to 80% of n-butanol. The highest value of the Φ(IMEP) function was obtained for a 50% share of n-butanol and was 43. It can also be seen that, for an engine powered by a mixture with B100, the range of IMEP variability is in a narrower range than it was for the engine powered by the mixture with D100. The presented parameters for assessing combustion stability have a direct impact on the engine’s performance, such as its efficiency or fuel consumption, as shown in Figure 17.
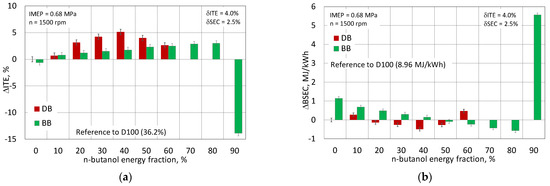
Figure 17.
Engine indicated efficiency (a) and specific energy consumption (b).
The engine powered by the DB mixture for the entire range of n-butanol content was more efficient than the engine powered by the reference fuel (D100). The engine achieved the highest efficiency for the DB40, 41%, and was 5% more efficient than the D100. For larger proportions of n-butanol, the efficiency began to decline due to increased combustion instability. In the BB-powered engine, up to 80% of the n-butanol energy content, an increase in efficiency was noted. For the BB80, the efficiency was close to 43% and was higher by 7.5% in relation to the engine powered by the reference fuel (B100). The benefits of using n-butanol in a mixture with other engine fuels can be seen on the BSEC, where practically for the entire range of n-butanol use (except BB90), a favorable trend of reducing the engine’s energy demand for fuel can be seen. It should also be noted that the fuels used, i.e., diesel fuel, biodiesel, and n-butanol, differ in density and viscosity to create the mixtures. In addition, the values of these two parameters significantly depend on the temperature. As research [49] shows, each of the fuels used has a different characteristic of lightness as a function of temperature, and in the analyzed cases, for different proportions of fuels, it is an even more complex process. Diesel fuel and n-butanol have similar viscosity, while biodiesel (RME) is more than 1.7 times more viscous than n-butanol. The viscosity and density of the base fuels and their mixtures affect the injection process, the range of the fuel stream, its distribution in the combustion chamber, as well as atomization and evaporation.
3.2. Emission
Diesel engines burn mainly heterogeneous mixtures and are characterized by increased HC and soot emissions [42]. In addition, compression-ignition engines operating with large excess air can also contribute to increased NOx emissions. Alcoholic fuels, due to the high latent heat of vaporization and high auto-ignition temperature, significantly delay fuel ignition and extend the premix period needed to create a homogeneous mixture. In addition, alcohols as oxygenated fuels contribute to a significant increase in the amount of active chemical oxygen in the cylinder of an internal combustion engine, helping to oxidize the fuel and burn off unoxidized exhaust components. As a result, they can contribute to the improvement of the ecological properties of the engine by reducing the emission of hydrocarbons, carbon monoxide, and soot [27]. As part of the research, the exhaust gas composition of a compression ignition engine powered by diesel/n-butanol (DB) and RME biodiesel/n-butanol (BB) mixtures was analyzed. The concentrations of nitrogen oxides (NOx), hydrocarbons (THC), carbon oxides (CO), carbon dioxide (CO2), and soot in the engine exhaust were measured.
3.2.1. NOx Emission
Nitrogen oxides (NOx) and soot are the most important components of a compression-ignition engine exhaust gas, which need to be reduced in order to meet modern, constantly stringent emission standards. NOx and soot are related to each other. Therefore, their simultaneous reduction requires, on the one hand, precise combustion control, and, on the other hand, the use of fuels with appropriate properties [50]. During the combustion of fuel in the cylinder of a piston engine, reactions take place that allow the formation of nitrogen oxide (NO), which, when discharged with the exhaust gases, undergoes additional oxidation to form nitrogen oxides (NOx). The formation of NO occurs mainly according to the Zeldovich thermal mechanism and the Fenimore prompt mechanism and depends on the oxygen concentration (excess air ratio), the temperature in the cylinder, and the residence time of the gas mixture in high temperature zones [31,32,51]. At temperatures above 1300 °C, nitrogen becomes highly reactive, which results in higher NOx emissions [29]. Figure 18a shows the NOx emissions of the engine fueled with mixtures of diesel fuel and n-butanol and mixtures of RME biodiesel and n-butanol related to the emissions of diesel fuel (D100) as the reference fuel (724 ppm). The literature presents a different effect of biodiesel on the emission of nitrogen oxides from a compression ignition engine. Some studies have reported significant reductions in NOx emissions from biodiesel [52], while other studies have shown an increase in NOx emissions with an increase in the percentage of this biofuel [26,53,54]. This inconsistency in the literature may result from different types of tested engines and their operational parameters, different types of biodiesel and different operating conditions [52]. The results of the research in this study showed that biodiesel has a beneficial effect on the emission of nitrogen oxides in a dual-fuel engine. Combustion of biodiesel alone was associated with lower emission by over 200 ppm (28%) compared to the combustion of diesel fuel. Moreover, the co-combustion of biodiesel and n-butanol, compared to the co-combustion of diesel fuel and n-butanol, was characterized by lower NOx emissions. Increasing the share of n-butanol for both diesel fuel/n-butanol (DB) and RME biodiesel/n-butanol (BB) mixtures resulted in an increase in NOx emissions [55]. In the case of co-combustion of diesel fuel and n-butanol, the highest emissions were recorded at DB40 and DB50 and they were higher than the emissions for D100 by over 300 ppm (41%). In the case of co-combustion of biodiesel and n-butanol, the highest concentration of NOx was obtained at BB80. It was over 100 ppm (14%) higher than the concentration obtained for the reference fuel. At DB60 and BB90, the NOx concentration in the exhaust gas decreased. Feeding the engine with biodiesel by increasing the amount of oxygen in the cylinder should improve the conditions for the formation of nitrogen oxides. However, thanks to the high CN, biodiesel did not retard ignition, limited the time to create a homogeneous combustible mixture, and contributed to the reduction of the maximum values of the heat release rate and the increase of maximum temperatures. As a result, the conditions necessary for NOx formation were not present in the cylinder. In Figure 6, it can be seen that the peak HRR values and thus the maximum temperatures (Figure 13) were much lower when combusting biodiesel blends. The increase in NOx emissions accompanying the increase in the share of n-butanol in the DB and BB mixtures was due to two reasons. First, alcohol, with a high latent heat of vaporization, increased the ignition delay, favoring the formation of a homogeneous combustible mixture which, after self-ignition, burned rapidly, mainly in the kinetic phase, with high HRR, ensuring a sufficiently high temperature in the cylinder for formation of nitrogen oxides [51]. Secondly, together with n-butanol, additional active oxygen was supplied to the cylinder, promoting the formation of NOx [32]. According to [50], in the case of high engine loads, the supply of alcoholic fuel, i.e., n-butanol, to the compression-ignition engine causes an increase in NOx emission caused by an increased share of the combustion phase of the pre-mixed charge, which results in a higher temperature in the cylinder. The decrease in nitrogen oxide emissions for the largest shares of n-butanol (DB60 and BB90) resulted from a significant delay in ignition, deterioration of combustion, and the shifting of this process to the expansion stroke, where low temperature prevented the formation of NOx.
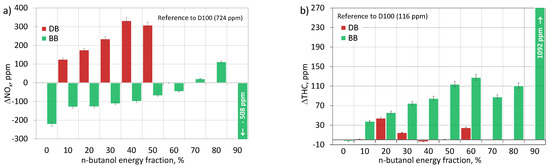
Figure 18.
Emission of NOx (a) and THC (b) for test engine powered by diesel/n-butanol and RME biodiesel/n-butanol blends.
3.2.2. THC Emission
THC is a product of the incomplete combustion of hydrocarbon fuel. There are many reasons for excessive THC emissions from a compression ignition engine. The heterogeneity of the air-fuel mixture, its excessive thickening (or excessive dilution) and low temperature cause an increase in THC emissions [42]. Incomplete fuel evaporation, accumulation of fuel in the crevices of the combustion chamber and deposition of the fuel layer on the walls of the chamber due to excessive and misdirected injection are also mentioned as important factors contributing to the formation of THC [32]. In addition, the fuel properties, such as cetane number, heat of vaporization, viscosity, and fuel composition (C/H2 and C/O2 ratio), also have an impact on the total THC emissions. Fuels with a lower cetane number favor the formation of hydrocarbon pollutants [26]. Under conditions of lower engine load, the emission of THC is higher than at full load because combustion takes place at a lower temperature, which is accompanied by a low temperature of the cylinder walls [29,53]. At high load, the rate of heat release is high and intense. Hence, the temperature in the cylinder is high and the emission of THC is low [51]. Figure 18b shows the THC emissions of the engine powered by mixtures of diesel fuel with n-butanol and mixtures of RME biodiesel with n-butanol related to the emissions of diesel fuel (D100) as the reference fuel (116 ppm). Comparing the emissions for diesel fuel alone and for biodiesel, a similar tendency of both fuels to create unburned hydrocarbons can be seen. Only after adding n-butanol, significant differences in the emission of this compound were recorded. The THC emitted from the combustion of biodiesel blends has a higher concentration in the exhaust gas than the THC from the combustion of diesel blends. In the available literature, one can often find studies indicating a different effect of biodiesel co-combusted with alcohols on the emissions of unburned hydrocarbons [26,32,54]. In the presented research, a slight increase in the proportion of alcohol in a mixture with diesel fuel, up to 20%, resulted in an increase in THC emissions compared to diesel fuel, by 56 ppm (48%). Further increasing the amount of n-butanol resulted in the reduction of hydrocarbon emissions to the values obtained for diesel fuel alone. Another increase in THC emissions was achieved for the highest share of n-butanol (DB60). The increase in the share of alcohol in the mixture with biodiesel, up to 60%, resulted in an increase in THC emissions, compared to diesel fuel, by 128 ppm (110%). Further increasing the amount of n-butanol, up to 70%, resulted in a reduction of hydrocarbon emissions, up to 88 ppm over diesel emissions. For BB80 and BB90, another increase in the concentration of hydrocarbons was observed, up to nine times (up to 1092 ppm in relation to D100). The higher THC emissions obtained for the RME biodiesel/n-butanol mixtures compared to the diesel/n-butanol mixtures were mainly due to the lower temperature in the cylinder during combustion, represented by lower maximum values of the heat release rate (HRRmax) (Figure 6). The initial increase in the share of n-butanol in mixtures of diesel fuel/n-butanol and biodiesel RME/n-butanol, increasing the ignition delay, extended the time of formation of the premixed mixture and increased the temperature in the engine cylinder, which should reduce the THC concentration in the exhaust gas [56]. However, under these conditions, other factors that caused the increase in THC emissions were of greater importance. These were slower evaporation of alcohol-containing mixtures, and thus slower and worse mixing of fuel with air due to the high latent heat of vaporization of n-butanol, as well as increased aerosol penetration of the fuel mixture (due to lower viscosity and lower density of n-butanol) causing an undesirable impact of the fuel against the walls of the chamber (and thus extinguishing the flame) [48,51]. The high heat of vaporization of n-butanol increased the probability of deposition of unburned charge on the walls and inside the crevices of the combustion chamber, contributing to an increase in THC emissions [42]. Although the content of molecular oxygen in mixtures with n-butanol was higher than in diesel fuel or biodiesel, its effect on the reduction of THC impurities was negligible [26]. The decrease in THC emission during the further increase in the share of n-butanol, above 20% for DB and 60% for BB, resulted from the dominant influence of the temperature increase in the cylinder caused by the combustion of a homogeneous mixture in the presence of active chemical oxygen. In addition, the additional oxygen improved combustion by diluting the mixture and eliminating fuel-rich zones in the engine cylinder. The re-increase in THC emissions for DB60 and BB90 was caused by a decrease in temperature and heat release rate in the engine cylinder due to too much delay in ignition and shift of combustion to the expansion stroke. Under these conditions, an intense process of extinguishing the flame in the combustion chamber took place, which led to an increase in HC emissions [26,57]. Moreover, the excess of oxygen in mixtures with such a high alcohol content led to excessive depletion of the combustible mixture and incomplete combustion [53].
3.2.3. CO Emission
Carbon monoxide (CO) is a toxic compound produced as a result of incomplete combustion of hydrocarbon fuel [29,57]. Excessive CO emissions arise for two reasons. Firstly, it can be due to oxygen deficiency in fuel-rich areas in the engine combustion chamber, caused by too much enrichment of the fuel mixture [26]. In the case of too rich mixtures, the fuel cannot mix with enough air to oxidize it. Secondly, it can be due to the excessive depletion of the fuel mixture and the lowering of the combustion temperature in the cylinder, which causes a cooling effect [53]. Under these conditions, the flame cannot effectively propagate in the engine cylinder, the fuel is decomposed and its partial oxidation to CO [32]. Fuel properties, such as viscosity, volatility, or density, are also important factors influencing CO emissions [29]. Figure 19a shows the CO emissions of the engine fueled with mixtures of diesel fuel and n-butanol as well as mixtures of RME biodiesel and n-butanol related to the emissions of diesel fuel (D100) as the reference fuel (0.53%). It can be seen that the combustion of biodiesel alone, compared to the combustion of diesel fuel alone, resulted in an increased CO emission of 0.12% (23%). Adding n-butanol to diesel and biodiesel further made the difference in CO emissions in favor of DB blends. Analyzing the effect of increasing the share of butyl alcohol co-combusted with diesel fuel, it can be seen that the addition of alcohol had a positive effect on the concentration of carbon monoxide in the engine exhaust [48]. The lowest emissions were recorded for DB50 and DB60 and were approximately 0.4% (75%) lower than the emissions for D100. When analyzing the impact of increasing the share of butyl alcohol co-combusted with biodiesel, it can be seen that a small addition of alcohol increased CO emissions to 20%. Only after exceeding the 20% share of n-butanol was there a decrease in the concentration of carbon monoxide by approximately 0.35% (66%) for BB80, compared to the value obtained for diesel fuel. For BB90, another increase in CO concentration in the exhaust gas was observed. The higher CO emissions achieved by the co-combustion of RME biodiesel/n-butanol blends can be explained by the lower combustion temperature (lower HRRmax) and higher fuel mass injected into the cylinder due to the lower calorific value of biodiesel compared to diesel [26]. Under these conditions, the combustion temperature in the cylinder was of greater importance than the additional oxygen supplied in the biodiesel. The decrease in CO emissions along with the increase in the share of n-butanol in the DB and BB mixture was influenced by: the increasing combustion temperature in the cylinder and the availability of chemically active oxygen supplied in the alcohol fuel, which promoted the oxidation of CO to CO2 and eliminated fuel-rich zones in the combustion chamber of the engine [31,57]. The increased ignition retardation also increased the time to homogenize the combustible mixture in which oxygen had sufficient time to reach each fuel particle. Not without significance is also the fact that, along with the increasing share of n-butanol, there was a decrease in the carbon content in the mixture, reflected in the decreasing C/H2 ratio (Figure 20a). For higher shares of n-butanol, there was a reduction in carbon monoxide concentration caused by a positive and dominant influence of a large amount of oxygen and a decrease in C/O2 and C/H2 (Figure 20) [29]. For BB90, there was a rapid increase in CO emissions, caused by a decrease in temperature, and heat release rate in the engine cylinder, caused by too much delay in ignition and a shift in combustion on the expansion stroke [54].
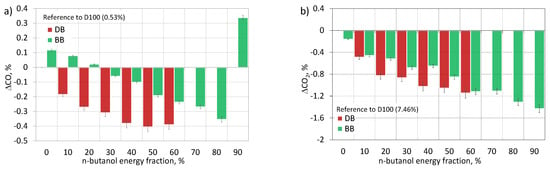
Figure 19.
Emission of CO (a) and CO2 (b) for test engine powered by diesel/n-butanol and RME biodiesel/n-butanol blends.
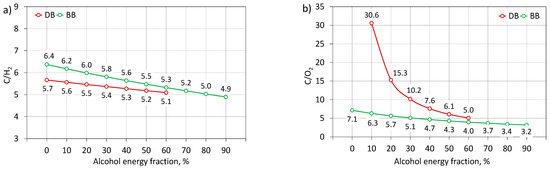
Figure 20.
C/H2 (a) and C/O2 (b) ratio for test engine powered by diesel/n-butanol and RME biodiesel/n-butanol blends.
3.2.4. CO2 Emission
Carbon dioxide (CO2) is the end product of combustion of any fuel that contains a carbon molecule in its atomic structure. In a compression-ignition engine, carbon dioxide is formed as a result of the complete combustion of fuel under high temperature and excess oxygen conditions. The availability of sufficient oxygen for complete combustion increases carbon dioxide emissions. With adequate oxygen availability, the hydroxyl radical OH (one of the main oxidants) converts CO into CO2 [53]. Figure 19b shows the CO2 emissions of the engine fueled with mixtures of diesel fuel and n-butanol as well as mixtures of RME biodiesel and n-butanol related to the emissions of diesel fuel alone (D100) as the reference fuel (7.46%). It can be seen that the addition and increase of n-butanol in the mixture of DB and BB reduced the concentration of carbon dioxide in the exhaust gas of a dual-fuel engine. The engine powered by the diesel/n-butanol mixture was characterized by a greater reduction in emissions. The lowest concentrations were recorded for DB60 and BB90 and they were respectively 1.14% (15%) and 1.43% (19%) below the concentration value obtained for the reference fuel. The decrease in CO2 emissions resulted mainly from the decrease in the amount of carbon supplied to the cylinder in mixtures with alcohol fuel, mapped by a decrease in the C/H2 ratio (Figure 20a). This factor turned out to have a greater effect on reducing the concentration of carbon dioxide in the exhaust gas than the additional amount of oxygen supplied in n-butanol (C/O2 in Figure 20b).
3.2.5. Soot Emission
Soot is one of the main components of particulate matter and indicates the propensity of the engine to form and emit them [32]. In compression-ignition engines, soot is formed due to excess fuel that creates fuel-rich zones in the combustion chamber that are not completely combusted in the engine cylinder. Soot particles are mainly formed in the initial stage of diffusion combustion as a result of fuel dissociation under high temperature and excess fuel conditions (low air excess ratio). Under the operating conditions of a compression ignition engine, part of the soot formed in the first stage of the combustion process is eliminated in subsequent stages of the cycle due to oxidation in the oxygen-rich areas of the combustion chamber [42]. In addition to the excess air ratio, the soot emissions are also influenced by: temperature and pressure in the engine cylinder, the degree of fuel-air mixing, fuel injection time and its orientation, and the composition of the fuel itself (C/H2 and C/O2 ratio) [53]. Figure 21 shows the soot emissions of the engine fueled with mixtures of diesel fuel and n-butanol and mixtures of RME biodiesel and n-butanol related to the emissions of diesel fuel alone (D100) as the reference fuel (519 mg/m3). It can be seen that the combustion of biodiesel alone, compared to the combustion of diesel fuel, was associated with an increased emission of soot, by approximately 73 mg/m3 (14%). Similarly, the combustion of BB mixtures, compared to the combustion of DB mixtures, was characterized by a higher concentration of soot in the exhaust gas. Analyzing the effect of increasing the share of butyl alcohol co-combusted with diesel fuel, it can be seen that the addition of alcohol had a positive effect on soot emissions. The lowest emissions were recorded for DB50 and DB60 and they were approximately 500 mg/m3 (96%) lower than the reference fuel emissions. When analyzing the impact of increasing the share of butyl alcohol co-combusted with biodiesel, it can be seen that a small addition of alcohol up to 10% increased soot emissions. Only after exceeding the 10% share of n-butanol was there a decrease in the soot concentration by approximately 490 mg/m3 (94%) for BB80, compared to the value obtained for diesel fuel. For BB90, soot emissions increased again, but still lower, by approximately 390 mg/m3 (75%), compared to D100. The higher soot emissions associated with the combustion of biodiesel and BB blends alone compared to diesel fuel and DB blends resulted from lower combustion temperatures (lower HRRmax in Figure 6). The reduction of soot emissions as a result of adding alcoholic fuel to diesel and biodiesel was due to the limitation of the possibility of the formation of fuel-rich regions in the engine cylinder due to the delayed ignition and increased time for the formation of a homogeneous combustible mixture [51]. Additionally, the pre-prepared mixture burned at a higher temperature, contributing to complete combustion and soot burning [32]. Other significant factors influencing the reduction of soot emissions were the increased amount of chemically active oxygen supplied in n-butanol, which favored the burning of the soot (decrease in the C/O2 ratio in Figure 20b) and the decreased share of carbon in the DB and BB mixtures (decrease in the C/H2 ratio) in Figure 20a) [48,52].
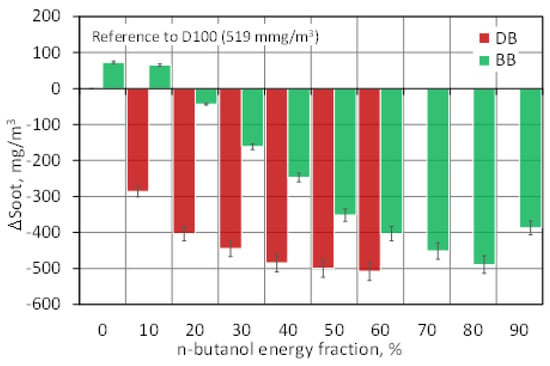
Figure 21.
Soot emission for test engine powered by diesel/n-butanol and RME biodiesel/n-butanol blends.
The above analyses show that increasing the share of n-butanol for biodiesel/n-butanol (BB) mixtures increased NOx emissions. A different effect was observed in the case of soot emissions, where the butanol concentration in BB significantly decreased with the increase in the share of butanol in the exhaust gas. Thus, a decrease in soot emissions is accompanied by an increase in NOx emissions, which confirms the trade-off between soot and NOx. Only for BB90, there is a decrease in the NOx concentration and a slight increase in the soot concentration in the exhaust gas, which resulted from a significant ignition delay, deterioration of combustion, and shifting of this process to the expansion stroke, where the low temperature prevented the formation of NOx.
4. Conclusions
The paper presents the results of a comparative analysis of diesel engine fueling with a mixture of diesel fuel and n-butanol as well as RME and n-butanol. The influence of the type of fuel on the engine performance, stability of the combustion process, and the emission of toxic exhaust components was assessed. The tests were carried out for the constant engine load, rotational speed, and the injection timing. Based on the obtained results, the following conclusions were formulated:
- −
- The maximum energy share of n-butanol in a mixture with diesel fuel, allowing for stable operation, is 50%, and with RME 80%.
- −
- For both analyzed cases, with up to 40% of the energy content of n-butanol, the COVIMEP index was at the same level of approximately 2%. In the engine powered by a mixture with RME, with up to 80% of the n-butanol content, the COVIMEP index was constant below 2%.
- −
- In the engine fueled with the BB mixture, the ignition delay time (ID) for the BB80 increased by 5 deg of CA in relation to RME. For the DB supply, for DB60, the ID increment was 7 deg of CA.
- −
- For DB60, the combustion time was shortened from 51 to 22 deg of CA, whereas for BB60 from 51 to 45 deg of CA, respectively.
- −
- For the engine fueled with DB mixture up to 50% of n-butanol share, there was a high repeatability of the CA10 combustion start and CA50 angle, whereas in the BB-powered engine up to 70% of n-butanol was achieved.
- −
- The spread of the conventional CA90 combustion end in an engine powered by DB increased with an increase in the share of n-butanol to 50%. In an engine powered by BB with up to 80% of the share of n-butanol, the repeatability of CA90 was higher than for the reference fuel.
- −
- The analysis of the uniqueness of pmax, the angle pmax, and CA50 showed that the engine powered by the BB mixture is characterized by greater combustion stability for the range of up to 80% of the energy share of n-butanol.
- −
- The share of n-butanol in the combusted mixture contributed to the increase in engine efficiency. A greater increase in efficiency was obtained for the engine powered by DB40 mixture and amounted to 5% in relation to the reference fuel supply.
- −
- Relating the NOx emission to the values obtained for the engine powered by diesel fuel, the increase in NOx emission was obtained for DB mixtures. The highest increase was for DB40, by over 300 ppm. For feeding BB, with up to 60% share of n-butanol, the achieved NOx emission was lower than for the reference fuel.
- −
- THC emission for both DB and BB was higher than for the reference fuel. The increase was small for DB supply, and for BB supply. The highest increase was for BB60 and amounted to 120 ppm.
- −
- Summing up the CO emission, it can be said that the share of n-butanol has a positive effect on the emission of CO, and the CO2 emission for the entire range of n-butanol share was lower than for the reference fuel.
- −
- The share of n-butanol has a positive effect on the reduction of soot emissions. Only for B100 and BB10 was the emission of soot higher by approximately 70 mg/m3 compared to the diesel fuel supply. The greatest soot reduction was recorded for the DB60 fuel by over 500 mg/m3.
The supply of a compression-ignition piston engine with a mixture of the basic fuel, i.e., diesel fuel or biodiesel with the participation of n-butanol, gives satisfactory results in terms of both the improvement of engine operating parameters, combustion stability, as well as in terms of exhaust emissions.
Author Contributions
Conceptualization, W.T., A.J., and K.G.-R.; data curation, W.T. and A.J.; formal analysis, W.T.; investigation, W.T., A.J., and K.G.-R.; methodology, W.T. and A.J.; writing—original draft, W.T.; writing—review & editing, W.T. and K.G.-R. All authors have read and agreed to the published version of the manuscript.
Funding
Research was financed by the Ministry of Science and Higher Education of Poland from the funds dedicated to scientific research No. BS/PB 1-100-316/2023/P.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Imtenan, S.; Varman, M.; Masjuki, H.H.; Kalam, M.A.; Sajjad, N.; Arbab, M.I.; Fattah, I.M.R. Impact of low temperature combustion attaining strategies on diesel engine emissions for diesel and biodiesels: A review. Energy Convers. Manag. 2014, 80, 329–356. [Google Scholar] [CrossRef]
- Morawski, A.P.; Rodrigues de Araújo, L.; Schiaffino, M.S.; Cristofori de Oliveira, R.; Chun, A.; Ribeiro, L.C.; Santos, J.J.C.S.; Donatelli, J.L.M.; Cunh, C.C.M. On the suitable superstructure thermoeconomic optimization of a waste heat recovery system for a Brazilian diesel engine power plant. Energy Convers. Manag. 2012, 34, 113947. [Google Scholar] [CrossRef]
- Ghadikolaei, M.A.; Wong, P.K.; Cheung, C.S.; Ning, Z.; Yung, K.F.; Zhao, J.; Gali, N.K.; Berenjestanaki, A.V. Impact of lower and higher alcohols on the physicochemical properties of particulate matter from diesel engines: A review. Renew. Sustain. Energy Rev. 2021, 143, 110970. [Google Scholar] [CrossRef]
- Tutak, W. Bioethanol E85 as a fuel for dual fuel diesel engine. Energy Convers. Manag. 2014, 86, 39–48. [Google Scholar] [CrossRef]
- Tutak, W.; Jamrozik, A.; Grab-Rogaliński, K. Effect of natural gas enrichment with hydrogen on combustion process and emission characteristic of a dual fuel diesel engine. Int. J. Hydrogen Energy 2020, 45, 9088–9097. [Google Scholar] [CrossRef]
- Huang, H.; Zhu, Z.; Chen, Y.; Chen, Y.; Lv, D.; Zhu, J.; Ouyang, T. Experimental and numerical study of multiple injection effects on combustion and emission characteristics of natural gas–diesel dual-fuel engine. Energy Convers. Manag. 2019, 183, 84–96. [Google Scholar] [CrossRef]
- Nour, M.; Attia, A.M.A.; Nada, S.A. Combustion, performance and emission analysis of diesel engine fuelled by higher alcohols (butanol, octanol and heptanol)/diesel blends. Energy Convers. Manag. 2019, 185, 313–329. [Google Scholar] [CrossRef]
- Wei, J.; He, C.; Fan, C.; Pan, S.; Wei, M.; Wang, C. Comparison in the effects of alumina, ceria and silica nanoparticle additives on the combustion and emission characteristics of a modern methanol-diesel dual-fuel CI engine. Energy Convers. Manag. 2021, 238, 114121. [Google Scholar] [CrossRef]
- Mofijur, M.; Rasul, M.G.; Hyde, J.; Bhuyia, M.M.K. Role of biofuels on IC engines emission reduction. Energy Procedia 2015, 75, 886–892. [Google Scholar] [CrossRef]
- EL-Seesy, A.I.; Kayatas, Z.; Takayama, R.; He, Z.; Kandasamy, S.; Kosaka, H. Combustion and emission characteristics of RCEM and common rail diesel engine working with diesel fuel and ethanol/hydrous ethanol injected in the intake and exhaust port: Assessment and comparison. Energy Convers. Manag. 2020, 205, 112453. [Google Scholar] [CrossRef]
- Jacob, A.; Ashok, B.; Alagumalai, A.; Chyuan, O.H.; Le, P.T.K. Critical review on third generation micro algae biodiesel production and its feasibility as future bioenergy for IC engine applications. Energy Convers. Manag. 2021, 228, 113655. [Google Scholar] [CrossRef]
- Frigo, S.; Pasini, G.; Caposciutti, G.; Antonelli, M.; Galletti, A.M.R.; Gori, S.; Costi, R.; Arnone, L. Utilisation of advanced biofuel in CI internal combustion engine. Fuel 2021, 297, 120742. [Google Scholar] [CrossRef]
- Duraisamy, G.; Rangasamy, M.; Govindan, N.A. Comparative study on methanol/diesel and methanol/PODE dual fuel RCCI combustion in an automotive diesel engine. Renew. Energy 2020, 145, 542–556. [Google Scholar] [CrossRef]
- Zapata-Mina, J.; Restrepo, A.; Romero, C.; Quintero, H. Exergy analysis of a diesel engine converted to spark ignition operating with diesel, ethanol, and gasoline/ethanol blends. Sustain. Energy Technol. Assess. 2020, 42, 100803. [Google Scholar] [CrossRef]
- Işık, M.Z.; Aydın, H. Analysis of ethanol RCCI application with safflower biodiesel blends in a high load diesel power generator. Fuel 2016, 184, 248–260. [Google Scholar] [CrossRef]
- Jamrozik, A. The effect of the alcohol content in the fuel mixture on the performance and emissions of a direct injection diesel engine fueled with diesel-methanol and diesel-ethanol blends. Energy Convers. Manag. 2017, 148, 461–476. [Google Scholar] [CrossRef]
- Kuszewski, H.; Jakubowski, M.; Jaworski, A.; Lubas, J.; Balawender, K. Effect of temperature on tribological properties of 1-butanol–diesel fuel blends–Preliminary experimental study using the HFRR method. Fuel 2021, 296, 120700. [Google Scholar] [CrossRef]
- Çelebi, Y.; Aydın, H. An overview on the light alcohol fuels in diesel engines. Fuel 2019, 236, 890–911. [Google Scholar] [CrossRef]
- Kuszewski, H. Effect of adding 2-ethylhexyl nitrate cetane improver on the autoignition properties of ethanol–diesel fuel blend–Investigation at various ambient gas temperatures. Fuel 2018, 224, 57–67. [Google Scholar] [CrossRef]
- Kumar, N.; Pali, H.S.; Sonthalia, A.; Sidharth. Higher Alcohols as Diesel Engine Fuel. In Advances in Energy and Combustion; Green Energy and Technology; Gupta, A.K., De, A., Aggarwal, S.K., Kushari, A., Runchal, A.K., Eds.; Springer: Singapore, 2022. [Google Scholar]
- Thomas, K.G.; Nath, B.; Jackson, R.J.; Sharon, H. Combustion characteristics assessment of diesel engine fueled by diesel-butanol-used cooking oil biodiesel blends. AIP Conf. Proc. 2020, 2225, 030001. [Google Scholar]
- Kuszewski, H. Experimental study of the autoignition properties of n-butanol–diesel fuel blends at various ambient gas temperatures. Fuel 2019, 235, 1316–1326. [Google Scholar] [CrossRef]
- Sidhu, M.S.; Roy, M.M.; Wang, W. Glycerine emulsions of diesel-biodiesel blends and their performance and emissions in a diesel engine. Appl. Energy 2018, 230, 148–159. [Google Scholar] [CrossRef]
- He, B.-Q.; Liu, M.-B.; Zhao, H. Comparison of combustion characteristics of n-butanol/ethanol–gasoline blends in a HCCI engine. Energy Convers. Manag. 2015, 95, 101–109. [Google Scholar] [CrossRef]
- Nagarajan, J.; Narayanasamy, B. Effect of pentanol-biodiesel blends on performance and emission characteristics of the diesel engine. Int. J. Ambient Energy 2019, 42, 900–906. [Google Scholar]
- Xiao, H.; Guo, F.; Wang, R.; Yang, X.; Li, S.; Ruan, J. Combustion performance and emission characteristics of diesel engine fueled with iso-butanol/biodiesel blends. Fuel 2020, 268, 117387. [Google Scholar] [CrossRef]
- Sahin, Z.; Durgun, O.; Aksu, O.N. Experimental investigation of n-butanol/diesel fuel blends and n-butanol fumigation–Evaluation of engine performance, exhaust emissions, heat release and flammability analysis. Energy Convers. Manag. 2015, 103, 778–789. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Y.; Luo, K. Biodiesel/butanol blends as a pure biofuel excluding fossil fuels: Effects on diesel engine combustion, performance, and emission characteristics. Proc. Inst. Mech. Eng. Part D Int. J. Automot. Eng. 2020, 234, 2988–3000. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Kumar, M. Impact of n-butanol as an additive with eucalyptus biodiesel-diesel blends on the performance and emission parameters of the diesel engine. Fuel 2020, 277, 118178. [Google Scholar] [CrossRef]
- Divakar Shetty, A.S.; Dineshkumar, L.; Koundinya, S.; Mane, S.K. Experimental investigation on CRDI engine using butanol-biodiesel-diesel blends as fuel. AIP Conf. Proc. 2017, 1859, 020028. [Google Scholar]
- Xiao, H.; Guo, F.; Li, S.; Wang, R.; Yang, X. Combustion performance and emission characteristics of a diesel engine burning biodiesel blended with n-butanol. Fuel 2019, 258, 115887. [Google Scholar] [CrossRef]
- Imtenan, S.; Masjuki, H.H.; Varman, M.; Fattah, I.M.R.; Sajjad, H.; Arbab, M.I. Effect of n-butanol and diethyl ether as oxygenated additives on combustion–emission-performance characteristics of a multiple cylinder diesel engine fuelled with diesel–jatropha biodiesel blend. Energy Convers. Manag. 2015, 94, 84–94. [Google Scholar] [CrossRef]
- Siwale, L.; Lukács, K.; Torok, A.; Bereczky, A.; Mbarawa, M.; Penninger, A.; Kolesnikov, A. Combustion and emission characteristics of n-butanol/diesel fuel blend in a turbo-charged compression ignition engine. Fuel 2013, 107, 409–418. [Google Scholar] [CrossRef]
- Mickevicius, T.; Slavinskas, S.; Labeckas, G. Performance and emissions of diesel engine operating on biodiesel and butanol blends. Eng. Rural Dev. 2020, 19, 1381–1386. [Google Scholar]
- Makarevičienė, V.; Kazancev, K.; Kazanceva, I. Possibilities for improving the cold flow properties of biodiesel fuel by blending with butanol. Renew. Energy 2015, 75, 805–807. [Google Scholar] [CrossRef]
- Tutak, W.; Jamrozik, A. Characteristics of the flow field in the combustion chamber of the internal combustion test engine. Chem. Process Eng. 2011, 2, 203–214. [Google Scholar] [CrossRef]
- Tsolakis, A.; Megaritis, A.; Wyszynski, M.L.; Theinnoi, K. Engine performance and emissions of a diesel engine operating on diesel-RME (rapeseed methyl ester) blends with EGR (exhaust gas recirculation). Energy 2007, 32, 2072–2080. [Google Scholar] [CrossRef]
- Jamrozik, A.; Tutak, W.; Pyrc, M.; Gruca, M.; Kočiško, M. Study on co-combustion of diesel fuel with oxygenated alcohols in a compression ignition dual-fuel engine. Fuel 2018, 221, 329–345. [Google Scholar] [CrossRef]
- Kiełczynski, P.; Ptasznik, S.; Szalewski, M.; Balcerzak, A.; Wieja, K.; Rostocki, A.J. Thermophysical properties of rapeseed oil methyl esters (RME) at high pressures and various temperatures evaluated by ultrasonic methods. Biomass Bioenergy 2017, 107, 113–121. [Google Scholar] [CrossRef]
- Liang, M.S. Assessing emission and power tradeoffs of biodiesel and n-Butanol in diesel blends for fuel sustainability. Fuel 2021, 283, 118861. [Google Scholar] [CrossRef]
- Han, J.; He, W.; Somers, L.M.T. Experimental investigation of performance and emissions of ethanol and n-butanol fuel blends in a heavy-duty diesel engine. Front. Mech. Eng. 2020, 6, 26. [Google Scholar] [CrossRef]
- Zhou, X.; Qian, W.; Pan, M.; Huang, R.; Xu, L.; Yin, Y. Potential of n-butanol/diesel blends for CI engines under post injection strategy and different EGR rates conditions. Energy Convers. Manag. 2020, 204, 112329. [Google Scholar] [CrossRef]
- Jamrozik, A.; Grab-Rogaliński, K.; Tutak, W. Hydrogen effects on combustion stability, performance and emission of diesel engine. Int. J. Hydrogen Energy 2020, 45, 19936–19947. [Google Scholar] [CrossRef]
- Grab-Rogaliński, K.; Tutak, W.; Jamrozik, A. Comparative analysis of combustion process of dual fuel diesel engine fueled by diesel/hydrogen and biodiesel/hydrogen. SAE Tech. Pap. 2020, 1, 2074. [Google Scholar]
- Heywood, J.B. Internal Combustion Engine Fundamentals, 2nd ed.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Halbe, M.H.; Fain, D.J.; Shaver, G.M.; Kocher, L.; Koeberlein, D. Control-oriented premixed charge compression ignition CA50 model for a diesel engine utilizing variable valve actuation. Int. J. Engine Res. 2017, 18, 847–857. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, L.; Wang, Z.; Hua, L.; Zhang, Q. Investigating the combustion stability of shale gas engines under HHO. Fuel 2021, 291, 120098. [Google Scholar] [CrossRef]
- Rakopoulos, D.C.; Rakopoulos, D.C.; Giakoumis, E.G.; Dimaratos, A.M.; Kyritsis, D.C. Effects of butanol–diesel fuel blends on the performance and emissions of a high-speed DI diesel engine. Energy Convers. Manag. 2010, 51, 1989–1997. [Google Scholar] [CrossRef]
- Trost, D.; Polcar, A.; Boldor, D.; Kumbar, V. Pour point and predictive models for the viscosity-temperature non-linear behaviour of ternary fuel blends for a compression ignition engine. BioResources 2023, 18, 653–677. [Google Scholar] [CrossRef]
- Nour, M.; Elseesy, A.I.; Attia, A.; Li, X.; Nada, S. Adding n-butanol, n-heptanol, and n-octanol to improve vaporization, combustion, and emission characteristics of diesel/used frying oil biodiesel blends in DICI engine. Environ. Prog. Sustain. Energy 2020, 40, e13549. [Google Scholar] [CrossRef]
- Zheng, Z.; Xia, M.; Liu, H.; Shang, R.; Ma, G.; Yao, M. Experimental study on combustion and emissions of n-butanol/biodiesel under both blended fuel mode and dual fuel RCCI mode. Fuel 2018, 226, 240–251. [Google Scholar] [CrossRef]
- Gillani, S.E.; Ikhlaq, M.; Khan, M.U.; Awan, D.; Hamza, A. Effects of butanol blending and fumigation with Jatropha biodiesel on combustion, performance, and emissions of diesel engine. Int. J. Environ. Sci. Technol. 2021, 18, 819–834. [Google Scholar] [CrossRef]
- Nanthagopal, K.; Ashok, B.; Saravanan, B.; Patel, D.; Sudarshan, B.; Ramasamy, R.A. An assessment on the effects of 1-pentanol and 1-butanol as additives with Calophyllum Inophyllum biodiesel. Energy Convers. Manag. 2018, 158, 70–80. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Abdullah, N.R.; Zawawi, Z.Z.; Rahim, A.A.; Kadir, H.A. Effect of n-butanol blends on engine performance and exhaust emission of compression ignition engine fuelled with diesel-palm oil methyl ester (B20). In Proceedings of the International Colloquium on Computational & Experimental Mechanics (ICCEM 2020), Selangor, Malaysia, 25–26 June 2020; IOP Conference Series: Materials Science and Engineering 2021. Volume 1062. [Google Scholar]
- Ibrahim, A. Performance and combustion characteristics of a diesel engine fuelled by butanol–biodiesel–diesel blends. Appl. Eng. 2016, 103, 651–659. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Huang, G.; He, Z.; Qian, Y.; Lu, X. Experimental study of butanol/biodiesel dual-fuel combustion in intelligent charge compression ignition (ICCI) mode: A systematic analysis at low load. Fuel 2021, 287, 119523. [Google Scholar] [CrossRef]
- Çelebi, Y.; Aydın, H. Investigation of the effects of butanol addition on safflower biodiesel usage as fuel in a generator diesel engine. Fuel 2018, 222, 385–393. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).