1. Introduction
The emergence of the SARS-CoV-2 virus has affected people’s lives worldwide in recent years. In addition, many people live or work in closed spaces. Therefore, the ventilation system is very important, and designing the right location for the inlet and exit of the ventilation airflow is critical because it can increase or prevent the spread of virus- carrying droplets in indoor spaces. It is also necessary to check the infection risk of people in indoor areas and, if possible, place people in locations to lower their chances of contracting this virus.
Many studies have been made on the virus structure and numerical prediction of virus transmission (particles created by coughing or sneezing). Bar et al. [
1] investigated the physical conditions of the virus and the size, volume, and mass of droplets containing the virus. Accordingly, the saliva droplets are mostly water with a density close to water. Mucus droplets have a lot of non-volatile compounds with a higher viscosity. Li et al. [
2] performed multiple simulations and examined the biological conditions of the virus. They provided a deep insight into the spreading and transmission of the COVID-19 virus. The results show that respiratory infection is the main route of virus transmission and the urban environment has a significant effect on the infection risk.
It is essential to use an appropriate turbulence model in calculations. Recently, Li et al. [
3] showed that the performance of supply/exhaust openings had a substantial impact on the distribution of pollutant particles in clean rooms. They used the RNG k-ε turbulence model and the Eulerian–Lagrangian approach for particle tracking. Redrow et al. [
4] examined the dispersion of droplets emitted from patients’ mouths using the k-ε RNG turbulence model. Their simulation included the evaporation and diffusion of sputum particles expelled by humans coughing or sneezing.
The results of the research on the influenza virus indicate that the spread of respiratory viruses in indoor environments is mainly caused by air circulation. Liu et al. [
5] performed a numerical analysis and investigated particle dispersion in laboratory conditions. They used the standard k-ε turbulence model and the Eulerian–Lagrangian approach for particle tracking. Miranda et al. [
6] studied the ventilation conditions in school classrooms. Analysis of different variables and their effect on particle emission was evaluated. Mirzaie et al. [
7] examined the distribution of droplets containing the SARS-CoV-2 virus in a classroom with and without seat partitions. They used the Lagrangian method to track droplets. Their results showed that using partitions could help reduce infections. In the absence of seat partitions, the concentration of respiratory droplets around the student seats close to the infected speaker was higher. Ahmadzadeh et al. [
8] studied particle dispersion in a classroom. They examined the conditions of the spread of Covid-19 in an indoor environment. They concluded that opening the classroom window had a significant effect on reducing virus-carrying particles. Asif et al. [
9] studied preventive measures and emerging technologies in detecting and preventing the spread of COVID-19 virus-carrying droplets. They showed that wearing a mask effectively prevented the spread of the disease. Kim et al. [
10] studied the effect of using a face mask and if it provides the necessary immunity against the virus. Additionally, making masks of recycled materials and modifying them chemically has a significant impact on reducing environmental problems. It also turns them into materials with a high added value. Motamedi et al. [
11] proposed a framework for assessing the infection risk in relation to both spatial and temporal characteristics. First, a verified CFD model of exhaled droplets is created, and then its performance is assessed using an office case study affected by various ventilation techniques, including cross ventilation, single ventilation, mechanical ventilation, and no ventilation cases. To assess the infection probability of the ventilation cases, two indicators of local and general infection risks were utilized. Their results show that the single ventilation case has the highest infection probability.
Nowadays, more research in the field of COVID-19 virus in order to finding a way to prevent further spread of this virus is very important. One of the issues that can affect the further spread and transmission of this virus is the discussion of how to design air ventilation systems in a specific space. Most modern buildings use mechanical ventilation systems. Mechanical ventilation systems use fans and ducts to bring the fresh air inside the target space. The main aim of this paper is to design a proper placement of inlet and exhaust ducts of a ventilation system in order to create a safe area in a space, so that the infection risk at this point reaches its lowest level. Various research and methods have been suggested to reduce the spread of virus particles in the spaces, such as leaving the doors and windows open, partitioning the area, and using an additional fan to remove the particles. These methods are not reasonable, as they waste more money and energy. In addition, there is a need for an independent ventilation system and a costly redesign of the interior of the room. This paper, however, presents a new method to design the appropriate location of the air ventilation ducts, so that it is a practical method and leads to logical results in order to reduce the spread of the virus, and it does not cause significant costs and energy waste like other methods. In other words, the new approach suggests suitable locations for the ventilation supply and exhaust registers that ensure proper space ventilation, reduce the spread of droplets and particles in the area, and decrease the possibility of occupants being infected with the virus. The simulation is conducted using an Eulerian–Lagrangian scheme, the infection risk is evaluated using the Wells–Riley equation, and, finally, a safe area is defined by changing the position of the ventilation supply and exhaust registers.
3. Results
This article examines the transport and distribution of respiratory droplets in buildings and indoor spaces (with a ventilation system). In addition, appropriate locations of the ventilation supply and exhaust registers are discussed, and the risks of infection for different cases are assessed. The cases considered are:
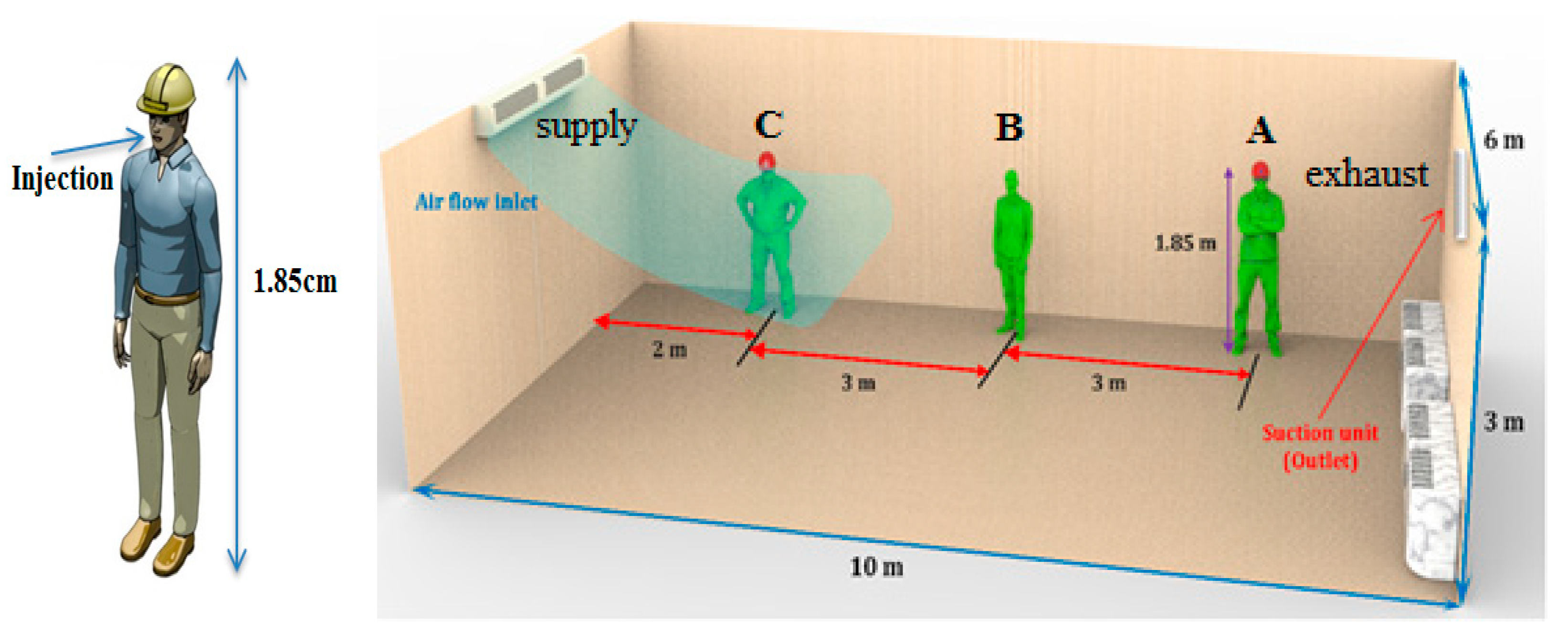
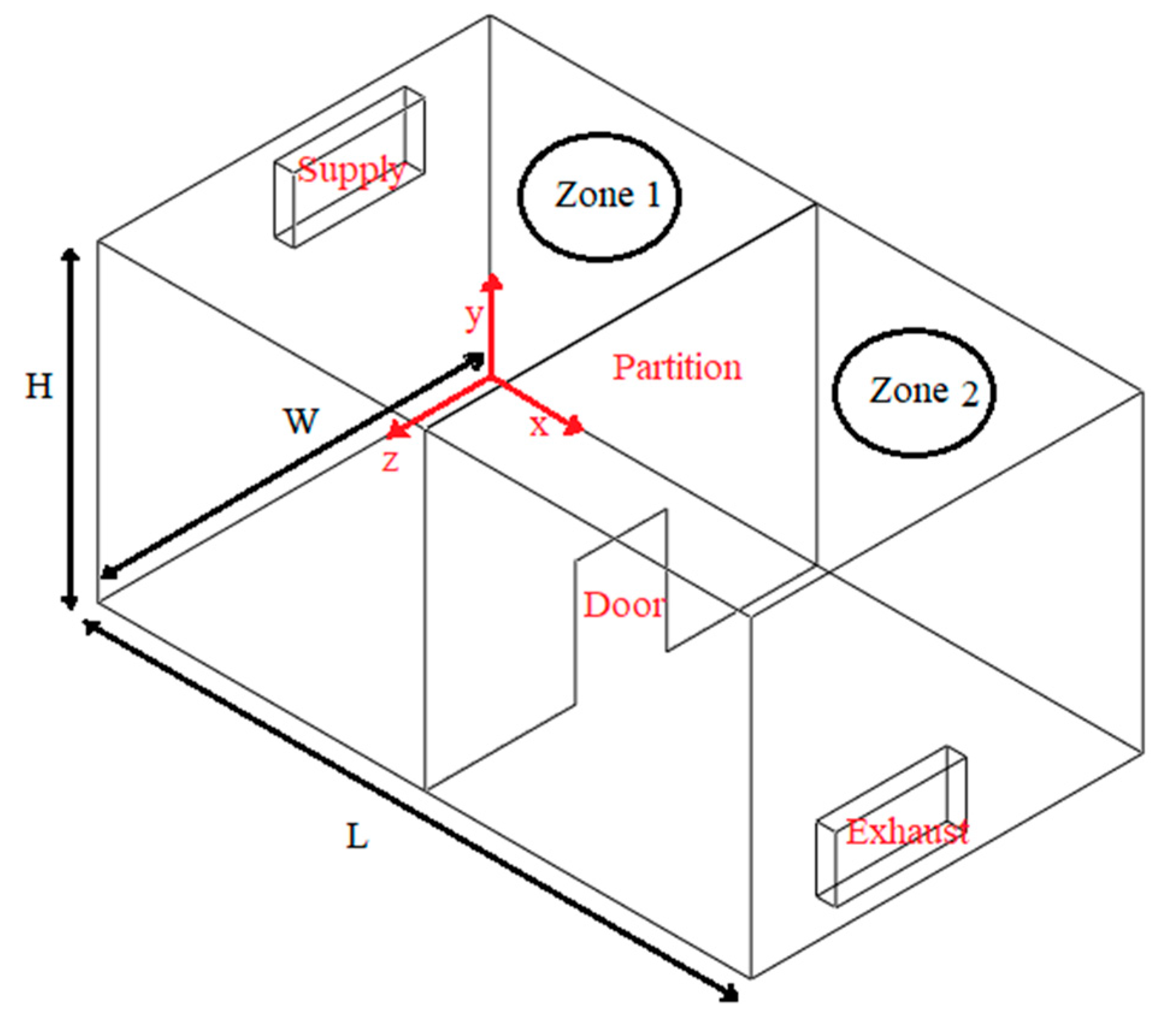
Case 1: Ventilation air flow along the length of the indoor space (
Figure 1).
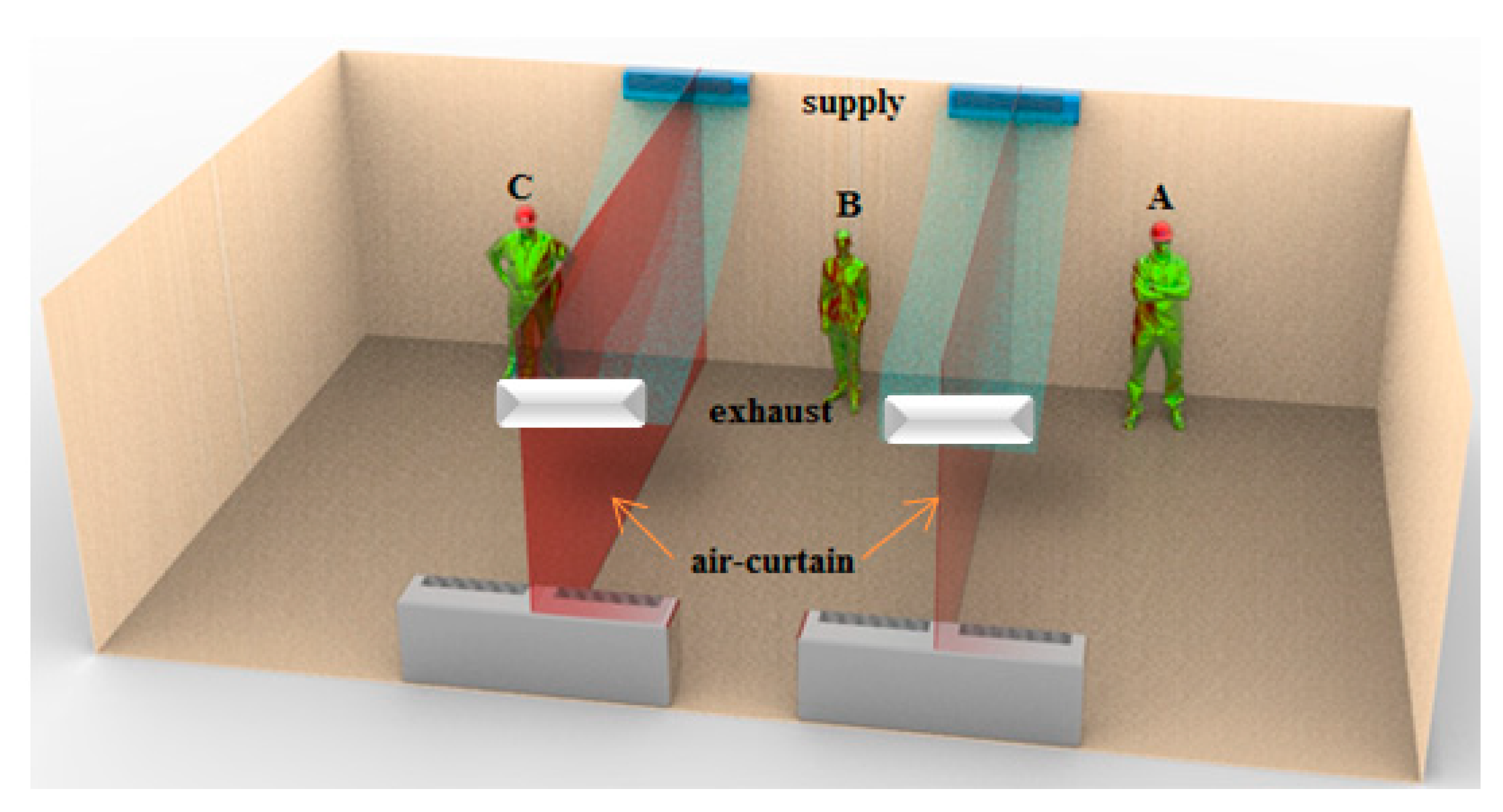
Case 2: Ventilation air flow across the width of the indoor space and creation of an air curtain-type flow pattern (
Figure 2).
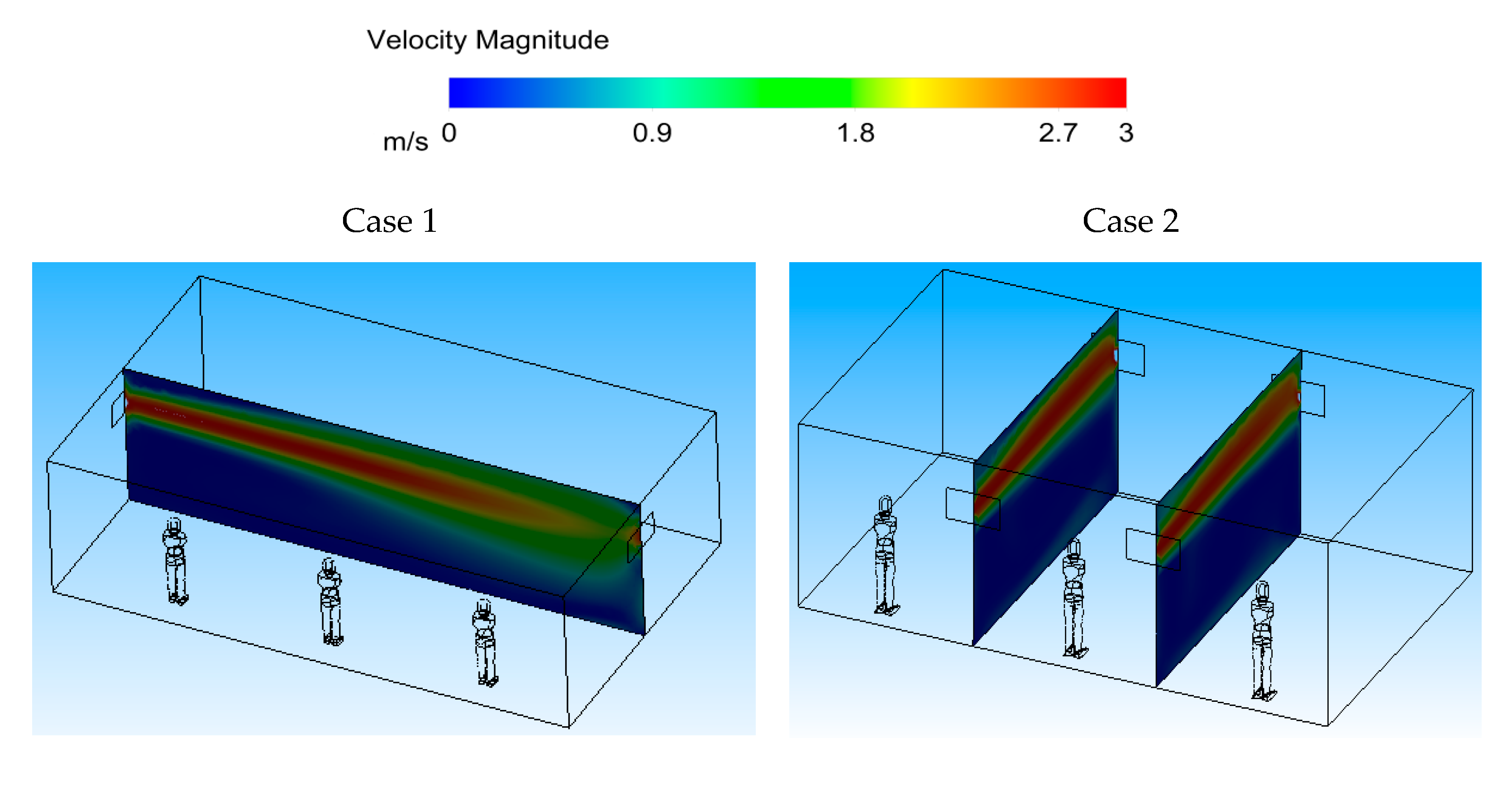
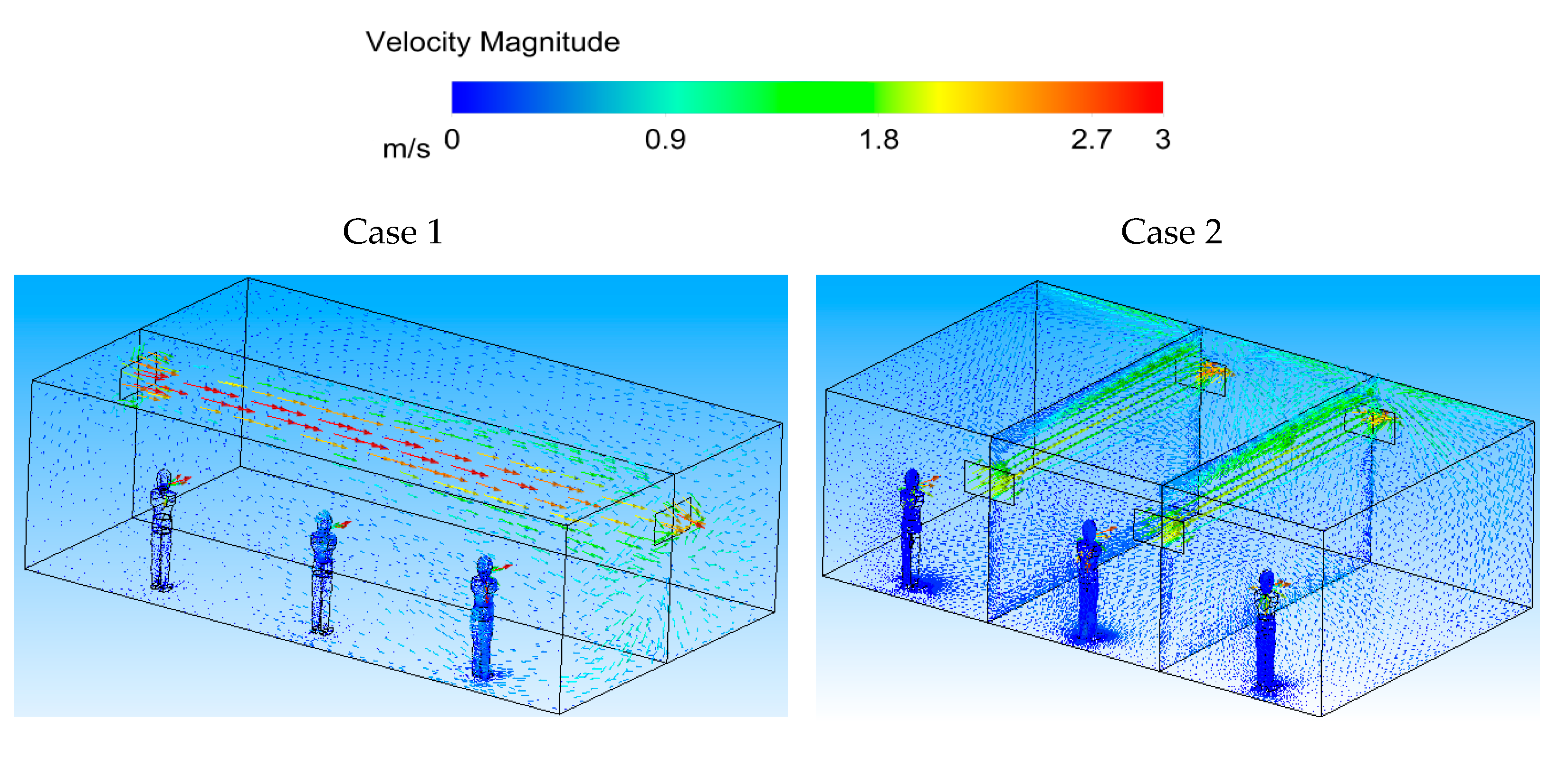
Figure 10 (Case 1) shows the velocity contour in the middle planes of the space in both cases, and at two sections across the width of the room for Case 2. It can be seen that the ventilation air flow entering the area spreads throughout the room for Case 1. For Case 2, however, the ventilation air flow spreads across the room width, creating an air-curtain-type flow pattern.
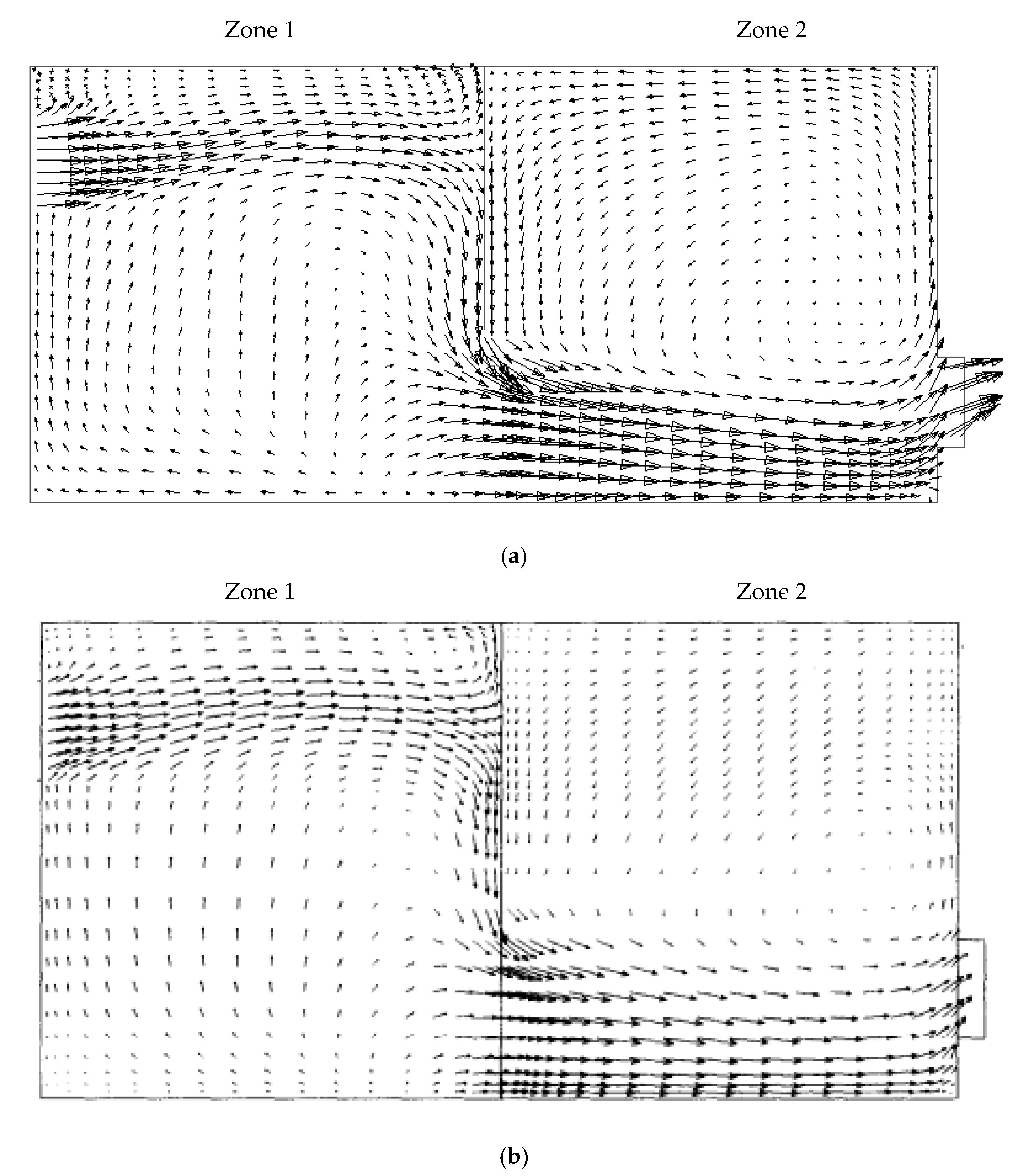
Figure 11 shows the velocity vector diagrams in the middle planes of the space along and across the area (input–output registers) for two cases. It is known that the ventilation air flow in the space proceeds under constant flow conditions.
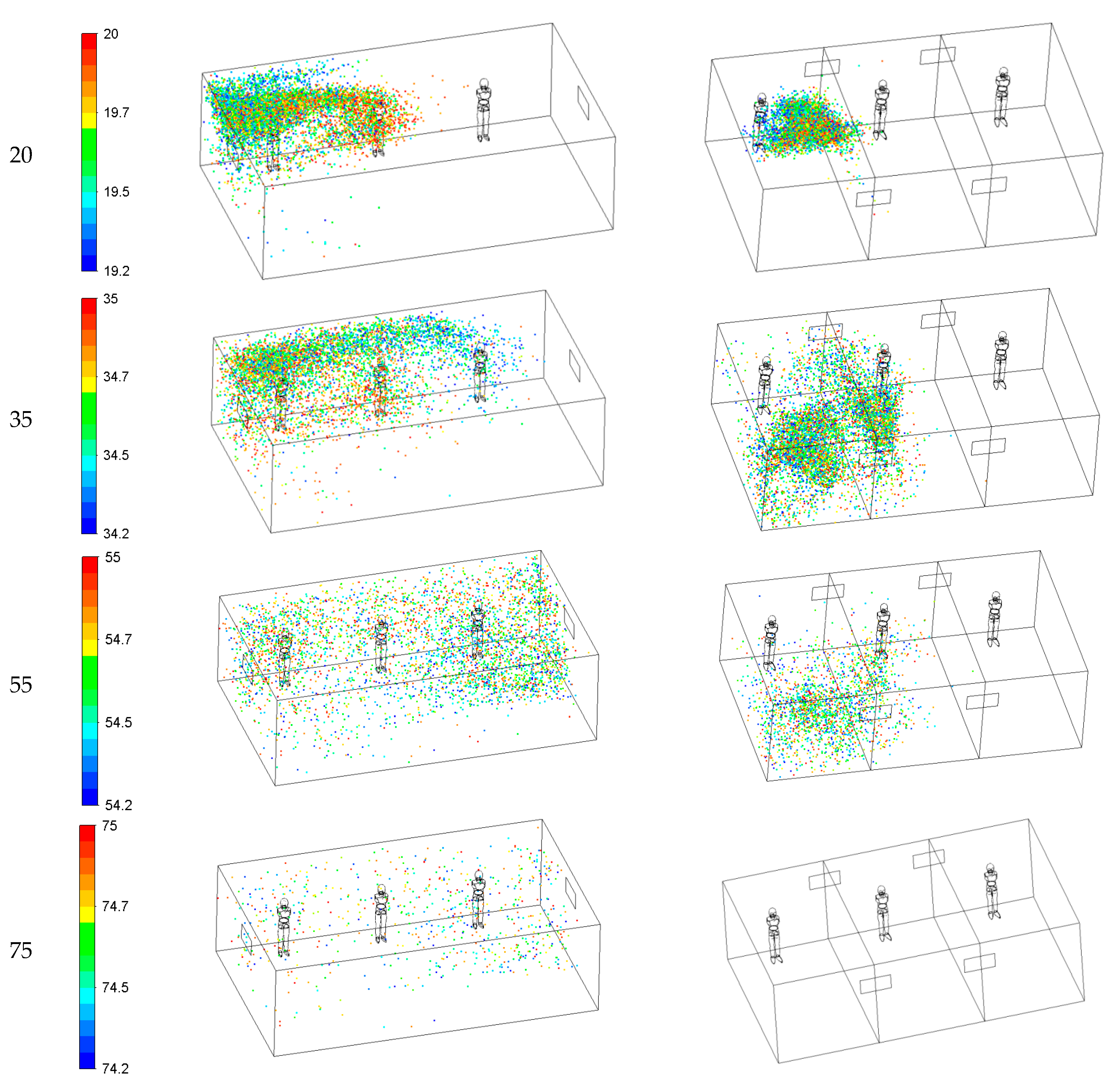
Figure 12 illustrates the dispersion of cough droplets in 2 ventilation systems at different times (Case 1 and 2). The droplet size changes due to evaporation affecting their movement, their residence time (the time for which the droplets remain suspended in the air), and their removal through the exhaust. Case 1 results show that at 1.5 s, the emitted droplets stay close to the patient’s face and then spread in the room. Twenty seconds after coughing, the drops spread over half of the room near the inlet, and their peak concentration moves toward the middle of the space. At t = 35 s, the respiratory droplets enter the other side of the room and spread throughout the space. Finally, the droplet concentration reduces significantly at 75 s after the coughing, while a small fraction of the respiratory droplets remains suspended in the air in the room.
Case 2 shows that, at 1.5 s after the coughing, the emitted droplets stay close to the patient’s face and then spread further away. The Case 2 ventilation system generates air curtain-type flow patterns in the room. At t = 20 s, the air curtain prevents the particles from spreading to the other side of the room. At 35s, Case 1 shows that the respiratory droplets enter the other side of the space and spread throughout the area. However, in Case 2, due to the air curtain, cough droplets do not penetrate the other side of the air curtain. Finally, at 75 s after the cough, there are almost no droplets in the room. As shown in
Figure 12, in Case 2, ventilation air flows across the room width and generates air-curtain flow patterns, and prevents the droplets from spreading to the other areas. This means that forming air curtains prevents droplets from spreading and creates safe spaces in the room.
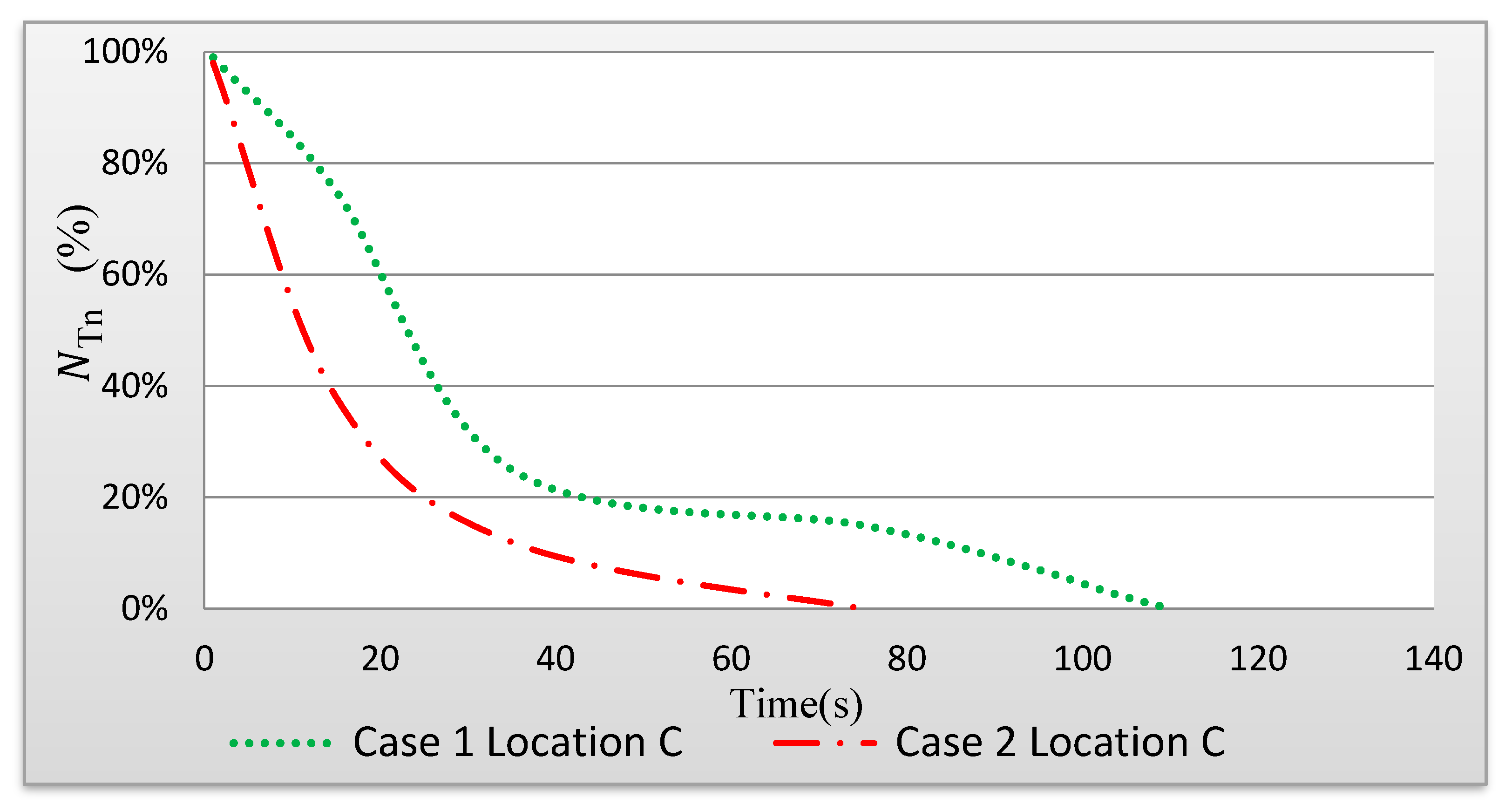
Figure 13 shows the time-dependent changes in the fraction of droplets remaining in the space (N
Tn). When the ventilation air flow is along the length of the indoor space (Case 1), it takes 115 s to clear the area of the droplets. However, when there is a ventilation air flow across the width of the indoor space (air curtain), it takes 75 s for the droplets to leave the area (Case 2).
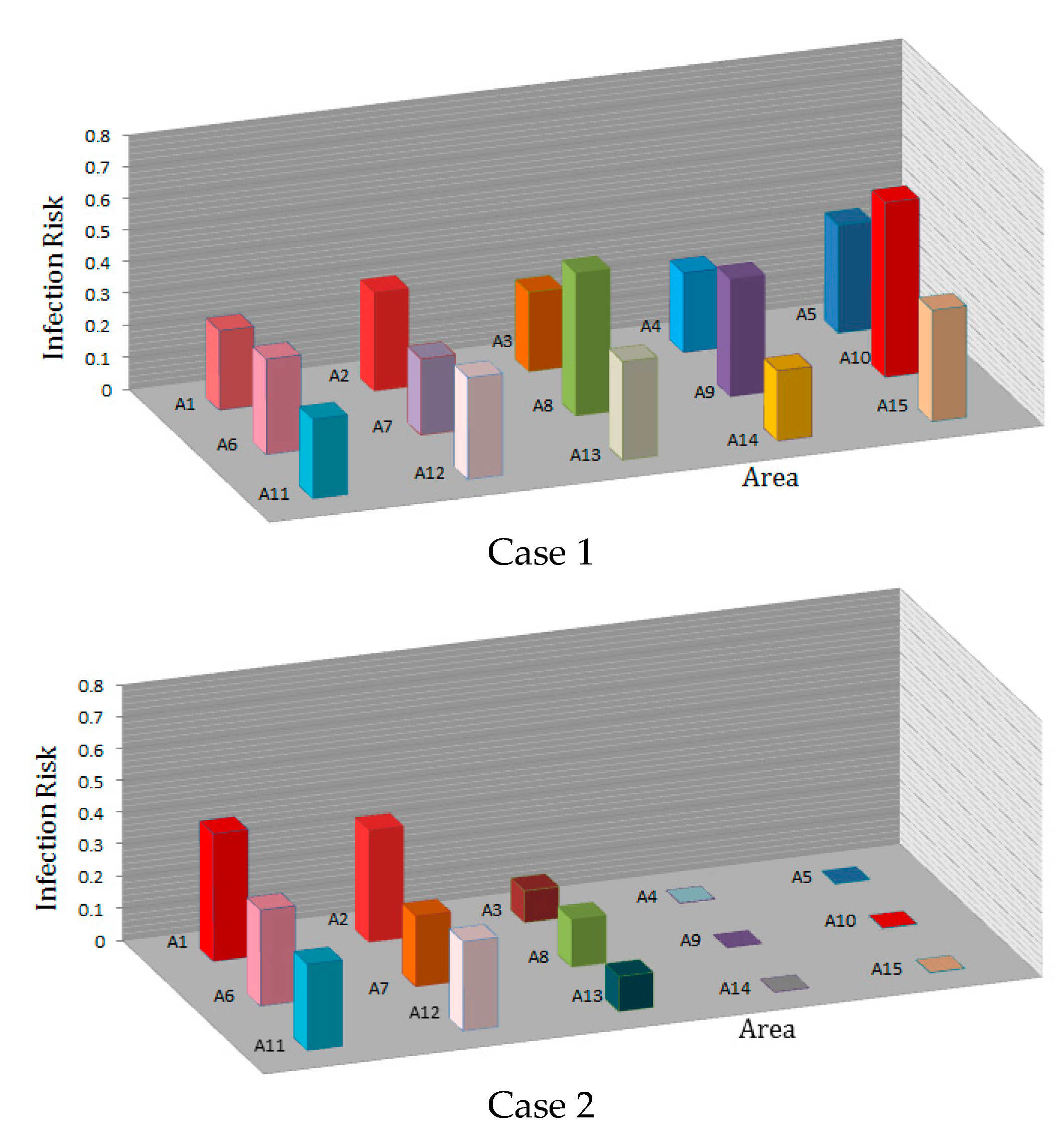
Figure 14 shows the infection risk based on the average droplet concentration in each area in the time interval from 0 to 115 s. Again, the distance between the coughing person and the ventilation outlet determines the residence time of the droplets before they escape through the outlet.
For the ventilation system in Case 1, the droplets remain around the patient’s face for about 3 s after the coughing and then spread in the room. In this case, the ventilation inlet and outlet are located along the room, the cough droplets spread throughout the room, and people in all areas are at high risk of infection. When the coughing person is at location C, and the ventilation air flow proceeds along the length of the indoor space (see
Figure 3), the infection risk in areas A1, A2, A7, A8, A9, and A10 are 23%, 27%, 19%, 33%, 30%, and 48%, respectively.
For the ventilation system in Case 2, the air curtain prevents and delays the spreading of the cough droplets in the room and the chance of infecting other occupants is reduced. When the coughing person is at location C, for the ventilation system in Case 2 with inlet and outlet registers on the side of the indoor space (see
Figure 3), the infection risk in areas A1, A2, A7, A8, A9, and A10 are 37%, 32%, 15%, 10%, 0.05%, and 0.05%, respectively. In this case, the infection risk in areas A4, A5, A9, A10, A14, and A15 significantly reduced.
The comparison between the infection risks for Case 1 and Case 2 ventilation systems shows that people on the other side of the air curtain generally run a lower risk of infection. In addition, the average infection risk of people in a space with air curtain-type air flow patterns significantly reduced compared to Case 1 ventilation with no air curtain. For example, when the coughing person is in area C, the infection risk of a person in area A10 decreases from 57% to 0.05%. When there is no air curtain-type air flow pattern, persons in all areas run a high risk of infection.
4. Conclusions
The dispersion of cough droplets was investigated using CFD simulations and assuming realistic conditions with the presence of 2 ventilation systems in the indoor space environment of a building. The results of the simulation of a patient’s cough, the dispersion of droplets, and the risk of infection are as follows:
In Case 1, it takes 115 s to clear the area of the droplets compared to 75 s in Case 2.
In Case 1, the droplets spread all over the room after a while, but, in Case 2, they remain only in a part of the space for all the time, so in Case 2, there is always a safe area with a low risk of infection of Covid-19 virus
When curtain-type air flow patterns are formed in the room, the infection risk of people at the other end of the room decreases significantly. For example, the risk in area A10 decreases from 48% in Case 1 to 0.05% in Case 2, and in area A9 the infection risk is reduced from 30% in Case 1 to 0.05% in Case 2.
To sum up, the presence of the curtain-type air flow pattern (Case2) causes less diffusion of droplets in the space, and the particles leave the area earlier. In Case 2, there is always a safe area with a low risk of infection of Covid-19 virus. Therefore, it can be concluded that proper design of the location of the supply and exhaust registers in a ventilation system may have a significant effect on the spread of virus particles in the area and also on the possibility of occupants being infected with the virus.