Investigating the Influence of Pore Shape on Shale Gas Recovery with CO2 Injection Using Molecular Simulation
Abstract
:1. Introduction
2. Methods
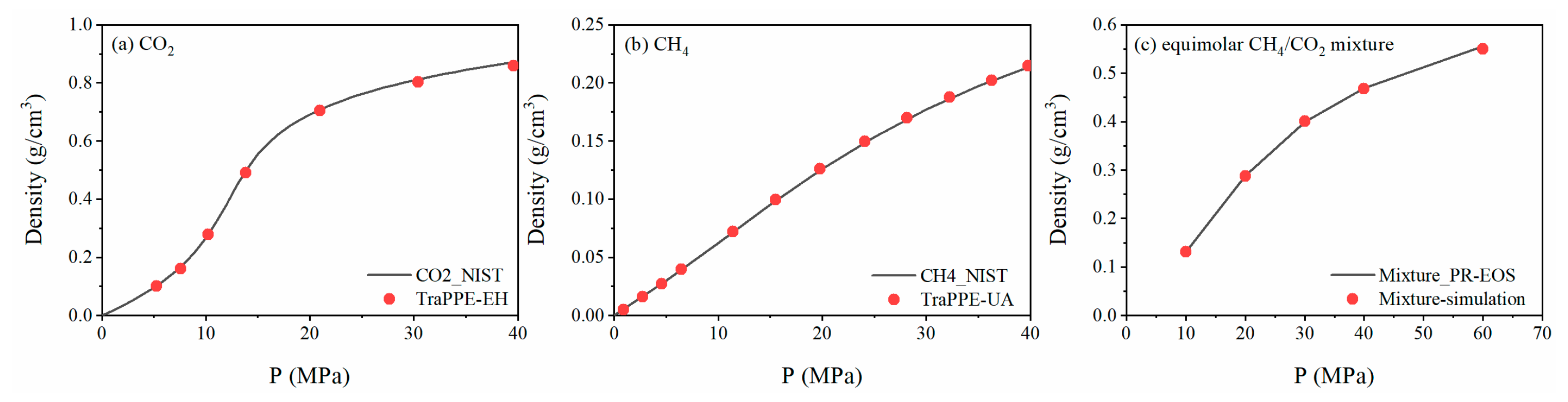
2.1. Simulation Methods
2.2. Simulation Setup
3. Results and Discussion
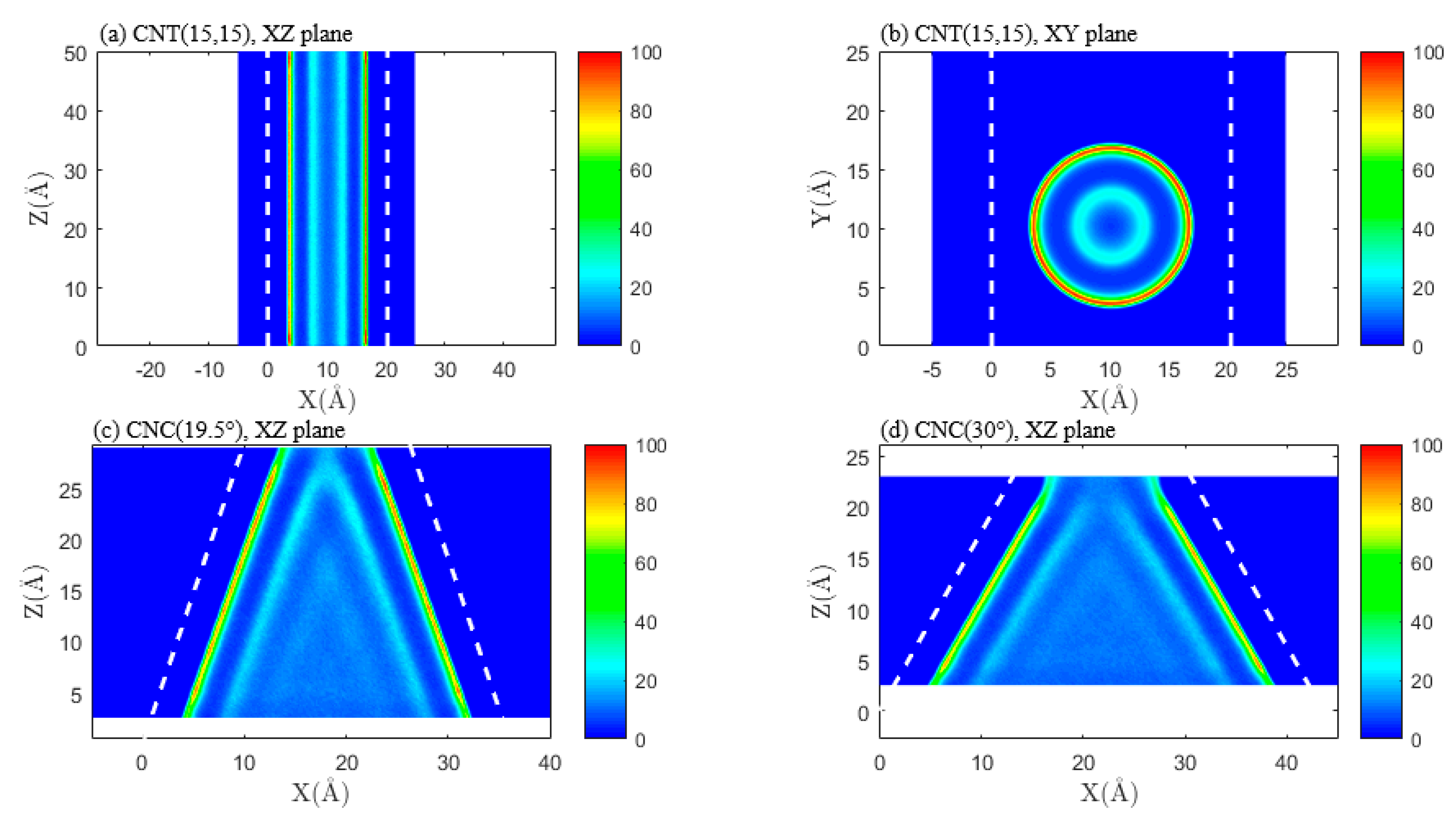
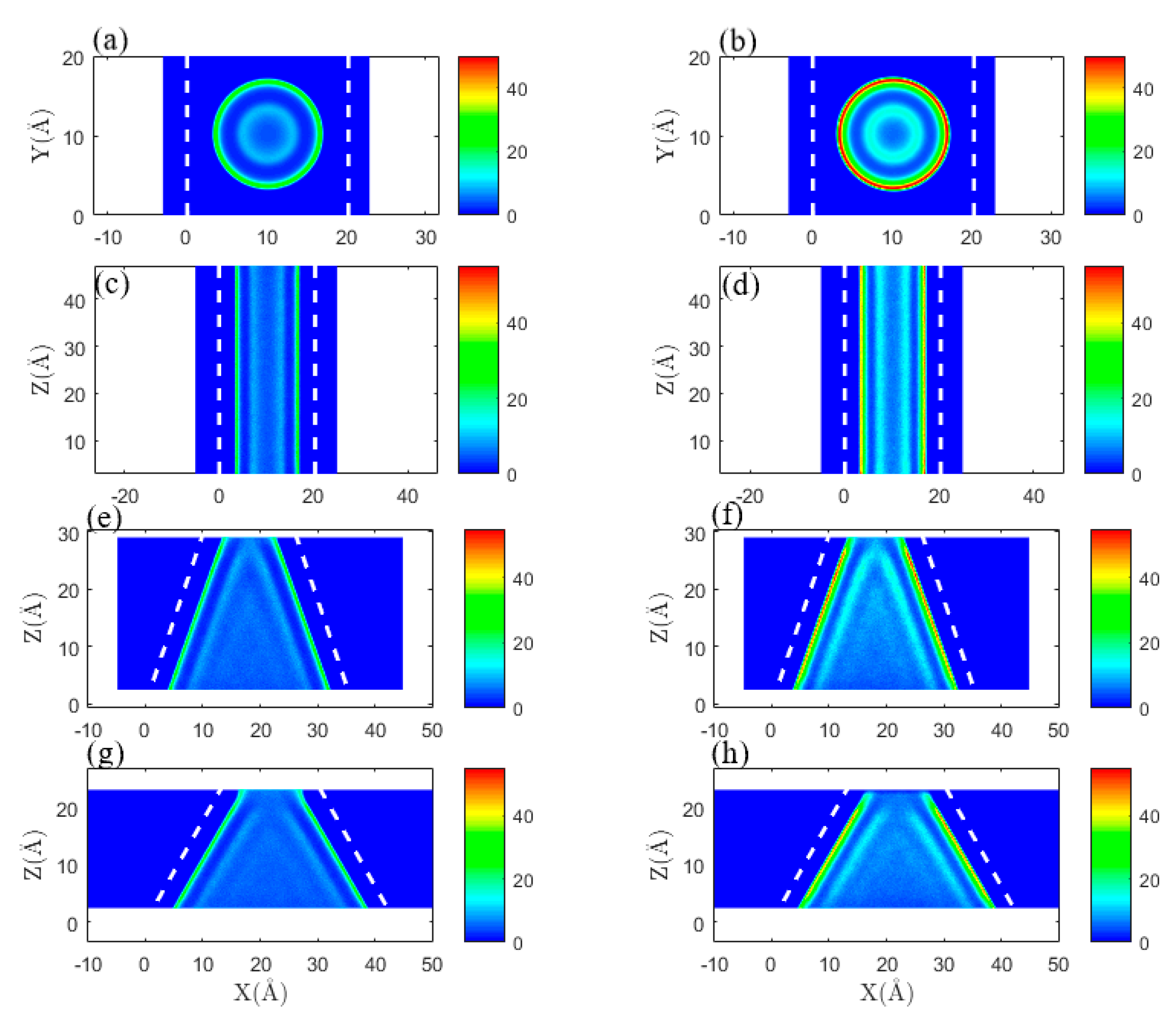
3.1. Effect of Pore Shape on Methane Adsorption Characteristics
3.2. Effect of Pore Shape on the CO2/CH4 Competitive Absorption
3.3. Effect of Pore Shape on Shale Gas Recovery Process
- 1.
- The conventional pressure drawdown method, in which the system pressure was reduced to 20 MPa;
- 2.
- The CO2 injection method, where the CO2 huff–puff process was simulated with a combination of the CO2 injection process and pressure drawdown process.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godec, M.; Koperna, G.; Petrusak, R.; Oudinot, A. Enhanced Gas Recovery and CO2 Storage in Gas Shales: A Summary Review of Its Status and Potential. Energy Procedia 2014, 63, 5849–5857. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.Y.; Song, Y.C.; Jian, W.W.; Li, D.; Hu, C.; Zhan, Y.C. Research Progress of CO2 Sequestration with Enhanced Gas Recovery. Adv. Mater. Res. 2013, 807–809, 1075–1079. [Google Scholar] [CrossRef]
- Li, X.; Wei, N.; Liu, Y.; Fang, Z.; Dahowski, R.T.; Davidson, C.L. CO2 Point Emission and Geological Storage Capacity in China. Energy Procedia 2009, 1, 2793–2800. [Google Scholar] [CrossRef]
- Van Der Meer, L.G.H.; Kreft, E.; Geel, C.; Hartman, J. K12-B a Test Site for CO2 Storage and Enhanced Gas Recovery. In Proceedings of the 67th EAGE Conference & Exhibition, Madrid, Spain, 13–16 June 2005; pp. 27–35. [Google Scholar] [CrossRef]
- Oldenburg, C.M.; Pruess, K.; Benson, S.M. Process Modeling of CO2 Injection into Natural Gas Reservoirs for Carbon Sequestration and Enhanced Gas Recovery. Energy Fuels 2001, 15, 293–298. [Google Scholar] [CrossRef]
- Ougier-Simonin, A.; Renard, F.; Boehm, C.; Vidal-Gilbert, S. Microfracturing and Microporosity in Shales. Earth-Sci. Rev. 2016, 162, 198–226. [Google Scholar] [CrossRef]
- Curtis, J.B. Fractured Shale-Gas Systems. Am. Assoc. Pet. Geol. Bull. 2002, 86, 1921–1938. [Google Scholar] [CrossRef]
- Ross, D.J.K.; Bustin, R.M. The Importance of Shale Composition and Pore Structure upon Gas Storage Potential of Shale Gas Reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Ross, D.J.K.; Bustin, R.M. Shale Gas Potential of the Lower Jurassic Gordondale Member, Northeastern British Columbia, Canada. Bull. Can. Pet. Geol. 2007, 55, 51–75. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, W.; Hu, Q.; Xia, X.; Zhang, C.; Zhang, H. Fluid Distribution and Gas Adsorption Behaviors in Over-Mature Shales in Southern China. Mar. Pet. Geol. 2019, 109, 223–232. [Google Scholar] [CrossRef]
- Zhang, T.; Ellis, G.S.; Ruppel, S.C.; Milliken, K.; Yang, R. Effect of Organic-Matter Type and Thermal Maturity on Methane Adsorption in Shale-Gas Systems. Org. Geochem. 2012, 47, 120–131. [Google Scholar] [CrossRef]
- Mastalerz, M.; He, L.; Melnichenko, Y.B.; Rupp, J.A. Porosity of Coal and Shale: Insights from Gas Adsorption and SANS/USANS Techniques. Energy Fuels 2012, 26, 5109–5120. [Google Scholar] [CrossRef]
- Yang, F.; Ning, Z.; Liu, H. Fractal Characteristics of Shales from a Shale Gas Reservoir in the Sichuan Basin, China. Fuel 2014, 115, 378–384. [Google Scholar] [CrossRef]
- Kurniawan, Y.; Bhatia, S.K.; Rudolph, V. Simulation of Binary Mixture Adsorption of Methane and CO2 at Supercritical Conditions in Carbons. AIChE J. 2006, 52, 957–967. [Google Scholar] [CrossRef]
- Sun, Z.; Li, X.; Liu, W.; Zhang, T.; He, M.; Nasrabadi, H. Molecular Dynamics of Methane Flow Behavior through Realistic Organic Nanopores under Geologic Shale Condition: Pore Size and Kerogen Types. Chem. Eng. J. 2020, 398, 124341. [Google Scholar] [CrossRef]
- Zeng, K.; Jiang, P.; Lun, Z.; Xu, R. Molecular Simulation of Carbon Dioxide and Methane Adsorption in Shale Organic Nanopores. Energy Fuels 2019, 33, 1785–1796. [Google Scholar] [CrossRef]
- Jessen, K.; Tang, G.Q.; Kovscek, A.R. Laboratory and Simulation Investigation of Enhanced Coalbed Methane Recovery by Gas Injection. Transp. Porous Media 2008, 73, 141–159. [Google Scholar] [CrossRef]
- Zhu, X.; Zhao, Y.P. Atomic Mechanisms and Equation of State of Methane Adsorption in Carbon Nanopores. J. Phys. Chem. C 2014, 118, 17737–17744. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhu, X.; Lin, K.; Zhao, Y.-P. Molecular Dynamics Simulations of the Enhanced Recovery of Confined Methane with Carbon Dioxide. Phys. Chem. Chem. Phys. 2015, 17, 31887–31893. [Google Scholar] [CrossRef]
- Zhang, B.; Shan, B.; Zhao, Y.; Zhang, L. Review of Formation and Gas Characteristics in Shale Gas Reservoirs. Energies 2020, 13, 5427. [Google Scholar] [CrossRef]
- Wang, R.; Sang, S.; Zhu, D.; Liu, S.; Yu, K. Pore Characteristics and Controlling Factors of the Lower Cambrian Hetang Formation Shale in Northeast Jiangxi, China. Energy Explor. Exploit. 2018, 36, 43–65. [Google Scholar] [CrossRef]
- Khangar, R.G.; Mendhe, V.A.; Kamble, A.D.; Ranjan Das, P.; Shukla, P.; Bannerjee, M.; Varma, A.K. Variation in Pore Structure and Associated Fractal Dimensions of Barakar and Barren Measures Carbon-Rich Gas Shales of Jharia Basin, India. ACS Omega 2021, 6, 28678–28698. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Xu, L.; Li, P.; Liu, Y.; Dang, W. Pore Structure and Fractal Characteristics of Deep Shale: A Case Study from Permian Shanxi Formation Shale, from the Ordos Basin. ACS Omega 2022, 7, 9229–9243. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, P.; Sun, Z.; Lu, Z.; Du, Z.; Liang, H.; Lu, D. A New Method for Analysis of Dual Pore Size Distributions in Shale Using Nitrogen Adsorption Measurements. Fuel 2017, 210, 446–454. [Google Scholar] [CrossRef]
- Potoff, J.J.; Siepmann, J.I. Vapor-Liquid Equilibria of Mixtures Containing Alkanes, Carbon Dioxide, and Nitrogen. AIChE J. 2001, 47, 1676–1682. [Google Scholar] [CrossRef]
- Martin, M.G.; Siepmann, J.I. Transferable Potentials for Phase Equilibria. 1. United-Atom Description of n-Alkanes. J. Phys.Chem. B 1998, 102, 2569–2577. [Google Scholar] [CrossRef]
- Dauber-Osguthorpe, P.; Roberts, V.A.; Osguthorpe, D.J.; Wolff, J.; Genest, M.; Hagler, A.T. Structure and Energetics of Ligand Binding to Proteins: Escherichia coli Dihydrofolate Reductase-Trimethoprim, a Drug-Receptor System. Proteins Struct. Funct. Bioinform. 1988, 4, 31–47. [Google Scholar] [CrossRef]
- Lorentz, H.A. Ueber Die Anwendung Des Satzes Vom Virial in Der Kinetischen Theorie Der Gase. Ann. Phys. 1881, 248, 127–136. [Google Scholar] [CrossRef]
- Peng, D.-Y.; Robinson, D.B. A New Equation of State. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Widom, B. Potential-Distribution Theory and the Statistical Mechanics of Fluids. J. Phys. Chem. 1982, 86, 869–872. [Google Scholar] [CrossRef]
- Wldom, B. Some Topics in the Theory of Fluids. J. Chem. Phys. 1963, 39, 2808–2812. [Google Scholar] [CrossRef]
- Martin, M.G. MCCCS Towhee: A Tool for Monte Carlo Molecular Simulation. Mol. Simul. 2013, 39, 1212–1222. [Google Scholar] [CrossRef]
- Zhou, J.; Jin, Z.; Luo, K.H. Effects of Moisture Contents on Shale Gas Recovery and CO2 Sequestration. Langmuir 2019, 35, 8716–8725. [Google Scholar] [CrossRef]
- Donohue, M.D.; Aranovich, G.L. Classification of Gibbs Adsorption Isotherms. Adv. Colloid Interface Sci. 1998, 76–77, 137–152. [Google Scholar] [CrossRef]
- Irving Lanowix, B. Adsorption of Gases on Glass, Mica and Platinum. the Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar]
- Michalec, L.; Lísal, M. Molecular Simulation of Shale Gas Adsorption onto Overmature Type II Model Kerogen with Control Microporosity. Mol. Phys. 2017, 115, 1086–1103. [Google Scholar] [CrossRef]
- Pang, W.; He, Y.; Yan, C.; Jin, Z. Tackling the Challenges in the Estimation of Methane Absolute Adsorption in Kerogen Nanoporous Media from Molecular and Analytical Approaches. Fuel 2019, 242, 687–698. [Google Scholar] [CrossRef]




| Atom Type | ε/kB 1 (K) | σ (nm) | q(e) |
|---|---|---|---|
| CH4 | 148 | 0.373 | 0 |
| C-CO2 | 27 | 0.280 | 0.70 |
| O-CO2 | 79 | 0.305 | −0.35 |
| C-CNT/CNC | 28.18 | 0.340 | 0 |
| Stage | P (MPa) | Bulk Gas Composition | |
|---|---|---|---|
| Pressure drawdown | Initial pressure | 30 | Pure CH4 |
| Pressure drawdown | 20 | Pure CH4 | |
| CO2 injection | Initial pressure | 30 | Pure CH4 |
| First pressure drawdown | 20 | Pure CH4 | |
| CO2 injection | 26.1 | 0.75CH4, 0.25CO2 | |
| Second pressure drawdown | 20 | 0.75CH4, 0.25CO2 |
| Pore Shape | pL (MPa) | ρL (mmol/cm3) | R2 |
|---|---|---|---|
| CNT (15,15) | 5.73 | 14.94 | 0.991 |
| CNC (19.5°) | 11.26 | 17.42 | 0.994 |
| CNC (30°) | 13.00 | 18.46 | 0.988 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Gao, S.; Liu, L.; Jing, T.; Mao, Q.; Zhu, M.; Zhao, W.; Du, B.; Zhang, X.; Shen, Y. Investigating the Influence of Pore Shape on Shale Gas Recovery with CO2 Injection Using Molecular Simulation. Energies 2023, 16, 1529. https://doi.org/10.3390/en16031529
Zhou J, Gao S, Liu L, Jing T, Mao Q, Zhu M, Zhao W, Du B, Zhang X, Shen Y. Investigating the Influence of Pore Shape on Shale Gas Recovery with CO2 Injection Using Molecular Simulation. Energies. 2023; 16(3):1529. https://doi.org/10.3390/en16031529
Chicago/Turabian StyleZhou, Juan, Shiwang Gao, Lianbo Liu, Tieya Jing, Qian Mao, Mingyu Zhu, Wentao Zhao, Bingxiao Du, Xu Zhang, and Yuling Shen. 2023. "Investigating the Influence of Pore Shape on Shale Gas Recovery with CO2 Injection Using Molecular Simulation" Energies 16, no. 3: 1529. https://doi.org/10.3390/en16031529
APA StyleZhou, J., Gao, S., Liu, L., Jing, T., Mao, Q., Zhu, M., Zhao, W., Du, B., Zhang, X., & Shen, Y. (2023). Investigating the Influence of Pore Shape on Shale Gas Recovery with CO2 Injection Using Molecular Simulation. Energies, 16(3), 1529. https://doi.org/10.3390/en16031529






