Abstract
Coal maceral composition has a great effect on gas adsorption and diffusion. The interaction between maceral composition and supercritical CO2 (SCCO2) fluid will affect gas diffusion behavior in coals. Thus, the diffusivity derived from adsorption kinetics of CH4 and CO2 in vitrinite- and inertinite-rich coals with low-violate bituminous rank collected from the Hancheng mine of the Weibei coalfield pre- and post-SCCO2 fluid exposure (SFE) were tested at the conditions of 45 °C and 0.9 MPa. In combination with pore distribution and functional group content, the possible mechanism of the alterations in gas diffusion characteristics in coals with various maceral compositions was addressed. The results show that for vitrinite-rich coals, SFE increases the macropore apparent diffusion coefficient of CH4, while this treatment decreases the micropore apparent diffusion coefficient of CH4. However, the reverse trend is found for CO2 diffusion–adsorption rate. For inertinite-rich coals post-SFE, CH4 diffusion–adsorption rate increases, while an increase and a decrease in diffusivity CO2 occur for macropore and micropore, respectively. Generally, SFE shows a stronger impact on CO2 adsorption rate than CH4 in coals. The results suggest that the diffusion of CH4 and CO2 in coals with different maceral compositions show selectivity to SCCO2 fluid. The possible reason can be attributed to the changes in pore structure and surface functional group content. SFE causes an increase in macro/mesopore volume of all samples. However, SFE induces a reduction in oxygen-containing species content and micropore volume of inertinite-rich coals, while the opposite trend occurs in vitrinite-rich coals. Thus, the changes in pore volume and surface functional group account for the difference in gas diffusivity of coals with different maceral compositions. With regard to the micropore diffusion–adsorption behavior of CH4 and CO2, the impact of oxygen-containing species is superior to pore volume. The oxygen-containing species favor CO2 diffusion–adsorption but go against CH4 transport. This effect accounts for the reduction in the micropore diffusion–adsorption rate of CH4 and the increase in micropore diffusivity of CO2 in vitrinite-rich coals, respectively. However, the aforementioned effect is the opposite for inertinite-rich coals. Overall, the changes in gas diffusion in coals with different maceral composition during the CO2-ECBM process requires further attention.
1. Introduction
The emission of carbon dioxide (CO2) has led to serious global warming, which has brought a series of global environmental issues [1]. Multiple research has confirmed that the geo-sequestration of CO2 in coal seams has become a potential technology to mitigate CO2 emissions and to enhance CH4 production (CO2-ECBM) [2,3,4], which can store CO2 in geologic time [5,6]. Most investigations suggest that the depth of CO2 sequestration in coal seams is always above 800 m [6], in which the reservoir conditions exceed the critical points of CO2 (31.05 °C and 7.38 MPa). As a result, as can be seen in Figure 1, CO2 is presented as supercritical CO2 fluid (SCCO2) [7]. In this situation, the thermophysical properties of CO2 (viscosity, density, surface tension, etc.) undergo a great change [8,9,10]. SCCO2 with low viscosity, low surface tension, and high density can extract hydrocarbons from the coal matrix [11]. The extraction effect and complex SCCO2-coal interaction can alter the physical and chemical characteristics of coals [12,13].
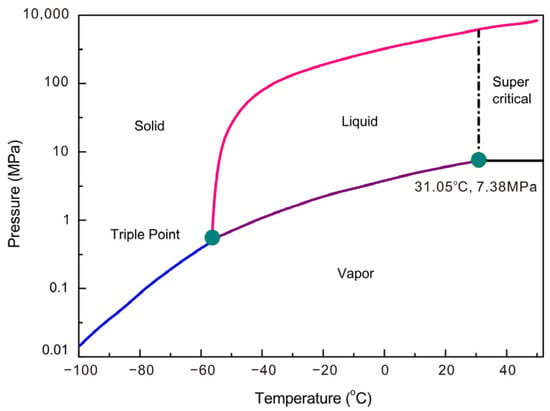
Figure 1.
Phase state of CO2 with varying temperature and pressure (modified from Holliday et al. [7]).
Sampath et al. [14] investigated the changes in coal pore parameters after different interaction time of SCCO2 with coal, and the results indicated that the reduction in pore parameters occurred after hours of SCCO2 treatment, but the increases were recorded after weeks of SCCO2 interaction. They concluded that the different alterations in pore structure at a given SCCO2 time were related to the comprehensive effects of multiple processes. Chen et al. [15] investigated the influence of SCCO2 on nanopore distribution of various rank coals at conditions of 12 MPa and 323.15 K. During SCCO2 for 10 days, they found that the micropore volume decreased but the macropore volume obviously increased. Additionally, the impact of SCCO2 showed selectivity to coal rank. Gao et al. [16] examined the change in the pore structure of anthracite coals after the CO2-H2O reaction at conditions of 5 MPa and 40 °C. The results revealed that the mineral compositions played an important role in the alterations in pore shape, pore type, pore volume, and connectivity. They suggested that the interaction of CO2-H2O with anthracite benefited pore modification. The work of Zhang et al. [17] investigated the change in the chemical structure of coals after SCCO2 treatment by synchrotron ATR-FTIR microspectroscopy. The results showed that the aromaticity of high-volatile bituminous coal and low-volatile bituminous coal increased by different degrees owing to the loss of alkanes. Their findings provided further evidence of SCCO2 extraction and reinforced the concept of plasticization of coal after SCCO2 exposure. In recent work, zhang et al. [18] studied the responses of microstructures and nanoscale mechanical properties of anthracite coal to SCCO2 by using optical microscopy and nanoindentation. The results of nanoindentation directly testified to the softening effects of SCCO2 by the changes in Young′s modulus and hardness. Overall, the CO2 adsorption capacity of coals changed owing to the alterations in physical and chemical structure [19,20,21,22].
Hitherto, the change in coal structure and CO2 adsorption capacity due to SCCO2-coal interaction is related to coal maceral composition and coal type [23,24]. Mastalerz et al. [25] investigated the change in pore distribution of vitrain, clarain, and fusain lithotypes after exposure to gaseous CO2. They found that the pore structure of clarain after gaseous CO2 exposure showed minor change, while the changing trend in pore properties of vitrain and fusain was the opposite. Cao et al. [26] analyzed the interaction between the chemical structure of coal lithotypes and gaseous CO2. They found that a decline in aromaticity and an increase in aliphatic carbons occurred in vitrain and fusain after CO2 adsorption, whereas reverse results were found in clarain and bright clarain. Karacan et al. [27] found that vitrinite exhibited matrix swelling after CO2 adsorption, while inertinite showed matrix compression. Maphala and Wagner [28] studied the interaction between sub-critical CO2 and maceral composition of high-volatile bituminous coals. The results demonstrated that no significant difference in pore structure was observed for inertinite-rich coals after CO2 exposure, while obvious alteration was found for vitrinite-rich coals. Nevertheless, the CO2 adsorption amounts of both inertinite- and vitrinite-rich coals increased after exposure to CO2. Mavhengere et al. [29] investigated the interactions of sub-critical CO2 and SCCO2 fluid with medium bituminous coals. They concluded that the changes in pore properties and chemical structure of vitrinite-rich coals and inertinite-rich coals are different. However, SCCO2 fluid improved CO2 adsorption capacity of all samples.
In summary, SCCO2 fluid exposure (SFE) alters the physicochemical properties of coals with various maceral compositions. Notwithstanding, the alterations in diffusivity of CH4 and CO2 in coals are also important to CO2-ECBM [30,31]. The diffusion–adsorption rate or diffusivity of CH4 and CO2 is crucial to the CO2 injection rate and CH4 recovery efficiency during the implementation of the CO2 sequestration process [32,33,34,35]. Keshavarz et al. [36] reported the influence of maceral composition on the CH4 and CO2 diffusivity of bituminous and sub-bituminous coals. They found that the gas diffusion rate increased with the increasing inertinite content. The work of Guang et al. [37] showed that CO2 pressure had a critical effect on gas diffusivity in sub-bituminous coals. Goodman et al. [38] studied the effect of gaseous CO2 on CO2 diffusion in high-volatile bituminous coals at low pressure (<0.62 MPa). Wang et al. [39] studied the diffusivity of CO2 and CH4 in high-volatile bituminous coals and anthracite pre- and post-SCCO2 exposure. However, little attention is paid to the role of maceral composition in the influence of SCCO2 fluid on CH4 and CO2 diffusion in low-volatile bituminous coals. Thus, as for low-volatile bituminous coals, the present study aims to analyze the influence of SFE on CH4 and CO2 diffusivity in vitrinite- and inertinite-rich coals. Moreover, the mechanisms of SFE dependence on gas diffusion are addressed.
2. Materials and Methods
2.1. Characteristics of Coal Samples
All the studied samples were obtained from the Hancheng coalmine of the Webei coalfield located in the southeastern margin of Ordos Basin. The detailed geological setting of the selected coal samples has been described in our previous work [40]. Among the samples, XS9 and XS1 samples were collected from the XS coal mine, and SSP17 and SSP16 samples were from the SSP coal mine (Table 1). According to GB/T 6948-2008 and GB/T 8899-2013 standards, the maximum vitrinite reflectance (Ro,max) and maceral composition content were measured, respectively. Based on the GB/T 212-2008 standard, the proximate analysis (moisture, ash, and volatile) of samples (air dried basis) is given in Table 1. The values of Ro,max and volatile matter yield indicate that all the samples belonged to low-volatile bituminous coals. Based on an arbitrary 50% inertinite boundary, as suggested by Unsworth et al. [41], vitrinite-rich coals and inertinite-rich coals could be identified. Thus, XS9 and XS1 samples were classified into vitrinite-rich coals, while SSP17 and SSP16 samples were inertinite-rich coals.

Table 1.
Information of coal samples including Ro,max, maceral composition, and proximate analysis.
2.2. Geochemical Interaction of SCCO2 Fluid with Coal Samples
A supercritical fluid interaction apparatus (Thar Process, Inc., Pittsburgh, PA, USA), shown in Figure 2, was employed to conduct the geochemical interaction of SCCO2 fluid with drying coal samples. The detailed apparatus information can be found in our previous work [42]. During the SCCO2-coal interaction, a long duration may be needed to achieve the SCCO2 equilibrium state for large particle sizes due to the long migration path [43,44], which may result in slow interaction efficiency between SCCO2 and coal. As suggested by Goodman et al. [38] and Wang et al. [39], in order to enhance the interaction efficiency, 80–120 mesh particle samples were sieved to conduct the experiment. Prior to the interaction measurement, the samples were put into a vacuum drying oven to remove moisture. The drying temperature and time were 105 °C and 24 h, respectively. Subsequently, 50 g of particle samples were moved into the interaction/extraction vessel to conduct the geochemical interaction of SCCO2 fluid with particle samples. The interaction temperature of 45 °C and pressure of 12 MPa were chosen as simulative reservoir conditions with a depth of 1200 m for geologic sequestration of CO2. The mass flow rate and exposure duration of SCCO2 fluid were set as 10 g/min and 12 h, respectively, according to previous works [39,45]. At the end of the exposure, all the samples were degassed in a vacuum drying oven at 105 °C for 24 h. Finally, all the samples were stored in a closed cell filled with inert helium prior to subsequent characterizations and measurements.
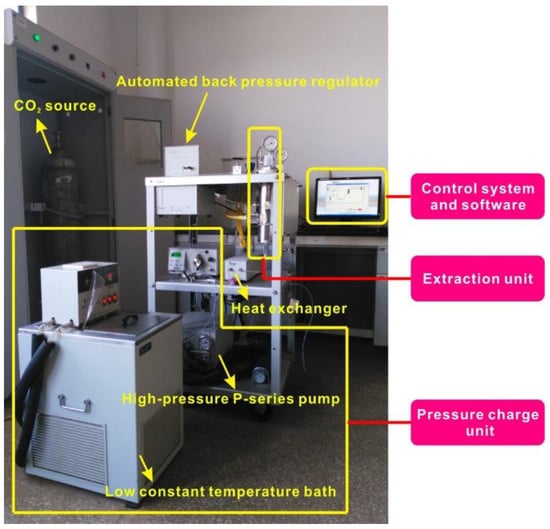
Figure 2.
Dynamic supercritical fluid geochemical interaction apparatus.
2.3. Pore Volume and Specific Surface Area Analysis
The pore volume (PV) and specific surface area (SSA) were measured by ASAP2460 adsorption apparatus (Micromeritics, Norcross, GA, USA). The remaining moisture and gas in samples pre- and post-SFE were removed by drying at 105 °C for 24 h before pore analysis. N2 and CO2 were selected as a molecular probe to detect macro/mesopore parameters and micropore parameters at 77 K and 273 K, respectively. The PV and SSA of macro/mesopores were determined by BET and BJH model, respectively. Both the PV and SSA of micropores were obtained by the DR model. The corresponding pore diameter range for macro-, meso- and micropores were divided into >50 nm, 2–50 nm, and <2 nm, as suggested by the work of Sing et al. [46].
2.4. X-ray Photoelectron Spectroscopy Measurement
The X-ray photoelectron spectroscopy (XPS) analysis of all samples was measured by PHI 5000 VersaProbe-II spectrometer. The surface element content including C1(s), O1(s), N1(s), S2(p), C1(s), Al2(p), Si2(p) was obtained with 36 scans. The test was performed at 200 W and 15 kV. The charge correction of the surface element was conducted based on the C1(s) peak at 284.8 eV. According to the work of Weitzsacker et al. [47], the inorganic O derived from SiO2 and AlO1.5 had a great effect on the distribution of organic O in coals. Thus, in order to obtain the organic O content in coals, the inorganic O concentration was subtracted from the total surface O1(s).
2.5. Diffusion–Adsorption Measurement
The diffusion–adsorption test of CH4 and CO2 in coals pre- and post-SFE was carried out using a volumetric technique through high-pressure isothermal adsorption apparatus. The detailed parameters of the experimental rig can be found in our previous work [42]. Before the test, all the samples were put into a vacuum drying oven to remove moisture at 105 °C for 24 h. Each sample with a mass of 40 ± 0.0005 g was used for gas diffusion–adsorption test. The experimental temperature of 45 °C and pressure of 0.9 MPa were selected to perform for CH4 and CO2 adsorption. Particularly, the recording frequency of pressure data was set as 1 s.
According to the principle of mass conservation, the time-based testing data of the adsorption amount can be calculated by:
where GSE is the experimentally observed Gibbsian surface excess amount, mmol/g; R is the universal gas constant, 8.314 J/mol/K; m is the mass of samples, g; T is the testing temperature, K; VRC and VV is the volume of the reference and adsorption cell, respectively, m3; P1 and P2 are the initial pressures of sample cell and a reference cell, respectively, MPa; P3 and P4 are the instantaneous pressures of the reference cell and adsorption at time of t, respectively, MPa; Z are the compressibility factors of CH4 or CO2. In this study, the compressibility factors of CH4 and CO2 were calculated by Wagner and Span-EOS [48] and Span and Wagner-EOS with high predictive accuracy [49], respectively. Based on the data log of pressure against t, the CH4 and CO2 adsorption capacity at a given t were obtained through Equation (1), followed by the CH4 or CO2 adsorption rate curves. It should be noted that the calculation of CH4 or CO2 adsorption rate incorporates the absolute adsorption amount (nabs). The relationship between nabs and GSE is:
where ρa and ρb are adsorbed phase density and bulk density of adsorbate, respectively, g/cm3. According to previous studies [50], ρa of adsorbate is always deemed as a pseudo-liquid state. Therefore, ρa of CH4 and CO2 were 0.421 g/cm3 and 1.227 g/cm3, respectively [51,52].
2.6. Gas Diffusivity Modelling
Compared to the unipore model, the bidisperse model can well represent the gas adsorption rate behavior in coal pores [53,54]. Therefore, the simplified bidisperse model was chosen to study the gas adsorption rate according to the study of Pan et al. [32]. The bidisperse model consists of a fast macropore diffusion stage and a slow micropore diffusion stage.
The first/fast stage occurs at the beginning of the experiment and can be expressed by:
where Ma is the total capacity of CH4 or CO2 adsorbed in macropore at t, mmol/g; Ra is the macropore radius, m; Da is the effective diffusivity at macro scale, m2/s; Da/Ra2 is the macropore apparent diffusion coefficients, s−1.
The slow stage can be expressed by:
where Mi is the total capacity of CH4 or CO2 adsorbed in micropore at t, mmol/g; Ri is the micropore radius, m; Di is the effective diffusivity at micro scale, m2/s; Di/Ri2 is the micropore apparent diffusion coefficients, s−1.
Combined with Equations (3) and (4), the whole diffusion–adsorption process uptake is given as:
where β is defined as the ratio of macropore adsorption to the total adsorption [30]. Thus, according to Equation (5), the parameters Da/Ra2, Di/Ri2, and β can be estimated by using nonlinear curve fitting.
3. Results and Discussions
3.1. Gas Diffusivity and Bidisperse Modelling
Figure 3 displays the experimental curves of CH4 and CO2 adsorption with time t. Take the case of sample SSP16, the repetitive experiments of CH4 adsorption with time t, shown in Figure 4, indicates good reproducibility of the diffusion–adsorption measurements. Typically, for both vitrinite- and inertinite-rich coals, the adsorption rate curves of CH4 and CO2 display fast adsorption within the initial 1000 s and then gradually approaches the equilibrium state. The bidispere model exhibits a good fitting with the experimental data of CH4 and CO2 adsorption rate. The bidisperse model fitting parameters of gas diffusivity of the coals pre- and post-SFE are listed in Table 2 and Table 3, respectively. The correlation coefficients R2 for all samples were over 0.98, thus implying that the bidisperse pore-diffusion model can well represent CH4 or CO2 diffusion behaviors in all samples. Within the whole experimental pressure range, the magnitude orders of Da/Ra2 (10−3–10−2) and Di/Ri2 (10−5–10−4) values of CH4 and CO2 apparent diffusion coefficients have a good agreement with the findings by Pan et al. [32] and Cui et al. [54]. Under similar equilibrium pressure, the slope of adsorption rate against time t of CO2 was higher than that of CH4 (Figure 3). It is found from Table 2 and Table 3 that the pore apparent diffusion coefficients of CO2 was one order of magnitude larger than that of CH4 on the same coal sample. The reasons may be related to the molecular kinetics diameter, adsorption interaction between adsorbate and adsorbent, and adsorption heat, which have been addressed in previous studies [54,55].
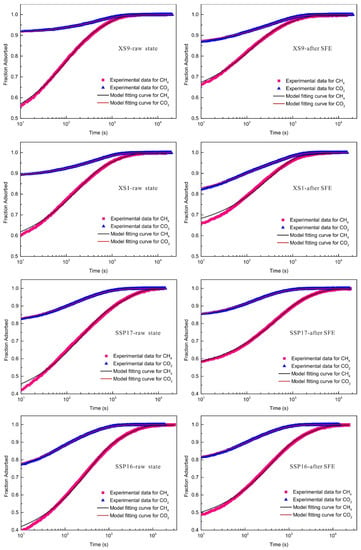
Figure 3.
Experimental and fitting curves of CH4 and CO2 adsorption rate in samples.
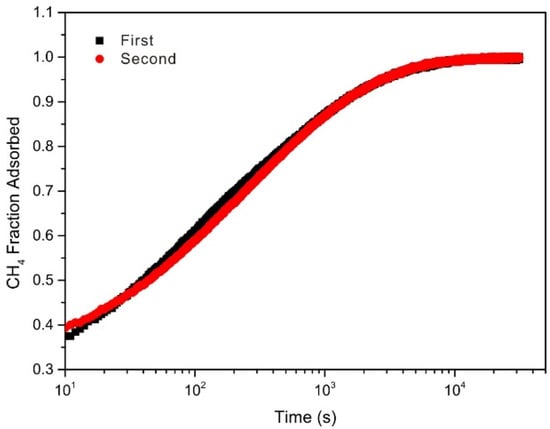
Figure 4.
Reproducibility of CH4 diffusion–adsorption results for SSP16 sample.

Table 2.
Statistical results of CH4 diffusion rate.

Table 3.
Statistical results of CO2 diffusion rate.
As presented in Table 2 and Table 3, the alterations in values of Da/Ra2 and Di/Ri2 of CH4 and CO2 show that the gas diffusion–adsorption rates of vitrinite- and inertinite-rich coals present different responses to SFE. For vitrinite-rich coals, SFE caused an increase in macropore diffusion coefficients of CH4 (Da/Ra2 increased by 12.19–65.34%), but induced a decline in micropore effective diffusion coefficients of CH4 (Di/Ri2 decreased by 8.75–17.79%). However, the reverse trend was found for the change in pore diffusion coefficients of CO2 due to SFE, which owned a decrease of 28.95–52.77% and an increase of 17.32–29.80% for Da/Ra2 and Di/Ri2, respectively. For inertinite-rich coals after SFE, both the macro- and micropore diffusivity of CH4 increase. In terms of pore diffusion coefficients of CO2, the Da/Ra2 and Di/Ri2 values are 1.25–1.40 times and 0.84–0.95 times, respectively, compared to the original samples. The aforementioned results indicate that macro- and micropore diffusivity of CH4 and CO2 vary due to SFE but differ in inertinite-rich coals and vitrinite-rich coals. This phenomenon implies that maceral composition played a dominate role in the change of gas adsorption kinetic behaviors due to SCCO2 interaction. Moreover, the impact of SFE on pore diffusion was more evident for CO2 than for CH4.
3.2. Influencing Mechanism of SFE on Gas Diffusion
For bidisperse diffusion, as described by Cui et al. [54] and Wei et al. [56], the gas diffusion in coal is mainly controlled by micropore surface diffusion and macro/mesopore diffusion. Many previous studies have shown that pore properties and surface functional groups have a significant impact on gas diffusion–adsorption behaviors [57,58,59,60]. The studies of Liu et al. [61] reported that mesopore volume determined the effective diffusion coefficient of CH4. Wang et al. [39] found that micropore diffusion coefficient was proportional to micropore surface area and was mainly controlled by surface diffusion. Therefore, the variation in the pore structure of inertinite- and vitrinite-rich coals after SCCO2 interaction should be concerned. Additionally, various kinds of functional groups exist on the coal surface. Numerous studies revealed that the oxygen-containing functional groups showed a great impact on gas adsorption behavior [62]. Thus, the variations in oxygen-containing functional group contents after exposure to SCCO2 would affect gas diffusion and adsorption in coals. In the present work, the pore parameters and surface oxygen-containing species distribution before and after SFE were analyzed to account for the possible influencing mechanisms of SFE on gas diffusion.
The PV and SSA of macro/mesopore and micropore are listed in Table 4. SFE increases PV and SSA at macro/mesopore scale of both vitrinite- and inertinite-rich coals (Table 4). SCCO2 interaction enhances the micropore development of vitrinite-rich coals, while degrading the microporosity of inertinite-rich coals (Table 4). The impact of SFE on pore parameters can be attributed to the physical and chemical reaction, such as coal matrix swelling and extraction effect between SCCO2 fluid and coal [20,23,25,63]. The distinction between microporosity of the vitrinite- and inertinite-rich coals pre- and post-SFE may be related to the differences in the chemical structure of maceral composition [26], which need further studies. The various SCCO2-caused changes in pore parameters illustrate the critical roles of maceral type in SCCO2-coal interaction. Owing to the increased macro/mesopore volume in post-SCCO2 samples, the macropore diffusivity of CH4 and CO2 improved except for the macropore diffusivity of CO2 in vitrinite-rich coals. However, as shown in Table 2, Table 3 and Table 4, the varying trend between micropore apparent diffusion coefficients and micropore surface area in the post-SCCO2 samples was different. For example, the micropore surface area of the inertinite-rich coals decreased while the micropore diffusion coefficients of CH4 increased. A similar phenomenon can be found in vitrinite-rich coals. This illustrates that the alterations in pore parameters between original and post-CO2 samples are inadequate to account for the impact of SFE on CH4 and CO2 diffusion.

Table 4.
Summary of PV and SSA of micro- and macro/mesopore in samples pre- and post-SFE.
Taking the case of the XS1 sample, Figure 5 depicts the curve-resolved fitting of the XPS C (1s) spectrum with a binding energy between 280 eV and 292 eV. In our study, the functional groups from C (1s) resolving are deconvoluted into C-C/C-H (284.8 eV), C-O (286.3 ± 0.2 eV), and C=O (287.5 ± 0.2 eV) according to previous studies [64,65]. The fitting results of C content, O oxygen content, and different surface functional groups, pre- and post-SFE, are displayed in Table 5. The functional groups of vitrinite-coals and inertinite-rich coals showed different responses to the reaction with SCCO2. For the post-SCCO2 vitrinite-rich coals, the C content and C-C/C-H group concentration showed a minor decrease, but the amount of both organic O, C-O, and C=O species increased. However, the opposite trend was recorded for the inertinite-rich coals after SCCO2 interaction. An evident reduction in C-O and C=O species was found, whereas an increase in C-C/C-H species was recorded (Table 5). The variations in surface functional groups of coals show that SFE made a change in the surface chemistry property, thereby altering diffusion behaviors of CH4 and CO2. Hitherto, the mechanisms of chemical interaction of SCCO2 with oxygen-containing species have still been ambiguous.
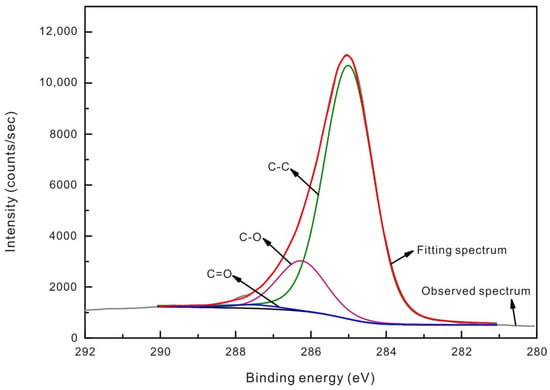
Figure 5.
Example of the curve-resolved fitting of XPS C (1s) for the XS1 sample.

Table 5.
Surface carbon and organic oxygen content and relative abundance of functional groups.
According to previous studies, the mechanisms are associated with the physical and chemical adsorption of CO2 on coals. The O atoms of oxygen-containing species own high electronegativity. It favors CO2 adsorption owing to an inductive effect, which makes CO2 stably physisorbed on coal surfaces [58]. The work of Huang et al. [66] showed that oxygen-containing species enhanced CO2 adsorption capacity by hydrogen bonds, which induces CO2 chemisorbed on coal surfaces. These may decrease the amounts of carbon-oxygen species [67]. Moreover, the mechanism relates to the extraction of SCCO2 fluid. SCCO2 fluid can mobilize polycyclic aromatic hydrocarbons, aliphatic species, and low-molecular-weight compounds [11], which can be testified by our XPS results with the decline in C-C/C-H groups of vitrinite-rich coals due to SFE (Table 5). Both the physical-chemical adsorption and extraction effects can contribute to the decrease of different functional group species. As shown in Table 5, the extraction interaction of SCCO2 with vitrinite-rich coals may be strong, which causes the decrease of C-C/C-H species and the relative enrichment of carbon-oxygen species. Compared to the vitrinite-rich coals, more oxygen-containing functional groups exist in the inertinite-rich coal surface, which provides more preferential polar sites for CO2 sorption. The chemical interaction of SCCO2 with carbon–oxygen species may be superior to the extraction effect, thus leading to an apparent reduction of C-O and C=O species concentrations of the inertinite-rich coals.
Many studies show that oxygen-containing species can weaken Van der Waals interactions between CH4 and pore surface on one hand and block pore on the other [68,69], thus decreasing CH4 adsorption capacity of coals. However, hydrogen bonds, high electronegativity, and electrostatic contribution between oxygen-containing species and CO2 favor CO2 adsorption [23,29,62,70]. The works of Prinz et al. [71] showed that the oxygen-containing groups might accommodate micropores (0.5–0.8 nm) of the coals with Ro,max above 1.5%. Thus, the redistribution of oxygen-containing species due to SCCO2 interaction would alter gas diffusion and adsorption behaviors on the coal micropore surface. According to the aforementioned analyses, in combination with data in Table 5, it can be inferred that the increasing oxygen-containing species of vitrinite-rich coals decreases the diffusivity of CH4 on the micropore surface but enhances CO2 diffusivity. Although SFE increases the micropore SSA of the vitrinite-rich coals, the effect of oxygen-containing species may be superior to the micropore surface area. Thus, the net result is that SCCO2 interaction leads to decreasing micropore diffusion coefficients of CH4 but increasing CO2 micropore diffusion coefficients in the vitrinite-rich coals. Furthermore, the migration of low-molecular-weight compounds and aliphatic species extracted by SCCO2 fluid may affect CH4 and CO2 diffusion. The extracted organic species in vitrinite-rich coals may migrate into the macro/mesopores [72]. These organic species can enhance the adsorption potential of CH4 on coals by increasing adsorption heat [73] but hinder pore entrance for CO2 diffusion [72]. This may be another reason for the decrease in the diffusion rate of CO2 and the increase in the diffusion rate of CH4 in the macro/mesopore of the vitrinite-rich coals. For the inertinite-rich coals after SFE, the reduction in both oxygen-containing species and micropore SSA improve the diffusivity of CH4 in micropore but weaken CO2 diffusion in micropores. Moreover, coal matrix swelling may occur when CO2 adsorbs on oxygen-containing species and micropore surface, which may compress micropore space and transform some micropores into mesopores. As a result, the concomitant reduction in micropore surface area and rise in mesopore volume occur. This phenomenon may improve macropore diffusion coefficients but degrade micropore diffusion coefficients of CO2 in inertinite-rich coals. These results obtained from this study indicate that whether vitrinite- or inertinite-rich coals, the comprehensive effect of pore parameters and in coals due to SCCO2 exposure contributes to the different changes in both CH4 and CO2 diffusion properties. However, the role of maceral composition in the change of pore and surface functional groups after SFE is different.
3.3. Implications of Maceral Composition for CO2 Sequestration
In our present study, CO2 exhibits a higher pore diffusivity than CH4, which manifests the viability of improving CH4 recovery by CO2 displacement [74]. As discussed in a previous analysis, pore parameters and surface functional groups related to maceral composition after SCCO2 interaction are of great significance in CH4 and CO2 diffusion. Thus, the influence of maceral composition on gas diffusivities after SCCO2-interaction can not be ignored during CO2-ECBM implementation. For the vitrinite-rich coals after SCCO2 interaction, the micropore diffusion coefficients of CH4 decrease while those of CO2 increase, which benefits displacement of CH4 by CO2 injection. However, the reduction in macropore diffusion coefficients of CO2 may slow CO2 diffusion and weaken CO2 displacement efficiency. Thus, for coal reservoirs with high content of vitrinite, it is necessary to consider how to improve the transport and diffusion capacity of CO2 in macro/mesopores during the CO2 injection process. In terms of the inertinite-rich coals after SCCO2 exposure, SCCO2 exposure obviously decreases diffusivities of CO2 in micropores, but increases diffusivities of CH4 in micropores. This result may impair CH4 recovery by CO2 injection; therefore, for coal reservoirs with high content of inertinite, it is required to consider how to mitigate the adverse influence of SFE on micropore structure and surface chemistry. Generally, the detrimental effect of SFE on CH4 recovery and CO2 diffusion in coals with different maceral compositions should be fully evaluated for target CO2-ECBM projects.
4. Conclusions
This study mainly addressed the influence of supercritical CO2 fluid on CH4 and CO2 diffusion in vitrinite-rich coals and inertinite-rich coals. The main conclusions are included below.
- (1)
- The bidisperse-pore diffusion model can well analyze CH4 and CO2 diffusion behaviors in all samples. In comparison with the original inertinite-rich coal samples, SFE increases the pore diffusivity of CH4 at both macro- and microscale and enhances the macropore diffusivity of CO2, but weakens the micropore diffusion rate of CO2. For the vitrinite-rich coal samples after SCCO2 interaction, an increase in macropore diffusivity and a decrease in micropore diffusivity of CH4 are recorded, while the opposite trend is noticed for macro- and micropore diffusion rate of CO2. The different SCCO2-induced changes in CH4 and CO2 diffusion in the vitrinite- and inertinite-rich coals illustrate the importance of maceral composition in SCCO2-coal interaction.
- (2)
- Pore parameter analysis shows that SFE increases macro/mesopore volume of all samples and enlarges micropore SSA of vitrinite-rich coals but degrades that of inertinite-rich coals. The XPS results show that SCCO2 interaction causes the increase of oxygen-containing species of vitrinite-rich coals, while it results in a decrease of oxygen-containing species of the inertinite-rich coals. The comprehensive result of pore parameters and functional group distribution in different maceral compositions accounting for the roles of SFE on CH4 and CO2 diffusion. As a result, different adverse effects may occur after the interaction of SCCO2 with vitrinite- and inertinite-rich coals. Therefore, the impact of SFE on CH4 and CO2 diffusion in coals with different maceral compositions should be fully evaluated in practical CO2-ECBM projects.
Author Contributions
Conceptualization and methodology, W.L. (Wei Li) and W.L. (Weili Lin); validation, H.L., W.L. (Wei Li) and X.S.; formal analysis, W.L. (Weili Lin); investigation, Z.W.; resources, Z.W.; data curation, W.L. (Weili Lin); writing—original draft preparation, W.L. (Wei Li); writing—review and editing, W.L. (Wei Li); visualization, H.L. and X.S.; supervision, Z.W.; project administration, Z.W.; funding acquisition, Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Foundation of PetroChina Company Limited, grant number 2021DQ0107-1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (No. 41902176), Outstanding Youth Fund of (No. YQ2021D005), University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (No. UNPYSCT-2020145), Heilongjiang Postdoctoral Financial Assistance (No. LBH-Z19121), and Youth Science Fund of Northeast Petroleum University (No. 2018QNL-24).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Blunden, J.; Arndt, D.S. State of the Climate in 2016. Bull. Am. Meteorol. Soc. 2017, 98, S1–S280. [Google Scholar] [CrossRef]
- Chabangu, N.; Beck, B.; Hicks, N.; Botha, G.; Viljoen, J.; Davids, S.; Cloete, M. The investigation of CO2 storage potential in the Zululand Basin in South Africa. Energy Procedia 2014, 63, 2789–2799. [Google Scholar] [CrossRef]
- Zhao, W.Z.; Su, X.B.; Xia, D.P.; Hou, S.H.; Wang, Q.; Zhou, Y.X. Enhanced coalbed methane recovery by the modification of coal reservoir under the supercritical CO2 extraction and anaerobic digestion. Energy 2022, 259, 124914. [Google Scholar] [CrossRef]
- Li, Y.; Pan, S.Q.; Ning, S.Z.; Shao, L.Y.; Jing, Z.H.; Wang, Z.S. Coal measure metallogeny: Metallogenic system and implication for resource and environment. Sci. China Earth Sci. 2022, 65, 1211–1228. [Google Scholar] [CrossRef]
- White, C.M.; Smith, D.H.; Jones, K.L.; Goodman, L.A.; Jikich, S.A.; LaCount, R.B.; DuBose, S.B.; Ozdemir, E.; Morsi, B.I.; Schroeder, K.T. Sequestration of carbon dioxide in coal with enhanced coalbed methane recovery: A review. Energy Fuels 2005, 19, 659–724. [Google Scholar] [CrossRef]
- Orr, F.M., Jr. Onshore Geologic Storage of CO2. Science 2009, 325, 1656–1658. [Google Scholar] [CrossRef]
- Holliday, D.W.; Williams, G.M.; Holloway, S.; Savage, D.; Bannon, M.P. A Preliminary Feasibility Study for the Underground Disposal of Carbon Dioxide in the UK; British Geological Survey: Nottingham, UK, 1991; p. 24. [Google Scholar]
- Heidaryan, E.; Hatami, T.; Rahimi, M.; Moghadasi, J. Viscosity of pure carbon dioxide at supercritical region: Measurement and correlation approach. J. Supercrit. Fluids 2011, 56, 144–151. [Google Scholar] [CrossRef]
- Jarrahian, A.; Heidaryan, E. A novel correlation approach to estimate thermal conductivity of pure carbon dioxide in the supercritical region. J. Supercrit. Fluids 2012, 64, 39–45. [Google Scholar] [CrossRef]
- Heidaryan, E.; Jarrahian, A. Modified Redlich-Kwong equation of state for supercritical carbon dioxide. J. Supercrit. Fluids 2013, 81, 92–98. [Google Scholar] [CrossRef]
- Kolak, J.J.; Burruss, R.C. The use of solvent extractions and solubility theory to discern hydrocarbon associations in coal, with application to the coal-supercritical CO2 system. Org. Geochem. 2014, 73, 56–69. [Google Scholar] [CrossRef]
- Ren, J.G.; Niu, Q.H.; Wang, Z.Z.; Wang, W.; Yuan, W.; Weng, H.B.; Sun, H.W.; Li, Y.C.; Du, Z.G. CO2 adsorption/desorption, induced deformation behavior, and permeability characteristics of different rank coals: Application for CO2-Enhanced coalbed methane recovery. Energy Fuels 2022, 36, 5709–5722. [Google Scholar] [CrossRef]
- Liu, X.Q.; Li, M.J.; Zhang, C.H.; Fang, R.H.; Zhong, N.N.; Xue, Y.; Zhou, Y.; Jiang, W.D.; Chen, X.Y. Mechanistic insight into the optimal recovery efficiency of CBM in sub-bituminous coal: Through molecular simulation. Fuel 2020, 266, 117137. [Google Scholar] [CrossRef]
- Sampath, K.H.S.M.; Sin, I.; Perera, M.S.A.; Matthai, S.K.; Ranjith, P.G.; Li, D.Y. Effect of supercritical-CO2 interaction time on the alterations in coal pore structure. J. Nat. Gas Sci. Eng. 2020, 76, 103214. [Google Scholar] [CrossRef]
- Chen, K.; Liu, X.F.; Wang, L.K.; Song, D.Z.; Nie, B.S.; Yang, T. Influence of sequestered supercritical CO2 treatment on the pore size distribution of coal across the rank range. Fuel 2021, 306, 121708. [Google Scholar] [CrossRef]
- Gao, S.S.; Jia, L.L.; Zhou, Q.J.; Cheng, H.F.; Wang, Y. Microscopic pore structure changes in coal induced by a CO2-H2O reaction system. J. Pet. Sci. Eng. 2022, 208, 109361. [Google Scholar] [CrossRef]
- Zhang, G.L.; Ranjith, P.G.; Li, Z.S.; Vongsvivut, J.; Gao, M.Z. Application of synchrotron ATR-FTIR microspectroscopy for chemical characterization of bituminous coals treated with supercritical CO2. Fuel 2021, 296, 120639. [Google Scholar] [CrossRef]
- Zhang, G.L.; Ranjith, P.G.; Lyu, Q. Direct evidence of CO2 softening effects on coal using nanoindentation. Energy 2022, 254, 124221. [Google Scholar] [CrossRef]
- Mirzaeian, M.; Hall, P.J.; Jirandehi, H.F. Study of structural change in Wyodak coal in high-pressure CO2 by small angle neutron scattering. J. Mater. Sci. 2010, 45, 5271–5281. [Google Scholar] [CrossRef]
- Liu, S.Q.; Sang, S.X.; Ma, J.S.; Wang, T.; Du, Y.; Fang, H.H. Effects of supercritical CO2 on micropores in bituminous and anthracite coal. Fuel 2019, 242, 96–108. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Z.C.; Xu, Y.; Fu, X.; Li, W.; Zhang, D.F. Impacts of long-term exposure to supercritical carbon dioxide on physicochemical properties and adsorption and desorption capabilities of moisture-equilibrated coals. Enegry Fuels 2021, 35, 12270–12287. [Google Scholar] [CrossRef]
- Zhang, D.F.; Li, C.; Zhangm, J.; Lun, Z.M.; Jia, S.Q.; Luo, C.J.; Jiang, W.P. Influences of dynamic entrainer-blended supercritical CO2 fluid exposure on high-pressure methane adsorption on coals. J. Nat. Gas Sci. Eng. 2019, 66, 180–191. [Google Scholar] [CrossRef]
- Mastalerz, M.; Drobniak, A.; Walker, R.; Morse, D. Coal lithotypes before and after saturation with CO2: Insights from micro- and mesoporosity, fluidity, and functional group distribution. Int. J. Coal Geol. 2010, 83, 467–474. [Google Scholar] [CrossRef]
- Sha, F.; Deng, B.Z.; Yin, G.Z.; Zhang, D.M.; Li, M.H.; Liu, P.; Liu, C. Kinetic behavior of heterogeneous sorption deformation on coal: Effect of maceral/micro-lithotype distribution. Int. J. Coal Geol. 2019, 216, 103324. [Google Scholar] [CrossRef]
- Mastalerz, M.; Drobniak, A.; Rupp, J. Meso- and micropore characteristics of coal lithotypes: Implications for CO2 adsorption. Energy Fuels 2008, 22, 4049–4061. [Google Scholar] [CrossRef]
- Cao, X.Y.; Mastalerz, M.; Chappell, M.A.; Miller, L.F.; Li, Y.; Mao, J.D. Chemical structures of coal lithotypes before and after CO2 adsorption as investigated by advanced solid-state 13C nuclear magnetic resonance spectroscopy. Int. J. Coal Geol. 2011, 88, 67–74. [Google Scholar] [CrossRef]
- Karacan, C.O.; Mitchell, G.D. Behavior and effect of different coal microlithotypes during gas transport for carbon dioxide sequestration into coal seams. Int. J. Coal Geol. 2003, 53, 201–217. [Google Scholar] [CrossRef]
- Maphala, T.; Wagner, N.J. Effects of CO2 storage in coal on coal properties. Energy Procedia 2012, 23, 426–438. [Google Scholar] [CrossRef]
- Mavhengere, P.; Maphala, T.; Wagner, N. Physical and structural effects of carbon dioxide storage on vitrinite-rich coal particles under subcritical and supercritical conditions. Int. J. Coal Geol. 2015, 150–151, 1–6. [Google Scholar] [CrossRef]
- Naveen, P.; Asif, M.; Ojha, K.; Panigrahi, D.C.; Vuthaluru, H.B. Sorption kinetics of CH4 and CO2 diffusion in coal theoretical and experimental study. Energy Fuels 2017, 31, 6825–6837. [Google Scholar] [CrossRef]
- Song, Y.; Quan, F.K.; Yuan, J.H. Diffusion of guest molecules in coal: Insights from simulation. Fuel 2022, 323, 124295. [Google Scholar]
- Pan, J.Z.; Connell, L.D.; Camilleri, M.; Connelly, L. Effects of matrix moisture on gas diffusion and flow in coal. Fuel 2003, 82, 1219–1229. [Google Scholar] [CrossRef]
- Bai, J.J.; Kang, Y.L.; Chen, M.J.; Liang, L.; You, L.J.; Li, X.C. Investigation of multi-gas transport behavior in shales via a pressure pulse method. Chem. Eng. J. 2019, 360, 1667–1677. [Google Scholar] [CrossRef]
- Liu, L.F.; Wang, Y.H.; Aryana, S.A. Insights into scale translation of methane transport in nanopores. J. Nat. Gas Sci. Eng. 2021, 96, 104220. [Google Scholar] [CrossRef]
- Liu, L.F.; Nieto-Draghi, C.; Lachet, V.; Heidaryan, E.; Aryana, S. A. Bridging confined phase behavior of CH4-CO2 binary systems across scales. J. Supercrit. Fluids 2022, 189, 105713. [Google Scholar] [CrossRef]
- Keshavarz, A.; Sakurovs, R.; Grigore, M.; Sayyafzadeh, M. Effect of maceral composition and coal rank on gas diffusion in Australian coals. Int. J. Coal Geol. 2017, 173, 65–75. [Google Scholar] [CrossRef]
- Guang, W.F.; Liu, X.Q.; Zhang, Z.Y.; Luo, P. Effects of sub- and supercritical CO2 on coal diffusivity and surface thermodynamics. Energy Fuels 2022, 36, 3737–3748. [Google Scholar] [CrossRef]
- Goodman, A.L.; Favors, R.N.; Larsen, J.W. Argonne coal structure rearrangement caused by sorption of CO2. Energy Fuels 2006, 20, 2537–2543. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Li, W.; Zhang, D.F.; Wang, H.H.; Jiang, W.P.; Zhu, L.; Tao, J.; Huo, P.L.; Zhang, J. Influence of CO2 exposure on adsorption kinetics of methane and CO2 on coals. J. Nat. Gas Sci. Eng. 2016, 34, 811–822. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.F.; Song, X.X. Multifractal analysis of Hg pore size distributions of tectonically deformed coals. Int. J. Coal Geol. 2015, 144–145, 138–152. [Google Scholar] [CrossRef]
- Unsworth, J.F.; Fowler, C.S.; Jones, L.F. Moisture in coal 2. Maceral effects on pore structure. Fuel 1989, 68, 18–26. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.F.; Song, X.X. Influence of fluid exposure on surface chemistry and pore-fracture morphology of various rank coals: Implications for methane recovery and CO2 storage. Energy Fuels 2017, 31, 12552–12569. [Google Scholar] [CrossRef]
- Busch, A.; Gensterblum, Y.; Krooss, B.M.; Littke, R. Methane and carbon dioxide adsorption-diffusion experiments on coal: Upscaling and modeling. Int. J. Coal Geol. 2004, 60, 151–168. [Google Scholar] [CrossRef]
- Wang, H.B.; Li, T.; Zou, Q.L.; Cheng, Z.H.; Yang, Z.K. Influences of path control effects on characteristics of gas migration in a coal reservoir. Fuel 2020, 267, 117212. [Google Scholar] [CrossRef]
- Goodman, A.L.; Busch, A.; Duffy, G.J.; Fitzgerald, J.E.; Gasem, K.A.M.; Gensterblum, Y.; Krooss, B.M.; Levy, J.; Ozdemir, E.; Pan, Z.; et al. An inter-laboratory comparison of CO2 isotherms measured on Argonne premium coal samples. Energy Fuels 2004, 18, 1175–1182. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Weitzsacker, C.L.; Gardella, J.A., Jr. Quantitative electron spectroscopic analysis of the surface chemistry of bituminous coals. Energy Fuels 1996, 10, 141–148. [Google Scholar] [CrossRef]
- Wanger, W.; Span, R. Special equations of state for methane, argon, and nitrogen for the temperature range from 270 to 350 K at pressures up to 30 MPa. Int. J. Thermophys. 1993, 14, 699–725. [Google Scholar]
- Span, R.; Wanger, W. A New equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data 1996, 25, 1509–1596. [Google Scholar] [CrossRef]
- Zhang, D.F.; Cui, Y.J.; Liu, B.; Li, S.G.; Song, W.L.; Lin, W.G. Supercritical pure methane and CO2 adsorption on various rank coals of China: Experiments and modeling. Energy Fuels 2011, 25, 1891–1899. [Google Scholar] [CrossRef]
- Harpalani, S.; Prusty, B.K.; Dutta, P. Methane/CO2 sorption modeling for coalbed methane production and CO2 sequestration. Energy Fuels 2006, 20, 1591–1599. [Google Scholar] [CrossRef]
- Siemons, N.; Busch, A. Measurement and interpretation of supercritical CO2 sorption on various coals. Int. J. Coal Geol. 2007, 69, 229–242. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Bustin, R.M. The effect of pore structure and gas pressure upon the transport properties of coal: A laboratory and modeling study. 2. Adsorption rate modeling. Fuel 1999, 78, 1345–1362. [Google Scholar] [CrossRef]
- Cui, X.J.; Bustin, R.M.; Dipple, G. Selective transport of CO2, CH4, and N2 in coals: Insights from modeling of experimental gas adsorption data. Fuel 2004, 83, 293–303. [Google Scholar] [CrossRef]
- Sakurovs, R.; Day, S.; Weir, S. Relationships between the critical properties of gases and their high pressure sorption behavior on coals. Energy Fuels 2010, 24, 1781–1787. [Google Scholar] [CrossRef]
- Wei, X.R.; Wand, G.X.; Massarotto, P.; Golding, S.D.; Rudolph, V. Numerical simulation of multicomponent gas diffusion and flow in coals for CO2 enhanced coalbed methane recovery. Chem. Eng. Sci. 2007, 62, 4193–4203. [Google Scholar] [CrossRef]
- Gensterblum, Y.; Busch, A.; Krooss, B.M. Molecular concept and experimental evidence of competitive adsorption of H2O, CO2 and CH4 on organic material. Fuel 2014, 115, 581–588. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wilcox, J. Molecular simulation studies of CO2 adsorption by carbon model compounds for carbon capture and sequestration applications. Environ. Sci. Technol. 2013, 47, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.J.; Kang, Y.L.; Chen, M.J.; Chen, Z.X.; You, L.J.; Li, X.C.; Chen, G. Impact of surface chemistry and pore structure on water vapor adsorption behavior in gas shale. Chem. Eng. J. 2020, 402, 126238. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.H.; Pan, Z.J.; Tong, W.S. Nanoscale pore structure and mechanical property analysis of coal: An insight combining AFM and SEM images. Fuel 2020, 260, 116352. [Google Scholar] [CrossRef]
- Liu, H.H.; Mou, J.H.; Cheng, Y.P. Impact of pore structure on gas adsorption and diffusion dynamics for long-flame coal. J. Nat. Gas Sci. Eng. 2015, 22, 203–213. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wilcox, J. Molecular simulation of CO2 adsorption in micro- and mesoporous carbons with surface heterogeneity. Int. J. Coal Geol. 2012, 104, 83–95. [Google Scholar] [CrossRef]
- Liu, C.J.; Sang, S.X.; Zhang, K.; Song, F.; Wang, H.W.; Fan, X.F. Effects of temperature and pressure on pore morphology of different rank coals: Implications for CO2 geological storage. J. CO2 Util. 2019, 34, 343–352. [Google Scholar] [CrossRef]
- Kelemen, S.R.; Afeworki, M.; Gorbaty, M.L. Characterization of organically bound oxygen forms in lignites, peats, and pyrolyzed peats by X-ray photoelectron spectroscopy (XPS) and solid-state 13C NMR methods. Energy Fuels 2002, 16, 1450–1462. [Google Scholar] [CrossRef]
- Kelemen, S.R.; Kwiatek, P.J. Quantification of organic oxygen species on the surface of fresh and reacted argonne premium coal. Energy Fuels 1995, 9, 841–848. [Google Scholar] [CrossRef]
- Huang, X.; Chu, W.; Sun, W.J.; Jiang, C.F.; Feng, Y.Y.; Xue, Y. Investigation of oxygen-containing group promotion effect on CO2-coal interaction by density functional theory. Appl. Surf. Sci. 2014, 299, 162–169. [Google Scholar] [CrossRef]
- Mirzaeian, M.; Hall, P.J. The interactions of coal with CO2 and its effects on coal structure. Energy Fuels 2006, 20, 2022–2027. [Google Scholar] [CrossRef]
- Hao, S.X.; Wen, J.; Yu, X.P.; Chu, W. Effect of the surface oxygen groups on methane adsorption on coals. Appl. Surf. Sci. 2013, 264, 433–442. [Google Scholar] [CrossRef]
- Cuervo, M.R.; Asedegbega-Nieto, E.; Díaz, E.; Ordóñeza, S.; Vega, A.; Dongil, A.B.; Rodríguez-Ramos, I. Modification of the adsorption properties of high surface area graphites by oxygen functional groups. Carbon 2008, 46, 2096–2106. [Google Scholar] [CrossRef]
- Lu, X.Q.; Jin, D.L.; Wei, S.X.; Zhang, M.M.; Zhu, Q.; Shi, X.F.; Deng, Z.G.; Guo, W.Y.; Shen, W.Z. Competitive adsorption of a binary CO2-CH4 mixture in nanoporous carbons: Effects of edge-functionalization. Nanoscale 2015, 7, 1002–1012. [Google Scholar] [CrossRef]
- Prinz, D.; Pyckhout-Hintzen, W.; Littke, R. Development of the meso- and macroporous structure of coals with rank as analysed with small angle neutron scattering and adsorption experiments. Fuel 2004, 83, 547–556. [Google Scholar] [CrossRef]
- Bae, J.-S.; Bhatia, S.K.; Rudolph, V.; Massarotto, P. Pore accessibility of methane and carbon dioxide in coals. Energy Fuels 2009, 23, 3319–3327. [Google Scholar] [CrossRef]
- Zhou, F.B.; Liu, S.Q.; Pang, Y.Q.; Li, J.L.; Xin, H.H. Effects of coal functional groups on adsorption microheat of coal bed methane. Energy Fuels 2015, 29, 1550–1557. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.B.; Wang, J.; Pan, Z.J. Variation in permeability during CO2-CH4 displacement in coal seams: Part 1—Experimental insights. Fuel 2020, 263, 116666. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).