Free-Base Porphyrin Aggregates Combined with Nickel Phosphite for Enhanced Alkaline Hydrogen Evolution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Electrode Manufacturing Procedure
2.3. Electrochemical Experiments
2.4. Morphostructural Characterization
3. Results
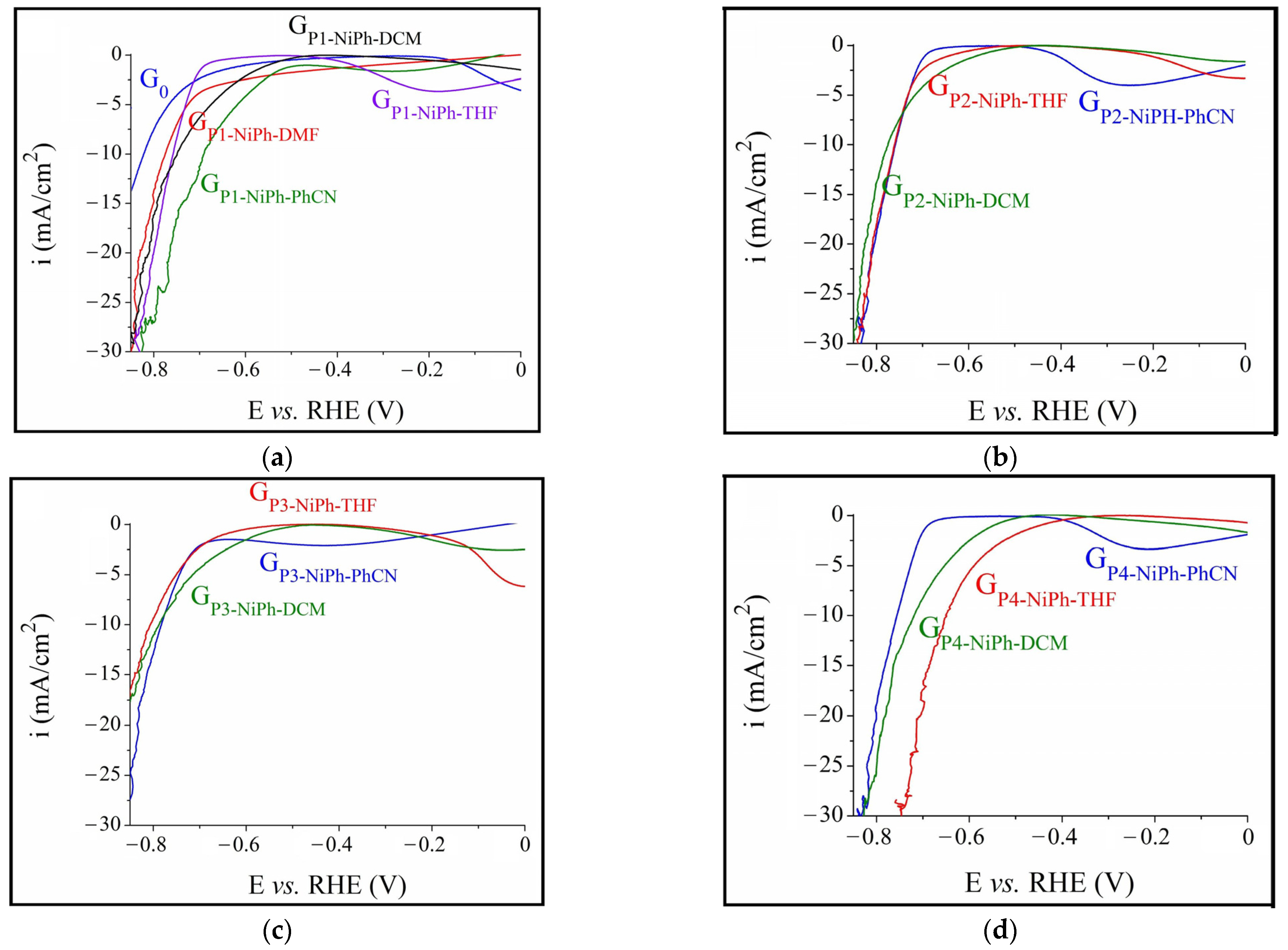
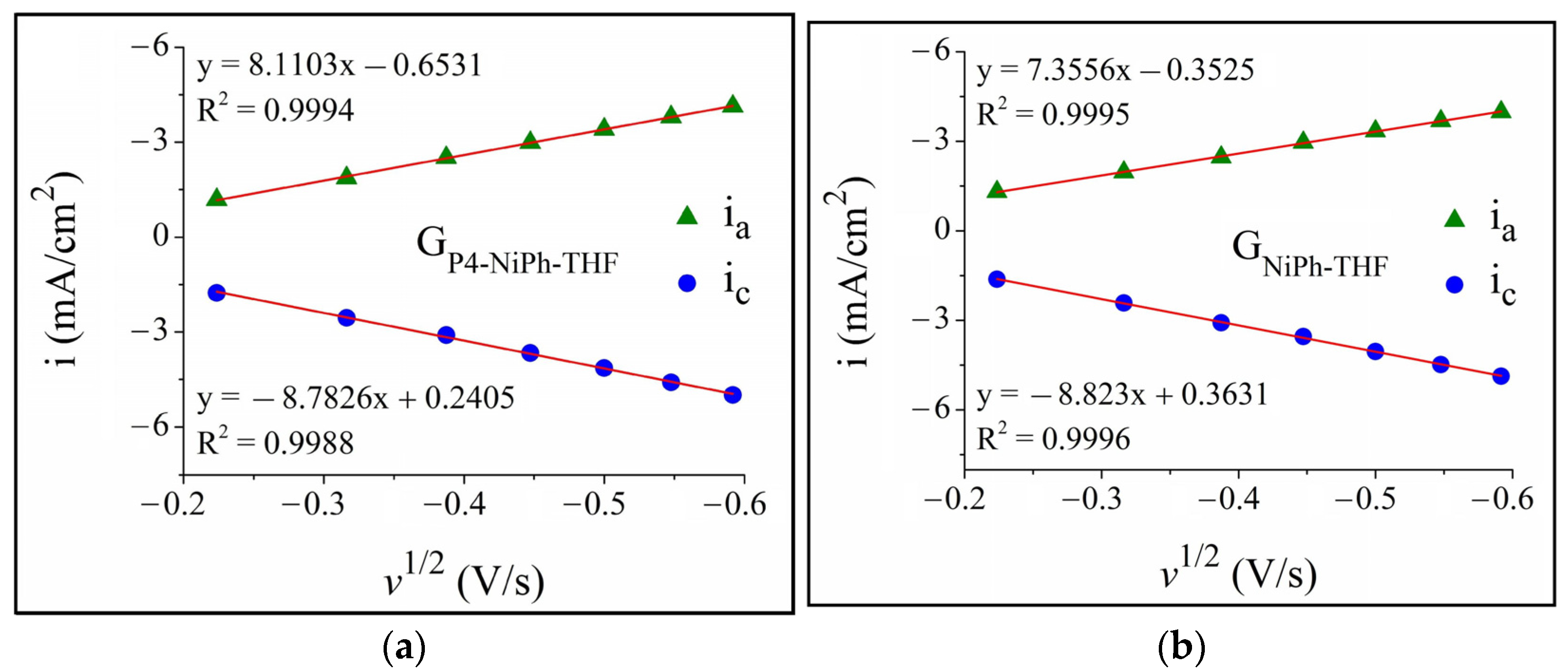
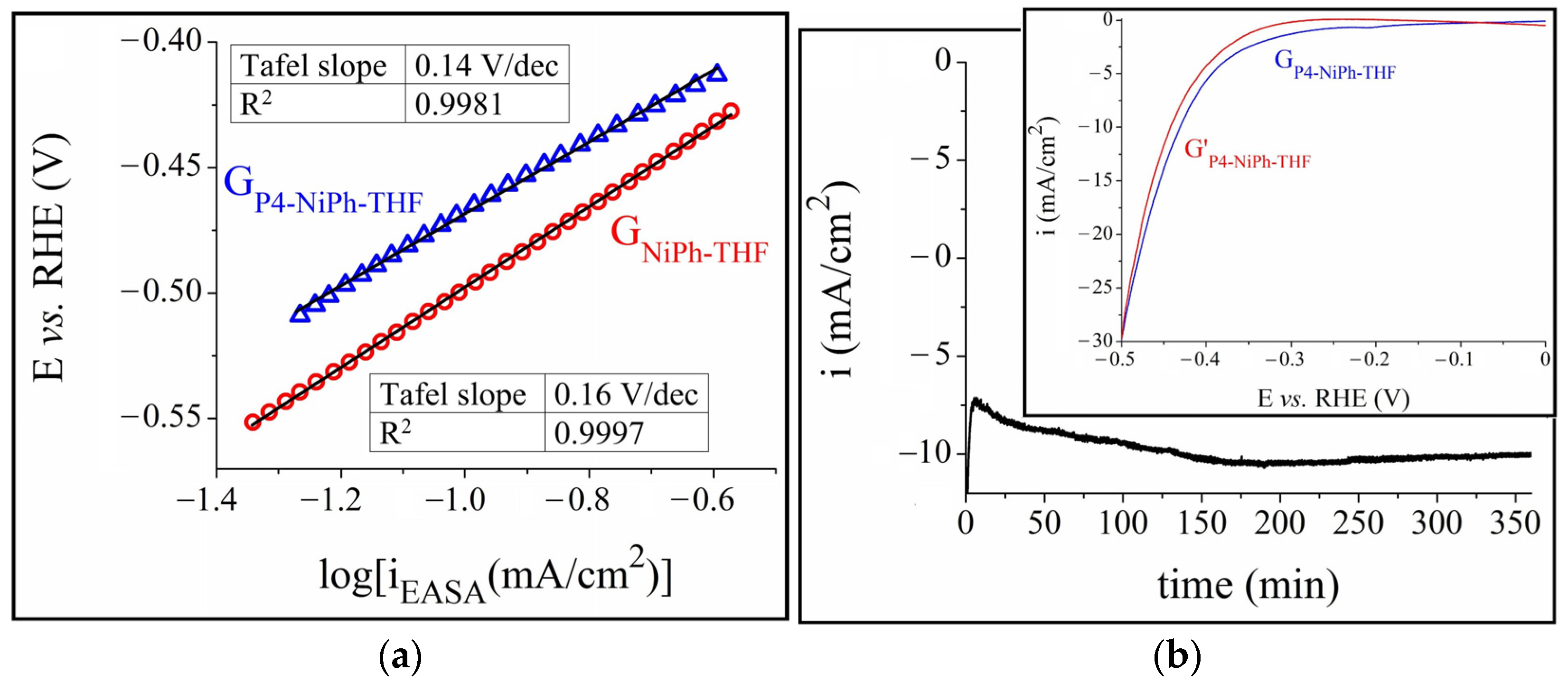
3.1. A-HER Electrocatalytic Activity of the Modified Electrodes
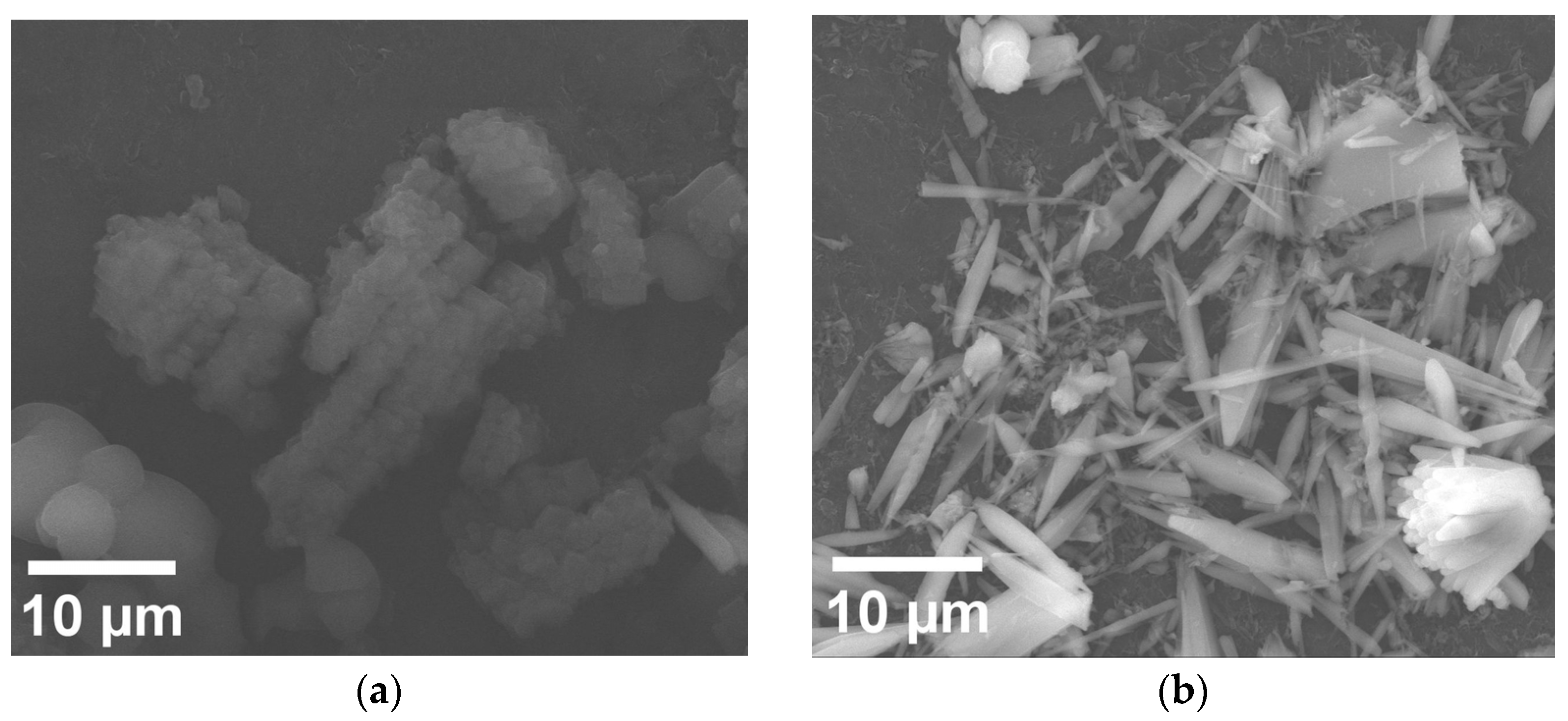
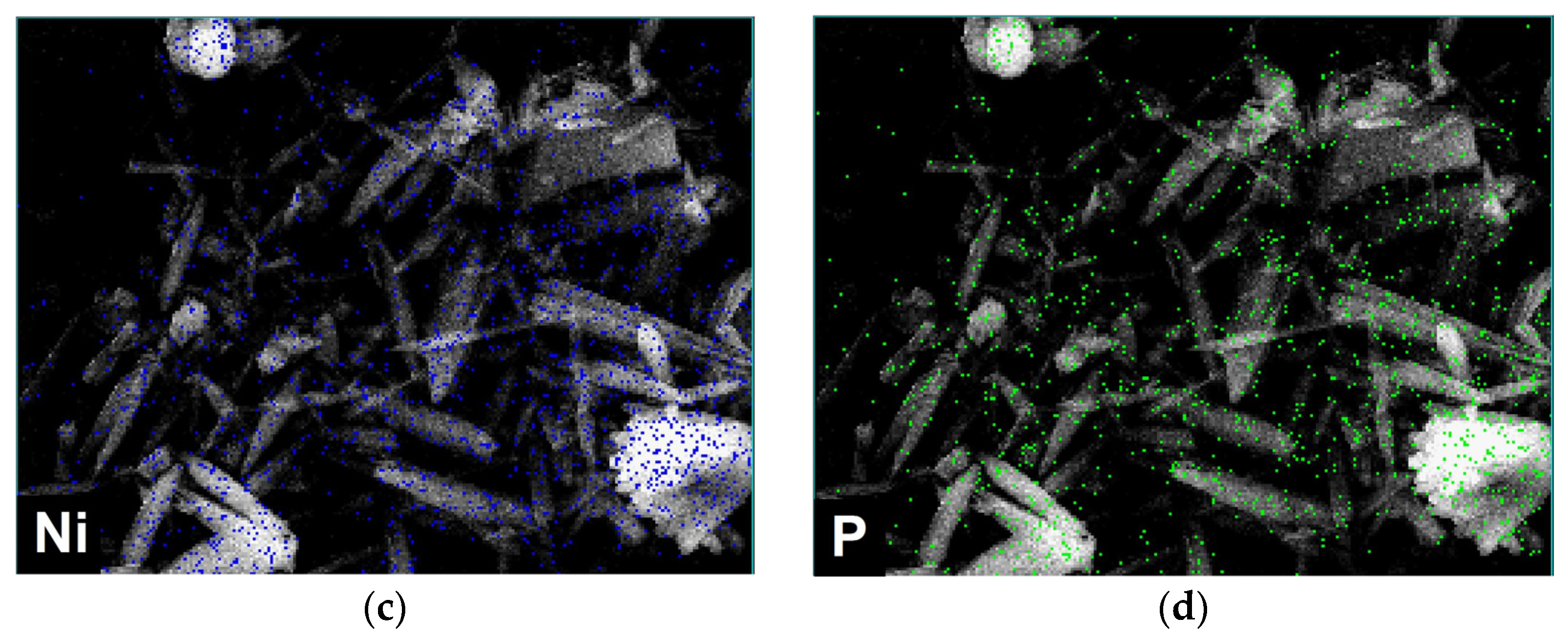
3.2. Morphostructural Study
3.3. Additional Considerations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, M.; Liu, D.; Zi, B.; Chen, Y.; Liu, D.; Du, X.; Li, F.; Zhou, P.; Ke, Y.; Li, J.; et al. Remarkable Synergistic Effect in Cobalt-Iron Nitride/Alloy Nanosheets for Robust Electrochemical Water Splitting. J. Energy Chem. 2022, 65, 405–414. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, P.; Lin, J.; Cao, J.; Qi, J. Modification Strategies on Transition Metal-Based Electrocatalysts for Efficient Water Splitting. J. Energy Chem. 2021, 58, 446–462. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Qin, Y.; Zhang, W.; Wang, L.; Luo, M.; Yang, H.; Guo, S. Recent Advances on Water-Splitting Electrocatalysis Mediated by Noble-Metal-Based Nanostructured Materials. Adv. Energy Mater. 2020, 10, 1903120. [Google Scholar] [CrossRef]
- Crabtree, G.W.; Dresselhaus, M.S. The Hydrogen Fuel Alternative. MRS Bull. 2008, 33, 421–428. [Google Scholar] [CrossRef]
- Hota, P.; Das, A.; Maiti, D.K. A Short Review on Generation of Green Fuel Hydrogen through Water Splitting. Int. J. Hydrog. Energy 2023, 48, 523–541. [Google Scholar] [CrossRef]
- Chakraborty, B.; Beltrán-Suito, R.; Hausmann, J.N.; Garai, S.; Driess, M.; Menezes, P.W. Enabling Iron-Based Highly Effective Electrochemical Water-Splitting and Selective Oxygenation of Organic Substrates through In Situ Surface Modification of Intermetallic Iron Stannide Precatalyst. Adv. Energy Mater. 2020, 10, 2001377. [Google Scholar] [CrossRef]
- Li, X.P.; Huang, C.; Han, W.K.; Ouyang, T.; Liu, Z.Q. Transition Metal-Based Electrocatalysts for Overall Water Splitting. Chin. Chem. Lett. 2021, 32, 2597–2616. [Google Scholar] [CrossRef]
- Li, S.; Li, E.; An, X.; Hao, X.; Jiang, Z.; Guan, G. Transition Metal-Based Catalysts for Electrochemical Water Splitting at High Current Density: Current Status and Perspectives. Nanoscale 2021, 13, 12788–12817. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, J.; Li, X.; Mu, S.; Verpoort, F.; Xue, J.; Kou, Z.; Wang, J. Nurturing the Marriages of Single Atoms with Atomic Clusters and Nanoparticles for Better Heterogeneous Electrocatalysis. Interdiscip. Mater. 2022, 1, 51–87. [Google Scholar] [CrossRef]
- Wang, T.; Wang, P.; Zang, W.; Li, X.; Chen, D.; Kou, Z.; Mu, S.; Wang, J. Nanoframes of Co3O4–Mo2N Heterointerfaces Enable High-Performance Bifunctionality toward Both Electrocatalytic HER and OER. Adv. Funct. Mater. 2022, 32, 2107382. [Google Scholar] [CrossRef]
- Si, C.; Zhang, W.; Lu, Q.; Guo, E.; Yang, Z.; Chen, J.; He, X.; Luo, J. Recent Advances in Perovskite Catalysts for Efficient Overall Water Splitting. Catalysts 2022, 12, 601. [Google Scholar] [CrossRef]
- Raja, D.S.; Lin, H.W.; Lu, S.Y. Synergistically Well-Mixed MOFs Grown on Nickel Foam as Highly Efficient Durable Bifunctional Electrocatalysts for Overall Water Splitting at High Current Densities. Nano Energy 2019, 57, 1–13. [Google Scholar] [CrossRef]
- Wan, W.; Wei, S.; Li, J.; Triana, C.A.; Zhou, Y.; Patzke, G.R. Transition Metal Electrocatalysts Encapsulated into N-Doped Carbon Nanotubes on Reduced Graphene Oxide Nanosheets: Efficient Water Splitting through Synergistic Effects. J. Mater. Chem. A 2019, 7, 15145–15155. [Google Scholar] [CrossRef]
- Li, W.; Wang, C.; Lu, X. Integrated Transition Metal and Compounds with Carbon Nanomaterials for Electrochemical Water Splitting. J. Mater. Chem. A 2021, 9, 3786–3827. [Google Scholar] [CrossRef]
- Ge, Y.; Lyu, Z.; Marcos-Hernández, M.; Villagrán, D. Free-Base Porphyrin Polymer for Bifunctional Electrochemical Water Splitting. Chem. Sci. 2022, 13, 8597–8604. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Lee, K.; Min, M.; Cho, Y.; Kim, M.; Lee, H. A Molecular Approach to an Electrocatalytic Hydrogen Evolution Reaction on Single-Layer Graphene. Nanoscale 2017, 9, 3969–3979. [Google Scholar] [CrossRef] [PubMed]
- Fagadar-Cosma, E.; Vlascici, D.; Fagadar-Cosma, G. Porfirinele de la Sinteză la Aplicații; Eurostampa: Timisoara, Romania, 2008; ISBN 978-973-687-680-6. [Google Scholar]
- Král, V.; Králová, J.; Kaplánek, R.; Bříza, T.; Martásek, P. Quo Vadis Porphyrin Chemistry? Physiol. Res. 2006, 55, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, F.; Linhardt, R.J. Porphyrin-Based Compounds and Their Applications in Materials and Medicine. Dye. Pigment. 2021, 188, 109136. [Google Scholar] [CrossRef]
- Wei, W.; Zhao, Z.X.; Xia, B.H.; Li, W. Theoretical Analysis of Expanded Porphyrins: Aromaticity, Stability, and Optoelectronic Properties. Front. Chem. 2022, 10, 1–10. [Google Scholar] [CrossRef]
- Magna, G.; Monti, D.; Di Natale, C.; Paolesse, R.; Stefanelli, M. The Assembly of Porphyrin Systems in Well-Defined Nanostructures: An Update. Molecules 2019, 24, 4307. [Google Scholar] [CrossRef]
- Martin, K.E.; Wang, Z.; Busani, T.; Garcia, R.M.; Chen, Z.; Jiang, Y.; Song, Y.; Jacobsen, J.L.; Vu, T.T.; Schore, N.E.; et al. Donor-Acceptor Biomorphs from the Ionic Self-Assembly of Porphyrins. J. Am. Chem. Soc. 2010, 132, 8194–8201. [Google Scholar] [CrossRef] [PubMed]
- Birdeanu, M.; Fagadar-Cosma, E. The Self-Assembly of Porphyrin Derivatives into 2D and 3D Architectures. In Quantum Nanosystems: Structure, Properties and Interactions; Putz, M.V., Ed.; Apple Academic Press: Toronto, ON, Canada, 2014; pp. 173–206. ISBN 9781774633144. [Google Scholar]
- Zhang, W.; Lai, W.; Cao, R. Energy-Related Small Molecule Activation Reactions: Oxygen Reduction and Hydrogen and Oxygen Evolution Reactions Catalyzed by Porphyrin- and Corrole-Based Systems. Chem. Rev. 2017, 117, 3717–3797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, L.; Wang, H.; Cao, R.; Wang, J.; Bai, F.; Fan, H. Self-Assembled One-Dimensional Porphyrin Nanostructures with Enhanced Photocatalytic Hydrogen Generation. Nano Lett. 2018, 18, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Beyene, B.B.; Hung, C.H. Recent Progress on Metalloporphyrin-Based Hydrogen Evolution Catalysis. Coord. Chem. Rev. 2020, 410, 213234. [Google Scholar] [CrossRef]
- Yao, B.; He, Y.; Wang, S.; Sun, H.; Liu, X. Recent Advances in Porphyrin-Based Systems for Electrochemical Oxygen Evolution Reaction. Int. J. Mol. Sci. 2022, 23, 6036. [Google Scholar] [CrossRef] [PubMed]
- Keshipour, S.; Asghari, A. A Review on Hydrogen Generation by Phthalocyanines. Int. J. Hydrog. Energy 2022, 47, 12865–12881. [Google Scholar] [CrossRef]
- Chen, L.; Sagar, R.U.R.; Chen, J.; Liu, J.; Aslam, S.; Nosheen, F.; Anwar, T.; Hussain, N.; Hou, X.; Liang, T. Cobalt Phthalocyanine as an Efficient Catalyst for Hydrogen Evolution Reaction. Int. J. Hydrog. Energy 2021, 46, 19338–19346. [Google Scholar] [CrossRef]
- Li, Q.; Bao, Y.; Bai, F. Porphyrin and Macrocycle Derivatives for Electrochemical Water Splitting. MRS Bull. 2020, 45, 569–573. [Google Scholar] [CrossRef]
- Wang, N.; Lei, H.; Zhang, Z.; Li, J.; Zhang, W.; Cao, R. Electrocatalytic Hydrogen Evolution with Gallium Hydride and Ligand-Centered Reduction. Chem. Sci. 2019, 10, 2308–2314. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Q.; Dou, Y.; Sudi, M.S.; Zhu, W.; Shang, D.; Li, L. Porphyrin Coordination Polymer Supported Transition-Metal Sulfides as Precious-Metal-Free Electrocatalysts for Efficient Overall Water Splitting. Dye. Pigment. 2022, 206, 110620. [Google Scholar] [CrossRef]
- Wang, Y.; Song, D.; Li, J.; Shi, Q.; Zhao, J.; Hu, Y.; Zeng, F.; Wang, N. Covalent Metalloporphyrin Polymer Coated on Carbon Nanotubes as Bifunctional Electrocatalysts for Water Splitting. Inorg. Chem. 2022, 61, 10198–10204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.; Wang, Y.; Yang, S.; Wu, X.; Lv, B.; Wang, N.; Gao, Y.; Xu, X.; Lei, H.; et al. Electropolymerization of Cobalt Porphyrins and Corroles for the Oxygen Evolution Reaction. Chin. Chem. Lett. 2021, 32, 3807–3810. [Google Scholar] [CrossRef]
- Menezes, P.W.; Indra, A.; Das, C.; Walter, C.; Göbel, C.; Gutkin, V.; Schmeißer, D.; Driess, M. Uncovering the Nature of Active Species of Nickel Phosphide Catalysts in High-Performance Electrochemical Overall Water Splitting. ACS Catal. 2017, 7, 103–109. [Google Scholar] [CrossRef]
- Menezes, P.W.; Panda, C.; Loos, S.; Bunschei-Bruns, F.; Walter, C.; Schwarze, M.; Deng, X.; Dau, H.; Driess, M. A Structurally Versatile Nickel Phosphite Acting as a Robust Bifunctional Electrocatalyst for Overall Water Splitting. Energy Environ. Sci. 2018, 11, 1287–1298. [Google Scholar] [CrossRef]
- Taranu, B.-O.; Ivanovici, M.G.; Svera, P.; Vlazan, P.; Sfirloaga, P.; Poienar, M. Ni11□ (HPO3)8(OH)6 Multifunctional Materials: Electrodes for Oxygen Evolution Reaction and Potential Visible-Light Active Photocatalysts. J. Alloy. Compd. 2020, 848, 156595. [Google Scholar] [CrossRef]
- Poienar, M.; Svera, P.; Taranu, B.-O.; Ianasi, C.; Sfirloaga, P.; Buse, G.; Veber, P.; Vlazan, P. Electrochemical Investigation of the OER Activity for Nickel Phosphite-Based Compositions and Its Morphology-Dependent Fluorescence Properties. Crystals 2022, 12, 1803. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Fagadar-Cosma, G.; Vasile, M.; Enache, C. Synthesis, Spectroscopic and Self-Assembling Characterization of Novel Photoactive Mixed Aryl-Substituted Porphyrin. Curr. Org. Chem. 2012, 16, 931–941. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Lascu, A.; Palade, A.; Creanga, I.; Birdeanu, M. Hybrid Material Based on 5-(4-Pyridyl)-10,15,20-Tris(4-Phenoxyphenyl)-Porphyrin and Gold Colloid for CO2 Detection. Dig. J. Nanomater. Biostruct. 2016, 11, 419–424. [Google Scholar]
- Birdeanu, A.V.; Birdeanu, M.; Fagadar-Cosma, E. Corrosion Protection Characteristics of Ceramics, Porphyrins and Hybrid Ceramics/Porphyrins, Deposited as Single and Sandwich Layers, by Pulsed Laser Deposition (PLD). J. Alloy. Compd. 2017, 706, 220–226. [Google Scholar] [CrossRef]
- Alexandrova, R.; Kalfin, R.; Tudose, R.; Fagadar-Cosma, E. Comparative Cytotoxicity Assays Performed Using a Free Porphyrin and Its Zn(II), Co(II) and Cu(II) Complexes. Influence of Optical and Aggregation Properties. Stud. Univ. Babes-Bolyai Chem. 2018, 63, 65–77. [Google Scholar] [CrossRef]
- Salageanu, L.; Muntean, D.; George, H.F.; Lascu, A.; Anghel, D.; Bagiu, I.C.; Fagadar-Cosma, E. Antimicrobial Activity of Different Substituted Meso-Porphyrin Derivatives. Rev. Romana Med. Lab. 2020, 28, 205–216. [Google Scholar] [CrossRef]
- Anghel, D.; Lascu, A.; Epuran, C.; Fratilescu, I.; Ianasi, C.; Birdeanu, M.; Fagadar-Cosma, E. Hybrid Materials Based on Silica Matrices Impregnated with Pt-Porphyrin or PtNPs Destined for CO2 Gas Detection or for Wastewaters Color Removal. Int. J. Mol. Sci. 2020, 21, 4262. [Google Scholar] [CrossRef] [PubMed]
- Taranu, B.O.; Fagadar-Cosma, E. Catalytic Properties of Free-Base Porphyrin Modified Graphite Electrodes for Electrochemical Water Splitting in Alkaline Medium. Processes 2022, 10, 611. [Google Scholar] [CrossRef]
- Vlascici, D.; Fagadar-Cosma, E.; Popa, I.; Chiriac, V.; Gil-Agusti, M. A Novel Sensor for Monitoring of Iron(III) Ions Based on Porphyrins. Sensors 2012, 12, 8193–8203. [Google Scholar] [CrossRef]
- Vlascici, D.; Popa, I.; Chiriac, V.A.; Fagadar-Cosma, G.; Popovici, H.; Fagadar-Cosma, E. Potentiometric Detection and Removal of Copper Using Porphyrins. Chem. Cent. J. 2013, 7, 111. [Google Scholar] [CrossRef]
- Taranu, B.O.; Fagadar-Cosma, E.; Popa, I.; Plesu, N.; Taranu, I. Adsorbed Functionalized Porphyrins on Polyaniline Modified Platinum Electrodes. Comparative Electrochemical Properties. Dig. J. Nanomater. Biostruct. 2014, 9, 667–679. [Google Scholar]
- Popa, I.; Fagadar-Cosma, G.; Taranu, B.O.; Birdeanu, A.V.; Taranu, I.; Vlascici, D.; Birdeanu, M.; Fagadar-Cosma, E. Electrochemical Behavior of Tetra(4-Methoxyphenyl)Porphyrin Thin Films Obtained by Laser Deposition on Graphite Electrode. Dig. J. Nanomater. Biostruct. 2014, 9, 1277–1287. [Google Scholar]
- Popa, I.; Fagadar-Cosma, E.; Taranu, B.-O.; Birdeanu, M.; Fagadar-Cosma, G.; Taranu, I. Corrosion Protection Efficiency of Bilayer Porphyrin-Polyaniline Film Deposited on Carbon Steel. Macromol. Symp. 2015, 352, 16–24. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Tarabukina, E.; Zakharova, N.; Birdeanu, M.; Taranu, B.; Palade, A.; Creanga, I.; Lascu, A.; Fagadar-Cosma, G. Hybrids Formed between Polyvinylpyrrolidone and an A3B Porphyrin Dye: Behaviour in Aqueous Solutions and Chemical Response to CO2 Presence. Polym. Int. 2016, 65, 200–209. [Google Scholar] [CrossRef]
- Taranu, B.-O.; Vlascici, D.; Sebarchievici, I.; Fagadar-Cosma, E. The Aggregation Behavior of an A3B Free Base Porphyrin and Its Application as Chromium(III)-Selective Membrane Sensor. Stud. Univ. Babes-Bolyai Chem. 2016, 61, 199–212. [Google Scholar]
- Taranu, B.O.; Sebarchievici, L.; Taranu, I.; Birdeanu, M.; Cosma, E.F. Electrochemical and Microscopic Characterization of Two Meso-Substituted A3B and A4 Porphyrins. Rev. Chim. 2016, 67, 892–896. [Google Scholar]
- Poienar, M.; Maignan, A.; Sfirloaga, P.; Malo, S.; Vlazan, P.; Guesdon, A.; Lainé, F.; Rouquette, J.; Martin, C. Polar Space Group and Complex Magnetism in Ni11□(HPO3)8(OH)6: Towards a New Multiferroic Material? Solid State Sci. 2015, 39, 92–96. [Google Scholar] [CrossRef]
- Dudas, Z.; Enache, C.; Fagadar-Cosma, G.; Armeanu, I.; Fagadar-Cosma, E. Hybrid Silica-Porphyrin Materials with Tailored Pore Sizes. Mater. Res. Bull. 2010, 45, 1150–1156. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Enache, C.; Armeanu, I.; Dascalu, D.; Fagadar-Cosma, G.; Vasile, M.; Grozescu, I. The Influence of PH over Topography and Spectroscopic Properties of Silica Hybrid Materials Embedding Meso-Tetratolylporphyrin. Mater. Res. Bull. 2009, 44, 426–431. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Enache, C.; Tudose, R.; Armeanu, I.; Mosoarca, E.; Vlascici, D.; Costisor, O. UV-VIS and Fluorescence Spectra of Meso-Tetraphenylporphyrin and Meso-Tetrakis-(4-Methoxyphenyl) Porphyrin in THF and THF-Water Systems. The Influence of PH. Rev. Chim. 2007, 58, 451–455. [Google Scholar]
- Snyder, L.R.; Kirkland, J.J.; Glajch, J.L. Practical HPLC Method Development, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1997; Volume 3, ISBN 978-0-471-00703-6. [Google Scholar]
- Szabadai, Z.; Sbarcea, L.; Udrescu, L. Analiza Fizică Și Chimică a Medicamentului; Victor Babes Publishing House: Timisoara, Romania, 2016; ISBN 978-606-786-020-7. [Google Scholar]
- Wang, S.; Lu, A.; Zhong, C.J. Hydrogen Production from Water Electrolysis: Role of Catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Durovic, M.; Hnat, J.; Bouzek, K. Electrocatalysts for the Hydrogen Evolution Reaction in Alkaline and Neutral Media. A Comparative Review. J. Power Sources 2021, 493, 229708. [Google Scholar] [CrossRef]
- Poienar, M.; Taranu, B.-O.; Svera, P.; Sfirloaga, P.; Vlazan, P. Disclosing the Thermal Behaviour, Electrochemical and Optical Properties of Synthetic Fe3(PO4)2(OH)2 Materials. J. Therm. Anal. Calorim. 2022, 147, 11839–11855. [Google Scholar] [CrossRef]
- Fratilescu, I.; Lascu, A.; Taranu, B.O.; Epuran, C.; Birdeanu, M.; Macsim, A.-M.; Tanasa, E.; Vasile, E.; Fagadar-Cosma, E. One A3B Porphyrin Structure—Three Successful Applications. Nanomaterials 2022, 12, 1930. [Google Scholar] [CrossRef]
- Taranu, B.-O.; Fagadar-Cosma, E. The PH Influence on the Water-Splitting Electrocatalytic Activity of Graphite Electrodes Modified with Symmetrically Substituted Metalloporphyrins. Nanomaterials 2022, 12, 3788. [Google Scholar] [CrossRef]
- Sebarchievici, I.; Taranu, B.O.; Birdeanu, M.; Rus, S.F.; Fagadar-Cosma, E. Electrocatalytic Behaviour and Application of Manganese Porphyrin/Gold Nanoparticle-Surface Modified Glassy Carbon Electrodes. Appl. Surf. Sci. 2016, 390, 131–140. [Google Scholar] [CrossRef]
- Taranu, B.O.; Vlazan, P.; Racu, A. Water Splitting Studies in Alkaline Medium Using Graphite Electrodes Modified with Transition Metal Oxides and Compositions Containing Them. Stud. Univ. Babes-Bolyai Chem. 2022, 67, 79–95. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.; Liu, Y.; Shen, G.; Yu, R. Platinum Nanoparticles-Doped Sol-Gel/Carbon Nanotubes Composite Electrochemical Sensors and Biosensors. Biosens. Bioelectron. 2006, 21, 1125–1131. [Google Scholar] [CrossRef]
- Taranu, B.-O.; Vlazan, P.; Svera, P.; Poienar, M.; Sfirloaga, P. New Functional Hybrid Materials Based on Clay Minerals for Enhanced Electrocatalytic Activity. J. Alloy. Compd. 2022, 892, 162239. [Google Scholar] [CrossRef]
- Sebarchievici, I.; Lascu, A.; Fagadar-Cosma, G.; Palade, A.; Fringu, I.; Birdeanu, M.; Taranu, B.; Fagadar-Cosma, E. Optical and Electrochemical-Mediated Detection of Ascorbic Acid Using Manganese Porphyrin and Its Gold Hybrids. Comptes Rendus Chim. 2018, 21, 327–338. [Google Scholar] [CrossRef]
- Gu, Z.; Zhang, Y.; Wei, X.; Duan, Z.; Ren, L.; Ji, J.; Zhang, X.; Zhang, Y.; Gong, Q.; Wu, H.; et al. Unveiling the Accelerated Water Electrolysis Kinetics of Heterostructural Iron-Cobalt-Nickel Sulfides by Probing into Crystalline/Amorphous Interfaces in Stepwise Catalytic Reactions. Adv. Sci. 2022, 9, 2201903. [Google Scholar] [CrossRef]
- Paul, R.; Zhai, Q.; Roy, A.K.; Dai, L. Charge Transfer of Carbon Nanomaterials for Efficient Metal-free Electrocatalysis. Interdiscip. Mater. 2022, 1, 28–50. [Google Scholar] [CrossRef]
- Zhou, Z.; Zaman, W.Q.; Sun, W.; Cao, L.M.; Tariq, M.; Yang, J. Cultivating Crystal Lattice Distortion in IrO2: Via Coupling with MnO2 to Boost the Oxygen Evolution Reaction with High Intrinsic Activity. Chem. Commun. 2018, 54, 4959–4962. [Google Scholar] [CrossRef]
- Liu, Y.; Xing, Y.; Zheng, X.; Xu, S.; Li, D.; Jiang, D. Synergistically Enhancing Electrocatalytic Activity of Co2P by Cr Doping and P Vacancies for Overall Water Splitting. Appl. Surf. Sci. 2022, 600, 154099. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Lu, W. Synergistic Effect: Hierarchical Ni3S2@Co(OH)2 Heterostructure as Efficient Bifunctional Electrocatalyst for Overall Water Splitting. Appl. Surf. Sci. 2018, 457, 156–163. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Yang, L.; Cheng, D.; Cao, D. Single-Atom Ru Doping Induced Phase Transition of MoS2 and S Vacancy for Hydrogen Evolution Reaction. Small Methods 2019, 3, 1900653. [Google Scholar] [CrossRef]
- Zou, X.; Huang, X.; Goswami, A.; Silva, R.; Sathe, B.R.; Mikmeková, E.; Asefa, T. Cobalt-Embedded Nitrogen-Rich Carbon Nanotubes Efficiently Catalyze Hydrogen Evolution Reaction at All PH Values. Angew. Chem.—Int. Ed. 2014, 53, 4372–4376. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Li, S.; Wu, F.; Saqib, M.; Luque, R.; Xu, G. Unprecedented Metal-Free 3D Porous Carbonaceous Electrodes for Full Water Splitting. Energy Environ. Sci. 2016, 9, 1210–1214. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.H.; Su, H.; Lv, L.B.; Zhao, T.J.; Ge, J.M.; Wei, X.; Wang, K.X.; Li, X.H.; Chen, J.S. Nitrogen-Doped Graphene Microtubes with Opened Inner Voids: Highly Efficient Metal-Free Electrocatalysts for Alkaline Hydrogen Evolution Reaction. Nano Res. 2016, 9, 2606–2615. [Google Scholar] [CrossRef]
- Cai, G.; Zeng, L.; He, L.; Sun, S.; Tong, Y.; Zhang, J. Imine Gels Based on Ferrocene and Porphyrin and Their Electrocatalytic Property. Chem.—Asian J. 2020, 15, 1963–1969. [Google Scholar] [CrossRef]
- Wang, A.; Cheng, L.; Zhao, W.; Shen, X.; Zhu, W. Electrochemical Hydrogen and Oxygen Evolution Reactions from a Cobalt-Porphyrin-Based Covalent Organic Polymer. J. Colloid Interface Sci. 2020, 579, 598–606. [Google Scholar] [CrossRef]
- Jia, H.; Yao, Y.; Gao, Y.; Lu, D.; Du, P. Pyrolyzed Cobalt Porphyrin-Based Conjugated Mesoporous Polymers as Bifunctional Catalysts for Hydrogen Production and Oxygen Evolution in Water. Chem. Commun. 2016, 52, 13483–13486. [Google Scholar] [CrossRef]
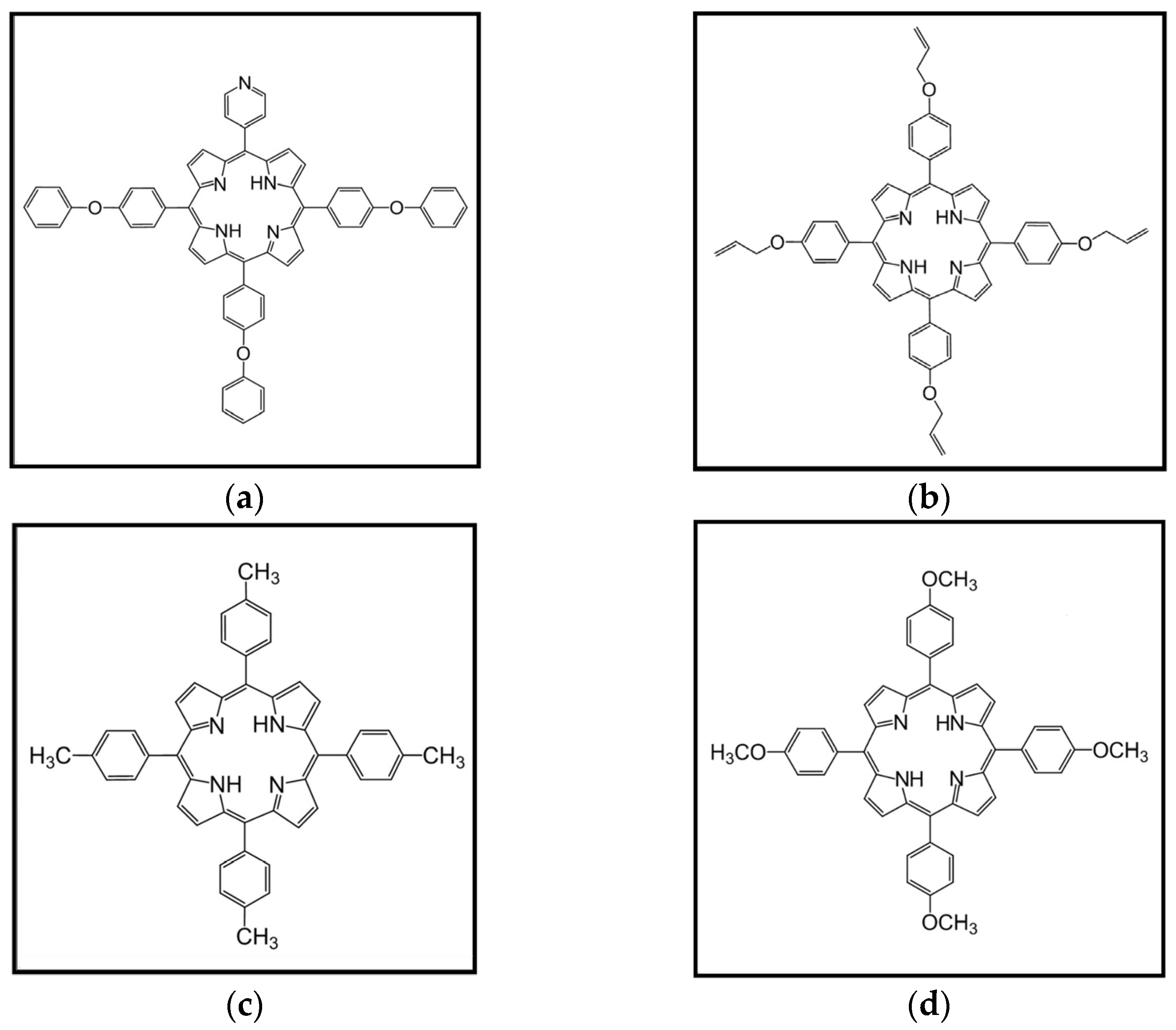


| Electrode Designation | Porphyrin | Solvent | NiPh (mg) in 0.5 mL Porphyrin Solution |
|---|---|---|---|
| G0 | - | - | - |
| GP1-NiPh-DMF | P1 | DMF | 1.5 |
| GP1-NiPh-PhCN | P1 | PhCN | 1.5 |
| GP1-NiPh-THF | P1 | THF | 1.5 |
| GP1-NiPh-DCM | P1 | DCM | 1.5 |
| GP2-NiPh-PhCN | P2 | PhCN | 1.5 |
| GP2-NiPh-THF | P2 | THF | 1.5 |
| GP2-NiPh-DCM | P2 | DCM | 1.5 |
| GP3-NiPh-PhCN | P3 | PhCN | 1.5 |
| GP3-NiPh-THF | P3 | THF | 1.5 |
| GP3-NiPh-DCM | P3 | DCM | 1.5 |
| GP4-NiPh-PhCN | P4 | PhCN | 1.5 |
| GP4-NiPh-THF | P4 | THF | 1.5 |
| GP4-NiPh-DCM | P4 | DCM | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taranu, B.-O.; Fagadar-Cosma, E.; Sfirloaga, P.; Poienar, M. Free-Base Porphyrin Aggregates Combined with Nickel Phosphite for Enhanced Alkaline Hydrogen Evolution. Energies 2023, 16, 1212. https://doi.org/10.3390/en16031212
Taranu B-O, Fagadar-Cosma E, Sfirloaga P, Poienar M. Free-Base Porphyrin Aggregates Combined with Nickel Phosphite for Enhanced Alkaline Hydrogen Evolution. Energies. 2023; 16(3):1212. https://doi.org/10.3390/en16031212
Chicago/Turabian StyleTaranu, Bogdan-Ovidiu, Eugenia Fagadar-Cosma, Paula Sfirloaga, and Maria Poienar. 2023. "Free-Base Porphyrin Aggregates Combined with Nickel Phosphite for Enhanced Alkaline Hydrogen Evolution" Energies 16, no. 3: 1212. https://doi.org/10.3390/en16031212
APA StyleTaranu, B.-O., Fagadar-Cosma, E., Sfirloaga, P., & Poienar, M. (2023). Free-Base Porphyrin Aggregates Combined with Nickel Phosphite for Enhanced Alkaline Hydrogen Evolution. Energies, 16(3), 1212. https://doi.org/10.3390/en16031212







