A Post-Mortem Study Case of a Dynamically Aged Commercial NMC Cell
Abstract
1. Introduction
2. Experimental Background
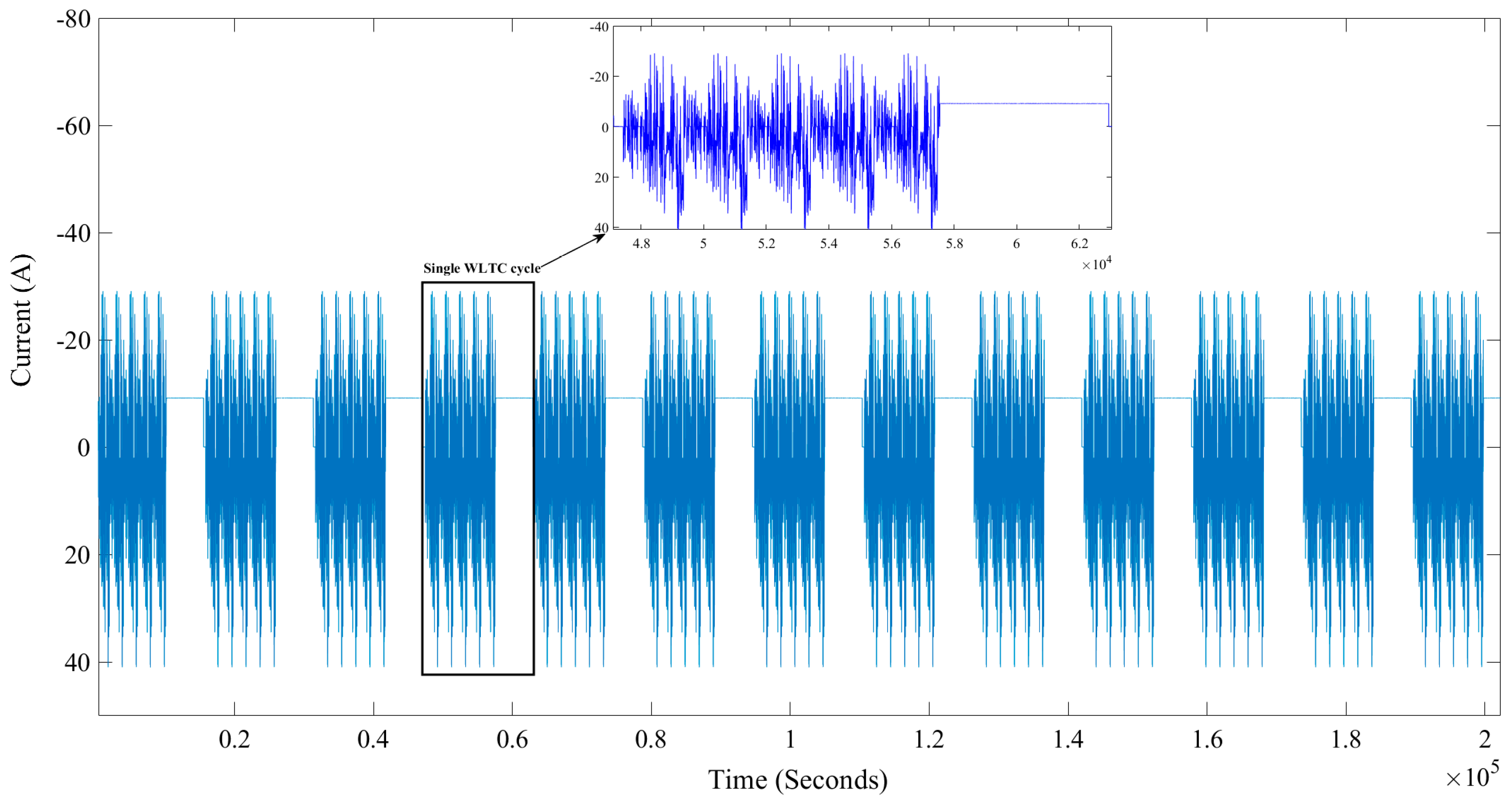
2.1. Historical Data
2.2. Selected Results
3. Post-Mortem Analysis
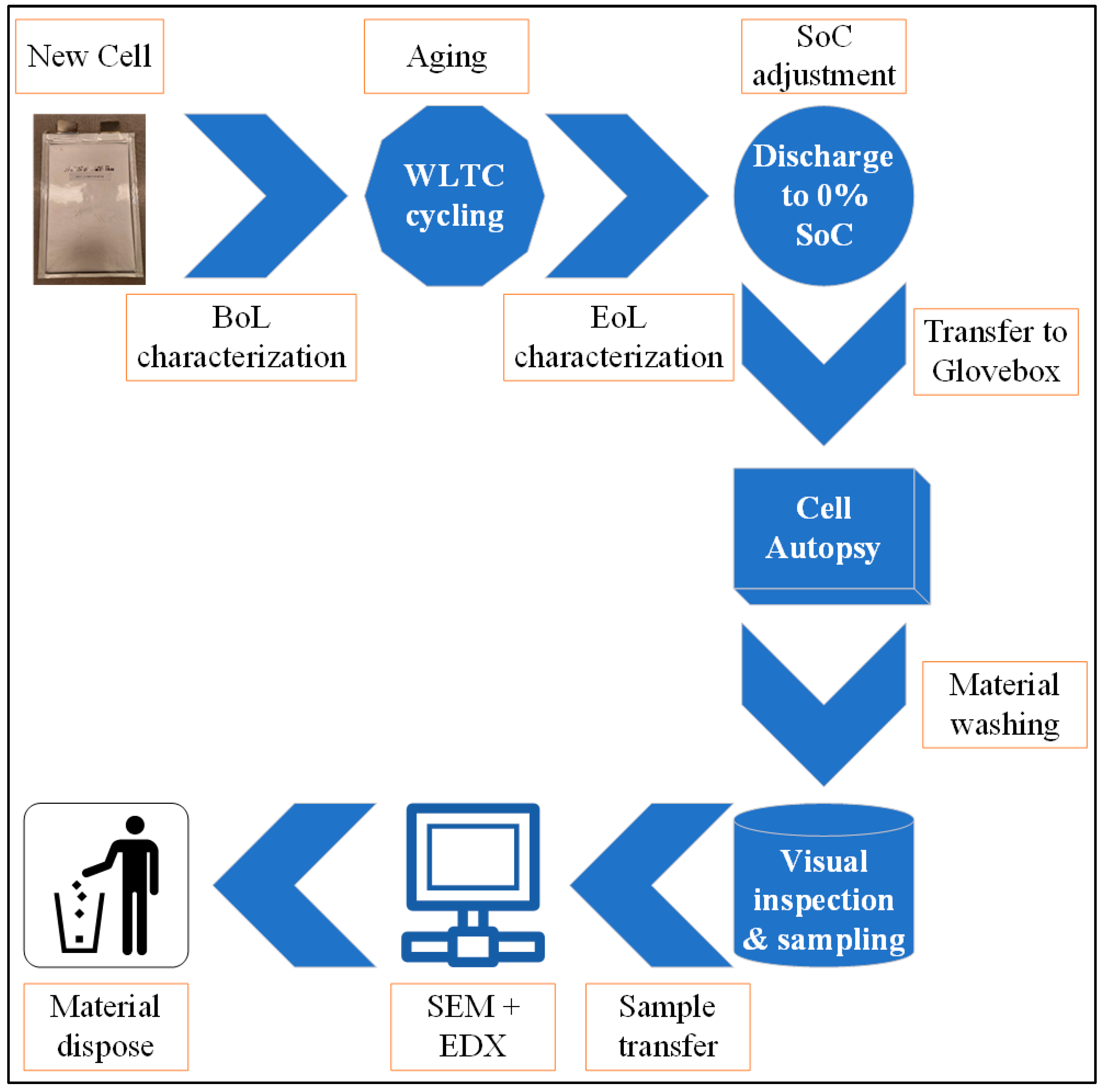
3.1. Autopsy Preparation
- Cell01 was first discharged completely as the post-mortem activity of the cell was conducted at 0% SoC. It was discharged with a very slow constant current rate (with C/20) and then the voltage was kept constant at 3 V (with C/200 as the cut-off). The intention was to ensure complete de-lithiation, keeping the voltage stable at around the minimum cut-off of 3.0 V (±0.01). A deep discharge also reduces the risk of any potential thermal runaway.
- The cell was transferred and disassembled inside a safe and stable environment. An Argon-rich glovebox with controlled environment (O2 < 0.1 ppm, H2O < 0.1 ppm) was used for the cell autopsy. This was because the metallic lithium and some cell components react with moisture content and/or oxygen when exposed. The pouch cells are usually easier to disassemble. In this case, ceramic scissors were used to cut the pouch edges and sides of the cell to avoid short-circuiting.
- After safely rolling the separator out, the anode and cathode sheets were detached from the stacked design for sampling purposes. Due to the long operational life at 45 °C, the electrolyte was found to be mostly dried out or decomposed. Thus, the focus of the study was kept on the negative and positive electrode analysis. Figure 5 displays the extracted anode and cathode sheets from which the samples were harvested, as shown in Figure 5c,f, respectively. The cell had a total of 19 cathodes and 20 anode sheets that were previewed to be degraded inhomogeneously. It was also found that the anode area was 11.6% larger than the coated cathode surface [22].
- The electrode samples were harvested from the 16th and 17th sheets for anode and cathode, respectively, as they looked visually interesting due to a few colored regions (bluish) or having distinguishable corners. The 15 mm cut samples were rinsed a few times with dimethyl carbonate (DMC) to remove electrolyte residuals and vacuum dried. The samples were left under vacuum in the anti-chamber of the glovebox overnight to dry completely considering the low contamination area.
- The dried samples were then transferred into bottles for further analysis within air-tight Ziploc bags that necessarily ensured no air contact.
3.2. Physico-Chemical Analysis
3.2.1. Scanning Electron Microscopy (SEM)
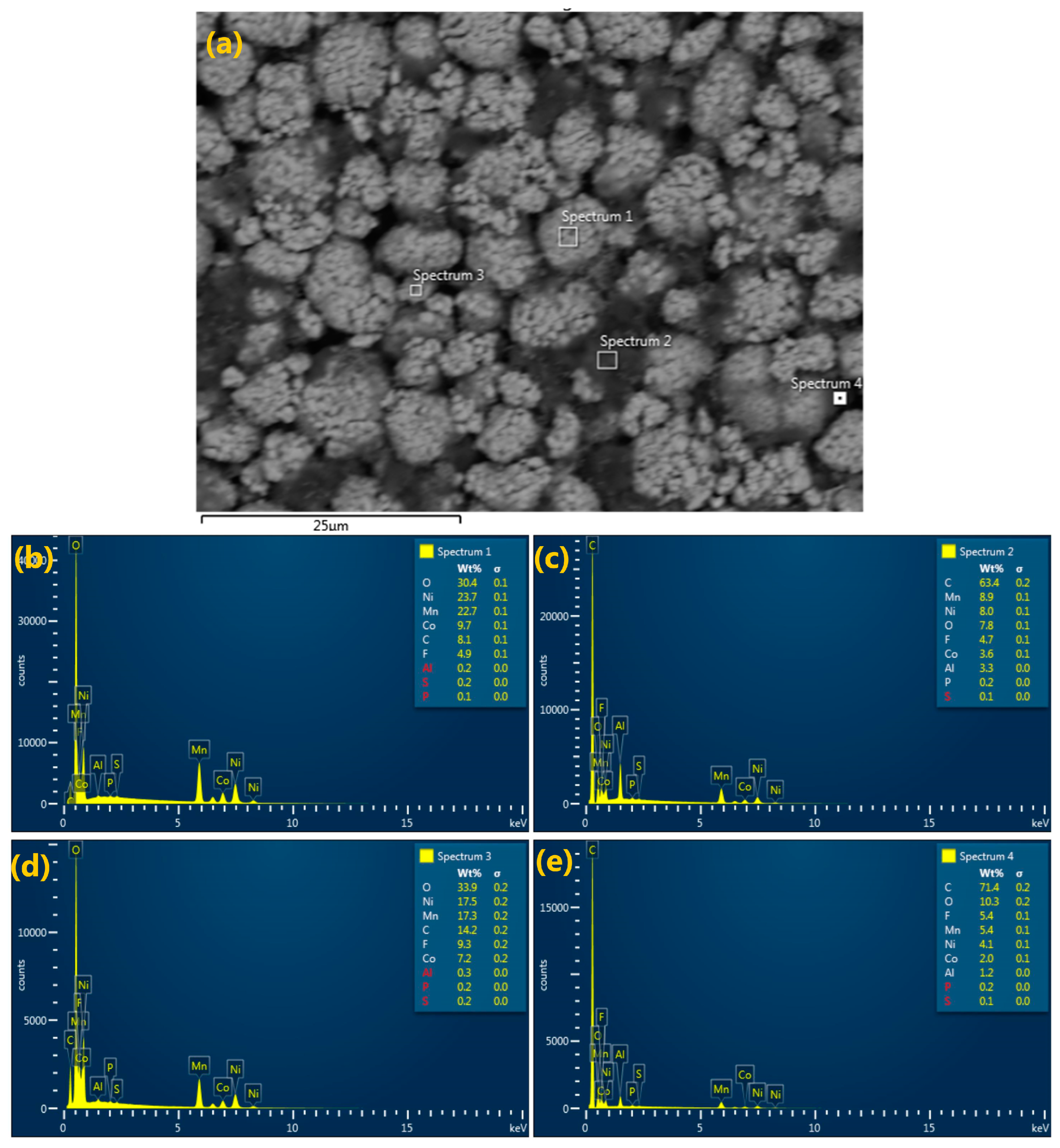
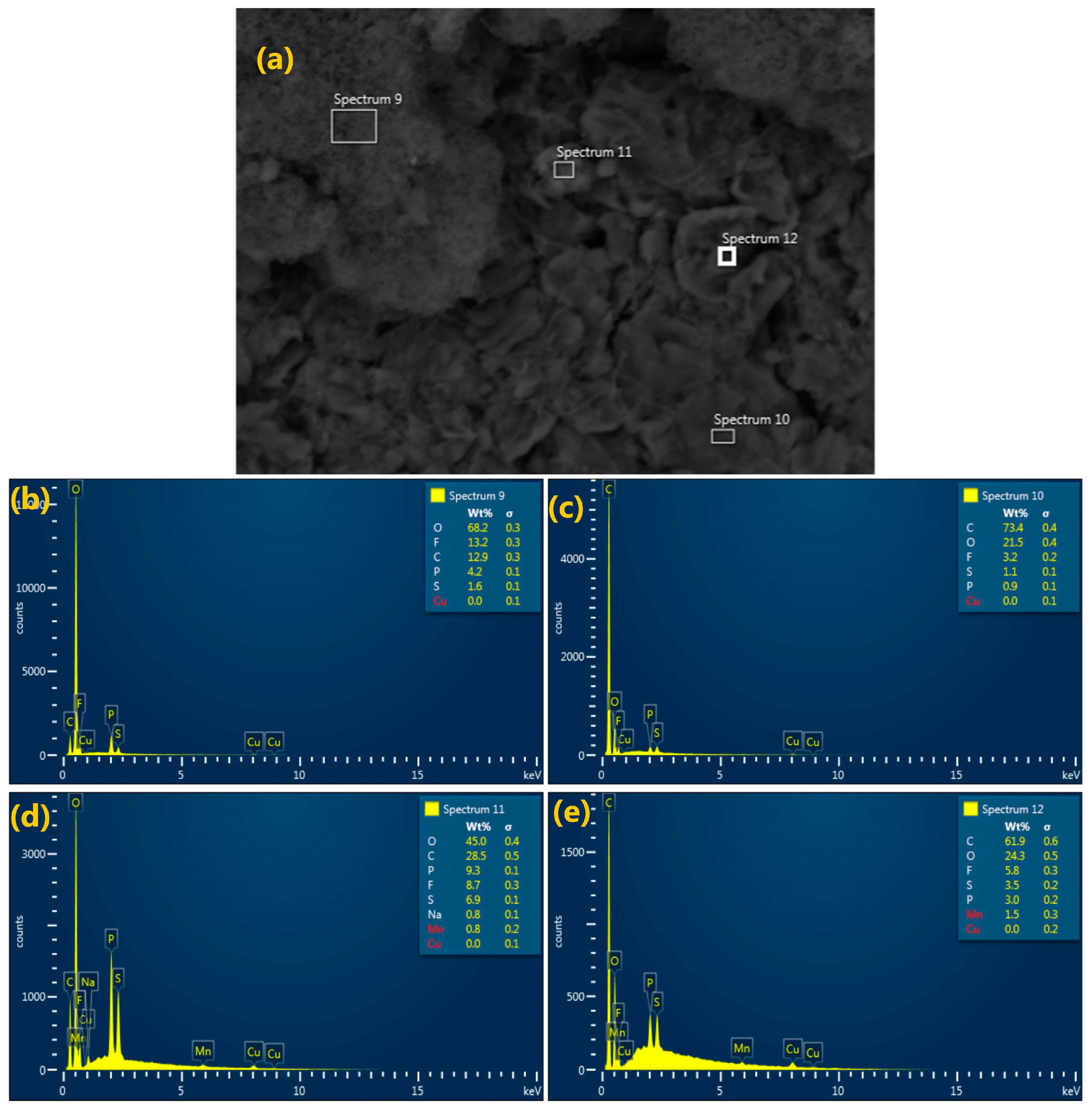
3.2.2. Energy Dispersive Spectroscopy (EDS)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sample Type | Sample Number | Volumetric Weight Percentile of the Elements (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| O | Ni | Mn | Co | C | F | Al | S | P | Na | ||
| Cathode | Spectrum 1 | 30.4 | 23.7 | 22.7 | 9.7 | 8.1 | 4.9 | 0.2 | 0.2 | 0.1 | - |
| Spectrum 2 | 7.8 | 8.0 | 8.9 | 3.6 | 63.4 | 4.7 | 3.3 | 0.1 | 0.2 | - | |
| Spectrum 3 | 33.9 | 17.5 | 17.3 | 7.2 | 14.2 | 9.3 | 0.3 | 0.2 | 0.2 | - | |
| Spectrum 4 | 10.3 | 4.1 | 5.4 | 2.0 | 71.4 | 5.4 | 1.2 | 0.1 | 0.2 | - | |
| Anode | Spectrum 9 | 68.2 | - | - | - | 12.9 | 13.2 | - | 1.6 | 4.2 | - |
| Spectrum 10 | 21.5 | - | - | - | 73.4 | 3.2 | - | 1.1 | 0.9 | - | |
| Spectrum 11 | 45.0 | - | 0.8 | - | 28.5 | 8.7 | - | 6.9 | 9.3 | 0.8 | |
| Spectrum 12 | 24.3 | - | 1.5 | - | 61.9 | 5.8 | - | 3.5 | 3 | - | |
References
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief History of Early Lithium-Battery Development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Dai, Q.; Gaines, L.; Hu, M.; Tukker, A.; Steubing, B. Future material demand for automotive lithium-based batteries. Commun. Mater. 2020, 1, 99. [Google Scholar] [CrossRef]
- Maheshwari, A.; Heck, M.; Santarelli, M. Cycle aging studies of lithium nickel manganese cobalt oxide-based batteries using electrochemical impedance spectroscopy. Electrochim. Acta 2018, 273, 335–348. [Google Scholar] [CrossRef]
- Hosen, S.; Jaguemont, J.; Van Mierlo, J.; Berecibar, M. Battery lifetime prediction and performance assessment of different modeling approaches. Iscience 2021, 24, 102060. [Google Scholar] [CrossRef] [PubMed]
- Jalkanen, K.; Karppinen, J.; Skogström, L.; Laurila, T.; Nisula, M.; Vuorilehto, K. Cycle aging of commercial NMC/graphite pouch cells at different temperatures. Appl. Energy 2015, 154, 160–172. [Google Scholar] [CrossRef]
- Khaleghi, S.; Hosen, S.; Karimi, D.; Behi, H.; Beheshti, S.H.; Van Mierlo, J.; Berecibar, M. Developing an online data-driven approach for prognostics and health management of lithium-ion batteries. Appl. Energy 2022, 308, 118348. [Google Scholar] [CrossRef]
- Hosen, S.; Kalogiannis, T.; Youssef, R.; Karimi, D.; Behi, H.; Jin, L.; Van Mierlo, J.; Berecibar, M. Twin-model framework development for a comprehensive battery lifetime prediction validated with a realistic driving profile. Energy Sci. Eng. 2021, 9, 2191–2201. [Google Scholar] [CrossRef]
- Sulzer, V.; Mohtat, P.; Aitio, A.; Lee, S.; Yeh, Y.T.; Steinbacher, F.; Khan, M.U.; Lee, J.W.; Siegel, J.B.; Stefanopoulou, A.G.; et al. The challenge and opportunity of battery lifetime prediction from field data. Joule 2021, 5, 1934–1955. [Google Scholar] [CrossRef]
- Hosen, S.; Pirooz, A.; Kalogiannis, T.; He, J.; Van Mierlo, J.; Berecibar, M. A Strategic Pathway from Cell to Pack-Level Battery Lifetime Model Development. Appl. Sci. 2022, 12, 4781. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation diagnostics for lithium ion cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Fleischhammer, M.; Waldmann, T.; Bisle, G.; Hogg, B.-I.; Wohlfahrt-Mehrens, M. Interaction of cyclic ageing at high-rate and low temperatures and safety in lithium-ion batteries. J. Power Sources 2015, 274, 432–439. [Google Scholar] [CrossRef]
- Waldmann, T.; Wilka, M.; Kasper, M.; Fleischhammer, M.; Wohlfahrt-Mehrens, M. Temperature dependent ageing mechanisms in Lithium-ion batteries—A Post-Mortem study. J. Power Sources 2014, 262, 129–135. [Google Scholar] [CrossRef]
- Klett, M.; Eriksson, R.; Groot, J.; Svens, P.; Högström, K.C.; Lindström, R.W.; Berg, H.; Gustafson, T.; Lindbergh, G.; Edström, K. Non-uniform aging of cycled commercial LiFePO4//graphite cylindrical cells revealed by post-mortem analysis. J. Power Sources 2014, 257, 126–137. [Google Scholar] [CrossRef]
- Storch, M.; Hahn, S.L.; Stadler, J.; Swaminathan, R.; Vrankovic, D.; Krupp, C.; Riedel, R. Post-mortem analysis of calendar aged large-format lithium-ion cells: Investigation of the solid electrolyte interphase. J. Power Sources 2019, 443, 227243. [Google Scholar] [CrossRef]
- Waldmann, T.; Iturrondobeitia, A.; Kasper, M.; Ghanbari, N.; Aguesse, F.; Bekaert, E.; Daniel, L.; Genies, S.; Gordon, I.J.; Löble, M.W.; et al. Review—Post-Mortem Analysis of Aged Lithium-Ion Batteries: Disassembly Methodology and Physico-Chemical Analysis Techniques. J. Electrochem. Soc. 2016, 163, A2149–A2164. [Google Scholar] [CrossRef]
- Edge, J.S.; O’Kane, S.; Prosser, R.; Kirkaldy, N.D.; Patel, A.N.; Hales, A.; Ghosh, A.; Ai, W.; Chen, J.; Yang, J.; et al. Lithium ion battery degradation: What you need to know. Phys. Chem. Chem. Phys. 2021, 23, 8200–8221. [Google Scholar] [CrossRef]
- Christensen, J.; Newman, J. Cyclable Lithium and Capacity Loss in Li-Ion Cells. J. Electrochem. Soc. 2005, 152, A818–A829. [Google Scholar] [CrossRef]
- Lin, X.; Khosravinia, K.; Hu, X.; Li, J.; Lu, W. Lithium Plating Mechanism, Detection, and Mitigation in Lithium-Ion Batteries. Prog. Energy Combust. Sci. 2021, 87, 100953. [Google Scholar] [CrossRef]
- Logan, E.; Dahn, J. Electrolyte Design for Fast-Charging Li-Ion Batteries. Trends Chem. 2020, 2, 354–366. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Warnecke, A.J. Degradation Mechanisms in NMC-Based Lithium-Ion Batteries. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2017. [Google Scholar]
- Gopalakrishnan, R.; Li, Y.; Smekens, J.; Barhoum, A.; Van Assche, G.; Omar, N. Electrochemical impedance spectroscopy characterization and parameterization of lithium nickel manganese cobalt oxide pouch cells: Dependency analysis of temperature and state of charge. Ionics 2019, 25, 111–123. [Google Scholar] [CrossRef]
- Hosen, S.; Karimi, D.; Kalogiannis, T.; Pirooz, A.; Jaguemont, J.; Berecibar, M.; Van Mierlo, J. Electro-aging model development of nickel-manganese-cobalt lithium-ion technology validated with light and heavy-duty real-life profiles. J. Energy Storage 2020, 28, 101265. [Google Scholar] [CrossRef]
- Fermín-Cueto, P.; McTurk, E.; Allerhand, M.; Medina-Lopez, E.; Anjos, M.F.; Sylvester, J.; dos Reis, G. Identification and machine learning prediction of knee-point and knee-onset in capacity degradation curves of lithium-ion cells. Energy AI 2020, 1, 100006. [Google Scholar] [CrossRef]
- Verma, P.; Maire, P.; Novák, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Pinson, M.B.; Bazant, M.Z.; Chavhan, P.M.; Reddy, V.; Solanki, P.R.; Malhotra, B.D.; Kim, C. Theory of SEI Formation in Rechargeable Batteries: Capacity Fade, Accelerated Aging and Lifetime Prediction. J. Electrochem. Soc. 2012, 160, A243–A250. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogg, B.-I.; Wohlfahrt-Mehrens, M. Li plating as unwanted side reaction in commercial Li-ion cells—A review. J. Power Sources 2018, 384, 107–124. [Google Scholar] [CrossRef]
- Waldmann, T.; Gorse, S.; Samtleben, T.; Schneider, G.A.; Knoblauch, V.; Wohlfahrtmehrens, M. A Mechanical Aging Mechanism in Lithium-Ion Batteries. J. Electrochem. Soc. 2014, 161, A1742–A1747. [Google Scholar] [CrossRef]
- Stiaszny, B.; Ziegler, J.C.; Krauß, E.E.; Schmidt, J.P.; Ivers-Tiffée, E. Electrochemical characterization and post-mortem analysis of aged LiMn2O4–Li(Ni0.5Mn0.3Co0.2)O2/graphite lithium ion batteries. Part I: Cycle aging. J. Power Sources 2014, 251, 439–450. [Google Scholar] [CrossRef]
- Liu, M.; Ren, Z.; Wang, D.; Zhang, H.; Bi, Y.; Shen, C.; Guo, B. Addressing Unfavorable Influence of Particle Cracking with a Strengthened Shell Layer in Ni-Rich Cathodes. ACS Appl. Mater. Interfaces 2021, 13, 18954–18960. [Google Scholar] [CrossRef]
- Xu, R.; Sun, H.; de Vasconcelos, L.S.; Zhao, K. Mechanical and Structural Degradation of LiNixMnyCozO2Cathode in Li-Ion Batteries: An Experimental Study. J. Electrochem. Soc. 2017, 164, A3333–A3341. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Guo, Z. Simulation of crack behavior of secondary particles in Li-ion battery electrodes during lithiation/de-lithiation cycles. Int. J. Mech. Sci. 2019, 155, 178–186. [Google Scholar] [CrossRef]
- Burns, J.C.; Kassam, A.; Sinha, N.N.; Downie, L.E.; Solnickova, L.; Way, B.M.; Dahn, J.R. Predicting and Extending the Lifetime of Li-Ion Batteries. J. Electrochem. Soc. 2013, 160, A1451–A1456. [Google Scholar] [CrossRef]
- Broussely, M.; Herreyre, S.; Biensan, P.; Kasztejna, P.; Nechev, K.; Staniewicz, R. Aging mechanism in Li ion cells and calendar life predictions. J. Power Sources 2001, 97–98, 13–21. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Riahi, A.R.; Alpas, A.T. Thermal cycling induced capacity enhancement of graphite anodes in lithium-ion cells. Carbon 2014, 67, 592–606. [Google Scholar] [CrossRef]
- Krämer, Y.; Birkenmaier, C.; Feinauer, J.; Hintennach, A.; Bender, C.L.; Meiler, M.; Schmidt, V.; Dinnebier, R.E.; Schleid, T. A New Method for Quantitative Marking of Deposited Lithium by Chemical Treatment on Graphite Anodes in Lithium-Ion Cells. Chem. A Eur. J. 2015, 21, 6062–6065. [Google Scholar] [CrossRef]
- Jankowski, P.; Lindahl, N.; Weidow, J.; Wieczorek, W.G.; Johansson, P. Impact of Sulfur-Containing Additives on Lithium-Ion Battery Performance: From Computational Predictions to Full-Cell Assessments. ACS Appl. Energy Mater. 2018, 1, 2582–2591. [Google Scholar] [CrossRef]





| Cycling Temperature | Performed Cycles | SoHcap | SoHIR | |
|---|---|---|---|---|
| Cell01 | 45 °C | 2184 | 72.1% | 476.2% |
| Cell02 | 45 °C | 3468 | 79.4% | 256.6% |
| Cell02 * | - | 3060 | 83.8% | 200.9% |
| Cell03 | 10 °C | 1428 | 101.6% | 142.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosen, M.S.; Yadav, P.; Van Mierlo, J.; Berecibar, M. A Post-Mortem Study Case of a Dynamically Aged Commercial NMC Cell. Energies 2023, 16, 1046. https://doi.org/10.3390/en16031046
Hosen MS, Yadav P, Van Mierlo J, Berecibar M. A Post-Mortem Study Case of a Dynamically Aged Commercial NMC Cell. Energies. 2023; 16(3):1046. https://doi.org/10.3390/en16031046
Chicago/Turabian StyleHosen, Md Sazzad, Poonam Yadav, Joeri Van Mierlo, and Maitane Berecibar. 2023. "A Post-Mortem Study Case of a Dynamically Aged Commercial NMC Cell" Energies 16, no. 3: 1046. https://doi.org/10.3390/en16031046
APA StyleHosen, M. S., Yadav, P., Van Mierlo, J., & Berecibar, M. (2023). A Post-Mortem Study Case of a Dynamically Aged Commercial NMC Cell. Energies, 16(3), 1046. https://doi.org/10.3390/en16031046









