Abstract
This review aimed to summarize the current knowledge regarding dibenzofulvene derivatives (DBF) investigated for photovoltaics and organic electronics applications. The work begins with a detailed analysis of the synthesis and modification methods for dibenzofulvene derivatives’ structure. Then, the physicochemical properties (thermal, electrochemical, and optical) of the selected compounds are discussed in detail. Moreover, this article also presents the DFT calculations performed so far. Finally, the review presents the latest research on the applications of dibenzofulvene derivatives as dyes for DSSC cells, hole transport materials (HTMs) for perovskite solar cells (PSCs), organic light-emitting diodes (OLEDs), and luminescent and electrochromic materials. Considering the above, this review may be helpful when designing new organic compounds for photovoltaic and organic electronic applications.
1. Introduction
In recent years, the way of life of people in many places around the world has changed significantly. This is mainly due to the dynamic development of electrical and electronic devices, ubiquitous digitization, and easy access to the Internet. However, this has primarily led to a very high dependence on electricity [1]. Today, electricity is both a commodity and a strategic service. A lack of it, known as energy poverty, causes extreme difficulties in everyday functioning [2]. In addition, its strategic nature also results from the significant problems in storing electricity. Therefore, energy must be supplied continuously, which is also a big problem. Currently, energy production in many places around the world is based on the exploitation of energy resources (coal, natural gas, and crude oil). A significant disadvantage with this solution is the generation of large amounts of waste that pollute the environment through combustion by-products [1,2,3]. This problem has been discussed for many years, resulting in various restrictions on greenhouse gas emissions. Additionally, energy resources are gradually depleting, which negatively affects the total costs of electricity [1]. Moreover, in recent years, further problems have emerged that have impacted energy prices. An example of this was the COVID-19 pandemic because, during it, there were many restrictions related to transport and to ways of working [2,4]. However, no one expected that the current energy crisis would begin with a war in Ukraine [2,4]. This conflict caused the prices of raw materials such as natural gas to increase significantly, which resulted in a dynamic increase in electricity prices. This contributed to initiating or accelerating the energy transformation in many countries worldwide (mainly Europe). This applies, in particular, to renewable energy sources and nuclear energy. Among renewable energy sources, solar cells deserve special attention because solar panels directly convert solar energy into electricity [4,5,6,7,8,9,10,11,12,13,14]. During this process, no pollutants are emitted into the atmosphere, and no waste is generated that requires disposal. Photovoltaic farms also do not disturb the natural environment during their operation. Moreover, photovoltaic panels are increasingly being installed on buildings and houses [5]. Thanks to this, electricity consumption in urban agglomerations can be significantly reduced. Solar cells using organic compounds are an extremely interesting solution that has been intensively researched recently [7,9,12,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. These include, among others, dye-sensitized solar cells (DSSCs) [16,17,18,25,26], perovskite solar cells (PVSs) [7,9,12,27,28], or even bulk heterojunction solar cells (BHJ) [15,19,20,29,30]. Many of the tested cells of this type can be installed in unusual places in buildings, such as windows or facades. Such cells are also much lighter compared to currently used devices. Additionally, they can be extremely aesthetic because their shape and color can be modified. However, for the further development of these solar cells, cheap, efficient, and stable organic materials are necessary. An example of organic compounds that enjoy great interest in the literature are fluorene derivatives [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. First of all, this is due to the many advantages of compounds of this type, such as the possibility of modification through many chemical reactions, excellent thermal stability, the high quantum yield of photoluminescence, relatively good features of molecular self-assembly, ambipolar properties of charge transport (holes and electrons), many peculiar features regarding nonlinear optical properties, as well as very good photostability [40,50]. A very interesting variant of the mentioned compounds are dibenzofulvene derivatives (DBFs) (Figure 1) [32,33,34,35,37,38,39,40,41,42,43,44,45,46,48,49,50]. These compounds largely retain the properties of fluorene and its derivatives. However, due to the carbon–carbon double bond (C=C) in the C9 position of fluorene and the additional substituent at the end of this bond, dibenzofulvene derivatives gain additional opportunities to modify their physicochemical properties. Thanks to this, dibenzofulvene derivatives are becoming increasingly popular compounds in various research [32,33,34,35,37,38,39,40,41,42,43,44,45,46,48,49,50].
Figure 1.
Dibenzofulvene derivatives (DBF).
In this review, we present the current knowledge regarding dibenzofulvene derivatives by investigating their applications in photovoltaic and organic electronics. In the article, we will discuss the synthesis, selected physicochemical properties, DFT calculations, and application research. We hope that this review will be helpful when designing new dibenzofulvene derivatives.
2. Synthesis
Dibenzofulvene derivatives (DBF) have been scarcely described in the literature. However, in the last decade, interest in them has increased significantly (Figure 2) [32,33,34,35,37,38,39,40,41,42,43,44,45,46,48,49,50].
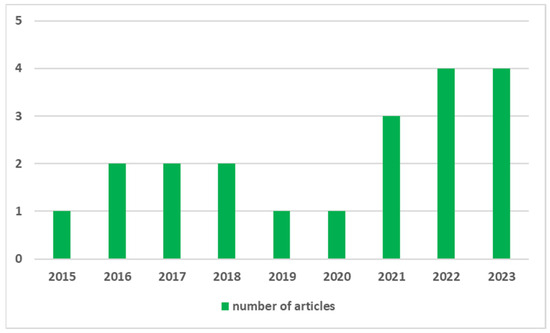
Figure 2.
Number of published articles on dibenzofulvene derivatives for their applications in photovoltaic and organic electronics.
This is largely due to the intensive search for new chemical compounds for applications in photovoltaic and organic electronics. All dibenzofulvene derivatives described in the literature in this respect are presented in Figure 3.
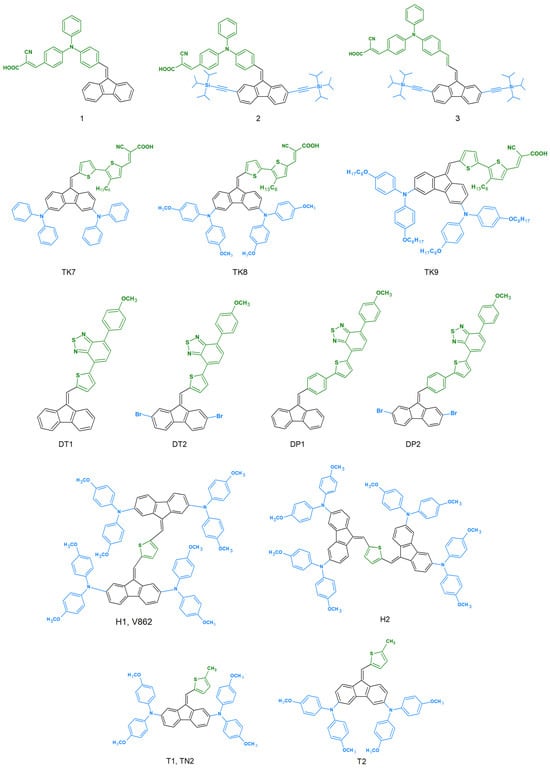
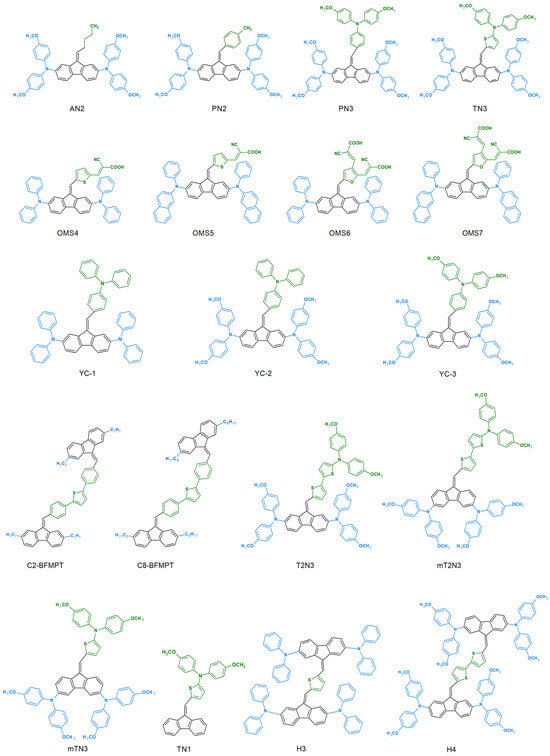
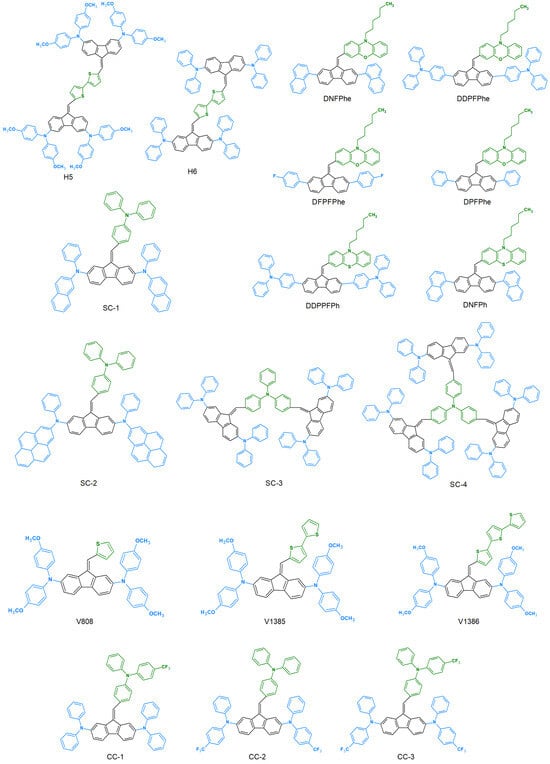
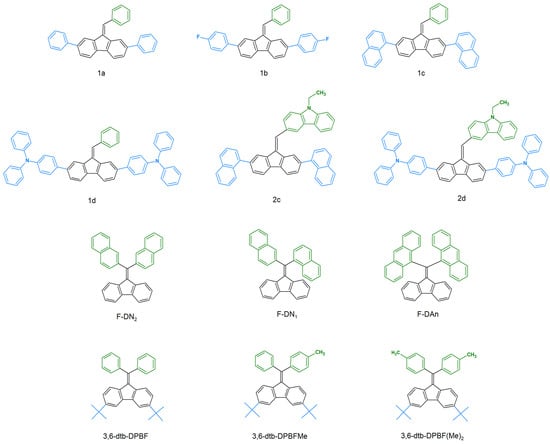
Figure 3.
Dibenzofulvene derivatives (DBF) tested for use in photovoltaic and organic electronics [32,33,34,35,37,38,39,40,41,42,43,44,45,46,48,49,50].
In terms of their synthetic aspect, dibenzofulvenes have many advantages. First of all, they can be obtained by a very simple and efficient Knoevenagel condensation (Figure 4) [32,33,34,35,39,40,42,43,44,45,46,48,49,50]. In the case of DBFs, this reaction involves the condensation of fluorene or its derivatives (usually bromo derivatives) with a selected aldehyde, dialdehyde, or trialdehyde [32,33,34,35,39,40,42,43,44,45,46,48,49,50]. As a result, a double bond (C=C) is formed between the carbon atom in the C9 position of fluorene and the carbon atom of the carbonyl group of the aldehyde. Additionally, an aliphatic, aromatic, or heteroaromatic substituent derived from an aldehyde is introduced into the structure of the compound (Figure 3) [32,33,34,35,39,40,42,43,44,45,46,48,49,50]. The mentioned Knoevenagel condensation reaction is most often carried out in the presence of potassium tert-butoxide (t-BuOK) and ethanol (EtOH) [33,34,35,38,39,40,43,44,49]. Less often, a mixture of tetrabutylammonium bromide (TBABr), 40% sodium hydroxide (NaOH), and toluene is used [45,48,50]. In the literature, you can also find single reports about the use of sodium hydroxide and ethanol [32], sodium tert-butoxide (t-BuONa), and dimethylformamide (DMF) [42] (or potassium tert-butoxide and tetrahydrofuran (THF) [46]). Moreover, selected literature reports indicate that the use of ultrasound during synthesis is extremely beneficial [33,35,38]. In the case of purification methods, in many cases, it is only recommended to filter the precipitate and wash it thoroughly with water and selected organic solvents, e.g., ethanol. An additional advantage of the discussed condensation is the generally high reaction yield.
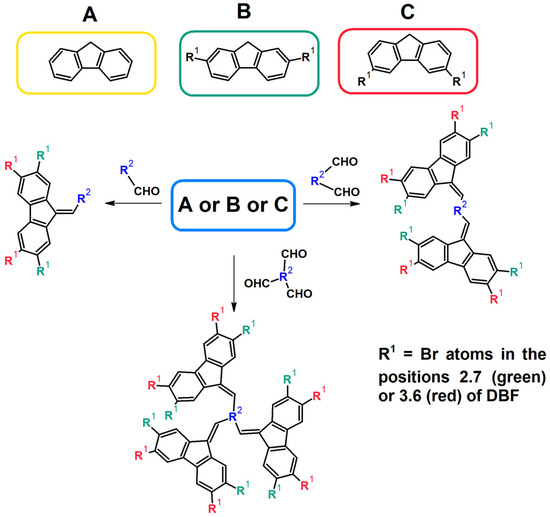
Figure 4.
Synthesis of dibenzofulvene derivatives (DBF) by the Knoevenagel condensation reaction [32,33,34,35,39,40,42,43,44,45,46,48,49,50].
Another advantage of dibenzofulvene derivatives is their great potential for the structural modification of this type of system [32,33,34,35,37,38,39,40,41,42,43,44,45,46,48,49,50]. Fluorene is a fragment that successfully undergoes a bromination reaction at the 2,7 [53,54] or 3,6 [55,56] position. Thanks to this, dibenzofulvene derivatives containing dibromofluorene (2,7 or 3,6) in their structure undergo the Buchwald–Hartwig [33,35,38,39,40,43,44,46,48,49], Suzuki [34,45,50], or Sonogashira [32] coupling reactions. The Buchwald–Hartwig coupling enables the introduction of aromatic amine systems into the structure of a dibenzofulvene derivative by creating a new carbon–nitrogen (C-N) bond (Figure 5) [33,35,38,39,40,43,44,46,48,49]. The mentioned reaction takes place between a bromo derivative of dibenzofulvene and an aromatic amine in the presence of a catalytic system consisting of palladium catalyst precursor bis(dibenzylideneacetone)palladium(0) ([Pd(dba)2]), tri(tert-butyl)phosphine ligand ((t-Bu)3P), and sodium tert-butoxide [33,35,38,39,40,43,44,46,48,49]. The literature also reports the use of palladium (II) acetate [PdOAc2] (catalyst precursor), tri-tertbutylphosphonium tetrafluoroborate [(t-Bu3)PH]BF4], and sodium tert-butoxide as a catalytic system [48]. The most commonly used solvent for a Buchwald–Hartwig coupling is toluene [33,35,38,39,40,43,44,46,48,49]. In the case of heating reaction mixtures during synthesis, microwave radiation (at a temperature of 110 °C) [33,35,38,43,44] or classical heating (at the boiling point of the reaction mixture) [39,40,46,48] are used. All compounds obtained via the mentioned coupling require purification by chromatography. Thanks to this, the vast majority of them are synthesized with good efficiency [33,35,38,39,40,43,44,46,48,49].
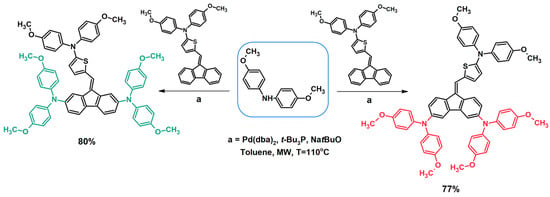
Figure 5.
Modification of dibenzofulvene derivatives using the Buchwald–Hartwig coupling method [43].
In turn, the Suzuki coupling allows for the introduction of further aromatic or heteroaromatic substituents into the structure of a dibenzofulvene derivative by creating a new carbon–carbon (C-C) bond (Figure 6) [34,45,50]. The most commonly discussed reaction occurs between a bromoderivative of dibenzofulvene and an aromatic or heteroaromatic boronic acid or ester. For efficient coupling, a catalytic system consists of a palladium catalyst tetrakis(triphenylphosphine)palladium(0) ([Pd(PPh3)4]) [34] or precursor such as bis(triphenylphosphine)palladium(II) dichloride ([PdCl2(PPh3)2]) [45,50], as well as potassium carbonate (K2CO3) [34] or potassium hydroxide (KOH) [45,50]. The solvent used for the Suzuki coupling is tetrahydrofuran (THF) with the addition of water [34,45,50]. All reactions of this type described in the literature were carried out at a temperature of 80°C for 0.5 h to 12 h [34,45,50]. As before, all compounds obtained using the Suzuki coupling required purification by chromatography. Most dibenzofulvene derivatives were synthesized by this method with good yields [34,45,50].
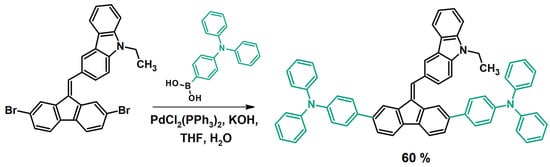
Figure 6.
Modification of dibenzofulvene derivatives using the Suzuki coupling method [50].
The last coupling reaction through which the structure of dibenzofulvene derivatives was modified was the Sonogashira coupling [32]. This reaction takes place between the bromo derivative of dibenzofulvene and the unsymmetrical (terminal) derivative of acetylene ((Triisopropylsilyl)acetylene - TIPSA), which results in the formation of a new carbon–carbon (C-C) bond (Figure 7) [32]. As in the case of the previous reactions, for an efficient Sonogashira coupling, a catalytic system is required consisting of the following: the palladium catalyst precursor bis(triphenylphosphine)palladium(II) dichloride ([PdCl2(PPh3)2]), the copper(I) iodide co-catalyst (CuI), and triphenylphosphine (PPh3) [32]. Triethylamine (NEt3) acts as both a base and a solvent [32]. Due to the use of bromo derivatives of dibenzofulvene for coupling, it was necessary to apply heating during the synthesis (70 °C or the boiling point of the reaction mixture depending on the compound being synthesized [32]). All DBFs obtained by a Sonogashira coupling were purified by chromatography. Through considering the above, they were synthesized with a good yield [32].
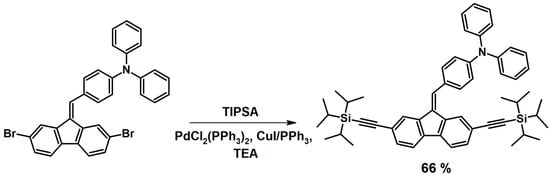
Figure 7.
Modification of dibenzofulvene derivatives using the Sonogashira coupling method [32].
Moreover, dibenzofulvene derivatives modified by coupling can successfully undergo further reactions. An example of this type of transformation is the synthesis of DBFs that have a carbonyl group (-CO-) in their structure [32,33,39]. In this case, dibenzofulvene derivatives after Sonogashira [32] or Buchwald–Hartwig [33,39] coupling are subjected to subsequent Knoevenagel condensation with cyanoacetic acid in the presence of ammonium acetate and acetic acid as a solvent (Figure 8) [32,33,39]. In this case, this condensation requires heating to 120 °C [33] or to the boiling point of the reaction mixture [32]. The duration of the syntheses largely depends on the structure of the dibenzofulvene derivative, as they can last 4 h [32] or 12 h [33]. The obtained products, despite their carboxyl group, are most often purified using chromatography [32,33].
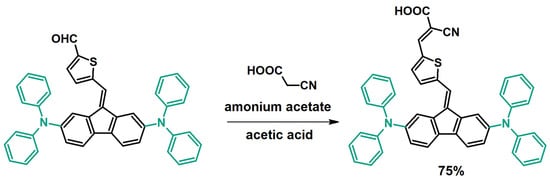
Figure 8.
Synthesis of a dibenzofulvene derivative that has a carboxyl group in its structure (-COOH) [32,33,39].
A completely different approach to synthesizing dibenzofulvene derivatives, which is also worth paying attention to, is using the Corey–Fuchs reaction and then the Suzuki coupling. This makes it possible to obtain dibenzofulvene derivatives that have two substituents attached to the double bond (Figure 9) [57]. The Corey–Fuchs reaction occurs between 9-fluorenone, carbon tetrabromide, and triphenylphosphine [57]. Dichloromethane is used as the solvent. Moreover, it does not require the use of radical reaction conditions. Gentle heating of the reaction mixture at 40 °C for 24 h is sufficient. Then, purified (by chromatography and crystallization) 9-(dibromomethylene)-9H-fluorene is subjected to a Suzuki coupling with a selected aromatic boronic acid (Figure 9) [57]. The mentioned coupling is carried out in the presence of a catalyst—tetrakis(triphenylphosphine)palladium(0) ([Pd(PPh3)4]), tetrabutylammonium hydrogen sulfate (C16H35N(HSO4)), and potassium carbonate (K2CO3) [57]. Toluene is used as a solvent. For efficient coupling, heating at 90 °C for 12 to 24 h is sufficient. Regarding methods for the purification of dibenzofulvene derivatives to be obtained in this way, chromatography is recommended in the literature [57].
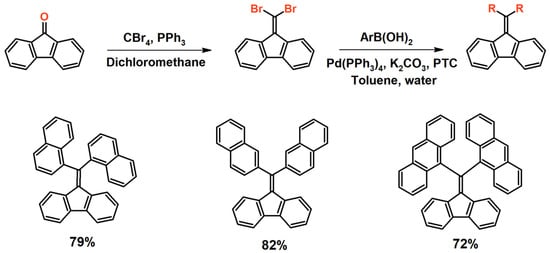
Figure 9.
Synthesis of a dibenzofulvene derivative using the Corey–Fuchs reaction and the Suzuki coupling [57].
3. Thermal Properties
The thermal properties of organic compounds are fundamental in their practical use in photovoltaic and organic electronics. This is mainly because the low thermal stability of the derivatives used may lead to impaired operation or complete damage to the devices. In the case of dibenzofulvene derivatives, their thermal properties have been tested only for selected compounds [39,40,42,45,46,48,49,50]. All thermal parameters presented in the literature were determined using differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) [39,40,42,45,46,48,49,50] (Table 1). Interestingly, the DBFs showed excellent thermal stability, as their 5% mass loss (Td) was most often observed above 300 °C. Only three of the described compounds were characterized by lower Td, namely YC-3 (206 °C) [40], CC-3 (204 °C) [49] and SC-4 (249 °C) [46]. Through analyzing the structure of the derivatives YC-1, YC-2, and YC-3, we can see that they show very high structural similarity. They differ only in the number of methoxy groups (-OCH3) on the phenyl rings. However, this has a significant impact on thermal stability because, by increasing the number of methoxy groups (-OCH3), Td decreases [40] (Figure 10). We can observe a similar relationship for CC-1, CC-2, and CC-3 derivatives. With a significant increase in the number of trifluoromethyl groups (-CF3) or a change in the place of their attachment in the structures of compounds, we observed a change in thermal parameters [49] (Figure 10). The number of carbon–carbon double bonds (C=C) formed due to the Knoevenagel condensation reaction between fluorene (or its derivatives) and dialdehyde or trialdehyde also had an unfavorable impact on thermal properties [46]. An example illustrating this relationship is the comparison of the SC-3 derivative with SC-4. As the number of the mentioned double bonds increased (from two to three), we observed a significant decrease in Td from 425 °C to 249 °C [46]. The SC-2 derivative had the highest thermal stability (Td = 464 °C). This was most likely due to the two pyrene substituents found in the structure of that compound [46]. Moreover, dibenzofulvene derivatives very often exhibit glass transition (Tg). We can observe them in a wide temperature range from 53 °C to 181 °C (Table 1). The melting temperature (Tm) for DBF is observed only for selected compounds (C2-BFMPT, C8-BFMPT, DDPFPhe, DFPFPhe, DPFPhe, DDPPFPhe, 1a, 1b, and 1d) in the range from 83 °C to 231 °C (Table 1).

Table 1.
Thermal properties of dibenzofulvene derivatives.
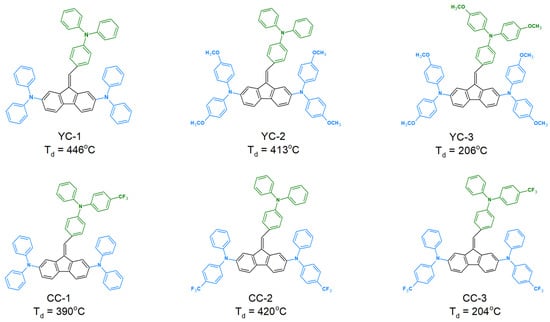
Figure 10.
The influence of methoxy groups (-OCH3) and trifluoromethyl groups (-CF3) on the thermal stability of dibenzofulvene derivatives (DBF).
4. DFT Calculations
Currently, theoretical calculations are an important element of scientific research. Thanks to them, experimental research has become more understandable. The theoretical determination of parameters, such as the values of HOMO and LUMO orbitals or absorption wavelengths, makes it possible to explain many processes and phenomena. To better understand the physicochemical properties of the discussed dibenzofulvene derivatives, calculations were carried out using density functional theory (DFT) and time-dependent density functional theory (TDDFT). In the case of functionals, CAM-B3LYP [32,34], B3LYP [32,34,39,45], APBE-D3 [33,35], PBE0 [35,43], and B3PW91 [50] were used. Bases such as 6-31G(d) [32,34,39,50], def2-TZVP [33,35,43], and 6-311G (d, p) [45] were also used. Calculations were performed using the Gaussian [39,45,50] or TURBOMOLE [33,35,43] methods. All of the calculation results described in the literature for the reviewed papers are presented in Table 2.

Table 2.
DFT calculation values for dibenzofulvene derivatives.
In 2015, calculations of dibenzofulvene derivatives (DBF) were presented for the first time in terms of applications in photovoltaics [32]. A. Tigreros et al. focused their attention on analyzing spatial configurations and characterizing electronic structures. In the obtained dyes (1–3), dihedral angles were determined between the fluorene motif and the substituent attached via a double bond (Table 2). In the case of Derivatives 1 and 2, a structural compromise was observed that minimized steric hindrance [32]. Compound 3, which had an additional double bond in its structure, had a flatter conformation, thus leading to a better electronic coupling. The location of the HOMO orbital for all of the given molecules was on the donor units (triarylamine and fluorene moieties), while the LUMO orbital mainly covered the acceptor units (cyanoacrylate fragments). The particular frontier molecular orbital distribution observed for Dye 3 revealed an effective π conjugation across the fluorene–triarylamine junction, which resulted from the more planar geometry of 3 than those of Dyes 1 and 2 [32]. Moreover, TD-DFT calculations were performed for Compounds 1–3, and the absorption maximum values were also determined (Table 2). These results were consistent with the experimental values obtained for the dyes in a THF solution. Calculations showed that the higher absorption values obtained in the experiment resulted from the charge transfer process in the tested dyes [32]. A year later, P. Gopikrishna and P. K. Iyer studied the aggregation-induced emission enhancement (AIEE) phenomenon in monosubstituted dibenzofulvene derivatives [34]. For this purpose, they performed DFT calculations showing that the presented structures did not have a planar configuration. For DT1 and DT2, the fragment containing the thiophene and benzothiadiazole motifs was located in one plane, while the paramethoxyphenyl group (dihedral angle of 121.68°) and DBF (dihedral angle of about 118°) were out of plane. The whole thing created the configuration of the boat. In the case of DP1 and DP2 molecules, we can observe similarities. The thiophene, benzothiadiazole, and phenyl fragments were almost in one plane, and the other two groups (DBF and paramethoxyphenyl) were out of a plane [34]. Interestingly, in this study, we were dealing with a chair configuration and dihedral angles of approximately 119° and 121.68°, respectively (Table 2). The theoretical data agree with the X-ray data of the single crystal, thus confirming the correctness of the calculations. The direct attachment of a substituent to the dibenzofulvene unit characterized the DT molecules. The HOMO orbital in these compounds was located on thiophene, as well as on the DBF unit and the paramethoxyphenyl group. In DP molecules with an additional phenyl, the HOMO orbital was situated on the thiophene, phenyl, and paramethoxyphenyl fragments, as well as being partially located on the dibenzofulvene unit. These results confirmed that, when DBF is directly attached to thiophene, it acts as a strong electron donor when compared to the phenyl, thus indicating that the phenyl unit between thiophene and DBF reduces the donating capacity of DBF moiety [34]. The LUMO orbital for all four compounds was located in the benzothiadiazole group. Theoretical UV-Vis spectra were obtained for the presented molecules. Each of them had two bands in the ranges of 352–370 nm and 495–512 nm. Transitions around 500 nm were attributed to ICT processes from the HOMO to LUMO orbitals. The bands in the 352-370 nm range were π−π* transitions from HOMO to LUMO+1. Moreover, the results of the theoretical calculation strongly supported the experimental observations that better conjugation in DT luminogens is the main reason for the redshift of the emission when compared to DP luminogens [34]. In the same year, new compounds of TK7-9 were tested. They noticed that the lowest energy absorption peak mainly describes the HOMO to LUMO transition, and that it had a charge transfer character [33]. In 2017, the authors did not present dihedral angles or frontier molecular orbitals but focused on the obtained spectra [35]. Their calculations concentrated on explaining the nature of the bands formed during the spectroelectrochemical process. The π−π* transition characterized molecules in a ground state, while oxidized molecules had an IVCT (intervalence charge transfer transition). To determine this, the authors used an analysis of the band shape, which was carried out with Hush’s theory. A. Beneduci et al. found that the presented structures behave like mixed valence compounds (MVs), and that their classification after band analysis allows them to be assigned to class III [35]. Considering the calculations for further oxidation processes, it was found that, despite the increase in H1 oxidation, the shape of the band did not change significantly. This proved that the main electron transfer pathway in H1 was the vertical one, even at high oxidation states. A different situation occurred for H2. The NIR spectrum changed significantly with increasing oxidation. It is possible that this was due to a strong orthogonal (vertical and horizontal) electronic coupling, which would be modulated by the potential change and would induce a reversal of the orbital ordering with respect to H1. In fact, the DFT calculations showed that, while in H1, there were two highly occupied orbitals localized on the dibenzofulvene moieties and one well separated bridge-localized orbital at lower energy. In H2, due to the stronger coupling of dibenzofulvene cores with the central bridge, the energy difference between the different orbitals was considerably smaller and the formerly bridge-localized orbital also displayed a significant amplitude on the amine centers. Thus, it acquired a partially mixed character and was shifted to a higher energy, thus becoming the highest occupied molecular orbital [35]. A very similar approach to DFT calculations was proposed by researchers in 2018. The molecules they presented were 2- and 3-center MVs belonging to class III or borderline class II/III [38]. Absorption bands between 900 and 2200 nm relating to cationic species were assigned to IVCT excitations. The higher energy bands for dication radicals in 2-center MVs were the N-to-bridge charge transfer (NBCT) transitions. For 3-center MVs, the higher-energy NIR absorption bands were assigned to a combination of NBCT and IVCT bands. This allowed for multiple electron coupling paths to exist in 3-site MVs. The HOMO and LUMO orbital values were not provided for the calculated compounds. However, the arrangement of the frontier orbitals for molecules in the neutral form is presented (Figure 11) [38].
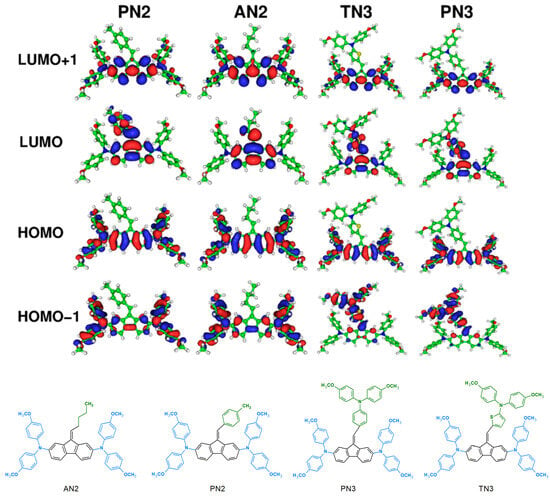
Figure 11.
Isodensity plots of the most relevant molecular orbitals of the neutral compounds. Reprinted (adapted) with permission from [38]. Copyright 2018 American Chemical Society.
The next tested compounds for which calculations were performed were OMS4-7. Dihedral angles were determined for all of their derivatives. The compounds of OMS4-5 containing a thiophene substituent had a larger dihedral angle value than the compounds of OMS6-7 containing furan (Table 2). The reason for this phenomenon was the effect of the steric hindrance of dionones and the lower resonance energy of the furan unit [39]. In the case of a larger dihedral angle, a charge transfer may be difficult, and the absorption bands will be in the region of lower values. Interestingly, OMS5 and OMS7 compounds have higher HOMO orbital values than their analogs (OMS4 and 6). The OMS4 molecule was characterized by the lowest dipole moment among the discussed group. This may result in a deterioration of the electron capture character. In subsequent years, the issue of the behavior of compounds during oxidation was also discussed. As before, the authors determined possible energy transfer routes and centers involved in a given process [43]. In 2022, M. M. Giangregorio et al. referred to the previously described H1 and H2 compounds. This time, the authors expanded the scope of their research to include four other compounds (H3–H6). Absorption results calculated using TD-DFT are presented for the entire series. Interestingly, the values given for H1 and H2 differed significantly (Table 2) [44]. An interesting group of compounds was described in 2022 by M. R. Nagar et al. [45]. These molecules exhibited a donor–acceptor architecture. This was confirmed by the fact that the HOMO orbital was located mainly on the phenoxazine motif. In turn, the LUMO covered more of the fluorene unit, touching only the phenoxazine rings. This fact showed that the phenoxazine ring and the fluorene were not in the same plane; thus, these compounds must have limited π-conjugation along the molecular backbone. Interestingly, in the case of DNFPh, the situation was different [45]. The LUMO orbital was mainly located on fluorene, while the HOMO was spread over the triphenylamine and fluorene unit. The difference in this case may have been due to the strong donor capacity of triphenylamine. Additionally, the singlet and triplet energy levels were determined for the given compounds (Table 2). Moreover, the calculated UV-Vis spectra can be attributed to the charge transfer phenomenon between the donor (D) and acceptor (A) groups [45]. Although theoretical research is gaining more and more supporters, they are not always used [40,46,48]. In summary, in most of the results presented in the literature, the authors focused on correlating theoretical and experimental results. For some compounds, the degree of rotation of the molecule’s core relative to the substituent was considered. In many cases, attempts were made to determine and assign transitions in the absorption bands for molecules oxidized at the first and higher stages. For a large group of molecules, it was possible to determine the values of the HOMO (in the range of −4.68 eV to −5.96 eV) and LUMO (in the range of −1.86 eV to −3.45 eV) orbitals.
5. Redox Behavior
Dibenzofulvene-based (DBF) molecules are an interesting class of compounds with potential applications in the fields of electrochemistry and photovoltaics. This is primarily because of the unique π-bond system that results from DBF, which can be regarded as a vinyl derivative of fluorene. The unique structural feature of DBF allows for various modifications, which, in turn, have a significant impact on its electrochemical properties. In this chapter, we focus on properties such as the reversibility of redox processes and polymerization reactions that occur through the coupling of vinyl or fluorene moieties.
Redox properties are significantly influenced by the nearly planar structure of the DBF molecule. This effect becomes particularly evident when comparing DBF with its most closely related derivative, 1,1-diphenylethylene (Figure 12) [59]. Unlike 1,1-diphenylethylene, DBF readily undergoes polymerization through its vinyl moiety under various conditions. This results in the synthesis of poly(dibenzofulvene) (poly(DBF)) when using anionic, free-radical, and cationic catalysts. This capability arises from the stabilization of the cations, anions, and radicals formed during the polymerization process, thereby leading to polymers with strong π-stacking properties [60,61,62].
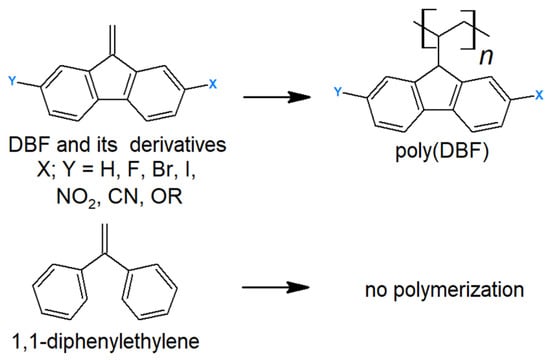
Figure 12.
DBF polymerization through vinyl moiety.
It is worth noting that this process is sensitive to the presence of oxygen, which becomes integrated into the polymer structure, thus forming an alternating copolymer [63]. Extensive studies on DBF derivatives with different substituents have demonstrated their ability to form vinyl polymers through the free-radical polymerization method (Figure 12) [64]. These studies reveal that the DBF structure can be subject to various modifications, thereby resulting in polymers with slightly different properties. Additionally, the use of other monomers such as acrylates can lead to the formation of various DBF copolymers [65].
Electrochemical studies of polymers obtained in this manner indicate the irreversible oxidation of poly(DBF) [61,64]. The high reactivity of vinyl groups explains why the electrochemical oxidation reactions of simple DBFs and their derivatives that lack vinyl substituents do not lead to the formation of an electroactive polymer film through irreversible oxidation reactions [66]. Such electrodeposited products could block the electrode surface due to limited conductivity. However, when substituents are introduced to vinyl moiety, the electrochemical oxidation properties of DBF derivatives change. The reversibility of the first oxidation process increases, thus allowing for subsequent oxidation states. For instance, electrochemical studies have demonstrated that the coupling of 9,9′-bifluorenylidene (F-DBF) occurs within a wide anodic range, and that it extends well beyond the first oxidation state [67]. This leads to the formation of poly(F-DBF), thus creating an electroactive and conductive layer on the working electrode surface. Notably, studies employing coulometric, IR, and NMR spectroscopic techniques indicate that, in such cases, conjugation occurs through the fluorene moieties while preserving the vinyl bond, as shown in Figure 13a.
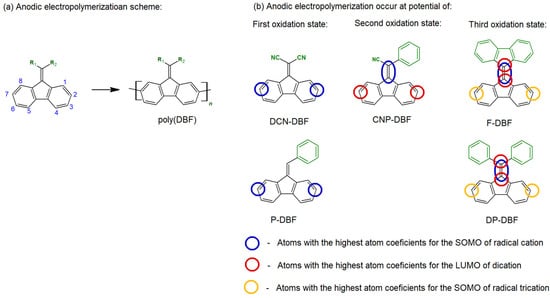
Figure 13.
(a) DBF polymerization through carbon atoms no. 2 and no. 7. (b) Differences in atom coefficients for the SOMO of radical cations, LUMO of dications, and SOMO of radical trications estimated from semi-empirical calculations.
The redox properties of DBF nitrile derivatives have been extensively tested by Rault-Berthelot [68]. Various compounds with different structures, as presented in Figure 13b, were compared. The results revealed significant differences in the electrochemical oxidation process depending on the structure of the monomer used. For example, the presence of two nitrile groups in the DCN-DBF compound led to a shift in the electron density from the vinyl to the fluorene group. As a result, this caused follow-up coupling reactions via the 2,7 positions of the fluorene moiety, which were already in the first oxidation state. Consequently, the polymerization process occurred via the fluorenes.
In the case of CNP-DBF, the deposit formation process was observed during the second oxidation stage, while, for F-DBF, it occurred during the third oxidation step. This variation was related to the differences in electron density, as determined from semi-empirical calculations of the SOMO orbitals of radical cations, the LUMO of dications, and the SOMO of radical trications. These findings aligned with previously published research, such as in article [69]. In this publication, the P-DBF derivative was substituted with a sulfamoyl moiety, thereby resulting in a compound with the highest spin density for the radical cation located on the vinyl group, thus rendering it highly reactive. This reactivity, in turn, led to the irreversible nature of an electrochemical oxidation that involved the vinyl group. These examples illustrate how the properties of DBF-based derivatives strongly depend on the type of substituents and their substitution patterns, with the substitution of the vinyl group significantly impacting the reactivity of the entire molecule.
The possibility of modifying redox properties through the nature and structure of substituents has a direct impact on potential electrochemical applications. One such example is the use of dye-sensitized solar cells (DSSCs). The introduction of functional groups that enhance stability can result in the formation of compounds with stable redox states [70,71]. For instance, the influence of diphenylamines has been extensively studied. Published research demonstrates that diphenylamines, when substituted at carbons C2 and C7, significantly enhance the reversibility of the oxidation process for radical cations and dications, as illustrated in Figure 14. Such substitutions effectively block the oxidative polymerization of the DBF core. It has been observed that the modification of diphenylamines with alkoxy chains can reduce the oxidation potential [33]. However, it is important to consider additional factors such as surface coverage and aggregation when evaluating these effects.
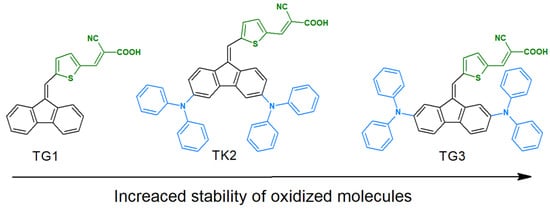
Figure 14.
The reversibility of oxidation processes.
In the literature related to dye-sensitized solar cells (DSSCs), studies have investigated the impact of the type of vinyl substitution used as a conjugation bridge between DBF and anchoring groups [32,39]. Analyzing the results in terms of electrochemical properties, theoretical computations, and their performance in devices revealed a significant contribution of these aspects to electrochemical behavior. What was particularly important was that, depending on the molecule structure, different parts of the molecule were primarily responsible for the oxidation process, which can have a significant impact on DSSC properties. It is worth noting that, when the fluorene part primarily participates in the HOMO orbital, the separation of positive and negative charges tends to occur more easily, thereby leading to improved device parameters.
Electrochemical reversible oxidation combined with the modulation of the transmission of visible and infrared radiation results in electrochromic materials. Electrochromism is the phenomenon of reversible color change during redox processes. Beneduci et al. [35] described these properties for DBF derivatives. Compounds H1, H2, T1, and T2 all comprise a DBF core, diphenylamines substituted at carbons C2 and C7, and a thiophene bridge connected through vinyl. A cyclic voltammetry (CV) of these molecules demonstrated reversible radical cation/neutral and radical cation/dication redox couples. Spectroelectrochemical measurements revealed significant changes in the UV-Vis-NIR spectra, and this was correlated with different oxidized states. This research strategy was also continued in subsequent publications by the same research group [38,43,44]. Compounds AN2, PN2, PN3, and TN3 were used as electrochromic materials, thereby allowing for the modulation of electromagnetic radiation in both the visible and near-infrared ranges [38].
In another study, the series of compounds H1-6 was examined, in which the influence of the thiophene or bithiophene bridge and the modification of diphenylamine substituents was focused on [44]. These studies confirmed that the substitution of C2 and C7 fluorene atoms results in an efficient coupling between diphenylamines. In the case of compounds TN3, T2N3, mTN3, and mT2N3, the effect of substituting three diphenylamines as redox centers was tested [43]. For derivatives with substitutions in positions 2 and 7, the results for the radical cations suggested a self-exchange in electrons between the N1 and N2 diphenylamine groups. For compounds with substitutions in positions 3 and 6, the N3 group was also involved in resonance.
Electrochemical properties are also important for other different types of photovoltaic [46,49] and optoelectronic applications [45]. They enable a quick estimation of the energy levels of HOMO and LUMO orbitals. While these estimates are approximate, they often prove to be highly practical for a range of compounds. EHOMO and ELUMO are summarized in Table 3.

Table 3.
Electrochemical data for the dibenzofulvene derivatives.
6. Optical Properties
Absorption and emission measurements are an essential element of research on chemical compounds. Thanks to them, the parameters and relationships that we can determine often show us the usefulness of molecules in further application research. The presented dibenzofulvene derivatives were examined in detail in terms of optical properties. The research was carried out in organic solvents [32,33,34,35,38,39,40,42,43,50] in a solid [34,43,50], which was in a crystalline form [42], as well as at low temperature [45]. The most frequently used concentration of solutions in which the samples were measured was 1 × 10−5 M. All data obtained in the tests are presented in Table 4.

Table 4.
Optical properties, including maximum absorption (λabs), emission (λPL), the molar extinction coefficient (ε), as well as the quantum yield (Ф) of dibenzofulvene derivatives.
In 2015, G.-F. Zhang et al. drew attention to dibenzofulvene derivatives in their article [57]. The authors presented studies in THF and in a solid state. They showed that the compounds had weak fluorescence when in THF alone. This prompted researchers to check whether they were examples of aggregation-induced emission (AIE). For F-DN1, F-DN2, and F-DAn, it was confirmed that the PL intensity increased significantly with the amount of water. In this case, this effect may be due to morphological transition and/or agglomeration. In addition to the AIE properties, the authors showed that F-DAn is characterized by piezofluorochromism [57]. Its fluorescence reversibly changed from green to red under alternating grinding and solvent fuming procedures. To confirm this effect, the authors recorded the spectra of green fluorescence (after grinding) and red fluorescence (after solvent evaporation). The emission maximums were 536 nm and 620 nm, respectively. Moreover, it was observed that reversible conversions can occur multiple times between both colors. The piezofluorochrome effect could also be observed in the change in quantum yield (0.63 for the green state and 0.11 for the red state). F-DAn may be a promising piezofluorochrome material due to the good reversibility of this effect and clear emission color changes with a high redshift [57]. The next of the presented studies on dibenzofulvene derivatives were carried out for compounds 1-3 [32]. Absorption measurements showed that these molecules had two main bands (Table 4). The first one, with the furthest shift toward higher values in the 422–460 nm range, was assigned to intramolecular charge transfer (ICT). The authors indicated that it resulted from the transfer of charge from the donor (triarylamine) to the acceptor (cyanoacrylic acid). The second band around 350 nm can be attributed to the π–π* transition. It refers to the transitions occurring on the fluorene and bis(ethynyl)fluorene units. When comparing 2 and 3, we see that the compound with an additional vinyl group had more red-shifted bands. Moreover, the maximum emission values and fluorescence quantum yields were determined for the entire group (Table 4). The data presented indicated that the excited state of Dye 3 was more stabilized than those of 1 and 2 [32]. In 2016, DT1, DT2, DP1, and DP2 molecules were tested in the context of AIEE [34]. Also, for these compounds, two absorption bands were demonstrated. First, as before, it was assigned to the intramolecular charge transfer (ICT) process in the region of higher values. The second band corresponded to π−π* transitions. Interestingly, P. Gopikrishna et al. also presented research results that were obtained in thin film. The results obtained in the solid state showed a large red shift compared to the values obtained in the THF solution. Interestingly, a slight structural modification differentiating DT1 from DT2 (when inserting bromine atoms into the molecular structure) resulted in a red shift of the obtained values (Table 4). In addition, the authors examined the effect of the additional phenyl ring on the properties (DP1 and DP2) [34]. The results indicated that the quantum efficiency increased for DP1 and DP2 (with an additional phenyl ring), but the emission values were blueshifted (Table 4). For the presented DT1, DT2, DP1, and DP2 series, measurements were performed in solvents of different polarities. P. Gopikrishna et al. showed that these compounds are subject to the effect of solvatochromism due to the strong dependence on the polarity of the solvent [34]. The AIEE effect was also investigated in the absorption and emission spectra for all compounds. Measurements were carried out in a mixture of THF and water. No changes were observed in the absorption spectra below a water addition of 60%. The bands showed a bathochromic shift after exceeding this value for DT1, DT2, and DP1. DP2 also showed a redshift, which caused the initial two bands to merge into one [34]. Changes in intensity and redshifts were observed in the emission spectra, which suggested the formation of J-type aggregations. The formation of nano-aggregates was additionally confirmed by DLS and TEM [34]. Subsequent studies also examined absorption spectra, thereby revealing two bands that were assigned to the charge transfer (CT) and π−π* transitions [33,38]. In 2018, E. Kasparavicius et al. decided to use the V862 derivative for research on PSC. In their study, they decided to check what happens to the compound after adding 4-tert-butylpyridine (tBP). It was found that the primary oxidized form generated after adding tBP disappears with time and according to the type of solvent polarity. After more detailed studies, it was determined that the ambient light irradiation was sufficient for accelerating the decomposition of the tested oxidized HTMs in solution [37]. Y. C. Chen et al., in their research, presented the UV-Vis results of OMS4-7 compounds. Although these molecules are similar, they have significant differences (Table 4). OSM4-5 have one absorption band that is derived from the combination of local and delocal π−π* transitions. OSM6-7 show two characteristic bands, the first around 420 nm (charge transfer character) and the second below 400 nm (π−π* transition). The introduction of vinylfuran and dianchores into the molecule structure resulted in a red shift of the absorption bands. Interestingly, the authors pointed out that the emission properties were difficult to measure due to the relatively low emission intensity [39]. In 2020, measurements were performed for the dibenzofulvene derivatives with a triphenylamine group (YC1-3). The presented compounds differed in the content of Ome groups in their structure. Interestingly, the molecules were characterized by poor solubility in the polar solvents (DMSO and DMF), while they dissolved well in THF and CH2Cl2. The research showed that the number of OMe groups did not affect the maximum of peaks above 400 nm (peak on the shoulder). The only difference was noticed for the spectra around 380 nm. For the YC-1 without OMe groups, the maximum of this peak was blueshifted by 10 nm when compared to YC-2 and 3 [40]. In 2021, the research results for C2-BFMPT and C8-BFMPT were interestingly described. Initially, absorption and emission tests were performed in a THF solution. It was quickly proven that these compounds had weak emissions in solution [42]. This was due to the high freedom of rotation and rapid relaxation of the excited state in the molecules. Performing a series of emission spectra with different percentages of water confirmed that these compounds exhibited the AIE effect. This prompted the researchers to research the obtained crystal forms. For C8-BFMPT, crystal form II was used due to its high structural disorder, defects within the sample, and its scattering effect in form I [42]. Both samples recorded an increase in quantum yields (Table 4). In addition, the authors decided to further investigate the influence of the environment on the behavior of molecules. For this purpose, they performed measurements in liquid nitrogen. No significant changes in quantum yield were noted for C2-BFMPT. However, a slight blueshift could be observed. Interestingly, C8-BFMPT demonstrated a structured PL spectrum, and the PL QY sharply increased up to 19% [42]. TN3, T2N3, mTN3, and mT2N3 displayed a similar absorption band in the 474–486 nm range. The authors defined this band as the amino-to-bridge (NTB) charge-transfer character (CT). It is interesting to note that the anchoring position of the redox centers on the DBF core significantly influenced the contribution of the different redox centers to this NTB excitation [43]. In the case of compounds TN1, TN3, and T2N3 having redox centers located only in the exocyclic or both exocyclic and 2,7- positions, only the N3 center is involved in the transfer of charge to the bridge. For mTN3 and mT2N3, all centers participate in charge transfer, which redox centers on exocyclic and 3,6-positions ensure. In the case of the absorption band around 383 nm, the anchoring position of the redox centers had a stronger effect. TN2, TN3, and T2N3 had a clear peak at this value. This band can be attributed to the DBF π−π* transition. Interestingly, for mTN3 and mT2N3, this peak did not occur. This can be explained by the fact that the coupling of redox centers in these molecules resulted in a greater involvement of molecular orbitals, which were delocalized on DBF and diphenylamine substituents. For these derivatives, we observed a transition around 265 nm, which refers to the mixed π−π*-CT transition (and also includes diphenylamines) [43]. All of the molecules also saw a peak in the range of 295–306 nm, which corresponded with the π−π* transitions of diphenylamine. M. M. Giangregorio et al., in their studies on compounds H1–H6, showed the same properties as those presented for compounds TN3, T2N3, mTN3, and mT2N3 [44]. In 2022, it was decided to determine the singlet and triplet energies for certain compounds (DNFPhe, DDPFPhe, DFPFPhe, DPFPhe, DDPPFPh, and DNFPh), which were used as hole transporting materials. For this purpose, in addition to standard measurements in solution and solid, the authors recorded emission results at a low temperature (77 K). The triplet energies were estimated from the first triplet peak of the low-temperature PL spectra. The obtained energies ranged from 2.56 to 2.69 eV. The compounds DDPPFPh and DNFPh displayed higher triplet-energies as compared to the other compounds, DNFPhe, DDPFPhe, DFPFPhe and DPFPhe, which may be attributed to their extended conjugation in molecular structure, while the other compounds exhibited an interrupted conjugation long chain [45]. In subsequent studies, scientists have continued to focus on absorption and emission measurements [46,48,50]. Moreover, fluorescence lifetimes were determined for the series of compounds 1a-d and 2c-d [50]. To summarize, dibenzofulvene derivatives have been studied relatively well so far. Research on this important group of compounds was conducted in the solvents of various polarities, both in the solid state and at low temperature. In addition, parameters such as maximum absorptions and emissions, quantum yields, and even lifetimes were determined. Most of the presented compounds showed emissions that were above 500 nm. Such precise characterizations allowed for estimating the usefulness of given compounds in application research.
7. Device Characteristics
Due to their physicochemical properties, dibenzofulvene derivatives have enormous application potential. The mentioned compounds have been investigated, among others, as dyes for dye-sensitized solar cells (DSSC), hole transport materials (HTM) for perovskite cells (PSC), organic light-emitting diodes (OLED), and even luminescent materials. Detailed information regarding the uses of dibenzofulvene derivatives is presented below.
7.1. Dibenzofulvene Derivatives as Dyes for Dye-Sensitized Solar Cells (DSSCs)
In 2015, dibenzofulvene derivatives were used as dyes in dye-sensitized solar cells (DSSCs) [32]. Devices containing Compounds 1–3 were characterized by overall conversion efficiencies (η) of 3.98%, 4.09%, and 4.73%, respectively. The N719 complex, often used as a standard dye in DSSC cells, has a conversion efficiency of (η) = 7.30%. It can, therefore, be concluded that the efficiency of Dye 3 was over 60% of the conversion of the device that had N719 as dye [32]. Despite a slight structural modification between the particles, we observed significant differences in their parameters relative to the devices (Table 5). Dye 3, when used with an additional vinyl group and two (triisopropylsilyl)acetylene (TIPSA) units, showed the greatest broadening of the incident photon conversion efficiency (IPCE) spectra. This was consistent with its bathochromic nature, which was shown in the solution spectra [32]. Surprisingly, it had the lowest FF (fill factors) (Table 5). When comparing Compounds 1–3 in terms of open-cell voltage (Voc), we saw an improvement in this parameter after adding TIPSA units to the structure. This may have resulted from the dark current generated by the reduction of I3− with the injected electrons due to the occurrence of sterically highly hindered groups on the TiO2 surface [32]. In 2016, A.-L. Capodilupo et al. also investigated a group of dibenzofulvene derivatives for DSSC cells [33]. The total conversion efficiency for TK7, TK8, and TK9 was 7.88%, 6.35%, and 6.14%, respectively. As one can see, the device with TK7 had the best parameters (Table 5). This was due to its highest photocurrent density, which is related to the molar extinction coefficient (ε). The trend observed for the Jsc values was the same as for the ε coefficient. The incident photon conversion efficiency (IPCE) spectra for TK7-9 molecules covered a wide range, i.e., from 350 to 750 nm [33]. TK7 had the best IPCE spectrum, and it showed the highest efficiency in the 400–550 nm range, thereby reaching approximately 80%. The next group of DBFs tested as dyes for DSSC cells was OMS4-7 [39]. These compounds had much worse parameters than their predecessors. The total conversion efficiency ranged from 0.41 to 2.42%. This group covered a small range of the incident photon conversion efficiency spectrum (350–600 nm). Moreover, these derivatives showed a low IPCE intensity. Weaker photocurrent densities in the case of the OMS series were caused by the arylamine substituted in the 2,7 positions. Of the entire presented group, OMS4 showed the best parameters. Therefore, Y. C. Chen et al. decided to test the device with this particular dye under lamps with different light intensities [39]. Interestingly, changing the parameters significantly improved the overall conversion efficiency of the device. The best results were obtained for the TL84-2500 lx lamp (8.78%). All results are presented in Table 5 [39]. All of the compounds used so far are presented in Figure 15.

Table 5.
Dibenzofulvene derivatives used as dyes in dye-sensitized solar cells (DSSCs).
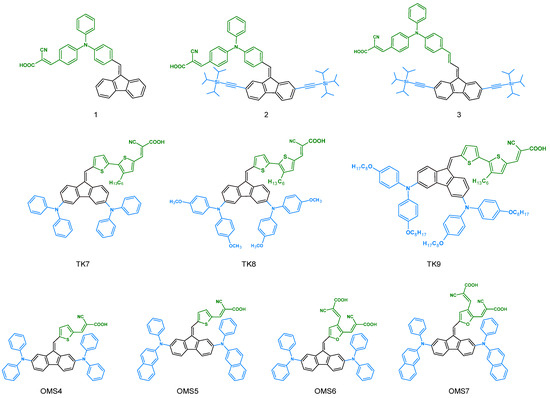
Figure 15.
Structures of the dibenzofulvene derivatives used as dyes in dye-sensitized solar cells (DSSCs).
7.2. Dibenzofulvene Derivatives as Hole Transport Materials (HTMs) in Perovskite Solar Cells (PSCs)
Another application of dibenzofulvene derivatives is as hole transport materials (HTMs) for perovskite cells (PSCs). The first mentions of the use of the compounds discussed as HTMs date back to 2018 [37]. E. Kasparavicius et al., in their research, presented results related to the stability of the oxidized forms of the V862 derivative. Thanks to UV-Vis tests, they were able to determine how long it takes for the compound in question to degrade under specific conditions, such as in high temperatures or in an aerobic or anaerobic environment [37]. In 2020, further research was published on the use of dibenzofulvene derivatives (YC-1-3) as hole transport materials [40]. Initially, the authors focused on describing the structure of the device and preliminary measurements. They created test devices with the structure of ITO/HTL/CH3NH3PbI3/PC61BM/BCP/Ag. PEDOT:PSS was used as a model, and YC-1, YC-2, and YC-3 were used as HTLs (hole transport layers). The first measurements were based on UV-Vis, photoluminescence (PL), time-resolved PL (TRPL) spectra, and X-ray diffraction (XRD). From the tests performed, it was observed that YC-1-3 had a similar perovskite absorption behavior as the previously used PEDOT:PSS. Thanks to this, it was assumed that a sufficient light flux should reach the light-absorbing layer to generate a photocurrent [40]. Photoluminescence measurements at a 550 nm excitation gave a peak near 770 nm, which was attributed to the perovskite’s PL. The decay time for the perovskite spin coated on YC-1 was shorter than that on PEDOT:PSS, whereas on YC-2 and YC-3 layers perovskite exhibited longer decay times when compared with the standard HTL. As a result, the hole extraction capability of YC-1 was superior to those of the others, thereby suggesting that its p–i interface featured the most efficient charge transfer [40]. Compounds YC-2 and YC-3 had lower hole extraction ability, which resulted from a poorer match of energy levels with the perovskite layer. Measurements carried out on ten individual devices were used to determine the final parameters of the device (Table 6). The device efficiency (PCE) for YC-1 was 15.78 ± 0.61%. This was the highest result achieved in the entire YC series. Moreover, this value was higher than the device with PEDOT:PSS as a HTM (12.80 ± 1.31). Better FF and Voc values were responsible for such a great result when compared to the standard. Devices containing YC-2 and YC-3 had worse parameters, which could result from a poorer matching of HOMO orbitals with the perovskite (Table 6). Moreover, in their research, the authors proved that YC-1 as a HTL was characterized by the fastest charge transfer. Cell stability tests showed that, after approximately 1300 h, the cell retained 97.3% of its original efficiency. Interestingly, this exceeded the efficiency of the standard cell (with PEDOT: PSS), whose efficiency in these conditions was 75.1%. This good performance may be due to the excellent interfacial contact, hydrophobicity, and dopant-free characteristics of the YC-1-based device [40]. To improve the parameters of the compounds YC as HTLs, Y.-C. Chen et al. decided to make a device in which a two-layer system would be used as an HTL. They chose the best from the YC-1 series as the organic layer, and the inorganic component was NiOx. The research showed that using such a system increased the efficiency of the device (19.37%). Moreover, the cell stability was 96.3% of the initial device efficiency. The authors found that the use of NiOx with YC-1 as an HTL resulted in an increase in PCE and good stability. Moreover, they concluded that this cell had commercial potential [40]. Such promising results of YC-1 prompted researchers to conduct further research on it [41]. This time YC-1 also served as an HTM, but the design of the device was different (ITO/SnO2 (~30 nm)/PCBM/MAPbI3 perovskite (~500 nm)/YC-1 (40 nm) or P3HT (~60 nm)/MoO3 (5 nm)/Ag (100 nm)), and P3HT was used as a standard. H.-C. Lin et al. presented extensive and detailed characteristics of the manufactured devices. Incident photon conversion efficiency (IPCE) spectra were also performed for the obtained cells. The spectral sensitivity range covered the area from 400 to 800 nm. The maximum IPCE value was 90.3% (YC-1) and 84.7% (P3HT). The PSC efficiency for YC-1 was 13.89 ± 1.12, and it was determined based on four devices. Detailed data are presented in Table 6. And, this time, the authors came to the conclusion that the cell containing YC-1 in its structure turned out to be better than the standard P3HT [41]. However, researchers do not always have the opportunity to determine the test parameters of devices. To check whether the designed particles can be hole transport materials, space-charge-limited current (SCLC) method tests can be performed [44]. This method makes it possible to measure charge mobility in a direction that is perpendicular to the film. Such tests for the series of Compounds H1-H6 were performed by M. M. Giangregorio et al. [44]. H1-H6 derivatives were not characterized by high hole mobility values. It was also impossible to indicate a correlation between the hole mobility measurements and the chemical structure [44]. As part of further research in 2022, SC-1-4 compounds were used to build test cells [46]. The design of the devices was as follows: ITO/NiOx/SC-1–4/CH3NH3PbI3/PC61BM/Bathocuproine(BCP)/Ag. The addition of SC-1-4 as HTMs between the perovskite layer and NiOx allowed for the improvement of the cell parameters. Among all the derivatives, SC-4, in combination with NiOx, turned out to be the best (Table 6). The maximum cell efficiency for this HTM was 19.86% (NiOx by itself was 18.97%). The improved cell performance was influenced by better hole mobility, morphological behavior, and time-resolved PL results [46]. Moreover, the HOMO orbital value for SC-4 was closest to that of perovskite. External quantum efficiency (EQE) spectra were performed for all devices. The spectra clearly showed the good intensity of the tested HTMs in the range of 300 to 800 nm. Additionally, the authors examined the long-term stability of the test devices. For this purpose, they used non-encapsulated PSC cells that were stored in an argon atmosphere or in air at a temperature of 25 °C. For NiOx, NiOx/SC-1, NiOx/SC-2 NiOx/SC-3, and NiOx/SC-4-based devices in an argon atmosphere, the stability after 80 days was 75%, 83%, 87%, 91%, and 80%, respectively. However, for the devices stored in air, it was 65%, 83%, 84%, 72%, and 91%, respectively, after 49 days. NiOx/SC-4, when kept in aerobic conditions, was characterized by the greatest stability, which may be due to its more hydrophobic nature [46]. Interesting research was also presented by S. Mandati et al. [48]. In their extensive work, they showed that new HTMs, marked as V808, V1385, and V1386, enable PSC constructions [48]. The cell efficiencies with these HTMs were 4.7%, 4.9%, and 4.5% (for V808, V1385, and V1386, respectively). These values were close to or better than the P3HT standard (4.68%). Interestingly, the authors determined the thickness of the HTM layers for individual cell structures. These studies showed that, in order to achieve an efficiency close to 4.7% for P3HT, a layer with a thickness of ≈100 nm must be applied. For comparison, similar yields for the V808, V1385, and V1386 derivatives were achieved for layers with a thickness of ≈25–30 nm [48]. In 2023, tests were carried out for the CC series [49]. The construction of the devices was as follows: ITO/NiOx/CC-1–3/CH3NH3PbI3/PC61BM/BCP/Ag. For all three derivatives, this structure was two layered. To compare the results and to assess the effectiveness of this architecture, tests were performed for a single-layer device based on CC-3 without the presence of -CF3 groups. The single-layer device gave much lower results than the two-layer device [49]. Among the CC derivatives used in the two-layer construction, CC-3 turned out to be the best. The cell with a NiOx/CC-3 architecture achieved a maximum efficiency of 19.82%. Achieving the best parameters was influenced by the appropriate energy level, a larger grain size, a smooth surface, and the hydrophobicity of NiOx/CC-3. In addition, the authors determined the external quantum efficiency (EQE) [49]. EQE spectra showed the greatest intensity in the 350–800 nm range. The authors of the article also tested the long-term stability of non-encapsulated cells. Test PSCs were stored in an argon atmosphere and in air at 25 °C. Stability tests showed that, after 40 days, the NiOx/CC-3 device retained 80% of the initial PCE value. This good result was attributed to the appropriate hydrophobicity of the device [49]. It is also worth paying attention to the research conducted by S. Kotowicz et al. In these studies, the amount of doped Li+ salt was investigated. The authors showed that, by increasing the concentration of lithium bis(trifluoromethanesulfonyl)imide (Li-TFSI), the PCE of the cell can be improved. Interestingly, a large excess of dopant does not have a positive effect on the cell efficiency [50]. J. G. Sánchez et al. decided to use the compounds T1, PN2, and PN3, which were already known from the literature [35,38]. The researchers performed application tests for them with respect to perovskite cells (PSCs) [72]. They constructed a device with the FTO/compact TiO2/LiTFSI-doped mesoporous TiO2/CsFAMA/HTM/Au structure. T1, PN2, and PN3 served as HTMs. The standard was Spiro-OMeTAD. Additionally, a triple cesium cation (CsFAMA) was used in the cell structure. The PN3 compound had the best parameters, thanks to which the tested cell showed an efficiency of 16.08% (with a JSC = 20.83 mA/cm2, a VOC = 1.046 V, and an FF = 73.82%). This result was slightly lower than the standard for which PCE was 17.75% (with parameters equal to a JSC = 21.86 mA/cm2, a VOC = 1.086 V, and an FF = 74.73%). PN2 also turned out to be slightly worse. Their PCE was 15.29%. This could be due to the lower VOC value (Table 6). The worst result from the entire series of compounds was obtained by T1 (PCE = 13.53%). This result may be due to a poorer match between the HOMO T1 energy level and the maximum of the CsFAMA [72] valence band. External quantum efficiency measurements were also performed for the manufactured devices. Through interpreting the results obtained in the spectra, it can be seen that the EQE for PN3 and T1 were very close to the standard in the range of 350–550 nm. Interestingly, in the same range, PN2 showed significantly higher values. In the range of 550–750 nm, the values of the tested devices decrease slightly and were lower than for Spiro-OMeTAD. In their work, the authors also presented studies on the impact of DBF-based HTMs on recombination mechanisms in photovoltaic devices. The obtained studies showed that trap-assisted recombination is suppressed in devices when using DBF-based HTMs more effectively compared to a recombination using Spiro-OMeTAD. The authors also suggested that there is a reduced surface defect and greater charge extraction capacity in a perovskite/HTM interface in devices that are prepared with PN3, PN2, and T1 [72]. The stability of the given devices was also tested. J. G. Sánchez et al. monitored the performance of the device under a continuous one-sun illumination in ambient conditions (15 ± 2 °C and 60 ± 5% RH). Typically, this stability is determined by the t80 parameter showing a 20% loss in performance. Interestingly, PSCs with DBF-based HTMs are more stable than the device with Spiro-OMeTAD. The device with Spiro-OMeTAD in its structure reached t80 after 50 min. For PSCs with PN2 and T1, t80 was achieved after 300 and 200 min, respectively. PSC with PN3 was the most stable, and the t80 parameter was not obtained during the measurement, which may indicate that its time may be longer than 1000 min. In addition, the stability of the devices over time was also examined. After 95 days, measurements showed that the devices’ PCE decreased in the PN3 (10%), PN2 (11%), T1 (14%), and Spiro-OMeTAD (19%) sequences [72]. All of the compounds used so far are presented in Figure 16.

Table 6.
Dibenzofulvene derivatives used as hole transport materials (HTMs) in perovskite solar cells (PSCs).
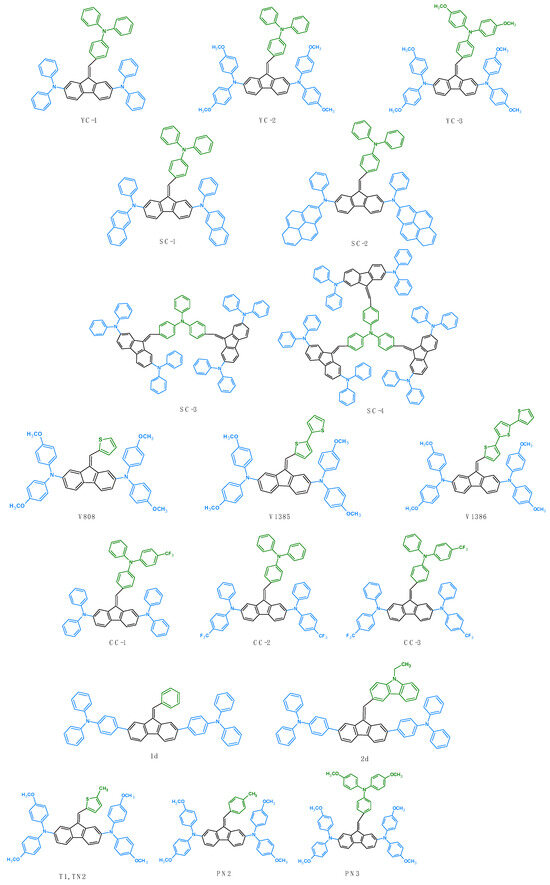
Figure 16.
Structures of dibenzofulvene derivatives used as hole transport materials (HTMs) in perovskite solar cells (PSCs).
7.3. Other Uses of Dibenzofulvene Derivatives
Dibenzofulvene derivatives have also been investigated as luminescent materials. In 2015, G.-F. Zhang et al. drew attention to dibenzofulvene derivatives. They showed that F-DN1, F-DN2, and F-DAn have an aggregation-induced emission (AIE) character. Moreover, one of them showed a strong phenomenon called piezofluorochromism with a shift of 80 nm. F-DAn changed the color from green to red under alternating grinding and solvent-fuming stimuli [57]. Already in 2016, there were reports that the compounds DT1, DT2, DP1, and DP2 exhibited the AIEE phenomenon [34]. DT1 and DT2 had a redshift of approximately 45 nm and 94 nm, respectively, in the aggregated state. Derivatives DP1 and DP2 exhibited dual-state emitting behavior [34]. A. A. Sonina et al. also showed that the C2-BFMPT and C8-BFMPT compounds have an aggregation-induced emission (AIE) character, which they presented in their work [42]. C. Cunha et al. also presented the AIE nature of their compounds. Moreover, they performed detailed studies of DBF in an aggregated state [58].
In 2018, G.A. Corrente et al. investigated DBF derivatives as electrochromic devices [38]. PN2, AN2, TN3, and PN3 were anodic components in constructing the EC devices. In the neutral state, the color of the molecules depends on the NBCT transition at approximately 450 nm. The DBF derivatives were enabled to obtain electrochromic films covering a wide range of off-state colors, from the almost colorless AN2, a pale pink PN2, and a weakly orange PN3 up to an orange-red TN3 [38]. It was also possible to obtain a black color. The absorption bands of these compounds, called MV, cover almost the entire VIS range in oxidized states. Moreover, this range can be extended to nearly the entire NIR. It was enough to apply switching potentials, thereby allowing for the radical cation and the dication species to coexist. Devices based on the presented derivatives (PN2, AN2, TN3, and PN3) can change from a transmissive state (i.e., almost completely permeable) to an opaque state under the influence of the activated pulse. The authors also studied the aging of test EC devices. It was found that, although the devices were manufactured in an air atmosphere and that no seals were used, the wear was still low. Only after 4500 cycles was a drop in transmittance below 8% observed [38].
In numerous works (mentioned in the previous Section 7.2.) on dibenzofulvene derivatives, they have been presented as good hole transport materials (HTMs). M. R. Nagar et al. decided to use this potential in organic light-emitting diodes (OLEDs) [45]. They decided to investigate the possibilities of the hole transport of their compounds (DNFPhe, DDPFPhe, DFPFPhe, DPFPhe, DDPPFPhe, and DNFPhe) using the example of yellow phosphorescent OLED and green TADF OLED. The results obtained from the measurements are presented in Table 7. Tests for yellow phosphorescent OLED showed that the newly used HTMs gave better results than the NPB standard. Many factors influenced this. Among other things, effective electron and exciton confinement within the emissive layer due to their shallower LUMO energy levels, particularly at high brightness or voltages, were essential. The surface of the materials was also important; in the case of compounds based on phenoxazine and phenothiazine, it turned out to be smooth. This helped prevent unwanted current leakage when measuring the device. The new HTMs showed high hole mobility and efficient high-triplet energies. It is also worth noting that the energy levels of the host and guest were consistent, which helped inject holes into the emission layer. In addition, it was decided to check the durability of the constructed test OLEDs [45]. The DDPPFPh derivative, having a phenothiazine in its structure at an initial brightness of 10,000 cd m−2, gave a lifetime of 11.7 h. For comparison, the NPB standard showed a service life of 3.8 h under the same conditions. When analyzing the results for the initial brightness of 1000 cd m−2, we also see a significant difference. The device with DDPPFPh gives a lifetime of 465 h, while the standard one is only 151 h. Similar results were obtained for a device with an architecture containing phenoxazine (DDPFPhe), for which the lifetime was 262 h, and this was also higher than for NPB. In the case of green TADF OLED, we can observe analogies. The best compound in the entire series turned out to be DDPPFPh. It achieved the highest efficiency of the device, which could be the result of high hole mobility, appropriate HOMO and LUMO energy levels, and adequately high triplet energy. LEDs with newly synthesized HTMs showed better parameters than the standard (Table 7). Furthermore, the use of dibenzofulvene derivatives with a phenothiazine or phenoxazine motif as HTMs provided improved device stability [45]. All of the compounds used so far are presented in Figure 17.

Table 7.
Dibenzofulvene derivatives used as hole transport materials (HTMs) in OLEDs.
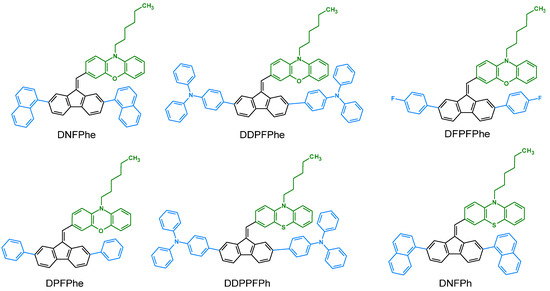
Figure 17.
Structures of dibenzofulvene derivatives used as hole transport materials (HTMs) in OLEDs.
8. Conclusions
This review summarizes the current knowledge regarding dibenzofulvene derivatives (DBF) in photovoltaic and organic electronic applications. Dibenzofulvene derivatives are obtained by simple and efficient Knoevenagel condensation. Then, their structure is modified using the Buchwald–Hartwig, Suzuki, or Sonogashira coupling reactions. This makes it possible to obtain a very wide group of compounds that differ in terms of substituents. Moreover, dibenzofulvene derivatives exhibit good thermal properties as their thermal stability is generally above 300 °C. These compounds mostly exhibit two main absorption bands, which are assigned as ICT (above 400 nm) and π–π*. They often present dependencies on the solvent used. They may also exhibit the AIEE phenomenon. Most of them have emissions above 500 nm. Many of the experimental studies described in the literature have often been confronted with DFT calculations. Thanks to this, it was possible to determine the location of the HOMO and LUMO orbitals. Moreover, in many cases, theoretical values of the absorption maximum were determined, thus confirming the compliance of the calculations with experimental studies. Due to their properties, dibenzofulvene derivatives are being tested as dyes for DSSC cells, hole transport materials (HTM) for PSC and OLED cells, and luminescent or electrochromic materials. Despite the group of dibenzofulvene derivatives presented in this article having been examined and described, these compounds still have a very large potential to be successfully used in further research. In the longer term, the development of the presented dibenzofulvene derivatives will be possible in the synthesis of more complex compounds in terms of substituents in Positions 2.7 and 3.6, as well as in the double bond. The huge number of available substrates allows for the design and preparation of new compounds that are tailored to selected applications. Moreover, thanks to such a wide range of substrates and new compounds, it will be possible to successfully determine the influence of substituents on the properties of dibenzofulvene derivatives. Currently, an essential aspect of research is a thorough knowledge of the produced compounds in terms of basic research, which helps select compounds for application. The abovementioned derivatives can also be used as monomers for the synthesis of polymers, which may also have a positive impact on the progress in the field of materials dedicated to photovoltaic and organic electronics. We hope that this review will be helpful in the design and research of subsequent dibenzofulvene derivatives.
Author Contributions
Conceptualization, A.S.-K., P.L. and S.K.; methodology, A.S.-K., P.L. and S.K.; investigation, A.S.-K., P.L., A.K. and S.K.; data curation, A.K. and S.K.; writing—original draft preparation, A.S.-K., P.L. and S.K.; writing—review and editing, A.S.-K., P.L. and S.K.; visualization, A.S-K, P.L., A.K. and S.K.; supervision, S.K.; project administration, S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Szlapa-Kula, A.; Kula, S. Progress on Phenanthroimidazole Derivatives for Light-Emitting Electrochemical Cells: An Overview. Energies 2023, 16, 5194. [Google Scholar] [CrossRef]
- Santillán, O.S.; Cedano, K.G. Energy Literacy: A Systematic Review of the Scientific Literature. Energies 2023, 16, 7235. [Google Scholar] [CrossRef]
- Rusănescu, C.O.; Rusănescu, M.; Istrate, I.A.; Constantin, G.A.; Begea, M. The Effect of Dust Deposition on the Performance of Photovoltaic Panels. Energies 2023, 16, 6794. [Google Scholar] [CrossRef]
- Milewska, B.; Milewski, D. The Impact of Energy Consumption Costs on the Profitability of Production Companies in Poland in the Context of the Energy Crisis. Energies 2023, 16, 6519. [Google Scholar] [CrossRef]
- Mohammad, A.K.; Garrod, A.; Ghosh, A. Do Building Integrated Photovoltaic (BIPV) Windows Propose a Promising Solution for the Transition toward Zero Energy Buildings? A Review. J. Build. Eng. 2023, 79, 107950. [Google Scholar] [CrossRef]
- Dada, M.; Popoola, P. Recent Advances in Solar Photovoltaic Materials and Systems for Energy Storage Applications: A Review. Beni-Suef Univ. J. Basic Appl. Sci. 2023, 12, 66. [Google Scholar] [CrossRef]
- Li, X.; Yu, H.; Liu, Z.; Huang, J.; Ma, X.; Liu, Y.; Sun, Q.; Dai, L.; Ahmad, S.; Shen, Y.; et al. Progress and Challenges Toward Effective Flexible Perovskite Solar Cells. Nano-Micro Lett. 2023, 15, 206. [Google Scholar] [CrossRef]
- Kumar, P.; You, S.; Vomiero, A. Recent Progress in Materials and Device Design for Semitransparent Photovoltaic Technologies. Adv. Energy Mater. 2023, 13, 2301555. [Google Scholar] [CrossRef]
- Liu, J.; Ye, T.; Yu, D.; Liu, S.; Yang, D. Recoverable Flexible Perovskite Solar Cells for Next-Generation Portable Power Sources. Angew. Chem. Int. Ed. 2023, 62, e202307225. [Google Scholar] [CrossRef]
- Maalouf, A.; Okoroafor, T.; Jehl, Z.; Babu, V.; Resalati, S. A Comprehensive Review on Life Cycle Assessment of Commercial and Emerging Thin-Film Solar Cell Systems. Renew. Sustain. Energy Rev. 2023, 186, 113652. [Google Scholar] [CrossRef]
- Sugiura, T.; Nakano, N. Review: Numerical Simulation Approaches of crystalline-Si Photovoltaics. Energy Sci. Eng. 2023, 11, 3888–3906. [Google Scholar] [CrossRef]
- Diouf, B.; Muley, A.; Pode, R. Issues, Challenges, and Future Perspectives of Perovskites for Energy Conversion Applications. Energies 2023, 16, 6498. [Google Scholar] [CrossRef]
- Sayed, E.; Olabi, A.; Alami, A.; Radwan, A.; Mdallal, A.; Rezk, A.; Abdelkareem, M. Renewable Energy and Energy Storage Systems. Energies 2023, 16, 1415. [Google Scholar] [CrossRef]
- Bei, Q.; Zhang, B.; Wang, K.; Zhang, S.; Xing, G.; Cabanetos, C. Benzothiadiazole-Based Materials for Organic Solar Cells. Chin. Chem. Lett. 2024, 35, 108438. [Google Scholar] [CrossRef]
- Rafique, S.; Abdullah, S.M.; Sulaiman, K.; Iwamoto, M. Fundamentals of Bulk Heterojunction Organic Solar Cells: An Overview of Stability/Degradation Issues and Strategies for Improvement. Renew. Sustain. Energy Rev. 2018, 84, 43–53. [Google Scholar] [CrossRef]
- Schoden, F.; Dotter, M.; Knefelkamp, D.; Blachowicz, T.; Schwenzfeier Hellkamp, E. Review of State of the Art Recycling Methods in the Context of Dye Sensitized Solar Cells. Energies 2021, 14, 3741. [Google Scholar] [CrossRef]
- Grifoni, F.; Bonomo, M.; Naim, W.; Barbero, N.; Alnasser, T.; Dzeba, I.; Giordano, M.; Tsaturyan, A.; Urbani, M.; Torres, T.; et al. Toward Sustainable, Colorless, and Transparent Photovoltaics: State of the Art and Perspectives for the Development of Selective Near-Infrared Dye-Sensitized Solar Cells. Adv. Energy Mater. 2021, 11, 2101598. [Google Scholar] [CrossRef]
- Bodedla, G.B.; Zhu, X.; Zhou, Z.; Wong, W.-Y. Small Molecules Containing Amphoteric Imidazole Motifs as Sensitizers for Dye-Sensitized Solar Cells: An Overview. Top. Curr. Chem. Z 2022, 380, 49. [Google Scholar] [CrossRef]
- Al-Ahmed, A.; Afzaal, M.; Mahar, N.; Khan, F.; Pandey, S.; Zahir, M.H.; Al-Suliman, F.A. The Synergy of Lead Chalcogenide Nanocrystals in Polymeric Bulk Heterojunction Solar Cells. ACS Omega 2022, 7, 45981–45990. [Google Scholar] [CrossRef]
- Göhler, C.; Deibel, C. The Role of Dynamic and Static Disorder for Charge-Transfer States in Organic Bulk Heterojunction Solar Cells. ACS Energy Lett. 2022, 7, 2156–2164. [Google Scholar] [CrossRef]
- Xie, Y.; Lu, H.; Huang, J.; Xie, H. Natural Materials for Sustainable Organic Solar Cells: Status and Challenge. Adv. Funct. Mater. 2023, 33, 2213910. [Google Scholar] [CrossRef]
- Solak, E.K.; Irmak, E. Advances in Organic Photovoltaic Cells: A Comprehensive Review of Materials, Technologies, and Performance. RSC Adv. 2023, 13, 12244–12269. [Google Scholar] [CrossRef] [PubMed]
- Mohamed El Amine, B.; Zhou, Y.; Li, H.; Wang, Q.; Xi, J.; Zhao, C. Latest Updates of Single-Junction Organic Solar Cells up to 20% Efficiency. Energies 2023, 16, 3895. [Google Scholar] [CrossRef]
- Tarique, W.B.; Uddin, A. A Review of Progress and Challenges in the Research Developments on Organic Solar Cells. Mater. Sci. Semicond. Process. 2023, 163, 107541. [Google Scholar] [CrossRef]
- Ebenezer Anitha, A.; Dotter, M. A Review on Liquid Electrolyte Stability Issues for Commercialization of Dye-Sensitized Solar Cells (DSSC). Energies 2023, 16, 5129. [Google Scholar] [CrossRef]
- Najm, A.S.; Alwash, S.A.; Sulaiman, N.H.; Chowdhury, M.S.; Techato, K. N719 Dye as a Sensitizer for Dye-sensitized Solar Cells (DSSCs): A Review of Its Functions and Certain Rudimentary Principles. Environ. Prog. Sustain. Energy 2023, 42, e13955. [Google Scholar] [CrossRef]
- Baumeler, T.; Saleh, A.A.; Wani, T.A.; Huang, S.; Jia, X.; Bai, X.; Abdi-Jalebi, M.; Arora, N.; Grätzel, M.; Dar, M.I. Champion Device Architectures for Low-Cost and Stable Single-Junction Perovskite Solar Cells. ACS Mater. Lett. 2023, 5, 2408–2421. [Google Scholar] [CrossRef]
- Han, C.; Xiao, X.; Zhang, W.; Gao, Q.; Qi, J.; Liu, J.; Xiang, J.; Cheng, Y.; Du, J.; Qiu, C.; et al. Impact and Role of Epitaxial Growth in Metal Halide Perovskite Solar Cells. ACS Mater. Lett. 2023, 5, 2445–2463. [Google Scholar] [CrossRef]
- Wei, Y.; Lan, C.; Zhou, S.; Li, C. Recent Advances in Photodetectors Based on Two-Dimensional Material/Si Heterojunctions. Appl. Sci. 2023, 13, 11037. [Google Scholar] [CrossRef]
- Moineau-Chane Ching, K.I. Impact of Alkyl-Based Side Chains in Conjugated Materials for Bulk Heterojunction Organic Photovoltaic Cells—A Review. Energies 2023, 16, 6639. [Google Scholar] [CrossRef]
- Beaupré, S.; Boudreault, P.T.; Leclerc, M. Solar-Energy Production and Energy-Efficient Lighting: Photovoltaic Devices and White-Light-Emitting Diodes Using Poly(2,7-fluorene), Poly(2,7-carbazole), and Poly(2,7-dibenzosilole) Derivatives. Adv. Mater. 2010, 22, E6–E27. [Google Scholar] [CrossRef] [PubMed]
- Tigreros, A.; Rivera-Nazario, D.M.; Ortiz, A.; Martin, N.; Insuasty, B.; Echegoyen, L.A. Fluoren-9-ylidene-Based Dyes for Dye-Sensitized Solar Cells. Eur. J. Org. Chem. 2015, 2015, 5537–5545. [Google Scholar] [CrossRef]
- Capodilupo, A.-L.; Giannuzzi, R.; Corrente, G.A.; De Marco, L.; Fabiano, E.; Cardone, A.; Gigli, G.; Ciccarella, G. Synthesis and Photovoltaic Performance of Dibenzofulvene-Based Organic Sensitizers for DSSC. Tetrahedron 2016, 72, 5788–5797. [Google Scholar] [CrossRef]
- Gopikrishna, P.; Iyer, P.K. Monosubstituted Dibenzofulvene-Based Luminogens: Aggregation-Induced Emission Enhancement and Dual-State Emission. J. Phys. Chem. C 2016, 120, 26556–26568. [Google Scholar] [CrossRef]
- Beneduci, A.; Corrente, G.A.; Fabiano, E.; Maltese, V.; Cospito, S.; Ciccarella, G.; Chidichimo, G.; Gigli, G.; Capodilupo, A.-L. Orthogonal Electronic Coupling in Multicentre Arylamine Mixed-Valence Compounds Based on a Dibenzofulvene–Thiophene Conjugated Bridge. Chem. Commun. 2017, 53, 8960–8963. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Q. Recent Progress in Non-Fullerene Small Molecule Acceptors in Organic Solar Cells (OSCs). J. Mater. Chem. C 2017, 5, 1275–1302. [Google Scholar] [CrossRef]
- Kasparavicius, E.; Magomedov, A.; Malinauskas, T.; Getautis, V. Long-Term Stability of the Oxidized Hole-Transporting Materials Used in Perovskite Solar Cells. Chem. A Eur. J. 2018, 24, 9910–9918. [Google Scholar] [CrossRef]
- Corrente, G.A.; Fabiano, E.; Manni, F.; Chidichimo, G.; Gigli, G.; Beneduci, A.; Capodilupo, A.-L. Colorless to All-Black Full-NIR High-Contrast Switching in Solid Electrochromic Films Prepared with Organic Mixed Valence Systems Based on Dibenzofulvene Derivatives. Chem. Mater. 2018, 30, 5610–5620. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Huang, G.-W.; Chang, Y.-J.; Wen, J.-J. Branched Dibenzofulvene-Based Organic Dyes for Dye-Sensitized Solar Cells under One Sun and Dim Light. J. Mater. Sci. Mater. Electron. 2019, 30, 12981–12991. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Li, Y.-H.; Chung, C.-L.; Hsu, H.-L.; Chen, C.-P. Triphenylamine Dibenzofulvene–Derived Dopant-free Hole Transporting Layer Induces Micrometer-sized Perovskite Grains for Highly Efficient near 20% for P-i-n Perovskite Solar Cells. Prog. Photovolt. 2020, 28, 49–59. [Google Scholar] [CrossRef]
- Lin, H.-C.; Chen, L.-Y.; Lu, C.-C.; Lai, J.-Y.; Chen, Y.-C.; Hung, Y.-J. Ambipolar Carrier Transport Properties of Triphenylamine/Dibenzofulvene Derivative and Its Application for Efficient n-i-p Perovskite Solar Cells. Org. Electron. 2021, 95, 106200. [Google Scholar] [CrossRef]
- Sonina, A.A.; Becker, C.S.; Kuimov, A.D.; Shundrina, I.K.; Komarov, V.Y.; Kazantsev, M.S. Alkyl-Substituted Bis(4-((9 H -Fluoren-9-Ylidene)Methyl)Phenyl)Thiophenes: Weakening of Intermolecular Interactions and Additive-Assisted Crystallization. CrystEngComm 2021, 23, 2654–2664. [Google Scholar] [CrossRef]
- Capodilupo, A.-L.; Fabiano, E.; Franco, L.; Gambino, S.; Leoncini, M.; Accorsi, G.; Gigli, G. Control of Electron Transfer Processes in Multidimensional Arylamine-Based Mixed-Valence Compounds by Molecular Backbone Design. J. Phys. Chem. A 2021, 125, 7840–7851. [Google Scholar] [CrossRef] [PubMed]
- Giangregorio, M.M.; Gambino, S.; Fabiano, E.; Leoncini, M.; Cardone, A.; Corrente, G.A.; Beneduci, A.; Accorsi, G.; Gigli, G.; Losurdo, M.; et al. Synthesis and Investigation of Electro-Optical Properties of H-Shape Dibenzofulvene Derivatives. Molecules 2022, 27, 1091. [Google Scholar] [CrossRef]
- Nagar, M.R.; Choudhury, A.; Tavgeniene, D.; Beresneviciute, R.; Blazevicius, D.; Jankauskas, V.; Kumar, K.; Banik, S.; Ghosh, S.; Grigalevicius, S.; et al. Solution-Processable Phenothiazine and Phenoxazine Substituted Fluorene Cored Nanotextured Hole Transporting Materials for Achieving High-Efficiency OLEDs. J. Mater. Chem. C 2022, 10, 3593–3608. [Google Scholar] [CrossRef]
- Lin, S.-C.; Cheng, T.-H.; Chen, C.-P.; Chen, Y.-C. Structural Effect on Triphenylamine Dibenzofulvene Based Interfacial Hole Transporting Materials for High-Performance Inverted Perovskite Solar Cells. Mater. Chem. Phys. 2022, 288, 126385. [Google Scholar] [CrossRef]
- Das, A.S.; Nair, A.R.; Sreekumar, A.; Sivan, A. Approaches to Obtaining Fluorenes: An Alternate Perspective. ChemistrySelect 2022, 7, e202201097. [Google Scholar] [CrossRef]
- Mandati, S.; Juneja, N.; Katerski, A.; Jegorovė, A.; Grzibovskis, R.; Vembris, A.; Dedova, T.; Spalatu, N.; Magomedov, A.; Karazhanov, S.; et al. 4.9% Efficient Sb2S3 Solar Cells from Semitransparent Absorbers with Fluorene-Based Thiophene-Terminated Hole Conductors. ACS Appl. Energy Mater. 2023, 6, 3822–3833. [Google Scholar] [CrossRef]
- Sholihah, N.; Cheng, H.-C.; Wang, J.-C.; Ni, J.-S.; Yu, Y.-Y.; Chen, C.-P.; Chen, Y.-C. Passivation of Inverted Perovskite Solar Cells by Trifluoromethyl-Group-Modified Triphenylamine Dibenzofulvene Hole Transporting Interfacial Layers. J. Phys. Chem. C 2023, 127, 6167–6178. [Google Scholar] [CrossRef]
- Kotowicz, S.; Tavgeniene, D.; Beresneviciute, R.; Zaleckas, E.; Krucaite, G.; Katarzyna Pająk, A.; Korzec, M.; Grzegorz Małecki, J.; Lipiński, M.; Grigalevicius, S.; et al. Effect of Substituent Structure in Fluorene Based Compounds: Experimental and Theoretical Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 300, 122832. [Google Scholar] [CrossRef]
- Wu, K.; Zheng, Y.; Chen, R.; Zhou, Z.; Liu, S.; Shen, Y.; Zhang, Y. Advances in Electrochemiluminescence Luminophores Based on Small Organic Molecules for Biosensing. Biosens. Bioelectron. 2023, 223, 115031. [Google Scholar] [CrossRef] [PubMed]
- Suman; Kovvuri, J.; Islavath, N. Molecular Modifications in Fluorene Core for Efficient Organic Photovoltaic Cells. J. Photochem. Photobiol. A Chem. 2024, 446, 115162. [Google Scholar] [CrossRef]
- He, X.; Yin, L.; Li, Y. Efficient Design and Structural Modifications for Tuning the Photoelectric Properties of Small-Molecule Acceptors in Organic Solar Cells. New J. Chem. 2019, 43, 6577–6586. [Google Scholar] [CrossRef]
- Göbel, D.; Clamor, N.; Nachtsheim, B.J. Regioselective Ortho-Functionalization of Bromofluorenecarbaldehydes Using TMPMgCl·LiCl. Org. Biomol. Chem. 2018, 16, 4071–4075. [Google Scholar] [CrossRef] [PubMed]
- Reger, D.; Haines, P.; Amsharov, K.Y.; Schmidt, J.A.; Ullrich, T.; Bönisch, S.; Hampel, F.; Görling, A.; Nelson, J.; Jelfs, K.E.; et al. A Family of Superhelicenes: Easily Tunable, Chiral Nanographenes by Merging Helicity with Planar π Systems. Angew. Chem. Int. Ed 2021, 60, 18073–18081. [Google Scholar] [CrossRef]
- Lemasson, F.; Berton, N.; Tittmann, J.; Hennrich, F.; Kappes, M.M.; Mayor, M. Polymer Library Comprising Fluorene and Carbazole Homo- and Copolymers for Selective Single-Walled Carbon Nanotubes Extraction. Macromolecules 2012, 45, 713–722. [Google Scholar] [CrossRef]
- Zhang, G.-F.; Aldred, M.P.; Chen, Z.-Q.; Chen, T.; Meng, X.; Zhu, M.-Q. Efficient green-red piezofluorochromism of bisanthracene-modified dibenzofulvene. RSC Adv. 2015, 5, 1079–1082. [Google Scholar] [CrossRef]
- Cunha, C.; Peixoto, M.S.; Santos, J.; Abreu, P.E.; Paixão, J.A.; Pineiro, M.; Seixas de Melo, J.S. Practical Design of 3,6-Di-tert-butyldiphenyldibenzofulvene Derivatives with Enhanced Aggregation-Induced Emission. ACS Appl. Opt. Mater. 2023, 1, 340–353. [Google Scholar] [CrossRef]
- Nakano, T.; Takewaki, K.; Yade, T.; Okamoto, Y. Dibenzofulvene, a 1,1-Diphenylethylene Analogue, Gives a π-Stacked Polymer by Anionic, Free-Radical, and Cationic Catalysts. J. Am. Chem. Soc. 2001, 123, 9182–9183. [Google Scholar] [CrossRef]
- Nakano, T.; Yade, T.; Fukuda, Y.; Yamaguchi, T.; Okumura, S. Free-Radical Polymerization of Dibenzofulvene Leading to a π-Stacked Polymer: Structure and Properties of the Polymer and Proposed Reaction Mechanism. Macromolecules 2005, 38, 8140–8148. [Google Scholar] [CrossRef]
- Nakano, T.; Yade, T. Synthesis, Structure, and Photophysical and Electrochemical Properties of a π-Stacked Polymer. J. Am. Chem. Soc. 2003, 125, 15474–15484. [Google Scholar] [CrossRef]
- Nakano, T. Synthesis and Properties of π-Stacked Vinyl Polymers. In π-Stacked Polymers and Molecules; Springer: Japan, Tokyo, 2014; pp. 1–49. [Google Scholar]
- Nakano, T.; Nakagawa, O.; Yade, T.; Okamoto, Y. Solid-State Polymerization of Dibenzofulvene Leading to a Copolymer with Oxygen. Macromolecules 2003, 36, 1433–1435. [Google Scholar] [CrossRef]
- Wong, M.Y.; Leung, L.M. A Comprehensive Study of Substituent Effects on Poly(Dibenzofulvene)s. New J. Chem. 2017, 41, 512–520. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Nakano, T. Free-Radical Copolymerization of Dibenzofulvene with (Meth)Acrylates Leading to π-Stacked Copolymers. Polymers 2018, 10, 654. [Google Scholar] [CrossRef]
- Eakins, G.L.; Cooper, M.W.; Gerasimchuk, N.N.; Phillips, T.J.; Breyfogle, B.E.; Stearman, C.J. Structural Influences Impacting the Role of the 9-Ylidene Bond in the Electronic Tuning of Structures Built upon 9-Fluorenylidene Scaffolds. Can. J. Chem. 2013, 91, 1059–1071. [Google Scholar] [CrossRef]
- Rault-Berthelot, J.; Le Deit, H.; Massaoudi, M.; Simonet, J. Anodic Oxidation of 9,9′-Bifluorenylidene: Electrochemical Behaviour and Physicochemical Properties of Relevant Polymers. J. Electroanal. Chem. 1995, 380, 237–247. [Google Scholar] [CrossRef]
- Rault-Berthelot, J.; Rozé, C.; Granger, M.M. Anodic Oxidation of 2(9H-Fluoren-9-Ylidene) Malononitrile and 2(9H-Fluoren-9-Ylidene)-2-Phenylacetonitrile. Electrochemical Behavior and Physicochemical Properties of the Derived Polymers. J. Electroanal. Chem. 1997, 436, 85–101. [Google Scholar] [CrossRef]
- Hubert, C.; Tran, K.; Hauquier, F.; Cougnon, C.; Pilard, J.-F.; Gosselin, P.; Rault-Berthelot, J.; Raoult, E. Anodic Behaviour of Methylidene-Cyclopentadiaryl Derivatives: Cyclic Voltammetry and Theoretical Study. New J. Chem. 2007, 31, 1730–1737. [Google Scholar] [CrossRef]
- Capodilupo, A.L.; Marco, L.D.; Fabiano, E.; Giannuzzi, R.; Scrascia, A.; Carlucci, C.; Corrente, G.A.; Cipolla, M.P.; Gigli, G.; Ciccarella, G. New Organic Dyes Based on a Dibenzofulvene Bridge for Highly Efficient Dye-Sensitized Solar Cells. J. Mater. Chem. A 2014, 2, 14181–14188. [Google Scholar] [CrossRef]
- Corrente, G.A.; Fabiano, E.; De Marco, L.; Accorsi, G.; Giannuzzi, R.; Cardone, A.; Gigli, G.; Ciccarella, G.; Capodilupo, A.-L. Effects of Donor Position on Dibenzofulvene-Based Organic Dyes for Photovoltaics. J. Mater. Sci. Mater. Electron. 2017, 28, 8694–8707. [Google Scholar] [CrossRef]
- Sánchez, J.G.; Aktas, E.; Martínez-Ferrero, E.; Capodilupo, A.L.; Corrente, G.A.; Beneduci, A.; Palomares, E. Increasing the stability of perovskite solar cells with dibenzofulvene-based hole transporting materials. Electrochim. Acta 2022, 432, 141190. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).