Research Progress on CO2 Capture, Utilization, and Storage (CCUS) Based on Micro-Nano Fluidics Technology
Abstract
1. Introduction
2. Micro–Nanofluidic Devices and Chip Manufacturing
3. Research on CO2 Capture
4. Research on CO2 Enhanced Oil and Gas Recovery
4.1. Phase Behavior
4.2. Seepage
4.3. Diffusion Mass Transfer Study
4.4. Minimum Miscibility Pressure
4.5. Displacement Efficiency
5. Research on CO2 Geological Sequestration
5.1. CO2-Enhanced Oil and Gas Recovery and Sequestration Technology
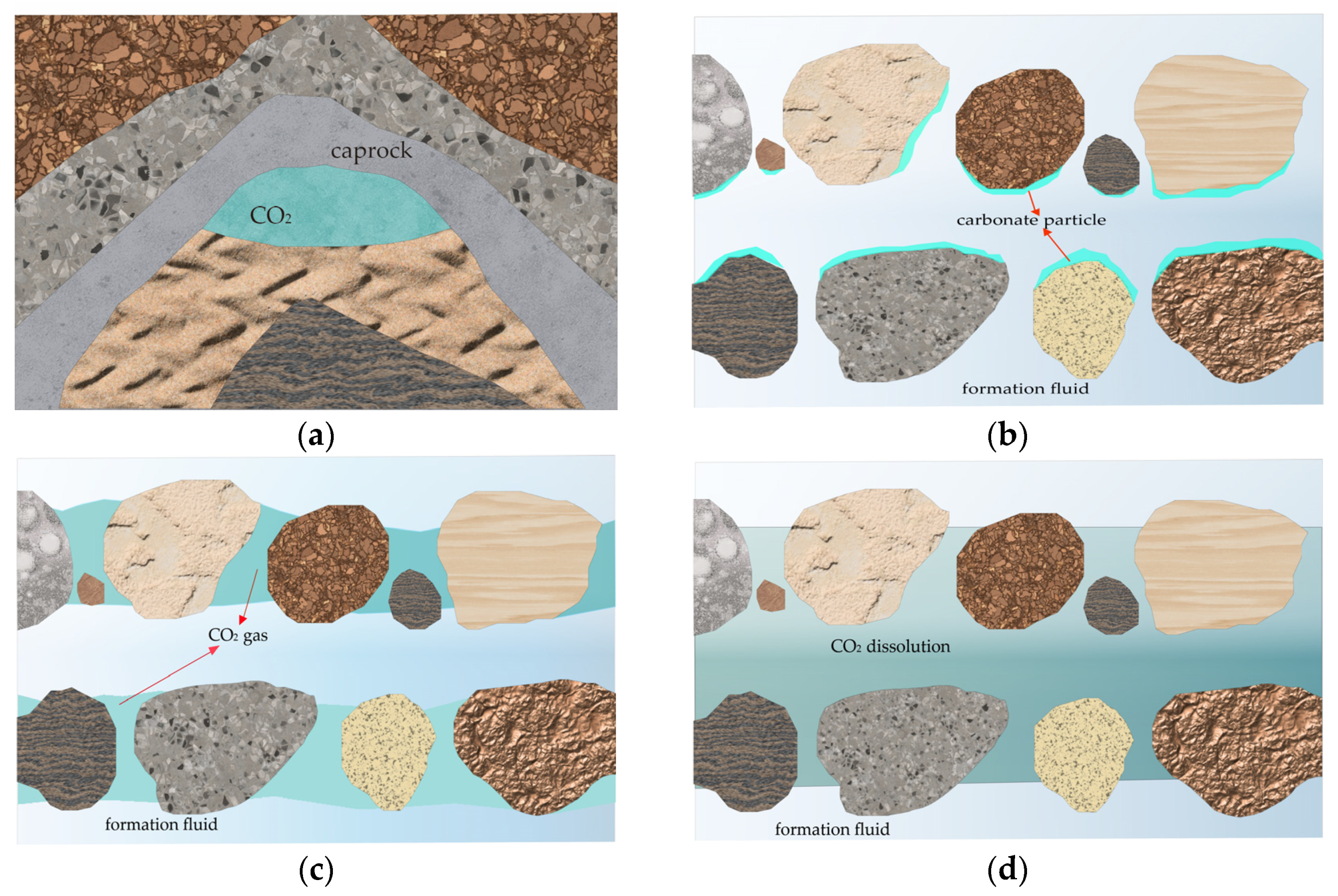
5.2. Saline Aquifer CO2 Sequestration Technology
6. Limitations
7. Prospects
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, C.; Tao, S.; Bai, B.; Yang, Z.; Zhu, R.; Hou, L.; Yuan, X.; Zhang, G.; Wu, S.; Pang, Z.; et al. Differences and Relations between Unconventional and Conventional Oil and Gas. China Pet. Explor. 2015, 20, 1. [Google Scholar]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C.; Jarvie, D.M. Morphology, Genesis, and Distribution of Nanometer-Scale Pores in Siliceous Mudstones of the Mississippian Barnett Shale. J. Sediment. Res. 2009, 79, 848–861. [Google Scholar] [CrossRef]
- Sheng, J.J. Enhanced oil recovery in shale reservoirs by gas injection. J. Nat. Gas Sci. Eng. 2015, 22, 252–259. [Google Scholar] [CrossRef]
- Bao, B.; Zhao, S.; Xu, J. Progress in studying fluid phase behaviours with micro- and nano-fluidic technology. CIESC J. 2018, 69, 4530–4541. [Google Scholar]
- Tan, S.P.; Qiu, X.; Dejam, M.; Adidharma, H. Critical point of fluid confined in nanopores: Experimental detection and measurement. J. Phys. Chem. C 2019, 123, 9824–9830. [Google Scholar] [CrossRef]
- Wang, Z.; Pereira, J.-M.; Gan, Y. Effect of Wetting Transition during Multiphase Displacement in Porous Media. Langmuir 2020, 36, 2449–2458. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Nestler, B. Wetting transition and phase separation on flat substrates and in porous structures. J. Chem. Phys. 2021, 154, 094704. [Google Scholar] [CrossRef]
- Alfi, M.; Nasrabadi, H.; Banerjee, D. Experimental investigation of confinement effect on phase behavior of hexane, heptane and octane using lab-on-a-chip technology. Fluid Phase Equilibria 2016, 423, 25–33. [Google Scholar] [CrossRef]
- Jin, Z.; Firoozabadi, A. Thermodynamic Modeling of Phase Behavior in Shale Media. SPE J. 2016, 21, 190–207. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Bonilla, M.R.; Nicholson, D. Molecular transport in nanopores: A theoretical perspective. Phys. Chem. Chem. Phys. 2011, 13, 15350–15383. [Google Scholar] [CrossRef]
- Parsa, E.; Yin, X.; Ozkan, E. Direct Observation of the Impact of Nanopore Confinement on Petroleum Gas Condensation. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 28–30 September 2015. D031S031R008. [Google Scholar]
- Yang, Q.; Bi, R.; Banerjee, D.; Nasrabadi, H. Direct Observation of the Vapor–Liquid Phase Transition and Hysteresis in 2 nm Nanochannels. Langmuir 2022, 38, 9790–9798. [Google Scholar] [CrossRef]
- Baek, S.; Akkutlu, I.Y. Enhanced Recovery of Nanoconfined Oil in Tight Rocks Using Lean Gas (C2H6 and CO2) Injection. SPE J. 2021, 26, 2018–2037. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, Z.; Sun, Z.; Yao, J.; Yang, Y.; Sun, H.; Zhang, L.; Zhang, K. Research advances in microscale fluid characteristics of shale reservoirs based on nanofluidic technology. Acta Pet. Sin. 2023, 44, 207. [Google Scholar]
- Yarin, A.L. Novel nanofluidic and microfluidic devices and their applications. Curr. Opin. Chem. Eng. 2020, 29, 17–25. [Google Scholar] [CrossRef]
- Wang, H.; Wei, B.; Hou, J.; Liu, Y.; Du, Q. Heavy oil recovery in blind-ends during chemical flooding: Pore scale study using microfluidic experiments. J. Mol. Liq. 2022, 368, 120724. [Google Scholar] [CrossRef]
- Onaka, Y.; Sato, K. Dynamics of pore-throat plugging and snow-ball effect by asphaltene deposition in porous media micromodels. J. Pet. Sci. Eng. 2021, 207, 109176. [Google Scholar] [CrossRef]
- Liang, T.; Xu, K.; Lu, J.; Nguyen, Q.; DiCarlo, D. Evaluating the Performance of Surfactants in Enhancing Flowback and Permeability after Hydraulic Fracturing through a Microfluidic Model. SPE J. 2020, 25, 268–287. [Google Scholar] [CrossRef]
- Bob, B.; Shi, J.; Feng, J.; Yang, Z.; Peng, B.; Zhao, S. Research progress of surfactant enhanced oil recovery based on microfluidics technology. Acta Pet. Sin. 2022, 43, 432–442+452. [Google Scholar]
- Zhao, X.; Feng, Y.; Liao, G.; Liu, W. Visualizing in-situ emulsification in porous media during surfactant flooding: A microfluidic study. J. Colloid Interface Sci. 2020, 578, 629–640. [Google Scholar] [CrossRef]
- Sugar, A.; Torrealba, V.; Buttner, U.; Hoteit, H. Assessment of Polymer-Induced Clogging Using Microfluidics. SPE J. 2020, 26, 3793–3804. [Google Scholar] [CrossRef]
- Li, J. Enhanced Oil Recovery Using Bubbles in a Reservoir-on-a-Chip (ROC). Ph.D. Thesis, Shandong University, Jinan, China, 2021. [Google Scholar]
- Kotdawala, R.R.; Kazantzis, N.; Thompson, R.W. Analysis of binary adsorption of polar and nonpolar molecules in narrow slit-pores by mean-field perturbation theory. J. Chem. Phys. 2005, 123, 244709. [Google Scholar] [CrossRef]
- Li, Z.; Jin, Z.; Firoozabadi, A. Phase Behavior and Adsorption of Pure Substances and Mixtures and Characterization in Nanopore Structures by Density Functional Theory. SPE J. 2014, 19, 1096–1109. [Google Scholar] [CrossRef]
- Travalloni, L.; Castier, M.; Tavares, F.W. Phase equilibrium of fluids confined in porous media from an extended Peng–Robinson equation of state. Fluid Phase Equilibria 2014, 362, 335–341. [Google Scholar] [CrossRef]
- Derouane, E.G. On the physical state of molecules in microporous solids. Microporous Mesoporous Mater. 2007, 104, 46–51. [Google Scholar] [CrossRef]
- Tan, S.P.; Piri, M. Equation-of-state modeling of confined-fluid phase equilibria in nanopores. Fluid Phase Equilibria 2015, 393, 48–63. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Y.; Nestler, B. Wetting Effect on Patterned Substrates. Adv. Mater. 2023, 35, e2210745. [Google Scholar] [CrossRef]
- Snustad, I.; Røe, I.T.; Brunsvold, A.; Ervik, Å.; He, J.; Zhang, Z. A review on wetting and water condensation—Perspectives for CO2 condensation. Adv. Colloid Interface Sci. 2018, 256, 291–304. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Zeng, Y.; Harrison, D.J. Self-Assembled Colloidal Arrays as Three-Dimensional Nanofluidic Sieves for Separation of Biomolecules on Microchips. Anal. Chem. 2007, 79, 2289–2295. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B.; Lesieur, S.; Mutafchieva, R.; Ollivon, M.; Bourgaux, C.; Willumeit, R.; Couvreur, P. Dynamic control of nanofluidic channels in protein drug delivery vehicles. J. Drug Deliv. Sci. Technol. 2008, 18, 41–45. [Google Scholar] [CrossRef]
- Abgrall, P.; Nguyen, N.T. Nanofluidic Devices and Their Applications. Anal. Chem. 2008, 80, 2326–2341. [Google Scholar] [CrossRef]
- Yang, H.Y.; Han, Z.J.; Yu, S.F.; Pey, K.L.; Ostrikov, K.; Karnik, R. Carbon nanotube membranes with ultrahigh specific adsorption capacity for water desalination and purification. Nat. Commun. 2013, 4, 2220. [Google Scholar] [CrossRef]
- Morais, S.; Cario, A.; Liu, N.; Bernard, D.; Lecoutre, C.; Garrabos, Y.; Ranchou-Peyruse, A.; Dupraz, S.; Azaroual, M.; Hartman, R.L.; et al. Studying key processes related to CO2 underground storage at the pore scale using high pressure micromodels. React. Chem. Eng. 2020, 5, 1156–1185. [Google Scholar] [CrossRef]
- Hele-Shaw, H.S. Flow of water. Nature 1898, 58, 520. [Google Scholar] [CrossRef]
- Engel, M.; Wunsch, B.H.; Neumann, R.F.; Giro, R.; Bryant, P.W.; Smith, J.T.; Steiner, M.B. Nanoscale Flow Chip Platform for Laboratory Evaluation of Enhanced Oil Recovery Materials. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 9–11 October 2017. D021S019R002. [Google Scholar]
- Zhang, Y.; Zhou, C.; Qu, C.; Wei, M.; He, X.; Bai, B. Fabrication and verification of a glass–silicon–glass micro-/nanofluidic model for investigating multi-phase flow in shale-like unconventional dual-porosity tight porous media. Lab A Chip 2019, 19, 4071–4082. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.G.; Doyle, P.S. Photopatterned oil-reservoir micromodels with tailored wetting properties. Lab A Chip 2015, 15, 3047–3055. [Google Scholar] [CrossRef]
- Wegner, J.; Ganzer, L. Rock-on-a-Chip Devices for High p, T Conditions and Wettability Control for the Screening of EOR Chemicals. In Proceedings of the SPE Europec Featured at 79th EAGE Conference and Exhibition, Paris, France, 12–15 June 2017. D041S010R007. [Google Scholar]
- Lignos, I.; Ow, H.; Lopez, J.P.; McCollum, D.L.; Zhang, H.; Imbrogno, J.; Shen, Y.; Chang, S.; Wang, W.; Jensen, K.F. Continuous Multistage Synthesis and Functionalization of Sub-100 nm Silica Nanoparticles in 3D-Printed Continuous Stirred-Tank Reactor Cascades. ACS Appl. Mater. Interfaces 2020, 12, 6699–6706. [Google Scholar] [CrossRef]
- Jacobs, T. Reservoir-on-a-Chip Technology Opens a New Window Into Oilfield Chemistry. J. Pet. Technol. 2019, 71, 25–27. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Ma, C.; Qiao, W.; Ling, L. Fabrication of hierarchical carbon nanosheet-based networks for physical and chemical adsorption of CO2. J. Colloid Interface Sci. 2018, 534, 72–80. [Google Scholar] [CrossRef]
- Khansary, M.A.; Aroon, M.A.; Shirazian, S. Physical adsorption of CO2 in biomass at atmospheric pressure and ambient temperature. Environ. Chem. Lett. 2020, 18, 1423–1431. [Google Scholar] [CrossRef]
- Williamson, I.; Nelson, E.B.; Li, L. Carbon dioxide sorption in a nanoporous octahedral molecular sieve. J. Phys. D Appl. Phys. 2015, 48, 335304. [Google Scholar] [CrossRef]
- Xie, L.; Jin, Z.; Dai, Z.; Zhou, T.; Zhang, X.; Chang, Y.; Jiang, X. Fabricating self-templated and N-doped hierarchical porous carbon spheres via microfluidic strategy for enhanced CO2 capture. Sep. Purif. Technol. 2023, 322, 124267. [Google Scholar] [CrossRef]
- Qi, Z.; Xu, L.; Xu, Y.; Zhong, J.; Abedini, A.; Cheng, X.; Sinton, D. Disposable silicon-glass microfluidic devices: Precise, robust and cheap. Lab A Chip 2018, 18, 3872–3880. [Google Scholar] [CrossRef] [PubMed]
- Towler, B.F. Fundamental Principles of Reservoir Engineering; Society of Petroleum Engineers: Houston, TX, USA, 2002. [Google Scholar]
- Nojabaei, B.; Johns, R.T.; Chu, L. Effect of Capillary Pressure on Phase Behavior in Tight Rocks and Shales. SPE Reserv. Eval. Eng. 2013, 16, 281–289. [Google Scholar] [CrossRef]
- Alfi, M. Experimental Study of Confinement Effect on Phase Behavior of Hydrocarbons in Nano-Slit Channels Using Nanofluidic Devices. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2019. [Google Scholar]
- Liu, Y.; Li, H.A.; Okuno, R. Phase behavior of fluid mixtures in a partially confined space. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dubai, United Arab Emirates, 27 September 2016; OnePetro: Richardson, TX, USA, 2016. [Google Scholar]
- Ustinov, E.A.; Do, D.D. Modeling of adsorption in finite cylindrical pores by means of density functional theory. Adsorption 2005, 11, 455–477. [Google Scholar] [CrossRef]
- Achour, S.H.; Okuno, R. Phase stability analysis for tight porous media by minimization of the Helmholtz free energy. Fluid Phase Equilibria 2020, 520, 112648. [Google Scholar] [CrossRef]
- Nichita, D.V. Volume-based phase stability analysis including capillary pressure. Fluid Phase Equilibria 2019, 492, 145–160. [Google Scholar] [CrossRef]
- Aljamaan, H.M. Multiscale and Multicomponent Flow and Storage Capacity Investigation of Unconventional Resources. Ph.D. Thesis, Stanford University, Stanford, CA, USA, 2017. [Google Scholar]
- Konno, M.; Shibata, K.; Saito, S. Adsorption of light hydrocarbon mixtures on molecular sieving carbon MSC-5A. J. Chem. Eng. Jpn. 1985, 18, 394–398. [Google Scholar] [CrossRef][Green Version]
- Yun, J.H.; Düren, T.; Keil, F.J.; Seaton, N.A. Adsorption of methane, ethane, and their binary mixtures on MCM-41: Experimental evaluation of methods for the prediction of adsorption equilibrium. Langmuir 2002, 18, 2693–2701. [Google Scholar] [CrossRef]
- Luo, S.; Lutkenhaus, J.L.; Nasrabadi, H. Experimental study of confinement effect on hydrocarbon phase behavior in nano-scale porous media using differential scanning calorimetry. In Proceedings of the SPE Annual Technical Conference and Exhibition, Houston, TX, USA, 28–30 September 2015; SPE: Kuala Lumpur, Malaysia, 2015. D031S043R003. [Google Scholar]
- Jin, B.; Nasrabadi, H. Phase behavior of multi-component hydrocarbon systems in nano-pores using gauge-GCMC molecular simulation. Fluid Phase Equilibria 2016, 425, 324–334. [Google Scholar] [CrossRef]
- Luo, S.; Lutkenhaus, J.L.; Nasrabadi, H. Use of differential scanning calorimetry to study phase behavior of hydrocarbon mixtures in nano-scale porous media. J. Pet. Sci. Eng. 2018, 163, 731–738. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, L.; Song, Y.; Zhao, Y.; Zhang, Y.; Wang, D. Estimation of minimum miscibility pressure (MMP) of CO2 and liquid n-alkane systems using an improved MRI technique. Magn. Reson. Imaging 2016, 34, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Piccinin, S.; Pellegrini, V.; de Gironcoli, S.; Tozzini, V. Nano-Scale Corrugations in Graphene: A Density Functional Theory Study of Structure, Electronic Properties and Hydrogenation. J. Phys. Chem. C 2015, 119, 7900–7910. [Google Scholar] [CrossRef]
- Jin, B.; Nasrabadi, H. Phase Behavior in Shale Organic/Inorganic Nanopores from Molecular Simulation. SPE Reserv. Eval. Eng. 2018, 21, 626–637. [Google Scholar] [CrossRef]
- Pitakbunkate, T.; Balbuena, P.B.; Moridis, G.J.; Blasingame, T.A. Effect of Confinement on Pressure/Volume/Temperature Properties of Hydrocarbons in Shale Reservoirs. SPE J. 2016, 21, 621–634. [Google Scholar] [CrossRef]
- Jin, B.; Bi, R.; Nasrabadi, H. Molecular simulation of the pore size distribution effect on phase behavior of methane confined in nanopores. Fluid Phase Equilibria 2017, 452, 94–102. [Google Scholar] [CrossRef]
- Luo, S.; Lutkenhaus, J.L.; Nasrabadi, H. Confinement-Induced Supercriticality and Phase Equilibria of Hydrocarbons in Nanopores. Langmuir 2016, 32, 11506–11513. [Google Scholar] [CrossRef]
- Luo, S.; Lutkenhaus, J.L.; Nasrabadi, H. Effect of nano-scale pore size distribution on fluid phase behavior of gas IOR in shale reservoirs. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 14–18 April 2018; SPE: Kuala Lumpur, Malaysia, 2018. D041S019R003. [Google Scholar]
- Liu, Y.; Jin, Z.; Li, H.A. Comparison of Peng-Robinson Equation of State WITH Capillary Pressure Model with Engineering Density-Functional Theory in Describing the Phase Behavior of Confined Hydrocarbons. SPE J. 2018, 23, 1784–1797. [Google Scholar] [CrossRef]
- Salahshoor, S.; Fahes, M. Experimental Investigation of the Effect of Pore Size on Saturation Pressure for Gas Mixtures. In Proceedings of the Annual Technical Conference and Exhibition, Orlando, FL, USA, 7–10 May 2018; SPE: Kuala Lumpur, Malaysia, 2018. D011S006R003. [Google Scholar]
- Molla, S.; Mostowfi, F. Microfluidic PVT--Saturation Pressure and Phase-Volume Measurement of Black Oils. SPE Reserv. Eval. Eng. 2016, 20, 233–239. [Google Scholar] [CrossRef]
- Bao, B.; Qiu, J.; Liu, F.; Fan, Q.; Luo, W.; Zhao, S. Capillary trapping induced slow evaporation in nanochannels. J. Pet. Sci. Eng. 2020, 196, 108084. [Google Scholar] [CrossRef]
- Wang, L.; Parsa, E.; Gao, Y.; Ok, J.T.; Neeves, K.; Yin, X.; Ozkan, E. Experimental study and modeling of the effect of nanoconfinement on hydrocarbon phase behavior in unconventional reservoirs. In Proceedings of the SPE Western Regional Meeting, Denver, CO, USA, 17–18 April 2014; SPE: Kuala Lumpur, Malaysia, 2014. SPE-169581-MS. [Google Scholar]
- Zhong, J.; Zhao, Y.; Lu, C.; Xu, Y.; Jin, Z.; Mostowfi, F.; Sinton, D. Nanoscale Phase Measurement for the Shale Challenge: Multicomponent Fluids in Multiscale Volumes. Langmuir 2018, 34, 9927–9935. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Zandavi, S.H.; Li, H.; Bao, B.; Persad, A.H.; Mostowfi, F.; Sinton, D. Condensation in One-Dimensional Dead-End Nanochannels. ACS Nano 2016, 11, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jin, B.; Banerjee, D.; Nasrabadi, H. Direct visualization and molecular simulation of dewpoint pressure of a confined fluid in sub-10 nm slit pores. Fuel 2018, 235, 1216–1223. [Google Scholar] [CrossRef]
- Zhang, K.; Jia, N.; Li, S.; Liu, L. Static and dynamic behavior of CO2 enhanced oil recovery in shale reservoirs: Experimental nanofluidics and theoretical models with dual-scale nanopores. Appl. Energy 2019, 255, 113752. [Google Scholar] [CrossRef]
- Jatukaran, A. Visualization of Fluid Dynamics in Nanoporous Media for Unconventional Hydrocarbon Recovery; University of Toronto: Toronto, ON, Canada, 2018. [Google Scholar]
- Jatukaran, A.; Zhong, J.; Abedini, A.; Sherbatian, A.; Zhao, Y.; Jin, Z.; Mostowfi, F.; Sinton, D. Natural gas vaporization in a nanoscale throat connected model of shale: Multi-scale, multi-component and multi-phase. Lab A Chip 2018, 19, 272–280. [Google Scholar] [CrossRef]
- Alfi, M.; Nasrabadi, H.; Banerjee, D. Effect of confinement on bubble point temperature shift of hydrocarbon mixtures: Experimental investigation using nanofluidic devices. In Proceedings of the Annual Technical Conference and Exhibition, Anaheim, CA, USA, 8–10 May 2017; SPE: Kuala Lumpur, Malaysia, 2017. D011S009R004. [Google Scholar]
- Washburn, E.W. The Dynamics of Capillary Flow. Phys. Rev. B 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Song, Y.; Song, Z.; Liu, Y.; Guo, J.; Bai, B.; Hou, J.; Bai, M.; Song, K. Phase behavior and minimum miscibility pressure of confined fluids in organic nanopores. In Proceedings of the Improved Oil Recovery Conference, Tulsa, OK, USA, 31 August–4 September 2020; SPE: Kuala Lumpur, Malaysia, 2020. D021S035R002. [Google Scholar]
- Li, H.; Zhong, J.; Pang, Y.; Zandavi, S.H.; Persad, A.H.; Xu, Y.; Mostowfi, F.; Sinton, D. Direct visualization of fluid dynamics in sub-10 nm nanochannels. Nanoscale 2017, 9, 9556–9561. [Google Scholar] [CrossRef]
- Lu, H.; Xu, Y.; Duan, C.; Jiang, P.; Xu, R. Experimental Study on Capillary Imbibition of Shale Oil in Nanochannels. Energy Fuels 2022, 36, 5267–5275. [Google Scholar] [CrossRef]
- Zuo, M.; Chen, H.; Xu, C.; Stephenraj, I.R.; Qi, X.; Yu, H.; Liu, X.Y. Study on Dynamic Variation Characteristics of Reservoir Fluid Phase Behavior During CO2 Injection in CO2 Based Enhanced Oil Recovery Process. In Proceedings of the IADC/SPE Asia Pacific Drilling Technology Conference and Exhibition, Bangkok, Thailand, 9–10 August 2022; SPE: Kuala Lumpur, Malaysia, 2022. D012S001R004. [Google Scholar]
- Rezk, M.G.; Foroozesh, J. Phase behavior and fluid interactions of a CO2-Light oil system at high pressures and temperatures. Heliyon 2019, 5, e02057. [Google Scholar] [CrossRef]
- Fu, T. Microfluidics in CO2 capture, sequestration, and applications. In Advances in Microfluidics-New Applications in Biology, Energy, and Materials Sciences; BoD—Books on Demand: Norderstedt, Germany, 2016; pp. 293–313. [Google Scholar]
- Belyaev, A.V.; Dedov, A.V.; Krapivin, I.I.; Varava, A.N.; Jiang, P.; Xu, R. Study of Pressure Drops and Heat Transfer of Nonequilibrial Two-Phase Flows. Water 2021, 13, 2275. [Google Scholar] [CrossRef]
- Du, L.; Liu, W.; Chen, X.; Qin, X.; Ren, X. Research Progress on the Diffusion of CO2 in Crude Oil. Oilfield Chem. 2019, 36, 372–380. [Google Scholar]
- Kuhn, S.; Jensen, K.F. A pH-sensitive laser-induced fluorescence technique to monitor mass transfer in multiphase flows in microfluidic devices. Ind. Eng. Chem. Res. 2012, 51, 8999–9006. [Google Scholar] [CrossRef]
- Qiu, J.; Bao, B.; Zhao, S.; Lu, X. Microfluidics-based determination of diffusion coefficient for gas-liquid reaction system with hydrogen peroxide. Chem. Eng. Sci. 2020, 231, 116248. [Google Scholar] [CrossRef]
- Hu, H.; Jiang, P.; Huang, F.; Xu, R. Role of trapped liquid in flow boiling inside micro-porous structures: Pore-scale visualization and heat transfer enhancement. Sci. Bull. 2021, 66, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Alfarge, D.; Alsaba, M.; Wei, M.; Bai, B. Miscible gases based EOR in unconventional liquids rich reservoirs: What we can learn. In Proceedings of the SPE International Heavy Oil Conference and Exhibition, Kuwait City, Kuwait, 10–12 December 2018; SPE: Kuala Lumpur, Malaysia, 2018. D022S034R002. [Google Scholar]
- Cronin, M.; Emami-Meybodi, H.; Johns, R.T. Diffusion-Dominated Proxy Model for Solvent Injection in Ultratight Oil Reservoirs. SPE J. 2018, 24, 660–680. [Google Scholar] [CrossRef]
- Bao, B.; Feng, J.; Qiu, J.; Zhao, S. Direct Measurement of Minimum Miscibility Pressure of Decane and CO2 in Nanoconfined Channels. ACS Omega 2020, 6, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Flock, D.; Nouar, A. Parametric Analysis on the Determination of The Minimum Miscibility Pressure In Slim Tube Displacements. J. Can. Pet. Technol. 1984, 23, 05. [Google Scholar] [CrossRef]
- Christiansen, R.L.; Haines, H.K. Rapid Measurement of Minimum Miscibility Pressure with the Rising-Bubble Apparatus. SPE Reserv. Eng. 1987, 2, 523–527. [Google Scholar] [CrossRef]
- Rao, D.N. A new technique of vanishing interfacial tension for miscibility determination. Fluid Phase Equilibria 1997, 139, 311–324. [Google Scholar] [CrossRef]
- Pereponov, D.; Tarkhov, M.; Dorhjie, D.B.; Rykov, A.; Filippov, I.; Zenova, E.; Krutko, V.; Cheremisin, A.; Shilov, E. Microfluidic Studies on Minimum Miscibility Pressure for n-Decane and CO2. Energies 2023, 16, 4994. [Google Scholar] [CrossRef]
- Zhang, K.; Jia, N.; Li, S.; Liu, L. Thermodynamic phase behaviour and miscibility of confined fluids in nanopores. Chem. Eng. J. 2018, 351, 1115–1128. [Google Scholar] [CrossRef]
- Peng, D.-Y.; Robinson, D.B. The Characterization of the Heptanes and Heavier Fractions for the GPA Peng-Robinson Programs; GPA Research Report RR-28; Gas Processors Association: Tulsa, OK, USA, 1978. [Google Scholar]
- van der Waals, J.D. Over de Continuiteit van der Gas-en Vloeistoftoestand. Ph.D. Thesis, Leiden University, Leiden, The Netherlands, 1873. (In Dutch). [Google Scholar]
- Zhang, Y. Fabrication of Micro-/Nanofluidic Models and Their Applications for Enhanced Oil Recovery Mechanism Study; Missouri University of Science and Technology: Rolla, MO, USA, 2020. [Google Scholar]
- Adyani Wan Razak, W.N.; Kechut, N.I. Advanced technology for rapid minimum miscibility pressure determination (part 1). In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Jakarta, Indonesia, 30 October–1 November 2007; SPE: Kuala Lumpur, Malaysia, 2007. SPE-110265-MS. [Google Scholar]
- Nguyen, P.; Mohaddes, D.; Riordon, J.; Fadaei, H.; Lele, P.; Sinton, D. Fast Fluorescence-Based Microfluidic Method for Measuring Minimum Miscibility Pressure of CO2 in Crude Oils. Anal. Chem. 2015, 87, 3160–3164. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Balhoff, M.T.; Mohanty, K.K. Effect of Reservoir Heterogeneity on Primary Recovery and CO2 Huff ‘n’ Puff Recovery in Shale-Oil Reservoirs. SPE Reserv. Eval. Eng. 2014, 17, 404–413. [Google Scholar] [CrossRef]
- Yu, W.; Lashgari, H.R.; Wu, K.; Sepehrnoori, K. CO2 injection for enhanced oil recovery in Bakken tight oil reservoirs. Fuel 2015, 159, 354–363. [Google Scholar] [CrossRef]
- Alharthy, N.; Teklu, T.W.; Kazemi, H.; Graves, R.M.; Hawthorne, S.B.; Braunberger, J.; Kurtoglu, B. Enhanced Oil Recovery in Liquid–Rich Shale Reservoirs: Laboratory to Field. SPE Reserv. Eval. Eng. 2017, 21, 137–159. [Google Scholar] [CrossRef]
- Hoffman, B.T. Comparison of various gases for enhanced recovery from shale oil reservoirs. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012; OnePetro: Richardson, TX, USA, 2012. [Google Scholar]
- Yu, Y.; Sheng, J.J. Experimental investigation of light oil recovery from fractured shale reservoirs by cyclic water injection. In Proceedings of the SPE Western Regional Meeting, Anchorage, AK, USA, 23–26 May 2016; SPE: Kuala Lumpur, Malaysia, 2016. SPE-180378-MS. [Google Scholar]
- Wang, L.; Tian, Y.; Yu, X.; Wang, C.; Yao, B.; Wang, S.; Winterfeld, P.H.; Wang, X.; Yang, Z.; Wang, Y.; et al. Advances in improved/enhanced oil recovery technologies for tight and shale reservoirs. Fuel 2017, 210, 425–445. [Google Scholar] [CrossRef]
- Luo, S. Experimental and Simulation Studies on Phase Behavior of Petroleum Fluids in Nanoporous Media. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2018. [Google Scholar]
- Jia, B.; Tsau, J.-S.; Barati, R. A review of the current progress of CO2 injection EOR and carbon storage in shale oil reservoirs. Fuel 2018, 236, 404–427. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, F.; Qiu, J.; Xu, Z.; Bao, B. Microscopic transport and phase behaviors of CO2 injection in heterogeneous formations using microfluidics. Energy 2022, 256, 124524. [Google Scholar] [CrossRef]
- Al-Kindi, I.; Babadagli, T. Revisiting Kelvin equation and Peng–Robinson equation of state for accurate modeling of hydrocarbon phase behavior in nano capillaries. Sci. Rep. 2021, 11, 6573. [Google Scholar] [CrossRef]
- Bao, B.; Zhao, S. A review of experimental nanofluidic studies on shale fluid phase and transport behaviors. J. Nat. Gas Sci. Eng. 2020, 86, 103745. [Google Scholar] [CrossRef]
- Lu, H.; Huang, F.; Jiang, P.; Xu, R. Exsolution effects in CO2 huff-n-puff enhanced oil recovery: Water-Oil-CO2 three phase flow visualization and measurements by micro-PIV in micromodel. Int. J. Greenh. Gas Control. 2021, 111, 103445. [Google Scholar] [CrossRef]
- Huang, F.; Xu, R.; Jiang, P.; Wang, C.; Wang, H.; Lun, Z. Pore-scale investigation of CO2/oil exsolution in CO2 huff-n-puff for enhanced oil recovery. Phys. Fluids 2020. [Google Scholar] [CrossRef]
- Jang, X.Z.J. Impacts of gettability on immiscible fluid flow pattern-Microfluidic chip experiment. China Pet. Process. Petrochem. Technol. 2019, 21, 80. [Google Scholar]
- Guo, Y.; Zhang, L.; Yang, Y.; Xu, Z.; Bao, B. Pore-scale investigation of immiscible displacement in rough fractures. J. Pet. Sci. Eng. 2021, 207, 109107. [Google Scholar] [CrossRef]
- Zhong, J.; Abedini, A.; Xu, L.; Xu, Y.; Qi, Z.; Mostowfi, F.; Sinton, D. Nanomodel visualization of fluid injections in tight formations. Nanoscale 2018, 10, 21994–22002. [Google Scholar] [CrossRef]
- Liu, T.; Ma, X.; Diao, Y.; Jin, X.; Fu, J.; Zhang, C. Research status of CO2 geological storage potential evaluation methods at home and abroad. Geol. Surv. China 2021, 8, 101–108. [Google Scholar]
- Zheng, X.; Mahabadi, N.; Yun, T.S.; Jang, J. Effect of capillary and viscous force on CO2 saturation and invasion pattern in the microfluidic chip. J. Geophys. Res. Solid Earth 2017, 122, 1634–1647. [Google Scholar] [CrossRef]
- Morais, S.; Liu, N.; Diouf, A.; Bernard, D.; Lecoutre, C.; Garrabos, Y.; Marre, S. Monitoring CO2 invasion processes at the pore scale using geological labs on chip. Lab A Chip 2016, 16, 3493–3502. [Google Scholar] [CrossRef]
- Liu, N.; Aymonier, C.; Lecoutre, C.; Garrabos, Y.; Marre, S. Microfluidic approach for studying CO2 solubility in water and brine using confocal Raman spectroscopy. Chem. Phys. Lett. 2012, 551, 139–143. [Google Scholar] [CrossRef]
- Hosseini, H.; Guo, F.; Ghahfarokhi, R.B.; Aryana, S.A. Microfluidic fabrication techniques for high-pressure testing of microscale supercritical CO2 foam transport in fractured unconventional reservoirs. JoVE J. Vis. Exp. 2020, 161, e61369. [Google Scholar] [CrossRef]
- McCourt, T.A.; Zhou, F.; Bianchi, V.; Pike, D.; Donovan, D. Chip-Firing on a Graph for Modelling Complex Geological Architecture in CO2 Injection and Storage. Transp. Porous Media 2019, 129, 281–294. [Google Scholar] [CrossRef]
- Diao, Y.; Zhang, S.; Guo, J.; Li, X.; Zhang, H. Geological safety evaluation method for CO2 geological storage in deep saline aquifer. Geol. China 2011, 38, 786–792. [Google Scholar]
- Gerami, A.; Alzahid, Y.; Mostaghimi, P.; Kashaninejad, N.; Kazemifar, F.; Amirian, T.; Mosavat, N.; Warkiani, M.E.; Armstrong, R.T. Microfluidics for Porous Systems: Fabrication, Microscopy and Applications. Transp. Porous Media 2018, 130, 277–304. [Google Scholar] [CrossRef]
- de Lima, V.; Einloft, S.; Ketzer, J.M.; Jullien, M.; Bildstein, O.; Petronin, J.-C. CO2 Geological storage in saline aquifers: Paraná Basin caprock and reservoir chemical reactivity. Energy Procedia 2011, 4, 5377–5384. [Google Scholar] [CrossRef]
- Zirrahi, M.; Hassanzadeh, H.; Abedi, J. Modeling of CO2 dissolution by static mixers using back flow mixing approach with application to geological storage. Chem. Eng. Sci. 2013, 104, 10–16. [Google Scholar] [CrossRef]
- Jafari, M.; Cao, S.C.; Jung, J. Geological CO2 sequestration in saline aquifers: Implication on potential solutions of China’s power sector. Resour. Conserv. Recycl. 2017, 121, 137–155. [Google Scholar] [CrossRef]
- Uemura, S.; Matsui, Y.; Noda, A.; Tsushima, S.; Hirai, S. Nanosized CO2 Droplets Injection for Stable Geological Storage. Energy Procedia 2013, 37, 5596–5600. [Google Scholar] [CrossRef][Green Version]
- Ho, T.H.M.; Tsai, P.A. Microfluidic salt precipitation: Implications for geological CO2 storage. Lab A Chip 2020, 20, 3806–3814. [Google Scholar] [CrossRef]
- Tirapu-Azpiroz, J.; Ferreira, M.E.; Silva, A.F.; Ohta, R.L.; Ferreira, R.N.B.; Giro, R.; Wunsch, B.; Steiner, M.B. Advanced optical on-chip analysis of fluid flow for applications in carbon dioxide trapping. In Proceedings of the Microfluidics, BioMEMS, and Medical Microsystems XX, San Francisco, CA, USA, 22–27 January 2022; SPE: Kuala Lumpur, Malaysia, 2022; Volume 11955, pp. 34–45. [Google Scholar]
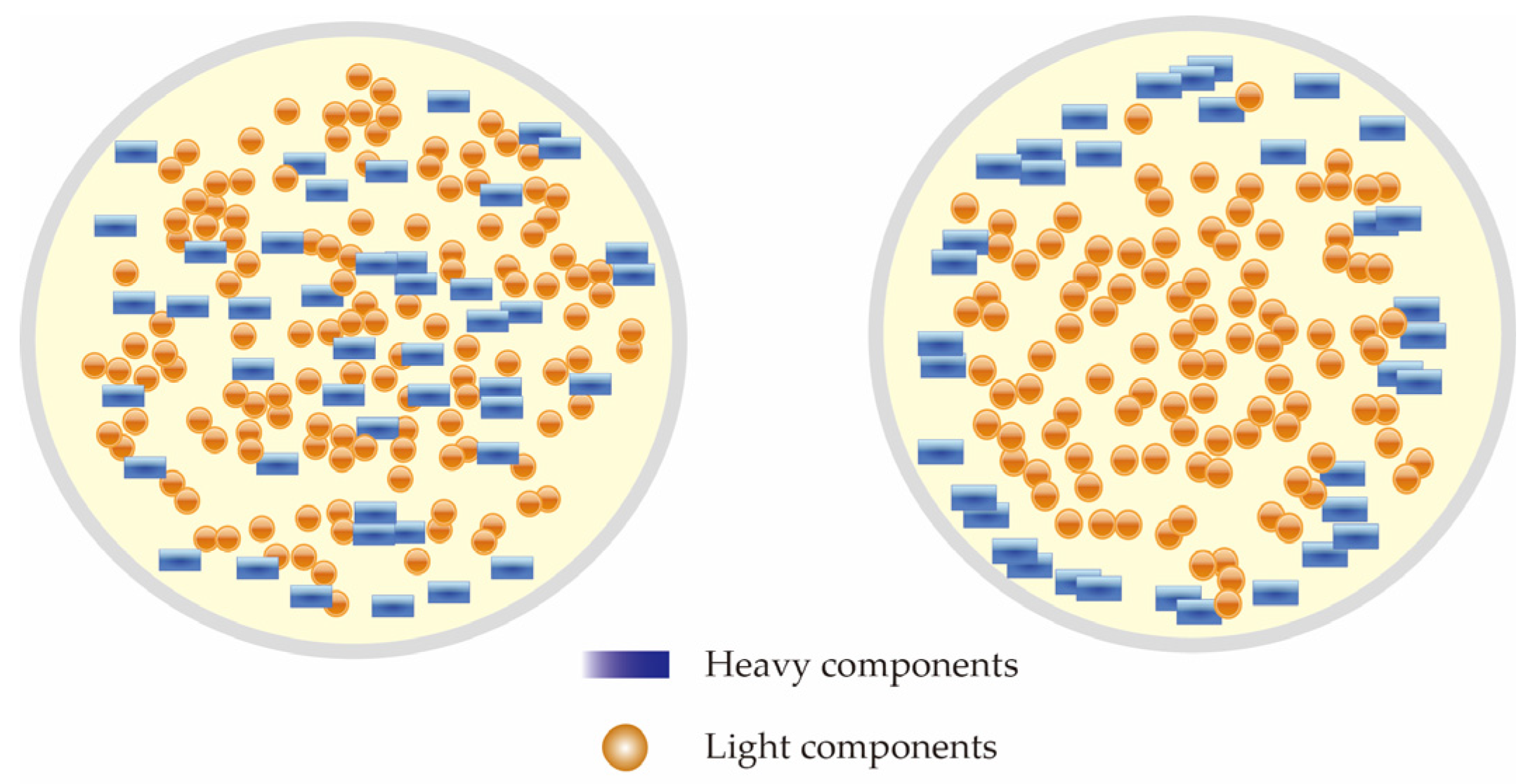
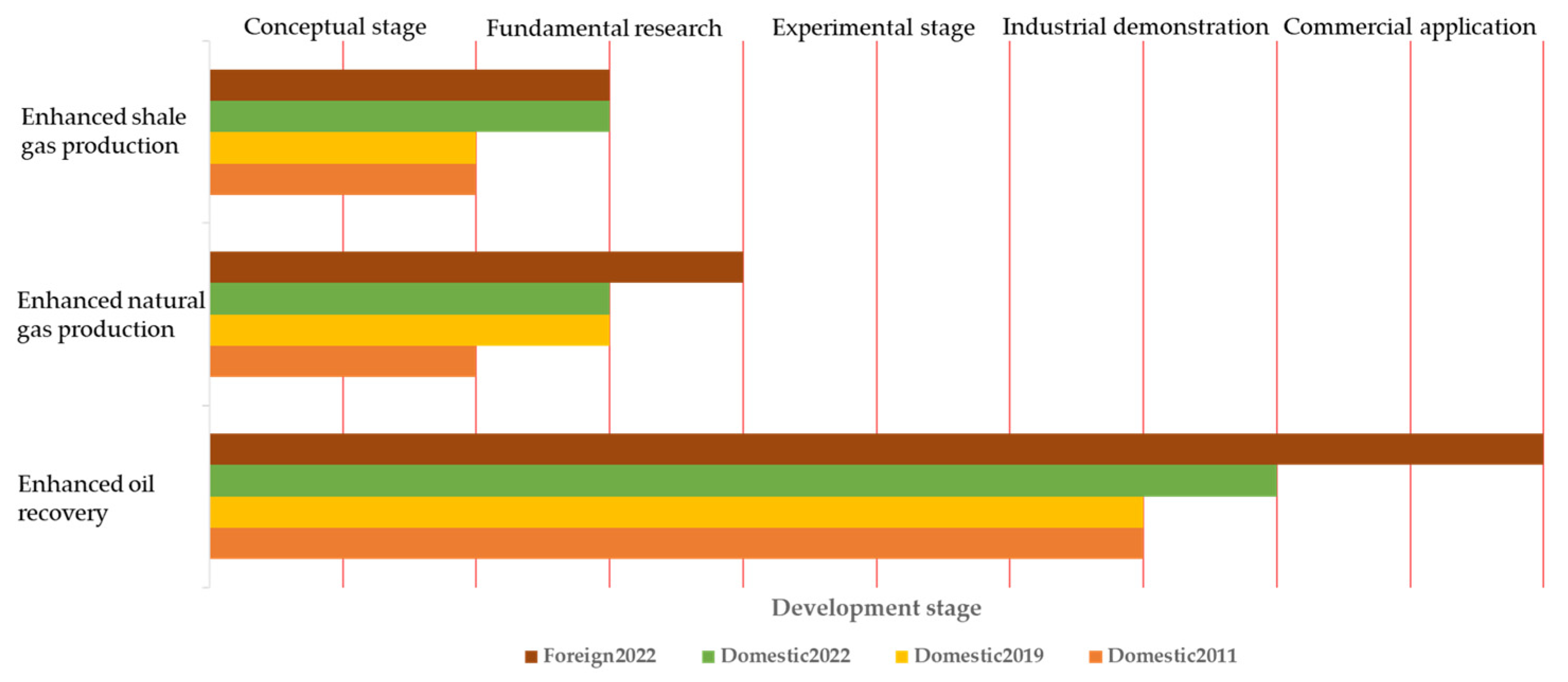



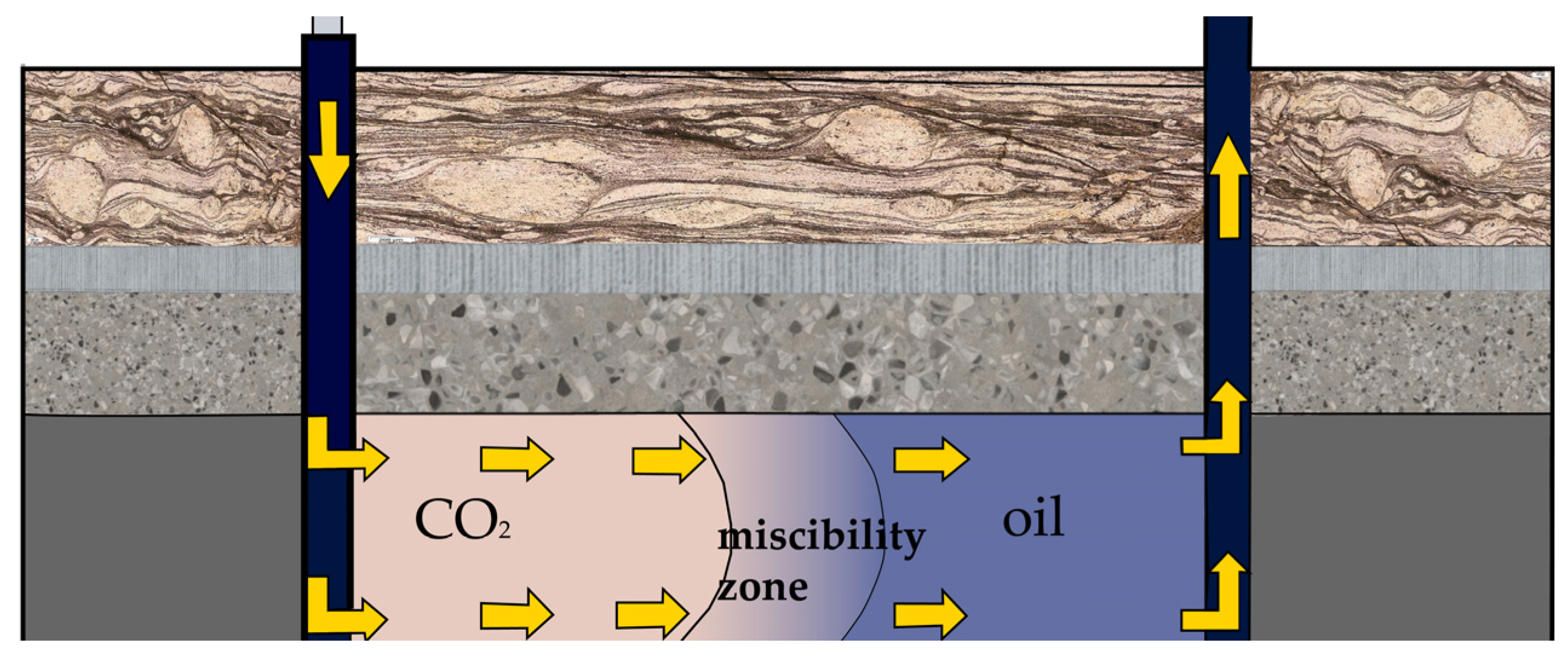
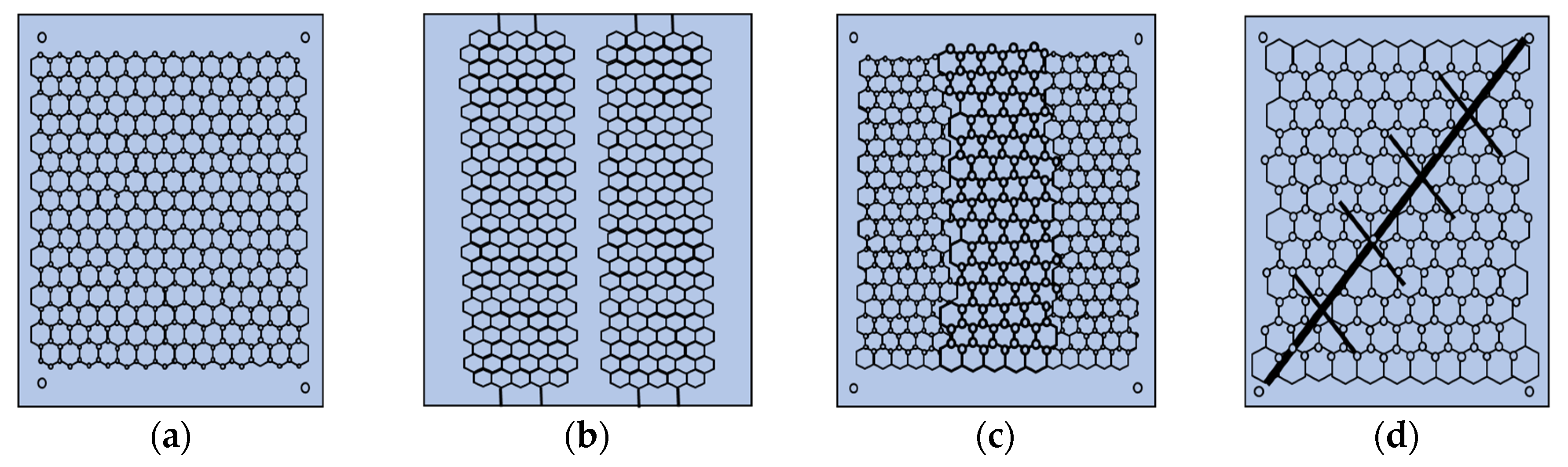
| Research Methods | Principles | Advantages | Disadvantages | ||
|---|---|---|---|---|---|
| Experimental methods | Rock core displacement experiment [55] | Analyze recovery rate and other data based on experimental parameters | Realistic rock core displacement with high fidelity | Long cycle, not visually observable, and poor repeatability | |
| Adsorption–desorption method [56,57] | Isotherm adsorption line | Direct approach to observe the phase transition point and critical behavior of hydrocarbons in nanoporous carbon | Most are non-realistic rock cores | ||
| Differential scanning calorimetry (DSC) [58] | Determining material thermal properties by measuring the rate of heat release or absorption during temperature increase | Simulating the bubble point temperature of confined hydrocarbons | Difficulty in controlling and measuring phase transition rates | ||
| Theoretical simulation | Molecular simulation | Molecular dynamics (MD) [59] | Numerically solving the classical equations of motion allows for the determination of the phase trajectory of a molecular system and the characterization of its macroscopic thermodynamic properties | High accuracy for simple components | High computational cost, difficulty in calculating near the critical point |
| Monte Carlo (MC) [12] | Repeatedly sample different configurations of a molecular system and calculate the total energy of each configuration | ||||
| The equation of state [27,51] | Phase equilibrium and force calculation | Consider capillary forces and critical displacement | Depend on the equation of state | ||
| Density functional theory (DFT) [52,60] | Fluid molecular density as the fundamental variable to describe the thermodynamics of a system | Exhibits minimal discrepancies with molecular simulation results | Limited to simple molecular density statistics | ||
| Instrumental analysis | Nuclear magnetic resonance online scanning (NMR) [61] | 1H and 13C nuclei resonate in the magnetic field | Full-scale pore observation in the range of nanometers to millimeters | In situ imaging of oil or water requires phase shielding, resulting in low imaging accuracy and high costs | |
| CT scanning | Three-dimensional image scanning and reconstruction | Digital rock | High sample requirements and high costs | ||
| Experimental Objective | Component | Size | Phenomenon |
|---|---|---|---|
| Evaporation | n-pentane [71] | 50 μm 145 nm | Nanoporous capillary confinement enhances liquid capture and significantly impedes liquid evaporation, reducing the evaporation rate by approximately 16 fold. |
| n-pentane [72] | 100 nm 5 μm | The confinement effect leads to the preferential evaporation of the fluid in microchannels over nanochannels. | |
| Condensation | propane [11] | 30 nm 50 nm 500 nm | The condensation pressure for the 50 nm chip is close to the prediction of the Kelvin equation, while for the 30 nm chip, the condensation pressure is significantly lower than the predicted value. |
| propane [73,74] | 70 nm 100 nm | There exist disparities between the two length scales. | |
| n-butane [12] | 2 nm | The deviation of the condensation pressure from the bulk phase reaches as high as 22.9%. | |
| Bubble point temperature | n-hexane, n-octane, n-heptane [50] | 4 nm 20 nm 50 nm 100 nm | The bubble point temperatures for 4 nm and 10 nm confinement exhibit significant deviations compared to those for 100 nm and 50 nm confinement. The confinement effect is more pronounced in the 4 nm channel, leading to a noticeable increase in the bubble point temperature. |
| Dew point pressure | n-butane [75] | 4 nm 10 nm 50 nm | At 4 nm, the dew point pressure exhibits a deviation of up to 14% compared to the bulk phase. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Sun, L.; Huo, X.; Feng, C.; Zhang, Z. Research Progress on CO2 Capture, Utilization, and Storage (CCUS) Based on Micro-Nano Fluidics Technology. Energies 2023, 16, 7846. https://doi.org/10.3390/en16237846
Pan X, Sun L, Huo X, Feng C, Zhang Z. Research Progress on CO2 Capture, Utilization, and Storage (CCUS) Based on Micro-Nano Fluidics Technology. Energies. 2023; 16(23):7846. https://doi.org/10.3390/en16237846
Chicago/Turabian StylePan, Xiuxiu, Linghui Sun, Xu Huo, Chun Feng, and Zhirong Zhang. 2023. "Research Progress on CO2 Capture, Utilization, and Storage (CCUS) Based on Micro-Nano Fluidics Technology" Energies 16, no. 23: 7846. https://doi.org/10.3390/en16237846
APA StylePan, X., Sun, L., Huo, X., Feng, C., & Zhang, Z. (2023). Research Progress on CO2 Capture, Utilization, and Storage (CCUS) Based on Micro-Nano Fluidics Technology. Energies, 16(23), 7846. https://doi.org/10.3390/en16237846






