Air-Blown Biomass Gasification Process Intensification for Green Hydrogen Production: Modeling and Simulation in Aspen Plus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Feedstock Selection and Characterization
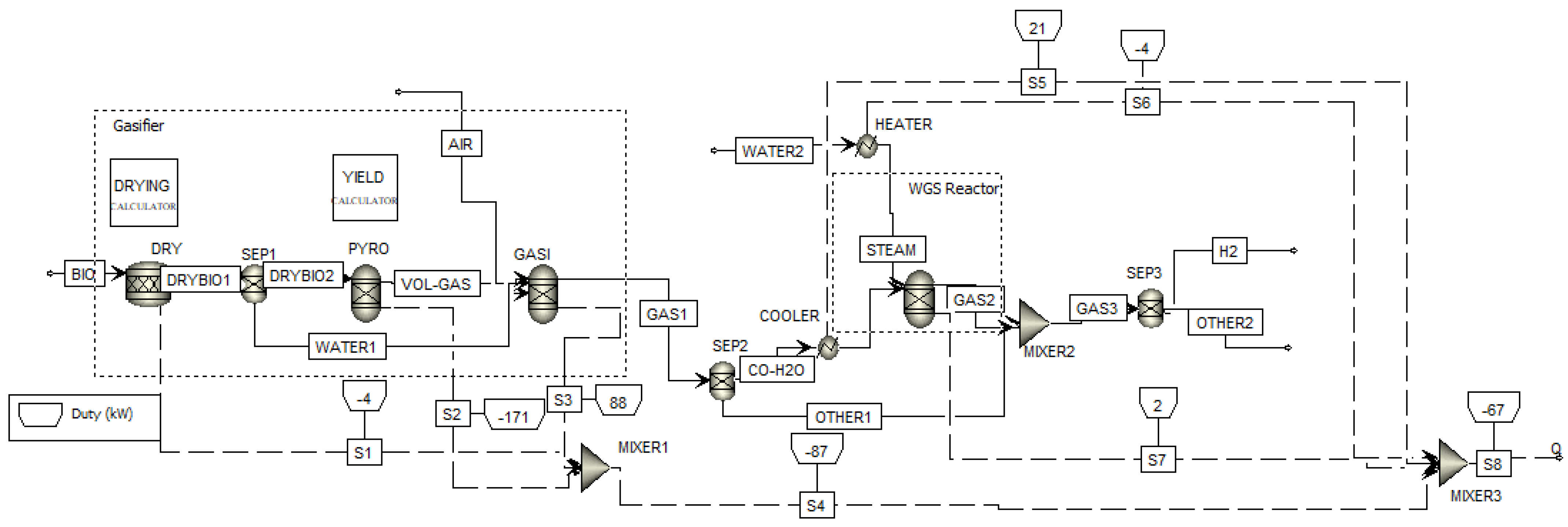
2.2. Aspen Plus Model Development and Validation
- Residence time is long enough for the equilibrium state to be achieved.
- Homogeneous mixing with uniform pressure and temperature.
- Kinetic and potential energies are neglected.
- The gasifier is considered zero-dimensional and adiabatic.
- Gasifying medium is enough to convert all carbon in the biomass.
- Nitrogen is considered inert.
- The produced gas comprises solely CO, H2, CO2, CH4, N2, and H2O.
- Tar, char, and ash contents are considered negligible.
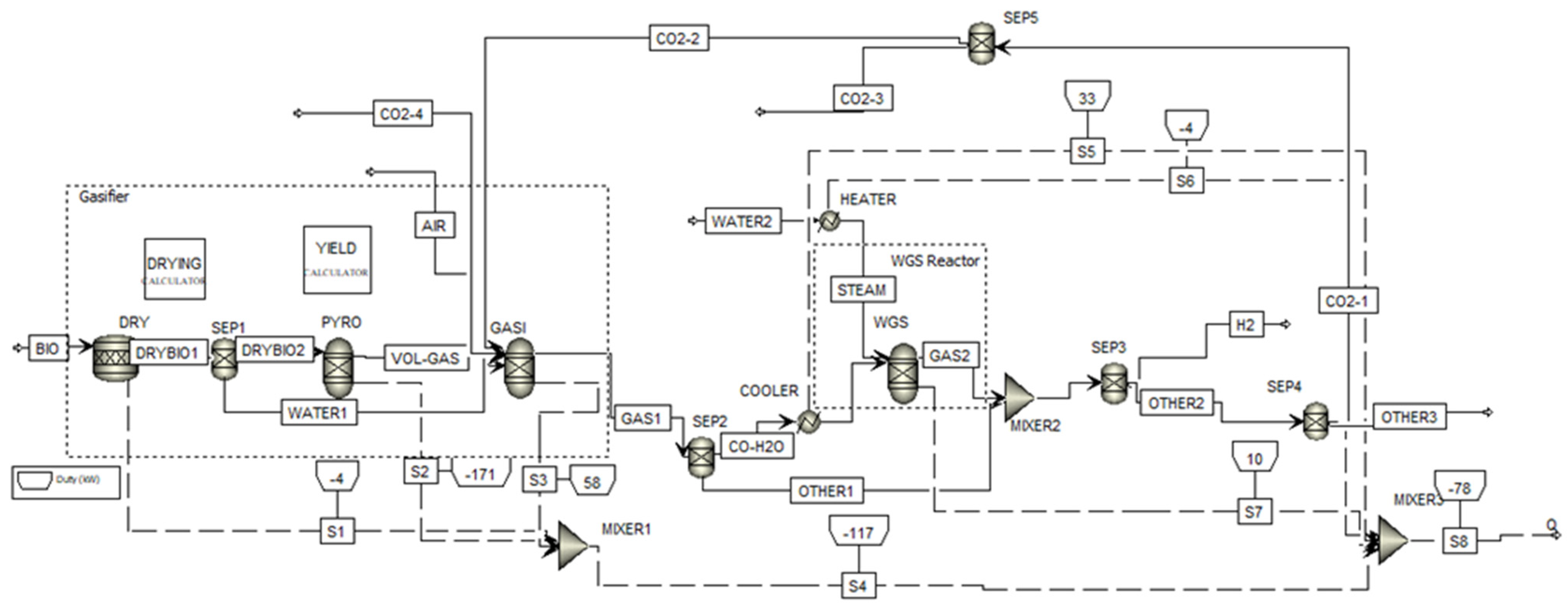
2.2.1. Aspen Plus Model Considering Carbon Dioxide Recycling
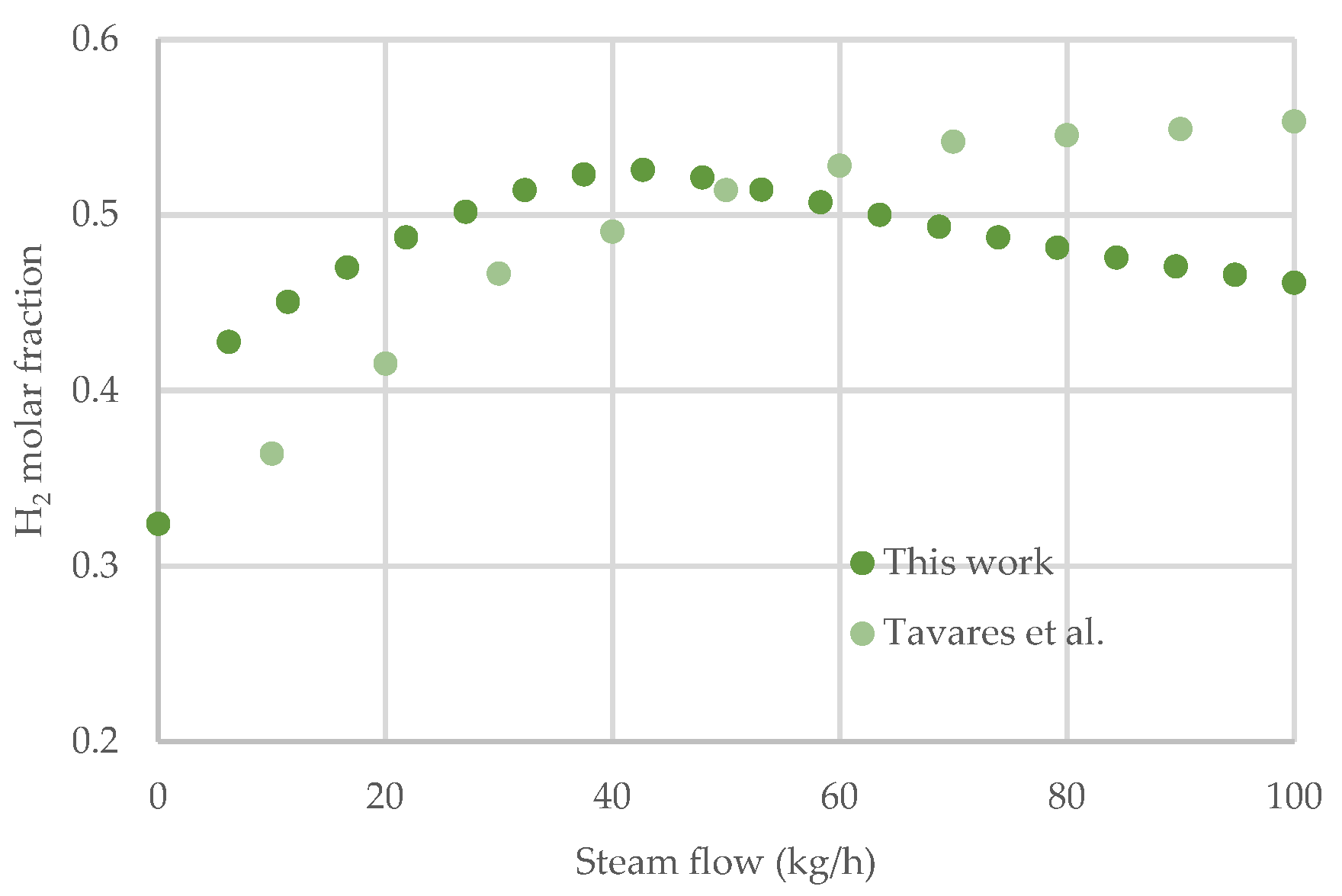
2.2.2. Gasification Model Validation
3. Results and Discussion
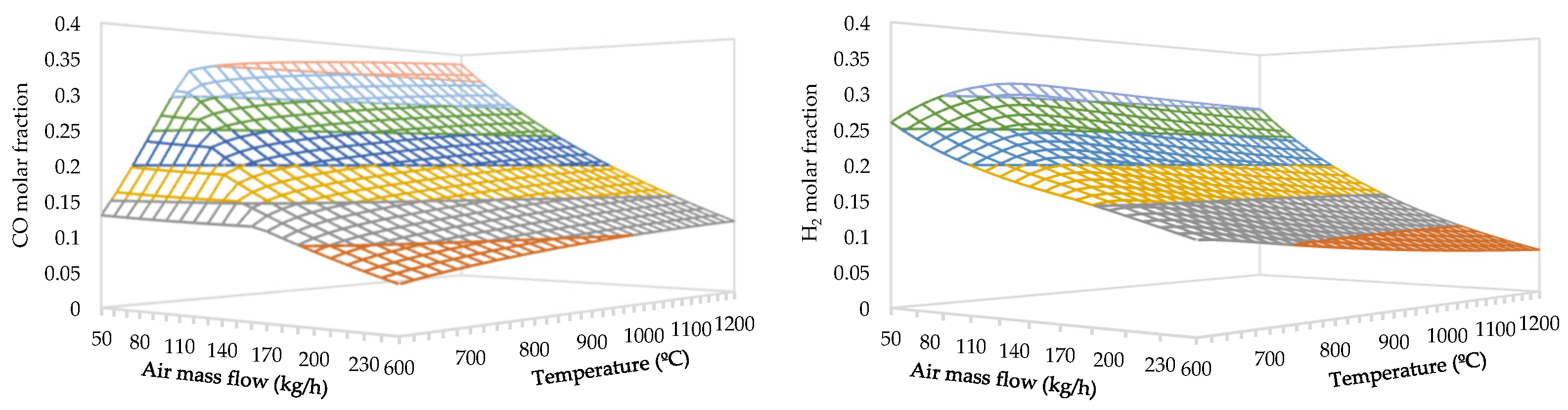
3.1. Effect of Air Flow Rate and Temperature
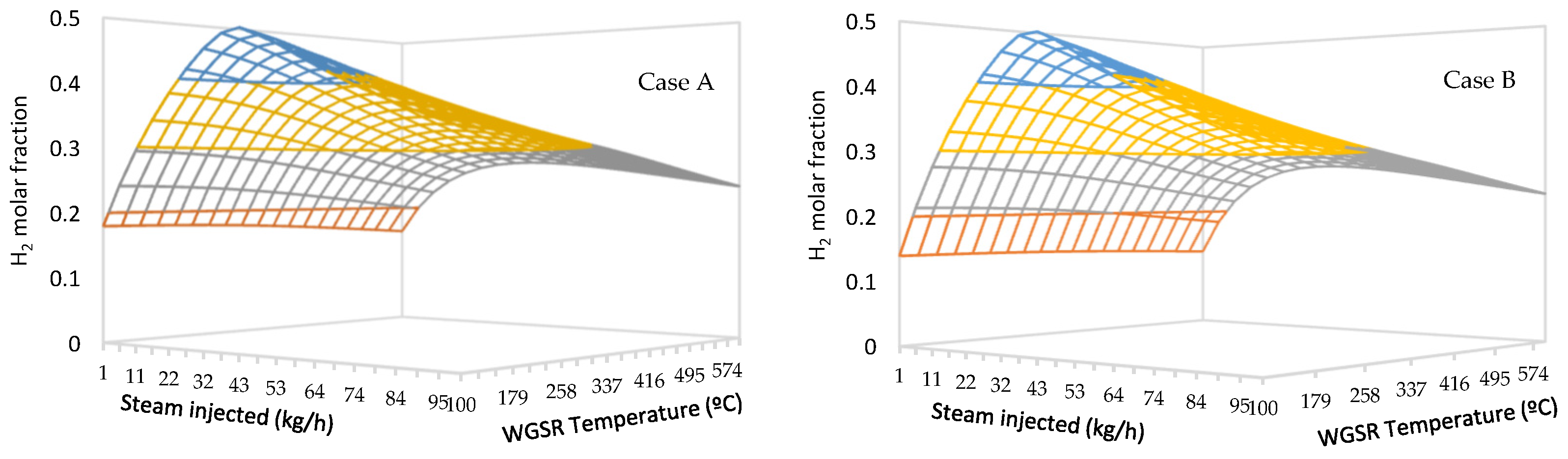
3.2. Effect of The Water–Gas Shift Reactor
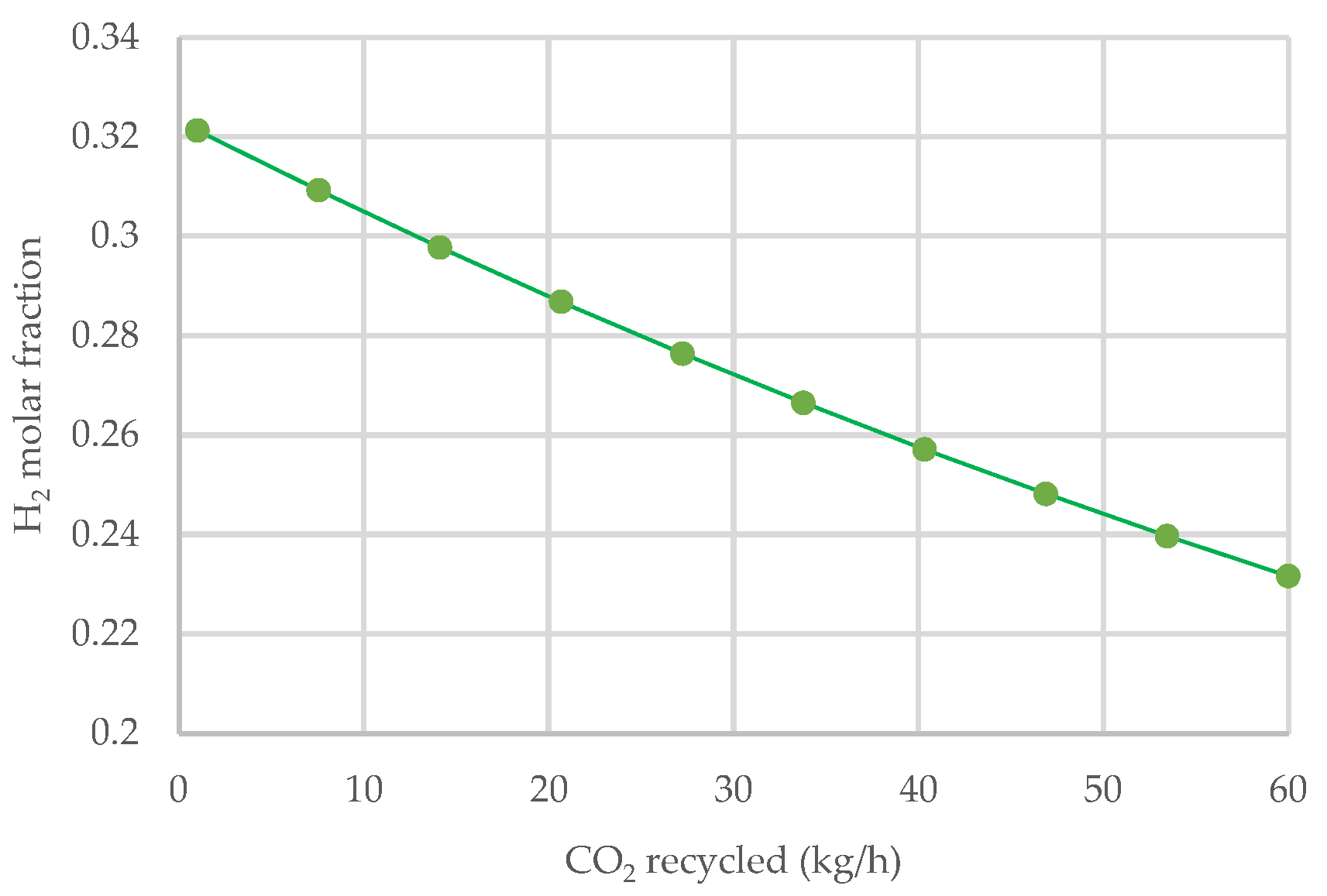
3.3. Effect of Carbon Dioxide Recycle Stream
4. Conclusions
- The maximum molar fraction of CO is obtained at a temperature of 1200 °C and an air flow rate of 50 kg/h, achieving 38.5%.
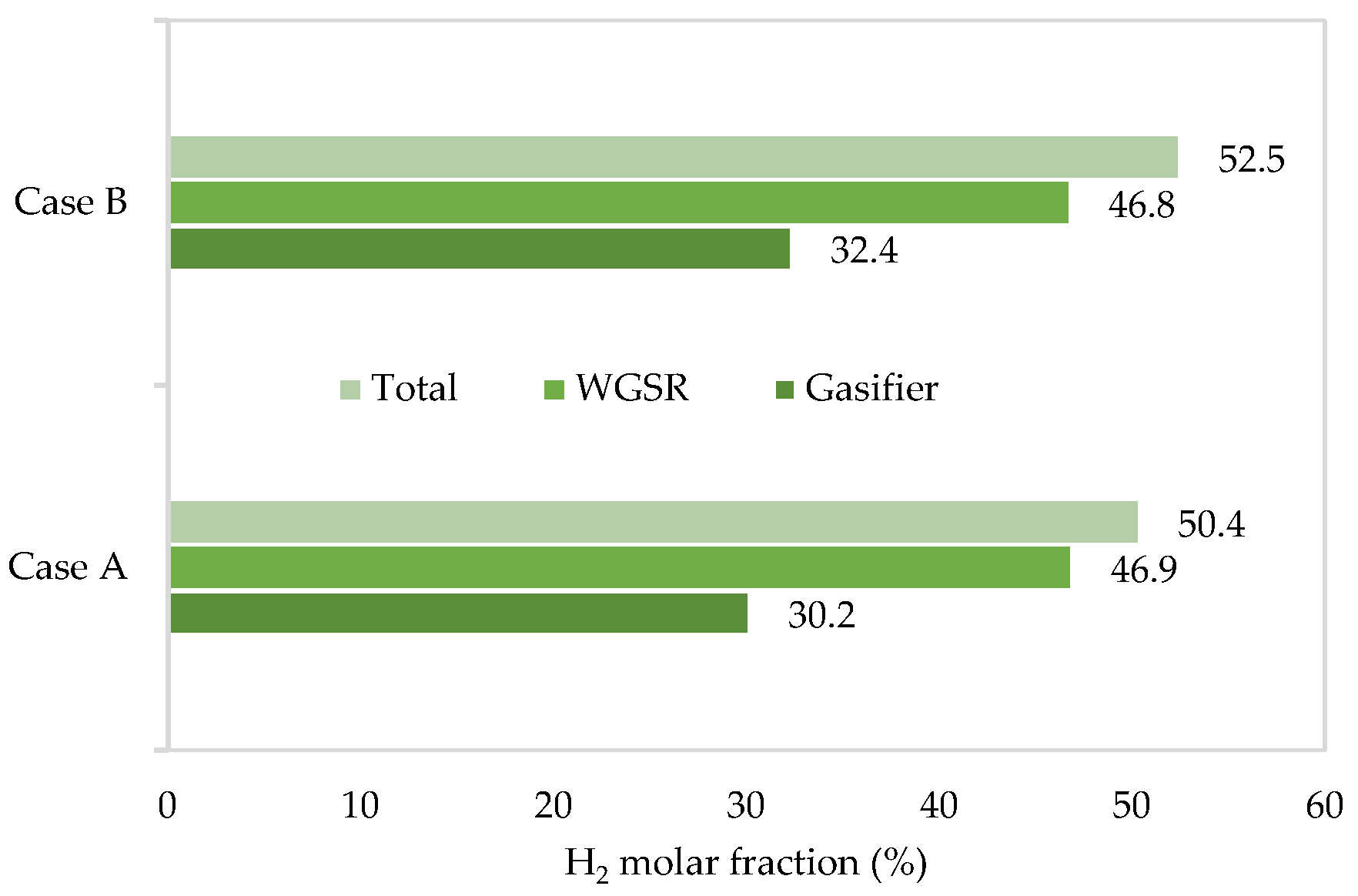
- The maximum molar fraction of H2 is obtained at a temperature of 780 °C and an air flow rate of 50 kg/h, attaining 32.4%.
- The Case B approach is more favorable for green hydrogen production, allowing for a 52.5% molar fraction thanks to the higher molar fraction of hydrogen at the exit of the gasifier block, with the same steam consumption in the WGS reactor as in Case A.
- The combined process implemented in this study allows for a greater hydrogen molar fraction at lower steam flow rates than a pure steam gasification process.
- The introduction of CO2 as an additional gasifying agent has no positive effect on the H2 molar fraction in a forest residue gasification process.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Monteiro, E.; Ferreira, S. Some Perspectives for the Gasification Process in the Energy Transition World Scenario. Energies 2023, 16, 5543. [Google Scholar] [CrossRef]
- Ramos, A.; Monteiro, E.; Rouboa, A. Numerical approaches and comprehensive models for gasification process: A review. Renew. Sustain. Energy Rev. 2019, 110, 188–206. [Google Scholar] [CrossRef]
- Tavares, R.; Monteiro, E.; Fouzi, T.; Rouboa, A. Numerical investigation of optimum operating conditions for syngas and hydrogen production from biomass gasification using Aspen Plus. Renew. Energy 2020, 146, 1309–1314. [Google Scholar] [CrossRef]
- González-Vázquez, M.P.; Rubiera, F.; Pevida, C.; Pio, D.T.; Tarelho, L.A.C. Thermodynamic Analysis of Biomass Gasification Using Aspen Plus: Comparison of Stoichiometric and Non-Stoichiometric Models. Energies 2021, 14, 189. [Google Scholar] [CrossRef]
- Martins, A.H.; Rouboa, A.; Monteiro, E. On the green hydrogen production through gasification processes: A techno-economic approach. J. Clean. Prod. 2023, 383, 135476. [Google Scholar] [CrossRef]
- Ren, R.; Wang, H.; You, C. Steam Gasification of Refuse-Derived Fuel with CaO Modification for Hydrogen-Rich Syngas Production. Energies 2022, 15, 8279. [Google Scholar] [CrossRef]
- Pitrez, P.; Monteiro, E.; Rouboa, A. Numerical analysis of plasma gasification of hazardous waste using Aspen Plus. Energy Rep. 2023, 9, 418–426. [Google Scholar] [CrossRef]
- Vikram, S.; Rosha, P.; Kumar, S.; Mahajani, S. Thermodynamic analysis and parametric optimization of steam-CO2 based biomass gasification system using Aspen Plus. Energy 2022, 241, 122854. [Google Scholar] [CrossRef]
- Ersoz, A.; DurakÇetin, Y.; Sarioglan, A.; Turan, A.Z.; Mert, M.S.; Yuksel, F.; Figen, H.E.; Guldal, N.O.; Karaismailoglu, M.; Baykara, S.Z. Investigation of a novel & integrated simulation model for hydrogen production from lignocellulosic biomass. Int. J. Hydrogen Energy 2018, 43, 1081–1093. [Google Scholar]
- Pala, L.P.R.; Wang, Q.; Kolb, G.; Hessel, V. Steam gasification of biomass with subsequent syngas adjustment using shift reaction for syngas production: An Aspen Plus model. Renew. Energy 2017, 101, 484–492. [Google Scholar] [CrossRef]
- Chu, H.; Li, Q.; Meng, A.; Zhang, Y. Investigation of hydrogen production from model bio-syngas with high CO2 content by water-gas shift reaction. Int. J. Hydrogen Energy 2015, 40, 4092–4100. [Google Scholar] [CrossRef]
- Ferreira, S.; Monteiro, E.; Brito, P.; Vilarinho, C. Biomass resources in Portugal: Current status and prospects. Renew. Sust. Energy Rev. 2017, 78, 1221–1235. [Google Scholar] [CrossRef]
- Silva, V.; Monteiro, E.; Couto, N.; Brito, P.; Rouboa, A. Analysis of syngas quality from Portuguese biomasses: An experimental and numerical study. Energy Fuels 2014, 28, 5766–5777. [Google Scholar] [CrossRef]
- Mutlu, O.Ç.; Zeng, T. Challenges and Opportunities of Modeling Biomass Gasification in Aspen Plus: A Review. Chem. Eng. Technol. 2020, 43, 1674–1689. [Google Scholar] [CrossRef]
- De, S.; Agarwal, A.K.; Moholkar, V.S.; Thallada, B. Coal and Biomass Gasification: Recent Advances and Future Challenges; Springer Nature: Singapore, 2018. [Google Scholar]
- Puig-Arnavat, M.; Bruno, J.C.; Coronas, A. Review and analysis of biomass gasification models. Renew. Sust. Energy Rev. 2010, 14, 2841–2851. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis, and Torrefaction: Practical Design and Theory, 3rd ed.; Academic Press, Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- La Villetta, M.; Costa, M.; Massarotti, N. Modelling approaches to biomass gasification: A review with emphasis on the stoichiometric method. Renew. Sust. Energ. Rev. 2017, 74, 71–88. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 3): Gasification technologies. Bioresour. Technol. 2002, 83, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Al Malah, K.I.M. Aspen Plus: Chemical Engineering Applications; John Wiley & Sons: New York, NY, USA, 2017. [Google Scholar]
- Aspen Technology Inc. Aspen Plus User Guide; Version 10.2; Aspen Technology Inc.: Cambridge, MA, USA, 2000. [Google Scholar]
- Towler, G.P.; Sinnott, R.K. ; Chemical Engineering Design: Principles, Practice, and Economics of Plant and Process Design, 2nd ed.; Butterworth-Heinemann: Oxford, UK; Waltham, MA, USA, 2013. [Google Scholar]
- Aspen Technology Inc. Getting Started Modeling Processes with Solids; Version 8.4; Aspen Technology, Inc.: Bedford, MA, USA, 2013. [Google Scholar]
- Mendiburu, A.Z.; Carvalho, J.A.; Rolando Zanzi, R.; Coronado, C.R.; Silveira, J.L. Thermochemical equilibrium modeling of a biomass downdraft gasifier: Constrained and unconstrained non-stoichiometric models. Energy 2014, 71, 624–637. [Google Scholar] [CrossRef]
- Pandey, B.; Sheth, P.N.; Prajapati, Y.K. Air-CO2 and oxygen-enriched air-CO2 biomass gasification in an autothermal downdraft gasifier: Experimental studies. Energy Convers. Manag. 2022, 270, 116216. [Google Scholar] [CrossRef]
- Chan, Y.H.; Rahman, S.N.F.S.A.; Lahuri, H.M.; Khalid, A. Recent progress on CO-rich syngas production via CO2 gasification of various wastes: A critical review on efficiency, challenges and outlook. Environ. Pollut. 2021, 278, 116843. [Google Scholar] [CrossRef]
- Jayah, T.H.; Aye, L.; Fuller, R.J.; Stewart, D.F. Computer simulation of a downdraft wood gasifier for tea drying. Biomass Bioenergy 2003, 25, 459–469. [Google Scholar] [CrossRef]
- Jarungthammachote, S.; Dutta, A. Thermodynamic equilibrium model and second law analysis of a downdraft waste gasifier. Energy 2007, 32, 1660–1669. [Google Scholar] [CrossRef]
- Ferreira, S.; Monteiro, E.; Brito, P.; Vilarinho, C. A Holistic Review on Biomass Gasification Modified Equilibrium Models. Energies 2019, 12, 160. [Google Scholar] [CrossRef]
- Ismail, T.M.; Monteiro, E.; Ramos, A.; El-Salam, M.A.; Rouboa, A. An Eulerian model for forest residues gasification in a plasma gasifier. Energy 2019, 182, 1069–1083. [Google Scholar] [CrossRef]
- Kangas, P.; Hannula, I.; Koukkari, P.; Hupa, M. Modelling super-equilibrium in biomass gasification with the constrained Gibbs energy method. Fuel 2014, 129, 631–653. [Google Scholar] [CrossRef]
- Ferreira, S.; Monteiro, E.; Calado, L.; Silva, V.; Brito, P.; Vilarinho, C. Experimental and modeling analysis of brewers’ spent grains gasification in a downdraft reactor. Energies 2019, 12, 4413. [Google Scholar] [CrossRef]
- Ferreira, S.; Monteiro, E.; Brito, P.; Vilarinho, C. Experimental Analysis of Brewers’ Spent Grains Steam Gasification in an Allothermal Batch Reactor. Energies 2019, 12, 912. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Zawawi, N.A.; Kasim, F.H.; Inayat, A.; Khasri, A. Assessing the gasification performance of biomass: A review on biomass gasification process conditions, optimization and economic evaluation. Renew. Sustain. Energy Rev. 2016, 53, 1333–1347. [Google Scholar] [CrossRef]
- Thomson, R.; Kwong, P.; Ahmad, E.; Nigam, K.D.P. Clean syngas from small commercial biomass gasifiers; a review of gasifier development, recent advances and performance evaluation. Int. J. Hydrogen Energy 2020, 45, 21087–21111. [Google Scholar] [CrossRef]
- Haryanto, A.; Fernando, S.D.; Filip, S.D.; Steele, P.H.; Pordesimo, L.; Adhikari, S. Hydrogen Production through the Water−Gas Shift Reaction: Thermodynamic Equilibrium versus Experimental Results over Supported Ni Catalysts. Energy Fuels 2009, 23, 3097–3102. [Google Scholar] [CrossRef]
- Idriss, H.; Scott, M.; Subramani, V. Introduction to hydrogen and its properties. In Compendium of Hydrogen Energy; Subramani, V., Basile, A., Veziroğlu, T.N., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2015; pp. 3–19. [Google Scholar]
- Couto, N.; Silva, V.; Monteiro, E.; Rouboa, A.; Brito, P. An experimental and numerical study on the Miscanthus gasification by using a pilot scale gasifier. Renew. Energy 2017, 109, 248–261. [Google Scholar] [CrossRef]
| Proximate Analysis (wt.%. d.b.) | Ultimate Analysis (wt.%, d.b.) | ||
|---|---|---|---|
| Volatile matter | 79.8 | C | 43.0 |
| Fixed carbon | 20.0 | H | 5.0 |
| Ash | 0.2 | O | 49.6 |
| N | 2.4 | ||
| Unit Operation | Model | Features |
|---|---|---|
| Yield reactor | RYield (PYRO) | Yield reactors are especially useful for modeling streams with pseudo components, solids with a particle size dispersion, or processes that create small quantities of various by-products. The yield shift reactor overcomes some of the disadvantages of existing reactor models by allowing the designer to select a yield pattern. |
| Conversion reactor | RStoic (DRY) | A conversion or stoichiometric reactor requires both a reaction stoichiometry and an extent of reaction, which is commonly described as the extent of a limiting reagent’s conversion. It can be used when the reaction kinetics are unknown or when it is known that the process will persist to complete conversion because no reaction kinetics information is required. |
| Gibbs reactor | RGibbs (GASI, WGS) | The Gibbs reactor, subject to the mass balance constraint, resolves the entire reaction and phase equilibrium of all species in the component list by minimizing the Gibbs free energy. A Gibbs reactor can be configured with constraints such as a specified conversion of one species or a temperature approach to equilibrium. |
| Stream mixing | Mixer (MIXER 1, 2) | Mixers join several streams (mass, heat, or work) into a single stream. |
| Component splitter | Sep (SEP 1, 2) | The separator separates the entering stream components into several exit streams based on the identified flows or split fractions. |
| Heater | Heater (HEATER) | Heaters are used to heat or cool a stream. |
| Proximate Analysis (wt.%. d.b.) | Ultimate Analysis (wt.%, d.b.) | ||
|---|---|---|---|
| Volatile matter | 80.1 | C | 50.6 |
| Fixed carbon | 19.2 | H | 6.5 |
| Ash | 0.7 | O | 42.0 |
| N | 0.2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novais, B.; Ramos, A.; Rouboa, A.; Monteiro, E. Air-Blown Biomass Gasification Process Intensification for Green Hydrogen Production: Modeling and Simulation in Aspen Plus. Energies 2023, 16, 7829. https://doi.org/10.3390/en16237829
Novais B, Ramos A, Rouboa A, Monteiro E. Air-Blown Biomass Gasification Process Intensification for Green Hydrogen Production: Modeling and Simulation in Aspen Plus. Energies. 2023; 16(23):7829. https://doi.org/10.3390/en16237829
Chicago/Turabian StyleNovais, Bernardino, Ana Ramos, Abel Rouboa, and Eliseu Monteiro. 2023. "Air-Blown Biomass Gasification Process Intensification for Green Hydrogen Production: Modeling and Simulation in Aspen Plus" Energies 16, no. 23: 7829. https://doi.org/10.3390/en16237829
APA StyleNovais, B., Ramos, A., Rouboa, A., & Monteiro, E. (2023). Air-Blown Biomass Gasification Process Intensification for Green Hydrogen Production: Modeling and Simulation in Aspen Plus. Energies, 16(23), 7829. https://doi.org/10.3390/en16237829









