Recycling Ash and Slag Waste from Thermal Power Plants to Produce Foamed Geopolymers
Abstract
:1. Introduction
- -
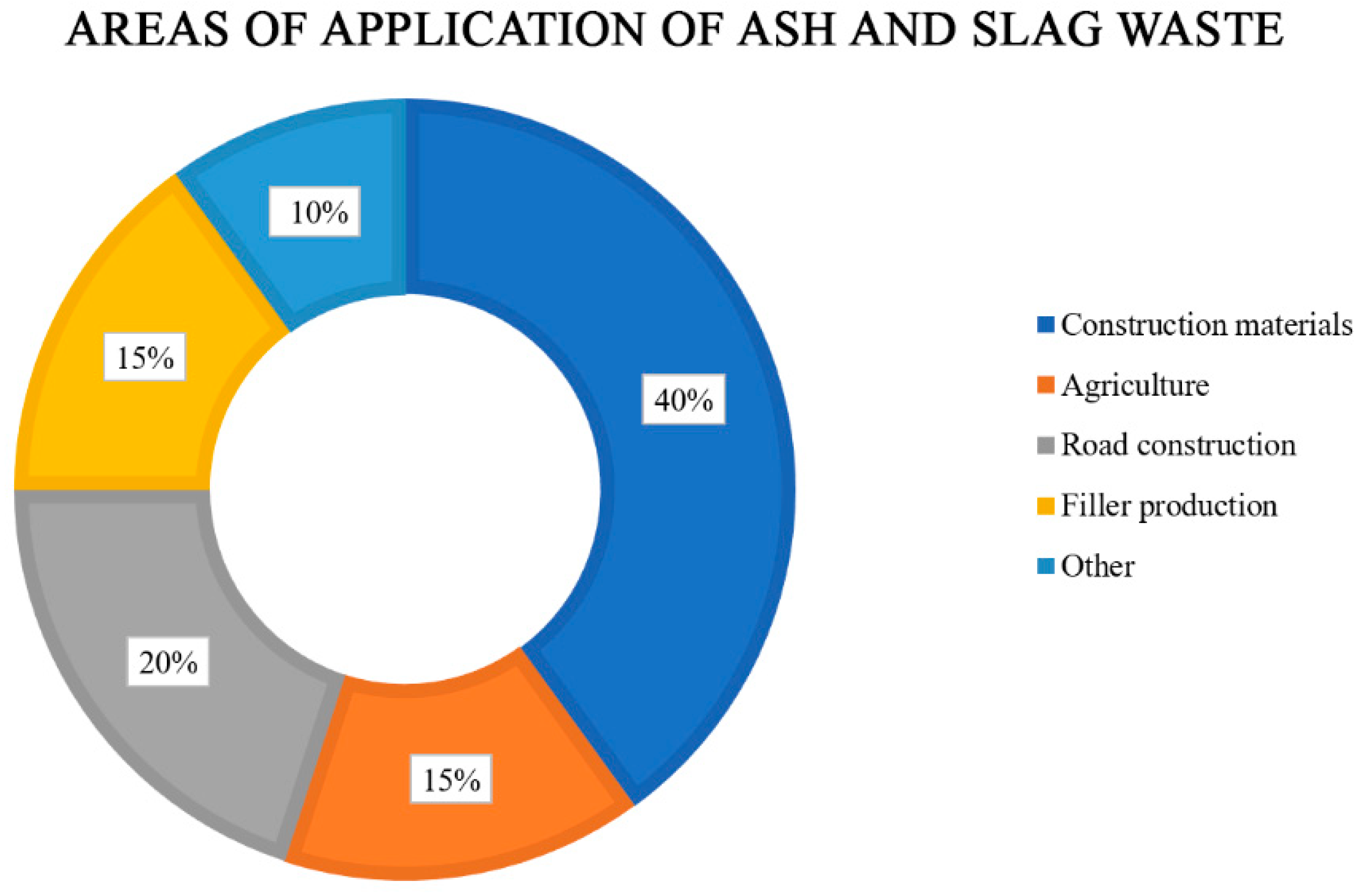
- Producing building materials (cement, bricks, blocks, and geopolymers);
- -
- Producing road construction materials;
- -
- Agriculture (soil stabilizers, fertilizers, etc.);
- -
- Producing various fillers;
- -
- Producing other materials (sorbents, zeolites, etc.).
2. Materials and Methods
2.1. Materials
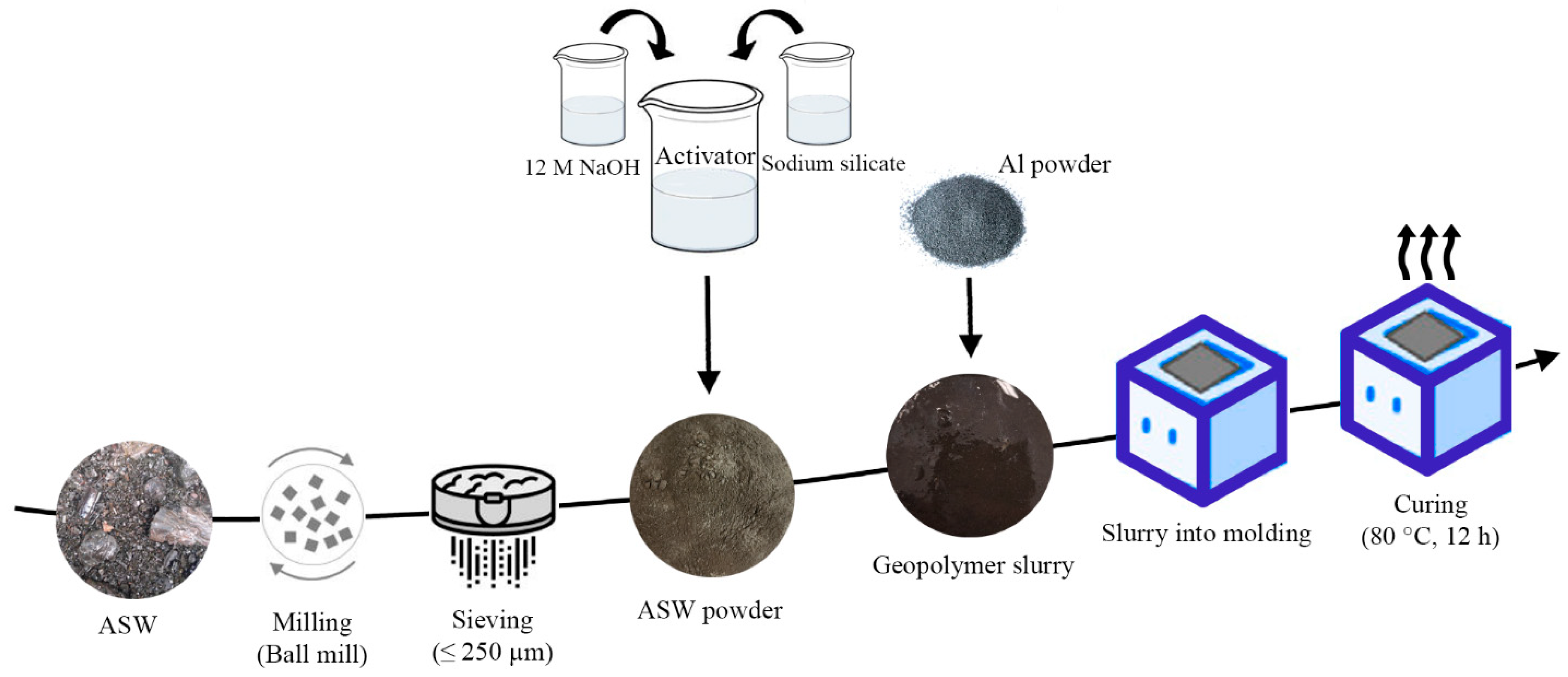
2.2. Synthesis of Foamed Geopolymer Materials
2.3. Methods
3. Results and Discussion
3.1. Technological Properties of Foamed Geopolymers
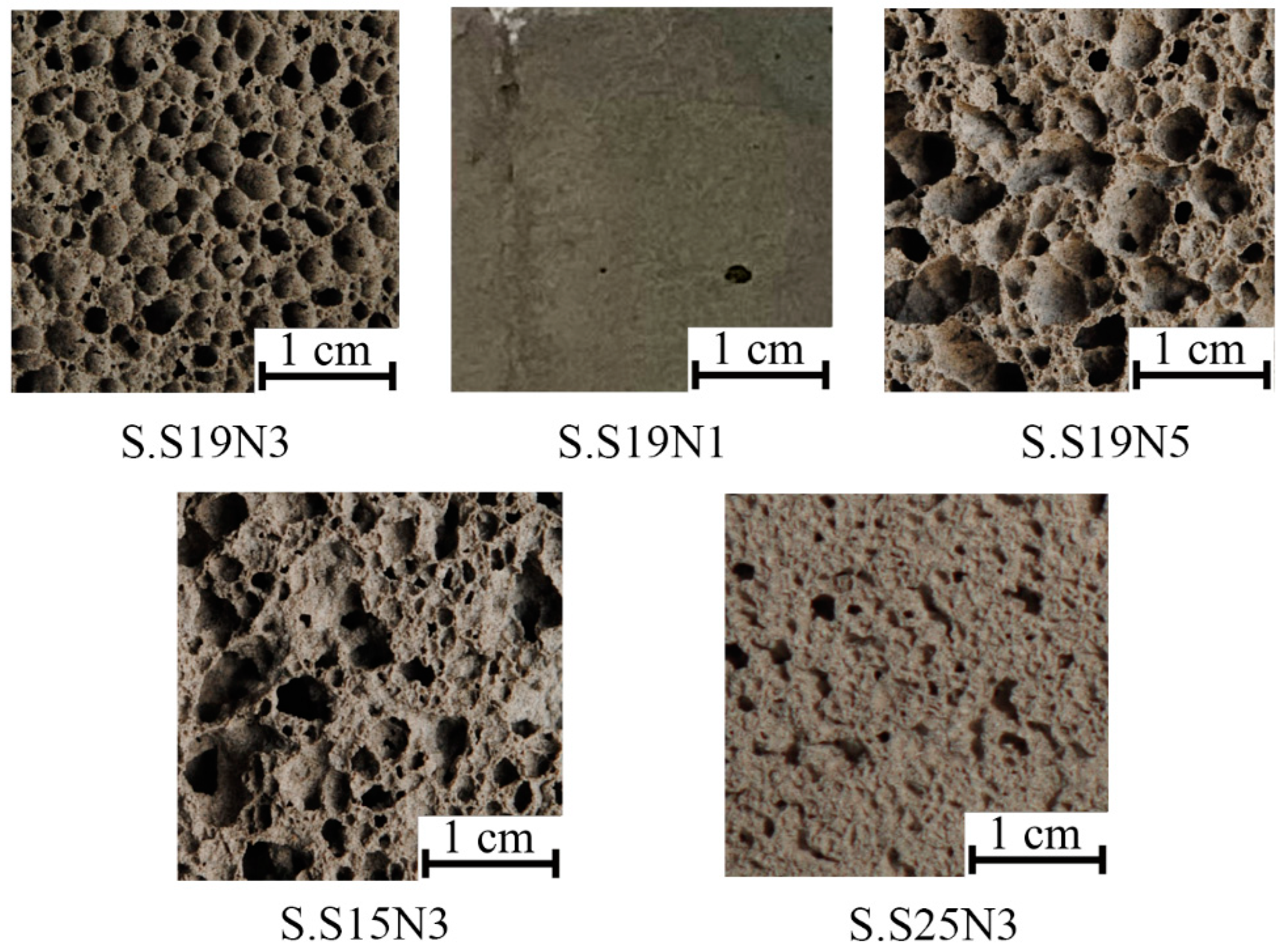
3.2. Phase Composition and Microstructure
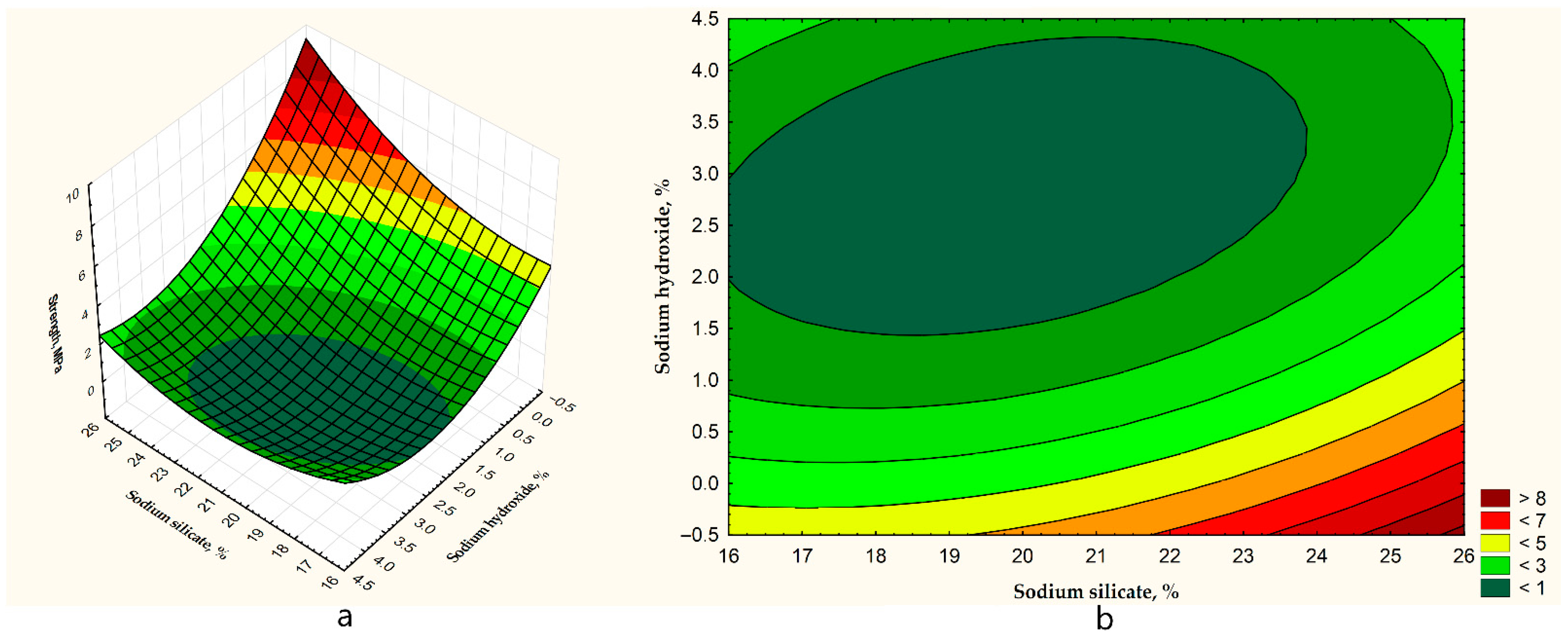
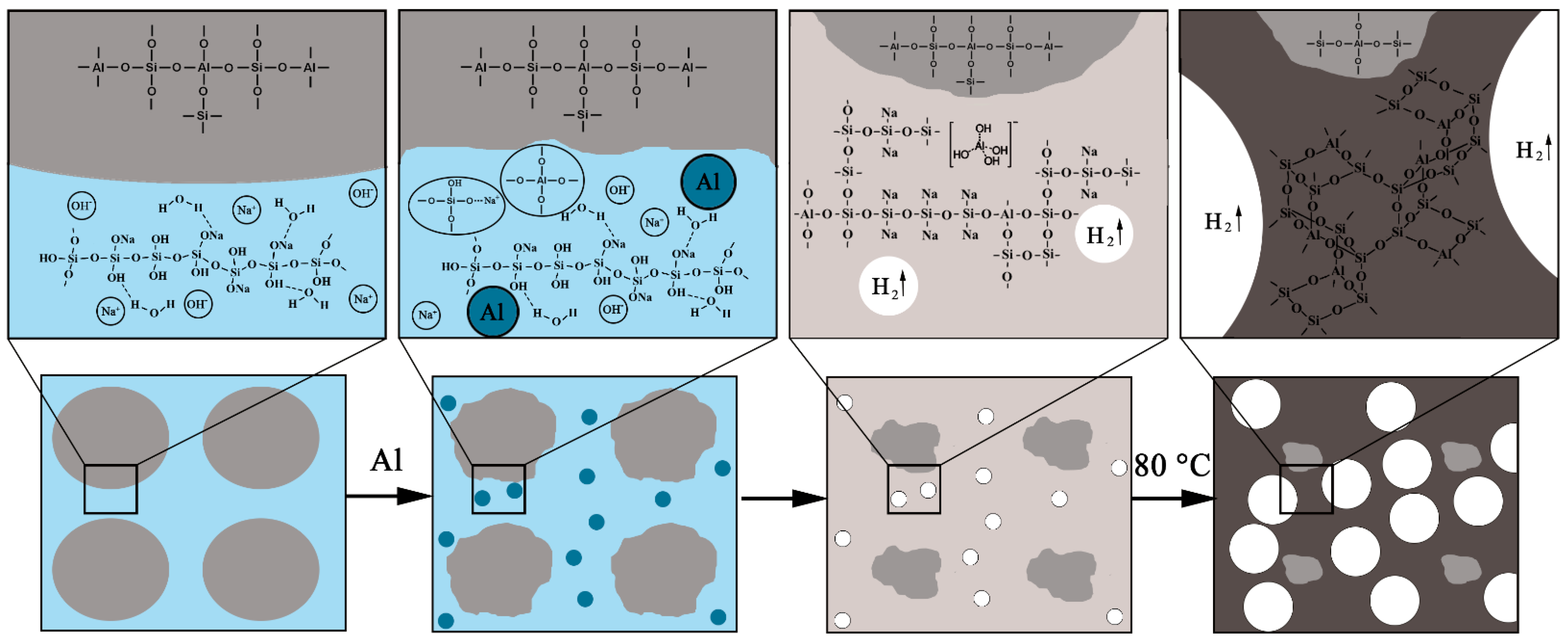
3.3. Mechanism of Porous Geopolymers Formation
3.4. Evaluation of the Developed Mechanism Applicability for ASW of Other TPP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Putilova, I.V. Current State of the Coal Ash Handling Problem in Russia and Abroad, Aspects of the Coal Ash Applications in Hydrogen Economy. Int. J. Hydrogen Energy 2023, 48, 31040–31048. [Google Scholar] [CrossRef]
- Chukaeva, M.A.; Matveeva, V.A.; Sverchkov, I.P. Complex Processing of High-Carbon Ash and Slag Waste. J. Min. Inst. 2022, 253, 97–104. [Google Scholar] [CrossRef]
- Ryabov, Y.V.; Delitsyn, L.M.; Ezhova, N.N.; Sudareva, S.V. Methods for Beneficiation of Ash and Slag Waste from Coal-Fired Thermal Power Plants and Ways for Their Commercial Use (a Review). Therm. Eng. 2019, 66, 149–168. [Google Scholar] [CrossRef]
- Yatsenko, E.A.; Goltsman, B.M.; Trofimov, S.V.; Novikov, Y.V.; Smoliy, V.A.; Ryabova, A.V.; Klimova, L.V. Influence of Various Coal Energy Wastes and Foaming Agents on Foamed Geopolymer Materials’ Synthesis. Materials 2023, 16, 264. [Google Scholar] [CrossRef] [PubMed]
- Yatsenko, E.A.; Goltsman, B.M.; Novikov, Y.V.; Kurdashov, V.M.; Klimova, L.V. Study into the Possibilities of Synthesis of Foamed Geopolymer Materials Based on Ash and Slag Waste from Thermal Power Plants of the Russian Federation’s Arctic Zone. Glas. Phys. Chem. 2022, 48, 429–435. [Google Scholar] [CrossRef]
- Cherkasova, T.G.; Cherkasova, E.V.; Tikhomirova, A.V.; Bobrovnikova, A.A.; Nevedrov, A.V.; Papin, A.V. Coal Waste as a Raw Material for Obtaining Rare and Trace Elements. Bull. Kuzbass State Technol. Univ. 2016, 185, 185–188. [Google Scholar]
- Dosmukhamedov, N.K.; Zholdasbay, E.E. Technology of Ash and Slag Waste Processing by Chloridizing Roasting. Metallurgist 2022, 66, 180–189. [Google Scholar] [CrossRef]
- Delitsyn, L.; Popel, O.; Kulumbegov, R.; Sulman, M.; Petropavlovskii, K. Main Ways of Disposal of Aluminosilicate Ash of Coal-Power Plants and Environmental Consequences. AIP Conf. Proc. 2023, 2526, 040041. [Google Scholar]
- Iatsyshyn, A.; Artemchuk, V.; Zaporozhets, A.; Popov, O.; Kovach, V. Mathematical Approaches for Determining the Level of Impact of Ash-Slag Dumps of Energy Facilities on the Environment. In Systems, Decision and Control in Energy I; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–13. [Google Scholar]
- Cherkasova, T.G.; Cherkasova, E.V.; Tikhomirova, A.V.; Gilyazidinova, N.V.; Klyuev, R.V.; Martyushev, N.V.; Karlina, A.I.; Skiba, V.Y. Study of Matrix and Rare Elements in Ash and Slag Waste of a Thermal Power Plant Concerning the Possibility of Their Extraction. Metallurgist 2022, 65, 1324–1330. [Google Scholar] [CrossRef]
- Lyapin, A.A.; Parinov, I.A.; Buravchuk, N.I.; Cherpakov, A.V.; Shilyaeva, O.V.; Guryanova, O.V. Improving Road Pavement Characteristics; Springer: Cham, Switzerland, 2020; Volume 10, pp. 973–978. [Google Scholar]
- Choudhary, J.; Kumar, B.; Gupta, A. Utilization of Solid Waste Materials as Alternative Fillers in Asphalt Mixes: A Review. Constr. Build. Mater. 2020, 234, 117271. [Google Scholar] [CrossRef]
- Abdila, S.R.; Abdullah, M.M.A.B.; Ahmad, R.; Burduhos Nergis, D.D.; Rahim, S.Z.A.; Omar, M.F.; Sandu, A.V.; Vizureanu, P. Syafwandi Potential of Soil Stabilization Using Ground Granulated Blast Furnace Slag (GGBFS) and Fly Ash via Geopolymerization Method: A Review. Materials 2022, 15, 375. [Google Scholar] [CrossRef]
- Yatsenko, E.A.; Goltsman, B.M.; Trofimov, S.V.; Kurdashov, V.M.; Novikov, Y.V.; Smoliy, V.A.; Ryabova, A.V.; Klimova, L.V. Improving the Properties of Porous Geopolymers Based on TPP Ash and Slag Waste by Adjusting Their Chemical Composition. Materials 2022, 15, 2587. [Google Scholar] [CrossRef] [PubMed]
- Akhmedov, I.; Khamidov, A.; Shavkat, Y.; Jalalov, Z.; Umarov, I.; Kazadayev, A. Research of Ash-Slag Mixtures for Production of Construction Materials. Spectr. J. Innov. Reforms Dev. 2022, 10, 85–91. [Google Scholar]
- Karim, M.R.; Zain, M.F.M.; Jamil, M.; Lai, F.C.; Islam, M.N. Use of Wastes in Construction Industries as an Energy Saving Approach. Energy Procedia 2011, 12, 915–919. [Google Scholar] [CrossRef]
- He, H.; Dong, Z.; Peng, Q.; Wang, X.; Fan, C.; Zhang, X. Impacts of Coal Fly Ash on Plant Growth and Accumulation of Essential Nutrients and Trace Elements by Alfalfa (Medicago Sativa) Grown in a Loessial Soil. J. Environ. Manag. 2017, 197, 428–439. [Google Scholar] [CrossRef]
- Tripathi, R.C.; Masto, R.E.; Ram, L.C. Bulk Use of Pond Ash for Cultivation of Wheat—Maize—Eggplant Crops in Sequence on a Fallow Land. Resour. Conserv. Recycl. 2009, 54, 134–139. [Google Scholar] [CrossRef]
- Menshov, P.V.; Khlupin, Y.V.; Nalesnik, O.I.; Makarovskikh, A.V. Ash and Slag Waste as a Secondary Raw Material. Procedia Chem. 2014, 10, 184–191. [Google Scholar] [CrossRef]
- Mounanga, P.; Khokhar, M.I.A.; El Hachem, R.; Loukili, A. Improvement of the Early-Age Reactivity of Fly Ash and Blast Furnace Slag Cementitious Systems Using Limestone Filler. Mater. Struct. 2011, 44, 437–453. [Google Scholar] [CrossRef]
- Palomo, A.; Maltseva, O.; Garcia-Lodeiro, I.; Fernández-Jiménez, A. Portland Versus Alkaline Cement: Continuity or Clean Break: “A Key Decision for Global Sustainability”. Front. Chem. 2021, 9, 705475. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Torgal, F.; Labrincha, J.; Leonelli, C.; Palomo, A.; Chindaprasit, P. Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Yatsenko, E.A.; Ryabova, A.V.; Vil’bitskaya, N.A.; Kurdashov, V.M.; Trofimov, S.V.; Golovko, D.A. Eco-Geopolymers Based on CHP Plant Ash-Slag Waste: Promising Materials for Road Construction in the Arctic Zone. Glas. Ceram. 2022, 78, 490–493. [Google Scholar] [CrossRef]
- Ziegler, D.; Formia, A.; Tulliani, J.-M.; Palmero, P. Environmentally-Friendly Dense and Porous Geopolymers Using Fly Ash and Rice Husk Ash as Raw Materials. Materials 2016, 9, 466. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, G.; Liang, K. Development of Fly Ash and Slag Based High-Strength Alkali-Activated Foam Concrete. Cem. Concr. Compos. 2022, 128, 104447. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer Foam Concrete: An Emerging Material for Sustainable Construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Bai, C.; Colombo, P. Processing, Properties and Applications of Highly Porous Geopolymers: A Review. Ceram. Int. 2018, 44, 16103–16118. [Google Scholar] [CrossRef]
- Moghadam, M.J.; Ajalloeian, R.; Hajiannia, A. Preparation and Application of Alkali-Activated Materials Based on Waste Glass and Coal Gangue: A Review. Constr. Build. Mater. 2019, 221, 84–98. [Google Scholar] [CrossRef]
- Zhao, J.; Tong, L.; Li, B.; Chen, T.; Wang, C.; Yang, G.; Zheng, Y. Eco-Friendly Geopolymer Materials: A Review of Performance Improvement, Potential Application and Sustainability Assessment. J. Clean. Prod. 2021, 307, 127085. [Google Scholar] [CrossRef]
- Kozub, B.; Bazan, P.; Gailitis, R.; Korniejenko, K.; Mierzwiński, D. Foamed Geopolymer Composites with the Addition of Glass Wool Waste. Materials 2021, 14, 4978. [Google Scholar] [CrossRef]
- Raza, S.; Orooji, Y.; Ghasali, E.; Hayat, A.; Karimi-Maleh, H.; Lin, H. Engineering Approaches for CO2 Converting to Biomass Coupled with Nanobiomaterials as Biomediated towards Circular Bioeconomy. J. CO2 Util. 2023, 67, 102295. [Google Scholar] [CrossRef]
- Cho, Y.-K.; Yoo, S.-W.; Jung, S.-H.; Lee, K.-M.; Kwon, S.-J. Effect of Na2O Content, SiO2/Na2O Molar Ratio, and Curing Conditions on the Compressive Strength of FA-Based Geopolymer. Constr. Build. Mater. 2017, 145, 253–260. [Google Scholar] [CrossRef]
- Liew, Y.-M.; Heah, C.-Y.; Kamarudin, H. Others Structure and Properties of Clay-Based Geopolymer Cements: A Review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Gharzouni, A.; Joussein, E.; Samet, B.; Baklouti, S.; Rossignol, S. Effect of the Reactivity of Alkaline Solution and Metakaolin on Geopolymer Formation. J. Non. Cryst. Solids 2015, 410, 127–134. [Google Scholar] [CrossRef]
- Hardjito, D.; Wallah, S.E.; Sumajouw, D.M.J.; Rangan, B.V. Fly Ash-Based Geopolymer Concrete. Aust. J. Struct. Eng. 2005, 6, 77–86. [Google Scholar] [CrossRef]
- Henon, J.; Alzina, A.; Absi, J.; Smith, D.S.; Rossignol, S. Porosity Control of Cold Consolidated Geomaterial Foam: Temperature Effect. Ceram. Int. 2012, 38, 77–84. [Google Scholar] [CrossRef]
- Moutaoukil, S.; Alehyen, S.; Sobrados, I.; Fadil, M.; Taibi, M. Effects of Temperature, Time and Alkaline Solution Content on the Mechanical Properties of Class C Fly Ash-Based Geopolymer Using Taguchi Method. Moroc. J. Chem. 2023, 11, 11. [Google Scholar]
- Paija, N.; Kolay, P.K.; Mohanty, M.; Kumar, S. Ground Bottom Ash Application for Conventional Mortar and Geopolymer Paste. J. Hazard. Toxic Radioact. Waste 2020, 24, 4019025. [Google Scholar] [CrossRef]
- Mohamed, O.; Ahmed, E.; Najm, O.; Al-Aribe, K.; Hijah, E. Water Absorption Characteristics and Rate of Strength Development of Mortar with Slag-Based Alkali-Activated Binder and 25% Fly Ash Replacement. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Mohamed, O.A.; Najm, O.; Ahmed, E. Alkali-Activated Slag & Fly Ash as Sustainable Alternatives to OPC: Sorptivity and Strength Development Characteristics of Mortar. Clean. Mater. 2023, 8, 100188. [Google Scholar]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Fly Ash-Based Geopolymers: The Relationship between Composition, Pore Structure and Efflorescence. Cem. Concr. Res. 2014, 64, 30–41. [Google Scholar] [CrossRef]
- Rajamma, R.; Labrincha, J.A.; Ferreira, V.M. Alkali Activation of Biomass Fly Ash—Metakaolin Blends. Fuel 2012, 98, 265–271. [Google Scholar] [CrossRef]
- Sharmila, S.; Natarajan, N.; Dineshkumar, S. Analysis of Microstructural Properties and Durability Characteristics of Geopolymer Mortar. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Novais, R.M.; Ascensão, G.; Buruberri, L.H.; Senff, L.; Labrincha, J.A. Influence of Blowing Agent on the Fresh-and Hardened-State Properties of Lightweight Geopolymers. Mater. Des. 2016, 108, 551–559. [Google Scholar] [CrossRef]
- Do\ugan-Sa\uglamtimur, N.; Öznur, H.Ö.; Bilgil, A.; Vural, T.; Süzgeç, E. The Effect of Alkali Activation Solutions with Different Water Glass/NaOH Solution Ratios on Geopolymer Composite Materials. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP: London, UK, 2019; Volume 660, p. 12003. [Google Scholar]
- Görhan, G.; Kürklü, G. The Influence of the NaOH Solution on the Properties of the Fly Ash-Based Geopolymer Mortar Cured at Different Temperatures. Compos. Part B Eng. 2014, 58, 371–377. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P.; Sanjayan, J. Regulating the Chemical Foaming Reaction to Control the Porosity of Geopolymer Foams. Mater. Des. 2017, 120, 255–265. [Google Scholar]
- Rao, P.R.; Momayez, M.; Runge, K.A.; Muralidharan, K. Recent Developments in Thermally Insulating Materials Based on Geopolymers—A Review Article. Min. Metall. Explor. 2020, 37, 995–1014. [Google Scholar] [CrossRef]
- Xu, H.; van Deventer, J.S.J. The Effect of Alkali Metals on the Formation of Geopolymeric Gels from Alkali-Feldspars. Colloids Surf. A Physicochem. Eng. Asp. 2003, 216, 27–44. [Google Scholar]
- Berardi, U.; Doan, H.; Landi, E. Salient Parameters Affecting the Performance of Foamed Geopolymers as Sustainable Insulating Materials. Constr. Build. Mater. 2021, 313, 125400. [Google Scholar]
- Al Bakri Abdullah, M.M.; Kamarudin, H.; Abdulkareem, O.A.K.A.; Ghazali, C.M.R.; Rafiza, A.R.; Norazian, M.N. Optimization of Alkaline Activator/Fly Ash Ratio on the Compressive Strength of Manufacturing Fly Ash-Based Geopolymer. Appl. Mech. Mater. 2011, 110–116, 734–739. [Google Scholar] [CrossRef]
- Hardjito, D.; Wallah, S.E. Study on Engineering Properties of Fly Ash-Based Geopolymer Concrete. J. Australas. Ceram. Soc. 2002, 38, 44–47. [Google Scholar]
- Patankar, S.V.; Ghugal, Y.M.; Jamkar, S.S. Effect of Concentration of Sodium Hydroxide and Degree of Heat Curing on Fly Ash-Based Geopolymer Mortar. Indian J. Mater. Sci. 2014, 2014, 938789. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, J.; Ye, G. The Pore Structure and Permeability of Alkali Activated Fly Ash. Fuel 2013, 104, 771–780. [Google Scholar] [CrossRef]
- Kamseu, E.; Nait-Ali, B.; Bignozzi, M.C.; Leonelli, C.; Rossignol, S.; Smith, D.S. Bulk Composition and Microstructure Dependence of Effective Thermal Conductivity of Porous Inorganic Polymer Cements. J. Eur. Ceram. Soc. 2012, 32, 1593–1603. [Google Scholar] [CrossRef]
- Yu, H.; Xu, M.; Chen, C.; He, Y.; Cui, X. A Review on the Porous Geopolymer Preparation for Structural and Functional Materials Applications. Int. J. Appl. Ceram. Technol. 2022, 19, 1793–1813. [Google Scholar] [CrossRef]
- Liu, X.; Hu, C.; Chu, L. Microstructure, Compressive Strength and Sound Insulation Property of Fly Ash-Based Geopolymeric Foams with Silica Fume as Foaming Agent. Materials 2020, 13, 3215. [Google Scholar] [CrossRef] [PubMed]
- Jaya, N.A.; Yun-Ming, L.; Cheng-Yong, H.; Abdullah, M.M.A.B.; Hussin, K. Correlation between Pore Structure, Compressive Strength and Thermal Conductivity of Porous Metakaolin Geopolymer. Constr. Build. Mater. 2020, 247, 118641. [Google Scholar] [CrossRef]
- Gu, G.; Xu, F.; Ruan, S.; Huang, X.; Zhu, J.; Peng, C. Influence of Precast Foam on the Pore Structure and Properties of Fly Ash-Based Geopolymer Foams. Constr. Build. Mater. 2020, 256, 119410. [Google Scholar] [CrossRef]
- Korniejenko, K.; Pławecka, K.; Bazan, P.; Figiela, B.; Kozub, B.; Mróz, K.; Łach, M. Green Building Materials for Circular Economy-Geopolymer Foams. Proc. Eng. Technol. Innov. 2023, 25, 26–34. [Google Scholar] [CrossRef]
- Huang, Y.; Gong, L.; Shi, L.; Cao, W.; Pan, Y.; Cheng, X. Experimental Investigation on the Influencing Factors of Preparing Porous Fly Ash-Based Geopolymer for Insulation Material. Energy Build. 2018, 168, 9–18. [Google Scholar] [CrossRef]
- Moni, S.M.F.K.; Ikeora, O.; Pritzel, C.; Görtz, B.; Trettin, R. Preparation and Properties of Fly Ash-Based Geopolymer Concrete with Alkaline Waste Water Obtained from Foundry Sand Regeneration Process. J. Mater. Cycles Waste Manag. 2020, 22, 1434–1443. [Google Scholar] [CrossRef]
- Al Bakri Abdullah, M.M.; Hussin, K.; Bnhussain, M.; Ismail, K.N.; Yahya, Z.; Razak, R.A. Fly Ash-Based Geopolymer Lightweight Concrete Using Foaming Agent. Int. J. Mol. Sci. 2012, 13, 7186–7198. [Google Scholar] [CrossRef]
- Korniejenko, K.; Kejzlar, P.; Louda, P. The Influence of the Material Structure on the Mechanical Properties of Geopolymer Composites Reinforced with Short Fibers Obtained with Additive Technologies. Int. J. Mol. Sci. 2022, 23, 2023. [Google Scholar] [CrossRef]




| Material | NaOH (Dry), wt.% | Na2SiO3 (Waterglass), wt.% | Na2SiO3/NaOH Ratio | Ref. |
|---|---|---|---|---|
| Fly ash | 2.5 | 20 | 2.5 | [37] |
| Fly ash | Not given | Not given | 1.5–3.0 | [38] |
| Slag + fly ash | Not given | Not given | 1.5–2.5 | [39] |
| Slag + fly ash | Not given | Not given | 1.5–2.5 | [40] |
| Fly ash | 3.5 | 17 | 1.5 | [41] |
| Metakaolin + fly ash | 3–7 | 11–26 | 0.5–2.5 | [42] |
| Slag + fly ash | Not given | Not given | 2.5 | [43] |
| Metakaolin + fly ash | 4–5 | 37 | 2.3 | [44] |
| Fly ash + sand | 1.5–4.5 | 8–27 | 1.0–3.0 | [45] |
| Fly ash + sand | 0.5–2 | 12.5–13.5 | 2.0 | [46] |
| Content | Na2O | K2O | CaO | MgO | MnO | Al2O3 | Fe2O3 | SiO2 | TiO2 | P2O5 | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASW (Novocherkassk SDPP) | 0.9 | 3.0 | 3.1 | 2.1 | 0.1 | 18.8 | 10.3 | 51.2 | 0.8 | 0.1 | 0.3 | 9.3 |
| ASW (Severodvinsk CHPP-1) | 3.6 | 2.3 | 2.1 | 2.8 | 0.1 | 17.9 | 6.8 | 61.6 | 0.8 | 0.2 | 0.3 | 2.3 |
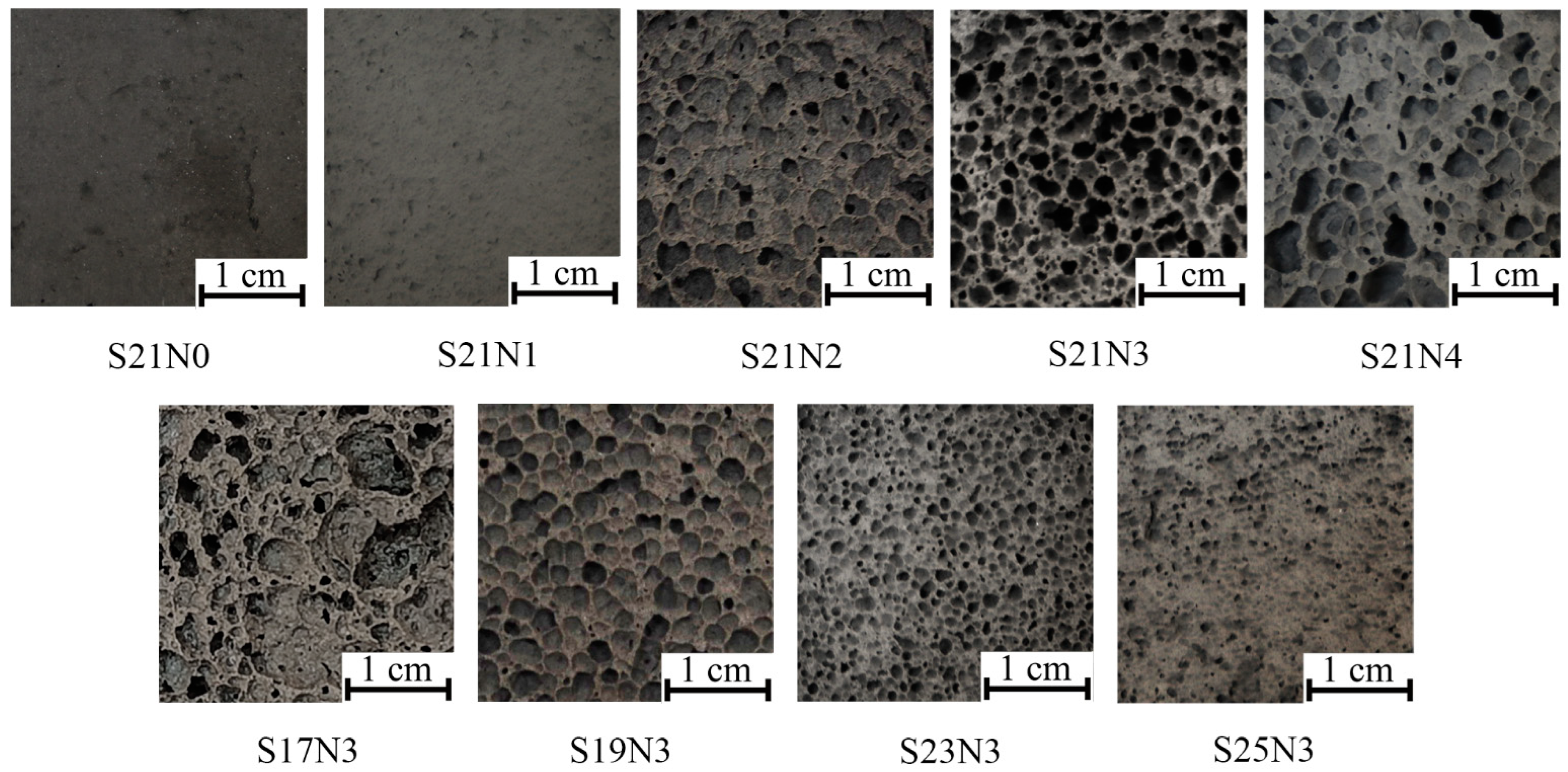
| Composition | ASW | Sodium Silicate | Sodium Hydroxide | Aluminum Powder | Water, over 100 |
|---|---|---|---|---|---|
| S21N0 | 77 | 21 | 0 | 2 | 0 |
| S21N1 | 76 | 21 | 1 | 2 | 2 |
| S21N2 | 75 | 21 | 2 | 2 | 4 |
| S21N3 | 74 | 21 | 3 | 2 | 6 |
| S21N4 | 73 | 21 | 4 | 2 | 8 |
| S17N3 | 78 | 17 | 3 | 2 | 6 |
| S19N3 | 76 | 19 | 3 | 2 | 6 |
| S23N3 | 72 | 23 | 3 | 2 | 6 |
| S25N3 | 70 | 25 | 3 | 2 | 6 |
| S.S19N3 | 76 | 19 | 3 | 2 | 6 |
| S.S19N1 | 78 | 19 | 1 | 2 | 6 |
| S.S19N5 | 74 | 19 | 5 | 2 | 6 |
| S.S15N3 | 80 | 15 | 3 | 2 | 6 |
| S.S25N3 | 70 | 25 | 3 | 2 | 6 |
| Composition | Density, kg/m3 | Compressive Strength, MPa | Porosity, % | Thermal Conductivity, W/(m·K) |
|---|---|---|---|---|
| S21N0 | 1202 ± 11 | 4.48 ± 0.19 | 48.4 ± 0.5 | 0.272 ± 0.003 |
| S21N1 | 786 ± 5 | 1.47 ± 0.07 | 66.3 ± 0.2 | 0.176 ± 0.001 |
| S21N2 | 373 ± 8 | 0.59 ± 0.07 | 84.0 ± 0.4 | 0.083 ± 0.002 |
| S21N3 | 349 ± 7 | 0.57 ± 0.02 | 84.9 ± 0.3 | 0.079 ± 0.002 |
| S21N4 | 319 ± 6 | 0.37 ± 0.02 | 86.3 ± 0.2 | 0.072 ± 0.001 |
| S17N3 | 389 ± 8 | 0.55 ± 0.03 | 83.3 ± 0.4 | 0.086 ± 0.002 |
| S19N3 | 335 ± 6 | 0.55 ± 0.03 | 85.6 ± 0.2 | 0.075 ± 0.001 |
| S23N3 | 512 ± 10 | 0.70 ± 0.04 | 78.0 ± 0.4 | 0.113 ± 0.002 |
| S25N3 | 702 ± 16 | 1.67 ± 0.07 | 69.9 ± 0.7 | 0.156 ± 0.004 |
| Element | S21N0 | S21N3 | S19N3 |
|---|---|---|---|
| O | 65.5 | 62.2 | 62.5 |
| Na | 6.7 | 11.2 | 11.3 |
| K | 1.2 | 1.1 | 0.9 |
| Ca | 1.2 | 1.2 | 1.2 |
| Mg | 0.9 | 0.9 | 0.9 |
| Al | 6.1 | 5.6 | 5.8 |
| Si | 15.9 | 14.7 | 14.9 |
| Ti | 0.2 | 0.3 | 0.2 |
| Fe | 2.3 | 2.8 | 2.3 |
| Composition | Density, kg/m3 | Compressive Strength, MPa | Porosity, % | Thermal Conductivity, W/(m·K) |
|---|---|---|---|---|
| S.S19N3 | 366 ± 11 | 0.58 ± 0.03 | 82.0 ± 0.5 | 0.081 ± 0.001 |
| S.S19N1 | 841 ± 12 | 1.41 ± 0.06 | 58.7 ± 0.6 | 0.185 ± 0.002 |
| S.S19N5 | 349 ± 6 | 0.33 ± 0.02 | 82.8 ± 0.3 | 0.079 ± 0.002 |
| S.S15N3 | 406 ± 8 | 0.62 ± 0.02 | 80.0 ± 0.4 | 0.089 ± 0.001 |
| S.S25N3 | 789 ± 19 | 1.88 ± 0.06 | 61.2 ± 0.9 | 0.161 ± 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yatsenko, E.A.; Goltsman, B.M.; Izvarin, A.I.; Kurdashov, V.M.; Smoliy, V.A.; Ryabova, A.V.; Klimova, L.V. Recycling Ash and Slag Waste from Thermal Power Plants to Produce Foamed Geopolymers. Energies 2023, 16, 7535. https://doi.org/10.3390/en16227535
Yatsenko EA, Goltsman BM, Izvarin AI, Kurdashov VM, Smoliy VA, Ryabova AV, Klimova LV. Recycling Ash and Slag Waste from Thermal Power Plants to Produce Foamed Geopolymers. Energies. 2023; 16(22):7535. https://doi.org/10.3390/en16227535
Chicago/Turabian StyleYatsenko, Elena A., Boris M. Goltsman, Andrey I. Izvarin, Viktor M. Kurdashov, Victoria A. Smoliy, Anna V. Ryabova, and Lyudmila V. Klimova. 2023. "Recycling Ash and Slag Waste from Thermal Power Plants to Produce Foamed Geopolymers" Energies 16, no. 22: 7535. https://doi.org/10.3390/en16227535
APA StyleYatsenko, E. A., Goltsman, B. M., Izvarin, A. I., Kurdashov, V. M., Smoliy, V. A., Ryabova, A. V., & Klimova, L. V. (2023). Recycling Ash and Slag Waste from Thermal Power Plants to Produce Foamed Geopolymers. Energies, 16(22), 7535. https://doi.org/10.3390/en16227535








