Torrefaction as a Way to Remove Chlorine and Improve the Energy Properties of Plant Biomass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Feedstock and Torrefaction Process
2.2. Chlorine Content Analysis
2.3. Physicochemical Analyses
2.4. Statistical Analysis
3. Results
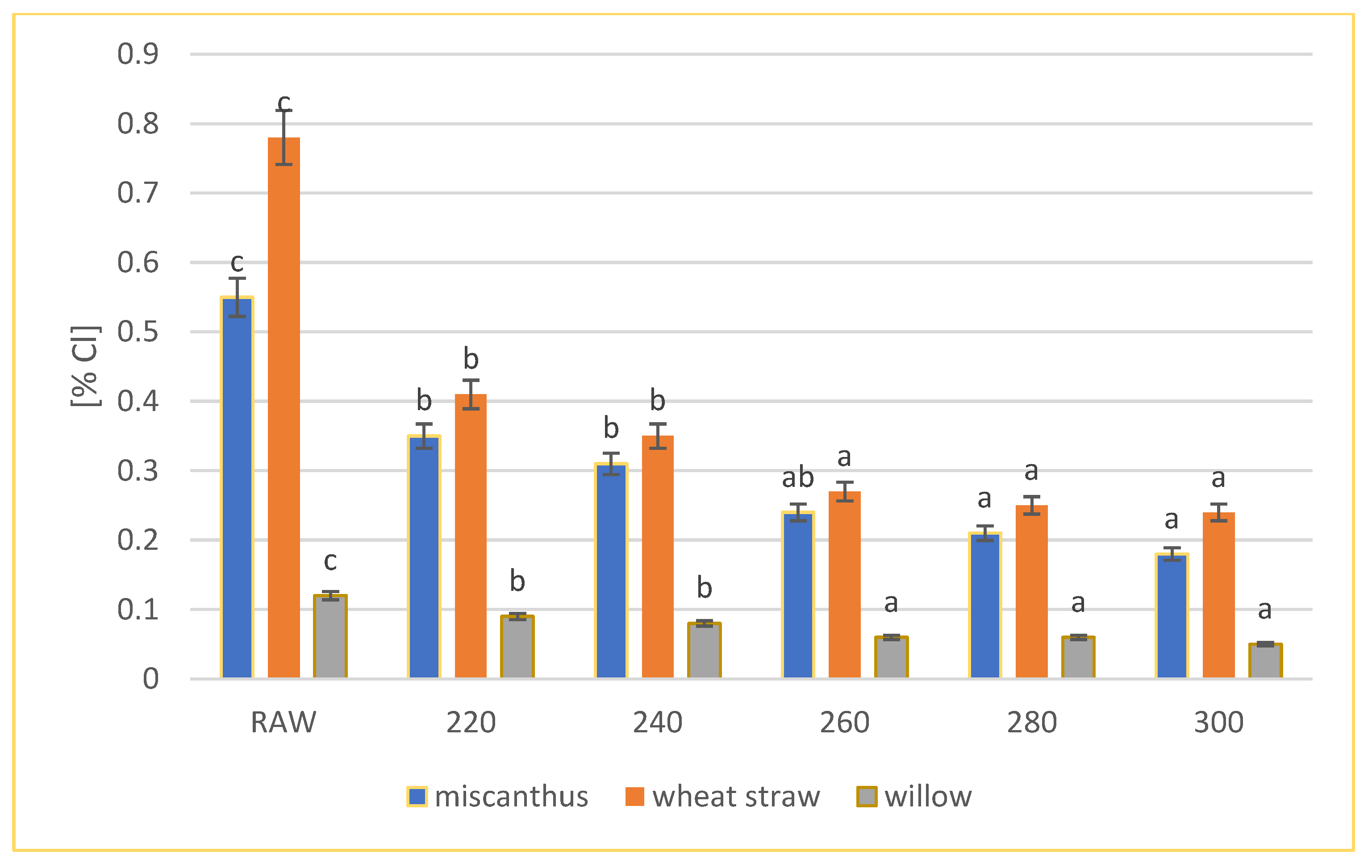
3.1. Total Chlorine Content Analysis
3.2. Determination of Energy Properties in the Tested Biomass
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mousavi, S.M.; Thorin, E.; Schmidt, F.M.; Sepman, A.; Bai, X.S.; Fatehi, H. Numerical Study and Experimental Verification of Biomass Conversion and Potassium Release in a 140 kW Entrained Flow Gasifier. Energy Fuels 2023, 37, 1116–1130. [Google Scholar] [CrossRef] [PubMed]
- He, Z.M.; Cao, J.P.; Zhao, X.Y. Review of Biomass Agglomeration for Fluidized-Bed Gasification or Combustion Processes with a Focus on the Effect of Alkali Salts. Energy Fuels 2022, 36, 8925–8947. [Google Scholar] [CrossRef]
- Hardy, T.; Kakietek, S.; Halawa, K.; Mościcki, K.; Janda, T. Determination of High Temperature Corrosion Rates of Steam Boiler Evaporators Using Continuous Measurements of Flue Gas Composition and Neural Networks. Energies 2020, 13, 3134. [Google Scholar] [CrossRef]
- Kawahara, Y. An Overview on Corrosion-Resistant Coating Technologies in Biomass/Waste-to-Energy Plants in Recent Decades. Coatings 2016, 6, 34. [Google Scholar] [CrossRef]
- Trzeszczyński, J.; Stanek, R. Managing the maintenance of the technical condition of pipes of heating surfaces of boilers based on risk analysis. Energetyka 2013, 6, 498–505. [Google Scholar]
- Wasielewski, R.; Hrabak, J. Influence of chlorine, sulfur and alkalis content in waste biomass on its use in power production. Arch. Waste Manag. Environ. Prot. 2015, 17, 1–8. [Google Scholar]
- Yildiz, C.; Richter, M.; Ströhle, J.; Epple, B. Release of Sulfur and Chlorine Gas Species during Combustion and Pyrolysis of Walnut Shells in an Entrained Flow Reactor. Energies 2023, 16, 5684. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Q.; Wan, B.; Xu, J.; Huang, Q.; Yan, J.; Tang, Y. Transformation of Phosphorus during Low-Temperature Co-Combustion of Sewage Sludge with Biowastes. ACS Sustain. Chem. Eng. 2021, 9, 3668–3676. [Google Scholar] [CrossRef]
- Wu, D.; Yuan, Z.; Liu, S.; Zheng, J.; Wei, X.; Zhang, C. Recent Development of Corrosion Factors and Coating Applications in Biomass Firing Plants. Coatings 2020, 10, 1001. [Google Scholar] [CrossRef]
- Komada, P. Analysis of the Biomass Thermal Processing Process; Publishing House of the Polish Academy of Sciences: Warsaw, Poland, 2019. [Google Scholar]
- Sobczyk, W.; Sobczyk, E.J. Varying the Energy Mix in the EU-28 and in Poland as a Step towards Sustainable Development. Energies 2021, 14, 1502. [Google Scholar] [CrossRef]
- Pronobis, M. Modernization of Power Boilers; PWN Scientific Publishing House: Warsaw, Poland, 2017. [Google Scholar]
- Ciukaj, S. The technical considerations of biomass co-firing in coal fired power boilers. Energy Soc. Politics 2017, 5, 83–114. [Google Scholar]
- Kanwal, F.; Ahmed, A.; Jamil, F.; Rafiq, S.; Ayub, H.M.U.; Ghauri, M.; Khurram, M.S.; Munir, S.; Inayat, A.; Bakar, M.S.A.; et al. Co-Combustion of Blends of Coal and Underutilised Biomass Residues for Environmental Friendly Electrical Energy Production. Sustainability 2021, 13, 4881. [Google Scholar] [CrossRef]
- Król, D.; Motyl, P.; Poskrobko, S. Chlorine Corrosion in a Low-Power Boiler Fired with Agricultural Biomass. Energies 2022, 15, 382. [Google Scholar] [CrossRef]
- Bis, Z. Fluidized Bed Boilers. Theory and Practice; Publishing House of the Częstochowa University of Technology: Częstochowa, Poland, 2010. [Google Scholar]
- Nowak, K.; Rabczak, S. Co-Combustion of Biomass with Coal in Gate Water Boilers at Low Load Boiler Operation. Energies 2021, 14, 2520. [Google Scholar] [CrossRef]
- Jaworski, T.J.; Kajda-Szcześniak, M. Study on the Similarity of the Parameters of Biomass and Solid Waste Fuel Combustion for the Needs of Thermal Power Engineering. Sustainability 2020, 12, 7894. [Google Scholar] [CrossRef]
- Brągoszewska, E.; Pawlak, M. Health Risks Associated with Occupational Exposure to Biological Air Pollutants Occurring during the Processing of Biomass for Energy Purposes: A Case Study. Energies 2021, 14, 2086. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Zhang, L.; Wang, Z.; Zhao, Z.; Zhu, W.; Ma, J.; Shen, B. Effects of Torrefaction Pretreatment on the Structural Features and Combustion Characteristics of Biomass-Based Fuel. Molecules 2023, 28, 4732. [Google Scholar] [CrossRef]
- Ratte, J.; Fardet, E.; Mateos, D.; Hery, J.S. Mathematical modelling of a continuous biomass torrefaction reactor: TORSPYD (TM) column. Biomass Bioenergy 2011, 35, 3481–3495. [Google Scholar] [CrossRef]
- Pečnik, J.G.; Zouari, M.; Schwarzkopf, M.; DeVallance, D.B. Utilization of Torrefied and Non-Torrefied Short Rotation Willow in Wood–Plastic Composites. Polymers 2023, 15, 3997. [Google Scholar] [CrossRef]
- Uslu, A.; Faaij, A.P.C.; Bergman, P.C.A. Pre-treatment technologies. and their effect on international bioenergy supply chain logistics. Techno-economic evaluation of torrefaction. fast pyrolysis and pelletisation. Energy 2008, 33, 1206–1223. [Google Scholar] [CrossRef]
- Chen, W.; Kuo, P. A study on torrefaction of various biomass materials and its impact on lignocellulosic structure simulated by a thermogravimetry. Energy 2010, 35, 2580–2586. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Skreiberg, Ø. Upgrading biomass fuels via wet torrefaction: A review and comparison with dry torrefaction. Renew. Sustain. Energy Rev. 2016, 54, 665–677. [Google Scholar] [CrossRef]
- He, C.; Tang, C.; Li, C.; Yuan, J.; Tran, K.-Q.; Bach, Q.-V.; Qiu, R.; Yang, Y. Wet torrefaction of biomass for high quality solid fuel production: A review. Renew. Sustain. Energy Rev. 2018, 91, 259–271. [Google Scholar] [CrossRef]
- Cardona, S.; Gallego, L.J.; Valencia, V.; Martínez, E.; Rios, L.A. Torrefaction of eucalyptus-tree residues: A new method for energy and mass balances of the process with the best torrefaction conditions. Sustain. Energy Technol. Assess. 2019, 31, 17–24. [Google Scholar] [CrossRef]
- PN-EN 15359:2012; Solid Recovered Fuels—Specifications and Classes. Polish Committee for Standardization: Warsaw, Poland, 2013.
- Banaś, J.; Utnik-Banaś, K. Wykorzystanie drewna jako odnawialnego surowca do produkcji energii w zrównoważonej gospodarce leśnej. Energies 2022, 15, 2264. [Google Scholar] [CrossRef]
- Long, H.; Li, X.; Wang, H.; Jia, J. Biomass resource and their bioenergy potential estimation: A review. Renew. Sustain. Energy Rev. 2013, 26, 344–352. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. Biomass energy and the environmental impacts associated with its production and utilization. Renew. Sustain. Energy Rev. 2010, 14, 919–937. [Google Scholar] [CrossRef]
- Saidur, R.; Abdelaziz, E.A.; Demirbas, A.; Hossain, M.S.; Mekhilef, S. A review on biomass as a fuel for boilers. Renew. Sustain. Energy Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- Mandley, S.J.; Daioglou, V.; Junginger, H.M.; van Vuuren, D.P.; Wicke, B. EU bioenergy development to 2050. Renew. Sustain. Energy Rev. 2020, 127, 109858. [Google Scholar] [CrossRef]
- Perea-Moreno, M.-A.; Samerón-Manzano, E.; Perea-Moreno, A.-J. Biomass as Renewable Energy: Worldwide Research Trends. Sustainability 2019, 11, 863. [Google Scholar] [CrossRef]
- Safarian, S. To what extent could biochar replace coal and coke in steel industries? Fuel 2023, 339, 127401. [Google Scholar] [CrossRef]
- Sarker, T.R.; Azargohar, R.; Dalai, A.K.; Venkatesh, M. Physicochemical and Fuel Characteristics of Torrefied Agricultural Residues for Sustainable Fuel Production. Energy Fuels 2020, 34, 14169–14181. [Google Scholar] [CrossRef]
- Zhang, C.; Ho, S.-H.; Chen, W.-H.; Fu, Y.; Chang, J.-S.; Bi, X. Oxidative torrefaction of biomass nutshells: Evaluations of energy efficiency as well as biochar transportation and storage. Appl. Energy 2019, 235, 428–441. [Google Scholar] [CrossRef]
- Li, N.; Wang, Y.; Cui, S.; Sun, D. Investigations on NO reduction with biomass char: Char structural changes during the heat treatment in N2 and subsequent NO/O2 gasification. Fuel 2021, 287, 119564. [Google Scholar] [CrossRef]
- Mian, I.; Li, X.; Dacres, O.D.; Wang, J.; Wei, B.; Jian, Y.; Zhong, M.; Liu, J.; Ma, F.; Rahman, N. Combustion kinetics and mechanism of biomass pellet. Energy 2020, 205, 117909. [Google Scholar] [CrossRef]
- Singh, R.K.; Sarkar, A.; Chakraborty, J.P. Effect of torrefaction on the physicochemical properties of pigeon pea stalk (Cajanus cajan) and estimation of kinetic parameters. Renew. Energy 2019, 138, 805–819. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, S.; Zhang, Z.; Zhang, Y.; Li, J.; Ahmed, S.; Yan, B.; Chen, G.; Li, N. Flue gas torrefaction of distilled spirit lees and the effects on the combustion and nitrogen oxide emission. Bioresour. Technol. 2021, 342, 125975. [Google Scholar] [CrossRef]
- Guragain, Y.N.; Vadlani, P.V. Renewable Biomass Utilization: A Way Forward to Establish Sustainable Chemical and Processing Industries. Clean Technol. 2021, 3, 243–259. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Singh, S.; Chakraborty, J.P.; Mondal, M.K. Optimization of process parameters for torrefaction of Acacia nilotica using response surface methodology and characteristics of torrefied biomass as upgraded fuel. Energy 2019, 186, 115865. [Google Scholar] [CrossRef]
- Chin, K.; H’ng, P.; Go, W.; Wong, W.; Lim, T.; Maminski, M.; Paridah, M.; Luqman, A. Optimization of torrefaction conditions for high energy density solid biofuel from oil palm biomass and fast growing species available in Malaysia. Ind. Crops Prod. 2013, 49, 768–774. [Google Scholar] [CrossRef]
- Werkelin, J.; Skrifvars, B.J.; Hupa, M. Ash-forming elements in four Scandinavian wood species. Part 1: Summer harvest. Biomass Bioenergy 2005, 29, 451–466. [Google Scholar] [CrossRef]
- Zhang, S.; Min, G.; Wang, J.; Zhu, S.; Zhang, H.; Liu, X. Release characteristics of potassium and chlorine for torrefied wheat straw during a combined pyrolysis-combustion system. Bioresour. Technol. 2020, 312, 123591. [Google Scholar] [CrossRef] [PubMed]
- Sá, L.C.R.; Loureiro, L.M.E.F.; Nunes, L.J.R.; Mendes, A.M.M. Torrefaction as a Pretreatment Technology for Chlorine Elimination from Biomass: A Case Study Using Eucalyptus globulus Labill. Resources 2020, 9, 54. [Google Scholar] [CrossRef]
- Wang, K.; Kong, G.; Zhang, G.; Zhang, X.; Han, L.; Zhang, X. Steam Gasification of Torrefied/Carbonized Wheat Straw for H2-Enriched Syngas Production and Tar Reduction. Int. J. Environ. Res. Public Health 2022, 19, 10475. [Google Scholar] [CrossRef]


| Parameters | Raw Willow | 220 °C | 240 °C | 260 °C | 280 °C | 300 °C | |
|---|---|---|---|---|---|---|---|
| x ± SD | |||||||
| C | % | 48.24 c ± 0.1 | 48.31 c ± 0.31 | 49.19 bc ± 0.21 | 52.44 b ± 0.35 | 54.03 ab ± 0.18 | 55.27 a ± 0.11 |
| H | 5.48 ab ± 0.02 | 5.92 a ± 0.02 | 5.99 a ± 0.06 | 4.37 c ± 0.1 | 3.99 cd ± 0.04 | 3.31 d ± 0.01 | |
| N | 0.51 d ± 0.01 | 1.24 a ± 0.04 | 1.21 b ± 0.03 | 1.07 c ± 0.02 | 1.01 c ± 0.04 | 0.91 b ± 0.03 | |
| Moisture content | 10.64 a ± 0.12 | 9.01 b ± 0.04 | 8.61 bc ± 0.03 | 8.02 bc ± 0.1 | 7.98 c ± 0.11 | 7.76 c ± 0.1 | |
| Ash content | 3.03 b ± 0.1 | 3.07 b ± 0.1 | 3.31 ab ± 0.05 | 3.49 a ± 0.1 | 3.6 a ± 0.1 | 3.69 a ± 0.12 | |
| Volatile matter | 22.51 c ± 0.22 | 23.14 d ± 0.16 | 24.85 bc ± 0.16 | 29.3 b ± 0.32 | 37.71 a ± 0.24 | 40.14 a ± 0.25 | |
| HHV | MJ·kg−1 | 17.22 c ± 0.16 | 19.05 b ± 0.05 | 20.03 b ± 0.1 | 21.19 a ± 0.14 | 21.72 a ± 0.1 | 21.77 a ± 0.1 |
| Raw Wheat Straw | 220 °C | 240 °C | 260 °C | 280 °C | 300 °C | ||
| C | % | 44.22 d ± 0.06 | 47.34 c ± 0.1 | 49.61 c ± 0.12 | 50.94 b ± 0.16 | 51.4 ab ± 0.11 | 53.26 a ± 0.08 |
| H | 6.94 a ± 0.07 | 5.55 b ± 0.06 | 4.91 bc ± 0.08 | 4.32 c ± 0.1 | 3.96 c ± 0.07 | 3.44 d ± 0.02 | |
| N | 0.26 d ± 0.03 | 1.01 c ± 0.03 | 1.05 c ± 0.02 | 1.24 b ± 0.04 | 1.3 b ± 0.1 | 1.16 a ± 0.08 | |
| Moisture content | 10.05 a ± 0.1 | 8.24 b ± 0.1 | 7.69 bc ± 0.1 | 6.55 c ± 0.13 | 5.94 c ± 0.15 | 4.12 d ± 0.1 | |
| Ash content | 3.95 d ± 0.1 | 4.88 c ± 0.11 | 6.72 c ± 0.17 | 8.84 b ± 0.16 | 9.32 a ± 0.1 | 9.41 a ± 0.1 | |
| Volatile matter | 14.1 d ± 0.09 | 18.62 c ± 0.22 | 22.37 bc ± 0.16 | 31.33 b ± 0.23 | 40.58 ab ± 0.2 | 44.77 a ± 0.2 | |
| HHV | MJ·kg−1 | 17.43 d ± 0.1 | 18.12 c ± 0.1 | 19.01 c ± 0.1 | 19.65 b ± 0.24 | 20.01 b ± 0.13 | 20.87 a ± 0.26 |
| Raw Miscantus | 220 °C | 240 °C | 260 °C | 280 °C | 300 °C | ||
| C | % | 43.16 d ± 0.05 | 45.31 c ± 0.1 | 47.12 c ± 0.11 | 50.12 b ± 0.2 | 51.75 ab ± 0.24 | 53.01 a ± 0.31 |
| H | 6.55 a ± 0.06 | 5.31 b ± 0.03 | 4.87 bc ± 0.08 | 4.02 c ± 0.1 | 3.34 c ± 0.06 | 3.03 d ± 0.02 | |
| N | 0.18 d ± 0.01 | 0.92 c ± 0.03 | 1.01 c ± 0.02 | 1.06 b ± 0.02 | 1.13 b ± 0.02 | 1.1 a ± 0.05 | |
| Moisture content | 9.06 a ± 0.1 | 8.63 b ± 0.1 | 7.58 bc ± 0.2 | 6.23 c ± 0.1 | 5.03 c ± 0.1 | 4.75 d ± 0.1 | |
| Ash content | 4.06 d ± 0.09 | 5.62 c ± 0.1 | 6.37 c ± 0.11 | 7.12 b ± 0.1 | 8.08 a ± 0.1 | 9.07 a ± 0.1 | |
| Volatile matter | 12.73 d ± 0.22 | 18.37 c ± 0.21 | 23.12 bc ± 0.17 | 31.54 b ± 0.22 | 40.14 ab ± 0.16 | 42.54 a ± 0.16 | |
| HHV | MJ·kg−1 | 17.51 d ± 0.1 | 18.34 c ± 0.11 | 19.36 c ± 0.21 | 20.16 b ± 0.21 | 21.11 b ± 0.1 | 21.62 a ± 0.16 |
| Torrefied | Torrefaction Temperature | Cl | Ash | HHV | References |
|---|---|---|---|---|---|
| °C | % | MJ·kg−1 | |||
| Wheat straw | 220 | 0.41 | 4.88 | 18.12 | This work |
| 240 | 0.35 | 6.72 | 19.01 | ||
| 260 | 0.27 | 8.84 | 19.65 | ||
| 280 | 0.25 | 9.32 | 20.01 | ||
| 300 | 0.24 | 9.41 | 20.87 | ||
| Wheat straw | 200 | - | 3.85 | 18.55 | [47] |
| 250 | - | 4.68 | 19.94 | ||
| 300 | - | 8.98 | 27.22 | ||
| Eucalyptus | 240 | 0.05 | 1.02 | - | [48] |
| 280 | 0 | 1.1 | - | ||
| 320 | 0 | 2.52 | - | ||
| Wheat straw | 220 | - | 9.33 | 18.9 | [49] |
| 240 | - | 10.26 | 20.11 | ||
| 260 | - | 13.44 | 22.36 | ||
| 280 | - | 17.15 | 23.33 | ||
| 300 | - | 17.52 | 23.58 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajcar, M.; Zardzewiały, M.; Saletnik, B.; Zaguła, G.; Puchalski, C.; Gorzelany, J. Torrefaction as a Way to Remove Chlorine and Improve the Energy Properties of Plant Biomass. Energies 2023, 16, 7365. https://doi.org/10.3390/en16217365
Bajcar M, Zardzewiały M, Saletnik B, Zaguła G, Puchalski C, Gorzelany J. Torrefaction as a Way to Remove Chlorine and Improve the Energy Properties of Plant Biomass. Energies. 2023; 16(21):7365. https://doi.org/10.3390/en16217365
Chicago/Turabian StyleBajcar, Marcin, Miłosz Zardzewiały, Bogdan Saletnik, Grzegorz Zaguła, Czesław Puchalski, and Józef Gorzelany. 2023. "Torrefaction as a Way to Remove Chlorine and Improve the Energy Properties of Plant Biomass" Energies 16, no. 21: 7365. https://doi.org/10.3390/en16217365
APA StyleBajcar, M., Zardzewiały, M., Saletnik, B., Zaguła, G., Puchalski, C., & Gorzelany, J. (2023). Torrefaction as a Way to Remove Chlorine and Improve the Energy Properties of Plant Biomass. Energies, 16(21), 7365. https://doi.org/10.3390/en16217365









