Influence of Interface Morphology on the Thermal Stress Distribution of SOFC under Inhomogeneous Temperature Field
Abstract
:1. Introduction
2. Materials and Methods
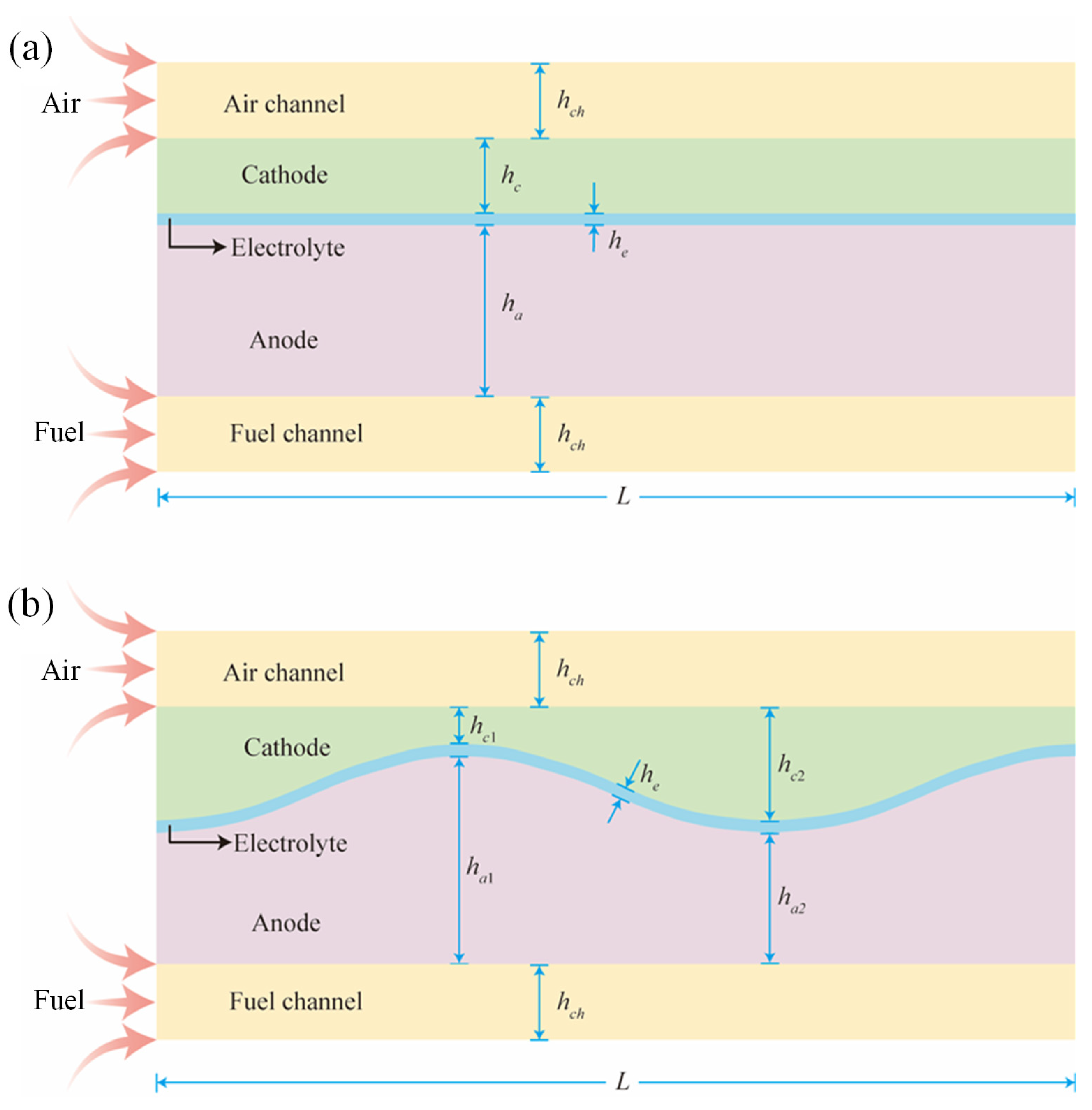
2.1. Geometry Structures
2.2. Material Models
2.3. Finite Element Model
3. Results and Discussion
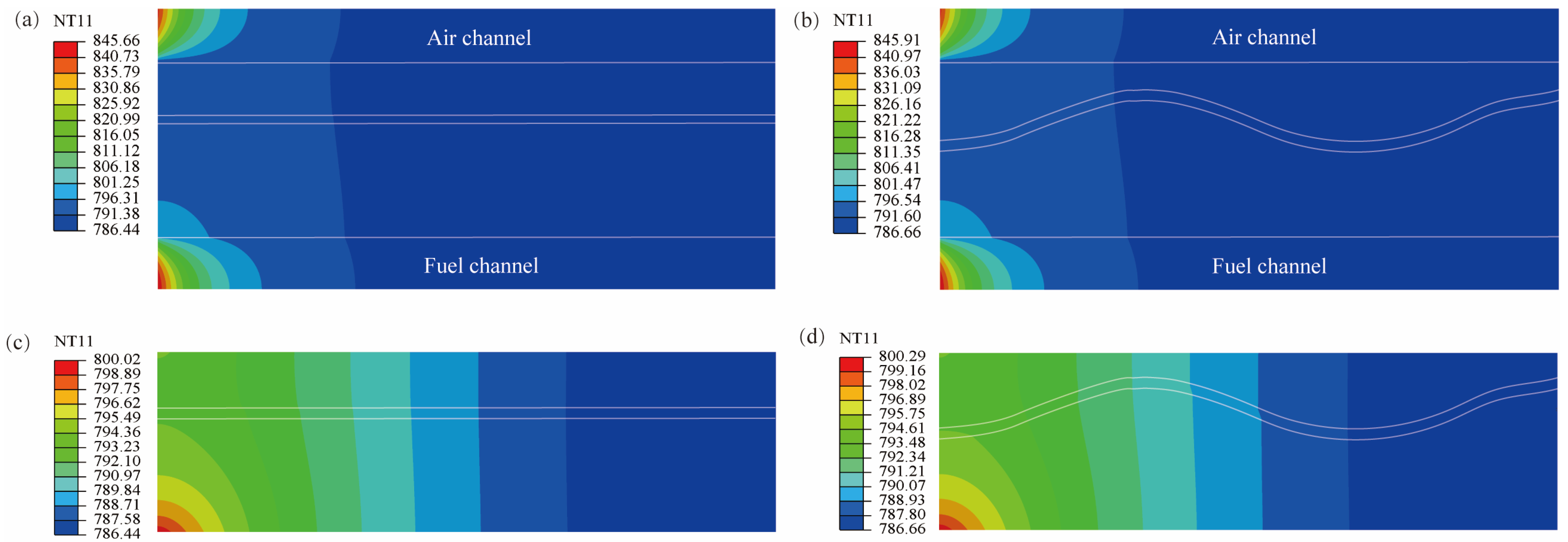
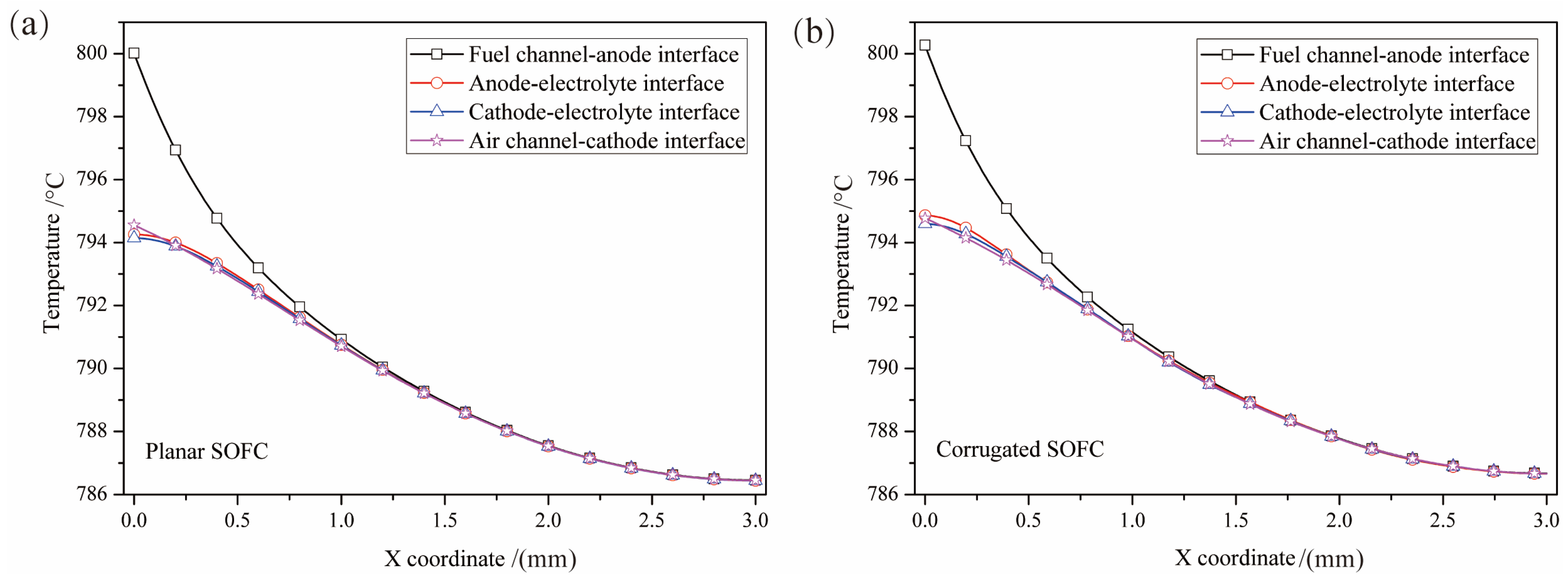
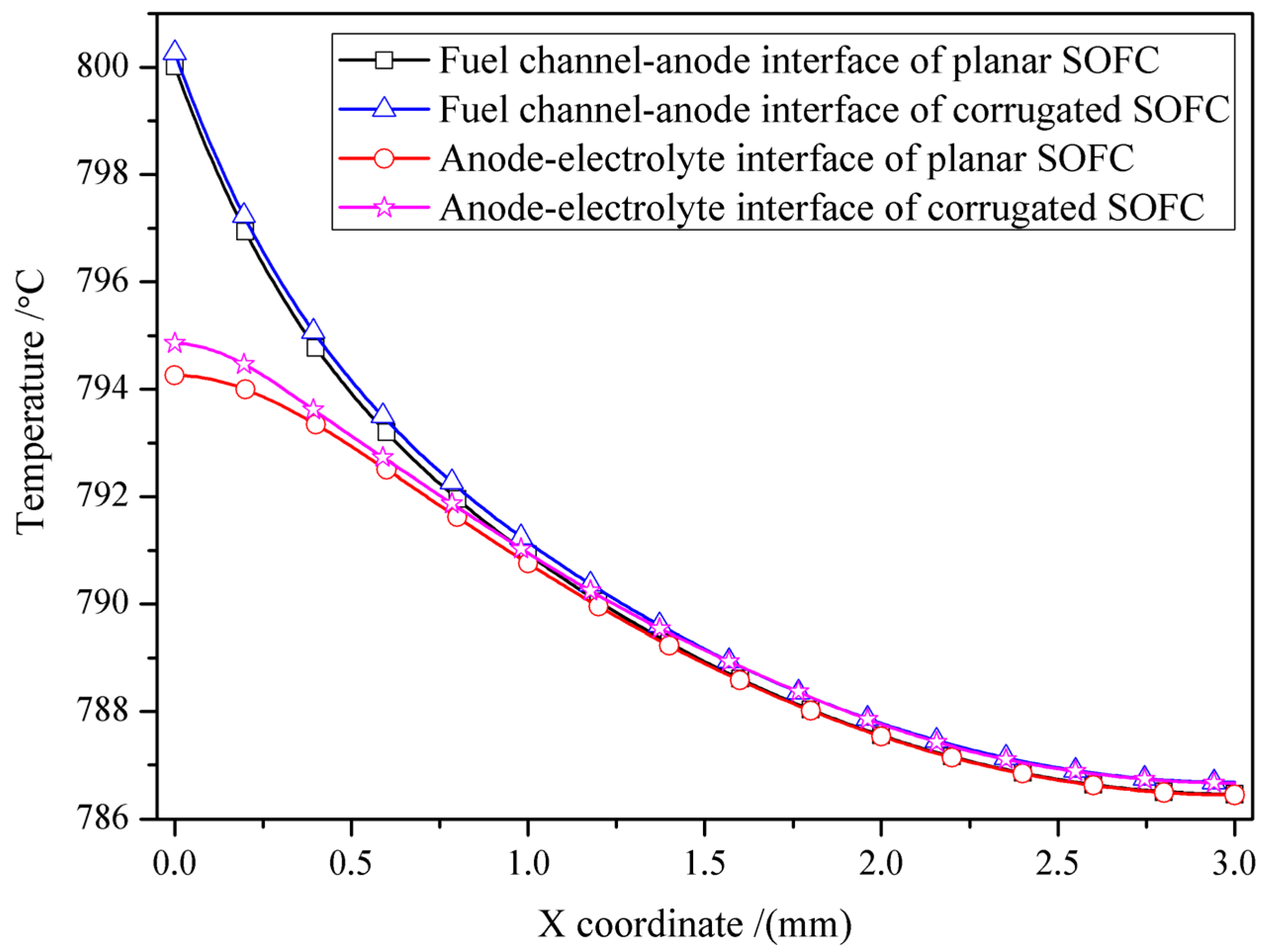
3.1. Influence of SOFC Interface Morphology on Temperature Distribution
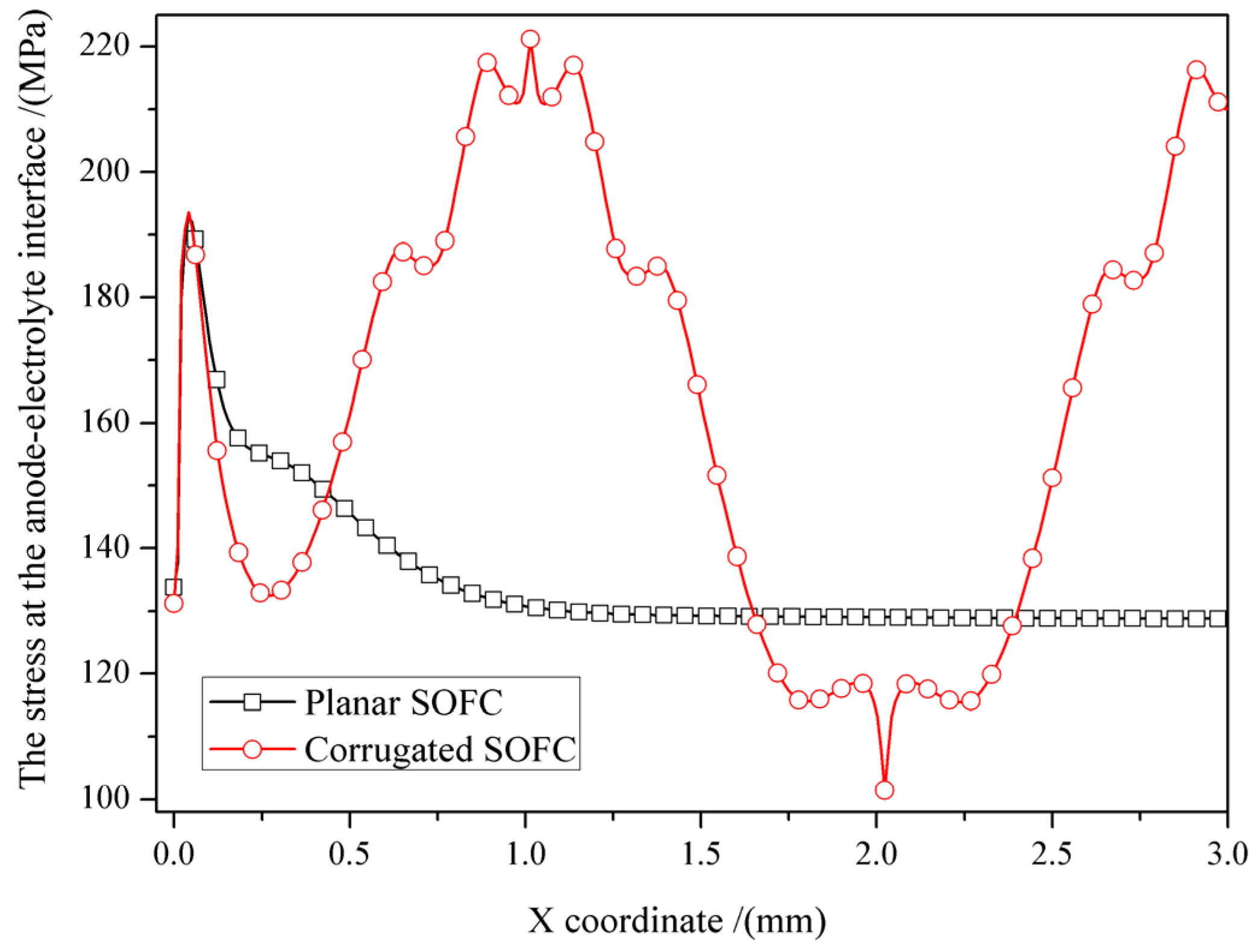
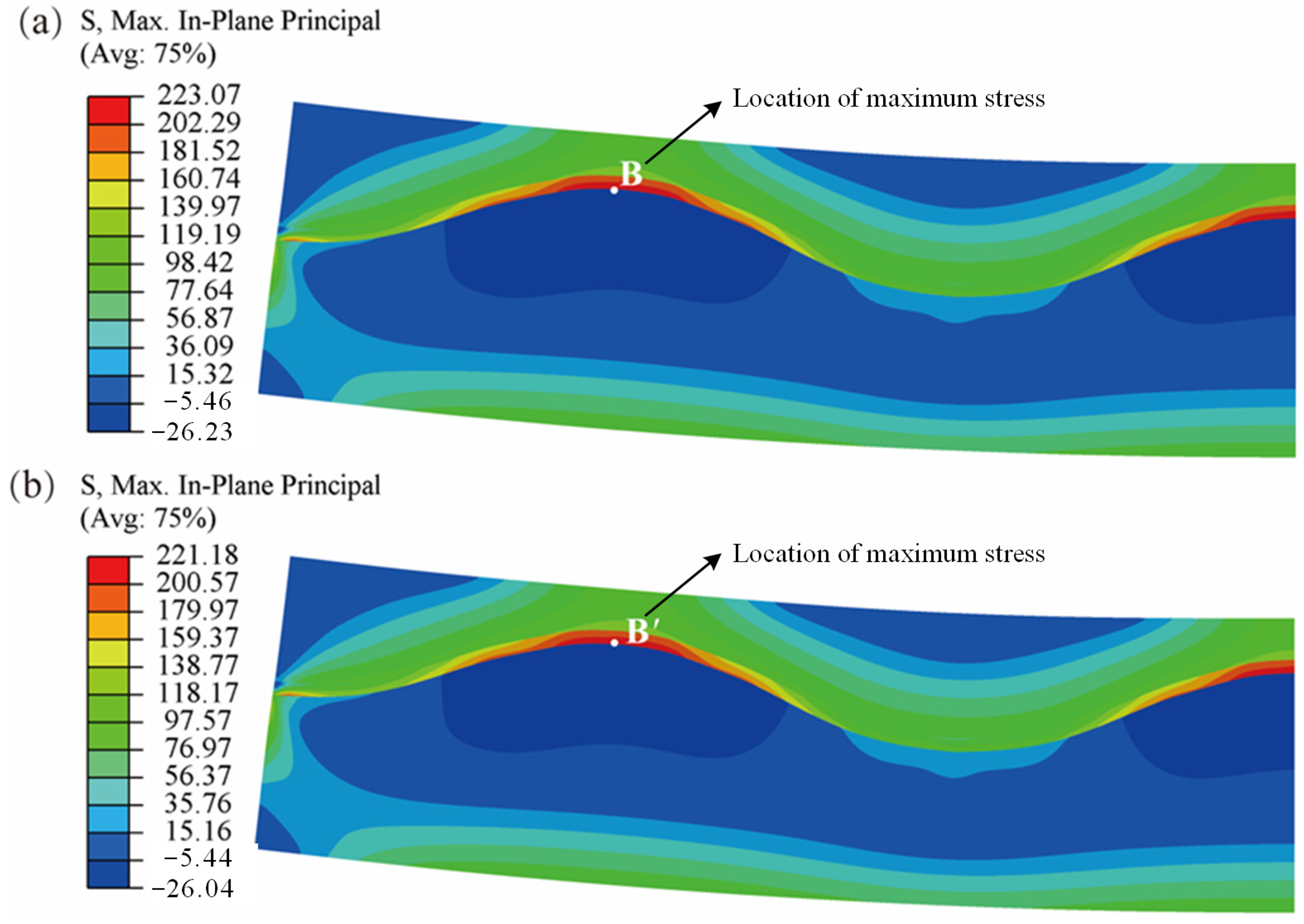
3.2. Influence of SOFC Interface Morphology on Stress Distribution under the Inhomogeneous Temperature Field
3.3. Contribution of Temperature Gradient to Stress Distribution of SOFC with Different Interface Morphology
4. Conclusions
- (1)
- The influence of interface morphology on the temperature distribution of SOFCs under heat flow is not very obvious. The overall temperature of the corrugated cell is slightly higher than that of the planar cell.
- (2)
- The influence of interface morphology on the stress distribution of SOFCs under the inhomogeneous temperature field is significant, including the extreme values of the cells and their locations. Compared to the planar SOFC, the corrugated SOFC has a higher maximum stress and a lower minimum stress, and more pronounced stress fluctuations. Because high stress can promote crack propagation, while low stress can restrain crack propagation, whether the interface morphology can enhance the stability of the cell is related to the position of the initial crack.
- (3)
- The contribution of the inhomogeneous temperature field to the planar SOFC is 5%, while the contribution to the corrugated SOFC is only 1%. And compared with the homogeneous temperature field, the inhomogeneous temperature field increases the stress of planar SOFC and decreases the stress of the corrugated SOFC. Therefore, ignoring the temperature gradient caused by heat conduction will underestimate the thermal stress of the planar SOFC, while it will overestimate for the corrugated SOFC. And if the calculation is simplified using a homogeneous temperature field, the stress of the corrugated SOFC tends to be conservative.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yusaf, T.; Faisal Mahamude, A.S.; Kadirgama, K.; Ramasamy, D.; Farhana, K.; Dhahad, H.A.; Talib, A.B.D.R.A. Sustainable hydrogen energy in aviation-A narrative review. Int. J. Hydrogen Energy 2023, in press. [Google Scholar] [CrossRef]
- Arsad, A.Z.; Hannan, M.A.; Al-Shetwi, A.Q.; Mansur, M.; Muttaqi, K.M.; Dong, Z.Y.; Blaabjerg, F. Hydrogen energy storage integrated hybrid renewable energy systems: A review analysis for future research directions. Int. J. Hydrogen Energy 2022, 47, 17285–17312. [Google Scholar]
- Xie, J.M.; Wei, X.Y.; Bo, X.Q.; Zhang, P.; Chen, P.Y.; Hao, W.Q.; Yuan, M.N. State of charge estimation of lithium-ion battery based on extended Kalman filter algorithm. Front. Energy Res. 2023, 11, 1180881. [Google Scholar]
- Hao, W.Q.; Xie, J.M. Reducing diffusion-induced stress of bilayer electrode system by introducing pre-strain in lithium-ion battery. J. Electrochem. Energy Convers. Storage 2021, 18, 20909. [Google Scholar]
- Zeng, Z.Z.; Qian, Y.P.; Zhang, Y.J.; Hao, C.K.; Dan, D.; Zhuge, W.L. A review of heat transfer and thermal management methods for temperature gradient reduction in solid oxide fuel cell (SOFC) stacks. Appl. Energy 2020, 280, 115899. [Google Scholar]
- Xie, J.M.; Hao, W.Q.; Wang, F.H. Parametric study on interfacial crack propagation in solid oxide fuel cell based on electrode material. Int. J. Hydrogen Energy 2022, 47, 7975–7989. [Google Scholar]
- Liso, V.; Olesen, A.C.; Nielsen, M.P.; Kær, S.K. Performance comparison between partial oxidation and methane steam reforming processes for solid oxide fuel cell (SOFC) micro combined heat and power (CHP) system. Energy 2011, 36, 4216–4226. [Google Scholar]
- Rokni, M. Thermodynamic analysis of SOFC (solid oxide fuel cell)-Stirling hybrid plants using alternative fuels. Energy 2013, 61, 87–97. [Google Scholar] [CrossRef]
- Komatsu, Y.; Kimijima, S.; Szmyd, J.S. Performance analysis for the part-load operation of a solid oxide fuel cell-micro gas turbine hybrid system. Energy 2010, 35, 982–988. [Google Scholar] [CrossRef]
- Dunbar, W.R.; Lior, N.; Gaggioli, R.A. Combing fuel cells with fuel fired power plants for improved exergy efficiency. Energy 1991, 16, 1259–1274. [Google Scholar] [CrossRef]
- Bang-Møller, C.; Rokni, M.; Elmegaard, B.; Ahrenfeldt, J.; Henriksen, U.B. Decentralized combined heat and power production by two-stage biomass gasification and solid oxide fuel cells. Energy 2013, 58, 527–537. [Google Scholar]
- Zitouni, B.; Moussa, H.B.; Oulmi, K.; Saighi, S.; Chetehouna, K. Temperature field, H2 and H2O mass transfer in SOFC single cell: Electrode and electrolyte thickness effects. Int. J. Hydrogen Energy 2009, 34, 5032–5039. [Google Scholar] [CrossRef]
- Deng, Z.H.; Cao, H.L.; Li, X.; Jiang, J.H.; Yang, J.; Qin, Y. Generalized predictive control for fractional order dynamic model of solid oxide fuel cell output power. J. Power Sources 2010, 195, 8097–8103. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Jiang, J.H.; Li, S.H.; Yang, J.; Li, J. Dynamic modeling and analysis of a 5-kW solid oxide fuel cell system from the perspectives of cooperative control of thermal safety and high efficiency. Int. J. Hydrogen Energy 2015, 40, 456–476. [Google Scholar] [CrossRef]
- Xie, J.M.; Hao, W.Q.; Wang, F.H. Crack propagation of planar and corrugated solid oxide fuel cells during cooling process. Int. J. Energy Res. 2019, 43, 3020–3027. [Google Scholar] [CrossRef]
- Biswas, S.; Nithyanantham, T.; Thangavel, S.N.; Bandopadhyay, S. High-temperature mechanical properties of reduced NiO-8YSZ anode-supported bi-layer SOFC structures in ambient air and reducing environments. Ceram. Int. 2013, 39, 3103–3111. [Google Scholar]
- Ivers-Tiffee, E.; Weber, Q.; Herbstritt, D. Materials and technologies for SOFC-components. J. Eur. Ceram. Soc. 2001, 21, 1805–1811. [Google Scholar] [CrossRef]
- Gazzarri, J.I.; Kesler, O. Short-stack modeling of degradation in solid oxide fuel cells: Part I. Contact degradation. J. Power Sources 2008, 176, 138–154. [Google Scholar] [CrossRef]
- Gazzarri, J.I.; Kesler, O. Non-destructive delamination detection in solid oxide fuel cells. J. Power Sources 2007, 167, 430–441. [Google Scholar] [CrossRef]
- Park, K.; Yu, S.; Bae, J.; Kim, H.; Ko, Y. Fast performance degradation of SOFC caused by cathode delamination in long-term testing. Int. J. Hydrogen Energy 2010, 35, 8670–8677. [Google Scholar] [CrossRef]
- LeMasters, J. Thermal Stress Analysis of LCA-Based Solid Oxide Fuel Cells; Master of Science in Mechanical Engineering, Georgia Institute of Technology: Atlanta, GA, USA, 2004. [Google Scholar]
- Valluru, S. Steady State Thermal Stress Analyses of Two-Dimensional and Three-Dimensional Solid Oxide Fuel Cells; Master of Science in Mechanical Engineering, West Virginia University: Morgantown, WV, USA, 2005. [Google Scholar]
- Srikar, V.T.; Turner, K.T.; AndrewIe, T.Y.; Mark, S.S. Structural design considerations for micro machined solid oxide fuel cells. J. Power Sources 2004, 125, 62–69. [Google Scholar] [CrossRef]
- Tseronis, K.; Bonis, I.; Kookos, I.K.; Theodoropoulos, C. Parametric and transient analysis of non-isothermal planar solid oxide fuel cells. Int. J. Hydrogen Energy 2012, 37, 530–547. [Google Scholar] [CrossRef]
- Dollard, W.J. Solid oxide fuel cell developments at Westinghouse. J. Power Sources 1992, 37, 133–139. [Google Scholar] [CrossRef]
- Singhal, S.C. Advances in tubular solid oxide fuel cell technology. ECS PV. Jan. 1995, 1995-1, 195–207. [Google Scholar] [CrossRef]
- Irvine, J.T.S.; Connor, P. Solid Oxide Fuels Cells: Facts and Figures: Past Present and Future Perspectives for SOFC Technologies; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Sarkar, P.; Yamarte, L.; Rho, H.; Johanson, L. Anode-supported tubular micro-solid oxide fuel cell. Int. J. Appl. Ceram. Technol. 2007, 4, 103–108. [Google Scholar] [CrossRef]
- Panthi, D.; Tsutsumi, A. Micro-tubular solid oxide fuel cell based on a porous yttria-stabilized zirconia support. Sci. Rep. 2014, 4, 5754. [Google Scholar] [CrossRef]
- Kendall, K. Progress in microtubular solid oxide fuel cells. Int. J. Appl. Ceram. Technol. 2010, 7, 1–9. [Google Scholar] [CrossRef]
- Funahashi, Y.; Shimamori, T.; Suzuki, T.; Fujishiro, Y.; Awano, M. Fabrication and characterization of components for cube shaped micro tubular SOFC bundle. J. Power Sources 2007, 163, 731–736. [Google Scholar] [CrossRef]
- Kendall, K.; Newton, J.; Kendall, M. Microtubular SOFC (mSOFC) System in Truck APU Application. ECS Trans. 2015, 68, 187–192. [Google Scholar] [CrossRef]
- Kendall, K.; Liang, B.; Kendall, M. Microtubular SOFC (mSOFC) system in mobile robot applications. ECS Trans. 2017, 78, 237–242. [Google Scholar] [CrossRef]
- Lee, S.B.; Lim, T.H.; Song, R.H.; Shin, D.R.; Dong, S.K. Development of a 700 W anode-supported micro-tubular SOFC stack for APU applications. Int. J. Hydrogen Energy 2008, 33, 2330–2336. [Google Scholar] [CrossRef]
- Serincan, M.F.; Pasaogullari, U.; Sammes, N.M. Computational thermal-fluid analysis of a microtubular solid oxide fuel cell. J. Electrochem. Soc. 2008, 155, B1117–B1127. [Google Scholar] [CrossRef]
- Jia, J.; Jiang, R.; Shen, S.; Abudula, A. Effect of operation parameters on performance of tubular solid oxide fuel cell. AIChE J. 2008, 54, 554–564. [Google Scholar] [CrossRef]
- Sánchez, D.; Chacartegui, R.; Muñoz, A.; Sánchez, T. Thermal and electrochemical model of internal reforming solid oxide fuel cells with tubular geometry. J. Power Sources 2006, 160, 1074–1087. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Wang, G.L.; Zhang, H.O.; Xia, W.S. Comparison of heat and mass transfer between planar and MOLB-type SOFCs. J. Power Sources 2008, 177, 426–433. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Wang, G.L.; Zhang, H.O.; Xia, W.S. Computational analysis of thermo-fluid and electrochemical characteristics of MOLB-type SOFC stacks. J. Power Sources 2007, 173, 233–239. [Google Scholar] [CrossRef]
- Hwang, J.J.; Chen, C.K.; Lai, D.Y. Detailed characteristic comparison between planar and MOLB-type SOFCs. J. Power Sources 2005, 143, 75–83. [Google Scholar] [CrossRef]
- Ramírez-Minguela, J.J.; Rodríguez-Muñoz, J.L.; Pérez-García, V.; Mendoza-Miranda, J.M.; Muñoz-Carpio, V.D.; Alfaro-Ayala, J.A. Solid oxide fuel cell numerical study: Modified MOLB-type and simple planar geometries with internal reforming. Electrochim. Acta 2015, 159, 149–157. [Google Scholar] [CrossRef]
- Konno, A.; Iwai, H.; Saito, M.; Yoshida, H. A corrugated mesoscale structure on electrode-electrolyte interface for enhancing cell performance in anode-supported SOFC. J. Power Sources 2011, 196, 7442–7449. [Google Scholar] [CrossRef]
- Konno, A.; Iwai, H.; Inuyama, K.; Kuroyanagi, A.; Saito, M.; Yoshida, H.; Kodani, K.; Yoshikata, K. Mesoscale-structure control at anode/electrolyte interface in solid oxide fuel cell. J. Power Sources 2011, 196, 98–109. [Google Scholar] [CrossRef]
- Kenjo, T.; Osawa, S.; Fujikawa, K. High temperature air cathodes containing ion conductive oxides. J. Electrochem. Soc. 1991, 138, 349–355. [Google Scholar] [CrossRef]
- Haanappel, V.A.C.; Mertens, J.; Rutenbeck, D.; Tropartz, C.; Herzhof, W.; Sebold, D.; Tietz, F. Optimisation of processing and microstructural parameters of LSM cathodes to improve the electrochemical performance of anode-supported SOFCs. J. Power Sources 2005, 141, 216–226. [Google Scholar] [CrossRef]
- Xie, J.M.; Hao, W.Q.; Wang, F.H. Interface strength analysis of the corrugated anode-electrolyte interface in solid oxide fuel cell characterized by peel force. J. Power Sources 2018, 396, 141–147. [Google Scholar] [CrossRef]
- Yang, C.L.; Li, W.; Zhang, S.Q.; Bi, L.; Peng, R.R.; Chen, C.S.; Liu, W. Fabrication and characterization of an anode-supported hollow fiber SOFC. J. Power Sources 2009, 187, 90–92. [Google Scholar] [CrossRef]
- Brahim, C.; Ringued, A.; Gourba, E.; Cassir, M.; Billard, A.; Briois, P. Electrical properties of thin bilayered YSZ/GDC SOFC electrolyte elaborated by sputtering. J. Power Sources 2006, 156, 45–49. [Google Scholar] [CrossRef]
- Han, M.F.; Tang, X.L.; Yin, H.Y.; Peng, S.P. Fabrication, microstructure and properties of a YSZ electrolyte for SOFCs. J. Power Sources 2007, 165, 757–763. [Google Scholar] [CrossRef]
- Xie, J.M.; Hao, W.Q.; Wang, F.H. The analysis of interfacial thermal stresses of solid oxide fuel cell applied for submarine power. Int. J. Energy Res. 2018, 42, 2010–2020. [Google Scholar] [CrossRef]
- Xie, J.M.; Hao, W.Q.; Wang, F.H. Analysis of anode functional layer for minimizing thermal stress in solid oxide fuel cell. Appl. Phys. A 2017, 123, 656. [Google Scholar] [CrossRef]
- Nakajo, A.; Mueller, F.; Brouwer, J.; Favrat, D. Mechanical reliability and durability of SOFC stacks. Part I: Modelling of the effect of operating conditions and design alternatives on the reliability. Int. J. Hydrogen Energy 2012, 37, 9249–9268. [Google Scholar] [CrossRef]
- Nakajo, A.; Mueller, F.; Brouwer, J.; Favrat, D. Mechanical reliability and durability of SOFC stacks. Part II: Modelling of mechanical failures during ageing and cycling. Int. J. Hydrogen Energy 2012, 37, 9269–9286. [Google Scholar] [CrossRef]
- Chatterjee, A.; Sharma, G.; Varshney, J.; Neogy, S.; Singh, R.N. Comparative study of mechanical properties of pure nanocrystalline Ni and Ni-Tf nanocomposite. Mater. Sci. Eng. A 2017, 684, 626–633. [Google Scholar] [CrossRef]
- Lin, Y.M.; Beale, S. Performance predictions in solid oxide fuel cells. In Proceedings of the Third International Conference on CFD in the Minerals and Process Industries CSIRO, Melbourne, VIC, Australia, 10–12 December 2003. [Google Scholar]
- Simulia, D.C.S. Abaqus 6.13 Help Documentation: Abaqus Analysis User’s Manual; Dassault Systems Simulia Corp: Waltham, MA, USA, 2013. [Google Scholar]


| Unit | Temperature | Anode (Ni-YSZ) | Electrolyte (YSZ) | Cathode (LSM) | |
|---|---|---|---|---|---|
| Thermal conductivity λ | W/(m·K) | - | 6.0 | 2.7 | 6.0 |
| Density ρ | kg/m3 | - | 6870 | 5900 | 6570 |
| Specific heat c | J/(kg·K) | - | 595 | 606 | 573 |
| Elastic modulus E | GPa | 293 K | 72.5 | 196.3 | 41.3 |
| 1073 K | 58.1 | 148.6 | 48.3 | ||
| Poisson’s ratio μ | - | 293 K | 0.36 | 0.31 | 0.33 |
| 1073 K | 0.36 | 0.31 | 0.33 | ||
| Thermal expansion coefficient α | ×10−6/K | 293 K | 12.41 | 10.0 | 9.8 |
| 1073 K | 12.60 | 10.5 | 11.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Li, J.; Hao, W.; Wang, F. Influence of Interface Morphology on the Thermal Stress Distribution of SOFC under Inhomogeneous Temperature Field. Energies 2023, 16, 7349. https://doi.org/10.3390/en16217349
Xie J, Li J, Hao W, Wang F. Influence of Interface Morphology on the Thermal Stress Distribution of SOFC under Inhomogeneous Temperature Field. Energies. 2023; 16(21):7349. https://doi.org/10.3390/en16217349
Chicago/Turabian StyleXie, Jiamiao, Jingyang Li, Wenqian Hao, and Fenghui Wang. 2023. "Influence of Interface Morphology on the Thermal Stress Distribution of SOFC under Inhomogeneous Temperature Field" Energies 16, no. 21: 7349. https://doi.org/10.3390/en16217349
APA StyleXie, J., Li, J., Hao, W., & Wang, F. (2023). Influence of Interface Morphology on the Thermal Stress Distribution of SOFC under Inhomogeneous Temperature Field. Energies, 16(21), 7349. https://doi.org/10.3390/en16217349





