Abstract
Low-temperature contact freezing refrigeration solutions available on the market typically contain refrigerants inside the plates, which may result in harmful emissions into the atmosphere and the contamination of food products in case of failure. However, a concept for a refrigeration plant with new coolant—a solution of potassium formate—is introduced in this paper. It comprises two systems: the primary one, where the compressor system is cooling down the coolant in the evaporator (as a heat and mass exchanger), and the secondary one, where the pump system is responsible for coolant circulation. As a coolant in the intermediate system, a 48% aqueous solution of potassium formate was utilized due to its numerous benefits. This liquid is neutral to the pump circuit installation materials and does not pose a threat to the food product in case of leakage, as it is also used as a food preservative additive. The use of liquid coolant ensures that any malfunction results in a visible leak, allowing for prompt intervention, unlike refrigerants that escape unnoticed into the atmosphere. Moreover, a refrigerant with a lower GWP than traditional solutions was chosen, making the system more eco-friendly.
1. Introduction
Preserving products through freezing is a popular technique that maintains their nutritional value, flavor, and aroma. There are different types of product freezing processes, which are categorized by feed media, process flow, or equipment design. The contact freezing method using a plate freezer is a primary way of freezing. It accelerates the freezing of certain food products into convenient forms for storage and post-processing. With no air layer between the freezing surface and the product, this method intensifies the process compared to conventional air-blast freezing. The concept of plate freezing is a method where the product is positioned between two plates filled with refrigerant. This results in a cuboid-shaped block of frozen products that is convenient for storage and processing after thawing, as required by the plant’s production technology [1,2].
Therefore, many authors have recently dealt with the topic of food preservation using plate freezers. One of the proposals is presented by Fernandez et al. [3]. The authors describe their innovative model for a horizontal plate freezer, which is based on a cascade refrigeration system, where ammonia—acting as an upper-order factor—is responsible for the condensation of CO2. Referring to the same energy distribution system, Dopazo et al. [4] describe their research on the freezing process in a horizontal plate freezer, focusing on determining the relationship between the temperature of the coolant inside the freezing plate and the freezing time of the product. A relationship is observed, indicating that lowering the temperature of the coolant inside the plate by 1K results in a reduction in the freezing time by 2 min. Cao et al. [5] postulate the possibility of using ejectors for each plate inside the freezer. However, such solutions can only be used in freezers operating in a direct expansion system. A classic system of a plate freezer with vertical loading powered by an ammonia system (diesel engine–ammonia system) used to freeze a catch of fish on a ship is described by Backi and Gravdahl [6]. The article defines a one-dimensional mathematical model and draws attention to the existence of a hidden heat of fusion. The topic of freezing food products immediately after fishing/shrimping is also discussed by Haddad et al., Lakshimisha et al., and Zhou et al. [7,8,9]. In these articles, products such as shrimps and mackerel frozen in a plate freezer immediately after being caught are subjected to sensory as well as qualitative analysis, and the results are promising.
The refrigeration industry has recently switched from synthetic refrigerants—HFCs, which have a negative impact on the environment, for example by increasing the greenhous effect—to natural refrigerants such as ammonia, carbon dioxide or saline solutions [3,4,10]. While these refrigerants are presently utilized in the refrigeration industry, they do have some technological limitations. In direct systems, both ammonia and carbon dioxide have certain shortcomings. The prevailing ammonia plants necessitate cooling systems with relatively high capacity, resulting in significant investment and operating expenses, which renders them unfeasible for small plant operations. Additionally, employing ammonia refrigeration equipment poses a risk of toxic and explosive refrigerant leakage from the system [11]. Although carbon dioxide can be used as a refrigerant, it has certain limitations due to its high sensitivity to temperature fluctuations. It is important to note that carbon dioxide has a critical temperature of 31.25 °C [12]. If the temperature of a product being frozen suddenly changes, there is a possibility of surpassing the critical temperature during the contact between the product and the plates. This can cause thermal convection, leading to a sudden rise in the temperature and pressure of carbon dioxide. Consequently, the refrigeration system capacity reduces sharply, and the freezing cycle is significantly extended. Additionally, carbon dioxide becomes highly poisonous to humans at 5% atmospheric content and lethal at 10% atmospheric content. Moreover, the refrigeration system operates at pressures ranging from 10 to 120 bar, which increases the risk of refrigerant leakage that can cause injuries in areas with people.
There is an alternative solution for cooling plates in a plate freezer that involves an indirect low-coagulation refrigeration system. This system utilizes a conventional low-temperature refrigeration system, which is supplied with either carbon dioxide or synthetic refrigerant on the primary side. Such a low-freezing liquid that can be used as a coolant is a solution of potassium formate. This solution, due to its properties, is used in refrigeration in devices such as heat pumps, air conditioning systems, heat exchangers, etc. [13,14,15]. Extensive studies on the physico-chemical properties of potassium salts tested on the basis of the precipitated film were presented in some articles [16,17]. The authors studied the film formed as a result of liquid flow and heat as well as mass transfer operations. By examining the relationship between the Reynolds number and Nusselts number for potassium formate, the authors pointed to the high dynamic viscosity of the coolant (relative to water) and confirmed more effective heat exchange with the environment through the so-called waves of liquid flow (non-laminar flow). In the study, the authors noted that at the same vapor pressure, the results of electrochemical tests showed that the potassium formate solution is much less aggressive than other salt solutions [18]. The reduction in the corrosive effect of this solution on steel was also demonstrated by [19].
The aim of the article was to present the possibilities of innovative use of potassium formate in an indirect system for food preservation in contact freezing. The use of appropriately selected working fluids (refrigerant and coolant) makes the system safe in the event of possible contact with food (leakage) and more eco-friendly. This innovative technology, which uses an indirect system 48% aqueous solution of potassium formate, has never been utilized before and is protected by patent PL 24012 [20].
2. Materials and Methods
2.1. The Refrigerant
When selecting a refrigerant, several important factors are considered. These include minimizing the environmental impact, ensuring safe usage, reducing energy consumption, utilizing reliable equipment to reduce maintenance costs, and complying with current regulations. It is crucial that the chosen refrigerant meets the standards outlined in Commission Implementing Regulation (EU) No 517/2014. This regulation prohibits the marketing of stationary refrigeration equipment containing HFCs with a GWP of 2500 or higher [21] after 1 January 2020. With these criteria in mind, the refrigerants listed in Section 3.2 were preselected.
The refrigerant selection process involved using the Bellinger method, which considers multiple criteria to determine the best refrigerant. This method involves ranking options based on predetermined sub-criteria and total rating values. The algorithm involves comparing the ratings of decision options against all criteria and bringing them to the same dimension for comparison. The Bellinger method algorithm is based on the distance between the upper and lower limit values. The method was chosen for decision-making support for the selection of refrigerants used in intermediate cooling systems, as it allows comparing a small number of options and comparing the decision options against all criteria.
The Bellinger method comprises eight successive stages: identifying the requirements and constraints for variants of solutions to the analyzed problem, defining the decision options available in a given situation, detailing the adopted evaluation criteria, adopting the units of measurement and the desired direction of changes within a given criterion (stimulants and destimulants) as well as lower and upper limits of changes for the analyzed subcriteria, establishing the weights assigned by the decision-maker to the adopted evaluation criteria, developing a matrix containing the actual values of the analyzed criteria from the point of view of individual variants, presenting all the numbers from the matrix from the previous stage as a percentage of the so-called path from the least to the most desirable state, multiplying all the numbers obtained at the sixth stage by the weights adopted at the fourth stage, determining the best variant on the basis of the sum of assessments assigned to individual variants taking into account all the analyzed criteria. A detailed description of the various stages of Bellinger’s multi-criteria analysis was provided by Górny (2019) [22].
2.2. Coolant
To analyze the coolant, the specific heat and thermal conductivity of a 48% aqueous solution of potassium formate need to be determined.
2.2.1. Pour Point Test
Samples were prepared with the following mass concentrations: 10%, 20%, 30%, 35%, 40%, 45%, 50%, 60%, and 75%.
Further to the Regulation [23], to accurately calculate the freezing point, the pour point of the substance must also be determined. The substance samples are first cooled to approximately −60 °C and then placed on a rack. The pour point is determined by observing the meniscus of the substance when the sample is tilted at a 45° angle. The temperature at which the first drop appears after tilting and the temperature at which the meniscus is completely tilted are noted.
2.2.2. Thermal Conductivity Test
Thermal conductivity testing in the temperature range from −50 to +80 °C was carried out using a bicalorimeter. The diagram of the measuring device is shown in Figure 1.
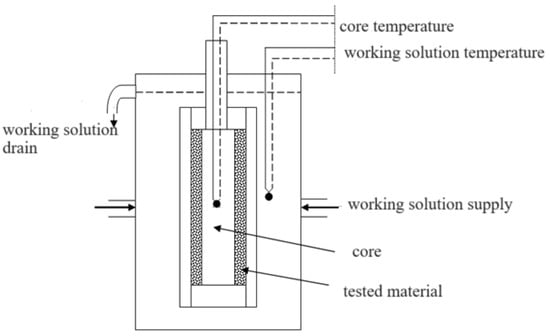
Figure 1.
Scheme of a bicalorimeter.
The thermal conductivity coefficient of the tested material was determined using Equation (1):
where m—the pace of cooling (1/s), δ—thickness of the material measured (m) (0.001 m), ρ—density of the nucleus material (kg/m3) (8440 kg/m3), c—specific heat of the nucleus material (J/kgK) (375 J/kgK), and l—characteristic dimension of the nucleus (m) (0.1 m).
λ = m ⋅ ϱ ⋅ c ⋅ l ⋅δ [W/mK]
2.2.3. Specific Heat Test
In order to determine the specific heat of an aqueous solution of potassium formate, the energy balance method was applied using a bicalorimeter. From the energy balance of the two mixing liquids in the calorimetric vessel, the specific heat values were determined from the following Equation (2):
where cc—specific heat of coolant (J/kgK); mw—mass of working fluid (kg); cw—specific heat of working fluid; mk—mass of calorimeter; ck—specific heat of calorimeter (J/kgK); T—final temperature temp (K); Tp—initial temperature (K); mc—mass of coolant (kg); Tc—coolant temperature (K).
3. Results and Discussion
3.1. Plant
A schematic diagram of the plant with a description of the individual components is shown in Figure 2.
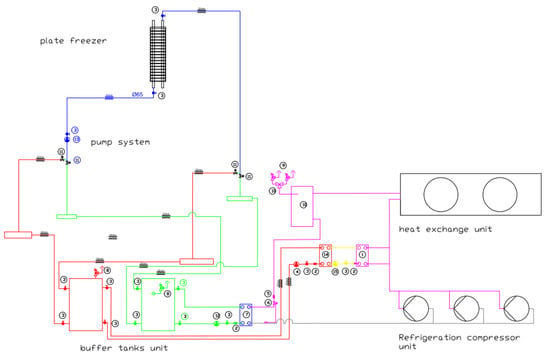
Figure 2.
Diagram of the freezer refrigeration system. 1—overheating plate heat exchanger; 2—pump filter; 3—flap shut-off valve; 4—primary coolant pump (heat recovery); 5—liquid filter HFC-HFD; 6—electronic expansion valve; 7—refrigerant evaporator (plate heat exchanger); 8—safety valve; 9—safety valve for refrigerant; 10—refrigerant reservoir; 11—flap control valve; 12—primary coolant pump; 13—secondary coolant pump; 14—indirect heat exchanger; 15—indirect glycol pump. Color lines: pink: liquid refrigerant; black: gas refrigerant; yellow: glycol; green: coolant cold side installation; red: coolant hot side installation; blue: coolant supply installation.
This installation employs a refrigeration system that utilizes the indirect circulation of a low-freezing liquid. The primary side comprises a conventional low-temperature refrigeration system that is supplied with a carefully chosen refrigerant (chosen using a multi-criteria method). The secondary side consists of a pumping system that injects the low-freezing liquid into the plates. The installation comprises three main components: a low-temperature chiller with a heat exchanger, a refrigerant/salt solution, a hydraulic module that is responsible for distributing the liquid between the heat exchanger and the plate freezer, and a contact freezer that is adapted to function with the salt solution as a coolant. In the described case, the salt solution being used is called potassium formate, which is a type of potassium salt. It is a 48% solution of potassium formate.
3.2. Refrigerant Selection
Based on the criteria listed in Section 2.1, the following refrigerants were selected for further multi-criteria analysis: refrigerant R452A, ammonia R717, and carbon dioxide R744. The refrigerants were chosen with reference to the properties of the previously commonly used refrigerant R404A. However, the refrigerants selected for analysis have a much lower GWP and can be successfully used in low- and medium-temperature systems. The above refrigerants are decision variants in Bellinger’s multi-criteria analysis, which was subsequently used as the optimal method to support the decision of selecting a refrigerant to be used in intermediate coolant cooling systems. The analysis was performed taking into account the steps of the Bellinger method discussed in Section 2.1. First, the requirements and constraints for the variant solutions to the problem under analysis were established. The following evaluation criteria were adopted: GWP creation (intensification) potential (K1), practical concentration limit (K2) (kg/m3), temperature slip (K3) (K), condensation temperature at 26 bar related to R404A (absolute difference in values for the selected refrigerant and R404A) (K4) (K), critical temperature related to R404A (absolute difference of values for the selected medium and R404A) (K5) (K), a normal boiling point related to R404A (absolute difference of values for the selected medium and R404A) (K6) (K), safety group (K7). Of the listed criteria, the first 6 (K1–K6) can be presented numerically, while for criterion K7—safety group, a numerical evaluation is proposed in Table 1.

Table 1.
Numerical scoring for criterion K7 for multi-criteria evaluation [4].
Criteria K4–K6 relate to the parameters of the R404A refrigerant to be replaced and represent the absolute difference in the value of a given parameter for the chosen refrigerant and R404A. These differences should be as low as possible. Within a given criterion, the destimulants are represented by criteria K1, K3 K4, K5, and K6. This means that the lowest numerical values within a criterion are the most desirable. On the other hand, within a given criterion, criteria K2 and K7 constitute stimulants. This means that the largest numerical values within a criterion are the most desirable. The lower and upper limits of each criterion are the smallest and largest numerical values, respectively. In order to account for decision-makers’ varying preferences, we have adopted three common attitudes: ecological, qualitative, and safety-oriented. Each attitude assigns different weights to individual criteria. Table 2 displays the corresponding set of weights for each attitude.

Table 2.
Weights for different types of the decision-maker’s approach.
When making decisions based on ecological impact, the criterion related to GWP was given the most weight (40% influence on the decision). The other criteria (K2–K7) were also considered but with less importance (10% influence on the decision each). On the other hand, in the quality option, the focus was on criteria related to thermodynamic parameters and GWP. Among these criteria, K1 and K3–K6 had an equal decision impact of 18% each. Weights of 5% were assigned to the remaining criteria (K2 and K7), while the safe option prioritized criteria related to safe usage (K2 and K7) with a weight of 35% each. Criterion K1 had a 10% influence on the decision, and the remaining criteria (K3–K6) were assigned weights of 5% each. A matrix was then created to display the actual values of each criterion for each option. Table 3 provides a summary of all numerical values, including the absolute difference in value for the selected refrigerant and R404A for criteria K4–K6.

Table 3.
Weights for different types of the decision-maker’s attitudes.
In the subsequent stage, the authors compiled all the numbers from the matrix of actual values regarding the analyzed criteria based on individual variants. This was calculated as a percentage of the path from the least to the most desirable state, which can be found in Table 4. To make the compilation, the relevant formulas for stimulants and destimulants were used [22].

Table 4.
A matrix of actual values expressed as a percentage of the so-called path from the least to the most desirable state.
Final calculations were then made to support the decision to select an environmentally friendly refrigerant that meets the requirements of Regulation 517/2014. Table 5, Table 6 and Table 7 show the products of the weights of the individual criteria and the percentages of the so-called path from the least to the most desirable state for three examples of the decision-maker’s attitudes. The best option was then selected based on the sum of the scores given to the individual options, taking into account all the analyzed criteria. The variants for the individual attitudes of the decision-maker were then ranked.

Table 5.
Ratings obtained by the individual variants, taking into account all criteria analyzed; the ecological attitude of the decision-maker.

Table 6.
Scores for individual variants, taking into account all the criteria analyzed; a qualitative approach.

Table 7.
Scores obtained by individual variants, taking into account all criteria analyzed; a safety approach.
For the case studied, the decision in the environmental variant is to choose the refrigerant R744 (evaluation score 0.675859). The next factors in the comprehensive assessment of the decision-maker from this variant are R717 (score of 0.59697) and the refrigerant R452A (score of 0.5). In the ecological approach, the order is not identical to that resulting from the criterion with the highest weighting, i.e., K1—GWP creation (intensification) potential (Table 5).
Based on a qualitative evaluation, factor R452A was chosen with a score of 0.64. The decision-maker also considered R717 (score 0.534545) and R744 (score 0.525427). In the qualitative approach, the ranking is based on criteria that best represent the 35 performance parameters of the R404A refrigerant to be replaced. However, the rank order differs from the ecological approach (Table 6).
For safety purposes, a refrigerant with a score of 0.85, documented as R452A, was chosen. The decision-maker assessed other refrigerants comprehensively and determined that the next best options are R744 with a score of 0.587298 and R717 with a score of 0.198485. The differences in the evaluation of these refrigerants are most noticeable in this particular category with the ranking being influenced by factors such as the risk of leakage and associated dangers (K2 and K7) (Table 7).
After analyzing the rankings, it was determined that refrigerant R452A had the highest rating in two out of three categories. Therefore, this refrigerant was chosen as the one for the refrigeration plant being researched.
3.3. Testing the Coolant’s Thermal Properties
3.3.1. Pour Point
In Figure 3, the temperature values are plotted against the mass concentration of potassium formate. Error bars represent the standard deviation from at least five measurements.
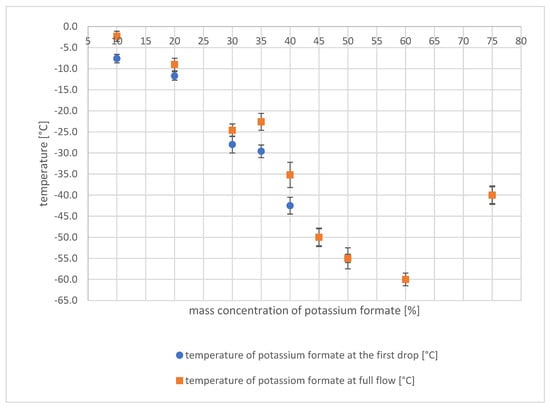
Figure 3.
Temperature values plotted against the mass concentration of potassium formate.
From the conducted tests, it was observed that the pour point decreases as the proportion of potassium formate in the solution increases. The lowest pour point of −60 °C was achieved at a concentration of 60%. However, as the concentration of potassium formate in the solution increases further, the pour point also increases. At a concentration of 75%, the pour point recorded was −40 °C. To achieve the lowest crystallization temperature in the intermediate circuit of a refrigeration plant, a concentration range of 45–60% is considered optimal. However, considering the system’s resistance, the ideal concentration range is between 45 and 50%, since potassium formate has a higher resistance than water. Hence, increasing its proportion in the solution beyond this range does not provide any significant benefit to the refrigeration system’s operation.
3.3.2. Thermal Conductivity
Figure 4 displays the thermal conductivity values of potassium formate, as determined by a bicalorimeter, across temperatures ranging from −50 to +80 °C.
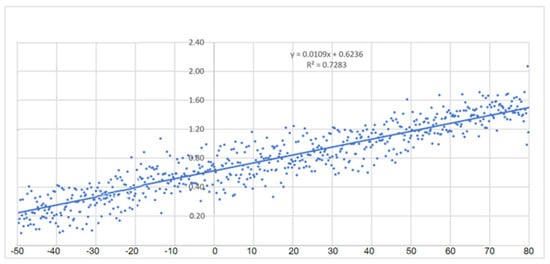
Figure 4.
Thermal conductivity values of potassium formate.
Through conducting their own research, the authors were able to determine a trend line for the thermal conductivity of a 48% mass concentration aqueous solution of potassium formate with the equation y = 0.0109x + 0.6236 and a coefficient of determination of R2 = 0.7283. This resulted in the creation of Table 8, which shows a consistent and linear increase in thermal conductivity values.

Table 8.
Thermal conductivity values for temperature range from −50 to +50 °C.
3.3.3. Specific Heat Capacity
The parameter values for determining specific heat are shown in Table 9.

Table 9.
Parameters for determining specific heat capacity.
The results of the study for the specific heat capacity of an aqueous solution of potassium formate with a mass concentration of 48% indicated an average value for this parameter (in the temperature range from −19.2 to 16.9 °C) of 3500 kJ/kgK. Due to the insignificant temperature-dependent difference in the value of the parameter investigated, it was assumed that at temperatures between −50 and +50 °C, this influence could also be neglected and the value obtained considered correct. In comparison to selected commercial coolants (Ergolid A—ethylene glycol and water based, Ergolid ECO—propylene glycol and water based, ProCOLD Super Cool—potassium formate and water based), the specific heat of the tested aqueous potassium formate solution with a mass concentration of 48% was higher, which is a positive result [24].
4. Conclusions
In the context of commercial application of plate freezers dedicated to individual installations (that are not part of a large plant), indirect systems supplied with potassium formate solution should be considered as a viable alternative to units supplied with carbon dioxide, ammonia or fluorocarbons. The simultaneous use of potassium formate solution as a coolant (pump circuit) and the refrigerant selected using the Bellinger method (compressor circuit) in an indirect system presented in the publication can be considered an innovative and ecological concept. This is due to the following reasons, i.e., the use of a coolant that is not aggressive to personnel and the processed product (can be used in contact with food) and also has appropriate thermal properties; the lower amount of refrigerant in the system (the reason is a division of the entire refrigeration system into two circuits; the use of a refrigerant (selected using the Bellinger method) with a lower GWP than traditional solutions (the reference refrigerant is R404A with GWP = 3922); the possibility of quickly noticing the location of a leak in the installation due to the use of liquid (not gaseous) coolant (visible leakage). The use of a plate freezer with an indirect refrigeration system is therefore a promising alternative solution, which appears to be free of the major drawbacks known from existing models.
5. Future Perspective
This article shows the possibility of an innovative use of solution of potassium formate in the context of an indirect contact freezing system. The next step will certainly be comprehensive testing of the resulting prototype installation, including efficiency determination, evaluation and ablation studies. Calculations related to the life cycle assessment of the installation will be carried out. It is planned to compare the usability of the created system with the existing ones. It should be emphasized, however, that the use of potassium formate (which in the event of leakage does not pose a threat to the stored food) is already at this stage an advantage over existing systems intended for food preservation.
6. Patents
Gaszek, K.; Gaszek, S. Techcool spółka z ograniczoną odpowiedzialnością, PL 240 127 B1. 2022.02.21. Układ chłodzący zamrażarki, zwłaszcza zamrażarki płytowej. Poland (patent application).
Author Contributions
Conceptualization, K.B. and K.P.; methodology, K.G. (Krzysztof Gaszek); software, K.G. (Kasper Górny); validation, T.B., K.G. (Krzysztof Gaszek) and K.P.; formal analysis, K.P.; investigation, T.B.; resources, K.B.; data curation, K.G. (Kasper Górny); writing—original draft preparation, Z.S.; writing—review and editing, K.B.; visualization, Z.S.; supervision, K.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The National Centre for Research and Development, Poland, grant number 5210201041400450414/NCBR/7918.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hessami, M.A. Study of the Efficiency of Plate Freezing vs. Blast Freezing of Boxed Boneless Meat in an Abattoir. In Proceedings of the ASME 2004 Heat Transfer/Fluids Engineering Summer Conference, Charlotte, NC, USA, 11–15 July 2004; Volume 1, pp. 1023–1030. [Google Scholar]
- Ladino Moreno, L.A.; Stetzer, O.; Lohmann, U. Contact freezing: A review of experimental studies. Atmos. Chem. Phys. 2013, 13, 9745–9769. [Google Scholar] [CrossRef]
- Fernández-Seara, J.; Dopazo, J.A.; Uhía, F.J.; Rubén Diz, F.J. Experimental Analysis of the Freezing Process in a Horizontal Plate Freezer with CO2 as Refrigerant in a Cascade Refrigeration System. Heat Transf. 2012, 33, 1170–1176. [Google Scholar] [CrossRef][Green Version]
- Dopazo, J.A.; Fernandez-Seara, J. Experimental evaluation of freezing processes in horizontal plate freezers using CO2 as refrigerant. Int. J. Refrig. 2012, 35, 2093–2101. [Google Scholar] [CrossRef]
- Cao, X.C.; Wan, J.Q.; Song, L.Y. Experimental Study of Plate Freezer with Ejector. Procedia Eng. 2015, 121, 1238–1244. [Google Scholar] [CrossRef][Green Version]
- Backi, C.; Gravdahl, J.T. Freezing of Fish by Vertical Platefreezers. Proceedings of the 17th Nordic Process Control Workshop; Jørgensen, J.B., Huusom, J.K., Sin, G., Eds.; Technical University of Denmark, DTU Informatics: Kogens Lyngby, Denmark, 2012; ISBN 978-87-643-0946-1. [Google Scholar]
- Haddad, N.A.; Watts, E.; Lively, J.A. Evaluation of Post- Harvest Producers for Quality Enhancememnt In the Louisiana Commercial Shrimp Industry. J. Aquat. Food Prod. Technol. 2021, 31, 96–111. [Google Scholar] [CrossRef]
- Lakshimisha, I.P.; Ravishankar, C.N.; Ninnan, G.; Mohan, C.O.; Gopal, T.K.S. Effect of Freezing Time on the Quality of Indian Mackerel (Rastrelliger kanagurta) during Frozen Storage. J. Food Sci. 2008, 73, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Chu, Y.; Ying, L.; Xie, J. Quality of Frozen Mackerel during Storage as Processed by Different Freezing Methods. Int. J. Food Prop. 2022, 25, 593–607. [Google Scholar] [CrossRef]
- Bolaji, B.O.; Huan, Z. Ozone depletion and global warming: Case for the use of natural refrigerant—A review. Renew. Sustain. Energy Rev. 2013, 18, 49–54. [Google Scholar] [CrossRef]
- Pearson, A. Carbon dioxide—New uses for an old refrigerant. Int. J. Refrig. 2005, 28, 1140–1148. [Google Scholar] [CrossRef]
- Haixia, W.; Jusheng, C.; Qingling, L. A Review of Pipeline Transportation Technology of Carbon Dioxide. IOP Conf. Ser. Earth Environ. Sci. 2019, 310, 032033. [Google Scholar]
- Bicui, Y.; Yaqi, D.; Zheng, W.; Guangming, C. Experimental evaluation of a variable-stage open absorption heat pump system utilizing aqueous KCOOH solution as absorbent. Int. J. Refrig. 2023, 148, 83–95. [Google Scholar]
- Kashish, K.; Alok, S.; Prem, K.C.; Kamal, K.P.; Vikas, P. Progressive development in hybrid liquid desiccant-vapour compression cooling system: A review. Sustain. Energy Technol. Assess. 2023, 55, 102960. [Google Scholar]
- Ge, T.S.; Zhang, J.Y.; Dai, Y.J.; Wang, R.Z. Experimental study on performance of silica gel and potassium formate composite desiccant coated heat exchanger. Energy 2017, 141, 149–158. [Google Scholar] [CrossRef]
- Tao, W.; Meng, W.; Yi, C.; Weifeng, H.; Yimo, L. Thermal properties study and performance investigation of potassium formate solution in a falling film dehumidifier/regenerator. Int. J. Heat. Mass. Transf. 2019, 134, 131–142. [Google Scholar]
- Yuqi, S.; Guangming, C. Experimental study of falling film absorption with potassium formate-Water as working pair. Int. J. Therm. Sci. 2022, 176, 107520. [Google Scholar]
- Tao, W.; Yimo, L.; Liyuan, S. Experimental study on the corrosion behavior and regeneration performance of KCOOH aqueous solution. Sol. Energy 2020, 201, 638–648. [Google Scholar]
- Tao, W.; Lin, L.; Mai, L.; Hong, Z. Comparative study of the regeneration characteristics of LiCl and a new mixed liquid desiccant solution. Energy 2018, 163, 992–1005. [Google Scholar]
- Gaszek, K.; Gaszek, S. Techcool Spółka z Ograniczoną Odpowiedzialnością. PL 240 127 B1, 21 February 2022. [Google Scholar]
- Regulation (EU) No 517/2014 of the European Parliament and of the Council of 16 April 2014 on Fluorinated Greenhouse Gases and Repealing Regulation (EC) No 842/2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32014R0517 (accessed on 5 May 2023).
- Górny, K. A method of decision support for the selection of lubricants for refrigeration compressors on the basis of tribological tests. Tribologia 2019, 3, 31–38. [Google Scholar] [CrossRef]
- Council Regulation (EC) No 440/2008 of 30 May 2008 laying down test methods pursuant to Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Available online: https://eur-lex.europa.eu/eli/reg/2008/440/oj (accessed on 5 May 2023).
- Chmielewska, M.; Chmielewski, W.; Gaziński, B.; Krzyżaniak, G. Płyny Instalacyjne ERGOLID; Boryszew S.A.: Sochaczew, Poland, 2005; Available online: https://procold.pl (accessed on 22 November 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).