Application of Alkali-Activated Sustainable Materials: A Step towards Net Zero Binder
Abstract
1. Introduction
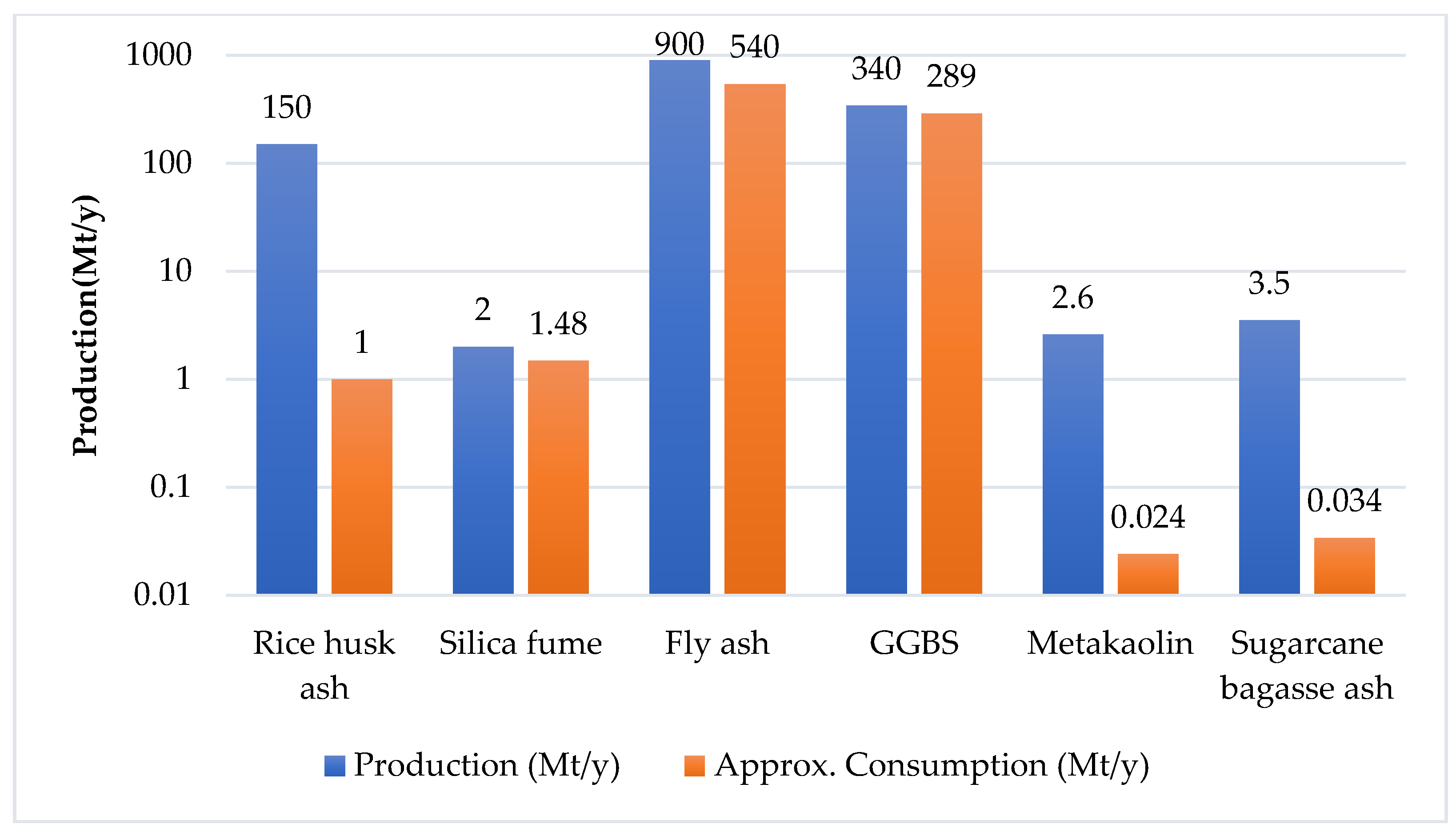
2. Supplementary Cementitious Materials (SCM)
3. Alkali-Activated Materials (AAM)
3.1. Precursors
3.2. Alkali Activators
3.3. Activator Solution
3.4. Alkali Activation Mechanism
3.4.1. Alkali Activation Mechanism for Low Calcium Precursors
3.4.2. Alkali Activation Mechanism for High Calcium Precursors
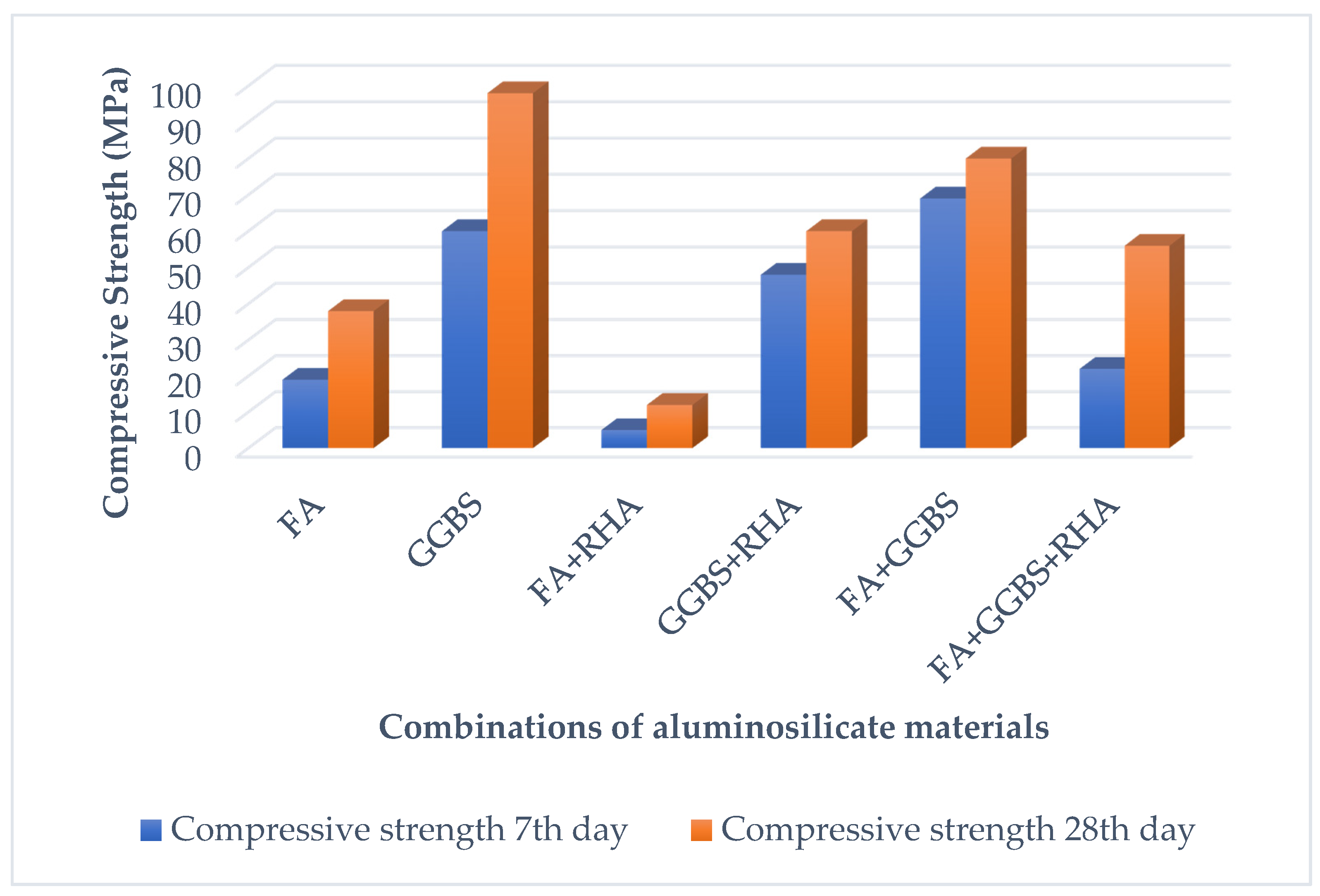
4. Properties of Alkali-Activated Materials
4.1. Physico-Mechanical Properties of AAM
4.2. Durability Properties of AAM
5. Admixtures Used in AAC
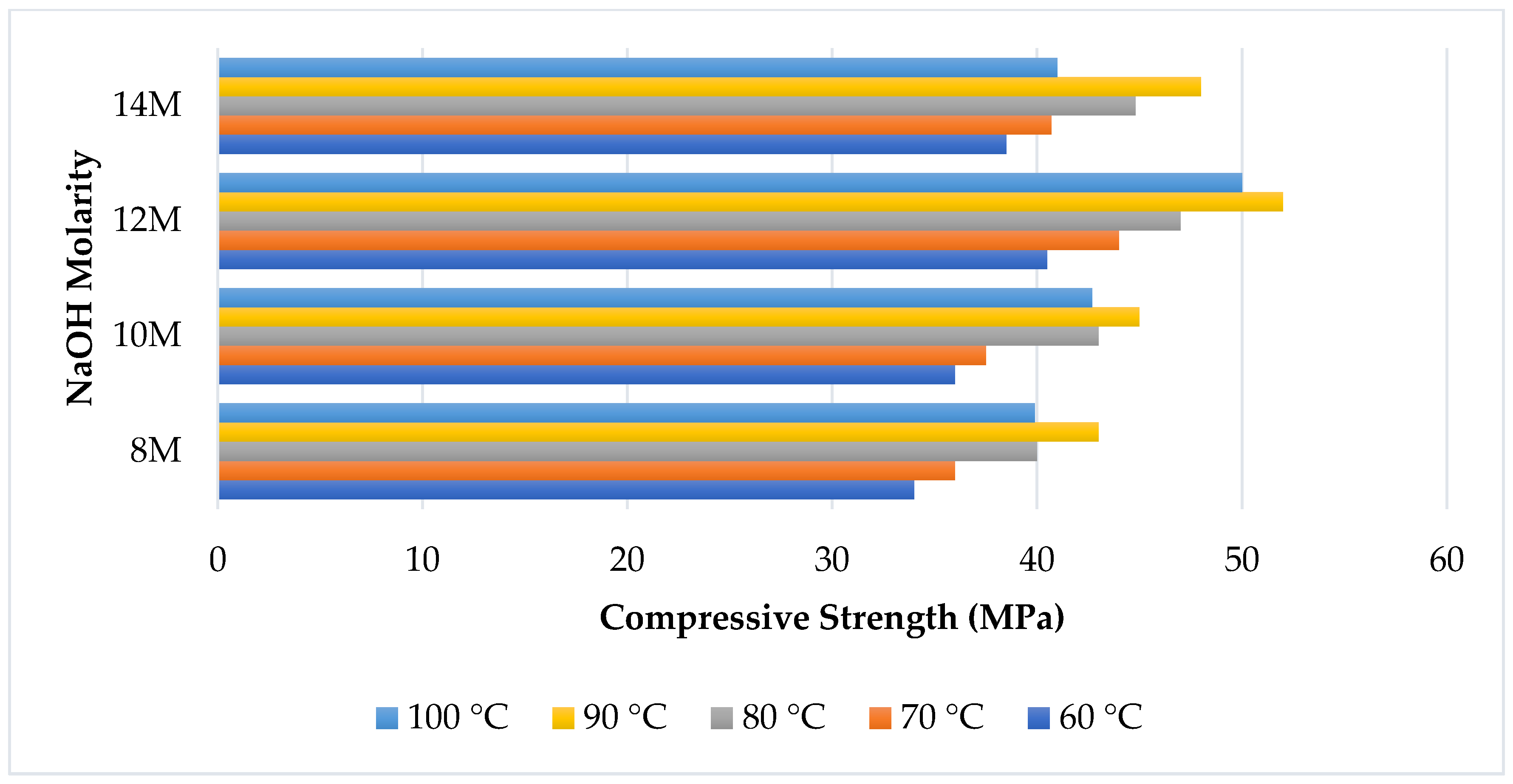
6. Curing Regime
7. Environmental Footprint of AAC Compared to OPC
8. Discussion
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ling, Y.; Fu, C.; Zhang, P.; Taylor, P. Advances in Sustainable Concrete System. Crystals 2022, 12, 698. [Google Scholar] [CrossRef]
- Hamilton, J.; Kennard, D.H.; Rapf, O.; Kockat, D.J.; Zuhaib, D.S.; Toth, D.Z.; Barrett, M.; Milne, C. 2022 Global Status Report for Buildings and Construction: Towards a Zero-Emission, Efficient and Resilient Buildings and Construction Sector; United Nations Environment Programme (2022); United Nations: Nairobi, Kenya, 2022; ISBN 978-92-807-3984-8. [Google Scholar]
- Chen, C.; Xu, R.; Tong, D.; Qin, X.; Cheng, J.; Liu, J.; Zheng, B.; Yan, L.; Zhang, Q. A Striking Growth of CO2 emissions from the Global Cement Industry Driven by New Facilities in Emerging Countries. Environ. Res. Lett. 2022, 17, 044007. [Google Scholar] [CrossRef]
- Huseien, G.F.; Shah, K.W. Durability and Life Cycle Evaluation of Self-Compacting Concrete Containing Fly Ash as GBFS Replacement with Alkali Activation. Constr. Build. Mater. 2020, 235, 117458. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-Efficient Cements: Potential Economically Viable Solutions for a Low-CO2 Cement-Based Materials Industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Ahmed, H.U.; Mosavi, A. Survey of Mechanical Properties of Geopolymer Concrete: A Comprehensive Review and Data Analysis. Materials 2021, 14, 4690. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.P.; Vo, D.H.; Hwang, C.L. Engineering and Durability Properties of Eco-Friendly Mortar Using Cement-Free SRF Binder. Constr. Build. Mater. 2018, 160, 145–155. [Google Scholar] [CrossRef]
- Olivier, J.G.J.; Peters, J.A.H.W. Trends in Global CO2 and Total Greenhouse Gas Emissions: 2019 Report; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2020; Volume 4068, pp. 1–70. [Google Scholar]
- Jittin, V.; Bahurudeen, A. Evaluation of Rheological and Durability Characteristics of Sugarcane Bagasse Ash and Rice Husk Ash Based Binary and Ternary Cementitious System. Constr. Build. Mater. 2022, 317, 125965. [Google Scholar] [CrossRef]
- Rasheed, R.; Tahir, F.; Afzaal, M.; Ahmad, S.R. Decomposition Analytics of Carbon Emissions by Cement Manufacturing—A Way Forward towards Carbon Neutrality in a Developing Country. Environ. Sci. Pollut. Res. 2022, 29, 49429–49438. [Google Scholar] [CrossRef]
- Amer, I.; Kohail, M.; El-Feky, M.S.; Rashad, A.; Khalaf, M.A. A Review on Alkali-Activated Slag Concrete. Ain Shams Eng. J. 2021, 12, 1475–1499. [Google Scholar] [CrossRef]
- Alsalman, A.; Assi, L.N.; Kareem, R.S.; Carter, K.; Ziehl, P. Energy and CO2 Emission Assessments of Alkali-Activated Concrete and Ordinary Portland Cement Concrete: A Comparative Analysis of Different Grades of Concrete. Clean. Environ. Syst. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Kumar, S.; Smith, S.R.; Fowler, G.; Velis, C.; Kumar, S.J.; Arya, S.; Rena; Kumar, R.; Cheeseman, C. Challenges and Opportunities Associated with Waste Management in India. R. Soc. Open Sci. 2017, 4, 160764. [Google Scholar] [CrossRef]
- Madurwar, M.V.; Mandavgane, S.A.; Ralegaonkar, R.V. Use of Sugarcane Bagasse Ash as Brick Material. Curr. Sci. 2014, 107, 1044–1051. [Google Scholar]
- Gavali, H.R.; Bras, A.; Faria, P.; Ralegaonkar, R.V. Development of Sustainable Alkali-Activated Bricks Using Industrial Wastes. Constr. Build. Mater. 2019, 215, 180–191. [Google Scholar] [CrossRef]
- Nodehi, M.; Taghvaee, V.M. Applying Circular Economy to Construction Industry through Use of Waste Materials: A Review of Supplementary Cementitious Materials, Plastics, and Ceramics; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; Volume 2, ISBN 0123456789. [Google Scholar]
- Rakhimova, N.R.; Rakhimov, R.Z. Toward Clean Cement Technologies: A Review on Alkali-Activated Fly-Ash Cements Incorporated with Supplementary Materials. J. Non Cryst. Solids 2019, 509, 31–41. [Google Scholar] [CrossRef]
- Miller, S.A.; John, V.M.; Pacca, S.A.; Horvath, A. Carbon Dioxide Reduction Potential in the Global Cement Industry by 2050. Cem. Concr. Res. 2018, 114, 115–124. [Google Scholar] [CrossRef]
- Gavali, H.R.; Ralegaonkar, R.V. Design Development of Sustainable Alkali-Activated Bricks. J. Build. Eng. 2020, 30, 101302. [Google Scholar] [CrossRef]
- Shelote, K.M.; Gavali, H.R.; Bras, A.; Ralegaonkar, R.V. Utilization of Co-Fired Blended Ash and Chopped Basalt Fiber in the Development of Sustainable Mortar. Sustainability 2021, 13, 1247. [Google Scholar] [CrossRef]
- Chilukuri, S.; Kumar, S.; Raut, A. Status of Agro-Industrial Waste Used to Develop Construction Materials in Andhra Pradesh Region—India. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1197, 012075. [Google Scholar] [CrossRef]
- Bras, A.; Ravijanya, C.; de Sande, V.T.; Riley, M.; Ralegaonkar, R.V. Sustainable and Affordable Prefab Housing Systems with Minimal Whole Life Energy Use. Energy Build. 2020, 220, 110030. [Google Scholar] [CrossRef]
- Chippagiri, R.; Bras, A.; Ralegaonkar, R.V. Development of Sustainable Prefabricated Housing System by Small-Scale Experimental Model. In Proceedings of the Institution of Civil Engineers-Engineering Sustainability; Thomas Telford Ltd.: London, UK, 2022; pp. 1–14. [Google Scholar] [CrossRef]
- Ram, S.; Ralegaonkar, R.V.; Pradhan, K. Use of Co-Fired Blended Ash in the Development of Sustainable Construction Materials. In Proceedings of the Institution of Civil Engineers-Engineering Sustainability; Thomas Telford Ltd.: London, UK, 2017; Volume 171, pp. 425–432. [Google Scholar] [CrossRef]
- Sakhare, V.; Raut, S.; Ralegaonkar, R. Performance Assessment of Sustainable Composite Roofing Assemblies Using Experimentation. Procedia Eng. 2015, 118, 268–275. [Google Scholar] [CrossRef]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-Activated Fly Ashes: A Cement for the Future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-Part Alkali-Activated Materials: A Review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Abhishek, H.S.; Prashant, S.; Kamath, M.V.; Kumar, M. Fresh Mechanical and Durability Properties of Alkali-Activated Fly Ash-Slag Concrete: A Review. Innov. Infrastruct. Solut. 2022, 7, 1–14. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Cheng, B. Mechanical Properties of Alkali-Activated Concrete Containing Crumb Rubber Particles. Case Stud. Constr. Mater. 2022, 16, e00803. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-Activated Materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Gavali, H.R.; Bras, A.; Ralegaonkar, R.V. Cleaner Construction of Social Housing Infrastructure with Load-Bearing Alkali-Activated Masonry. Clean Technol. Environ. Policy 2021, 23, 2303–2318. [Google Scholar] [CrossRef]
- Nodehi, M.; Taghvaee, V.M. Alkali-Activated Materials and Geopolymer: A Review of Common Precursors and Activators Addressing Circular Economy. Circ. Econ. Sustain. 2022, 2, 165–196. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Cizer, Ö.; Pontikes, Y.; Heath, A.; Patureau, P.; Bernal, S.A.; Marsh, A.T.M. Advances in Alkali-Activation of Clay Minerals. Cem. Concr. Res. 2020, 132, 106050. [Google Scholar] [CrossRef]
- Elahi, M.M.A.; Hossain, M.M.; Karim, M.R.; Zain, M.F.M.; Shearer, C. A Review on Alkali-Activated Binders: Materials Composition and Fresh Properties of Concrete. Constr. Build. Mater. 2020, 260, 119788. [Google Scholar] [CrossRef]
- Patel, Y.J.; Shah, N. Enhancement of the Properties of Ground Granulated Blast Furnace Slag Based Self Compacting Geopolymer Concrete by Incorporating Rice Husk Ash. Constr. Build. Mater. 2018, 171, 654–662. [Google Scholar] [CrossRef]
- Ahmad, S.; Bahraq, A.A.; Shaqraa, A.A.; Khalid, H.R.; Al-Gadhib, A.H.; Maslehuddin, M. Effects of Key Factors on the Compressive Strength of Metakaolin and Limestone Powder-Based Alkali-Activated Concrete Mixtures: An Experimental and Statistical Study. Case Stud. Constr. Mater. 2022, 16, e00915. [Google Scholar] [CrossRef]
- Hossain, M.M.; Karim, M.R.; Elahi, M.M.A.; Islam, M.N.; Zain, M.F.M. Long-Term Durability Properties of Alkali-Activated Binders Containing Slag, Fly Ash, Palm Oil Fuel Ash and Rice Husk Ash. Constr. Build. Mater. 2020, 251, 119094. [Google Scholar] [CrossRef]
- Moraes, J.C.B.; Tashima, M.M.; Akasaki, J.L.; Melges, J.L.P.; Monzó, J.; Borrachero, M.V.; Soriano, L.; Payá, J. Increasing the Sustainability of Alkali-Activated Binders: The Use of Sugar Cane Straw Ash (SCSA). Constr. Build. Mater. 2016, 124, 148–154. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Abd Elmoaty, A.E.M.; Emam, M.A. Factors Affecting the Mechanical Properties of Alkali Activated Ground Granulated Blast Furnace Slag Concrete. Constr. Build. Mater. 2019, 197, 339–355. [Google Scholar] [CrossRef]
- Ahmad, J.; Kontoleon, K.J.; Al-Mulali, M.Z.; Shaik, S.; El Ouni, M.H.; El-Shorbagy, M.A. Partial Substitution of Binding Material by Bentonite Clay (BC) in Concrete: A Review. Buildings 2022, 12, 634. [Google Scholar] [CrossRef]
- Laidani, Z.E.-A.; Ouldkhaoua, Y.; Sahraoui, M.; Benabed, B. Feasibility of Marble Powder and Calcined Bentonite in SCM as Partial Substitution of Cement for Sustainable Production. Epa. -J. Silic. Based Compos. Mater. 2022, 74, 61–66. [Google Scholar] [CrossRef]
- Charitha, V.; Athira, V.S.; Jittin, V.; Bahurudeen, A.; Nanthagopalan, P. Use of Different Agro-Waste Ashes in Concrete for Effective Upcycling of Locally Available Resources. Constr. Build. Mater. 2021, 285, 122851. [Google Scholar] [CrossRef]
- Ruan, Y.; Jamil, T.; Hu, C.; Gautam, B.P.; Yu, J. Microstructure and Mechanical Properties of Sustainable Cementitious Materials with Ultra-High Substitution Level of Calcined Clay and Limestone Powder. Constr. Build. Mater. 2022, 314, 125416. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.; Chen, Q.; Lawi, A.S.; Chen, J. Effect of Partial Substitution of Cement with Dolomite Powder on Glass-Fiber-Reinforced Mortar. Constr. Build. Mater. 2022, 344, 128201. [Google Scholar] [CrossRef]
- Banu, S.S.; Kartikeyan, J.; Jayabalan, P. Influence of Using Agro-Waste as a Partial Replacement in Cement on the Compressive Strength of Concrete—A Statistical Approach. Constr. Build. Mater. 2020, 250, 118746. [Google Scholar] [CrossRef]
- Chinnu, S.N.; Minnu, S.N.; Bahurudeen, A.; Senthilkumar, R. Influence of Palm Oil Fuel Ash in Concrete and a Systematic Comparison with Widely Accepted Fly Ash and Slag: A Step towards Sustainable Reuse of Agro-Waste Ashes. Clean. Mater. 2022, 5, 100122. [Google Scholar] [CrossRef]
- Tong, Y.; Seibou, A.O.; Li, M.; Kaci, A.; Ye, J. Bamboo Sawdust as a Partial Replacement of Cement for the Production of Sustainable Cementitious Materials. Crystals 2021, 11, 1593. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Giri, J.P. Use of Recycled Foundry Sand for the Development of Green Concrete and Its Quantification. J. Build. Eng. 2022, 52, 104474. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, P.; Parashar, A.K. Use of Waste Glass and Demolished Brick as Coarse Aggregate in Production of Sustainable Concrete. Mater. Today Proc. 2022, 62, 4030–4035. [Google Scholar] [CrossRef]
- Kumar Parashar, A.; Sharma, P.; Sharma, N. A Study of GGBS Based Cement Concrete with the Inclusion of Waste Foundry Sand on Mechanical Properties. Mater. Today Proc. 2022, 62, 4134–4139. [Google Scholar] [CrossRef]
- Jha, P.; Sachan, A.K.; Singh, R.P. Investigation on the Durability of Concrete with Partial Replacement of Cement by Bagasse Ash (BAH) and Sand by Stone Dust (SDT). Mater. Today Proc. 2020, 43, 237–243. [Google Scholar] [CrossRef]
- Jha, P.; Sachan, A.K.; Singh, R.P. Agro-Waste Sugarcane Bagasse Ash (ScBA) as Partial Replacement of Binder Material in Concrete. Mater. Today Proc. 2021, 44, 419–427. [Google Scholar] [CrossRef]
- Liang, G.; Liu, T.; Li, H.; Wu, K. Shrinkage Mitigation, Strength Enhancement and Microstructure Improvement of Alkali-Activated Slag/Fly Ash Binders by Ultrafine Waste Concrete Powder. Compos. Part B Eng. 2022, 231, 109570. [Google Scholar] [CrossRef]
- Huseien, G.F.; Ismail, M.; Khalid, N.H.A.; Hussin, M.W.; Mirza, J. Compressive Strength and Microstructure of Assorted Wastes Incorporated Geopolymer Mortars: Effect of Solution Molarity. Alex. Eng. J. 2018, 57, 3375–3386. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, G.; Liang, K. Development of Fly Ash and Slag Based High-Strength Alkali-Activated Foam Concrete. Cem. Concr. Compos. 2022, 128, 104447. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Goodier, C.I.; Austin, S.A. Factors Affecting the Slump and Strength Development of Geopolymer Concrete. Constr. Build. Mater. 2020, 261, 119945. [Google Scholar] [CrossRef]
- Elyamany, H.E.; Abd Elmoaty, A.E.M.; Elshaboury, A.M. Setting Time and 7-Day Strength of Geopolymer Mortar with Various Binders. Constr. Build. Mater. 2018, 187, 974–983. [Google Scholar] [CrossRef]
- Alomayri, T.; Adesina, A.; Das, S. Influence of Amorphous Raw Rice Husk Ash as Precursor and Curing Condition on the Performance of Alkali Activated Concrete. Case Stud. Constr. Mater. 2021, 15, e00777. [Google Scholar] [CrossRef]
- Jittin, V.; Madhuri, P.; Santhanam, M.; Bahurudeen, A. Influence of Preconditioning and Curing Methods on the Durability Performance of Alkali-Activated Binder Composites. Constr. Build. Mater. 2021, 311, 125346. [Google Scholar] [CrossRef]
- Hwang, C.L.; Huynh, T.P. Effect of Alkali-Activator and Rice Husk Ash Content on Strength Development of Fly Ash and Residual Rice Husk Ash-Based Geopolymers. Constr. Build. Mater. 2015, 101, 1–9. [Google Scholar] [CrossRef]
- Yurt, Ü.; Bekar, F. Comparative Study of Hazelnut-Shell Biomass Ash and Metakaolin to Improve the Performance of Alkali-Activated Concrete: A Sustainable Greener Alternative. Constr. Build. Mater. 2022, 320, 126230. [Google Scholar] [CrossRef]
- Marvila, M.T.; de Azevedo, A.R.G.; Vieira, C.M.F. Reaction Mechanisms of Alkali-Activated Materials. Rev. IBRACON Estrut. Mater. 2021, 14, 1–26. [Google Scholar] [CrossRef]
- Ram, S.; Tare, M.S.; Aswath, P.B.; Ralegaonkar, R.V. Potential of Co-Fired Fly Ashes as a Construction Material—A Review; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; ISBN 9780128035818. [Google Scholar]
- Madurwar, M.; Sakhare, V.; Ralegaonkar, R. Suitability and Sustainability of Sugarcane Bagasse Ash Bricks. Proc. Inst. Civ. Eng. Eng. Sustain. 2018, 171, 115–122. [Google Scholar] [CrossRef]
- Saini, G.; Vattipalli, U. Assessing Properties of Alkali Activated GGBS Based Self-Compacting Geopolymer Concrete Using Nano-Silica. Case Stud. Constr. Mater. 2020, 12, e00352. [Google Scholar] [CrossRef]
- Yaswanth, K.K.; Revathy, J.; Gajalakshmi, P. Strength, Durability and Micro-Structural Assessment of Slag-Agro Blended Based Alkali Activated Engineered Geopolymer Composites. Case Stud. Constr. Mater. 2022, 16, e00920. [Google Scholar] [CrossRef]
- Sajjad, U.; Sheikh, M.N.; Hadi, M.N.S. Incorporation of Graphene in Slag-Fly Ash-Based Alkali-Activated Concrete. Constr. Build. Mater. 2022, 322, 126417. [Google Scholar] [CrossRef]
- Ghafoor, M.T.; Khan, Q.S.; Qazi, A.U.; Sheikh, M.N.; Hadi, M.N.S. Influence of Alkaline Activators on the Mechanical Properties of Fly Ash Based Geopolymer Concrete Cured at Ambient Temperature. Constr. Build. Mater. 2021, 273, 121752. [Google Scholar] [CrossRef]
- Luan, C.; Shi, X.; Zhang, K.; Utashev, N.; Yang, F.; Dai, J.; Wang, Q. A Mix Design Method of Fly Ash Geopolymer Concrete Based on Factors Analysis. Constr. Build. Mater. 2021, 272, 121612. [Google Scholar] [CrossRef]
- Padmakar, M.; Barhmaiah, B.; Priyanka, M.L. Characteristic Compressive Strength of a Geo Polymer Concrete. Mater. Today Proc. 2020, 37, 2219–2222. [Google Scholar] [CrossRef]
- Parihar, H.S.; Verma, M. Analysis on Morality of Polymer Concrete for Enhancing Ambient Temperature. Mater. Today Proc. 2021, 45, 3312–3317. [Google Scholar] [CrossRef]
- Malkawi, A.B.; Habib, M.; Alzubi, Y.; Aladwan, J. Engineering Properties of Lightweight Geopolymer Concrete Using Palm Oil Clinker Aggregate. Int. J. GEOMATE 2020, 18, 132–139. [Google Scholar] [CrossRef]
- Kumar, M.L.; Revathi, V. Microstructural Properties of Alkali-Activated Metakaolin and Bottom Ash Geopolymer. Arab. J. Sci. Eng. 2020, 45, 4235–4246. [Google Scholar] [CrossRef]
- Sheen, Y.N.; Le, D.H. Innovative Use of Sugarcane Bagasse Ash in Green Alkali-Activated Slag Material: Effects of Activator Concentration on the Blended Pastes. Sugar Tech 2022, 24, 1037–1051. [Google Scholar] [CrossRef]
- Reddy, R.S.R.; Anand Sagar, B. Experimental Study on Mechanical and Durability Properties of Recycled Aggregate Based Geo-Polymer Concrete. Mater. Today Proc. 2022, 52, 649–654. [Google Scholar] [CrossRef]
- Nikolić, I.; Karanović, L.; Častvan, I.J.; Radmilović, V.; Mentus, S.; Radmilović, V. Improved Compressive Strength of Alkali Activated Slag upon Heating. Mater. Lett. 2014, 133, 251–254. [Google Scholar] [CrossRef]
- Mathew, G.; Issac, B.M. Effect of Molarity of Sodium Hydroxide on the Aluminosilicate Content in Laterite Aggregate of Laterised Geopolymer Concrete. J. Build. Eng. 2020, 32, 101486. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. The Effects of Ground Granulated Blast-Furnace Slag Blending with Fly Ash and Activator Content on the Workability and Strength Properties of Geopolymer Concrete Cured at Ambient Temperature. Mater. Des. 2014, 62, 32–39. [Google Scholar] [CrossRef]
- Gomaa, E.; Sargon, S.; Kashosi, C.; Gheni, A.; ElGawady, M.A. Mechanical Properties of High Early Strength Class C Fly Ash-Based Alkali Activated Concrete. Transp. Res. Rec. 2020, 2674, 430–443. [Google Scholar] [CrossRef]
- Aydin, S.; Baradan, B. Effect of Activator Type and Content on Properties of Alkali-Activated Slag Mortars. Compos. Part B Eng. 2014, 57, 166–172. [Google Scholar] [CrossRef]
- Parashar, A.K.; Sharma, P.; Sharma, N. An Investigation on Properties of Concrete with the Adding of Waste of Ceramic and Micro Silica. Mater. Today Proc. 2022, 62, 4036–4040. [Google Scholar] [CrossRef]
- Hadi, M.N.S.; Farhan, N.A.; Sheikh, M.N. Design of Geopolymer Concrete with GGBFS at Ambient Curing Condition Using Taguchi Method. Constr. Build. Mater. 2017, 140, 424–431. [Google Scholar] [CrossRef]
- Rachmansyah; Hardjasaputra, H.; Cornelia, M. Experimental Study of Effect Additional Water on High Performance Geopolymer Concrete. MATEC Web Conf. 2019, 270, 01004. [Google Scholar] [CrossRef]
- Niş, A. Compressive Strength Variation of Alkali Activated Fly Ash/Slag Concrete with Different NaOH Concentrations and Sodium Silicate to Sodium Hydroxide Ratios. J. Sustain. Constr. Mater. Technol. 2019, 4, 351–360. [Google Scholar] [CrossRef]
- Murugesan, T.; Vidjeapriya, R.; Bahurudeen, A. Development of Sustainable Alkali Activated Binder for Construction Using Sugarcane Bagasse Ash and Marble Waste. Sugar Tech 2020, 22, 885–895. [Google Scholar] [CrossRef]
- Das, S.K.; Shrivastava, S. Influence of Molarity and Alkali Mixture Ratio on Ambient Temperature Cured Waste Cement Concrete Based Geopolymer Mortar. Constr. Build. Mater. 2021, 301, 124380. [Google Scholar] [CrossRef]
- Rekha, Y.; Suriya, S.; Hamedul Irshad, H.M. Comparative Study on Oven Curing of Geo-Polymer Concrete over Conventional Concrete. Mater. Today Proc. 2021, 55, 462–469. [Google Scholar] [CrossRef]
- Gómez-Casero, M.A.; Pérez-Villarejo, L.; Castro, E.; Eliche-Quesada, D. Effect of Steel Slag and Curing Temperature on the Improvement in Technological Properties of Biomass Bottom Ash Based Alkali-Activated Materials. Constr. Build. Mater. 2021, 302, 124205. [Google Scholar] [CrossRef]
- Fu, Q.; Xu, W.; Zhao, X.; Bu, M.X.; Yuan, Q.; Niu, D. The Microstructure and Durability of Fly Ash-Based Geopolymer Concrete: A Review. Ceram. Int. 2021, 47, 29550–29566. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Sun, B.; Ye, G.; de Schutter, G. A Review: Reaction Mechanism and Strength of Slag and Fly Ash-Based Alkali-Activated Materials. Constr. Build. Mater. 2022, 326, 126843. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, L.; Li, Y.; Zha, J.; Tian, G.; Wang, F.; Zhang, H.; Liang, J. Synthesis of Zeolites by In-Situ Conversion of Geopolymers and Their Performance of Heavy Metal Ion Removal in Wastewater:A Review. J. Clean. Prod. 2022, 349, 131441. [Google Scholar] [CrossRef]
- Candamano, S.; Policicchio, A.; Conte, G.; Abarca, R.; Algieri, C.; Chakraborty, S.; Curcio, S.; Calabro, V.; Crea, F.; Agostino, R.G. Preparation of Foamed and Unfoamed Geopolymer/NaX Zeolite/Activated Carbon Composites for CO2 Adsorption. J. Clean. Prod. 2022, 330, 129843. [Google Scholar] [CrossRef]
- Duxson, P.; Mallicoat, S.W.; Lukey, G.C.; Kriven, W.M.; van Deventer, J.S.J. The Effect of Alkali and Si/Al Ratio on the Development of Mechanical Properties of Metakaolin-Based Geopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 8–20. [Google Scholar] [CrossRef]
- Candamano, S.; Crea, F.; Iorfida, A. Mechanical Characterization of Basalt Fabric-Reinforced Alkali-Activated Matrix Composite: A Preliminary Investigation. Appl. Sci. 2020, 10, 2865. [Google Scholar] [CrossRef]
- Angulo-Ramírez, D.E.; Mejía de Gutiérrez, R.; Puertas, F. Alkali-Activated Portland Blast-Furnace Slag Cement: Mechanical Properties and Hydration. Constr. Build. Mater. 2017, 140, 119–128. [Google Scholar] [CrossRef]
- Karim, M.R.; Zain, M.F.M.; Jamil, M.; Lai, F.C. Fabrication of a Non-Cement Binder Using Slag, Palm Oil Fuel Ash and Rice Husk Ash with Sodium Hydroxide. Constr. Build. Mater. 2013, 49, 894–902. [Google Scholar] [CrossRef]
- Mounika, G.; Ramesh, B.; Kalyana Rama, J.S. Experimental Investigation on Physical and Mechanical Properties of Alkali Activated Concrete Using Industrial and Agro Waste. Mater. Today Proc. 2020, 33, 4372–4376. [Google Scholar] [CrossRef]
- Nodehi, M.; Ozbakkaloglu, T.; Gholampour, A.; Mohammed, T.; Shi, X. The Effect of Curing Regimes on Physico-Mechanical, Microstructural and Durability Properties of Alkali-Activated Materials: A Review. Constr. Build. Mater. 2022, 321, 126335. [Google Scholar] [CrossRef]
- Chithambaram, S.J.; Kumar, S.; Prasad, M.M. Thermo-Mechanical Characteristics of Geopolymer Mortar. Constr. Build. Mater. 2019, 213, 100–108. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Rao, R.; Wang, C.; Fang, C. Effects of Combined Usage of GGBS and Fly Ash on Workability and Mechanical Properties of Alkali Activated Geopolymer Concrete with Recycled Aggregate. Compos. Part B Eng. 2019, 164, 179–190. [Google Scholar] [CrossRef]
- Kishore, K.; Gupta, N. Mechanical Characterization and Assessment of Composite Geopolymer Concrete. Mater. Today Proc. 2021, 44, 58–62. [Google Scholar] [CrossRef]
- Wu, Y.H.; Huang, R.; Tsai, C.J.; Lin, W.T. Utilizing Residues of CFB Co-Combustion of Coal, Sludge and TDF as an Alkali Activator in Eco-Binder. Constr. Build. Mater. 2015, 80, 69–75. [Google Scholar] [CrossRef]
- Thunuguntla, C.S.; Gunneswara Rao, T.D. Effect of Mix Design Parameters on Mechanical and Durability Properties of Alkali Activated Slag Concrete. Constr. Build. Mater. 2018, 193, 173–188. [Google Scholar] [CrossRef]
- Babu, D.V. Assessing the Performance of Molarity and Alkaline Activator Ratio on Engineering Properties of Self-Compacting Alkaline Activated Concrete at Ambient Temperature. J. Build. Eng. 2018, 20, 137–155. [Google Scholar] [CrossRef]
- Bernardo, E.; Elsayed, H.; Mazzi, A.; Tameni, G.; Gazzo, S.; Contrafatto, L. Double-Life Sustainable Construction Materials from Alkali Activation of Volcanic Ash/Discarded Glass Mixture. Constr. Build. Mater. 2022, 359, 129540. [Google Scholar] [CrossRef]
- Thomas, R.J.; Peethamparan, S. Alkali-Activated Concrete: Engineering Properties and Stress-Strain Behavior. Constr. Build. Mater. 2015, 93, 49–56. [Google Scholar] [CrossRef]
- Cai, R.; Tian, Z.; Ye, H. Durability Characteristics and Quantification of Ultra-High Strength Alkali-Activated Concrete. Cem. Concr. Compos. 2022, 134, 104743. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Zhang, Z.; Ou, Z. Durability of Alkali-Activated Materials in Aggressive Environments: A Review on Recent Studies. Constr. Build. Mater. 2017, 152, 598–613. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Cheng, Y.; Huseien, G.F.; Shah, K.W. Shrinkage Mechanisms and Shrinkage-Mitigating Strategies of Alkali-Activated Slag Composites: A Critical Review. Constr. Build. Mater. 2022, 318, 125993. [Google Scholar] [CrossRef]
- Mastali, M.; Kinnunen, P.; Dalvand, A.; Mohammadi Firouz, R.; Illikainen, M. Drying Shrinkage in Alkali-Activated Binders—A Critical Review. Constr. Build. Mater. 2018, 190, 533–550. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Candamano, S.; Crea, F.; Gazzaniga, G.; Pastore, T. The Combined Use of Admixtures for Shrinkage Reduction in One-Part Alkali Activated Slag-Based Mortars and Pastes. Constr. Build. Mater. 2020, 248, 118682. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Shrinkage Mitigation Strategies in Alkali-Activated Slag. Cem. Concr. Res. 2017, 101, 131–143. [Google Scholar] [CrossRef]
- Zhang, W.; Xue, M.; Lin, H.; Duan, X.; Jin, Y.; Su, F. Effect of Polyether Shrinkage Reducing Admixture on the Drying Shrinkage Properties of Alkali-Activated Slag. Cem. Concr. Compos. 2023, 136, 104865. [Google Scholar] [CrossRef]
- Thomas, R.J.; Lezama, D.; Peethamparan, S. On Drying Shrinkage in Alkali-Activated Concrete: Improving Dimensional Stability by Aging or Heat-Curing. Cem. Concr. Res. 2017, 91, 13–23. [Google Scholar] [CrossRef]
- Hojati, M.; Rajabipour, F.; Radlińska, A. Creep of Alkali-Activated Cement Mixtures. Case Stud. Constr. Mater. 2022, 16, e00954. [Google Scholar] [CrossRef]
- Chi, M. Effects of the Alkaline Solution/Binder Ratio and Curing Condition on the Mechanical Properties of Alkali-Activated Fly Ash Mortars. Sci. Eng. Compos. Mater. 2017, 24, 773–782. [Google Scholar] [CrossRef]
- Yuan, X.H.; Chen, W.; Lu, Z.A.; Chen, H. Shrinkage Compensation of Alkali-Activated Slag Concrete and Microstructural Analysis. Constr. Build. Mater. 2014, 66, 422–428. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W. Influence of Partial Replacement of Fly Ash by Metakaolin on Mechanical Properties and Microstructure of Fly Ash Geopolymer Paste Exposed to Sulfate Attack. Ceram. Int. 2016, 42, 3504–3517. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Abdollahnejad, Z.; Camões, A.F.; Jamshidi, M.; Ding, Y. Durability of Alkali-Activated Binders: A Clear Advantage over Portland Cement or an Unproven Issue? Constr. Build. Mater. 2012, 30, 400–405. [Google Scholar] [CrossRef]
- Raju, T.; Ramaswamy, K.P.; Saraswathy, B. A Review on the Effects of Chemical Admixtures on Alkali Activated Concrete. Mater. Today Proc. 2022, 65, 846–851. [Google Scholar] [CrossRef]
- Tong, S.; Yuqi, Z.; Qiang, W. Recent Advances in Chemical Admixtures for Improving the Workability of Alkali-Activated Slag-Based Material Systems. Constr. Build. Mater. 2021, 272, 121647. [Google Scholar] [CrossRef]
- Al Makhadmeh, W.; Soliman, A. On the Mechanisms of Shrinkage Reducing Admixture in Alkali Activated Slag Binders. J. Build. Eng. 2022, 56, 104812. [Google Scholar] [CrossRef]
- Aredes, F.G.M.; Campos, T.M.B.; MacHado, J.P.B.; Sakane, K.K.; Thim, G.P.; Brunelli, D.D. Effect of Cure Temperature on the Formation of Metakaolinite-Based Geopolymer. Ceram. Int. 2015, 41, 7302–7311. [Google Scholar] [CrossRef]
- Mo, B.H.; Zhu, H.; Cui, X.M.; He, Y.; Gong, S.Y. Effect of Curing Temperature on Geopolymerization of Metakaolin-Based Geopolymers. Appl. Clay Sci. 2014, 99, 144–148. [Google Scholar] [CrossRef]
- Loganayagan, S.; Bhagavath, B.A.; Kalaiyarasan, U.; Arasan, E. Experimental Investigation on Characteristics of Fly-Ash Based Geo-Polymer Mixed Concrete. Mater. Today Proc. 2021, 45, 1559–1562. [Google Scholar] [CrossRef]
- Habert, G.; Ouellet-Plamondon, C. Recent Update on the Environmental Impact of Geopolymers. RILEM Tech. Lett. 2016, 1, 17. [Google Scholar] [CrossRef]
- Chippagiri, R.; Bras, A.; Sharma, D.; Ralegaonkar, R.V. Technological and Sustainable Perception on the Advancements of Prefabrication in Construction Industry. Energies 2022, 15, 7548. [Google Scholar] [CrossRef]
- Gevaudan, J.P.; Srubar, W.V., III. Energy Performance of Alkali-Activated Cement-Based Concrete Buildings. In AEI 2017; American Society of Civil Engineers; Reston, VR, USA, 2017; pp. 311–323. [Google Scholar]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer Foam Concrete: An Emerging Material for Sustainable Construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Ligero, C. Development of Sustainable, Innovative and Energy-Efficient Concrete, Based on the Integration of All-Waste Materials: SUS-CON Panels for Building Applications. In Proceedings of the 9th International Concrete Conference, Dundee, UK, 4–6 July 2016. [Google Scholar]
- Turner, L.K.; Collins, F.G. Carbon Dioxide Equivalent (CO2-e) Emissions: A Comparison between Geopolymer and OPC Cement Concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Meshram, R.B.; Kumar, S. Comparative Life Cycle Assessment (LCA) of Geopolymer Cement Manufacturing with Portland Cement in Indian Context. Int. J. Environ. Sci. Technol. 2022, 19, 4791–4802. [Google Scholar] [CrossRef]
- Van Den Heede, P.; De Belie, N. Environmental Impact and Life Cycle Assessment (LCA) of Traditional and “green” Concretes: Literature Review and Theoretical Calculations. Cem. Concr. Compos. 2012, 34, 431–442. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.; Mejía-Arcila, J.; Mejía de Gutiérrez, R.; Martínez, E. Life Cycle Assessment (LCA) of an Alkali-Activated Binary Concrete Based on Natural Volcanic Pozzolan: A Comparative Analysis to OPC Concrete. Constr. Build. Mater. 2018, 176, 103–111. [Google Scholar] [CrossRef]
- Nikoloutsopoulos, N.; Sotiropoulou, A.; Kakali, G.; Tsivilis, S. Physical and Mechanical Properties of Fly Ash Based Geopolymer Concrete Compared to Conventional Concrete. Buildings 2021, 11, 178. [Google Scholar] [CrossRef]
- Wang, A.; Zheng, Y.; Zhang, Z.; Liu, K.; Li, Y.; Shi, L.; Sun, D. The Durability of Alkali-Activated Materials in Comparison with Ordinary Portland Cements and Concretes: A Review. Engineering 2020, 6, 695–706. [Google Scholar] [CrossRef]



| Agro-Industrial Waste Materials | Binder | Substitution Percentage | Compressive Strength (MPa) | Remarks | Source |
|---|---|---|---|---|---|
| Marble Powder waste (MP) + Calcined Bentonite (CB) | MP + Cement | 5–20% Marble Powder waste + 5–20% Calcined Bentonite | 40 | Water absorption increases with in percentage of marble powder and calcined bentonite, 15–20% MP as a partial substitution of cement, and 15–20% of CB as partial substitution of fine aggregate is feasible | [41] |
| RHA+SBA | SBA+ RHA+ Cement | 10–30% SBA + 5–15% RHA | 67 | 10% SBA with 5% RHA substitution gave the desired results | [9] |
| Calcined Clay + Limestone Powder | Calcined Clay + Limestone Powder + Cement | 50–80% Calcined Clay + Limestone Powder | 54 | 50–70% substitution is recommended to increase the toughness of cementitious materials, but there was no effect on the compressive strength | [44] |
| Dolomite powder waste (DP) | DP + Cement | 0–20% | 56 | Flexural strength and compressive strength increased, and water absorption rate and drying shrinkage decreased with 10% substitution of DP | [45] |
| Waste foundry sand | Cement | 0–50% | 29.06 | 20% substitution of foundry sand offered improved strength compared to that of control mix | [49] |
| Crushed water glass aggregate (WGA) + demolished bricks (DB) | Cement | 0%, 25%, 50%, and 100% | 26 | Workability of concrete decreases with increasing amounts of WGA and demolished brick as substitution of coarse aggregate, 50% replacement of WGA and DB achieved desired results for hardened properties | [50] |
| Ground granulated blast furnace slag (GGBS) + waste foundry sand | GGBS + Cement | 5–20% GGBS + 10–40% WFS | 58 | M30 grade concrete with 15% GGBS as partial substitution for cement plus 30% waste foundry sand as partial substitution for sand gave satisfactory results | [51] |
| Sugarcane bagasse ash (SBA) + Stone Dust (SD) | SBA+ Cement | 10% SBA + 0–50% SD | - | M25 grade concrete prepared with 10% substitution of SBA as cementitious material and 40% substitution of SD as a filler material showed a better resistance to both hydrochloric and sulphuric acids attack | [52] |
| Sugarcane bagasse ash (SBA) | SBA+ Cement | 0-25% | 35 | Compressive strength improved at 10% substitution of SBA for M25 grade concrete | [53] |
| Element (%) | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | TiO2 | P2O5 | SO3 | LOI | Source |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | 53 | 30.6 | 3.8 | 4.6 | 1.3 | 0.5 | 1.4 | 1.1 | - | - | 2.3 | [54] |
| 57.2 | 28.8 | 3.67 | 5.16 | 1.48 | 0.08 | 0.94 | - | - | 0.1 | 0.12 | [55] | |
| 49.46 | 34.53 | 6.34 | 3.57 | 0.52 | 0.42 | 1.38 | 1.92 | 0.4 | 1.14 | 6.1 | [56] | |
| GGBS | 30.8 | 10.9 | 0.64 | 51.8 | 4.57 | 0.45 | 0.36 | - | - | 0.06 | 0.22 | [55] |
| 36.5 | 11.6 | 0.43 | 39.46 | 8.14 | 0.39 | 0.68 | 0.84 | 0.15 | 0.73 | 0.03 | [57] | |
| SF | 96.81 | 0.25 | 0.45 | 0.16 | 0.26 | 0.14 | 0.28 | - | - | 0.14 | 1.3 | [58] |
| SBA | 71.4 | 3.39 | 3.51 | 6.74 | - | - | 0.74 | - | - | 2.44 | - | [9] |
| RHA | 96.7 | 0.3 | 1.26 | 0.57 | 0.27 | 0.05 | 0.45 | 0.02 | 0.31 | - | - | [59] |
| 85.79 | 0.67 | 0.97 | 2.94 | - | - | 4.65 | - | - | 2.89 | - | [60] | |
| 95.6 | - | 0.24 | 0.7 | - | - | 2.666 | 0.02 | 0.52 | 0.15 | - | [61] | |
| WBG | 70.57 | 2.48 | 0.28 | 5.59 | 3.05 | 14.49 | 1.35 | - | - | 0.19 | 0.95 | [55] |
| WC | 72.8 | 12.2 | 0.54 | 0.01 | 1 | 13.5 | - | - | - | - | - | [55] |
| MK | 64.86 | 20.56 | 0.31 | 1.24 | 0.22 | 0.11 | 0.5 | 0.37 | 1.01 | 10.82 | [62] | |
| HSA | 12.36 | 3.83 | 5.34 | 48.52 | 4.67 | 2.17 | 16.42 | - | - | 0.4 | 6.29 | [62] |
| MP | 0.51 | 0.13 | 0.06 | 56.24 | 0.1 | 0.45 | 0.01 | - | - | 0.01 | 42.49 | [41] |
| CB | 63.04 | 17.15 | 3.17 | 1.93 | 4.12 | 2.64 | 1.47 | - | - | 0.38 | - | [41] |
| Precursors | Molarity of NaOH | Na2SiO3/NaOH | Curing Condition | Compressive Strength (MPa) | Tensile Strength (Mpa) | Flexural Strength (Mpa) | Remark | Source |
|---|---|---|---|---|---|---|---|---|
| WBG, FA, GBFS, and waste ceramic | 2 M–16 M | 3 | Ambient curing | 54 | - | - | - | [55] |
| Blast Furnace Slag (BFS) + RHA | 12 M | 2 | Ambient curing Thermal Curing | 55 58 | - - | 6.9 7 | - | [59] |
| GGBS+FA | 16 M | 2.5 | - | 71 | 4.8 | 7.1 | - | [66] |
| RHA, GGBS, M-Sand, and Copper Slag | 8 M | 1:2.5 | Ambient | 31.1 | 1.95 | - | Inclusion of copper slag of up to 40% is beneficial in chloride aggressive environment | [67] |
| GGBS+FA | 14 M | 1:2.5 | Ambient temperature (27 ± 2 °C) | 54 | 3.8 | 4.9 | Addition of 0.5% of graphene can improve the compressive strength | [68] |
| FA | 8 M–16 M | - | Oven curing | 21.5 | - | 5 | Mechanical properties increased with increase in molarity and decreased with higher Na2SiO3/NaOH ratio | [69] |
| FA | 11.5–13.5 M | - | Ambient | 92.86 | - | - | Strength decreases linearly with the increase in water to alkali activators ratio | [70] |
| GGBS+SF | 13 M | - | - | 32.4 | - | - | M25 grade concrete prepared with 13 Molar NaOH, 40% Na2SiO3 | [71] |
| GGBS+FA (40:60) | 8 M | 1.5 | Ambient temperature (27 ± 2 °C) | 60 | - | - | - | [72] |
| FA | 14 M | 2 | 23 ± 2 °C and Relative humidity 80 ± 5% | 60 | - | - | Replaced conventional aggregate by palm oil clinker aggregate | [73] |
| Metakaolin + Bottom Ash | 8 M | 2 | Ambient curing | 58.95 | 5.96 | 6.94 | - | [74] |
| Material | Molarity | Curing Condition | Compressive Strength (MPa) | Source |
|---|---|---|---|---|
| SCBA + GGBS | 6M | Ambient | 68.22 | [86] |
| SCBA + GGBS | 8M | Ambient | 71.21 | [86] |
| SCBA + GGBS | 10M | Ambient | 76.17 | [86] |
| SCBA + GGBS | 6M | Heat- 65 °C (24 h) | 48.93 | [86] |
| SCBA + GGBS | 8M | Heat- 65 °C (24 h) | 61.04 | [86] |
| SCBA + GGBS | 10M | Heat- 65 °C (24 h) | 61.78 | [86] |
| GGBS + RHA | 10M | Ambient | 60 | [99] |
| FA + GGBS + RHA | 10M | Ambient | 56 | [99] |
| FA + GGBS + RHA | - | Steam curing | 37.2 | [126] |
| Materials | Energy (GJ/t) | Emission (t-CO2/t) |
|---|---|---|
| OPC | 4.53 | 0.84 |
| FA | 0.033 | 0.004 |
| MK | 2.5 | 0.33 |
| GGBS | 0.857 | 0.052 |
| SF | 0.036 | 0.014 |
| Aggregates | 0.083 | 0.0048 |
| Parameters | Remarks | Source |
|---|---|---|
| Setting Time | AAC exhibits lower setting time as compared to that of OPC concrete | [122,123] |
| Compressive Strength | Higher compressive strength achieved for AAC as compared to that of OPC concrete | [135] |
| Tensile Strength | Similar strength was achieved as compared to that of OPC concrete | [136] |
| Flexural Strength | Higher strength was observed as compared to that of OPC | [136] |
| Durability | AAC was found to be more durable than OPC concrete was (depends on raw materials used) | [137], [121] |
| Environmental Impact | ||
| CO2 Emission | 44.7% less emissions than OPC | [135] |
| Energy | 43% less energy consumption than OPC | [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanjewar, B.A.; Chippagiri, R.; Dakwale, V.A.; Ralegaonkar, R.V. Application of Alkali-Activated Sustainable Materials: A Step towards Net Zero Binder. Energies 2023, 16, 969. https://doi.org/10.3390/en16020969
Lanjewar BA, Chippagiri R, Dakwale VA, Ralegaonkar RV. Application of Alkali-Activated Sustainable Materials: A Step towards Net Zero Binder. Energies. 2023; 16(2):969. https://doi.org/10.3390/en16020969
Chicago/Turabian StyleLanjewar, Bhagyashri A., Ravijanya Chippagiri, Vaidehi A. Dakwale, and Rahul V. Ralegaonkar. 2023. "Application of Alkali-Activated Sustainable Materials: A Step towards Net Zero Binder" Energies 16, no. 2: 969. https://doi.org/10.3390/en16020969
APA StyleLanjewar, B. A., Chippagiri, R., Dakwale, V. A., & Ralegaonkar, R. V. (2023). Application of Alkali-Activated Sustainable Materials: A Step towards Net Zero Binder. Energies, 16(2), 969. https://doi.org/10.3390/en16020969






