The Effects of Demineralization on Reducing Ash Content in Corn and Soy Biomass with the Goal of Increasing Biofuel Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Shredding
2.2. Ash Content
2.3. Demineralization
2.4. Micro and Macro Element
3. Results and Discussion
3.1. Ash Content
3.1.1. The Influence of Sieving
3.1.2. The Influence of Demineralization
3.2. Micro and Macro Elements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Demirbaş, A. Demineralization of Agricultural Residues by Water Leaching. Energy Sources 2003, 25, 679–687. [Google Scholar] [CrossRef]
- Šafran, B.; Jug, M.; Radmanović, K.; Hasan, M.; Kristijan, A.; Vučković, K.; Stjepan, R. Contribution to the research on wood pellet characteristics from Turopolje area. Šumarski List 2018, 142, 149–158. [Google Scholar] [CrossRef]
- Khan, A.; de Jong, W.; Jansens, P.; Spliethoff, H. Biomass combustion in fluidized bed boilers: Potential problems and remedies. Fuel Process. Technol. 2009, 90, 21–50. [Google Scholar] [CrossRef]
- Abreu, P.; Casaca, C.; Costa, M. Ash deposition during the co-firing of bituminous coal with pine sawdust and olive stones in a laboratory furnace. Fuel 2010, 89, 4040–4048. [Google Scholar] [CrossRef]
- Liu, X.; Bi, X.T. Removal of inorganic constituents from pine barks and switchgrass. Fuel Process. Technol. 2011, 92, 1273–1279. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Heracleous, E.; Patiaka, D.T.; Kalogiannis, K.G.; Michailof, C.M.; Lappas, A.A. Optimization of bio-oil yields by demineralization of low quality biomass. Biomass Bioenergy 2015, 83, 105–115. [Google Scholar] [CrossRef]
- Alcazar-Ruiz, A.; Dorado, F.; Sanchez-Silva, L. Influence of Temperature and Residence Time on Torrefaction Coupled to Fast Pyrolysis for Valorizing Agricultural Waste. Energies 2022, 15, 7914. [Google Scholar] [CrossRef]
- Jie, C.; Zhen, X.; Wu, J.H. Research Progress on Biomass Pyrolysis Kinetics. Adv. Mater. Res. 2013, 805–806, 240–246. [Google Scholar] [CrossRef]
- Balat, M. Mechanisms of Thermochemical Biomass Conversion Processes. Part 3: Reactions of Liquefaction. Energy Sources Part A Recover. Util. Environ. Eff. 2008, 30, 649–659. [Google Scholar] [CrossRef]
- Li, G.; Hse, C.; Qin, T. Wood liquefaction with phenol by microwave heating and FTIR evaluation. J. For. Res. 2015, 26, 1043–1048. [Google Scholar] [CrossRef]
- Antonović, A.; Jambreković, V.; Jaroslav, K.; Španić, N.; Medved, S. Influence of Urea-Formaldehyde Resin Modification with Liquefied Wood on Particleboard Properties. Drv. Ind. 2010, 61, 5–14. [Google Scholar]
- Jovičić, N.; Antonović, A.; Matin, A.; Antolović, S.; Kalambura, S.; Krička, T. Biomass Valorization of Walnut Shell for Liquefaction Efficiency. Energies 2022, 15, 495. [Google Scholar] [CrossRef]
- Trubetskaya, A. Reactivity Effects of Inorganic Content in Biomass Gasification: A Review. Energies 2022, 15, 3137. [Google Scholar] [CrossRef]
- Guo, Q.; Cheng, Z.; Chen, G.; Yan, B.; Li, J.; Hou, L.; Ronsse, F. Assessment of biomass demineralization on gasification: From experimental investigation, mechanism to potential application. Sci. Total. Environ. 2020, 726, 138634. [Google Scholar] [CrossRef] [PubMed]
- Banks, S.; Nowakowski, D.; Bridgwater, A. Fast pyrolysis processing of surfactant washed Miscanthus. Fuel Process. Technol. 2014, 128, 94–103. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, S.; Sun, L.-S.; Su, S.; Xu, K.; He, L.-M.; Xiang, J. Influence of different demineralization treatments on physicochemical structure and thermal degradation of biomass. Bioresour. Technol. 2013, 146, 254–260. [Google Scholar] [CrossRef]
- Pattiya, A.; Chaow-U-Thai, A.; Rittidech, S. The Influence of Pretreatment Techniques on Ash Content of Cassava Residues. Int. J. Green Energy 2013, 10, 544–552. [Google Scholar] [CrossRef]
- Li, Y.; Deng, B.; Hou, Y.; Wang, S.; Zeng, F.; Luo, Y.; Ge, J.; Yao, S. Dissolution kinetics of calcium ions in hydrothermal demineralization of eucalyptus. Bioresources 2022, 17, 2849–2863. [Google Scholar] [CrossRef]
- Ge, J.; Wu, Y.; Han, Y.; Qin, C.; Nie, S.; Liu, S.; Wang, S.; Yao, S. Effect of hydrothermal pretreatment on the demineralization and thermal degradation behavior of eucalyptus. Bioresour. Technol. 2020, 307, 123246. [Google Scholar] [CrossRef]
- Li, W.Y.; Zhang, Z.B.; Zhao, L.Q.; Lu, Q. Thermogravimetric Analysis of Raw and Demineralized Biomass Materials. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2013. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, S.; Ding, K.; Zhu, S.; Zhang, H.; Liu, X. Pyrolysis behavior of raw/torrefied rice straw after different demineralization processes. Biomass Bioenergy 2018, 119, 229–236. [Google Scholar] [CrossRef]
- Eom, I.-Y.; Kim, J.-Y.; Lee, S.-M.; Cho, T.-S.; Yeo, H.; Choi, J.-W. Comparison of pyrolytic products produced from inorganic-rich and demineralized rice straw (Oryza sativa L.) by fluidized bed pyrolyzer for future biorefinery approach. Bioresour. Technol. 2013, 128, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Lateef, H.U.; Kazmi, M.; Tabish, A.N.; Cheema, I.I.; Rashid, M.I. Effect of demineralization on physiochemical and thermal characteristics of wheat straw. Energy Sources Part A Recover. Util. Environ. Eff. 2020. [Google Scholar] [CrossRef]
- Namkung, H.; Park, J.-H.; Lee, Y.-J.; Song, G.-S.; Choi, J.W.; Park, S.-J.; Kim, S.; Liu, J.; Choi, Y.-C. Performance evaluation of biomass pretreated by demineralization and torrefaction for ash deposition and PM emissions in the combustion experiments. Fuel 2021, 292, 120379. [Google Scholar] [CrossRef]
- Asadieraghi, M.; Daud, W.M.A.W. Characterization of lignocellulosic biomass thermal degradation and physiochemical structure: Effects of demineralization by diverse acid solutions. Energy Convers. Manag. 2014, 82, 71–82. [Google Scholar] [CrossRef]
- Dã az-Vã¡zquez, L.M.; Rojas-Pérez, A.; Fuentes-Caraballo, M.; Robles, I.V.; Jena, U.; Das, K.C. Demineralization of Sargassum spp. Macroalgae Biomass: Selective Hydrothermal Liquefaction Process for Bio-Oil Production. Front. Energy Res. 2015, 3, 6. [Google Scholar] [CrossRef]
- Gwirtz, J.A.; Garcia-Casal, M.N. Processing maize flour and corn meal food products. Ann. N. Y. Acad. Sci. 2013, 1312, 66–75. [Google Scholar] [CrossRef]
- Xiong, S.; Öhman, M.; Zhang, Y.; Lestander, T. Corn Stalk Ash Composition and Its Melting (Slagging) Behavior during Combustion. Energy Fuels 2010, 24, 4866–4871. [Google Scholar] [CrossRef]
- Statistical Yearbook of Repulic of Coratia. 2018. Available online: https://web.dzs.hr/Hrv/Archive/arh_stat_year.htm (accessed on 5 September 2022).
- Matin, A.; Krička, T.; Grubor, M.; Leto, J.; Bilandžija, N.; Voća, N.; Jurišić, V.; Zmaić, K.; Kiš, D.; Kopilović, I. Iskoristivost poslježetvenih ostataka za proizvodnju zelene energije, 1st ed.; Josip Juraj Srossmayer Univesrity of Osijek: Osijek, Croatia, 2019; pp. 48–50. [Google Scholar]
- Liu, Z.; Cao, Y.; Wang, Z.; Ren, H.; Amidon, T.E.; Lai, Y. The Utilization of Soybean Straw. I. Fiber Morphology and Chemical Characteristics. Bioresources 2015, 10, 2266–2280. [Google Scholar] [CrossRef]
- SIS-CEN/TS 15149-2:2006; Solid Biofuels—Methods for the Determination of Particle Size Distribution—Part 2: Vibrating Screen Method Using Sieve Apertures of 3.15 mm and Below. SIST: Ljubljana, Slovenia, 2006; 3.
- SIS-CEN/TS 14775:2004; Solid Biofuels—Method for the Determination of Ssh Content. SIST: Ljubljana, Slovenia, 2004.
- HRN EN ISO 16967:2015; Solid Biofuels—Determination of Major Elements—Al, Ca, Fe, Mg, P, K, Si, Na. HRN: Zagreb, Croatia, 2015.
- HRN EN ISO 16968:2015; Solid Biofuels—Determination of Minor Elements. HRN: Zagreb, Croatia, 2015.
- Chin, K.; H’Ng, P.; Maminski, M.; Go, W.; Lee, C.; Raja-Nazrin, R.; Khoo, P.; Ashikin, S.; Halimatun, I. Additional additives to reduce ash related operation problems of solid biofuel from oil palm biomass upon combustion. Ind. Crops Prod. 2018, 123, 285–295. [Google Scholar] [CrossRef]
- Parmar, K. Biomass-An Overview on Composition Characteristics and Properties. IRA-Int. J. Appl. Sci. 2017, 7, 42–51. [Google Scholar] [CrossRef]
- Álvarez, P.; Santamaría, R.; Blanco, C.; Granda, M. Thermal degradation of lignocellulosic materials treated with several acids. J. Anal. Appl. Pyrolysis 2005, 74, 337–343. [Google Scholar] [CrossRef]
- Masiá, A.A.T.; Buhre, B.J.P.; Gupta, R.P.; Wall, T.F. Characterising ash of biomass and waste. Fuel Process. Technol. 2007, 88, 1071–1081. [Google Scholar] [CrossRef]
- Obernberger, I.; Brunner, T.; Bärnthaler, G. Chemical properties of solid biofuels—Significance and impact. Biomass Bioenergy 2006, 30, 973–982. [Google Scholar] [CrossRef]
- Van Loo, S.; Koppejan, J. Handbook of Biomass Combustion and Co-Firing; IEA: Paris, France, 2002; ISBN 9036517737. [Google Scholar]
- Niu, Y.; Tan, H.; Hui, S. Ash-related issues during biomass combustion: Alkali-induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures. Prog. Energy Combust. Sci. 2016, 52, 1–61. [Google Scholar] [CrossRef]
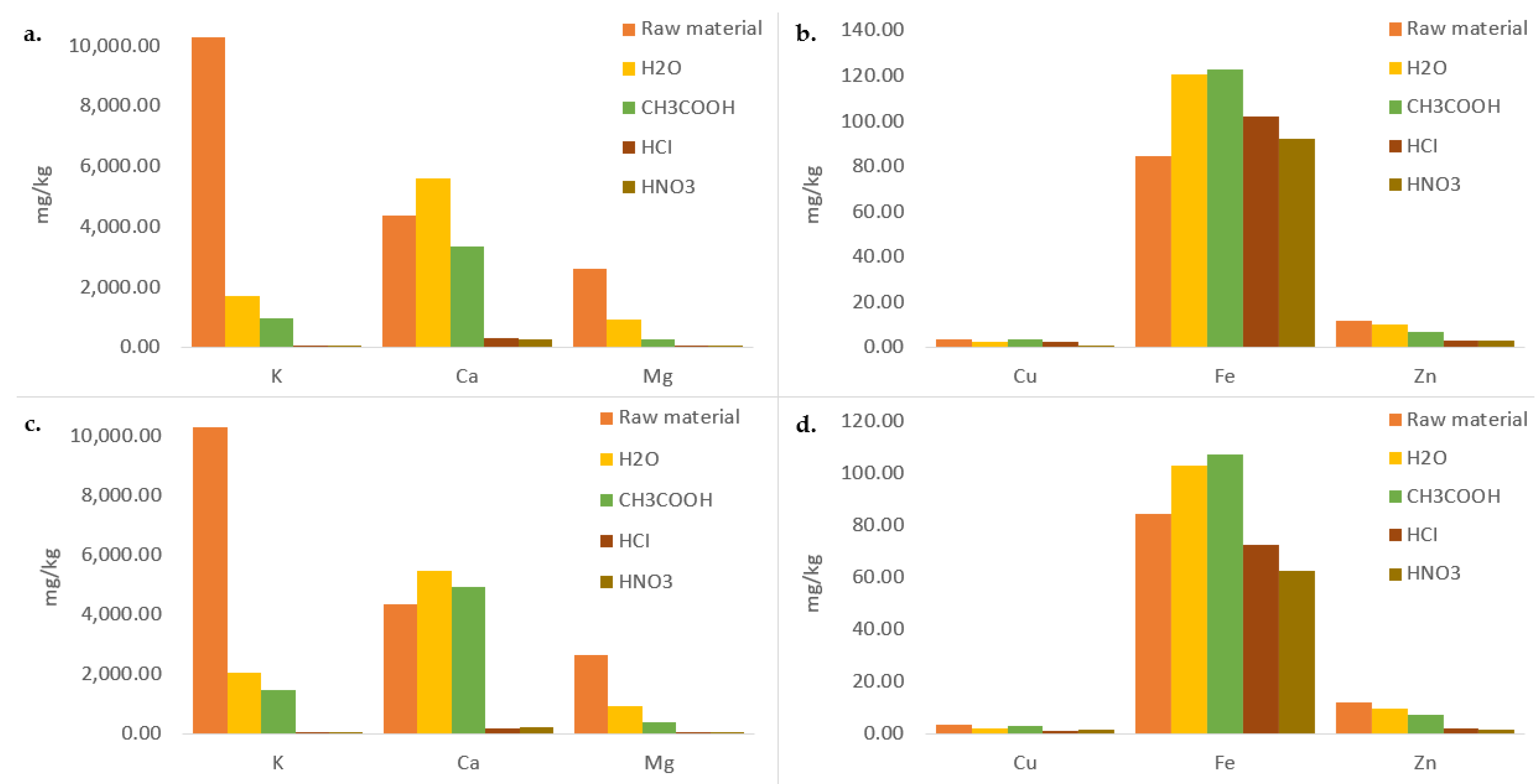
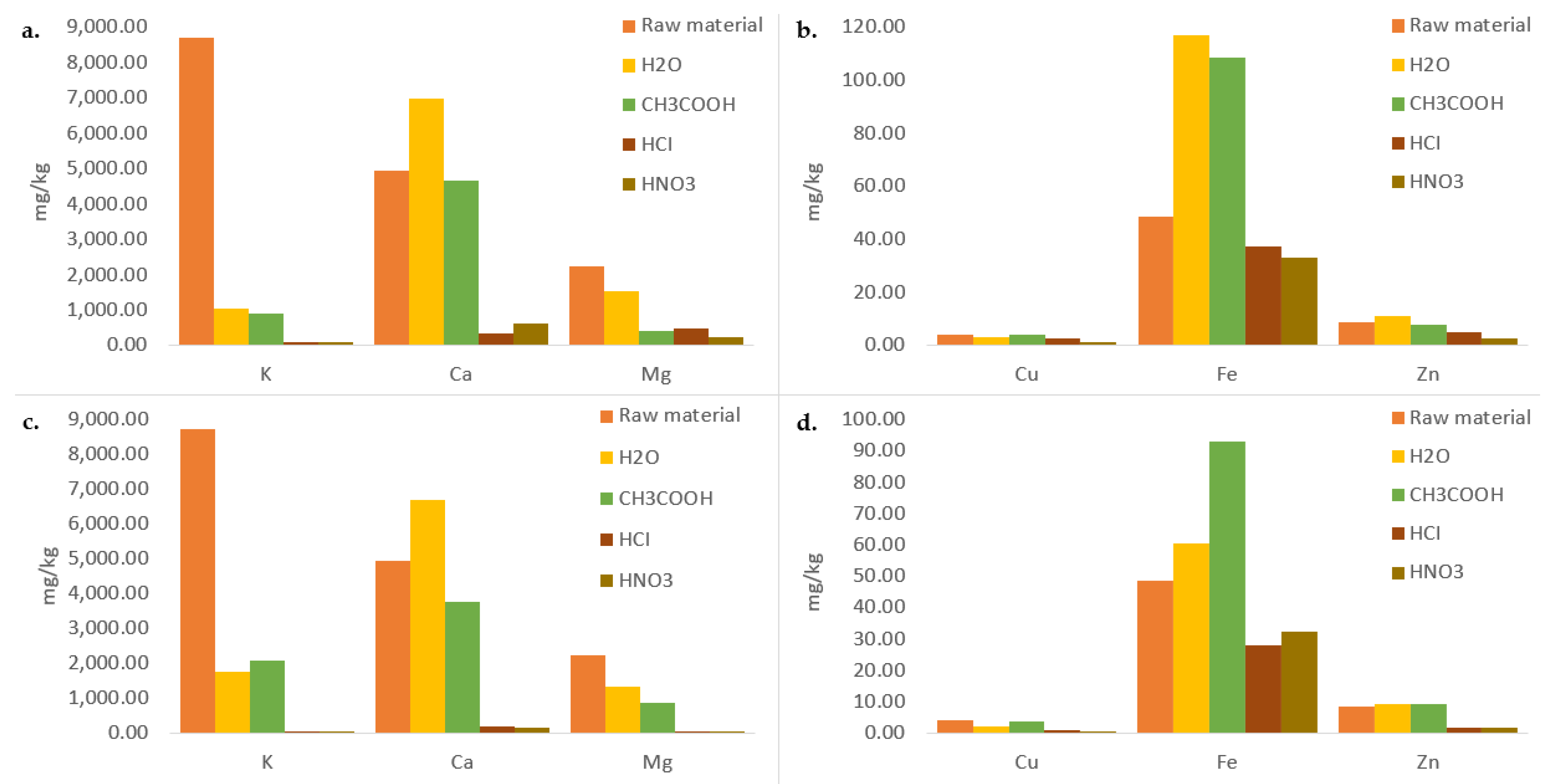

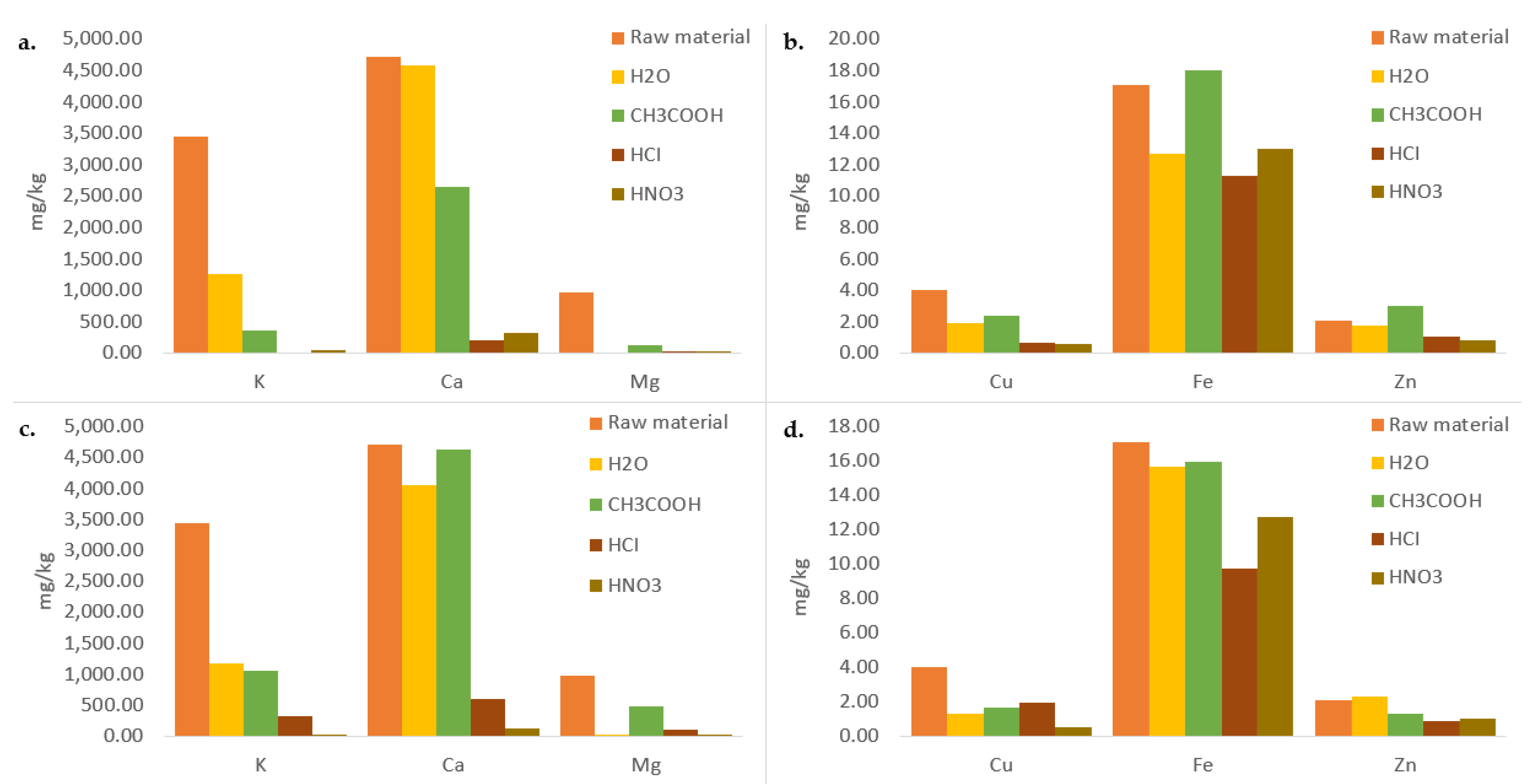
| Sample | Ash (%) |
|---|---|
| Soybean x | 3.36 ± 0.18 a |
| Soybean I | 2.94 ± 0.77 b |
| Soybean II Corn x Corn I Corn II Statistical significance Minimum Maximum Average | 1.55 ± 0.22 a 8.17 ± 0.05 b 6.73 ± 0.03 c 6.37 ± 0.02 c * 1.55 8.17 4.85 |
| Sample | Time | Solvent | Ash (%) |
|---|---|---|---|
| Soybean I | 30 | dH2O | 1.38 ± 0.06 f |
| CH3COOH | 0.75 ± 0.04 cde | ||
| HCl | 0.11 ± 0.02 ab | ||
| HNO3 | 0.18 ± 0.05 ab | ||
| 240 | dH2O | 1.17 ± 0.1 def | |
| CH3COOH | 0.59 ± 0.13 bc | ||
| HCl | 0.04 ± 0.01 a | ||
| HNO3 | 0.1 ± 0.04 ab | ||
| Soybean II | 30 | dH2O | 0.85 ± 0.06 cde |
| CH3COOH | 0.44 ± 0.06 abc | ||
| HCl | 0.1 ± 0.02 ab | ||
| HNO3 | 0.12 ± 0.03 ab | ||
| 240 | dH2O | 0.72 ± 0.06 cd | |
| CH3COOH | 0.61 ± 0.08 bc | ||
| HCl | 0.02 ± 0.01 a | ||
| HNO3 | 0.03 ± 0.02 a | ||
| Corn I | 30 | dH2O | 4.46 ± 0.1 ijk |
| CH3COOH | 4.36 ± 0.35 ijk | ||
| HCl | 3.45 ± 0.13 g | ||
| HNO3 | 3.85 ± 0.1 ghi | ||
| 240 | dH2O | 4.18 ± 0.26 hij | |
| CH3COOH | 3.62 ± 0.26 g | ||
| HCl | 3.35 ± 0.21 g | ||
| HNO3 | 3.45 ± 0.12 g | ||
| Corn II | 30 | dH2O | 4.71 ± 0.15 k |
| CH3COOH | 3.4 ± 0.32 g | ||
| HCl | 1.46 ± 0.02 f | ||
| HNO3 | 1.62 ± 0.34 f | ||
| 240 | dH2O | 4.18 ± 0.14 hij | |
| CH3COOH | 3.78 ± 0.23 gh | ||
| HCl | 1.26 ± 0.17 ef | ||
| HNO3 | 1.25 ± 0.35 ef | ||
| Statistical significance | * | ||
| Minimum | 0.02 | ||
| Maximum | 4.71 | ||
| Average | 1.86 | ||
| SS | ||
|---|---|---|
| Effect | DF | Ash (%) |
| Time | 1 | 0.77 n.s. |
| Solvent | 3 | 36.41 * |
| Time*Solvent | 3 | 0.17 n.s. |
| Error | 88 | 227.91 |
| Time (min) | Solid | Grain Size Group | |
|---|---|---|---|
| - | - | I | II |
| 30 | dH2O | 33.76 | 26.11 |
| 30 | CH3COOH 1% | 35.30 | 46.66 |
| 30 | HCl 1% | 48.86 | 77.13 |
| 30 | HNO3 1% | 42.79 | 74.68 |
| 240 | dH2O | 38.01 | 54.78 |
| 240 | CH3COOH | 48.86 | 40.77 |
| 240 | HCl 1% | 50.26 | 80.27 |
| 240 | HNO3 1% | 48.74 | 80.38 |
| Time (min) | Solid | Grain Size Group | |
|---|---|---|---|
| - | - | I | II |
| 30 | dH2O | 53.37 | 45.13 |
| 30 | CH3COOH 1% | 74.71 | 72.07 |
| 30 | HCl 1% | 96.60 | 94.08 |
| 30 | HNO3 1% | 94.04 | 92.37 |
| 240 | dH2O | 60.44 | 53.66 |
| 240 | CH3COOH | 80.22 | 60.72 |
| 240 | HCl 1% | 98.90 | 98.73 |
| 240 | HNO3 1% | 96.62 | 98.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukuruzović, J.; Matin, A.; Kontek, M.; Krička, T.; Matin, B.; Brandić, I.; Antonović, A. The Effects of Demineralization on Reducing Ash Content in Corn and Soy Biomass with the Goal of Increasing Biofuel Quality. Energies 2023, 16, 967. https://doi.org/10.3390/en16020967
Kukuruzović J, Matin A, Kontek M, Krička T, Matin B, Brandić I, Antonović A. The Effects of Demineralization on Reducing Ash Content in Corn and Soy Biomass with the Goal of Increasing Biofuel Quality. Energies. 2023; 16(2):967. https://doi.org/10.3390/en16020967
Chicago/Turabian StyleKukuruzović, Juraj, Ana Matin, Mislav Kontek, Tajana Krička, Božidar Matin, Ivan Brandić, and Alan Antonović. 2023. "The Effects of Demineralization on Reducing Ash Content in Corn and Soy Biomass with the Goal of Increasing Biofuel Quality" Energies 16, no. 2: 967. https://doi.org/10.3390/en16020967
APA StyleKukuruzović, J., Matin, A., Kontek, M., Krička, T., Matin, B., Brandić, I., & Antonović, A. (2023). The Effects of Demineralization on Reducing Ash Content in Corn and Soy Biomass with the Goal of Increasing Biofuel Quality. Energies, 16(2), 967. https://doi.org/10.3390/en16020967







