Abstract
This paper presents an analysis of the energy consumption of a continuous flow ohmic heater (CFOH) with advanced process controls for heating operations in the food and drinks industry. The study was carried out by using operational data collected from a CFOH pilot plant that was designed and constructed at the National Centre of Excellence for Food Engineering (NCEFE), Sheffield Hallam University. The CFOH is controlled by a PC and includes an onboard Programmable Logic Controller (PLC) and a Human Machine Interface (HMI) so that it can be operated as a stand-alone unit with basic on/off and power setting control but without any advanced control features. The technical solution presented in this paper for heating foods demonstrates significant energy saving compared with conventional heating methods. Using the CFOH, the electric current generated in the food products by the Joule effect produces a rapid temperature increase with very high energy efficiency. This technique eliminates the low efficiency of heat transfer from the surface of vessels typically used to heat and cook food products. The analysis presented in this paper describes the energy consumption of the CFOH and compares the efficiency of the CFOH when different advanced process control techniques are used. Experimental results and analysis have shown that the CFOH can achieve an energy efficiency conversion of at least 87.9%. It has also shown that the energy conversion percentage can be increased by applying advanced controllers such as model predictive control (MPC) or adaptive model predictive control (AMPC).
1. Introduction
Despite the progress being made in recent years, the food and drinks industry in the United Kingdom (U.K.) is still using a significant number of low-energy efficiency processes based on fossil fuel sources of energy, which produces high levels of CO2 emissions. The U.K.’s food and drink sector was responsible for 165 million tons of carbon emissions in 2019. This equates to about 17% of the U.K.’s carbon footprint. The application of OH in the food industry is a very energy-efficient method of converting electrical energy into heat energy for heating foods from a 50 Hz voltage supply [1]. OH involves the passing of electric current through food products, heat is generated within the food substance and dissipated directly in the medium with very high efficiency (typically > 90%) by the Joule effect [2,3,4,5]. In contrast, other conventional heating methods such as radiation, convection, and conduction are time-consuming and have significantly (about 50%) lower energy efficiency [6,7].
In OH, the food material—which can be in the form of a homogenous mixture or heterogenous mixture with particulates—acts as a resistor in an ohmic heating process and directly converts electrical energy to thermal energy with short heating times [8]. The presence of free ions and water molecules within the food substance creates resistance to their movement and increases their kinetic energy, thereby heat is volumetrically produced within the food substance due to the ionic motion [9,10].
The advantages of OH in food processing include faster heating times compared with those of other conventional heating methods [11,12], unlimited heating depth of foods unlike microwave heating or other conventional methods [13], and volumetric heating [5]. Some applications of OH in food processing include cooking [14,15], preheating [16], extraction [5,17], blanching [18], sterilization [19], and pasteurization [20,21].
In designing the ohmic heater structure, several researchers have proposed different physical structures for the OH system, but a fundamental design structure of the OH systems is such that two or more electrodes are used to pass current through the food substance [22]. The ohmic heater can be designed as a containing vessel (batch) or a continuous flow system depending on the location and position of the electrodes [23]. In the batch OH system, the food product is in a heating chamber where the electrodes are in constant contact with the food product throughout heating, unlike a continuous flow ohmic heater (CFOH), where food product flows from an infeed reservoir tank through a heating chamber with electrodes and the heated food product is collected in an outfeed tank. Some of the published design configurations are batch ohmic heaters with two rectangular electrodes [24,25], cylindrical batch ohmic heaters with electrodes at the closed ends [26,27], continuous flow heaters with variable electrode positions [23], and continuous flow heaters with parallel spaced electrodes [28].
Some authors have presented their work on the energy consumption efficiency of ohmic heating of liquid food products in a batch system [4,5,29]. However, there is a gap in the current literature for energy consumption analysis of the CFOH. This paper is therefore focused on the energy consumption analysis of the CFOH and the energy consumption when different advanced process control techniques are applied to the CFOH. In summary, the following work has been conducted in this paper to address the gaps in the literature:
- Energy consumption analysis of a CFOH.
- Energy consumption analysis using a range of control techniques:
- ○
- Proportional, integral, and derivative (PID) control.
- ○
- Model predictive control (MPC).
- ○
- Adaptive model predictive control (AMPC).
2. Materials and Methods
2.1. Materials
The food products used were orange juice of the ‘Country Range’ brand purchased at a local supermarket and prepared saline solution of known electrical conductivity using table salt. The initial temperature of the refrigerated orange juice was between 9 and 10 °C and the prepared saline solution was between 18 and 20 °C. The initial electrical conductivity of the orange juice at start-up and the initial product temperature were measured using an Aprea PC60 Premium Multiparameter.
2.2. Experimental Apparatus
Experiments were carried out in the continuous flow ohmic heater shown in Figure 1 which consists of the following parts:
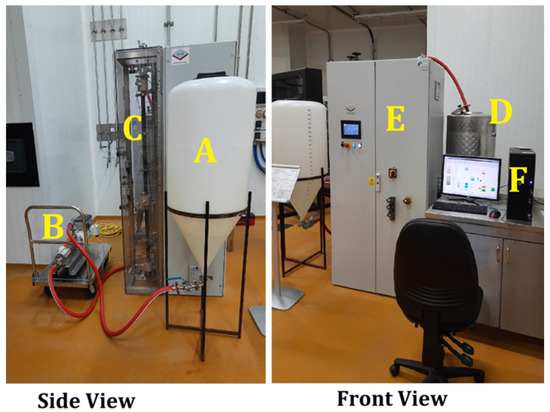
Figure 1.
Continuous flow ohmic heating system pilot plant designed at NCEFE in collaboration with Ohm-E Technology (UK).
- A: infeed tank
- B: infeed pump
- C: electrodes and electrode housing
- D: outfeed tank
- E: control panel
- F: PC control
Both the infeed tank (A) and the outfeed tank (D) have a reserve capacity of 200 L. The infeed pump has a capacity of 60 L/hr. The applicators are ceramic with a bore diameter of 20 mm. The electrodes are made of titanium oxide. In the control panel (E), the following components are present: a Mitsubishi PLC, high voltage (HV) transformer, HV thyristor, HV meter, current meter, contactors, and isolator. The applicator comprises an inline colinear arrangement of electrodes for continuous in-pipe heating.
From the CFOH unit in Figure 1 above, the pilot plant has the following features:
- Heating liquid food products to circa 100 °C by nominally delivering 10 kW into a wide range of product conductivities from 0.15 to 0.9 S/m (infeed) operating at voltages of up to 4.2 kV;
- Comprising a continuous process applicator;
- Being integrated with and being controlled from a PC- MATLAB or LabVIEW platform;
- Driving a suitable pump with pump speed control for product flow rate control.
The applicator enclosure is assembled in a vertical column comprising three electrode housings with insulating spacing spools between the housings shown in Figure 2.
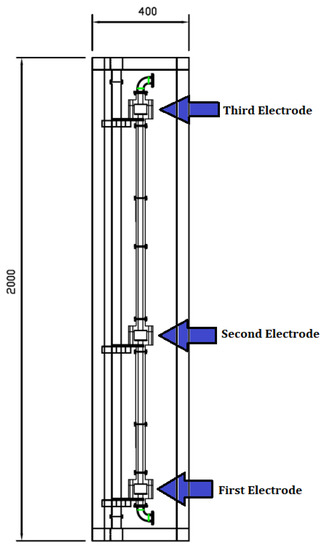
Figure 2.
Applicator arrangement and electrode position.
2.3. Energy Analysis
The conservation of energy governing ohmic heating systems presented by [13] is given as:
where is the volumetric heat (W/) generated by the applied electric field (E); T is the temperature term in K; is the initial temperature; and k, 𝜌, c, and are the temperature-dependent thermophysical properties of the food—the thermal conductivity (W/mK), the density (kg/), the specific heat capacity (J/kg K), and the electrical conductivity (S/m), respectively.
In Equation (1), ∇∙(k∙∇T) represents the heat transferred by conduction through the fluid, and represents the volumetric heat generation, which is important in Ohmic heating but negligible in conventional heating processes. The predicted temperature is described by Equation (3) as a function of the conductivity (), where is the temperature coefficient (1/K). The fourth term (𝜌∇·v) in Equation (1) is the work done by the fluid on its surrounding, which is 0 for an incompressible fluid. The fifth term () represents the radiative heat transfer [13].
The energy balance equation can be expressed as the total energy input to total energy output given below:
where ∑ is the sum of the energy in the CFOH, ∑ is the sum of energy as a result of the ohmic heating effect and thermo-physical properties. is the electrical energy into the ohmic heater, is the mass flowrate, and is the heat capacity of the heated food product. is the inlet temperature of the food product from the infeed tank. is the room temperature and is the outlet temperature recorded.
The energy losses considered are due to thermal conduction to the applicators and heat loss at the titanium electrodes. Therefore, heat loss (is given by:
where is the heat capacity of titanium while is the initial temperature of the electrodes. The latent heat of vaporization is not considered because the only opening is from the applicator outlet. The energy efficiency ῃ has been described by [29] and is given by:
2.4. Methodology
The steps taken in the analysis of the energy efficiency of the CFOH are described below. During the experiment, two separate food materials were used. The saline solution consisted of table salt diluted with tap water at an initial temperature of 18 °C and measured electrical conductivity of 0.3 S/m at room temperature. The other food material used was refrigerated orange juice with an initially measured electrical conductivity of 0.35 S/m at 9–10 °C. The steps in which the experiment was carried out were identical for the different food samples.
2.5. Open Platform Communication (OPC) between the Pilot Plant and Lab-Based Personal Computer (PC)
To implement process control on a PLC with MATLAB/SIMULINK environment, the open platform communication (OPC) server and client protocol was used. The OPC server and client protocol enable the exchange of data in real-time between the PLC on which the ohmic heater is based and a stand-alone lab-based computer so that simple and advanced model-based control can be applied. With the OPC established, the following tasks were performed:
- Read/write to and from the PLC.
- Trend and real-time data collection and storage on the lab-based PC.
- Implementation of classical and advanced controllers.
The OPC server KEPServerEX was configured to communicate with the MITSUBISHI FX5U PLC with an Ethernet module using an Ethernet communication protocol. The input/output (I/O) tags written to the PLC were sent to the OPC server for MATLAB to read and write to it.
In this study, the OPC server KEPServerEX was configured to exchange data over a local area network (LAN). It also can be accessed over the Internet, therefore the controllers can be operated remotely for an Internet of Things (IoT)-based approach to be implemented, which could provide a powerful management tool to enhance food safety [30].
2.6. Implementation of PID, MPC, and AMPC
The advanced process controllers including PID, MPC, and AMPC were developed prior to the experiment. The author will briefly discuss the development of the advanced process controllers and the interface between MATLAB and the PLC-based CFOH unit.
2.6.1. PID Control of the Continuous Flow Ohmic Heater
The PID controller is a very common controller used in the control of loop processes, to implement the PID control, we assume a single input single output (SISO) system where the only controlled variable is the output temperature. Other process parameters such as the mass flow rate are kept constant. The equation of the PID controller is expressed as:
The term is known as the proportional term and is based on the difference between the set point (desired output temperature) and the actual value (recorded temperature), in this case, it represents a temperature error. The integral term, , is the summation of the error values and is the derivative of the proportional error, which determines if the error is diminishing. Therefore, the proportional term decreases the rise time, the integral term reduces the steady state error, while the derivative term reduces the settling time and overshoot. The PID controller was tuned to the following settings: the proportional (P) term to 2.5, the integral (I) term to 0.2, and the derivative (D) term to 0. The closed-loop control of the CFOH using the PID control technique is shown below in Figure 3.
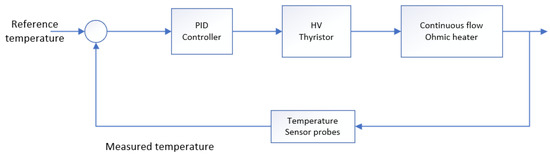
Figure 3.
Closed loop PID control of the continuous flow ohmic heater.
The product flow rate was kept constant throughout the heating process. The desired output temperature was given as a reference to the PID controller. The temperature at the output was measured by two optic fiber temperature probes at the outlet of the heater. An additional thermocouple was placed outside the heating region to further validate the readings by the optic fiber probes. The measured temperature was compared with the reference temperature and the error generated feeds into the PID controller. The PID controller then provided the HV thyristor a dimensionless value ranging from 0 to 100. This dimensionless value received by the HV thyristor corresponds to an HV voltage reading which was applied to the electrodes. The desired performance of the PID controller was ensured by proper tunning of the controller gains.
2.6.2. MPC and AMPC Implementation on the Continuous Flow Ohmic Heater
Model-based predictive control (MPC) describes a set of advanced control methods, which make use of a process model to predict the future behavior of the controlled system [31]. In this prediction, the MPC determines an optimal solution for the output by solving a constrained optimization problem. At each time step, the prediction and optimization are computed; therefore, the MPC is also referred to as “receding horizon” control. In essence, the idea is that a short-term (predictive) optimization achieves optimality over a long time.
Short-term prediction over a long time achieves optimal results because the error forecast is small compared with a distant prediction. The combination of prediction and optimization is the main difference from conventional control approaches, which use precomputed control laws [32].
The MPC is represented by the form:
where , , , , and are matrices parameters that can vary with time. is the time index, is the plant model states, is the control input (manipulated variable (MV)), is the measured disturbance, is the unmeasured disturbance, and is the plant output.
A traditional MPC controller includes a linearized and fixed nominal operating point (at which the plant model applies the optimal control solution (). For the MPC used in this work, the properties are given below:
and . Sampling time = 0.3 s, prediction horizon = 30, and control horizon = 3. Manipulated variable rate ( = . Output variable weight = .
In AMPC, as time evolves the nominal operating point will be updated and time variance will be consistent with the updated plant model. AMPC can be written in the form:
where , and are the parameter matrices to be updated, is the nominal operating point of the AMPC. are the nominal state increments, is the nominal input, and is the nominal output. For the AMPC used in this research, the same properties of the MPC were used, with the only additional variables being the nominal state increase of , , and .
The electrical power delivered by the ohmic heater was recorded by an onboard power meter in real-time in the MATLAB environment. For temperature measurements, within the electrodes, two pairs of optic fiber temperature probes were placed side by side at the midpoint of the heating chamber between the first and second electrodes and just before the third electrode. Two sets of thermocouples were used. The first thermocouple was placed at the infeed into the ohmic heating chamber just before the first electrode to measure the product inlet temperature. At the outlet, a second thermocouple was placed after the third electrode to further validate and measure the outlet temperature. Special care was taken to avoid placing unshielded thermocouples between the electric field. The steps taken are described below:
- Ensure a minimum of 20 L of product is in the infeed tank (e.g., saline or orange juice);
- An appropriate flow rate of 60 L/hr is set;
- The desired process controller is chosen (e.g., PID, MPC, or AMPC);
- A target temperature of 90 °C is set;
- Heating commences and the process runs for 200 s;
- Data are being recorded in real-time;
- The process can be repeated.
3. Results and Discussion
3.1. Heating Rate of Saline and Energy Efficiency
In the figures below, the product temperature as a function of the applied voltage is presented at a constant flow rate of 60 L/hr. The performance of the choice of advanced process control is also shown by the deviation of the outfeed temperature from the setpoint heating temperature of 90 °C. The energy efficiency of heating saline to 90 °C in the ohmic heater is also presented. The energy efficiency is derived from the application of Equation (8).
Heating Rate of Saline and Energy Efficiency with PID, MPC, and AMPC Control
In Figure 4, the plot shows the overlapped temperature output profiles from the CFOH and the voltage profiles when PID, MPC, and AMPC controllers were independently used in heating saline solution.
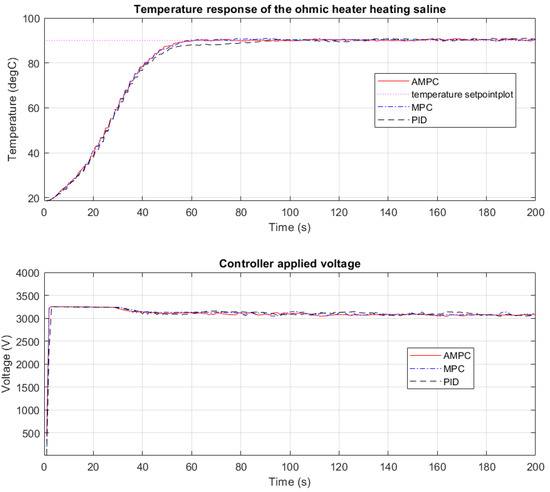
Figure 4.
Temperature response heating saline using PID, MPC, and AMPC controller.
As can be seen from Figure 4, rapid heating is an advantage of ohmic heating. The outlet temperature reached the setpoint temperature from 18 °C to 90 °C and settled within 1.5 min of heating. A steady-state error of about 2 °C is observed from the PID temperature profile. The use of the MPC controller gives a more desirable outfeed temperature compared with the use of the PID controller. A reduced steady-state error of about 0.8 °C is observed compared with 2 °C when a PID controller was used. When the AMPC controller was used, the steady state error observed is about 0.6 °C. This demonstrates that the AMPC controller has better performance in terms of tracking reference temperature setpoints than both the PID and MPC controllers. Comparing the voltage response of the AMPC controller, a smoother voltage profile is seen compared with an oscillatory pattern with the PID (Figure 4) and aggressive short steps with the MPC. The real-time recorded electrical power consumption and energy efficiency are shown in Figure 5 below.
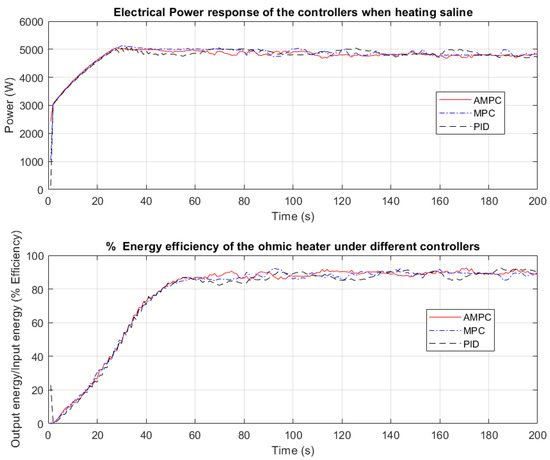
Figure 5.
Electrical power and energy efficiency of the ohmic heater while heating saline.
In Figure 5, the electrical power input to the CFOH is the smoothest when AMPC is used compared with that of PID and MPC. The PID electrical power input is observed to oscillate when the desired setpoint temperature was reached from 60 s to 200 s. The absence of oscillation on the electrical power input in Figure 5 when MPC and AMPC are used suggests that they are a more robust controller than the PID controller. Even though there is no significant change in the applied electrical power, the energy efficiency when a PID controller was used is the lowest. The AMPC is observed to have the highest energy efficiency.
Table 1 shows the electrical power consumed and the energy efficiency of the ohmic heater between 1–60 s and 60 s to 200 s. The efficiency is evaluated from 0 to 60 s and from 60 s to 200 s. This is because 0 to 60 s represents the temperature build-up within the heating chamber.

Table 1.
Energy consumption and energy efficiency of heated saline using PID, MPC, and AMPC controller.
According to Table 1, the use of the MPC controller increases the energy efficiency of the ohmic heater. This can be seen from the temperature plot in Figure 5. The conversion of input energy to heat for the CFOH is more efficient using an MPC controller than a PID controller. However, there is no significant change in the electrical energy consumed by both controllers. The energy efficiency achieved by AMPC is the highest when compared with PID and MPC controllers. This means that using AMPC on the ohmic heater gives a higher temperature conversion rate from the applied power.
3.2. Heating Rate of Orange Juice and Energy Efficiency
Heating Rate of Orange Juice and Energy Efficiency with PID, MPC, and AMPC Control
A similar procedure to Section 3.1 was repeated for orange juice at an infeed temperature of 9–10 °C.
In Figure 6, the temperature response while heating orange juice to 90 °C when a PID, MPC, and AMPC controller were used is shown. The orange juice in the infeed tank was at 10 °C.
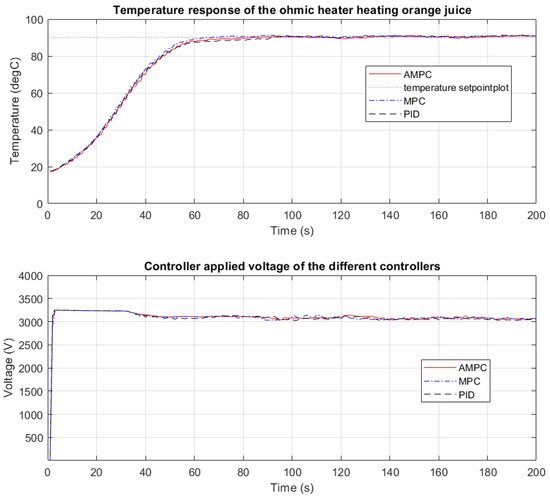
Figure 6.
Temperature response heating orange juice using PID, MPC, and AMPC controller.
In Figure 6, the rapid heating of the CFOH is seen as the temperature rises from 10 °C to 90 °C and settles in just over 70 s. From Figure 6, voltage oscillation is observed between 30 s and 70 s, this is because the PID controller struggles to keep the output temperature at the desired setpoint of 90 °C. When the MPC control was used, the temperature response of the CFOH was more desirable. The steady-state error is less than 0.8 °C and the output temperature settles within 60 s. The settling time when AMPC was used to heat orange juice to 90 °C is about 65 s. The voltage profile of the AMPC is less aggressive compared with the response of the MPC and reduced voltage oscillation is seen when compared with the PID response.
In Figure 7, the electrical power response profiles and energy efficiency of the CFOH when PID, MPC, and AMPC controllers were used to heat orange juice is shown.
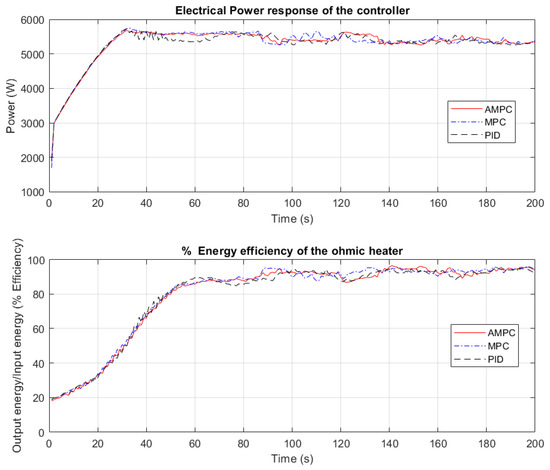
Figure 7.
Electrical power and energy efficiency of the ohmic heater.
According to Figure 7, undesirable sustained oscillation is observed for the electrical power input when the PID controller was used. This sustained oscillation is a result of the PID performance. Between 30 s to 70 s, the PID controller struggles to keep the output temperature close to the desired setpoint temperature. It can be seen in Figure 7 from the electrical power input that the MPC takes aggressive control actions to keep the output temperature close to the desired temperature of 90 °C. In Figure 7, the electrical power input to the CFOH when the AMPC controller was used appears to be aggressive, albeit not as aggressive as the MPC. The energy efficiency of the CFOH when AMPC was used decreases just after 120 s and then increases. This trend is consistent with both the PID and MPC and can be attributed to product mixing within the heating chamber.
Table 2 presents the energy consumption and energy efficiency of the CFOH when the PID controller was used.

Table 2.
Energy consumption and energy efficiency of heated orange juice using PID, MPC, and AMPC controller.
According to Table 2, reduced energy efficiency is observed in the first 60 s of operation. The reduced energy efficiency, which is the conversion of electrical energy to heat, can be attributed to the low inlet product temperature of 9–10 °C. In Table 2, the MPC has a higher energy efficiency when compared with that of the PID controller. This can be attributed to the aggressive nature of the controller action to keep the output temperature close to the desired setpoint temperature. From Table 2, no significant change in the electrical power consumed during heating is seen when compared with PID and MPC. A general trend of lower energy efficiency is observed from 0 to 60 s. Compared with PID, the AMPC has a higher energy efficiency, but not more than the MPC does.
3.3. Discussion
From the results above, it was observed that while heating saline solution using PID, MPC, and AMPC, there is no significant change in the electrical power consumed during heating. However, the energy efficiency conversion from electrical power to heat improves when an AMPC controller is used. While heating saline, PID has the lowest energy efficiency (87.9%), MPC has an energy efficiency of 88.40%, and AMPC has an energy efficiency of 89.1%.
For the heating of orange juice, a low energy efficiency of less than 53% was observed in the first 60 s of operation. This is due to the low input product temperature of 9 °C. After the first 60 s of operation, the temperature built up within the heating chamber significantly increased, hence the increased energy efficiency of 91.0%, 92.2%, and 91.8% for PID, MPC, and AMPC, respectively. Also, no significant difference in the electrical power is observed. In Figure 8, the energy efficiency comparison chart of the controllers when applied to saline and orange juice is shown.
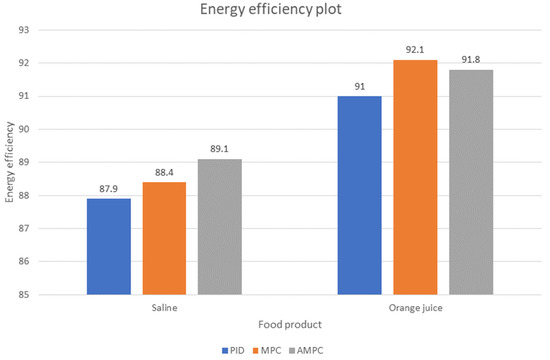
Figure 8.
Energy efficiency plot of the ohmic heater for different controllers.
It was observed that the MPC has a higher energy efficiency than PID and AMPC when heating from a much lower temperature of 9 °C. However, the AMPC has a higher energy efficiency than the PID and MPC when heating starts from a higher temperature of 18 °C. The authors are tempted to attribute this characteristic to the very aggressive control of the MPC to keep the output temperature close to the desired setpoint value, but future studies will be conducted to answer this question.
In this study, the food products used were a saline solution and orange juice, which are uniform and homogenous in nature. When the CFOH is used, the heating times are drastically reduced to less than 1 min for a flow rate of 1 L/min with a minimum efficiency of 87.9% for saline and 91% for orange juice. The energy efficiency achieved is very similar to the work reported in [12], where a non-homogenous food product consisting of fish samples was heated using a smaller scale batch OH (2.5 kW), and a similar trend of fast heating time and energy efficiency of 89.89% were recorded.
Due to the lack of published work using CFOH, it is not possible to directly compare with other results in the literature. However, Atuonwu et al. [33] produced a detailed analysis of energy performance for several innovative and conventional food preservation technologies including high-pressure processing (HPP), microwave volumetric heating (MVH), batch ohmic heating (OH), and conventional thermal treatment (UHT) where orange juice was heated from ambient temperature (approx. 20 °C) to 76 °C. For a fair comparison, the best results from each of the heating methods with different set-ups were selected to compare with the results produced in this paper. In [33], a hypothetical continuous OH (Cont. OH) was also used in the comparison where the continuous OH process, without voltage switching temperature control, was expected to achieve an energy efficiency of 95%, in theory. The comparison chart can be seen in Figure 9.
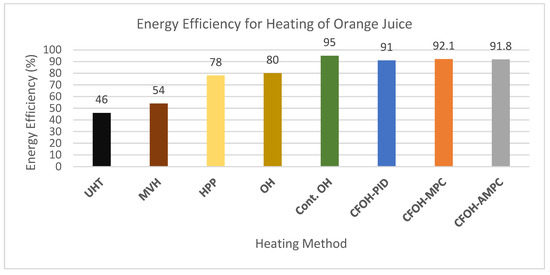
Figure 9.
Energy efficiency comparison with different heating methods. Note: the first 5 data were quoted from [33].
As shown in Figure 9, not surprisingly, the conventional technology UHT—which was represented by an electrically powered hot water-to-orange juice heat exchanger—had the lowest energy efficiency of 46% because of the heat loss during heat transfer. The MVH system, even when the magnetron cooling energy was discounted, could only achieve 54% of energy efficiency. As a non-thermal method, HPP could produce 78% of energy efficiency at a 95% filling ratio, which was a huge improvement from 31% of energy efficiency at a 36% filling ratio. The batch OH process had the highest energy efficiency of 80% among all the thermal processes. It is clear that the CFOH process in this work compares favorably with all the heating technologies reported in [33], even when the starting temperature was much lower at 10 °C. The average energy efficiency of 91.6% for all three controllers is not far off from the 95% claimed in the hypothetical continuous OH process.
4. Conclusions
In this work, the gap in the literature analyzing the energy consumption of a continuous flow ohmic heater was discussed and addressed. The energy consumption analysis was based on the novel development of a state-of-the-art CFOH with advanced controllers. Experiments were carried out for both saline solution and orange juice. The advanced MPC and AMPC controllers were designed based on a validated mathematical model and innovatively implemented using an OPC server through MATLAB/Simulink for real-time control. This is the first time such experimental results are being obtained and reported. From the experimental results, it was established that the CFOH has an energy efficiency conversion percentage of at least 87.9%. The analysis has also shown that the energy conversion percentage can be increased by applying MPC and AMPC controllers, or other advanced controllers.
When analyzing the energy consumed during the heating process, it was observed that there are no significant electrical power differences for both saline solution and orange juice, and that the energy efficiency observed was a function of the controller performance.
Future work will continue to analyze the energy efficiency of the CFOH with other process parameters such as the flow rate, varying infeed temperatures, setpoint temperature, and for different food materials, especially those of heterogeneous mixtures. IoT-enabled remote control facilities will also be explored and developed to improve the operation and management of the processes. Other applications of the CFOH, such as pasteurization and fermentation, will also be investigated together with further analysis of heat recovery during the cooling phase of the food materials.
Author Contributions
Conceptualization, H.Z., M.H., O.O.-o. and X.X.; methodology, O.O.-o.; software, O.O.-o.; validation, O.O.-o., H.Z., M.H. and X.X.; writing—original draft preparation, O.O.-o.; supervision, H.Z., M.H. and X.X.; project administration, H.Z. and M.H.; funding acquisition H.Z. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the EU Horizon 2020 SUSFOOD 2 MEFPROC project under grant agreement No. 727473. For the purpose of open access, the authors have applied a Creative Commons Attribution (CC BY) license to any Author Accepted Manuscript version arising from this submission.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Sheffield Hallam University. Ethic Review ID: ER35263715, 4/10/2021.
Informed Consent Statement
Not applicable.
Data Availability Statement
The experimental data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
The authors would like to thank Ohm-E Technology (UK), Jonny Shepherd, and the NCEFE team at Sheffield Hallam University for the guidance received when performing experiments.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Maloney, N.; Harrison, M. Advanced Heating Technologies for Food Processing. In Innovation and Future Trends. In Food Manufacturing and Supply Chain Technologies; Leadley, C.E., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 203–256. [Google Scholar]
- Sastry, S. Ohmic heating and moderate electric field processing. Food Sci. Technol. Int. 2008, 14, 419–422. [Google Scholar] [CrossRef]
- Ramaswamy, H.S.; Marcotte, M.; Sastry, S.; Abdelrahim, K. Ohmic Heating in Food Processing; CRC Press: Boca Raton, FL, USA, 2014; pp. 15–36. [Google Scholar]
- Gavahian, M.; Chu, Y.; Farahnaky, A. Effects of ohmic and microwave cooking on textural softening and physical properties of rice. J. Food Eng. 2019, 243, 114–124. [Google Scholar] [CrossRef]
- Sofi’i, I.; Arifin, Z. Energy consumption for patchouli oil extraction using ohmic heating. IOP Conf.Ser. Earth Environ. Sci. 2022, 1012, 012062. [Google Scholar] [CrossRef]
- Silva, V.L.M.; Santos, L.M.; Silva, A.M.S. Ohmic heating: An emerging concept in organic synthesis. Chem. Eur. J. 2017, 23, 7853–7865. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Farahnaky, A. Ohmic-assisted hydrodistillation technology: A review. Trends Food Sci. Technol 2018, 72, 153–161. [Google Scholar] [CrossRef]
- Cappato, L. Ohmic heating in dairy processing: Relevant aspects for safety and quality. Trends Food Sci. Technol. 2017, 62, 104–112. [Google Scholar] [CrossRef]
- Paul Singh, R.; Heldman, D.R. Introduction to Food Engineering, 4th ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 369–376. [Google Scholar]
- Kumar, T.A. Review on Ohmic Heating Technology: Principle, Applications and Scope. Int. J. Agric. Environ. Biotechnol. 2018, 11, 679–687. [Google Scholar] [CrossRef]
- Zell, M.; Lyng, J.G.; Cronin, D.A.; Morgan, D.J. Ohmic cooking of whole beef muscle—Evaluation of the impact of a novel rapid ohmic cooking method on product quality. Meat Sci. 2010, 86, 258–263. [Google Scholar] [CrossRef]
- Aydin, C.; Kurt, Ü.; Kaya, Y. Comparison of the Effects of Ohmic and Conventional Heating Methods on Some Quality Parameters of the Hot-smoked Fish Pâté. J. Aquat. Food Prod. Technol. 2020, 29, 407–416. [Google Scholar] [CrossRef]
- Marcotte, M. Ohmic Heating of Viscous Liquid Foods. Ph.D. Thesis, McGill University, Sainte-Anne-de-Bellevue, QC, Canada, 1999. [Google Scholar]
- Lakshmi, S.; Chakkaravarthi, A.; Subramanian, R.; Singh, V. Energy consumption in microwave cooking of rice and its comparison with other domestic appliances. J. Food Eng. 2007, 78, 715–722. [Google Scholar] [CrossRef]
- Tumpanuvatr, T.; Jittanit, W. Quality improvement of refrigerated ready-to-eat cooked brown rice by adding gellan gum and trehalose with ohmic heating compared to conventional cooking method. J. Food Process. Preserv. 2022, 46, e16443. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Kang, D. Application of continuous-type pulsed ohmic heating system for inactivation of foodborne pathogens in buffered peptone water and tomato juice. Lwt-Food Sci. Technol. 2018, 93, 316–322. [Google Scholar] [CrossRef]
- Cabas, B.M.; Icier, F. Ohmic Heating–Assisted Extraction of Natural Color Matters from Red Beetroot. Food Bioprocess Technol. 2021, 14, 2062–2077. [Google Scholar] [CrossRef]
- Sarang, S.; Sastry, S.K.; Gaines, J.; Yang, T.C.S.; Dunne, P. Product Formulation for Ohmic Heating: Blanching as a Pretreatment Method to Improve Uniformity in Heating of Solid-Liquid Food Mixtures. J. Food Sci. 2007, 72, E227–E234. [Google Scholar] [CrossRef] [PubMed]
- Pelacci, M.; Malavasi, M.; Cattani, L.; Gozzi, M.; Tedeschi, F.; Vignali, G.; Rainieri, S.; George, S.; Zuber, F.; Mathiot, P.; et al. Impact of indirect and ohmic heating sterilization processes on quality parameters of apple puree: Application in a real industrial line. J.Phys. Conf. Ser. 2021, 1868, 012004. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Gholamhosseinpour, A.; Niakousari, M. Application of microwave and ohmic heating for pasteurization of cantaloupe juice: Microbial inactivation and chemical properties. J. Sci. Food Agric. 2019, 99, 4276–4286. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Cabral, L.; Guimarães, J.T.; Noronha, M.F.; Cappato, L.P.; Cruz, A.G.; Sant’Ana, A.S. Conventional and ohmic heating pasteurization of fresh and thawed sheep milk: Energy consumption and assessment of bacterial microbiota during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2022, 76, 102947. [Google Scholar] [CrossRef]
- Sakr, M.; Liu, S. A comprehensive review on applications of ohmic heating (OH). Renew. Sustain. Energy Rev. 2014, 39, 262–269. [Google Scholar] [CrossRef]
- Icier, F.; Ilicali, C. The use of tylose as a food analog in ohmic heating studies. J. Food Eng. 2005, 69, 67–77. [Google Scholar] [CrossRef]
- Somavat, R.; Kamonpatana, P.; Mohamed, H.M.H.; Sastry, S.K. Ohmic sterilization inside a multi-layered laminate pouch for long-duration space missions. J. Food Eng. 2012, 112, 134–143. [Google Scholar] [CrossRef]
- Choi, W.; Kim, S.; Park, S.; Ahn, J.; Kang, D. Numerical analysis of rectangular type batch ohmic heater to identify the cold point. Food science & nutrition. Food Sci. Nutr. 2020, 8, 648–658. [Google Scholar] [PubMed]
- Sagita, D.; Darmajana, D.A.; Hidayat, D.D.; Novrinaldi; Sitorus, A. Design and performance of ohmic-based fermentor model for controlling fermentation process. IOP Conf. Ser. Earth Environ. Sci. 2020, 542, 012033. [Google Scholar] [CrossRef]
- Pesso, T.; Piva, S. Laminar forced convection in a cylindrical collinear ohmic sterilizer. Therm. Sci. 2017, 21, 2217–2226. [Google Scholar] [CrossRef]
- Kamonpatana, P.; Mohamed, H.M.H.; Shynkaryk, M.; Heskitt, B.; Yousef, A.E.; Sastry, S.K. Mathematical modeling and microbiological verification of ohmic heating of a solid–liquid mixture in a continuous flow ohmic heater system with electric field perpendicular to flow. J. Food Eng. 2013, 118, 312–325. [Google Scholar] [CrossRef]
- Darvishi, H.; Hosainpour, A.; Nargesi, F.; Fadavi, A. Exergy and energy analyses of liquid food in an Ohmic heating process: A case study of tomato production. Innov. Food Sci. Emerg. Technol. 2015, 31, 73–82. [Google Scholar] [CrossRef]
- Lavelli, V. Circular food supply chains Impact on value addition and safety. Trends Food Sci. Technol. 2021, 114, 323–332. [Google Scholar] [CrossRef]
- Schwenzer, M.; Ay, M.; Bergs, T.; Abel, D. Review on model predictive control: An engineering perspective. Int. J. Adv. Manuf. Technol. 2021, 117, 1327–1349. [Google Scholar] [CrossRef]
- Mayne, D.Q.; Rawlings, J.B.; Rao, C.V.; Scokaert, P.O.M. Constrained model predictive control: Stability and optimality. Automatica 2000, 36, 789–814. [Google Scholar] [CrossRef]
- Atuonwu, J.C.; Leadley, C.; Bosman, A.; Tassou, S.A.; Lopez-Quiroga, E.; Fryer, P.J. Comparative assessment of innovative and conventional food preservation technologies: Process energy performance and greenhouse gas emissions. Innov. Food Sci. Emerg. Technol. 2018, 50, 174–187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).