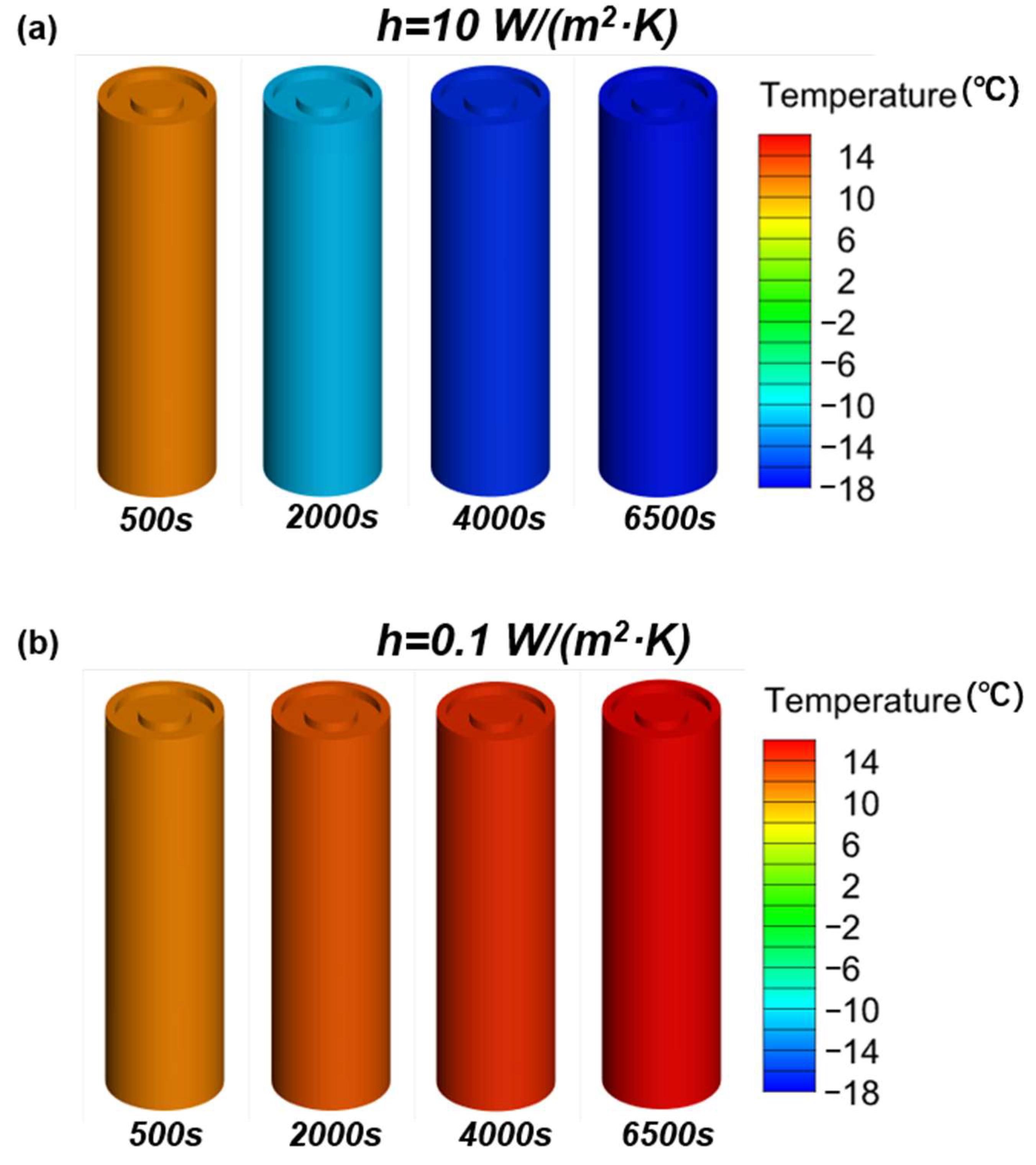
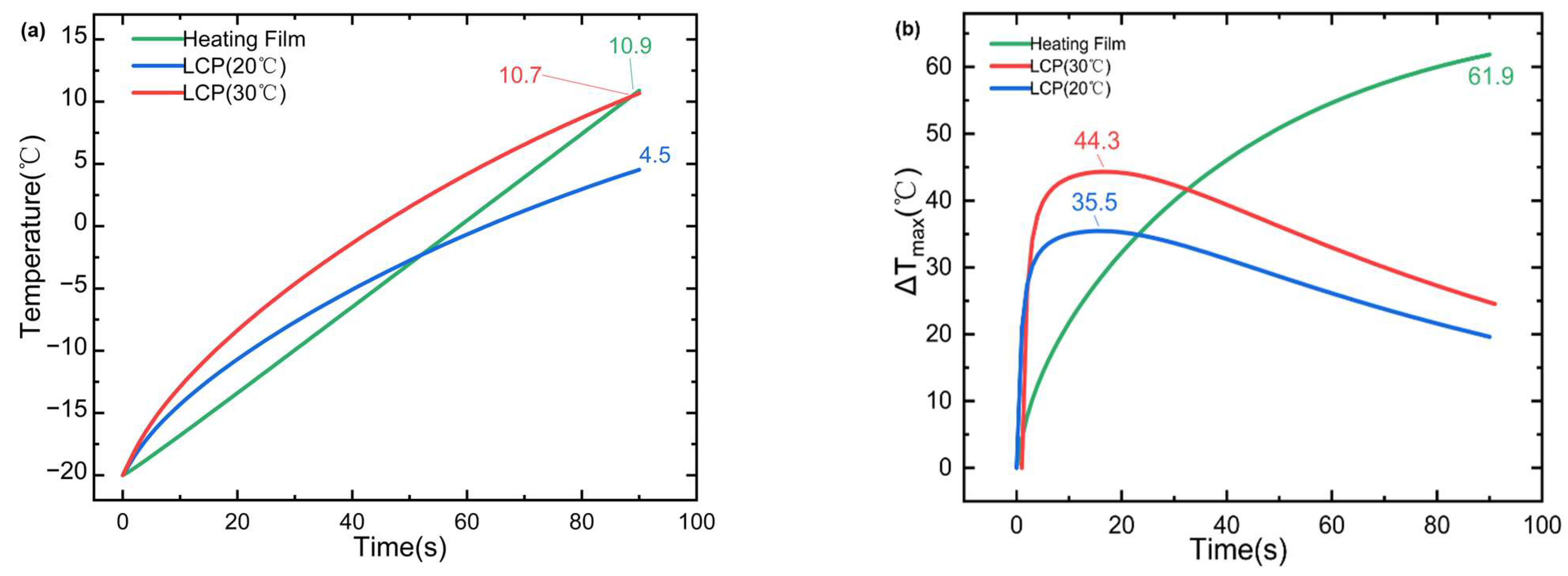
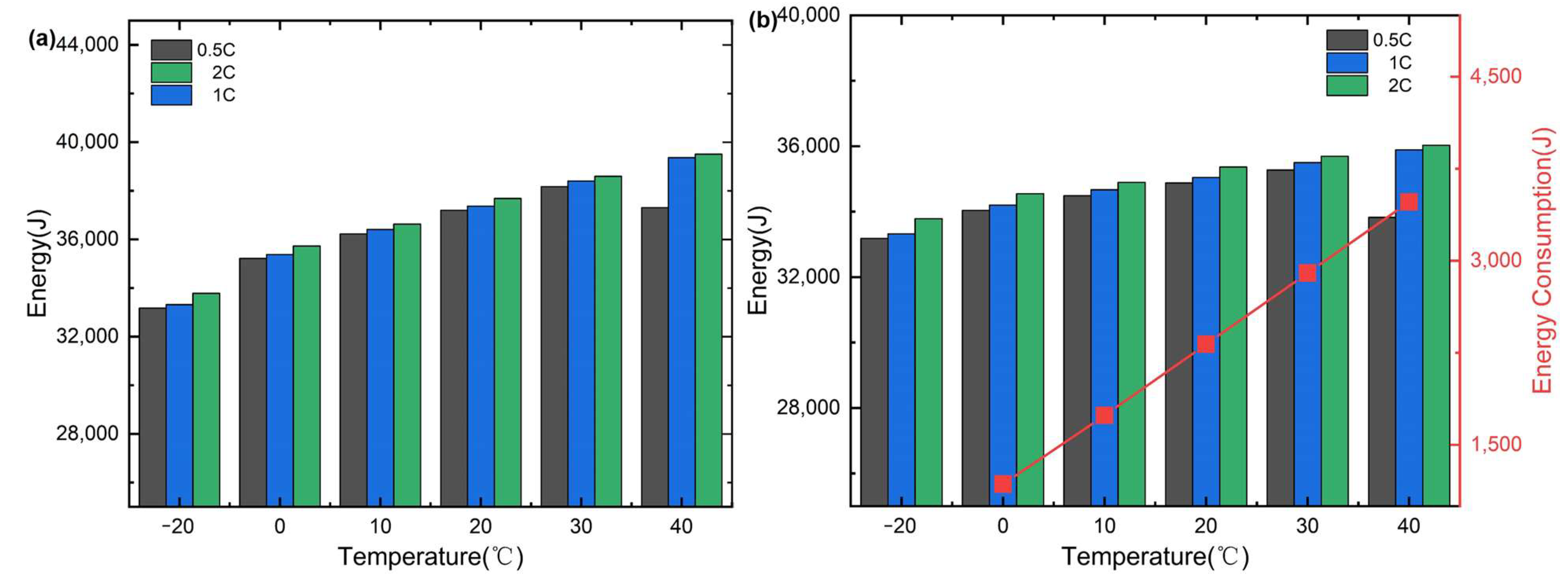
Evidence suggests that the use of internal combustion engine vehicles (ICE) powered by fossil fuels is among the most important factors for dramatic climate changes around the world [
1]. Hybrid electric vehicles (HEVs), plug-in electric vehicles (PHEVs), and pure electric vehicles (EVs) are gradually gaining market share in order to alleviate the air pollution and global warming problems that come with them [
2]. Compared to other types of batteries, lithium-ion batteries (LIB) have higher specific energy, power density, and cycle stability, and are therefore ideal as a driving energy source for electric vehicles [
3]. However, the power, discharge capacity, and cycle life of LIBs are extremely sensitive to the operating temperature, and in order for LIBs to better exhibit excellent cycling performance, Pesaran et al. [
4] limited the optimal operating temperature range of LIBs within 15~35 °C. At high temperatures, the accumulated heat from discharge of LIBs can seriously weaken cycle life and power performance, and excessive temperatures can cause chemical chain exothermic reactions within the LIB [
5], leading to a thermal runaway or even explosion. When the temperature is below 0 °C, the conductivity and diffusivity of the liquid phase is severely reduced [
6,
7], making the electrolyte resistance larger. The decrease in the electrochemical inverse rate of the active particle surface and the solid-phase diffusion coefficient of li-ions [
8] also lead to a larger electrochemical polarization resistance and charge transfer resistance [
9], and these temperature-dependent electrochemical parameters caused the performance of LIBs at low temperatures to fail to meet commercial requirements. In addition, Li et al. [
10] predicted that when the amount of Li
+ involved in charge transfer reactions on the surface of graphite particles during low temperature charging of LIBs is greater than the amount diffusing to the interior of the particles, there is a possibility of precipitation of solid phase Li at the negative electrode/electrolyte interface, which grows into dendrites capable of piercing the diaphragm to cause internal short-circuiting or even explosion of LIBs [
11]. Therefore, how to preheat the battery to a suitable operating temperature at low temperatures has received increased attention across a number of disciplines in recent years. One of the main focuses of current research is the development of a preheated thermal management system (BTMS) with high efficiency and low energy consumption; this is because the preheating method is simple and easy to operate, and there is a lack of battery materials that perform well at both low and normal temperatures [
6,
12].
According to the energy transfer law of the heating process, low-temperature BTMS is classified as either internal or external preheating. External preheating distinguishes four different types, i.e., air preheating, liquid preheating, phase change material (PCM) preheating, and electric heating element preheating. The use of air preheating is a tried-and-true method to heat the battery, with the benefits of simple installation and disassembly, good efficiency, and high safety. Ji et al. [
13] compared the heating time for heating a lithium battery from −20 °C to 20 °C with different load resistances using a coupled heating method of air preheating and lithium-ion battery self-discharge. As the heating resistance drops, the data reveal that the heating time drops from 201 s to 85 s. Wang et al. [
14] designed a heating box with parallel resistive wires inside, and 20 °C air was preheated through the box and then heated the lithium-ion battery. The experimental results presented here suggest that the approach might increase the ambient air temperature by 70 °C in just 8 min. However, the method’s complex structure and lackluster economics make it unattractive. What is more, the high voltage and density of parallel resistance pose a significant risk of accidental electrocution. Although most studies have improved on the simple air preheating method, it is still difficult to improve the heating efficiency due to the low thermal conductivity of air, and in large battery modules, a large temperature gradient is generated in the air flow direction, which has a significant impact on the lifetime of lithium-ion battery modules [
15]. Due to the high specific heat capacity and heat transfer coefficient of the liquid, the battery is able to achieve a greater increase in heating temperature and greater temperature uniformity. In BTMS, the liquid is usually pumped through a pre-set flow channel of the clamping block or plate to heat the battery indirectly [
16], which is used in the market for electric cars like the Volt and Tesla because it works well and is safe [
17]. Li et al. [
18] developed a battery heat power model to simulate the LCP thermal management capability based on the heat generation source term and heat transfer control equation for Li-ion battery. The simulation findings indicate that, with a coolant input temperature of 10 °C, the LCP heating system can heat the battery pack from −30 °C to coolant temperature and sustain a maximum temperature difference of 1 °C within 1 h. Rao et al. [
19] designed a microchannel LCP BTMS with variable contact surface for cylindrical Li-ion battery modules. Inlet velocity of 0.05 m/s is required to produce the cooling effect of a continuous contact surface system, and the simulation results show that the temperature difference may be reduced by 28% using this heating approach. Boiling heat transfer with two-phase flow has higher heat transfer coefficient and better temperature uniformity than single-phase flow. In the literature [
20], a cooling system was designed to immerse the battery cells in a hydrofluoric ether liquid and then fill the cells with porous dielectric material and use a fan to improve the cooling performance of the system, and the results showed that the temperature could be maintained at about 35 °C all the time, even when the batteries were operated at a very high charge/discharge rate. Saw et al. [
21] introduced distilled water mist into dry air and allowed the water mist to evaporate and absorb heat on the cell surface to improve the cooling performance. Results from experiments and computer simulations demonstrated that mist cooling can give a battery pack a more acceptable temperature and homogeneity than dry air cooling. However, complexity of equipment, large power requirements, and the possibility of a battery short-circuit are all drawbacks of the liquid preheating method [
22]. In contrast to air and liquid preheating, phase change materials (PCM) are of interest because they release/absorb a large amount of heat stored as latent heat when they undergo phase change [
23]. Zhong et al. [
24] used composite phase change material (CPCM) to improve the heat dissipation performance of PCM at high temperature and wound resistive wire outside the cylindrical Li-ion battery module to heat the battery at low temperature. The connection mechanism can raise the battery pack’s central temperature by 40 °C in 300 s, according to experiments. Additionally, it can keep the temperature of a 5C battery pack that has been discharged to below 45 °C in an operating environment of 40 °C. This has a high heating rate and temperature control performance. Bai et al. [
25] designed a PCM/microchannel water-cooled plate thermal management structure with upper and lower layered structure in the middle of the square battery pack, which can limit the battery pack temperature to below 40 °C at 2C discharge multiplier, but the disadvantage is that the temperature difference of the battery pack is not limited to 5 °C. PCM’s poor heat dissipation at high temperatures is a result of its low thermal conductivity, and its shortcomings of heavy weight and high cost have prevented it from gaining popularity in the EV industry. In exterior heating, electric heating element heating is a system with greater heating efficiency, lower cost, and a simpler structure. Most other BTMS also require electric heating components for low-temperature heat transmission. Mostafavi et al. [
26] used a thermoelectric cooling film using the Peltier effect for dual thermal management of a Li-ion battery pack. Experiment results showed that at 5C, the cooling film could almost completely absorb the heat released by the battery pack, and the temperature rise rate of the heated battery could reach 2 °C/s by reversing the electrodes of the cooling film. Kang et al. [
27] placed a cylindrical Li-ion battery pack into an aluminum square battery tray with resistance heaters attached to both sides of the tray. The heaters heated the battery pack at a heating boundary condition of 20 W/m
2 at −32 °C and further reduced the temperature difference from 10 °C to 1 °C by optimizing the aluminum tray design and heating area. Internal heating is a method of producing heat spontaneously by applying excitation to a lithium-ion battery and utilizing the battery’s high internal resistance at low temperatures [
28]. Unlike the external heating method, the internal heating method does not have to deal with too much thermal conductivity and thermal resistance. It is also not limited by the shape of the cell and can get a more even temperature [
29]. So far, self-heating, mutual pulse heating, and AC heating are the three main ways that BTMS has investigated for heating the inside. A widely studied strategy applied to the fast preheating of Li-ion batteries is the self-heating structure. Wang et al. [
30] proposed the structure of embedding thin nickel sheets inside the lithium-ion battery to form a self-heating circuit of the battery. This method can turn on/off the heating function at any time and can heat the battery from −30 °C to 0 °C in 29.6 s with high heating efficiency, but it is not popular now because of the change of the internal structure of the battery, which makes it difficult for mass production and quality inspection in assembly line production. In order to achieve a low decay rate fast charge in 10 min, the above “sandwich” self-heating structure proposed by Wang’s team is applied to the proposed asymmetric temperature modulated fast charging idea by Yang et al. [
31,
32]. The result is that the 10 min fast charging method at 60 °C is able to increase the cycle life from 60 cycles to 1700 cycles compared to 6 C charging at 26 °C. Additionally, for the fast cold start, Ye et al. [
33] designed a self-heating structure by adding uniform ultra-thin nickel sheets as thermal regulators inside an all-solid-state battery, and a 5 kWh square battery pack was able to uniformly increase the battery temperature from room temperature to up to 90 °C in 10.5 s with an energy consumption of only 5.86% of the battery capacity. One of the most efficient heating methods is alternating current (AC) heating, which, unlike self-heating and inter-pulse heating, employs an external power source applied to the internal resistance of the Li-ion battery to generate heat [
34]. Li et al. [
35] simulated the heating of 18650 cylindrical Li-ion batteries using sinusoidal alternating current (SAC) signals based on a hierarchical thermal model, and the capacity and power of the batteries could be increased by 45% and 12% after preheating the cells using AC signals within 100 Hz at −20 °C. However, a note of caution is a temperature difference of 6 °C between the inside and outside of the battery. Besides, charging and discharging LIBs at low temperatures will speed up the deposition of lithium ions inside the battery. How the internal heating makes the battery produce heat depends entirely on the multidimensional coupled electrochemical reactions inside the battery. The lithium plating of the negative electrode and the battery’s cycle life are two aspects of their utilization that necessitate investigation using electrochemical modeling owing to this restriction.
Thermal modeling of Li-ion batteries can avoid complicated experimental operations and safety issues and reduce time costs. The electrochemical-thermal coupling model (ECT), equivalent circuit model (ECM), and NTGK empirical model are widely used mathematical models to describe the charging and discharging and heat generation characteristics of Li-ion battery. Zhang et al. [
36] established an equivalent circuit model (ECM) to simulate the preheating of 18650 battery at low temperature using sinusoidal AC signal, analysis showed that at a rate of 1 °C/min, there is essentially no capacity loss or temperature change within the battery. In literature [
37], H
2O-Al
2O
3 nanofluid has been used to submerge and cool cylindrical lithium battery modules, which were externally supplemented with a fan to improve the cooling performance of the system, and the cooling effect of the cooling system was simulated by a 1D electrochemical model and a 3D CFD model for different cell arrays and airflow inlet and outlet locations. The results showed that the cooling strategy could reduce the maximum temperature of the battery stack by 16–24 K. Patil et al. [
38] used a multi-scale multi-domain (MSMD) approach to simulate a square battery pack immersed in a flowing dielectric using the NTGK mathematical model, and showed that the maximum battery pack temperature can still be limited to 40 °C at a discharge multiplier of 5C. Although internal heating can bring good temperature rise rate and temperature uniformity, it has not been applied in practice because its effect on the safety of Li-ion battery has not been fully studied.
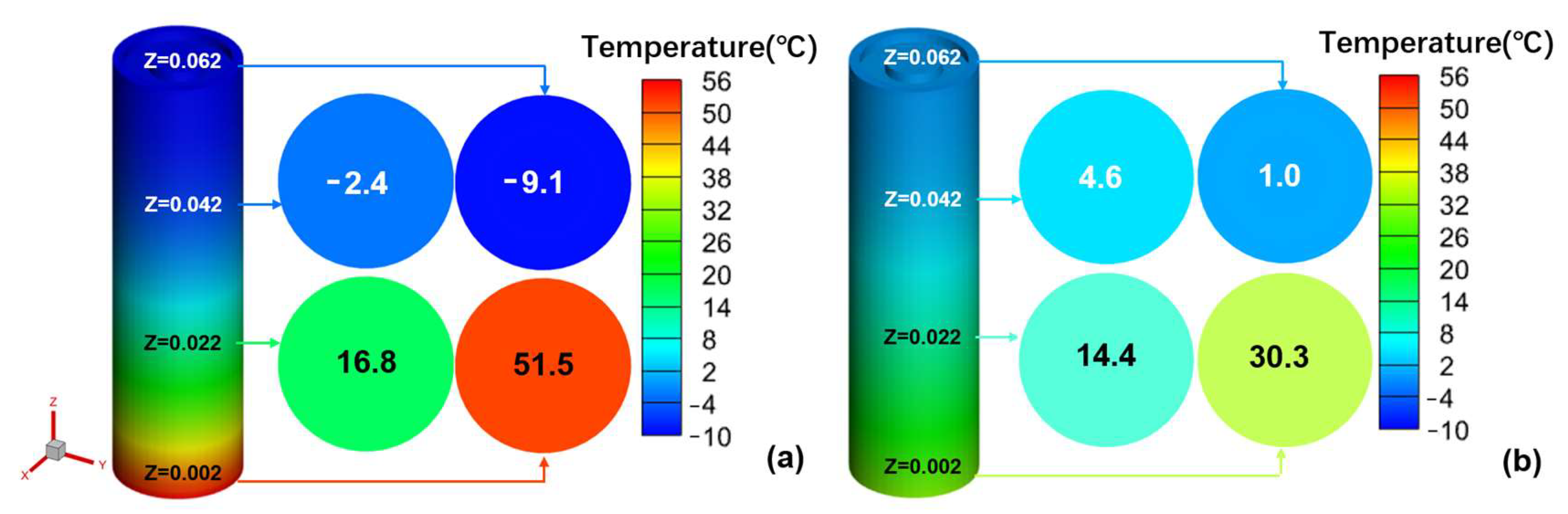
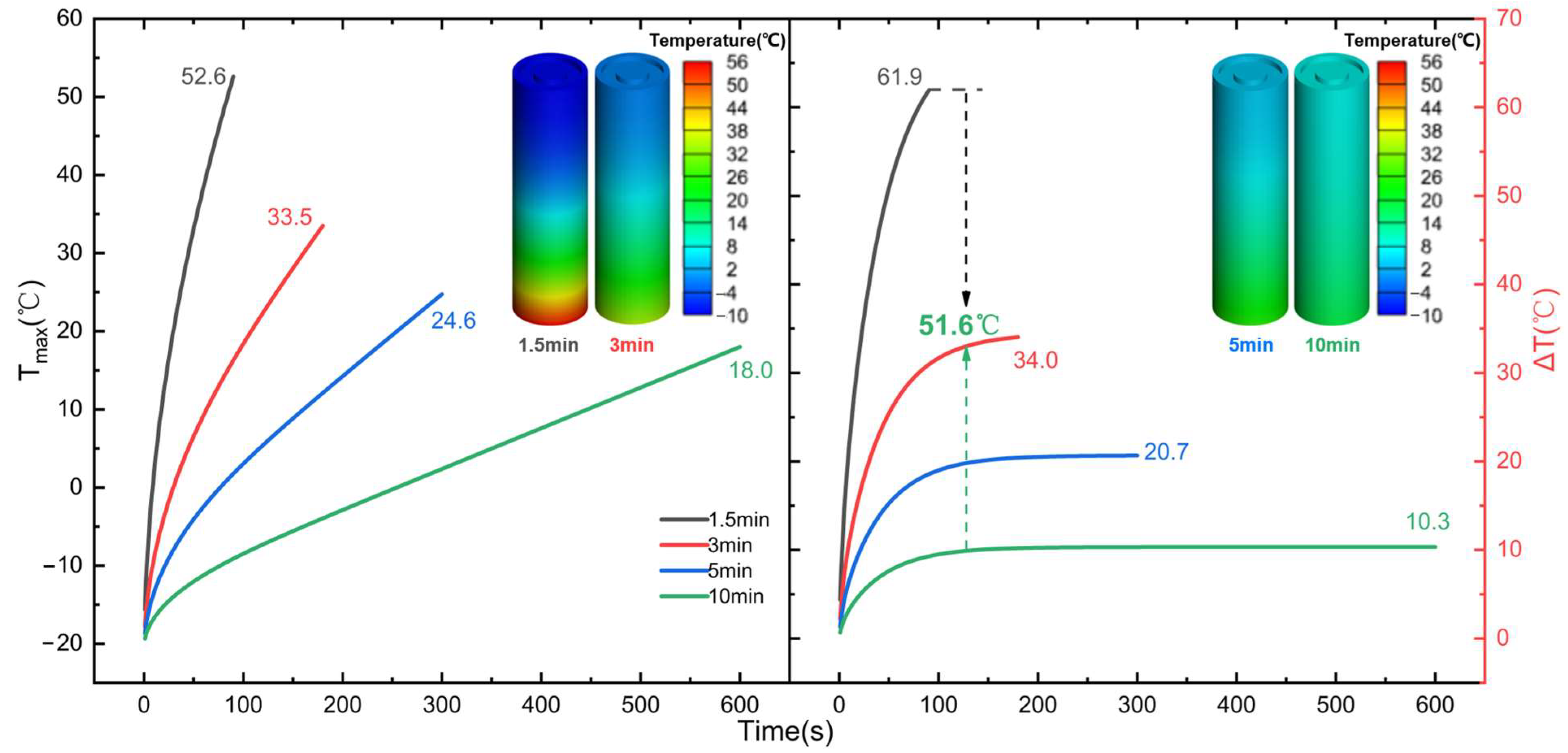
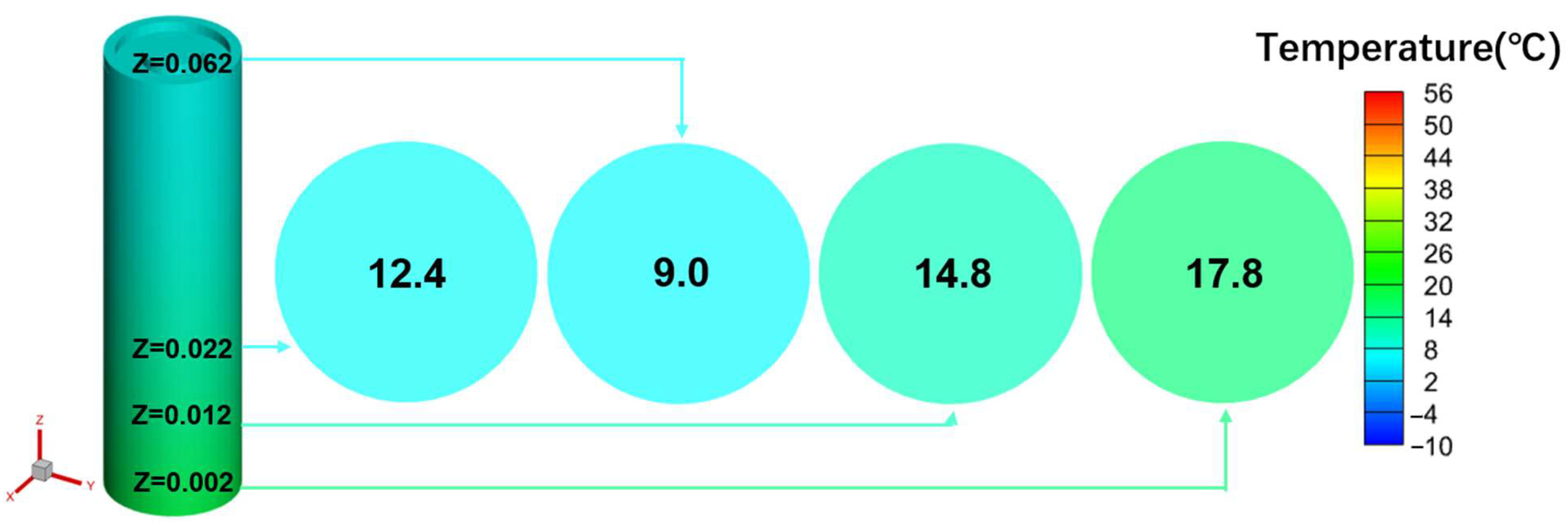
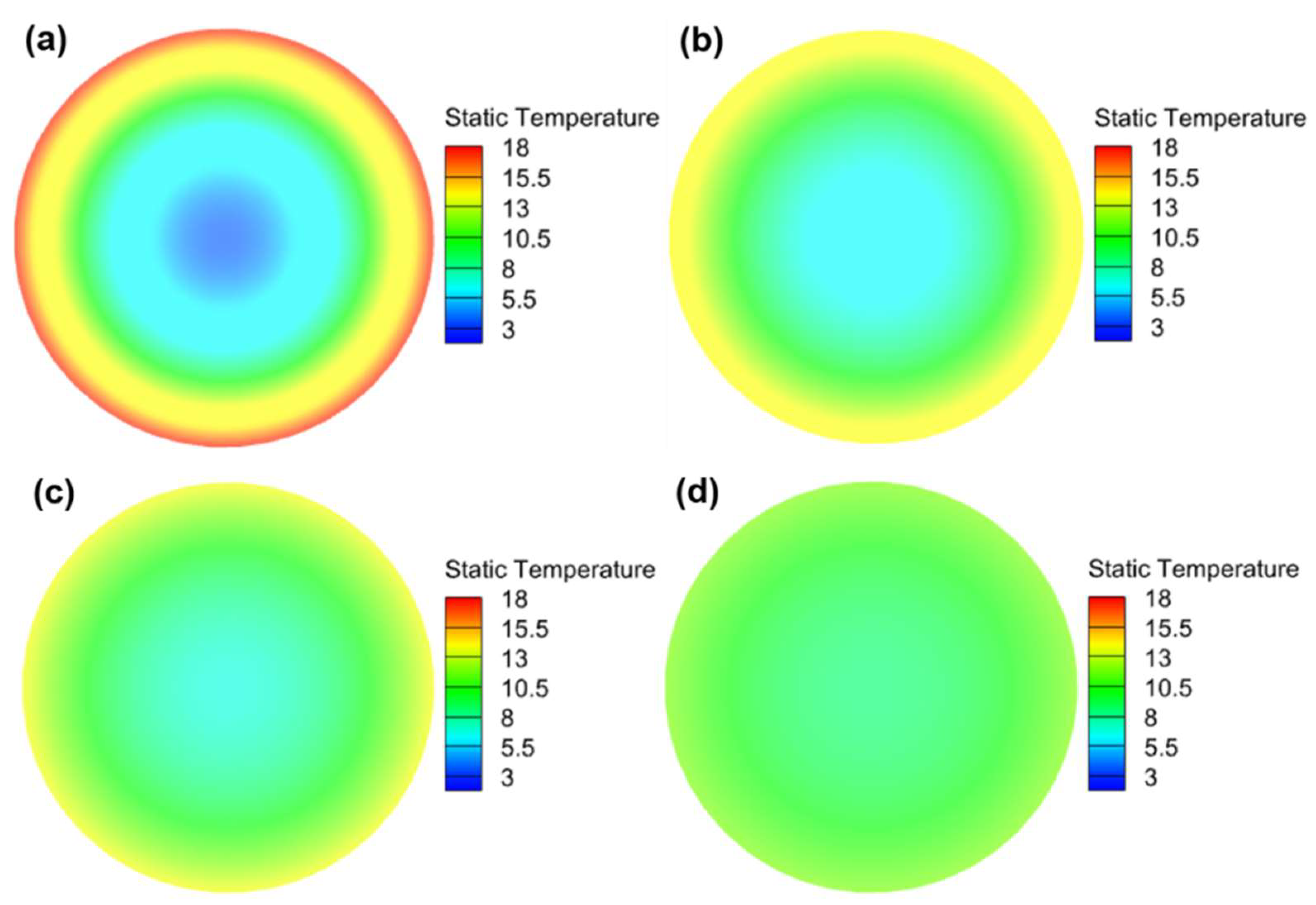
Most studies on low-temperature preheating ignore the problem of the battery’s energy balance; therefore, this paper selects the LCP preheating and HF preheating methods, which are more maturely applied in practice, to analyze the feasibility of low-temperature cold start of Li-ion battery from the standpoint of energy balance. The ECM, NTGK, and Newman models can be used as electrochemical models, and the Newman model, also known as the P2D model, provides a more complete reduction of the transport processes of lithium ions inside the electrode and electrolyte and the electrochemical reactions when the electrode particles are removed/inserted, and the calculated results are more accurate. However, parameters are hard to modify, and the calculation is sophisticated, both of which reduce the tool’s effectiveness. Therefore, this study models the lithium-ion battery in the electrochemical domain using the NTGK empirical model because of its simplicity, its easily modifiable parameters, and its high efficiency of numerical solution. Thermal modeling of Li-ion battery was performed within the MSMD framework to calculate the temperature and energy of Li-ion battery at various temperatures and discharge rates, and the model’s feasibility was tested experimentally. Following that, the parameters influencing the cell temperature uniformity of HF heating were investigated. Finally, the problem of precise temperature control encountered with conventional electric heating film heating was explored, and the variables and optimal level combinations that significantly affect the temperature difference and temperature rise rate were derived using an orthogonalization test method.