The Effects of Cracking Ratio on Ammonia/Air Non-Premixed Flames under High-Pressure Conditions Using Large Eddy Simulations
Abstract
:1. Introduction
2. Experimental and Numerical Setup
2.1. Experimental Details
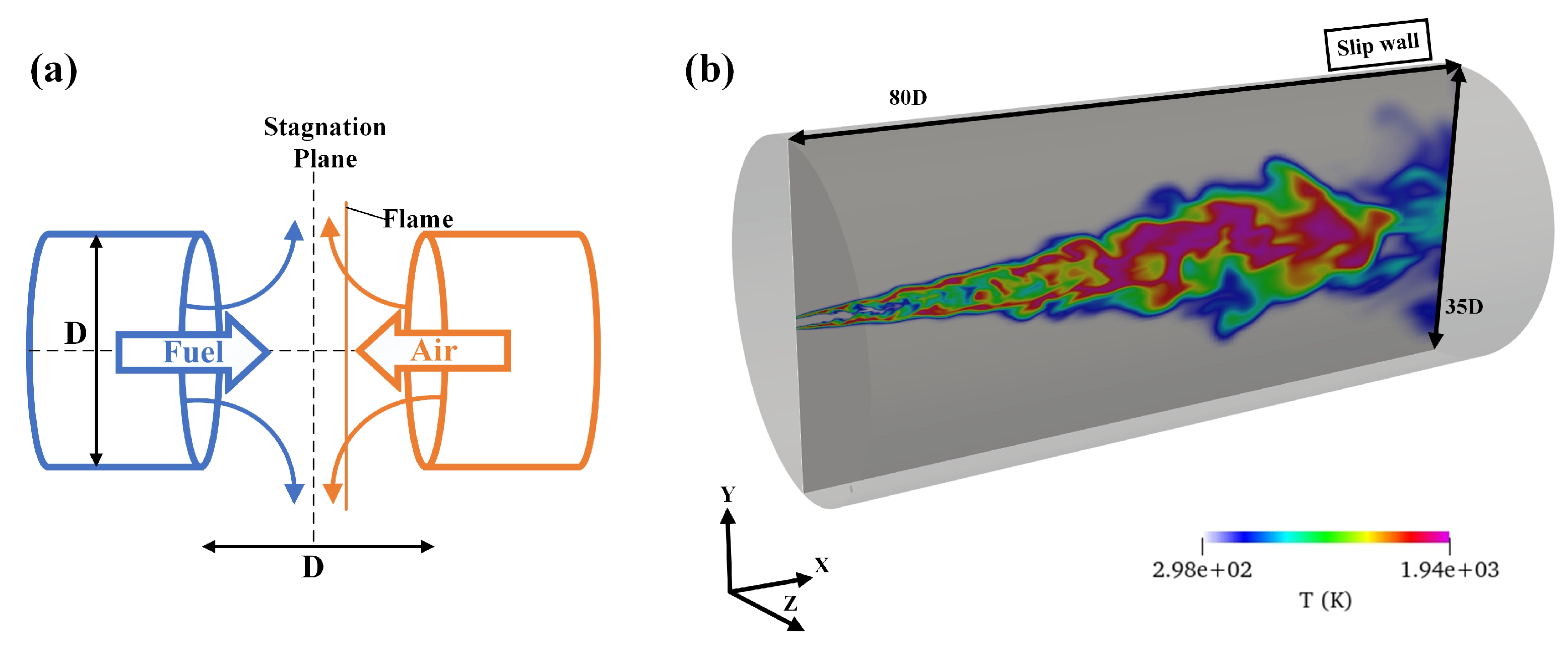
2.2. Computational Setup
3. Numerical Methodology
3.1. Conservation Equations for LES
3.2. Flamelet/Progress Variable (FPV) Model
3.3. The NO Prediction Model
4. Results and Discussions
4.1. 1D Laminar Counterflow Flame Simulation
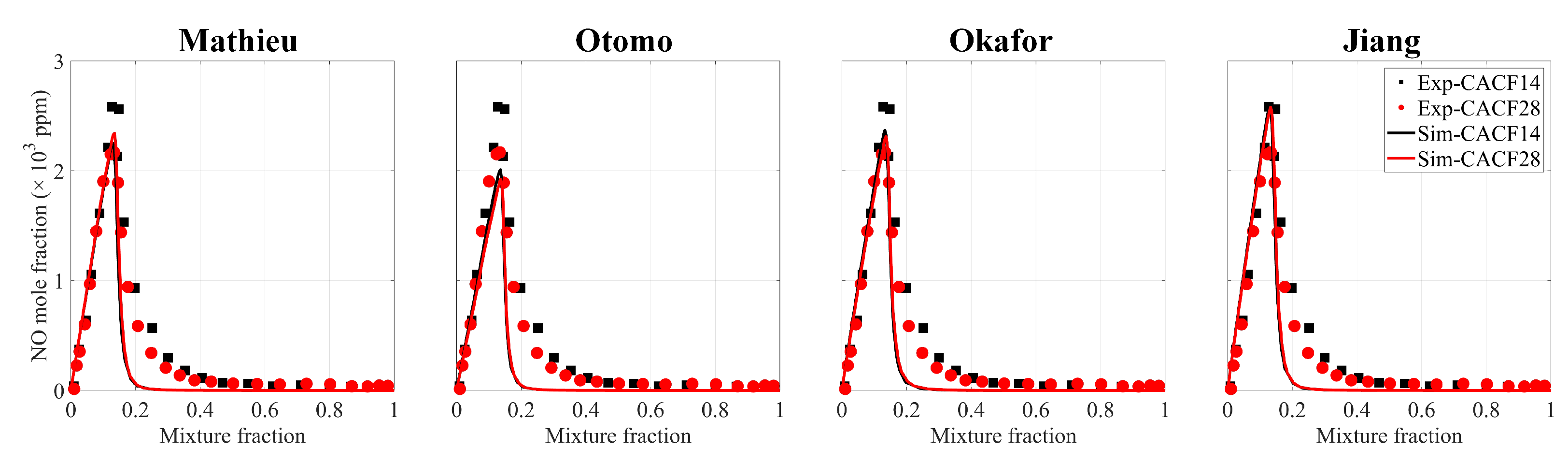
4.1.1. Mechanism Comparison
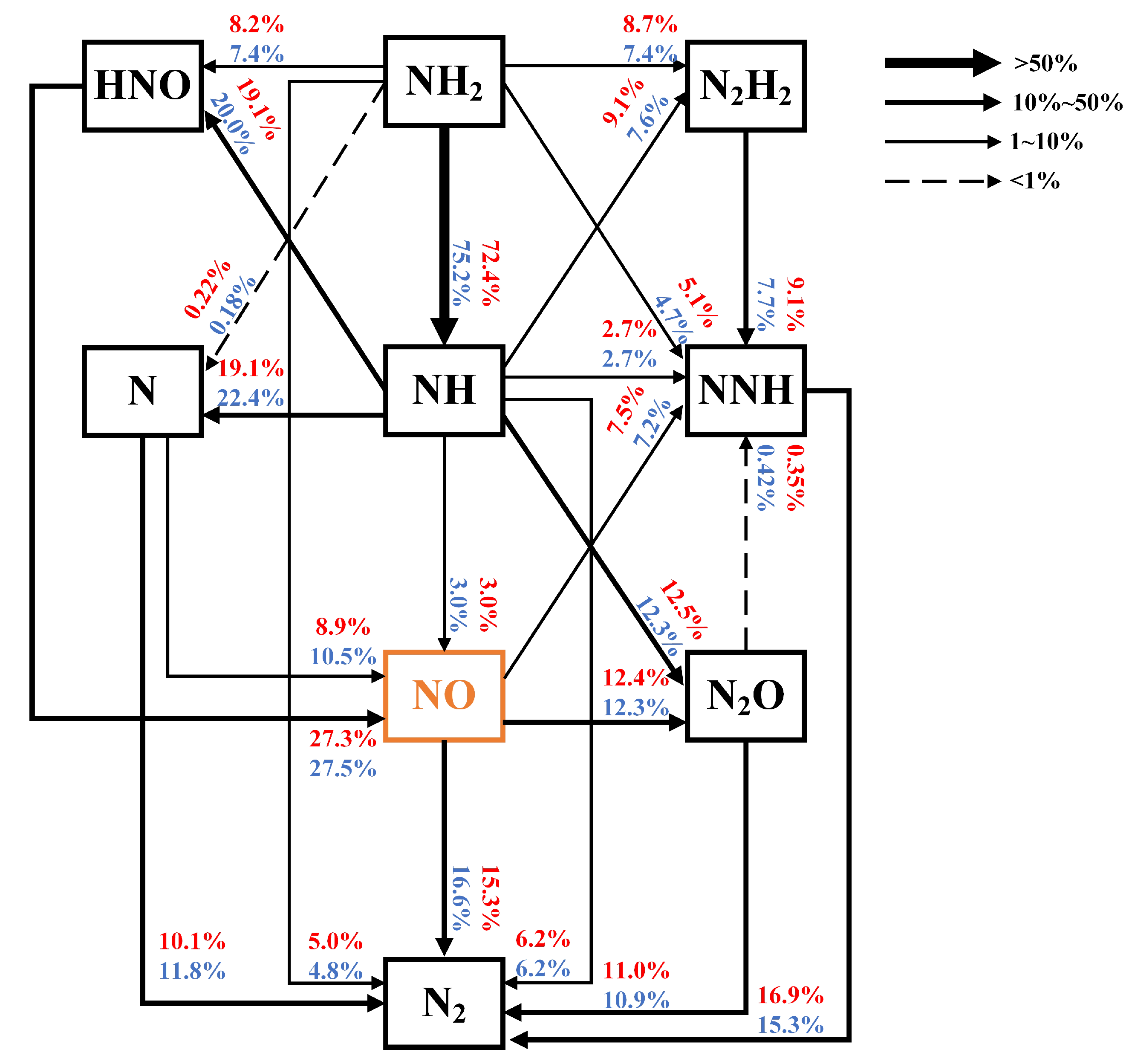
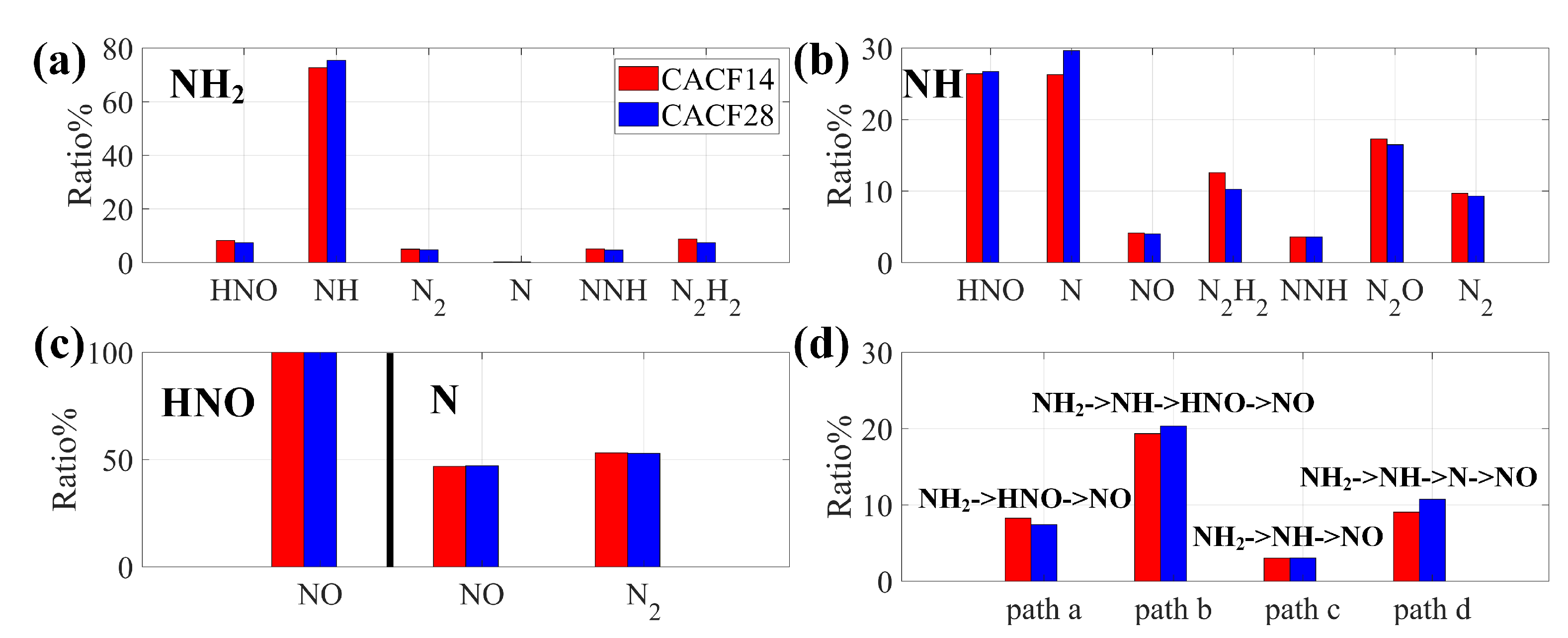
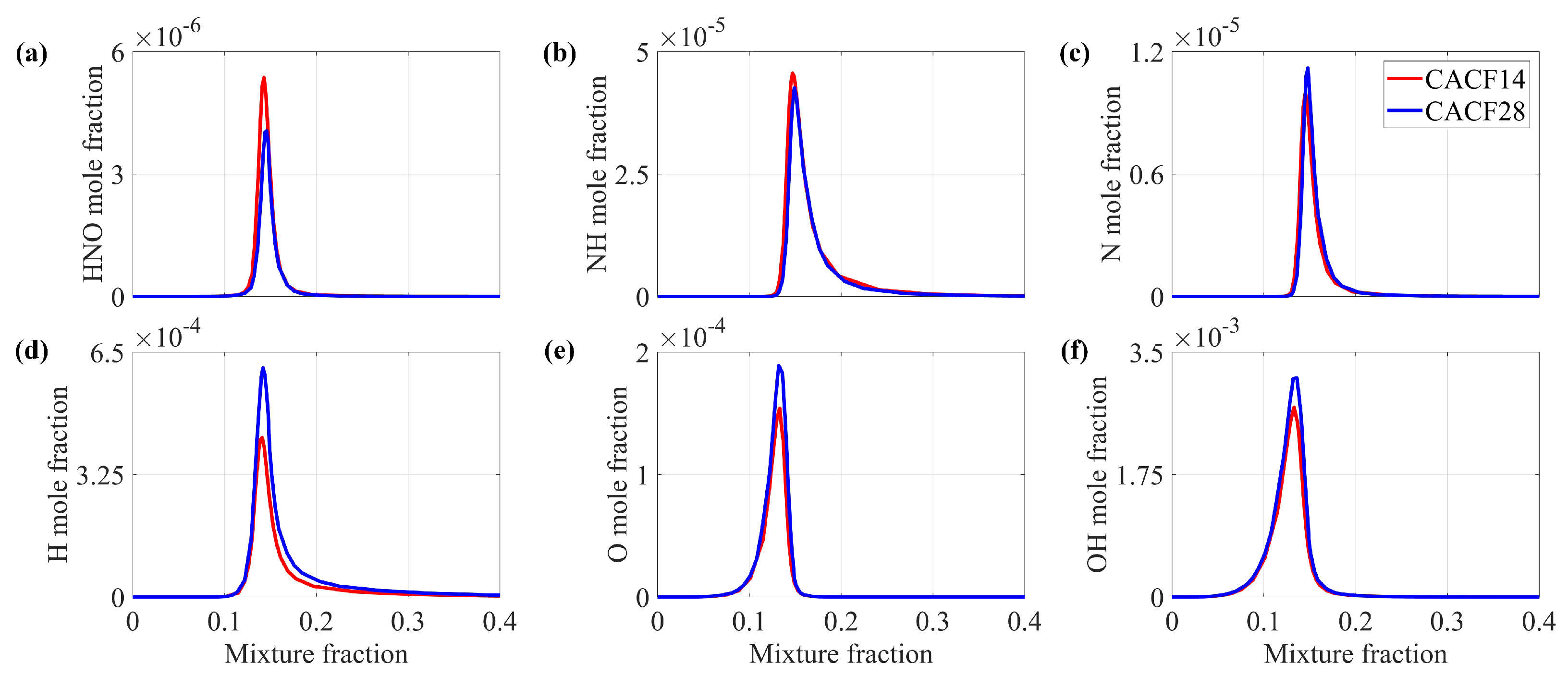
4.1.2. Reaction Path Analysis
4.2. 3D Turbulent Jet Flame Simulation
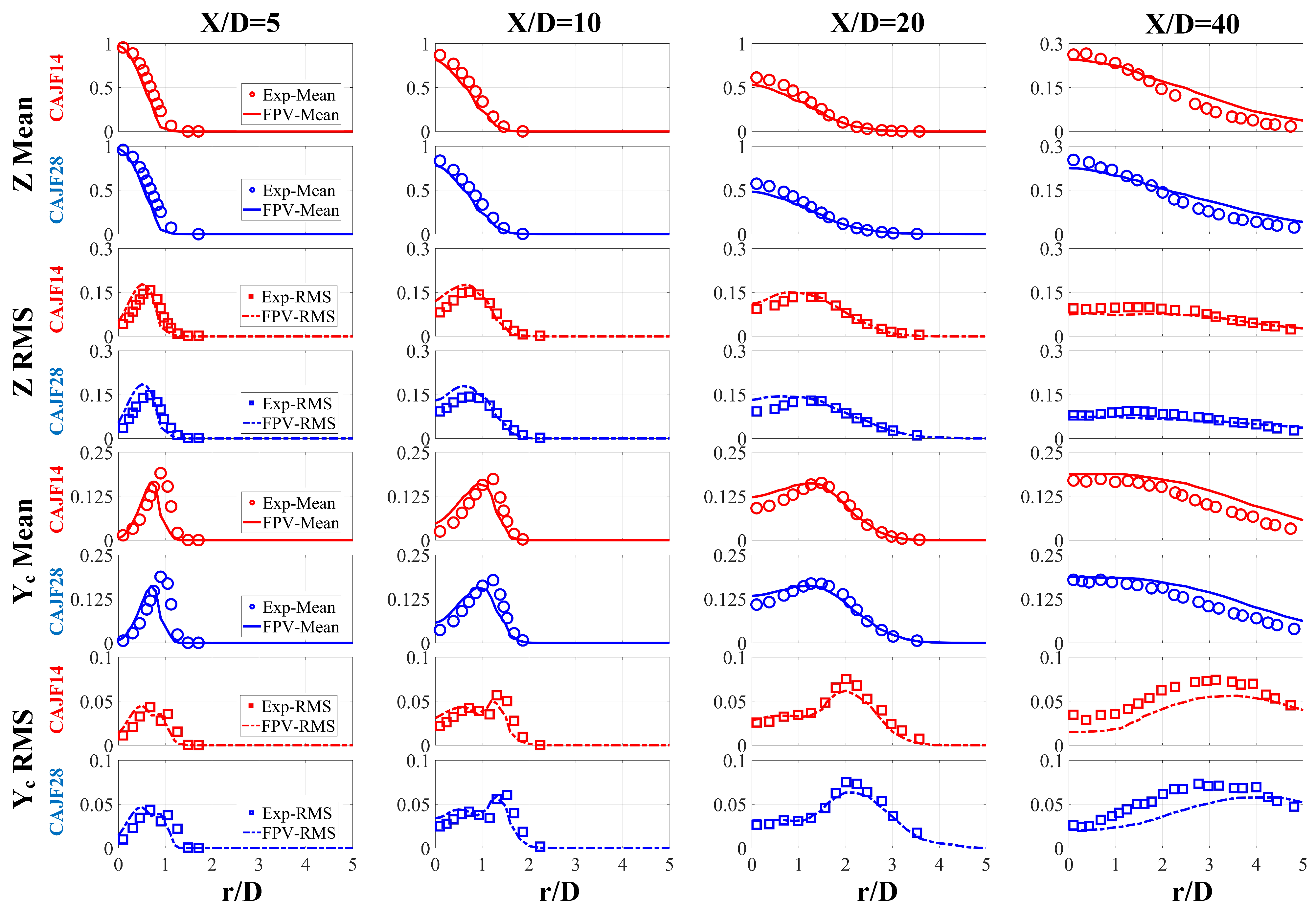
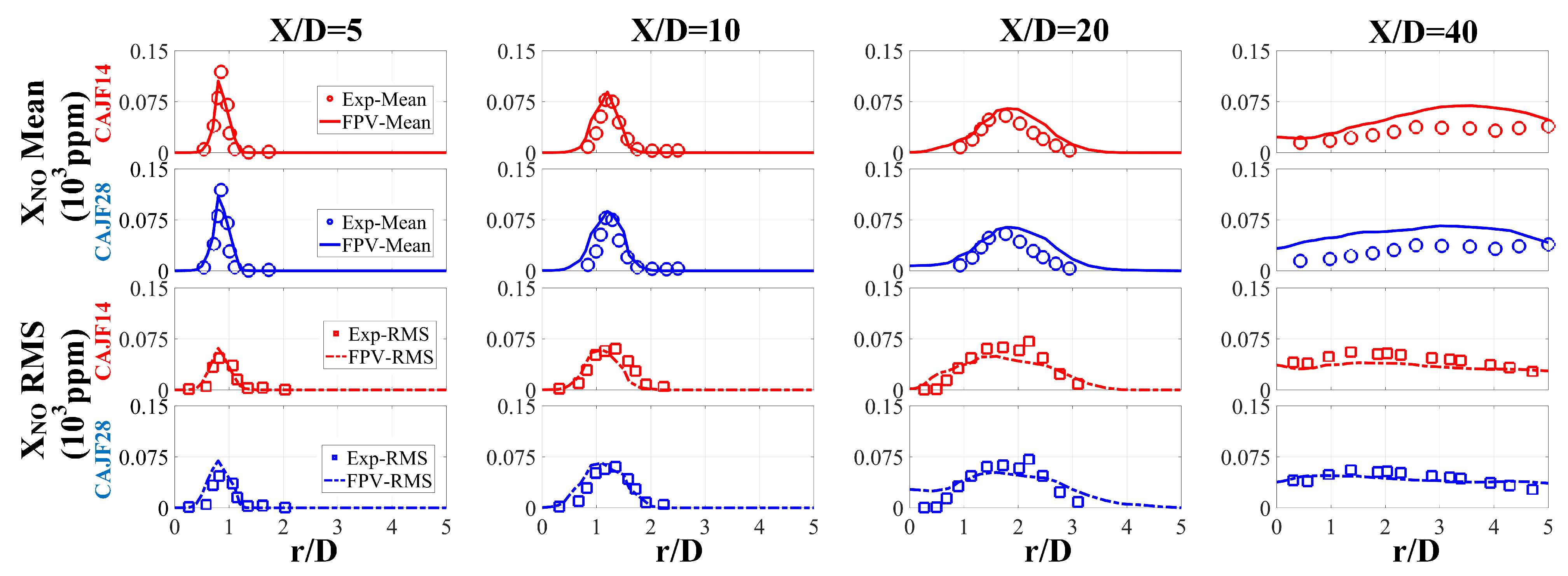
4.2.1. Simulation Verification
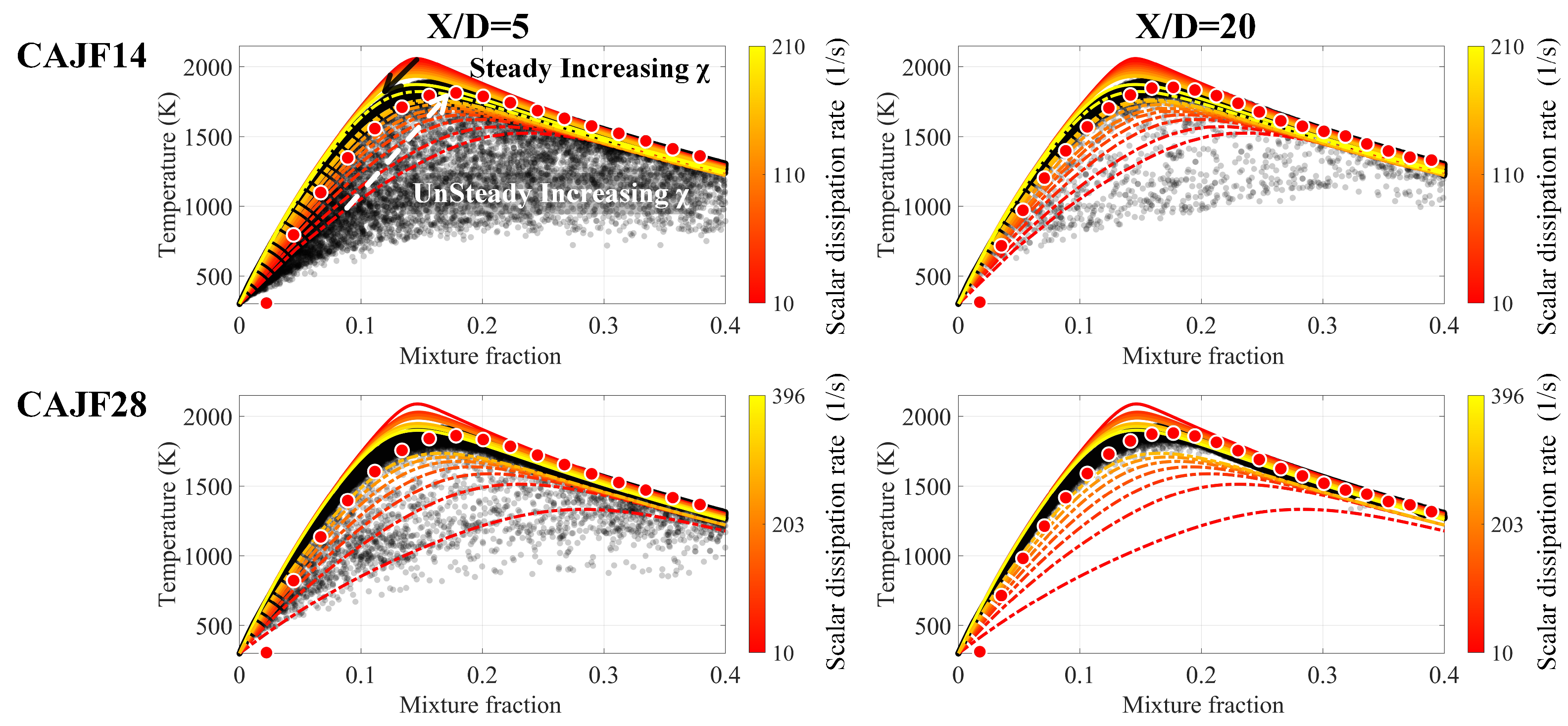
4.2.2. Flame structure analysis
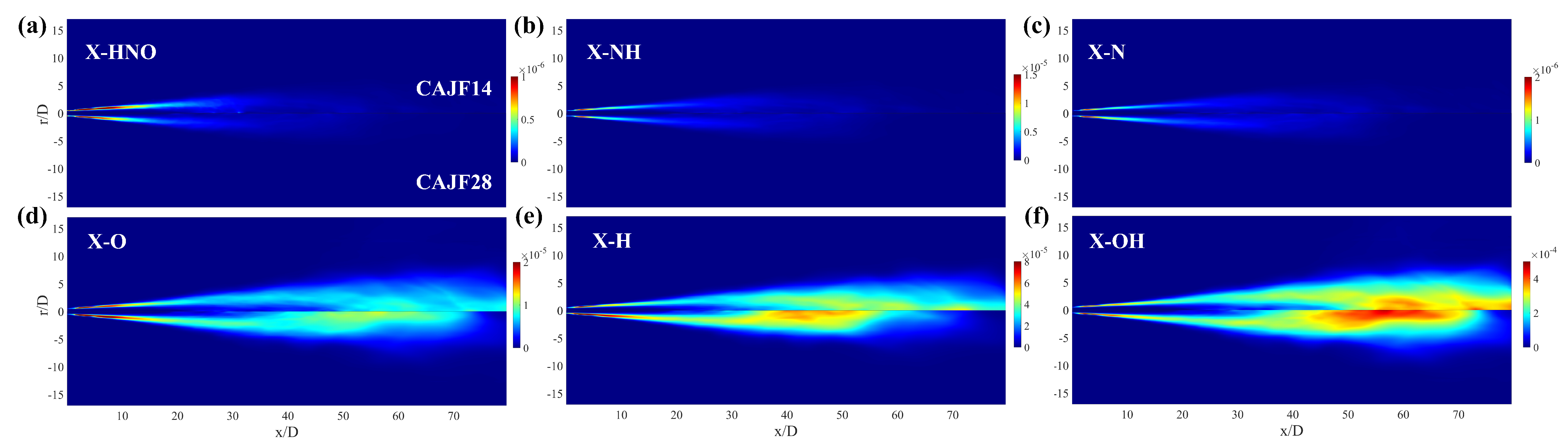
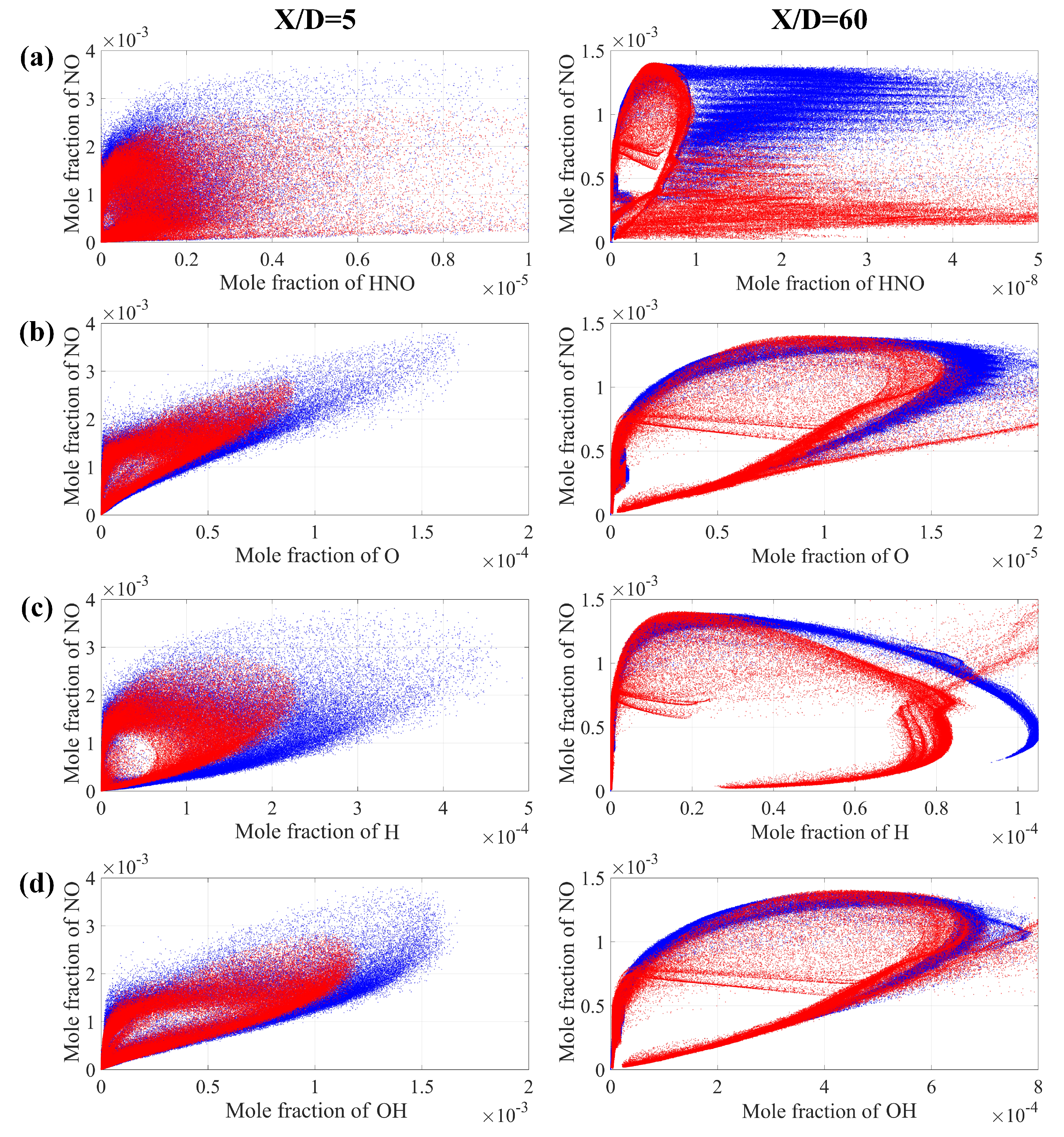
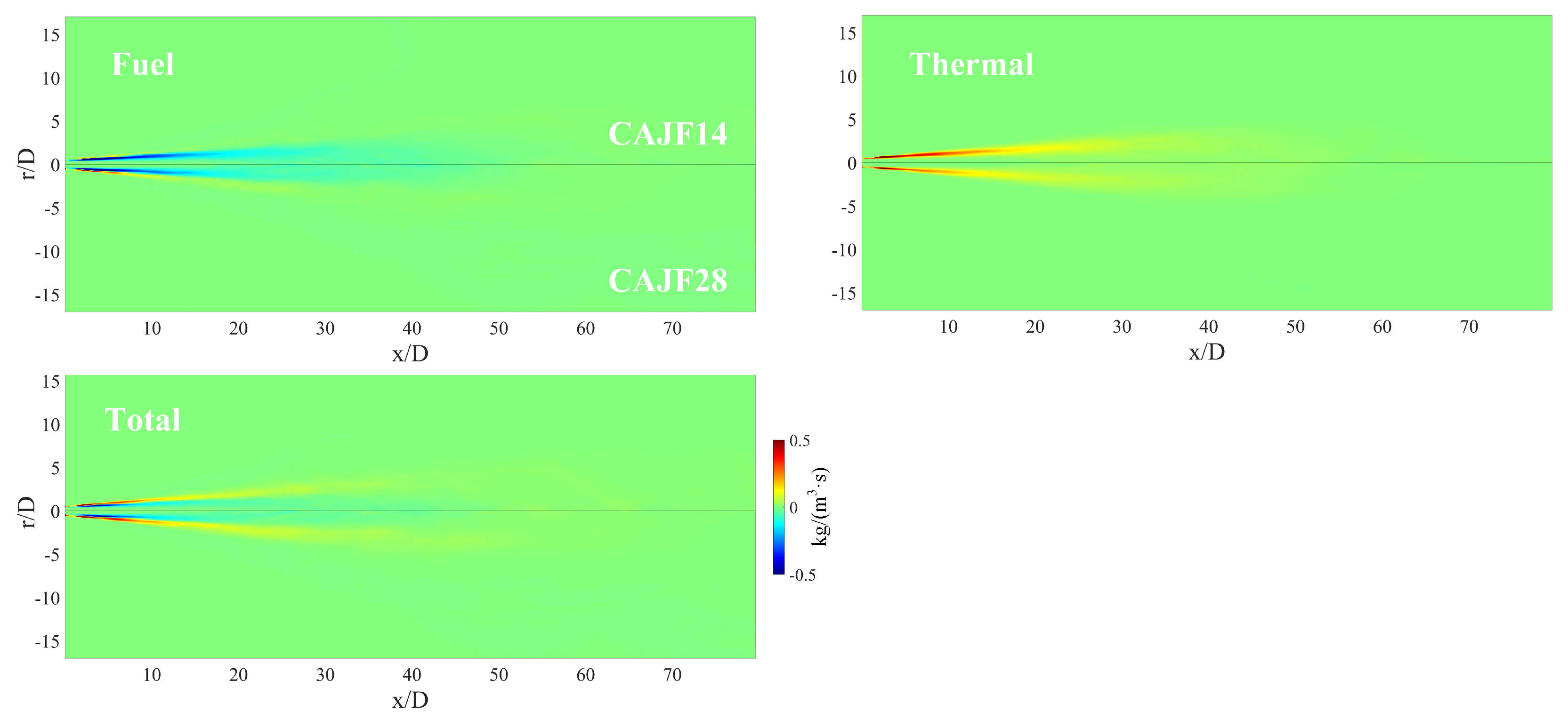
4.2.3. Formation Mechanism
5. Conclusions
- Through nitrogen flow analyses, the conversion of nitrogen-containing species and the NO pathways were examined. It was found that the species that contributes the most to generation is , and the most important pathway for NO generation is .
- The simulation and experimental results agree quite well, and the simulations provided fairly accurate predictions for , proving the applicability of the FPV method in hydrogen–ammonia flames. The flame structure and formation of turbulent jet flames were examined. It was found that with increasing cracking ratio, not only is the flame reactivity enhanced but the generation of is also increased for the turbulent flames.
- The correlation between important radical concentrations and concentration was examined. We showed that the correlation between and is weaker when compared to that of and radicals such as , and throughout the entire field. Moreover, the increased cracking ratio also results in a higher level of radical concentrations.
- The distribution of source terms were also explored. It was found that the source term has a higher absolute value upstream, and the absolute value rapidly decreases with the increase in the streamwise distance. The total source term is positive in the fuel-lean zone and shows negative values in the fuel-rich zone. In addition, the fuel in most regions is negative, while the thermal is positive.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and Future Role of Haber–Bosch Ammonia in a Carbon-Free Energy Landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Yapicioglu, A.; Dincer, I. A Review on Clean Ammonia as a Potential Fuel for Power Generators. Renew. Sustain. Energy Rev. 2019, 103, 96–108. [Google Scholar] [CrossRef]
- Awad, O.I.; Zhou, B.; Harrath, K.; Kadirgama, K. Characteristics of NH3/H2 Blend as Carbon-Free Fuels: A Review. Int. J. Hydrogen Energy 2022. is press. [Google Scholar] [CrossRef]
- Hayakawa, A.; Goto, T.; Mimoto, R.; Arakawa, Y.; Kudo, T.; Kobayashi, H. Laminar Burning Velocity and Markstein Length of Ammonia/Air Premixed Flames at Various Pressures. Fuel 2015, 159, 98–106. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.K.A.; Okafor, E.C. Science and Technology of Ammonia Combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Kobayashi, N.; He, Z.; Nagai, Y. Study on Using Hydrogen and Ammonia as Fuels: Combustion Characteristics and NOx Formation: Hydrogen and Ammonia as Fuels. Int. J. Energy Res. 2014, 38, 1214–1223. [Google Scholar] [CrossRef]
- Mathieu, O.; Petersen, E.L. Experimental and Modeling Study on the High-Temperature Oxidation of Ammonia and Related NOx Chemistry. Combust. Flame 2015, 162, 554–570. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Zhou, H.; Ren, Z. Analysis of Air-Staged Combustion of NH3/CH4 Mixture with Low NOx Emission at Gas Turbine Conditions in Model Combustors. Fuel 2019, 237, 50–59. [Google Scholar] [CrossRef]
- Xiao, H.; Lai, S.; Valera-Medina, A.; Li, J.; Liu, J.; Fu, H. Study on Counterflow Premixed Flames Using High Concentration Ammonia Mixed with Methane. Fuel 2020, 275, 117902. [Google Scholar] [CrossRef]
- Kurata, O.; Iki, N.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Kobayashi, H.; Hayakawa, A. Performances and Emission Characteristics of NH3–Air and NH3CH4–Air Combustion Gas-Turbine Power Generations. Proc. Combust. Inst. 2017, 36, 3351–3359. [Google Scholar] [CrossRef]
- Khateeb, A.A.; Guiberti, T.F.; Zhu, X.; Younes, M.; Jamal, A.; Roberts, W.L. Stability Limits and Exhaust NO Performances of Ammonia-Methane-Air Swirl Flames. Exp. Therm. Fluid Sci. 2020, 114, 110058. [Google Scholar] [CrossRef]
- Otomo, J.; Koshi, M.; Mitsumori, T.; Iwasaki, H.; Yamada, K. Chemical Kinetic Modeling of Ammonia Oxidation with Improved Reaction Mechanism for Ammonia/Air and Ammonia/Hydrogen/Air Combustion. Int. J. Hydrogen Energy 2018, 43, 3004–3014. [Google Scholar] [CrossRef]
- Frigo, S.; Gentili, R. Analysis of the Behaviour of a 4-Stroke Si Engine Fuelled with Ammonia and Hydrogen. Int. J. Hydrogen Energy 2013, 38, 1607–1615. [Google Scholar] [CrossRef]
- Comotti, M.; Frigo, S. Hydrogen Generation System for Ammonia–Hydrogen Fuelled Internal Combustion Engines. Int. J. Hydrogen Energy 2015, 40, 10673–10686. [Google Scholar] [CrossRef]
- Mei, B.; Zhang, J.; Shi, X.; Xi, Z.; Li, Y. Enhancement of Ammonia Combustion with Partial Fuel Cracking Strategy: Laminar Flame Propagation and Kinetic Modeling Investigation of NH3/H2/N2/Air Mixtures up to 10 Atm. Combust. Flame 2021, 231, 111472. [Google Scholar] [CrossRef]
- Shohdy, N.N.; Alicherif, M.; Lacoste, D.A. Transfer Functions of Ammonia and Partly Cracked Ammonia Swirl Flames. Energies 2023, 16, 1323. [Google Scholar] [CrossRef]
- Mercier, A.; Mounaïm-Rousselle, C.; Brequigny, P.; Bouriot, J.; Dumand, C. Improvement of SI Engine Combustion with Ammonia as Fuel: Effect of Ammonia Dissociation Prior to Combustion. Fuel Commun. 2022, 11, 100058. [Google Scholar] [CrossRef]
- Kim, N.; Lee, M.; Park, J.; Park, J.; Lee, T. A Comparative Study of NOx Emission Characteristics in a Fuel Staging and Air Staging Combustor Fueled with Partially Cracked Ammonia. Energies 2022, 15, 9617. [Google Scholar] [CrossRef]
- Tang, H.; Yang, C.; Wang, G.; Krishna, Y.; Guiberti, T.F.; Roberts, W.L.; Magnotti, G. Scalar Structure in Turbulent Non-Premixed NH3/H2/N2 Jet Flames at Elevated Pressure Using Raman Spectroscopy. Combust. Flame 2022, 244, 112292. [Google Scholar] [CrossRef]
- Wang, G.; Tang, H.; Yang, C.; Magnotti, G.; Roberts, W.L.; Guiberti, T.F. Quantitative Laser-Induced Fluorescence of NO in Ammonia-Hydrogen-Nitrogen Turbulent Jet Flames at Elevated Pressure. Proc. Combust. Inst. 2023, 39, 1465–1474. [Google Scholar] [CrossRef]
- Abdelwahid, S.; Malik, M.R.; Al Kader Hammoud, H.A.; Hernández-Pérez, F.E.; Ghanem, B.; Im, H.G. Large Eddy Simulations of Ammonia-Hydrogen Jet Flames at Elevated Pressure Using Principal Component Analysis and Deep Neural Networks. Combust. Flame 2023, 253, 112781. [Google Scholar] [CrossRef]
- Tang, J. Study and Application of NOx Prediction Model and Flamelet Tabulation Method for Aeroengine Combustion Chamber. Ph.D. Thesis, Northwestern Polytechnical University, Xi’an, China, 2018. [Google Scholar]
- Tang, Y.; Raman, V. Large Eddy Simulation of Premixed Turbulent Combustion Using a Non-Adiabatic, Strain-Sensitive Flamelet Approach. Combust. Flame 2021, 234, 111655. [Google Scholar] [CrossRef]
- An, Z.; Zhang, M.; Zhang, W.; Mao, R.; Wei, X.; Wang, J.; Huang, Z.; Tan, H. Emission Prediction and Analysis on CH4/NH3/Air Swirl Flames with LES-FGM Method. Fuel 2021, 304, 121370. [Google Scholar] [CrossRef]
- Honzawa, T.; Kai, R.; Okada, A.; Valera-Medina, A.; Bowen, P.J.; Kurose, R. Predictions of NO and CO Emissions in Ammonia/Methane/Air Combustion by LES Using a Non-Adiabatic Flamelet Generated Manifold. Energy 2019, 186, 115771. [Google Scholar] [CrossRef]
- Kai, R.; Tokuoka, T.; Nagao, J.; Pillai, A.L.; Kurose, R. LES Flamelet Modeling of Hydrogen Combustion Considering Preferential Diffusion Effect. Int. J. Hydrogen Energy 2022, 48, 11086–11101. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, J.; Wei, Z.; Quadarella, E.; Im, H.G.; Liu, Z. A Species-Weighted Flamelet/Progress Variable Model with Differential Diffusion Effects for Oxy-Fuel Jet Flames. Combust. Flame 2023, 251, 112674. [Google Scholar]
- Gao, Z.; Wang, H.; Song, C.; Luo, K.; Fan, J. Large-Eddy Simulation of Hydrothermal Flames Using Extended Flamelet/Progress Variable Approach. J. Supercrit. Fluids 2020, 163, 104843. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Luo, K.; Fan, J. The Effects of Differential Diffusion on Turbulent Non-Premixed Flames LO2/CH4 under Transcritical Conditions Using Large-Eddy Simulation. Energies 2023, 16, 1065. [Google Scholar] [CrossRef]
- Boyette, W.R.; Guiberti, T.F.; Magnotti, G.; Roberts, W.L. Structure of Turbulent Nonpremixed Syngas Flames at High Pressure. Proc. Combust. Inst. 2019, 37, 2207–2214. [Google Scholar] [CrossRef]
- Tang, H.; Yang, C.; Wang, G.; Guiberti, T.F.; Magnotti, G. Raman Spectroscopy for Quantitative Measurements of Temperature and Major Species in High-Pressure Non-Premixed NH3/H2/N2 Counterflow Flames. Combust. Flame 2022, 237, 111840. [Google Scholar] [CrossRef]
- Design, R. CHEMKIN-PRO 18.2; ANSYS, Inc.: San Diego, CA, USA, 2017. [Google Scholar]
- Kee, R.J.; Miller, J.A.; Evans, G.H.; Dixon-Lewis, G. A Computational Model of the Structure and Extinction of Strained, Opposed Flow, Premixed Methane-Air Flames. Symp. (Int.) Combust. 1989, 22, 1479–1494. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Experimental and Numerical Study of the Laminar Burning Velocity of CH4–NH3–Air Premixed Flames. Combust. Flame 2018, 187, 185–198. [Google Scholar] [CrossRef]
- Jiang, Y.; Gruber, A.; Seshadri, K.; Williams, F. An Updated Short Chemical-kinetic Nitrogen Mechanism for Carbon-free Combustion Applications. Int. J. Energy Res. 2020, 44, 795–810. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, H.; Xiao, H.; Luo, K.; Fan, J. Emission Characteristics and Heat Release Rate Surrogates for Ammonia Premixed Laminar Flames. Int. J. Hydrogen Energy 2021, 46, 13461–13470. [Google Scholar] [CrossRef]
- Chai, W.S.; Bao, Y.; Jin, P.; Tang, G.; Zhou, L. A Review on Ammonia, Ammonia-Hydrogen and Ammonia-Methane Fuels. Renew. Sustain. Energy Rev. 2021, 147, 111254. [Google Scholar] [CrossRef]
- Song, Y.; Hashemi, H.; Christensen, J.M.; Zou, C.; Marshall, P.; Glarborg, P. Ammonia Oxidation at High Pressure and Intermediate Temperatures. Fuel 2016, 181, 358–365. [Google Scholar] [CrossRef]
- Xu, L.; Chang, Y.; Treacy, M.; Zhou, Y.; Jia, M.; Bai, X.S. A Skeletal Chemical Kinetic Mechanism for Ammonia/n-Heptane Combustion. Fuel 2023, 331, 125830. [Google Scholar] [CrossRef]
- Alnasif, A.; Mashruk, S.; Shi, H.; Alnajideen, M.; Wang, P.; Pugh, D.; Valera-Medina, A. Evolution of Ammonia Reaction Mechanisms and Modeling Parameters: A Review. Appl. Energy Combust. Sci. 2023, 15, 100175. [Google Scholar]
- Han, X.; Wang, Z.; Costa, M.; Sun, Z.; He, Y.; Cen, K. Experimental and Kinetic Modeling Study of Laminar Burning Velocities of NH3/Air, NH3/H2/Air, NH3/CO/Air and NH3/CH4/Air Premixed Flames. Combust. Flame 2019, 206, 214–226. [Google Scholar] [CrossRef]
- Petrova, M.; Williams, F. A Small Detailed Chemical-Kinetic Mechanism for Hydrocarbon Combustion. Combust. Flame 2006, 144, 526–544. [Google Scholar] [CrossRef]
- Weller, H.G.; Tabor, G.; Jasak, H.; Fureby, C. A Tensorial Approach to Computational Continuum Mechanics Using Object-Oriented Techniques. Comput. Phys. 1998, 12, 620. [Google Scholar] [CrossRef]
- Peters, N. Turbulent Combustion. Meas. Sci. Technol. 2001, 12, 2022. [Google Scholar] [CrossRef]
- Pierce, C.D.; Moin, P. Progress-Variable Approach for Large-Eddy Simulation of Non-Premixed Turbulent Combustion. J. Fluid Mech. 2004, 504, 73–97. [Google Scholar] [CrossRef]
- Lilly, D.K. A Proposed Modification of the Germano Subgrid-scale Closure Method. Phys. Fluids A Fluid Dyn. 1992, 4, 633–635. [Google Scholar] [CrossRef]
- Bilger, R.; Stårner, S.; Kee, R. On reduced mechanisms for methane-air combustion in nonpremixed flames. Combust. Flame 1990, 80, 135–149. [Google Scholar] [CrossRef]
- Domingo, P.; Vervisch, L.; Veynante, D. Large-Eddy Simulation of a Lifted Methane Jet Flame in a Vitiated Coflow. Combust. Flame 2008, 152, 415–432. [Google Scholar] [CrossRef]
- Pitsch, H. A C++ Computer Program for 0-D and 1-D Laminar Flame Calculations; RWTH: Aachen, Germany, 1998. [Google Scholar]
- Ihme, M.; Shunn, L.; Zhang, J. Regularization of Reaction Progress Variable for Application to Flamelet-Based Combustion Models. J. Comput. Phys. 2012, 231, 7715–7721. [Google Scholar] [CrossRef]
- Peters, N. Laminar Diffusion Flamelet Models in Non-Premixed Turbulent Combustion. Prog. Energy Combust. Sci. 1984, 10, 319–339. [Google Scholar] [CrossRef]
- Coelho, P.J.; Peters, N. Unsteady Modelling of a Piloted Methane/Air Jet Flame Based on the Eulerian Particle Flamelet Model. Combust. Flame 2001, 124, 444–465. [Google Scholar] [CrossRef]
- Ihme, M.; Pitsch, H. Modeling of Radiation and Nitric Oxide Formation in Turbulent Nonpremixed Flames Using a Flamelet/Progress Variable Formulation. Phys. Fluids 2008, 20, 055110. [Google Scholar] [CrossRef]
- Wang, H.; Hawkes, E.R.; Savard, B.; Chen, J.H. Direct Numerical Simulation of a High Ka CH4/Air Stratified Premixed Jet Flame. Combust. Flame 2018, 193, 229–245. [Google Scholar] [CrossRef]
- Okafor, E.C.; Somarathne, K.D.K.A.; Ratthanan, R.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Tsujimura, T.; Furutani, H.; Kobayashi, H. Control of NOx and Other Emissions in Micro Gas Turbine Combustors Fuelled with Mixtures of Methane and Ammonia. Combust. Flame 2020, 211, 406–416. [Google Scholar] [CrossRef]
- Somarathne, K.D.K.A.; Okafor, E.C.; Sugawara, D.; Hayakawa, A.; Kobayashi, H. Effects of OH Concentration and Temperature on NO Emission Characteristics of Turbulent Non-Premixed CH4/NH3/Air Flames in a Two-Stage Gas Turbine like Combustor at High Pressure. Proc. Combust. Inst. 2021, 38, 5163–5170. [Google Scholar] [CrossRef]
- Somarathne, K.D.K.A.; Okafor, E.C.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Kobayashi, H. Emission Characteristics of Turbulent Non-Premixed Ammonia/Air and Methane/Air Swirl Flames through a Rich-Lean Combustor under Various Wall Thermal Boundary Conditions at High Pressure. Combust. Flame 2019, 210, 247–261. [Google Scholar] [CrossRef]
- Duynslaegher, C.; Jeanmart, H.; Vandooren, J. Flame Structure Studies of Premixed Ammonia/Hydrogen/Oxygen/Argon Flames: Experimental and Numerical Investigation. Proc. Combust. Inst. 2009, 32, 1277–1284. [Google Scholar] [CrossRef]





| Flame Type | Flame Case | (m/s) | (m/s) | |||
|---|---|---|---|---|---|---|
| Laminar | CACF14 | 0.754 | 0.184 | 0.062 | 0.36 | 0.29 |
| CACF28 | 0.563 | 0.328 | 0.109 | 0.36 | 0.29 | |
| Turbulent | CAJF14 | 0.754 | 0.184 | 0.062 | 8.6 | 0.24 |
| CAJF28 | 0.563 | 0.328 | 0.109 | 10.1 | 0.24 |
| Sequence | Reaction | Sequence | Reaction |
|---|---|---|---|
| R220: | N + NO = + O | R255: | + HNO = + NO |
| R221: | N + = NO + O | R256: | + NO = + |
| R222: | N + OH = NO + H | R257: | + NO = NNH + OH |
| R224: | + O = 2 NO | R263: | NNH + O = NH + NO |
| R228: | + NO = + OH | R269: | NNH + NO = + HNO |
| R229: | NO + O + M = + M | R271: | H + NO + M = HNO + M |
| R230: | + O = NO + | R272: | HNO + O = NO + OH |
| R231: | + H = NO + OH | R273: | HNO + H = + NO |
| R233: | NH + O = NO + H | R274: | HNO + OH = NO + |
| R237: | NH + = NO + OH | R275: | HNO + = + NO |
| R240: | NH + NO = + H | R276: | HNO + = HONO + NO |
| R241: | NH + NO = + H | R290: | + O = + NO |
| R242: | NH + NO = + OH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wang, H.; Luo, K.; Fan, J. The Effects of Cracking Ratio on Ammonia/Air Non-Premixed Flames under High-Pressure Conditions Using Large Eddy Simulations. Energies 2023, 16, 6985. https://doi.org/10.3390/en16196985
Wang C, Wang H, Luo K, Fan J. The Effects of Cracking Ratio on Ammonia/Air Non-Premixed Flames under High-Pressure Conditions Using Large Eddy Simulations. Energies. 2023; 16(19):6985. https://doi.org/10.3390/en16196985
Chicago/Turabian StyleWang, Chengming, Haiou Wang, Kun Luo, and Jianren Fan. 2023. "The Effects of Cracking Ratio on Ammonia/Air Non-Premixed Flames under High-Pressure Conditions Using Large Eddy Simulations" Energies 16, no. 19: 6985. https://doi.org/10.3390/en16196985
APA StyleWang, C., Wang, H., Luo, K., & Fan, J. (2023). The Effects of Cracking Ratio on Ammonia/Air Non-Premixed Flames under High-Pressure Conditions Using Large Eddy Simulations. Energies, 16(19), 6985. https://doi.org/10.3390/en16196985







