Gas Turbine Combustion Technologies for Hydrogen Blends
Abstract
1. Introduction
2. Effects of Hydrogen on Combustion
- It has a higher adiabatic flame temperature than methane (see Table 3 where the values of the adiabatic flame temperature () of selected fuels at stoichiometric conditions are reported [19,28]). This temperature also defines the combustion efficiency and the cooling requirememts of the liner; thus, it is important for the GT design [29].
- The higher adiabatic temperature can lead to an increase in levels.
- Mixing with conventional fuels reduces CO emissions since the carbon input decreases at a constant equivalence ratio; the oxidation of CO and is increased by the higher concentration of OH and H radicals [30]. Working with the excessive lean mixture, the temperature decreases and CO emissions increase [25].
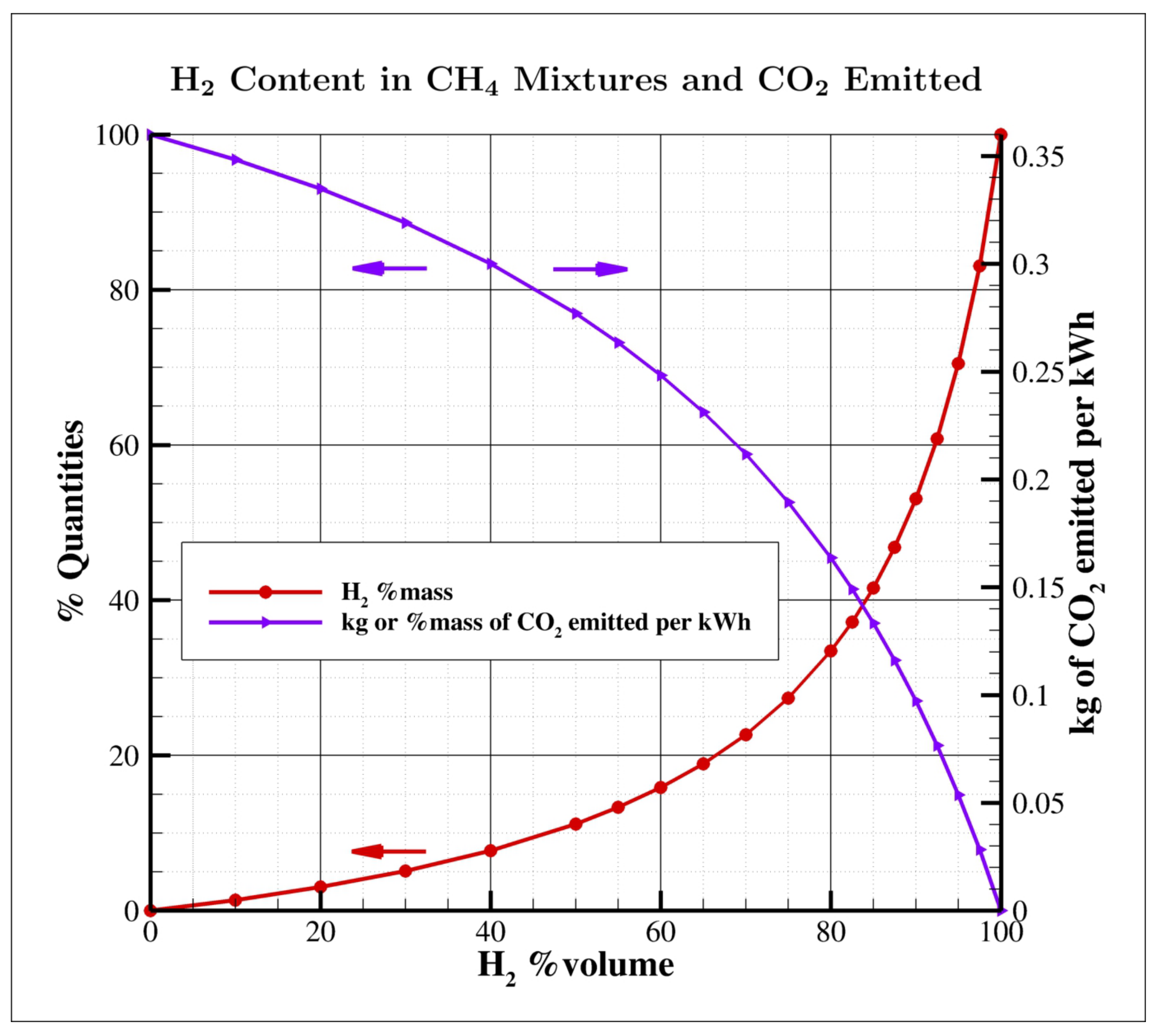
- As shown in Table 3, the lower calorific value of hydrogen, on a volumetric basis, is approximately of that of natural gas; therefore, fuel supply systems capable of providing a higher volumetric flow rate are required.
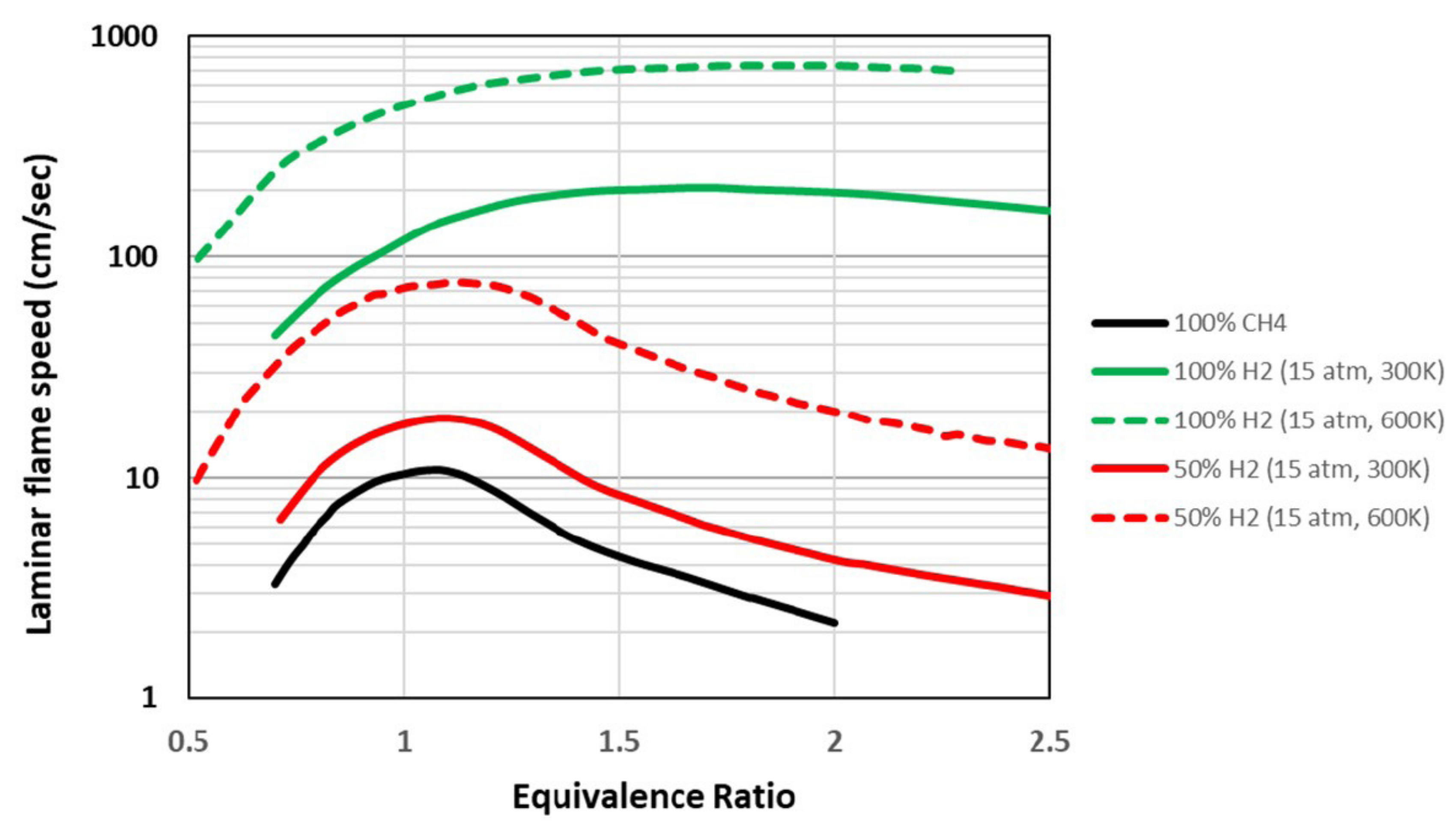
- Hydrogen has a reactivity about a hundred times greater than natural gas and a higher flame speed of about an order of magnitude [31]. Even mixtures containing hydrogen in various percentages have higher laminar flame speeds than methane alone: a mixture of hydrogen has twice the flame speed compared to methane [25,32,33]. Figure 3 shows the trend of the laminar flame speed as a function of the equivalence ratio.
- The higher flame speed (due to the increased production of OH, H, O radicals and the higher molecular diffusivity of [36]) increases the risk of dynamic instabilities (i.e., flashbacks where turbulent flame speed exceeds the flow velocity, typically in the flow boundary layer, allowing flame propagation inside the mixer, with obvious problems of material resistance and safety). Furthermore, enrichment increases the critical strain rate and minimizes the quenching possibilities of the flame [37,38].
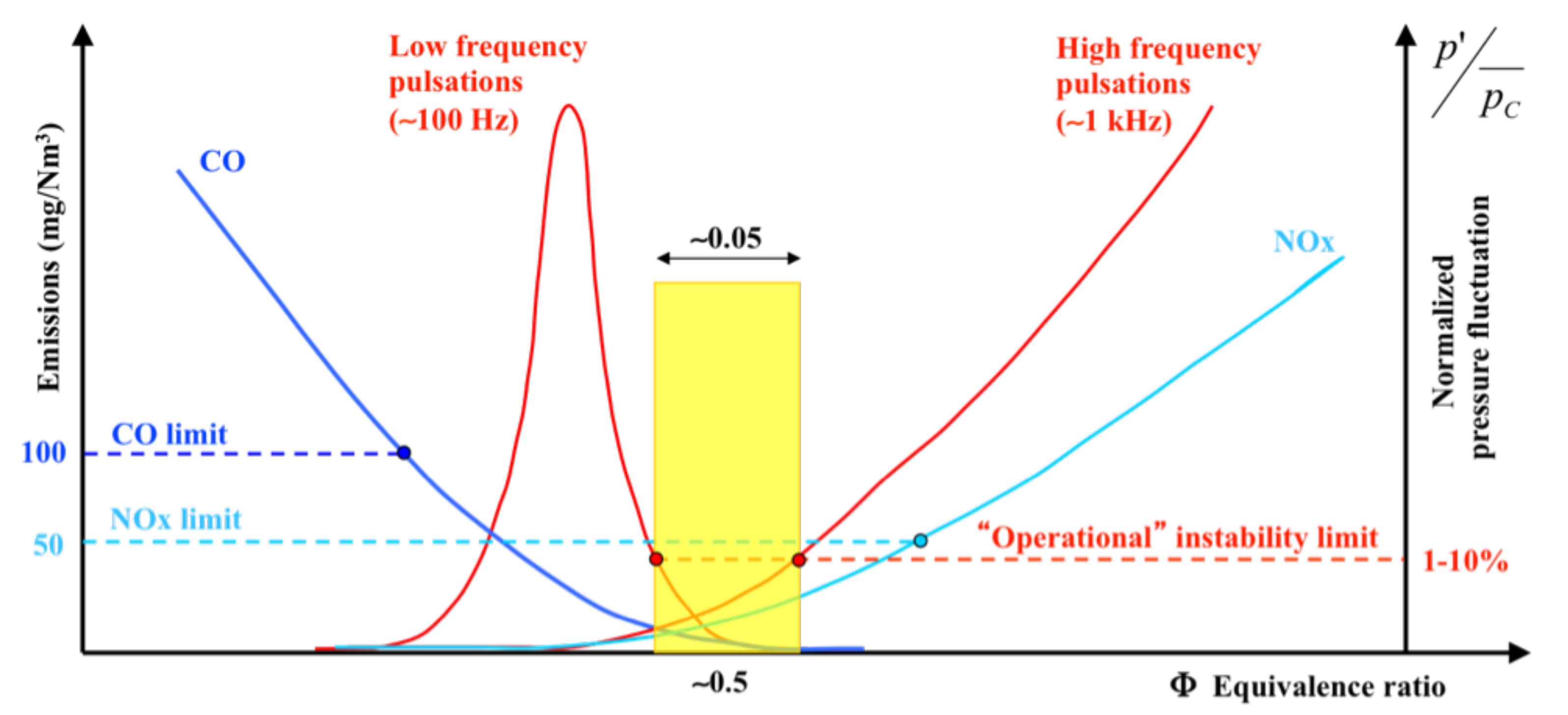
- High content increases the risk of thermo-acoustic instabilities characterized by large amplitude pressure oscillations that are driven by unsteadiness in the phase heat release, which strongly alters the combustion process. Shorter convective time scales of the methane–hydrogen mixture and faster reaction rates affect the phase relationship between the fluctuating pressure and unsteady heat release [39,40,41,42,43,44] Although, for many years, both the research and industrial worlds have investigated combustion instabilities [45,46,47], the problem is still not completely solved. The strategies employed to avoid or reduce its effects are substantially based on empirical methods. In addition, lean flames are highly thermo-diffusively unstable, which means that wrinkles in the flame will tend to become more pronounced, thus justifying the higher turbulent flame speed of lean flames compared to other lean mixtures [48].
- The combustion of hydrogen produces flames that are not very bright; therefore, most of the energy is transmitted by convection, by the sensible heat in the exhausted gas [49].
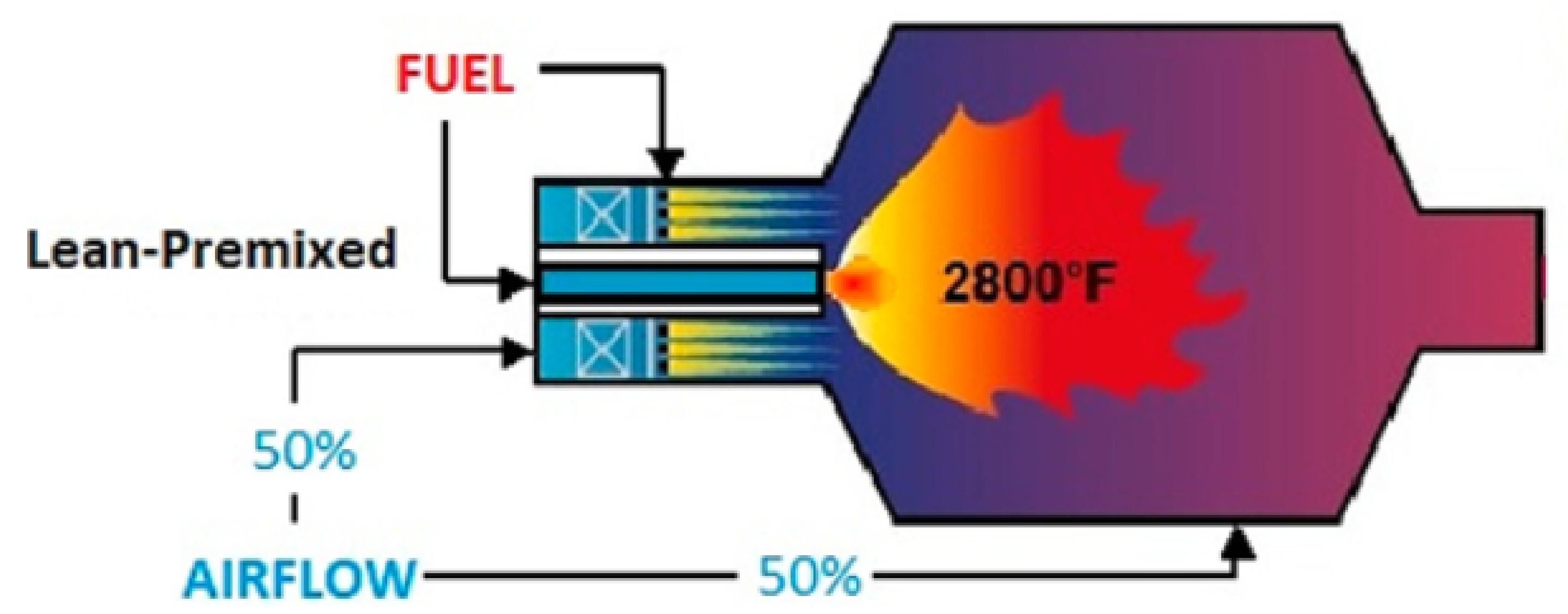
3. Low -Ready Combustion Technologies
- (1)
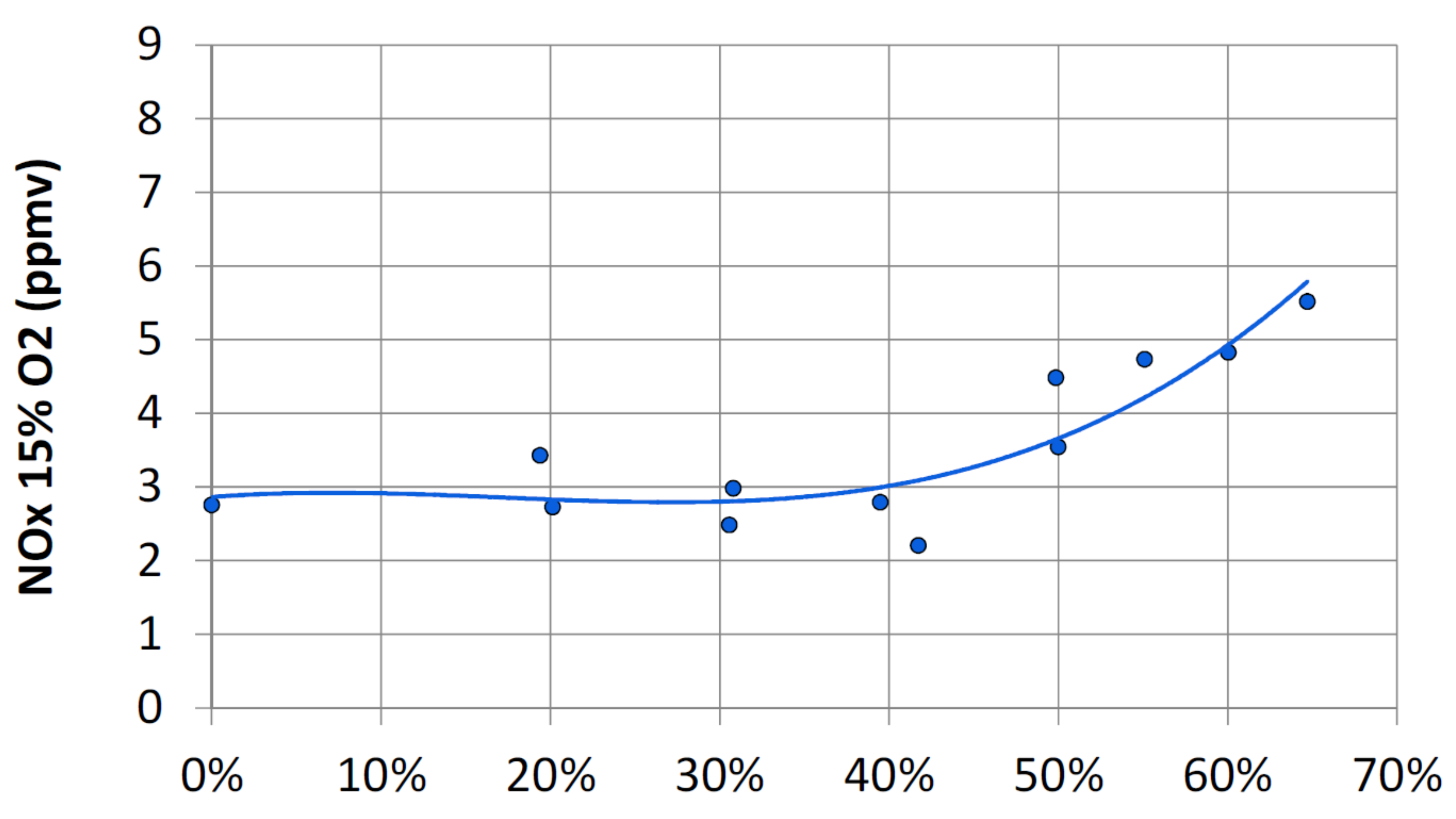
- Thermal NO or Zeldovich mechanism [52]. The reactions of this mechanism require a high activation energy, thus high temperatures, to activate them [53,54].R1:R2:R3:The rate of NO formation strongly depends on temperature, pressure, and residence time; decreasing any of these three reduces NO, but the exponential dependence on temperature makes the reduction of the combustion temperature the key strategy for low combustion. Fortunately, thermal formation rates are relatively slow, and therefore, equilibrium concentrations are never reached in the combustion devices. It is also dependent on the square root of the pressure [55]. emissions can be reduced by lowering the flame temperature or by reducing the time that the mixture spends in the combustor. With bland fuel, it is possible to simultaneously reduce the length of the combustor and working with a leaner mixture due to the higher flame speed and to the lower flammability limit compared to natural gas.
- (2)
- R4:R5:R6:R7:
- (3)
- NNH pathway [59,60]. This mechanism is important at higher temperature conditions in the fuel-rich side of the flame zone as well as in the reaction zone.R8:R9:R10:
- (4)
- pathway [61]. The pathway seems to be significant at high pressures and moderate temperatures in the fuel-lean flame conditions.R11:R12:R13:
- (5)
- Fuel NO [51,56]. When nitrogen is chemically bound to fuel (e.g., ), it converts almost completely to in the exhaust gases. From the thermal decomposition of fuel-bound compounds in the reaction zone, radicals such as can be formed and converted to [62]. While most gaseous fuels, such as natural gas, do not contain fuel-bound nitrogen, nitrogen is frequently present in liquid and solid fuels. Unprocessed fuel oil, for instance, can contain more than 1000 parts per million (ppm) of bound nitrogen, resulting in over 40 ppm of nitrogen oxides () in the exhaust gases solely due to this mechanism. Additionally, the sulfur content in liquid fuels can contribute to acid rain and harm the catalysts used to control emissions in automobiles. Fortunately, refining processes designed to remove sulfur also eliminate nitrogen from the fuel. Ultra-low sulfur diesel (ULSD), as defined by ASTM D975 and containing less than 15 ppm of sulfur, typically contains less than 10 ppm of fuel-bound nitrogen, resulting in less than 1 ppm of being added to the exhaust. Low sulfur diesel (LSD) contains less than 500 ppm of sulfur and can introduce a few ppm of into the exhaust. In contrast, high sulfur diesel, with sulfur levels of up to 5000 ppm (0.5%), also contains a significant amount of fuel-bound nitrogen, which can contribute an additional 50 ppm of . Such levels are generally considered unacceptable in regions where emissions are regulated.
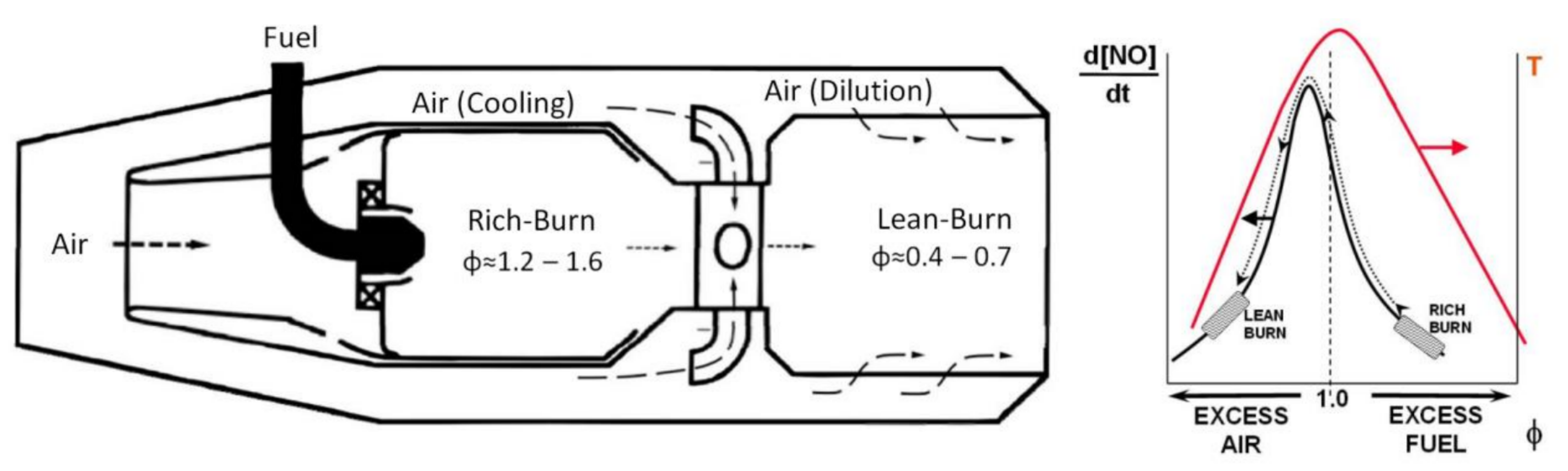
- combustion aerodynamically stabilized by propagation;
- combustion stabilized by self-ignition;
- staged combustion, stabilized with different methodologies (by propagation and/or self-ignition); and
- micro-mixing combustion, with many small premixed, partially premixed or diffusive flames.
3.1. Aerodynamically Stabilized Combustion
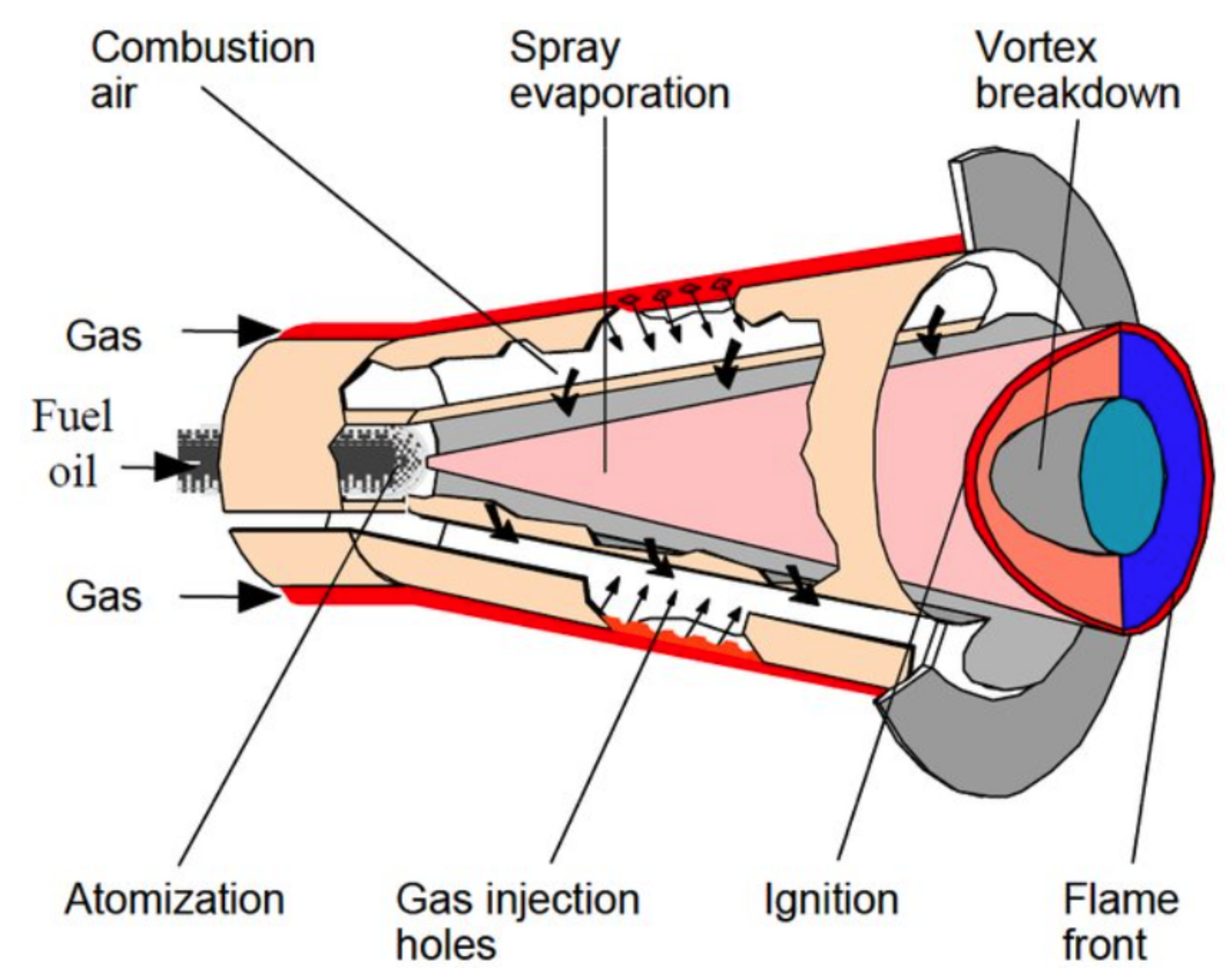
3.2. Stabilized Combustion for Self-Ignition
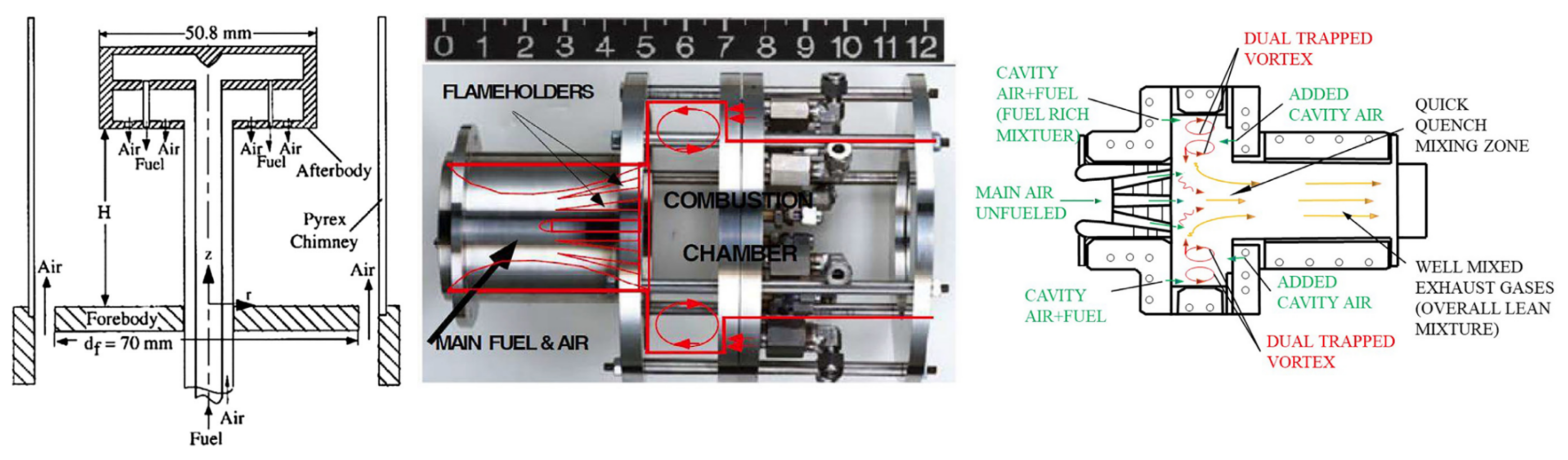
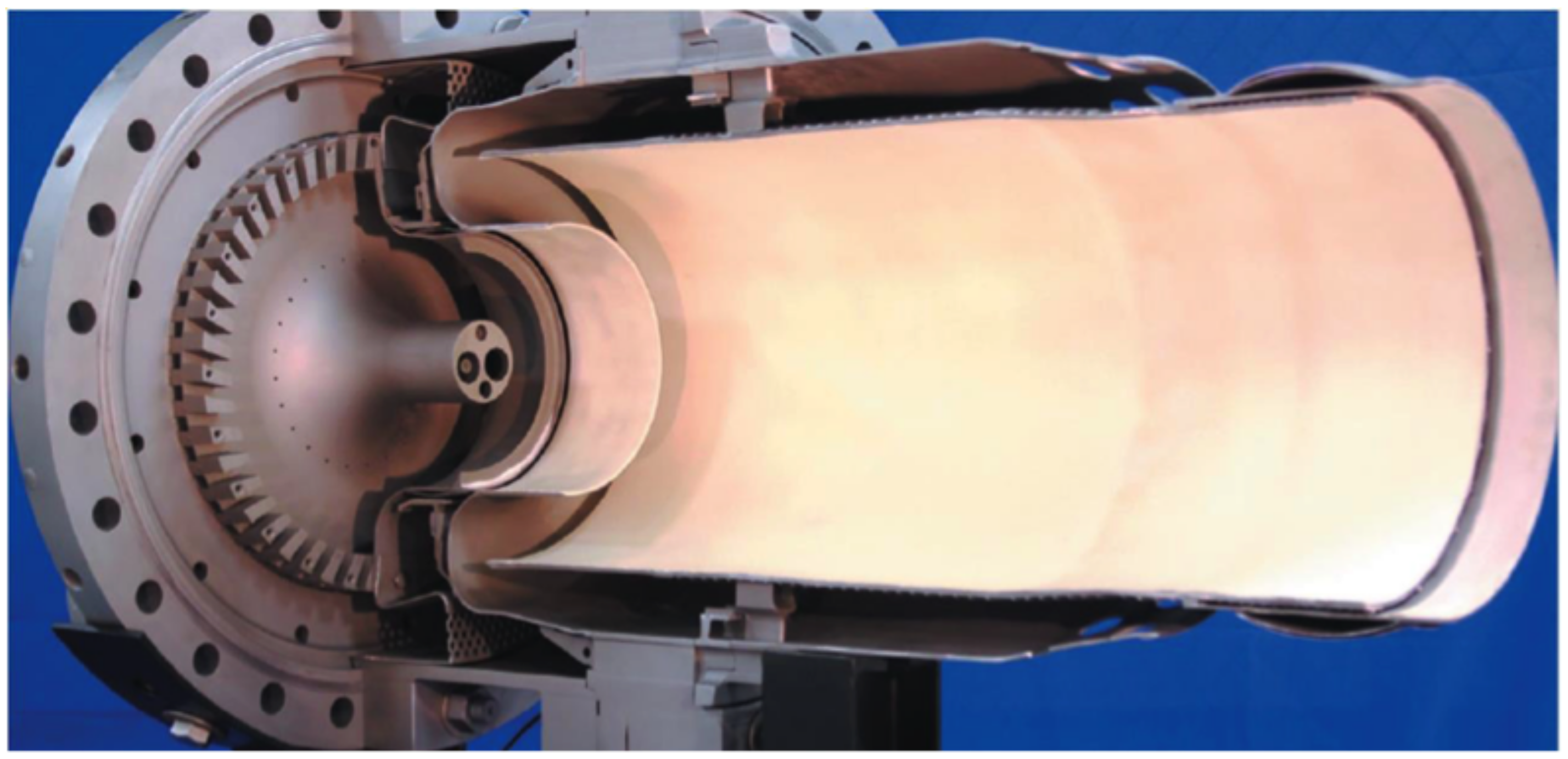
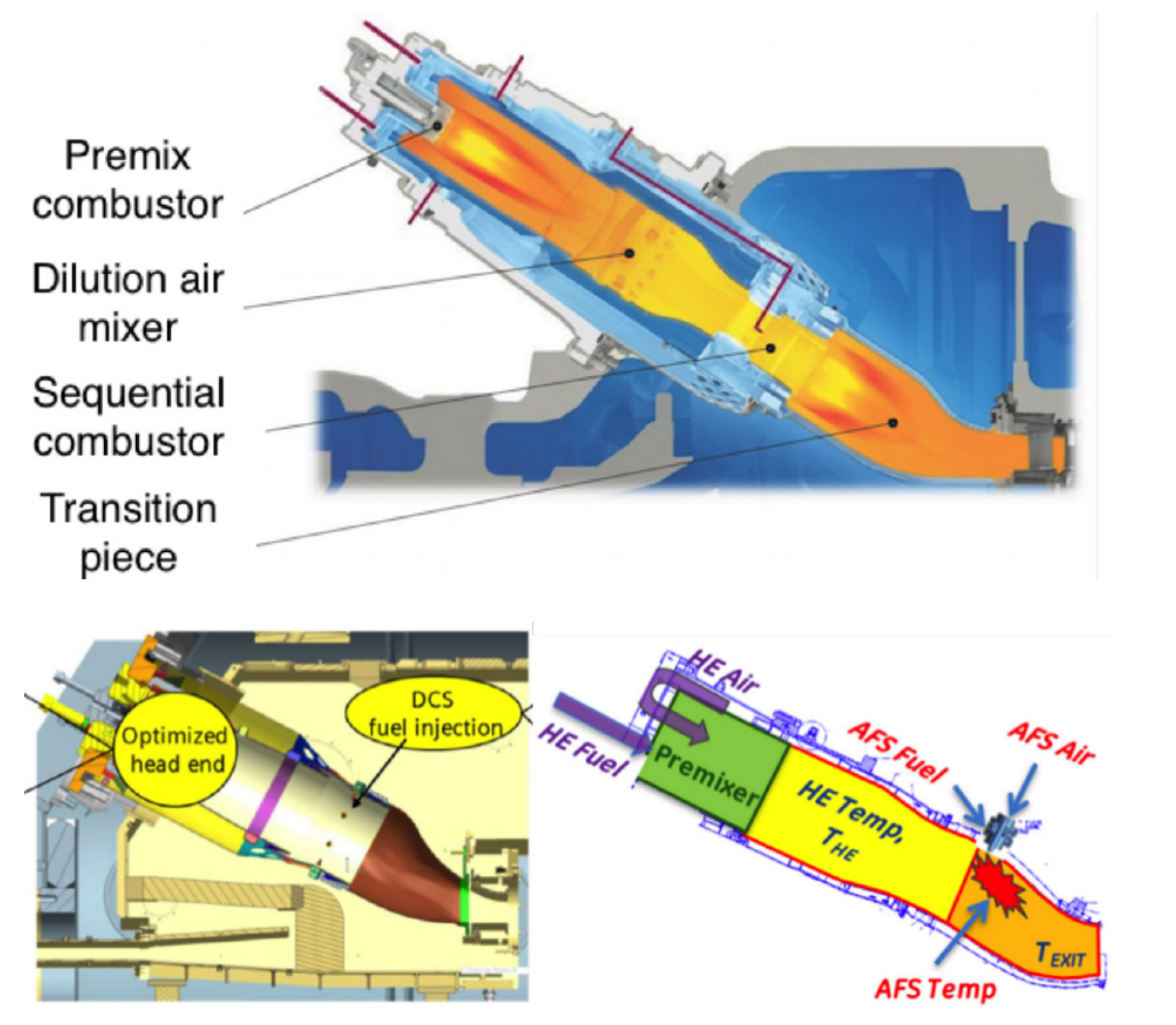
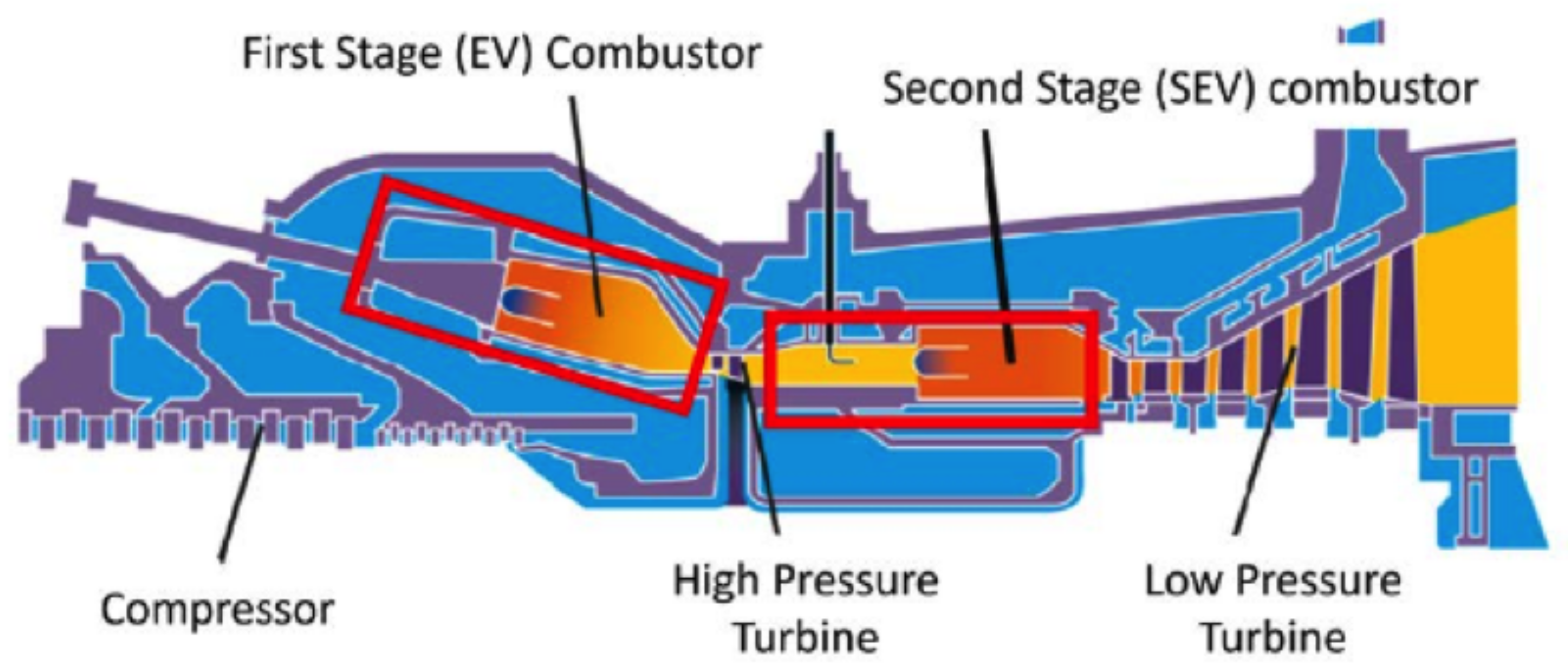
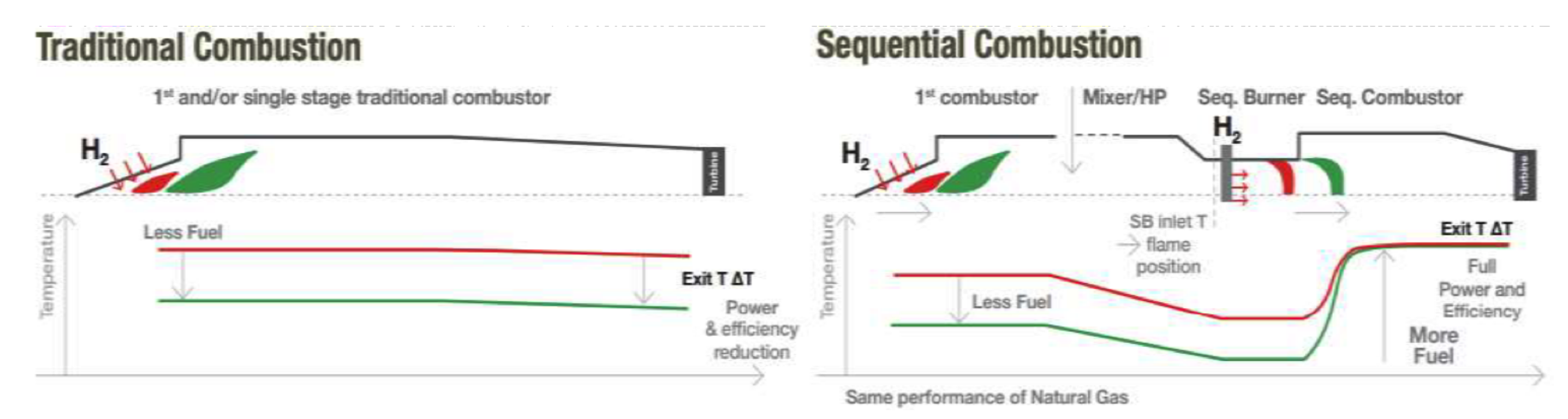
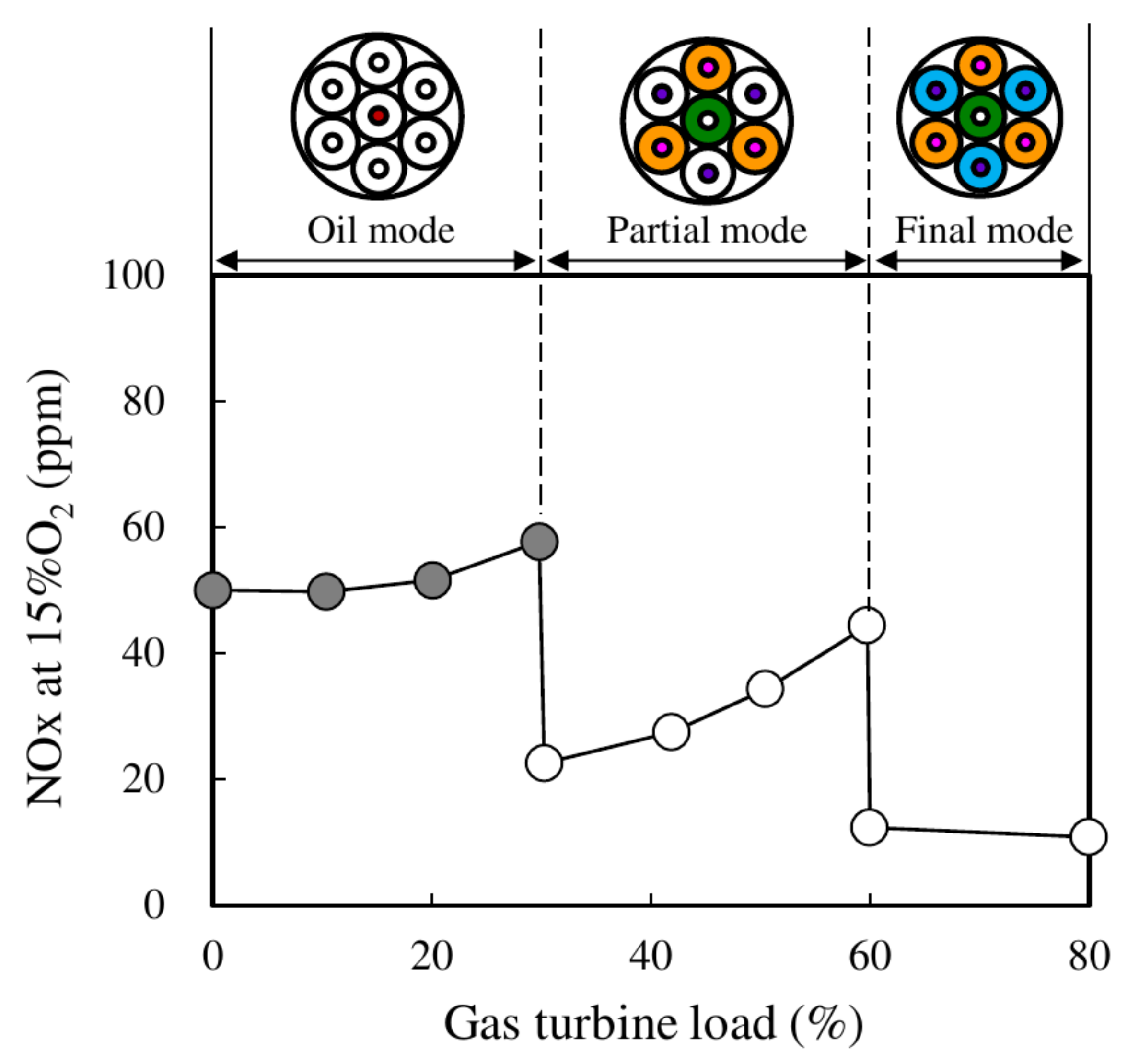
3.3. Staged Combustion
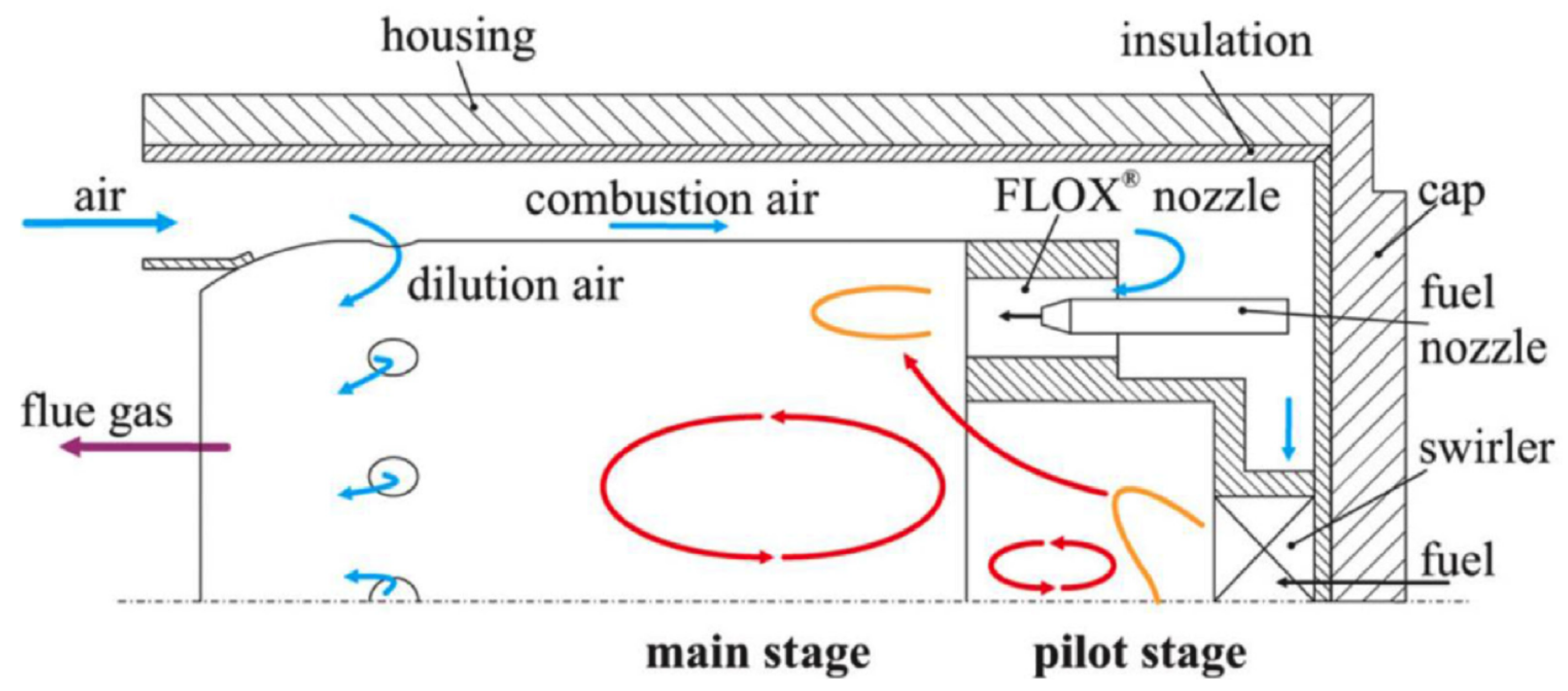
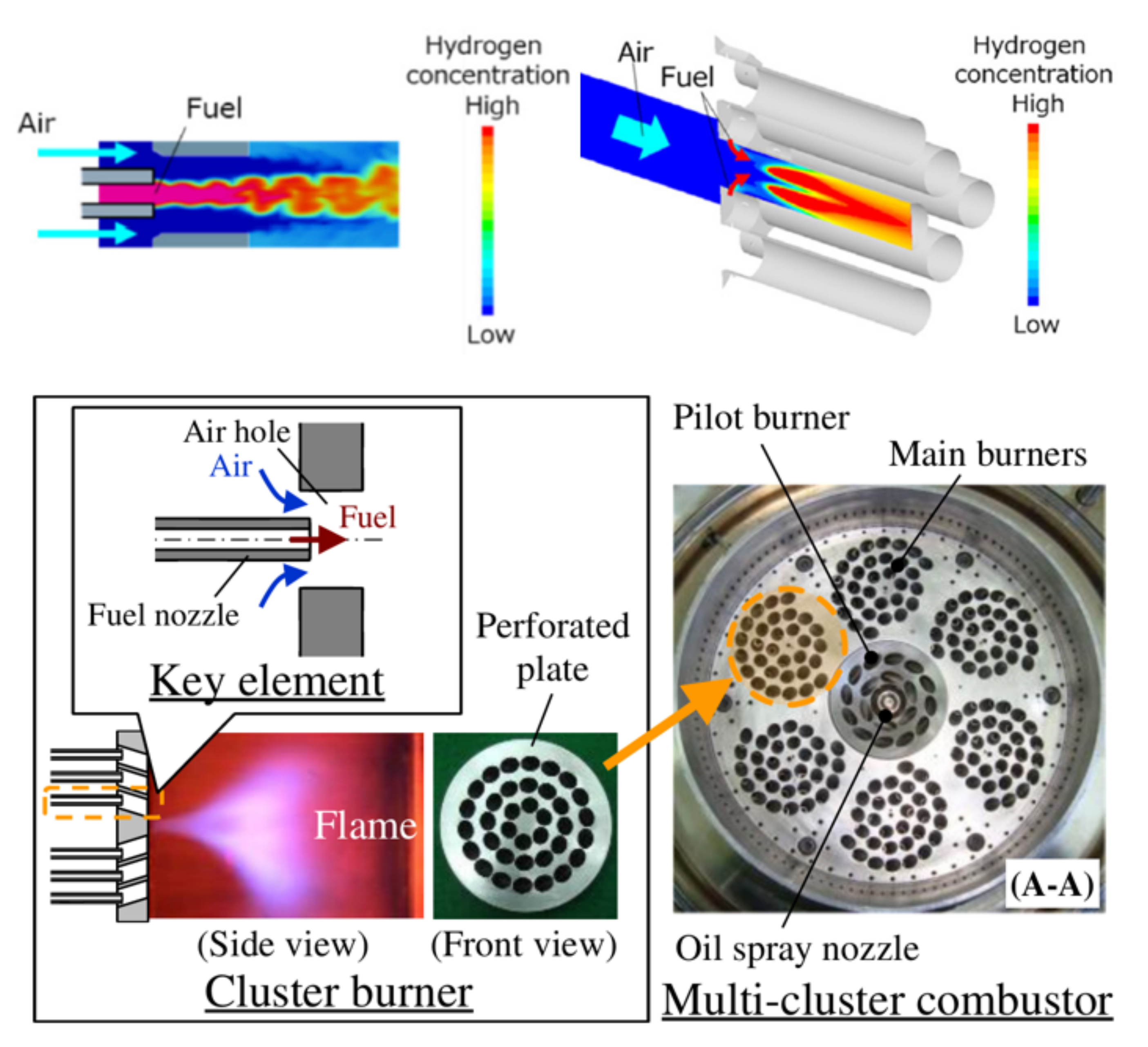
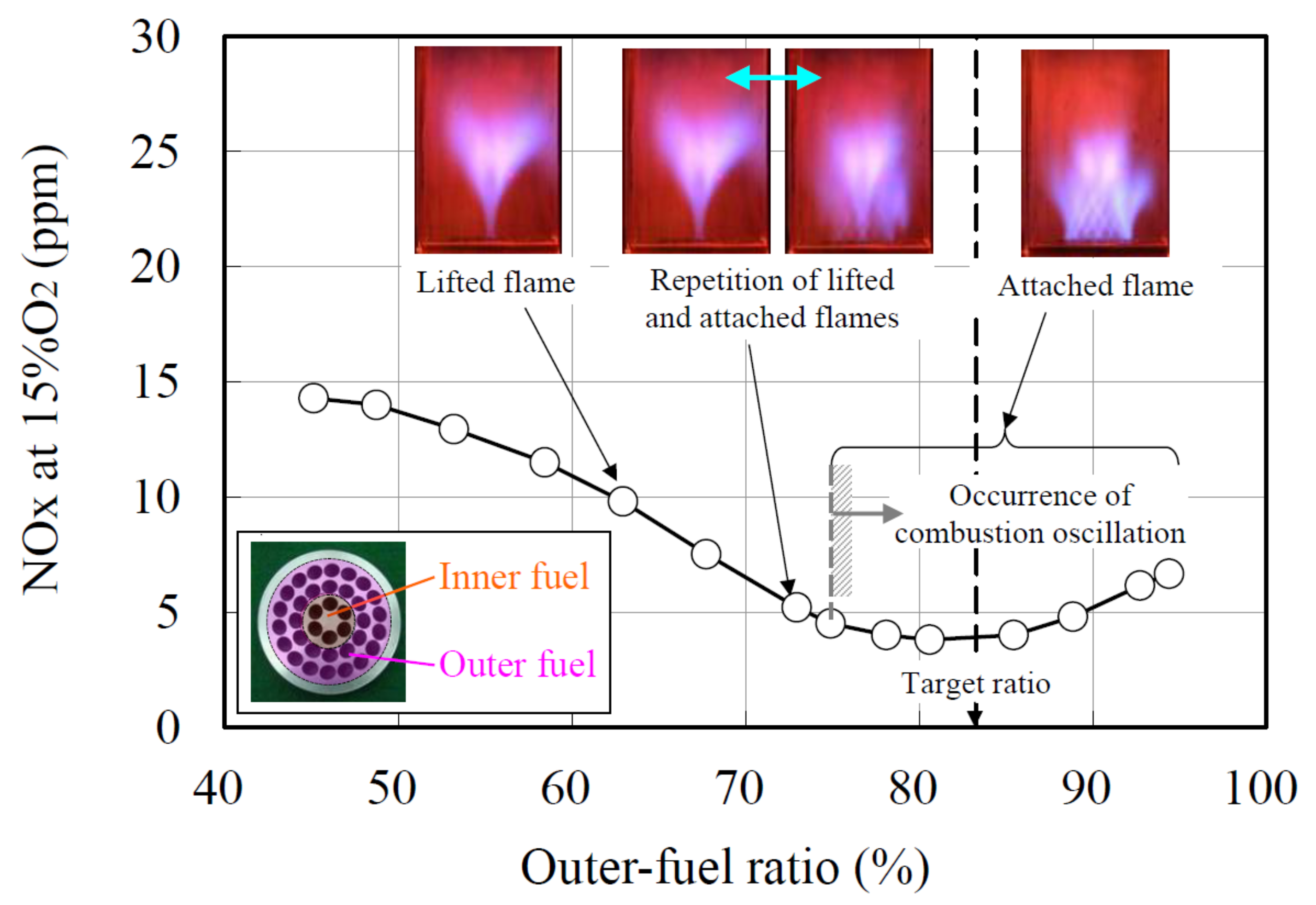
3.4. Micro-Mixing
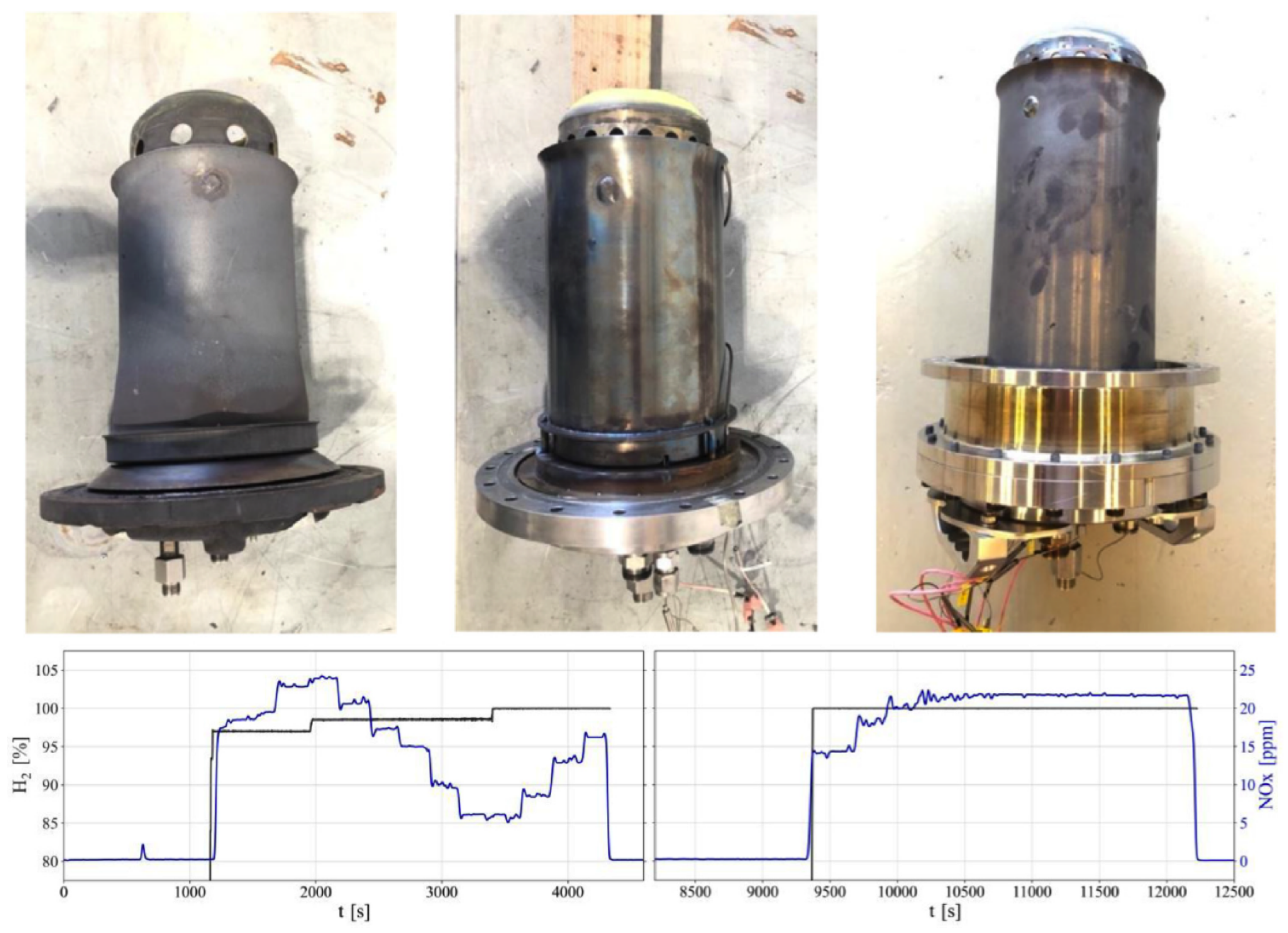
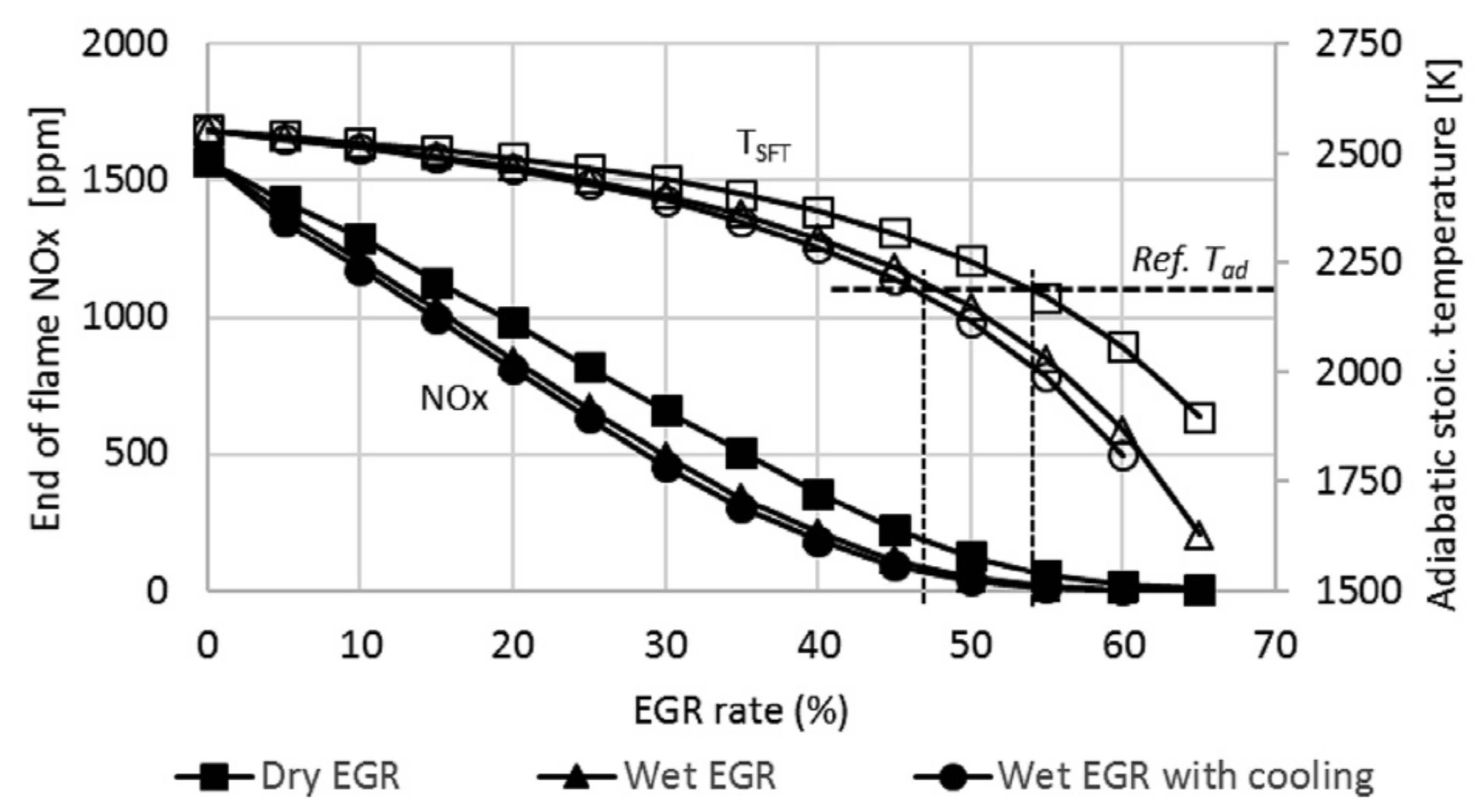
3.5. Exhaust Gas Recirculation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Russ, M. Cost-effective strategies for an optimised allocation of carbon dioxide emission reduction measures. In Unwelttechnik; Verlag-Shaker: Aachen, Germany, 1994. [Google Scholar]
- Ausfelder, F.; Bazzanella, A. Hydrogen in the chemical industry. In Hydrogen Science and Engineering: Materials, Processes, Systems and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Karakaya, E.; Nuur, C.; Assbring, L. Potential transitions in the iron and steel industry in Sweden: Towards a hydrogen based future? J. Clean. Prod. 2018, 195, 651–663. [Google Scholar] [CrossRef]
- Otto, A.A.; Robinius, M.; Grube, T.; Schiebahn, S.; Praktiknjo, A.; Stolten, D. Power-to-steel: Reducing CO2 through the integration of renewable energy and hydrogen into the German steel industry. Energies 2017, 10, 451. [Google Scholar] [CrossRef]
- International Energy Agency. World Energy Outlook. pp. 1–810. Available online: https://www.iea.org/reports/world-energy-outlook-2019 (accessed on 16 August 2023).
- Giacomazzi, E.; Messina, G. Hydrogen and the Fuel-Flexibility Dilemma in Gas Turbines. In Energia, Ambiente e Innovazione; ENEA: Stockholm, Sweden, 2021; Volume 1, pp. 125–129. [Google Scholar]
- Kumar, A.; Singh, R.; Sinha, A.S.K. Catalyst modification strategies to enhance the catalyst activity and stability during steam reforming of acetic acid for hydrogen production. Int. J. Hydrog. Energy 2019, 44, 12983–13010. [Google Scholar] [CrossRef]
- Shiva, K.S.; Himabindu, V. Hydrogen production by PEM water electrolysis-a review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Nahar, G.; Mote, D.; Dupont, V. Hydrogen production from reforming of biogas:review of technological advances and an Indian perspective. Renew. Sustain. Energy Rev. 2017, 76, 1032–1052. [Google Scholar] [CrossRef]
- Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Wahid, A.Z. Outlook of fermentative hydrogen production techniques: An overview of dark, photo and integrated dark photo fermentative approach to biomass. Energy Strateg. Rev. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- Pandey, Y.K.B.; Prajapati, P.N. Sheth, Recent progress in thermochemical techniques to produce hydrogen gas from biomass: A state of the art review. Int. J. Hydrog. Energy 2019, 44, 25384–25415. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Fermentative hydrogen production using various biomass-based materials as feedstock. Renew. Sustain. Energy Rev. 2019, 92, 284–306. [Google Scholar] [CrossRef]
- Basheer, A.A.; Ali, I. Water photo splitting for green hydrogen energy by green nanoparticles. Int. J. Hydrog. Energy 2019, 44, 11564–11573. [Google Scholar] [CrossRef]
- Kadier, A.; Kalil, M.S.; Abdeshahian, P.; Chandrasekhar, K.; Mohamed, A.; Azman, N.F.; Logroño, W.; Simayi, Y.; Hamid, A.A. Recent advances and emerging challenges in microbial electrolysis cells (MECs) for microbial production of hydrogen and value-added chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- L’Orange Seigo, S.; Dohle, S.; Siegrist, M. Public Perception of Carbon Capture and Storage (CCS): A Review. Renew. Sustain. Energy Rev. 2014, 38, 848–863. [Google Scholar] [CrossRef]
- Carbon Capture and Storage Association (CCSA). What is CCS? CCSA: London, UK, 2020; Available online: https://www.ccsassociation.org/ (accessed on 20 August 2023).
- Black, S.; Roll, P.; Grames, M.; Goodwin, M.; Biccum, J.; Legault, R.; Soares, B.; Dorman, D. Dual Fuel Direct Ignition Burners. U.S. Patent 20190162410 A1, 30 May 2019. [Google Scholar]
- Richards, G.A.; McMillian, M.M.; Gemmen, R.S.; Rogers, W.A.; Cully, S.R. Issues for Low-Emission, Fuel-Flexible Power Systems. Prog. Energy Combust. Sci. 2001, 27, 141–169. [Google Scholar] [CrossRef]
- Döbbeling, K.; Hellat, J.; Koch, H. 25 Years of BBC/ABB/ Alstom Lean Premix Combustion Technologies. J. Eng. Gas Turbines Power 2007, 129, 2–12. [Google Scholar] [CrossRef]
- Davis, L.B.; Black, S.H. Dry Low NOx Combustion Systems for GE Heavy-Duty Gas Turbines; GER-3568G; GE Power Systems: Schenectady, NY, USA, 2000. [Google Scholar]
- Clean Hydrogen Partnership. Clean Hydrogen Joint Undertaking, Strategic Research and Innovation Agenda 2021–2027, Annex to GB decision no. CleanHydrogen-GB-2022-02. Available online: https://www.clean-hydrogen.europa.eu (accessed on 23 August 2023).
- ETN Global. Addressing the Combustion Challenges of Hydrogen Addition to Natural Gas. 2022. Available online: https://etn.global/tag/news/ (accessed on 25 August 2023).
- York, W.D.; Ziminsky, W.S.; Yilmaz, E. Development and Testing of a Low NOx Hydrogen Combustion System for Heavy Duty Gas Turbines. In Proceedings of the ASME Turbo Expo 2012: Turbine Technical Conference and Exposition, Copenhagen, Denmark, 11–15 June 2012; pp. 1395–1405. [Google Scholar]
- Taamallah, S.; Vogiatzaki, K.; Alzahrani, F.M.; Mokheimer, E.M.; Habib, M.A.; Ghoniem, A.F. Fuel Flexibility, Stability and Emissions in Premixed Hydrogen-Rich Gas Turbine Combustion: Technology, Fundamentals, and Numerical Simulations. Appl. Energy 2015, 154, 1020–1047. [Google Scholar] [CrossRef]
- Du Toit, M.H.; Avdeenkov, A.V.; Bessarabov, D. Reviewing H2 Combustion: A Case Study for Non-Fuel-Cell Power Systems and Safety in Passive Autocatalytic Recombiners. Energy Fuels 2018, 32, 6401–6422. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, Y.; Huang, Z. Progress in combustion investigations of hydrogen enriched hydrocarbons. Renew. Sustain. Energy Rev. 2014, 30, 195–216. [Google Scholar] [CrossRef]
- Azazul Haque, M.; Nemitallah, M.A.; Abdelhafez, A.; Mansir, I.B.; Habib, M.A. Review of Fuel/Oxidizer-Flexible Combustion in Gas Turbines. Energy Fuels 2020, 34, 10459–10485. [Google Scholar] [CrossRef]
- Nemitallah, M.A.; Abdelhafez, A.A.; Ali, A.; Mansir, I.; Habib, M.A. Frontiers in Combustion Techniques and Burner Designs for Emissions Control and CO2 Capture: A Review. Int. J. Energy Res. 2019, 43, 7790–7822. [Google Scholar]
- Zhen, H.S.; Leung, C.W.; Cheung, C.S.; Huang, Z.H. Characterization of biogas-hydrogen premixed flames using Bunsen burner. Int. J. Hydrog. Energy 2014, 39, 13292–13299. [Google Scholar] [CrossRef]
- Kim, H.S.; Arghode, V.K.; Gupta, A.K. Flame Characteristics of Hydrogen-Enriched Methane-Air Premixed Swirling Flames. Int. J. Hydrog. Energy 2009, 34, 1063–1073. [Google Scholar] [CrossRef]
- Tang, C.; Huang, Z.; Jin, C.; He, J.; Wang, J.; Wang, X.; Miao, H. Laminar Burning Velocities and Combustion Characteristics of Propane-Hydrogen-Air Premixed Flames. Int. J. Hydrog. Energy 2008, 33, 4906–4914. [Google Scholar] [CrossRef]
- Hu, E.; Huang, Z.; He, J.; Jin, C.; Zheng, J. Experimental and Numerical Study on Laminar Burning Characteristics of Premixed Methane-Hydrogen-Air Flames. Int. J. Hydrog. Energy 2009, 34, 4876–4888. [Google Scholar] [CrossRef]
- Gersen, S.; Anikin, N.B.; Mokhov, A.V.; Levinsky, H.B. Ignition Properties of Methane/Hydrogen Mixtures in a Rapid Compression Machine. Int. J. Hydrog. Energy 2008, 33, 1957–1964. [Google Scholar] [CrossRef]
- Park, S. Hydrogen addition effect on NO formation in methane/air lean-premixed flames at elevated pressure. Int. J. Hydrog. Energy 2021, 46, 25712–25725. [Google Scholar] [CrossRef]
- Sankaran, R.; Im, H.R. Effects of Hydrogen Addition on the Markstein Length and Flammability Limit of Stretched Methane/Air Premixed Flames. Combust. Sci. Technol. 2006, 178, 1585–1611. [Google Scholar] [CrossRef]
- Imteyaz, B.A.; Nemitallah, M.A.; Abdelhafez, A.A.; Habib, M.A. Combustion Behavior and Stability Map of Hydrogen-Enriched Oxy-Methane Premixed Flames in a Model Gas Turbine Combustor. Int. J. Hydrog. Energy 2018, 43, 16652–16666. [Google Scholar] [CrossRef]
- Nemitallah, M.A.; Imteyaz, B.; Abdelhafez, A.; Habib, M.A. Experimental and Computational Study on Stability Characteristics of Hydrogen-Enriched Oxy-Methane Premixed Flames. Appl. Energy 2019, 250, 433–443. [Google Scholar] [CrossRef]
- Janus, M.C.; Richards, R.A.; Yip, M.J.; Robey, E.H. Effects of Ambient Conditions and Fuel Composition on Combustion Stability. In Proceedings of the 1997 American Society of Mechanical Engineers (ASME)/International Gas Turbine Institute (IGTI) Turbo Expo Meeting, Orlando, FL, USA, 2–5 June 1997; p. 13. [Google Scholar]
- Figura, L.; Lee, J.G.; Quay, B.D.; Santavicca, D.A. The Effects of Fuel Composition on Flame Structure and Combustion Dynamics in a Lean Premixed Combustor. In Turbo Expo: Power for Land, Sea, and Air; ASMEDC: Montreal, QC, Canada, 2007; Volume 47918, pp. 181–187. [Google Scholar]
- Schefer, R.W.; Wicksall, D.M.; Agrawal, A.K. Combustion of Hydrogen-Enriched Methane in a Lean Premixed Swirl-Stabilized Burner. Proc. Combust. Inst. 2002, 29, 843–851. [Google Scholar] [CrossRef]
- Zhang, J.; Ratner, A. Experimental Study on the Excitation of Thermoacoustic Instability of Hydrogen-Methane/Air Premixed Flames under Atmospheric and Elevated Pressure Conditions. Int. J. Hydrog. Energy 2019, 44, 21324–21335. [Google Scholar] [CrossRef]
- Karlis, E.; Liu, Y.; Hardalupas, Y.; Taylor, A.M.K.P. H2 Enrichment of CH4 Blends in Lean Premixed Gas Turbine Combustion: An Experimental Study on Effects on Flame Shape and Thermoacoustic Oscillation Dynamics. Fuel 2019, 254, 115524. [Google Scholar] [CrossRef]
- Beita, J.; Talibi, M.; Sadasivuni, S.; Balachandran, R. Thermoacoustic Instability Considerations for High Hydrogen Combustion in Lean Premixed Gas Turbine Combustors: A Review. Hydrogen 2021, 2, 33–57. [Google Scholar] [CrossRef]
- Lieuwen, T.; Torres, H.; Johnson, C.; Zinn, B.T. A Mechanism for Combustion Instabilities in Premixed Gas Turbine Combustors. ASME J. Eng. Gas Turbines Power 2001, 123, 182–190. [Google Scholar] [CrossRef]
- Gonzalez-Juez, E.; Lee, J.G.; Santavicca, D.A. A Study of Combustion Instabilities Driven by Flame-Vortex Interactions. In Proceedings of the 41st AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Tucson, AZ, USA, 10–13 July 2005. [Google Scholar]
- Lee, J.G.; Santavicca, D.A. Experimental Diagnostics for the Study of Combustion Instabilities in Lean Premixed Combustors. J. Propul. Power 2003, 19, 735–750. [Google Scholar] [CrossRef]
- LaPenna, P.E.; Berger, L.; Attili, A.; Lamioni, R.; Fogla, N.; Pitsch, H.; Creta, F. Data-driven subfilter modelling of thermo-diffusively unstable hydrogen-air premixed flames. Combust. Theory Model. 2021, 25, 1064–1085. [Google Scholar] [CrossRef]
- Lam, K.K.; Geipel, P.; Larfeldt, J. Hydrogen Enriched Combustion Testing of Siemens Industrial SGT-400 at Atmospheric Conditions. In Turbo Expo: Power for Land, Sea, and Air; ASME: Dusseldorf, Germany, 2014; pp. 1–10. [Google Scholar]
- Turbomachinery International. Available online: https://www.turbomachinerymag.com/view/solving-the-challenge-of-lean-hydrogen-premix-combustion-with-highly-reactive-fuels (accessed on 25 August 2023).
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Zeldovich, Y.B. The oxidation of nitrogen in combustion and explosions. Acta Physicochem. USSR 1946, 21, 577–628. [Google Scholar]
- Iverach, D.; Basden, K.S.; Kirov, N.Y. Formation of nitric oxide in fuel-lean and fuel-rich flames. Symp. (Int.) Combust. 1976, 14, 767–775. [Google Scholar] [CrossRef]
- Harris, R.J.; Nasralla, M.; Williams, A. Formation of oxides of nitrogen in high-temperature CH4-O2-N2 flames. Combust. Sci. Technol. 1976, 14, 85–94. [Google Scholar] [CrossRef]
- Richards, G.; Weiland, N.; Straket, P. The Gas Turbine Handbook; National Energy Technology Laboratory: Pittsburgh, PA, USA, 2006; pp. 203–208. [Google Scholar]
- Fenimore, C.P. Formation of nitric oxide in premixed hydrocarbon flames. Symp. (Int.) Combust. 1971, 13, 373–380. [Google Scholar] [CrossRef]
- Hayhurst, A.N.; Vince, I.M. Nitric-oxide formation from N2 in flames-the importance of prompt NO. Prog. Energy Combust. Sci. 1980, 6, 35–51. [Google Scholar] [CrossRef]
- Lamoureux, N.; El Merhubi, H.; Pillier, L.; de Persis, S.; Desgroux, P. Modeling of NO formation in low pressure premixed flames. Combust. Flame 2016, 163, 557–575. [Google Scholar] [CrossRef]
- Bozzelli, J.W.; Dean, A.M. O + NNH: A possible new route for NOx formation in flames. Int. J. Chem. Kinet. 1995, 27, 1097–1109. [Google Scholar] [CrossRef]
- Day, M.S.; Bell, J.B.; Gao, X.; Glarborg, P. Numerical simulation of nitrogen oxide formation in lean premixed turbulent H2/O2/N2 flames. Proc. Combust. Inst. 2011, 33, 1591–1599. [Google Scholar] [CrossRef]
- Malte, P.; Pratt, D.T. Measurements of atomic oxygen and nitrogen oxides in jet-stirred combustion. Symp. (Int.) Combust. 1975, 15, 1061–1070. [Google Scholar] [CrossRef]
- Houser, T.J.; Hull, M.; Alway, R.; Biftu, T. Kinetics of formation of HCN during pyridine pyrolysis. Int. J. Chem. Kinet. 1980, 12, 579. [Google Scholar] [CrossRef]
- Ghoniem, A.F.; Park, S.; Wachsman, A.; Annaswamy, A.; Wee, D.; Murat Altay, H. Mechanism of Combustion Dynamics in a Backward-Facing Step Stabilized Premixed Flame. Proc. Combust. Inst. 2005, 30, 1783–1790. [Google Scholar] [CrossRef]
- Murat Altay, H.; Hudgins, D.E.; Speth, R.L.; Annaswamy, A.M.; Ghoniem, A.F. Mitigation of Thermoacoustic Instability Utilizing Steady Air Injection near the Flame Anchoring Zone. Combust. Flame 2010, 157, 686–700. [Google Scholar] [CrossRef]
- Kurz, R.; Brun, K.; Meher-Homji, C.; Moore, J.; Gonzalez, F. Gas Turbine Performance and Maintenance. In Proceedings of the 42nd Turbomachinery Symposium, Houston, TX, USA, 1–3 October 2013. [Google Scholar]
- Lieuwen, T. Fuel Flexibility Influences on Premixed Combustor Blowout, Flashback, Autoignition, and Stability. J. Eng. Gas Turbines Power 2008, 130, 011506. [Google Scholar] [CrossRef]
- Emerson, B.; Wu, D.; Lieuwen, T.; Shepperd, S.; Noble, D.; Angello, L. Assessment of Current Capabilities and Near-Term Availability of Hydrogen-Fired Gas Turbines Considering a Low-Carbon Future. In Proceedings of the ASME Turbo Expo 2020 Turbomachinery Technical Conference and Exposition GT 2020, Virtual, 21–25 September 2020. [Google Scholar]
- Sattelmayer, T.; Felchlin, M.P.; Haumann, J.; Hellat, J.; Styner, D. Second-Generation Low-Emission Combustors for ABB Gas Turbines: Burner Development and Tests at Atmospheric Pressure. J. Eng. Gas Turbines Power 1992, 114, 118–125. [Google Scholar] [CrossRef]
- Joos, F.; Brunner, P.; Stalder, M.; Tschirren, S. Field Experience with the Sequential Combustion System of the GT24/GT26. ABB Rev. 1998, 5, 12–20. [Google Scholar]
- Hiddemann, M.; Stevens, M.; Hummel, F. Increased Operational Flexibility from the GT26 (2011) Upgrade; Power-Gen Asia: Bangkok, Thailand, 2012. [Google Scholar]
- Cowell, L.H.; Etheridge, C.; Smith, K.O. Ten Years of DLE Industrial Gas Turbine Operating Experiences; GT-2002-30280; ASME: New York, NY, USA, 2002. [Google Scholar]
- Cong, T.L.; Dagaut, P. Experimental and detailed kinetic modeling of the oxidation of methane and methane/syngas mixtures and effect of carbon dioxide addition. Combust. Sci. Technol. 2008, 180, 2046–2091. [Google Scholar] [CrossRef]
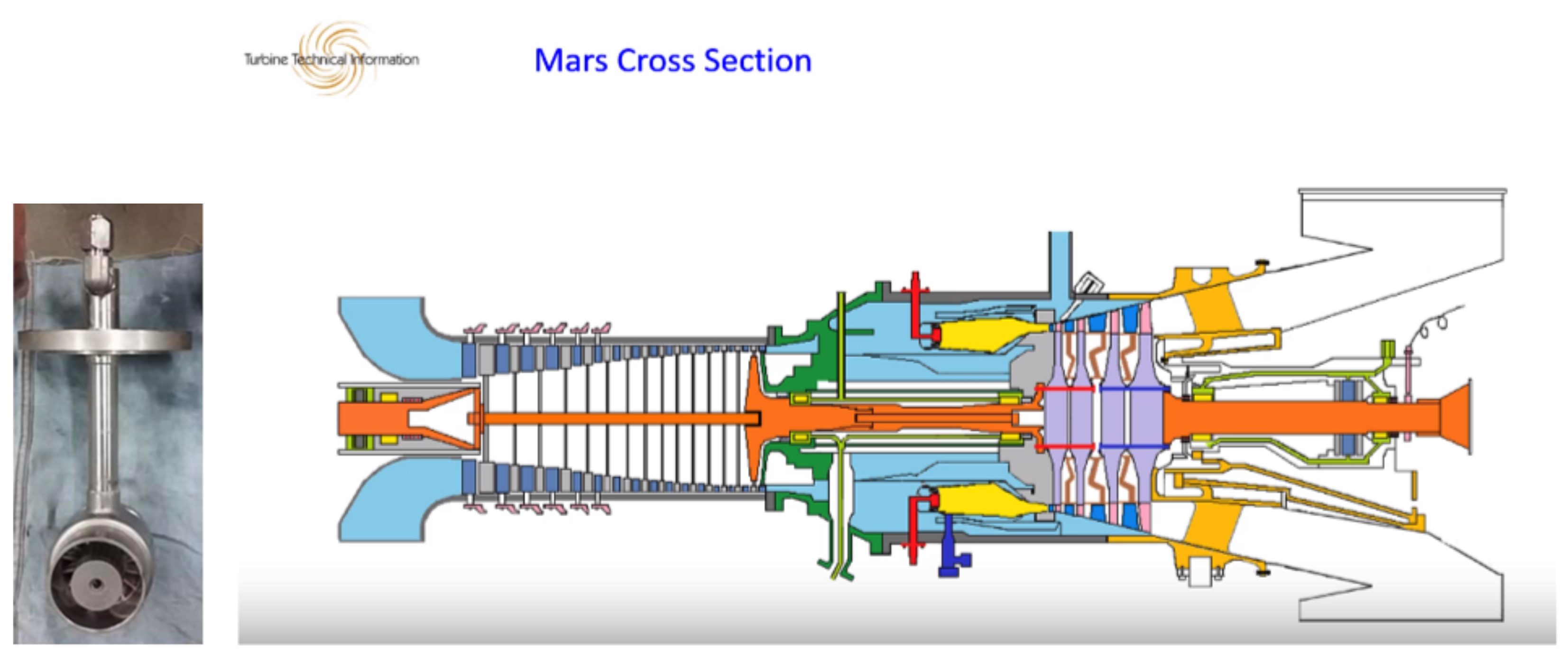
- Etheridge, C.J. Mars SoLoNOx: Lean Premixed Combustion Technology in Production. In Proceedings of the ASME International Gas Turbine and Aeroengine Congress and Exposition, The Hague, The Netherlands, 13–16 June 1994. [Google Scholar]
- Solar Turbines Mars Training. Available online: https://www.dmba5411.com/solar-mars-cross-section/ (accessed on 27 August 2023).
- Zhao, D.; Gutmark, E.; De Goey, P. A review of cavity-based trapped vortex, ultra-compact, high-g, inter-turbine combustors. Prog. Energy Combust. Sci. 2018, 66, 42–82. [Google Scholar] [CrossRef]
- Stuttaford, P.; Rizkalla, H.; Oumejjoud, K.; Demougeot, N.; Bosnoian, J.; Hernandez, F.; Yaquinto, M.; Mohammad, A.P.; Terrell, D.; Weller, R. FlameSheetTM Combustor Engine and Rig Validation for Operational and Fuel Flexibility with Low Emissions, GT2016-56696. In Proceedings of the ASME Turbo Expo: Turbomachinery Technical Conference and Exposition GT2016, Seoul, Republic of Korea, 13–17 June 2016. [Google Scholar]
- Axelsson, L.U.; Bouten, T.; Stuttaford, P.; Jansen, D.; Koomen, J.; Flein, S.; Laagland, G.; van der Ploeg, H.; Ghenai, C.; Zbeeb, K.; et al. High Hydrogen Gas Turbine Retrofit Solution to Eliminate Carbon Emissions, Paper ID 15-IGTC21, Gas Turbines in a Carbon-Neutral Society. In Proceedings of the 10th International Gas Turbine Conference, Virtual, 11–15 October 2021. [Google Scholar]
- Rizkalla, H.; Hernandez, F.; Bullard, T.; Benoit, J.; Stuttaford, P.; Jansen, D. Future-Proofing Today’s Industrial Gas Turbines: Combustion System Fuel Flexibility Improvements For Hydrogen Consumption in a Renewable Dominated Marketplace. In Proceedings of the 9th International Gas Turbine Conference, Brussels, Belgium, 10–11 October 2018. [Google Scholar]
- Erickson, D.M.; Day, S.A.; Doyle, R. Design Considerations for Heated Gas Fuel; GER-4189B; GE Power Systems: Greenville, SC, USA, 2003. [Google Scholar]
- PSM. FlameSheetTM: A Revolution in Combustion Technology for Power Generation Gas Turbines. Available online: https://www.psm.com/ (accessed on 27 August 2023).
- Noor, M.M.; Wande, A.P.; Yusaf, T. MILD Combustion: The Future for Lean and Clean Combustion Technology. Int. Rev. Mech. Eng. IREME 2014, 8, 252–257. [Google Scholar] [CrossRef][Green Version]
- Galletti, C.; Parente, A.; Derudi, M.; Rota, R.; Tognotti, L. Numerical and experimental analysis of NO emissions from a lab-scale burner fed with hydrogen-enriched fuels and operating in MILD combustion. Int. J. Hydrog. Energy 2009, 34, 8339–8351. [Google Scholar] [CrossRef]
- Cavaliere, A.; de Joannon, M. Mild Combustion. Prog. Energy Combust. Sci. 2004, 30, 329–366. [Google Scholar] [CrossRef]
- Wunning, J. Flameless Oxidation. In Proceedings of the 6th HiTACG Symposium, Essen, Germany, 17–19 October 2005. [Google Scholar]
- Banihabib, R.; Lingstädt, T.; Wersland, M.; Kutne, P.; Assadi, M. Development and testing of a 100 kW fuel-flexible micro gas turbine running on 100% hydrogen. Int. J. Hydrog. Energy 2023, 6, 317. [Google Scholar]
- Perpignan, A.A.V.; Rao, A.G.; Roekaerts, D.J.E.M. Flameless combustion and its potential towards gas turbines. Prog. Energy Combust. Sci. 2018, 69, 28–66. [Google Scholar] [CrossRef]
- Sharma, S.; Chowdhury, A.; Kumar, S. A novel air injection scheme to achieve MILD combustion in a can-type gas turbine combustor. Energy 2020, 194, 116819. [Google Scholar] [CrossRef]
- Fortunato, V.; Giraldo, A.; Rouabah, M.; Nacereddine, R.; Delanaye, M.; Parente, A. Experimental and Numerical Investigation of a MILD Combustion Chamber for Micro Gas Turbine Applications. Energies 2018, 11, 3363. [Google Scholar] [CrossRef]
- Banihabib, R.; Assadi, M. A Hydrogen-Fueled Micro Gas Turbine Unit for Carbon-Free Heat and Power Generation. Sustainability 2022, 14, 13305. [Google Scholar] [CrossRef]
- Mosier, S.A.; Pierce, R.M. Advanced Combustor Systems for Stationary Gas Turbine Engines, Phase I. Review and Preliminary Evaluation; Contract 68-02-2136, FR-11405, Final Report; U.S. Environmental Protection Agency: Washington, DC, USA, 1980; Volume I.
- Ayed, A.H. Numerical Characterization and Development of the Dry Low NOx High Hydrogen Content Fuel Micromix Combustion for Gas Turbine Applications. Ph.D. Thesis, Aachen University, Aachen, Germany, 2017. [Google Scholar]
- Kobayashi, H.; Hayakawa, A.; Kunkuma, K.D.; Somarathne, A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Indlekofer, T.; Gruber, A.; Wiseman, S.; Nogenmyr, K.J.; Larfeldt, J. Numerical Investigation of Rich-Lean Staging in SGT-750 Scaled DLE Burner with Partially-Decomposed Ammonia; ASME TurboExpo: Rotterdam, The Netherlands, 2022. [Google Scholar]
- Eroglu, A.; Larfeldt, J.; Klapdor, E.V.; Yilmaz, E.; Prasad, V.N.; Prade, B.; Witzel, B.; Koeni, M. Hydrogen Capabilities of Siemens Energy Gas Turbines, an OEM Perspective, Paper ID Number: 7-IGTC21, Gas turbines in a carbon-neutral society. In Proceedings of the 10th International Gas Turbine Conference, Virtual, 11–15 October 2021. [Google Scholar]
- Noble, D.; Wu, D.; Emerson, B.; Sheppard, S.; Lieuwen, T.; Angello, L. Assessment of Current Capabilities and Near-Term Availability of Hydrogen-Fired Gas Turbines Considering a Low-Carbon Future. J. Eng. Gas Turbines Power 2021, 143, 041002. [Google Scholar] [CrossRef]
- Aditya, K.; Gruber, A.; Xu, C.; Lu, T.; Krisman, A.; Bothien, M.R.; Chen, J.H. Direct Numerical Simulation of a flame stabilization assisted by autoignition in a reheat gas turbine combustor. Proc. Combust. Inst. 2019, 37, 2635–2642. [Google Scholar] [CrossRef]
- ANSALDO Energia. Time to Face Our World’s Biggest CH2allenge, Leaflet. 2018. Available online: https://www.ansaldoenergia.com/ (accessed on 28 August 2023).
- Funke, H.H.W.; Beckmann, N.; Abanteriba, S. An overview on dry low NOx micromix combustor development for hydrogen-rich gas turbine applications. Int. J. Hydrog. Energy 2019, 44, 6978–6990. [Google Scholar] [CrossRef]
- Ayed, A.H.; Kusterer, K.; Funke, H.H.-W.; Keinz, J.; Striegan, C.; Bohn, D. Experimental and numerical investigations of the dry-low-NOx hydrogen micromix combustion chamber of an industrial gas turbine. Propuls. Power Res. 2015, 4, 123–131. [Google Scholar] [CrossRef]
- Kim, D. Review on the Development Trend of Hydrogen Gas Turbine Combustion Technology. J. Korean Soc. Combust. 2019, 24, 1–10. [Google Scholar]
- Weiland, N.T.; Sidwell, T.G.; Strakey, P.A. Testing of a Hydrogen Diffusion Flame Array Injector at Gas Turbine Conditions. Combust. Sci. Technol. 2013, 185, 1132–1150. [Google Scholar] [CrossRef]
- Devriese, C.; Pennings, W.; de Reuver, H.; Penninx, G.; de Ruiter, G.; Bastiaans, R.; De Paepe, W. The Design and Optimisation of a Hydrogen Combustor for a 100 Kw Micro Gas Turbine, Paper ID Number: 23-IGTC21, Gas turbines in a carbon-neutral society. In Proceedings of the 10th International Gas Turbine Conference, ETN, Brussels, Belgium, 11–15 October 2021. [Google Scholar]
- Hussain, M.; Abdelhafez, A.; Nemitallah, M.A.; Araoye, A.A.; Mansour, R.B.; Habib, M.A. A highly diluted oxy-fuel micromixer combustor with hydrogen enrichment for enhancing turndown in gas turbines. Appl. Energy 2020, 279, 115818. [Google Scholar] [CrossRef]
- Tekin, N.; Horikawa, T.A.; Ashikaga, M.; Funke, H. Kawasaki Hydrogen Road–Development of Innovative Hydrogen Combustion Systems for Industrial Gas Turbines, Paper ID Number: 2-IGTC21, Gas turbines in a carbon-neutral society. In Proceedings of the 10th International Gas Turbine Conference, ETN, Brussels, Belgium, 11–15 October 2021. [Google Scholar]
- Ayed, A.H.; Kusterer, K.; Funke, H.H.W.; Keinz, J.; Bohn, D. CFD Based Exploration of the Dry-Low-NOx Hydrogen Micromix Combustion Technology at Increased Energy Densities. Propuls. Power Res. 2017, 6, 15–24. [Google Scholar] [CrossRef]
- Marek, C.J.; Smith, T.D.; Kundu, K. Low Emission Hydrogen Combustors for Gas Turbines Using Lean Direct Injection, AIAA–2005–3776. In Proceedings of the 41st AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Tucson, AZ, USA, 10–13 July 2005. [Google Scholar]
- Holdeman, J.D.; Smith, T.D.; Clisset, J.R.; Lear, W.E. A spreadsheet for the Mixing of a Row of Jets with a Confined Crossflow, NASA/TM–2005-213137. Available online: http://gltrs.grc.nasa.gov (accessed on 16 August 2023).
- Börner, S.; Funke, H.; Hendrick, P.; Recker, E. LES of Jets In Cross-Flow and Application to the “Micromix” Hydrogen Combustion. In Proceedings of the XIX International Symposium on Air Breathing Engines, Montreal, QC, Canada, 7–11 September 2009. [Google Scholar]
- Dodo, S.; Asai, T.; Koizumi, H.; Takahashi, H.; Yoshida, S.; Inoue, H. Combustion Characteristics of a Multiple-Injection Combustor for Dry Low-NOx Combustion of Hydrogen-Rich Fuels Under Medium Pressure. In Proceedings of the ASME 2011 Turbo Expo: Turbine Technical Conference and Exposition, Vancouver, BC, Canada, 6–10 June 2011; pp. 467–476. [Google Scholar]
- Funke, H.H.W.; Dickhoff, J.; Keinz, J.; Haj Ayed, A.; Parente, A.; Hendrick, P. Experimental and Numerical Study of the Micromix Combustion Principle Applied for Hydrogen and Hydrogen Rich Syngas as Fuel with Increased Energy Density for Industrial Gas Turbine Applications. Energy Procedia 2014, 61, 1736–1739. [Google Scholar] [CrossRef]
- Funke, H.H.W.; Keinz, J.; Kusterer, K.; Haj Ayed, A.; Kazari, M.; Kitajima, J.; Horikawa, A.; Okada, K. Experimental and Numerical Study on Optimizing the DLN Micromix Hydrogen Combustion Principle for Industrial Gas Turbine Applications. In Proceedings of the ASME Turbo Expo 2015: Turbine Technical Conference and Exposition, Montreal, QC, Canada, 15–19 June 2015. [Google Scholar]
- York, W.D.; Ziminsky, W.S.; Yilmaz, E. Development and Testing of a Low NOx Hydrogen Combustion System for Heavy-Duty Gas Turbines. J. Eng. Gas Turbines Power 2013, 135, 1–8. [Google Scholar] [CrossRef]
- Asai, T.; Miura, K.; Matsubara, Y.; Akiyama, Y.; Karishuku, M.; Dodo, S.; Okazaki, T.; Tanimura, S. Development Of Gas Turbine Combustors For Fuel Flexibility. In Proceedings of the 8th International Gas Turbine Conference, the Future of Gas Turbine Technology, Brussels, Belgium, 12–13 October 2016. [Google Scholar]
- Asai, T.; Akiyama, Y.; Yonoki, K.; Horii, N.; Miura, K.; Karishuku, M.; Dodo, S. Development Of Fuel-Flexible Gas Turbine Combustor. In Proceedings of the 45th Turbomachinery & 32ND Pump Symposia, Houston, TX, USA, 13–15 September 2016. [Google Scholar]
- Ditaranto, M.; Heggset, T.; Berstad, D. Concept of hydrogen fired gas turbine cycle with exhaust gas recirculation: Assessment of process performance. Energy 2020, 192, 116646. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakao, M.; Ito, E.; Nose, M.; Saitoh, K.; Tsukagoshi, K. Development of Low NOx Combustion System with EGR for 1700 °C-class Gas Turbine. MITSUBISHI-HITACHI Heavy Ind. Tech. Rev. 2013, 50, 1–6. [Google Scholar]
- Rao, A.; Wu, Z.; Mehra, R.K.; Duan, H.; Ma, F. Effect of hydrogen addition on combustion, performance and emission of stoichiometric compressed natural gas fueled internal combustion engine along with exhaust gas recirculation at low, half and high load conditions. Fuel 2021, 304, 121358. [Google Scholar] [CrossRef]
- Hunicz, J.; Mikulski, M.; Shukla, P.C. Partially premixed combustion of hydrotreated vegetable oil in a diesel engine: Sensitivity to boost and exhaust gas recirculation. Fuel 2021, 307, 121910. [Google Scholar] [CrossRef]
- Gong, C.; Si, X.; Liu, F. Combustion and emissions behaviors of a stoichiometric GDI engine with simulated EGR (CO2) at low load and different spark timings. Fuel 2021, 295, 120614. [Google Scholar] [CrossRef]
- Gong, C.; Si, X.; Wang, Y.; Liu, F. Effect of CO2 dilution on combustion and emissions of a GDI engine under the peak NOX generation mixture. Fuel 2021, 295, 120613. [Google Scholar] [CrossRef]
- Wang, S.; Zhai, Y.; Wang, Z.; Hou, R.; Zhang, T.; Ji, C. Comparison of air and EGR with different water fractions dilutions on the combustion of hydrogen-air mixtures. Fuel 2022, 324, 124686. [Google Scholar] [CrossRef]
- Finney, K.N.; De Santis, A.; Best, T.; Clements, A.G.; Diego, M.E.; Porkashanian, M. Exhaust Gas Recycling for Enhanced CO2 Capture: Experimental and CFD Studies on a Micro-Gas Turbine, Industrial Combustion. J. Int. Flame Res. Found. 2016, 201610. [Google Scholar]
- Li, H.L.; Ditaranto, M.; Berstad, D. Technologies for increasing CO2 concentration in exhaust gas from natural gas-fired power production with postcombustion, amine-based CO2 capture. Energy 2011, 36, 1124–1133. [Google Scholar] [CrossRef]
- Calvert, J.; MacKenzie, K. Start-Up and Shut-Down Times of Power CCUS Facilities, Department for Business, Energy & Industrial Strategy; BEIS Research Paper Number 2020/031; AECOM Limited: Dallas, TX, USA, 2020. [Google Scholar]
- Sundkvist, S.G.; Dahlquist, A.; Janczewski, J.; Sjodin, M.; Bysveen, M.; Ditaranto, M. Concept for a combustion system in oxyfuel gas turbine combined cycles. J. Eng. Gas Turbines Power 2014, 136, 101513. [Google Scholar] [CrossRef]
- Ditaranto, M.; Li, H.L.; Lovas, T. Concept of hydrogen fired gas turbine cycle with exhaust gas recirculation: Assessment of combustion and emissions performance. Int. J. Greenh. Gas Control 2015, 37, 377–383. [Google Scholar] [CrossRef]
- Wrinkler, D.; Muller, P.; Reimer, S.; Griffin, T.; Burdet, A.; Mantzaras, J.; Ghermay, Y. Improvement of gas turbine combustion reactivity under flue gas recirculation condition with in-situ hydrogen addition. In Proceedings of the ASME Turbo Expo 2009: Power for Land, Sea and Air GT 2009, Orlando, FL, USA, 8–12 June 2009. [Google Scholar]

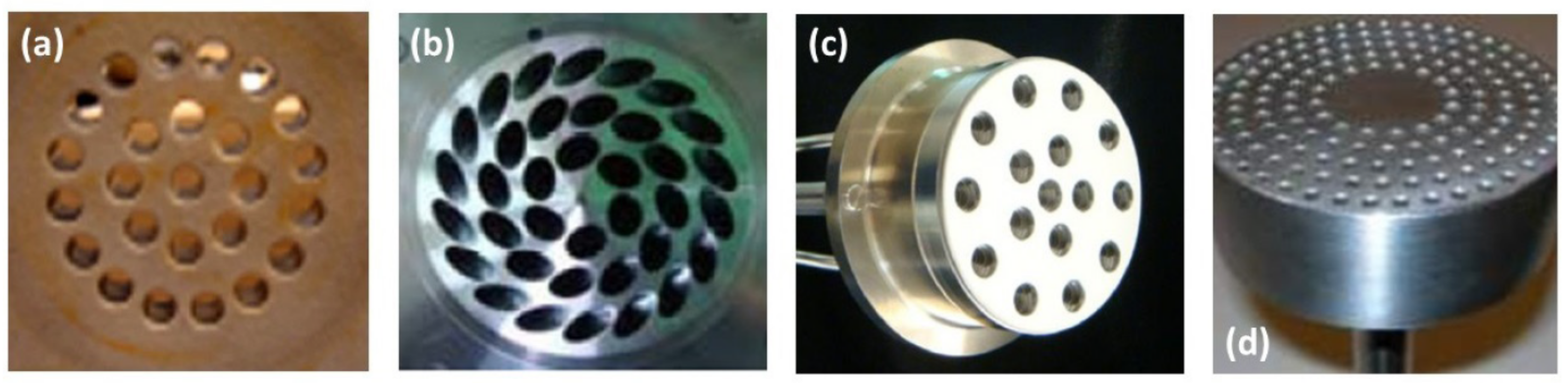
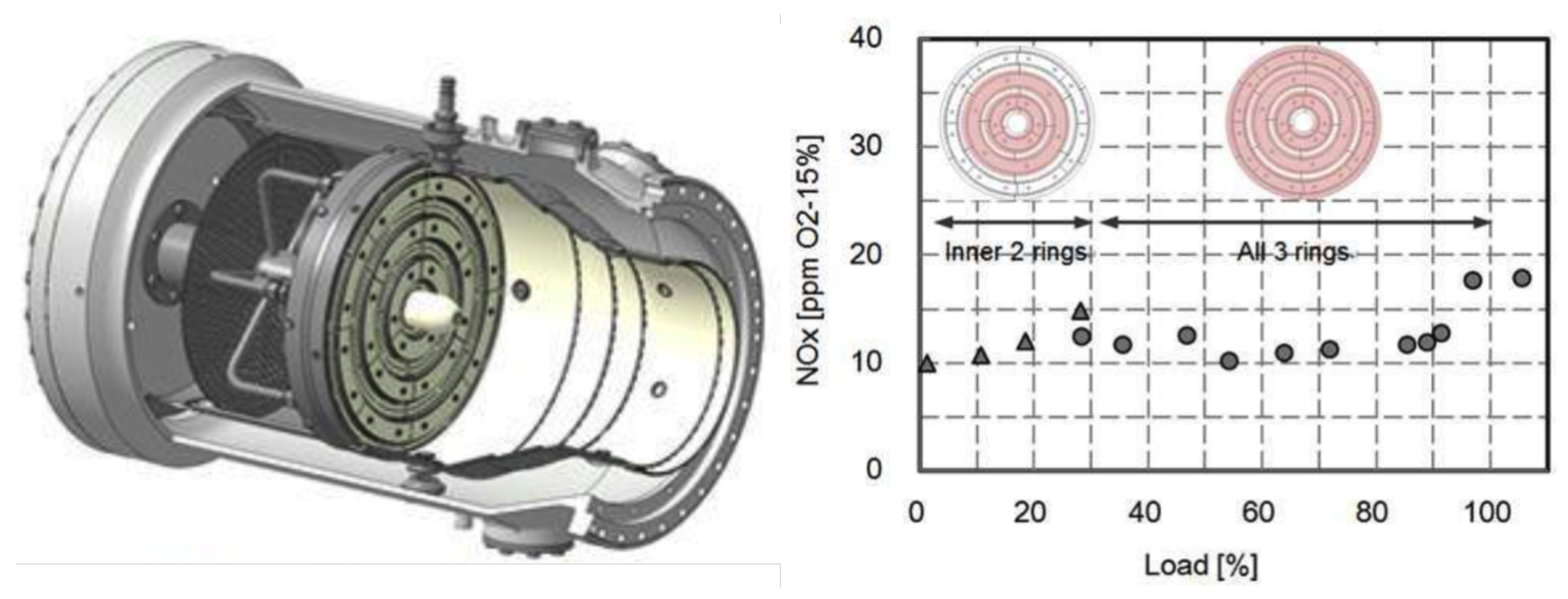

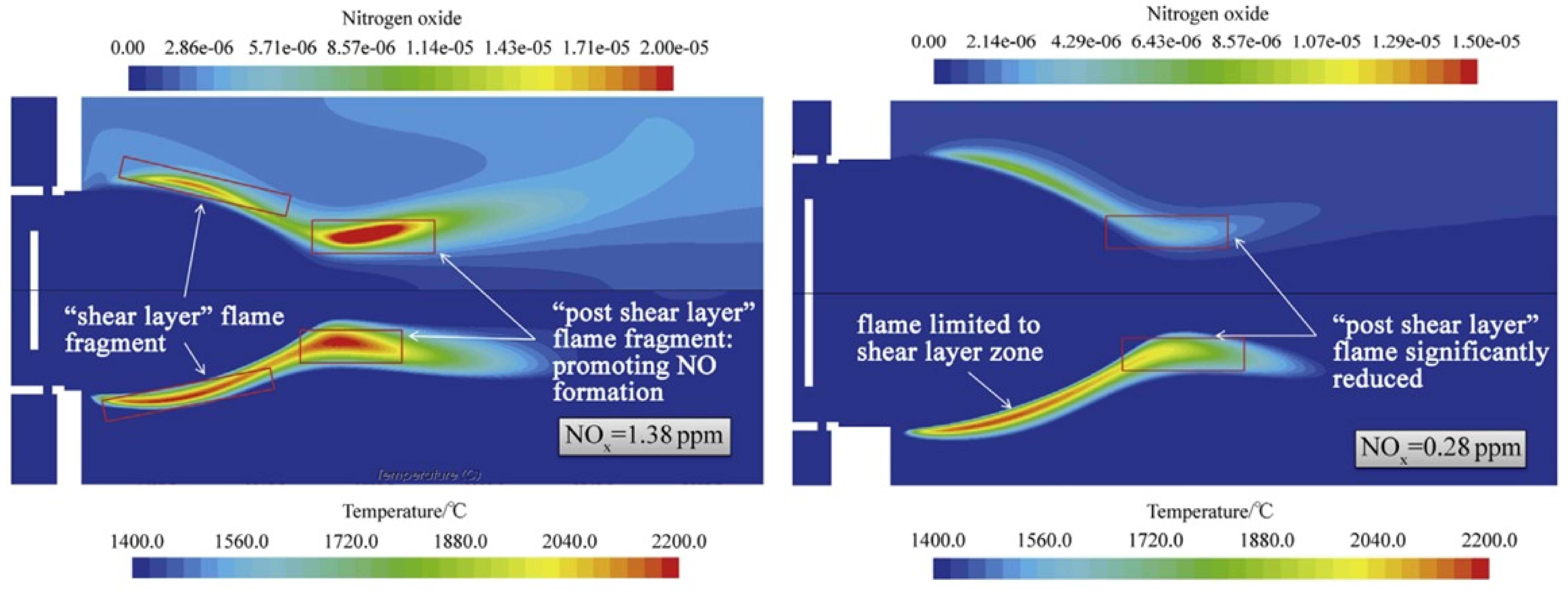
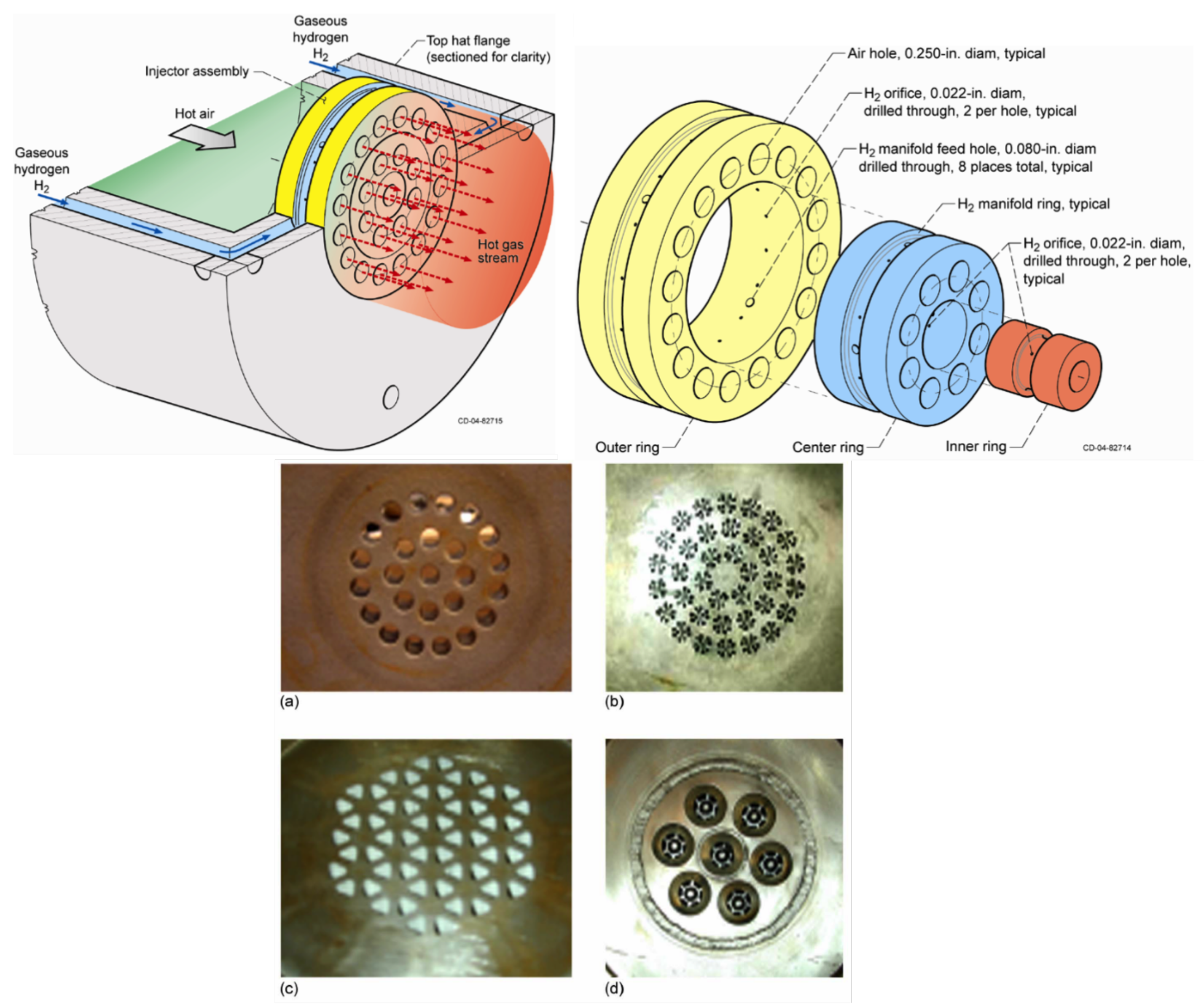
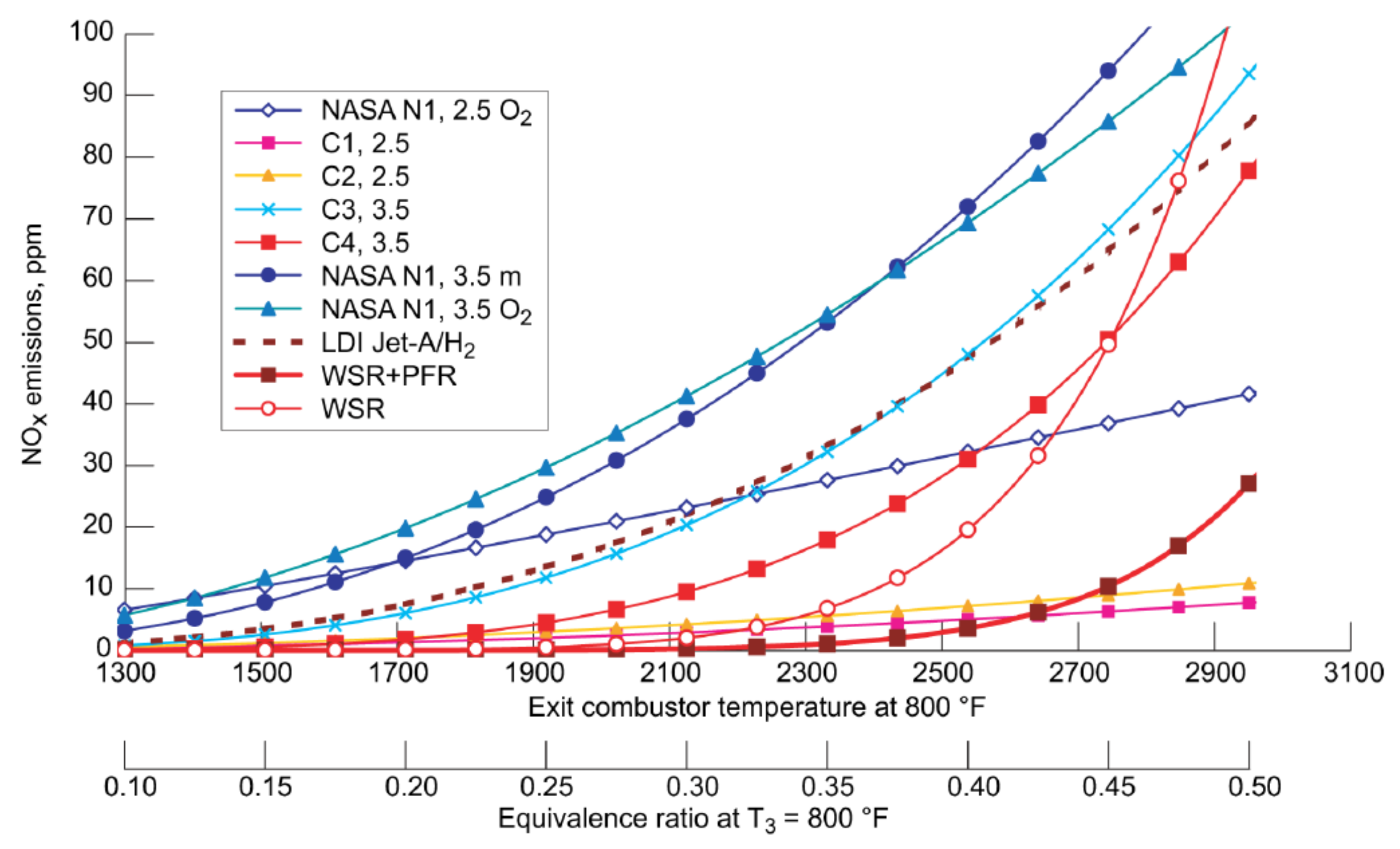
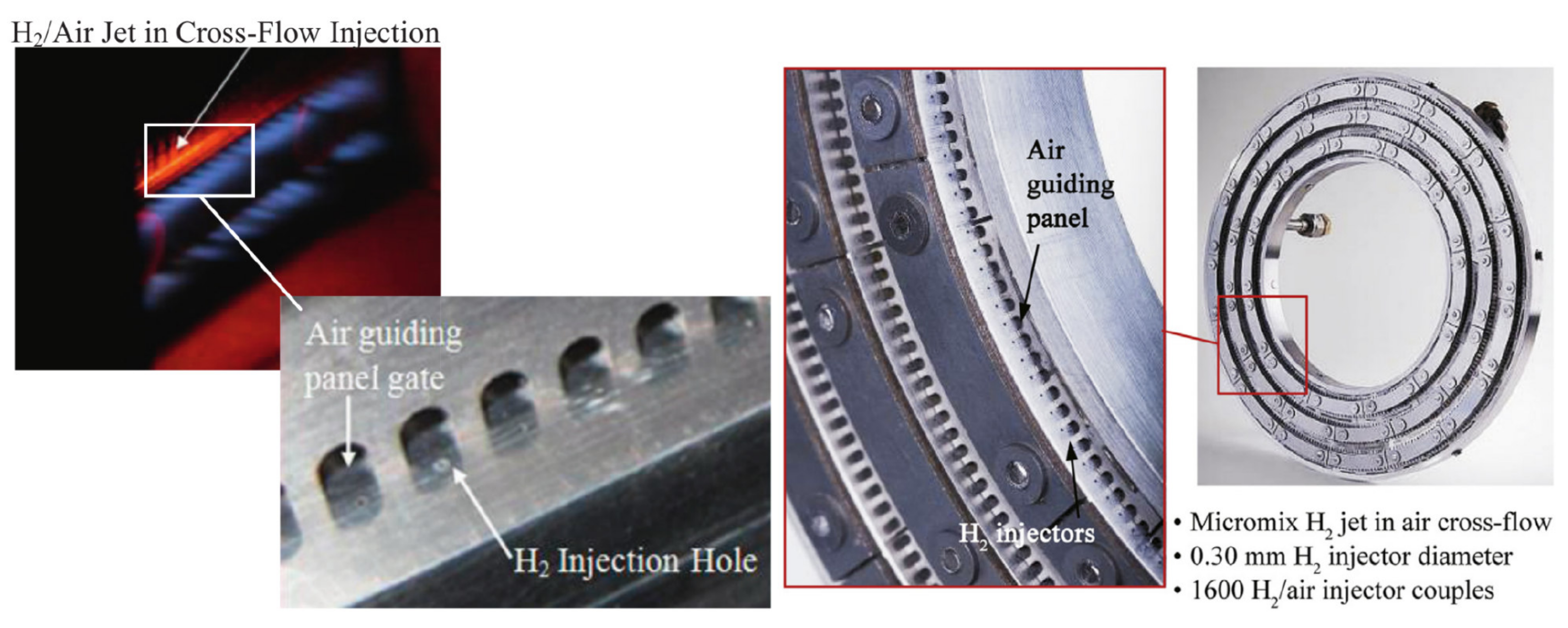
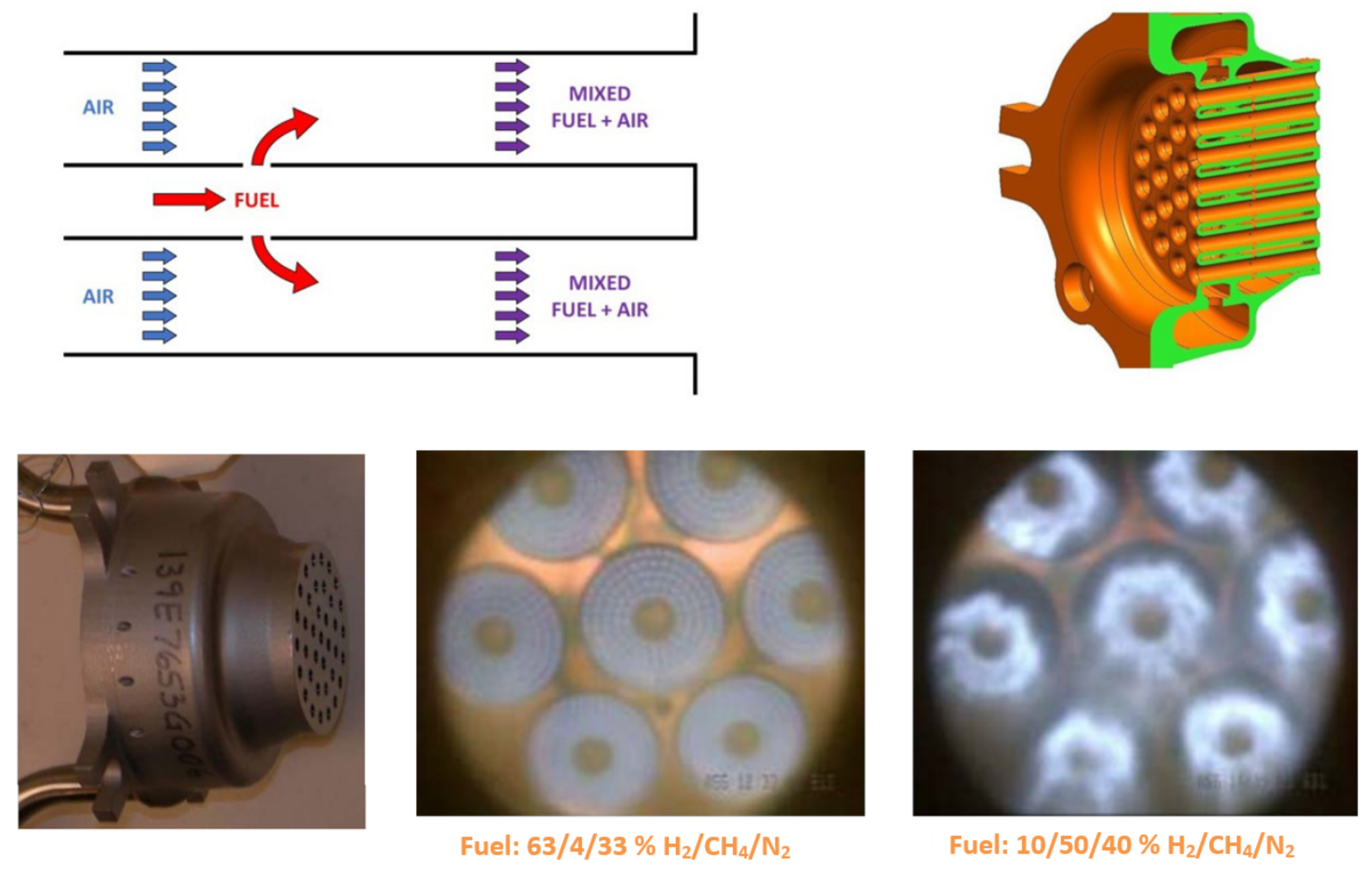
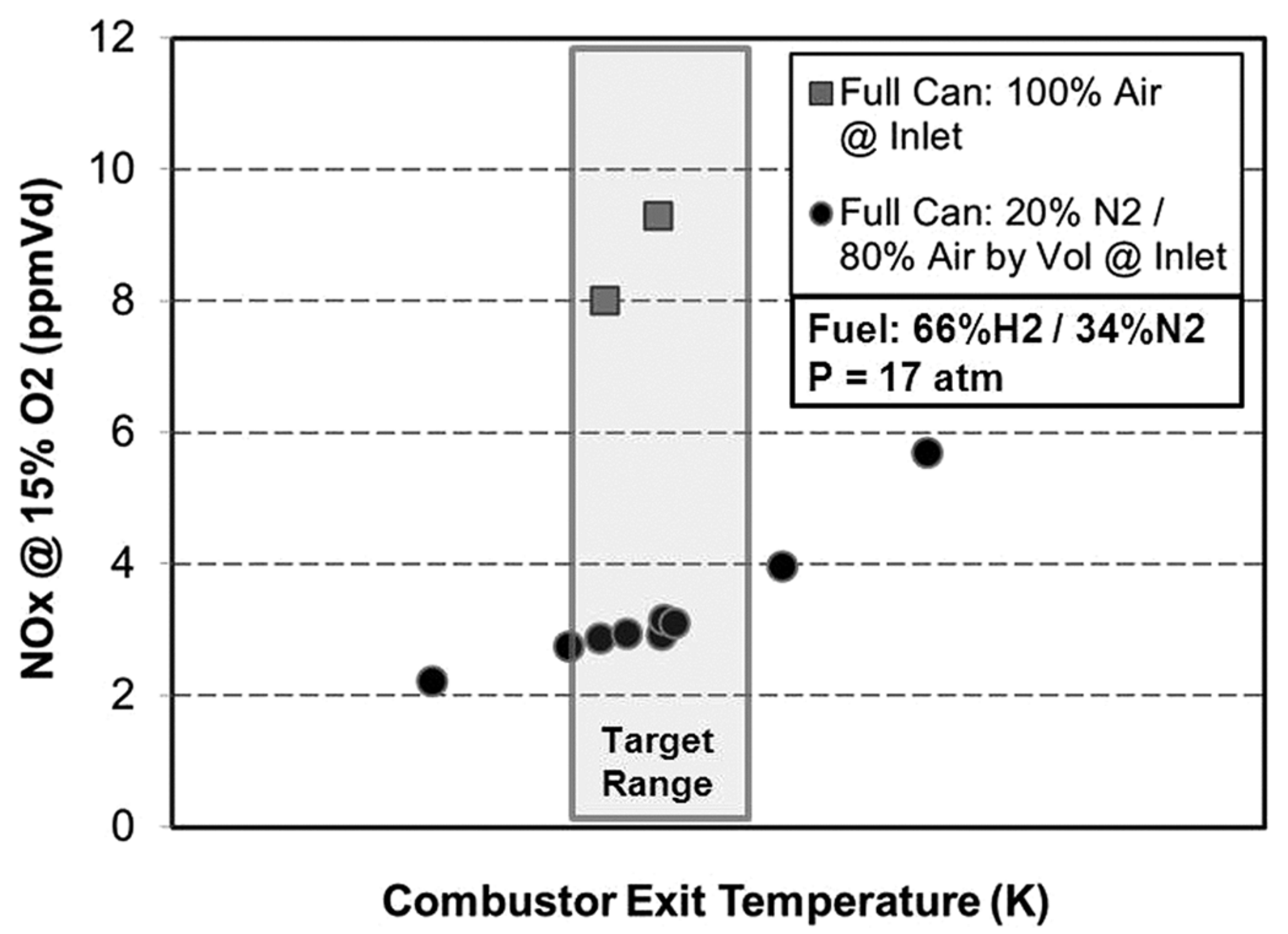
| Combustion | NO | Complexity | CAPEX/OPEX | |
|---|---|---|---|---|
| Diffusive/diluted | 0–100% | Higher | Higher | Higher |
| Dry Low Emission (DLE) Lean Premixed Combustion | 44–63% heavy duty (100–500 MWe) 43–55% industrial (30–100 MWe) 35% aero-derivative (1–30 MWe) 20–32 microturbine (0.1–1 MWe) | - | - | - |
| KPI | Unit | SoA 2020 | Target 2024 | Target 2030 |
|---|---|---|---|---|
| fuel content | % by mass | 0–5 | 0–23 | 0–100 |
| % by volume | 0–30 | 0–70 | 0–100 | |
| emissions | ppmv at 15% dry | <25 at 30% vol. | <25 at 70% vol. | <25 at 100% vol. |
| <31 at 30% vol. | <29 at 70% vol. | <24 at 100% vol. | ||
| Max. content at start-up | % by mass | 0.7 | 3 | 100 |
| % by volume | 5 | 20 | 100 | |
| Max. electrical efficiency loss | % points | 10 at 30% vol. | 10 at 70% vol. | 10 at 100% vol. |
| Min. ramp rate | % load/minute | 10 at 30% vol. | 10 at 70% vol. | 10 at 100% vol. |
| accepted fluctuations | % by mass/minute | |||
| % by volume/minute |
| - | Jet-A | |||
|---|---|---|---|---|
| 2.016 | 16.04 | 44.097 | ∼168 | |
| 0.0838 | 0.6512 | 1.87 | 775–840 | |
| 845–858 | 813–905 | 760–766 | 483 | |
| 0.02 | 0.29–0.33 | 0.26–0.305 | 20 | |
| 4–75 | 5–15 | 2.1–10 | 0.6–7 | |
| 0.1–7.1 | 0.4–1.6 | 0.56–2.7 | - | |
| 29.53 | 9.48 | 4.02 | 15 | |
| 2318–2400 | 2158–2226 | 2198–2267 | 2366 | |
| 118.8–120.3 | 50 | 46.35 | 43 | |
| 141.75 | 55.5 | 50.4 | 46.2 | |
| 10.78 | 35.8 | 91.21 | - | |
| 12.75 | 39.72 | 99.03 | - | |
| 40.7 | 47.94 | 73.3 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecere, D.; Giacomazzi, E.; Di Nardo, A.; Calchetti, G. Gas Turbine Combustion Technologies for Hydrogen Blends. Energies 2023, 16, 6829. https://doi.org/10.3390/en16196829
Cecere D, Giacomazzi E, Di Nardo A, Calchetti G. Gas Turbine Combustion Technologies for Hydrogen Blends. Energies. 2023; 16(19):6829. https://doi.org/10.3390/en16196829
Chicago/Turabian StyleCecere, Donato, Eugenio Giacomazzi, Antonio Di Nardo, and Giorgio Calchetti. 2023. "Gas Turbine Combustion Technologies for Hydrogen Blends" Energies 16, no. 19: 6829. https://doi.org/10.3390/en16196829
APA StyleCecere, D., Giacomazzi, E., Di Nardo, A., & Calchetti, G. (2023). Gas Turbine Combustion Technologies for Hydrogen Blends. Energies, 16(19), 6829. https://doi.org/10.3390/en16196829